- 1School of Basic Medicine, Jiamusi University, Jiamusi, Heilongjiang, China
- 2Department of Biochemistry and Molecular Biology, School of Basic Medical Sciences, Peking University, Beijing, China
- 3The Seventh People’s Hospital affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China
GPR68 is a proton-sensing G protein-coupled receptor with an activation threshold at extracellular pH values between 6.5 and 7.0. It is widely expressed in diverse cell types, particularly in fibroblasts and cancer cells, within inflammatory and tumor microenvironments. In inflammatory bowel disease patients, GPR68 expression is also significantly increased in macrophages and monocytes. GPR68 primarily modulates inflammatory responses through the Gq/11–phospholipase C–inositol 1,4,5-trisphosphate/Ca2+ signaling axis. Extracellular acidification first promotes GPR68 coupling with Gq/11, subsequently enhancing phospholipase Cβ activity and increasing IP3 production; IP3 then mediates Ca2+ release from the endoplasmic reticulum, activating calmodulin-dependent kinase and calcineurin, ultimately inducing NF-κB and NFAT nuclear translocation to upregulate inflammatory mediators such as IL-6, TNF-α, and COX-2. This cascade activates inflammatory signaling pathways, thereby driving cellular and tissue senescence and creating favorable conditions for the progression of age-related diseases. However, its long-term causal relationship requires further validation through prospective studies. Abnormal GPR68 expression is closely associated with chronic inflammation, acidosis, and fibrosis in diseases including osteoarthritis, atherosclerosis, chronic kidney disease, Alzheimer’s disease, Parkinson’s disease, glioblastoma (GBM), and pancreatic cancer. In GBM, knocking down GPR68 or using the GPR68 inhibitor ogremorphin significantly reduces tumor cell survival. Despite its potential as a therapeutic target, challenges remain, such as the unresolved crystal structure, the lack of in vivo causality, cell-type specificity, and context-dependent signaling mechanisms. Targeting GPR68 may offer novel therapeutic strategies for these pathological processes.
1 Introduction
In recent years, the relationship between aging and disease progression has gradually been clarified, and factors such as inflammation and extracellular acidification are considered to be crucial to the aging process (Arnett, 2003; Jha et al., 2024; Zhang et al., 2024). As a pH-sensing G protein-coupled receptor (pH-GPCR), GPR68 serves a crucial function in sensing acidic changes in the extracellular environment and regulating inflammatory responses (Matsingos et al., 2024; Justus et al., 2024; Wiley et al., 2018; Merchant and Ling, 2023). The pH-GPCR family was first described in 2003 by Ludwig et al. and consists of GPR4, GPR65 and GPR68 (Ludwig et al., 2003; Justus et al., 2013; Foord et al., 2005). These receptors belong to the GPCR family. GPCRs are seven-transmembrane receptors and the largest family of cell signaling receptors. GPCRs are encoded by more than 800 genes in the human genome, accounting for about 3% of the human genes, responding to a variety of cell signals, and regulating multiple physiological processes (Vassilatis et al., 2003).GPR68 function links it to the pathogenesis of multiple age-related diseases, highlighting its potential as a therapeutic target (Rowe et al., 2021). To more clearly illustrate the relationship between GPR68 and age-related diseases, we provide descriptions through both charts and text, as shown in Figures 1, 2.
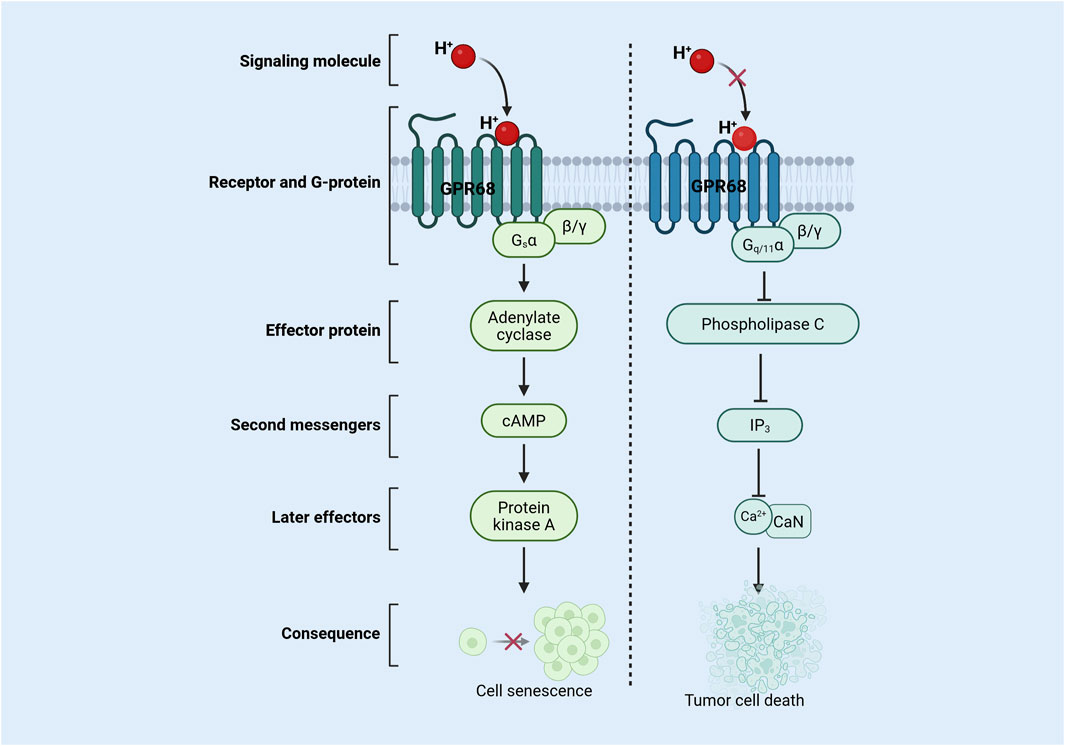
Figure 1. Mechanism of action of GPR68 in senescent cells and tumor cells. During aging, extracellular acidification activates the GPR68 signaling pathway, leading to restricted cell proliferation and inducing cellular senescence. In tumors, reduced extracellular acidification inhibits GPR68 pathway activation, thereby inducing tumor cell death. Abbreviations: CaN, calcineurin; cAMP, cyclic adenosine monophosphate; Gq/11, G protein alpha-q/11 subunit; Gsα, protein alpha-s subunit; H+, hydrogen ion; GPR68, G protein-coupled receptor 68; IP3, inositol 1,4,5-trisphosphate; β/γ, beta/Gamma Subunit.
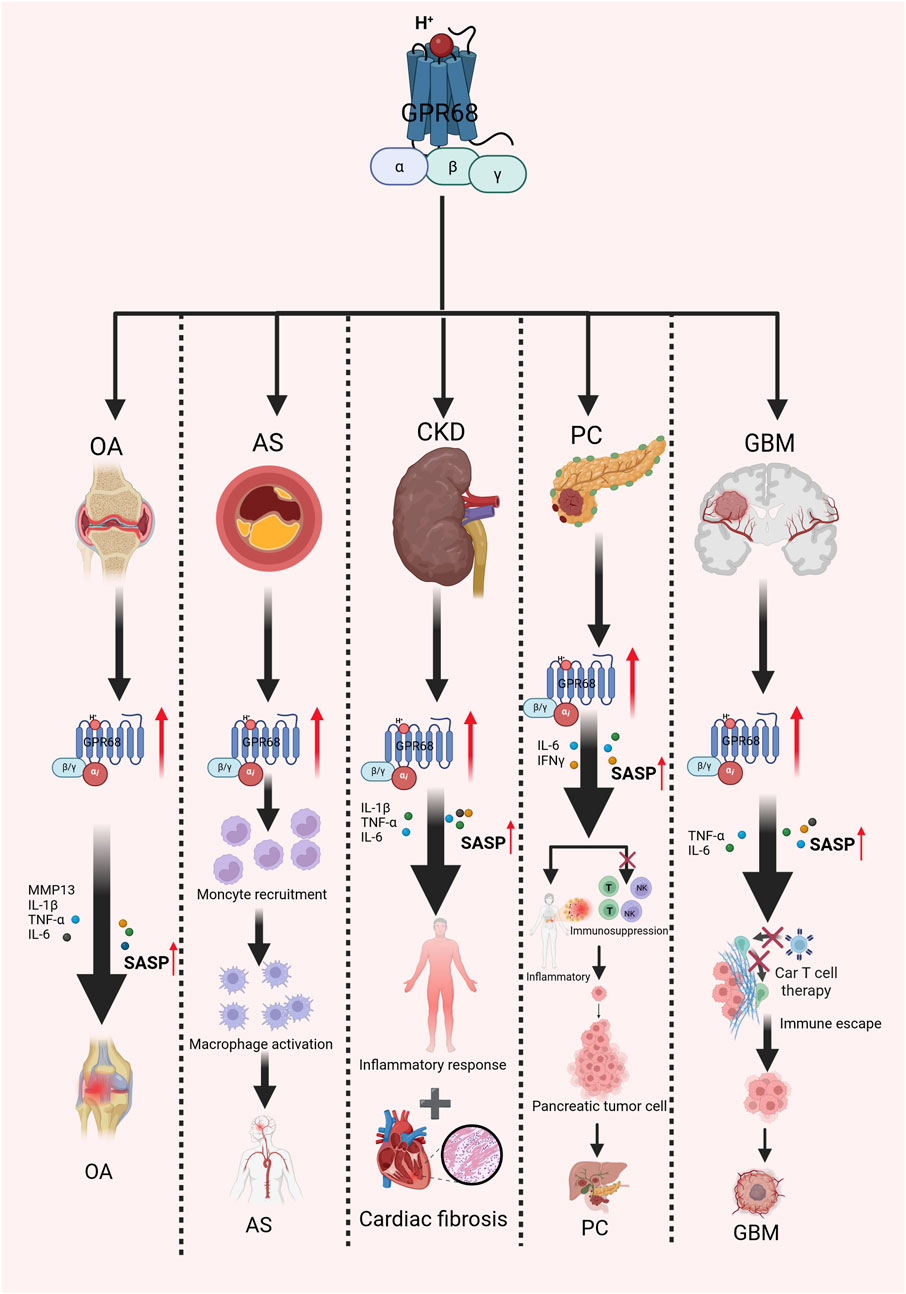
Figure 2. Mechanism of action of GPR68 in aging-related diseases. It explores the association between GPR68 and multiple age-related diseases. Activation of GPR68 releases multiple inflammatory mediators, intensifying inflammatory responses and thereby accelerating the progression of several age-related diseases to a certain extent. Abbreviations: AS, atherosclerosis; CKD, chronic kidney disease; GBM, glioblastoma; IFN-γ, interferon-γ; NK, natural killer; OA, osteoarthritis; SASP, senescence-associated secretory phenotype; T, T lymphocyte cell; TNF-α, tumor necrosis factor alpha.
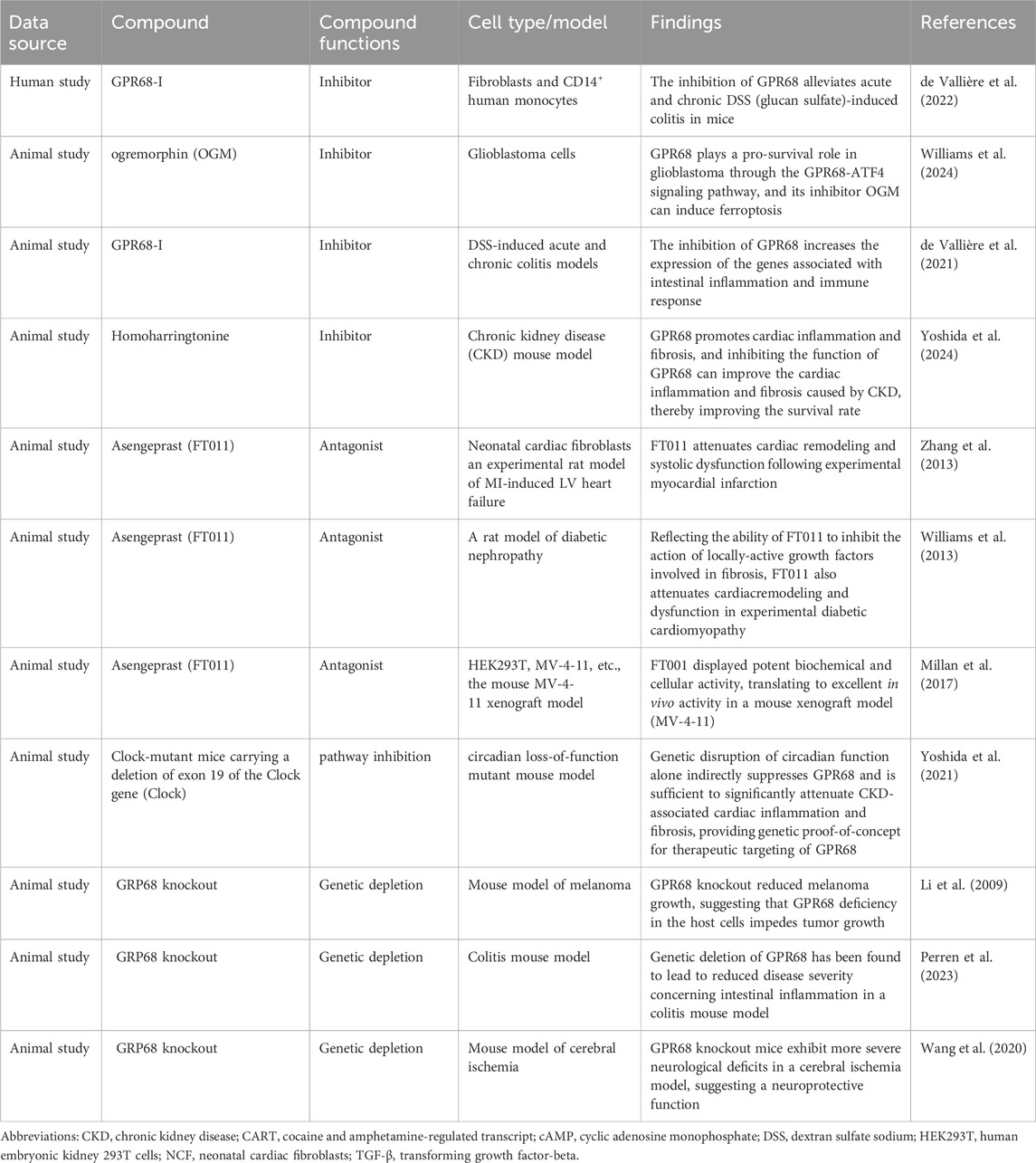
Changes in extracellular proton concentration have an important impact on the cells physiological and pathological processes. Because the GPR68 receptor acts as a proton sensor, it can respond to changes in extracellular proton concentration and regulate various biological functions by stimulating the Gq/11–PLC–IP3/Ca2+ axis (Yu et al., 2024). Research indicates that GPR68 is significantly associated with the progression of multiple diseases, particularly in maintaining homeostasis and functional integrity of hematopoietic stem cells during aging (Wiley et al., 2018; He et al., 2025). Aging accelerates the secretion of inflammatory factors (including cytokines and chemokines), especially IL-1, IL-6, and TNF-α. These cytokines are known as senescence-associated secretory phenotype (SASP) factors, and during aging, their release induces long-term persistent low-grade chronic inflammation (Maciel-Barón et al., 2016). Aging-related diseases (e.g., cardiovascular diseases, diabetes, and neurodegenerative diseases) are often accompanied by local acidification in the affected tissues. GPR68 can sense changes in extracellular pH and may be involved in the regulation of inflammatory responses through signal transduction pathways (de Vallière et al., 2022). To more clearly describe how GPR68 influences disease progression by regulating cytokines, we have organized the results into a summary table, as shown in Table 1. Endothelial dysfunction is an important feature of the inflammatory response, and it is associated with a variety of aging-related diseases, such as heart failure, cardiovascular disease, and diabetes (Zuchi et al., 2020; Xu et al., 2021). Research suggests that GPR68 contributes to endothelial function regulation, with investigations demonstrating that GPR68 activation is related to an increase in endothelial cell permeability and inflammatory response, and GPR68 inhibitors may help protect the endothelial barrier function (Tomura et al., 2005).
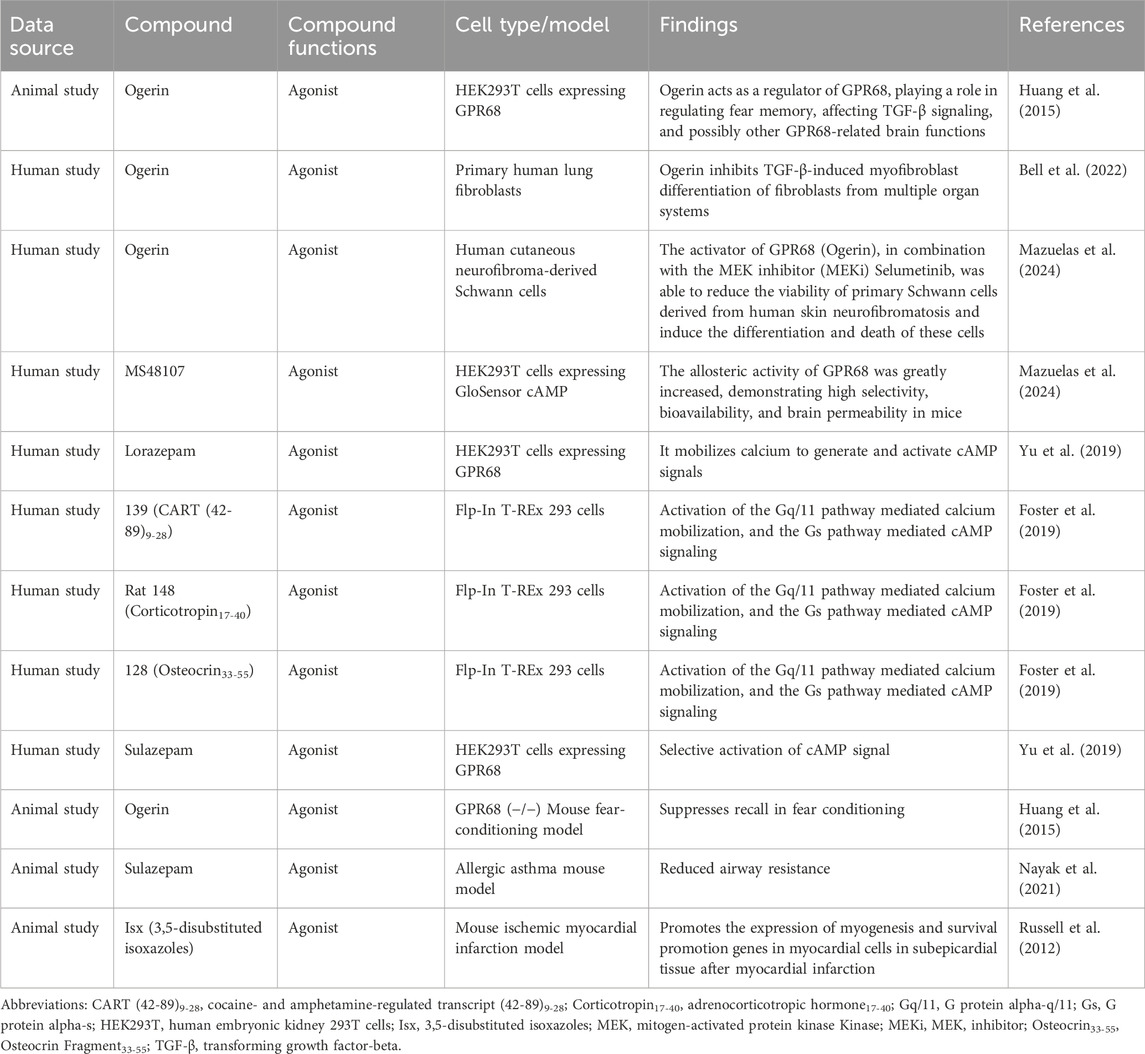
The relationship between GPR68 and inflammation is complex, encompassing diverse cell types and signaling cascades. This review describes the structure and function of GPR68 in detail, exploring the mechanism by which GPR68 regulates the development of aging-related diseases and analyzing the clinical application potential of GPR68 in disease treatment and prognosis assessment, with the goal of promoting the development of novel therapeutic strategies based on GPR68 receptors. We illustrate the therapeutic potential of GPR68 in age-related diseases in Figure 3.
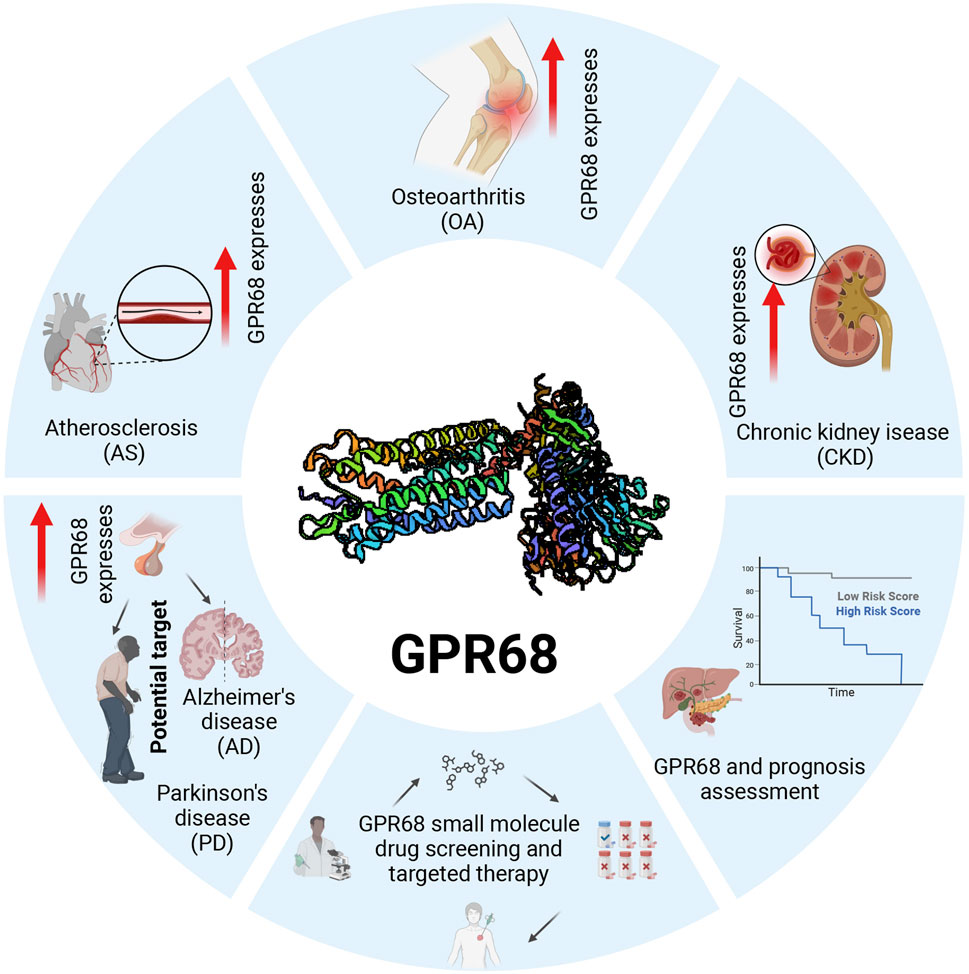
Figure 3. Expression and clinical application potential of GPR68 inaging-related diseases. GPR68 is upregulated in multiple age-related diseases, indicating its potential value as a therapeutic target. Screening for specific small-molecule drugs targeting GPR68 holds promise for treating various age-related conditions. Prospective evaluation of treatment regimens for age-related diseases can validate the specific therapeutic effects of GPR68-targeting small-molecule drugs. Abbreviations: AS, atherosclerosis; CKD, chronic kidney disease; GPR68, G protein-coupled receptor 68; OA, osteoarthritis.
2 The proton-sensing receptor—GPR68
GPR68 primarily activates downstream signaling pathways by sensing extracellular acidity changes, thereby participating in the regulation of physiological and pathological processes such as inflammation, cell proliferation, and apoptosis.
2.1 The discovery and development of GPR68
GPR68, alternatively referred to as ovarian cancer G protein-coupled receptor 1, was initially identified in a human ovarian cancer cell line HEY. As research continued, the scientific community gradually adopted the name GPR68 to more accurately reflect its identity as a member of the GPCR family (He et al., 2025; Imenez Silva and Wagner, 2022). Associated research has demonstrated that the GPR68 gene resides on chromosome 14 and possesses three experimentally validated transcript variants; these variants code for identical GPR68 protein, with variations existing solely in the 5′untranslated region. The distinctions could affect the mRNA expression levels, subcellular localization, and response to specific signals. GPR68 has an open reading frame of 1095 nucleotides and can encode a protein of 365 amino acids (Xu and Casey, 1996).
In recent years, some researchers have classified GPR68 as a pH-GPCR whose activation depends on the extracellular pH value (Imenez Silva and Wagner, 2022). The activation state of GPR68 is measured by inositol phosphate formation (Imenez Silva and Wagner, 2022). The discovery of GPR68 was made in the context of intensive research into a family of GPCRs that respond to a variety of ligands, including light, odor, taste, hormones, and neurotransmitters (Vassilatis et al., 2003; Hauser et al., 2017). As molecular biology and genomics techniques continue to advance, GPCRs are becoming increasingly important in research on disease progression, and GPR68 is one of them.
2.2 The structure of GPR68
GPR68 is a member of the GPCR superfamily, which includes an extracellular N-terminal, an intracellular C-terminal, and seven transmembrane structures with three extracellular and three intracellular loops, as shown in Figure 4 (Sisignano et al., 2021). The N terminal of GPR68 lies outside the cell and contains histidine residues that are critical for proton sensing. In the unprotonated state, histidine residues interact with each other through hydrogen bonding to maintain the deactivated state of the receptor. After protonation, these residues may lose their hydrogen bonds, resulting in a conformational change of the receptor. The C-terminal of GPR68 is located inside the cell and is involved in interaction with G proteins, as well as receptor regulation and signal transduction, and the C-terminal may also be involved in the receptor internalization and degradation process.
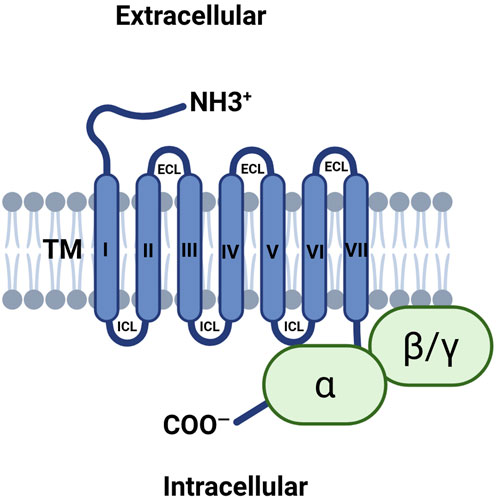
Figure 4. Illustrating the transmembrane structure of GPR68. GPR68 includes an extracellular N-terminal, an intracellular C-terminal, and seven transmembrane structures with three extracellular and three intracellular loops. Abbreviations: ECL, extracellular loop; ICL, intracellular loop; TM, transmembrane.
As a unique GPCR, the crystal or cryo-electron microscope structure of GPR68 has not yet been determined, in stark contrast to the situation with many other GPCRs. As early as 2003, a three-dimensional (3D) prediction model for GPR68 was proposed (Ludwig et al., 2003), and in the past decade, different methods have been used to analyze the structure of GPR68. In 2021, the SWISS-MODEL server was used to analyze the 3D structure of the GPR68 protein, and AlphaFold modeling was used to observe the 3D structure of the generated GPR68 protein. However, the structure of GPR68 has not been fully analyzed (Jumper et al., 2021; Studer et al., 2021). Although studies have reported that GPR68 may affect the development of aging by regulating the acidic microenvironment, the relevant regulatory mechanisms have not been well elucidated, which may be attributed to the fact that the structure of GPR68 has not been thoroughly studied (Chandra et al., 2016). Therefore, it is of great significance to strengthen studies on its structure and function in the future, specifically in relation to the activation of GPR68 in acidic microenvironments. For example, the relevant researchers inserted a ring-arranged fluorescent protein into the third intracellular ring of GPR68 to engineer a genetically encoded fluorescence reporter gene activated by GPR68, called “iGlow.” (Ozkan et al., 2021).
2.3 GPR68 expression levels
The levels of GPR68 expression in different tissues of the normal human body are different. For example, it is expressed in the heart, spleen, lung, kidney, small intestine, brain, testis, and placenta, but not in the skeletal muscles, liver, thymus, pancreas, prostate, ovary, and colon (Wiley et al., 2019). For some specific cell types, GPR68 is expressed in peripheral blood leukocytes (Xu and Casey, 1996) (including T cells (McAleer et al., 2018) and neutrophils (Murata et al., 2009)), thyroid cells (Afrasiabi et al., 2006), liver cells (Matsingos et al., 2024), aortic smooth muscle cells (Liu et al., 2010), and airway smooth muscle cells (Ichimonji et al., 2010; Saxena et al., 2012). In addition, GPR68 is most richly expressed in the pituitary gland, followed by the esophageal mucosa, cerebellum, and lung (Wiley et al., 2019). The mechanism by which GPR68 exerts its effects depends not only on expression levels but primarily through changes in extracellular acidity and tension stimuli. We cannot judge its function based solely on expression levels; instead, we must rely on specific environmental triggers. High expression merely indicates that functional responses will be more rapid and agile under specific triggering conditions. The reason for the differential expression of GPR68 in different tissues may be that it performs unique functions in different tissues.
Moreover, GPR68 exhibits notable elevation in multiple aging-related diseases, such as cancer, osteoarthritis (OA), and others. In pancreatic cancer (PC), it was found that the average expression level of GPR68 in 147 patients with PC was 10.5 times higher than that in 165 healthy individuals (Wiley et al., 2018). Through clinical data analysis, GPR68 was found to be highly expressed in head-and-neck squamous cell carcinoma and to correlate with disease progression and patient survival (Zhang et al., 2020). Therefore, GPR68 may be a potential therapeutic target and prognostic marker for the management of head and neck squamous cell carcinoma. In studies on OA, GPR68 was found to be expressed in human cartilage and was associated with the degradation of the extracellular matrix during OA development. Studies have also shown that the high expression of GPR68 is linked to the severity of extracellular matrix degeneration and OA progression; thus, enhanced GPR68 expression may aggravate the progression of OA and lead to serious dysfunction (Khan et al., 2023).
3 Pathogenic mechanisms involving GPR68
Inflammatory sites exhibit localized acidification due to heightened metabolic activity or tissue damage, leading to decreased extracellular pH. H+ (hydrogen ion) directly binds to the N-terminal domain of GPR68, inducing conformational changes that activate Gq/11 proteins. This activation subsequently modulates inflammatory responses through multiple pathways—including Ca2+ signaling channels, the MAPK/ERK pathway, and ion channels—thereby influencing disease progression under specific conditions.
3.1 GPR68 and the inflammatory response
Studies indicate that activation of the GPR68 receptor facilitates inflammatory signaling, particularly in intestinal inflammation and age-related chronic inflammation (Ichimonji et al., 2010; Khan et al., 2023; Perren et al., 2023; Yoshida et al., 2024; Matsuzaki et al., 2011; Hutter et al., 2018). In a colitis experiment on mice, the GPR68 small molecule inhibitor ogremorphin (OGM) was used to diminish pro-inflammatory cytokine levels, including TNF and IL-6, and fibrotic factor TGF-β1, and reduce the degree of colon inflammation in mice, suggesting that GPR68 may regulate the inflammatory response through the release of regulatory factors/inflammatory factors (de Vallière et al., 2021).
GPR68 promotes certain aging-related pathological processes under specific conditions. For example, in chronic kidney disease (CKD), the increased expression of GPR68 on human monocytes promotes the release of TNF-α, IL-6, and other pro-inflammatory cytokines, which aggravate systemic inflammation and fibrosis (Sarakpi et al., 2023). TNF-α and IL-6 demonstrate strong connections with numerous inflammatory conditions, encompassing inflammatory bowel disease (IBD), rheumatoid arthritis, multiple sclerosis and atherosclerosis (AS) (Sarakpi et al., 2023; Gonzalez Caldito, 2023; Tanaka et al., 2014; Hirano, 2021). The above studies suggest that the role of GPR68 in chronic inflammation in aging-related diseases may be achieved by regulating the SASP. The composition of the SASP is complex and dynamic, including a variety of pro-inflammatory factors, immune regulatory factors, cell growth factors, and matrix proteases (Xiong et al., 2024). These factors work together to affect the behavior of surrounding cells, including inducing the senescence of surrounding cells, and participate in the chronic inflammatory response of tissues. The expression level of the SASP usually increases with aging, and it exhibits strong associations with the development of multiple age-related disorders, encompassing cardiovascular diseases (Ferroni et al., 2003), diabetes (Pradhan et al., 2001), and cancer (Cuollo et al., 2020; Ortiz-Montero et al., 2017; Faget et al., 2019).
Relevant studies have shown that the removal of senescent cells can reduce the level of pro-inflammatory cytokines in the SASP in vivo, thereby reducing aging-related inflammation and tissue damage (Birch and Gil, 2020). During the aging process, GPR68 promotes the expression and secretion of pro-inflammatory factors like TNF-α and IL-8, resulting in a persistent chronic inflammatory response in the body and a variety of aging-related diseases (Wiley et al., 2018; Chandra et al., 2016; Tylutka et al., 2024; Pan et al., 2023; Lin et al., 2023; Bruunsgaard et al., 2000).
Given the role of GPR68 in chronic inflammation, investigating its modulatory impact on inflammatory processes holds substantial medical importance, as it could advance research into age-associated disorders and facilitate the identification of innovative therapeutic targets.
3.2 GPR68 and the immune response
The expression of GPR68 may affect immune cell function, including macrophages, neutrophils, dendritic cells, and T cells, and thereby affect the inflammatory response (Justus et al., 2024). Relevant studies have shown that macrophages and granulocytes are GPR68 positive in immunohistochemical clinical samples of human duodenum, indicating that GPR68 is expressed in macrophages, granulocytes, and other immune cells (de Vallière et al., 2022). In a clinical study of IBD, it was discovered that GPR68 mRNA expression levels within the affected mucosal tissue of IBD patients exhibited notably elevated values than those observed in non-IBD controls. In situ hybridization experiments on non-inflammatory and inflammatory tissues of patients with IBD and mucosal samples of non-IBD subjects found that compared with non-inflammatory tissues, the expression of GPR68 in the macrophages of inflamed tissues was increased (de Vallière et al., 2022). Related studies have shown that GPR68 is involved in the inflammatory response mediated by dendritic cells (Aoki et al., 2013) and macrophages (Okajima, 2013).
Tumor studies have shown that GPR68 may regulate immune cells through cytokines/inflammatory factors and thus affect tumor progression and initiation. For instance, in a mouse melanoma model, GPR68 knockout was noted to elevate the number of effector CD8+ T cells, induce T cell proliferation and migration enhancement, and increase the synthesis of cytokines interferon-γ (IFN-γ), TNF-α, and granzyme B, ultimately reducing the degree of melanoma invasion (Cao et al., 2021). Activation of GPR68 may inhibit the activity of some immune cells, thus providing good conditions for the immune escape of tumor cells and the occurrence and development of tumors. For example, the collection of melanoma tissue from wild-type mice and GPR68-knockout mice and analysis of tissue infiltration by immune cells, such as lymphocytes, monocytes, neutrophils, and natural killer (NK) cells, revealed a low accumulation of CD8+ T cells and NK cells in wild-type mouse melanoma tissues, while a high accumulation of CD8+ T cells and NK cells was observed in GPR68-knockout mouse melanoma tissues. This indicates that GPR68 expression inhibited the infiltration of melanoma tissues by CD8+ T cells and NK cells (Ye et al., 2023). GPR68 is expressed in cancer-associated fibroblasts (CAFs) and immune cells and serves a pivotal function in a variety of diseases by regulating cytokine production. These cells release a variety of cytokines into the tumor microenvironment that affect the function of nearby immune cells. For example, in pancreatic and colorectal cancers, CAFs express GPR68, which increases the secretion of proinflammatory cytokines, facilitates inflammatory signaling, and affects the tumor immune response (Wiley et al., 2019).
3.3 The GPR68 signaling pathway associated with aging progression
The regulatory effect of GPR68 can be realized through a variety of intracellular signaling pathways, including Gq/11–PLCβ–IP3/Ca2+, adenylate cyclase (AC)/cAMP/PKA/cAMP response element-binding protein (CREB). When human pancreatic cells are exposed to an acidic microenvironment, GPR68 activates the NF-κB signaling pathway and promotes the secretion of the pro-inflammatory factor IL-8, suggesting that GPR68 plays a catalytic role in the inflammatory response of human pancreatic cells (Chandra et al., 2016). IL-8 is a strong and important pro-inflammatory factor during aging that may cause tissue damage and functional decline (Tylutka et al., 2024; Lin et al., 2023). Therefore, the correlation between GPR68 and the NF-κB signaling pathway is related to the inflammatory response, and GPR68 may affect the aging process through the inflammatory factors TNF-α and IL-8, thereby affecting the occurrence and development of aging-related diseases. The AC/cAMP signaling pathway is also one of the important signaling pathways of GPR68. GPR68 activates AC through the Gs protein to increase the concentration of cAMP. cAMP activates PKA, which regulates several downstream effector molecules and transcription factors. The AC/cAMP signaling pathway serves a pivotal function in a variety of physiological and pathological processes, especially in inflammatory responses during cell proliferation and aging (Zeng et al., 2020).
These pathways are not only involved in cell proliferation, survival, and inflammatory response, but also closely related to the occurrence and development of a variety of aging-related diseases. Overall, the potential of GPR68 as a drug target for the treatment of aging-related diseases is gradually being recognized, but drug development targeting GPR68 faces great challenges. Future studies are needed to further elucidate the specific mechanisms of action of GPR68 in different aging-related diseases and how to effectively modulate its activity to treat the disease.
4 Effects on aging-related diseases
Given the important role of GPR68 in inflammation and immunity, focusing on the effects of GPR68 on aging-related diseases and their mechanisms is critical.
4.1 Osteoarthritis
OA is a chronic inflammatory aging-related disease characterized by joint inflammation, cartilage loss, and joint pain (Jeon et al., 2018). More than 80% of people over 65 years of age have OA (Wieland et al., 2005). To some extent, GPR68 modulates the progression of OA. Experimental studies on mouse OA models found that the expression of GPR68 is positively correlated with matrix metallopeptidase 13 levels, indicating that the activation of GPR68 could affect the development of OA chondrocytes through catabolism and matrix degradation, thereby contributing to disease progression (Khan et al., 2023). GPR68 may serve a function in the development of OA through the following signaling pathways: the NF-κB signaling pathway and the Ras homolog family member A/Rho-associated coiled-coil containing protein kinase pathway. Furthermore, activation of GPR68 may promote the activation of NF-κB, leading to the release of pro-inflammatory cytokines, thereby enhancing inflammatory signal transduction and cartilage degradation (Khan et al., 2023; Tang et al., 2022). GPR68 influences the development of OA by regulating the expression of multiple cytokines, such as TNF-α and IL-1β (Sok et al., 2013; Akeson and Malemud, 2017). In one study, researchers studied the effects of GPR68 in a mouse model of OA. The results showed that mice lacking GPR68 had lower levels of T cell activation and markedly reduced arthritis symptoms. This suggests that the presence of GPR68 is closely related to the proliferation and activation of T cells, further confirming the importance of GPR68 in the degree of inflammation and immune response in OA.
4.2 Atherosclerosis
Vascular aging refers to the structural and functional changes that occur in the vascular system due to aging. Dysfunction of the vascular system can lead to various aging-related diseases such as giant cell arteritis and AS (Guo et al., 2022). In the pathogenesis of AS, GPR68 may be involved in the occurrence and development of the disease by affecting the function of vascular endothelial cells and the inflammatory response. In the early stage of AS, the aggregation of monocytes and macrophages is a typical feature. Activation of GPR68 can promote the migration of monocytes to the inflammatory site, stimulate the activation of macrophages, and increase the inflammatory response. Studies have shown that GPR68 is essential for the physiological function of blood vessels, especially in sensing hemodynamic changes (Xu et al., 2018). Changes in hemodynamics can induce endothelial cells to release pro-inflammatory cytokines, further aggravate the local inflammatory response, promote intra-arterial lipid deposition, and exacerbate AS (Ma et al., 2024). The role of GPR68 in regulating cellular response to acidosis, as well as the acidic characteristics of the atherosclerotic plaque microenvironment (Xiao et al., 2023), suggests that GPR68 may serve an important function in the pathogenesis of AS.
4.3 Chronic kidney disease
CKD is a common inflammatory disease in the elderly. It is a long-term condition of reduced kidney function that usually manifests as a decreased estimated glomerular filtration rate (eGFR) or the presence of protein in the urine. With the increasing aging of the global population, the incidence of CKD has increased markedly in the elderly population, especially in adults over 65 years of age, with almost 40% affected (Merchant and Ling, 2023). Relevant studies have shown that the expression of GPR68 in CKD tissues is markedly increased, suggesting that GPR68 may be involved in the regulation of the pathophysiological process of CKD. It has been reported that CKD upregulates GPR68 expression in monocytes and enhances the secretion of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6), thereby promoting inflammatory signaling and, under certain conditions, amplifying systemic inflammation and cardiac fibrosis (Sarakpi et al., 2023; Yoshida et al., 2021). The effect of GPR68 on CKD may be mediated by the Gq and Gs signaling pathways, through which GPR68 activates the conversion of PI to diacylglycerol and IP3, resulting in an increase in intracellular calcium ion concentration. This increase in calcium concentration enhances the expression of pro-inflammatory cytokines, which contribute to the inflammatory response in CKD (Guo et al., 2022).
4.4 Alzheimer’s disease
Alzheimer’s Disease (AD) is a progressive neurodegenerative disorder primarily characterized by memory impairment, cognitive decline, and behavioral changes. AD is the most common type of dementia among the elderly, accounting for approximately 60%–70% of all dementia cases (Dolphin et al., 2024). GPR68 may play a regulatory role in AD. Although no direct evidence currently exists to prove GPR68’s involvement in AD, researchers have noted its expression in mammalian brains and speculate that it may participate in the pathological processes of AD (Khan and He, 2017). GPR68 and GPR4 both belong to the pH–GPCR family and are classified as Class A orphan receptors, exhibiting numerous similarities. Through the integration of transcriptomic association analysis and weighted gene co-expression network analysis, GPR4 was identified as one of the predictive biomarkers for AD, providing statistical evidence for its significant association with AD risk (Zhou et al., 2023). Although no direct therapeutic studies targeting GPR68 in AD have been reported to date, existing research on GPR4 and its predictive value in AD provides a rationale. Future work will therefore use additional technical approaches to explore the potential of GPR68 as a predictive biomarker for AD.
4.5 Parkinson’s disease
Parkinson’s disease (PD) is a chronic, progressive neurological disorder that primarily affects motor function. Its pathological features include degeneration of dopaminergic neurons in the substantia nigra and the formation of Lewy bodies (Koeglsperger et al., 2023). The typical symptoms of PD include tremor, muscle rigidity, bradykinesia, and postural instability (Harrison et al., 2025). Additionally, non-motor symptoms such as cognitive impairment, depression, hyposmia, and sleep disorders are also commonly observed in PD patients (Chahine et al., 2025; Chen, 2018; Sohail et al., 2017). GPR68 may exert a regulatory effect on PD. Although no direct evidence currently exists to prove GPR68’s role in PD, researchers have proposed that GPR68 may participate in the pathological processes of PD (Khan and He, 2017). GPR68 shares numerous similarities with GPR4, as both belong to the pH–GPCR family and are classified as Class A orphan receptors. Findings from GPR4 research may provide important insights for future studies targeting GPR68 in PD. Researchers discovered that at the cellular level, activation of GPR4 is closely associated with neuronal apoptosis. Knockout of GPR4 significantly reduces neuronal apoptosis (Haque et al., 2020). Research has found that the GPR4 inhibitor NE52-QQ57 significantly reduces neuronal damage and improves motor function and spatial memory in mouse PD models (Haque et al., 2021). Although no direct experimental studies targeting GPR68 in PD have been reported to date, research on GPR4 in this disease suggests that GPR68 is likely to emerge as a potential therapeutic target for PD in the future.
4.6 Glioblastoma
Glioblastoma (GBM) is one of the most common primary malignant brain tumors of the adult central nervous system. GBM poses a serious health challenge to patients due to the proliferation of tumor cells and their invasion of normal tissues, which usually results in a poor prognosis and survival times of less than 1 year. Recent studies have shown that the activation of GPR68 under acidic conditions activates the endoplasmic reticulum stress activating transcription factor 4 (ATF4) signaling pathway, which is a novel survival pathway in glioblastoma. The activation of the GPR68-ATF4 signaling pathway can promote the production of the pro-inflammatory factors IL-6 and TNF-α and helps glioblastoma cells to evade immune surveillance, thus enhancing the growth and survival of glioblastoma cells (Kumari et al., 2021; Li et al., 2024). GPR68 is expressed in macrophages, and the activation of GPR68 may lead to the polarization of macrophages into the M2 phenotype, which usually has immunosuppressive properties and contributes to tumor growth and metastasis (Justus et al., 2024). One study used OGM, a novel small molecule inhibitor of GPR68, to explore the role of GPR68 in GBM cells and found that blocking the GPR68 signaling pathway led to ferroptosis of tumor cells in GBM cell lines. These findings suggest that GPR68 may be a new target for the treatment of GBM (Williams et al., 2024).
4.7 Pancreatic cancer
PC is a malignant tumor characterized by aggressive growth, local invasion, and distant metastasis. A study has shown that the expression of GPR68 in PC is markedly higher than that in most normal pancreatic cells (Wiley et al., 2019). This high expression may be closely related to tumor development, possibly due to the involvement of GPR68 in the interaction between tumor cells and the tumor microenvironment. In PC tissues, the GPR68 expression levels correlate with tumor aggressiveness and prognosis, making it a potential biomarker and therapeutic target. In an acidic microenvironment, GPR68 activation in pancreatic CAFs upregulates IL-6 via the cAMP/PKA/CREB axis (Wiley et al., 2019). Furthermore, relevant studies have shown that GPR68 functionally inhibits the expression of IFN-γ in tumor-infiltrating CD8+ T cells and NK cells, which may lead to tumor immune escape and further promote tumor growth (Ye et al., 2023).
GPR68 is involved in the pathogenesis of a variety of aging-related diseases, including inflammatory diseases and cancer. By studying the relationship between GPR68 and inflammatory factors, researchers hope to develop new therapeutic strategies to target the inflammatory mechanisms of these diseases (Sarakpi et al., 2023; Elemam et al., 2022). As an important pH-GPCR, GPR68 is involved in the regulation of the inflammatory response and cytokine production. Given its central role, GPR68 is a promising therapeutic target, and future research is expected to translate its mechanism of action into novel treatments for aging-related diseases.
5 Clinical application potential of GPR68
Considering that GPR68 can influence the progression of multiple aging-related diseases, it has excellent potential for clinical application in early diagnosis, targeted therapy, and prognosis evaluation.
5.1 Early diagnosis
GPR68 plays an essential role in regulating cellular functions, especially inflammatory responses. Specifically, the activation of GPR68 can induce the release of cytokines, chemokines, and other inflammatory mediators, thereby triggering or aggravating the inflammatory response (van Setten, 2022). In chronic inflammation, the expression of GPR68 is usually markedly elevated, which is positively correlated with the severity of inflammation and the clinical score (Justus et al., 2024). GPR68 expression is markedly increased in multiple tumors and correlates with poor patient prognosis, implying that GPR68 is a potential prognostic marker for cancer. Specifically, through the analysis of tumor samples, it was found that the expression level of GPR68 in breast cancer tissues was positively correlated with the metastasis and recurrence of the tumor; therefore, GPR68 expression provides important information for the early detection and diagnosis of cancer (Elemam et al., 2022). In addition, GPR68 expression in neuroendocrine tumors shows its potential as a diagnostic marker. For instance, GPR68 expression in pheochromocytoma and cervical adenocarcinoma was shown to be positively correlated with patient survival, suggesting its value in diagnosing these cancers (Herzig et al., 2019).
5.2 Targeted therapy
Given its involvement in regulating inflammation, tumors, and fibrosis, inhibiting GPR68 activity is a potential therapeutic strategy for aging-related diseases.
In CKD, GPR68 has been found to promote the activation of the inflammasome by increasing the intracellular calcium ion concentration, thus aggravating the inflammatory response. Interestingly, the GPR68 inhibitor OGM suppresses this inflammatory response, reducing tissue damage and improving the prognosis of chronic diseases. GPR68 is closely associated with tumor proliferation, invasion, and metastasis. In clinical studies, the inhibition of GPR68 expression and blocking of GPR68 signaling slowed the growth and spread of tumor cells. For example, in GBM, a novel small molecule drug, OGM, inhibits GPR68 by activating ATF4, which induces ferroptosis and inhibits tumor progression (Williams et al., 2024). Similarly, GPR68 facilitates tumor escape from immune surveillance, and knockout of GPR68 in melanoma and prostate cancer inhibits tumor proliferation (Yan et al., 2014; Li et al., 2009) Chronic inflammation is one of the primary drivers of fibrosis. Certa Therapeutics is investigating the GPR68 antagonist FT011 for the treatment of renal fibrosis (Cornell et al., 2024). FT011 has shown significant efficacy in multiple fibrosis-related disease models; in diabetic cardiomyopathy and myocardial infarction models, it markedly reduced cardiac fibrosis and improved function (Zhang et al., 2013; Zhang et al., 2012). GPR68 expression is significantly elevated in the inflamed mucosa of patients with IBD, particularly in those with Crohn’s disease and ulcerative colitis, where its expression levels correlate positively with disease severity (de Vallière et al., 2022). Research indicates that treatment with the GPR68 inhibitor OGM in acute and chronic mouse colitis models reduces mucosal inflammatory responses, providing the first evidence that targeting GPR68 ameliorates mouse colitis (de Vallière et al., 2021). GPR68 also plays an important regulatory role in the hematopoietic system. Researchers using hematopoietic cell-specific GPR68 conditional knockout mice have shown that targeting GPR68 enhances the function of aging hematopoietic stem cells, providing a novel therapeutic strategy for age-related hematopoietic decline (He et al., 2025).
In summary, GPR68 is a potential therapeutic target for inflammatory diseases, cancer, fibrosis, and age-related decline in hematopoietic function. Targeting GPR68 could provide new therapeutic options for patients who do not respond well to conventional therapies. To better evaluate the therapeutic efficacy of targeting GPR68 across age-related diseases, we have compiled the relevant results in Table 2. To gain a deeper understanding of the mechanism of action of GPR68, we systematically compiled information on GPR68 agonists and their biological function, as shown in Table 3.
5.3 Prognostic evaluation
GPR68 contributes to the evaluation of the prognosis of patients with chronic inflammation and tumors. In one study, it was found that GPR68 mRNA expression at the site of inflammation in the colon was markedly elevated in patients with IBD, and this elevation was positively correlated with disease severity and the clinical prognostic score (Justus et al., 2024). Moreover, activation of GPR68 promoted inflammation and fibrosis of the heart, which suggests that GPR68 is a potential player in the occurrence and progression of heart disease, and its high expression may be associated with poor prognosis (Yoshida et al., 2024). In oncology, several studies have shown a close relationship between GPR68 and tumor prognosis, drug resistance, and tumor metastasis. By assessing GPR68 expression in a patient’s tumor, it is possible to more accurately predict patient survival and tumor development. For example, in patients with breast cancer, high GPR68 expression is associated with higher recurrence and mortality rates, which confirms the prognostic potential of GPR68 and provides a basis for individualized treatment plans for patients (Elemam et al., 2022). Notably, in oral cancer studies, GPR68 deletion can aggravate chemically induced oral dysplasia, indicating that GPR68 activation may have a protective effect in oral cancer (Shore et al., 2023). The role of GPR68 may vary across different tumors, potentially inhibiting tumor growth or promoting its progression. Potential reasons include tissue-specific cellular characteristics and differences in the tumor microenvironment. As a potential therapeutic target, GPR68 should not be approached with a uniform treatment strategy across all diseases. We must thoroughly investigate its specific functions in different pathological states to fully unlock its potential as a therapeutic agent.
GPR68 is not only a promising tumor biomarker but also a potential therapeutic target for chronic inflammatory and neoplastic diseases, and it can be used to evaluate the prognosis of patients and select appropriate therapeutic strategies. Future studies should focus on revealing the specific mechanisms underlying the effects of GPR68 and its role in chronic inflammation and different types of cancer to develop more effective treatment strategies for improving patient survival and quality of life.
6 Conclusions and future perspectives
Currently, research on GPR68 has gradually increased and revealed the important function of GPR68 in regulating aging-related diseases. However, there are still many challenges that limit a more comprehensive understanding of GPR68. Here we provide some highly representative, but not exhaustive, examples of the challenges facing research on GPR68.
6.1 From structure to mechanism: deepening our understanding of GPR68
The structure of GPR68 is crucial; without a high-resolution structure, the lack of in vivo causality between GPR68 activation and downstream pathological outcomes remains unresolved. Although the crystal or cryo-electron microscopic structures of many GPCRs have been determined, the exact structure of GPR68 requires further elucidation due to the absence of key experimental data. Advances in technology have opened up the possibility of utilizing multiple approaches to study the specific structure of GPR68. For example, in molecular dynamics simulation, a three-dimensional model of GPR68 has been proposed based on the homology modeling of other known GPCRs. Using the C-X-C chemokine receptor 4 architecture as a template, researchers first generated a 3D model of GPR68. Subsequently, they produced a set of 2,900 additional models through elastic network extension. In total, a repository of 3,307 models has been established, laying the foundation for further research on the specific structure of GPR68 (Rowe et al., 2021).
In the future, the structure of GPR68 can be studied by X-ray crystallography, nuclear magnetic resonance, and the accuracy of the structure of GPR68 can be improved by combining various experimental techniques. Importantly, combining structural studies of GPR68 with computer prediction and validation through virtual screening and mutation analysis is a powerful strategy for elucidating the structural function of GPR68. For example, computer-aided drug design was used to screen possible GPR68 ligands and predict GPR68 binding patterns. Site-specific mutation of key amino acid residues of GPR68 was performed, and the effect of mutation on protein activity was analyzed by functional experiments (Basak, 2013). These research strategies will help to further understand the structure and function of GPR68 and promote the application of GPR68 in the biomedical field.
Considering the effects of GPR68 on aspects of immune cell function, the inflammatory microenvironment, and the tumor immune microenvironment, future studies should focus on the multiple roles of GPR68 in mechanism, immunity, and signal transduction. For instance, we focus on how to regulate the GPR68 status change when the microenvironment pH changes. In a weakly alkaline microenvironment, GPR68 is stabilized in a deactivated state by hydrogen bonding. With decreased pH and acidification of the microenvironment, GPR68 activity increases, and this response exhibits pronounced signal context-dependency, meaning that the same proton stimulus can trigger distinct G-protein coupling or β-arrestin recruitment patterns depending on cell type, receptor density, and concurrent signaling inputs. Consequently, GPR68 activation may affect immune cell function in inflammation and tumors in a context-dependent manner. Therefore, the future mechanism research can focus on the following aspects: In the tumor microenvironment, how GPR68 affects cell signaling in response to pH changes in the tumor microenvironment should be explored. Furthermore, the interaction between GPR68 and other signaling pathways is also the focus of research, because GPR68 may affect the tumor immune microenvironment by regulating the function of immune cells. Moreover, other aspects of the research are equally noteworthy, such as how GPR68 affects the proliferation, activation, and apoptosis of immune cells in inflammatory and tumor microenvironments. Additionally, how GPR68 regulates the activity of T cells by changing the acidic environment of cells should be studied. These studies could elucidate the underlying mechanisms by which GPR68 affects chronic inflammation and the immune microenvironment and provide a theoretical basis for the development of novel chronic inflammation therapies and cancer immunotherapies.
6.2 Toward clinical translation: challenges and strategies
Advancing the clinical use of GPR68 in inflammatory diseases and cancer represents an urgent goal. The development of small molecule drugs faces several challenges, such as poor compound selectivity, low drug efficiency, and considerable side effects. Several strategies are proposed to address these issues: First, high-throughput screening techniques are used to screen compounds that specifically bind to GPR68. Second, studying how small molecule drugs selectively target GPR68 could help in designing more effective treatments. Finally, novel drug delivery systems to improve the accuracy of small molecule drugs that target GPR68 should also be developed. In terms of biomarker identification, GPR68 may be used as an inflammatory and tumor-specific marker because GPR68 is upregulated at inflammatory sites and in multiple tumor cell lines. Future studies should focus on the identification of biomarkers for age-related diseases, which could help improve the efficiency of early diagnosis and effectively evaluate the prognosis of patients with tumors. By evaluating patient prognosis, we can provide patients with an individualized treatment plan, which would improve the accuracy and effectiveness of treatment. In terms of technological innovation, emerging biotechnology, such as clustered regularly interspaced short palindromic repeats (CRISPR) gene editing and single-cell sequencing, should be used to deeply study the function of GPR68 and its role in inflammation and tumors. In addition, early-stage clinical trials of GPR68 to evaluate its therapeutic potential in chronic inflammation and different types of cancer should be designed and performed. In clinical trials, long-term follow-up should be used to evaluate the efficacy and safety of GPR68-targeted therapy. This will help build more reliable clinical evidence.
We systematically reviewed and synthesized current knowledge regarding the challenges associated with GPR68, including specificity, off-target effects, drug delivery, and experimental data.
To address the low specificity of GPR68, we conducted an analysis focusing on the complexity of its ligand recognition and signaling mechanisms. The primary ligand for GPR68 is extracellular H+, with its activity dependent on changes in extracellular pH. Additionally, its ligand recognition involves specific amino acid residues, such as extracellular histidine and acidic residues, whose protonation states are crucial for receptor activation. Another significant reason for its low specificity is the cross-reactivity with other pH-GPCRs, such as GPR4 and GPR65.
Off-target effects of GPR68 small-molecule drugs primarily stem from the high structural and functional similarity among members of the GPCR family. This similarity may cause small-molecule drugs to interact with other GPCRs while binding to GPR68. Addressing the off-target effects of GPR68-targeting drugs demands a multi-pronged approach, which includes optimizing drug selectivity, evaluating mechanisms of action, developing specific modulators, integrating genetic analysis, and utilizing animal models to validate safety. These strategies not only enhance drug efficacy but also minimize side effects, providing safer treatment options for patients.
Small-molecule drugs targeting GPR68 face numerous challenges in drug delivery and efficacy. GPR68 is expressed in multiple tissues and cell types, but its expression levels and function vary significantly across different environments. This heterogeneity makes precise targeting of specific tissues or cells difficult, thereby reducing delivery efficiency. Most small-molecule drugs targeting GPR68 are prone to degradation and inactivation in the in vivo environment, particularly under acidic conditions. This instability limits effective accumulation at target sites, resulting in poor drug stability. Although GPR68 plays a critical role in specific barrier tissues, small-molecule drugs often struggle to penetrate these barriers, hindering drug delivery to the intended location.
The systematic analysis of GPR68 trial data requires a multi-faceted approach. First, as a pH-GPCR, it plays crucial roles in diverse physiological and pathological processes, including neuroprotection, inflammation regulation, and tumor microenvironment modulation. Experimental designs can employ techniques such as molecular dynamics simulations, gene expression analysis, Western blotting, and flow cytometry to investigate GPR68 activation mechanisms under varying pH conditions and its downstream signaling pathways. Additionally, its role in inflammatory bowel disease warrants attention, as its expression correlates with disease activity and its inhibition reduces colonic inflammation. The synthesis of experimental data should integrate in vivo and in vitro models, including mouse models, cell cultures, and clinical sample analyses, to comprehensively evaluate its function and potential therapeutic value. Integrating multidimensional data can provide deeper insights into the physiological and pathological mechanisms of GPR68.
6.3 Concretization of future work: a comprehensive research case study of GPR68 in atherosclerosis
To thoroughly investigate the function of GPR68 and its mechanisms in inflammation and tumorigenesis, we present a detailed overview across four key areas: CRISPR knockout application, in vivo validation of inhibitors, high-throughput screening, and clinical trial design. We chose AS as an example to show the relationship between GPR68 and AS. GPR68 knockout mice can be generated via CRISPR-Cas9. An AS model will be established by feeding mice a high-fat diet for over 16 weeks. The formation of atherosclerotic plaques will be compared between GPR68 knockout mice, wild-type mice, and wild-type mice treated with the GPR68 inhibitor OGM. Serum levels of inflammatory mediators (IL-6, TNF-α) will be measured by ELISA to validate the effects of GPR68 knockout and pharmacological inhibition on atherosclerotic progression. High-throughput screening of multiple GPR68 small-molecule inhibitors will be conducted using a HEK293 cell line stably expressing GPR68 and the calcium indicator GCaMP6s as the screening platform. The Enamine compound library will be selected for screening, with automated screening and virtual screening assisting in the efficient batch screening of GPR68 small-molecule inhibitors. Clinical evidence supporting GPR68 as an AS biomarker remains insufficient. A prospective cohort study and biomarker validation will be conducted, enrolling 300 AS patients and 200 healthy controls. Blood samples will be collected to measure GPR68 expression in blood cells and serum levels of the inflammatory cytokine IL-6. Patients underwent follow-up for up to 3 years. Subsequently, the relationship between GPR68 expression and AS progression will be assessed.
Continuous efforts in the above aspects will broaden the research and application prospects of GPR68. Future research will not only help reveal its potential in the treatment of inflammation and cancer but may also advance the development of treatment options for other age-related diseases associated with GPR68, fully realizing its potential as a therapeutic target.
Author contributions
JW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Writing – original draft, Writing – review and editing. FC: Data curation, Methodology, Writing – original draft, Writing – review and editing. ZH: Data curation, Methodology, Writing – original draft, Writing – review and editing. ZL: Formal Analysis, Methodology, Writing – original draft, Writing – review and editing. ZM: Formal Analysis, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review and editing. PZ: Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from the Heilongjiang Natural Science Foundation of China (Nos LH2022H090, PL2024H013), Innovative Team Project of Heilongjiang Provincial Education Department (2024-KYYWF-0617), Jiamusi University “East Pole” Academic Team Program (DJXSTD202404), Collaborative Innovation Achievement Program for “Double First Class” Disciplines in Heilongjiang Province (LJGXCG2023-089), Heilongjiang Province “double firstclass” new round of construction discipline support platform construction project (TSXK14702350), Jiamusi University 2022 annual Youth innovative Talent Training Support program project (JMSUQP2022006), Jiamusi University National Fund Cultivation Project (Grant No. JMSUGPZR2022-010).
Acknowledgments
We thank Fusheng Zhang for his helpful suggestions during the preparation of this review. We thank BioRender (www.BioRender.com) for expert assistance in the pattern drawing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AC, adenylate cyclase; AS, atherosclerosis; ATF4, activating transcription factor 4; 3D, three-dimensional; CAFs, cancer-associated fibroblasts; CKD, chronic kidney disease; CREB, cAMP response element-binding protein; CRISPR, clustered regularly interspaced short palindromic repeats; GBM, glioblastoma; IBD, inflammatory bowel disease; IFN-γ, interferon-γ; NK, natural killer; OA, osteoarthritis; OGM, ogremorphin; PC, pancreatic cancer; pH-GPCR, pH-sensing G protein-coupled receptor; SASP, senescence-associated secretory phenotype.
References
Afrasiabi, E., Blom, T., Ekokoski, E., Tuominen, R. K., and Törnquist, K. (2006). Sphingosylphosphorylcholine enhances calcium entry in thyroid FRO cells by a mechanism dependent on protein kinase C. Cell. Signal. 18, 1671–1678. doi:10.1016/j.cellsig.2006.01.005
Akeson, G., and Malemud, C. J. (2017). A role for soluble IL-6 receptor in osteoarthritis. J. Funct. Morphol. Kinesiol. 2, 27. doi:10.3390/jfmk2030027
Aoki, H., Mogi, C., Hisada, T., Nakakura, T., Kamide, Y., Ichimonji, I., et al. (2013). Proton-sensing ovarian cancer G protein-coupled receptor 1 on dendritic cells is required for airway responses in a murine asthma model. PloS One 8, e79985. doi:10.1371/journal.pone.0079985
Arnett, T. (2003). Regulation of bone cell function by acid-base balance. Proc. Nutr. Soc. 62, 511–520. doi:10.1079/pns2003268
Basak, S. C. (2013). Recent developments and future directions at current computer aided drug design. Curr. Computer-Aided Drug Des. 9, 1. doi:10.2174/1573409911309010001
Bell, T. J., Nagel, D. J., Woeller, C. F., and Kottmann, R. M. (2022). Ogerin mediated inhibition of TGF-β(1) induced myofibroblast differentiation is potentiated by acidic pH. PloS One 17, e0271608. doi:10.1371/journal.pone.0271608
Birch, J., and Gil, J. (2020). Senescence and the SASP: many therapeutic avenues. Genes and Dev. 34, 1565–1576. doi:10.1101/gad.343129.120
Bruunsgaard, H., Skinhøj, P., Pedersen, A. N., Schroll, M., and Pedersen, B. K. (2000). Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin. Exp. Immunol. 121, 255–260. doi:10.1046/j.1365-2249.2000.01281.x
Cao, L., Li, W., Yang, X., Zhang, W., Li, M., Zhang, H., et al. (2021). Inhibition of host Ogr1 enhances effector CD8(+) T-cell function by modulating acidic microenvironment. Cancer Gene Ther. 28, 1213–1224. doi:10.1038/s41417-021-00354-0
Chahine, L., Rosso, A. L., Troidl, I., Ganguli, M., Newman, A. B., Lopresti, B. J., et al. (2025). Features of prodromal parkinson disease and dopaminergic neurotransmission in community-dwelling older adults. Neurology 105, e214037. doi:10.1212/wnl.0000000000214037
Chandra, V., Karamitri, A., Richards, P., Cormier, F., Ramond, C., Jockers, R., et al. (2016). Extracellular acidification stimulates GPR68 mediated IL-8 production in human pancreatic β cells. Sci. Rep. 6, 25765. doi:10.1038/srep25765
Chen, H. (2018). Author response: olfaction and incident parkinson disease in US white and Black older adults. Neurology 90, 941. doi:10.1212/wnl.0000000000005515
Cornell, J., Rea, S., Neitzel, L. R., Williams, C. H., and Hong, C. C. (2024). Proton sensing GPCR's: the missing link to Warburg's oncogenic legacy? J. Cancer Biol. 5, 65–75. doi:10.46439/cancerbiology.5.066
Cornwell, A. C., Tisdale, A. A., Venkat, S., Maraszek, K. E., Alahmari, A. A., George, A., et al. (2023). Lorazepam stimulates IL6 production and is associated with poor survival outcomes in pancreatic cancer. Clin. Cancer Res. Official J. Am. Assoc. Cancer Res. 29, 3793–3812. doi:10.1158/1078-0432.Ccr-23-0547
Cuollo, L., Antonangeli, F., Santoni, A., and Soriani, A. (2020). The senescence-associated secretory phenotype (SASP) in the challenging future of cancer therapy and age-related diseases. Biology 9, 485. doi:10.3390/biology9120485
de Vallière, C., Cosin-Roger, J., Simmen, S., Atrott, K., Melhem, H., Zeitz, J., et al. (2016). Hypoxia positively regulates the expression of pH-Sensing G-Protein-Coupled receptor OGR1 (GPR68). Cell. Mol. Gastroenterology Hepatology 2, 796–810. doi:10.1016/j.jcmgh.2016.06.003
de Vallière, C., Bäbler, K., Busenhart, P., Schwarzfischer, M., Maeyashiki, C., Schuler, C., et al. (2021). A novel OGR1 (GPR68) inhibitor attenuates inflammation in murine models of colitis. Inflamm. Intest. Dis. 6, 140–153. doi:10.1159/000517474
de Vallière, C., Cosin-Roger, J., Baebler, K., Schoepflin, A., Mamie, C., Mollet, M., et al. (2022). pH-Sensing G protein-coupled receptor OGR1 (GPR68) expression and activation increases in intestinal inflammation and fibrosis. Int. J. Mol. Sci. 23, 1419. doi:10.3390/ijms23031419
Dolphin, H., Dyer, A. H., Morrison, L., Shenkin, S. D., Welsh, T., and Kennelly, S. P. (2024). New Horizons in the diagnosis and management of alzheimer's disease in older adults. Age Ageing 53, afae005. doi:10.1093/ageing/afae005
Elemam, N. M., Youness, R. A., Hussein, A., Shihab, I., Yakout, N. M., Elwany, Y. N., et al. (2022). Expression of GPR68, an acid-sensing orphan G protein-coupled receptor, in breast cancer. Breast Cancer, Front. Oncol. 12, 847543. doi:10.3389/fonc.2022.847543
Faget, D. V., Ren, Q., and Stewart, S. A. (2019). Unmasking senescence: context-dependent effects of SASP in cancer. Nat. Rev. Cancer 19, 439–453. doi:10.1038/s41568-019-0156-2
Ferroni, P., Basili, S., Martini, F., Cardarello, C. M., Ceci, F., Di Franco, M., et al. (2003). Serum metalloproteinase 9 levels in patients with coronary artery disease: a novel marker of inflammation. J. Investigative Med. Official Publ. Am. Fed. Clin. Res. 51, 295–300. doi:10.1136/jim-51-05-17
Foord, S. M., Bonner, T. I., Neubig, R. R., Rosser, E. M., Pin, J. P., Davenport, A. P., et al. (2005). International union of pharmacology. XLVI. G protein-coupled receptor list. Pharmacol. Rev. 57, 279–288. doi:10.1124/pr.57.2.5
Foster, S. R., Hauser, A. S., Vedel, L., Strachan, R. T., Huang, X. P., Gavin, A. C., et al. (2019). Discovery of human signaling systems: pairing peptides to G protein-coupled receptors. Cell 179, 895–908.e21. doi:10.1016/j.cell.2019.10.010
Gonzalez Caldito, N. (2023). Role of tumor necrosis factor-alpha in the central nervous system: a focus on autoimmune disorders. Front. Immunol. 14, 1213448. doi:10.3389/fimmu.2023.1213448
Guo, J., Huang, X., Dou, L., Yan, M., Shen, T., Tang, W., et al. (2022). Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 7, 391. doi:10.1038/s41392-022-01251-0
Haque, M. E., Akther, M., Azam, S., Choi, D. K., and Kim, I. S. (2020). GPR4 knockout improves the neurotoxin-induced, caspase-dependent mitochondrial apoptosis of the dopaminergic neuronal cell. Int. J. Mol. Sci. 21, 7517. doi:10.3390/ijms21207517
Haque, M. E., Azam, S., Akther, M., Cho, D. Y., Kim, I. S., and Choi, D. K. (2021). The neuroprotective effects of GPR4 inhibition through the attenuation of caspase mediated apoptotic cell death in an MPTP induced mouse model of parkinson's disease. Int. J. Mol. Sci. 22, 4674. doi:10.3390/ijms22094674
Harrison, E. C., Tueth, L. E., Haussler, A. M., Rawson, K. S., and Earhart, G. M. (2025). Personalized auditory rhythmic cues to optimize gait in older adults and people with parkinson disease. J. Neurologic Phys. Ther. JNPT 49, 162–170. doi:10.1097/npt.0000000000000508
Hauser, A. S., Attwood, M. M., Rask-Andersen, M., Schiöth, H. B., and Gloriam, D. E. (2017). Trends in GPCR drug discovery: new agents, targets and indications. Nat. Rev. Drug Discov. 16, 829–842. doi:10.1038/nrd.2017.178
He, X., Hawkins, C., Lawley, L., Phan, T. M., Park, I., Joven, N., et al. (2025). Loss of G-protein coupled receptor 68 in hematopoietic tissues enhances long-term hematopoietic stem cell function upon aging. Stem Cell Res. and Ther. 16, 408. doi:10.1186/s13287-025-04506-z
Herzig, M., Dasgupta, P., Kaemmerer, D., Sänger, J., Evert, K., Schulz, S., et al. (2019). Comprehensive assessment of GPR68 expression in normal and neoplastic human tissues using a novel rabbit monoclonal antibody. Int. J. Mol. Sci. 20, 5261. doi:10.3390/ijms20215261
Hirano, T. (2021). IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 33, 127–148. doi:10.1093/intimm/dxaa078
Huang, X. P., Karpiak, J., Kroeze, W. K., Zhu, H., Chen, X., Moy, S. S., et al. (2015). Allosteric ligands for the pharmacologically dark receptors GPR68 and GPR65. Nature 527, 477–483. doi:10.1038/nature15699
Hutter, S., van Haaften, W. T., Hünerwadel, A., Baebler, K., Herfarth, N., Raselli, T., et al. (2018). Intestinal activation of pH-Sensing receptor OGR1 [GPR68] contributes to fibrogenesis. J. Crohn's and Colitis 12, 1348–1358. doi:10.1093/ecco-jcc/jjy118
Ichimonji, I., Tomura, H., Mogi, C., Sato, K., Aoki, H., Hisada, T., et al. (2010). Extracellular acidification stimulates IL-6 production and Ca(2+) mobilization through proton-sensing OGR1 receptors in human airway smooth muscle cells. Lung Cell. Mol. Physiology 299, L567–L577. doi:10.1152/ajplung.00415.2009
Imenez Silva, P. H., and Wagner, C. A. (2022). Physiological relevance of proton-activated GPCRs. Pflugers Archiv Eur. J. Physiology 474, 487–504. doi:10.1007/s00424-022-02671-1
Jeon, O. H., David, N., Campisi, J., and Elisseeff, J. H. (2018). Senescent cells and osteoarthritis: a painful connection. J. Clin. Investigation 128, 1229–1237. doi:10.1172/jci95147
Jha, S. K., De Rubis, G., Devkota, S. R., Zhang, Y., Adhikari, R., Jha, L. A., et al. (2024). Cellular senescence in lung cancer: molecular mechanisms and therapeutic interventions. Ageing Res. Rev. 97, 102315. doi:10.1016/j.arr.2024.102315
Jumper, J., Evans, R., Pritzel, A., Green, T., Figurnov, M., Ronneberger, O., et al. (2021). Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589. doi:10.1038/s41586-021-03819-2
Justus, C. R., Dong, L., and Yang, L. V. (2013). Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front. Physiology 4, 354. doi:10.3389/fphys.2013.00354
Justus, C. R., Marie, M. A., Sanderlin, E. J., and Yang, L. V. (2024). The roles of proton-sensing G-Protein-Coupled receptors in inflammation and cancer. Genes 15, 1151. doi:10.3390/genes15091151
Khan, M. Z., and He, L. (2017). Neuro-psychopharmacological perspective of orphan receptors of rhodopsin (class A) family of G protein-coupled receptors. Psychopharmacology 234, 1181–1207. doi:10.1007/s00213-017-4586-9
Khan, N. M., Diaz-Hernandez, M. E., Martin, W. N., Patel, B., Chihab, S., and Drissi, H. (2023). pH-sensing G protein-coupled orphan receptor GPR68 is expressed in human cartilage and correlates with degradation of extracellular matrix during OA progression. PeerJ 11, e16553. doi:10.7717/peerj.16553
Koeglsperger, T., Rumpf, S. L., Schließer, P., Struebing, F. L., Brendel, M., Levin, J., et al. (2023). Neuropathology of incidental lewy body and prodromal parkinson's disease. Mol. Neurodegener. 18, 32. doi:10.1186/s13024-023-00622-7
Kumari, N., Reabroi, S., and North, B. J. (2021). Unraveling the molecular nexus between GPCRs, ERS, and EMT. Mediat. Inflamm. 2021, 6655417. doi:10.1155/2021/6655417
Li, H., Wang, D., Singh, L. S., Berk, M., Tan, H., Zhao, Z., et al. (2009). Abnormalities in osteoclastogenesis and decreased tumorigenesis in mice deficient for ovarian cancer G protein-coupled receptor 1. PloS One 4, e5705. doi:10.1371/journal.pone.0005705
Li, M. S., Wang, X. H., and Wang, H. (2024). Immunomodulation of proton-activated G protein-coupled receptors in inflammation. Curr. Med. Sci. 44, 475–484. doi:10.1007/s11596-024-2872-4
Lin, Z. C., Hsu, C. Y., Hwang, E., Wang, P. W., and Fang, J. Y. (2023). The role of cytokines/chemokines in an aging skin immune microenvironment. Mech. Ageing Dev. 210, 111761. doi:10.1016/j.mad.2022.111761
Liu, J. P., Komachi, M., Tomura, H., Mogi, C., Damirin, A., Tobo, M., et al. (2010). Ovarian cancer G protein-coupled receptor 1-dependent and -independent vascular actions to acidic pH in human aortic smooth muscle cells. Am. J. Physiology. Heart Circulatory Physiology 299, H731–H742. doi:10.1152/ajpheart.00977.2009
Ludwig, M. G., Vanek, M., Guerini, D., Gasser, J. A., Jones, C. E., Junker, U., et al. (2003). Proton-sensing G-protein-coupled receptors. Nature 425, 93–98. doi:10.1038/nature01905
Ma, S., Xie, X., Yuan, R., Xin, Q., Miao, Y., Leng, S. X., et al. (2024). Vascular aging and atherosclerosis: a perspective on aging. Aging Dis. 16, 33–48. doi:10.14336/ad.2024.0201-1
Maciel-Barón, L. A., Morales-Rosales, S. L., Aquino-Cruz, A. A., Triana-Martínez, F., Galván-Arzate, S., Luna-López, A., et al. (2016). Senescence associated secretory phenotype profile from primary lung mice fibroblasts depends on the senescence induction stimuli. Age Dordr. Neth. 38, 26. doi:10.1007/s11357-016-9886-1
Matsingos, C., Howell, L. A., McCormick, P. J., and Fornili, A. (2024). Elucidating the activation mechanism of the proton-sensing GPR68 receptor. J. Mol. Biol. 436, 168688. doi:10.1016/j.jmb.2024.168688
Matsuzaki, S., Ishizuka, T., Yamada, H., Kamide, Y., Hisada, T., Ichimonji, I., et al. (2011). Extracellular acidification induces connective tissue growth factor production through proton-sensing receptor OGR1 in human airway smooth muscle cells. Biochem. Biophysical Res. Commun. 413, 499–503. doi:10.1016/j.bbrc.2011.08.087
Mazuelas, H., Magallón-Lorenz, M., Uriarte-Arrazola, I., Negro, A., Rosas, I., Blanco, I., et al. (2024). Unbalancing cAMP and Ras/MAPK pathways as a therapeutic strategy for cutaneous neurofibromas. JCI Insight 9, e168826. doi:10.1172/jci.insight.168826
McAleer, J. P., Fan, J., Roar, B., Primerano, D. A., and Denvir, J. (2018). Cytokine regulation in human CD4 T cells by the aryl hydrocarbon receptor and Gq-Coupled receptors. Sci. Rep. 8, 10954. doi:10.1038/s41598-018-29262-4
Merchant, A. A., and Ling, E. (2023). An approach to treating older adults with chronic kidney disease. CMAJ Can. Med. Assoc. J. = J. De l'Association Medicale Can. 195, E612–e618. doi:10.1503/cmaj.221427
Millan, D. S., Kayser-Bricker, K. J., Martin, M. W., Talbot, A. C., Schiller, S. E. R., Herbertz, T., et al. (2017). Design and optimization of benzopiperazines as potent inhibitors of BET bromodomains. ACS Med. Chem. Lett. 8, 847–852. doi:10.1021/acsmedchemlett.7b00191
Murata, N., Mogi, C., Tobo, M., Nakakura, T., Sato, K., Tomura, H., et al. (2009). Inhibition of superoxide anion production by extracellular acidification in neutrophils. Cell. Immunol. 259, 21–26. doi:10.1016/j.cellimm.2009.05.008
Nayak, A. P., Deshpande, D. A., Shah, S. D., Villalba, D. R., Yi, R., Wang, N., et al. (2021). OGR1-dependent regulation of the allergen-induced asthma phenotype. Lung Cell. Mol. physiology 321, L1044–l1054. doi:10.1152/ajplung.00200.2021
Okajima, F. (2013). Regulation of inflammation by extracellular acidification and proton-sensing GPCRs. Cell. Signal. 25, 2263–2271. doi:10.1016/j.cellsig.2013.07.022
Ortiz-Montero, P., Londoño-Vallejo, A., and Vernot, J. P. (2017). Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun. Signal. CCS 15, 17. doi:10.1186/s12964-017-0172-3
Ozkan, A. D., Gettas, T., Sogata, A., Phaychanpheng, W., Zhou, M., and Lacroix, J. J. (2021). Mechanical and chemical activation of GPR68 probed with a genetically encoded fluorescent reporter. J. Cell Sci. 134, jcs255455. doi:10.1242/jcs.255455
Pan, H., Li, H., Guo, S., Wang, C., Long, L., Wang, X., et al. (2023). The mechanisms and functions of TNF-α in intervertebral disc degeneration. Exp. Gerontol. 174, 112119. doi:10.1016/j.exger.2023.112119
Perren, L., Busch, M., Schuler, C., Ruiz, P. A., Foti, F., Weibel, N., et al. (2023). OGR1 (GPR68) and TDAG8 (GPR65) have antagonistic effects in models of colonic inflammation. Int. J. Mol. Sci. 24, 14855. doi:10.3390/ijms241914855
Pradhan, A. D., Manson, J. E., Rifai, N., Buring, J. E., and Ridker, P. M. (2001). C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. Jama 286, 327–334. doi:10.1001/jama.286.3.327
Rowe, J. B., Kapolka, N. J., Taghon, G. J., Morgan, W. M., and Isom, D. G. (2021). The evolution and mechanism of GPCR proton sensing. J. Biol. Chem. 296, 100167. doi:10.1074/jbc.RA120.016352
Russell, J. L., Goetsch, S. C., Aguilar, H. R., Coe, H., Luo, X., Liu, N., et al. (2012). Regulated expression of pH sensing G Protein-coupled receptor-68 identified through chemical biology defines a new drug target for ischemic heart disease. ACS Chem. Biol. 7, 1077–1083. doi:10.1021/cb300001m
Sarakpi, T., Mesic, A., and Speer, T. (2023). Leukocyte-endothelial interaction in CKD. Clin. kidney J. 16, 1845–1860. doi:10.1093/ckj/sfad135
Saxena, H., Deshpande, D. A., Tiegs, B. C., Yan, H., Battafarano, R. J., Burrows, W. M., et al. (2012). The GPCR OGR1 (GPR68) mediates diverse signalling and contraction of airway smooth muscle in response to small reductions in extracellular pH. Br. J. Pharmacol. 166, 981–990. doi:10.1111/j.1476-5381.2011.01807.x
Shore, D., Griggs, N., Graffeo, V., Amin, A., Zha, X. M., Xu, Y., et al. (2023). GPR68 limits the severity of chemical-induced oral epithelial dysplasia. Sci. Rep. 13, 353. doi:10.1038/s41598-023-27546-y
Sisignano, M., Fischer, M. J. M., and Geisslinger, G. (2021). Proton-sensing GPCRs in health and disease. Cells 10, 2050. doi:10.3390/cells10082050
Sohail, S., Yu, L., Schneider, J. A., Bennett, D. A., Buchman, A. S., and Lim, A. S. P. (2017). Sleep fragmentation and parkinson's disease pathology in older adults without parkinson's disease. Mov. Disord. Official J. Mov. Disord. Soc. 32, 1729–1737. doi:10.1002/mds.27200
Sokolove, J., and Lepus, C. M. (2013). Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 5, 77–94. doi:10.1177/1759720x12467868
Studer, G., Tauriello, G., Bienert, S., Biasini, M., Johner, N., and Schwede, T. (2021). ProMod3-A versatile homology modelling toolbox. PLoS Comput. Biol. 17, e1008667. doi:10.1371/journal.pcbi.1008667
Tanaka, T., Narazaki, M., and Kishimoto, T. (2014). IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 6, a016295. doi:10.1101/cshperspect.a016295
Tang, S., Nie, X., Ruan, J., Cao, Y., Kang, J., and Ding, C. (2022). Circular RNA circNFKB1 promotes osteoarthritis progression through interacting with ENO1 and sustaining NF-κB signaling. Cell Death and Dis. 13, 695. doi:10.1038/s41419-022-05148-2
Tomura, H., Wang, J. Q., Komachi, M., Damirin, A., Mogi, C., Tobo, M., et al. (2005). Prostaglandin I(2) production and cAMP accumulation in response to acidic extracellular pH through OGR1 in human aortic smooth muscle cells. J. Biol. Chem. 280, 34458–34464. doi:10.1074/jbc.M505287200
Tylutka, A., Walas, Ł., and Zembron-Lacny, A. (2024). Level of IL-6, TNF, and IL-1β and age-related diseases: a systematic review and meta-analysis. Front. Immunol. 15, 1330386. doi:10.3389/fimmu.2024.1330386
van Setten, G. B. (2022). GPR-68 in human lacrimal gland. Detection and possible role in the pathogenesis of dry eye disease. J. Francais D'ophtalmologie 45, 921–927. doi:10.1016/j.jfo.2022.02.009
Vassilatis, D. K., Hohmann, J. G., Zeng, H., Li, F., Ranchalis, J. E., Mortrud, M. T., et al. (2003). The G protein-coupled receptor repertoires of human and mouse. Proc. Natl. Acad. Sci. U. S. A. 100, 4903–4908. doi:10.1073/pnas.0230374100
Wang, T., Zhou, G., He, M., Xu, Y., Rusyniak, W. G., Xu, Y., et al. (2020). GPR68 is a neuroprotective proton receptor in brain ischemia. Stroke 51, 3690–3700. doi:10.1161/strokeaha.120.031479
Wieland, H. A., Michaelis, M., Kirschbaum, B. J., and Rudolphi, K. A. (2005). Osteoarthritis - an untreatable disease? Nat. Rev. Drug Discov. 4, 331–344. doi:10.1038/nrd1693
Wiley, S. Z., Sriram, K., Liang, W., Chang, S. E., French, R., McCann, T., et al. (2018). “GPR68, a proton-sensing GPCR, mediates interaction of cancer-associated fibroblasts and cancer cells”, 32. official publication of the Federation of American Societies for Experimental Biology, 1170–1183. doi:10.1096/fj.201700834RFASEB J.
Wiley, S. Z., Sriram, K., Salmerón, C., and Insel, P. A. (2019). GPR68: an emerging drug target in cancer. Int. J. Mol. Sci. 20, 559. doi:10.3390/ijms20030559
Williams, S. J., Zammit, S. C., Cox, A. J., Shackleford, D. M., Morizzi, J., Zhang, Y., et al. (2013). 3',4'-Bis-difluoromethoxycinnamoylanthranilate (FT061): an orally-active antifibrotic agent that reduces albuminuria in a rat model of progressive diabetic nephropathy. Bioorg. and Med. Chem. Lett. 23, 6868–6873. doi:10.1016/j.bmcl.2013.09.100
Williams, C. H., Neitzel, L. R., Cornell, J., Rea, S., Mills, I., Silver, M. S., et al. (2024). GPR68-ATF4 signaling is a novel prosurvival pathway in glioblastoma activated by acidic extracellular microenvironment. Exp. Hematol. and Oncol. 13, 13. doi:10.1186/s40164-023-00468-1
Xiao, Q., Hou, R., Xie, L., Niu, M., Pan, X., and Zhu, X. (2023). Macrophage metabolic reprogramming and atherosclerotic plaque microenvironment: fostering each other? Clin. Transl. Med. 13, e1257. doi:10.1002/ctm2.1257
Xiong, J., Dong, L., Lv, Q., Yin, Y., Zhao, J., Ke, Y., et al. (2024). Targeting senescence-associated secretory phenotypes to remodel the tumour microenvironment and modulate tumour outcomes. Clin. Transl. Med. 14, e1772. doi:10.1002/ctm2.1772
Xu, Y., and Casey, G. (1996). Identification of human OGR1, a novel G protein-coupled receptor that maps to chromosome 14. Genomics 35, 397–402. doi:10.1006/geno.1996.0377
Xu, J., Mathur, J., Vessières, E., Hammack, S., Nonomura, K., Favre, J., et al. (2018). GPR68 senses flow and is essential for vascular physiology. Cell 173, 762–775.e16. doi:10.1016/j.cell.2018.03.076
Xu, S., Ilyas, I., Little, P. J., Li, H., Kamato, D., Zheng, X., et al. (2021). Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol. Rev. 73, 924–967. doi:10.1124/pharmrev.120.000096
Yamanaka, T., Harimoto, N., Yokobori, T., Muranushi, R., Hoshino, K., Hagiwara, K., et al. (2021). Conophylline inhibits hepatocellular carcinoma by inhibiting activated cancer-associated fibroblasts through suppression of G protein-coupled receptor 68. Mol. Cancer Ther. 20, 1019–1028. doi:10.1158/1535-7163.Mct-20-0150
Yan, L., Singh, L. S., Zhang, L., and Xu, Y. (2014). Role of OGR1 in myeloid-derived cells in prostate cancer. Oncogene 33, 157–164. doi:10.1038/onc.2012.566
Ye, S., Zhu, Y., Zhong, D., Song, X., Li, J., Xiao, F., et al. (2023). G protein-coupled receptor GPR68 inhibits lymphocyte infiltration and contributes to gender-dependent melanoma growth. Front. Oncol. 13, 1202750. doi:10.3389/fonc.2023.1202750
Yoshida, Y., Matsunaga, N., Nakao, T., Hamamura, K., Kondo, H., Ide, T., et al. (2021). Alteration of circadian machinery in monocytes underlies chronic kidney disease-associated cardiac inflammation and fibrosis. Nat. Commun. 12, 2783. doi:10.1038/s41467-021-23050-x
Yoshida, Y., Fukuoka, K., Sakugawa, M., Kurogi, M., Hamamura, K., Hamasaki, K., et al. (2024). Inhibition of G protein-coupled receptor 68 using homoharringtonine attenuates chronic kidney disease-associated cardiac impairment. J. Laboratory Clin. Med. 269, 31–46. doi:10.1016/j.trsl.2024.02.004
Yu, X., Huang, X. P., Kenakin, T. P., Slocum, S. T., Chen, X., Martini, M. L., et al. (2019). Design, synthesis, and characterization of Ogerin-Based positive allosteric modulators for G protein-coupled receptor 68 (GPR68). J. Med. Chem. 62, 7557–7574. doi:10.1021/acs.jmedchem.9b00869
Yu, S., Liu, D., Yan, C., Yuan, C., Zhang, C., and Zheng, S. (2024). A novel mutation in GPR68 causes hypomaturation amelogenesis imperfecta. Archives Oral Biol. 164, 105991. doi:10.1016/j.archoralbio.2024.105991
Zeng, Z., Mukherjee, A., Varghese, A. P., Yang, X. L., Chen, S., and Zhang, H. (2020). Roles of G protein-coupled receptors in inflammatory bowel disease. World J. Gastroenterology 26, 1242–1261. doi:10.3748/wjg.v26.i12.1242
Zhang, Y., Edgley, A. J., Cox, A. J., Powell, A. K., Wang, B., Kompa, A. R., et al. (2012). FT011, a new anti-fibrotic drug, attenuates fibrosis and chronic heart failure in experimental diabetic cardiomyopathy. Eur. J. Heart Fail. 14, 549–562. doi:10.1093/eurjhf/hfs011
Zhang, Y., Elsik, M., Edgley, A. J., Cox, A. J., Kompa, A. R., Wang, B., et al. (2013). A new anti-fibrotic drug attenuates cardiac remodeling and systolic dysfunction following experimental myocardial infarction. Int. J. Cardiol. 168, 1174–1185. doi:10.1016/j.ijcard.2012.11.067
Zhang, W., Han, Y., Li, W., Cao, L., Yan, L., Qin, C., et al. (2020). Clinical data analysis reveals the role of OGR1 (GPR68) in head and neck squamous cancer. Animal Models Exp. Med. 3, 55–61. doi:10.1002/ame2.12105
Zhang, X., Ge, R., Wu, J., Cai, X., Deng, G., Lv, J., et al. (2024). Structural characterization and improves cognitive disorder in ageing mice of a glucomannan from Dendrobium huoshanense. Int. J. Biol. Macromol. 269, 131995. doi:10.1016/j.ijbiomac.2024.131995
Zhou, S., Ma, G., Luo, H., Shan, S., Xiong, J., and Cheng, G. (2023). Identification of 5 potential predictive biomarkers for alzheimer's disease by integrating the unified test for molecular signatures and weighted gene coexpression network analysis. journals gerontology. Ser. A, Biol. Sci. Med. Sci. 78, 653–658. doi:10.1093/gerona/glac179
Keywords: GPR68, chronic inflammation, immune response, aging-related diseases, therapeutic target
Citation: Wei J, Cui F, Huang Z, Li Z, Mao Z and Zhang P (2025) Acid sensing to inflammaging: mechanisms and therapeutic promise of GPR68 (OGR1) in aging-related diseases. Front. Aging 6:1684450. doi: 10.3389/fragi.2025.1684450
Received: 12 August 2025; Accepted: 10 October 2025;
Published: 20 October 2025.
Edited by:
Minglei Guo, Eastern Virginia Medical School, United StatesReviewed by:
Mithlesh Kumar Temre, National Institute on Aging (NIH), United StatesWenhui Hu, Virginia Commonwealth University, United States
Copyright © 2025 Wei, Cui, Huang, Li, Mao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pengxia Zhang, cGVuZ3hpYXpAMTYzLmNvbQ==; Zebin Mao, emViaW5tYW9AcGt1LmVkdS5jbg==
 Jianlei Wei
Jianlei Wei Fengxin Cui3
Fengxin Cui3 Zebin Mao
Zebin Mao Pengxia Zhang
Pengxia Zhang