- 1Department of Chemical and Biochemical Engineering, The University of Western Ontario, London, ON, Canada
- 2Department of Chemistry, The University of Western Ontario, London, ON, Canada
- 3Department of Bioengineering, King Fahd University of Petroleum and Minerals, Dhahran, Saudi Arabia
- 4Interdisciplinary Research Center for Bio Systems and Machines, King Fahd University of Petroleum & Minerals, Dhahran, Saudi Arabia
- 5Department of Mechanical and Industrial Engineering, Qatar University, Doha, Qatar
Stimuli-responsive, or “smart”, injectable hydrogels respond to real-time stimuli through physical or chemical changes. This allows hydrogels to be dynamic within their environment in the presence of internal or external stimuli. Owing to this, smart injectable hydrogels have gained noticeable implications within the field of biomedicine. Over the past decade, stimuli-responsive injectable hydrogels have been extensively studied for wound healing and cancer therapies but remain largely unexplored for bone healing applications. In this mini-review, we aim to explore the role of smart injectable hydrogels and assess their current and future implications within the field of bone healing. Specifically, we discuss the physicochemical and biological aspects that must be taken into consideration when developing a material in this field, as well as the various strategies for designing such a material. Additionally, we discuss the current role of stimuli-responsive injectable hydrogels for an array of bone healing applications and their potential for successful clinical translation.
1 Introduction
Injectable hydrogels represent a transformative class of biomaterials and have gained significant attention in the biomedical field due to their unique properties and remarkable versatility. Injectable hydrogels are 3-dimensional (3D), highly swelling polymeric networks that are stable in physiological conditions (Yu and Ding, 2008). Accordingly, injectable hydrogels can be administered as liquids and subsequently form gels in situ or be administered as shear-thinning gels, making them well-suited for non-invasive therapies (Alonso et al., 2021; Shamiya et al., 2024). The significance of injectable hydrogels lies in their capacity to closely mimic the natural extracellular matrix (ECM), which is a 3D network of macromolecules within the body that provides structural and biochemical support to the surrounding cells in tissues. In this manner, injectable hydrogels provide a biomimetic environment that supports critical biological processes (Yang et al., 2014).
However, traditional hydrogels are often limited by their static nature. Their fixed physical and chemical properties can act as limiting factors to their adaptability in real-time physiological environments (Lavrador et al., 2021). Stimuli-responsive, or “smart”, injectable hydrogels have recently been developed to address this limitation. Smart hydrogels are designed to react to external and internal stimuli, such as pH (Ghauri et al., 2021; Shi H. et al., 2024; Li M. et al., 2024), temperature (Liu et al., 2022; Tallapaneni et al., 2023; Liu X. et al., 2024), enzymes (Carlini et al., 2019; Kumar et al., 2023a; Kumar et al., 2023b), or others (Rybak et al., 2024; Liu L. et al., 2024; Shi et al., 2024b; Choi et al., 2022), such that they offer dynamic interactions with their environment. This allows them to have precise spatial and temporal control over their therapeutic actions.
The development of smart injectable hydrogels has been extensively studied for many biological applications, including wound healing (Yang Y. et al., 2023; Rasool et al., 2019; Chen Q. et al., 2024; Shi et al., 2024c; Li S. et al., 2024), myocardial infarctions (Carlini et al., 2019; Matsumura et al., 2019; Zhang F. et al., 2024), and cancer therapies (Zhou et al., 2021; Jia et al., 2020; Augustine et al., 2021). However, the study of these stimuli-responsive materials for bone healing applications has been limited. This is largely due to their limited mechanical properties and lack of inherent osteogenic factors. Bone healing is a complicated process that requires synchronization between various growth factors, cells, and the ECM, and requires that this synchronization occur in a series of stages in response to bone healing (Zhang et al., 2020; Coyle et al., 2025). Recently, stimuli-responsive hydrogels have been studied for bone healing specifically for their minimally invasive properties and their ability to adapt to a changing environment. Smart materials have been developed to respond to external and internal stimuli to induce changes in stiffness and other mechanical properties, as well as to release osteogenic therapies such as growth factors, small molecule drugs, or nanoparticles with osteogenic potential (Choi et al., 2025).
This review explores the various types of stimuli-responsive injectable hydrogels, highlighting their classification based on different stimuli, their mechanisms of action, and their recent advances in a variety of bone healing applications. Further, we evaluate the strengths and limitations of using smart injectable hydrogels and their future clinical potential to repair bone fractures and defects.
2 Physical, chemical, biological considerations for injectable hydrogels
Bone is a structurally complex and mechanically demanding tissue, composed of cortical (compact) and trabecular (spongy) bone, each with distinct mechanical and biological microenvironments. Its ECM is a biphasic system, consisting of approximately one-third organic components, primarily type I collagen fibers and two-thirds inorganic minerals, mainly hydroxyapatites (Hassan et al., 2023). In contrast to hydrogels intended for soft tissue applications, like skin or myocardium, those designed for bone regeneration must emulate the significantly higher stiffness of bone (in cortical bone), along with its viscoelastic properties and mineralized ECM. Designing an effective injectable hydrogel for bone regeneration requires careful consideration of several key factors (Lee and Shin, 2007; Bai et al., 2018). The hydrogel must be inherently biocompatible, noncytotoxic, and nonimmunogenic to prevent adverse immune responses. It should possess osteoinductive, osteoconductive, osteogenic, and osteocompatible properties to actively promote new bone formation. Mimicking the natural ECM is crucial to support cell adhesion, proliferation, and osteogenic differentiation. Additionally, the hydrogel must degrade in harmony with tissue ingrowth, creating space for new bone tissue. It should maintain sufficient structural integrity and mechanical strength to withstand load-bearing conditions. Tunable pore size and interconnected porosity modulated through polymer composition and crosslinking density are essential for enhancing cell interactions, regulating the release of bioactive factors, and ensuring efficient exchange of nutrients.
The performance and functionality of injectable hydrogels are inherently governed by a combination of physical, chemical, and biological factors (Figure 1). Physically, these hydrogels must exhibit suitable viscosity and shear-thinning behavior to allow for facile injection, followed by rapid gelation under physiological conditions (Alonso et al., 2021). This facilitates minimally invasive administration and stable in situ formation. Chemically, the constituent materials should be stable, non-toxic, and capable of undergoing crosslinking under mild, physiologically relevant conditions, often triggered by stimuli such as pH, temperature, or enzymatic activity (Bustamante-Torres et al., 2021). Biologically, the hydrogels must be biocompatible and biodegradable, supporting cellular viability and tissue integration while avoiding adverse immune responses (Revete et al., 2022). These hydrogels are fabricated using natural and/or synthetic polymers that offer tunable mechanical properties, degradation, and shape. However, it is important to note that injectable hydrogels are still limited in their mechanical integrity, and they are often restricted to non-load bearing defect sites.
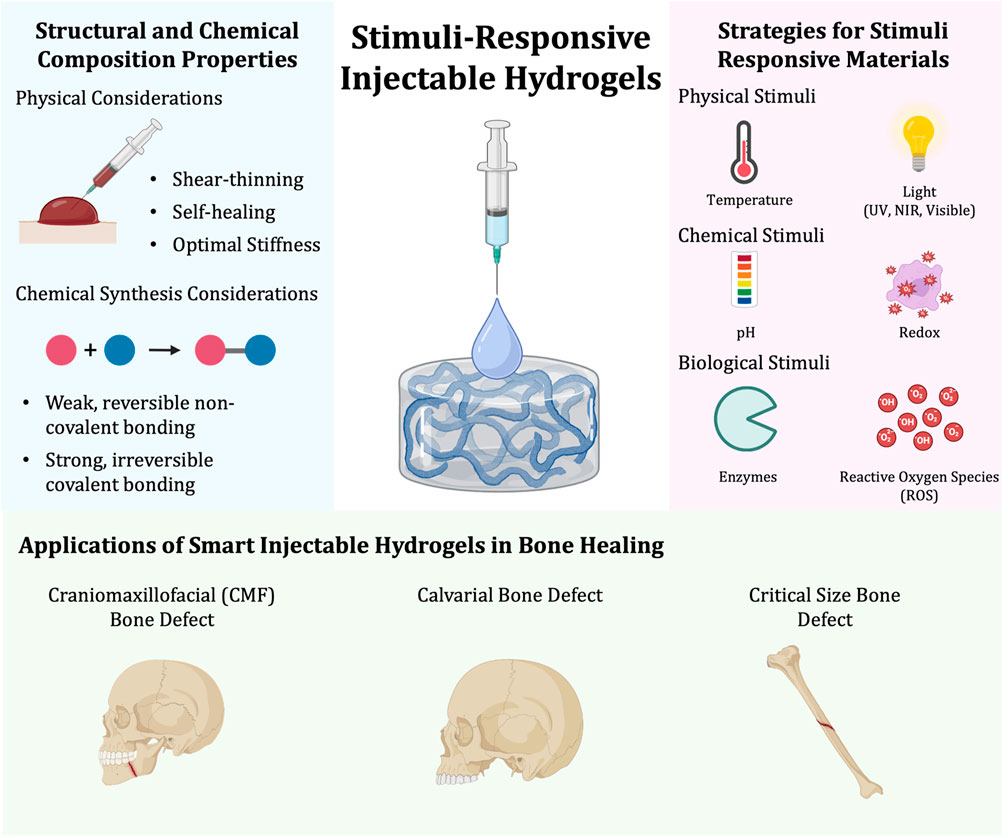
Figure 1. Synthesis of stimuli-responsive injectable hydrogels and their applications in bone healing. The structural and chemical composition of stimuli-responsive injectable hydrogels needs to be considered and can be synthesized by taking physical and chemical properties into account (left). Stimuli-responsive hydrogels can be synthesized in a variety of ways. Some strategies for developing a stimuli-responsive material can be classified to be responsive to physical, chemical, and/or biological stimuli (right). Lastly, there are a variety of bone healing applications in which smart injectable hydrogels can be applied to. Some of these include craniomaxillofacial, calvarial, and critical size bone defects (bottom).
Injectable hydrogels can be broadly classified into in situ forming and shear-thinning systems. In situ forming hydrogels transition from a liquid to a gel state upon administration, without the use of toxic reagents or heat (Chen et al., 2018). Shear-thinning hydrogels, such as nanocomposite systems incorporating materials like laponite, can flow under applied stress during injection and subsequently retain their gel structure once the stress is removed (Liu et al., 2017). Injectable hydrogels are highly suitable for bone healing due to their minimally invasive delivery, ability to conform to irregular defects, and in situ gelation without toxic triggers. Their biocompatibility, biodegradability, tissue adhesiveness, and porosity support cell infiltration and new tissue growth (Liu B. et al., 2020; Park et al., 2022; Zheng J. et al., 2023; Liu C. et al., 2020). Shear-thinning hydrogels, especially those with nanomaterials with osteogenic potential, enhance structural stability post-injection and promote osteogenic activity, making them effective scaffolds for bone regeneration (Zandi et al., 2021).
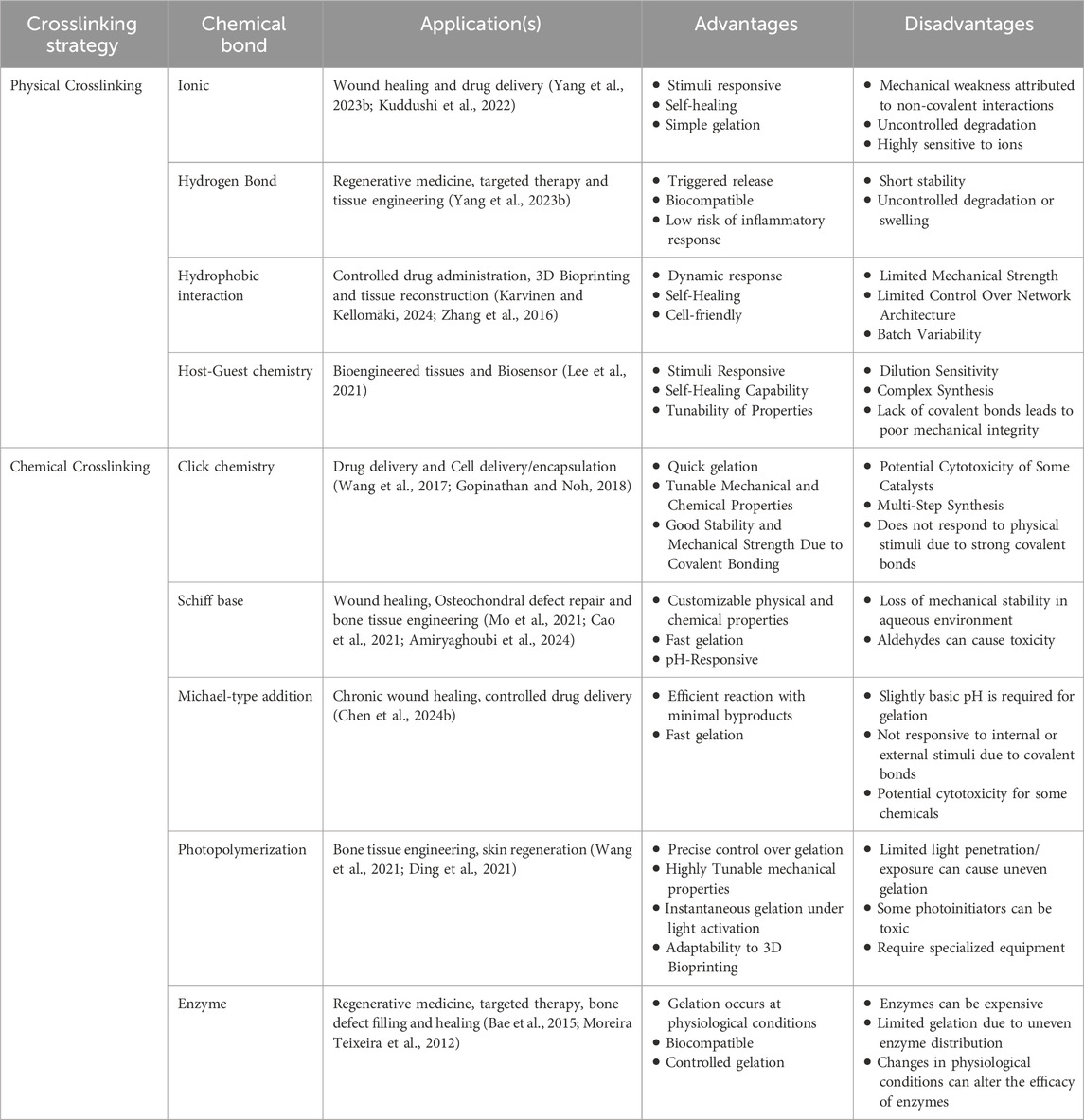
Crosslinking strategies play a pivotal role in defining the structural and functional properties of injectable hydrogels. Physically crosslinked hydrogels rely on non-covalent interactions, including electrostatic forces, hydrogen bonding, hydrophobic interactions, van der Waals forces, and host–guest chemistry (Rizzo and Kehr, 2021). These networks are reversible and often responsive to environmental stimuli such as temperature, pH, or light. In contrast, chemically crosslinked hydrogels involve covalent bond formation through methods such as click chemistry, Michael-type addition, Schiff base reactions, photopolymerization, or enzymatic catalysis (Li et al., 2021; Basu et al., 2018). These covalent networks are typically irreversible and offer enhanced mechanical stability, prolonged retention at the target site, and controlled drug release. The robustness of chemically crosslinked hydrogels minimizes premature degradation and drug diffusion, thus enabling precise control over gelation kinetics, degradation profiles, and biofunctionalization. This ensures consistent and predictable in vivo and in vitro performance, which is critical for therapeutic success. The advantages, disadvantages, and possible applications of various crosslinking strategies for injectable hydrogels are considered in Table 1.
3 Classification of stimuli-responsive injectable hydrogels
There are a variety of strategies to synthesize stimuli-responsive material. These strategies are commonly classified under physical, chemical, and/or biological stimuli and encompass most materials making up stimuli-responsive injectable hydrogels (Figure 1).
3.1 Physical stimulus
3.1.1 Temperature responsive
Temperature-responsive hydrogels alter their volume in response to temperature changes. The variation in temperature affects any hydrophobic interactions and hydrogen bonding between polymer chains, leading to structural and volume changes (Xue et al., 2002; Mah and Ghosh, 2013). This behavior occurs at the hydrogel’s lower critical solution temperature (LCST) or upper critical solution temperature (UCST) (Pardeshi et al., 2022). Depending on the ratio of hydrophobic to hydrophilic groups, two outcomes are possible:
(1) Number of hydrophilic groups > Number of hydrophobic groups: This is a case of positive thermosensitive hydrogels, where the water solubility of the hydrogel increases with rising temperatures. This means that temperature increases cause the hydrogel to swell, and temperature decreases cause the hydrogel to shrink.
(2) Number of hydrophilic groups < Number of hydrophobic groups: This is a case of negative thermosensitive hydrogels, where the hydrogels shrink above the LCST. This is due to the stronger hydrophobic interactions, which reduce the contact area with water (Huang et al., 2019). Below the LCST, hydrogen bonding between the hydrophilic groups and water dominates and leads to swelling.
Commonly used thermoresponsive hydrogels are prepared from natural polymers, proteins or polypeptides, pluronics, and copolymers based on polycaprolactone, poly(N-isopropylacrylamide), poly(D, L-lactide), polyethylene glycol, and poly(amino ester urethane) (Tanga et al., 2023).
3.1.2 Light responsive
Light-responsive injectable hydrogels are commonly engineered for their easy accessibility and controllable external stimuli. Clinically, this offers a technology which is easy, non-invasive, and allows for precise spatiotemporal control. In this regard, the properties of light-responsive hydrogels change upon the irradiation of light, including visible light, ultraviolet (UV), and near-infrared radiation (NIR) (Li et al., 2019).
Specifically, there are two primary mechanisms that drive these responses:
(1) Photothermal effects, wherein certain substances that can be integrated into injectable hydrogels have the ability to absorb and emit light radiation, resulting in the production of heat (Anugrah et al., 2019). One such example is indocyanine green, a photothermal agent approved by the U.S Food and Drug Administration, which can generate and transfer heat in response to NIR.
(2) Photodegradation, wherein hydrogel chains are functionalized with photosensitive functional groups (Zhao et al., 2018). Here, photochemical reactions take place to induce phase transitions of the hydrogel. One such example is the use of o-methoxy-nitro-benzene family monomers—these functional groups can be grafted onto synthetic polymers before in situ gelation, such that they result in the rapid cleavage and degradation of the hydrogel upon exposure to UV light.
3.2 Chemical stimulus
3.2.1 pH responsive
pH-responsive hydrogels undergo volume changes in response to shifts in the external pH, enabling them to swell and degrade. Over recent decades, various pH-sensitive hydrogels have been developed. These hydrogels typically contain ionizable groups, such as acidic (e.g., carboxylic and sulfonic acids) or basic (e.g., ammonium salts) side chains (Jabeen et al., 2017). When the pH of the surrounding environment changes, the ionization of these groups is affected, altering the crosslinking density of the gel network and consequently impacting the hydrogel’s swelling behavior. Various pH-responsive self-healing hydrogels, employing borate ester and imine bonds as typical pH-responsive reversible dynamic covalent bonds, hold promise for targeting the acidic tumor microenvironment while concurrently exhibiting self-healing properties, thereby showing broad application prospects in the field of medicine (Gu et al., 2022).
pH-responsive hydrogels are extensively utilized in biomedical applications, particularly in targeted drug delivery systems, where their ability to respond to subtle pH changes enables precise and controlled release of therapeutic agents (Liu et al., 2023; Zheng Z. et al., 2023). They are especially valuable in cancer therapy, as the slightly acidic extracellular environment of tumors (pH 5.4–6.0) can trigger drug release from these hydrogels, minimizing off-target effects and enhancing therapeutic efficacy (Thambi et al., 2023). They are also widely explored for wound healing, as the slightly alkaline pH of infected wounds or chronic wounds (pH 7.2–8.0) can trigger hydrogel degradation or drug release, promoting tissue regeneration and infection control (Bennison et al., 2017). Additionally, these hydrogels are utilized in regenerative medicine for pH-triggered delivery of growth factors or stem cells to injury sites, as well as in oral and gastrointestinal drug delivery, where acidic pH along the digestive tract (pH 1.2) can be leveraged for site-specific release (Thambi et al., 2023). While these materials find applications across various biomedical fields, drug delivery and cancer therapy remain the primary areas of research and development due to their significant potential in improving targeted treatment strategies.
3.2.2 Redox responsive
Injectable hydrogels can be designed to respond to oxidation and reduction cues in the cellular environment. Redox-responsive hydrogels are normally achieved through redox-sensitive chemical linkages (He et al., 2021). This includes disulfide bonds (-S-S-), thioketals, or selenium-containing moieties (Grocke et al., 2021). In an oxidative environment, such as those with high reactive oxygen species (ROS) levels, these linkages undergo a cleavage or transformation, triggering the degradation of the hydrogel and the release of any loaded cargo. In a reducing environment, such as those with high levels of intracellular glutathione, disulfide bonds can be broken, which can lead to gel dissolution or structural remodeling.
Redox-responsive hydrogels can be commonly used for targeted drug delivery via site-specific degradation. Here, injectable hydrogels can be designed such that their networks are interconnected through disulfide crosslinking (Altinbasa et al., 2022). This approach allows for the hydrogel network to be stable under normal physiological conditions and degrade only in areas with high ROS or glutathione levels, such as in tumor microenvironments or injury sites.
3.3 Biological stimulus
3.3.1 Enzyme responsive
Injectable hydrogels can be designed with an enzyme-responsive moiety that can undergo specific reactions when exposed to a specific enzyme. These reactions can result in the formation or degradation of a hydrogel network, enabling reversible or irreversible gel-sol transitions (Coulter et al., 2024). These hydrogels can be prepared for enzyme-initiated in situ gelation, allowing them to solidify in the presence of the enzyme at the target location. Contrastingly, these hydrogels are often designed with enzyme-triggered degradation in response to enzymatic activity. This allows for smart injectable hydrogels, where the therapeutic agent carried by the hydrogel is released in response to the presence of specific enzymes (Coulter et al., 2024; Vera-González et al., 2024).
Enzyme-responsive injectable hydrogels can be engineered using a variety of strategies:
(1) Peptide sequences can be used to serve as the substrate for enzymes (i.e.,; matrix metalloproteinase (MMP)-sensitive peptides, elastase-sensitive peptides) (Carlini et al., 2019).
(2) Using crosslinkers with enzyme-cleavable bonds (i.e.,; amide, ester, or thiol bonds) (Joshi et al., 2018).
(3) Polymer backbones can be grafted with enzyme-sensitive units (i.e.,; polyethylene glycol, hyaluronic acid, gelatin) (Soeriyadi et al., 2014).
(4) Using self-assembling peptide amphiphiles of block copolymers that degrade upon enzymatic activity (Xiao and Huang, 2024).
These strategies encompass a variety of materials that can act as building blocks for injectable hydrogels, including, but not limited to, peptides, synthetic polymers, fatty acid amphiphiles, and DNA. These hydrogels can be further fine-tuned for their responsiveness to the target enzyme by modifying the structure and concentration of the moieties present in the hydrogel.
3.3.2 ROS responsive
Reactive oxygen species (ROS) are a highly reactive group of oxygen-containing chemicals and exhibit significantly higher reactivity than the ground state oxygen. Their impact is often deemed dual in nature—beneficial under physiological conditions, but harmful under pathological conditions (Yang et al., 2025). While ROS can play essential roles in enhancing and supporting cellular functions, excessive production of ROS can inhibit cellular activity. Beyond the cellular antioxidant capacity, ROS leads to oxidative stress and damaged macromolecules within the cell, leading to cell death and/or carcinogenesis.
Recently, smart injectable hydrogels have been developed to be triggered by an excess of ROS, including hydroxyl radicals, superoxide anions, hydrogen peroxide, and others (Yu et al., 2022). These hydrogels are engineered with ROS-sensitive linkages or moieties, such as borate ester bonds and thioketals. Here, structural integrity of the hydrogel is maintained under physiological conditions but is degraded in response to elevated ROS levels. This degradation occurs by consuming excess ROS and can also help facilitate controlled and localized drug release.
4 Applications of stimuli responsive injectable hydrogels for bone healing
Smart injectable hydrogels have been extensively studied for many biomedical applications, but remain largely unexplored for bone healing applications. Stem cell therapy, growth factor delivery, and drug-free mineral-based delivery are some strategies that have been thoroughly studied for osteogenesis. Here, we will review some of these strategies that have since been paired with stimuli-responsive materials for optimal cargo delivery and/or inherent bone healing (Figure 1). The articles discussed herein are summarized in Table 2.
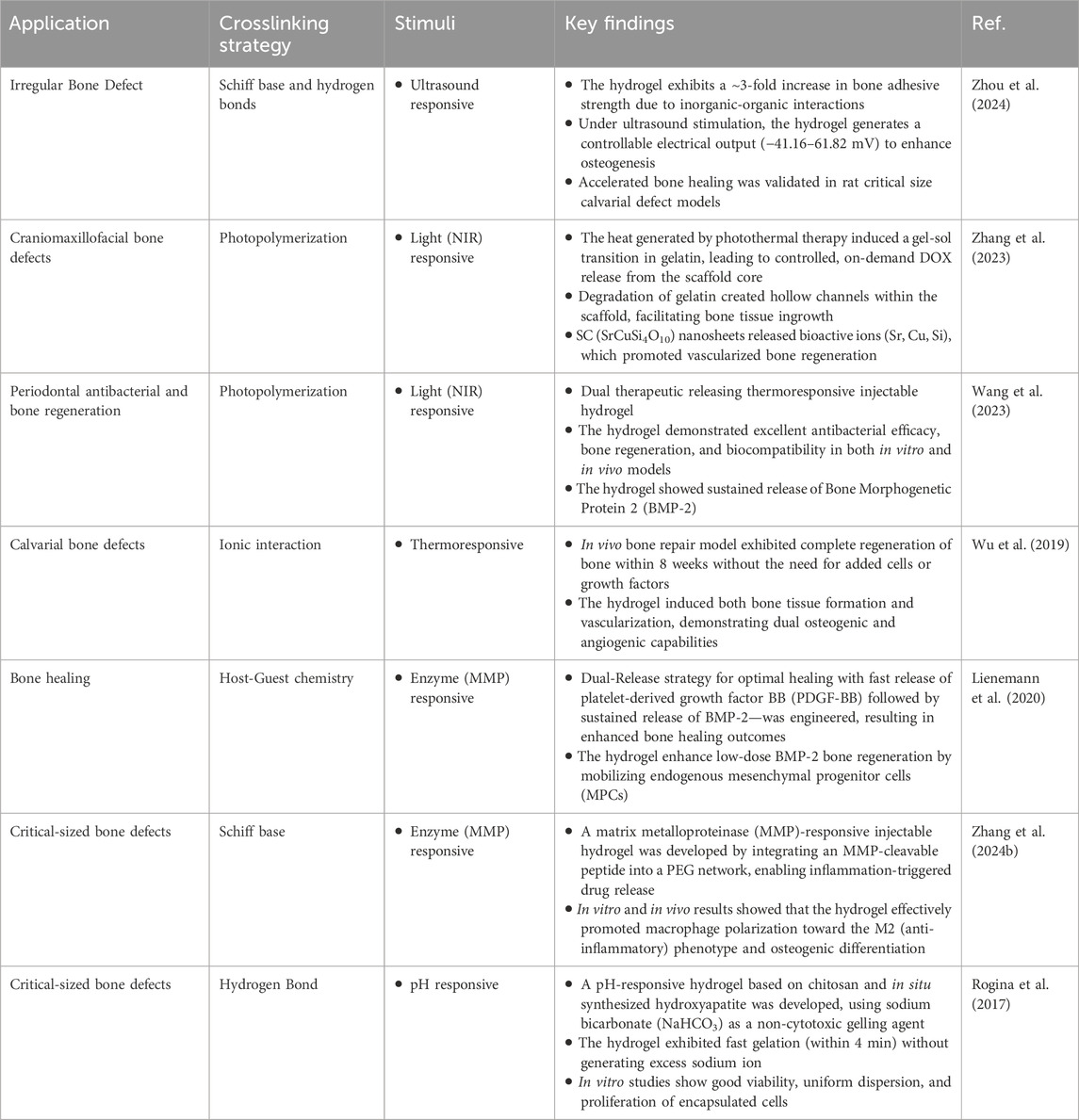
Table 2. Stimuli-responsive injectable hydrogels for bone healing: applications, crosslinking strategies and outcomes.
4.1 Craniomaxillofacial (CMF) bone defects
Craniomaxillofacial (CMF) bone defects are a major clinical challenge wherein portions of bone are missing from the skull or jaw. Traditionally, CMF bone defects require surgical intervention for repair (Dewey and Harley, 2021). Injectable hydrogels have offered an alternative approach for CMF bone defects due to their ability to conform to complex and irregularly shaped defects. Moreso suitable for CMF bone regeneration, stimuli-responsive injectable hydrogels can interact dynamically with the physiological environment and enable tissue regeneration when needed. Additionally, due to their biocompatibility and potential to induce osteogenesis, some bioceramics and biopolymers are currently approved by the United States Food and Drug Administration (FDA) for craniomaxillofacial utilization. In this regard, bioceramics have been used in conjunction with a gelatin-based hydrogel and loaded with doxorubicin, beta-tricalcium phosphate (B-TCP) and SrCuSi4O10 nanosheets (Zhang et al., 2023). The SrCuSi4O10 nanosheets allowed the hydrogel to absorb NIR light and convert it to heat, triggering a series of events: (i) a gel-sol transition of the hydrogel, which (ii) on-demand releases the loaded doxorubicin and B-TCP, and (iii) breaks down the SrCuSi4O10 nanosheets into their bioactive ions, all of which partake in bone healing. Another approach for CMF bone defects is loading an injectable hydrogel with a photosensitizer and an osteoinductive agent, bone morphogenetic protein 2 (BMP-2), was usable for photothermal and photodynamic therapy in the treatment of periodontitis (Wang et al., 2023). When irradiated with NIR light, the composite hydrogel was able to absorb the light and convert it to heat, raising the local temperature enough to release BMP-2 through hydrogel degradation and kill surrounding pathogens. Further, upon exposure to NIR, the photosensitizer produced ROS, decreasing local inflammation and further enhancing bone healing.
4.2 Calvarial bone defects
Calvarial defects are critical-sized cranial injuries that are defined by a localized absence or deficiency of bone. Calvarial defects do not heal spontaneously and are therefore a commonly used method to assess the efficacy of materials for bone healing applications (Alvarez Echazú et al., 2022). Wu et al. have developed a thermo-sensitive in situ-forming injectable hydrogel, wherein the system undergoes a sol-gel transition in response to physiological temperature (Wu et al., 2019). Here, the hydrogel system is mainly composed of ionic interactions between chitosan and glycerophosphate, the nature of which are dictated by the environmental temperature. At low temperatures (ie., room temperature), the hydrogel remains in a liquid state; however, as the temperature rises to a physiological temperature (37°C), the number of ionic interactions between the two materials increases, leading to gelation. The adaptability of the hydrogel allowed the authors to apply the hydrogel system to a calvarial bone defect in mice, such that the hydrogel precisely conformed to the defect site before stabilizing into a solid scaffold. Further, the authors incorporated bioactive glass nanoparticles to stimulate osteogenesis through the release of active ions and silk fibroin to add structural integrity. Taken together, this allowed the calvarial bone defect to fully heal within 8 weeks, as opposed to the untreated group, which did not heal at all. In another instance, authors developed a polyethylene glycol-based hydrogel with MMP-sensitive peptide linkers to co-deliver growth factors in a sequential time-controlled manner (Lienemann et al., 2020). First, these hydrogels were able to fast release platelet-derived growth factors that were weakly encapsulated in the hydrogel via non-covalent interactions. This was then followed by a sustained and enzyme-triggered release of a low dose of BMP-2, which was encapsulated within the hydrogel and held together by MMP-sensitive peptide crosslinkers. By tailoring the number of MMP-degradable linkages within the hydrogel network, the authors were able to design the hydrogel to degrade and release BMP-2 in a sustained manner and at the site of injury. Taken together, this hydrogel was able to mimic the events of the healing cascade—cell mobilization first, followed by cell differentiation—using a combination of MMP activity (biological stimulus) and engineered timing (material design).
4.3 Critical size bone defects
Critical size bone defects have been a long-standing clinical challenge due to delayed healing, risk of infection, and inadequate vascularization (Alvarez Echazú et al., 2022). Traditional treatment, often involving metallic fixation devices and bone grafts, are invasive and may not fully restore the structural and/or functional integrity of the bone. Smart injectable hydrogels have recently become a strategy to improve upon traditional methods. However, injectable hydrogels are limited in their mechanical strength, and as such, are best suited for non-load-bearing areas or as adjuncts to structural implants. Zhang et al. studied the in vitro and in vivo effects of using a matrix metalloproteinase (MMP)-responsive injectable hydrogel for osteogenic differentiation (Zhang M. et al., 2024). The authors integrated an MMP-cleavable peptide into a polyethylene glycol network, such that in instances of inflammation and thus elevated levels of MMPs, the hydrogel would degrade and release loaded cargo—in this case, pro-regenerative phosphatidylserine. It was found that the hydrogel was both mechanically and biologically adaptable to the defect site by promoting anti-inflammatory macrophage polarization and osteogenic differentiation in response to MMP-driven degradation. In another approach, Zhou et al. developed an injectable nanocomposite hydrogel composed of piezoelectric amino-modified barium titanate nanoparticles embedded within a gelatin-based matrix for the treatment of critical-sized bone defects (Zhou et al., 2024). The embedded nanoparticles are able to respond to external ultrasound stimulus by converting mechanical energy into electrical signals. These electrical signals, localized by the hydrogel, mimic the natural bioelectric environment of bone healing and are simultaneously able to stimulate osteogenic differentiation of surrounding cells.
5 Conclusions and future perspectives
There is an increasing need for effective, safe, and minimally invasive strategies to treat a variety of bone defects. Non-load bearing, craniomaxillofacial, and calvarial bone defects affect millions of patients each year (Aghali, 2021; Gaihre et al., 2017). Current treatments are effective to a certain degree; however, they carry the potential to delay bone healing, introduce pathogens, and can result in repeat surgeries (Masters et al., 2019). As mentioned, stimuli responsive injectable hydrogels can be used as an alternative or as adjuncts to existing strategies to better attend to patient needs.
Over the past decade, smart injectable hydrogels have shown great potential, specifically for (1) their ability to on-demand deliver therapeutic agents, such as cells, and (2) their ability to conform and adapt to defect sites due to their stimuli responsive sol-gel transitions. However, there are design difficulties limiting the ability of these hydrogels to be used for clinical bone healing applications, specifically regarding their mechanical properties, or lack thereof, when applied to load-bearing defects. Some strategies to circumvent these issues include increasing crosslinking density or material stiffness, which can also be useful for osteodifferentiation. However, this creates an unfavorable environment and reduces the efficacy for encapsulated cells to proliferate and migrate.
Recently, hydrogels have been designed to react to spatiotemporal mechanical cues for optimal cell-assisted bone regeneration. Xue et al. have developed a new type of macroporous hydrogel for stem cell-assisted bone healing (Xue et al., 2025). This hydrogel is developed with a rigid shell for sustained mechanical cues in guiding stem cell osteodifferentiation and to withstand mechanical load. Further, the developed hydrogel has a soft matrix with tunable degradation rates capable of synchronizing with new tissue deposition to allow for proliferation and migration of cells. Taken together, this new “smart” spatiotemporal system can facilitate bone regeneration in vivo models and address the mechanical limitations of current technologies.
Another alternative to current technologies is using bioprinted 4D hydrogels. 4D bioprinted hydrogels are another form of “smart” technologies—it is a combination of 3D bioprinted technologies that can respond to external or internal stimuli and the fourth dimension, time (Prakash et al., 2024). Unlike injectable hydrogels, these materials can be prefabricated yet still allow for post-printing shape transformations and functional adjustments in the same way injectability can. This up-and-coming technology has the potential for structural flexibility, incorporation of a wide array of bioactive molecules, and the development of neural networks. Smart 4D bioprinted scaffolds have very recently been studied in relation to other biomedical applications (Hann et al., 2023; McLoughlin et al., 2023; Joshi et al., 2023); however, studies for bone healing applications are currently limited. This technology is projected to be most useful for engineering tissues, development of prosthetics, and for surgical implants (Bodaghi et al., 2024).
Despite significant advancements with stimuli-responsive injectable hydrogels, several challenging issues still need to be resolved before these materials can be moved to the clinics. Some of the potential limitations include – (a) toxicity arising from the degradation products of the hydrogels (Stewart et al., 2024), (b) uncontrolled off-target release of cargo molecules (therapeutic or diagnostic agents) (Decuzzi and Cook, 2021; Guo et al., 2025), (c) relatively low mechanical strength and poor physical stability of most stimuli-responsive hydrogels (Guo et al., 2025). Nonetheless, smart injectable hydrogels have made significant preclinical advancements for diverse orthopedic applications. Developing multi-stimuli responsive hydrogels (e.g., hydrogels that respond to light, pH, redox agents, enzymes or metal ions) and hydrogels that are primarily composed of natural polymers (e.g., gelatin, hyaluronic acid, decellularized extracellular matrix) might help overcome some of the above-mentioned obstacles. Use of naturally-derived polymers might help reduce the potential cytotoxic effects arising from degradation products of synthetic hydrogels, as well as assessing in vitro cytotoxicity by inducing stimuli-responsive degradation and evaluation cell viability in the presence of these by-products. Further, the use of multi-stimuli triggered hydrogels with programable functions might better facilitate target-specific drug release from hydrogel carriers. Lastly, exploring dynamic covalent bonds to create smart injectable hydrogels, instead of using non-covalent bonds, might be another promising strategy to improve the rigidity and long-term stability for these hydrogels for broader biomedical applications such as in soft robotics, tissue engineering, drug delivery, and as coating materials for medical devices including bone graft substitutes.
Author contributions
AA: Visualization, Conceptualization, Investigation, Writing – original draft, Writing – review and editing. YS: Writing – original draft, Writing – review and editing, Investigation, Project administration, Visualization. AH: Writing – review and editing. AP: Investigation, Writing – original draft, Writing – review and editing, Funding acquisition, Visualization, Conceptualization, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Arghya Paul is thankful to the following funding agencies for providing support: Early Research Award (ERA) from the Province of Ontario, Canada Research Chairs Program of the Natural Sciences and Engineering Research Council (NSERC) of Canada (CRC-2024-00196), Wolfe-Western Fellowship At-Large for Outstanding Newly Recruited Research Scholar, and Canadian Institutes of Health Research Operating Grant (CIHR – IMHA, Grant no: 185629). Yasmeen Shamiya would like to acknowledge the funding and support from NSERC Canada Graduate Scholarship—Doctoral Program (CGS D). MD Anwarul Hasan and Arghya Paul gratefully acknowledges the support received from the Qatar Research, Development and Innovation (QRDI) Council through the Academic Research Grant (ARG01-0531-230440).
Acknowledgments
The authors would also like to thank BioRender, as some of the images and illustrations were created with BioRender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aghali, A. (2021). Craniofacial bone tissue engineering: current approaches and potential therapy. Cells 10, 2993. doi:10.3390/cells10112993
Alonso, J. M., Del Olmo, J. A., Gonzalez, R. P., and Saez-martinez, V. (2021). Injectable hydrogels: from laboratory to industrialization. Polym. (Basel) 13, 650. doi:10.3390/polym13040650
Altinbasak, I., Kocak, S., Sanyal, R., and Sanyal, A. (2022). Fast-forming dissolvable redox-responsive hydrogels: exploiting the orthogonality of thiol-maleimide and thiol-disulfide exchange chemistry. Biomacromolecules 23, 3525–3534. doi:10.1021/acs.biomac.2c00209
Alvarez Echazú, M. I., Perna, O., Olivetti, C. E., Antezana, P. E., Municoy, S., Tuttolomondo, M. V., et al. (2022). Recent advances in synthetic and natural biomaterials-based therapy for bone defects. Macromol. Biosci. 22, 2100383. doi:10.1002/mabi.202100383
Amiryaghoubi, N., Fathi, M., Safary, A., Javadzadeh, Y., and Omidi, Y. (2024). In situ forming alginate/gelatin hydrogel scaffold through schiff base reaction embedded with curcumin-loaded chitosan microspheres for bone tissue regeneration. Int. J. Biol. Macromol. 256, 128335. doi:10.1016/j.ijbiomac.2023.128335
Anugrah, D. S. B., Ramesh, K., Kim, M., Hyun, K., and Lim, K. T. (2019). Near-infrared light-responsive alginate hydrogels based on diselenide-containing cross-linkage for on demand degradation and drug release. Carbohydr. Polym. 223, 115070. doi:10.1016/j.carbpol.2019.115070
Augustine, R., Mamun, A.Al, Hasan, A., Salam, S. A., Chandrasekaran, R., Ahmed, R., et al. (2021). Imaging cancer cells with nanostructures: prospects of nanotechnology driven non-invasive cancer diagnosis. Adv. Colloid Interface Sci. 294, 102457. doi:10.1016/j.cis.2021.102457
Bae, J. W., Choi, J. H., Lee, Y., and Park, K. D. (2015). Horseradish peroxidase-catalysed in situ-forming hydrogels for tissue-engineering applications. J. Tissue Eng. Regen. Med. 9, 1225–1232. doi:10.1002/term.1917
Bai, X., Gao, M., Syed, S., Zhuang, J., Xu, X., and Zhang, X. Q. (2018). Bioactive hydrogels for bone regeneration. Bioact. Mater 3, 401–417. doi:10.1016/j.bioactmat.2018.05.006
Basu, S., Pacelli, S., Feng, Y., Lu, Q., Wang, J., and Paul, A. (2018). Harnessing the noncovalent interactions of DNA backbone with 2D silicate nanodisks to fabricate injectable therapeutic hydrogels. ACS Nano 12, 9866–9880. doi:10.1021/acsnano.8b02434
Bennison, L., Miller, C., Summers, R., Minnis, A., Sussman, G., and McGuiness, W. (2017). The PH of wounds during healing and infection: a descriptive literature review. Wound practice and research, 25. Australia: Cambridge Media.
Bodaghi, M., Wang, L., Zhang, F., Liu, Y., Leng, J., Xing, R., et al. (2024). 4D printing roadmap. Smart Mater Struct. 33, 113501. doi:10.1088/1361-665X/AD5C22
Bustamante-Torres, M., Romero-Fierro, D., Arcentales-Vera, B., Palomino, K., Magaña, H., and Bucio, E. (2021). Hydrogels classification according to the physical or chemical interactions and as stimuli-sensitive materials. Gels 7, 182. doi:10.3390/gels7040182
Cao, Z., Bai, X., Wang, C., Ren, L., and Ma, D. (2021). A simple polysaccharide based injectable hydrogel compositing nano-hydroxyapatite for bone tissue engineering. Mater Lett. 293, 129755. doi:10.1016/j.matlet.2021.129755
Carlini, A. S., Gaetani, R., Braden, R. L., Luo, C., Christman, K. L., and Gianneschi, N. C. (2019). Enzyme-responsive progelator cyclic peptides for minimally invasive delivery to the heart post-myocardial infarction. Nat. Commun. 10, 1735. doi:10.1038/s41467-019-09587-y
Chen, T., Hou, K., Ren, Q., Chen, G., Wei, P., and Zhu, M. (2018). Nanoparticle–polymer synergies in nanocomposite hydrogels: from design to application. Macromol. Rapid Commun. 39, 1800337. doi:10.1002/marc.201800337
Chen, Q., Yang, Z. R., Du, S., Chen, S., Zhang, L., and Zhu, J. (2024a). Polyphenol-sodium alginate supramolecular injectable hydrogel with antibacterial and anti-inflammatory capabilities for infected wound healing. Int. J. Biol. Macromol. 257, 128636. doi:10.1016/j.ijbiomac.2023.128636
Chen, Y., Chen, B., Dong, J., Yang, D., Tang, H., Wen, L., et al. (2024b). A tough and bioadhesive injectable hydrogel formed with maleimidyl alginate and pristine gelatin. Carbohydr. Polym. 334, 122011. doi:10.1016/j.carbpol.2024.122011
Choi, C. E., Chakraborty, A., Coyle, A., Shamiya, Y., and Paul, A. (2022). Contact-free remote manipulation of hydrogel properties using light-triggerable nanoparticles: a materials science perspective for biomedical applications. Adv. Healthc. Mater 11, 2102088. doi:10.1002/ADHM.202102088
Choi, C. E., Shamiya, Y., Luo, W., and Paul, A. (2025). NIR-responsive ZIF-8 metal-organic framework nanohybrids with photothermal, antimicrobial, and osteoinductive properties to prevent implant infection. Macromol. Biosci. 25, 2400594. doi:10.1002/MABI.202400594
Coulter, S. M., Pentlavalli, S., An, Y., Vora, L. K., Cross, E. R., Moore, J. V., et al. (2024). In situ forming, enzyme-responsive peptoid-peptide hydrogels: an advanced long-acting injectable drug delivery system. J. Am. Chem. Soc. 146, 21401–21416. doi:10.1021/jacs.4c03751
Coyle, A., Chakraborty, A., Huang, J., Shamiya, Y., Luo, W., and Paul, A. (2025). In vitro engineered ECM-incorporated hydrogels for osteochondral tissue repair: a cell-free approach. Adv. Healthc. Mater 14, 2402701. doi:10.1002/ADHM.202402701
Decuzzi, P., and Cook, A. B. (2021). Harnessing endogenous stimuli for responsive materials in theranostics. ACS Nano 15, 2068–2098. doi:10.1021/acsnano.0c09115
Dewey, M. J., and Harley, B. A. C. (2021). Biomaterial design strategies to address obstacles in craniomaxillofacial bone repair. RSC Adv. 11, 17809–17827. doi:10.1039/d1ra02557k
Ding, H., Li, B., Liu, Z., Liu, G., Pu, S., Feng, Y., et al. (2021). Nonswelling injectable chitosan hydrogel via UV crosslinking induced hydrophobic effect for minimally invasive tissue engineering. Carbohydr. Polym. 252, 117143. doi:10.1016/j.carbpol.2020.117143
Gaihre, B., Uswatta, S., and Jayasuriya, A. (2017). Reconstruction of craniomaxillofacial bone defects using tissue-engineering strategies with injectable and non-injectable scaffolds. J. Funct. Biomater. 8, 49. doi:10.3390/jfb8040049
Ghauri, Z. H., Islam, A., Qadir, M. A., Gull, N., Haider, B., Khan, R. U., et al. (2021). Development and evaluation of PH-Sensitive biodegradable ternary blended hydrogel films (Chitosan/Guar Gum/PVP) for drug delivery application. Sci. Rep. 11, 21255. doi:10.1038/s41598-021-00452-x
Gopinathan, J., and Noh, I. (2018). Click chemistry-based injectable hydrogels and bioprinting inks for tissue engineering applications. Tissue Eng. Regen. Med. 15, 531–546. doi:10.1007/s13770-018-0152-8
Grocke, G. L., Zhang, H., Kopfinger, S. S., Patel, S. N., and Rowan, S. J. (2021). Synthesis and characterization of redox-responsive disulfide cross-linked polymer particles for energy storage applications. ACS Macro Lett. 10, 1637–1642. doi:10.1021/acsmacrolett.1c00682
Gu, J., Zhao, G., Yu, J., Xu, P., Yan, J., Jin, Z., et al. (2022). Injectable PH-Responsive hydrogel for combinatorial chemoimmunotherapy tailored to the tumor microenvironment. J. Nanobiotechnology 20, 372. doi:10.1186/s12951-022-01561-z
Guo, A., Cao, Q., Fang, H., and Tian, H. (2025). Recent advances and challenges of injectable hydrogels in drug delivery. J. Control. Release 385, 114021. doi:10.1016/J.JCONREL.2025.114021
Hann, S. Y., Cui, H., Esworthy, T., and Zhang, L. G. (2023). 4D thermo-responsive smart HiPSC-CM cardiac construct for myocardial cell therapy. Int. J. Nanomedicine 18, 1809–1821. doi:10.2147/IJN.S402855
Hassan, H. T., Ismaeel, G. L., Jalil, A. T., Hadi, W. H., Jasim, I. K., Almulla, A. F., et al. (2023). Advanced injectable hydrogels for bone tissue regeneration. Biophys. Rev. 15, 223–237. doi:10.1007/s12551-023-01053-w
He, Z., Xu, Q., Newland, B., Foley, R., Lara-Sáez, I., Curtin, J. F., et al. (2021). Reactive oxygen species (ROS): utilizing injectable antioxidative hydrogels and ROS-producing therapies to manage the double-edged sword. J. Mater Chem. B 9, 6326–6346. doi:10.1039/d1tb00728a
Huang, H., Qi, X., Chen, Y., and Wu, Z. (2019). Thermo-sensitive hydrogels for delivering biotherapeutic molecules: a review. Saudi Pharm. J. 27, 990–999. doi:10.1016/j.jsps.2019.08.001
Jabeen, S., Islam, A., Ghaffar, A., Gull, N., Hameed, A., Bashir, A., et al. (2017). Development of a novel PH sensitive silane crosslinked injectable hydrogel for controlled release of neomycin sulfate. Int. J. Biol. Macromol. 97, 218–227. doi:10.1016/j.ijbiomac.2017.01.014
Jia, Y. P., Shi, K., Yang, F., Liao, J. F., Han, R. X., Yuan, L. P., et al. (2020). Multifunctional nanoparticle loaded injectable thermoresponsive hydrogel as NIR controlled release platform for local photothermal immunotherapy to prevent breast cancer postoperative recurrence and metastases. Adv. Funct. Mater 30, 2001059. doi:10.1002/adfm.202001059
Joshi, N., Yan, J., Levy, S., Bhagchandani, S., Slaughter, K. V., Sherman, N. E., et al. (2018). Towards an arthritis flare-responsive drug delivery system. Nat. Commun. 9, 1275. doi:10.1038/s41467-018-03691-1
Joshi, A., Choudhury, S., Baghel, V. S., Ghosh, S., Gupta, S., Lahiri, D., et al. (2023). 4D printed programmable shape-morphing hydrogels as intraoperative self-folding nerve conduits for sutureless neurorrhaphy. Adv. Healthc. Mater 12, 2300701. doi:10.1002/adhm.202300701
Karvinen, J., and Kellomäki, M. (2024). 3D-Bioprinting of self-healing hydrogels. Eur. Polym. J. 209, 112864. doi:10.1016/j.eurpolymj.2024.112864
Kuddushi, M., Pandey, D. K., Singh, D. K., Mata, J., and Malek, N. (2022). An ionic hydrogel with stimuli-responsive, self-healable and injectable characteristics for the targeted and sustained delivery of doxorubicin in the treatment of breast cancer. Mater Adv. 3, 632–646. doi:10.1039/d1ma00835h
Kumar, A., Ali, A., Kanika, N., Vyawahare, A., Ahmad, A., Mishra, R. K., et al. (2023a). Highly biocompatible smart injectable hydrogel for the management of rheumatoid arthritis. ACS Biomater. Sci. Eng. 9, 5312–5321. doi:10.1021/acsbiomaterials.3c00514
Kumar, A., Kanika, N., Kumar, V., Ahmad, A., Mishra, R. K., Nadeem, A., et al. (2023b). Colon-adhering delivery system with inflammation responsiveness for localized therapy of experimental colitis. ACS Biomater. Sci. Eng. 9, 4781–4793. doi:10.1021/acsbiomaterials.3c00480
Lavrador, P., Esteves, M. R., Gaspar, V. M., and Mano, J. F. (2021). Stimuli-responsive nanocomposite hydrogels for biomedical applications. Adv. Funct. Mater 31, 2005941. doi:10.1002/adfm.202005941
Lee, S. H., and Shin, H. (2007). Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug Deliv. Rev. 59, 339–359. doi:10.1016/j.addr.2007.03.016
Lee, S. Y., Jeon, S. I., Sim, S. B., Byun, Y., and Ahn, C.-H. (2021). A supramolecular host-guest interaction-mediated injectable hydrogel system with enhanced stability and sustained protein release. Acta Biomater. 131, 286–301. doi:10.1016/j.actbio.2021.07.004
Li, L., Scheiger, J. M., and Levkin, P. A. (2019). Design and applications of photoresponsive hydrogels. Adv. Mater. 31, 1807333. doi:10.1002/adma.201807333
Li, Y., Yang, H. Y., and Lee, D. S. (2021). Advances in biodegradable and injectable hydrogels for biomedical applications. J. Control. Release 330, 151–160. doi:10.1016/j.jconrel.2020.12.008
Li, M., Mu, Y., Xu, Q., Jin, L., and Fu, Y. (2024a). Injectable, rapid self-healing, antioxidant and antibacterial nanocellulose-tannin hydrogels formed via metal-ligand coordination for drug delivery and wound dressing. Ind. Crops Prod. 208, 117876. doi:10.1016/j.indcrop.2023.117876
Li, S., Li, X., Xu, Y., Fan, C., Li, Z. A., Zheng, L., et al. (2024b). Collagen fibril-like injectable hydrogels from self-assembled nanoparticles for promoting wound healing. Bioact. Mater 32, 149–163. doi:10.1016/j.bioactmat.2023.09.012
Lienemann, P. S., Vallmajo-Martin, Q., Papageorgiou, P., Blache, U., Metzger, S., Kiveliö, A. S., et al. (2020). Smart hydrogels for the augmentation of bone regeneration by endogenous mesenchymal progenitor cell recruitment. Adv. Sci. 7, 1903395. doi:10.1002/advs.201903395
Liu, M., Zeng, X., Ma, C., Yi, H., Ali, Z., Mou, X., et al. (2017). Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 5, 17014. doi:10.1038/boneres.2017.14
Liu, B., Li, J., Lei, X., Miao, S., Zhang, S., Cheng, P., et al. (2020a). Cell-loaded injectable Gelatin/Alginate/LAPONITE® nanocomposite hydrogel promotes bone healing in a critical-size rat calvarial defect model. RSC Adv. 10, 25652–25661. doi:10.1039/d0ra03040f
Liu, C., Wu, J., Gan, D., Li, Z., Shen, J., Tang, P., et al. (2020b). The characteristics of mussel-inspired NHA/OSA injectable hydrogel and repaired bone defect in rabbit. J. Biomed. Mater Res. B Appl. Biomater. 108, 1814–1825. doi:10.1002/jbm.b.34524
Liu, W., Xie, R., Zhu, J., Wu, J., Hui, J., Zheng, X., et al. (2022). A temperature responsive adhesive hydrogel for fabrication of flexible electronic sensors. Flex. Electron. 6, 68. doi:10.1038/s41528-022-00193-5
Liu, T., Du, Y., Yan, Y., Song, S., Qi, J., Xia, X., et al. (2023). PH-Responsive dual-functional hydrogel integrating localized delivery and anti-cancer activities for highly effective therapy in PDX of OSCC. Mater. Today 62, 71–97. doi:10.1016/j.mattod.2022.12.009
Liu, X., Shen, J., Wang, Y., Li, M., and Fu, S. (2024a). Photoinduced metal-free atom transfer radical polymerization for the modification of cellulose with Poly(N-Isopropylacrylamide) to create thermo-responsive injectable hydrogels. Int. J. Mol. Sci. 25, 2867. doi:10.3390/ijms25052867
Liu, L., Wang, W., Huang, L., Xian, Y., Ma, W., Fan, J., et al. (2024b). Injectable pathological microenvironment-responsive anti-inflammatory hydrogels for ameliorating intervertebral disc degeneration. Biomaterials 306, 122509. doi:10.1016/j.biomaterials.2024.122509
Mah, E., and Ghosh, R. (2013). Thermo-responsive hydrogels for stimuli-responsive membranes. Processes 1, 238–262. doi:10.3390/pr1030238
Masters, E. A., Trombetta, R. P., de Mesy Bentley, K. L., Boyce, B. F., Gill, A. L., Gill, S. R., et al. (2019). Evolving concepts in bone infection: redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy”. “Acute vs. Chronic Osteomyelitis”, “the Immune Proteome” “Local Antibiotic Therapy.” Bone Res 7, 20. doi:10.1038/s41413-019-0061-z
Matsumura, Y., Zhu, Y., Jiang, H., D’Amore, A., Luketich, S. K., Charwat, V., et al. (2019). Intramyocardial injection of a fully synthetic hydrogel attenuates left ventricular remodeling post myocardial infarction. Biomaterials 217, 119289. doi:10.1016/j.biomaterials.2019.119289
McLoughlin, S. T., McKenna, A. R., and Fisher, J. P. (2023). 4D bioprinting via molecular network contraction for membranous tissue fabrication. Adv. Healthc. Mater 12, 2300642. doi:10.1002/adhm.202300642
Mo, C., Xiang, L., and Chen, Y. (2021). Advances in injectable and self-healing polysaccharide hydrogel based on the schiff base reaction. Macromol. Rapid Commun. 42, 2100025. doi:10.1002/marc.202100025
Moreira Teixeira, L. S., Feijen, J., van Blitterswijk, C. A., Dijkstra, P. J., and Karperien, M. (2012). Enzyme-catalyzed crosslinkable hydrogels: emerging strategies for tissue engineering. Biomaterials 33, 1281–1290. doi:10.1016/j.biomaterials.2011.10.067
Pardeshi, S., Damiri, F., Zehravi, M., Joshi, R., Kapare, H., Prajapati, M. K., et al. (2022). Functional thermoresponsive hydrogel molecule to material design for biomedical applications. Polym. (Basel) 14, 3126. doi:10.3390/polym14153126
Park, Y. B., Lin, S., Bai, Y., Moeinzadeh, S., Kim, S., Huang, J., et al. (2022). Dual delivery of BMP2 and IGF1 through injectable hydrogel promotes cranial bone defect healing. Tissue Eng. Part A 28, 760–769. doi:10.1089/ten.tea.2022.0002
Prakash, A., Malviya, R., Sridhar, S. B., and Shareef, J. (2024). 4D printing in dynamic and adaptive bone implants: progress in bone tissue engineering. Bioprinting 44, e00373. doi:10.1016/J.BPRINT.2024.E00373
Rasool, A., Ata, S., and Islam, A. (2019). Stimuli responsive biopolymer (chitosan) based blend hydrogels for wound healing application. Carbohydr. Polym. 203, 423–429. doi:10.1016/j.carbpol.2018.09.083
Revete, A., Aparicio, A., Cisterna, B. A., Revete, J., Luis, L., Ibarra, E., et al. (2022). Advancements in the use of hydrogels for regenerative medicine: properties and biomedical applications. Int. J. Biomater. 2022, 1–16. doi:10.1155/2022/3606765
Rizzo, F., and Kehr, N. S. (2021). Recent advances in injectable hydrogels for controlled and local drug delivery. Adv. Healthc. Mater 10, 2001341. doi:10.1002/adhm.202001341
Rogina, A., Ressler, A., Matić, I., Gallego Ferrer, G., Marijanović, I., Ivanković, M., et al. (2017). Cellular hydrogels based on PH-Responsive chitosan-hydroxyapatite system. Carbohydr. Polym. 166, 173–182. doi:10.1016/j.carbpol.2017.02.105
Rybak, D., Rinoldi, C., Nakielski, P., Du, J., Haghighat Bayan, M. A., Zargarian, S. S., et al. (2024). Injectable and self-healable nano-architectured hydrogel for NIR-light responsive Chemo- and photothermal bacterial eradication. J. Mater Chem. B 12, 1905–1925. doi:10.1039/d3tb02693k
Shamiya, Y., Chakraborty, A., Zahid, A. A., Bainbridge, N., Guan, J., Feng, B., et al. (2024). Ascorbyl palmitate nanofiber-reinforced hydrogels for drug delivery in soft tissues. Commun. Mater. 5 (5), 197–12. doi:10.1038/s43246-024-00641-x
Shi, H., Ma, D., Wu, D., Qiu, X., Yang, S., Wang, Y., et al. (2024a). A PH-Responsive, injectable and self-healing chitosan-coumarin hydrogel based on schiff base and hydrogen bonds. Int. J. Biol. Macromol. 255, 128122. doi:10.1016/j.ijbiomac.2023.128122
Shi, C., Zhang, Y., Wu, G., Zhu, Z., Zheng, H., Sun, X., et al. (2024b). Hyaluronic acid-based reactive oxygen species-responsive multifunctional injectable hydrogel platform accelerating diabetic wound healing. Adv. Healthc. Mater 13, 2302626. doi:10.1002/ADHM.202302626
Shi, C., Zhang, Y., Wu, G., Zhu, Z., Zheng, H., Sun, X., et al. (2024c). Hyaluronic acid-based reactive oxygen species-responsive multifunctional injectable hydrogel platform accelerating diabetic wound healing. Adv. Healthc. Mater 13, 2302626. doi:10.1002/adhm.202302626
Soeriyadi, A. H., Gupta, B., Reece, P. J., and Gooding, J. J. (2014). Optimising the enzyme response of a porous silicon photonic crystal via the modular design of enzyme sensitive polymers. Polym. Chem. 5, 2333–2341. doi:10.1039/c3py01638b
Stewart, C. L., Hook, A. L., Zelzer, M., Marlow, M., and Piccinini, A. M. (2024). PLGA-PEG-PLGA hydrogels induce cytotoxicity in conventional in vitro assays. Cell Biochem. Funct. 42, e4097. doi:10.1002/CBF.4097
Tallapaneni, V., Mude, L., Pamu, D., Palanimuthu, V. R., Magham, S. V., Karri, V. V. S. R., et al. (2023). Growth factor loaded thermo-responsive injectable hydrogel for enhancing diabetic wound healing. Gels 9, 27. doi:10.3390/gels9010027
Tanga, S., Aucamp, M., and Ramburrun, P. (2023). Injectable thermoresponsive hydrogels for cancer therapy: challenges and prospects. Gels 9, 418. doi:10.3390/gels9050418
Thambi, T., Jung, J. M., and Lee, D. S. (2023). Recent strategies to develop PH-Sensitive injectable hydrogels. Biomater. Sci. 11, 1948–1961. doi:10.1039/d2bm01519f
Vera-González, N., Deusenbery, C., LaMastro, V., and Shukla, A. (2024). Fungal enzyme-responsive hydrogel drug delivery platform for triggered antifungal release. Adv. Healthc. Mater 13, 2401157. doi:10.1002/adhm.202401157
Wang, X., Li, Z., Shi, T., Zhao, P., An, K., Lin, C., et al. (2017). Injectable dextran hydrogels fabricated by metal-free click chemistry for cartilage tissue engineering. Mater. Sci. Eng. C 73, 21–30. doi:10.1016/j.msec.2016.12.053
Wang, J. H., Tsai, C. W., Tsai, N. Y., Chiang, C. Y., Lin, R. S., Pereira, R. F., et al. (2021). An injectable, dual crosslinkable hybrid pectin methacrylate (PECMA)/gelatin methacryloyl (GelMA) hydrogel for skin hemostasis applications. Int. J. Biol. Macromol. 185, 441–450. doi:10.1016/j.ijbiomac.2021.06.162
Wang, W., Zhang, G., Wang, Y., Ran, J., Chen, L., Wei, Z., et al. (2023). An injectable and thermosensitive hydrogel with nano-aided NIR-II phototherapeutic and chemical effects for periodontal antibacteria and bone regeneration. J. Nanobiotechnology 21, 367. doi:10.1186/s12951-023-02124-6
Wu, J., Zheng, K., Huang, X., Liu, J., Liu, H., Boccaccini, A. R., et al. (2019). Thermally triggered injectable chitosan/silk fibroin/bioactive glass nanoparticle hydrogels for in-situ bone formation in rat calvarial bone defects. Acta Biomater. 91, 60–71. doi:10.1016/j.actbio.2019.04.023
Xiao, X., and Huang, J. (2024). Enzyme-responsive supramolecular self-assembly in small amphiphiles. Langmuir 17, 21. doi:10.1021/ACS.LANGMUIR.4C01762/ASSET/IMAGES/LARGE/LA4C01762_0013.JPEG
Xue, W., Hamley, I. W., and Huglin, M. B. (2002). Rapid swelling and deswelling of thermoreversible hydrophobically modified Poly(N-Isopropylacrylamide) hydrogels prepared by freezing polymerisation. Polym. Guildf. 43, 5181–5186. doi:10.1016/S0032-3861(02)00396-8
Xue, B., Xu, Z., Li, L., Guo, K., Mi, J., Wu, H., et al. (2025). Hydrogels with programmed spatiotemporal mechanical cues for stem cell-assisted bone regeneration. Nat. Commun. 16 (1), 3633. doi:10.1038/s41467-025-59016-6
Yang, J.-A., Yeom, J., Hwang, B. W., Hoffman, A. S., and Hahn, S. K. (2014). In situ-forming injectable hydrogels for regenerative medicine. Prog. Polym. Sci. 39, 1973–1986. doi:10.1016/j.progpolymsci.2014.07.006
Yang, Y., Li, M., Pan, G., Chen, J., and Guo, B. (2023a). Multiple stimuli-responsive nanozyme-based cryogels with controlled NO release as self-adaptive wound dressing for infected wound healing. Adv. Funct. Mater 33, 2214089. doi:10.1002/adfm.202214089
Yang, K., Zhao, X., Wei, W., Lin, C. X., Sun, L., Wei, Z., et al. (2023b). A novel injectable and self-biodegradable Poly(Aspartic acid) hydrogel. Mater Des. 226, 111662. doi:10.1016/j.matdes.2023.111662
Yang, H., Wang, W., Xiao, J., Yang, R., Feng, L., Xu, H., et al. (2025). ROS-responsive injectable hydrogels loaded with exosomes carrying MiR-4500 reverse liver fibrosis. Biomaterials 314, 122887. doi:10.1016/J.BIOMATERIALS.2024.122887
Yu, L., and Ding, J. (2008). Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 37, 1473. doi:10.1039/b713009k
Yu, J., Zhang, R., Chen, B., Liu, X., Jia, Q., Wang, X., et al. (2022). Injectable reactive oxygen species-responsive hydrogel dressing with sustained nitric oxide release for bacterial ablation and wound healing. Adv. Funct. Mater 32, 2202857. doi:10.1002/adfm.202202857
Zandi, N., Sani, E. S., Mostafavi, E., Ibrahim, D. M., Saleh, B., Shokrgozar, M. A., et al. (2021). Nanoengineered shear-thinning and bioprintable hydrogel as a versatile platform for biomedical applications. Biomaterials 267, 120476. doi:10.1016/j.biomaterials.2020.120476
Zhang, J., Muirhead, B., Dodd, M., Liu, L., Xu, F., Mangiacotte, N., et al. (2016). An injectable hydrogel prepared using a PEG/vitamin E copolymer facilitating aqueous-driven gelation. Biomacromolecules 17, 3648–3658. doi:10.1021/acs.biomac.6b01148
Zhang, L. Y., Bi, Q., Zhao, C., Chen, J. Y., Cai, M. H., and Chen, X. Y. (2020). Recent advances in biomaterials for the treatment of bone defects. Organogenesis 16, 113–125. doi:10.1080/15476278.2020.1808428
Zhang, X., Wei, H., Dong, C., Wang, J., Zhang, T., Huang, L., et al. (2023). 3D printed hydrogel/bioceramics core/shell scaffold with NIR-II triggered drug release for chemo-photothermal therapy of bone tumors and enhanced bone repair. Chem. Eng. J. 461, 141855. doi:10.1016/j.cej.2023.141855
Zhang, F., Zhang, Y., Qian, S., Qian, X., Jiao, J., Ma, B., et al. (2024a). Injectable and conductive nanomicelle hydrogel with α-Tocopherol encapsulation for enhanced myocardial infarction repair. ACS Nano 18, 10216–10229. doi:10.1021/acsnano.4c00509
Zhang, M., Yu, T., Li, J., Yan, H., Lyu, L., Yu, Y., et al. (2024b). Matrix metalloproteinase-responsive hydrogel with On-Demand release of phosphatidylserine promotes bone regeneration through immunomodulation. Adv. Sci. 11, 2306924. doi:10.1002/advs.202306924
Zhao, D., Tang, Q., Zhou, Q., Peng, K., Yang, H., and Zhang, X. (2018). A photo-degradable injectable self-healing hydrogel based on star Poly(Ethylene Glycol)-: b-Polypeptide as a potential pharmaceuticals delivery carrier. Soft Matter 14, 7420–7428. doi:10.1039/c8sm01575a
Zheng, J., Wang, Y., Wang, Y., Duan, R., and Liu, L. (2023a). Gelatin/hyaluronic acid photocrosslinked double network hydrogel with nano-hydroxyapatite composite for potential application in bone repair. Gels 9, 742. doi:10.3390/gels9090742
Zheng, Z., Yang, X., Zhang, Y., Zu, W., Wen, M., Liu, T., et al. (2023b). An injectable and PH-Responsive hyaluronic acid hydrogel as metformin carrier for prevention of breast cancer recurrence. Carbohydr. Polym. 304, 120493. doi:10.1016/j.carbpol.2022.120493
Zhou, L., Chen, F., Hou, Z., Chen, Y., and Luo, X. (2021). Injectable self-healing CuS nanoparticle complex hydrogels with antibacterial, anti-cancer, and wound healing properties. Chem. Eng. J. 409, 128224. doi:10.1016/j.cej.2020.128224
Keywords: stimuli-responsive, injectable, hydrogels, bone healing, biomaterials
Citation: Al Mamun A, Shamiya Y, Hasan A and Paul A (2025) Unlocking the potential of stimuli-responsive injectable hydrogels for bone healing applications. Front. Biomater. Sci. 4:1641339. doi: 10.3389/fbiom.2025.1641339
Received: 04 June 2025; Accepted: 23 July 2025;
Published: 13 August 2025.
Edited by:
Dietmar Werner Hutmacher, Queensland University of Technology, AustraliaReviewed by:
Bram Soliman, University of New South Wales, AustraliaCopyright © 2025 Al Mamun, Shamiya, Hasan and Paul. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arghya Paul, YXJnaHlhLnBhdWxAdXdvLmNh
†Present address: Center for Biosystems and Machines, King Fahd University of Petroleum & Minerals, Dhahran, 31261, Saudi Arabia
‡These authors have contributed equally to this work
 Abdulla Al Mamun1‡
Abdulla Al Mamun1‡ Anwarul Hasan
Anwarul Hasan Arghya Paul
Arghya Paul