Introduction
Currently, there is an array of established aging clocks that incorporate various factors such as epigenetic modifications, proteomic changes, inflammatory and immune pathways, neuroimaging techniques, and alterations related to the microbiome. These aging clocks possess significant therapeutic potential for alleviating the effects of chronic inflammation and associated diseases (Min et al., 2024).
Moreover, comprehensive multidisciplinary scoping reviews pertaining to the nitric oxide (NO)-mediated hypovascularity hypoxia hypothesis may shed light on the interconnected nature of biological aging clocks by integrating reductionist, holistic, and systems biology approaches. These longitudinal biological investigations are essential for elucidating persistent changes and big picture causal relationship patterns that underlie the primary biological mechanisms driving aging clocks (Phua, 2021; Phua, 2023; Phua, 2024).
Reductionist, holistic and system biology
Reductionism is characterized by the breakdown of systems into their constituent parts to analyze individual functions, whereas holism underscores the interconnectedness and emergent properties of the system as a whole (Gatherer, 2010). As the landscape of scientific inquiry progresses, there is an increasing acknowledgment of the necessity for holistic approaches that recognize the importance of intricate interactions and emergent attributes (Delker and Mann, 2017).
Furthermore, the focus of systems-based geroscience is to accelerate research into the drivers of biological mechanisms that underlie aging, with the aim of developing improved clinical interventions for diseases and chronic conditions commonly experienced by the elderly population (Sierra, 2016; de Magalhães, 2024).
Various medical approaches to the management of chronic diseases
Temporal medicine, which is inherently time-centric, examines the dynamics of disease progression over time, the effects of treatments at various stages of a patient’s illness, and the influence of intervention timing on patient health outcomes (Choudhary and Fränti, 2023; Rodenkirchen et al., 2025).
Integrative medicine combines established medical practices with evidence-based complementary therapies and lifestyle modifications to improve overall health and wellbeing. This holistic approach is particularly effective in preventing chronic diseases and managing existing conditions (Teut and Ortiz, 2021).
The future trajectory of precision geromedicine encompasses proactive, preventive, and interceptive strategies aimed at enhancing the healthspan (Amalaraj et al., 2025; Kroemer et al., 2025).
The human biological aging clock
The concept of the biological aging clock posits that aging is an orderly and predictable process governed by intrinsic biological mechanisms, rather than merely a reflection of chronological age. This perspective indicates that aging adheres to a defined program, characterized by measurable markers that indicate an organism’s age and its proximity to mortality (Palmer, 2022).
In addition, the field of OMICS—which encompasses the comprehensive analysis and interpretation of multi-omics data (large datasets) representing the structure and function of biological systems at various levels—has significantly transformed our approaches to studying biological systems (Ahmed, 2022; Chen et al., 2023). This paradigm shift includes “top-down” methodologies, greatly influenced by the advancements in OMICS, integrated with “bottom-up” strategies, thereby providing a comprehensive toolkit to facilitate effective biological system investigations (Dai and Shen, 2022).
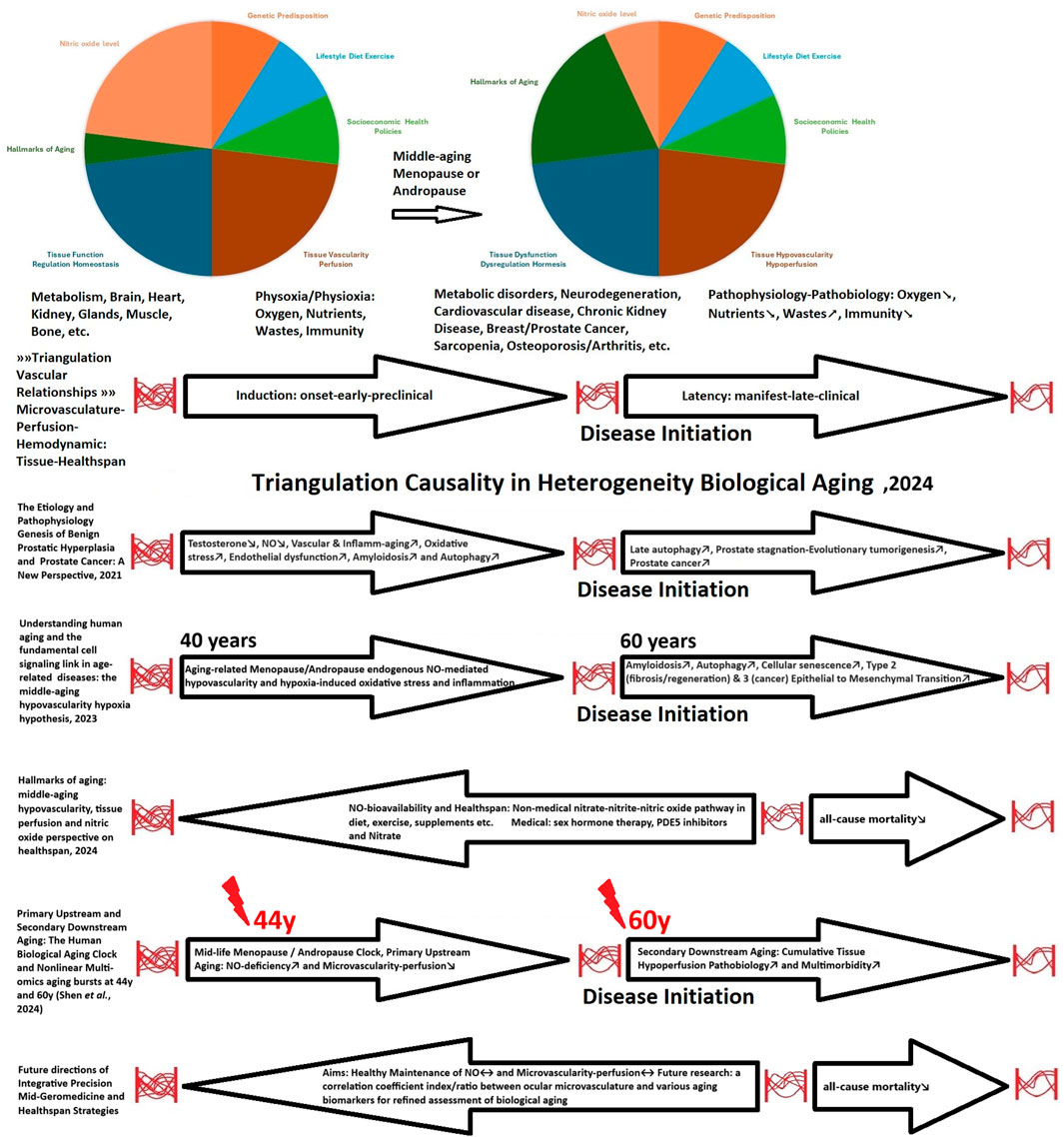
The emergent characteristics of multi-omics datasets highlight a deeper understanding of the predictable biological and cellular dynamics linked to the mid-life hormonal clock associated with menopause and andropause. Furthermore, they highlight the primary aging-related NO deficiency; a critical gasotransmitter, and micro-angiopathy; characterized by reducing microvascularity–perfusion function. Additionally, these attributes reflect the secondary cumulative pathobiology of tissue hypoperfusion, which involves hypoxia and chronic inflammation (Figure 1) (Phua, 2021; Phua, 2023; Phua, 2024).
Healthspan strategies and precision middle-aged geromedicine: mid-geromedicine
The field of gerontology typically examines aging in individuals aged 65 and older (Lyu et al., 2024). It is important to note that the human biological aging clock is initiated in midlife, between the ages of 40 and 60 (Levine et al., 2016; Huang et al., 2025).
Consequently, public health prevention efforts should prioritize the middle-aged demographic as it presents the greatest opportunity for intervention at the upstream level. Woods et al. (2024); coined the term “precision middle-aged geromedicine—Mid-Geromedicine” (Figure 1).
The dynamic progression of aging (Ferrucci et al., 2020), particularly concerning early-stage (induction) microvascularity-perfusion networks, would demonstrate superior tissue functionality recovery in comparison to late-stage (latency) dysfunction, which is often compounded by cumulative pathobiological pathologies (Figure 1) (Phua, 2024). This transition dynamism (Yang et al., 2024) is linked to the positive outcomes associated with early sex hormone replacement therapies in alignment with the timing hypothesis (El Khoudary et al., 2020; Hodis and Mack, 2022), correlated with reductions in all-cause mortality during menopause (Arayici et al., 2024; Liu et al., 2024) and andropause (Jaiswal et al., 2024; Yeap et al., 2024), and increased production of NO (Jokela et al., 2003; Gur et al., 2020).
Furthermore, significant nonlinear multi-omics aging bursts are at observed at 44 and 60 years of age (Shen et al., 2024), coinciding with a decline in sex hormone levels and increasing inflammation (Ketchem et al., 2023; Allahverdiyeva et al., 2024; Lombardo et al., 2024) and the onset of increased multimorbidity in individuals aged 65 and older (Figure 1) (Divo et al., 2014; Song et al., 2023).
Empirical perspective of endogenous nitric oxide (NO) as a gasotransmitter in biological aging
NO serves as a crucial empirical link between aging-related sex hormones and the vascular-aging process within the microvascularity–perfusion mechanistic network, a concept integral to the human biological aging clock and its beneficial health effects (Figure1) (Phua, 2023; 2024). Experimental reduction in sex hormone levels leads to the development of micro-angiopathy, while the supplementation of sex hormones reverses this condition, promoting micro-angiogenesis (Yura et al., 2020; Wang et al., 2022). NO actively promotes angiogenesis (Zhang et al., 2023). However, as individuals age, there is a notable decrease in overall NO production (Siervo et al., 2024), accompanied by reduced blood vessel density and altered dynamics of endothelial cell populations, within the aging endocrine system (Chen J. et al., 2021).
A paradoxical relationship exists between hypertension and capillary rarefaction (Frost et al., 2021), which characterizes microvascularity–perfusion aging, with NO acting as a principal driver in this process (da Silva et al., 2021; Bryan, 2022). This relationship is particularly evident in the context of chronic diseases (Bryan et al., 2023). In China, nearly one in four adults experiences multimorbidity, with hypertensive conditions frequently co-occurring with other health issues (Hu et al., 2024). Longitudinal accelerated aging is associated with the incidence of circulatory diseases (vascular-aging), related chronic conditions, and both all-cause and cause-specific mortality (Liu et al., 2023). Furthermore, studies involving human biopsies, autopsies, and imaging revealed a 32% reduction in median microvascular density (hypovascularity) (Querfeld et al., 2020).
In a state of health, the NO-cyclic 3′–5′ guanosine–monophosphate signaling pathway is vital for regulating smooth muscle tone, platelet function, cardiac contractility, renal operation, fluid homeostasis, and cellular growth (Mónica et al., 2016). Dysregulation of NO signaling is a common feature across various significant disorders, including cardiovascular disease, diabetes, and cancer (Lundberg and Weitzberg, 2022).
Research findings underscore the independent role of gasotransmitters in mediating age-related physiological and pathological processes, encompassing diabetes (van den Born et al., 2016), immune responses (Fagone et al., 2018), mitochondrial function (Hendriks et al., 2019), fibrotic diseases (Chen Y. et al., 2021), cardiovascular protection (Pagliaro et al., 2024), inflammatory edema (Coavoy-Sanchez et al., 2024), and age-associated oxidative stress (Munteanu et al., 2025).
In the modern era, individuals are increasingly seeking straightforward and natural methods to enhance their wellbeing and support both physical and mental health. This trend includes participation in activities at gyms and specialized wellness studios, as well as the adoption of healthy dietary practices. Many of these lifestyle interventions, often referred to as bio-hacking, are associated with the production of NO. Activities such as exercise (Arefirad et al., 2022) and exposure to natural sunlight (Hazell et al., 2022) play a crucial role in this process. Furthermore, gym supplements like L-arginine/-citrulline (Kiani et al., 2022), along with super-foods such as beetroots (Zamani et al., 2021) and pumpkin seeds (Akomolafe et al., 2025), are recognized for their potential to boost NO levels. Specialized wellness centers that offer hyperbaric oxygen therapy (Yamamoto et al., 2020), cold therapy (Wiecek et al., 2021), and light therapy (Kashiwagi et al., 2024) also contribute to this focus on health. Healthy dietary practices encompass consuming dietary nitrates as in following a Mediterranean diet (Mohajeri and Cicero, 2023). Conversely, the consumption of ultra-processed foods has been linked to a decline in NO production (Babalola et al., 2025). This also encompasses the effects of environmental and climatic factors on the processes of physiological aging (Zhang et al., 2024).
Moreover, two genetic variants GCH1 (Guo et al., 2025) and EPAS1 (Li C. et al., 2019) have been identified that enhance the adaptability of Tibetans residing at high altitudes in low-oxygen environments. The particular variant, GCH1, is associated with reduced expression levels, leading to increased NO production and enhanced oxygen delivery. Additionally, evidence indicates that these highland communities experience lower mortality rates from circulatory diseases and cancer (Wander et al., 2020; Burtscher et al., 2021).
Integrative precision mid-geromedicine and prostate aging degeneration hypothesis
Integrative Precision Mid-Geromedicine leverages existing knowledge within its scientific domain to explore potential approaches for investigating pertinent issues or challenges, thereby providing deeper insights (McGregor and Frodsham, 2023).
The Prostate Aging Degeneration Hypothesis (Phua, 2021) predictable aging-related degeneration serves as a key illustration of mechanistic reasoning strategies aimed at addressing the underlying causes of symptoms (Flowers et al., 2023). Dysfunctions in prostate tissue remodeling result from alterations in smooth muscle function, prostate growth, enlargement, fibrosis, (Liu et al., 2019), and localized inflammation (Magri et al., 2019).
Benign prostatic hyperplasia (BPH/prostate enlargement) is a prevalent condition affecting approximately 50% of men over the age of 50, often accompanied by lower urinary tract symptoms (LUTS) (Haile et al., 2024), chronic pelvic pain syndrome (CPPS), chronic prostatitis (Pena et al., 2021), and incidence of prostate cancer exceeding 1 in 2 in men aged 65 and older (Rosario and Rosario, 2025).
The observation of abundant deposits of prostatic pro-inflammatory corpora amylacea (wasteosome-starch-like-bodies) (DuPre et al., 2018; Riba et al., 2022) is largely overlooked in conventional medicine. This neglect represents a research gap as the alternative explanations for BPH/enlargement-symptomology within a confined suprapubic space: the reduction in bladder capacity and urethral compression in LUTS and the pressure-related CPPS in the adjacent sensitive fascia (Gatt et al., 2025).
A hypoxic low-grade inflammation microenvironment is a characteristic feature of many tumors (Korbecki et al., 2021), largely resulting from inadequate vascular networks that fail to sufficiently supply oxygen (Alimoradi et al., 2016). To tackle this issue, the sex hormone bioavailability is essential for maintaining microvascularity–density health (Wang et al., 2022). However, testosterone therapy has been shown not to alleviate LUTS, but it does improve markers of prostatitis (anti-inflammatory) in men with BPH (Rastrelli et al., 2022). As such, a dual medical approach is warranted, one that addresses the aging-related NO-deficiency alongside complementary therapies such as regular prostatic drainage/massage to eliminate starch-like-wasteosomes (Sun and Bao, 2013), thereby mitigating prostatic size, pressure-related pain, and inflammation. This underscores the holistic dimension of integrative medicine and its potential role in the emerging field of Integrative-Precision-Mid-Geromedicine.
The eradication of localized chronic inflammation serves as a pivotal strategy in preventive medicine aimed at mitigating the risk of cervical cancer linked to human papillomavirus through vaccination initiatives (Jain et al., 2023). Similarly, this extends to the management of Helicobacter pylori infections and stomach cancer prevention (Li W.-Q. et al., 2019).
Importantly, testosterone and its reduced metabolites, 5α- and 5β-dihydrotestosterone, exert vasodilatory effects (Sánchez-Fernández et al., 2024). Testosterone has not been shown to increase the risk of prostate cancer (Siltari et al., 2023) and is anti-fibrotic (Chung et al., 2021). In fact, elevated testosterone levels are correlated with smaller prostate size (Xia et al., 2021). Conversely, the use of 5α-reductase inhibitors has been linked to an increased risk of acute coronary syndrome (Chou et al., 2015), sexual dysfunction (Corona et al., 2017), type-2 diabetes (Wei et al., 2019), age-related macular degeneration (Su et al., 2024), and suicide (Kim et al., 2025).
In a similar vein, phosphodiesterase-5 inhibitors restore NO-signaling (Lee et al., 2022) and exhibit anti-fibrotic (Li et al., 2024) and anti-inflammatory (Isidori et al., 2021) properties.
Discussion
The future trajectories of Integrative-Precision-Mid-Geromedicine hold the promise of substantially diminishing the incidence of chronic diseases, guiding us toward paradigms of preventive geromedicine (Thomas et al., 2023; Hoenders et al., 2024). This approach empowers individuals to take charge of their healthspan strategies through proactive health education programs, ultimately leading to reduced healthcare costs and enhanced sustainability within healthcare systems.
Author contributions
TP: Conceptualization, Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review and editing, Validation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views expressed are those of the author’s knowledge of the scientific background and not necessarily those of the Department of Health or the Institution.
References
Ahmed, Z. (2022). Precision medicine with multi-omics strategies, deep phenotyping, and predictive analysis. Prog. Mol. Biol. Transl. Sci. 190 (1), 101–125. doi:10.1016/bs.pmbts.2022.02.002
Akomolafe, S. F., Akinjiyan, M. O., Asogwa, N. T., and Elekofehinti, O. O. (2025). Evaluation of the protective effects of raw and roasted pumpkin (Cucurbita pepo L.) seed-supplemented diets on cisplatin-induced cardiotoxicity in rats. J. Mol. histology 56 (3), 157. doi:10.1007/s10735-025-10432-4
Alimoradi, H., Matikonda, S. S., Gamble, A. B., Giles, G. I., and Greish, K. (2016). Hypoxia responsive drug delivery systems in tumor therapy. Curr. Pharm. Des. 22 (19), 2808–2820. doi:10.2174/1381612822666160217130049
Allahverdiyeva, S., Geyer, C. E., Veth, J., de Vries, L. M., de Taeye, S. W., van Gils, M. J., et al. (2024). Testosterone and estradiol reduce inflammation of human macrophages induced by anti-SARS-CoV-2 IgG. Eur. J. Immunol. 54 (12), e2451226. doi:10.1002/eji.202451226
Amalaraj, J. J. P., Island, L., Ong, J. Y. Y., Wang, L., Valderas, J. H. M., Dunn, M., et al. (2025). Towards precision geromedicine in Singapore. GeroScience. doi:10.1007/s11357-025-01686-7
Arayici, M. E., Kilic, M. E., and Yilmaz, M. B. (2024). The impact of hormone replacement therapy on the risk of heart failure in postmenopausal women: a meta-analysis of clinical and observational studies. Pharmacoepidemiol. drug Saf. 33 (10), e70029. doi:10.1002/pds.70029
Arefirad, T., Seif, E., Sepidarkish, M., Mohammadian Khonsari, N., Mousavifar, S. A., Yazdani, S., et al. (2022). Effect of exercise training on nitric oxide and nitrate/nitrite (NOx) production: a systematic review and meta-analysis. Front. physiology 13, 953912. doi:10.3389/fphys.2022.953912
Babalola, O. O., Akinnusi, E., Ottu, P. O., Bridget, K., Oyubu, G., Ajiboye, S. A., et al. (2025). ‘The impact of ultra-processed foods on cardiovascular diseases and cancer: epidemiological and mechanistic insights’, Aspects Mol. Med., 5, p. 100072. doi:10.1016/j.amolm.2025.100072
Bryan, N. S. (2022). Nitric oxide deficiency is a primary driver of hypertension. Biochem. Pharmacol. 206, 115325. doi:10.1016/j.bcp.2022.115325
Bryan, N. S., Ahmed, S., Lefer, D. J., Hord, N., and von Schwarz, E. R. (2023). Dietary nitrate biochemistry and physiology. An update on clinical benefits and mechanisms of action. Nitric oxide Biol. Chem. 132, 1–7. doi:10.1016/j.niox.2023.01.003
Burtscher, J., Millet, G. P., and Burtscher, M. (2021). Does living at moderate altitudes in Austria affect mortality rates of various causes? An ecological study. BMJ open 11 (6), e048520. doi:10.1136/bmjopen-2020-048520
Chen, J., Lippo, L., Labella, R., Tan, S. L., Marsden, B. D., Dustin, M. L., et al. (2021). Decreased blood vessel density and endothelial cell subset dynamics during ageing of the endocrine system. EMBO J. 40 (1), e105242. doi:10.15252/embj.2020105242
Chen, Y., Yuan, S., Cao, Y., Kong, G., Jiang, F., Li, Y., et al. (2021). Gasotransmitters: potential therapeutic molecules of fibrotic diseases. Oxidative Med. Cell. Longev. 2021, 3206982. doi:10.1155/2021/3206982
Chen, C., Wang, J., Pan, D., Wang, X., Xu, Y., Yan, J., et al. (2023). Applications of multi-omics analysis in human diseases. MedComm 4 (4), e315. doi:10.1002/mco2.315
Chou, C.-H., Lin, M. C., Sung, F. C., and Kao, C. H. (2015). 5α-Reductase inhibitors increase acute coronary syndrome risk in patients with benign prostate hyperplasia. J. Endocrinol. investigation 38 (7), 799–805. doi:10.1007/s40618-015-0263-1
Choudhary, G. I., and Fränti, P. (2023). Predicting onset of disease progression using temporal disease occurrence networks. Int. J. Med. Inf. 175, 105068. doi:10.1016/j.ijmedinf.2023.105068
Chung, C.-C., Lin, Y. K., Kao, Y. H., Lin, S. H., and Chen, Y. J. (2021). Physiological testosterone attenuates profibrotic activities of rat cardiac fibroblasts through modulation of nitric oxide and calcium homeostasis. Endocr. J. 68 (3), 307–315. doi:10.1507/endocrj.EJ20-0344
Coavoy-Sanchez, S. A., da Costa Marques, L. A., Costa, S. K. P., and Muscara, M. N. (2024). Role of gasotransmitters in inflammatory edema. Antioxidants and redox Signal. 40 (4–6), 272–291. doi:10.1089/ars.2022.0089
Corona, G., Tirabassi, G., Santi, D., Maseroli, E., Gacci, M., Dicuio, M., et al. (2017). Sexual dysfunction in subjects treated with inhibitors of 5α-reductase for benign prostatic hyperplasia: a comprehensive review and meta-analysis. Andrology 5 (4), 671–678. doi:10.1111/andr.12353
da Silva, G. M., da Silva, M. C., Nascimento, D. V. G., Lima Silva, E. M., Gouvêa, F. F. F., de França Lopes, L. G., et al. (2021). Nitric oxide as a central molecule in hypertension: focus on the vasorelaxant activity of new nitric oxide donors. Biology 10 (10), 1041. doi:10.3390/biology10101041
Dai, X., and Shen, L. (2022). Advances and trends in omics technology development. Front. Med. 9, 911861. doi:10.3389/fmed.2022.911861
de Magalhães, J. P. (2024). Distinguishing between driver and passenger mechanisms of aging. Nat. Genet. 56 (2), 204–211. doi:10.1038/s41588-023-01627-0
Delker, R. K., and Mann, R. S. (2017). From reductionism to holism: toward a more complete view of development through genome engineering. Adv. Exp. Med. Biol. 1016, 45–74. doi:10.1007/978-3-319-63904-8_3
Divo, M. J., Martinez, C. H., and Mannino, D. M. (2014). Ageing and the epidemiology of multimorbidity. Eur. Respir. J. 44 (4), 1055–1068. doi:10.1183/09031936.00059814
DuPre, N. C., Flavin, R., Sfanos, K. S., Unger, R. H., To, S., Gazeeva, E., et al. (2018). Corpora amylacea in prostatectomy tissue and associations with molecular, histological, and lifestyle factors. Prostate 78 (15), 1172–1180. doi:10.1002/pros.23692
El Khoudary, S. R., Aggarwal, B., Beckie, T. M., Hodis, H. N., Johnson, A. E., Langer, R. D., et al. (2020). Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American heart association. Circulation 142 (25), e506–e532. doi:10.1161/CIR.0000000000000912
Fagone, P., Mazzon, E., Bramanti, P., Bendtzen, K., and Nicoletti, F. (2018). Gasotransmitters and the immune system: mode of action and novel therapeutic targets. Eur. J. Pharmacol. 834, 92–102. doi:10.1016/j.ejphar.2018.07.026
Ferrucci, L., Gonzalez-Freire, M., Fabbri, E., Simonsick, E., Tanaka, T., Moore, Z., et al. (2020). Measuring biological aging in humans: a quest. Aging cell 19 (2), e13080. doi:10.1111/acel.13080
Flowers, S., Holder, K. H., Rump, G. K., and Gardner, S. M. (2023). Missed connections: exploring features of undergraduate biology students’ knowledge networks relating gene regulation, cell-cell communication, and phenotypic expression. CBE life Sci. Educ. 22 (4), ar44. doi:10.1187/cbe.22-03-0041
Frost, S., Nolde, J. M., Chan, J., Joyson, A., Gregory, C., Carnagarin, R., et al. (2021). Retinal capillary rarefaction is associated with arterial and kidney damage in hypertension. Sci. Rep. 11 (1), 1001. doi:10.1038/s41598-020-79594-3
Gatherer, D. (2010). So what do we really mean when we say that systems biology is holistic? BMC Syst. Biol. 4, 22. doi:10.1186/1752-0509-4-22
Guo, Y., Zheng, W., Yue, T., Baimakangzhuo, , Qi, X., Liu, K., et al. (2025). GCH1 contributes to high-altitude adaptation in tibetans by regulating blood nitric oxide. J. Genet. genomics = Yi chuan xue bao. doi:10.1016/j.jgg.2025.04.005
Gur, S., Alzweri, L., Yilmaz-Oral, D., Kaya-Sezginer, E., Abdel-Mageed, A. B., Dick, B., et al. (2020). Testosterone positively regulates functional responses and nitric oxide expression in the isolated human corpus cavernosum. Andrology 8, 1824–1833. doi:10.1111/andr.12866
Haile, E. S., Sotimehin, A. E., and Gill, B. C. (2024). Medical management of benign prostatic hyperplasia. Clevel. Clin. J. Med. 91 (3), 163–170. doi:10.3949/ccjm.91a.23027
Hazell, G., Khazova, M., Cohen, H., Felton, S., and Raj, K. (2022). Post-exposure persistence of nitric oxide upregulation in skin cells irradiated by UV-A. Sci. Rep. 12 (1), 9465. doi:10.1038/s41598-022-13399-4
Hendriks, K. D., Maassen, H., van Dijk, P. R., Henning, R. H., van Goor, H., and Hillebrands, J. L. (2019). Gasotransmitters in health and disease: a mitochondria-centered view. Curr. Opin. Pharmacol. 45, 87–93. doi:10.1016/j.coph.2019.07.001
Hodis, H. N., and Mack, W. J. (2022). Menopausal hormone replacement therapy and reduction of all-cause mortality and cardiovascular disease: it is about time and timing. Cancer J. (Sudbury, Mass.) 28 (3), 208–223. doi:10.1097/PPO.0000000000000591
Hoenders, R., Ghelman, R., Portella, C., Simmons, S., Locke, A., Cramer, H., et al. (2024). A review of the WHO strategy on traditional, complementary, and integrative medicine from the perspective of academic consortia for integrative medicine and health. Front. Med. 11, 1395698. doi:10.3389/fmed.2024.1395698
Hu, Y., Wang, Z., He, H., Pan, L., Tu, J., and Shan, G. (2024). Prevalence and patterns of multimorbidity in China during 2002-2022: a systematic review and meta-analysis. Ageing Res. Rev. 93, 102165. doi:10.1016/j.arr.2023.102165
Huang, W., Deng, L., Wen, Q., Zhang, Z., Yue, J., Zhang, C., et al. (2025). Dynamics of serum testosterone and biological aging in men: insights from Chinese, American, and British populations. EClinicalMedicine 82, 103178. doi:10.1016/j.eclinm.2025.103178
Isidori, A. M., Giannetta, E., Pofi, R., Venneri, M. A., Gianfrilli, D., Campolo, F., et al. (2021). Targeting the NO-cGMP-PDE5 pathway in COVID-19 infection. The DEDALO project. Andrology 9 (1), 33–38. doi:10.1111/andr.12837
Jain, M., Yadav, D., Jarouliya, U., Chavda, V., Yadav, A. K., Chaurasia, B., et al. (2023). Epidemiology, molecular pathogenesis, immuno-pathogenesis, immune escape mechanisms and vaccine evaluation for HPV-associated carcinogenesis. Pathog. Basel, Switz. 12 (12), 1380. doi:10.3390/pathogens12121380
Jaiswal, V., Sawhney, A., Nebuwa, C., Borra, V., Deb, N., Halder, A., et al. (2024). Association between testosterone replacement therapy and cardiovascular outcomes: a meta-analysis of 30 randomized controlled trials. Prog. Cardiovasc. Dis. 85, 45–53. doi:10.1016/j.pcad.2024.04.001
Jokela, H., Dastidar, P., Rontu, R., Salomäki, A., Teisala, K., Lehtimäki, T., et al. (2003). Effects of long-term estrogen replacement therapy versus combined hormone replacement therapy on nitric oxide-dependent vasomotor function. J. Clin. Endocrinol. metabolism 88 (9), 4348–4354. doi:10.1210/jc.2003-030029
Kashiwagi, S., Yokomizo, S., Bragin, D. E., Perle, S. J., Kastanenka, K. V., Gerashchenko, D., et al. (2024). Therapeutic potentials of near-infrared II photobiomodulation to treat cerebrovascular diseases via nitric oxide signalling. Adv. Exp. Med. Biol. 1463, 195–200. doi:10.1007/978-3-031-67458-7_33
Ketchem, J. M., Bowman, E. J., and Isales, C. M. (2023). Male sex hormones, aging, and inflammation. Biogerontology 24 (1), 1–25. doi:10.1007/s10522-022-10002-1
Kiani, A. K., Bonetti, G., Medori, M. C., Caruso, P., Manganotti, P., Fioretti, F., et al. (2022). Dietary supplements for improving nitric-oxide synthesis. J. Prev. Med. Hyg. 63 (2 Suppl. 3), E239–E245. doi:10.15167/2421-4248/jpmh2022.63.2S3.2766
Kim, J., Jang, S.-Y., and Park, E.-C. (2025). Differential association between cumulative dose of 5α-reductase inhibitors and mortality. Sci. Rep. 15 (1), 10962. doi:10.1038/s41598-025-95583-w
Korbecki, J., Simińska, D., Gąssowska-Dobrowolska, M., Listos, J., Gutowska, I., Chlubek, D., et al. (2021). Chronic and cycling hypoxia: drivers of cancer chronic inflammation through HIF-1 and NF-κB activation: a review of the molecular mechanisms. Int. J. Mol. Sci. 22 (19), 10701. doi:10.3390/ijms221910701
Kroemer, G., Maier, A. B., Cuervo, A. M., Gladyshev, V. N., Ferrucci, L., Gorbunova, V., et al. (2025). From geroscience to precision geromedicine: understanding and managing aging. Cell 188 (8), 2043–2062. doi:10.1016/j.cell.2025.03.011
Lee, M.-K., Lee, J. H., Sohn, S. Y., Lee, S. Y., Jeong, T. Y., and Kim, S. C. (2022). Effect of low-dose tadalafil once daily on glycemic control in patients with type 2 diabetes and erectile dysfunction: a randomized, double-blind, placebo-controlled pilot study. Diabetology and metabolic syndrome 14 (1), 56. doi:10.1186/s13098-022-00825-w
Levine, M. E., Lu, A. T., Chen, B. H., Hernandez, D. G., Singleton, A. B., Ferrucci, L., et al. (2016). Menopause accelerates biological aging. Proc. Natl. Acad. Sci. U. S. A. 113 (33), 9327–9332. doi:10.1073/pnas.1604558113
Li, C., Li, X., Xiao, J., Liu, J., Fan, X., Fan, F., et al. (2019). Genetic changes in the EPAS1 gene between Tibetan and Han ethnic groups and adaptation to the Plateau hypoxic environment. PeerJ 7, e7943. doi:10.7717/peerj.7943
Li, W.-Q., Zhang, J. Y., Ma, J. L., Li, Z. X., Zhang, L., Zhang, Y., et al. (2019). Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a randomized intervention trial. BMJ Clin. Res. ed. 366, l5016. doi:10.1136/bmj.l5016
Li, T., Zhang, Y., Zhou, Z., Guan, L., Zhang, Y., Zhou, Z., et al. (2024). Phosphodiesterase type 5 inhibitor tadalafil reduces prostatic fibrosis via MiR-3126-3p/FGF9 axis in benign prostatic hyperplasia. Biol. direct 19 (1), 61. doi:10.1186/s13062-024-00504-y
Liu, T. T., Thomas, S., Mclean, D. T., Roldan-Alzate, A., Hernando, D., Ricke, E. A., et al. (2019). Prostate enlargement and altered urinary function are part of the aging process. Aging 11 (9), 2653–2669. doi:10.18632/aging.101938
Liu, W.-S., You, J., Ge, Y. J., Wu, B. S., Zhang, Y., Chen, S. D., et al. (2023). Association of biological age with health outcomes and its modifiable factors. Aging cell 22 (12), e13995. doi:10.1111/acel.13995
Liu, K., He, Y., Li, Q., Sun, S., Mei, Z., and Zhao, J. (2024). Impact of hormone replacement therapy on all-cause and cancer-specific mortality in colorectal cancer: a systematic review and dose‒response meta-analysis of observational studies. J. evidence-based Med. 17 (2), 377–389. doi:10.1111/jebm.12622
Lombardo, G., Mondelli, V., Worrell, C., Sforzini, L., Mariani, N., Nikkheslat, N., et al. (2024). Disturbed sex hormone milieu in males and females with major depressive disorder and low-grade inflammation. J. Affect. Disord. 356, 167–176. doi:10.1016/j.jad.2024.03.018
Lundberg, J. O., and Weitzberg, E. (2022). Nitric oxide signaling in health and disease. Cell 185 (16), 2853–2878. doi:10.1016/j.cell.2022.06.010
Lyu, Y.-X., Fu, Q., Wilczok, D., Ying, K., King, A., Antebi, A., et al. (2024). Longevity biotechnology: bridging AI, biomarkers, geroscience and clinical applications for healthy longevity. Aging 16 (20), 12955–12976. doi:10.18632/aging.206135
Magri, V., Boltri, M., Cai, T., Colombo, R., Cuzzocrea, S., De Visschere, P., et al. (2019). Multidisciplinary approach to prostatitis. Arch. Ital. Urol. Androl. organo Uff. Soc. Ital. Ecogr. Urol. Nefrol. 90 (4), 227–248. doi:10.4081/aiua.2018.4.227
McGregor, D., and Frodsham, S. (2023). Scientific intelligence: recognising it to nurture it. J. Intell. 11 (4), 60. doi:10.3390/jintelligence11040060
Min, M., Egli, C., Dulai, A. S., and Sivamani, R. K. (2024). Critical review of aging clocks and factors that May influence the pace of aging. Front. aging 5, 1487260. doi:10.3389/fragi.2024.1487260
Mohajeri, M., and Cicero, A. F. G. (2023). Adherence to the mediterranean diet association with serum levels of nitric oxide, prostacyclin, and thromboxane B(2) among prinzmetal angina patients and healthy persons. Nutrients 15 (3), 738. doi:10.3390/nu15030738
Mónica, F. Z., Bian, K., and Murad, F. (2016). “The endothelium-dependent nitric Oxide–cGMP pathway,” in Advances in pharmacology (United States), 1–27. doi:10.1016/bs.apha.2016.05.001
Munteanu, C., Galaction, A. I., Onose, G., Turnea, M., and Rotariu, M. (2025). Harnessing gasotransmitters to combat age-related oxidative stress in smooth muscle and endothelial cells. Pharm. Basel, Switz. 18 (3), 344. doi:10.3390/ph18030344
Pagliaro, P., Weber, N. C., Femminò, S., Alloatti, G., and Penna, C. (2024). Gasotransmitters and noble gases in cardioprotection: unraveling molecular pathways for future therapeutic strategies. Basic Res. Cardiol. 119 (4), 509–544. doi:10.1007/s00395-024-01061-1
Palmer, R. D. (2022). Aging clocks and mortality timers, methylation, glycomic, telomeric and more. A window to measuring biological age. Aging Med. Milt. N.S.W 5 (2), 120–125. doi:10.1002/agm2.12197
Pena, V. N., Engel, N., Gabrielson, A. T., Rabinowitz, M. J., and Herati, A. S. (2021). Diagnostic and management strategies for patients with chronic prostatitis and chronic pelvic pain syndrome. Drugs and aging 38 (10), 845–886. doi:10.1007/s40266-021-00890-2
Phua, T. J. (2021). The etiology and pathophysiology genesis of benign prostatic hyperplasia and prostate cancer: a new perspective. Med. Basel, Switz. 8 (6), 30. doi:10.3390/medicines8060030
Phua, T. J. (2023). Understanding human aging and the fundamental cell signaling link in age-related diseases: the middle-aging hypovascularity hypoxia hypothesis. Front. aging 4, 1196648. doi:10.3389/fragi.2023.1196648
Phua, T. J. (2024). Hallmarks of aging: middle-aging hypovascularity, tissue perfusion and nitric oxide perspective on healthspan. Front. aging 5, 1526230. doi:10.3389/fragi.2024.1526230
Querfeld, U., Mak, R. H., and Pries, A. R. (2020). Microvascular disease in chronic kidney disease: the base of the iceberg in cardiovascular comorbidity. Clin. Sci. Lond. Engl. 1979 134 (12), 1333–1356. doi:10.1042/CS20200279
Rastrelli, G., Cipriani, S., Lotti, F., Cellai, I., Comeglio, P., Filippi, S., et al. (2022). Testosterone does not affect lower urinary tract symptoms while improving markers of prostatitis in men with benign prostatic hyperplasia: a randomized clinical trial. J. Endocrinol. investigation 45 (7), 1413–1425. doi:10.1007/s40618-022-01776-9
Riba, M., Del Valle, J., Molina-Porcel, L., Pelegrí, C., and Vilaplana, J. (2022). Wasteosomes (Corpora amylacea) as a hallmark of chronic glymphatic insufficiency. Proc. Natl. Acad. Sci. U. S. A. 119 (48), e2211326119. doi:10.1073/pnas.2211326119
Rodenkirchen, J., Hoyer, A., and Brinks, R. (2025). Modeling the temporal prevalence peak drift of chronic diseases. BMC Med. Res. Methodol. 25 (1), 65. doi:10.1186/s12874-025-02517-1
Sánchez-Fernández, D., Eguibar, A., López, C., Cuesta, Á. M., Albiñana, V., Rogers-Ezewuike, S., et al. (2024). Effect of 5β-dihydrotestosterone on vasodilator function and on cell proliferation. PloS one 19 (10), e0312080. doi:10.1371/journal.pone.0312080
Shen, X., Wang, C., Zhou, X., Zhou, W., Hornburg, D., Wu, S., et al. (2024). Nonlinear dynamics of multi-omics profiles during human aging. Nat. aging 4 (11), 1619–1634. doi:10.1038/s43587-024-00692-2
Sierra, F. (2016). The emergence of geroscience as an interdisciplinary approach to the enhancement of health span and life span. Cold Spring Harb. Perspect. Med. 6 (4), a025163. doi:10.1101/cshperspect.a025163
Siervo, M., Hussin, A. M., Calella, P., Ashor, A., Shannon, O. M., Mendes, I., et al. (2024). Associations between aging and vitamin D status with whole-body nitric oxide production and markers of endothelial function. J. Nutr. 154 (2), 469–478. doi:10.1016/j.tjnut.2023.12.002
Siltari, A., Murtola, T. J., Kausz, J., Talala, K., Taari, K., Tammela, T. L., et al. (2023). Testosterone replacement therapy is not associated with increased prostate cancer incidence, prostate cancer-specific, or cardiovascular disease-specific mortality in Finnish men. Acta Oncol. Stockh. Swed. 62, 1898–1904. doi:10.1080/0284186X.2023.2278189
Song, D., Liu, D., Ning, W., Chen, Y., Yang, J., Zhao, C., et al. (2023). Incidence, prevalence and characteristics of multimorbidity in different age groups among urban hospitalized patients in China. Sci. Rep. 13 (1), 18798. doi:10.1038/s41598-023-46227-4
Su, Y.-C., Shen, C. Y., Shao, S. C., Lai, C. C., Hsu, S. M., Lee, C. N., et al. (2024). Risk of age-related macular degeneration in men receiving 5α-reductase inhibitors: a population-based cohort study. Age ageing 53 (7), afae155. doi:10.1093/ageing/afae155
Sun, Z., and Bao, Y. (2013). Eliminating sedimentation for the treatment of chronic pelvic pain syndrome. Exp. Ther. Med. 5 (5), 1339–1344. doi:10.3892/etm.2013.982
Teut, M., and Ortiz, M. (2021). Integrative medicine and ageing. Complementary Med. Res. 28. 383–386. doi:10.1159/000519159
Thomas, S. A., Browning, C. J., Charchar, F. J., Klein, B., Ory, M. G., Bowden-Jones, H., et al. (2023). Transforming global approaches to chronic disease prevention and management across the lifespan: integrating genomics, behavior change, and digital health solutions. Front. public health 11, 1248254. doi:10.3389/fpubh.2023.1248254
van den Born, J. C., Hammes, H. P., Greffrath, W., van Goor, H., and Hillebrands, J. L.DFG GRK International Research Training Group 1874 Diabetic Microvascular Complications DIAMICOM (2016). Gasotransmitters in vascular complications of diabetes. Diabetes 65 (2), 331–345. doi:10.2337/db15-1003
Wander, K., Su, M., Mattison, P. M., Sum, C. Y., Witt, C. C., Shenk, M. K., et al. (2020). High-altitude adaptations mitigate risk for hypertension and diabetes-associated anemia. Am. J. Phys. Anthropol. 172 (2), 156–164. doi:10.1002/ajpa.24032
Wang, B., Pan, D., Ban, Y., Sun, Z., Tian, Y., and Luo, G. (2022). The relationship between prostatic microvessel density and different concentrations of oestrogen/androgen in sprague-dawley rats. Eur. J. Med. Res. 27 (1), 87. doi:10.1186/s40001-022-00719-7
Wei, L., Lai, E. C. C., Kao-Yang, Y. H., Walker, B. R., MacDonald, T. M., and Andrew, R. (2019). Incidence of type 2 diabetes mellitus in men receiving steroid 5α-reductase inhibitors: population based cohort study. BMJ Clin. Res. ed. 365, l1204. doi:10.1136/bmj.l1204
Wiecek, M., Szygula, Z., Gradek, J., Kusmierczyk, J., and Szymura, J. (2021). Whole-body cryotherapy increases the activity of nitric oxide synthase in older men. Biomolecules 11 (7), 1041. doi:10.3390/biom11071041
Woods, T., Palmarini, N., Corner, L., and Siow, R. (2024). Quantum healthy longevity from cells to cities. Front. Aging 5, 1416447. doi:10.3389/fragi.2024.1416447
Xia, B.-W., Zhao, S. C., Chen, Z. P., Chen, C., Liu, T. S., Yang, F., et al. (2021). Relationship between serum total testosterone and prostate volume in aging men. Sci. Rep. 11 (1), 14122. doi:10.1038/s41598-021-93728-1
Yamamoto, N., Oyaizu, T., Enomoto, M., Horie, M., Yuasa, M., Okawa, A., et al. (2020). VEGF and bFGF induction by nitric oxide is associated with hyperbaric oxygen-induced angiogenesis and muscle regeneration. Sci. Rep. 10 (1), 2744. doi:10.1038/s41598-020-59615-x
Yang, J. L., Hodara, E., Sriprasert, I., Shoupe, D., and Stanczyk, F. Z. (2024). Estrogen deficiency in the menopause and the role of hormone therapy: integrating the findings of basic science research with clinical trials. Menopause (New York, N.Y.) 31 (10), 926–939. doi:10.1097/GME.0000000000002407
Yeap, B. B., Marriott, R. J., Dwivedi, G., Adams, R. J., Antonio, L., Ballantyne, C. M., et al. (2024). Associations of testosterone and related hormones with all-cause and cardiovascular mortality and incident cardiovascular disease in men: individual participant data meta-analyses. Ann. Intern. Med. 177 (6), 768–781. doi:10.7326/M23-2781
Yura, E. M., Bury, M. I., Chan, Y., Morey, A. F., Sharma, A. K., and Hofer, M. D. (2020). Reversing urethral hypovascularity through testosterone and estrogen supplementation. Urology 146, 242–247. doi:10.1016/j.urology.2020.06.103
Zamani, H., de Joode, M. E. J. R., Hossein, I. J., Henckens, N. F. T., Guggeis, M. A., Berends, J. E., et al. (2021). The benefits and risks of beetroot juice consumption: a systematic review. Crit. Rev. food Sci. Nutr. 61 (5), 788–804. doi:10.1080/10408398.2020.1746629
Zhang, J., Li, C., Zhang, Y., Wu, J., and Huang, Z. (2023). Therapeutic potential of nitric oxide in vascular aging due to the promotion of angiogenesis. Chem. Biol. Drug. Des. 102 (2), 395–407. doi:10.1111/cbdd.14248
Keywords: biological clock, biological aging, nitric oxide, hypoxia, chronic inflammation, healthspan, benign prostatic hyperplasia, prostate cancer
Citation: Phua TJ (2025) The human biological clock and aging—a comprehensive approach integrating reductionism, holism, and geromedicine for proactive healthspan strategies. Front. Aging 6:1658952. doi: 10.3389/fragi.2025.1658952
Received: 03 July 2025; Accepted: 25 July 2025;
Published: 18 August 2025.
Edited by:
George A. Garinis, University of Crete, GreeceReviewed by:
Qingfeng Li, Shanghai Jiao Tong University, ChinaChengmeng Zhang, Peking University, China
Copyright © 2025 Phua. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Teow J. Phua, dGVvd2pwaHVhQGdtYWlsLmNvbQ==
 Teow J. Phua
Teow J. Phua