- 1Department of Neurology, Oslo University Hospital, Rikshospitalet, Oslo, Norway
- 2Letten Centre and GliaLab, Division of Anatomy, Department of Molecular Medicine, Institute of Basic Medical Sciences, University of Oslo, Oslo, Norway
Epilepsy is one of the most common neurological disorders – estimated to affect at least 65 million worldwide. Most of the epilepsy research has so far focused on how to dampen neuronal discharges and to explain how changes in intrinsic neuronal activity or network function cause seizures. As a result, pharmacological therapy has largely been limited to symptomatic treatment targeted at neurons. Given the expanding spectrum of functions ascribed to the non-neuronal constituents of the brain, in both physiological brain function and in brain disorders, it is natural to closely consider the roles of astrocytes in epilepsy. It is now widely accepted that astrocytes are key controllers of the composition of the extracellular fluids, and may directly interact with neurons by releasing gliotransmitters. A central tenet is that astrocytic intracellular Ca2+ signals promote release of such signaling substances, either through synaptic or non-synaptic mechanisms. Accruing evidence suggests that astrocytic Ca2+ signals play important roles in both seizures and epilepsy, and this review aims to highlight the current knowledge of the roles of this central astrocytic signaling mechanism in ictogenesis and epileptogenesis.
Introduction
Epilepsy is one of the most common neurological disorders – estimated to affect around 1% of the world’s population (Hesdorffer et al., 2011; Neligan et al., 2012; Beghi, 2016). It is a chronic disorder, characterized by sudden, violent perturbations of normal brain function, causing social stigma, morbidity, and risk of premature death. In spite of a multitude of drugs for the treatment of epilepsy, about 30% of patients are not able to control their seizures with seizure suppressing medication (French, 2007; Perucca and Gilliam, 2012).
There is a striking lack of knowledge of the pathophysiological cellular mechanisms at play in epilepsy. For instance, the process transforming normal brain matter to a focus for epileptic seizures – the process of epileptogenesis – is not well understood. Also, the central question of what sets in motion an epileptic seizure – ictogenesis – remains unanswered. Most of the epilepsy research has so far focused on how to dampen neuronal discharges and to explain how changes in intrinsic neuronal activity or neuronal network function cause seizures. As a result, pharmacological therapy has been limited to symptomatic treatment aiming at neuronal targets. Given the expanding spectrum of roles ascribed to the non-neuronal constituents of the brain, it is natural to take a closer look at astrocytes as potential targets for epilepsy treatment.
Astrocytes are critical homeostatic controllers of extracellular glutamate and K+ levels (Rothstein et al., 1996; Larsen et al., 2014; Danbolt et al., 2016). Numerous studies have also demonstrated that astrocytes have important roles in supporting the neurons metabolically (Pellerin and Magistretti, 1994; Lundgaard et al., 2015) and that they have the capability of altering the vascular tone (Mulligan and MacVicar, 2004; Haydon and Carmignoto, 2006; Gordon et al., 2008). Increasing evidence suggests that astrocytes play important roles in brain state transitions and maintenance (Paukert et al., 2014; Poskanzer and Yuste, 2016; Szabó et al., 2017; Bojarskaite et al., 2020). Notably, astrocytes seem to also directly partake in brain signaling by releasing substances that affect neurons at the so-called tripartite synapse (Perea et al., 2009; Bindocci et al., 2017; Martin-Fernandez et al., 2017). A central tenet is that astroglial intracellular Ca2+ signals promote such “gliotransmitter” release, either through synaptic or non-synaptic mechanisms (Perea et al., 2014; Bazargani and Attwell, 2016). Glutamate, purines and D-serine are examples of transmitter substances that are thought to be released from astrocytes in a Ca2+ dependent manner (ibid.).
Perturbation of astrocytic Ca2+ signaling has been demonstrated in seizures and in epileptic tissue, potentially affecting both the homeostatic functions and signaling functions of astrocytes. These downstream mechanisms are largely speculative in the context of epilepsy but reflect the knowledge of roles of astrocytic Ca2+ signaling in physiology. Here, we discuss the relatively limited body of studies directly assessing astrocytic Ca2+ signaling in epilepsy, and briefly discuss potential downstream effects (Table 1). For the sake of structure and simplification, we arrange the topic into paragraphs on ictogenesis (i.e., the emergence of seizure activity), and epileptogenesis (i.e., the process by which the brain develops the predisposition of generating spontaneous seizures). These two processes are highly interconnected (Blauwblomme et al., 2014), but animal studies are often designed to study one of these two facets of epilepsy, and hence provide a framework for the further discussion.
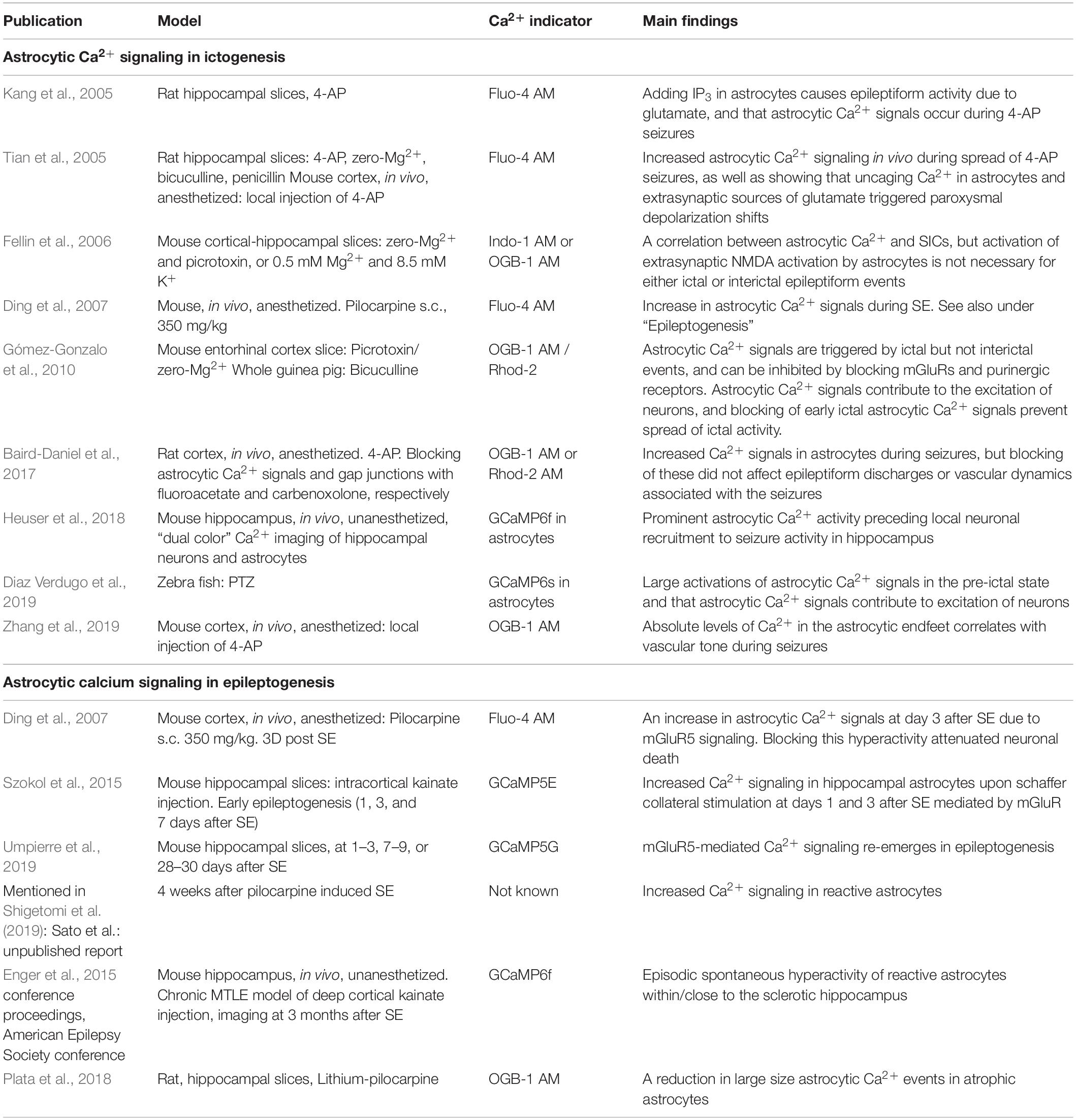
Table 1. Key publications investigating the roles of astrocytic Ca2 + signalling in ictogenesis and epileptogenesis.
Astrocytic Ca2+ Signaling and Ictogenesis
Ictogenesis describes the emergence of seizure activity (Blauwblomme et al., 2014). The interaction between astrocytes and neurons in ictogenesis has only sparsely been investigated and findings are to some extent ambiguous or contradictory, potentially due to different experimental models (Table 1; Tian et al., 2005; Fellin et al., 2006; Gómez-Gonzalo et al., 2010; Baird-Daniel et al., 2017; Heuser et al., 2018; Diaz Verdugo et al., 2019). Astrocytes express a plethora of functionally important receptors, transporters and channels, and a role of these cells in ictogenesis is highly suggestive (Agulhon et al., 2008; Patel et al., 2019; Caudal et al., 2020). Several known astrocyte-neuron interactions involving Ca2+ signaling can partake in ictogenesis or in the maintenance of hypersynchronous neuronal activity, possibly by creating excitatory feedback loops (Figure 1; Gómez-Gonzalo et al., 2010; Henneberger, 2017).
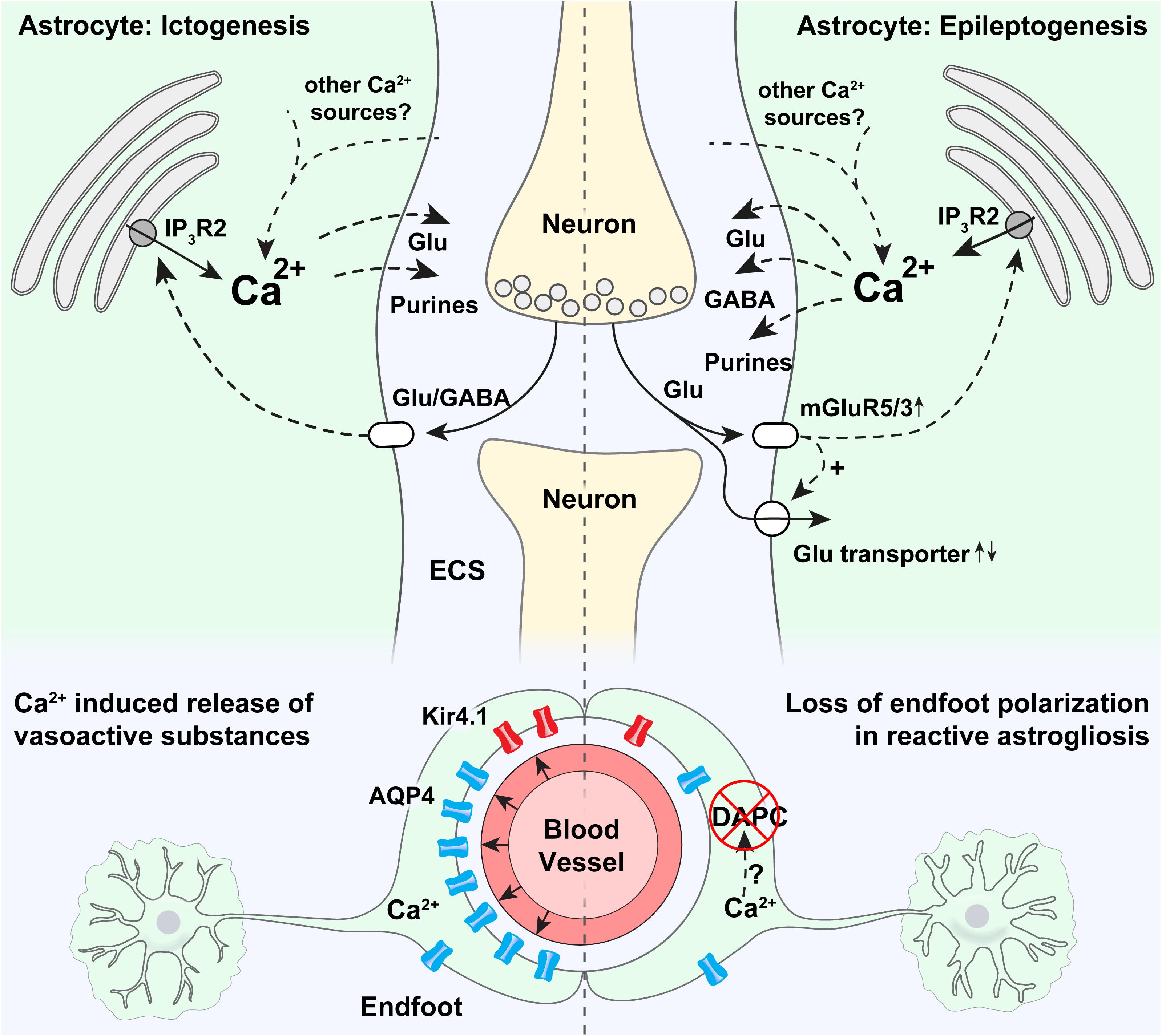
Figure 1. Potential roles of astrocytic Ca2+ signaling in epilepsy. Strong astrocytic Ca2+ signals have been shown to occur in the emergency of acute seizures (in ictogenesis), that are probably triggered by neurotransmitters released by neurons. Ca2+ increases at the onset of seizures are known to be partly mediated by release through IP3R2 from the endoplasmic reticulum, even though pronounced Ca2+ signaling is present also in mice devoid of IP3R2. It is thought that intracellular Ca2+ increases may trigger proconvulsive gliotransmitter release. In astrocytic endfeet, increased Ca2+ signaling has been shown to correlate with ictal vasodilation. Epileptogenesis triggers a pronounced increase in mGluR5 expression, mGluR5-mediated Ca2+ signaling, and increased glutamate uptake. An increase in astrocytic Ca2+ signaling has been demonstrated in the days after status epilepticus, and aberrant Ca2+ signaling at later time points in the epileptogenesis has been anecdotally reported. Increased Ca2+ signaling could potentially cause both the release of glutamate (pro-convulsive), purines (pro-convulsive), and GABA (anti-convulsive, through Bestrophin-1 channels). In astrocytic endfeet in epileptic tissue a pronounced loss of aquaporin-4 (AQP4) and the K+ inwardly rectifying channel Kir4.1 can potentially be due to Ca2+ activated proteases causing a disassembly of the dystrophin associated protein complex (DAPC) tethering AQP4 and Kir4.1 to perivascular endfeet.
Building upon seminal studies demonstrating that astrocytes are able to directly interact with neurons (Nedergaard, 1994; Parpura et al., 1994; Araque et al., 1998; Parpura and Haydon, 2000; Parri et al., 2001; Angulo et al., 2004), Fellin et al. (2006), found that eliciting astrocytic Ca2+ signals by photolysis of caged Ca2 + and by application of ATP agonist and mGluR5 agonist triggered slow inward currents (SICs) in nearby neurons that were unaffected by application of the neuronal sodium channel blocker tetrodotoxin (Fellin et al., 2004). Soon thereafter, Tian et al. (2005) demonstrated that Ca2+ mediated glutamate release from astrocytes during experimentally induced seizure activity triggered slow inward currents (SICs) in neurons. These findings proposed a role for astrocytes in synchronizing neuronal activity and contributing to seizure generation (Tian et al., 2005). Further exploring which astrocytic Ca2+ signaling mechanisms were involved in this context, Kang et al. applied IP3 into astrocytes of the CA1 hippocampal region in rats, and were able to trigger epileptiform discharges in adjacent neurons (Kang et al., 2005). Later, Ding et al. (2007) were able to demonstrate increased astrocytic Ca2+ signaling in an in vivo pilocarpine epilepsy model. They proposed that this increase in Ca2+ signaling was due to activation of astrocytic metabotropic glutamate receptors, and that this activation led to the release of glutamate from astrocytes that could contribute to neuronal SICs through the activation of extrasynaptic neuronal NMDA receptors. By applying simultaneous patch-clamp recordings and Ca2+ imaging in cortical slices of the rat entorhinal cortex, Gómez-Gonzalo et al. (2010) found that Ca2+ elevations in astrocytes correlate with initiation and maintenance of focal seizure-like discharges, and postulated a recurrent excitatory loop between neurons and astrocytes in ictogenesis, where astrocytes play a role in recruiting neurons to ictal events, possibly through the release of gliotransmitters (Gómez-Gonzalo et al., 2010).
By using two-photon microscopy and simultaneous astrocyte and neuron Ca2+ imaging in the hippocampal CA1 region of awake mice, we were able to show that prominent astrocytic Ca2+ transients preceded local hypersynchronous neuronal activity in the emergence of kainate induced generalized epileptic seizures (Heuser et al., 2018). These findings were in agreement with the earlier results from the study of Tian et al. (2005), who also observed stereotypical astrocytic Ca2+ signals typically preceding local neurons in the spread of cortical seizure activity. A later work by Diaz Verdugo et al. (2019) similarly demonstrated large and synchronized astrocytic Ca2+ signals preceding ictal onset in zebrafish, and proposed that this signaling modulated neural excitation through glutamate release, by gap junction dependent mechanisms. In another in vivo study, Zhang et al. (2019), provided evidence, although correlative, that increased Ca2+ concentration in astrocytic endfeet governed precapillary arteriole dilation during epileptic events, suggesting a role for astrocytes in the metabolic support of neurons in seizures. In contrast to these previously mentioned studies, data from another model for focal neocortical seizures in anesthetized rats using bulk-loaded synthetic Ca2+ indicators found the astrocytic Ca2+ activation to lag behind neuronal activation and to be unnecessary for ictogenesis and the accompanying vascular dynamics (Baird-Daniel et al., 2017).
An extensive array of stimuli and corresponding signaling pathways have been shown to trigger intracellular Ca2+ signals in astrocytes (Zhang et al., 2019; Caudal et al., 2020). To discuss all of them would go beyond the scope of this review. One important pathway is mediated by the Inositol 1,4,5-trisphosphate (IP3) receptor in the endoplasmic reticulum, of which the isoform 2 (IP3R2) is thought to be the key functional IP3 receptor in astrocytes (Figure 1; Sharp et al., 1999; Parri and Crunelli, 2003; Volterra and Steinhäuser, 2004; Scemes and Giaume, 2006; Foskett et al., 2007). Lack of IP3R2 has been shown to abolish a large proportion of astrocytic Ca2+ signals (Petravicz et al., 2008; Guerra-Gomes et al., 2020). In spite of the importance of IP3 as a second messenger involved in astrocytic Ca2+ dynamics, mice lacking this receptor are overtly normal (Petravicz et al., 2008). Accordingly, studies have questioned the physiological importance of IP3-mediated astrocytic Ca2+ signaling, by for instance demonstrating normal synaptic transmission and plasticity in mice devoid of IP3R2 (Agulhon et al., 2010; Nizar et al., 2013; Petravicz et al., 2014). Conversely, we have demonstrated attenuated seizure activity in mice devoid of IP3R2 compared to WT mice following low dose intraperitoneal kainate, suggesting a proconvulsant role of astrocytic IP3R2 mediated Ca2+ elevations (Heuser et al., 2018). However, seizure activity in this study was only collected for 1 h after initiation of seizures, encouraging further investigation of the role of IP3R2 at later time points during epileptogenesis and in chronic epilepsy. Interestingly, even though a sizable amount of Ca2+ signals were still present in the knockout mice, we found that the early activation of astrocytic Ca2+ signals in the emergence of seizures, as discussed above, was dependent on IP3R2 (Heuser et al., 2018). These two observations underscore the potential importance of IP3R2 in ictogenesis.
Another pathway involved in astrocytic Ca2+ signaling attracting increasing attention for a role in epilepsy is glial purinergic signaling (Ding et al., 2007; Wellmann et al., 2018; Alves et al., 2019; Nikolic et al., 2020). Activation of astrocytic purinergic receptors triggers intracellular Ca2+ signals that could promote astrocytic release of gliotransmitters like glutamate or ATP, which acts on neurons and modulates excitation [reviewed in Nikolic et al. (2020)]. Importantly, Nikolic et al. (2018) provided evidence for TNFα-driven autocrine astrocyte purinergic signaling as a trigger of glutamatergic gliotransmission in a model of mesial temporal lobe epilepsy (mTLE), highlighting the complex interplay between astrocytes and microglia in epilepsy pathogenesis, discussed elsewhere (Bedner and Steinhäuser, 2019).
Most of the studies above explored the role for astrocytic Ca2+ signals in seizures in relation to gliotransmission, i.e., that astrocytes release transmitters that directly signal to neurons. A growing body of evidence suggests that astrocytic Ca2+ signals also play important roles in the control of the homeostatic functions of astrocytes. For instance they have been shown to be involved in the uptake of extracellular K+ through modulation of the Na+/K+ ATPase, and through the breakdown of glycogen (Wang et al., 2012; Müller et al., 2014). These mechanisms remain poorly explored in the context of epilepsy but could be important downstream effects of astrocytic Ca2+ signaling.
Astrocytic Ca2+ Signaling and Epileptogenesis
Epileptogenesis refers to the gradual process by which a normal brain develops a propensity for recurrent seizure activity. A range of pathophysiological changes have been shown to occur during epileptogenesis, including inflammation, neurodegeneration, aberrant neurogenesis and dendritic plasticity, impaired blood-brain-barrier, epigenetic changes and alterations of the molecular composition and function of ion channels, receptors and transporters, and more (van Vliet et al., 2007; Vezzani et al., 2011; Steinhäuser and Seifert, 2012; Dingledine et al., 2014; Jessberger and Parent, 2015; Hauser et al., 2018; Escartin et al., 2021).
A common denominator of astrocytic pathophysiology associated with epileptogenesis is the process of reactive astrogliosis (Burda and Sofroniew, 2014; Pekny and Pekna, 2016). This is a graded response to a wide array of insults, which is a hallmark of many neurological disorders (Burda and Sofroniew, 2014; Ferlazzo et al., 2016; Glushakov et al., 2016; Pekny and Pekna, 2016; Fordington and Manford, 2020; Galovic et al., 2021).
Reactive astrocytes are characterized by morphological and molecular changes (Figure 1). Specifically they proliferate, undergo hypertrophy and increase their expression of intermediary filament proteins like glial fibrillary acid protein (GFAP) and vimentin (Yang et al., 1994; Pekny and Nilsson, 2005; Sofroniew, 2009; Cregg et al., 2014; Escartin et al., 2021). In extremis, these changes may lead to the formation of a glial scar (Miller, 2005; Barres, 2008; Sofroniew, 2009; Burda and Sofroniew, 2014; Ferlazzo et al., 2016; Glushakov et al., 2016; Pekny and Pekna, 2016; Fordington and Manford, 2020; Galovic et al., 2021). Reactive astrogliosis can be observed in several acquired forms of epilepsy but has mostly been investigated in the context of mTLE (Wieser and ILAE Commission on Neurosurgery of Epilepsy., 2004; Blümcke et al., 2013; Cendes et al., 2014).
There is ample evidence that reactive astrocytes display aberrant Ca2+ signaling at least in the early phase of epileptogenesis (Table 1). Ding et al. (2007) found increased astrocytic Ca2+ activity in the days following pilocarpine-induced SE in mice. In the same study both in vitro and in vivo pharmacological approaches demonstrated that these Ca2+ signals could contribute to neuronal death, linking astrocytic hyperactivity to a key hallmark of epileptogenesis (Ding et al., 2007). We confirmed the astrocytic hyperactivity following SE by employing genetically encoded Ca2+ indicators in acute hippocampal slices from a mouse model of mTLE, and found that stimulation-evoked Ca2+ transients in astrocytic endfeet even outlasted those in cell bodies during the latent phase of epileptogenesis (Szokol et al., 2015).
Increased astrocytic Ca2+ activity has been anecdotally reported at even later time points after the initial insult (Enger et al., 2015; Shigetomi et al., 2019). These increased Ca2+ signals are likely stimuli- and stage specific and may reflect the degree of the reactive astrogliosis (Kuchibhotla et al., 2009; Fordsmann et al., 2019), as others have shown attenuated astrocytic Ca2+ activity in atrophic astrocytes in chronic epilepsy (Plata et al., 2018).
The degree, development and underlying mechanisms involved in aberrant Ca2+ signaling in epileptogenesis are still unknown, but it is plausible that several of the physiological signaling pathways involved in astrocytic Ca2+ dynamics (Caudal et al., 2020), could be perturbed. A major pathway for eliciting astrocytic Ca2+ signals is the activation of the Gq G-protein coupled receptors (GqPCRs) and subsequent release of Ca2+ from the endoplasmic reticulum via IP3R2 as discussed in “Astrocyte Ca2 + signaling and Ictogenesis” (Figure 1; Foskett et al., 2007). Astrocytes express several GqPCRs, of which mGluR5 has attracted most attention due to an upregulation in epileptic tissue and potential involvement in an excitatory loop comprising glutamate induced Ca2+ dependent glutamate release from astrocytes (Umpierre et al., 2019). While astrocytes in the adult brain are almost depleted of mGluR5 (Sun et al., 2013), the receptor is consistently expressed in chronic epilepsy models and resected tissue from patients with epilepsy (Aronica et al., 2000, 2003), and a recent study has shown that mGluR5 expression and mGluR5-dependent Ca2+ transients reemerge during epileptogenesis along with an increase in glutamate uptake (Umpierre et al., 2019). This reemergence of astrocytic mGluR5 could potentially be a compensatory anti-epileptic mechanism to handle the elevated glutamate levels in epileptic tissue but could possibly also represent a pro-epileptic feature triggering downstream Ca2+ mediated gliotransmission.
Apart from these perturbations in glutamate dynamics, it has been shown that reactive astrocytes exhibit a tonic release of GABA, presumably through Bestrophin-1 channels (Pandit et al., 2020). Bestrophin-1 channels are Ca2+ activated anion channels, and increased GABA release could hence be a downstream effect of increased Ca2+ signaling in reactive astrocytes (Lee et al., 2010). In support of this conjecture is the finding of an accumulation of GABA in reactive astrocytes in a model of mTLE (Müller et al., 2020). Potentially, this is a protective aspect of reactive astrocytes to curb epileptiform activity in this pathological tissue.
Moreover, as mentioned in “Ictogenesis” astrocytic Ca2+ signaling has been suggested to be involved in homeostatic mechanisms of astrocytes. These mechanisms could be important downstream effects of astrocytic Ca2+ dyshomeostasis in epileptic tissue, but these effects are so far rudimentarily investigated in epilepsy.
Loss of astrocytic gap junction coupling has been shown to occur during early epileptogenesis in experimental models of mTLE and in specimens of resected hippocampi from patients with mTLE (Bedner et al., 2015; Deshpande et al., 2017, 2020; Henning et al., 2021). It is believed that this loss of astrocytic coupling in epilepsy may perturb the ability of astrocytes to remove K+ from the extracellular space through the process of K+ spatial buffering (Nwaobi et al., 2016). Notably, astrocytic gap junctions may also allow Ca2+ signals to propagate from cell to cell, at least during pathological conditions like seizure activity (Scemes and Giaume, 2006). It is tempting to hypothesize that such propagating Ca2+ waves could play a role in neuronal synchronization and seizure generation. Potentially a loss of astrocytic gap junctions as seen in epileptic tissue, may be a compensatory mechanism to prevent intercellular spread of astrocytic Ca2+ waves. Even so, to the best of our knowledge, no direct study of astrocytic Ca2+ signaling in gap junction deficient mice has been performed.
Loss of the highly concentrated expression of key membrane channels in astrocytic endfoot processes, i.e., loss of astrocyte polarization, is another pathological hallmark, which could be a consequence of perturbed glial Ca2+ dynamics (Figure 1). For instance AQP4 and Kir4.1 are normally densely expressed in astrocytic endfeet, kept in place by the so-called dystrophin associated protein complex (DAPC) (Nagelhus et al., 1998; Enger et al., 2012), and in tissue resectates from patients with mTLE, a striking loss of this polarized expression of both AQP4 and Kir4.1 have been shown (Eid et al., 2005; Heuser et al., 2012). It is possible that prolonged epileptic activity and increased Ca2+ signaling in astrocytic endfeet, as we demonstrated in Szokol et al. (2015), activate Ca2+ dependent proteases like calpain (Nagelhus and Ottersen, 2013), that shows affinity to dystrophin and could cleave the DAPC (Figure 1; Shields et al., 2000).
Even though the evidence is indirect, it has been suggested that this loss of astrocyte endfoot polarization could contribute to epileptogenesis and hyperexcitation (Binder et al., 2012; Binder and Carson, 2013; Crunelli et al., 2015). Notably, the loss of the astrocyte endfoot Kir4.1 channels in tissue from mTLE patients (Heuser et al., 2012) is expected to cause impaired K+ handling and resultant neuronal hyperexcitation due to the role of Kir4.1 in K+ homeostasis (Bordey and Sontheimer, 1998; Hinterkeuser et al., 2000; Kivi et al., 2000; Neusch et al., 2001; Djukic et al., 2007; Bockenhauer et al., 2009; Scholl et al., 2009; Steinhäuser et al., 2012).
Conclusion and Future Perspectives
Here we have discussed the role of astrocyte Ca2+ signaling in ictogenesis and epileptogenesis. These terms are used to describe two different features of epilepsy, but do not necessarily imply two separate processes, as mechanisms crucial in ictogenesis could also be an integral part of epileptogenesis, or vice versa. While we often associate astrocytic dysfunction in epileptogenesis with the appearance of reactive astrogliosis (Escartin et al., 2021), the term ictogenesis seems typically to be used when studying the interplay between neurons and astrocytes independent of pre-existing tissue pathology. Therefore, we may overlook the fact that ictogenesis most often would occur in tissue that has undergone pathological transformation typical for epileptogenesis, i.e., not normal, healthy tissue. On the other hand, epileptogenesis comprises many pathological changes beyond reactive astrogliosis, like alterations in transcriptional regulation, morphological, biochemical, metabolic and physiological remodeling ultimately resulting in gain or loss of function (Escartin et al., 2021).
Astrocytic Ca2+ signals are today considered a main readout of astrocytic activity and there are reasons to believe that they play important roles in epilepsy. Evidence suggests that such signals are neither necessary nor sufficient to maintain epileptiform activity, but rather should be seen as modulators of the pathophysiological process. The literature directly investigating the role of astrocytic Ca2+ signaling in epilepsy is still sparse and at some points contradictory, and for most proposed mechanisms only a small subset of the signaling pathways involved are identified. A major challenge will be to disentangle the potentially beneficial from detrimental consequences of the different modes of astrocyte Ca2+ signaling in reactive astrogliosis. It is even probable that astrocyte Ca2+ signaling may carry different roles in the large variety of epileptic entities. To decipher the roles of astrocyte Ca2+ signaling in epilepsy, next steps should include a rigorous study of the mechanisms mentioned above in vivo in adult mice, leveraging new developments in both imaging and genetics, with the aim of identifying promising targets for future pharmacological therapy of epilepsy.
Author Contributions
KH and RE reviewed the literature, conceptualized the manuscript, and wrote the manuscript. Both authors contributed to the article and approved the submitted version.
Funding
KH has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement no. 722053.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the Department of Neurology, Oslo University Hospital, and especially Erik Taubøll, leader of the Epilepsy Research Group, Oslo, https://www.ous-research.no/ergo/, for constant support. We would also like to thank all members of the EU Glia Ph.D. Consortium, http://www.eu-gliaphd.eu for long-lasting friendship and discussions on glial pathology in epilepsy, and the Letten Foundation.
References
Agulhon, C., Fiacco, T. A., and McCarthy, K. D. (2010). Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2 signaling. Science 327, 1250–1254. doi: 10.1126/science.1184821
Agulhon, C., Petravicz, J., McMullen, A. B., Sweger, E. J., Minton, S. K., Taves, S. R., et al. (2008). What is the role of astrocyte calcium in neurophysiology? Neuron 59, 932–946. doi: 10.1016/j.neuron.2008.09.004
Alves, M., De Diego Garcia, L., Conte, G., Jimenez-Mateos, E. M., D’Orsi, B., Sanz-Rodriguez, A., et al. (2019). Context-specific switch from anti- to pro-epileptogenic function of the P2Y receptor in experimental epilepsy. J. Neurosci. 39, 5377–5392. doi: 10.1523/jneurosci.0089-19.2019
Angulo, M. C., Kozlov, A. S., Charpak, S., and Audinat, E. (2004). Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J. Neurosci. 24, 6920–6927. doi: 10.1523/jneurosci.0473-04.2004
Araque, A., Sanzgiri, R. P., Parpura, V., and Haydon, P. G. (1998). Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J. Neurosci. 18, 6822–6829. doi: 10.1523/jneurosci.18-17-06822.1998
Aronica, E., Gorter, J. A., Jansen, G. H., van Veelen, C. W. M., van Rijen, P. C., Ramkema, M., et al. (2003). Expression and cell distribution of group I and group II metabotropic glutamate receptor subtypes in taylor-type focal cortical dysplasia. Epilepsia 44, 785–795. doi: 10.1046/j.1528-1157.2003.54802.x
Aronica, E., van Vliet, E. A., Mayboroda, O. A., Troost, D., da Silva, F. H., and Gorter, J. A. (2000). Upregulation of metabotropic glutamate receptor subtype mGluR3 and mGluR5 in reactive astrocytes in a rat model of mesial temporal lobe epilepsy. Eur. J. Neurosci. 12, 2333–2344. doi: 10.1046/j.1460-9568.2000.00131.x
Baird-Daniel, E., Daniel, A. G. S., Wenzel, M., Li, D., Liou, J.-Y., Laffont, P., et al. (2017). Glial calcium waves are triggered by seizure activity and not essential for initiating ictal onset or neurovascular coupling. Cereb. Cortex 27, 3318–3330. doi: 10.1093/cercor/bhx072
Barres, B. A. (2008). The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60, 430–440. doi: 10.1016/j.neuron.2008.10.013
Bazargani, N., and Attwell, D. (2016). Astrocyte calcium signaling: the third wave. Nat. Neurosci. 19, 182–189. doi: 10.1038/nn.4201
Bedner, P., Dupper, A., Hüttmann, K., Müller, J., Herde, M. K., Dublin, P., et al. (2015). Astrocyte uncoupling as a cause of human temporal lobe epilepsy. Brain 138, 1208–1222. doi: 10.1093/brain/awv067
Bedner, P., and Steinhäuser, C. (2019). TNFα-driven astrocyte purinergic signaling during epileptogenesis. Trends Mol. Med. 25, 70–72. doi: 10.1016/j.molmed.2018.12.001
Beghi, E. (2016). Addressing the burden of epilepsy: many unmet needs. Pharmacol. Res. 107, 79–84. doi: 10.1016/j.phrs.2016.03.003
Binder, D. K., and Carson, M. J. (2013). Glial cells as primary therapeutic targets for epilepsy. Neurochem. Int. 63, 635–637. doi: 10.1016/j.neuint.2013.09.004
Binder, D. K., Nagelhus, E. A., and Ottersen, O. P. (2012). Aquaporin-4 and epilepsy. Glia 60, 1203–1214. doi: 10.1002/glia.22317
Bindocci, E., Savtchouk, I., Liaudet, N., Becker, D., Carriero, G., and Volterra, A. (2017). Three-dimensional Ca imaging advances understanding of astrocyte biology. Science 356:eaai8185. doi: 10.1126/science.aai8185
Blauwblomme, T., Jiruska, P., and Huberfeld, G. (2014). Mechanisms of ictogenesis. Int. Rev. Neurobiol. 114, 155–185. doi: 10.1016/b978-0-12-418693-4.00007-8
Blümcke, I., Thom, M., Aronica, E., Armstrong, D. D., Bartolomei, F., Bernasconi, A., et al. (2013). International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a Task Force report from the ILAE commission on diagnostic methods. Epilepsia 54, 1315–1329. doi: 10.1111/epi.12220
Bockenhauer, D., Feather, S., Stanescu, H. C., Bandulik, S., Zdebik, A. A., Reichold, M., et al. (2009). Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N. Engl. J. Med. 360, 1960–1970.
Bojarskaite, L., Bjørnstad, D. M., Pettersen, K. H., Cunen, C., Hermansen, G. H., Åbjørsbråten, K. S., et al. (2020). Astrocytic Ca signaling is reduced during sleep and is involved in the regulation of slow wave sleep. Nat. Commun. 11:3240.
Bordey, A., and Sontheimer, H. (1998). Properties of human glial cells associated with epileptic seizure foci. Epilepsy Res. 32, 286–303. doi: 10.1016/s0920-1211(98)00059-x
Burda, J. E., and Sofroniew, M. V. (2014). Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81, 229–248. doi: 10.1016/j.neuron.2013.12.034
Caudal, L. C., Gobbo, D., Scheller, A., and Kirchhoff, F. (2020). The paradox of astroglial Ca2+ signals at the interface of excitation and inhibition. Front. Cell. Neurosci. 14:609947. doi: 10.3389/fncel.2020.609947
Cendes, F., Sakamoto, A. C., Spreafico, R., Bingaman, W., and Becker, A. J. (2014). Epilepsies associated with hippocampal sclerosis. Acta Neuropathol. 128, 21–37. doi: 10.1007/s00401-014-1292-0
Cregg, J. M., DePaul, M. A., Filous, A. R., Lang, B. T., Tran, A., and Silver, J. (2014). Functional regeneration beyond the glial scar. Exp. Neurol. 253, 197–207. doi: 10.1016/j.expneurol.2013.12.024
Crunelli, V., Carmignoto, G., and Steinhäuser, C. (2015). Novel astrocyte targets: new avenues for the therapeutic treatment of epilepsy. Neuroscientist 21, 62–83. doi: 10.1177/1073858414523320
Danbolt, N. C., Furness, D. N., and Zhou, Y. (2016). Neuronal vs glial glutamate uptake: resolving the conundrum. Neurochem. Int. 98, 29–45. doi: 10.1016/j.neuint.2016.05.009
Deshpande, T., Li, T., Henning, L., Wu, Z., Müller, J., Seifert, G., et al. (2020). Constitutive deletion of astrocytic connexins aggravates kainate−induced epilepsy. Glia 68, 2136–2147. doi: 10.1002/glia.23832
Deshpande, T., Li, T., Herde, M. K., Becker, A., Vatter, H., Schwarz, M. K., et al. (2017). Subcellular reorganization and altered phosphorylation of the astrocytic gap junction protein connexin43 in human and experimental temporal lobe epilepsy. Glia 65, 1809–1820. doi: 10.1002/glia.23196
Diaz Verdugo, C., Myren-Svelstad, S., Aydin, E., Van Hoeymissen, E., Deneubourg, C., Vanderhaeghe, S., et al. (2019). Glia-neuron interactions underlie state transitions to generalized seizures. Nat. Commun. 10: 3830.
Ding, S., Fellin, T., Zhu, Y., Lee, S.-Y., Auberson, Y. P., Meaney, D. F., et al. (2007). Enhanced astrocytic Ca2+ signals contribute to neuronal excitotoxicity after status epilepticus. J. Neurosci. 27, 10674–10684. doi: 10.1523/jneurosci.2001-07.2007
Dingledine, R., Varvel, N. H., and Dudek, F. E. (2014). When and how do seizures kill neurons, and is cell death relevant to epileptogenesis? Adv. Exp. Med. Biol. 813, 109–122. doi: 10.1007/978-94-017-8914-1_9
Djukic, B., Casper, K. B., Philpot, B. D., Chin, L.-S., and McCarthy, K. D. (2007). Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J. Neurosci. 27, 11354–11365. doi: 10.1523/jneurosci.0723-07.2007
Eid, T., Lee, T.-S. W., Thomas, M. J., Amiry-Moghaddam, M., Bjornsen, L. P., Spencer, D. D., et al. (2005). Loss of perivascular aquaporin 4 may underlie deficient water and K homeostasis in the human epileptogenic hippocampus. Proc. Natl. Acad. Sci. U.S.A. 102, 1193–1198. doi: 10.1073/pnas.0409308102
Enger, R., Gundersen, G. A., Haj-Yasein, N. N., Eilert-Olsen, M., Thoren, A. E., Vindedal, G. F., et al. (2012). Molecular scaffolds underpinning macroglial polarization: an analysis of retinal Müller cells and brain astrocytes in mouse. Glia 60, 2018–2026. doi: 10.1002/glia.22416
Enger, R., Heuser, K., Nome, C., Tang, W., Jensen, V., Helm, P. J., et al. (2015). “Abnormal astrocytic Ca2+ signaling in the sclerotic hippocampus of awake mice: a two-photon imaging study using the unilateral intracortical kainate injection model of mesial temporal lobe epilepsy,” in Proceedings of the AES 2015 Annual Meeting Abstract Database, (Chicago: American epilepsy society).
Escartin, C., Galea, E., Lakatos, A., O’Callaghan, J. P., Petzold, G. C., Serrano-Pozo, A., et al. (2021). Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 24, 312–325.
Fellin, T., Gomez-Gonzalo, M., Gobbo, S., Carmignoto, G., and Haydon, P. G. (2006). Astrocytic glutamate is not necessary for the generation of epileptiform neuronal activity in hippocampal slices. J. Neurosci. 26, 9312–9322. doi: 10.1523/jneurosci.2836-06.2006
Fellin, T., Pascual, O., Gobbo, S., Pozzan, T., Haydon, P. G., and Carmignoto, G. (2004). Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43, 729–743. doi: 10.1016/j.neuron.2004.08.011
Ferlazzo, E., Gasparini, S., Beghi, E., Sueri, C., Russo, E., Leo, A., et al. (2016). Epilepsy in cerebrovascular diseases: review of experimental and clinical data with meta-analysis of risk factors. Epilepsia 57, 1205–1214. doi: 10.1111/epi.13448
Fordington, S., and Manford, M. (2020). A review of seizures and epilepsy following traumatic brain injury. J. Neurol. 267, 3105–3111. doi: 10.1007/s00415-020-09926-w
Fordsmann, J. C., Murmu, R. P., Cai, C., Brazhe, A., Thomsen, K. J., Zambach, S. A., et al. (2019). Spontaneous astrocytic Ca activity abounds in electrically suppressed ischemic penumbra of aged mice. Glia 67, 37–52. doi: 10.1002/glia.23506
Foskett, J. K., White, C., Cheung, K.-H., and Mak, D.-O. D. (2007). Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 87, 593–658. doi: 10.1152/physrev.00035.2006
French, J. A. (2007). Refractory epilepsy: clinical overview. Epilepsia 48(Suppl. 1), 3–7. doi: 10.1111/j.1528-1167.2007.00992.x
Galovic, M., Ferreira-Atuesta, C., Abraira, L., Döhler, N., Sinka, L., Brigo, F., et al. (2021). Seizures and epilepsy after stroke: epidemiology, biomarkers and management. Drugs Aging. 38, 285–299. doi: 10.1007/s40266-021-00837-7
Glushakov, A. V., Glushakova, O. Y., Doré, S., Carney, P. R., and Hayes, R. L. (2016). Animal Models of Posttraumatic Seizures and Epilepsy. Methods Mol. Biol. 1462, 481–519.
Gómez-Gonzalo, M., Losi, G., Chiavegato, A., Zonta, M., Cammarota, M., Brondi, M., et al. (2010). An excitatory loop with astrocytes contributes to drive neurons to seizure threshold. PLoS Biol. 8:e1000352. doi: 10.1371/journal.pbio.1000352
Gordon, G. R. J., Choi, H. B., Rungta, R. L., Ellis-Davies, G. C. R., and MacVicar, B. A. (2008). Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 456, 745–749. doi: 10.1038/nature07525
Guerra-Gomes, S., Cunha-Garcia, D., Marques Nascimento, D. S., Duarte-Silva, S., Loureiro-Campos, E., Morais Sardinha, V., et al. (2020). IP R2 null mice display a normal acquisition of somatic and neurological development milestones. Eur. J. Neurosci. doi: 10.1111/ejn.14724 [Epub ahead of print].
Hauser, R. M., Henshall, D. C., and Lubin, F. D. (2018). The Epigenetics of Epilepsy and Its Progression. Neuroscientist 24, 186–200. doi: 10.1177/1073858417705840
Haydon, P. G., and Carmignoto, G. (2006). Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 86, 1009–1031. doi: 10.1152/physrev.00049.2005
Henneberger, C. (2017). Does rapid and physiological astrocyte-neuron signalling amplify epileptic activity? J. Physiol. 595, 1917–1927. doi: 10.1113/jp271958
Henning, L., Steinhäuser, C., and Bedner, P. (2021). Initiation of experimental temporal lobe epilepsy by early astrocyte uncoupling is independent of TGFβR1/ALK5 signaling. Front. Neurol. 12:660591. doi: 10.3389/fneur.2021.660591
Hesdorffer, D. C., Logroscino, G., Benn, E. K. T., Katri, N., Cascino, G., and Hauser, W. A. (2011). Estimating risk for developing epilepsy: a population-based study in Rochester, Minnesota. Neurology 76, 23–27. doi: 10.1212/wnl.0b013e318204a36a
Heuser, K., Eid, T., Lauritzen, F., Thoren, A. E., Vindedal, G. F., Taubøll, E., et al. (2012). Loss of perivascular Kir4.1 potassium channels in the sclerotic hippocampus of patients with mesial temporal lobe epilepsy. J. Neuropathol. Exp. Neurol. 71, 814–825. doi: 10.1097/nen.0b013e318267b5af
Heuser, K., Nome, C. G., Pettersen, K. H., Åbjørsbråten, K. S., Jensen, V., Tang, W., et al. (2018). Ca2+ Signals in astrocytes facilitate spread of epileptiform activity. Cereb. Cortex 28, 4036–4048. doi: 10.1093/cercor/bhy196
Hinterkeuser, S., Schröder, W., Hager, G., Seifert, G., Blümcke, I., Elger, C. E., et al. (2000). Astrocytes in the hippocampus of patients with temporal lobe epilepsy display changes in potassium conductances. Eur. J. Neurosci. 12, 2087–2096. doi: 10.1046/j.1460-9568.2000.00104.x
Jessberger, S., and Parent, J. M. (2015). Epilepsy and adult neurogenesis. Cold Spring Harb. Perspect. Biol. 7:a020677. doi: 10.1101/cshperspect.a020677
Kang, N., Xu, J., Xu, Q., Nedergaard, M., and Kang, J. (2005). Astrocytic glutamate release-induced transient depolarization and epileptiform discharges in hippocampal CA1 pyramidal neurons. J. Neurophysiol. 94, 4121–4130. doi: 10.1152/jn.00448.2005
Kivi, A., Lehmann, T.-N., Kovács, R., Eilers, A., Jauch, R., Meencke, H. J., et al. (2000). Effects of barium on stimulus-induced rises of [K]oin human epileptic non-sclerotic and sclerotic hippocampal area CA1. Eur. J. Neurosci. 12, 2039–2048. doi: 10.1046/j.1460-9568.2000.00103.x
Kuchibhotla, K. V., Lattarulo, C. R., Hyman, B. T., and Bacskai, B. J. (2009). Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science 323, 1211–1215. doi: 10.1126/science.1169096
Larsen, B. R., Assentoft, M., Cotrina, M. L., Hua, S. Z., Nedergaard, M., Kaila, K., et al. (2014). Contributions of the Na+/K+-ATPase, NKCC1, and Kir4.1 to hippocampal K+ clearance and volume responses. Glia 62, 608–622. doi: 10.1002/glia.22629
Lee, S., Yoon, B.-E., Berglund, K., Oh, S.-J., Park, H., Shin, H.-S., et al. (2010). Channel-mediated tonic GABA release from glia. Science 330, 790–796. doi: 10.1126/science.1184334
Lundgaard, I., Li, B., Xie, L., Kang, H., Sanggaard, S., Haswell, J. D. R., et al. (2015). Direct neuronal glucose uptake heralds activity-dependent increases in cerebral metabolism. Nat. Commun. 6:6807.
Martin-Fernandez, M., Jamison, S., Robin, L. M., Zhao, Z., Martin, E. D., Aguilar, J., et al. (2017). Synapse-specific astrocyte gating of amygdala-related behavior. Nat. Neurosci. 20, 1540–1548. doi: 10.1038/nn.4649
Miller, G. (2005). Neuroscience. The dark side of glia. Science 308, 778–781. doi: 10.1126/science.308.5723.778
Müller, J., Timmermann, A., Henning, L., Müller, H., Steinhäuser, C., and Bedner, P. (2020). Astrocytic GABA accumulation in experimental temporal lobe epilepsy. Front. Neurol. 11:614923. doi: 10.3389/fneur.2020.614923
Müller, M. S., Fox, R., Schousboe, A., Waagepetersen, H. S., and Bak, L. K. (2014). Astrocyte glycogenolysis is triggered by store-operated calcium entry and provides metabolic energy for cellular calcium homeostasis. Glia 62, 526–534. doi: 10.1002/glia.22623
Mulligan, S. J., and MacVicar, B. A. (2004). Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431, 195–199. doi: 10.1038/nature02827
Nagelhus, E. A., and Ottersen, O. P. (2013). Physiological roles of aquaporin-4 in brain. Physiol. Rev. 93, 1543–1562. doi: 10.1152/physrev.00011.2013
Nagelhus, E. A., Veruki, M. L., Torp, R., Haug, F. M., Laake, J. H., Nielsen, S., et al. (1998). Aquaporin-4 water channel protein in the rat retina and optic nerve: polarized expression in Müller cells and fibrous astrocytes. J. Neurosci. 18, 2506–2519. doi: 10.1523/jneurosci.18-07-02506.1998
Nedergaard, M. (1994). Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science 263, 1768–1771. doi: 10.1126/science.8134839
Neligan, A., Hauser, W. A., and Sander, J. W. (2012). The epidemiology of the epilepsies. Handb. Clin. Neurol. 107, 113–133. doi: 10.1016/b978-0-444-52898-8.00006-9
Neusch, C., Rozengurt, N., Jacobs, R. E., Lester, H. A., and Kofuji, P. (2001). Kir4.1 potassium channel subunit is crucial for oligodendrocyte development and in vivo myelination. J. Neurosci. 21, 5429–5438. doi: 10.1523/jneurosci.21-15-05429.2001
Nikolic, L., Nobili, P., Shen, W., and Audinat, E. (2020). Role of astrocyte purinergic signaling in epilepsy. Glia 68, 1677–1691. doi: 10.1002/glia.23747
Nikolic, L., Shen, W., Nobili, P., Virenque, A., Ulmann, L., and Audinat, E. (2018). Blocking TNFα-driven astrocyte purinergic signaling restores normal synaptic activity during epileptogenesis. Glia 66, 2673–2683. doi: 10.1002/glia.23519
Nizar, K., Uhlirova, H., Tian, P., Saisan, P. A., Cheng, Q., Reznichenko, L., et al. (2013). In vivo stimulus-induced vasodilation occurs without IP3 receptor activation and may precede astrocytic calcium increase. J. Neurosci. 33, 8411–8422. doi: 10.1523/jneurosci.3285-12.2013
Nwaobi, S. E., Cuddapah, V. A., Patterson, K. C., Randolph, A. C., and Olsen, M. L. (2016). The role of glial-specific Kir4.1 in normal and pathological states of the CNS. Acta Neuropathol. 132, 1–21. doi: 10.1007/s00401-016-1553-1
Pandit, S., Neupane, C., Woo, J., Sharma, R., Nam, M.-H., Lee, G.-S., et al. (2020). Bestrophin1-mediated tonic GABA release from reactive astrocytes prevents the development of seizure-prone network in kainate-injected hippocampi. Glia 68, 1065–1080. doi: 10.1002/glia.23762
Parpura, V., Basarsky, T. A., Liu, F., Jeftinija, K., Jeftinija, S., and Haydon, P. G. (1994). Glutamate-mediated astrocyte-neuron signalling. Nature 369, 744–747. doi: 10.1038/369744a0
Parpura, V., and Haydon, P. G. (2000). Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc. Natl. Acad. Sci. U.S.A. 97, 8629–8634. doi: 10.1073/pnas.97.15.8629
Parri, H. R., and Crunelli, V. (2003). The role of Ca2+ in the generation of spontaneous astrocytic Ca2+ oscillations. Neuroscience 120, 979–992. doi: 10.1016/s0306-4522(03)00379-8
Parri, H. R., Gould, T. M., and Crunelli, V. (2001). Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat. Neurosci. 4, 803–812. doi: 10.1038/90507
Patel, D. C., Tewari, B. P., Chaunsali, L., and Sontheimer, H. (2019). Neuron-glia interactions in the pathophysiology of epilepsy. Nat. Rev. Neurosci. 20, 282–297. doi: 10.1038/s41583-019-0126-4
Paukert, M., Agarwal, A., Cha, J., Doze, V. A., Kang, J. U., and Bergles, D. E. (2014). Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 82, 1263–1270. doi: 10.1016/j.neuron.2014.04.038
Pekny, M., and Nilsson, M. (2005). Astrocyte activation and reactive gliosis. Glia 50, 427–434. doi: 10.1002/glia.20207
Pekny, M., and Pekna, M. (2016). Reactive gliosis in the pathogenesis of CNS diseases. Biochim. Biophys. Acta 1862, 483–491. doi: 10.1016/j.bbadis.2015.11.014
Pellerin, L., and Magistretti, P. J. (1994). Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. U.S.A. 91, 10625–10629. doi: 10.1073/pnas.91.22.10625
Perea, G., Navarrete, M., and Araque, A. (2009). Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 32, 421–431. doi: 10.1016/j.tins.2009.05.001
Perea, G., Sur, M., and Araque, A. (2014). Neuron-glia networks: integral gear of brain function. Front. Cell. Neurosci. 8:378. doi: 10.3389/fncel.2014.00378
Perucca, P., and Gilliam, F. G. (2012). Adverse effects of antiepileptic drugs. Lancet Neurol. 11, 792–802. doi: 10.1016/s1474-4422(12)70153-9
Petravicz, J., Boyt, K. M., and McCarthy, K. D. (2014). Astrocyte IP3R2-dependent Ca2+ signaling is not a major modulator of neuronal pathways governing behavior. Front. Behav. Neurosci. 8:384. doi: 10.3389/fnbeh.2014.00384
Petravicz, J., Fiacco, T. A., and McCarthy, K. D. (2008). Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J. Neurosci. 28, 4967–4973. doi: 10.1523/jneurosci.5572-07.2008
Plata, A., Lebedeva, A., Denisov, P., Nosova, O., Postnikova, T. Y., Pimashkin, A., et al. (2018). Astrocytic atrophy following parallels reduced Ca2+ activity and impaired synaptic plasticity in the rat hippocampus. Front. Mol. Neurosci. 11:215. doi: 10.3389/fnmol.2018.00215
Poskanzer, K. E., and Yuste, R. (2016). Astrocytes regulate cortical state switching in vivo. Proc. Natl. Acad. Sci. U.S.A. 113, E2675–E2684.
Rothstein, J. D., Dykes-Hoberg, M., Pardo, C. A., Bristol, L. A., Jin, L., Kuncl, R. W., et al. (1996). Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16, 675–686. doi: 10.1016/s0896-6273(00)80086-0
Scemes, E., and Giaume, C. (2006). Astrocyte calcium waves: what they are and what they do. Glia 54, 716–725. doi: 10.1002/glia.20374
Scholl, U. I., Choi, M., Liu, T., Ramaekers, V. T., Häusler, M. G., Grimmer, J., et al. (2009). Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc. Natl. Acad. Sci. U. S. A. 106, 5842–5847. doi: 10.1073/pnas.0901749106
Sharp, A. H., Nucifora, F. C. Jr., Blondel, O., Sheppard, C. A., Zhang, C., Snyder, S. H., et al. (1999). Differential cellular expression of isoforms of inositol 1,4,5-triphosphate receptors in neurons and glia in brain. J. Comp. Neurol. 406, 207–220. doi: 10.1002/(sici)1096-9861(19990405)406:2<207::aid-cne6>3.0.co;2-7
Shields, D. C., Schaecher, K. E., Hogan, E. L., and Banik, N. L. (2000). Calpain activity and expression increased in activated glial and inflammatory cells in penumbra of spinal cord injury lesion. J. Neurosci. Res. 61, 146–150. doi: 10.1002/1097-4547(20000715)61:2<146::AID-JNR5>3.0.CO;2-C
Shigetomi, E., Saito, K., Sano, F., and Koizumi, S. (2019). Aberrant calcium signals in reactive astrocytes: a key process in neurological disorders. Int. J. Mol. Sci. 20, 996. doi: 10.3390/ijms20040996
Sofroniew, M. V. (2009). Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 32, 638–647. doi: 10.1016/j.tins.2009.08.002
Steinhäuser, C., and Seifert, G. (2012). “Astrocyte dysfunction in epilepsy,” in Jasper’s Basic Mechanisms of the Epilepsies, eds J. L. Noebels, M. Avoli, M. A. Rogawski, R. W. Olsen, and A. V. Delgado-Escueta (Bethesda, MD: National Center for Biotechnology Information).
Steinhäuser, C., Seifert, G., and Bedner, P. (2012). Astrocyte dysfunction in temporal lobe epilepsy: K+ channels and gap junction coupling. Glia 60, 1192–1202. doi: 10.1002/glia.22313
Sun, W., McConnell, E., Pare, J.-F., Xu, Q., Chen, M., Peng, W., et al. (2013). Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science 339, 197–200. doi: 10.1126/science.1226740
Szabó, Z., Héja, L., Szalay, G., Kékesi, O., Füredi, A., Szebényi, K., et al. (2017). Extensive astrocyte synchronization advances neuronal coupling in slow wave activity in vivo. Sci. Rep. 7:6018.
Szokol, K., Heuser, K., Tang, W., Jensen, V., Enger, R., Bedner, P., et al. (2015). Augmentation of Ca2+ signaling in astrocytic endfeet in the latent phase of temporal lobe epilepsy. Front. Cell. Neurosci. 9:49. doi: 10.3389/fncel.2015.00049
Tian, G.-F., Azmi, H., Takano, T., Xu, Q., Peng, W., Lin, J., et al. (2005). An astrocytic basis of epilepsy. Nat. Med. 11, 973–981.
Umpierre, A. D., West, P. J., White, J. A., and Wilcox, K. S. (2019). Conditional knock-out of mGluR5 from astrocytes during epilepsy development impairs high-frequency glutamate uptake. J. Neurosci. 39, 727–742. doi: 10.1523/jneurosci.1148-18.2018
van Vliet, E. A., da Costa Araújo, S., Redeker, S., van Schaik, R., Aronica, E., and Gorter, J. A. (2007). Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain 130, 521–534. doi: 10.1093/brain/awl318
Vezzani, A., French, J., Bartfai, T., and Baram, T. Z. (2011). The role of inflammation in epilepsy. Nat. Rev. Neurol. 7, 31–40.
Volterra, A., and Steinhäuser, C. (2004). Glial modulation of synaptic transmission in the hippocampus. Glia 47, 249–257. doi: 10.1002/glia.20080
Wang, F., Smith, N. A., Xu, Q., Fujita, T., Baba, A., Matsuda, T., et al. (2012). Astrocytes modulate neural network activity by Ca2 -dependent uptake of extracellular K+. Sci. Signal. 5:ra26. doi: 10.1126/scisignal.2002334
Wellmann, M., Álvarez-Ferradas, C., Maturana, C. J., Sáez, J. C., and Bonansco, C. (2018). Astroglial Ca-dependent hyperexcitability requires P2Y purinergic receptors and pannexin-1 channel activation in a chronic model of epilepsy. Front. Cell. Neurosci. 12:446. doi: 10.3389/fncel.2018.00446
Wieser, H.-G. ILAE Commission on Neurosurgery of Epilepsy. (2004). ILAE Commission Report. Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia 45, 695–714. doi: 10.1111/j.0013-9580.2004.09004.x
Yang, H. Y., Lieska, N., Shao, D., Kriho, V., and Pappas, G. D. (1994). Proteins of the intermediate filament cytoskeleton as markers for astrocytes and human astrocytomas. Mol. Chem. Neuropathol. 21, 155–176. doi: 10.1007/bf02815349
Zhang, C., Tabatabaei, M., Bélanger, S., Girouard, H., Moeini, M., Lu, X., et al. (2019). Astrocytic endfoot Ca correlates with parenchymal vessel responses during 4-AP induced epilepsy: an in vivo two-photon lifetime microscopy study. J. Cereb. Blood Flow Metab. 39, 260–271. doi: 10.1177/0271678x17725417
Keywords: astrocyte, epilepsy, calcium signaling, IP3, epileptogenesis, ictogenesis, astrogliosis
Citation: Heuser K and Enger R (2021) Astrocytic Ca2+ Signaling in Epilepsy. Front. Cell. Neurosci. 15:695380. doi: 10.3389/fncel.2021.695380
Received: 14 April 2021; Accepted: 16 June 2021;
Published: 15 July 2021.
Edited by:
Leonid Savtchenko, University College London, United KingdomReviewed by:
Ulyana Lalo, Immanuel Kant Baltic Federal University, RussiaReno Cervo Reyes, Holy Names University, United States
Copyright © 2021 Heuser and Enger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kjell Heuser, a2hldXNlckBvdXMtaGYubm8=; ZHIuaGV1c2VyQGdtYWlsLmNvbQ==; Rune Enger, cnVuZS5lbmdlckBtZWRpc2luLnVpby5ubw==
 Kjell Heuser
Kjell Heuser Rune Enger2*
Rune Enger2*