- 1Department of Biomedical and Diagnostic Sciences, University of Tennessee, College of Veterinary Medicine, Knoxville, TN, United States
- 2Center for Wildlife Health, School of Natural Resources, University of Tennessee Institute of Agriculture, Knoxville, TN, United States
Introduction: Batrachochytrium salamandrivorans (Bsal) was discovered a decade ago in Europe, where it is emerging and decimating salamander populations. North America, a global hotspot for salamander biodiversity, faces risk of Bsal introduction through trade or other pathways. An abundant salamander species in these systems, the eastern newt Notophthalmus viridescens, is highly susceptible to Bsal and may play an important role in Bsal epidemiology if the pathogen is introduced. However, we know very little about the physiological mechanisms contributing to the pathogenesis of Bsal chytridiomycosis. This limits our ability to treat infection on an individual level and predict the evolutionary responses of resistance and tolerance on the population level following Bsal invasion.
Methods: We tested the hypothesis that morbidity and mortality of Bsal-infected individuals are directly related to skin lesions, after controlling for Bsal infection intensity. To test this, we compared Bsal-induced lesions in eastern newts among four Bsal zoospore doses (5x103–6 per 10 mL) and maintained at three environmental temperatures (6, 14, and 22°C). Following euthanasia, animals were processed for histologic examination and Bsal-associated lesions were counted and graded for severity on a scale of 1–5. Additionally, dermal glands were examined for Bsal invasion and all internal organs were assessed.
Results: Newts exposed at 22°C did not become infected by Bsal. Newts exposed at 14°C had more lesions compared to those exposed at 6°C across all zoospore doses. For the lowest three zoospore exposure doses, as zoospore dose increased, so did lesion count. Additionally, there was a strong negative relationship between lesion count and survival, after accounting for Bsal infection intensity, suggesting that lesions are contributing to Bsal pathogenesis beyond infection intensity. Lesions were most abundant in the hindlimbs, cloacal region, and tail. There were no Bsal-related abnormalities in internal organs; further supporting our hypothesis that morbidity and mortality in infected individuals are directly related to skin lesions.
Discussion: This is the first Bsal susceptibility study which has evaluated the number, distribution, and severity of histologic lesions in Bsal-infected hosts across multiple temperatures. These results provide insight into the pathogenesis of Bsal chytridiomycosis, and how environmental temperature can impact disease progression. Additionally, these results indicate swabbing the hindlimbs, cloacal region, and tail might increase detection of Bsal on infected animals due to locally increased lesion prevalence.
1 Introduction
Emerging fungal diseases have recently caused unprecedented declines and extinctions in both plant and wildlife species (Fisher et al., 2012). Among affected wildlife species, amphibians have experienced the most devastating losses (Scheele et al., 2019). An emerging fungal disease involved in these declines is chytridiomycosis caused by two species of chytrid fungus, Batrachochytrium dendrobatidis (Bd) and Batrachochytrium salamandrivorans (Bsal) (Scheele et al., 2019).
Bd was discovered in 1998 and is the cause of the majority of these declines (Berger et al., 1999; Yap et al., 2015). Bsal, which was discovered more recently in 2013, is more pathogenic to salamanders, and is emerging quickly (Scheele et al., 2019). Bsal is presumed to have originated in Asia and spread to Europe through trade of infected animals (Martel et al., 2014; Yap et al., 2017), where it is currently causing large die-offs in multiple salamander species (Martel et al., 2014; Lotters et al., 2020; Martel et al., 2020).
Bsal has not yet been identified in North America (Waddle et al., 2020). However, due to multiple factors including environmental suitability in the wild, species susceptibility, and presence of Bsal in trade, it is likely only a matter of time before Bsal spreads to regions other than Asia and Europe (Gray et al., 2015; Grant et al., 2017; Yap et al., 2017; Carter et al., 2019; Carter et al., 2021; Gray et al., 2023). We know little about how Bsal chytridiomycosis manifests and progresses in susceptible species under varying environmental conditions. Addressing this knowledge gap will inform disease identification in susceptible individuals and can be used to better understand the mechanisms of host resistance and tolerance to this emerging pathogen (Raberg et al., 2009). It also can provide insight into the likelihood of infection being maintained and disease developing in captive amphibian trade.
Environmental temperature is especially important for amphibians as many physiologic functions, including the immune response, can be significantly affected by changes in temperature (Wells, 2007; Rollins-Smith, 2017; Rollins-Smith, 2020). Previous studies have shown the pathogenicity of Bsal can change with changing temperatures in both eastern newts as well as fire salamanders (Salamandra salamandra) (Blooi et al., 2015a; Stegen et al., 2017; Beukema et al., 2021; Carter et al., 2021). Carter et al (Carter et al., 2021). evaluated the survival rate of Bsal-infected adult eastern newts across 6, 14, and 22°C and revealed a difference in survivability as well as Bsal qPCR load at time of necropsy between the three temperatures. However, no previous study has evaluated the number, distribution, and severity of histologic lesions in Bsal-infected hosts across multiple temperatures.
Incorporating histologic analysis of skin lesions in Bsal chytridiomycosis studies is important for two reasons. First, positive qPCR results alone do not confirm that infection with a pathogen is inducing disease. Some urodelan species are much more tolerant to Bsal infection than others (Martel et al., 2014; Wilber et al., 2021; Gray et al., 2023). Therefore, a Bsal qPCR load that corresponds with severe disease in one species or individual, may correspond with mild or no disease in another. Consequently, it is important to correlate qPCR results with histologic lesion analysis to determine disease causality and severity.
Second, it has been shown that for multiple urodelan species, number of Bsal-induced skin lesions visible grossly is not predictive of survival and only has a weak positive correlation with Bsal qPCR load (Wilber et al., 2021). This may be because lesions are being missed without the use of histology. For Bd, qPCR-positive amphibians with no clinical signs or grossly apparent skin lesions can still have chytrid-induced skin lesions histologically (Borteiro et al., 2019). Therefore, incorporating histologic lesion counts and grades can provide a better overall picture of disease progression in an animal than gross examination alone (Thomas et al., 2018).
A previous study investigating site predilection for Bsal-induced skin lesions did not incorporate lesion grade (Ossiboff et al., 2019). Higher lesion grade typically corresponds with increased numbers of intralesional Bsal organisms. Therefore, by incorporating lesion grade into histologic analysis, we can more effectively determine the best anatomical locations to collect diagnostic samples in order to optimize pathogen detection in Bsal-infected amphibians.
Previous studies have investigated the effect of various Bsal zoospore exposure doses on survival rate and determined that this pathogen causes dose-dependent mortality in multiple species (Carter et al., 2019; Carter et al., 2021). Utilizing preserved specimens from Carter et al (Carter et al., 2021), which were exposed to varying Bsal doses at three temperatures, we analyzed how disease induced mortality rate and Bsal infection load relate to histological lesion counts and grades. Overall, the aim of this study was to better understand how Bsal causes morbidity in susceptible amphibian species.
2 Methods
2.1 Ethics statement
Husbandry as well as euthanasia procedures are described in detail in Carter et al (Carter et al., 2021), and followed recommendations provided by the American Veterinary Medical Association and the Association of Zoos and Aquariums. Additionally, they were approved by the University of Tennessee Institutional Animal Care and Use Committee (protocol #2623). Notophthalmus viridescens that reached euthanasia endpoints were humanely euthanized via transdermal exposure to benzocaine hydrochloride.
2.2 Animals
A total of 75 N. viridescens (eastern newts) were used in this study. Eastern newts were chosen as they have a wide distribution throughout North America (Nature IUfCo, 2020), and are known to be susceptible to Bsal chytridiomycosis (Martel et al., 2014; Longo et al., 2019; Carter et al., 2021). Animals were collected from Tennessee (TN Scientific Collection Permit #1504).
At arrival into the laboratory, animals were heat-treated at a temperature of 30 °C for 10 days to clear any potential Bd infection obtained in the wild (Chatfield and Richards-Zawacki, 2011; Bletz, 2013). After heat treatment, animals were skin swabbed following a standardized protocol of 10 swipes along the ventrum and 5 swipes along the plantar surface of each foot. DNA was extracted from the swabs, and Bd qPCR was performed following a previously described protocol (Boyle et al., 2004). All animals were confirmed to be qPCR negative for Bd and underwent a one-week acclimation period to their assigned experimental temperature prior to the start of the study.
2.3 Experimental design
Animals were randomly assigned to one of four zoospore exposure doses (5x103, 5x104, 5x105, or 5x106 per 10 mL) along with one of three temperatures (6, 14, and 22°C). Five animals were assigned to each treatment group along with five control animals (n=25 for each temperature trial). Animals were monitored twice daily for development of clinical signs and were euthanized when they reached humane disease endpoints (including loss of righting ability or unresponsiveness) or at the end of the study. Study duration was 60 days.
2.4 Experimental infection
Bsal was isolated from a fire salamander (Salamandra salamandra) in the Netherlands (isolate AMFP13/1 (Martel et al., 2013),). Cultures were maintained at the University of Tennessee Center for Wildlife Health laboratory and zoospores were enumerated according to previously described methods by Carter, et al (Carter et al., 2019).
Experimental infection was performed according to previously described methods by Carter, et al (Carter et al., 2021). Bsal-exposed individuals were exposed to either a dose of 5x103, 5x104, 5x105, or 5x106 zoospores per 10 mL. This was done by separately placing each individual into a 100-mL plastic tube which contained a mixture of Bsal zoospores and 9-mL of autoclaved dechlorinated water for 24 hours in the environmental incubators. Control animals were placed into similar tubes containing 10-mL of autoclaved dechlorinated water under identical time and environmental conditions as the exposed animals.
2.5 Histopathology
Methods for processing carcasses for histopathologic assessment were similar to those used by Sheley, et al., 2023 (Sheley et al., 2022). Carcasses were stored in 10% neutral buffered formalin for at least 48 hours prior to processing. Transverse sections through the body were taken at approximately 3-mm intervals and legs were removed at either the scapulohumeral or coxofemoral joint. Ten standardized sections (Table 1 & Figure 1) were placed into tissue cassettes. Cassettes containing tissues were decalcified for 24 hours using a solution containing formic acid. Tissues were then processed and stained routinely with hematoxylin and eosin (H&E). Histologic examination consisted of assessing all tissues for abnormalities as well as performing Bsal lesion counts and lesion grading in the skin for each of the 10 sections. Lesion grading consisted of scoring each lesion on a scale of 1–5 based on severity (Grade 1 = Bsal organisms within the stratum corneum, Grade 2 = Bsal organisms/associated cellular damage extending to the mid-epidermis, Grade 3 = Bsal organisms/associated cellular damage extending through the epidermis to the basement membrane, Grade 4 = coalescing/large Bsal lesions extending through the epidermis to the basement membrane which were <1 millimeter long, Grade 5 = coalescing/large Bsal lesions extending through the epidermis to the basement membrane which were ≥1 millimeter long; Table 2; Figure 2). Excelis Accu-Scope software was used to measure the perimeter of each section to standardize measures of lesion counts to counts per unit area of cross section.
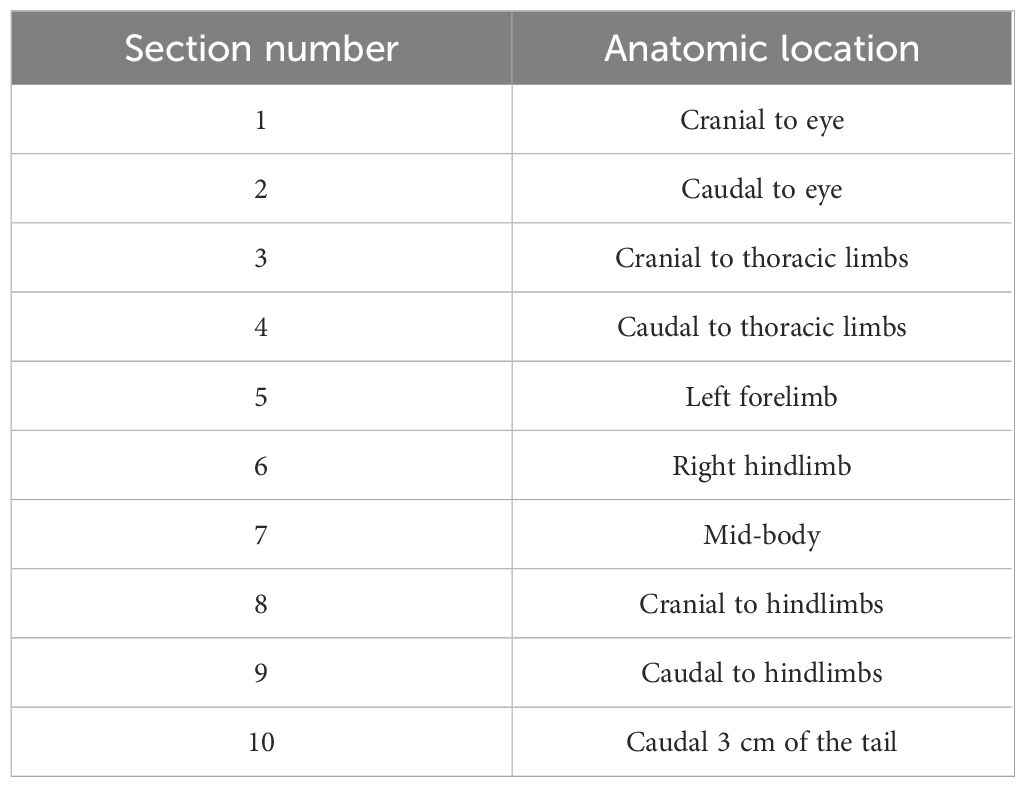
Table 1. Anatomic location associated with each of ten standardized sections examined histologically for lesion counts and grading in Batrachochytrium salamandrivorans infected N. viridescens.
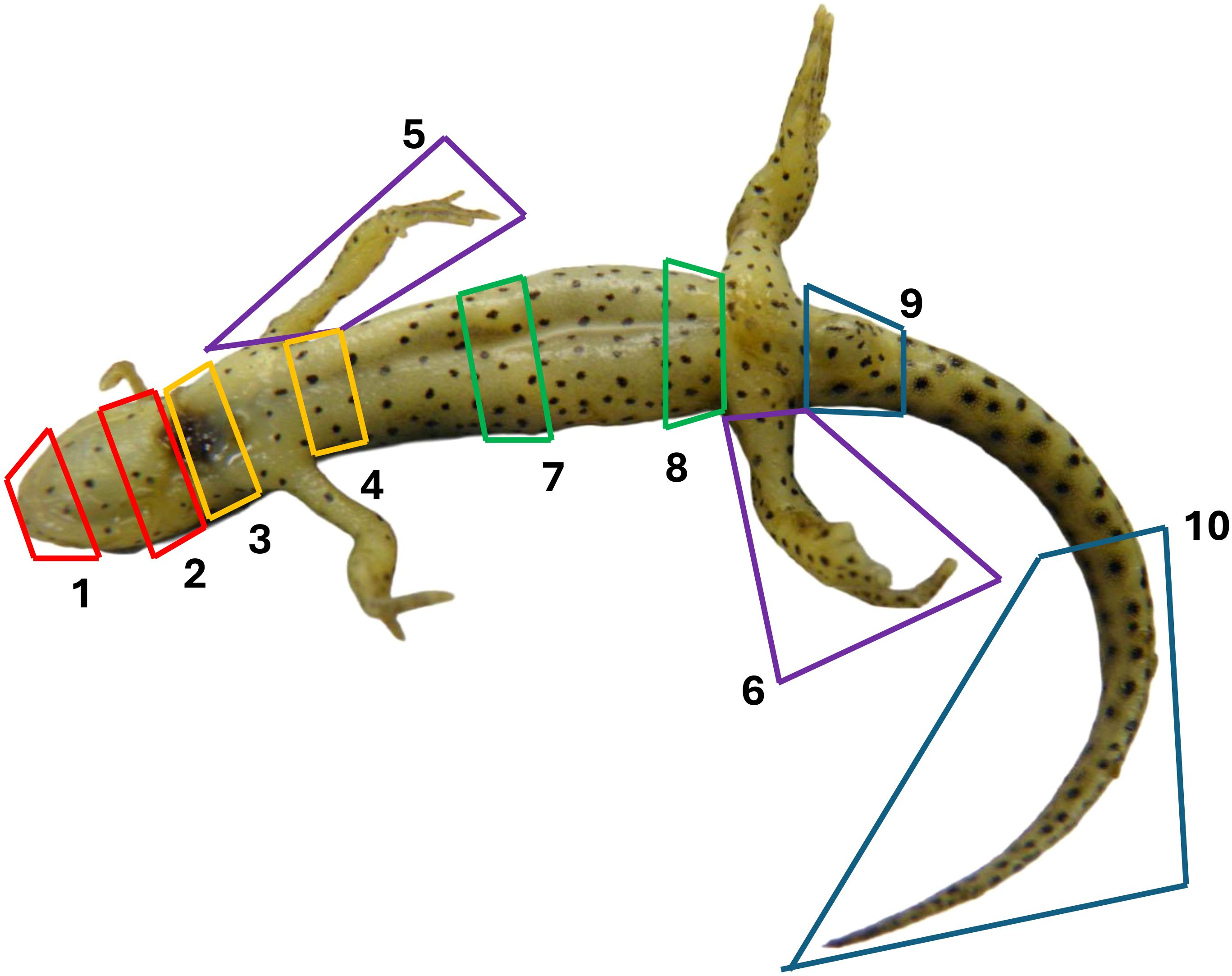
Figure 1. Anatomic location associated with each of the ten standardized sections examined histologically for lesion counts and grading in Batrachochytrium salamandrivorans infected N. viridescens.
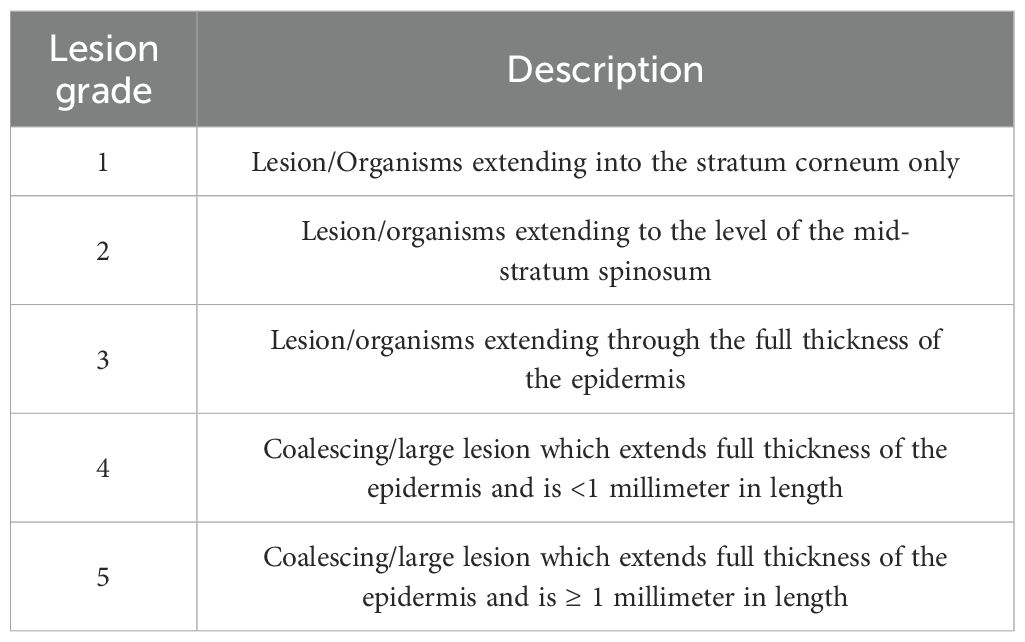
Table 2. Description of histologic lesion grading system used in Batrachochytrium salamandrivorans infected N. viridescens.
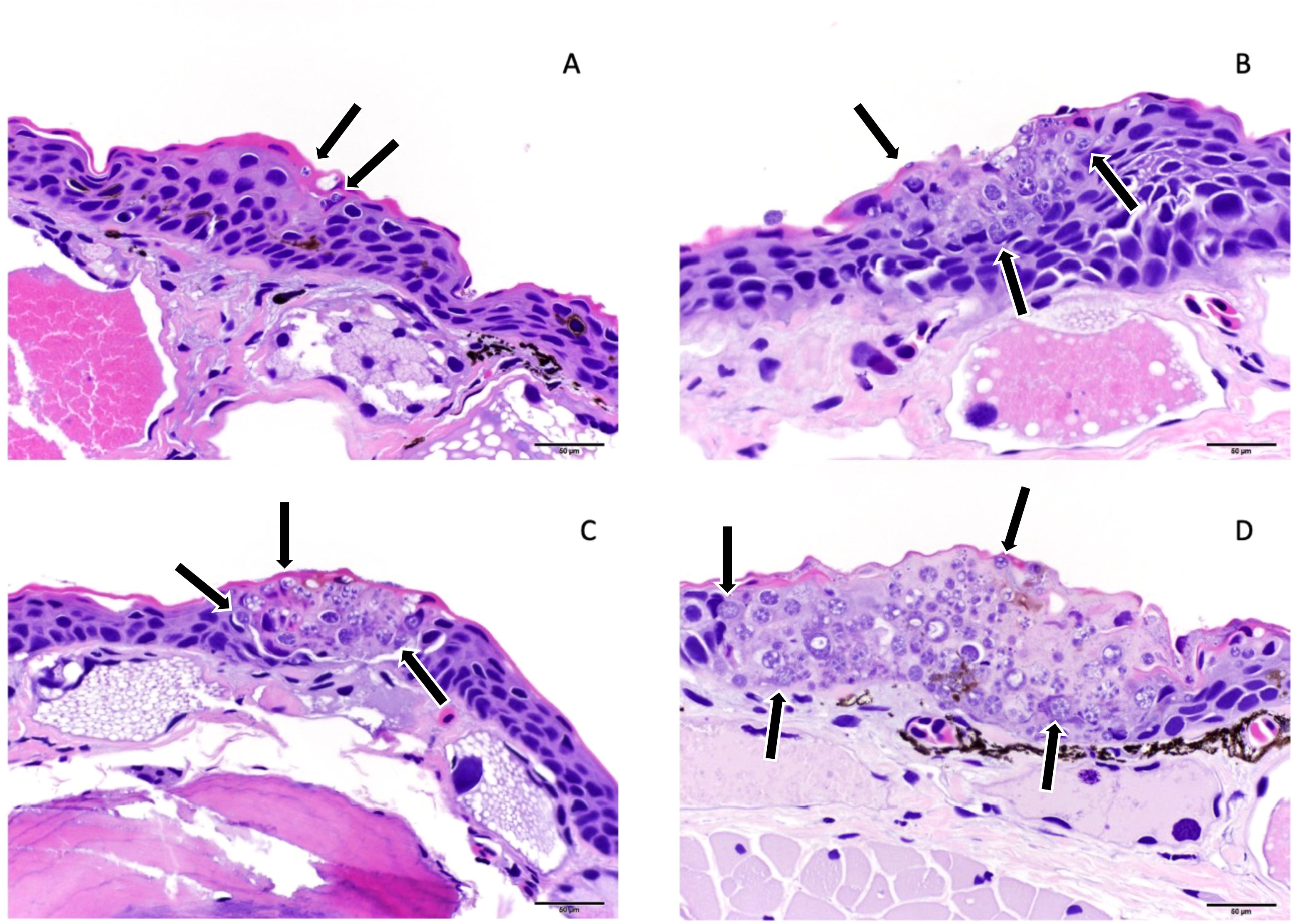
Figure 2. Examples of histologic lesion grading scheme used for N. viridescens infected with Batrachochytrium salamandrivorans (Bsal) including Grade 1 (A), Grade 2 (B), Grade 4 (C), and Grade 5 (D). Grade 1 lesions were limited to Bsal organisms (black arrows) within the stratum corneum, grade 2 lesions included Bsal organisms (black arrows) and associated cellular damage which extended into the mid-epidermis, grade 4 = coalescing/large lesions with Bsal organisms (black arrows) and associated cellular damage which extended through the epidermis to the basement membrane which were <1 millimeter in length, grade 5 lesions included coalescing/large lesions with Bsal organisms (black arrows) and associated cellular damage which extended full thickness through the epidermis and were ≥1 millimeter in length.
2.6 Quantitative polymerase chain reaction
At the time of necropsy, animals were skin swabbed following the standardized protocol previously mentioned of 10 swipes along the ventrum and 5 swipes along the plantar surface of each foot. To detect Bsal and estimate loads, genomic DNA was extracted from each skin swab using the Qiagen DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany) and qPCR performed similar to previously described methods (Blooi et al., 2015a) using the Applied Biosystems Quantstudio 6 Flex qPCR instrument (Thermo Fisher Scientific Inc). Samples were all run in duplicate and confirmed positive if both replicates reached cycle threshold prior to 50 amplification cycles (Carter et al., 2019).
2.7 Statistical analysis
2.7.1 Lesion counts
To determine how temperature and Bsal zoospore exposure dose affected the number of lesions per histologic cross section, a negative binomial generalized linear model was fit where the response variable was lesion count per cross section perimeter and the predictor variables were temperature, Bsal zoospore exposure dose, an interaction term between temperature and Bsal zoospore exposure dose, and section number. Environmental temperature included 6°C and 14°C, Bsal zoospore exposure dose included four levels 5x103–6, and section number included sections 1–10, corresponding to various anatomical sites of the animal’s body (Table 1; Figure 1). Initial analyses showed that lesion counts were over-dispersed relative to a Poisson distribution. Therefore, a negative binomial distribution was used to describe lesions. Additionally, lesion counts were obtained from histological cross sections with varying perimeters. This meant that a larger perimeter might lead to a higher number of counted lesions than a smaller perimeter even if the density of lesions per unit perimeter were the same. To account for this, a log (total perimeter) offset term was included in the negative binomial regression (Faraway et al., 2016). The lesion count model was fit using the brms package in R (Burkner, 2017), with a weakly regularizing prior on the effect sizes for the predictor variables temperature, Bsal zoospore exposure dose, and section number. Convergence was ensured when fitting the models by visually examining chains and checking that R_hat (the potential scale reduction factor) < 1.01 and effective sample size > 400 for all parameters (Vehtari et al., 2020). Five candidate models were fit to the data and best predictive model was selected using Pareto smoothed importance sampling leave-one-out information (PSIS-LOO (Vehtari et al., 2016),). The lower the PSIS-LOO of the model, the better the predictive performance. The models we compared to the final model described above included the following combinations of predictors: 1) temperature and Bsal zoospore exposure dose, 2) temperature, Bsal zoospore exposure dose, and temperature and Bsal zoospore exposure dose as an interaction term, and 3) temperature, Bsal zoospore exposure dose, and section number.
2.7.2 Probability of lesion presence
To determine how temperature and Bsal zoospore exposure dose affected the probability of lesion presence, a Bernoulli generalized linear model was fit where the response variable was lesion presence (1 = lesions present, 0 = lesions absent) and the predictor variables were Bsal zoospore exposure dose, temperature, an interaction term between Bsal zoospore exposure dose and temperature, and the scaled log (perimeter) (where we scaled by subtracting the mean and dividing by the standard deviation) as a covariate rather than an offset term in this analysis. However, the log(perimeter) covariate effectively acted as an offset, accounting for the fact that larger cross sections had a higher probability of lesion presence, all else being equal. The model was fit using the brms package in R (Burkner, 2017), with a weakly regularizing prior on the effect sizes for the predictor variables temperature, Bsal zoospore exposure dose, and perimeter. When fitting the models, model convergence was ensured using the previously described methods. Four candidate models were fit to the data and the best predictive model was selected using PSIS-LOO. Models compared to the final model described above included the following combinations of predictors: 1) the scaled log(perimeter) (where we scaled by subtracting the mean and dividing by the standard deviation), 2) Bsal zoospore exposure dose, temperature, and scaled log (perimeter), 3) Bsal zoospore exposure dose, temperature, Bsal zoospore exposure dose and temperature as an interaction term, scaled log(perimeter), and section number.
2.7.3 Lesion grades
To determine how temperature and Bsal zoospore exposure dose affected the expected lesion density of each lesion grade per unit cross section, a generalized linear model was fit where the response variable was lesion count, and the predictor variables were lesion grade, the interaction term of lesion grade and temperature, the interaction term of lesion grade and Bsal zoospore exposure dose, the interaction term of lesion grade and section number, and the random effect of animal ID. Lesion grade consisted of lesion grades 1–5. This model was an extension of a multinomial regression model where lesion grades were the multinomial categories, re-expressed as a Poisson or negative binomial log-linear model (Faraway et al., 2016). Originally, lesion grades 1–5 were included in the model; however, grade 5 lesions were dropped from the analysis due to their relative rarity leading to issues with separability. Models were fit with both a Poisson and negative binomial distribution of lesion counts, and the negative binomial was preferred due to the overdispersion of lesion counts. Additionally, an offset term of log(total perimeter) was included in the log-linear regression as described in the lesion count analysis.
The model was fit using the brms package in R (Burkner, 2017), with a weakly regularizing prior on the effect sizes for the predictor variables temperature, Bsal zoospore exposure dose, and lesion grade. All continuous covariates were standardized to a mean of 0 and a standard deviation of 1 before fitting. When fitting the models, model convergence was ensured using the previously described methods. Eight candidate models were fit to the data and the best predictive model was selected using PSIS-LOO. Models compared to the final model described above included the following combinations of predictors (using both a negative binomial distribution and a Poisson distribution for each): 1) lesion grade and individual ID as a random effect, 2) lesion grade, lesion grade and temperature as an interaction term, lesion grade and Bsal zoospore exposure dose as an interaction term, and individual ID as a random effect, 3) lesion grade, lesion grade and temperature as an interaction term, lesion grade and Bsal zoospore exposure dose as an interaction term, the combination of lesion grade, temperature, and Bsal zoospore exposure dose as an interaction term, and individual ID as a random effect. For potentially influential observations with a Pareto smoothing k > 0.7, these values were temporarily excluded to see if they affected model selection. Model selection was unaffected by these potentially influential observations, and they were included in the model inference. Finally, Bsal zoospore exposure dose was replaced with Bsal qPCR load at the time of necropsy in all models and were compared to original models using PSIS-LOO.
2.7.4 Survival analysis
To determine how Bsal zoospore exposure dose and lesion count affected survival rate, a Cox proportional hazards model was fit where the response variable was days survived and the predictor variables were Bsal zoospore exposure dose and lesion count. We standardized as lesion counts using the following approach. Lesion counts and perimeters were added together across all cross sections (sections 1 – 10) and total lesion count was divided by total perimeter for each individual, yielding the quantity total lesion count per unit length of cross section. This quantity was then multiplied by the average perimeter of a cross section. This allowed us to interpret the resulting coefficient from the survival model as the change in log hazard given an increase of one lesion in the average cross section. Cox proportional hazards models were also used in Carter et al (Carter et al., 2021). However, they only examined the relationship of survival to exposure dose. We were specifically interested in whether lesion count explained additional variability in survival after accounting for Bsal load. We fit two models i) survival ~ Bsal load at death/censoring + standardized_lesion_count and ii) survival ~ (Bsal load at death/censoring + standardized_lesion)*temperature. The second model allowed us to test whether temperature affected the effect of lesion count or Bsal load on host survival. If N. viridescens were euthanized after reaching humane endpoints, they were designated as right-censored in the analysis.
When fitting the models, it was ensured that the proportional hazards assumption was met by confirming a non-significant relationship between Schoenfeld residuals for each covariate and time (Klein and Moeschberger, 2003). Overall goodness-of-fit was checked by plotting cumulative hazard against Cox-Snell residuals and visually assessing for clear outliers by plotting deviance residuals against observation number. All models were fit with using the coxph function in the `survival` package in R [version 4.1.1 (Therneau and Lumley, 2015)].
3 Results
3.1 Lesion counts
Across all Bsal zoospore exposure doses, N. viridescens exposed at 14°C had a higher lesion count per average cross section than those exposed at 6°C (Figure 3), though the effect of temperature changed with zoospore exposure dose (Difference in PSIS-LOO for models with and without the temperature by zoospore exposure dose interaction = 41.2; Figure 3). N. viridescens exposed to the 5x105 zoospore dose at 14 °C had the overall highest lesion count per average cross section (number of lesions per average cross section for the 5x105/14°C treatment: Mean (E): 77.24, 95% Credible Interval (CI): 55.70–109.15; Figure 3). Due to the significant interaction between temperature and zoospore exposure dose, individuals exposed to the 5x106 zoospore dose at 14°C had lower lesion counts than those at 5x105 doses (Difference between 5x105 and 5x106 at 14°C: E: 41.92, 95%CI: 22.31–69.78; Figure 3). Across all zoospore exposure dose and temperature combinations, sections 6 (hindlimb), 9 (cloacal region) and 10 (tail) had the highest lesion counts relative to the baseline of Section 1 (effect sizes relative to Section 1: [(section 6 = E: 0.57; 95%CI: 0.2–0.95); (section 9 = E: 0.57; 95%CI: 0.18–0.98); (section 10 = E: 0.85; 95%CI: 0.48–1.23)]; Figure 3).
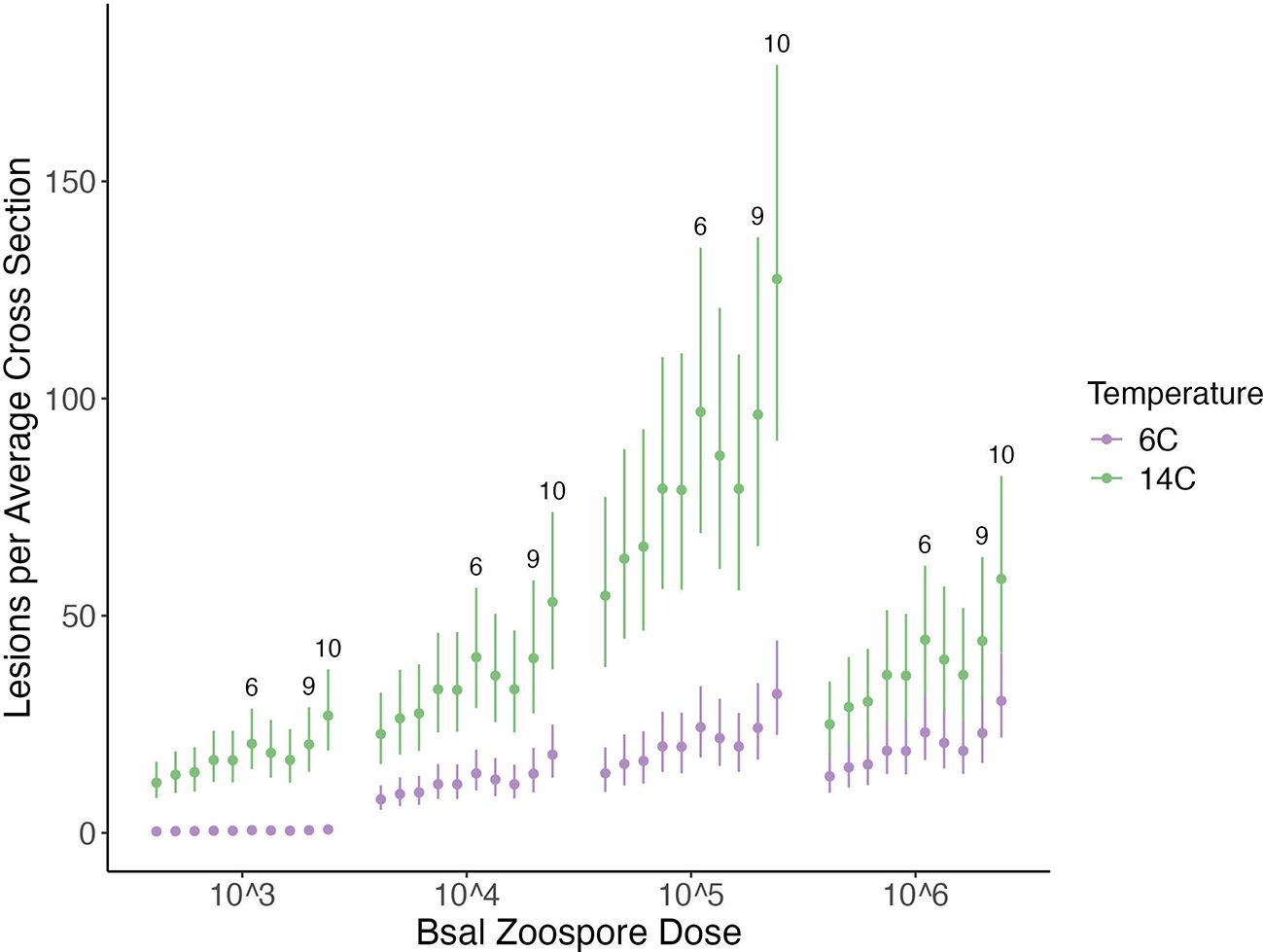
Figure 3. Plot of the histologic lesions per average cross section (y-axis) for each Batrachochytrium salamandrivorans (Bsal) zoospore exposure dose (x-axis) and temperature (6 °C = purple; 14 °C = green) treatment combination in Bsal-infected N. viridescens. Each point within a zoospore exposure dose represents a section in numerical order from section 1–section 10. Error bars are showing the 95% credible intervals around the predicted response.
3.2 Probability of lesion presence
N. viridescens exposed to the two lowest zoospore doses had a greater probability of having Bsal-associated skin lesions at 14°C than at 6°C (Estimate of difference between the probability of lesion presence at 14 °C minus the probability of lesion presence at 6°C for 5x103: E: 0.62, 95% CI: 0.46–0.75; Estimate of difference between the probability of lesion presence at 14°C minus the probability of lesion presence at 6°C for 5x104: E: 0.15, 95%CI: 0.07–0.27; Figure 4). N. viridescens exposed to the two highest zoospore doses had an approximately equal probability of having Bsal-associated skin lesions regardless of temperature (95% CI of difference between the probability of lesion presence at 14°C minus the probability of lesion presence at 6°C for 5x105: -0.02–0.03; 95% CI of difference between the probability of lesion presence at 14°C minus the probability of lesion presence at 6°C at 5x106: -0.02–0.02; Figure 4). Finally, N. viridescens exposed to zoospore doses 5x104–6 were more likely to have lesions present than those exposed to a zoospore dose of 5x103 regardless of the temperature (Figure 4).
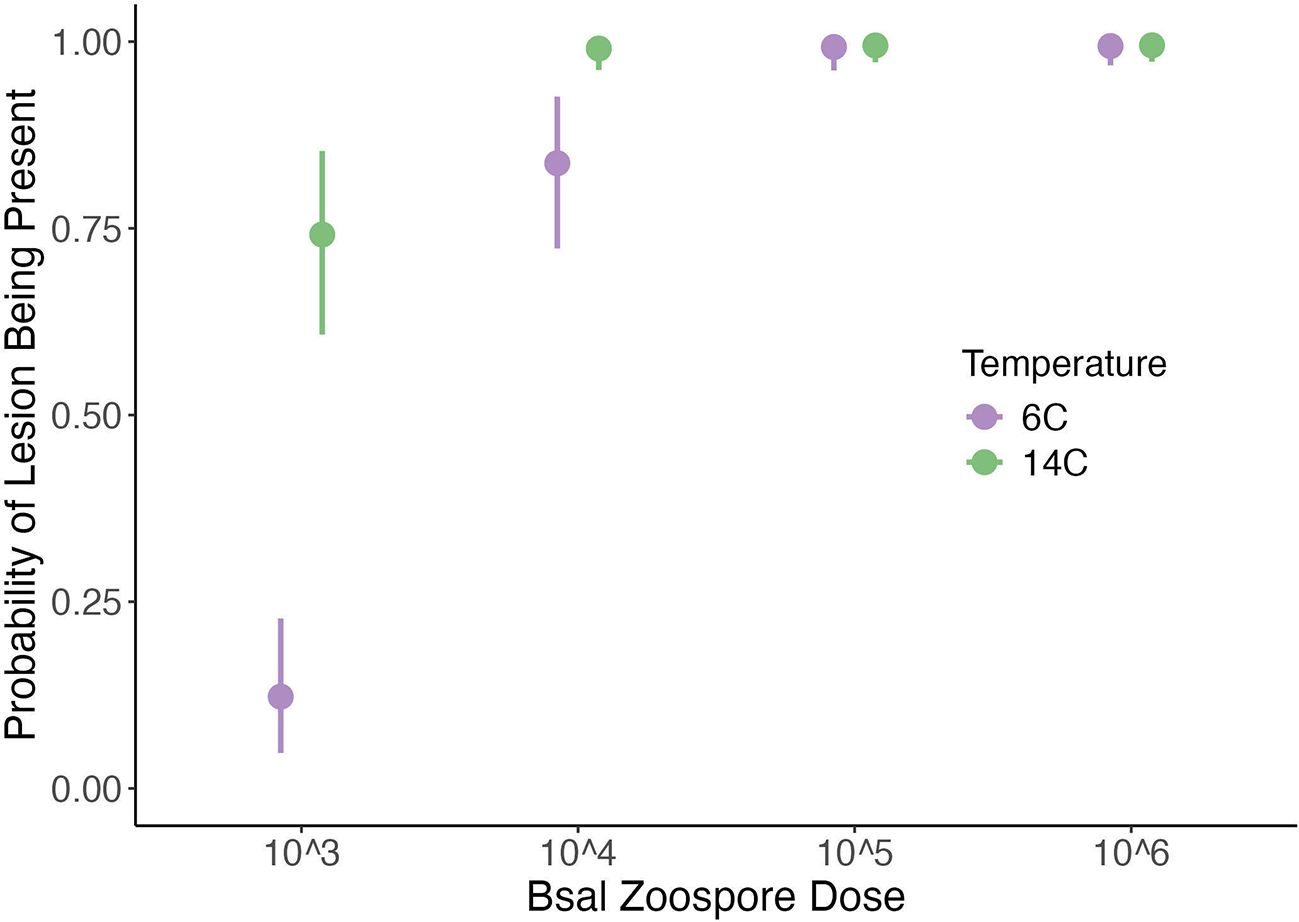
Figure 4. Plot of the probability of histologic lesions being present (y-axis) across each Batrachochytrium salamandrivorans (Bsal) zoospore exposure dose (x-axis) and temperature (6°C = purple; 14°C = green) treatment combination in Bsal-infected N. viridescens. Error bars are showing the 95% credible intervals around the predicted response.
3.3 Lesion grade
Regardless of temperature, Grade 1 lesions were the most common at the lowest zoospore exposure dose, Grade 2 lesions were the most common at the 5x105 zoospore exposure dose, and Grade 3 lesions were the most common at the 5x104 and 5x106 zoospore exposure doses (Figure 5). Grade 5 lesions were the least common grade of lesion across all zoospore exposure dose/temperature combinations (Total number of Grade 5 lesions observed from N. viridescens across all zoospore exposure dose/temperature combinations = 83). Grade 4 lesions were the second least common grade of lesions across all zoospore exposure dose/temperature combinations (Figure 5). Similar to the analysis on overall lesion counts, section number (sections 6 and 10) was an important predictor of lesion grade, particularly for Grade 4 and Grade 5 lesions (Difference in PSIS-LOO for models with and without Section as a factor = 579.9). As determined previously, temperature also had a significant effect on lesion count; however, the magnitude of the effect varied with lesion grade (Figure 5).
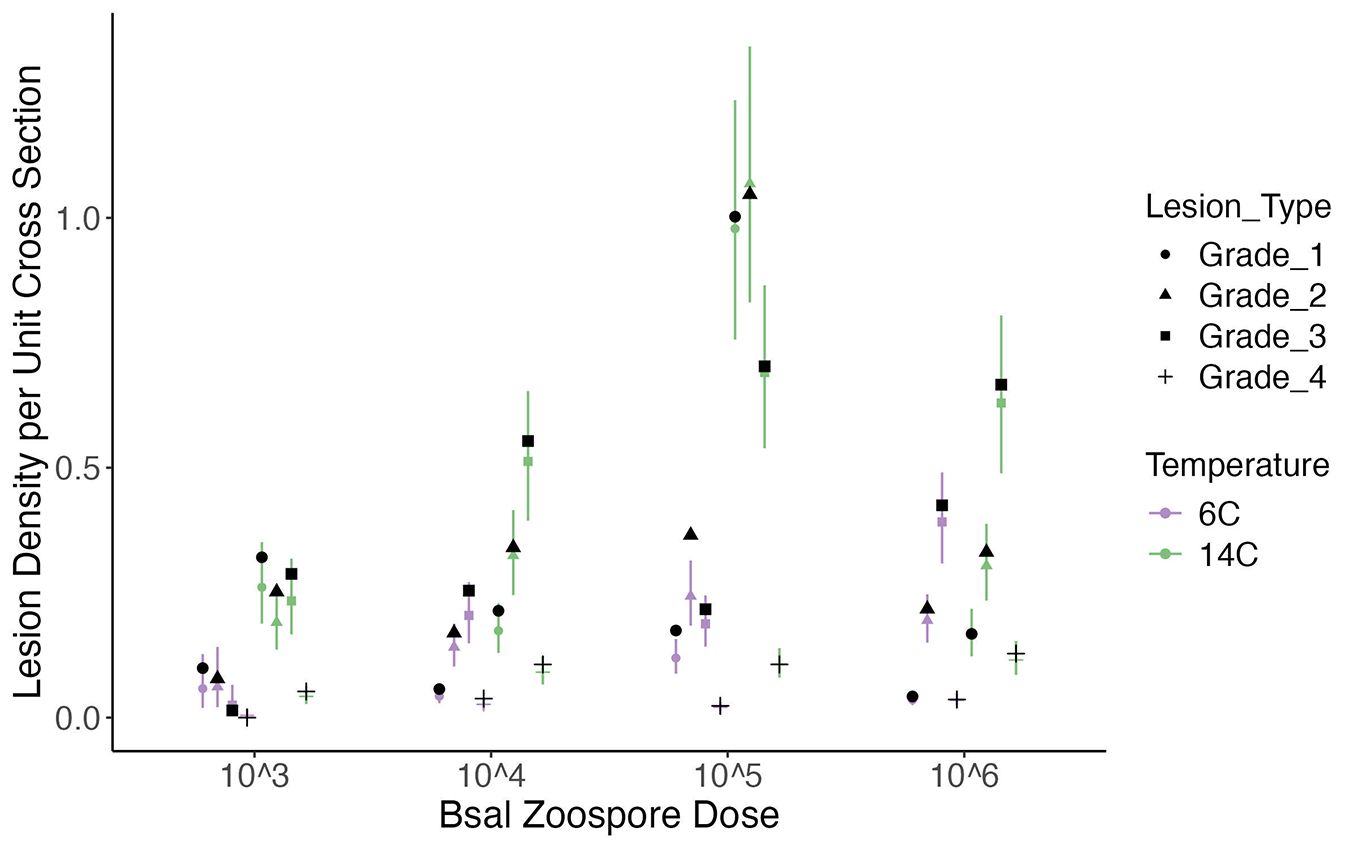
Figure 5. Plot of the histologic lesion density per unit of cross section (y-axis) for each Batrachochytrium salamandrivorans (Bsal) zoospore exposure dose (x-axis) and temperature (6°C = purple; 14°C = green) treatment combination in Bsal-infected N. viridescens. Each point within a zoospore exposure dose represents a lesion grade in numerical order from Grade 1–Grade 4 along with an associated shape (Grade 1 = circle; Grade 2 = triangle; Grade 3 = square; Grade 4 = +). Black points represent the observed mean lesion density for each lesion grade within a zoospore exposure dose and temperature. Error bars are showing the 95% credible intervals around the predicted response.
3.4 Survival analysis
A strong negative relationship was identified between lesion count and survival duration, where an increase in one lesion per average cross section increased the hazard rate by 1.06 (Hazard ratio: 1.06; 95%CI: 1.03-1.09). The average lesion count per cross section was 27 lesions. Our model predicted that increasing lesion count by 27 on the average cross section would increase the hazard rate 5.3 times. This strong relationship persisted after accounting for Bsal infection intensity at necropsy, despite increasing Bsal infection intensity also strongly increased the hazard rate of individuals (Hazard ratio for an increase in 1000 zoospore equivalents: 1.01, 95% CI: 1.005 – 1.03). Overall, this analysis shows that histological lesions describe variability in host survival beyond infection intensity. Including temperature as an interactive effect with lesion count and Bsal load did not significantly affect the model fit.
3.5 Internal organ and dermal gland examination
No lesions associated with Bsal chytridiomycosis were identified in internal organs. The only abnormalities identified in both control and exposed individuals included variable amounts of melanomacrophage hyperplasia in the liver as well as various parasites within the coelomic cavity, gastrointestinal tract, and skeletal muscle. Parasites included mesomycetozoans, trematodes, nematodes, and cestodes, and were associated with minimal to no inflammatory response. For the 6°C animals, dermal gland invasion was identified in 1–2 dermal glands in one 5x103, one 5x104, two 5x105, and one 5x106 individual(s). For the 14 °C animals, dermal gland invasion was identified in 1–4 dermal glands in two 5x104, one 5x105, and two 5x106 individual(s). In all regions of dermal gland invasion by Bsal organisms, there was accompanying cellular damage affecting the overlying dermis and epidermis.
4 Discussion
The main objective of this study was to determine how environmental temperature and pathogen dose relate to disease progression in Bsal-exposed N. viridescens. This study is unique in that it is the first study to evaluate Bsal disease progression in infected hosts using histologic evaluation of the number, distribution, and severity of lesions at different temperatures. Environmental temperature as well as Bsal zoospore exposure dose were both shown to have a significant effect on histologic lesion development. N. viridescens exposed at 22°C did not become infected at any Bsal zoospore exposure dose. N. viridescens exposed at 14°C had increased lesion counts compared to those exposed at 6°C across all zoospore exposure doses. These findings support those from a previous study (Carter et al., 2021), which determined that N. viridescens exposed to Bsal at 14°C had overall lower survival rates than those exposed at 6°C or 22°C. Lower survival rates in environmental conditions associated with higher lesion counts supports the hypothesis of Bsal chytridiomycosis having a similar pathogenesis to Bd chytridiomycosis, involving pathogen-induced epithelial damage being the primary cause of morbidity and mortality in infected individuals (Voyles et al., 2009).
The reason for these differences between exposure temperatures is likely multifactorial and due to variations in both the host response to infection and the pathogenicity of the fungus. Carter et al (Carter et al., 2021) showed that N. viridescens exposed at 6°C tend to survive longer and with a lower Bsal qPCR load at necropsy than those exposed at 14°C, likely indicating a decreased infection resistance at 14°C. A similar trend was also identified in a separate study involving S. salamandra (Stegen et al., 2017). Our results build on this result in two ways. First, our results show that reductions in lesion counts for a given Bsal load have significant positive effects on host survival, indicating that host control of lesions is a mechanism of tolerance. Second, we found that the joint effect of temperature and lesion count on newt survival was near zero. In general, this result suggests that while temperature directly affects host resistance (i.e., reductions in load), it does not affect the strength of this potential form of tolerance. Note that changes in Bsal load at 6°C compared to 14°C are also related to the thermal niche of the Bsal isolate used in this study in addition to resistance mechanisms of the host. However, the tolerance mechanism we identified (i.e., reducing lesions increased host survival) is independent of temperature and conditional on Bsal load, suggesting that this mechanism is largely a property of the host and not the pathogen.
Carter et al (Carter et al., 2021). identified another potential host response to Bsal infection in that N. viridescens housed at 6°C had a larger amount of total recovered proteins on their skin than those housed at 14°C. Carter et al (Carter et al., 2021). determined these proteins have inhibitory effects against Bsal zoospores which could have led to decreased rate of disease progression in the 6°C animals. Decreased histologic lesion counts at 6 degrees C compared to 14°C may also support this hypothesis. By incorporating histologic lesion counts in this study, we have gained an understanding of disease progression that Carter et al (Carter et al., 2021). was not able to assess without the use of histology. Host microbiome shifts and immune function differences between the two temperatures may have also influenced the disease progression. Carter et al (Carter et al., 2021). also found differences in richness and community structure on N. viridescens housed at 6, 14 and 22°C. Immune gene expression at varying temperatures should be explored further.
In regard to changes in the pathogenicity of the fungus, the strain of Bsal used in this experiment has an optimal in vitro growth temperature between 10°C and 15°C, with reduced growth at lower temperatures (Martel et al., 2013). Therefore, at 6°C, Bsal replication rate may have been reduced compared to at 14°C. This may have also contributed to decreased lesion numbers on N. viridescens exposed at this temperature. Previous studies have shown that S. salamandra housed at 25°C can clear Bsal infection with heat treatment alone (Blooi et al., 2015a), and at 20°C in combination with antibiotic and antifungal agents (Blooi et al., 2015b). This study as well as a study by Carter et al (Carter et al., 2021). also confirmed no infection when N. viridescens were exposed to Bsal at 22°C. It is important to note that not all strains of Bsal have the same thermal niche (Kelly et al., 2024), therefore, the results presented here should not be generalized across infection with all strains of Bsal. Future studies infecting hosts with additional strains of the pathogen at varying temperatures based on the thermal niche of the pathogen and the host would be needed to explore these potential differences.
The probability of lesion presence being equal between 6°C and 14°C at the two highest Bsal zoospore exposure doses indicates that when N. viridescens are exposed to a high enough load of the pathogen, they will become infected regardless of the environmental temperature at 6 or 14°C. This is supported by the decreased survival rate seen in the two highest zoospore exposure doses in this study. Additionally, similar survival rate findings were reported in a previous study (Carter et al., 2021).
Across all 6°C and 14°C temperature and zoospore exposure dose combinations, grade 5 lesions were the rarest, followed by grade 4 lesions. These lesion grades occurred most at the higher zoospore exposure doses in the 14°C temperature group. As these are the most severe lesion grades, it is likely that once lesions this severe begin to develop, mortality occurs shortly after.
The hindlimbs, cloacal region, and tail were identified as the most common sites for lesion development across all 6°C and 14°C temperature and zoospore exposure dose combinations. Additionally, the hindlimbs and tail were where the most severe lesions occurred. The hindlimbs and tail were also identified to be predilection sites for Bsal-associated lesions histologically in a previous study in N. viridescens; however, this study did not investigate lesion grade (Ossiboff et al., 2019). This information is very important for disease surveillance efforts as it provides knowledge regarding the best anatomic location to collect diagnostic samples to obtain the highest chance of detecting the pathogen in Bsal-infected animals. Additionally, these sections are of particular importance when examining an animal grossly for Bsal-associated skin lesions. Since these sites have the most numerous and most severe lesions, they are more likely to be visible without microscopic examination. It is important to note that additional studies investigating Bsal skin lesion distribution in naturally infected animals should be performed as exposure method could affect lesion distribution in experimental studies.
At 14°C, N. viridescens exposed to the second highest zoospore exposure dose had the greatest expected lesion density per cross section. This is an interesting finding as the highest zoospore exposure dose might be expected to have the highest lesion count. However, the reasoning for this is that although more lesions were present in animals at the 5x105 exposure dose, the lesions were more commonly less severe than those present in the 5x106 animals. For example, 20 of the 63 grade five lesions were found in 5x106 animals. Therefore, the 5x106 animals had a lower expected lesion density with overall more severe lesions.
Histologic lesion count was determined to have a strong negative correlation with survival time after accounting for Bsal infection intensity, with increasing lesion counts leading to decreased probability of survival. A previous study assessing gross rather than histologic lesion count showed that lesion count was not predictive of survival, after accounting for Bsal intensity (Wilber et al., 2021). This highlights the importance of incorporating histology into Bsal chytridiomycosis studies to fully understand the impact of infection and the mechanisms underlying resistance and tolerance (Thomas et al., 2018).
Invasion of dermal glands with Bsal zoosporangia was identified in at least one N. viridescens from the majority of zoospore exposure dose treatments within the 6°C and 14°C treatment groups. Amphibians possess two types of glands within their dermis classified as either granular glands or mucous glands. These glands have many important functions including maintaining moisture and permeability of the skin, producing and secreting antimicrobial peptides as a key component of the innate immune response, and producing substances to deter predators (Varga et al., 2018). Infection of dermal glands in Bsal chytridiomycosis has been reported previously (Ossiboff et al., 2019), and has been proposed as a potential unique component of Bsal pathogenesis (Sheley et al., 2023), as this does not occur in cases of Bd chytridiomycosis (Longcore et al, 1999; Berger et al., 2005). Carter et al (Carter et al., 2021). found that at two months post Bsal exposure, N. viridescens exposed at 6°C had significantly less defensive hydrophobic molecules produced through skin secretions than control N. viridescens, which could also be supportive of damage to these glands being an aspect of disease pathogenesis (Carter et al., 2021). Secretion of these defensive molecules has also been shown to be associated with stress as well as other types of injury and infection (Varga et al., 2018). Therefore, future studies assessing impaired function of these glands associated with Bsal infection are warranted.
As in other chytridiomycosis studies, no internal lesions directly related to chytridiomycosis were identified. Varying amounts of melanomacrophage hyperplasia were noted within the liver of both control and exposed N. viridescens; however, this is a non-specific finding. Multiple types of parasites were documented within the coelomic cavity, gastrointestinal tract, and skeletal muscle; however, they incited no to minimal inflammatory response and were considered incidental. Lack of internal lesions may provide further support to the proposed pathogenesis of chytridiomycosis primarily involving damage to the skin cells leading to electrolyte imbalances and cardiac arrest (Voyles et al., 2009; Sheley et al., 2022). Death associated with electrolyte imbalances is often very acute, and therefore, histologic lesions frequently do not have time to develop in internal organs such as the heart. Alternatively, skin lesions may create a portal of entry into the body for secondary bacterial infections which lead to bacteremia and contribute to mortality. No lesions associated with bacteremia were present in internal organs; however, in acute cases of bacteremia lesions may not have time to manifest histologically. Although no significant invasion of the epidermis by bacteria was noted in these animals on routine histologic staining with H&E, future studies may incorporate special staining for bacteria such as a Gram stain to highlight bacteria within the lesions histologically. Also, sterile culturing of internal organs could be useful in determining if acute bacteremia may play a role in morbidity and mortality in these infected animals.
Overall, findings show that histologic lesion count and grade are influenced by both environmental temperature as well as zoospore exposure dose in Bsal-infected N. viridescens. Additionally, these results provide support for the pathogenesis of chytridiomycosis primarily involving damage to the epidermal cells (Sheley et al., 2023). Also, further evidence that dermal gland invasion could potentially play a role in Bsal chytridiomycosis disease pathogenesis was provided (Ossiboff et al., 2019), as invasion of glands was identified in multiple individuals across treatment groups.
Future studies should assess the effect of temperature and zoospore exposure dose on histologic lesion count in additional amphibian species, as each species responds differently to infection (Martel et al., 2014; Gray et al., 2023). This will further our knowledge of host-pathogen interactions as well as increase our understanding of pathogen tolerance (Wilber et al., 2021). Additionally, future studies incorporating other diagnostic methods such as complete blood cell counts, blood gas evaluation, plasma protein electrophoresis and three-dimensional soft tissue imaging would be useful to better understand what disturbances are occurring in the host and leading to morbidity and mortality associated with skin lesions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by University of Tennessee Institutional Animal Care and Use Committee (protocol #2623). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
WS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. MW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. EC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. MG: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing, Resources. DM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding for this study was provided by the joint NSF-NIH-NIFA Ecology and Evolution of Infectious Disease award #1814520, the U.S. Department of Agriculture NIFA Hatch Project #1012932, and the University of Tennessee College of Veterinary Medicine Comparative and Experimental Medicine program.
Acknowledgments
The authors would like to thank Dr. Bobby Simpson and Alex Anderson of the University of Tennessee Research and Education Center for providing laboratory facilities and support. We also thank all of the current and former members of the UTIA Amphibian Disease Lab, specifically Adri Tompros, Dr. Ana Towe, Joe DeMarchi, Dr. Tan Watcharaanantapong, Markese Bohanon, Christian Yarber, Carlin Frost, Megan Wilson, Caleb Keoho, Alex Funk, Merrie Urban, and Carmen Merolle for their support and assistance with animal care. All animal procedures were approved by the University of Tennessee Institutional Animal Care and Use Committee under protocol #2623.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Berger L., Hyatt A. D., Speare R., and Longcore J. E. (2005). Life cycle stages of the amphibian chytrid batrachochytrium dendrobatidis. Dis. Aquat Organ 68, 51–63. doi: 10.3354/dao068051
Berger L., Speare R., and Hyatt A. (1999). “Chytrid fungi and amphibian declines: overview, implications and future directions,” in Declines and disappearances of Australian frog (Environment Australia, Canberra ACT), 22–33.
Beukema W., Pasmans F., Van Praet S., Ferri-Yanez F., Kelly M., Laking A. E., et al. (2021). Microclimate limits thermal behaviour favourable to disease control in a nocturnal amphibian. Ecol. Lett. 24, 27–37. doi: 10.1111/ele.13616
Bletz M. C. (2013). “Probiotic bioaugmentation of an anti-bd bacteria, janthinobacterium lividum, on the amphibian, notophthalmus viridescens: transmission efficacy and persistence of the probiotic on the host and non-target effects of probiotic addition on ecosystem components,” (James Madison University, Harrisonburg, VA).
Blooi M., Martel A., Haesebrouck F., Vercammen F., Bonte D., and Pasmans F. (2015a). Treatment of urodelans based on temperature dependent infection dynamics of batrachochytrium salamandrivorans. Sci. Rep. 5, 8037. doi: 10.1038/srep08037
Blooi M., Pasmans F., Rouffaer L., Haesebrouck F., Vercammen F., and Martel A. (2015b). Successful treatment of batrachochytrium salamandrivorans infections in salamanders requires synergy between voriconazole, polymyxin E and temperature. Sci. Rep. 5, 11788. doi: 10.1038/srep11788
Borteiro C., Kolenc F., Verdes J. M., Martinez Debat C., and Ubilla M. (2019). Sensitivity of histology for the detection of the amphibian chytrid fungus batrachochytrium dendrobatidis. J. Vet. Diagn. Invest. 31, 246–249. doi: 10.1177/1040638718816116
Boyle D. G., Boyle D. B., Olsen V., Morgan J. A., and Hyatt A. D. (2004). Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time taqman pcr assay. Dis. Aquat Organ 60, 141–148. doi: 10.3354/dao060141
Burkner P. (2017). Brms: an R package for bayesian multilevel models using stan. J. Stat. Software 80, 1–28. doi: 10.18637/jss.v080.i01
Carter E. D., Bletz M. C., Le Sage M., LaBumbard B., Rollins-Smith L. A., Woodhams D. C., et al. (2021). Winter is coming-temperature affects immune defenses and susceptibility to batrachochytrium salamandrivorans. PloS Pathog. 17, e1009234. doi: 10.1371/journal.ppat.1009234
Carter E. D., Miller D. L., Peterson A. C., Sutton W. B., Cusaac J. P. W., Spatz J. A., et al. (2019). Conservation risk of batrachochytrium salamandrivorans to endemic lungless salamanders. Conserv. Lett. 13, e12675. doi: 10.1111/conl.12675
Chatfield M. W. and Richards-Zawacki C. L. (2011). Elevated temperature as a treatment for batrachochytrium dendrobatidis infection in captive frogs. Dis. Aquat Organ 94, 235–238. doi: 10.3354/dao02337
Faraway J. J.. (2016). Extending the linear model with R: generalized linear, mixed effects and nonparametric regression models Taylor & Francis group. Eds. Dominici F., Faraway J. J., Tanner M., and Zidek J. (Boca Raton, FL: Chapman & Hall/CRC).
Fisher M. C., Henk D. A., Briggs C. J., Brownstein J. S., Madoff L. C., McCraw S. L., et al. (2012). Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186–194. doi: 10.1038/nature10947
Grant E. H. C., Muths E., Katz R. A., Canessa S., Adams M. J., Ballard J. R., et al. (2017). Using decision analysis to support proactive management of emerging infectious wildlife diseases. Front. Ecol. Environ. 15, 214–221. doi: 10.1002/fee.1481
Gray M. J., Carter E. D., Piovia-Scott J., Cusaac J. P. W., Peterson A. C., Whetstone R. D., et al. (2023). Broad host susceptibility of north american amphibian species to batrachochytrium salamandrivorans suggests high invasion potential and biodiversity risk. Nat. Commun. 14, 3270. doi: 10.1038/s41467-023-38979-4
Gray M. J., Lewis J. P., Nanjappa P., Klocke B., Pasmans F., Martel A., et al. (2015). Batrachochytrium salamandrivorans: the north american response and a call for action. PloS Pathog. 11, e1005251. doi: 10.1371/journal.ppat.1005251
Kelly M., Cuomo C. A., Beukema W., Carranza S., Erens J., Foubert M., et al. (2024). High phenotypic diversity correlated with genomic variation across the european batrachochytrium salamandrivorans epizootic. PloS Pathog. 20, e1012579. doi: 10.1371/journal.ppat.1012579
Klein J. P. and Moeschberger M. L. (2003). Survival analysis: techniques for censored and truncated data. 2nd ed (New York: Springer).
Longcore J. E., Nichols D. K., and Pessier A. P. Batrachochytrium Dendrobatidis Gen. Et Sp. Nov., a Chytrid Pathogenic to Amphibians Mycologia 1999 9doi: 10.1080/00275514.1999.12061011
Longo A., Fleischer R. C., and Lips K. R. (2019). Double trouble: co-infections of chytrid fungi will severely impact widely distributed N. Viridescens. Biol. Invasions 21. doi: 10.1007/s10530-019-01973-3
Lotters S., Veith M., Wagner N., Martel A., and Pasmans F. (2020). Bsal-driven salamander mortality pre-dates the european index outbreak. Salamandra 56, 239–242.
Martel A., Blooi M., Adriaensen C., Van Rooij P., Beukema W., Fisher M. C., et al. (2014). Recent introduction of a chytrid fungus endangers western palearctic salamanders. Science 346, 630–631. doi: 10.1126/science.1258268
Martel A., Spitzen-van der Sluijs A., Blooi M., Bert W., Ducatelle R., Fisher M. C., et al. (2013). Batrachochytrium salamandrivorans sp. Nov. Causes lethal chytridiomycosis in amphibians. Proc. Natl. Acad. Sci. U.S.A. 110, 15325–15329. doi: 10.1073/pnas.1307356110
Martel A., Vila-Escale M., Fernandez-Giberteau D., Martinez-Silvestre A., Canessa S., Van Praet S., et al. (2020). Integral chain management of wildlife diseases. Conserv. Lett. 13, e12707. doi: 10.1111/conl.12707
Nature IUfCo (2020). The iucn red list of threatened species. Available online at: https://www.iucnredlist.org (Accessed March 3,2022).
Ossiboff R. J., Towe A. E., Brown M. A., Longo A. V., Lips K. R., Miller D. L., et al. (2019). Differentiating batrachochytrium dendrobatidis and B. Salamandrivorans in amphibian chytridiomycosis using rnascope((R)) in situ hybridization. Front. Vet. Sci. 6. doi: 10.3389/fvets.2019.00304
Raberg L., Graham A. L., and Read A. F. (2009). Decomposing health: tolerance and resistance to parasites in animals. Philos. Trans. R Soc. Lond B Biol. Sci. 364, 37–49. doi: 10.1098/rstb.2008.0184
Rollins-Smith L. A. (2017). Amphibian immunity-stress, disease, and climate change. Dev. Comp. Immunol. 66, 111–119. doi: 10.1016/j.dci.2016.07.002
Rollins-Smith L. A. (2020). Global amphibian declines, disease, and the ongoing battle between batrachochytrium fungi and the immune system. Herpetologica 76, 178–188. doi: 10.1655/0018-0831-76.2.178
Scheele B. C., Pasmans F., Skerratt L. F., Berger L., Martel A., Wouter B., et al. (2019). Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Wildlife Dis. 363, 1459–1463. doi: 10.1126/science.aav0379
Sheley W. C., Gray M. J., Wilber M. Q., Cray C., Carter E. D., and Miller D. L. (2023). Electrolyte imbalances and dehydration play a key role in batrachochytrium salamandrivorans chytridiomycosis. Front. Vet. Sci. 9. doi: 10.3389/fvets.2022.1055153
Stegen G., Pasmans F., Schmidt B. R., Rouffaer L. O., Van Praet S., Schaub M., et al. (2017). Drivers of salamander extirpation mediated by batrachochytrium salamandrivorans. Nature 544, 353–356. doi: 10.1038/nature22059
Thomas V., Blooi M., Van Rooij P., Van Praet S., Verbrugghe E., Grasselli E., et al. (2018). Recommendations on diagnostic tools for batrachochytrium salamandrivorans. Transbound Emerg. Dis. 65, e478–ee88. doi: 10.1111/tbed.12787
Varga J. F. A., Bui-Marinos M. P., and Katzenback B. A. (2018). Frog skin innate immune defences: sensing and surviving pathogens. Front. Immunol. 9. doi: 10.3389/fimmu.2018.03128
Vehtari A., Gelman A., Simpson D., Carpenter B., and Burkner P. (2020). Rank-normalization, folding, and localization: an improved R.Hat for assessing convergence of mcmc (with discussion). Bayesian Anal. 16, 667–718. doi: 10.1214/20-BA1221
Vehtari A., Mononen T., Tolvanen V., and Sivula T. (2016). Bayesian leave-one-out cross-validation approximations for gaussian latent variable models. J. Mach. Learn. Res. 17, 1–38. doi: 10.1214/20-BA1221
Voyles J., Young S., Berger L., Campbell C., Voyles W. F., Dinudom A., et al. (2009). Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science 326, 582–585. doi: 10.1126/science.1176765
Waddle J. H., Grear D. A., Mosher B. A., Grant E. H. C., Adams M. J., Backlin A. R., et al. (2020). Batrachochytrium salamandrivorans (Bsal) not detected in an intensive survey of wild north american amphibians. Sci. Rep. 10, 13012. doi: 10.1038/s41598-020-69486-x
Wells D. K. (2007). The ecology and behavior of amphibians: world scientific. Chicago, IL: The University of Chicago Press.
Wilber M. Q., Carter E. D., Gray M. J., and Briggs C. J. (2021). Putative resistance and tolerance mechanisms have little impact on disease progression for an emerging salamander pathogen. Funct. Ecol. 35, 847–859. doi: 10.1111/1365-2435.13754
Yap T. A., Koo M. S., Ambrose R. F., Wake D. B., and Vredenburg V. T. (2015). Averting a north american biodiversity crisis. Science 349, 481–482. doi: 10.1126/science.aab1052
Keywords: Bsal, chytridiomycosis, histopathology, newt, salamander
Citation: Sheley WC, Wilber MQ, Carter ED, Gray MJ and Miller DL (2025) Environmental temperature and pathogen dose affect histologic lesion count and severity in Notophthalmus viridescens infected with Batrachochytrium salamandrivorans. Front. Amphib. Reptile Sci. 3:1628070. doi: 10.3389/famrs.2025.1628070
Received: 27 June 2025; Accepted: 13 August 2025;
Published: 01 September 2025.
Edited by:
Luisa Maria Diele Viegas, Federal University of Bahia (UFBA), BrazilReviewed by:
Federico Castro Monzon, National Autonomous University of Mexico, MexicoAmadeus Plewnia, University of Trier, Germany
Copyright © 2025 Sheley, Wilber, Carter, Gray and Miller. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wesley C. Sheley, d2VzbGV5LnNoZWxleUB3c3UuZWR1
 Wesley C. Sheley
Wesley C. Sheley Mark Q. Wilber
Mark Q. Wilber Edward Davis Carter
Edward Davis Carter Matthew J. Gray
Matthew J. Gray Debra L. Miller
Debra L. Miller