- 1Faculty of Superior Studies Cuautitlan (FES Cuautitlan), National Autonomous University of Mexico, Mexico, Mexico
- 2Doctoral Program in Animal Production and Health Sciences, National Autonomous University of Mexico, Cuautitlan Izcalli, Mexico
The Mexican axolotl (Ambystoma mexicanum) is widely used in laboratory research around the world, even though it is classified as critically endangered in nature. We review and describe the development of effective management strategies for this species, focusing on cryopreservation and research purposes under laboratory conditions. We share our experiences and challenges in maintaining water quality, feeding, and fostering natural reproduction in captive axolotls. Results indicate that the Mexican axolotl shows promise as a model for biobanking of endangered amphibian species.
Introduction
It is well known that amphibian species are threatened worldwide, with more than 40% classified in some risk category (Parra-Olea et al., 2014). Mexico is not an exception, as >43% of amphibian species are threatened. It is important to take effective conservation actions for Mexican species, which may include the use of assisted reproduction techniques (ARTs) for germplasm conservation (Holt and Pickard, 1999). One of the most iconic amphibians is the Mexican axolotl (Ambystoma mexicanum), a urodele species that can regenerate some organs and limbs. This endemic species has also held local cultural importance since the time of the Aztecs. Mexican axolotl has become a popular pet worldwide over the past decade, even though it is classified as critically endangered in its native habitat by the International Union for Conservation of Nature (IUCN, 2020). Population declines are the result of habitat loss and the introduction of invasive species (Semarnat, 2018). In Mexico, some of the strategies currently underway for the conservation of the axolotl include environmental education, workshops and collaborations among stakeholders, eradication of invasive species, construction of shelters, captive breeding, and research on embryo cryopreservation (Servin et al., 2023). Our research group is working on cryopreservation to conserve valuable genetic material that can be used in future reintroduction programs.
Unfortunately, information on the management of captive axolotl populations is sparse; there are a few published manuals for their management in zoos and aquariums. Regarding management of axolotls under laboratory conditions, useful older information can be found in the “Compendium of Axolotl Husbandry Methods” from the Axolotl Newsletter (Kim et al., 1997) and the optimized axolotl husbandry protocol (Khattak et al., 2014). A recent publication by Yandulskaya and Monaghan (2023) describes housing strategies for a new axolotl colony. Generally, individual laboratories establish their own consistent and controlled working conditions, but these may not be transferable to other research groups.
In 2021, an axolotl breeding area was installed at the vivarium of the Faculty of Superior Studies Cuautitlan, National Autonomous University of Mexico, for research on axolotl embryo conservation (see Servin et al., 2023). In order to maintain a population of healthy animals capable of reproducing in captivity, special conditions for housing, management, and breeding were identified and standardized beforehand. In this overview, we describe how we achieved these laboratory conditions. We review i) the management of Mexican axolotl populations in the laboratory, ii) captive breeding, and iii) the future use of cryopreservation in research and conservation.
Material and methods
Management of axolotls as a laboratory animal
Housing
As aquatic animals, axolotls are housed in tanks or aquariums. Duhon (1992) stated that an adult axolotl needs to be held in tanks >6 L. In our facility, we use plastic tanks with a capacity of 15 L.
We controlled temperature and photoperiod, and to reduce stress, we provided hiding places such as artificial or natural plants to enrich the environment. By providing such housing conditions, following recognized standards established for the use of laboratory animals (National Research Council, 2011), we maintained healthy animals that reproduce in the laboratory without hormone treatment. Our axolotl colony had produced several batches of fertilized eggs with a hatching rate of approximately 71%, similar to the patterns reported for other colonies (Servin et al., 2023).
Water quality
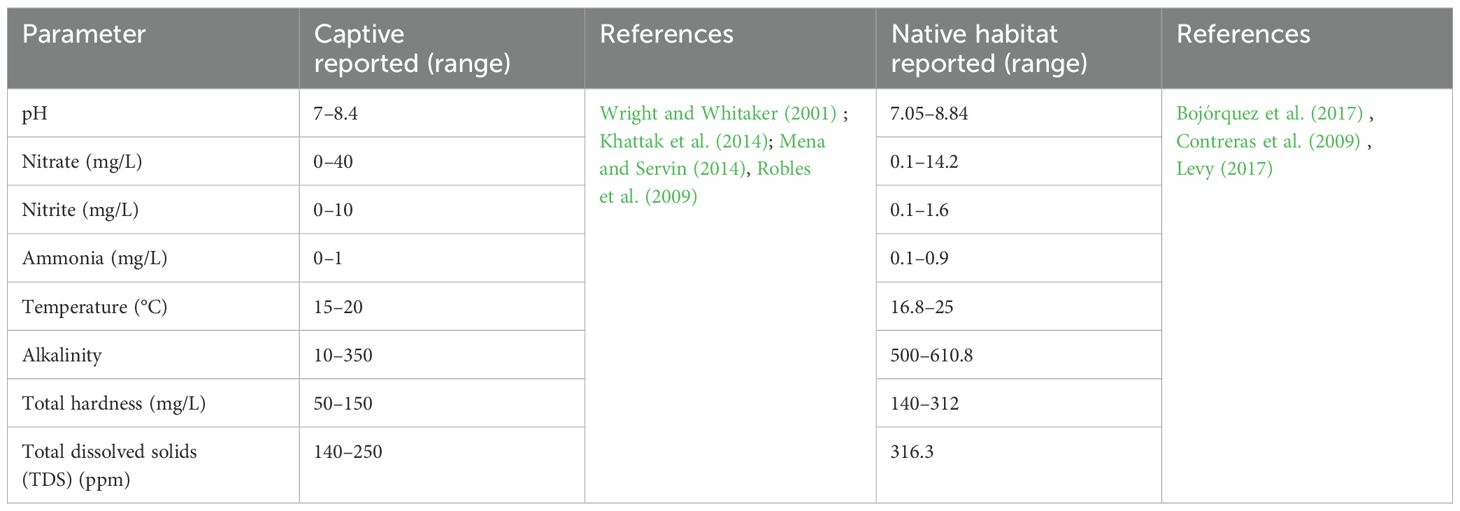
The main challenge to keeping axolotls under laboratory conditions is to maintain ideal water quality. Without this, animals can develop skin and metabolic diseases (Wright and Whitaker, 2001). Water quality includes chemical, physical, and biological characteristics as measured by pH; levels of nitrites, nitrates, and ammonia; and total dissolved solids (TDS). Various studies have reported a range of values for these parameters in captive and laboratory holding facilities as well as in native habitats (Table 1).
Sometimes, the source of water is the main husbandry-related problem. For example, in some places in Latin America, tap water does not offer safe conditions for keeping axolotls. This is why measurement of water quality is needed in laboratories. We tested water taken at different points in the vivarium at the Faculty of Superior Studies Cuautitlan, where we house axolotls, and also monitored tap water in our laboratory. To measure the physiochemical parameters in the tanks, we used the commercial API Freshwater Master Test™ for aquaria. To measure TDS and conductivity, we employed the digital test pen KTOG™. Results showed that tap water (from the laboratory and vivarium) had very high levels of hardness and TDS (Table 2), which can be dangerous for axolotls’ health (Duhon, 1992). However, the other water quality parameters were appropriate for axolotl husbandry.
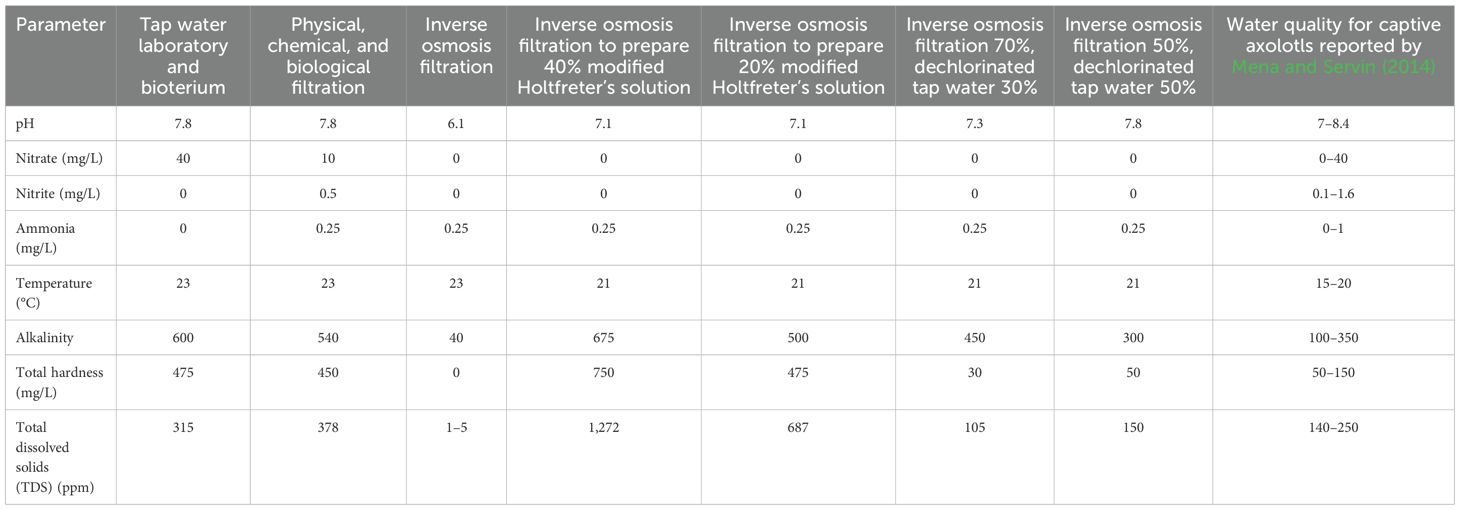
Table 2. Physical–chemical parameters of tap water from the laboratory and vivarium, at the Faculty of Superior Studies Cuautitlan, National Autonomous University of Mexico, before and after different treatments tested to find appropriate water quality conditions for holding Mexican axolotls.
We tested two methods to improve water quality before axolotls were introduced to our facilities. The first method consisted of a physical, chemical, and biological filtration system. This involved two types of external filters: a waterfall filter (AquaClear 30 Power Filter™) and a canister filter (Fluval 107™). Filters were fitted with 30 and 100 g, respectively, of activated carbon from coconut shells (Acuario Lomas™), ceramic rings (Aquajet Acuario Lomas™), and a polyurethane sponge (Black 25 PPI). In addition, we added commercial bacteria at doses of 10 mL per 40 L (BioPro Nitrobacter™). This system was tested in three 40 L aquariums, prior to introducing axolotls to the tanks. Filters were allowed to work for 1 month, without water changes. Water quality was measured once per week. In addition, a fine cotton fiber with a 0.25-μm mesh was added to the filter to catch particulates in order to reduce hardness and TDS.
In the second method, tap water was filtered using a five-step reverse osmosis filter (Rotoplas™). At this initial stage, tanks were simply aerated to determine whether water quality was constant after 1 week. Filtered water was then used as follows: i) to prepare 40% modified Holtfreter’s solution (NaCl 0.059 M, KCl 0.0067 M, CaCl2 0.00076 M, and NaHCO3 0.0024 M) (Nugas and Bryant, 1996), ii) to prepare 20% modified Holtfreter’s solution, iii) mixed 70%/30% (v/v) of reverse osmosis filtered water with dechlorinated tap water, and iv) mixed 50%/50% (v/v) of reverse osmosis filtered with dechlorinated tap water. Water was dechlorinated by adding 0.07 mL/L of potassium thiosulfate (Clorkill Biomaa™, 1.0 g/10 mL). The two different Holtfreter’s solutions were used to hold either larvae and adults (40% solution) or embryos (20% solution) (Nugas and Bryant, 1996). Each treatment was replicated three times (Table 2).
Axolotls were acquired from five different hatcheries to ensure genetic variability. We started with a population of 12 females (from three hatcheries) and 6 males (from two different hatcheries). All animals were approximately 1 year old, and each axolotl was identified by a name and number, as well as their housing tank. Females and males were kept separately and united only when reproduction was scheduled. They were housed in plastic tanks with a capacity of 15 L with aeration and artificial plants.
Ambient temperature
Maintaining appropriate temperatures for axolotls in our facility has been a challenge; since 2023, there have been several heatwaves where the ambient temperature reached 28.5°C to 31°C. The usual temperature in the area is 23°C to 25°C. We have used fans, coolers, and water chillers to maintain a constant temperature of 21°C, which is just over the limit recommended for the species (Mena and Servin, 2014). Shaffer (1989) found that water temperature in the native habitat was 16°C–20°C.
Feeding
Axolotls are strict carnivores, which means dietary protein levels must be high. Few reports exist regarding nutritional requirements for the species in captivity. We used the recommendations found in Manjarrez-Alcívar et al. (2022) of >45% protein in juvenile axolotl’s diet. Live food, such as teleost fishes, Tubifex tubifex, Artemia salina, and Daphnia sp., can be used as an option; however, under laboratory conditions, these organisms can be difficult to obtain or impractical for feeding a large number of individuals. Pelleted food may be the best option. Some brands of trout or turtle pellets can be used (Duhon, 1992; Khattak et al., 2014).
One of the problems when axolotls are fed pellets is that animals have to learn how to eat them. The use of stainless steel long tweezers (feeding tongs) helps solve this problem. We put a pellet at the tip of the feeding tongs, and we positioned it near the mouth of the axolotl. We found it advisable to employ operant conditioning, teaching the axolotl to associate feeding tongs with food and to eat pellets without stress. Axolotls can learn to eat pellets on their own, but care has to be taken to avoid uneaten food polluting holding tanks.
In our laboratory, axolotls are fed turtle pellets (Tortuguetas Normal™) containing >30% crude protein, 5% crude fat, 2% crude fiber, and 10% moisture; also, we offer live and freeze-dried tubifex (Tubifex Azoo™) containing >52% crude protein, 12% crude fat, 5% crude fiber, 12% crude ash, and 5% moisture. In addition, Daphnia and Artemia are offered to young axolotls <9 months old. With this diet, axolotls are kept healthy and can express natural behavior capturing small live food.
Captive breeding of the Mexican axolotl
The Mexican axolotl naturally reproduces in winter when temperatures range between 12°C and 14°C, day length is approximately 11 h of light and 13 h of darkness, and water quality is good and stable (see Table 2). In the laboratory, the environment for reproduction relies on artificial systems and activities, such as timers to control daylight hours, efficient filtering systems, programmable water chillers, and constant monitoring of physical–chemical parameters. In our facility, high temperatures due to climate change and several unusual heat waves created an additional challenge.
The reproduction tank must be large, preferably allowing for 40 L of water per individual. It must have bottom substrate, such as small stones for the placement of spermatophores, and natural or artificial plants to allow the collection of embryos or oocytes for cryopreservation. The breeding tank should be prepared several days, or weeks, prior to the collection of biological samples.
The future use of cryopreservation in research and conservation
ARTs may be a useful tool for the conservation of germplasm of endangered species (Wildt et al., 2001). Creation of biobanks, allowing the storage of genetic material, is of great importance for the future of conservation in cases, such as the Mexican axolotl, where it is not currently possible to reintroduce a species to its natural habitat owing to habitat destruction and the presence of invasive species. Cryopreservation of oocytes, sperm, and embryos has helped to preserve several endangered species. These include programs for the Amur tiger (Panthera tigris altaica) (Swanson et al., 2007), the Mexican wolf (Canis lupus baileyi) (Rosales, 2015), and the California condor (Gymnogyps californianus) (Gee et al., 2004). Sperm cryopreservation, in particular, helps maintain a genetic bank for future captive breeding efforts; however, the application of this technique to amphibians is relatively recent. Therefore, many techniques for obtaining samples and protocols for cryopreservation are under development in various parts of the world (Clulow et al., 2022).
We work with amphibian embryos, which is a challenge due to the presence of yolk and reduced permeability of the gelatinous layers that surround the embryo. In our ongoing work, we have cooled axolotl embryos to subzero temperatures (−6°C) and obtained hatching rates from 18% to 53% (unpublished data). Stopping or slowing embryonic development may be useful for translocation projects and to conduct research where embryo manipulation is necessary.
Results
Water quality
Water quality parameters for the vivarium, measured before and after different treatments, allowed for assessment as to whether water conditions were suitable for axolotls (Table 2). The first method—physical, chemical, and biological filtration—did not reduce hardness and TDS, but it improved water quality with regard to nutrients (such as nitrates, nitrites, and ammonia) and concentrations of organic matter (Table 2).
In the second method, water filtration using reverse osmosis, hardness and TDS dropped drastically, as well as nutrients (Table 2). Values for water quality metrics taken directly from the filter and water kept in the tank for 1 week were similar; however, none of these parameters were ideal for holding axolotls. Application of modified Holtfreter’s solution (40%) resulted in neutral pH, nitrites, nitrates, and ammonia in the appropriate range, but there was a dangerous rise in hardness and TDS. The reduction of concentrations to 20% produced better results, but values for TDS and hardness were still inappropriate. We tested the mix 70/30 (v/v) with dechlorinated tap water and found that the ranges of nutrients and pH were acceptable; however, hardness and TDS were lower than expected (Table 2). The mix 50%/50% (v/v) of reverse osmosis filter water with dechlorinated tap water provided appropriate water quality values, similar to those reported in Mena and Servin (2014). This water was adopted for routine housing of axolotls, and tank water was changed three times per week.
Feeding
We fed axolotls turtle pellets (Tortuguetas Normal™) and live and freeze-dried tubifex (Tubifex Azoo™). In addition, Daphnia and Artemia were offered to young axolotls <9 months old. With this diet, axolotls remained healthy and were able to express natural feeding behavior. We observed a low prevalence of disease, with just five cases in 3 years.
Breeding
Axolotls in our colony reproduced successfully in a 3-year period. We recorded 24 cases of egg laying that led to hatching. We started with a population of 12 females and 6 males; at present, the population has grown to 64 adult axolotls. Reproductive behavior was observed 24 to 72 h after pairs were placed in the breeding tank, and egg laying was observed 48 to 72 h after courtship. Most of the embryos produced were used for research on cryopreservation.
Discussion
Keeping and breeding animals for conservation purposes is always a challenge. Diverse biological information is required in order to recognize optimal conditions and then maintain animals within these environmental constraints. Although the Mexican axolotl is a species housed in research laboratories in many parts of the world, we faced many issues in establishing a new colony for research. Our general approach and the findings reported here should prove useful for establishing programs to rear other species of aquatic salamanders under laboratory conditions for conservation initiatives. For example, the Amphibian Conservation Action Plan (2024) notes that applied ex-situ research populations could be linked to ex-situ and in-situ conservation and research programs (IUCN, 2024). Such ex-situ populations can help aquatic salamanders, such as Chinese giant salamanders (Andrias spp.), where reproduction and housing in captivity could conserve genetic diversity and significantly aid conservation strategies (Jing et al., 2024).
Works such as those of Marcec et al. (2014) and Silla and Langhome (2022) have shown that cryopreservation may be a viable alternative for the genetic conservation of endangered amphibian species, and the technique deserves further study. The Mexican axolotl could serve as a model species for cryopreservation research and as a “flagship species” for the conservation of Mexican amphibian species employing captive breeding.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the Internal Committee for the Care and Use of Experimental Animals (CICUAE) of the National Autonomous University of Mexico. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
ES: Writing – original draft, Writing – review & editing. AM: Writing – review & editing, Writing – original draft. AA-R: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors thank the National Council of Humanities, Sciences and Technologies (CONAHCyT) of Mexico, the UNAM-PAPIIT IN206524, FESC-CI 2441, and COMECYT-FICDTEM-2023-08 for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bojórquez C., Rosiles R., Arana F., Esquivel A., Latournerie J., and Soto R. (2017). Chemical and biological contamination in the lake area of Xochimilco (Mexico: Universidad Autonoma Metropolitana (Mexico), 130.
Clulow J., Upton R., and Clulow S. (2022). “Cryopreservation of amphibian genomes: targeting the Holy Grail, cryopreservation of maternal-haploid and embryonic diploid genomes,” in Reproductive Technologies and biobanking for conservation of amphibians (Taylor & Francis, London UK), 362–400.
Contreras V., Martínez-Meyer E., Valiente E., and Zambrano L. (2009). Recent decline and potential distribution in the last remnant area of the microendemic Mexican axolotl (Ambystoma mexicanum). Biol. Conserv. 142, 2881–2885. doi: 10.1016/j.biocon.2009.07.008
Duhon S. T. (1992). Spawning Axolotls at UI: A ten-year history. Axolotl Newsletter. Summer 21, 29–36.
Gee G. F., Bakst M. R., and Donoghue A. M. (2004). Cryopreservation of avian semen: A review. J. Zoo Wildlife Med. 35 (3), 137–149.
Holt W. V. and Pickard A. R. (1999). Role of reproductive technologies and genetic resource banks in animal conservation. Rev. Reprod. 4, 143–150. doi: 10.1530/revreprod/4.3.143
IUCN SSC Amphibian Specialist Group. (2020). Ambystoma mexicanum. In: The IUCN red list of threatened species e. Available online at: https://dx.doi.org/10.2305/IUCN.UK.2020-3.RLTS.T1095A53947343.en (Accessed July 2, 2025).
IUCN SSC Amphibian Specialist Group. (2024). “Amphibian conservation action plan: A status review and roadmap for global amphibian conservation,” in IUCN SSC occasional paper, no 57. Eds. Wren S., Borzée. A., Marcec-Greaves R., and Angulo A.(Gland, Switzerland: IUCN).
Jing M., Liu C., Zhao S., Fan Z., Wang X., Luo J., et al. (2024). A conservation action plan for Chinese giant salamanders. Available online at: https://www.iucnamphibians.org/a-conservation-action-plan-for-chinese-giant-salam (Accessed June 28, 2025).
Khattak S., Murawala P., Andreas H., Kappert V., Schuez M., Sandoval-Guzman T., et al. (2014). Optimized axolotl (Ambystoma mexicanum) husbandry, breeding, metamorphosis, transgenesis and tamoxifen-mediated recombination. Nat. Protoc. 9, 529–540. doi: 10.1038/nprot.2014.040
Kim W., Nugas C., Henning A., Crawford K., Egar P., Muske L., et al. (1997). Compendium of axolotl husbandry methods. Axolotl Newslett. 25, 1–24.
Levy K. (2017). Distribución actual de Ambystoma mexicanum y su relación con las variables limnéticas de los canales de Xochimilco. (Tesis de Maestría) (México. Recuperado de: Universidad Nacional Autónoma de México). Available online at: https://repositorio.unam.mx/contenidos/65167.
Manjarrez-Alcívar I., Vega-Villasante F., Montoya-Martínez C., López-Félix E. F., Badillo-Zapata D., and Martínez-Cárdenas L. (2022). New findings in the searching of an optimal diet for the axolotl Ambystoma mexicanum: protein levels (Mexico: Agro Productividad https://doi). doi: 10.32854/agrop.v14i6.2188
Marcec R., Langhorne C., Vance C., Kouba A., and Willard S. (2014). Cryopreservation of spermic milt in the model species Ambystoma tigrinum (Tiger salamander) for application in endangered salamanders. Cryobiology 69, 515. doi: 10.1016/j.cryobiol.2014.09.342
Mena H. and Servin E. (2014). Manual básico para el cuidado en cautiverio del Axolote de Xochimilco (Ambystoma mexicanum) (Mexico: Institute of Biology. National Autonomous University of Mexico).
National Research Council (2011). Guide for the care and use of laboratory animals (Washington, DC: The National Academies Press). doi: 10.17226/12910
Parra-Olea G., Flores-Villela O., and Mendoza-Almeralla C. (2014). Biodiversidad de anfibios en México. Rev. Mexicana Biodiversidad 85, Suppl. 1, 460–466. doi: 10.7550/rmb.32027
Robles C., García- Basilio C., and Venegas-Perez R. (2009). Maintenance media for the axolotl Ambystoma mexicanum juveniles (Amphibia: Caudata). Hidrobiológica 19, 205–210.
Semarnat. (2018). Programa de Accion para la Conservacion de las Especies Ambystoma spp (Mexico: SEMARNAT/CONANP (Mexico)). Available online at: https://www.gob.mx/cms/uploads/attachment/file/444128/PACE_Ambystoma2.pdf.
Servin E., Alvarez-Guerrero A., Alcantar-Rodriguez A., Mercado-Marquez C., and Medrano A. (2023). “First approach for cryopreservation of axolotl embryos (Ambystoma mexicanum) as a conservation strategy,” in 1st european symposium on animal reproduction. Reproduction in domestic animals. 58, 194.
Shaffer H. B. (1989). Natural history, ecology, and the evolution of the Mexican “axolotls. Axolotl Newslett. 18, 5–11.
Silla A. and Langhome C. (2022). “Protocols for hormonally induced spermiation, and the cold storage, activation, and assessment of amphibian sperm,” in Reproductive Technologies and Biobanking for the conservation of amphibians (Taylor & Francis, London UK), 262–304.
Swanson W. F., Magarey G., and Herrick J. (2007). Cryopreservation of sperm in endangered felids: Developing linkage of in situ-ex situ populations. Soc. Reprod. Fertil. Suppl. 65, 417–432.
Rosales J. (2015). Comparación del uso de dos diluyentes para la criopreservación de semen de lobo mexicano (Canis lupus baileyi). Tesis de Maestría. Universidad Nacional Autónoma de México, Mexico. Recuperado de Universidad Nacional Autónoma de México.
Wildt D. E., Swanson W. F., Brown J. L., and Roth T. L. (2001). “Reproductive sciences in animal conservation,” in Reproductive sciences and integrated conservation (Cambridge University Press, London UK), 1–24.
Wright K. and Whitaker B. (2001). Amphibian medicine and husbandry (Florida USA: Krieger Publishing Company).
Keywords: Ambystoma mexicanum, laboratory husbandry, amphibian, water quality, axolotl
Citation: Servín E, Alcantar-Rodriguez A and Medrano A (2025) Experiences with the management and breeding of Mexican axolotl (Ambystoma mexicanum), for research and biobanking purposes, in a vivarium. Front. Amphib. Reptile Sci. 3:1620078. doi: 10.3389/famrs.2025.1620078
Received: 29 April 2025; Accepted: 14 July 2025;
Published: 14 August 2025.
Edited by:
Cynthia A. Paszkowski, University of Alberta, CanadaReviewed by:
Ivan Lazcano, National Autonomous University of Mexico, MexicoCopyright © 2025 Servín, Alcantar-Rodriguez and Medrano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erika Servín, ZXJpa2FzZXJ2aW4yNjhAZ21haWwuY29t
 Erika Servín
Erika Servín Alicia Alcantar-Rodriguez
Alicia Alcantar-Rodriguez Alfredo Medrano
Alfredo Medrano