- 1U.S. Geological Survey, Western Fisheries Research Center, Seattle, WA, United States
- 2Three Rivers Chapter, Trout Unlimited, Issaquah, WA, United States
- 3College of Fisheries and Ocean Sciences, University of Alaska Fairbanks, Fairbanks, AK, United States
- 4Department of Biology, Pacific Lutheran University, Tacoma, WA, United States
- 5College of Arts and Sciences - Biology, Saint Martin’s University, Lacey, WA, United States
- 6Lake Washington Basin Program, Trout Unlimited, Issaquah, WA, United States
- 7Aquatic Invasive Species Division, Fish Program, Washington State Department of Fish and Wildlife, Olympia, WA, United States
- 8Science Division, Habitat Program, Washington State Department of Fish and Wildlife, Olympia, WA, United States
- 9Wildlife Program, District 12 (Region 4), Washington State Department of Fish and Wildlife, Mill Creek, WA, United States
- 10Division of Biological Sciences, University of Washington, Bothell, WA, United States
- 11Southwestern Native Aquatic Resources and Recovery Center, US Fish and Wildlife Service, Dexter, NM, United States
- 12Department of Biological Sciences, California State University - East Bay, Hayward, CA, United States
- 13Wildlife Program, Washington State Department of Fish and Wildlife, Olympia, WA, United States
- 14Genetic and Health Laboratories, Washington State Department of Fish and Wildlife, Olympia, WA, United States
The African clawed frog (ACF, Xenopus laevis), which is indigenous to sub-Saharan Africa, is an aquatic invasive species known to have severe ecological impacts on native fauna when introduced into non-endemic regions. In 2015, ACFs were detected in Washington State, U.S. for the first time, and the species is now documented at three cities across western Washington: Lacey, Bothell, and Issaquah. We cataloged the known ACF occurrences, early management efforts, biological data about the frogs, and status of these invasive populations at the three sites from 2015–2023. The ACFs appear to be established in at least three watersheds in the Puget Sound region despite substantial effort at eradicating them at one site. Presence of ACFs in watersheds that lack surface connectivity implies independent introduction events, and the capture of frogs in multiple subbasins in the same watershed may reflect the potential for further spread. Because the ACF is nocturnal and otherwise behaviorally and visually highly cryptic, other established populations may go undetected. Where the ACFs are largely confined to stormwater ponds — as many of our current observations suggest — eradication may still be possible, though a substantial, focused effort would be required. In addition, significant refinement of eradication approaches will be needed to ensure effectiveness in topographically and vegetatively complex Pacific Northwest aquatic environments.
1 Introduction
Aquatic invasive species are a significant threat to freshwater ecosystems globally and particularly in North America (Lucy and Panov, 2014; Havel et al., 2015). Although climate-driven range shifts and land use change have facilitated some invasions, other anthropogenic activities like fishing, the pet trade, and the movement of garden and landscaping materials have also resulted in the introduction and establishment of many harmful invasive amphibian species outside of their native ranges (Lockwood et al., 2019; Gervais et al., 2020; Poland et al., 2021; Hossack et al., 2022). African clawed frogs (ACFs, Xenopus laevis), which are native to sub-Saharan Africa, are one of the most widely distributed invasive amphibian species globally (Williams, 2011; Van Sittert and Measey, 2016). Starting in the 1930s, ACFs were reared in laboratories for use as human pregnancy tests, and they have since become a model organism for scientific research and a common pet (Gurdon and Hopwood, 2000; Burggren and Warburton, 2007). Because of their prevalence in these global industries and their longevity, ACFs have been released into numerous waterways of the United States (U.S.) (U.S. Fish and Wildlife Service, 2017). ACFs may threaten native biodiversity through predation, competition, and/or as carriers of novel pathogens (Vredenburg et al., 2013; Duffus et al., 2015; Schoeman et al., 2020).
In the U.S., ACFs were first documented in the state of Florida in 1964, and subsequently in the states of Arizona, California, Colorado, Massachusetts, North Carolina, Texas, Virginia, and Wisconsin from 1965–1996 (Crayon, 2005; Somma and Freedman, 2023). Unsubstantiated sightings of ACFs have also been reported in Nevada, New Mexico, Utah, and Wyoming. Once established, ACF population levels typically do not decline unless extirpated by early, labor-intensive eradication efforts, as occurred in Texas, or when confronted with extreme cold weather events (Measey et al., 2012; Tinsley et al., 2015; Somma and Freedman, 2023). Despite attempts to control ACFs in some locations – including restricting the species in the pet and biomedical trade – the global spread of ACFs is likely to continue due to additional releases in concert with regional climate shifts that favor ACF persistence (Measey et al., 2012; Andersen et al., 2021).
In July 2015, two ACF populations were independently discovered in Washington State, marking the species’ first detection in the state (Ojala-Barbour et al., 2021). Since then, an additional population has been documented, and ACFs are now known to occupy three different urbanized areas of the Puget Sound region. All three cities with ACF introductions (Figure 1) are found in distinct, hydrologically divided watersheds in western Washington. Multiple organizations have responded to the discovery of ACFs in Washington, with early efforts focused on eradication of the ACFs and subsequent work aimed at scientific monitoring to determine the extent of ACF establishment, breeding, and/or potential spread.
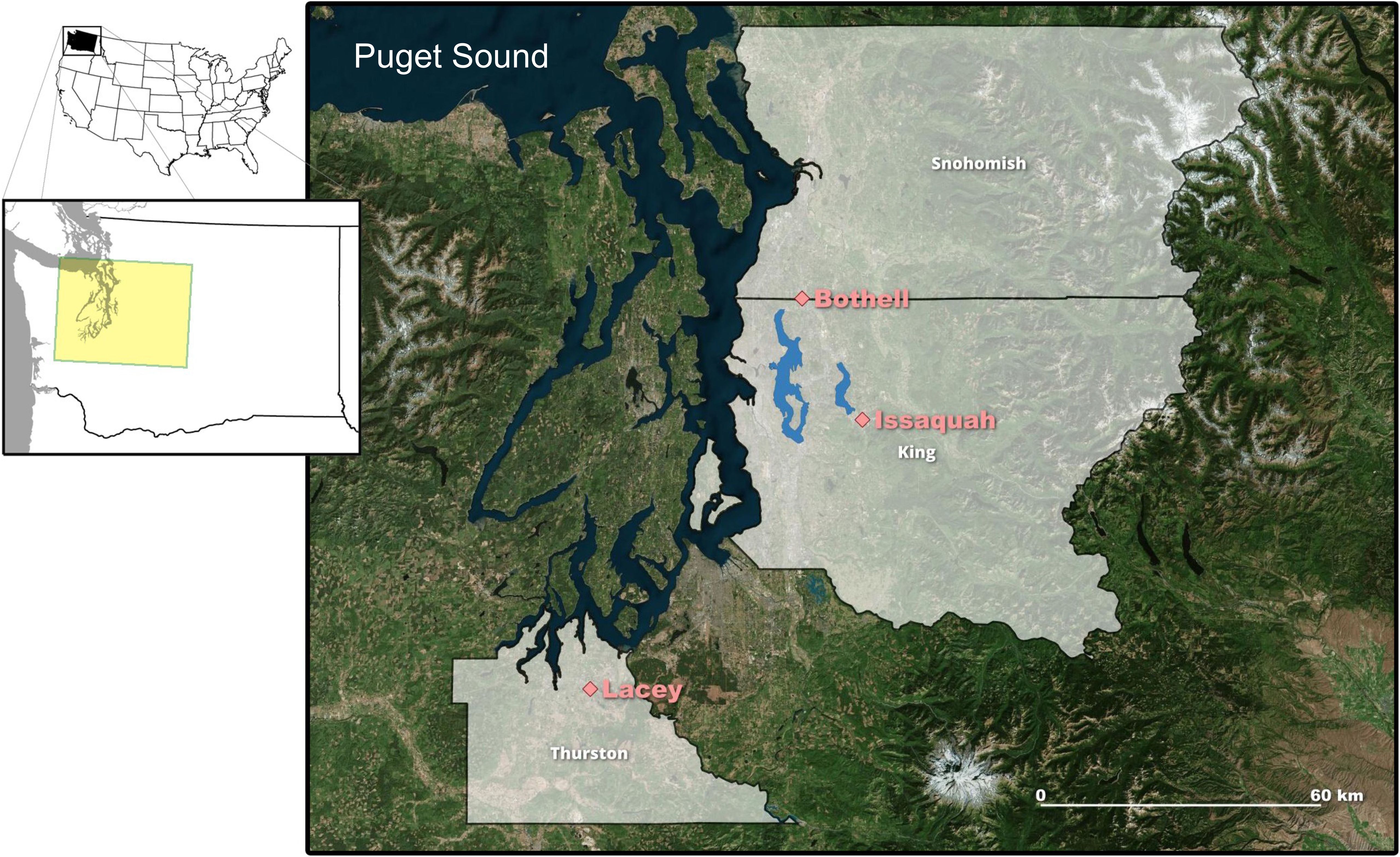
Figure 1. Inset maps of the contiguous United States with Washington (WA) State delineated and the western WA study area highlighted in yellow. Satellite map of western WA, along the Puget Sound coastal waters (shown in dark blue), displaying the locations of the first three African clawed frog populations discovered within Thurston, Snohomish, and King counties (gray shading) in the cities of Lacey, Bothell, and Issaquah, respectively (pink diamonds). Lake Washington, the second largest lake in WA State, is southwest of Bothell, and Lake Sammamish is northwest of Issaquah (interior waterbodies shown in light blue).
Here, we catalog the known ACF occurrences, early management efforts, and additional data collected on invasive ACFs in Washington from 2015–2023. We build on a report by Ojala-Barbour et al. (2021), which detailed management history and concerns related to the Washington ACF invasion. Multiple entities and individuals were responsible for information gathered after the Ojala-Barbour et al. (2021) report; ACF collection data are cataloged by the Washington Department of Fish and Wildlife. Our goal was to briefly summarize this report and expand upon it with details on subsequent ACF research and management efforts in Washington State, with suggestions for future directions.
2 Review and updates on African clawed frog populations in Washington State
Because ACF surveys at the three western Washington sites had various objectives (e.g., control and eradication in service of conservation goals, live animal collections for research projects, and monitoring for educational purposes), intensity and duration of trapping effort were inconsistent across sites. Aquatic funnel traps of various configurations, typically containing bait (cat food, tuna, or raw chicken), were primarily utilized because of their success in capturing a wide variety of amphibian species at varying levels of efficacy (Adams et al., 1997; Kronshage and Glandt, 2014; Swartz and Miller, 2018). The captured invasive frogs were humanely euthanized following procedures recommended by the American Veterinary Medical Association (AVMA) as described by Underwood and Anthony (2020).
2.1 Lacey, WA (first ACF detection site)
In Thurston County in July 2015, a state government employee spotted a frog and recognized it as a non-native species in a city-owned stormwater retention facility located in Lacey, WA (Figure 1; Boone, 2017). The stormwater facility, comprised of three ponds, is located near Saint Martin’s University (Figure 2). Ponds 1, 2, and 3 are 4,200, 14,600, and 4,600 m2 in size, respectively. Ponds 1 (the stormwater receiving pond) and 2 (a storage pond) have a permanent water linkage via a valved pipe. Further, water from Ponds 2 and 3 intermittently mix during stormwater overflow events (Ojala-Barbour et al., 2021). All three ponds receive water primarily from a network of stormwater pipes that are part of the Thurston County drainage system within the Woodland Creek basin (Berris, 1995). Pond 2 has a connection to the creek via an overflow standpipe (Figure 2). The City of Lacey erected a silt fence around each of the three stormwater ponds in 2015. These fences were intended to minimize ACF dispersal out of the ponds until potential future management efforts emerged. From 2015–2017, various organizations seined, trapped, drained, and/or experimentally salted the ponds in attempts to remove ACFs (Ojala-Barbour et al., 2021). These eradication endeavors resulted in over 6,200 ACFs removed from the three ponds (Table 1), with managers hoping that ACFs were eliminated from Pond 1, the only pond that had been salted, and their numbers reduced in the other two ponds at the Lacey site.
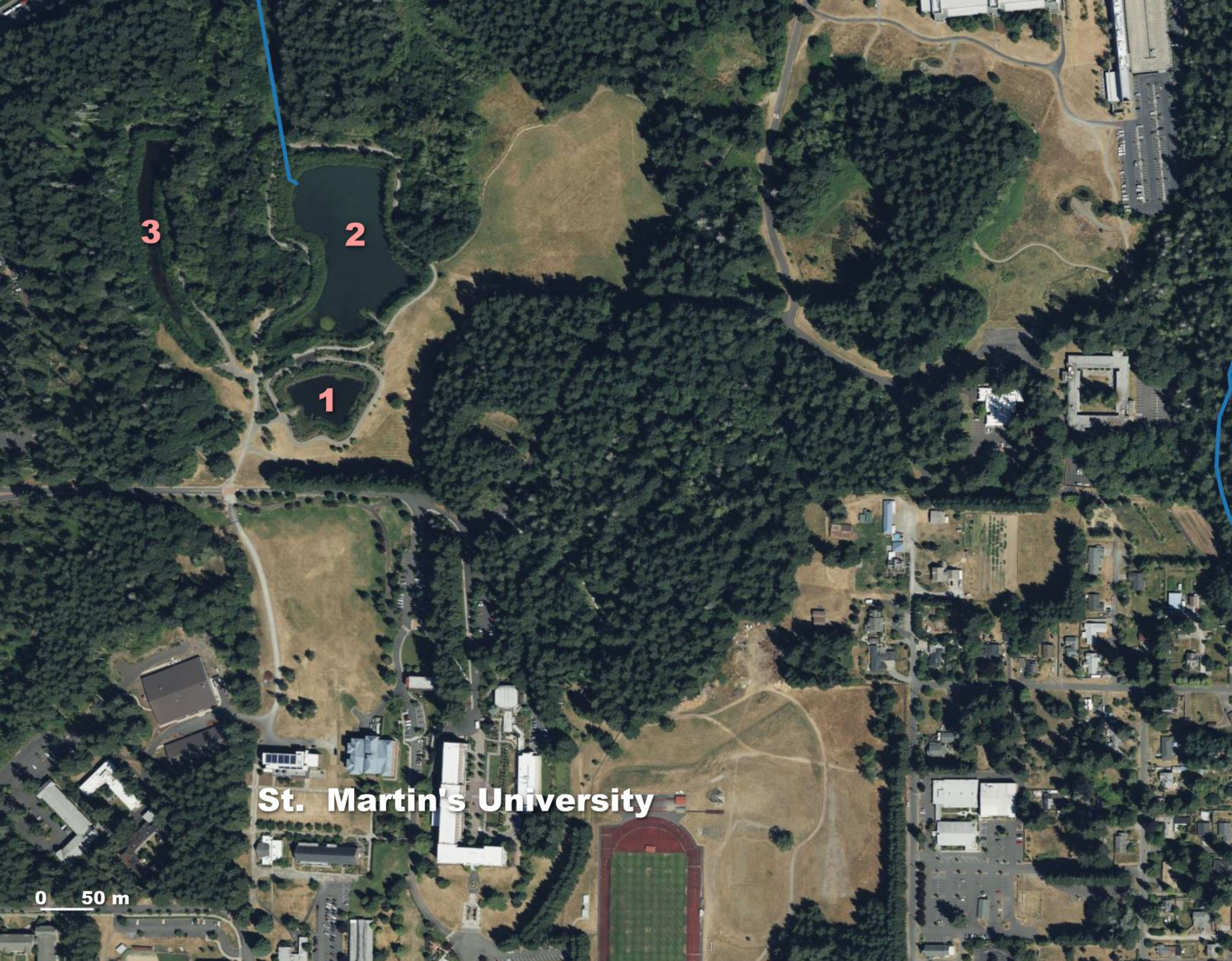
Figure 2. Zoomed in map of Lacey, Washington site. First detection of African clawed frogs (ACFs) in Washington State occurred at the City of Lacey Stormwater Ponds, referred to as the College Regional Ponds 1, 2, and 3, located near Saint (St.) Martin's University in July 2015 (pond numbers in pink font on satellite map). There is a connection from Pond 2 to Woodland Creek (streamline delineated in blue). This ACF site is located entirely within the Woodland Creek-Frontal Henderson Inlet basin.
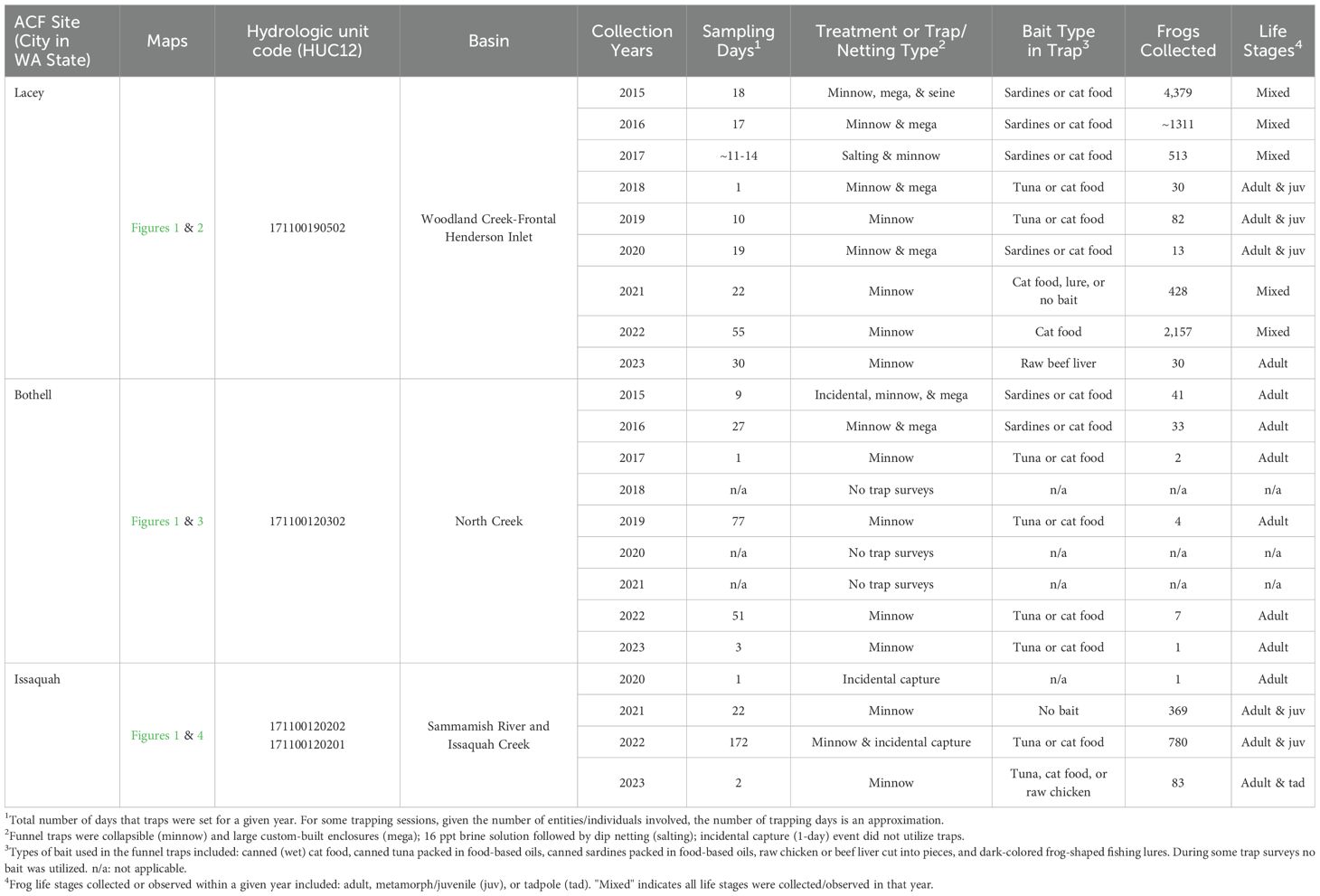
Table 1. Summarized highlights from the response to the initial detections of invasive African clawed frogs (ACFs, Xenopus laevis) in western Washington (WA) State from 2015–2023.
In 2018, ACFs were found in the storm sewers connected to the ponds. The discovery of frogs in the sewer suggested that the sewer pipes were utilized as additional habitat from which the ACFs were able to recolonize the ponds (Ojala-Barbour et al., 2021). The recolonization by ACFs was confirmed by the capture of 27 young adult frogs and three metamorphs (n = 30) during a single day of trapping in Pond 1 in June 2018. These captured animals were used in a pilot study to determine ACF susceptibility to fish rhabdoviruses (E.J. Emmenegger, personal communication 2024). Additionally, these frogs were screened for chytrid fungi, Batrachochytrium dendrobatidis (Bd) and Batrachochytrium salamandrivorans (Bsal), via skin swabs using standard DNA extraction and molecular (qPCR) testing protocols (Boyle et al., 2004; Blooi et al., 2013). All frogs captured in 2018 were Bsal-negative. However, they had 76.67% prevalence for the Bd fungus (23 Bd-positive frogs/30 tested).
Over a 10-day trapping event in July 2019, an additional 82 live frogs were collected from Ponds 1 and 2 for further fish rhabdovirus susceptibility testing (E.J. Emmenegger, personal communication 2024). Because ACFs were found to disperse within the storm sewers feeding into the three-pond complex in Lacey (Ojala-Barbour et al., 2021), surveys of 35 additional (adjacent) waterbodies within a 1-km radius of these sites were proposed in 2020, of which 23 sites had sufficient water to place traps. Thirteen ACFs were detected in Lacey Ponds 1 and 2, but no frogs were found in any of the adjacent ponds over a 19-day trapping period with typically 12 traps set at each site for 3 consecutive nights. A set of tissue samples (n = 174) collected in 2020, the majority from ACFs but also including some tissues from invasive American bullfrog Lithobates catesbeianus and native rough-skinned newt Taricha granulosa, were screened for ranaviruses (an iridovirus) via qPCR assays (Mao et al., 1997; Stilwell et al., 2018). No ranaviruses were found in the animal tissues screened from the Lacey ponds.
In the latter half of 2021, trapping was reinitiated at the Lacey site for a research study testing different trap bait efficacies (M.R. Friesen et al. unpublished 2024) and to develop a preliminary population estimate prior to further eradication attempts. From early June through late October 2021, intermittent trapping was conducted in all three ponds for a 22-day period. Captured ACFs, primarily collected from Pond 1, were marked with toe clips and released. Although 428 total frogs (418 juveniles/adults and 10 tadpoles) were captured, too few frogs were recaptured to sufficiently model population size. Trapping resumed, primarily in Pond 1, in early February 2022 and continued until mid-October, with 55 total days of trap monitoring. High trap success was observed again in 2022, with 2,157 ACFs collected in total (2,060 juveniles/adults and 97 tadpoles). All tadpoles were captured in the month of July and most juvenile/adult frogs (n = 1,977) were caught in the last 4 weeks of trapping. In July 2023, traps were set for 2 nights, for a global ACF microbiome research project (John Measey of Stellenbosch University, personal communication 2023), with a total of 30 frogs caught in Pond 1. During annual sewer maintenance, the City of Lacey has continued to find multiple ACFs in the storm sewer system that drains into these ponds (Doug Christenson, City of Lacey, personal communication 2020), suggesting future spread is possible.
2.2 Bothell, WA (second ACF detection site)
A second Washington State ACF population was found in July 2015 within Snohomish County in the city of Bothell. Bothell is located approximately 16.1 km northeast of Seattle, WA and lies within both Snohomish and King counties (Figure 1). A single ACF was captured by children fishing in a stormwater retention pond (Figure 3) located in the North Creek drainage on the Snohomish County side of the city (Ojala-Barbour et al., 2021). The mainstem of North Creek is 16 km long and runs north to south through a commercial zone and residential areas before discharging near Bothell into the Sammamish River, which eventually flows into Lake Washington, the second largest lake in the state (Scheffer and Robinson, 1939; Fevold et al., 2001).
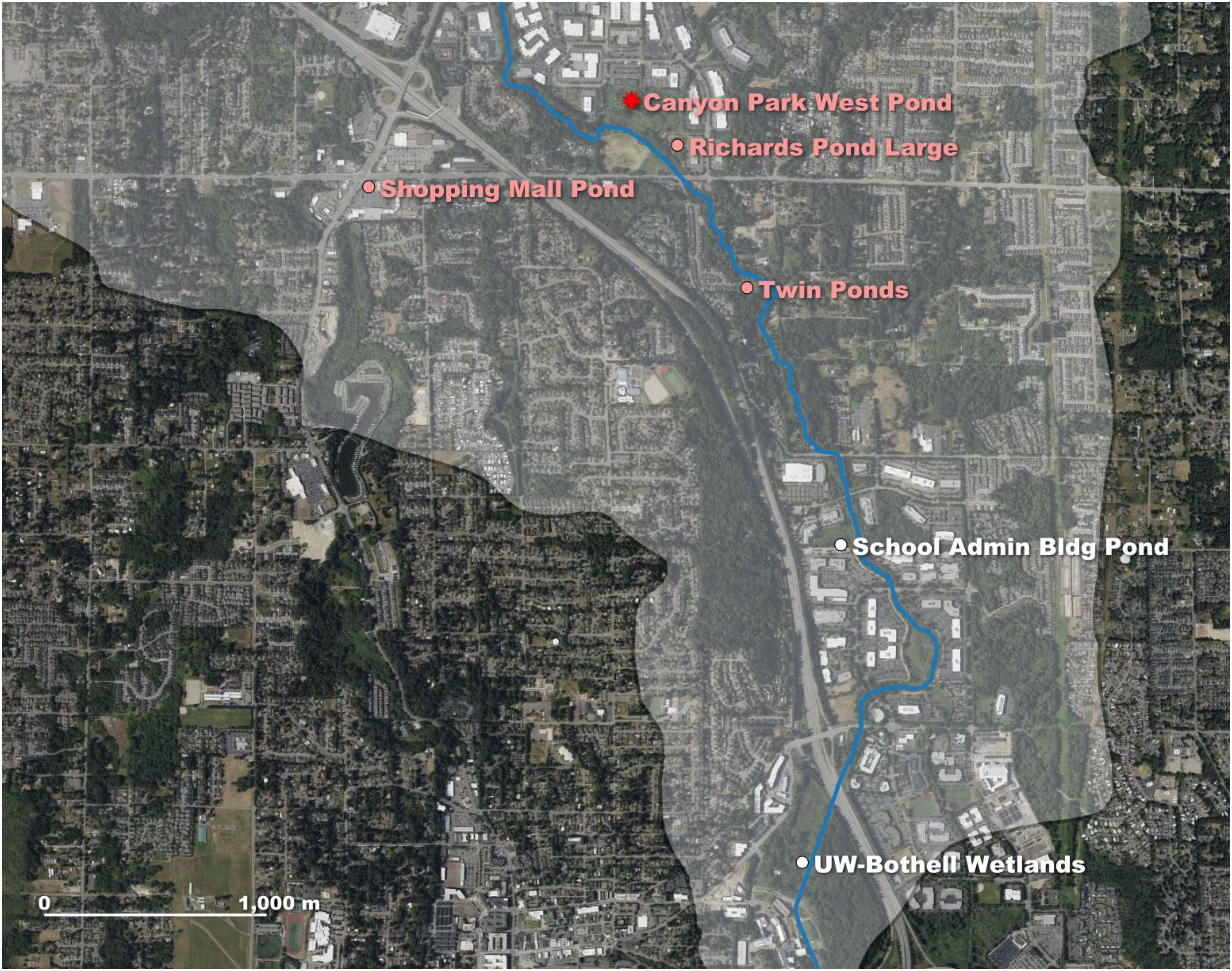
Figure 3. Zoomed in map of Bothell, Washington site. Later in July of 2015, a second African clawed frog (ACF) population was found in Washington State at the Canyon Park West Pond (red star on satellite map), also known as the ‘Ground Zero’ pond, in the North Creek subbasin (streamline delineated in blue and basin shaded in translucent light gray) within the city of Bothell, WA. The presence of ACFs was detected at three additional locations, Richards Pond Large, Shopping Mall Pond and Twin Ponds (labeled pink circles), during subsequent trap surveys from 2016–2023. No frogs were captured at the two downstream sites near the North Shore School District Administrative building and University of Washington (UW)-Bothell Wetlands area (labeled white circles).
From September to November 2015, traps were deployed intermittently (n = 8 field days) at the Canyon Park West Pond where the first ACF was observed (this location was informally termed ‘Ground Zero Pond’ by Washington Department of Fish and Wildlife in Ojala-Barbour et al., 2021). This effort captured 40 ACFs, confirming the presence of a new ACF population (Table 1). The Canyon Park West Pond is a 6000 m2 pond in the Canyon Park business development of Bothell and is connected to the main channel of North Creek via three outlet pipes.
From April to August 2016, intermittent surveys (n = 27 days in total) were conducted at eight stormwater retention pond sites within a 3.2-km radius from the Canyon Park West Pond along North Creek (Ojala-Barbour et al., 2021). ACFs were detected in three ponds. These ponds included re-detection at the Canyon Park West Pond and new detections at “Richards Pond Large” (300 m2 in size and connected to the Canyon Park West Pond by a culvert) and the “Twin Ponds” site (two small, linked ponds, 44 and 28 m2 in size, respectively, that are 1.5 km downstream from the Canyon Park West Pond). Despite this substantive effort, only 33 ACFs were captured, suggesting a relatively low density in the Bothell sites. In July 2017, six minnow traps were placed for one night in the Canyon Park West Pond, and only two ACFs were captured. No trapping surveys were conducted in 2018.
Trapping and removal efforts, using only minnow traps, were reconvened in 2019 by Trout Unlimited and the U.S. Geological Survey (USGS). One trap each was placed in the Canyon Park West and Richards Pond Large ponds in mid-April. Traps were checked every 2–3 days until early July. After 3.5 months of trapping in 2019, only four ACFs were removed from Richards Pond Large (all captured on a single day), supporting the previous speculation that there is a low density of ACFs in this region. No trapping or monitoring of ACFs occurred in the North Creek system in 2020 or 2021.
In 2022, traps were again placed in the two Canyon Park ponds from March to mid-May (3–8 traps each at the Canyon Park West and Richards Pond Large sites), capturing 7 frogs in total. Two frogs captured in May 2022 were screened for chytrid fungus (Bd), and both tested negative. An additional isolated pond, in a busy shopping complex of Bothell that does not run adjacent, nor connected, to North Creek but is 1.2 km directly SW from the Canyon Park West Pond, also had 2–6 traps deployed from April–May 2022, and 9 ACFs were removed (Figure 3). As part of a University of Washington-Bothell (UW-Bothell) biology class project, trapping surveys were initiated to monitor the potential spread of the ACF into the lower North Creek subbasin. For about one month starting in early April 2022, four traps each were placed 1) in a pond near the North Shore School District Building, 2.2 km downstream from the Canyon Park West Pond, and 2) at the UW-Bothell wetlands, which are approximately 1.9 km farther downstream and 0.9 km from North Creek’s confluence with the Sammamish River (Figure 3). No frogs were detected in the downstream pond and wetland sites located closer to the Sammamish River confluence.
Lastly, in July 2023, six minnow traps in total were set at the shopping complex pond and Richards Pond Large (Figure 3) for 3 nights of trapping with only 1 frog captured. Collectively, these intermittent trap surveys in Bothell further document an apparently low population density of ACFs in the North Creek system and suggest an absent or limited downstream migration to the Sammamish River.
2.3 Issaquah, WA (third ACF detection site)
In July 2020, an ACF was detected in King County, WA by a private contractor who incidentally caught one frog during a fish capture and relocation effort at a pond located in the City of Issaquah. Issaquah is approximately 27.4 km east from downtown Seattle and surrounds the southern end of Lake Sammamish (Figure 1). The contractor reported the finding to the Washington Invasive Species Council (The Watershed Company, 2020). This first ACF was identified in a sediment pond near Tibbetts Valley Park, located within the Tibbetts Creek subbasin (Figure 4; Table 1). The Tibbetts, Issaquah, Laughing Jacobs, Lewis, Inglewood, and Pine Lake Creeks are a series of subbasins that all flow into Lake Sammamish (King County, 2008). The city of Issaquah has a network of stormwater retention ponds and ditches that drain into Lake Sammamish to aid in the movement of rainwater during high overflow events (City of Issaquah, 2004; King County, 2014). No follow up surveys occurred in 2020 due to the coronavirus disease of 2019 (COVID-19) pandemic.
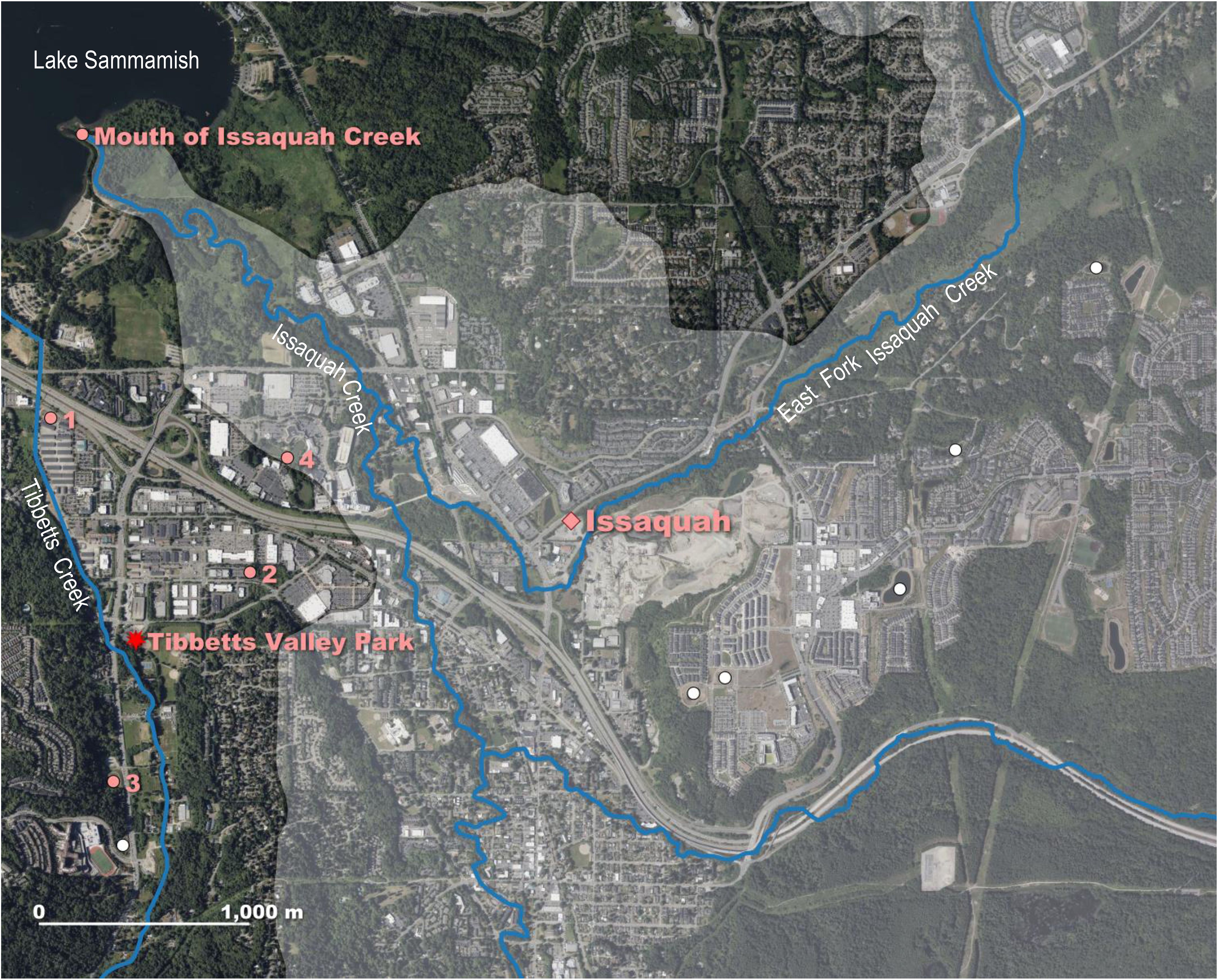
Figure 4. Zoomed in map of Issaquah, Washington site. Third African clawed frog (ACF) population found in 2020 at a pond near Tibbetts Valley Park (red star on satellite map) in Issaquah, WA within the Tibbetts Creek drainage of Lake Sammamish-Sammamish River subbasin (non-shaded area). Trap surveys in 2021 at 10 sites in the Tibbetts Creek and Issaquah Creek subbasins captured ACFs at four ponds (pink circles labeled 1, 2, 3, and 4) with no frogs detected at the other six ponds (unlabeled white circles). Frogs were found again in 2022 at the four sites with two additional ACFs collected near the confluence (labeled pink circle) of Issaquah Creek and Lake Sammamish. Streamlines delineated in blue and Issaquah Creek drainage area shaded in translucent light gray.
In June and July of 2021, as part of an undergraduate research project at Pacific Lutheran University, trapping surveys were performed in 10 natural and constructed ponds within the Tibbetts Creek and Issaquah Creek subbasins (n = 20 unbaited minnow traps per night per pond). ACFs were detected at four of the constructed stormwater ponds, designated Ponds 1, 2, 3, and 4 (Figure 4). Ponds 1, 2, and 3 are within the Tibbetts Creek subbasin, and Pond 4 is in the Issaquah Creek subbasin. Four nights of trapping at Ponds 1 and 4 captured 206 and 4 frogs, respectively. At Pond 2, a single night of trapping removed 56 frogs, while 12 nights of trapping at Pond 3 collected 103 frogs. The observed ACF size distribution changed seasonally, with smaller, recently metamorphosed frogs only captured in late July. This cluster of ACFs (total n = 369 frogs), described from multiple locations within the Tibbetts and Issaquah Creek subbasins in 2021, is considered the third population of ACFs found in western Washington.
In 2022, Trout Unlimited, with assistance from USGS, continued trapping and eradication efforts in Issaquah by using an assortment of funnel traps with variable trap densities among sites (Figure 4). Monitoring started in mid-January 2022 at Pond 1 and continued through the summer. Water temperatures ranged from 5.1˚C in the winter to 20–22 ˚C in all four ponds when trapping ended in July. The first ACFs captured (n = 36), were caught from January to early March utilizing 1 – 3 traps at Pond 1 per day, and then 4–7 traps were deployed daily until early July with 210 more frogs removed. Surveillance of ACFs at the other three Issaquah ponds occurred from April to July 2022, with 6–9 traps deployed at Pond 2, 4–8 traps set at Pond 3, and 2–12 traps placed in Pond 4. A total of 339, 164, and 29 frogs were captured in Ponds 2, 3, and 4, respectively. Some of the frogs collected in May 2022 (n = 6) were swabbed and screened for the Bd fungus as part of a Pacific Northwest Bd genetic lineage study (T.S. Jenkinson et al. unpublished 2024), and 2 tested positive. A total of 778 ACFs were removed from the four Issaquah ponds during the intensive 2022 trapping effort. Additionally, in April 2022, two adult ACFs were incidentally captured during fish sampling at the southern end of Lake Sammamish near the mouth of Issaquah Creek, located just east of Tibbetts Creek (Figure 4; Joseph Short of the Washington Department of Fish and Wildlife, personal communication 2022). In July 2023, eight traps each were deployed at Ponds 1 and 2 (Figure 4). During the 2-night trapping activity, approximately 81 adult frogs and 2 tadpoles were captured and removed from these two Issaquah ponds. The ACFs introduced into the Issaquah and Tibbetts Creeks appear to have reproducing populations that are thriving and may be dispersing downstream.
3 Discussion
ACFs appear to be well-established in at least three watersheds in the Puget Sound region of Washington State. Multiple entities have now identified robust ACF populations in the disparate urban regions of Issaquah and Lacey, with possibly sparser ACF densities observed in Bothell. Despite intensive albeit ad hoc removal efforts, no eradication attempt has had long-term success in any water body in Washington. The apparent failure to meaningfully control ACF populations despite significant collaborative effort over eight years underscores the challenges with studying and managing invasive ACFs. Further, the full extent of ACF occurrence in the Puget Sound region is poorly understood due to a lack of systematic sampling and the cryptic behavior of this highly invasive aquatic species. Additional ACF sightings in the region have come from incidental reports from the public but have not been investigated.
An open question is whether the three ACF populations represent multiple independent introductions in Washington State or an older invasion that has been spreading undocumented for some time. However, the fact that ACFs are in widely spaced and hydrologically discrete watersheds within western Washington State may suggest independent introduction events. Microsatellites (nuclear DNA), mitochondrial DNA, and genome sequencing are complementary molecular techniques that have been used to confirm the species associated with invasive frog colonizations and generate ACF phylogenies to discern genetic lineages/population structures of introduced ACF populations (Evans et al., 2015; Furman et al., 2015; De Busschere et al., 2016; Goodman et al., 2021; Pauwels et al., 2023; Premachandra et al., 2023). Based on trade records, ACFs introduced into the U.S. are likely of Asian origin, but also potentially from Chile or South Africa (Measey, 2017). It may be beneficial for regulatory and management response strategies to determine the genetic lineages of ACF populations in Washington State, which could provide clues to the route of their introduction pathway (e.g. international pet trade, laboratory/supplier breeding stock, domestic/regional translocation, or other sources).
Globally, many initial introductions of non-native ACF populations occurred in bioclimatic zones that have dry summers and mild wet winters, like the Mediterranean regions of Portugal, Italy and Chile, because they are considered optimal niches for ACF establishment (Measey et al., 2012; Lobos et al., 2013; Sousa et al., 2018). More recently northern intrusions of ACF populations into climatic areas initially thought of as less suitable have been discovered, such as in France and Belgium. This ACF invasion expansion may be potentially linked with regional climate shifts in conjunction with ACFs’ high capacity for adaptation and phenotypic plasticity (Ihlow et al., 2016; Courant et al., 2018; Kruger et al., 2022; Pauwels et al., 2023). Similarly in North America, some of the earliest ACF populations established were in the state of California on the southwest coast of the U.S. (Measey et al., 2012), and the northern spread of ACFs into coastal Washington State seems to be following a similar invasion pattern occurring in other parts of the world. The primary dispersal mechanisms of ACFs include anthropogenic-assisted movement, overland migration, and disturbed/constructed aquatic habitats (agricultural ponds, stormwater retention ponds, irrigation channels, etc.) that have flow linkages to streams/rivers. The latter mechanism appears to be the most frequently reported means of invasive ACF establishment and subsequent dispersal (Measey et al., 2012; Measey, 2016; De Villiers and Measey, 2017; Vimercati et al., 2024).
In our case study, the presence of ACFs in multiple subbasins in the same watershed is another indicator of a species capable of spreading on its own. Further, the incidental capture of two ACFs within Lake Sammamish opens the possibility of movement of this invasive species between the waterbodies in Issaquah, through Lake Sammamish, to similar waterbodies in Bothell, like North Creek, which drain into the Sammamish River. All ACF-invaded watersheds in Bothell and Issaquah ultimately flow into Lake Washington. Invasive ACFs that occupy constructed stormwater ponds, which are typically linked to natural waterways, may be flushed downstream during high water events (heavy rain, flooding, combined sewer overflows, etc.) significantly expanding their range of intrusion (Van Dijk, 1977; Le Viol et al., 2012). Overland movement of ACFs has been observed near the Lacey site (Ojala-Barbour et al., 2021). The presence of chytrid fungus (Bd) in two of the ACF populations in western Washington (Lacey and Issaquah) may also be of concern if these ACF-associated Bd lineages are novel or become disseminated because ACFs are known asymptomatic carriers of the fungus (Weldon et al., 2004; Wilson et al., 2018).
Managing ACFs may be more difficult if the species exhibits a “hydra effect” (Grosholz et al., 2021). ACFs are known to have a diverse diet, including cannibalism of larval conspecifics (Measey et al., 2015; Thorp et al., 2019). The hydra phenomenon, wherein eradication efforts of older life stages reduces cannibalistic pressure on younger life stages, potentially leads to an explosive population rebound that may exceed original population densities (Grosholz et al., 2021). Two observations in Washington State in 2022 suggest the hydra effect may occur in the invasive ACF system. First, in Issaquah, after continued removal of juvenile and adult ACFs using minnow traps at Pond 2, swarms of schooling ACF larvae were observed in the center of the pond in early fall. Second, in Lacey, for reasons associated with prior trapping removal efforts and/or some stochastic event, a collapse in the ACF population at this site seems to have occurred, because we caught few frogs for much of 2022 (February to mid-September). We eventually began catching numerous frogs at the end of the trapping season (mid-October), and all were recently metamorphosed juveniles and young adults. This observation may suggest that a small number of remaining ACF adults successfully bred, and that the Lacey population reestablished in late summer with a newly-metamorphosed cohort (Ojala-Barbour et al., 2021). Alternatively, the complex habitat of the Lacey stormwater ponds, such as the interconnecting pipes or water flows between ponds, sediment escape refugees, and gaps in the containment fence, could also cause a resurgence in juvenile frogs. Both hypotheses may help explain why prior eradication efforts of ACFs in Washington were unsuccessful.
Our limited knowledge of the extent of the ACF invasion in Washington coupled with the apparent failure of removing frogs by mechanical methods, suggest a challenging path forward for ACF management in the state. More robust inventory efforts, such as eDNA sampling, could help to elucidate how widespread ACF populations are in the region (Morisette et al., 2021). Whether ACF eradication in Washington State is a possibility at this point is unclear, especially given the limitations of the initial and ongoing scope and effort. If ACFs are largely confined to stormwater ponds – as most of our current observations suggest – it may still be possible to contain the species through suppression, although substantial effort, far exceeding prior efforts, would likely be required to achieve eradication at site, basin and statewide scales. We highlight the need to develop a national or regional strategy to reduce the risk of ACF introduction and spread in other jurisdictions, as currently, no ACF or invasive frog management strategy or plan exists. A western or national coordinated strategy could help to identify and prioritize actions to reduce risks and facilitate responses across larger scales.
Data availability statement
The datasets presented in this study can be found in online repositories. Non-proprietary raw data sets of the ACF collections from the three field sites are publicly available by request from the Washington Department of Fish and Wildlife as part of their scientific collection annual reports archive. The geocoordinate data file used to generate the figure maps displaying trap and ACF-populated pond locations can be found at the USGS ScienceBase digital repository at https://doi.org/10.5066/P134GH4R.
Ethics statement
The animal study was conducted following the animal welfare practices approved by the Institutional Animal Care and Use Committee (IACUC) of the USGS Western Fisheries Research Center for research involving aquatic animals under the guidelines described in the Guide for the Care and Use of Laboratory Animals and the American Veterinary Medical Association.
Author contributions
EE: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. RL: Conceptualization, Formal analysis, Investigation, Project administration, Writing – original draft, Writing – review & editing. ES: Investigation, Writing – review & editing. VT: Investigation, Writing – review & editing. EE: Conceptualization, Formal analysis, Investigation, Project administration, Resources, Visualization, Writing – review & editing. MF: Formal analysis, Investigation, Project administration, Resources, Writing – review & editing. MT: Investigation, Project administration, Writing – review & editing. EB: Investigation, Methodology, Writing – review & editing. DK: Investigation, Project administration, Resources, Writing – review & editing. JS: Formal analysis, Investigation, Project administration, Resources, Writing – review & editing. AP: Investigation, Project administration, Writing – review & editing. RV: Investigation, Resources, Writing – review & editing. RO: Conceptualization, Formal analysis, Investigation, Writing – review & editing. CA: Investigation, Project administration, Resources, Writing – review & editing. JJ: Formal analysis, Investigation, Project administration, Resources, Writing – review & editing. MK: Methodology, Resources, Writing – review & editing. TJ: Investigation, Methodology, Writing – review & editing. KH: Data curation, Investigation, Methodology, Writing – review & editing. TC: Methodology, Writing – review & editing. KW: Methodology, Resources, Writing – review & editing. TQ: Investigation, Project administration, Resources, Writing – review & editing. JB: Investigation, Resources, Writing – review & editing. ML: Conceptualization, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Pacific Lutheran University's researchers were supported with funding through their Natural Sciences Summer Undergraduate Research Program (NSSURP). Trout Unlimited and its local Three Rivers Chapter of Trout Unlimited provided funding to support purchasing equipment and staff time to support this project. U.S. Geological Survey scientists received base funds from the Ecosystems Mission Area Biological Threats and Invasive Species Research Program to participate in this study. Washington Department of Fish and Wildlife (WDFW) also provided funding to support management and aspects of this research. WDFW funding included revenue generated from personalized license plate sales which is dedicated to nongame wildlife management in the state of Washington (RCW 46.68.435). Research conducted by Saint Martin’s University received some financial support from WDFW in addition to the university’s backing for their work on this project.
Acknowledgments
We are grateful to all private landowners and companies that allowed us to enter their properties to monitor frog populations. We appreciate Alexis S. Harrison’s contributions involving the diagnostic screening of the frogs for chytrid fungi from the Lacey site. We acknowledge Marc Hayes for many contributions to the management and research of ACFs in Washington State from 2015–2020 and his review of the manuscript draft. We thank Blake E. Feist for his GIS-wizardry skills in helping to generate the figure maps. We are also grateful to student support from Katie Augsburger, Daniel Hardman, Olivia Cervantez, Jacie Fabela, and Dave Anderson. The City of Lacey supported management activities by providing supplies and staff support. We want to recognize Ashley H. MacKenzie for her assistance in completing the USGS internal peer and policy reviews, and data management. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. This article has been peer reviewed and approved for publication consistent with U.S. Geological Survey Fundamental Science Practices (https://pubs.usgs.gov/circ/1367/).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adams M. J., Richter K. O., Leonard W. P. (1997). “Surveying and monitoring amphibians using aquatic funnel traps,” in Sampling Amphibians in Lentic Habitats: Northwest Fauna Series 4. Eds. Olson D. H., Leonard W. P., Bury R. B. (Olympia, Washington USA: Society for Northwestern Vertebrate Biology), 47–54.
Andersen D., Borzée A., Jang Y. (2021). Predicting global climatic suitability for the four most invasive anuran species using ecological niche factor analysis. Global Ecol. Conserv. 25, e01433. doi: 10.1016/j.gecco.2020.e01433
Berris S. N. (1995). Conceptualization and simulation of runoff generation from rainfall for three basins in Thurston County, Washington. Water-Resources Investigations Rep. 94, 4038. doi: 10.3133/wri944038
Blooi M., Pasmans F., Longcore J. E., Spitzen-Van-Der-Sluijs A., Vercammen F., Martel A. (2013). Duplex real-time PCR for rapid simultaneous detection of Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans in amphibian samples. J. Clin. Microbiol. 51, 4173–4177. doi: 10.1128/jcm.02313-13
Boone R. (2017). Thousands of African clawed frogs killed in thurston county, centralia chronicle. Available online at: https://www.chronline.com/stories/thousands-of-african-clawed-frogs-killed-in-thurston-county,23054 (Accessed 9 December 2023).
Boyle D. G., Boyle D. B., Olsen V., Morgan J. A. T., Hyatt A. D. (2004). Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis. Aquat. Organisms 60, 141–148. doi: 10.3354/dao060141
Burggren W. W., Warburton S. (2007). Amphibians as animal models for laboratory research in physiology. ILAR 48, 260–269. doi: 10.1093/ilar.48.3.260
City of Issaquah (2004). Stormwater Management Plan Year 2022 (Issaquah, Washington USA: City of Issaquah Public Works Engineering Department), 174. June 2004 Final.
Courant J., Secondi J., Vollette J., Herrel A., Thirion J. M. (2018). Assessing the impacts of the invasive frog, Xenopus laevis, on amphibians in western France. Amphibia-Reptilia 39, 219–227.
Crayon J. J. (2005). “Species account: Xenopus laevis,” in Amphibian Declines: The Conservation Status of United States species. Ed. Lannoo M. J. (University of California Press, Berkeley), 522–526.
De Busschere C., Courant J., Herrel A., Rebelo R., Rödder D., Measey G. J., et al. (2016). Unequal contribution of native South African phylogeographic lineages to the invasion of the African clawed frog, Xenopus laevis, in Europe. PeerJ 4, e1659.
De Villiers F. A., Measey J. (2017). Overland movement in African clawed frogs (Xenopus laevis): empirical dispersal data from within their native range. PeerJ 5, e4039. doi: 10.7717/peerj.4039
Duffus A. L., Waltzek T. B., Stöhr A. C., Allender M. C., Gotesman M., Whittington R. J., et al. (2015). “Distribution and host range of ranaviruses,” in Ranaviruses: Lethal pathogens of ectothermic vertebrates. Eds. Gray M. J., Chinchar V. G. (Springer International, AG, Switzerland), 9–57.
Evans B. J., Carter T. F., Greenbaum E., Gvoždík V., Kelley D. B., McLaughlin P. J., et al. (2015). Genetics, morphology, advertisement calls, and historical records distinguish six new polyploid species of African clawed frog (Xenopus, Pipidae) from West and Central Africa. PloS One 10, e0142823. doi: 10.1371/journal.pone.0142823
Fevold K., May C., Berge H., Ostergaard E. (2001). 1999 habitat inventory and assessment of three tributaries to the Sammamish River: North, Swamp, and Little Bear Creeks. (Seattle, Washington, USA: King County Department of Natural Resources) 126.
Furman B. L., Bewick A. J., Harrison T. L., Greenbaum E., Gvoždík V., Kusamba C., et al. (2015). Pan-African phylogeography of a model organism, the African clawed frog ‘Xenopus laevis’. Mol. Ecol. 24, 909–925. doi: 10.1111/mec.2015.24.issue-4
Gervais J. A., Kovach R., Sepulveda A., Al-Chokhachy R., Joseph Giersch J., Muhlfeld C. C. (2020). Climate-induced expansions of invasive species in the Pacific Northwest, North America: a synthesis of observations and projections. Biol. Invasions 22, 2163–2183. doi: 10.1007/s10530-020-02244-2
Goodman C. M., Jongsma G. F., Hill J. E., Stanley E. L., Tuckett Q. M., Blackburn D. C., et al. (2021). A case of mistaken identity: Genetic and anatomical evidence reveals the cryptic invasion of Xenopus tropicalis in central Florida. J. Herpetology 55, 62–69. doi: 10.1670/20-083
Grosholz E., Ashton G., Bradley M., Brown C., Ceballos-Osuna L., Chang A., et al. (2021). Stage-specific overcompensation, the hydra effect, and the failure to eradicate an invasive predator. Proc. Natl. Acad. Sci. United States America 118, e2003955118. doi: 10.1073/pnas.2003955118
Gurdon J. B., Hopwood N. (2000). The introduction of Xenopus laevis into developmental biology: of empire, pregnancy testing and ribosomal genes. Int. J. Dev. Biol. 44, 43–50.
Havel J. E., Kovalenko K. E., Thomaz S. M., Amalfitano S., Kats L. B. (2015). Aquatic invasive species: challenges for the future. Hydrobiologia 750, 147–170. doi: 10.1007/s10750-014-2166-0
Hossack B. R., Oja E. B., Owens A. K., Hall D., Cobos C., Crawford C. L., et al. (2022). Empirical evidence for effects of invasive American Bullfrogs on occurrence of native amphibians and emerging pathogens. Ecol. Appl. 33, e2785. doi: 10.1002/eap.2785
Ihlow F., Courant J., Secondi J., Herrel A., Rebelo R., Measey G. J., et al. (2016). Impacts of climate change on the global invasion potential of the African clawed frog Xenopus laevis. PloS One 11, e0154869. doi: 10.1371/journal.pone.0154869
King County (2008). Development of a 3-Dimensional Hydrodynamic Model of Lake Sammamish. Prepared by Curtis DeGasperi (Seattle, Washington: Water and Land Resources Division).
King County (2014). “Lake sammamish water quality response to land use change,” in Prepared by Dr. Eugene B. Welch, Emeritus Professor, University of Washington, and Consulting Limnologist, and Debra Bouchard, Science and Technical Support Section (King County Water and Land Resources Division, Seattle, WA).
Kronshage A., Glandt D. (2014). “Minnow traps from North America as tools for monitoring amphibians–first results from European newt populations,” in Wasserfallen für Amphibien–praktische Anwendung im Artenmonitoring, vol. 77. (Recke, Germany: Abhandlungen aus dem Westfälischen Museum für Naturkunde), 51–76.
Kruger N., Secondi J., Du Preez L., Herrel A., Measey J. (2022). Phenotypic variation in Xenopus laevis tadpoles from contrasting climatic regimes is the result of adaptation and plasticity. Oecologia 200, 37–50. doi: 10.1007/s00442-022-05240-6
Le Viol I., Chiron F., Julliard R., Kerbiriou C. (2012). More amphibians than expected in highway stormwater ponds. Ecol. Eng. 47, 146–154. doi: 10.1016/j.ecoleng.2012.06.031
Lobos G., Cattan P., Estades C., Jaksic F. M. (2013). Invasive African clawed frog Xenopus laevis in southern South America: key factors and predictions. Stud. Neotropical Fauna Environ. 48, 1–12. doi: 10.1080/01650521.2012.746050
Lockwood J. L., Welbourne D. J., Romagosa C. M., Cassey P., Mandrak N. E., Strecker A., et al. (2019). When pets become pests: the role of the exotic pet trade in producing invasive vertebrate animals. Front. Ecol. Environ. 17, 323–330. doi: 10.1002/fee.2019.17.issue-6
Lucy F. E., Panov V. E. (2014). Keep beating the drum: ICAIS confirms aquatic invasive species are of continuing concern. Aquat. Invasions 9, 239–242. doi: 10.3391/ai.2014.9.3.01
Mao J., Hedrick R. P., Chinchar V. G. (1997). Molecular characterization, sequence analysis, and taxonomic position of newly isolated fish iridoviruses. Virology 229, 212–220. doi: 10.1006/viro.1996.8435
Measey J. (2016). Overland movement in African clawed frogs (Xenopus laevis): a systematic review. PeerJ 4, e2474. doi: 10.7717/peerj.2474
Measey J. (2017). Where do African clawed frogs come from? An analysis of trade in live Xenopus laevis imported into the USA. Salamandra 53, 398–404.
Measey G. J., Rödder D., Green S. L., Kobayashi R., Lillo F., Lobos G., et al. (2012). Ongoing invasions of the African clawed frog, Xenopus laevis: a global review. Biol. Invasions 14, 2255–2270. doi: 10.1007/s10530-012-0227-8
Measey G. J., Vimercati G., De Villiers F. A., Mokhatla M. M., Davies S. J., Edwards S., et al. (2015). Frog eat frog: exploring variables influencing anurophagy. PeerJ 3, e1204. doi: 10.7717/peerj.1204
Morisette J., Burgiel S., Brantley K., Daniel W. M., Darling J., Davis J., et al. (2021). Strategic considerations for invasive species managers in the utilization of environmental DNA (eDNA): steps for incorporating this powerful surveillance tool. Manage. Biol. invasions: Int. J. Appl. Res. Biol. invasions 12, 747. doi: 10.3391/mbi.2021.12.3.15
Ojala-Barbour R., Visser R., Quinn T., Lambert M. (2021). African Clawed Frog (Xenopus laevis) Risk Assessment, Strategic Plan, and Past Management for Washington State Department of Fish and Wildlife. Available online at: https://wdfw.wa.gov/sites/default/files/publications/02267/wdfw02267.pdf (Accessed 9 December 2023).
Pauwels O. S., Brecko J., Baeghe D., Venderickx J., Vanderheyden A., Backeljau T. (2023). Morphological, acoustic and genetic identification of a reproducing population of the invasive African clawed frog Xenopuslaevis (Anura, Pipidae) recently discovered in Belgium. ZooKeys 1184, 41–64. doi: 10.3897/zookeys.1184.103702
Poland T. M., Patel-Weynand T., Finch D. M., Miniat C. F., Hayes D. C., Lopez V. M. (2021). Invasive species in forests and rangelands of the United States: A comprehensive science synthesis for the United States forest sector. (Cham, Switzerland: Springer Nature), 455.
Premachandra T., Cauret C. M., Conradie W., Measey J., Evans B. J. (2023). Population genomics and subgenome evolution of the allotetraploid frog Xenopus laevis in southern Africa. G3 13, jkac325. doi: 10.1093/g3journal/jkac325
Scheffer V. B., Robinson R. J. (1939). A limnological study of Lake Washington. Ecol. Monogr. 9, 95–143. doi: 10.2307/1943256
Schoeman A. L., Joubert T. L., du Preez L. H., Svitin R. (2020). Xenopus laevis as UberXL for nematodes. Afr. Zoology 55, 7–24. doi: 10.1080/15627020.2019.1681295
Somma L. A., Freedman J. A. (2023). Xenopus laevis (Daudin 1802): U.S. Geological Survey (Gainesville, FL: Nonindigenous Aquatic Species Database). Available online at: https://nas.er.usgs.gov/queries/factsheet.aspx?SpeciesID=67 (Accessed 23 May 2023).
Sousa M., Maurício A., Rebelo R. (2018). “The xenopus laevis invasion in Portugal: an improbable connection of science, mediterranean climate and river neglect,” in Histories of Bioinvasions in the Mediterranean. Environmental History, vol. 8 . Eds. Queiroz A., Pooley S. (Springer, Cham), 133–148.
Stilwell N. K., Whittington R. J., Hick P. M., Becker J. A., Ariel E., Van Beurden S., et al. (2018). Partial validation of a TaqMan real-time quantitative PCR for the detection of ranaviruses. Dis. Aquat. Organisms 128, 105–116. doi: 10.3354/dao03214
Swartz T. M., Miller J. R. (2018). Trapping amphibians and their predators: tradeoffs in trap design and performance. Herpetological Rev. 49, 238–243.
The Watershed Company (2020).Keeping an eye out for invasive, non-native wildlife species: African Clawed Frog (Xenopus laevis). In: Blog on Science and Design. Available online at: https://www.watershedco.com/blog/keeping-an-eye-out-for-invasive-non-native-wildlife-species-african-clawed-frog-xenopus-laevis (Accessed 25 May 2023).
Thorp C. J., Vonesh J. R., Measey J. (2019). Cannibalism or congeneric predation? The African clawed frog, Xenopus laevis (Daudin), preferentially predates on larvae of Cape platannas, Xenopus gilli Rose and Hewitt. Afr. J. Ecol. 57, 59–65. doi: 10.1111/aje.2019.57.issue-1
Tinsley R. C., Stott L. C., Viney M. E., Mable B. K., Tinsley M. C. (2015). Extinction of an introduced warm-climate alien species, Xenopus laevis, by extreme weather events. Biol. Invasions 17, 3183–3195. doi: 10.1007/s10530-015-0944-x
Underwood W., Anthony R. (2020). AVMA guidelines for the euthanasia of animals: 2020 edition (Schaumburg, IL: American Veterinary Medical Association).
U.S. Fish and Wildlife Service (2017). African Clawed Frog (Xenopus laevis). Ecological Risk Screening Summary. Available online at: https://www.fws.gov/sites/default/files/documents/Ecological-Risk-Screening-Summary-African-Clawed-Frog.pdf (Accessed July 24, 2024).
Van Dijk D. (1977). Habitats and dispersal of southern African Anura. Afr. Zoology 12, 169–181. doi: 10.1080/00445096.1977.11447556
Van Sittert L., Measey G. J. (2016). Historical perspectives on global exports and research of African clawed frogs (Xenopus laevis). Trans. R. Soc. South Afr. 71, 157–166. doi: 10.1080/0035919X.2016.1158747
Vimercati G., Rödder D., Vuilleumier S., Berronneau M., Secondi J. (2024). Large-landscape connectivity models for pond-dwelling species: methods and application to two invasive amphibians of global concern. Landscape Ecol. 39, 76. doi: 10.1007/s10980-024-01858-4
Vredenburg V. T., Felt S. A., Morgan E. C., McNally S. V., Wilson S., Green S. L. (2013). Prevalence of Batrachochytrium dendrobatidis in Xenopus collected in Africa, (1871–2000) and in California, (2001–2010). PloS One 8, e63791. doi: 10.1371/journal.pone.0063791
Weldon C., Du Preez L. H., Hyatt A. D., Muller R., Speare R. (2004). Origin of the amphibian chytrid fungus. Emerging Infect. Dis. 10, 2100. doi: 10.3201/eid1012.030804
Williams G. (2011). 100 Alien Invaders: Animals and Plants That Are Changing Our World (Guilford, Connecticut (USA: The Globe Pequot Press Inc.), 160.
Keywords: African clawed frog, Xenopus laevis, Washington State, aquatic invasive species, amphibian
Citation: Emmenegger EJ, Lavier RA, Struck EJ, Tyurina VP, Eskew EA, Friesen MR, Taylor MA, Bueren EK, Kyle DR, Schultz JM, Pleus A, Visser RH II, Ojala-Barbour R, Anderson CD, Jensen JS, Keller M, Jenkinson TS, Haman KH, Capps TR, Warheit KI, Quinn T, Bush J and Lambert MR (2025) Invasive African clawed frogs (Xenopus laevis) in Washington State: status, response efforts, and lessons learned. Front. Amphib. Reptile Sci. 3:1524644. doi: 10.3389/famrs.2025.1524644
Received: 07 November 2024; Accepted: 14 March 2025;
Published: 22 April 2025.
Edited by:
Melissa Miller, University of Florida, United StatesReviewed by:
Michael J. Jowers, University of Granada, SpainSarah Kupferberg, Colorado State University, United States
Copyright © 2025 Emmenegger, Lavier, Struck, Tyurina, Eskew, Friesen, Taylor, Bueren, Kyle, Schultz, Pleus, Visser, Ojala-Barbour, Anderson, Jensen, Keller, Jenkinson, Haman, Capps, Warheit, Quinn, Bush and Lambert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Max R. Lambert, bWF4LmxhbWJlcnRAdG5jLm9yZw==
†Present addresses: Emily J. Struck, James Tarpo Jr. and Margaret Tarpo Department of Chemistry, Purdue University, West Lafayette, IN, United States Evan A. Eskew, Institute for Interdisciplinary Data Sciences, University of Idaho, Moscow, ID, United StatesMegan R. Friesen, Department of Biology, Wenatchee Valley College, Wenatchee, WA, United StatesEmma K. Bueren, Department of Biology, Indiana University, Bloomington, IN, United StatesMartha Keller, Animal and Plant Health Inspection Service, US Department of Agriculture, Kansas City, MO, United StatesMax R. Lambert, The Nature Conservancy in Washington, Seattle, WA, United States
‡These authors share first authorship
§ORCID: Martha Keller, orcid.org/0000-0002-8045-9342
 Eveline J. Emmenegger
Eveline J. Emmenegger Rebecca A. Lavier2,3‡
Rebecca A. Lavier2,3‡ Reed Ojala-Barbour
Reed Ojala-Barbour Christopher D. Anderson
Christopher D. Anderson Katherine H. Haman
Katherine H. Haman Timothy Quinn
Timothy Quinn Justin Bush
Justin Bush