- 1Institute for Superconducting and Electronic Materials (ISEM), Faculty of Engineering and Information Sciences, Innovation Campus, University of Wollongong, Wollongong, NSW, Australia
- 2School of Advanced Materials Science and Engineering, Sungkyunkwan University, Suwon-si, Republic of Korea
- 3SKKU Institute of Energy Science and Technology (SIEST), Sungkyunkwan University, Suwon-si, Republic of Korea
Development of advanced battery technologies for electric vehicles (EVs) has primarily focused on achieving high energy density, non-flammability, and fast charging capability. While commercial batteries have served as the backbone for EVs, numerous material challenges still remain to achieve these desired advancements. This perspective presents an overview of state-of-the-art strategies and recent breakthroughs aimed at overcoming the limitations of next-generation EV batteries.
1 Introduction
The transition to electric vehicles (EVs) is driven by the need for sustainable transportation solutions and growing concerns about their environmental impact (Tomaszewska et al., 2019). The main approach to accelerate this transition is to advance battery technologies that offer high-energy density, ultra-fast charging, and enhanced safety (Wu et al., 2019; Kim, 2022). These features are key to improving user convenience and ensuring reliable performance under practical conditions. Developing next-generation EV batteries requires a comprehensive understanding of material selection, electrode design, optimization, and in-depth characterization (Wu et al., 2020a). However, significant material-related challenges remain in simultaneously achieving these performance goals.
Silicon (Si) and lithium (Li) metal are considered ideal anode candidates due to their high theoretical capacities—3,579 and 3,860 mAh g−1, respectively—and, in the case of Li, the lowest redox potential (−3.04 V vs. standard hydrogen electrode) (Obrovac and Krause, 2006; Xu et al., 2014). Yet, both Si and Li metal anodes suffer from severe volume changes during cycling, which compromise interfacial stability and cycle performance. To overcome these intrinsic limitations, various strategies have been developed, such as using electrolyte additives, applying protective surface layers to stabilize the SEI, and designing 3D architectures to suppress volume expansion (Zhao et al., 2024; Yuan et al., 2022). On the cathode side, nickel (Ni)-rich oxides offer high energy density but exhibit structural instability during high-voltage cycling (Li et al., 2015). To achieve higher specific capacities and energy, Li excess cathode materials, such as disordered rocksalt, lithiated spinel, Li-rich, Mn-rich, and sulfur cathodes have gained significant attention (Fergus, 2010; Chen et al., 2024). In particular, Li-rich oxide materials, which are advantageous under high-voltage operations, are being explored to support high-energy density Li-ion batteries (LIBs) and compensate for Li loss (Johnson et al., 2008).
Furthermore, ultra-fast charging technologies capable of achieving a 90% state of charge within 10 min are crucial to approach the convenience of conventional fueling. Achieving this goal requires innovations in electrode architecture, ion transport kinetics, and thermal management to safely and efficiently accommodate the high current demands (Li et al., 2021). From a safety viewpoint, the replacement of flammable organic electrolytes with solid-state alternatives is inevitable. These non-flammable solid-state electrolytes (SEs) significantly enhance thermal stability and reduce explosion risk, marking a critical step toward safe and high-performance EV batteries (Jonderian and McCalla, 2021). This perspective outlines key materials challenges and highlights emerging strategies that will enable the widespread adoption of EVs in terms of LIBs, helping pave the way toward next-generation energy storage.
2 High-energy density batteries
Achieving high-energy density is a fundamental requirement for next-generation LIBs, especially to extend EV driving range and reduce battery size (Khan et al., 2023). From a materials perspective, selecting high-capacity anode and cathode materials and ensuring harmony between the two is necessary.
Si is highly desirable as a high-capacity anode, offering a theoretical capacity nearly ten times that of commercially used graphite (372 mAh g−1) (Shu et al., 1993). Notably, Si is abundant, cost-effective, and environmentally friendly, making it attractive for practical applications. However, its utilization is hindered by intrinsic properties. During lithiation, Si particle undergoes substantial volume expansion (∼300%), causing particle pulverization, continuous SEI rupture/reformation, and irreversible Li loss, all contributing to rapid capacity fading (McBrayer et al., 2023). Recent studies have focused on nano-sized Si particles embedded within a porous carbon framework, which effectively buffers volume expansion and suppresses unstable SEI formation (Cho et al., 2025). This composite maintained structural integrity over extended cycling and improved electrochemical reversibility under high currents. This hybrid architecture verified a promising strategy for next-generation Si anode, where mechanical accommodation, electron/ion transport pathways, and interface stability are simultaneously engineered to enable practical high-energy LIBs (Lee et al., 2019).
From another perspective, Li metal is also an ideal anode material for high-energy batteries. However, its practical application is limited by high reactivity and interfacial instability (Wang et al., 2020). During repeated Li plating and stripping, severe volume changes in the metallic Li electrode disrupt and reform the SEI, promoting dendritic Li growth that penetrates the separator and results in internal short circuits (Bassett et al., 2023). In addition to safety risks, Li dendrites form electrically isolated ‘dead Li’, accelerating performance degradation. One of the most effective strategies to suppress dendrite formation is the formation of a robust, ionically conductive artificial SEI through electrolyte additives, which promotes uniform Li-ion transport (Cheng et al., 2016). For instance, incorporating LiNO3 into ether-based dual-salt electrolytes has been reported to induce the formation of a homogeneous SEI with high ionic conductivity (∼10–4 S cm−1) and improved ion flux uniformity (Vu et al., 2021). Nonetheless, the poor solubility of LiNO3 in carbonate-based electrolytes remains a key limitation. To overcome this, alternative compounds, such as Li2S, Li3P, and LiAl5O8, have been explored (Park et al., 2023a).
Cathode materials are key to determining overall battery performance. Among them, LiNixCoyMnzO2 layered oxides with Ni content >80% (Ni-rich NCM) cathode materials have emerged as dominant cathode materials in commercial EV batteries, offering higher energy density (>200 mAh g−1) compared to lithium cobalt oxide (LiCoO2) (140 mAh g−1) (Sun et al., 2009; Chen and Dahn, 2003). Increasing the nickel content in NCM materials enhances reversible capacity as Ni undergoes a redox transition from +2 to +4, allowing the transfer of more electrons than other transition metals. However, the high-voltage operation of Ni-rich NCM materials induces abrupt changes in the c-axis lattice parameter, generating microcrack formation along secondary particles (Hong et al., 2024). These microcracks further enlarge the interface between cathode material and electrolytes, which increases internal resistance and reduces the rate performance. Recent studies show that surface coating, doping, and electrolyte optimization can mitigate these issues and enhance both rate capability and cycling stability (Sofian et al., 2024). In this context, Li-rich layered oxide [LLO, Li1+xTM1−xO2 (TM = Mn, Ni, Co., etc., 0 < x ≤ 0.33)] has gained significant attention as next-generation cathode material due to their high average voltage (>3.6 V) (He et al., 2023). Moreover, LLO exhibits higher specific capacity (>300 mAh g−1) benefiting from additional capacity attributions through anionic redox activity at high potential (>4.5 V). While LLO achieves high capacities through anionic redox activity, this mechanism induces structural degradation, voltage fading, and oxygen release during cycling, which can compromise both rate capability and safety (Jang et al., 2024). Managing the atomic composition in LLO has been proposed as an effective strategy to simultaneously activate both cationic and anionic redox reactions, which helps suppress voltage decay and enhance rate performance (Liu et al., 2025).
From a broader perspective, realizing high-energy LIBs requires the successful integration of advanced anode and cathode materials with stable interfaces and engineered structures that can accommodate mechanical and chemical instabilities during cycling.
3 Ultra-fast charging batteries
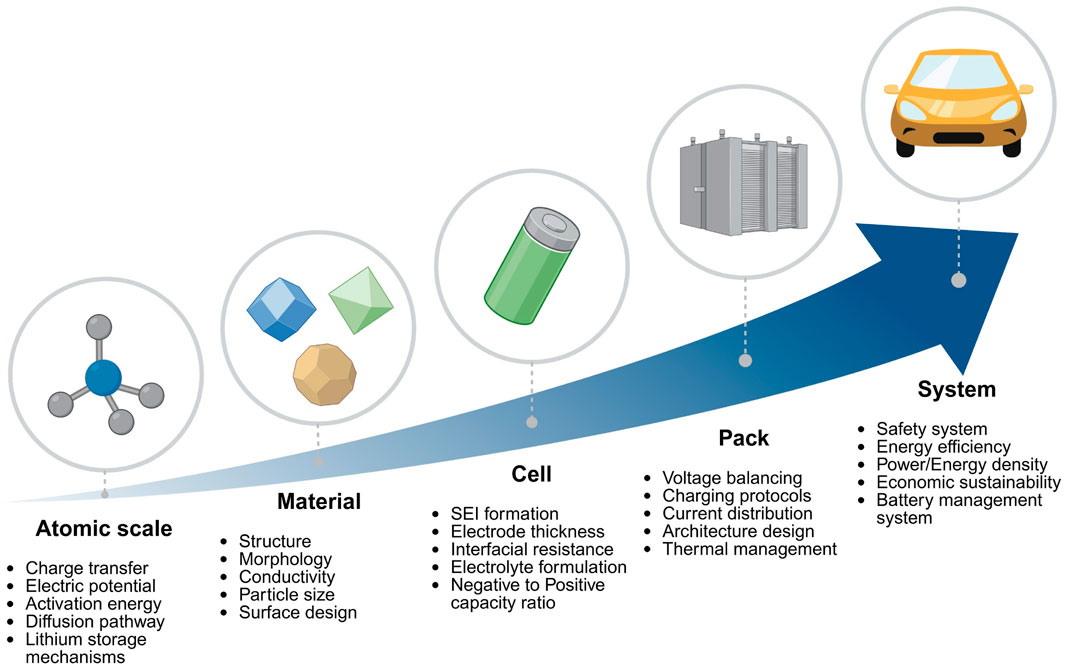
Under ultra-fast charging conditions, conventionally available graphite anodes meet significant limitations in uniformly accommodating Li-ions (Liu et al., 2023). Fast charging of batteries involves the rapid extraction of Li-ions from the cathode and their subsequent intercalation into the anode. During this process, the intercalation of Li-ions into graphite requires Li-ions desolvation from the solvent shell, which is considered a rate-determining step (Weiss et al., 2021; Zhong et al., 2023). Faster intercalation at edge planes compared to basal planes causes non-uniform ion distribution and a sharp increase in overpotential. As polarization rises, the local potential drops below the Li/Li+ redox potential, triggering Li plating and dendritic growth. These dendrites react with the electrolyte to form unstable SEI layers, increase interfacial resistance, and risk internal short circuits (Liu et al., 2024). As shown in Figure 1, improving fast charging capability in LIBs requires optimization across multiple scales. Among these, material-level innovations are particularly critical for enhancing ionic transport, structural stability, and interfacial properties.
Overcoming the limitations of graphite under ultra-fast charging conditions requires a comprehensive understanding of interfacial dynamics to secure both safety and reliability in LIBs. Recent research has been directed toward interfacial engineering to prevent Li plating and improve charge transfer without sacrificing energy density. Surface coatings, particularly those based on transition metal-based compounds, have shown effectiveness in enhancing Li-ion transport, stabilizing interface, and reducing undesirable side reactions. The desired effects can be achieved with minimal coating thickness and content, preserving overall energy density of LIBs. For example, TiO2 coatings improve both thermal stability and conductivity (Rhee et al., 2020), while Al2O3 coatings enhance electrolyte wettability, thereby promoting faster Li-ion transport throughout the electrode. (Kim et al., 2019). MoOx–MoPx/graphite composites reduce interfacial resistance and facilitate rapid Li intercalation, achieving 80% capacity in under 10 min with stable cycling (Lee et al., 2021). Transition metals, such as Co2P and MoS2, also improve interfacial stability by reducing overpotentials and forming a robust SEI (Jeong et al., 2024; Suh et al., 2025). Additionally, zeolitic imidazolate framework (ZIF)-8-derived porous carbon nanoparticles create a three-dimensional interface that enhances ion access and reduces overpotential, improving performance during fast charging (Han et al., 2024).
Continued efforts in surface chemistry engineering, SEI composition optimization, and electrode microstructure design are necessary to realize safe and reliable fast charging LIBs. The ultra-fast charging technologies must proceed in parallel with achieving high energy density, highlighting the need for advanced interfacial strategies tailored to high-capacity anodes, such as Si and Li metal.
4 Non-flammable batteries
Leakage and irreversible decomposition of liquid electrolytes during battery cycling present serious safety risks, including the potential for explosion. While additives can reduce the flammability of liquid electrolytes to some extent, they still pose safety concerns under extreme conditions (Wang et al., 2019). As a result, the adoption of solid-state electrolytes has become crucial for ensuring thermal and electrochemical safety in LIBs (Zhao et al., 2020). They possess non-flammability, high-temperature resistance, and non-volatilization to eliminate hidden safety risks (Lv et al., 2019). For practical application in batteries, SEs must possess several key criteria: (i) high ionic conductivity, (ii) strong moisture resistance, and (iii) low interfacial impedance (Park et al., 2023b). Among these, achieving high ionic conductivity is the most critical because the primary role of SEs is to facilitate Li-ion transport between the anode and cathode.
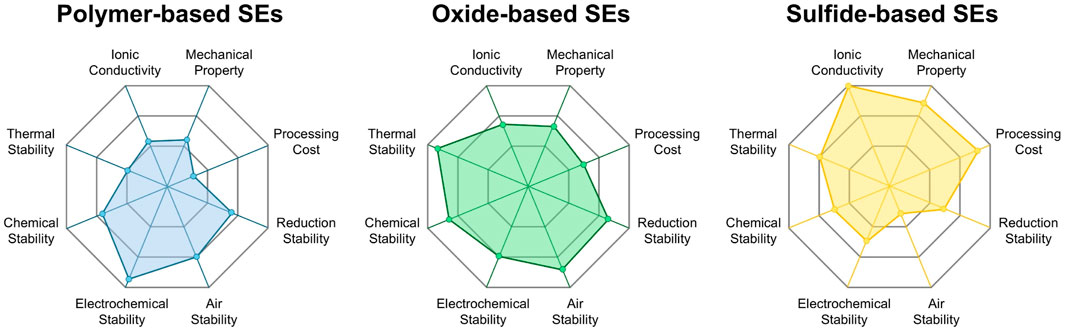
SEs are well known to be categorized into three types, mainly including polymers, oxides, and sulfides, each offering distinct advantages for specific applications (Figure 2). Polymer-based SEs are cost-effective, as they share similar properties and manufacturing processes with conventional liquid electrolytes (Du et al., 2024). Notably, among the three SEs introduced, polymer-based SEs demonstrate the broadest electrochemical stability window, indicating their enhanced electrochemical stability. Nevertheless, a persistent trade-off exists between mechanical robustness and Li-ion conductivity (Lu et al., 2023). One promising approach to overcome this is the incorporation of active fillers. For example, black phosphorus generates an in-situ Li-ion conducting interfacial layer, which promotes interfacial stability and ion transport (Wu et al., 2020b). Oxide-based SEs are another prominent category, offering high chemical stability due to their resistance to reaction with Li metal at the anode and transition metal oxide cathodes (Ohta et al., 2012). However, their brittleness results in poor interfacial contact, requiring further engineering solutions (Wolfenstine et al., 2018). This issue can be managed through thickness optimization of the SE layer (Balaish et al., 2021). Importantly, sulfide-based SEs, specifically Li6PS5X (X = Cl, Br, I), have garnered significant attention due to their high ionic conductivity (1.3 × 10−3 S cm−1), which is comparable to that of liquid electrolytes. (Walther et al., 2020). Despite this promising attribute, they face critical challenges including the formation of byproducts caused by reactions with SEs and cathode materials and poor moisture resistance, thereby limiting their practical application. To address this issue, moisture-absorbing additives have been proposed. For example, ZIF-8 effectively suppresses H2S gas generation by adsorbing residual moisture (Jung et al., 2023).
The development and optimization of SEs with enhanced ionic conductivity, improved interfacial stability, and greater mechanical robustness represents crucial advancements toward enabling safer, high-performance ASSBs. Advanced synchrotron-based analysis and data-driven modeling approaches are expected to play a critical role in probing interfacial phenomena, guiding material optimization, and accelerating the development of next-generation energy storage systems.
5 Perspective
As previously discussed, advanced battery systems for EVs must feature several key characteristics: high-energy density, fast charging capability, and non-flammability. The introduction of high-capacity anode and cathode materials offers a promising route to enhance energy density, but their successful implementation requires overcoming challenges. Simultaneously, achieving reliable ultra-fast charging requires not only fast ion diffusion in electrode materials but also the design of low-resistance and stable interfaces that can support rapid Li-ion transport without promoting Li dendrite formation. On the electrolyte side, solid-state systems offer a compelling pathway to non-flammable battery architectures. High ionic conductivity must be achieved alongside electrochemical, mechanical, and moisture stability to ensure overall performance, which calls for advanced composite design and interfacial engineering.
Looking ahead, breakthroughs will not arise from single-component optimization alone, but from the co-engineered integration of functional electrode materials across all components. Success will depend on the development of scalable, compatible, and robust material systems that combine fast ion transport, interfacial stability, and mechanical resilience. Ultimately, the future of LIBs lies in the rational design of materials at multiple scales, from the atomic-level tuning of surfaces and interfaces to the system-level assembly of high-performance, safe, and manufacturable energy storage devices.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
HL: Writing – review and editing, Conceptualization, Writing – original draft, Visualization. JS: Writing – original draft, Software, Visualization, Conceptualization. RC: Conceptualization, Data curation, Formal Analysis, Writing – original draft. JM: Supervision, Writing – review and editing. JK: Funding acquisition, Supervision, Writing – original draft, Writing – review and editing, Visualization, Project administration, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by ARC National Intelligence and Security Discovery Research Grants (NI240100355).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Balaish, M., Gonzalez-Rosillo, J. C., Kim, K. J., Zhu, Y., Hood, Z. D., and Rupp, J. L. (2021). Processing thin but robust electrolytes for solid-state batteries. Nat. Energy 6, 227–239. doi:10.1038/s41560-020-00759-5
Bassett, K. L., Small, K. A., Long, D. M., Merrill, L. C., Warren, B., and Harrison, K. L. (2023). Interfacial pressure improves calendar aging of lithium metal anodes. Front. Batter. Electrochem. 2, 1292639. doi:10.3389/fbael.2023.1292639
Chen, R., Leung, C. L. A., and Huang, C. (2024). Exploring the properties of disordered rocksalt battery cathode materials by advanced characterization. Adv. Funct. Mater. 34, 2308165. doi:10.1002/adfm.202308165
Chen, Z., and Dahn, J. (2004). Improving the capacity retention of LiCoO2 cycled to 4.5 V by heat-treatment. Electrochem. Solid-State Lett. 7, A11. doi:10.1149/1.1628871
Cheng, X. B., Zhang, R., Zhao, C. Z., Wei, F., Zhang, J. G., and Zhang, Q. (2016). A review of solid electrolyte interphases on lithium metal anode. Adv. Sci. 3, 1500213. doi:10.1002/advs.201500213
Cho, C.-H., Jeong, W. U., Jeong, J. S., Lim, C.-H., Yoon, J.-K., Bon, C. Y., et al. (2025). Porous silicon/carbon composites fabricated via microwave-assisted carbothermal shock for lithium-ion batteries. J. Energy Storage 107, 114983. doi:10.1016/j.est.2024.114983
Du, A., Lu, H., Liu, S., Chen, S., Chen, Z., Li, W., et al. (2024). Breaking the trade-off between ionic conductivity and mechanical strength in solid polymer electrolytes for high-performance solid lithium batteries. Adv. Energy Mater. 14, 2400808. doi:10.1002/aenm.202400808
Fergus, J. W. (2010). Recent developments in cathode materials for lithium ion batteries. J. Power Sources 195, 939–954. doi:10.1016/j.jpowsour.2009.08.089
Han, S. A., Suh, J. H., Kim, J., Park, S., Jeong, W. U., Shimada, Y., et al. (2024). 3D pathways enabling highly-efficient lithium reservoir for fast-charging batteries. Small 20, 2310201. doi:10.1002/smll.202310201
He, H., Li, H., Bai, X., Chang, Z., Gao, Z., Zhang, X., et al. (2023). Comparative study of high-temperature cycling and thermal stability of LLOs and NCMs under medium–high voltage. Energy fuels. 37, 6854–6864. doi:10.1021/acs.energyfuels.3c00845
Hong, M., Ho, V.-C., and Mun, J. (2024). Comprehensive review of single-crystal Ni-rich cathodes: single-crystal synthesis and performance enhancement strategies. Front. Batter. Electrochem. 3, 1338069. doi:10.3389/fbael.2024.1338069
Jang, H.-Y., Eum, D., Cho, J., Lim, J., Lee, Y., Song, J.-H., et al. (2024). Structurally robust lithium-rich layered oxides for high-energy and long-lasting cathodes. Nat. Commun. 15, 1288. doi:10.1038/s41467-024-45490-x
Jeong, W. U., Suh, J. H., Kim, D. K., Hong, Y., Lee, S.-M., and Park, M.-S. (2024). Controlled interfacial reactions with Co2P nanoparticles onto natural graphite anode for fast-charging lithium-ion batteries. Chem. Eng. J. 482, 148805. doi:10.1016/j.cej.2024.148805
Johnson, C. S., Li, N., Lefief, C., Vaughey, J. T., and Thackeray, M. M. (2008). Synthesis, characterization and electrochemistry of lithium battery electrodes: XLi2MnO3·(1−x)LiMn0. 333Ni0. 333Co0. 333O2 (0≤ x ≤ 0.7). Chem. Mat. 20, 6095–6106. doi:10.1021/cm801245r
Jonderian, A., and McCalla, E. (2021). The role of metal substitutions in the development of li batteries, part II: solid electrolytes. Mat. Adv. 2, 2846–2875. doi:10.1039/d1ma00082a
Jung, J. Y., Han, S. A., Kim, H.-S., Suh, J. H., Yu, J.-S., Cho, W., et al. (2023). Dry-electrode all-solid-state batteries fortified with a moisture absorbent. ACS Nano 17, 15931–15941. doi:10.1021/acsnano.3c04014
Khan, F. M. N. U., Rasul, M. G., Sayem, A., and Mandal, N. (2023). Maximizing energy density of lithium-ion batteries for electric vehicles: a critical review. Energy Rep. 9, 11–21. doi:10.1016/j.egyr.2023.08.069
Kim, D. S., Kim, Y. E., and Kim, H. (2019). Improved fast charging capability of graphite anodes via amorphous Al2O3 coating for high power lithium ion batteries. J. Power Sources 422, 18–24. doi:10.1016/j.jpowsour.2019.03.027
Kim, J. H. (2022). Grand challenges and opportunities in batteries and electrochemistry. Front. Batter. Electrochem. 1, 1066276. doi:10.3389/fbael.2022.1066276
Lee, J., Moon, J., Han, S. A., Kim, J., Malgras, V., Heo, Y.-U., et al. (2019). Everlasting living and breathing gyroid 3D network in Si@ SiOx/C nanoarchitecture for lithium ion battery. ACS Nano 13, 9607–9619. doi:10.1021/acsnano.9b04725
Lee, S.-M., Kim, J., Moon, J., Jung, K.-N., Kim, J. H., Park, G.-J., et al. (2021). A cooperative biphasic MoOx–MoPx promoter enables a fast-charging lithium-ion battery. Nat. Commun. 12, 39. doi:10.1038/s41467-020-20297-8
Li, J., Downie, L. E., Ma, L., Qiu, W., and Dahn, J. (2015). Study of the failure mechanisms of LiNi0.8Mn0.1Co0.1O2 cathode material for lithium ion batteries. J. Electrochem. Soc. 162, A1401–A1408. doi:10.1149/2.1011507jes
Li, L., Zhang, D., Deng, J., Gou, Y., Fang, J., Cui, H., et al. (2021). Carbon-based materials for fast charging lithium-ion batteries. Carbon 183, 721–734. doi:10.1016/j.carbon.2021.07.053
Liu, S., Gu, B., Chen, Z., Zhan, R., Wang, X., Feng, R., et al. (2024). Suppressing dendritic metallic li formation on graphite anode under battery fast charging. J. Energy Chem. 91, 484–500. doi:10.1016/j.jechem.2024.01.009
Liu, Y., Shi, H., and Wu, Z.-S. (2023). Recent status, key strategies and challenging perspectives of fast-charging graphite anodes for lithium-ion batteries. Energy Environ. Sci. 16, 4834–4871. doi:10.1039/d3ee02213g
Liu, Z., Wu, Z., Wang, H., Zhang, X., Chen, Y., Liu, Y., et al. (2025). Boosting cationic and anionic redox activity of Li-rich layered oxide cathodes via Li/Ni disordered regulation. J. Energy Chem. 100, 533–543. doi:10.1016/j.jechem.2024.09.015
Lu, G., Zhang, Y., Zhang, J., Du, X., Lv, Z., Du, J., et al. (2023). Trade-offs between ion-conducting and mechanical properties: the case of polyacrylate electrolytes. Carbon Energy 5, e287. doi:10.1002/cey2.287
Lv, F., Wang, Z., Shi, L., Zhu, J., Edström, K., Mindemark, J., et al. (2019). Challenges and development of composite solid-state electrolytes for high-performance lithium ion batteries. J. Power Sources 441, 227175. doi:10.1016/j.jpowsour.2019.227175
McBrayer, J. D., Harrison, K. L., Allcorn, E., and Minteer, S. D. (2023). Chemical contributions to silicon anode calendar aging are dominant over mechanical contributions. Front. Batter. Electrochem. 2, 1308127. doi:10.3389/fbael.2023.1308127
Obrovac, M., and Krause, L. (2007). Reversible cycling of crystalline silicon powder. J. Electrochem. Soc. 154, A103. doi:10.1149/1.2402112
Ohta, S., Kobayashi, T., Seki, J., and Asaoka, T. (2012). Electrochemical performance of an all-solid-state lithium ion battery with garnet-type oxide electrolyte. J. Power Sources 202, 332–335. doi:10.1016/j.jpowsour.2011.10.064
Park, S., Chaudhary, R., Han, S. A., Qutaish, H., Moon, J., Park, M.-S., et al. (2023a). Ionic conductivity and mechanical properties of the solid electrolyte interphase in lithium metal batteries. Energy Mater 3, 300005. doi:10.20517/energymater.2022.65
Park, S. S., Han, S. A., Chaudhary, R., Suh, J. H., Moon, J., Park, M.-S., et al. (2023b). Solid electrolyte: strategies to address the safety of all solid-state batteries. Adv. Energy Sustain. Res. 4, 2300074. doi:10.1002/aesr.202300074
Rhee, D. Y., Kim, J., Moon, J., and Park, M.-S. (2020). Off-stoichiometric TiO2−x-decorated graphite anode for high-power lithium-ion batteries. J. Alloys Compd. 843, 156042. doi:10.1016/j.jallcom.2020.156042
Shu, Z., Mcmillan, R., and Murray, J. (1993). Electrochemical intercalation of lithium into graphite. J. Electrochem. Soc. 140, 922–927. doi:10.1149/1.2056228
Sofian, A. D. A. B. A., Imaduddin, I. S., Majid, S., Kurniawan, T. A., Chew, K. W., Lay, C.-H., et al. (2024). Nickel-rich nickel–cobalt–manganese and nickel–cobalt–aluminum cathodes in lithium-ion batteries: pathways for performance optimization. J. Clean. Prod. 435, 140324. doi:10.1016/j.jclepro.2023.140324
Suh, J. H., Han, S. A., Yang, S. Y., Lee, J. W., Shimada, Y., Lee, S.-M., et al. (2025). Toward fast-charging and dendritic-free li growth on natural graphite through intercalation/conversion on MoS2 nanosheets. Adv. Mater. 37, 2414117. doi:10.1002/adma.202414117
Sun, Y.-K., Myung, S.-T., Park, B.-C., Prakash, J., Belharouak, I., and Amine, K. (2009). High-energy cathode material for long-life and safe lithium batteries. Nat. Mater. 8, 320–324. doi:10.1038/nmat2418
Tomaszewska, A., Chu, Z., Feng, X., O'kane, S., Liu, X., Chen, J., et al. (2019). Lithium-ion battery fast charging: a review. ETransportation 1, 100011. doi:10.1016/j.etran.2019.100011
Vu, T. T., Kim, B. G., Kim, J. H., and Moon, J. (2021). Suppression of dendritic lithium-metal growth through concentrated dual-salt electrolyte and its accurate prediction. J. Mat. Chem. A 9, 22833–22841. doi:10.1039/d1ta06294h
Walther, F., Randau, S., Schneider, Y., Sann, J., Rohnke, M., Richter, F. H., et al. (2020). Influence of carbon additives on the decomposition pathways in cathodes of lithium thiophosphate-based all-solid-state batteries. Chem. Mat. 32, 6123–6136. doi:10.1021/acs.chemmater.0c01825
Wang, Q., Jiang, L., Yu, Y., and Sun, J. (2019). Progress of enhancing the safety of lithium ion battery from the electrolyte aspect. Nano Energy 55, 93–114. doi:10.1016/j.nanoen.2018.10.035
Wang, R., Cui, W., Chu, F., and Wu, F. (2020). Lithium metal anodes: present and future. J. Energy Chem. 48, 145–159. doi:10.1016/j.jechem.2019.12.024
Weiss, M., Ruess, R., Kasnatscheew, J., Levartovsky, Y., Levy, N. R., Minnmann, P., et al. (2021). Fast charging of lithium-ion batteries: a review of materials aspects. Adv. Energy Mater. 11, 2101126. doi:10.1002/aenm.202101126
Wolfenstine, J., Allen, J. L., Sakamoto, J., Siegel, D. J., and Choe, H. (2018). Mechanical behavior of li-ion-conducting crystalline oxide-based solid electrolytes: a brief review. Ionics 24, 1271–1276. doi:10.1007/s11581-017-2314-4
Wu, F., Maier, J., and Yu, Y. (2020a). Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 49, 1569–1614. doi:10.1039/c7cs00863e
Wu, N., Li, Y., Dolocan, A., Li, W., Xu, H., Xu, B., et al. (2020b). In situ formation of Li3P layer enables fast li+ conduction across Li/solid polymer electrolyte interface. Adv. Funct. Mater. 30, 2000831. doi:10.1002/adfm.202000831
Wu, Y., Wang, W., Ming, J., Li, M., Xie, L., He, X., et al. (2019). An exploration of new energy storage system: high energy density, high safety, and fast charging lithium ion battery. Adv. Funct. Mater. 29, 1805978. doi:10.1002/adfm.201805978
Xu, W., Wang, J., Ding, F., Chen, X., Nasybulin, E., Zhang, Y., et al. (2014). Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 7, 513–537. doi:10.1039/c3ee40795k
Yuan, H., Ding, X., Liu, T., Nai, J., Wang, Y., Liu, Y., et al. (2022). A review of concepts and contributions in lithium metal anode development. Mater. Today 53, 173–196. doi:10.1016/j.mattod.2022.01.015
Zhao, H., Li, J., Zhao, Q., Huang, X., Jia, S., Ma, J., et al. (2024). Si-based anodes: advances and challenges in Li-ion batteries for enhanced stability. Electrochem. Energy Rev. 7, 11. doi:10.1007/s41918-024-00214-z
Zhao, Q., Stalin, S., Zhao, C.-Z., and Archer, L. A. (2020). Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mat. 5, 229–252. doi:10.1038/s41578-019-0165-5
Keywords: lithium-ion battery, material, high-energy, fast charging, non-flammability
Citation: Lee H, Suh JH, Chaudhary R, Mun J and Kim JH (2025) Materials challenges in high-energy batteries enabling ultra-fast charging and non-flammable performance for electric vehicles. Front. Batter. Electrochem. 4:1636618. doi: 10.3389/fbael.2025.1636618
Received: 28 May 2025; Accepted: 11 July 2025;
Published: 21 July 2025.
Edited by:
Hakim H. Iddir, Argonne National Laboratory (DOE), United StatesReviewed by:
Anh Vu, Argonne National Laboratory (DOE), United StatesAnika Tabassum Promi, Virginia Tech, United States
Copyright © 2025 Lee, Suh, Chaudhary, Mun and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junyoung Mun, bXVuanlAc2trdS5lZHU=; Jung Ho Kim, amhrQHVvdy5lZHUuYXU=
†These authors have contributed equally to this work and share first authorship
 Hyojoo Lee
Hyojoo Lee Joo Hyeong Suh
Joo Hyeong Suh Rashma Chaudhary
Rashma Chaudhary Junyoung Mun
Junyoung Mun Jung Ho Kim
Jung Ho Kim