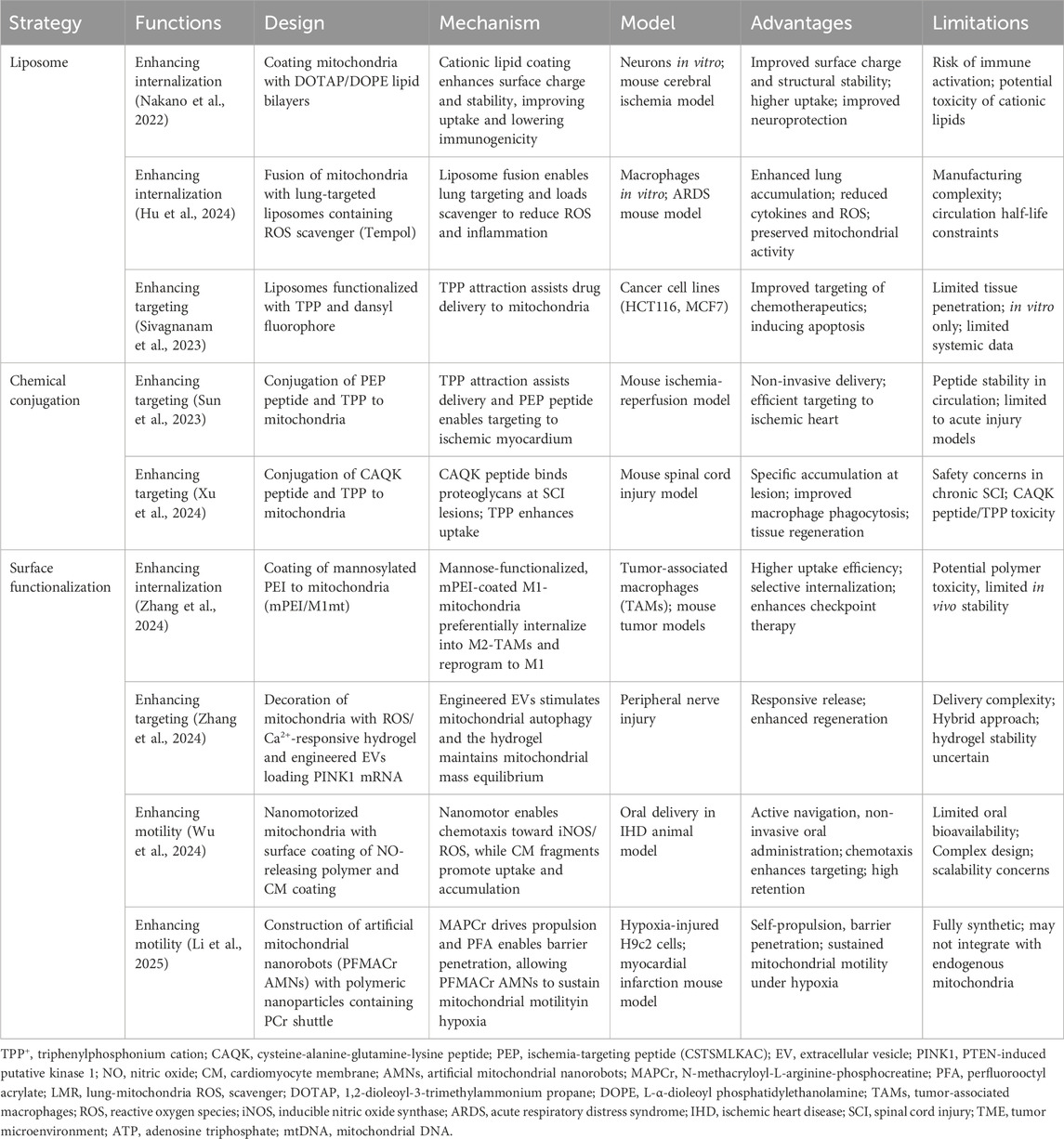
- 1BMI Center for Biomass Materials and Nanointerfaces, National Engineering Laboratory for Clean Technology of Leather Manufacture, Key Laboratory of Leather Chemistry and Engineering (Sichuan University), Ministry of Education, College of Biomass Science and Engineering, Sichuan University, Chengdu, Sichuan, China
- 2Tea Refining and Innovation Key Laboratory of Sichuan Province, College of Horticulture, Sichuan Agricultural University, Chengdu, Sichuan, China
- 3Division of 64K Cellssense, Vitagenix Asia Research & Innovation, Hong Kong SAR, China
- 4State Key Laboratory of Polymer Materials Engineering, Sichuan University, Chengdu, Sichuan, China
- 5Department of Chemical and Biological Engineering, Bioproducts Institute, The University of British Columbia, Vancouver, BC, Canada
Aging is a multifactorial process and a major risk factor for chronic disease. Among its hallmarks, mitochondrial dysfunction plays a central role, driven by impaired respiration and accumulated mitochondrial DNA mutations that disrupt energy metabolism and redox balance. Conventional mitochondrial transplantation has been explored as a therapeutic strategy, but its emphasis on increasing mitochondrial quantity without restoring function has limited success. Recent advances in nanoengineered mitochondria that integrate isolated mitochondria with functional nanomaterials, offer new opportunities to enhance organelle quality, boost metabolic activity, and achieve targeted delivery. Preclinical studies highlight their promise in cardiovascular, neurodegenerative, and other age-related disorders. In this mini-review, mitochondrial dysfunction in aging is first introduced, followed by the summary of rational designed strategies for engineering mitochondrial biohybrids and their emerging applications, and finally translational challenges are further discussed. By bridging materials science and mitochondrial therapy, nanoengineered mitochondria may represent a next-generation approach to anti-aging interventions.
1 Introduction
Aging represents one of the most pressing global challenges in the coming decades (Li et al., 2024). According to the World Health Organization, the population aged 60 years and older is projected to double from 1 billion in 2020 to 2.1 billion by 2050, highlighting the immense scale of global aging. The development of age-related pathologies not only seriously impacts the health of the elderly population but also imposes a socioeconomic burden, driving the urgent need for effective anti-aging strategies (Tuttle et al., 2021). A growing body of research has revealed that aging is driven by the accumulation of diverse cellular insults, including mitochondrial dysfunction, genomic instability, and oxidative stress. Specifically, mitochondria are essential regulators of energy metabolism, signaling, and cell fate (Guo et al., 2023; Suomalainen and Nunnari, 2024), but their function declines with age, marked by impaired oxidative phosphorylation (OXPHOS), accumulation of mitochondrial deoxyribonucleic acid (mtDNA) mutations, dysregulation of the tricarboxylic acid (TCA) cycle, and elevated levels of reactive oxygen species (ROS) (Sun et al., 2016). This functional decline is not merely a measurement of aging but actively drives the aging, positioning mitochondria as both triggers and amplifiers of cellular senescence.
Naturally derived mitochondrial modulators emerge as prominent anti-aging strategies, with mainstream antioxidant components including calcium α-ketoglutarate (AKG) (Ames, 2018; Zhang et al., 2023), ergothioneine (EGT) (Chin et al., 2014; Zhao et al., 2024c), ubiquinone-10 (Díaz-Casado et al., 2019), selenium (Bjørklund et al., 2022; Huang et al., 2024), and resveratrol (Lagouge et al., 2006; Drago et al., 2024). AKG, a key intermediate in the TCA cycle, and EGT, a thiol-histidine derivative, act synergistically to support mitochondrial homeostasis and systemic resilience against age-associated stress. However, their delivery efficiency and targeting precision remain largely underdeveloped. Meanwhile, conventional mitochondrial transplantation (MT), a therapeutic process involving the isolation and delivery of healthy exogenous mitochondria to damaged cells or organs to restore bioenergetics and promote repair, typically relies on the direct injection or infusion of isolated, unmodified mitochondria (Headley and Tsao, 2023). MT has been shown to restore cardiomyocyte and neuronal function across various disease contexts. Preclinical studies further demonstrate that MT can revive adenosine triphosphate (ATP) production, alleviate oxidative stress, and improve organ function in models of ischemia and neurodegeneration (Sun et al., 2020; Mukherjee et al., 2021). Although conventional MT increases mitochondrial quantity, this unmodified delivery approach fails to enhance the quality and activity of individual mitochondria, thereby limiting its therapeutic potential against aging and related pathologies (Riou et al., 2025).
Inspired by cell surface engineering, we propose nanoengineered mitochondria, which are biohybrid systems formed by integrating synthetic nanomaterials or biomolecules with isolated mitochondria to confer new functionalities (He et al., 2024; He et al., 2025; Liu et al., 2025). This emerging strategy operates at the interface of bioengineering and mitochondrial biology and aims to overcome the limitations of conventional MT (Guo et al., 2014; Qiu et al., 2021). These tailored nanobiohybrid systems have the potential to improve mitochondrial quality, boost metabolic activity, and reduce ROS levels (Chen et al., 2025; Wang et al., 2025a; Wang et al., 2025c). Moreover, these systems can enhance the targeting efficiency and motility of mitochondria, which is achieved through mitochondrial ligand-receptor recognition (e.g., triphenylphosphonium cation (TPP+)-modified nanoparticles and mitochondrial membrane potentials) (Zeng et al., 2025), stimulus-responsive navigation (e.g., pH/ROS-sensitive polymers guiding mitochondria to inflammatory sites), and external field-driven propulsion (e.g., magnetically steered nanocapsules). This mini-review therefore focuses specifically on the emerging of nanoengineered mitochondria, moving beyond the scope of earlier reviews that centered primarily on conventional transplantation. We first discuss the central role of mitochondrial dysfunction in aging and present recent advances in the rational design of nanoengineered mitochondria. We then highlight their therapeutic potential in treating age-related diseases and conclude by critically evaluating translational challenges and future directions (Figure 1). Collectively, we envision nanoengineered mitochondria as a next-generation platform for precise anti-aging interventions.
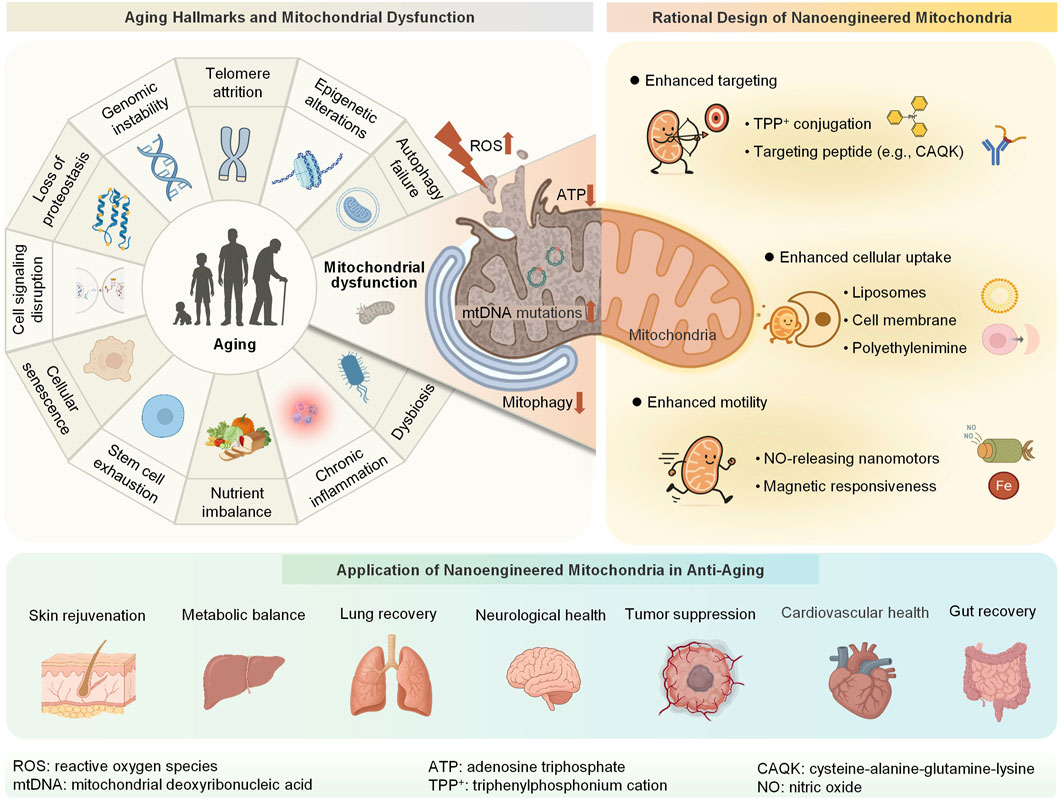
Figure 1. Schematic overview of the relationship between aging and mitochondrial dysfunction, the design of nanoengineered mitochondria, and their applications in anti-aging therapy. ROS, reactive oxygen species; mtDNA, mitochondrial deoxyribonucleic acid; ATP, adenosine triphosphate; TPP+, triphenylphosphonium cation; NO, nitric oxide; CAQK, cysteine-alanine-glutamine-lysine (Created with BioRender.com).
2 Mechanisms of mitochondrial dysfunction in aging
Aging is a complex and multifactorial biological process marked by the gradual and irreversible loss of cellular homeostasis and regenerative capacity, serving as a major risk factor for chronic diseases such as neurodegeneration, cardiovascular dysfunction, metabolic disorders, and cancer (Guo et al., 2022). In 2023, López-Otín et al. proposed twelve hallmarks of aging (López-Otín et al., 2023). Among these, mitochondrial dysfunction represents a major driver of aging, arising through a multilayered chain of causes and effects (Somasundaram et al., 2024). The accumulation of mtDNA mutations reduces electron transport chain efficiency and elevates ROS production, and a commonly proposed hypothesis is that ROS-induced damage further amplifies mitochondrial dysfunction, forming a self-reinforcing cycle (Nandi et al., 2019; Houldsworth, 2024). However, it remains unclear whether this cycle represents a primary driver of systemic aging or merely constitutes a secondary consequence of other pathological processes, a fundamental question that remains a major research gap in the field. In parallel, reduced activity of the PTEN-induced putative kinase 1 (PINK1)–PARKIN RBR E3 ubiquitin ligase (PARKIN) pathway impairs mitophagy, allowing defective mitochondria to persist and thereby exacerbating neurodegeneration and inflammation (Pickrell and Youle, 2015; Fang et al., 2019; Picca et al., 2023; Narendra and Youle, 2024). Furthermore, declines in respiratory chain function and ATP supply particularly compromise homeostasis in high-energy-demand tissues such as the brain and heart (Desler et al., 2012; Gasmi et al., 2024). Notably, the relative contribution of bioenergetic deficit versus oxidative damage to aging phenotypes across different tissues is still not fully quantified. Disruption of the AMP-activated protein kinase (AMPK)–Sirtuin 1 (SIRT1)–Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) signaling axis further impedes mitochondrial biogenesis and metabolic flexibility, promoting inflammation and senescence (Toyama et al., 2016; Zhu et al., 2025).
Collectively, these mitochondrial impairments converge to accelerate aging, underscoring the urgent need for integrated strategies to restore mitochondrial quality and function. By contrast, nutrient supplements such as AKG and EGT mitigate dysfunction through multiple mechanisms. AKG fuels energy metabolism, scavenges ROS, protects membranes, and supports Fe2+/AKG-dependent dioxygenases, thereby driving epigenetic remodeling and delaying senescence. It also activates the AMPK–SIRT1–PGC-1α axis, promoting mitochondrial biogenesis (Cheng et al., 2024). EGT accumulates in mitochondria, scavenges ROS, preserves mtDNA integrity, and maintains cristae structure while enhancing respiration through sulfur-transferase activity and redox cycling (Chin et al., 2014; Ames, 2018).
3 Rational design of nanoengineered mitochondria
While nutritional supplements such as AKG and EGT offer a promising, multi-mechanistic approach to ameliorate mitochondrial dysfunction, their efficacy remains constrained by poor bioavailability, insufficient tissue-specific targeting, and the complexity of age-related damage. To overcome these challenges, nanoengineered mitochondria have emerged as a promising alternative. Nanoengineered mitochondria are biohybrid systems in which isolated mitochondria are integrated with functional nanomaterials, including inorganic particles, organic polymers, and biomacromolecules. This integration confers improved targeting, enhanced motility, and greater cellular internalization. In practice, mitochondrial modification is commonly achieved through small peptide labeling, liposome transfection, vesicle packaging, or polymer coating (Zhao et al., 2024). These approaches provide versatile platforms for tailoring mitochondrial functions and can be categorized into strategies that enhance targeting, motility, and cellular internalization, with representative examples summarized in Table 1.
3.1 Enhancing mitochondrial targeting function
Liposomes functionalized with mitochondrial-targeting moieties represent a prominent platform for targeted drug delivery and mitochondrial modulation (Gajbhiye et al., 2023). TPP+ possesses a cationic and lipophilic nature and can accumulate in mitochondria while maintaining low reactivity and ease of synthesis (Kulkarni et al., 2021; Kaneko et al., 2023; Pawar et al., 2023). The conjugation of TPP+ enables specific targeting to cancer cell mitochondria, facilitating the delivery of drugs to trigger apoptosis (Reshetnikov et al., 2018). For instance, liposomes of TPP+-functionalized 10,12-pentacosadiynoic acid in phospholipids provide a stable cationic ligand with fluorescent labeling, enabling both targeting and visualization of mitochondrial interactions (Sivagnanam et al., 2023). To further improve targeting specificity, TPP+ was conjugated with cysteine-alanine-glutamine-lysine (CAQK) peptides (Xu et al., 2024). This design facilitated strong mitochondrial binding by inserting into the outer mitochondrial membrane to form the Mito-TPP-CAQK compound, thereby improving delivery precision. Building on the previous development as a versatile linker, TPP+ was used to connect the ischemia-targeting peptide (PEP, CSTSMLKAC) to mitochondria (Sun et al., 2023), to generate a PEP-TPP-mitochondria compound, where an enhanced cellular internalization into AC16 cardiomyocytes was observed compared with unmodified mitochondria, highlighting the potential of functionalized liposomes for cardiac applications. Beyond synthetic liposomes, adaptive hydrogel systems have also been explored to construct nanoengineered mitochondria to regulate the functions. In a model of Wallerian degeneration (WD) post-peripheral nerve injury, adaptive hydrogels have been used to deliver engineered extracellular vesicles (E-EV-P@HPCEP) carrying PINK1 mRNA (Zhao et al., 2024a). These EVs target senescent Schwann cells, stimulate mitochondrial autophagy, and maintain mitochondrial mass balance, thereby mitigating WD progression and improving nerve repair outcomes. Taken together, these findings suggest that liposomes equipped with mitochondrial-targeting ligands, especially TPP+-based constructs, offer a versatile and efficient strategy for mitochondrial drug delivery.
3.2 Enhancing mitochondrial motility
Nanoengineered mitochondria offer a promising approach to enhance mitochondrial motility, which played a significant role in the therapeutic outcomes in cardiac tissues (Sun et al., 2025). Mitochondria modified with nitric oxide (NO)-releasing nanomotors, to generate nanomotorized mitochondria (NM/Mito), can be guided toward cardiac lesions via chemotactic migration, characterized by high levels of inducible nitric oxide synthase (iNOS) and ROS (Wu et al., 2024). Further coating the cardiomyocyte membrane (CM) fragments asymmetrically to the surface of NM/Mito to generate CM/NM/Mito and further loading into pH-responsive enteric capsules to generate CM/NM/Mito@Cap, a non-invasive mitochondrial transplant strategy was developed via oral administration. CM/NM/Mito showed time-dependent fluorescence enhancement in high ROS/iNOS reservoirs, indicating active chemotaxis along the concentration gradient. Encapsulated in enteric-coated capsules for oral delivery, the formulation avoids gastric acid degradation and targets myocardial tissues, aiding ischemic heart disease (IHD) treatment. In parallel, monomers with motility (N-methacryloyl-L-arginine-phosphocreatine, MAPCr) and trans-barrier (Perfluorooctyl acrylate, PFA) units were employed to synthesize artificial mitochondrial nanorobots (PFMACr AMNs) (Li et al., 2025). The design of PFMACr AMNs enables the provision of high-energy phosphate bonds to damaged mitochondria, and enhances motility within pathological microenvironments.
3.3 Enhancing mitochondrial internalization
Surface functionalization with targeting ligands and bioactive molecules have been widely explored to enhance mitochondrial internalization, thereby improving the therapeutic efficacy of transplanted mitochondria in tissue repair (Wang et al., 2024a). A representative example is the development of a lung-targeted, mitochondria-based ROS scavenging system, termed LMR (lung-mitochondria ROS scavenger). LMR was fabricated by fusing mitochondria-targeting liposomes with lung-targeting liposomes encapsulating the ROS scavenger Tempol (Hu et al., 2024). The liposomes were prepared from dimethyldioctadecylammonium bromide, cholesterol, and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine–polyethylene glycol 2000-Tempol (DSPE-PEG2000-Tempol) using a method of solvent evaporation. In an ARDS model, LMR showed enhanced lung accumulation, preserved mitochondrial activity, and alleviated inflammation compared with unmodified mitochondria. An alternative strategy to enhance mitochondrial delivery is coating isolated mitochondria with a cationic artificial lipid membrane. Using a modified inverted emulsion method, mitochondria were encapsulated with 1,2-dioleoyl-3-trimethylammonium propane/L-α-dioleoyl phosphatidyl ethanolamine (DOTAP/DOPE) lipids, generating artificial membrane-coated mitochondria (AM-mito) with improved surface charge and structural stability. This coating not only preserved key mitochondrial proteins and membrane potential but also significantly enhanced cellular internalization and neuroprotective efficacy in vitro and in vivo (Nakano et al., 2022). Compared to unmodified mitochondria, AM-mito demonstrated uptake by neurons under ischemic conditions and reduced brain infarct volume following cerebral ischemia-reperfusion injury. By further exploring mitochondrial surface engineering, low-molecular-weight polyethyleneimine (mPEI) was used to coat isolated mitochondria from M1 macrophages, resulting in mannose-functionalized mitochondria (mPEI/M1mt) (Zhang et al., 2024). This modification enhanced mitochondrial uptake by tumor-associated macrophages (TAMs) and promoted their phenotypic shift toward pro-inflammatory M1 macrophages.
4 Application of nanoengineered mitochondria in anti-aging
Building on these rational design strategies, nanoengineered mitochondria have been applied in diverse preclinical models to rejuvenate mitochondrial performance and restore cellular homeostasis. Integrated with functional nanomaterials, these systems extend the therapeutic scope of conventional mitochondrial transplantation and have shown promise across a wide range of pathologies, including cancer, acute respiratory distress syndrome (ARDS), spinal cord injury, and peripheral nerve damage (Hu et al., 2024; Wang et al., 2025b; Yang et al., 2025). In the context of aging, their applications can be broadly categorized into three strategies: activation of mitochondrial autophagy, nanoengineered approaches in mitochondrial transplantation, and enhancement of ATP production capacity.
4.1 Activation of mitochondrial autophagy
Reduction of mitochondrial autophagy contributes to aging and is generally associated with the accumulation of damaged mitochondria, further linked to various age-related diseases such as pulmonary fibrosis, erectile dysfunction, and cardiovascular diseases. A recent study developed mitophagy-enhanced nanoengineered mitochondria (Mito-MEN) by anchoring PARKIN mRNA-loaded nanoparticles to healthy mitochondria, which not only improved exogenous mitochondrial delivery but also activated mitophagy to eliminate endogenous damaged mitochondria (Wang et al., 2024b). In a pulmonary fibrosis model, Mito-MEN restored mitochondrial pool homeostasis and significantly alleviated fibrosis symptoms, demonstrating the potential of nanoengineered mitochondria in treating aging-related lung disease. A schematic design of a piezoelectric nanosystem was developed for the treatment of diabetes-related erectile dysfunction (Wang et al., 2025b). In an erectile dysfunction model, the piezoelectric co-loaded nanosystem induced current that promoted mitochondrial autophagy and reduced ROS generation, thereby decreasing blood glucose levels and protecting mitochondria from damage. Each component of this nanosystem functions independently or cooperatively, thereby promoting penile repair and restoring erectile function.
4.2 Improvement of mitochondrial transplantation
MT technology, by delivering healthy mitochondria to damaged cells, tissues, or organs, has emerged as a potential therapeutic approach for treating mtDNA-related diseases and restoring the mitochondrial function of diseased cells in recent years (Zhao et al., 2024b). For example, PARKIN mRNA-loaded nanoparticle-engineered mitochondria (mNP-Mito) enhanced the delivery efficiency of healthy exogenous mitochondria and the mitophagy of damaged mitochondria, thereby restoring the function of complex I in treating Leber hereditary optic neuropathy (Wang et al., 2024c). Moreover, surface-engineered mitochondria that reprogram TAMs from pro-tumor M2 to anti-tumor M1 phenotype (mPEI/M1mt) through immunometabolic modulation can significantly enhance checkpoint inhibitor therapy in murine cancer models (Zhang et al., 2024). The transplantation of mPEI/M1mt reshapes the tumor immune microenvironment by promoting the activation and infiltration of CD8+ and CD4+ T cells. Specifically, CD8+ T cells act as the principal cytotoxic effectors, releasing perforin and granzymes to directly induce apoptosis of tumor cells (Koh et al., 2023). In parallel, CD4+ T helper cells orchestrate immune responses by secreting cytokines such as Interleukin-2 (IL-2) and Interferon-gamma (IFN-γ), which sustain CD8+ T cell proliferation and function, while also supporting dendritic cell maturation and antigen presentation (Topchyan et al., 2023). The coordinated action of cytotoxic and helper T cells is further strengthened by surface-engineered mitochondrial transplantation, which restores bioenergetics and promotes immune reprogramming, thereby synergizing with anti-Programmed Death-Ligand 1 (PD-L1) treatment to achieve superior antitumor efficacy.
4.3 Enhancement of mitochondrial ATP production
Damage to the ATP-producing capacity of mitochondria directly affects the energy supply of cells, leading to a series of diseases. Mitochondria-based nanorobots (PFMACr AMNs) can offer sufficient energy by manipulating the internal phosphate bond to effectively treat ischemic heart disease (Li et al., 2025). By co-incubating PFMACr AMNs with hypoxia-injured H9c2 cells, it was found that PFMACr AMNs were able to synthesize ATP for 12 h, keeping the ATP level in hypoxia-injured H9c2 cells comparable to that in normal H9c2 cells. In a myocardial infarction mouse model, PFMACr AMNs sustained ATP levels and reduced infarct size, demonstrating superior therapeutic potential. This innovative design opens up a new path for the construction of an artificial energy delivery system in the body. Despite these advances, most applications of nanoengineered mitochondria remain limited to rodent models, with large-animal and early human data still lacking. This gap represents a major barrier to clinical translation.
5 Conclusion
Functional nanomaterials, ranging from versatile inorganic and organic materials to biomacromolecules, have been integrated with mitochondria to construct engineered systems endowed with enhanced targeting, motility, and internalization efficiency. In addition to increasing mitochondrial biogenesis, this strategy also strengthens mitochondrial respiration and energy production. We reviewed mitochondrial dysfunction in aging, outlined design strategies of nanoengineered mitochondria, and summarized recent advances in their applications to age-related diseases. Collectively, these advances pave the way for the development of next-generation subcellular therapies targeting aging and its related disorders. Nevertheless, the clinical translation of nanoengineered mitochondria is still at an early stage, and several major challenges remain.
5.1 Delivery efficiency and stability
Mitochondria are fragile organelles, and preserving their structural and functional integrity during systemic administration remains technically challenging. Oral delivery, while advantageous for patient compliance, still requires improved tissue penetration, biological stability, and bioavailability. Potential strategies include polymeric or lipid coatings, encapsulation within hydrogels or microcapsules, and vesicle-based delivery systems to protect mitochondria and improve biodistribution. In addition, artificial intelligence-assisted design may help optimize material-mitochondria interactions and guide the development of more efficient delivery formulations.
5.2 Safety and immunogenicity
Long-term biosafety and immunogenicity need careful evaluation in both autologous and allogeneic contexts. While surface functionalization improves targeting efficiency, it may also cause off-target accumulation, immune responses, or interference with host metabolism. Future research should therefore emphasize systematic biosafety assessment and the development of low-immunogenic coatings or immune-evasive surface modifications to ensure safe translation.
5.3 Mechanistic understanding and therapeutic enhancement
The molecular mechanisms by which nanoengineered mitochondria interact with host signaling pathways remain incompletely understood. It is critical to clarify their roles in mitochondrial biogenesis, mitophagy, the AMPK–SIRT1–PGC-1α axis, and immunometabolic reprogramming. In parallel, incorporating widely studied anti-aging components such as AKG, EGT, selenium, ubiquinone-10, and resveratrol into nanoengineered mitochondrial systems may enhance mitochondrial quality, improve redox homeostasis, and expand therapeutic potential. Advanced tools such as organoid-based disease models, gene editing, and systems-level analyses can further accelerate mechanistic insights and support rational design.
Author contributions
SD: Formal Analysis, Investigation, Writing – original draft. YR: Investigation, Writing – original draft. QZ: Investigation, Writing – original draft. QL: Formal Analysis, Writing – review and editing. JL: Visualization, Writing – review and editing. KP: Visualization, Writing – review and editing. MM: Visualization, Writing – review and editing. TM: Visualization, Writing – review and editing. CY: Visualization, Writing – review and editing. YH: Conceptualization, Funding acquisition, Supervision, Writing – review and editing. JG: Conceptualization, Funding acquisition, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors declare that financial support was received for the preparation and publication of this article. The author acknowledges the financial support from National Key R&D Program of China (2022YFA0912800), National Excellent Young Scientists Fund (00308054A1045), National Natural Science Foundation of China (22178233, 22408241), Talents Program of Sichuan Province, Double First-Class University Plan of Sichuan University, State Key Laboratory of Polymer Materials Engineering (sklpme 2020-03-01), Sichuan Tianfu Emei Project (2022-EC02-00073-CG), and Fundamental Research Funds for the Central Universities (SCU2025D014) for the stipend during the preparation of this mini review article.
Acknowledgments
The authors acknowledge the suggestions from Gonghua Hong and the supports from the Key Laboratory of Leather Chemistry and Engineering (Sichuan University), Ministry of Education, and National Engineering Research Center of Clean Technology in Leather Industry. Figure 1 created in BioRender https://BioRender.com.
Conflict of interest
Authors KP, MM, TM, and CY were employed by Division of 64K Cellssense, Vitagenix Asia Research & Innovation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ames, B. N. (2018). Prolonging healthy aging: longevity vitamins and proteins. Proc. Natl. Acad. Sci. 115 (43), 10836–10844. doi:10.1073/pnas.1809045115
Bjørklund, G., Shanaida, M., Lysiuk, R., Antonyak, H., Klishch, I., Shanaida, V., et al. (2022). Selenium: an antioxidant with a critical role in anti-aging. Molecules 27 (19), 6613. doi:10.3390/molecules27196613
Chen, M., Dai, M., Hong, G., Li, F., Wu, Y., Pu, Y., et al. (2025). Personalized cervical plug combines mechanical and biological regulation for enhanced embryo implantation and live births. Matter 8 (6), 102043. doi:10.1016/j.matt.2025.102043
Cheng, D., Zhang, M., Zheng, Y., Wang, M., Gao, Y., Wang, X., et al. (2024). α-Ketoglutarate prevents hyperlipidemia-induced fatty liver mitochondrial dysfunction and oxidative stress by activating the AMPK-pgc-1α/Nrf2 pathway. Redox Biol. 74, 103230. doi:10.1016/j.redox.2024.103230
Chin, R. M., Fu, X., Pai, M. Y., Vergnes, L., Hwang, H., Deng, G., et al. (2014). The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature 510 (7505), 397–401. doi:10.1038/nature13264
Desler, C., Hansen, T. L., Frederiksen, J. B., Marcker, M. L., Singh, K. K., and Juel Rasmussen, L. (2012). Is there a link between mitochondrial reserve respiratory capacity and aging? J. Aging Res. 2012 (1), 192503. doi:10.1155/2012/192503
Díaz-Casado, M. E., Quiles, J. L., Barriocanal-Casado, E., González-García, P., Battino, M., López, L. C., et al. (2019). The paradox of coenzyme Q10 in aging. Nutrients 11 (9), 2221. doi:10.3390/nu11092221
Drago, L., Ciprandi, G., Brindisi, G., Brunese, F. P., Dinardo, G., Gori, A., et al. (2024). Certainty and uncertainty in the biological activities of resveratrol. Food Front. 5 (3), 849–854. doi:10.1002/fft2.375
Fang, E. F., Hou, Y., Palikaras, K., Adriaanse, B. A., Kerr, J. S., Yang, B., et al. (2019). Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer's disease. Nat. Neurosci. 22 (3), 401–412. doi:10.1038/s41593-018-0332-9
Gajbhiye, K. R., Salve, R., Narwade, M., Sheikh, A., Kesharwani, P., and Gajbhiye, V. (2023). Lipid polymer hybrid nanoparticles: a custom-tailored next-generation approach for cancer therapeutics. Mol. Cancer 22 (1), 160. doi:10.1186/s12943-023-01849-0
Gasmi, A., Bjørklund, G., Mujawdiya, P. K., Semenova, Y., Piscopo, S., and Peana, M. (2024). Coenzyme Q10 in aging and disease. Crit. Rev. Food. Sci. 64 (12), 3907–3919. doi:10.1080/10408398.2022.2137724
Guo, J., Ping, Y., Ejima, H., Alt, K., Meissner, M., Richardson, J. J., et al. (2014). Engineering multifunctional capsules through the assembly of metal–phenolic networks. Angew. Chem. Int. Ed. 53 (22), 5546–5551. doi:10.1002/anie.201311136
Guo, J., Huang, X., Dou, L., Yan, M., Shen, T., Tang, W., et al. (2022). Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 7 (1), 391. doi:10.1038/s41392-022-01251-0
Guo, Y., Guan, T., Shafiq, K., Yu, Q., Jiao, X., Na, D., et al. (2023). Mitochondrial dysfunction in aging. Ageing Res. Rev. 88, 101955. doi:10.1016/j.arr.2023.101955
He, X., Gong, G., Chen, M., Zhang, H., Zhang, Y., Richardson, J. J., et al. (2024). Metal-phenolic nanocloaks on cancer cells potentiate STING pathway activation for synergistic cancer immunotherapy. Angew. Chem. Int. Ed. 63 (12), e202314501. doi:10.1002/anie.202314501
He, Y., Liu, Q., He, Y., Deng, S., and Guo, J. (2025). Engineering live cell surfaces with polyphenol-functionalized nanoarchitectures. Chem. Sci. 16 (9), 3774–3787. doi:10.1039/d4sc07198k
Headley, C. A., and Tsao, P. S. (2023). Building the case for mitochondrial transplantation as an anti-aging cardiovascular therapy. Front. Cardiovasc. Med. 10, 1141124. doi:10.3389/fcvm.2023.1141124
Houldsworth, A. (2024). Role of oxidative stress in neurodegenerative disorders: a review of reactive oxygen species and prevention by antioxidants. Brain Commun. 6 (1), fcad356. doi:10.1093/braincomms/fcad356
Hu, H., Zhang, W., Zhou, Y., Zhao, K., Kuang, J., Liu, X., et al. (2024). Engineered mitochondrial ROS scavenger nanocomplex to enhance lung biodistribution and reduce inflammation for the treatment of ARDS. Adv. Compos. Hybrid. Mater. 7 (6), 194. doi:10.1007/s42114-024-00989-1
Huang, L., Song, L., Hao, C., Xu, C., Kuang, H., and Qu, A. (2024). Combination of chiral selenium and manganese nanoparticles for alleviating aging through antioxidation and antiinflammation. Nano Res. 18 (6), 94907467. doi:10.26599/NR.2025.94907467
Kaneko, M., Yamazaki, H., Ono, T., Horie, M., and Ito, A. (2023). Effective magnetic hyperthermia induced by mitochondria-targeted nanoparticles modified with triphenylphosphonium-containing phospholipid polymers. Cancer Sci. 114 (9), 3750–3758. doi:10.1111/cas.15895
Koh, C.-H., Lee, S., Kwak, M., Kim, B.-S., and Chung, Y. (2023). CD8 T-cell subsets: heterogeneity, functions, and therapeutic potential. Exp. Mol. Med. 55 (11), 2287–2299. doi:10.1038/s12276-023-01105-x
Kulkarni, C. A., Fink, B. D., Gibbs, B. E., Chheda, P. R., Wu, M., Sivitz, W. I., et al. (2021). A novel triphenylphosphonium carrier to target mitochondria without uncoupling oxidative phosphorylation. J. Med. Chem. 64 (1), 662–676. doi:10.1021/acs.jmedchem.0c01671
Lagouge, M., Argmann, C., Gerhart-Hines, Z., Meziane, H., Lerin, C., Daussin, F., et al. (2006). Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127 (6), 1109–1122. doi:10.1016/j.cell.2006.11.013
Li, Y., Tian, X., Luo, J., Bao, T., Wang, S., and Wu, X. (2024). Molecular mechanisms of aging and anti-aging strategies. Cell Commun. Signal. 22 (1), 285. doi:10.1186/s12964-024-01663-1
Li, N., Zhou, M., Wu, Z., Chen, Y., Duan, Y., Zhang, Z., et al. (2025). Artificial mitochondrial nanorobots deliver energy in vivo by oral administration. Adv. Mater 37, 2500495. doi:10.1002/adma.202500495
Liu, Q., Wu, Y., Fan, Q., Liu, J., Chen, Y., He, Y., et al. (2025). Oral nanoarmored live bacterial biotherapeutics bearing polyphenol-based supraparticles enhance chemotherapy via reestablishing immuno-oncology-microbiome axis. ACS Nano 19 (26), 23575–23591. doi:10.1021/acsnano.5c01158
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., and Kroemer, G. (2023). Hallmarks of aging: an expanding universe. Cell 186 (2), 243–278. doi:10.1016/j.cell.2022.11.001
Mukherjee, A., Calixto, A. D. B., Chavez, M., Delgado, J. P., and Soto, C. (2021). Mitochondrial transplant to replenish damaged mitochondria: a novel therapeutic strategy for neurodegenerative diseases? Prog. Mol. Biol. Transl. Sci. 177, 49–63. doi:10.1016/bs.pmbts.2020.10.001
Nakano, T., Nakamura, Y., Park, J.-H., Tanaka, M., and Hayakawa, K. (2022). Mitochondrial surface coating with artificial lipid membrane improves the transfer efficacy. Commun. Biol. 5 (1), 745. doi:10.1038/s42003-022-03719-9
Nandi, A., Yan, L.-J., Jana, C. K., and Das, N. (2019). Role of catalase in oxidative stress-and age-associated degenerative diseases. Oxid. Med. Cell. Longev. 2019 (1), 9613090. doi:10.1155/2019/9613090
Narendra, D. P., and Youle, R. J. (2024). The role of PINK1–Parkin in mitochondrial quality control. Nat. Cell Biol. 26 (10), 1639–1651. doi:10.1038/s41556-024-01513-9
Pawar, A., Korake, S., Pawar, A., and Kamble, R. (2023). Delocalized lipophilic cation triphenyl phosphonium: promising molecule for mitochondria targeting. Curr. Drug Deliv. 20 (9), 1217–1223. doi:10.2174/1567201819666220525092527
Picca, A., Faitg, J., Auwerx, J., Ferrucci, L., and D’Amico, D. (2023). Mitophagy in human health, ageing and disease. Nat. Metab. 5 (12), 2047–2061. doi:10.1038/s42255-023-00930-8
Pickrell, A. M., and Youle, R. J. (2015). The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron 85 (2), 257–273. doi:10.1016/j.neuron.2014.12.007
Qiu, X., Wang, X., He, Y., Liang, J., Liang, K., Tardy, B. L., et al. (2021). Superstructured mesocrystals through multiple inherent molecular interactions for highly reversible sodium ion batteries. Sci. Adv. 7 (37), eabh3482. doi:10.1126/sciadv.abh3482
Reshetnikov, V., Daum, S., Janko, C., Karawacka, W., Tietze, R., Alexiou, C., et al. (2018). ROS-responsive N-alkylaminoferrocenes for cancer-cell-specific targeting of mitochondria. Angew. Chem. Int. Ed. 57 (37), 11943–11946. doi:10.1002/anie.201805955
Riou, A., Broeglin, A., and Grimm, A. (2025). Mitochondrial transplantation in brain disorders: achievements, methods, and challenges. Neurosci. Biobehav. Rev. 169, 105971. doi:10.1016/j.neubiorev.2024.105971
Sivagnanam, S., Das, K., Pan, I., Stewart, A., Barik, A., Maity, B., et al. (2023). Engineered triphenylphosphonium-based, mitochondrial-targeted liposomal drug delivery system facilitates cancer cell killing actions of chemotherapeutics. RSC Chem. Biol. 5 (3), 236–248. doi:10.1039/d3cb00219e
Somasundaram, I., Jain, S. M., Blot-Chabaud, M., Pathak, S., Banerjee, A., Rawat, S., et al. (2024). Mitochondrial dysfunction and its association with age-related disorders. Front. Physiol. 15, 1384966. doi:10.3389/fphys.2024.1384966
Sun, N., Youle, R. J., and Finkel, T. (2016). The mitochondrial basis of aging. Mol. Cell 61 (5), 654–666. doi:10.1016/j.molcel.2016.01.028
Sun, X., Gao, R., Li, W., Zhao, Y., Yang, H., Chen, H., et al. (2020). Alda-1 treatment promotes the therapeutic effect of mitochondrial transplantation for myocardial ischemia-reperfusion injury. Bioact. Mater. 6 (7), 2058–2069. doi:10.1016/j.bioactmat.2020.12.024
Sun, X., Chen, H., Gao, R., Qu, Y., Huang, Y., Zhang, N., et al. (2023). Intravenous transplantation of an ischemic-specific Peptide-TPP-mitochondrial compound alleviates myocardial ischemic reperfusion injury. ACS Nano 17 (2), 896–909. doi:10.1021/acsnano.2c05286
Sun, M., van Oss, L., Wan, C., and Wilson, D. A. (2025). Communicative nanomotors reprogram cancer cell death via pyroptosis. Angew. Chem. Int. Ed. 64, e202510014. doi:10.1002/anie.202510014
Suomalainen, A., and Nunnari, J. (2024). Mitochondria at the crossroads of health and disease. Cell 187 (11), 2601–2627. doi:10.1016/j.cell.2024.04.037
Topchyan, P., Lin, S., and Cui, W. (2023). The role of CD4 T cell help in CD8 T cell differentiation and function during chronic infection and cancer. Immune Netw. 23 (5), e41. doi:10.4110/in.2023.23.e41
Toyama, E. Q., Herzig, S., Courchet, J., Lewis Jr, T. L., Losón, O. C., Hellberg, K., et al. (2016). Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 351 (6270), 275–281. doi:10.1126/science.aab4138
Tuttle, C. S., Luesken, S. W., Waaijer, M. E., and Maier, A. B. (2021). Senescence in tissue samples of humans with age-related diseases: a systematic review. Ageing Res. Rev. 68, 101334. doi:10.1016/j.arr.2021.101334
Wang, C., Su, Y., and Xie, J. (2024a). Advances in electrospun nanofibers: versatile materials and diverse biomedical applications. Acc. Mater. Res. 5 (8), 987–999. doi:10.1021/accountsmr.4c00145
Wang, Y., Hu, L. F., Liu, N. H., Yang, J. S., Xing, L., Jeong, J. H., et al. (2024b). Mitophagy-enhanced nanoparticle-engineered mitochondria restore homeostasis of the mitochondrial pool for alleviating pulmonary fibrosis. ACS Nano 47 (18), 32705–32722. doi:10.1021/acsnano.4c10328
Wang, Y., Liu, N., Hu, L., Yang, J., Han, M., Zhou, T., et al. (2024c). Nanoengineered mitochondria enable ocular mitochondrial disease therapy via the replacement of dysfunctional mitochondria. Acta Pharm. Sin. B 14 (12), 5435–5450. doi:10.1016/j.apsb.2024.08.007
Wang, S., Wang, Z., Zang, Z., Liang, X., Jia, B., Ye, T., et al. (2025a). A mitochondrion-targeting piezoelectric nanosystem for the treatment of erectile dysfunction via autophagy regulation. Adv. Mater. 37 (5), 2413287. doi:10.1002/adma.202413287
Wang, W., Yao, S.-Y., Luo, J., Ding, C., Huang, Q., Yang, Y., et al. (2025b). Engineered hypoxia-responsive albumin nanoparticles mediating mitophagy regulation for cancer therapy. Nat. Commun. 16 (1), 596. doi:10.1038/s41467-025-55905-y
Wang, Y., He, Y., Hong, G., Wang, X., and Guo, J. (2025c). Conversion of plant polyphenols into high-value products and multi-disciplinary applications. Sci. Sin. Chim. 55 (1), 37–49. doi:10.1360/SSC-2024-0209
Wu, Z., Chen, L., Guo, W., Wang, J., Ni, H., Liu, J., et al. (2024). Oral mitochondrial transplantation using nanomotors to treat ischaemic heart disease. Nat. Nanotechnol. 19 (9), 1375–1385. doi:10.1038/s41565-024-01681-7
Xu, J., Shi, C., Yuan, F., Ding, Y., Xie, Y., Liu, Y., et al. (2024). Targeted transplantation of engineered mitochondrial compound promotes functional recovery after spinal cord injury by enhancing macrophage phagocytosis. Bioact. Mater. 32, 427–444. doi:10.1016/j.bioactmat.2023.10.016
Yang, G., Dong, C., Wu, Z., Wu, P., Yang, C., Li, L., et al. (2025). Single-cell RNA sequencing-guided engineering of mitochondrial therapies for intervertebral disc degeneration by regulating mtDNA/SPARC-STING signaling. Bioact. Mater. 48, 564–582. doi:10.1016/j.bioactmat.2025.02.036
Zeng, J., Yan, Z., Wang, D., He, T., Tong, Z., Miao, J., et al. (2025). Mitochondria-targeted MXene@ MnO2-TPP nanoheterostructures for synergistic enhancement of sonodynamic therapy and immunotherapy in osteosarcoma. Bioact. Mater. 54, 450–465. doi:10.1016/j.bioactmat.2025.08.029
Zhang, W., Ding, L., Zhang, M., Zheng, S., Ma, R., Gong, J., et al. (2023). Dietary intake of α-ketoglutarate ameliorates α-synuclein pathology in mouse models of Parkinson’s disease. Cell. Mol. Life Sci. 80 (6), 155. doi:10.1007/s00018-023-04807-7
Zhang, C. J., Li, J. M., Xu, D., Wang, D. D., Qi, M. H., Chen, F., et al. (2024). Surface molecularly engineered mitochondria conduct immunophenotype repolarization of tumor-associated macrophages to potentiate cancer immunotherapy. Adv. Sci. 11 (38), 2403044. doi:10.1002/advs.202403044
Zhao, R., Deng, X., Tang, Y., Yang, X., Ge, Z., Wang, D., et al. (2024a). Mitigating critical peripheral nerve deficit therapy with reactive oxygen species/Ca2+-responsive dynamic hydrogel-mediated mRNA delivery. ACS Nano 18 (26), 16556–16576. doi:10.1021/acsnano.3c13102
Zhao, R., Dong, C., Liang, Q., Gao, J., Sun, C., Gu, Z., et al. (2024b). Engineered mitochondrial transplantation as an anti-aging therapy. Aging Dis. 16 (4), 1918–1945. doi:10.14336/AD.2024.0231
Zhao, X., Yang, X., Du, C., Hao, H., Liu, S., Liu, G., et al. (2024c). Up-regulated succinylation modifications induce a senescence phenotype in microglia by altering mitochondrial energy metabolism. J. Neuroinflamm. 21 (1), 296. doi:10.1186/s12974-024-03284-4
Keywords: anti-aging, nanoengineered mitochondrial biohybrids, surface functionalization, mitochondrial function restoration, age-related diseases
Citation: Deng S, Ren Y, Zhang Q, Liu Q, Long J, Picard K, Martin M, Miller T, Yuan C, He Y and Guo J (2025) Nanoengineered mitochondria for mitochondrial dysfunction and anti-aging interventions. Front. Aging 6:1688482. doi: 10.3389/fragi.2025.1688482
Received: 19 August 2025; Accepted: 15 September 2025;
Published: 26 September 2025.
Edited by:
Ryan Varghese, Saint Joseph’s University, United StatesReviewed by:
Mayank Sharma, SVKM’s Narsee Moonjee Institute of Management & Studies (NMIMS), IndiaRavi Vamsi Peri, Saint Joseph’s University, United States
Copyright © 2025 Deng, Ren, Zhang, Liu, Long, Picard, Martin, Miller, Yuan, He and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunxiang He, eXhoZUBzY3UuZWR1LmNu
 Siqi Deng1
Siqi Deng1 Chaofan Yuan
Chaofan Yuan Yunxiang He
Yunxiang He Junling Guo
Junling Guo