- 1Taronga Institute of Science and Learning, Taronga Conservation Society Australia, Mosman, NSW, Australia
- 2Environmental Futures, School of Science, University of Wollongong, Wollongong, NSW, Australia
- 3Centre for Integrative Ecology, School of Life and Environmental Science, Deakin University, Waurn Ponds, VIC, Australia
- 4Department of Ecosystem Management, Climate and Biodiversity, Institute of Zoology, University of Natural Resources and Life Sciences, Vienna, Austria
As the global biodiversity crisis worsens, Conservation Breeding Programs (CBPs) are proving critical for safeguarding threatened species, yet the influence of the ex situ rearing environment on phenotypic expression remains poorly understood. For amphibian CBPs, understanding the impacts of ultraviolet radiation (UVR) on various fitness-determining traits has come into focus. The aim of the present study was to investigate the effect of ecologically-relevant UVR levels on post-metamorphic colouration in the critically endangered northern corroboree frog (Pseudophryne pengilleyi). This species is characterised by striking lime/yellow and black colouration and shows inter-individual colour variation, but potential impacts of UVR are yet to be investigated. UVR was provided at one of two ecologically appropriate levels: lower UVR (∼0.2 UVI) and higher UVR (∼0.7 UVI). Each treatment included 10 replicate containers housing five frogs, and individuals remained under the same conditions for an experimental period of 16 weeks, spanning the first growth phase prior to hibernation. Frogs in the higher UVR treatment did not display significant differences in hue, though displayed slightly lower chroma (significant at weeks 4 and 12), and slightly higher luminance (significant at week 12 and 16). Underpinning these differences, frogs in both treatment groups displayed a significant change in colour during post metamorphic development. Hue became more yellow-green shifted, and there was an increase in luminance, chroma, and the proportion of yellow colouration. These findings indicate that the range of UVR levels we tested induced minor yet detectable colour changes, and that corroboree frogs display ontogenetic colour change. We conclude that the UVR levels in the range of ∼0.2-0.7 UVI do not cause extreme colour change in northern corroboree frogs and discuss the value of this knowledge for refining CBPs for corroboree frogs and other threatened amphibians.
Introduction
Biodiversity is declining at an accelerating rate with unprecedented loss reported for all vertebrate classes (Cardinale et al., 2012). In response to this biodiversity crisis, numerous conservation approaches have been developed, with one of the most prominent being the establishment of Conservation Breeding Programs (CBPs) (Mallinson, 1995). The primary goal of CBPs is to establish ex situ breeding and reintroduction regimes that facilitate the recovery of threatened species (Harley et al., 2018). In their simplest form, CBPs involve the strategic collection of wild individuals from declining populations to establish zoo-based insurance populations, where they are ultimately bred to produce colonies that protect against a species’ extinction (Dobson and Lyles, 2000). With this outcome in mind, CBPs, coupled with reintroduction programs, are now the recommended conservation strategy for many hundreds of species globally (IUCN SSC Amphibian Specialist Group, 2024).
While CBPs are a valuable management tool for a diversity of species, they require some core considerations in the pursuit of effective species recovery. Arguably one of the most significant being how best to simulate abiotic and biotic environmental conditions that generate individual phenotypes comparable to those observed in nature (Crates et al., 2023). Many species display remarkable phenotypic plasticity, whereby rearing conditions directly influence and alter the expression of morphological, physiological, and/or behavioural traits (Lisboa et al., 2025). The risk here is that animals generated in breeding programs develop phenotypes that perform sufficiently well within a CBP but may be maladapted to natural environments (Crates et al., 2023). With growing evidence that the phenotypic quality of zoo-bred animals is critical for their reintroduction success (Berger-Tal et al., 2020), there is increasing pressure to understand the impacts of zoo-based conditions on trait expression. Given the multitude of variables that can potentially influence an individual’s phenotype, it will always be challenging to understand and control the impacts of captivity on fitness. However, a logical first step towards meeting this objective is to experimentally investigate the impacts of individual environmental variables on traits expected to have a disproportionate impact on viability.
For amphibian CBPs, one abiotic variable that stands to greatly influence individual phenotypes and viability is ultraviolet radiation (UVR). UVR stimulates various photochemical reactions that are critical for survival, yet over exposure can have harmful effects that reduce performance and lifetime fitness (Fu et al., 2022). Some of the more harmful effects reported include lethal cellular and DNA damage (Londero et al., 2019), impaired development (Croteau et al., 2008a), impaired immune function (Cramp and Franklin, 2018), and reduced behavioural performance (Kats et al., 2000). Amphibians employ a range of defence mechanisms to minimise harmful impacts of UVR exposure, including behavioural avoidance of high UV environments (van de Mortel and Buttemer, 1998), DNA repair mechanisms (Londero et al., 2019), and the production and accumulation of protective UVR screening pigments by specialised chromatophore cells (Fu et al., 2022). Typically, UVR screening pigments are black to brown melanin pigments synthesised by melanophores, and yellow to red pteridine or carotenoid pigments synthesised or selectively stored by xanthophores (Suga and Munesada, 1988). In this regard, exposure to UVR can potentially cause significant changes in amphibian colouration and patterning (Rudh and Qvarnström, 2013). The effects of UVR variation on amphibian colouration is not well studied, but there is emerging evidence for UVR-induced colour change. Experimentally controlled UVR exposure has been shown to increase pigmentation production and promote skin darkening in larval newts and salamanders (Belden and Blaustein, 2002; Garcia et al., 2004; Ascanio et al., 2025), as well as larval and adult frogs (Langhelle et al., 1999; Franco-Belussi et al., 2016; Tang et al., 2025). There is also evidence for abnormal pigmentation in juvenile frogs exposed to UV as tadpoles (Lundsgaard et al., 2025).
Experimental and comparative studies have also shown that elevated UVR is related to elevated melanin pigmentation and colouration on the surface of organs (Franco-Belussi et al., 2016, 2017). Because melanophore cells have long dendrites that extend to the skin surface, increased dispersion of melanin over the top of xanthophores can conceal yellow and red colours contained in these cells, reducing their visibility and altering hues (Aspengren et al., 2008; Rojas et al., 2023; Visconti, 2024). Critically, colouration in anurans can have direct impacts on fitness in the wild. Beyond UVR protection, colour is known to support thermoregulation and osmoregulation, facilitate conspecific communication, and reduce predation risk by enhancing concealment or conspicuous warning displays (Rudh and Qvarnström, 2013). If colour expression is not optimised, individuals may therefore suffer reduced fitness in captivity and/or post release (Umbers et al., 2020). Therefore, studying the effects of UVR exposure on anuran colouration in species targeted for CBPs should be a priority.
The northern corroboree frog (Pseudophryne pengilleyi) is one of Australia’s most endangered vertebrates and has been the focus of a multi-institutional CBP since 2003 (McFadden et al., 2016). Over two thousand frogs are currently maintained in ex situ insurance colonies and hundreds of animals have been released into the wild, though survival remains low due to ongoing impacts of chytrid fungus infection. The species is characterised by striking lime/yellow and black markings and is known to be toxic to predators, thought to be controlled by the biosynthesis of pseudophrynamines and the sequestration of dietary pumilotoxin alkaloids (Daly et al., 1990). Given these characteristics, it is assumed that the yellow colouration serves an aposematic function (Umbers et al., 2020). Critically, the UVR environment during development appears to influence the expression of this colouration as preliminary observations have shown that rearing frogs at high UVR can lead to excess melanin production. Specifically, when frogs are reared at an ultraviolet radiation index (UVI) level of 1.4, an increase in black pigmentation has been observed, with some individuals becoming almost entirely black in colour (M. McFadden, unpublished data). This ‘black morphotype’ has never been observed in nature, so it is presumed to be maladaptive. In support of this supposition, a field study with corroboree frog clay models demonstrated that entirely ‘black frogs’ are more likely to be attacked by avian predators. By comparison, entirely ‘yellow frogs’ were afforded a high level of protection (Umbers et al., 2020). Given this knowledge, there is an urgent need to better understand the relationships between UVR and corroboree frog colouration in zoo-based environments.
The aim of the present study was to conduct a manipulative laboratory experiment with zoo-based northern corroboree frogs to investigate the influence of ecologically-appropriate levels of UVR on colour variation. We were interested in examining effects on colour appearance (hue), colour purity (chroma), colour brightness (luminance), and ratios of black to yellow colouration. We were also interested in examining whether colour expression increased during early development, as reported in the southern corroboree frog (Pseudophryne corroboree) (Walton et al., 2021). Ultimately, we were focussed on identifying UVR levels that do not stimulate the over production of skin melanin and the generation of unnaturally dark frogs. Because both our lower and higher UVR treatments were in the range expected for corroboree frogs (see methods), we predicted that we would not see major changes in colour tied to enhanced pigment production. However, based on the assumption that the higher UVR treatment would stimulate at least some increase in the production and/or storage of protective pigments, we predicted that frogs exposed to the higher UVR treatment would: 1) have a higher chroma (due to increased pigment concentration), 2) have a lower luminance (due to increased pigmentation absorbing light and reducing reflectance and colour brightness), 3) have a higher ratio of black to yellow colour (due to increased production of melanin), and 4) show a reduction in yellow hue (due to increased melanin concealing yellow pigments contained in chromatophores resulting in a lighter shade of yellow).
Methods
The procedures outlined above were performed following evaluation and approval by the Taronga Conservation Society Australia’s Animal Ethics Committee (approval number 3c/12/22).
Study species
The northern corroboree frog, P. pengilleyi is a small (snout-vent-length= 25-30mm) terrestrial frog with distinct longitudinal lime green/yellow and black dorsal colouration (Figure 1). The species is currently listed both nationally and internationally as critically endangered. Under the recommendation of the NSW Department of Climate Change, Energy, the Environment and Water (DCCEEW), a CBP for the species was established at Taronga Conservation Society Australia (Taronga Zoo, Sydney, Australia) in 2010 (McFadden et al., 2016). The breeding program has had excellent breeding and rearing success over a number of years, however, no investigations have been conducted into the optimal UVR conditions for rearing. Previously, it has been established that healthy frogs have been produced when reared under UVB emitting fluorescent tubes at 20-30µW/cm2, though some frogs have produced an increase in black pigmentation, and damaged dorsal skin surfaces, when reared at a UVI of over 1.4 (Figure 1) (McFadden et al., 2013).

Figure 1. Northern corroboree frog, Pseudophryne pengilleyi. Image shows an adult with typical colouration for the species (positioned on the left) and an adult with abnormal pigmentation due to UV-induced skin damage (positioned on the right; reared under UVI >1.4).Photograph courtesy of Michael McFadden.
Experimental animals
For this study, 100 juvenile, zoo-bred frogs were utilised from the CBP for this species at Taronga Zoo, Sydney. All frogs were produced from within the Northern Brindabella genetic management unit for this species (Morgan et al., 2008). Experimental animals were reared from 12 full-sibling clutches (each produced by separate male-female pairs) oviposited during March 2022. Eggs were held on a layer of moistened sphagnum moss in closed 600ml plastic containers and received no UV light for approximately three months until the point of hatching. Tadpoles were communally reared in two large tanks and fed ad libitum on a diet of natural silt, frozen endive and powdered Fluval vegetarian fish flake. Throughout larval development, tadpoles were bottom dwelling (water depth = 20cm) and had access to very low levels of UVR (<0.1UVI) produced from UV lights (Reptisun T5HO 5.0 UVB bulb, Pet Pacific Australia) suspended 35cm above the water level. New globes were installed one week prior to tadpole rearing to allow UV levels to stabilise. All experimental animals completed metamorphosis between November and December 2022. Individual metamorphs were able to be identified by their unique dorsal and ventral patterns and were entered into experimental treatments at approximately 15–40 days post metamorphosis.
Experimental design
To investigate the influence of UVR level on frog colouration, individual P. pengilleyi were reared under one of two UVR treatments following metamorphosis: a higher UVR treatment (∼0.7 UVI) and a lower UVR treatment(∼0.2 UVI). The two UVR treatment levels were selected because they represented upper and lower ends of the Ferguson Zone (Zone 1, 0-0.7 UVI) applicable to the natural behavioural activity of a primarily nocturnal species with crepuscular activity or diurnal activity under shaded conditions (Ferguson et al., 2010; Baines et al., 2016). Fergusson Zones have been created as a tool to select optimal UVR conditions for reptiles and amphibians in captivity, based on behavioural data for 15 species in the wild (Ferguson et al., 2010). The UVI scale is measured on a scale of 0 (extremely low) to 11+ (extremely high). The UVR treatments showed significant differences in UVI levels, UVB levels (W/m2), and UVA levels (W/m2)(for treatment means, standard deviations and ANOVA outputs see Table 1). UVI levels were measured from underneath the experimental container lid using a Solarmeter 6.5 (USA) while UVB and UVA levels were measured with an Alliance Technologies SpectroSense 2 (+) radiometer. Weekly measurements ensured consistency in UVR output throughout the study period. On day one of the experiment, individuals were digitally photographed (see ‘2.4 Photography’ below) and randomly assigned to a treatment group (lower or higher UVR) and an experimental container. Each of the two treatment groups had a sample size of 50 frogs (N = 100 experimental animals in total), with frogs housed in groups of five individuals. Frogs were housed in plastic enclosures (30cm x 19cm and 20cm high), with a ventilated flyscreen mesh lid to permit the desired transmission of UVR light. The 20 experimental containers (10 per treatment) were maintained within a single biosecure, climate-controlled facility across four adjacent shelves. There were 5 containers housed on each shelf, with two shelves allocated per treatment. Every four weeks, each experimental container was shifted two positions to the right, within their treatment, to avoid any potential room-position spatial effects. Each container experienced all possible positions within the two respective treatment shelves throughout the course of the experimental period. The experimental period consisted of 16 continuous weeks, commencing December 22, 2022 and finishing April 18, 2023.
Animal husbandry
The biosecure facility where experimental animals were reared was artificially climate-controlled, with temperature maintained at 20 °C during the day (7am to 7pm) and 17 °C at night (7pm – 7am). Temperature was constant across all treatment containers. The facility has skylights and a large window to permit a natural dawn and dusk. In addition to natural lighting, artificial lighting was provided above the enclosures using UVB emitting fluorescent tubes (Reptisun T5HO 5.0 UVB bulb; Pet Pacific, Australia) suspended approximately 25 cm above each container. These were controlled via a light-sensitive switch to replicate natural photoperiod, resulting in approximately 12 hours of UVR light per day, from 7am to 7pm. The greater level of UVR provided to frogs in the higher UVR treatment was achieved by placing a reflector above the tube throughout the duration of the experimental period. These conditions were in accordance with corroboree frog husbandry procedures developed by Taronga Zoo.
Each container had a 2cm layer of aquarium gravel, above a container base with twenty-four holes evenly drilled, to permit excellent drainage. Half of the floor space of each container was covered in a 5cm layer of rehydrated sphagnum moss (Brunnings, Australia), that was replaced every four weeks to maintain hygiene once photographs were taken. The sphagnum moss allowed frogs to burrow and provided an opportunity for shielding against UVR exposure. Enclosures were sprayed twice per week on Tuesday and Saturday with reverse-osmosis water to flush through uneaten food, faeces and nitrogenous waste. Frogs were fed three times per week with 2–4 day old house crickets (Acheta domestica) ad libitum. At each feed, crickets were dusted with calcium and multivitamin supplementation as required for healthy growth and development (Repashy Calcium Plus, United States).
Photography
To determine the effect of UVR light treatment (higher or lower) on frog colouration, individual P. pengilleyi were digitally photographed over a 16-week experimental period starting from the 22nd of December 2022 until 18th of April 2023. Frogs were photographed at the commencement of the experiment, immediately prior to being placed into experimental treatment containers (Week 0) and then every four weeks thereafter (Weeks 4, 8, 12, and 16). Frogs were digitally photographed to enable the quantification of their dorsal colouration following the methodology described in Kelleher et al. (2022) and Walton et al. (2021). During each sampling week, each frog was photographed individually inside a custom-built, portable photography light arena which was constructed from two opaque cylindrical containers stacked on top of each other, with a viewing hole cut at the top of the arena which allowed the camera lens to be placed through. The arena contained integrated white LED strip lighting that lined the inside walls of the arena, which created a standardised lighting environment for all photos. An X-rite ColorChecker Passport (X-rite, USA) was included in all photographs and was placed on the bottom of the arena, next to the focal frog (following Walton et al., 2021; Kelleher et al., 2022). The X-rite ColorChecker pad is an array of 24 coloured and grayscale squares against which the colours in each photo are standardised during image processing (see Figure 2). Photographs were taken with a Canon EOS 70D digital camera with a standard lens (Canon EFS 18–55 mm lens) that was positioned on top of the arena with the following settings: ISO = 400, f = 11, shutter speed = 1/200 (following Kelleher et al., 2022). The distance between the camera lens and the frog was approximately 28 cm. All photographs were taken and saved in raw format (.CR2) to prevent colour change issues that may be associated with file formatting (Frey and Haworth, 2014).
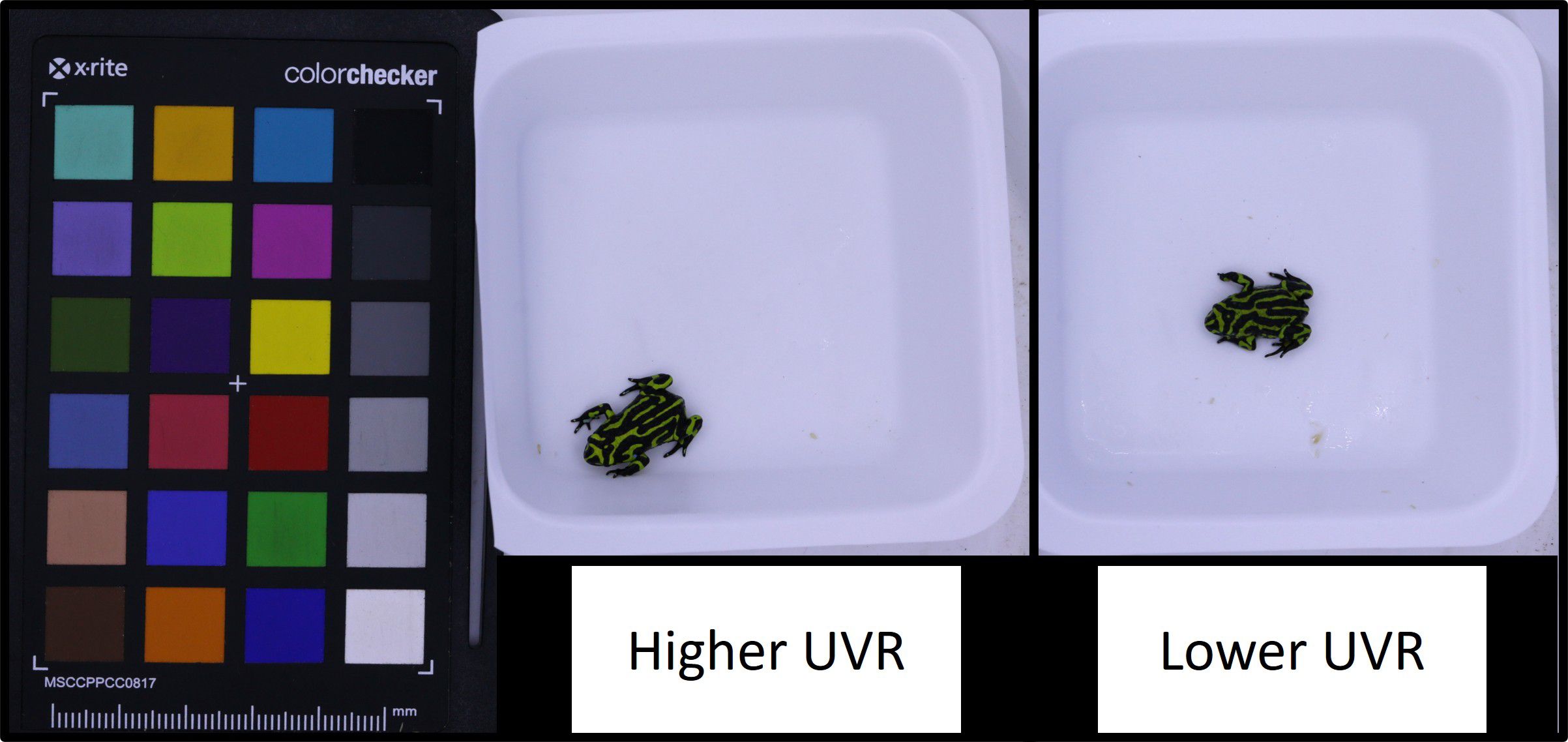
Figure 2. Northern corroboree frog (Pseudophryne pengilleyi) juveniles after 12 experimental weeks at different UVI levels. Image provides an example of a frog reared under higher UVR (∼0.7 UVI) and a frog reared under lower UVR (∼0.2 UVI). Of note, experimental frogs reared under these UVR conditions did not show any signs of abnormal skin pigmentation.
Colour analysis
To quantify aspects of P. pengilleyi dorsal colouration, all photographs were later analysed using a custom MATLAB script (Mathworks Inc., Natick, MA, USA) written by John Endler. The methods for the analysis have previously been described in detail (see Kelleher et al., 2021; Walton et al., 2021; Kelleher et al., 2022). In brief, using the colour standards from the X-rite ColorChecker in each photo as a calibration, we calculated the average red (R), blue (B), and green (G) pixel values from the yellow stripes on the entire dorsal surface (head, body, and legs) of each individual frog using the custom MATLAB script. After obtaining the standardised R, G, B values for each photo, we used a second custom MATLAB script (also written by John Endler) to determine a two-dimensional colour space and calculate four colour variables: (1) hue, (2) chroma, (3) luminance, and (4) the proportion of the total yellow colouration on the dorsal surface (as opposed to black).
Hue represents the colour or shade, and is measured as the angle relative to the colour space axis (Endler, 1990; Cadena et al., 2017). In this case, because we are measuring colour photographically and there are three channels (R,G,B) the colour space is triangular. Setting the centre of the triangle to the origin, hue can be represented as degrees on a circular scale around the origin, in which red = 0 deg (or 360 deg), green = 120 deg and blue = 240 deg (Walton et al., 2021). Chroma is the saturation or purity of the colouration, and is calculated as the distance from the colour space origin (Endler, 1990; Cadena et al., 2017). Luminance is the brightness of the colouration and is calculated by the summation of the standardised RGB values. The fraction of the body yellow was calculated by first thresholding the digital image, then calculating the total number of pixels in the frog’s body (entire dorsal surface), then calculating the number of yellow (white after thresholding) pixels. The fraction yellow is then the number of yellow pixels divided by the total number of pixels in the frog’s body.
Statistical analyses
To analyse the effects of UVR treatment (lower or higher), developmental time (week) as well as their interaction on hue (in degrees on the colour wheel) we used the MANOVA approach for circular data (Landler et al., 2022). The UV-treatment, the sample week (0, 4, 8, 12, 16) and their interaction were used as fixed variables, while frog ID was nested in container in an error term (i.e., Landler et al., submitted), accounting for multiple measures from the same individual as well as the nested design. For the model, hue was transformed to radians using the function rad from circular (Agostinelli and Lund, 2017), and split in their x (cosine) and y (sine) components. Both resulting response variables were used in the MANOVA, using the function manova. We calculated the circular mean for hue (in degrees) using the R function mean.circular as well as a circular confidence interval derived from the function mle.vonmises.bootstrap.ci (both from the package circular (Agostinelli and Lund, 2017). We performed MANOVA using Pillai’s trace as the test statistic. The F-statistics reported are approximate, as they are derived from the transformation of the Pillai trace statistic to an F-distribution.
To determine the effect of UVR treatment (lower or higher) on chroma, luminance, and proportion of yellow colouration, we used linear mixed effects models (LMM) with Gaussian error distribution and restricted likelihood (REML) parameter estimation, using the function lme in the R package lme4 version 1.1-35.2 (Bates et al., 2015). For the models, UVR treatment (lower or higher) sample week (0, 4, 8, 12, 16) and their interaction were entered as fixed categorical variables. Experimental container (1-20) was entered as a random effect. Frog ID was also entered as a random effect to account for repeated sampling of the same individuals across the experimental period (following Walton et al., 2021). Prior to analysis, chroma and the proportion of yellow colouration were arcsine transformed (asin(sqrt[x])) to improve normality. For each model, we assessed normality via visual inspection of normal quantile plots, and assessed model fit by visually examining diagnostic plots of residual vs. fitted values using the qqnorm function with R base plotting. The significance of fixed effects was obtained using Wald tests, using the function ‘Anova’ in the package car (Version 3.1-2; Fox and Weisberg, 2019). Post-hoc comparisons were made using Tukey’s pairwise tests using the function emmeans. Due to an image processing fault, two photographs from Week 12 had to be excluded from analyses. Therefore, total sample sizes for analysis for each treatment across each experimental week were as follows: Weeks 0, 4, 8 and 16: Higher UVR - N = 50, Lower UVR - N = 50. Week 12: Higher UVR – N = 50, Lower UVR - N = 48. All statistical analyses were conducted in R Version 4.4.3 (R Core Team, 2024). For all analyses, the threshold for statistical significance was p < 0.05.
Results
Neither of the UVR treatments caused the expression of black morphotypes or damage to dorsal skin surfaces previously observed when rearing frogs at a UVI over 1.4 (Figure 1). Changes in colouration were subtle (Figure 2), though differences between treatments, and temporal changes, were observed among several variables.
Hue
There was no effect of UVR treatment on hue (F2,16 = 0.148, p = 0.864), and no significant interaction between UVR treatment and sampling week (F2,455 = 2.192, p = 0.113). However, there was a significant effect of sampling week on hue (F2,455 = 125.469, p < 0.001). Over the 16-week experimental period, mean hue shifted towards a more yellow-green colour for individuals in both UVR treatment groups (week 0 mean range, 72.89 – 73.24°; week 16 mean range, 76.74 – 77.66°), with a trend for hue to continue to increase for frogs in the higher UVR treatment, while starting to plateau for frogs in the lower UVR treatment (Figure 3A).
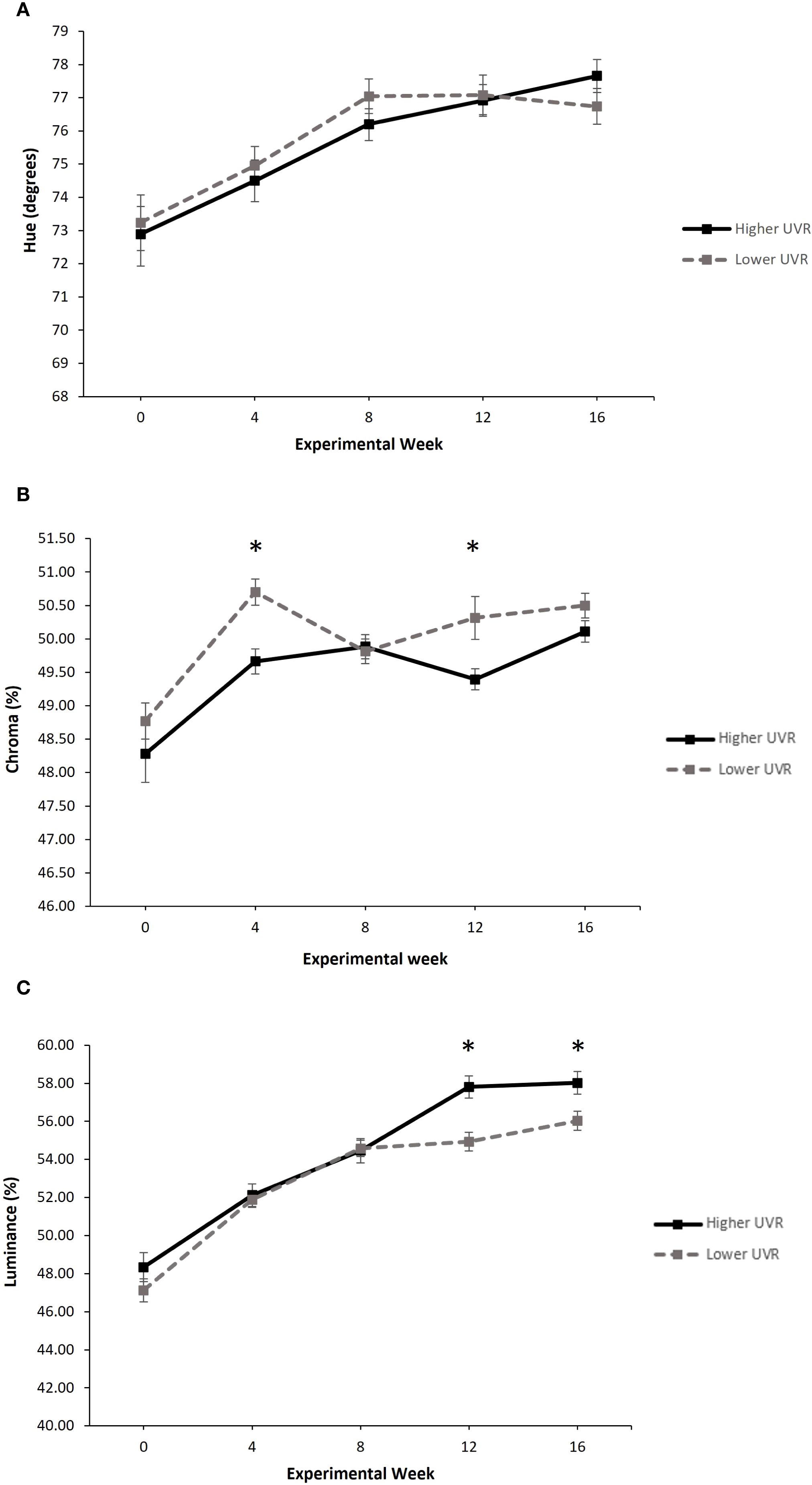
Figure 3. The effect of UVR treatment on (A) hue (degrees), (B) chroma (%) and (C) luminance (%) in P. pengilleyi over a 16-week experimental period. Data shown for hue are circular means ± circular confidence intervals. Data shown for chroma and luminance are untransformed means ± SEM. * indicates a significant difference in the treatment means at that experimental week (Tukey’s pairwise post hoc tests, p < 0.05).
Chroma
Overall, there was a significant effect of UVR treatment on chroma (LMM: = 7.5134, p = 0.0061). Frogs from the lower UVR treatment typically had higher chroma values than those from the higher UVR treatment, and these differences were statistically different at weeks 4 and 12 (Tukey’s pairwise posthoc tests, p < 0.05; Figure 3B). Sampling week also had a significant effect on chroma (LMM: = 84.1838, p < 0.001). Over the 16-week experimental period, chroma increased for frogs in both treatment groups (week 0 mean range, 48.28% – 48.77%; week 16 mean range, 50.11% – 50.50%) with a pronounced increased in chroma between week 0 to week 4 (Figure 3B). There was no significant interaction between UVR treatment and sampling week (LMM: = 8.0497, p = 0.0898).
Luminance
Overall, there was no significant effect of UVR treatment on luminance (LMM: = 3.5408, p = 0.0599). However, there was a significant effect of sampling week on luminance (LMM: = 698.7827, p = < 0.001), as well as a significant interaction between UVR treatment and sampling week (LMM: = 17.0494, p = 0.0019). Over the 16-week experimental period, luminance increased for frogs in both treatment groups (week 0 mean range, 47.13% – 48.34%; week 16 mean range, 56.03% – 58.02%), though began to diverge between treatments after week 8. At weeks 12 and 16, frogs from the higher UVR treatment had significantly higher luminance values than frogs from the lower UVR treatment (Tukey’s pairwise posthoc tests, p<0.05; Figure 3C).
Proportion of yellow colouration
Overall, there was no significant effect of UVR treatment on the proportion of total yellow colouration (compared to black colouration) over the 16-week experimental period (LMM: = 0.0061, p = 0.9377). There was a significant effect of sampling week (LMM: = 343.3258, p = < 0.001) and a significant interaction between UVR treatment and sampling week on the proportion of yellow colouration (LMM: = 11.7664, p = 0.0192). For individuals in both UVR treatment groups, the proportion of yellow colouration increased from week 0 to week 4, then declined and plateaued from Week 4 to Week 16. Frogs in the lower UVR treatment had a slightly higher proportion of yellow until week 8, after which point frogs in the higher UVR treatment had a slightly higher proportion of yellow (Figure 4).
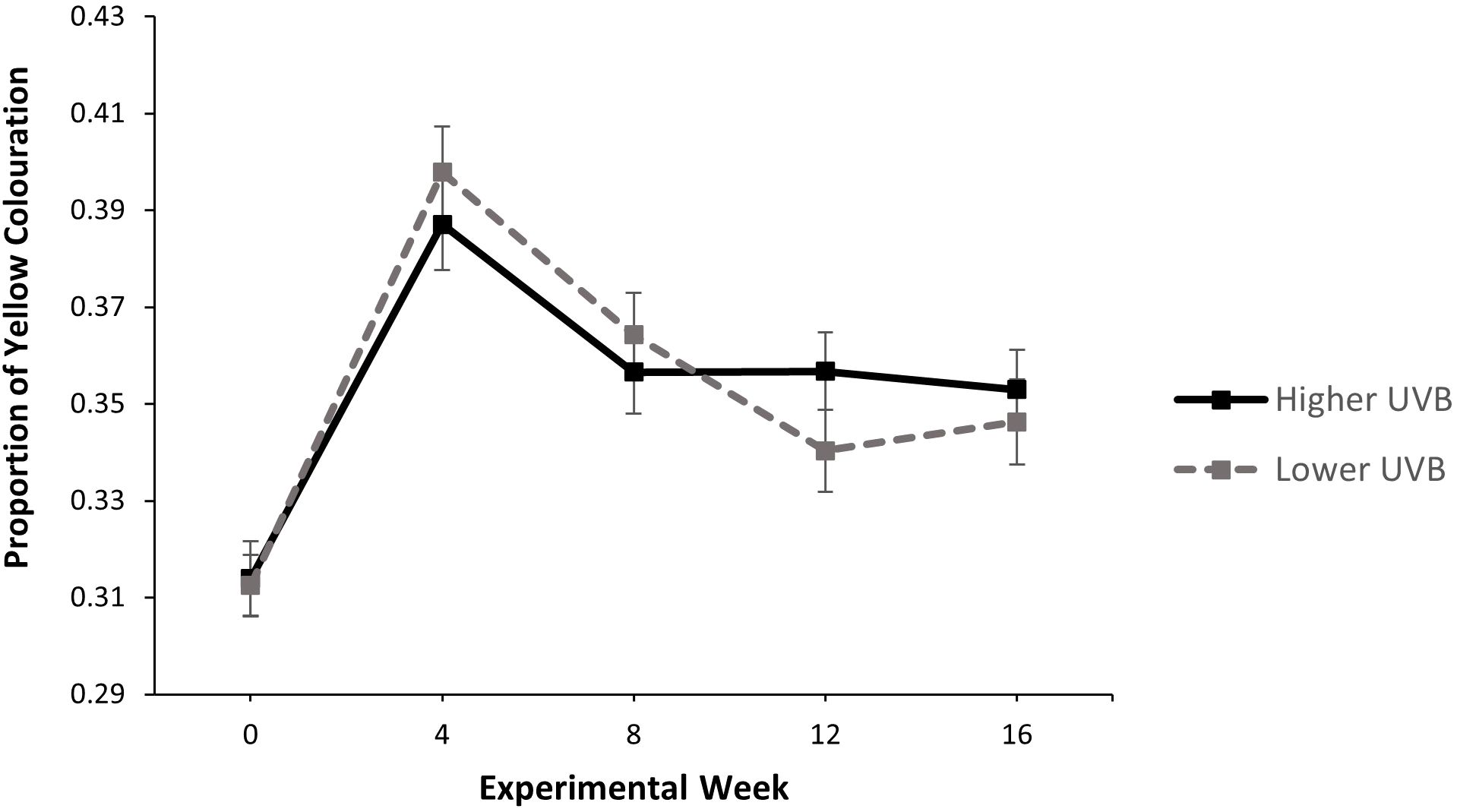
Figure 4. The effect of UVR treatment on the proportion of total yellow colouration across the total dorsal surface in P. pengilleyi over a 16-week experimental period. Data shown are untransformed means ± SEM.
Discussion
As threats to amphibian biodiversity worsen, and more threatened amphibian species are placed into CBPs, the influence of UVR on amphibian fitness is being increasingly investigated (Croteau et al., 2008b; Ceccato et al., 2016; Alton and Franklin, 2017; Lundsgaard et al., 2020). UVR is expected to have strong impacts on the colouration of zoo-based amphibians by stimulating the production and/or storage of protective pigments, yet knowledge in this area remains limited (Langhelle et al., 1999; Belden and Blaustein, 2002; Garcia et al., 2004; Franco-Belussi et al., 2016; Ascanio et al., 2025; Lundsgaard et al., 2025; Tang et al., 2025). The aim of the present study was to conduct a manipulative laboratory experiment with zoo-based P. pengilleyi to investigate the influence of UVR on colouration expressed during post-metamorphic development. With the overarching objective of identifying UVR levels that would avoid the expression of extreme melanin levels and unnaturally black morphotypes, we intentionally selected ecologically-appropriate UVR levels expected to induce only small to moderate changes in colouration. As expected, changes in colouration were not extreme (with no black colour morphotypes observed), though there were some differences observed between treatment groups, as well as changes in colouration over time. Specifically, while we found no difference in hue between treatment groups, frogs in the higher UVR treatment displayed slightly lower chroma (significant at weeks 4 and 12), and slightly higher luminance (significant at week 12 and 16). Underpinning these differences, we observed a pronounced change in colour over the 16-week experimental period, with frogs in both UVR treatments developing a higher hue (more green-yellow shifted), higher chroma, and higher luminance (measured for the yellow patches).
Effects of UVR treatment on colouration
Even though we did not expect a strong treatment effect, we did expect to see frogs at the higher UVR level show; i) at least some shift in hue towards a dull yellow colour (assuming that an increased production of melanin would reduce the visibility of yellow pigments), ii) a change in chroma (to more pure colours due to higher pigment concentration), iii) a change in luminance (to lower levels due to more pigments absorbing light and reducing reflectance), and iv) a higher ratio of black to yellow colour (due to increased production of melanin). Surprisingly, the patterns observed were not in this direction. Instead, we saw hue remain statistically unchanged, chroma to be typically lower in the higher UVR-treatment (significant at weeks 4 and 12), and luminance to be typically higher in the higher UVR-treatment (significant at weeks 12 and 16). Within these results there were significant interactions between UVR treatment and sampling week, reflecting that frogs in the higher UVR treatment showed lower luminance and proportion of yellow colouration (compared to those in the lower UVR treatment) early in the experimental period, yet higher values after experimental week 8. These results are more in line with what would be expected if frogs in the higher UVR treatment were in fact receiving a lower overall amount of UVR, with chronic effects of lower UVR exposures beginning to show by the end of the experimental period.
Accordingly, the most likely explanation for this outcome is that frogs were behaviourally regulating their UVR exposure. In a recent study, we reported that northern corroboree frogs exposed to the higher UVR treatment displayed lower day-time surface activity, spending significantly more time under a substrate refuge (McFadden et al., 2025). We speculated that this behavioural change (similarly observed in the frogs in the present study) may have reduced UVR exposure and obfuscated the detection of any effects of UVR on growth and development. This explanation may also apply to colouration. If frogs in the higher UVR treatment were avoiding UVR, the overall amount of UVR received may have fallen below that received by frogs in the lower UVR treatment. If this was the case, frogs in the higher UVR treatment would have been less stimulated to synthesise melanin or pteridines and/or store carotenoids. In turn, lower pigment in the skin may have decreased chroma (due to lower pigment concentrations and saturation), and increased light reflectance and luminance (due to less surface pigmentation resulting in higher reflectance). Cognisant of this possibility, we strongly recommend that future studies aiming to understand the relationship between UVR exposure and colouration in corroboree frogs, and other amphibians, restrict the availability of UVR refuges during experimental periods. This would allow for a more accurate assessment of the direct impacts of UVR on colouration, and a better understanding of the role of behaviour in regulating UVR exposure and phenotypic change. Though we emphasise that care should be taken to avoid causing harm to experimental animals caused by extreme UVR exposure, necessitating carefully planned experimental designs and UVR delivery methods.
Ontogenetic colour change
Despite detecting limited differences in colouration between the two UVR treatments, we recorded significant changes in colouration over the 16-week experimental period. Across both UVR treatments, frogs showed an approximate five-degree shift in hue (from approximately 73 to 78 degrees, indicating a shift towards a more yellow-green colour), a slight increase in chroma (from 48.28%/48.77% - 50.11%/50.50%), and a moderate increase in luminance (from 47.13%/48.34% -56.03%/58.02%). These changes suggest that northern corroboree frogs progressively develop colouration during the post metamorphic life stage. We recently reported a similar developmental change in the southern corroboree frog (P. corroboree), a sister species to P. pengilleyi (Walton et al., 2021). In a study investigating the effects of dietary β-carotene on colouration over 32-weeks post metamorphosis, we found that frogs in all treatment groups (including a control with negligible levels of dietary β-carotene) changed colour over time. Specifically, we observed that hue became slightly more yellow shifted (from 68.36/69.10 - 69.14/70.46), chroma increased (from 45.11%/48.82% - 50.79%/51.39%) and luminance increased (from 64.59%/67.35% - 72.09%/73.31%) (Walton et al., 2021). Taken together, the findings from these two studies suggest that corroboree frogs display intrinsic ontogenetic colour change, whereby individuals express increasingly strong colour signals in the three-to-four-month period following metamorphosis.
At present there is limited data on the exact biochemical mechanisms controlling corroboree frog colouration. However, it is known that frogs can develop their yellow colour in the absence of dietary carotenoids, suggesting that individuals synthesise their own pigments, likely pteridines synthesised during purine production (Walton et al., 2021). It is also known that the provision of dietary carotenoids can shift corroboree frog skin colour from ‘yellow’ to ‘yellow-orange’, indicating that dietary pigments enhance colour expression (Umbers et al., 2020). With this in mind, we speculate that ontogenetic colour change is the outcome of a progressive maturation and differentiation of xanthophores, accompanied by the production and accumulation of pteridine pigment granules, and potentially the increased accumulation of carotenoids (Walton et al., 2021). This suggestion is in line with explanations (and supporting evidence) for ontogenetic colour change in other amphibians (Bagnara et al., 1968). Importantly, however, given our observation that the colour of northern corroboree frogs progressively shifted to a more ‘yellow-green’ hue, other bio-mechanical changes must also be occurring. The expression of green hues in frogs is typically controlled by crystals present in iridophores (sandwiched between the inner melanophores and the outer xanthophores), which reflect light past yellow pigments in xanthophores to produce various shades of green (Gould and McHenry, 2024). Therefore, it seems logical to presume that juvenile northern corroboree frogs undergo progressive changes in iridophore structure during early development. Saying this, the possibility exists that the increased expression of green colour might be linked to a different mechanistic change. Recently, it has been shown that green colour in frogs can be controlled by the accumulation of a bile pigment (biliverdin) and an associated binding protein (serpin) which act together to modify reflectance spectra to produce shades of green in soft tissues (Taboada et al., 2020). Given this potential, to elucidate the mechanisms controlling northern corroboree frog colouration it will be necessary to biochemically characterise the structural components and pigments of both the Dermal Chromatophore Unit (DCU) and the outer skin layer at multiple points throughout development (Suga and Munesada, 1988; Yasutomi and Yamada, 1998; Bonansea et al., 2017).
From an evolutionary perspective, the progressive, and somewhat synchronous, developmental colour change we report raises the interesting question of why corroboree frogs have been selected to change colour soon after metamorphosis. One possibility is that a rapid increase in UVR exposure experienced by metamorphs as they move from ponds/bogs to more terrestrial habitats with higher UVR exposure has selectively favoured increased investment in the production and accumulation of protective UVR-screening pigments (Belden and Blaustein, 2002). This seems plausible given that terrestrial alpine habitats are characterised by very high UVR levels in summer (Fu et al., 2022). Another possibility is that ontogenetic colour change reflects a change in anti-predator defence strategy across life stages. For most of the larval life stage, tadpoles are jet black in colour. The characteristic yellow colouration only starts to show in the days immediately prior to metamorphosis, with our data suggesting it is maximally expressed in the three-to-four-month period post metamorphosis. In parallel, the larval and post metamorphic life stages experience very different light environments, with differences in predation pressure also presumed. Tadpoles reside in dark pools and are well hidden, whereas metamorphs move through terrestrial habitats and are comparatively conspicuous, especially during periods of diurnal activity. Survival benefits associated with blending into the background and reducing detection by predators (crypsis) may have favoured dark tadpoles (Davis et al., 2020), whilst benefits associated with being conspicuous and signalling toxicity to predators (aposematism) may have favoured the rapid development of black and yellow patterning post metamorphosis (Mappes et al., 2005; Rudh and Qvarnström, 2013). If true, however, it is not then clear why northern corroboree frogs progressively develop a more yellow-green hue, rather than a striking and highly conspicuous yellow or yellow-orange colouration typically associated with aposematic signals (Mappes et al., 2005; Blount et al., 2012). One possibility is that the function of colour (and pattern) may change depending on viewing distance by potential predators. At close distance, the colour and pattern may deter predators via an aposematic function, but at long distance there may be a concealing camouflaging effect, as reported in other frog species (Tullberg et al., 2005; Rojas, 2017; Barnett et al., 2018; Umbers et al., 2020). These are intriguing evolutionary questions that warrant further investigation, especially given that northern corroboree frogs display a more yellow-green hue than southern corroboree frogs (Walton et al., 2021), and that these species have evolved in habitats that differ considerably in vegetation type and colour. Northern corroboree frogs often occupy ‘green coloured’ grassy habitats in subalpine heath and forests, while southern corroboree frogs often occupy ‘yellow coloured’ sphagnum bogs. Such species-specific habitat differences (and presumed associated differences in predator-prey sensory dynamics) may have driven evolutionary divergence in defence strategies and colouration. Beyond investigating species-specific differences, if the development of yellow coloration in corroboree frogs functions as a warning signal, we should expect a concomitant increase in toxicity post metamorphosis. At present we have no data on whether corroboree frog toxins accumulate during early life, so this would be a valuable avenue for future research.
Conservation implications for P. pengilleyi
Our findings have important conservation implications. Northern corroboree frogs have suffered catastrophic population declines since the 1980s and the species is now listed as critically endangered. With ex situ breeding and reintroduction deemed a priority conservation action (Skerratt et al., 2016; McFadden et al., 2018), identifying environmental conditions that maximise the health and viability of frogs produced within the CBP will be critical for recovery. Importantly, decisions concerning the provision of UVR must consider the risk of UVR-induced damage balanced against the potential for beneficial effects. Rearing corroboree frogs in environments devoid of UVB places them at high risk of developing metabolic-bone disease, which can be a fatal infliction (Antwis and Browne, 2009). Therefore, provision of at least some UVR must be considered essential for viability. Our results help to identify an appropriate UVR range for rearing P. pengilleyi within the CBP. Recently we have shown that rearing frogs between UVIs of ∼0.2 UVI and ∼0.7 UVI can ensure high survival and appropriate growth and development, with the caveat that frogs have access to a refuge to allow behavioural regulation of UVR exposure (McFadden et al., 2025). Here we show that this same UVR range (again with the provision of a refuge) does not lead to extreme melanin production or skin damage, as previously observed at UVIs above 1.4. While it would be valuable to know how the colour of our experimental frogs compares to wild frogs from the source population, this data is not currently available. However, comparison to wild northern corroboree frogs from a different population located approximately 30 km away and at a slightly lower altitude indicates the experimental frogs have a slightly higher hue, chroma, and luminance. Specifically, frogs in the wild population displayed a hue of 61.1°, a chroma of 41.02%, and a luminance of 39.34% (see Kelleher et al., 2021). Such differences should be expected due to genetic or environmental differences (e.g. wild frogs experiencing a higher intake of dietary carotenoids or undergoing UVR hardening) and underscore an ongoing need to learn more about corroboree frog colouration under natural conditions. Nevertheless, the differences between the zoo-based and wild frogs are not extreme. Therefore, our current recommendation is to supply UVR in the ∼0.2 - 0.7 UVI range when breeding northern corroboree frogs. When considering reintroduction protocols, our finding that colour takes approximately four months to be maximally expressed is valuable. It is unclear if more colourful frogs will be better protected from UVR related damage, but this can be assumed given these associations have been reported for other frog species (Fu et al., 2022). We also don’t know if more colourful frogs will be more protected from predators, but again this can be assumed because a study that deployed clay models of southern corroboree frogs at historical breeding sites indicates that pure yellow frogs were less predated than black and yellow striped models or pure black models (Umbers et al., 2020). As such, to maximise colour expression, and the potential for adaptive benefits and improved survival, we recommend rearing northern corroboree frogs at UVI’s of ∼0.2 - 0.7 for at least four months prior to release.
One caveat to our recommendations is that ongoing research will be essential to improve the likelihood that we identify UVR levels needed to enhance the long-term viability of the northern corroboree frog CBP. A number of factors should be considered. First, we emphasise that UVR exposure may impact a diversity of fitness-determining traits that contribute to lifetime fitness, necessitating a broad multivariate-fitness analysis. Critical insights will come from experimental work aimed at elucidating effects of variable UVR exposure regimes on oxidative stress (Tang et al., 2025), cellular damage (Londero et al., 2019), DNA damage, immune function (Cramp and Franklin, 2018), vitamin-D synthesis (Antwis and Browne, 2009), and behavioural performance (Kats et al., 2000). Second, UVR is known to modulate the effects of other environmental stressors, including temperature, disease, pH, salinity, and heavy metals (reviewed by Hird et al., 2024). Understanding the potential for synergistic effects may be especially important for informing reintroduction programs, justifying long-term monitoring of how UVR interacts with co-occurring environmental stressors in the natural environment. Third, UVR sensitivity can be strongly life-stage dependent, with early amphibian life stages (eggs and larvae) known to be particularly susceptible to increased UVR exposure (Bancroft et al., 2008). With this in mind, future research investigating effects of UVR exposure should strive to encompass multiple life stages. This is especially important given recent evidence for UVR induced carryover effects in anuran amphibians. Directly relevant to the present study, Lundsgaard et al. (2023) reported that acute changes to the UVR exposure regime experienced by larvae in the striped marsh frog (Limnodynastes peronii) impacted several traits in later development, including time to metamorphosis and levels of skin pigmentation post-metamorphosis. Studying effects throughout development (and over longer temporal scales) is also encouraged because there is potential for chronic exposure to exacerbate effects of UVR exposure once thresholds are surpassed (as suggested by the interactive effects we observed between UVR treatment and time). From a methodological perspective, careful consideration should be given to lighting parameters used in experimental studies. When manipulating UVR it may be challenging to equalise levels of other electromagnetic wavelengths emitted by UV lights across UVR treatments, confounding effects of the UVR spectrum of interest. For instance, in the present study, the observed effects of the higher UVR treatment on colouration may have been linked to associated increases in visible light that induced similar colour changes, but for different reasons (e.g. to manage thermoregulatory requirements) (Bertolesi et al., 2020). In future studies, this matter could be overcome by using specialised light filters to control the spectral composition of different UVR treatments, with a focus on manipulating UVR levels whilst holding visible light wavelengths relatively constant (Hird et al., 2024).
Conservation implications for endangered amphibians
Globally, amphibians are the most threatened vertebrate group, with greater than two hundred species now being supported by CBPs (Harding et al., 2016; Tapley et al., 2022). For many of these species there remains no, or very limited, understanding of how UVR conditions influence the viability of individuals and populations. Our study draws attention to the value of stepping away from the traditional trial-and-error approach to ex situ breeding and using empirical data to inform management decisions. This adds to a growing body of literature highlighting the critical need for adaptive management of zoo-bred phenotypes (Crates et al., 2023). Moving forward, we encourage conservation managers to collect information on UVR levels found in natural habitats and use these data to design experiments that investigate the impacts of ecologically-appropriate UVR conditions on various fitness-determining traits, including colouration. We forecast that a stronger evidence base across species will greatly improve our capacity to manage threatened amphibians and help stem biodiversity loss.
Conclusions
In conclusion, the aim of this study was to conduct a manipulative laboratory experiment with zoo-based northern corroboree frogs to investigate the influence of ecologically-appropriate levels of UVR on colour variation over a 16-week experimental period post metamorphosis. While there was no evidence for a treatment effect on hue, frogs in the higher UVR treatment displayed slightly lower chroma (significant at weeks 4 and 12), and slightly higher luminance (significant at week 12 and 16). For both treatment groups, frogs displayed a significant change in colour during development. Hue became more yellow-green shifted, and we also observed increases in luminance, chroma, and the proportion of yellow colouration. These findings indicate that the higher UVR level tested induced subtle but not major changes in colouration, and that corroboree frogs display ontogenetic colour change. We conclude that the UVR levels used (UVI’s of ∼0.2 - 0.7) are appropriate for breeding northern corroboree frogs and recommend retaining frogs under these conditions for several months after metamorphosis to allow maximal colour expression prior to release. The only caveat being that ongoing research will be needed to assess impacts of UVR on the various traits that contribute to lifetime fitness, how UVR interacts with other environmental stressors to alter fitness, and whether effects are life-stage dependent. More broadly, as the amphibian-extinction crisis worsens and more species are entered into CBPs, we encourage conservation managers to employ experimental research to help identify UVR conditions that optimise the health and viability of individuals and populations, both in captivity and following reintroduction.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Taronga Conservation Society Australia’s Animal Ethics Committee (approval number 3c/12/22). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. AS: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing. SK: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JE: Funding acquisition, Software, Writing – review & editing. LL: Formal Analysis, Writing – review & editing. PB: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by grants from the Australian Research Council (linkage grant LP170100351) and NSW Environmental Trust (grant no. 2017/RD/0138) awarded to P.G.B, A.J.S, M.S.M., and J.A.E. with ancillary funding provided by Taronga Conservation Society Australia. A.J.S. was also in receipt of an ARC DECRA fellowship (DE210100812) at the time of the study.
Acknowledgments
We thank the staff in the Herpetofauna division at Taronga Zoo, in particular Del Leong and Gemma Chaudhuri for their contributions to husbandry and maintenance of the frogs throughout the experimental period and beyond. We also acknowledge the support of the New South Wales Department of Climate Change, Energy, the Environment and Water (DCCEEW), Senior Threatened Species Officer Dr David Hunter, who facilitates the integration of ex situ and in situ conservation efforts for this species.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agostinelli C. and Lund U. (2017). R package ‘Circular’: circular statistics (Ca: Department of Environmental Sciences, Informatics and Statistics). 0.4–93 ed.
Alton L. A. and Franklin C. E. (2017). Drivers of amphibian declines: effects of ultraviolet radiation and interactions with other environmental factors. Climate Change Responses 4, 1–26. doi: 10.1186/s40665-017-0034-7
Antwis R. and Browne R. (2009). Ultraviolet radiation and Vitamin D3 in amphibian health, behaviour, diet and conservation. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 154, 184–190. doi: 10.1016/j.cbpa.2009.06.008
Ascanio A., Fitzgerald V., Altomari P., Bracken J. T., and Jezkova T. (2025). Larval pigmentation reveals environmental and genetic influences in hybridizing ambystoma salamanders. Ecol. Evol. 15, e71911. doi: 10.1002/ece3.71911
Aspengren S., Hedberg D., Sköld H. N., and Wallin M. (2008). New insights into melanosome transport in vertebrate pigment cells. Int. Rev. Cell Mol. Biol. 272, 245–302. doi: 10.1016/S1937-6448(08)01606-7
Bagnara J. T., Taylor J. D., and Hadley M. E. (1968). The dermal chromatophore unit. J. Cell Biol. 38, 67–79. doi: 10.1083/jcb.38.1.67
Baines F. M., Chattell J., Dale J., Garrick D., Gill I., Goetz M., et al. (2016). How much UVB does my reptile need? The UV-Tool, a guide to the selection of UV lighting for reptiles and amphibians in captivity. J. Zoo Aquarium Res. 4, 42–63. doi: 10.19227/jzar.v4i1.150
Bancroft B. A., Baker N. J., and Blaustein A. R. (2008). A meta-analysis of the effects of ultraviolet B radiation and its synergistic interactions with pH, contaminants, and disease on amphibian survival. Conserv. Biol. 22, 987–996. doi: 10.1111/j.1523-1739.2008.00966.x
Barnett J. B., Michalis C., Scott-Samuel N. E., and Cuthill I. C. (2018). Distance-dependent defensive coloration in the poison frog Dendrobates tinctorius, Dendrobatidae. Proc. Natl. Acad. Sci. 115, 6416–6421. doi: 10.1073/pnas.1800826115
Bates D., Maechler M., Bolker B., and Walker S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48. doi: 10.18637/jss.v067.i01
Belden L. K. and Blaustein A. R. (2002). Population differences in sensitivity to UV-B radiation for larval long-toed salamanders. Ecology 83, 1586–1590. doi: 10.1890/0012-9658(2002)083[1586:PDISTU]2.0.CO;2
Berger-Tal O., Blumstein D. T., and Swaisgood R. R. (2020). Conservation translocations: a review of common difficulties and promising directions. Anim. Conserv. 23, 121–131. doi: 10.1111/acv.12534
Bertolesi G. E., Atkinson-Leadbeater K., Mackey E. M., Song Y. N., Heyne B., and McFarlane S. (2020). The regulation of skin pigmentation in response to environmental light by pineal Type II opsins and skin melanophore melatonin receptors. J. Photochem. Photobiol. B: Biol. 212, 112024. doi: 10.1016/j.jphotobiol.2020.112024
Blount J. D., Rowland H. M., Drijfhout F. P., Endler J. A., Inger R., Sloggett J. J., et al. (2012). How the ladybird got its spots: effects of resource limitation on the honesty of aposematic signals. Funct. Ecol. 26, 334–342. doi: 10.1111/j.1365-2435.2012.01961.x
Bonansea M. I., Heit C., and Vaira M. (2017). Pigment composition of the bright skin in the poison toad, Melanophryniscus rubriventris (Anura: Bufonidae) from Argentina. Salamandra 53, 142–147.
Cadena V., Rankin K., Smith K. R., Endler J. A., and Stuart-Fox D. (2017). Temperature-induced colour change varies seasonally in bearded dragon lizards. Biol. J. Linn. Soc. 123, 422–430. doi: 10.1093/biolinnean/blx152
Cardinale B. J., Duffy J. E., Gonzalez A., Hooper D. U., Perrings C., Venail P., et al. (2012). Biodiversity loss and its impact on humanity. Nature 486, 59–67. doi: 10.1038/nature11148
Ceccato E., Cramp R. L., Seebacher F., and Franklin C. E. (2016). Early exposure to ultraviolet-B radiation decreases immune function later in life. Conserv. Physiol. 4, cow037. doi: 10.1093/conphys/cow037
Cramp R. L. and Franklin C. E. (2018). Exploring the link between ultraviolet B radiation and immune function in amphibians: implications for emerging infectious diseases. Conserv. Physiol. 6, coy035. doi: 10.1093/conphys/coy035
Crates R., Stojanovic D., and Heinsohn R. (2023). The phenotypic costs of captivity. Biol. Rev. 98, 434–449. doi: 10.1111/brv.12913
Croteau M. C., Davidson M. A., Lean D., and Trudeau V. (2008a). Global increases in ultraviolet B radiation: potential impacts on amphibian development and metamorphosis. Physiol. Biochem. Zoology 81, 743–761. doi: 10.1086/591949
Croteau M. C., Martyniuk C. J., Trudeau V. L., and Lean D. R. (2008b). Chronic exposure of Rana pipiens tadpoles to UVB radiation and the estrogenic chemical 4-tert-octylphenol. J. Toxicol. Environ. Health Part A 71, 134–144. doi: 10.1080/15287390701613330
Daly J., Garraffo H., Pannell L., Spande T., Severini C., and Erspamer V. (1990). Alkaloids from Australian frogs (Myobatrachidae): pseudophrynamines and pumiliotoxins. J. Natural Products 53, 407–421. doi: 10.1021/np50068a020
Davis A. L., Thomas K. N., Goetz F. E., Robison B. H., Johnsen S., and Osborn K. J. (2020). Ultra-black camouflage in deep-sea fishes. Curr. Biol. 30, 3470–3476. doi: 10.1016/j.cub.2020.06.044
Dobson A. and Lyles A. (2000). Black-footed ferret recovery. Science 288, 985–988. doi: 10.1126/science.288.5468.985
Endler J. A. (1990). On the measurement and classification of colour in studies of animal colour patterns. Biol. J. Linn. Soc. 41, 315–352. doi: 10.1111/j.1095-8312.1990.tb00839.x
Ferguson G. W., Brinker A. M., Gehrmann W. H., Bucklin S. E., Baines F. M., and Mackin S. J. (2010). Voluntary exposure of some western-hemisphere snake and lizard species to ultraviolet-B radiation in the field: how much ultraviolet-B should a lizard or snake receive in captivity? Zoo Biol. 29, 317–334. doi: 10.1002/zoo.20255
Fox J. and Weisberg S. (2019). An R Companion to Applied Regression. 3rd edition. Sage Publications, Thousand Oaks. Available online at: https://socialsciences.mcmaster.ca/jfox/Books/Companion/.
Franco-Belussi L., Nilsson Sköld H., and De Oliveira C. (2016). Internal pigment cells respond to external UV radiation in frogs. J. Exp. Biol. 219, 1378–1383. doi: 10.1242/jeb.134973
Franco-Belussi L., Provete D. B., and De Oliveira C. (2017). Environmental correlates of internal coloration in frogs vary throughout space and lineages. Ecol. Evol. 7, 9222–9233. doi: 10.1002/ece3.3438
Frey T. G. and Haworth D. (2014). DSLR double star astrometry using an Alt-Az telescope. J. Double Star Observations 10, 223–231.
Fu T.-T., Sun Y.-B., Gao W., Long C.-B., Yang C.-H., Yang X.-W., et al. (2022). The highest-elevation frog provides insights into mechanisms and evolution of defenses against high UV radiation. Proc. Natl. Acad. Sci. 119, e2212406119. doi: 10.1073/pnas.2212406119
Garcia T. S., Stacy J., and Sih A. (2004). Larval salamander response to UV radiation and predation risk: color change and microhabitat use. Ecol. Appl. 14, 1055–1064. doi: 10.1890/02-5288
Gould J. and McHenry C. (2024). It’s not easy being green: Comparing typical skin colouration among amphibians with colour abnormalities associated with chromatophore deficits. Ecol. Evol. 14, e11438. doi: 10.1002/ece3.11438
Harding G., Griffiths R. A., and Pavajeau L. (2016). Developments in amphibian captive breeding and reintroduction programs. Conserv. Biol. 30, 340–349. doi: 10.1111/cobi.12612
Harley D., Mawson P. R., Olds L., McFadden M., and Hogg C. (2018). “The contribution of captive breeding in zoos to the conservation of Australia’s threatened fauna,” in Recovering Australia’s threatened species: A book of hope. Eds. Garnett S., Latch P., Lindenmayer D., and Woinarski J. (CSIRO Publishing, Clayton, Victoria), 281–294.
Hird C., Lundsgaard N. U., Downie A. T., Cramp R. L., and Franklin C. E. (2024). Considering ultraviolet radiation in experimental biology: a neglected pervasive stressor. J. Exp. Biol. 227, jeb247231. doi: 10.1242/jeb.247231
IUCN and SSC Amphibian Specialist Group (2024). Amphibian conservation action plan: A status review and roadmap for global amphibian conservation. IUCN, Gland, Switzerland. doi: 10.2305/QWVH2717
Kats L. B., Kiesecker J. M., Chivers D. P., and Blaustein A. R. (2000). Effects of UV-B radiation on anti-predator behavior in three species of amphibians. Ethology 106, 921–931. doi: 10.1046/j.1439-0310.2000.00608.x
Kelleher S. R., Silla A. J., Hertel A. G., Dingemanse N. J., and Byrne P. G. (2021). Mate preference plasticity in a critically endangered frog: implications for conservation breeding. Front. Conserv. Sci. 2, 748104. doi: 10.3389/fcosc.2021.748104
Kelleher S. R., Silla A. J., McFadden M. S., Stares M. G., Endler J. A., and Byrne P. G. (2022). Multiple phenotypic traits predict male mating success in a critically endangered frog. Behav. Ecol. Sociobiology 76, 40. doi: 10.1007/s00265-021-03119-9
Landler L., Ruxton G. D., and Malkemper E. P. (2022). The multivariate analysis of variance as a powerful approach for circular data. Movement Ecol. 10, 21. doi: 10.1186/s40462-022-00323-8
Langhelle A., Lindell M. J., and Nyström P. (1999). Effects of ultraviolet radiation on amphibian embryonic and larval development. J. Herpetology 33, 449–456. doi: 10.2307/1565642
Lisboa C. S., de Carvalho J. E., de Barros F. C., da Cruz J. B., and Brasileiro C. A. (2025). Influence of captive breeding environment on the locomotor performance and metabolism of the threatened Alcatraz Snouted Treefrog, Ololygon alcatraz. J. Zoology 325, 61–70. doi: 10.1111/jzo.13228
Londero J. E. L., Dos Santos M. B., and Schuch A. P. (2019). Impact of solar UV radiation on amphibians: focus on genotoxic stress. Mutat. Research/Genetic Toxicol. Environ. Mutagenesis 842, 14–21. doi: 10.1016/j.mrgentox.2019.03.003
Lundsgaard N. U., Cramp R. L., and Franklin C. E. (2020). Effects of ultraviolet-B radiation on physiology, immune function and survival is dependent on temperature: implications for amphibian declines. Conserv. Physiol. 8, coaa002. doi: 10.1093/conphys/coaa002
Lundsgaard N. U., Hird C., Doody K. A., Franklin C. E., and Cramp R. L. (2023). Carryover effects from environmental change in early life: An overlooked driver of the amphibian extinction crisis?. Global Change Biology 29, 3857–3868. doi: 10.1111/gcb.16726
Lundsgaard N. U., Franklin C. E., and Cramp R. L. (2025). Older amphibian larvae are more sensitive to ultraviolet radiation and experience more sublethal carryover effects post-metamorphosis. J. Exp. Zoology Part A: Ecol. Integr. Physiol. 343, 197–210. doi: 10.1002/jez.2882
Mallinson J. J. C. (1995). Conservation breeding programmes: an important ingredient for species survival. Biodiversity Conserv. 4, 617–635. doi: 10.1007/BF00222518
Mappes J., Marples N., and Endler J. A. (2005). The complex business of survival by aposematism. Trends Ecol. Evol. 20, 598–603. doi: 10.1016/j.tree.2005.07.011
McFadden M. S., Gilbert D., Bradfield K., Evans M., Marantelli G., and Byrne P. (2018). “The role of ex-situ amphibian conservation in Australia,” in Status of conservation and decline of amphibians: Australia, New Zealand, and pacific islands. Eds. Heatwole H. and Rowley J. J. L. (CSIRO Publishing, Clayton, Victoria), 125–140.
McFadden M., Hobbs R., Marantelli G., Harlow P., Banks C., and Hunter D. (2013). Captive management and breeding of the critically endangered southern corroboree frog (Pseudophryne corroboree)(Moore 1953) at Taronga and Melbourne Zoos. Amphibian Reptile Conserv. 5, 70–87.
McFadden M., Hunter D., Evans M., Scheele B., Pietsch R., and Harlow P. (2016). “Re-introduction of the northern corroboree frog in the Northern Brindabella Mountains, New South Wales, Australia,” in Global Re-introduction Perspectives: 2016. Case-studies from around the globe. Ed. Soorae P. S. (IUCN/SSC Re-introduction Specialist Group, Abu Dhabi, UAE), 35–39.
McFadden M. S., Silla A. J., Kelleher S. R., and Byrne P. G. (2025). Effects of ultraviolet radiation on the activity, survival, and growth of the critically endangered northern corroboree frog. Biol. Open 14, bio061827. doi: 10.1242/bio.061827
Morgan M. J., Hunter D., Pietsch R., Osborne W., and Keogh J. S. (2008). Assessment of genetic diversity in the critically endangered Australian corroboree frogs, Pseudophryne corroboree and Pseudophryne pengilleyi, identifies four evolutionarily significant units for conservation. Mol. Ecol. 17, 3448–3463. doi: 10.1111/j.1365-294X.2008.03841.x
R Core Team (2024). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at: https://www.R-project.org/.
Rojas B. (2017). Behavioural, ecological, and evolutionary aspects of diversity in frog colour patterns. Biol. Rev. 92, 1059–1080. doi: 10.1111/brv.12269
Rojas B., Lawrence J., and Márquez R. (2023). “Amphibian coloration: Proximate mechanisms, function, and evolution,” in Evolutionary ecology of amphibians. Eds. Moreno-Rueda G. and Comas M. (CRC Press, Boca Raton), 219–258.
Rudh A. and Qvarnström A. (2013). Adaptive colouration in amphibians. Semin. Cell Dev. Biol. 24, 553–561. doi: 10.1016/j.semcdb.2013.05.004
Skerratt L. F., Berger L., Clemann N., Hunter D. A., Marantelli G., Newell D. A., et al. (2016). Priorities for management of chytridiomycosis in Australia: saving frogs from extinction. Wildlife Res. 43, 105–120. doi: 10.1071/WR15071
Suga T. and Munesada K. (1988). The pigments in the dorsal skin of frogs. J. Natural Products 51, 713–718. doi: 10.1021/np50058a008
Taboada C., Brunetti A. E., Lyra M. L., Fitak R. R., Faigon Soverna A., Ron S. R., et al. (2020). Multiple origins of green coloration in frogs mediated by a novel biliverdin-binding serpin. Proc. Natl. Acad. Sci. 117, 18574–18581. doi: 10.1073/pnas.2006771117
Tang X., Wu J., Zhang H., Zhong L., Su R., Ma M., et al. (2025). UVB radiation and amphibian resilience: Analyzing skin color, immune suppression and oxidative stress in Rana kukunoris from different elevations. Ecotoxicology Environ. Saf. 294, 118075. doi: 10.1016/j.ecoenv.2025.118075
Tapley B., Johnson K., Michaels C. J., Bradfield K., Barber D., Randall L., et al. (2022). “Conservation breeding,” in Amphibian conservation action plan. Eds. Wren S., Borzée A., Marcec-Greaves R., and Angulo A. (IUCN, Gland, Switzerland), 267–283.
Tullberg B. S., Merilaita S., and Wiklund C. (2005). Aposematism and crypsis combined as a result of distance dependence: functional versatility of the colour pattern in the swallowtail butterfly larva. Proc. R. Soc. B: Biol. Sci. 272, 1315–1321. doi: 10.1098/rspb.2005.3079
Umbers K. D. L., Riley J. L., Kelly M. B. J., Taylor-Dalton G., Lawrence J. P., and Byrne P. G. (2020). Educating the enemy: Harnessing learned avoidance behavior in wild predators to increase survival of reintroduced southern corroboree frogs. Conserv. Sci. Pract. 2, e139. doi: 10.1111/csp2.139
van de Mortel T. F. and Buttemer W. A. (1998). Avoidance of ultraviolet-B radiation in frogs and tadpoles of the species Litoria aurea, L. dentata and L. peronii. Proc. Linn. Soc. New South Wales 119, 173–179.
Visconti M. A. (2024). Color change: neural and hormonal control of pigmentation. Front. Endocrinol. 15, 1476470. doi: 10.3389/fendo.2024.1476470
Walton S. J., Silla A. J., Endler J. A., and Byrne P. G. (2021). Does dietary β-carotene influence ontogenetic colour change in the southern corroboree frog? J. Exp. Biol. 224, jeb243182. doi: 10.1242/jeb.243182
Keywords: ultraviolet, UVR, captive breeding, ex situ, zoo-based, husbandry, amphibian, fitness
Citation: McFadden MS, Silla AJ, Kelleher SR, Endler JA, Landler L and Byrne PG (2025) Optimising conservation breeding efforts: investigating the effects of ultraviolet radiation on colouration of the northern corroboree frog (Pseudophryne pengilleyi). Front. Amphib. Reptile Sci. 3:1669704. doi: 10.3389/famrs.2025.1669704
Received: 20 July 2025; Accepted: 18 September 2025;
Published: 03 October 2025.
Edited by:
Lee Kats, Pepperdine University, United StatesReviewed by:
Jill Awkerman, United States Environmental Protection Agency (EPA), United StatesNiclas Lundsgaard, Queensland Department of Science, Information Technology and Innovation, Australia
Copyright © 2025 McFadden, Silla, Kelleher, Endler, Landler and Byrne. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael S. McFadden, bW1jZmFkZGVuQHpvby5uc3cuZ292LmF1
 Michael S. McFadden
Michael S. McFadden Aimee J. Silla
Aimee J. Silla Shannon R. Kelleher1
Shannon R. Kelleher1 John. A. Endler
John. A. Endler Phillip G. Byrne
Phillip G. Byrne