- 1Integrated Design Research Lab, David R. Ravin School of Architecture, University of North Carolina at Charlotte, Charlotte, NC, United States
- 2EcoClosure, Charlotte, NC, United States
- 3Department of Biological Sciences, University of North Carolina at Charlotte, Charlotte, NC, United States
- 4Integrated Design Research Lab, David R. Ravin School of Architecture and Infrastructure and Environmental Systems (INES), University of North Carolina at Charlotte, Charlotte, NC, United States
The rapid urbanization, population growth, and technological advancement have exacerbated global warming and environmental impacts. Buildings are one of the primary contributors to anthropogenic pollution and climate change. While net-zero energy buildings powered by renewable energy systems can help alleviate carbon emissions, a major challenge remains in addressing embodied carbon throughout the entire life cycle of buildings, including material processing, manufacturing, construction, and end-of-life phases. Therefore, it is important to implement carbon removal strategies in buildings and cities. Carbon removal strategies primarily include two categories: nature-based solutions such as reforestation, wetland restoration, and blue carbon strategies and technology-based carbon removal such as carbon capture and storage and direct air capture. This paper outlines nature-based solutions suitable for a built environment while still actively improving additional environmental benefits such as human wellbeing and overall ecosystem health. In particular, the research focuses on multifunctional microalgae-integrated building enclosures for efficient carbon sequestration. Due to their strong photosynthetic capability and rapid growth, microalgae have received significant attention for their carbon capture potential. Photobioreactors fabricated into buildings and industrial infrastructure allow microalgae to sequester more carbon while simultaneously producing biomass feedstock and other valuable biomass commodities. This study tested a microalgae photobioreactor-integrated window system using Chlorella and Chlorococcum. Our results indicated that Chlorella’s daily yield in a microalgae window was 175 mg/L-day, while Chlorococcum’s daily yield was 80 mg/L-day, which are consistent with the data published in the literature. These results demonstrate the viability of microalgae building enclosures for real-world carbon capture. The research suggests that a range of microalgae strains coupled with well-controlled growing environments has potential for cost-effective large scale carbon sequestration in the built environment; however, the combination of regulating ideal growing environmental conditions with the building energy efficiency of the microalgae enclosure system are areas of future research. Algal carbon sequestration, when combined with net-zero buildings, can address global warming and help sustainable urban development.
1 Introduction
It is expected that two out of every three people worldwide will reside in cities, leading to more construction and demolition. As the global population continues to grow, alongside the rapid urbanization, improved living conditions, and the expanding use of technology, the frequency of catastrophic climate disasters resulting from global warming is expected to intensity. Before the Industrial Era began in the late 18th century, historical atmospheric CO2 levels were consistently below 300 parts per million (ppm), today numbers are increasing at over 400 ppm. The CO2 increase is primarily attributed to anthropogenic activities, including increased construction and demolition, manufacturing, and consumption. Today, with the artificial intelligence (AI) era, data centers have further contributed to environmental impacts, accounting for approximately 4.4% of total U.S. electricity use and with cooling systems consuming up to 40% of this energy usage (Lawrence Berkeley National Laboratory, 2024; U.S. Department of Energy, 2023). To limit global warming to below 1.5°C above pre-industrial levels, it is critical to reduce CO2 emissions by approximately 45% from 2010 levels by 2030 and achieve carbon neutrality by 2050 (IPCC, 2018).
Energy-efficient and even net zero energy buildings are key measures toward carbon neutrality. For example, if all buildings, including data centers, are built to zero energy standards supplied by renewable energy systems, it would help to greatly lower CO2 emissions. At the same time, it is important to recognize the embodied carbon inherent in all building materials, as they entail embodied carbon emissions, even when their operations are powered by clean energy. Embodied carbon is the total greenhouse gas emissions (GHG emission) during the whole life cycle of materials and systems. It encompasses emissions from ore mining, their manufacture to transportation, construction, and the end phase of the system. Even renewable energy technologies such as solar panels, wind turbines, and hydronic energy systems, entail embodied carbon as a result of their production, transportation and installation. Concrete and steel, two of the most used construction materials, contribute to around 10% of the international carbon emissions (IEA, 2022).
Therefore, after minimizing carbon (C) emissions through net-zero energy building strategies, improved manufacturing processes, and the recycling of materials throughout a building’s life cycle, it becomes essential to incorporate carbon sink methods to offset the unavoidable embodied carbon. Carbon removal can be achieved through two methods: 1) nature-based carbon sequestration and 2) technology-based carbon sequestration such as carbon capture and storage (CCS) and direct air capture (DAC). Nature-based carbon removal includes forestation and reforestation, soil carbon sequestration, restoration of wetlands, and blue carbon strategies. This allows the ecosystem to grow and store more CO2 over a given period. For example, plants use sunlight and CO2 during photosynthesis, generating glucose and oxygen. In other words, CO2 serves as an essential external substance for the plant to produce energy, which subsequently supports plant growth. Nature-based carbon removals are reliable and symbiotic whereas CCS and DAC processes pose technological challenges, such as scalability and longevity in real-world applications (Tripathi et al., 2023).
For this paper, the authors examined the nature-based solutions in the built environment, focusing on microalgae building systems. By embedding plant systems into buildings, site surroundings, and communities, nature-based solutions achieve carbon reduction while providing additional benefits. People will be attached to natural environments as these solutions support biophilic effects leading to positive user experience (Kellert, 2018)
2 Nature-based carbon removal
Global warming has been caused by the imbalance between anthropogenic emissions and natural carbon sinks. The global carbon cycle is important in storing carbon in the Earth’s systems–atmosphere, lithosphere, biosphere, and hydrosphere. These primary Earth systems not only store carbon but also engage in carbon exchanges. According to the Intergovernmental Panel on Climate Change (IPCC) Sixth Assessment Report (202), nearly 5.1 GtC is accumulating annually in the atmosphere, which accelerates global warming. This accumulation translates to approximately 18.7 GtCO2 annually using the conversion ratio, further emphasizing the urgent need for international mitigation efforts.
2.1 Terrestrial carbon sinks
The biggest carbon flux in the biosphere is terrestrial photosynthesis, or gross primary production (GPP), although its global scale and spatiotemporal dynamics are still not fully understood (Anav et al., 2015). The quantity of CO2 that is extracted from the atmosphere annually to support photosynthesis is known as GPP (Prentice et al., 2024). Terrestrial ecosystems have absorbed approximately 30% of anthropogenic CO2 emissions through photosynthesis during the past 60 years (Friedlingstein et al., 2022). However, in recent research, correlations have been observed between global warming and the efficiency of photosynthesis and carbon uptake capabilities. Yuan et al. (2025) integrated several NEE datasets from climate datasets, Earth system models, eddy-covariance data-driven techniques, and atmospheric CO2 inversions to demonstrate that, over the past 40 years, tropical regions have contributed 81% ± 48% of the total global carbon update, but this capacity has reduced during extreme heat events (Yuan et al., 2025). Other research assessed how climate, growing season length, and maximum seasonal photosynthesis affect the yearly GPP in plant areas of the Loess Plateau using multi-source remote sensing and climatic data from 2001 to 2021 (Li et al., 2024). The results enhance the assessment of future terrestrial carbon cycle dynamics and the direction of landscape management in semi-arid ecosystems by indicating that drought limits ecosystem carbon sequestration by limiting photosynthetic capacity than through changes in plant phenology.
Soil is another important carbon reservoir. An estimated 1,500 Gt of soil organic carbon (SOC), or about twice as much carbon as the atmosphere, is found in soils worldwide (Schlesinger, 2000). Land alterations have played a large role in the increase of CO2 in the atmosphere, even if the burning of fossil fuels has been the primary source. According to some estimates, many American soils have lost between 30 and 50 percent of their pre-cultivation carbon content (Kucharik et al., 2001). Forest soils have a major influence on the global or regional cycling of carbon (C) and are the largest sink of terrestrial C. The persistence of plant residues or the stimulation of soil microbial activity and the increase in the contribution of microbial necromass to the slow cycling soil organic matter (OM) pools are the two ways that belowground input contributes to C storage. Therefore, important characteristics that regulate the quantity and placement of C inputs in the soil profile include plant architecture and rooting depth of species (Beniston et al., 2014).
Plants have two effects on soil organic matter. First, because they are autotrophic, plants are the primary source of organic carbon soil through litter deposition (both shoots and roots), the release of root exudates (both passive and active), and symbiotic functions (nitrogen-fixing and mycorrhizal relationships). Second, through the production of low degradable chemicals and promotion of stable aggregation formation, plants support the stabilization of soil organic matter. About one-third of all litter inputs in grassland soils and half in forest soils originates from root litter (Freschet et al., 2013). Recent research indicates that belowground inputs are the main contributors to long term OM stability in soils (Balesdent and Balabane, 1996). In addition, there was typically little chance that deteriorated grassland soils might store more organic carbon (OC) (Wiesmeier et al., 2015). The comparatively significant contribution of labile soil organic carbon (SOC), which is preferentially lost during soil deterioration, may be the cause of this. Additionally, silt and clay particles are significantly lost due to wind erosion, which directly reduces the capacity to stabilize more OC. According to research, heavy land use-induced SOC loss in semi-arid areas is mostly irreversible. It is not possible to consider observed increases in SOC following improved land management as a contribution to long-term OC sequestration since they primarily lead to the buildup of labile SOC that is susceptible to changes in land use and climate.
2.2 Blue carbon sinks
To keep global warming below the 1.5°C threshold established by the Paris Agreement; nature-based climate solutions have become essential approaches in the fight against climate change (Girardin et al., 2021). The phrase “blue carbon,” which was first used by Nellemann (2009) in the interagency report “Blue carbon: the role of healthy oceans in binding carbon” highlighted the enormous potential of coastal and marine ecosystems for carbon sequestration. While earlier carbon efforts focused mainly on terrestrial green systems, blue carbon has gained recognition for its substantial sequestration potential (Chen et al., 2024). Because of their significant carbon sink, long-term carbon storage, and effective removal of greenhouse gases, blue carbon ecosystems have attracted attention (Macreadie et al., 2021). However, the carbon cycle of natural ecosystems is expected to be severely impacted by significant environmental changes, such as sea level rising and global warming, as the atmospheric CO2 rises (Fang and Guo, 2007). The basic biological processes—soil accretion, carbon sequestration, and primary productivity—that govern the productivity of coastal wetlands are being impacted by these changes (Langley et al., 2013). Coastal such as seagrass bed meadows, salt marshes, and mangroves are especially important as they store carbon in sediments, above-ground (leaves, stems, and branches), below-ground (roots), and non-living biomass (dead wood) (Mcleod et al., 2011). Together, these ecosystems absorb over 70% of the organic carbon stored in marine sediments, making them essential carbon sinks even though they make up less than 0.2% of the world’s ocean area (Duarte et al., 2005). Blue carbon ecosystems play an important role in the global carbon cycle because of their high efficiency in converting CO2 to plant biomass, which results in high productivity and net carbon uptake (Bouillon et al., 2008); the fact that they are found in depositional environments makes it easier for both autochthonous and allochthonous carbon sources to accumulate (Saintilan et al., 2013); the fact that seawater inundates them maintains high sulfate concentrations in the sediment, which lowers methane emissions (Poffenbarger et al., 2011); and the anaerobic and waterlogged conditions significantly slow down decomposition processes (Atwood et al., 2017) so that organic carbon deposits can be stored over millennial time-scales through vertical accretion (Rogers et al., 2019). These ecosystems absorb 75–220 Tg C (terragrams of carbon), or 270–820 Tg CO2 (terragrams of CO2 equivalent), which accounts for 0.7%–2.3% of the world’s current fossil fuel emissions (Serrano et al., 2019).
2.3 Algal carbon capture
Microalgae live in freshwater, seawater or saline water, and convert sunlight and CO2 into chemical energy. As a result, they play a key role in the global carbon cycle as well as serve as the foundation of aquatic food chains (Barsanti and Gualtieri, 2022). The fact that microalgae are responsible for almost half of the carbon fixation on Earth, despite making up less than 1% of the world’s photosynthetic biomass, helps us visualize the scale of their contribution (Poorter et al., 2015). Microalgae can be classified into eukaryotic algae and prokaryotic algae. Eukaryotic algae have a more complex cellular structure with a nucleus enclosed by a nuclear membrane. They include green algae, diatoms, and dinoflagellates. Prokaryotic algae are blue-green algae (cyanobacteria) and do not have a nuclear membrane.
2.3.1 Photosynthetic efficiency
The carbon uptake rate is directly related to their photosynthetic efficiency. Microalgae’s single or multi-cells are tailored for capturing and converting light energy into chemical energy at a high rate. One of the efficient photosynthetic attributes is their higher surface area-to-volume ratio. The large surface-to-volume ratio enhances their carbon dioxide and nutrient uptake efficiently, hence leading to fast growth rates in microalgae. Most microalgae use the C3 photosynthetic pathway, but some species exhibit characteristics similar to C4 photosynthesis, particularly under carbon-limited conditions. The C4 pathway is characterized by concentrating CO2 around the enzyme Rubisco, thereby reducing photorespiration and increasing carbon fixation efficiency (Kupriyanova et al., 2023). Their superior photosynthetic capabilities absorb significant amounts of CO2. With optimum growing environments, microalgae can double their biomass in a few hours due to their highly efficient cellular structures (Benedetti et al., 2018).
2.3.2 Carbon sequestration potential and environmental benefits
13 million acres of microalgae can uptake an average of 0.5 gigatons of CO2 out of the atmosphere, while producing in excess of 300 tons of biomass during microalgal farming (Tripathi et al., 2023). In other words, the area of North Carolina, for example, could sequester 5 gigatons of CO2 per year, that the entire United States is responsible for. Once biomass is obtained, it can be converted into value-added products and biofuels. In addition, microalgae can serve as a nature-based solutions for wastewater treatment while removing carbon dioxide. Using CO2 available in the water or air for photosynthesis increases the concentration of carbon in biomass. Carbon retains a significant portion in the biomass, taking up 1.8 g of CO2 relative to 1 g of dry biomass assuming 50% of the biomass is carbon. The molecular weight of carbon is 12 g/mol while CO2 is 44 g/mol, which means 3.67 g of CO2 are needed to fix 1 g of carbon. Because we assume 0.5 g of carbon is contained in 1 g of biomass, the calculated amount of CO2 absorbed for every gram of dry biomass with 0.5 g of carbon results in 0.5 × 3.67 equaling approximately 1.8. This confirms that the microalgal biomass captures contributes significantly to CO2 from the atmosphere.
2.3.3 Applications and integration
Microalgae cultivation can be integrated at many biological levels and incorporated into wider applications, such as building enclosures, infrastructure, and waste treatment sites. Urban or industrial areas with space constraints will benefit greatly from microalgae applications since they use less land area than terrestrial plants to achieve similar carbon sequestration performance. When a photobioreactor directly integrates with the chimney or machine in manufacturing or power plants, industrial and power facilities can feed CO2 emissions directly, thereby improving their sequestration efficiency (Dineshbabu et al., 2019). Another benefit of microalgae application is its high-performance carbon uptake and biomass production per unit area compared to other terrestrial plants.
2.3.4 Year around growing environments
Photobioreactors allow microalgae to grow year-round because they provide optimum growing environments, unlike terrestrial plants, which exhibit different growth responses depending on the season or climate changes. The photobioreactor enables the growing environment to be efficiently adjusted in optimum settings in light intensity, temperature, CO2 concentrations, and nutrient concentrations, leading to the achievement of optimum carbon sequestration. As an end-of-life cycle of the photobioreactor, harvested biomass can have diverse economic uses in the form of biofuels, bioplastics, animal feed, fertilizers, and biochemical compounds. As a result, the biomass that stores carbon remains sequestered in the ecosystem.
Microalgae offer an effective nature-based solution for carbon dioxide removal through their fast growth and excellent photosynthetic capability. The coupling of microalgae photobioreactors with building applications and industrial processes offers the possibility of significant uptake of carbon emissions as well as the production of biomass for biofuel and high value-added applications.
2.4 Microalgae integrated building enclosures
The integration of microalgae systems into architectural design has garnered increasing attention in the pursuit of environmentally responsive and energy-efficient buildings. Algae façades serve as dynamic building envelopes capable of reducing carbon emissions, enhancing indoor comfort, and generating biomass for biofuels or other uses. Initial implementations of algae façades in New South Wales, Australia, highlighted both the promise of energy reduction and on-site biofuel production and the significant challenges related to design integration, construction logistics, and long-term maintenance (Wilkinson et al., 2017). The application of algae as a material system has been examined for infill design, noting its role in improving indoor environmental quality and visual connectivity with nature (Martokusumo et al., 2017). Algae façades have also been evaluated as viable strategies for green building regulation compliance and aesthetic integration, particularly in office and commercial settings (Poerbo et al., 2017). From a techno-economic perspective, algae building technology has been evaluated for its lifecycle benefits within net-zero energy frameworks, highlighting cost savings over the lifecycle and emphasizing algae façades’ compatibility with sustainable architectural design (Biloria and Thakkar, 2020). Further supporting its relevance, algae-powered buildings have been positioned as integral to closed-loop regenerative systems, providing both waste management and energy production (Sedighi, Pourmoghaddam Qhazvini and Amidpour, 2023). These authors estimated that microalgae façades can sequester an average of 5 g of CO2 per square foot per day under optimal conditions.
Advancements in cultivation techniques have expanded the potential of algae in architecture. For example, plasma-enhanced algae cultivation has emerged as a promising method to boost biomass productivity in high-density urban settings by optimizing light absorption and biochemical reactions within microalgae cells (Çelekli and Zarić, 2024a). In parallel, the integration of artificial wetlands and algal biofilters into building systems has been proposed to increase biodiversity and enhance air and water purification, showcasing interdisciplinary approaches to environmental design (Zarić and Çelekli, 2024). In a more visionary context, algae’s role in terraforming Mars has been proposed, based on its potential to contribute to atmospheric formation in extraterrestrial environments (Çelekli and Zariç, 2024b). The same study indicated that algae’s biotechnological contributions—including CO2 capture, nutrient recycling, wastewater remediation, and biomass valorization—position it as a key tool for sustainable development. This speculative application reinforces the biological and environmental adaptability of algae. Domestically, the future of algae technology in green buildings has been reviewed with emphasis on its capacity for thermal insulation, carbon uptake, and visual integration (Çelekli, Yeşildağ and Zariç, 2024).
3 Microalgae carbon removal experiments
The primary objectives of this experiment were to measure the carbon removal potential and the viability of the system within a real building environment.
3.1 Experiment setup
A performance mock-up of an algae façade was installed behind existing west-facing windows to provide effective shading, control visual glare during sunset hours, and remove CO2. The algae facade measured 3.7 m wide by 2.5 m tall and integrated modular X-shape photobioreactors (Figure 1). This modular photobioreactor design enabled rapid installation and efficient adaptability for various window shapes and sizes. The microalgae growth in the system was supported by various operational systems. For CO2, the algae façade utilized room air and biogenic CO2 produced by users. CO2-enriched room air was drawn through an intake box equipped with an air pump, mounted on one side of the system. The intake air was then distributed to the algae facade via air distribution pipes and aeration stones located at the base of the facade system. The oxygen-rich, clean air filtered by the algae facade was collected at the top of the system and returned to the interior space via the outtake air box installed on the opposite side of the system. Both the intake and outtake boxes were equipped with air quality sensors to compare the carbon removal efficiency and air quality improvements of the microalgae system. Additionally, UV-C lights were installed in both the intake and outtake boxes to prevent the introduction of unwanted microorganisms into the system and room air. For operation of the system, young algae and nutrient medium were introduced through the top inlet while grown algae were harvested at the bottom outlet through a gravity-driven harvesting technique. Microalgae samples were also collected from the bottom outlet.

Figure 1. Microalgae window demonstrating carbon removal and shading efficacy (left). The system comprises bioreactor modules designed for quick installation and easy adaptation to various window sizes and shapes (right).
3.2 Microalgae species and cultivation conditions in microalgae facade
The microalgae façade system was designed to grow six different microalgae species: Chlorella and Chlorococcum in addition to other algae with different end use potentials. The experiment focused on the carbon removal performance of Chlorella and Chlorococcum. Chlorella has been studied for its potential use in wastewater treatment and carbon capture, with recent studies leveraging AI-assisted environmental optimization and species selection to enhance environmental performance and operational efficiency (Abuhasheesh et al., 2025). Additionally, its ability to grow in municipal and industrial effluents can be utilized for nutrient recovery and environmental pollution reduction (Abuhasheesh et al., 2025). As a result of its fast growth and high lipid content, it has proven to be a promising candidate for sustainable biofuel energy sources (de Oliveira et al., 2024). Similarly, Chlorococcum, a single-celled green microalgae has a great biofuel potential due to its high lipid concentration. The species also helps with bioremediation, as it can uptake effluent nutrients in wastewater treatment. The free access to nutrients could significantly lower the cost of biofuel production (Mahapatra and Ramachandra, 2013).
The Chlorella and Chlorococcum algae were individually inoculated into each layer of the algae bioreactor. The bioreactor layers were then filled with fresh growth medium and gently bubbled with sterile filtered room air to move the microalgae and provide biogenic CO2 for photosynthesis under sunlight. The growth medium used for the algae facade was Alga-Gro™, a proprietary medium (Carolina Biological Supply Co., Burlington, NC). Illumination was primarily sunlight penetrating from the west-facing window and by ambient overhead fluorescent room light. In addition to air quality sensors, the algae facade was equipped with photosynthetically activated radiation (PAR sensor measuring 400–700 nm), visible light (measuring 380–770 nm), and solar radiation (measuring 350–1100 nm) as well as pH and water temperature sensors to monitor the environmental conditions supporting microalgae growth. The algae façade was installed on the interior side, drawing in room temperature air and ambient relative humidity. As a result, the algae water temperature remained relatively stable without significant fluctuations. Similarly, the daily sunlight exposure on the algae façade was relatively lower compared to outdoor installations.
3.3 Sampling and analysis
Each bioreactor layer was sampled twice per week over 4 weeks by draining approximately 10 mL from the bottom port. The samples were then brought to a biology laboratory and measured for optical density at 600 nm (OD 600) and dry biomass. The OD 600 was measured using a Bio-Rad Smart Spec Plus UV/VIS Spectrophotometer, which determine the cell concentration per mL. For measuring dry cell weight (DCW), the weight of a dry filter was measured first using a precision scale. The filter was then loaded with microalgae cells and dried in the oven at 110°C for 4 hours. The weight of the filter with the dried microalgae cells was measured after drying. The DCW was then calculated by subtracting the weight of the dry filter from the weight of the filter with the dried microalgae cells. The same procedure was performed twice per week over 4 weeks and the measured dry mass was used to track biomass productivity over time.
3.4 Results
At the start and end of the experiment, the data indicated stagnant or negative growth. These days were excluded from the average daily growth rate, focusing only on the periods with positive growth. As a result, the total number of experimental days used for our analysis were 25 days for Chlorella and 28 days for Chlorococcum respectively (Figure 2). The averaged daily biomass was calculated by dividing the total biomass growth by the total experimental days, expressed in mg/L-day. Daily biomass yield of Chlorella and Chlorococcum overall was 175 mg/L-day and 80 mg/L-day respectively, which were good compared to the data published in literature (Table 1).
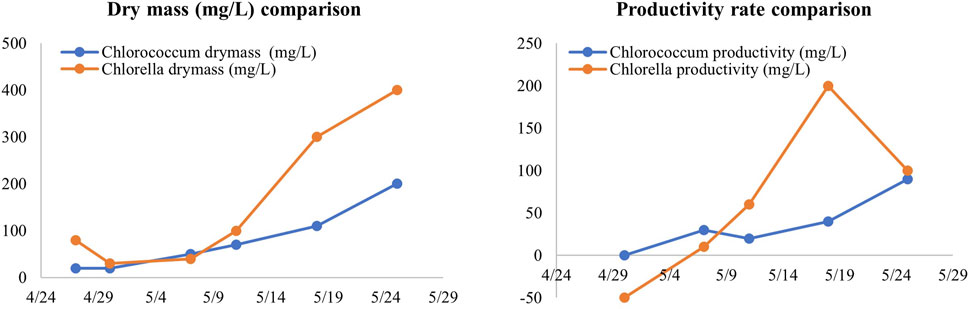
Figure 2. Dry mass (mg/L) comparisons (left) and the biomass productivity rates (mg/L/day) of Chlorococcum and Chlorella over time (right).
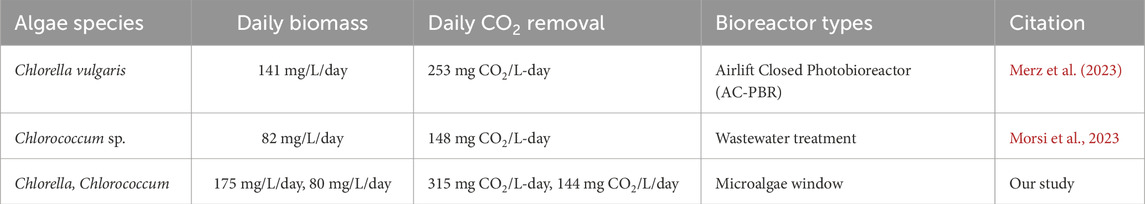
Table 1. Comparison of daily biomass yields and CO2 removal for Chlorella and Chlorococcum across different algae growing systems.
The daily yield is indicative of the efficiency of capturing and removing CO2 from the built environment. Microalgal biomass typically consists of approximately 50% carbon. This equates that a dry weight of 1 g of biomass removes 0.5 g of carbon or 1.8 g of CO2 using the molecular ratio of C (12 g/mol) and CO2 (44g/mol). Therefore, Chlorella’s daily yield (175 mg/L-day) and Chlorococcum’s daily yield (80 mg/L-day) translates into a CO2 removal rate of up to 315 mg CO2/L-day for Chlorella and 144 mg CO2/L/day for Chlorococcum. As microalgae grow, they convert atmospheric or dissolved CO2 into biomass through photosynthesis. This makes the observed growth rates directly related to their carbon removal capabilities.
The productivity rate was calculated by assessing the changes in biomass between consecutive days, expressed in milligrams per liter per day (mg/L-day). Faster productivity rates are visually represented by steeper slopes in the graphs, signifying quicker biomass accumulation. These growth rates served as indicators of whether the bioreactor provided an optimal environment for rapid cell division and overall microalgal productivity. For Chlorococcum, growth rates were observed to range from 0 mg/L/day to a peak of 13 mg/L/day. These findings highlight the distinct characteristics of the two microalgae species. Both Chlorococcum and Chlorella offered consistent carbon removal. It is important to provide good environmental conditions and cultivation practices to maximize environmental benefits. Figure 2 shows the comparison of dry mass and biomass productivity rate of Chlorococcum and Chlorella.
4 Conclusion
The study highlights the effectiveness of microalgae building enclosures in curtailing carbon emissions in the built environment. The microalgae enclosures incorporated Chlorella and Chlorococcum, allowing for the measurement of their growth and carbon sequestration performance in real-world settings. The system achieved a good carbon removal rate, leveraging the algae’s ability to convert atmospheric or dissolved CO2 into biomass via photosynthesis. The study demonstrated that Chlorella in our algae facade achieved a daily biomass yield of 175 mg/L-day while Chlorococcum reached 80 mg/L-day. These yields translate to estimated CO2 removal potentials of approximately 315 mg CO2/L-day for Chlorella and 144 mg CO2/L-day for Chlorococcum. Faster growth periods, marked by steeper slopes in biomass productivity rate graphs, indicated optimal growth environments and efficient resource utilization. The biomass productivity could be further improved by optimizing growing conditions such as light, nutrient levels, and CO2 availability.
The study also underscores the potential for microalgae-based photobioreactors not only to enhance urban carbon capture but also to provide additional benefits, such as building energy efficiency, better user experience, and biomass production for biofuels or other high-value bioproducts. Although our study focuses on measuring the carbon capture potential of microalgae-enclosed building systems, understanding the overall environmental and economic impact of the system remains crucial. In upcoming studies, we will conduct a life cycle assessment (LCA) to evaluate resource inputs such as water and nutrient medium as well as energy consumption from aeration and harvesting cycles. Alongside this, a techno-economic analysis (TEA) will be performed to assess costs pertaining to installation, operation, and maintenance against economic benefits from carbon capture, building energy savings, and biomass production. The LCA and TEA will enable us to understand the sustainability and commercial feasibility of the system at a large scale. Reflecting on these analyses, we intend to refine system designs and implementation strategies for widespread application in urban and industrial settings. In addition, future studies will include the evaluation of their long-term performance and maintenance requirements. Key aspects such as biofouling prevention, cleaning frequency, nutrient replenishment cycles, and system durability over time are essential to determine operational feasibility in algae window applications. Future research will involve multi-seasonal testing to monitor the effects of environmental fluctuations and usage patterns over extended periods. These efforts will help inform system design refinements, maintenance protocols, and lifecycle expectations to support the broader deployment of algae-integrated façades in sustainable urban infrastructure. This research highlights the multiple co-benefits of carbon removal and resource generation potential through nature-based solutions in the built environment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
KK: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review and editing. MP: Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing. PK: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Science Foundation (Award number: 2012157).
Acknowledgments
The authors extend their gratitude to Damani Lawes for his assistance with data collection and to Chengde Wu and Garrett Herbst for their contributions to the prototyping, fabrication, and operation of the algae facade.
Conflict of interest
Author KK was employed by EcoClosure.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abuhasheesh, Y., Ghazal, A., Tang, D. Y. Y., Banat, F., Hasan, S. W., and Show, P. L. (2025). Advances in Chlorella microalgae for sustainable wastewater treatment and bioproduction. Chem. Eng. J. Adv. 22, 100715. doi:10.1016/j.ceja.2025.100715
Anav, A., Friedlingstein, P., Beer, C., Ciais, P., Harper, A., Jones, C., et al. (2015). Spatiotemporal patterns of terrestrial gross primary production: a review. Rev. Geophys. 53 (3), 785–818. doi:10.1002/2015rg000483
Atwood, T. B., Connolly, R. M., Almahasheer, H., Carnell, P. E., Duarte, C. M., Ewers Lewis, C. J., et al. (2017). Global patterns in mangrove soil carbon stocks and losses. Nat. Clim. Change 7, 523–528. doi:10.1038/nclimate3326
Balesdent, J., and Balabane, M. (1996). Major contribution of roots to soil carbon storage inferred from maize cultivated soils. Soil Biol. Biochem. 28 (9), 1261–1263. doi:10.1016/0038-0717(96)00112-5
Barsanti, L., and Gualtieri, P. (2022). Algae: anatomy, biochemistry, and biotechnology. Florida, United states: CRC Press.
Benedetti, M., Vecchi, V., Barera, S., and Dall’Osto, L. (2018). Biomass from microalgae: the potential of domestication towards sustainable biofactories. Microb. Cell Factories 17 (1), 173–218. doi:10.1186/s12934-018-1019-3
Beniston, J. W., DuPont, S. T., Glover, J. D., Lal, R., and Dungait, J. A. J. (2014). Soil organic carbon dynamics 75 Years after land-use change in perennial grassland and annual wheat agricultural systems. Biogeochemistry 120, 37–49. doi:10.1007/s10533-014-9980-3
Biloria, N., and Thakkar, Y. (2020). Integrating algae building technology in the built environment: a cost and benefit perspective. Front. Archit. Res. 9 (2), 370–384. doi:10.1016/j.foar.2019.12.004
Bouillon, S., Borges, A. V., Castañeda-Moya, E., Diele, K., Dittmar, T., Duke, N. C., et al. (2008). Mangrove production and carbon sinks: a revision of global budget estimates. Glob. Biogeochem. Cycles 22 (2). doi:10.1029/2007gb003052
Çelekli, A., Yeşildağ, İ., and Zariç, Ö. E. (2024). Green building future: algal application technology. J. Sustain. Constr. Mater. Technol. 9 (2), 8–18. doi:10.47481/jscmt.1348260
Çelekli, A., and Zariç, Ö. E. (2024a). “Plasma-enhanced microalgal cultivation: a sustainable approach for biofuel and biomass production,” in Emerging applications of plasma science in allied technologies (IGI Global Scientific Publishing), 243–263.
Çelekli, A., and Zariç, Ö. E. (2024b). “Breathing life into Mars: terraforming and the pivotal role of algae in atmospheric genesis,” in Life sciences in space research.
Chen, T.-Y., Chen, J.-J., and Chou, W.-C. (2024). Rethinking blue carbon: unlocking invisible carbon sinks. Environ. Res. Lett. 19, 101001. doi:10.1088/1748-9326/ad7044
de Oliveira, K. L., da Silva Oliveira, J. L., Moraes, E. A., dos Santos Pires Cavalcante, K. M., de Oliveira, M. L. M., and Alves, C. R. (2024). Cultivation of microalgae Chlorella vulgaris, Monoraphidium sp., and Scenedesmus obliquus in wastewater from the household appliance industry for bioremediation and biofuel production. 3 Biotech. 14 (12), 294–314. doi:10.1007/s13205-024-04142-z
Dineshbabu, G., Vijayan, D., Uma, V. S., Makut, B. B., and Das, D. (2019). Microalgal systems for integrated carbon sequestration from flue gas and wastewater treatment. Appl. Microalgae Wastewater Treat. 2, 339–370. doi:10.1007/978-3-030-13909-4_15
Duarte, C. M., Middelburg, J. J., and Caraco, N. (2005). Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2, 1–8. doi:10.5194/bg-2-1-2005
Fang, J.-Y., and Guo, Z. D. (2007). Looking for missing carbon sinks from terrestrial ecosystems. Chin. J. Nat. 29 (1).
Freschet, G. T., Cornwell, W. K., Wardle, D. A., Elumeeva, T. G., Liu, W., Jackson, B. G., et al. (2013). Linking litter decomposition of above- and below-ground organs to plant–soil feedbacks worldwide. J. Ecol. 101 (4), 943–952. doi:10.1111/1365-2745.12092
Friedlingstein, P., Jones, M. W., O’Sullivan, M., Andrew, R. M., Bakker, D. C. E., Hauck, J., et al. (2022). Global carbon budget 2021. Glob. Clim. Syst. Data Prod. 14 (4), 1917–2005. doi:10.5194/essd-14-1917-2022
Girardin, C. A. J., Jenkins, S., Seddon, N., Allen, M., Lewis, S. L., Wheeler, C. E., et al. (2021). Nature-based solutions can help cool the planet — if we act now. Nature 593, 191–194. doi:10.1038/d41586-021-01241-2
IEA (International Energy Agency) (2022). Materials for construction and climate change. Available online at: https://www.iea.org.
IPCC (Intergovernmental Panel on Climate Change) (2018). Global warming of 1.5 ºC: summary for policymakers. Available online at: https://www.ipcc.ch/sr15/chapter/spm/.
Kellert, S. R. (2018). Nature by design: the practice of biophilic design. New Haven, CT: Yale University Press.
Kucharik, C. J., Brye, K. R., Norman, J. M., Foley, J. A., Gower, S. T., and Bundy, L. G. (2001). Measurements and modeling of carbon and nitrogen cycling in agroecosystems of southern Wisconsin: potential for SOC sequestration during the next 50 years. Ecosystems 4, 237–258. doi:10.1007/s10021-001-0007-2
Kupriyanova, E. V., Pronina, N. A., and Los, D. A. (2023). Adapting from low to high: an update to CO2-concentrating mechanisms of cyanobacteria and microalgae. Plants 12 (7), 1569. doi:10.3390/plants12071569
Langley, J. A., Mozdzer, T. J., Shepard, K. A., Hagerty, S. B., and Megonigal, J. P. (2013). Tidal marsh plant responses to elevated CO2, nitrogen fertilization, and sea level rise. Global Change Biology 19 (5), 1495–1503.
Lawrence Berkeley National Laboratory (2024). United States data center energy usage report. Berkeley, CA: U.S. Department of Energy. Available online at: https://eta-publications.lbl.gov/sites/default/files/2024-12/lbnl-2024-united-states-data-center-energy-usage-report.pdf.
Li, D., Li, X., Li, Z., Fu, Y., Zhang, J., Zhao, Y., et al. (2024). Drought limits vegetation carbon sequestration by affecting photosynthetic capacity of semi-arid ecosystems on the Loess Plateau. Science of the Total Environment 912.
Macreadie, P. I., Costa, M. D. P., Atwood, T. B., Friess, D. A., Kelleway, J. J., Kennedy, H., et al. (2021). Blue carbon as a natural climate solution. Nat. Rev. Earth and Environ. 2, 826–839. doi:10.1038/s43017-021-00224-1
Mahapatra, D. M., and Ramachandra, T. V. (2013). Algal biofuel: bountiful lipid from Chlorococcum sp. proliferating in municipal wastewater. Curr. Sci., 47–55.
Martokusumo, W., Koerniawan, M. D., Poerbo, H. W., Ardiani, N. A., and Krisanti, S. H. (2017). Algae and building façade revisited: a study of façade system for infill design. J. Archit. Urbanism 41 (4), 296–304. doi:10.3846/20297955.2017.1411847
Mcleod, E., Chmura, G. L., Bouillon, S., Salm, R., Björk, M., Duarte, C. M., et al. (2011). A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Frontiers in Ecology and the Environment 9 (10), 552–560.
Merz, C. R., Arora, N., Welch, M., Lo, E., and Philippidis, G. P. (2023). Microalgal cultivation characteristics using commercially available air-cushion packaging material as a photobioreactor. Sci. Rep. 13 (1), 3792. doi:10.1038/s41598-023-30080-6
Morsi, H. H., El-Sheekh, M. M., Eladel, H., Al-Tuwaijri, M. M., El-Sabbagh, S. M., Maher, A., et al. (2023). Screening the pollution-tolerant Chlorococcum sp. grown in municipal wastewater for simultaneous nutrient removal and biodiesel production. Water 15 (9), 1723. doi:10.3390/w15091723
Nellemann, C. (2009). Blue carbon: a unep rapid response assessment. United Nations Environment Programme.
Poerbo, H. W., Martokusumo, W., Koerniawan, M. D., Ardiani, N. A., and Krisanti, S. (2017). Algae façade as green building method: application of algae as a method to meet the green building regulation. IOP Conf. Ser. Earth Environ. Sci. 99 (1), 012012. doi:10.1088/1755-1315/99/1/012012
Poffenbarger, H. J., Needelman, B. A., and Megonigal, J. P. (2011). Salinity Influence on Methane Emissions from Tidal Marshes. Wetlands 31, 831–842.
Poorter, H., Jagodzinski, A. M., Ruiz-Peinado, R., Kuyah, S., Luo, Y., Oleksyn, J., et al. (2015). How does biomass distribution change with size and differ among species? An analysis for 1200 plant species from five continents. New Phytologist 208 (3), 736–749.
Prentice, I. C., Balzarolo, M., Bloomfield, K. J., Chen, J. M., Dechant, B., Ghent, D., et al. (2024). Principles for satellite monitoring of vegetation carbon uptake. Nat. Rev. Earth and Environ. 5, 818–832. doi:10.1038/s43017-024-00601-6
Rogers, K., Kelleway, J. J., Saintilan, N., Megonigal, J. P., Adams, J. B., Holmquist, J. R., et al. (2019). Wetland carbon storage controlled by millennial-scale variation in relative sea-level rise. Nature 567, 91–95.
Saintilan, N., Rogers, K., Mazumder, D., and Woodroffe, C. (2013). Allochthonous and autochthonous contributions to carbon accumulation and carbon store in southeastern Australian coastal wetlands. Estuarine, Coastal and Shelf Science 128, 84–92.
Schlesinger, W. H., and Andrews, J. A. (2000). Soil respiration and the global carbon cycle. Biogeochemistry 48, 7–20. doi:10.1023/a:1006247623877
Sedighi, M., Pourmoghaddam Qhazvini, P., and Amidpour, M. (2023). Algae-powered buildings: a review of an innovative, sustainable approach in the built environment. Sustainability 15 (4), 3729. doi:10.3390/su15043729
Serrano, O., Kelleway, J. J., Lovelock, C., and Lavery, P. S. (2019). Chapter 28 - conservation of blue carbon ecosystems for climate change mitigation and adaptation. Coastal Wetlands (Second Edition), Elsevier, 965–996.
Tripathi, S., Choudhary, S., Meena, A., and Poluri, K. M. (2023). Carbon capture, storage, and usage with microalgae: a review. Environ. Chem. Lett. 21 (4), 2085–2128. doi:10.1007/s10311-023-01609-y
U.S. Department of Energy (2023). DOE announces $40 million for more efficient cooling in data centers. Available online at: https://www.energy.gov/articles/doe-announces-40-million-more-efficient-cooling-data-centers.
Wiesmeier, M., Munro, S., Barthold, F., Steffens, M., Schad, P., and Kögel-Knabner, I. (2015). Carbon storage capacity of semi-arid grassland soils and sequestration potentials in northern China. Global Change Biology 21 (10), 3836–3845.
Wilkinson, S., Stoller, P., Ralph, P., Hamdorf, B., Catana, L. N., and Kuzava, G. S. (2017). Exploring the feasibility of algae building technology in NSW. Procedia Eng. 180, 1121–1130. doi:10.1016/j.proeng.2017.04.272
Yuan, X., Chen, X., Ochege, F. U., Hamdi, R., Tabari, H., Li, B., et al. (2025). Weakening of global terrestrial carbon sequestration capacity under increasing intensity of warm extremes. Nat. Ecol. and Evol. 9, 124–133. doi:10.1038/s41559-024-02576-5
Keywords: microalgae integrated building enclosures, carbon sequestration, regenerative resources, climate mitigation, resilient buildings and cities
Citation: Kim KH, Parrow MW and Kheirkhah Sangdeh P (2025) Microalgae-integrated building enclosures: a nature-based solution for carbon sequestration. Front. Built Environ. 11:1574582. doi: 10.3389/fbuil.2025.1574582
Received: 17 February 2025; Accepted: 23 April 2025;
Published: 26 May 2025.
Edited by:
Roberto Alonso González-Lezcano, CEU San Pablo University, SpainReviewed by:
Özgür Eren Zariç, University of Gaziantep, TürkiyeVitta Ibrahim, Pyramids Higher Institute for Engineering and Technology, Egypt
Copyright © 2025 Kim, Parrow and Kheirkhah Sangdeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyoung Hee Kim, a2tpbTMzQGNoYXJsb3R0ZS5lZHU=
 Kyoung Hee Kim
Kyoung Hee Kim Matthew W. Parrow
Matthew W. Parrow Parham Kheirkhah Sangdeh
Parham Kheirkhah Sangdeh