- 1Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan
- 2Department of Biological Sciences, Columbia University in the City of New York, New York, NY, United States
The cerebellum plays an important role in both motor control and cognition. The cerebellar cortex is neuron-rich and composed of characteristic folia and fissures. Defective cerebellar development leads to movement disorders and developmental delay. During early morphogenesis, cellular signaling programs orchestrate simultaneous cerebellar growth and foliation. Aberrant signaling causes various degrees of cerebellar hypoplasia. Based on mouse genetic studies, we discuss several developmental signaling pathways that drive cerebellar morphogenesis. Notably, hypoplasia of vermal lobules VI-VII has been linked to autism spectrum disorder and is in part attributed to brain-derived neurotrophic factor (BDNF)/tropomyosin receptor kinase B signaling. This review also discusses how BDNF biogenesis is critical for cerebellar foliation and whether restoring BDNF signaling could reverse cerebellar developmental disorders.
1 Introduction
The cerebellum is a neuron-rich structure residing at the base of the brain, connecting with the cerebrum, the brainstem and the spinal cord. Long recognized for its central role in movement control and coordination, the cerebellum is increasingly implicated in cognitive functions, including social behavior, reward circuitry, emotional and language processing (Carta et al., 2019; Wagner and Luo, 2020; Stoodley et al., 2021). A range of neurodevelopmental disorders, such as Joubert syndrome and autism spectrum disorders (ASD), arises from disruptions in cerebellar development of varying severity. Unraveling how genetic disruptions affect cerebellar morphogenesis is crucial. To gain insights into developmental origins of brain disorders, we conducted an extensive literature and database search to identify gene deficiencies that affect cerebellar fissure formation in mouse models. Through such an analysis, this review offers unique insights into how cerebellar fissure morphogenesis is controlled by cellular signaling programs. Moreover, we discuss the potential of small molecule compounds that modulate signaling pathways for reversing cerebellar developmental disorders, particularly hypoplasia of the vermal lobules VI and VII associated with ASD.
2 The cerebellum and its functional architecture
The cerebellum consists of two hemispheres connected by a narrow midline region called the vermis. The foliation pattern is symmetrical to the midline and gives rise to 10 lobules I–X that run perpendicular to the anterior–posterior axis along the vermis (Sillitoe and Joyner, 2007; Farini et al., 2021) (Figure 1A, upper panel). The cerebellar cortex has a laminar organization, i.e., the molecular layer (ML), Purkinje cell layer (PCL), and granule cell layer (GCL), going from the outer to inner direction (Figure 1A, lower panel). Nearly 99% of cerebellar neurons are granule cells (GCs). During development, GC precursors (GCPs) proliferate at the external granule layer (EGL), which is an outermost and transient layer (Figure 1B). After mitosis, GCs migrate inward to populate the GCL (for the detail, see below). The somata of Purkinje cells (PCs) form a monolayer called PCL. The molecular layer (ML) is largely cell-free but contains neuronal microcircuits, including GC axons, PC dendrites, and their synapses, as well as interneurons and glia (Apps and Hawkes, 2009) (Figure 1B). In the ML, axons of GCs ascend from the soma, forming parallel fibers which have extensive connections with dendrites of PCs. Besides, two major excitatory afferents, mossy fibers and climbing fibers, from other brain areas and spinal cord terminate in GCs (Legué et al., 2016). Embedded within the white matter under the cerebellar cortex, the deep cerebellar nuclei primarily receive afferents from the GABAergic PCs and generate the principal outputs of the cerebellum (Figures 1A,B). Precise organization of neurites and their connectivities constitute the cerebellar circuitry (Legué et al., 2016).
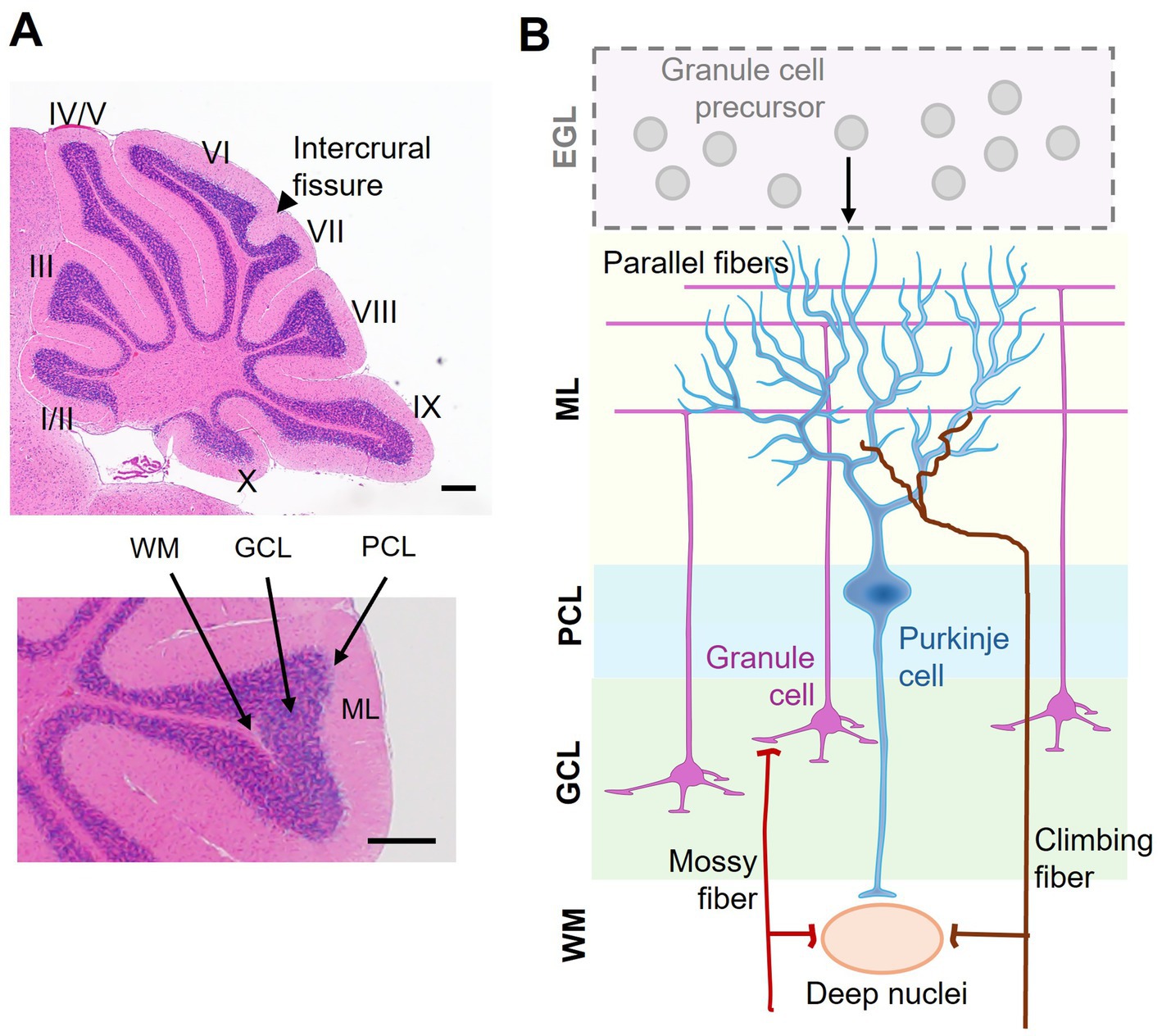
Figure 1. Laminar organization of the cerebellum. (A) A sagittal view of a mouse cerebellum stained with hematoxylin and eosin. Upper: the 10 lobules (I–X) and the fissure between lobules VI and VII, namely the intercrural fissure, are indicated. Lower: distinct layers of the cerebellar cortex, including the molecular layer (ML), Purkinje cell layer (PCL), granule cell layer (GCL), and white matter (WM). (B) Schematic representation of cerebellar cortical organization and connectivity. During early development, granule cell precursors (GCPs) proliferate in the external granule layer (EGL). The EGL disappears after granule cells (GCs) radially migrate inward to form the GCL in the mature cerebellum. The ML contains Purkinje cell dendrites and granule cell axons (i.e., parallel fibers). The PCL consists of a monolayer of Purkinje cells (PCs), acting as the output neurons of the cortex. Excitatory inputs to the cerebellum include mossy fibers, which relay sensory and motor information via granule cells, and climbing fibers, which form synaptic connections with PCs. The deep nuclei located in the WM integrate inhibitory signals from PCs and excitatory afferents, serving as the principal output hub of the cerebellum. Scale bar: 200 μm.
The cerebellum processes information from functionally diverse regions of the cerebral cortex to its support motor, cognitive, and affective functions (Stoodley et al., 2012). An early study reported that birth date-related PC clustering may correlate with functional compartmentalization along the mediolateral axis in the adult cerebellum (Hashimoto and Mikoshiba, 2003). Task-based neuroimaging studies have more recently revealed distinct functional territories of the cerebellum, i.e., sensorimotor (lobules II–VI, VIIIB), vestibular (lobules IX–X), oculomotor (lobules VI–VII, IX–X), visual (lobule VI), and auditory (lobules V–VI) zones (Stoodley and Schmahmann, 2018; Raymond and Medina, 2018) (Figure 1A). Based on spatiotemporal transcriptomics, it is now evident that the cerebellum exhibits regional specialization physiological and anatomical properties across subregions (i.e., folia) and neuronal subtypes (Cerminara et al., 2015; Kozareva et al., 2021; Lanore et al., 2021; Sepp et al., 2024). As a prime example of non-uniform microcircuitry, the longitudinal zebrin II+/− stripes in PCs reveal molecular heterogeneity that restricts specific splicing variant expression that influences PC plasticity, firing properties, and input–output connections (Lin et al., 2020). Therefore, cerebellar development is a highly regulated and genetically influenced process, rather than a uniform one. Nevertheless, more research is needed to determine how molecular factors align with functional specialization.
3 Lobules VI–VII: what is special?
Gene deficiencies affect cerebellar morphogenesis to varying degrees. Certain developmental defects contribute to hypoplasia of the vermian lobules VI–VII, a feature associated with ASD (Courchesne et al., 1988). Developmentally, lobules VI and VII expand significantly in the lateral hemisphere forming crus I in the rodent cerebellum, the homologous region of human crus I/II implicated in cognitive and visuomotor functions (Sugihara, 2018). Notably, lobules VI-VII exhibited a delayed developmental timeline in comparison with that of other lobules (Legué et al., 2016). Functional topography has revealed that lobules VI and VII are embryologically and phylogenetically distinct from the anterior lobules I–V (Courchesne et al., 1988). Tract-tracing studies revealed reciprocal connection from this posterior vermal region to associative and paralimbic cortices, providing anatomical substrate for cognitive functions (Kelly and Strick, 2003). Of note, lobule VII accounts for 47.70% gray matters of the human cerebellar volume (Diedrichsen et al., 2009). In vivo electrophysiological studies in the mouse cerebellum underscored an interplay of intrinsic cell properties and input–output profiles underpinning a zonal distribution of various cerebellar functions. For example, mossy fiber burst inputs to GCs and PC firing rates are characteristically different for lobules VI–VII versus X. This difference in input–output regularity may support the neural processing required by distinct tasks each subregion is involved in: an oscillation-based communication with cerebral cortex (lobules VI–VII) versus an always-on mechanism for vestibular functions (lobule X) (Witter and De Zeeuw, 2015).
4 Cerebellar development: cortical lamination and foliation
The pattern of vermis foliation is generally conserved across mammalian species. The human cerebellum begins to develop at gestational week 4 and ends around 2–3 years after birth (Carletti and Rossi, 2008; Iskusnykh and Chizhikov, 2022). In mice, cerebellar development begins at embryonic day (E) 9 and ends in the third postnatal week. The cerebellar primordium emerges in the roof of the fourth ventricle, comprising two primary germinal zones, i.e., the ventricular zone (VZ) and the rhombic lip (RL) (Leto et al., 2016) (Figure 2A, E12.5). VZ progenitor cells produce GABAergic PCs and Bergmann glia precursors. The RL produces glutamatergic GC precursors (GCPs). Foliation occurs concurrently with the formation of the cortical cell layers. Fissure formation is initiated around E16.5 when GCP expands at the outermost EGL, a transient secondary germinal zone, and move inwards to the inner EGL (Figure 2A, E18.5 and Figure 2B). GCP proliferation peaks between postnatal day 5 and 8 (P5-8). Subsequently, postmitotic GCs undergoes radial migration from the EGL across the PCL to populate the internal granule layer (IGL) (Figure 2B). The radial glia in the VZ transforms into Bergmann glia during E14.5-E18.5. Bergmann glial cells are essential for the folding of the cerebellar surfaces (Sudarov and Joyner, 2007; Leung and Li, 2018). As the anchoring points, Bergmann glia located next to the PC layer emit their radial fibers toward the pial surface for GC migration (Rahimi-Balaei et al., 2018) (Figure 2B). GC maturation and migration complete by P15, and proliferative EGL disappears (Figure 2A, P15) (Carletti and Rossi, 2008; Iskusnykh and Chizhikov, 2022). Notably, the kinetics of GC accumulation varies across different zones of the cerebellum. Maximal GC production is delayed in the lobules VI and VII compared to other zones (Legué et al., 2016). Whether such a delay results in the formation of the fissure (namely the intercrural fissure, see below) between lobules VI and VII particularly sensitive to certain cellular signals or neurotransmitters remains as an intriguing question.
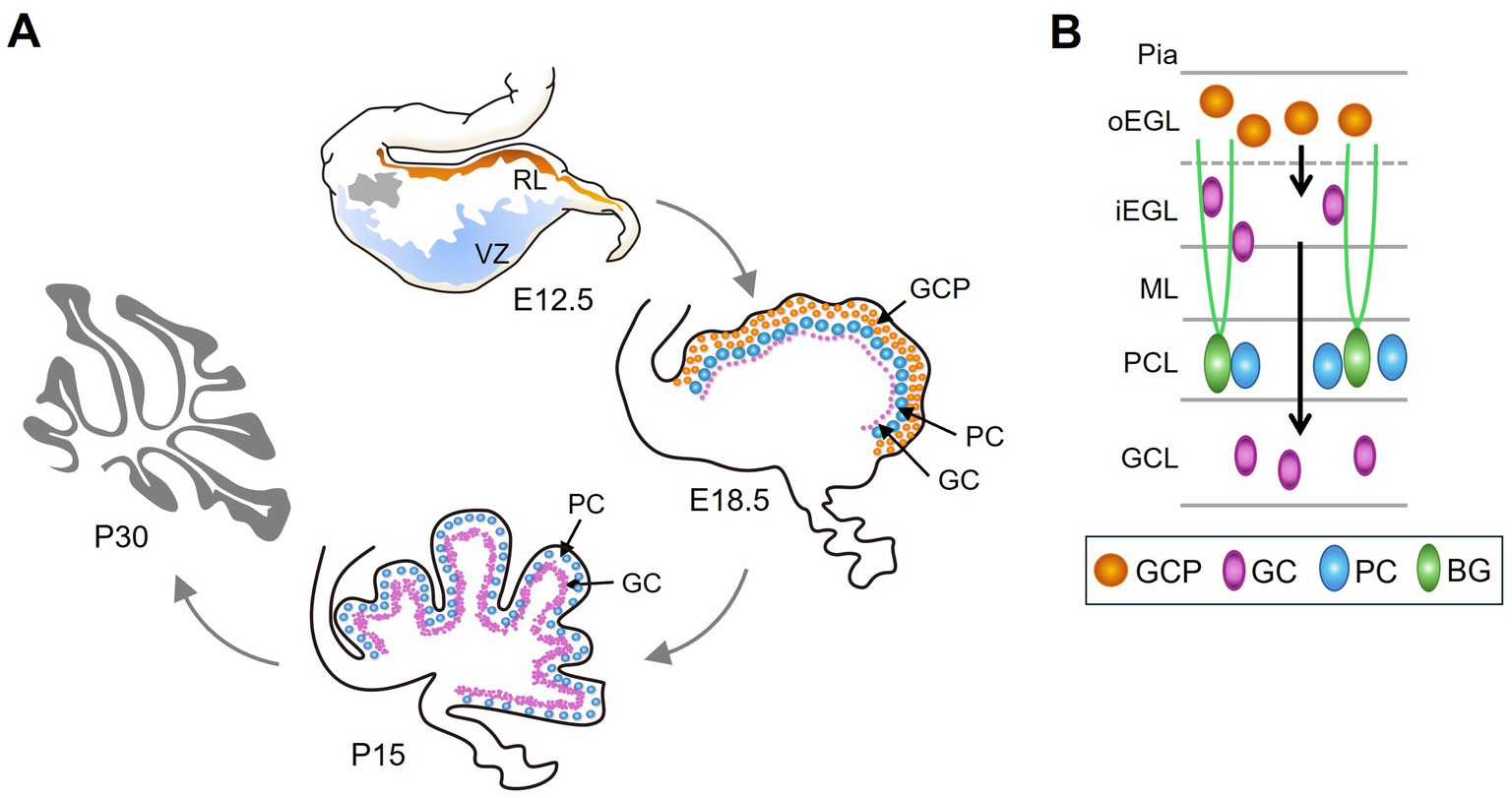
Figure 2. The development of the cerebellum. (A) Cerebellar development begins at E9 in mice. At E12.5, the cerebellar primordium forms in the roof of the fourth ventricle, consisting of two primary germinal zones: the ventricular zone (VZ) (blue) and the rhombic lip (RL) (orange). Grey indicates the nuclear transitory zone, where deep nuclear neurons develop. At E18.5, GCPs proliferate in the outermost layer, i.e., EGL (orange). PCs and postmitotic GCs (purple) are found beneath the EGL. By P15, GC maturation and migration are complete, leading to accumulation in the GCL, which is inside of the PCL. (B) Schematic of different cell types and its location in the developing cerebellum. GCPs proliferate at outer EGL (oEGL); after exiting the cell cycle, GCs migrate inward to inner GCL (iEGL). Subsequently, GCs migrate radially on Bergmann glial (BG) fibers to populate the interior of the cerebellum and ultimately form the GCL.
Cerebellar development is orchestrated through a program of transcriptional regulation and signaling pathways. Recently, single-cell transcriptomic profiling has identified the expression of nearly 200 cell-type specific transcription factors in the developing cerebellum and determined their respective regulon activities (Sepp et al., 2024). On the basis of mutant-induced phenotypic changes (see Section 6), this review discusses signaling pathways essential for cerebellar development. As early as E8.5, Wnt1 drives the expression of FGF8, a morphogen that specifies hindbrain and controls the onset of cerebellar development (Spassky et al., 2008). Bone morphogenetic proteins (BMPs) function before the formation of the cerebellar primordium to control stem cell specification in the anterior rhombic lip (Tong et al., 2015). Notch signaling preserves a pool of neural progenitor cells in an undifferentiated state. However, Notch levels differ between daughter cells after cell division. Although high levels of Notch maintain the progenitor fate, the intermediate and low levels of Notch activities may, respectively, generate inhibitory and excitatory neurons (Zhang et al., 2021). From E17.5 to postnatal days, PCs express Sonic hedgehog (Shh) to promote GCP proliferation in the EGL (Wallace, 1999). Reciprocally, Reelin released by GCPs disperses PC clusters into the monolayer (Miyata et al., 1997). Dysregulation of these cellular signaling pathways contributes to a range of cerebellar disorders (see below).
5 Cerebellar malformations in human disorders
Cerebellar malformations underpin a spectrum of debilitating disorders, from ataxia to autism. Accumulating animal-based evidence highlights loss of function genes and defective signaling that give rise to abnormal cellular patterning and foliation defects underlying disease progression. In this review, we describe several cerebellar developmental disorders and their associated susceptibility genes that have been confirmed in animal studies.
5.1 Dandy Walker malformation
Dandy Walker malformation (DWM) is the most common cerebellar malformation in human live births, characterized by dilation of fourth ventricle and vermis hypoplasia. DWM patients exhibit symptoms ranging from intellectual disability to autism (Carletti and Rossi, 2008). Genetic aberrations of DWM have been described in a variety of genes including ZIC1, ZIC4 and FOXC1 (Grinberg et al., 2004; Aldinger et al., 2009). The phenotypes of mice with a heterozygous deletion of Zic1 and Zic4 closely resemble DWM (Grinberg et al., 2004). Hypomorphic Foxc1 mutant mice exhibited similar defects in cerebellar foliation to DWM individuals with FOXC1 locus deletions (Haldipur et al., 2017).
5.2 Joubert syndrome-related disorders
Characterized by ataxia and delayed development, patients with Joubert syndrome similarly suffer from cerebellar vermis hypoplasia (Valente et al., 2006). Joubert syndrome-related disorders exhibit clinical heterogeneity due to their various genetic causes, including three genes (CEP290, AHI1/JBTS3, NPHP1/JBTS4) and two loci (JBTS1 and JBTS2) (Valente et al., 2005; Valente et al., 2006). Nevertheless, as with Joubert syndrome individuals, Ahi1 knockout mice exhibited hypoplasia in lobules VI-VII. Ahi1 has been linked to Wnt-β-catenin signaling (Lancaster et al., 2009), and a partial reversal of the cerebellar defects was achieved by treating these mice with lithium, a Wnt agonist (Lancaster et al., 2011).
5.3 CHARGE syndrome
CHARGE syndrome is characterized by multiple organ defects and commonly associated with CHD7 mutations (Zentner et al., 2010). Notably, individuals with CHARGE syndrome display cerebellar vermis hypoplasia and foliation defects. Similarly to CHARGE individuals, mice with Chd7 haploinsufficiency displayed foliation defects, developmental delay, and motor deficits caused by impaired epigenomic regulation of GCP differentiation and foliation anomalies (Zentner et al., 2010; Reddy et al., 2021).
5.4 Autism spectrum disorder
Autism spectrum disorder (ASD) represents a heterogeneous group of disorders characterized by social deficits and repetitive behaviors. Strong correlative evidence between abnormal cerebellar development and ASD has been established. CHD8 is one of the most frequently mutated genes in individuals with ASD (Talkowski et al., 2012; Neale et al., 2012; O'Roak et al., 2012). Ablation of Chd8 in mouse GCP resulted in pronounced foliation defects, vermis hypoplasia, and motor defects (Kawamura et al., 2021). Other transgenic mouse studies also converge on the association between cerebellar malformation and ASD-like phenotypes (see below).
5.5 Spinocerebellar ataxia
Spinocerebellar ataxia (SCA) is caused by variants in many different genes. A subset of hereditary cerebellar ataxia exhibits impaired PC dendritic arborization, zebrin-II stripe degradation, and climbing fiber dysfunction (Bartelt et al., 2024). The review will not discuss SCA, since it is an adult-onset neuro-degenerative disorder.
6 Mouse genes critical for cerebellar foliation
Over the past three decades, studies of mouse disease models have provided substantial information regarding cerebellar development and implication for human disorders associated with cerebellar malformations (Manto and Jissendi, 2012; Butts et al., 2014; Haldipur and Millen, 2019). Moreover, our understanding of cerebellar morphogenesis may be sped up by unexpected discoveries of genes associated with disease phenotypes. A comprehensive database has compiled a total of 543 mouse genes critical for cerebellar development and 630 human mutant loci associated with cerebellar phenotypes (Ramirez et al., 2022). Recent studies using single-cell RNA-seq of the cerebellum across developmental stages and species have revealed its cellular architecture, evolutionary differences, and insights into cerebellar diseases (Cerminara et al., 2015; Carter et al., 2018; Haldipur et al., 2019; Aldinger et al., 2021). To date, we still lack a complete understanding of the mechanisms governing cerebellar morphogenesis and how their dysregulation contributes to cerebellar disorders.
To improve our understanding of cerebellar foliation, we collected mouse genes that have been reported in cerebellar foliation from Mouse Genome Informatics (MGI)1 and PubMed.2 In the MGI database, 69 genes are categorized as having abnormal cerebellar fissure morphology or lobule formation, or with reduced or absent foliation in the cerebellum (Table 1, MGI). A PubMed search for “cerebellar hypoplasia” identified 52 genes whose knockout reduces cerebellum size and 19 genes whose knockout impairs intercrural fissure formation (Table 1, PubMed). Intriguingly, only 15 genes from this search were identified in the above categories of MGI (Table 1, footnotes). PubMed also revealed that approximately 30 genes whose knockout does not significantly affect cerebellar morphology, but several of them affect PC arborization or function, such as Bcl7a, Pten and Tsc2 (Tsai et al., 2012; Cupolillo et al., 2016; Wischhof et al., 2017).
7 Signaling pathways critical for cerebellar foliation
Having identified genes essential for cerebellar foliation, we turn to the molecular signaling pathways that affect cerebellar cytoarchitecture. From the 125 genes identified in our MGI and PubMed analyses pertaining to cerebellar hypoplasia and intercrural fissure defects, 28 emerge as key players in developmental signaling, neurotransmission, and cell survival (Table 2). Their disruption profoundly alters cerebellar morphology, often with striking specificity. Here, we explore how core pathways like BMP, Wnt, and Shh, alongside subtle modulators like BDNF, orchestrate foliation and reveal vulnerabilities in developmental disorders (Table 2).
7.1 Core developmental signaling pathways
7.1.1 Bone morphogenetic protein signaling
The BMPs are secreted signaling molecules expressed before the formation of cerebellum primordia. BMP signaling is important for the specification of neural stem cells in the anterior rhombic lip, as well as for the generation and differentiation of GCs (Tong et al., 2015). BMPs transduce signals by binding to the BMP receptor (BMRP1/BMPR2) complex. BMPR1 phosphorylates associated R-Smads, which subsequently form a heteromeric complex with co-Smad and translocate into the nucleus for transcriptional regulation. Bmpr1a/Bmpr1b double knockout results in severe cerebellar patterning defects (Qin et al., 2006). ZNF423 mutations are associated with Joubert syndrome (Chaki et al., 2012). Knockout of Zfp423 impairs cerebellar development (Warming et al., 2006; Alcaraz et al., 2006). Zfp423 is a zinc finger transcription factor that integrates BMP and Notch signaling to regulate the expression of neuronal differentiation factor Hes5 (Masserdotti et al., 2010).
7.1.2 Reelin signaling
Reeler mice with Reln mutations exhibit an ataxic gait, partly due to cerebellar underdevelopment. Dab1 mutations in scrambler mice result in phenotypes similar to those in reeler mice (Cendelin, 2014). Reelin, secreted by GCs in the EGL, controls the migration of posterior-born PCs to form the primordial PCL. As Reelin binds to its receptors apolipoprotein E receptor 2 (ApoER2) and very-low-density lipoprotein receptor (VLDLR), it triggers signal transduction through the adaptor Disabled-1 (Dab1), thereby modulating cytoskeletal rearrangement that direct neuronal migration (Jossin, 2020). Notably, aberrant activation of mTOR signaling results in ubiquitination and destruction of phosphorylated Dab1, and pharmacological inhibition of mTOR restores Reelin-Dab1 signaling and cell migration (Moon et al., 2015).
7.1.3 Wnt signaling
During cerebellar development, Wnt/β-catenin activity is present transiently at the embryonic rhombic lip before shifting to the cerebellar ventricular zone, where it promotes neural stem cell proliferation (Selvadurai and Mason, 2011; Pei et al., 2012). Wnt5a knockout leads to cerebellar hypoplasia and the depletion of both GABAergic and glutamatergic neurons (Subashini et al., 2017). Wnt ligands bind to the Frizzled receptors (Fzds) and co-receptors, preventing β-catenin phosphorylation and degradation. Increased β-catenin signaling activates genes including the engrailed transcription factors (EN1/2) that are critical for cerebellar development. Wnt activity is reduced in Ahi1-mutant mice, a model of Joubert syndrome (Lancaster et al., 2011). Additionally, Wnt/β-catenin activity is differentially affected by Frizzled-like receptor Tmem67 (Abdelhamed et al., 2019), the oxidoreductase Wwox (Cheng et al., 2020), and retinoid-related orphan receptor RORα (Lee et al., 2010). Notably, these Wnt regulators are implicated in ASD or Joubert syndrome, reinforcing their roles in cerebellar development.
7.1.4 Sonic hedgehog signaling
Shh is secreted by PCs in the ventricular zone and represents the main mitogenic factor driving postnatal GCP expansion in the EGL. In addition, Shh signaling affects Bergmann glial differentiation (De Luca et al., 2016). In the absence of hedgehog ligands, the transmembrane receptor Patched binds and inhibits the activity of Smoothened (Smo). Binding of Shh to Patched activates its downstream signaling. Smo releases the GLI family of transcription factors from sequestration by Suppressor of fused (Sufu), enabling their nuclear translocation and transactivation (De Luca et al., 2016). Gli proteins subsequently promote the expression of genes involved in cell proliferation, such as N-Myc and cyclins (Consalez et al., 2021). Homozygous missense variants in SUFU have been identified in Joubert syndrome (De Mori et al., 2017). Sufu deficiency causes severe mispatterning of the cerebellum (Kim et al., 2011).
7.1.5 Notch signaling
Notch signaling is also crucial for cerebellar development (Engler et al., 2018). Notch activity maintains the multipotency of cerebellar Sox2+ progenitors and its level regulates the ratio of inhibitory to excitatory neuron cell fates from common progenitor cells (Zhang et al., 2021). Notch signaling can be antagonized by the endocytic adaptor Numb. Intriguingly, Numb has multiple splice isoforms that may exert different effects in Notch signaling (Dho et al., 2025). Numb is, however, involved in diverse cellular processes. For example, conditional knockout of Numb in PCs impairs motor coordination due to downregulating metabotropic glutamate 1 receptor (mGlu1) on the cell surface (Zhou et al., 2015).
7.2 Neurotransmitters, growth factors, and hormones
In addition to the aforementioned factors, cerebellar foliation also involves neurotransmitters and hormones. γ-aminobutyric acid (GABA) is primarily known for its role as a synaptic neurotransmitter, but it also regulates cell proliferation, migration, and differentiation during brain development (Owens and Kriegstein, 2002). GABA depolarizes GCPs via ionotropic GABAA receptors and causes their cell cycle exit (Dave and Bordey, 2009). Gabrb3-KO mice exhibited deficits in social and exploratory behaviors, concomitant with a reduced intercrural fissure (DeLorey et al., 2008).
7.2.1 Neurotrophins
Neurotrophins represent a group of peptide growth factors including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and neurotrophin-3/4/5 (NT-3/4/5). These signaling molecules bind to their respective Trk family receptors to activate downstream MAP kinase pathways (Allen et al., 2013). In general, neurotrophins function in neuronal differentiation, neuroprotection, and synaptic plasticity. Bdnf-deficient mice displayed ataxia, decreased PC complexity and the loss of the intercrural fissure in their cerebellum (Schwartz et al., 1997). BDNF is not crucial for GCP proliferation, but it may contribute to GC maturation and maintenance (Gao et al., 1995). Notably, knockout of Ntrk2, which encodes the BDNF/NT-4 receptor TrkB, also caused similar phenotypic changes in the cerebellum as Bdnf knockouts (Minichiello et al., 1998). Knockout studies revealed that intercrural fissure formation is also affected by several genes involved in BDNF biogenesis or signaling, including Cadps2, Vav3 and Pex13/14 (Sadakata et al., 2007; Quevedo et al., 2010; Müller et al., 2011; Abe et al., 2018). Ca2+-dependent activator protein 2 (Cadps2) promotes BDNF secretion (Shinoda et al., 2011). Knockout of Vav3, a Rac/RhoA guanine nucleotide exchange factor, slightly compromised BDNF/TrkB signaling, but how it regulates BDNF remains unclear (Quevedo et al., 2010). Peroxisome biogenesis deficiency attenuates BDNF–TrkB pathway-mediated development (Müller et al., 2011; Abe et al., 2018). Despite paradoxically elevated levels of BDNF, Pex14 knockout compromised BDNF–TrkB signaling, likely due to an increase in truncated TrkB (Abe et al., 2018). Besides BDNF, NT-3 and its receptor TrkC are, respectively, regulated by the transcription factors Barhl1 and NeuroD1 (Li et al., 2004; Cho and Tsai, 2006). Interestingly, Barhl1 knockout also causes intercrural fissure deficiency (Li et al., 2004).
7.2.2 Thyroid hormones
Cerebellar development is sensitive to thyroid hormone levels (Neveu and Arenas, 1996). Hypothyroidism causes cerebellar dysfunction. Biologically active triiodothyronine (T3) binds to intracellular thyroid hormone receptors (TRs) to regulate target genes. Mice harboring ligand-binding mutant Thrb∆337 T exhibited reduced intercrural fissure (Portella et al., 2010). Moreover, the level of thyroid hormones can be differently regulated by three iodothyronine deiodinases (Dios). Dio3 knockout increased the T3 level and, intriguingly, abolished intercrural fissure formation (Peeters et al., 2013). Notably, thyroid deficiency reduces BDNF expression (Chakraborty et al., 2012), suggesting a link between thyroid and BDNF.
Our analysis indicated that disruption of core developmental signaling pathways, including BMP, Reelin, Wnt and Shh, substantially impairs cerebellar development, resulting in smaller cerebella. In contrast, ablation of non-core signals, such as BDNF, GABA, and thyroid, disrupts intercrural fissure formation without impairing the overall cerebellar morphogenesis (Figure 3).
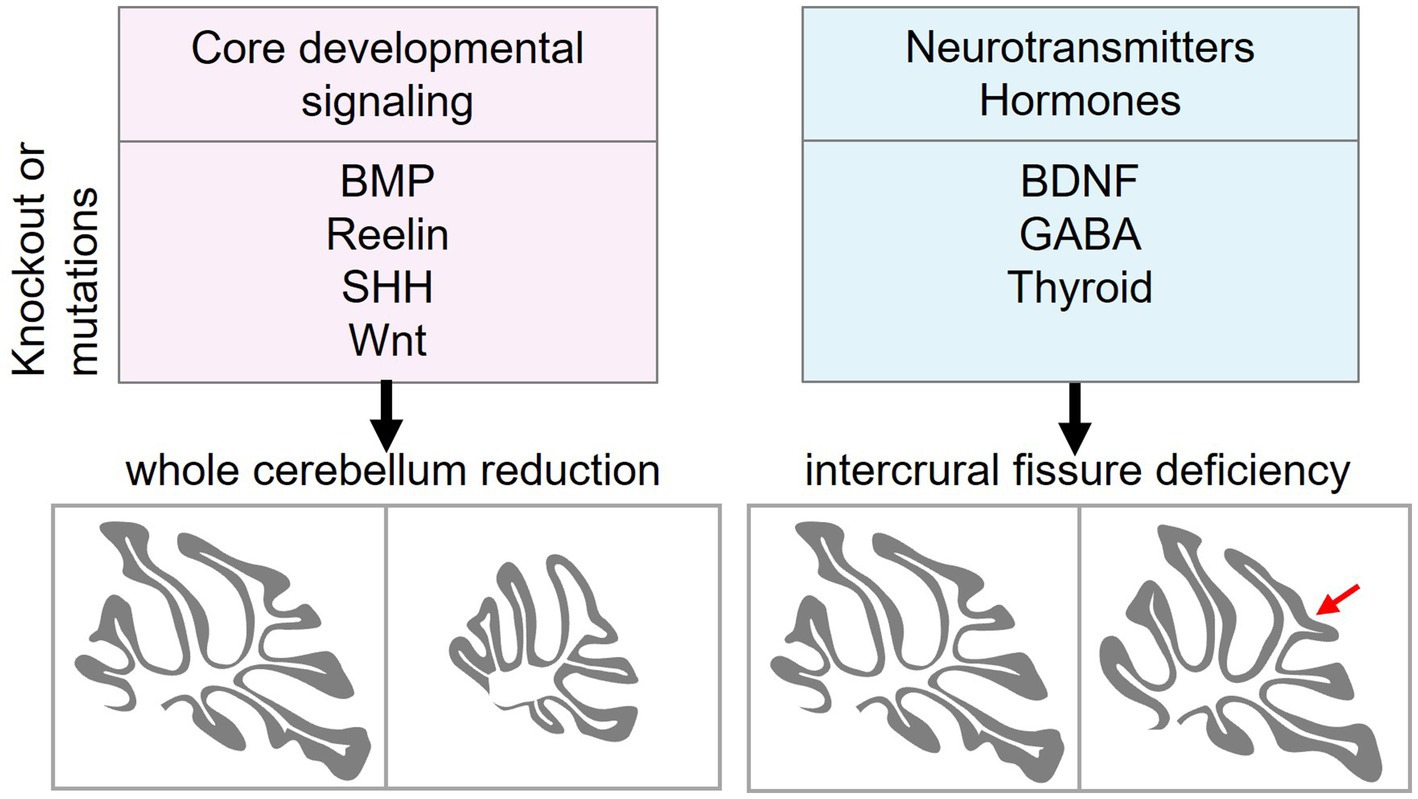
Figure 3. Distinct roles of core developmental signaling and neurotransmitter/hormone signaling in cerebellar morphogenesis. Core developmental signaling pathways, including BMP, Reelin, Shh, and Wnt, regulate the overall growth and patterning of the cerebellum. A Disruption of these pathways reduces the size of the cerebellum. In contrast, ablation of neurotransmitters and hormones such as BDNF (and other neurotrophins), GABA, and thyroid hormone impairs the intercrural fissure (red arrow) formation without affecting gross cerebellar morphology. BMP, bone morphogenetic protein; Shh, sonic hedgehog; BDNF, brain-derived neurotrophic factor; GABA, γ-aminobutyric acid.
8 BDNF is crucial for the formation of the fissure between lobules VI and VII
Since ASD is associated with abnormalities in vermal lobules VI and VII, identifying the key pathways that shape the intercrural fissure is essential. Although BDNF has been implicated in the formation of the intercrural fissure, strong evidence has been lacking. Strikingly, conventional knockout of two paralogous copies of Rbm4 gene results in the loss of the intercrural fissure and a significant reduction in BDNF levels (Tsai et al., 2023). RBM4 is an alternative splicing regulator (Markus and Morris, 2009). RNA-seq analysis of Rbm4 knockout brains revealed intron retention in Hsf1, which encodes a transcriptional activator for Bdnf (Shen et al., 2024). Intron retention leads to downregulation of HSF1 protein, and hence BDNF reduction. Prenatal re-expression of HSF1 in Rbm4 knockout brains restored BDNF levels and the intercrural fissure. Similar results were obtained with prenatal supplementation of 7,8-dihydroxyflavone, a TrkB agonist (Tsai et al., 2023), indicating that BDNF plays a crucial role in the formation of the intercrural fissure. Moreover, activation of N-methyl-D-aspartate (NMDA) receptors induces a kinase cascade involving Ca2+/Calmodulin-dependent protein kinase II (CaMKII) and SR protein kinase 1 (SRPK1), leading to phosphorylation of RBM4. Phosphorylated RBM4 translocates into the nucleus, where it promotes Hsf1 intron excision (Figure 4). Such stimulus-activated splicing mechanism is in line with the report that neuronal activation promotes the removal of retained introns (Mazille et al., 2022). It is noteworthy that, unlike Hsf1 intron excision upon NMDA stimulation, acute stress induces nuclear translocation of HSF1 for Bdnf transactivation in the hippocampus (Franks et al., 2023). Thus, developmental cues and cellular stress activate HSF1 via different mechanisms. Taken altogether, BDNF plays a crucial role in intercrural fissure formation. Further studies are needed to determine why this fissure is especially sensitive to BDNF signaling and whether BDNF is crucial for functional circuit formation particularly in the central cerebellar vermis during development.
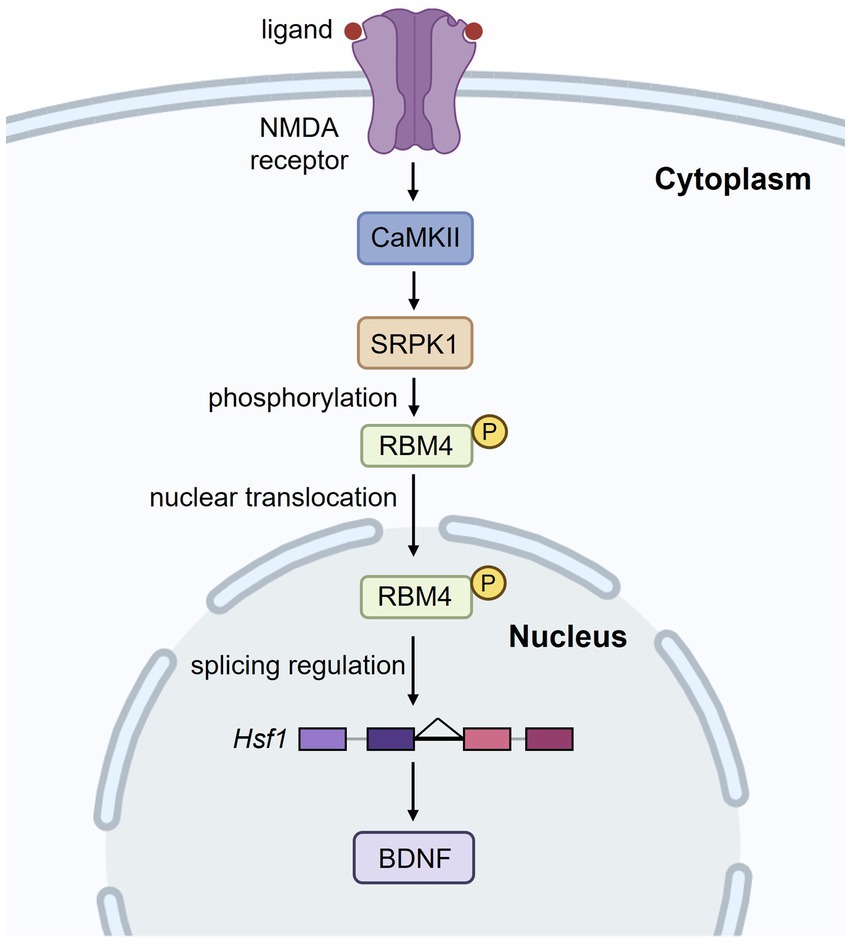
Figure 4. N-methyl-D-aspartate (NMDA) receptor activation regulates RBM4-mediated splicing of Hsf1. Upon NMDA receptor activation, a signaling cascade involving CaMKII and SRPK1 leads to the phosphorylation of RBM4. Phosphorylated RBM4 translocates into the nucleus, where it regulates the intron excision of Hsf1. This, in turn, enhances BDNF expression, which is crucial for neuronal function and development.
9 Interventions for cerebellar dysfunction
Mouse model with cerebellar hypoplasia can recapitulate key features of human cerebellar disorders (Section 5), making them a viable tool for developing interventions against neurodevelopmental disorders. For example, Joubert syndrome mouse model with defective Wnt signaling exhibit cerebellar midline fusion that can be partially reversed with lithium treatment, an agonist of Wnt signaling (Lancaster et al., 2011). Moreover, a mutation of the glia cell-line derived neurotrophic factor Ret gene causes cerebellar hypoplasia in mice that mimics Down’s syndrome. Such a mutation impairs Shh-mediated development of GCs and glial fibers, and a Smo agonists can rescue these neuronal defects (Ohgami et al., 2021). Mice with mutant methyl-CpG-binding protein 2 gene provide a Rett syndrome model and exhibit deficient BDNF–TrkB activity. A small molecule TrkB agonist, LM22A-4, can alleviate the motor learning deficits in these mice (Medeiros et al., 2024). Cerebellar BDNF expression is reduced in postmortem SCA6 human tissues (Takahashi et al., 2012). Consistent with this finding, reduced TrkB–BDNF signaling is evident in the early disease stage of a SCA6 mouse model (SCA684Q/84Q). Prolonged administration of 7,8-dihydroxyflavone improved ataxic phenotypes and PC firing rate (Cook et al., 2022). As described above, prenatal administration of 7,8-dihydroxyflavone restored cerebellar development and motor learning in Rbm4 knockout mice (Tsai et al., 2023). Given the effects of HSF1 overexpression in Rbm4 knockout mice (Shen et al., 2024), using small-molecule compounds to activate HSF1 for intervention is possible. HSF1 is targeted by multiple stress-induced signaling cascades (Hooper et al., 2016). Tanespimycin (17-AAG), a derivative of the antibiotic geldanamycin, can de-repress HSF1 from sequestration by its molecular chaperone HSP90 (Chen et al., 2014). 17-AAG can restore synaptic protein levels such as PSD95 and BDNF in Alzheimer’s disease models (Chen et al., 2014). Therefore, it may be possible to treat developmentally disordered brains with low BDNF, such as those with Rbm4 knockout, with 17-AAG or similar functional molecules. These findings suggest that pharmacological restoration of signaling activities may be useful for treating developmental disorders in the future.
10 Conclusion
This review summarizes key developmental signaling pathways and neuromodulators involved in cerebellar development. Dysregulation of these pathways results in cerebellar malformation, ranging from hypoplasia to local foliation defects. It is noteworthy that BDNF plays a pivotal role in shaping the intercrural fissure between lobules VI-VII—a structure implicated in ASD. Prenatal restoration of BDNF biogenesis or signaling can prevent cerebellar deficits in BDNF deficient mouse models. Additionally, it is possible to reverse other BDNF-deficient-caused deficits in the cerebellum through activation of TrkB. Unraveling how these pathways converge across species and disorders promises deeper insights into cerebellar morphogenesis and innovative therapies for neurodevelopmental challenges.
Author contributions
C-LS: Visualization, Writing – original draft, Writing – review & editing. Y-YT: Writing – original draft, Writing – review & editing. W-YT: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Grant 113WIA0110177 and 113-2311-B-001-020-MY3 from the National Science and Technology Council of Taiwan to W-YT.
Acknowledgments
We are grateful to Teiichi Furuichi (Tokyo University of Science) for his valuable suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Abdelhamed, Z. A., Abdelmottaleb, D. I., El-Asrag, M. E., Natarajan, S., Wheway, G., Inglehearn, C. F., et al. (2019). The ciliary frizzled-like receptor Tmem67 regulates canonical Wnt/β-catenin signalling in the developing cerebellum via Hoxb5. Sci. Rep. 9:5446. doi: 10.1038/s41598-019-41940-5
Abe, Y., Honsho, M., Itoh, R., Kawaguchi, R., Fujitani, M., Fujiwara, K., et al. (2018). Peroxisome biogenesis deficiency attenuates the BDNF-TrkB pathway-mediated development of the cerebellum. Life Sci. Alliance 1:e201800062. doi: 10.26508/lsa.201800062
Alcaraz, W. A., Gold, D. A., Raponi, E., Gent, P. M., Concepcion, D., and Hamilton, B. A. (2006). Zfp423 controls proliferation and differentiation of neural precursors in cerebellar vermis formation. Proc. Natl. Acad. Sci. USA 103, 19424–19429. doi: 10.1073/pnas.0609184103
Aldinger, K. A., Lehmann, O. J., Hudgins, L., Chizhikov, V. V., Bassuk, A. G., Ades, L. C., et al. (2009). FOXC1 is required for normal cerebellar development and is a major contributor to chromosome 6p25.3 Dandy-Walker malformation. Nat. Genet. 41, 1037–1042. doi: 10.1038/ng.422
Aldinger, K. A., Thomson, Z., Phelps, I. G., Haldipur, P., Deng, M., Timms, A. E., et al. (2021). Spatial and cell type transcriptional landscape of human cerebellar development. Nat. Neurosci. 24, 1163–1175. doi: 10.1038/s41593-021-00872-y
Allen, S. J., Watson, J. J., Shoemark, D. K., Barua, N. U., and Patel, N. K. (2013). GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther. 138, 155–175. doi: 10.1016/j.pharmthera.2013.01.004
Apps, R., and Hawkes, R. (2009). Cerebellar cortical organization: a one-map hypothesis. Nat. Rev. Neurosci. 10, 670–681. doi: 10.1038/nrn2698
Bartelt, L. C., Switonski, P. M., Adamek, G., Longo, F., Carvalho, J., Duvick, L. A., et al. (2024). Dysregulation of zebrin-II cell subtypes in the cerebellum is a shared feature across polyglutamine ataxia mouse models and patients. Sci. Transl. Med. 16:eadn5449. doi: 10.1126/scitranslmed.adn5449
Butts, T., Green, M. J., and Wingate, R. J. (2014). Development of the cerebellum: simple steps to make a 'little brain'. Development 141, 4031–4041. doi: 10.1242/dev.106559
Carletti, B., and Rossi, F. (2008). Neurogenesis in the cerebellum. Neuroscientist 14, 91–100. doi: 10.1177/1073858407304629
Carta, I., Chen, C. H., Schott, A. L., Dorizan, S., and Khodakhah, K. (2019). Cerebellar modulation of the reward circuitry and social behavior. Science 363:581. doi: 10.1126/science.aav0581
Carter, R. A., Bihannic, L., Rosencrance, C., Hadley, J. L., Tong, Y., Phoenix, T. N., et al. (2018). A single-cell transcriptional atlas of the developing murine cerebellum. Curr. Biol. 28, 2910–2920.e2. doi: 10.1016/j.cub.2018.07.062
Cendelin, J. (2014). From mice to men: lessons from mutant ataxic mice. Cerebellum Ataxias 1:4. doi: 10.1186/2053-8871-1-4
Cerminara, N. L., Lang, E. J., Sillitoe, R. V., and Apps, R. (2015). Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nat. Rev. Neurosci. 16, 79–93. doi: 10.1038/nrn3886
Chaki, M., Airik, R., Ghosh, A. K., Giles, R. H., Chen, R., Slaats, G. G., et al. (2012). Exome capture reveals ZNF423 and CEP164 mutations, linking renal ciliopathies to DNA damage response signaling. Cell 150, 533–548. doi: 10.1016/j.cell.2012.06.028
Chakraborty, G., Magagna-Poveda, A., Parratt, C., Umans, J. G., MacLusky, N. J., and Scharfman, H. E. (2012). Reduced hippocampal brain-derived neurotrophic factor (BDNF) in neonatal rats after prenatal exposure to propylthiouracil (PTU). Endocrinology 153, 1311–1316. doi: 10.1210/en.2011-1437
Chen, Y., Wang, B., Liu, D., Li, J. J., Xue, Y., Sakata, K., et al. (2014). Hsp90 chaperone inhibitor 17-AAG attenuates aβ-induced synaptic toxicity and memory impairment. J. Neurosci. 34, 2464–2470. doi: 10.1523/jneurosci.0151-13.2014
Cheng, Y. Y., Chou, Y. T., Lai, F. J., Jan, M. S., Chang, T. H., Jou, I. M., et al. (2020). Wwox deficiency leads to neurodevelopmental and degenerative neuropathies and glycogen synthase kinase 3β-mediated epileptic seizure activity in mice. Acta Neuropathol. Commun. 8:6. doi: 10.1186/s40478-020-0883-3
Cheng, Y., Sudarov, A., Szulc, K. U., Sgaier, S. K., Stephen, D., Turnbull, D. H., et al. (2010). The engrailed homeobox genes determine the different foliation patterns in the vermis and hemispheres of the mammalian cerebellum. Development 137, 519–529. doi: 10.1242/dev.027045
Cho, J. H., and Tsai, M. J. (2006). Preferential posterior cerebellum defect in BETA2/NeuroD1 knockout mice is the result of differential expression of BETA2/NeuroD1 along anterior-posterior axis. Dev. Biol. 290, 125–138. doi: 10.1016/j.ydbio.2005.11.024
Consalez, G. G., Goldowitz, D., Casoni, F., and Hawkes, R. (2021). Origins, development, and compartmentation of the granule cells of the cerebellum. Front. Neural Circuits 14:611841. doi: 10.3389/fncir.2020.611841
Cook, A. A., Jayabal, S., Sheng, J., Fields, E., Leung, T. C. S., Quilez, S., et al. (2022). Activation of TrkB-Akt signaling rescues deficits in a mouse model of SCA6. Sci. Adv. 8:eabh3260. doi: 10.1126/sciadv.abh3260
Courchesne, E., Yeung-Courchesne, R., Press, G. A., Hesselink, J. R., and Jernigan, T. L. (1988). Hypoplasia of cerebellar vermal lobules VI and VII in autism. N. Engl. J. Med. 318, 1349–1354. doi: 10.1056/nejm198805263182102
Cupolillo, D., Hoxha, E., Faralli, A., De Luca, A., Rossi, F., Tempia, F., et al. (2016). Autistic-like traits and cerebellar dysfunction in Purkinje cell PTEN knock-out mice. Neuropsychopharmacology 41, 1457–1466. doi: 10.1038/npp.2015.339
Dave, K. A., and Bordey, A. (2009). GABA increases Ca2+ in cerebellar granule cell precursors via depolarization: implications for proliferation. IUBMB Life 61, 496–503. doi: 10.1002/iub.185
De Luca, A., Cerrato, V., Fucà, E., Parmigiani, E., Buffo, A., and Leto, K. (2016). Sonic hedgehog patterning during cerebellar development. Cell. Mol. Life Sci. 73, 291–303. doi: 10.1007/s00018-015-2065-1
De Mori, R., Romani, M., D'Arrigo, S., Zaki, M. S., Lorefice, E., Tardivo, S., et al. (2017). Hypomorphic recessive variants in SUFU impair the sonic hedgehog pathway and cause Joubert syndrome with cranio-facial and skeletal defects. Am. J. Hum. Genet. 101, 552–563. doi: 10.1016/j.ajhg.2017.08.017
DeLorey, T. M., Sahbaie, P., Hashemi, E., Homanics, G. E., and Clark, J. D. (2008). Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: a potential model of autism spectrum disorder. Behav. Brain Res. 187, 207–220. doi: 10.1016/j.bbr.2007.09.009
Dho, S. E., Othman, K., Zhang, Y., and McGlade, C. J. (2025). NUMB alternative splicing and isoform-specific functions in development and disease. J. Biol. Chem. 301:108215. doi: 10.1016/j.jbc.2025.108215
Diedrichsen, J., Balsters, J. H., Flavell, J., Cussans, E., and Ramnani, N. (2009). A probabilistic MR atlas of the human cerebellum. NeuroImage 46, 39–46. doi: 10.1016/j.neuroimage.2009.01.045
Dussault, I., Fawcett, D., Matthyssen, A., Bader, J. A., and Giguère, V. (1998). Orphan nuclear receptor ROR alpha-deficient mice display the cerebellar defects of staggerer. Mech. Dev. 70, 147–153. doi: 10.1016/s0925-4773(97)00187-1
Engler, A., Zhang, R., and Taylor, V. (2018). Notch and Neurogenesis. Adv. Exp. Med. Biol. 1066, 223–234. doi: 10.1007/978-3-319-89512-3_11
Farini, D., Marazziti, D., Geloso, M. C., and Sette, C. (2021). Transcriptome programs involved in the development and structure of the cerebellum. Cell. Mol. Life Sci. 78, 6431–6451. doi: 10.1007/s00018-021-03911-w
Franks, H., Wang, R., Li, M., Wang, B., Wildmann, A., Ortyl, T., et al. (2023). Heat shock factor HSF1 regulates BDNF gene promoters upon acute stress in the hippocampus, together with pCREB. J. Neurochem. 165, 131–148. doi: 10.1111/jnc.15707
Gao, W. Q., Zheng, J. L., and Karihaloo, M. (1995). Neurotrophin-4/5 (NT-4/5) and brain-derived neurotrophic factor (BDNF) act at later stages of cerebellar granule cell differentiation. J. Neurosci. 15, 2656–2667. doi: 10.1523/jneurosci.15-04-02656.1995
Grinberg, I., Northrup, H., Ardinger, H., Prasad, C., Dobyns, W. B., and Millen, K. J. (2004). Heterozygous deletion of the linked genes ZIC1 and ZIC4 is involved in Dandy-Walker malformation. Nat. Genet. 36, 1053–1055. doi: 10.1038/ng1420
Haldipur, P., Aldinger, K. A., Bernardo, S., Deng, M., Timms, A. E., Overman, L. M., et al. (2019). Spatiotemporal expansion of primary progenitor zones in the developing human cerebellum. Science 366, 454–460. doi: 10.1126/science.aax7526
Haldipur, P., Dang, D., Aldinger, K. A., Janson, O. K., Guimiot, F., Adle-Biasette, H., et al. (2017). Phenotypic outcomes in mouse and human Foxc1 dependent Dandy-Walker cerebellar malformation suggest shared mechanisms. eLife 6:898. doi: 10.7554/eLife.20898
Haldipur, P., and Millen, K. J. (2019). What cerebellar malformations tell us about cerebellar development. Neurosci. Lett. 688, 14–25. doi: 10.1016/j.neulet.2018.05.032
Hashimoto,, and Mikoshiba, (2003). Mediolateral compartmentalization of the cerebellum is determined on the "birth date" of Purkinje cells. J. Neurosci. 23, 11342–11351. doi: 10.1523/JNEUROSCI.23-36-11342.2003
Hong, S. E., Shugart, Y. Y., Huang, D. T., Shahwan, S. A., Grant, P. E., Hourihane, J. O., et al. (2000). Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat. Genet. 26, 93–96. doi: 10.1038/79246
Hooper, P. L., Durham, H. D., Török, Z., Hooper, P. L., Crul, T., and Vígh, L. (2016). The central role of heat shock factor 1 in synaptic fidelity and memory consolidation. Cell Stress Chaperones 21, 745–753. doi: 10.1007/s12192-016-0709-1
Iskusnykh, I. Y., and Chizhikov, V. V. (2022). Cerebellar development after preterm birth. Front. Cell Dev. Biol. 10:1068288. doi: 10.3389/fcell.2022.1068288
Jossin, Y. (2020). Reelin functions, mechanisms of action and signaling pathways during brain development and maturation. Biomol. Ther. 10:964. doi: 10.3390/biom10060964
Kawamura, A., Katayama, Y., Kakegawa, W., Ino, D., Nishiyama, M., Yuzaki, M., et al. (2021). The autism-associated protein CHD8 is required for cerebellar development and motor function. Cell Rep. 35:108932. doi: 10.1016/j.celrep.2021.108932
Kelly, R. M., and Strick, P. L. (2003). Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J. Neurosci. 23, 8432–8444. doi: 10.1523/jneurosci.23-23-08432.2003
Kim, J. J., Gill, P. S., Rotin, L., van Eede, M., Henkelman, R. M., Hui, C. C., et al. (2011). Suppressor of fused controls mid-hindbrain patterning and cerebellar morphogenesis via GLI3 repressor. J. Neurosci. 31, 1825–1836. doi: 10.1523/jneurosci.2166-10.2011
Kozareva, V., Martin, C., Osorno, T., Rudolph, S., Guo, C., Vanderburg, C., et al. (2021). A transcriptomic atlas of mouse cerebellar cortex comprehensively defines cell types. Nature 598, 214–219. doi: 10.1038/s41586-021-03220-z
Lancaster, M. A., Gopal, D. J., Kim, J., Saleem, S. N., Silhavy, J. L., Louie, C. M., et al. (2011). Defective Wnt-dependent cerebellar midline fusion in a mouse model of Joubert syndrome. Nat. Med. 17, 726–731. doi: 10.1038/nm.2380
Lancaster, M. A., Louie, C. M., Silhavy, J. L., Sintasath, L., Decambre, M., Nigam, S. K., et al. (2009). Impaired Wnt-beta-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nat. Med. 15, 1046–1054. doi: 10.1038/nm.2010
Lanore, F., Cayco-Gajic, N. A., Gurnani, H., Coyle, D., and Silver, R. A. (2021). Cerebellar granule cell axons support high-dimensional representations. Nat. Neurosci. 24, 1142–1150. doi: 10.1038/s41593-021-00873-x
Lee, J. M., Kim, I. S., Kim, H., Lee, J. S., Kim, K., Yim, H. Y., et al. (2010). RORalpha attenuates Wnt/beta-catenin signaling by PKCalpha-dependent phosphorylation in colon cancer. Mol. Cell 37, 183–195. doi: 10.1016/j.molcel.2009.12.022
Legué, E., Gottshall, J. L., Jaumouillé, E., Roselló-Díez, A., Shi, W., Barraza, L. H., et al. (2016). Differential timing of granule cell production during cerebellum development underlies generation of the foliation pattern. Neural Dev. 11:17. doi: 10.1186/s13064-016-0072-z
Leto, K., Arancillo, M., Becker, E. B., Buffo, A., Chiang, C., Ding, B., et al. (2016). Consensus paper: cerebellar development. Cerebellum 15, 789–828. doi: 10.1007/s12311-015-0724-2
Leung, A. W., and Li, J. Y. H. (2018). The molecular pathway regulating Bergmann glia and folia generation in the cerebellum. Cerebellum 17, 42–48. doi: 10.1007/s12311-017-0904-3
Li, S., Qiu, F., Xu, A., Price, S. M., and Xiang, M. (2004). Barhl1 regulates migration and survival of cerebellar granule cells by controlling expression of the neurotrophin-3 gene. J. Neurosci. 24, 3104–3114. doi: 10.1523/jneurosci.4444-03.2004
Lin, Y. C., Hsu, C. H., Wang, P. N., Lin, C. P., and Chang, L. H. (2020). The relationship between Zebrin expression and cerebellar functions: insights from neuroimaging studies. Front. Neurol. 11:315. doi: 10.3389/fneur.2020.00315
Manto, M. U., and Jissendi, P. (2012). Cerebellum: links between development, developmental disorders and motor learning. Front. Neuroanat. 6:1. doi: 10.3389/fnana.2012.00001
Markus, M. A., and Morris, B. J. (2009). RBM4: a multifunctional RNA-binding protein. Int. J. Biochem. Cell Biol. 41, 740–743. doi: 10.1016/j.biocel.2008.05.027
Masserdotti, G., Badaloni, A., Green, Y. S., Croci, L., Barili, V., Bergamini, G., et al. (2010). ZFP423 coordinates notch and bone morphogenetic protein signaling, selectively up-regulating Hes5 gene expression. J. Biol. Chem. 285, 30814–30824. doi: 10.1074/jbc.M110.142869
Mazille, M., Buczak, K., Scheiffele, P., and Mauger, O. (2022). Stimulus-specific remodeling of the neuronal transcriptome through nuclear intron-retaining transcripts. EMBO J. 41:e110192. doi: 10.15252/embj.2021110192
Medeiros, D., Ayala-Baylon, K., Egido-Betancourt, H., Miller, E., Chapleau, C., Robinson, H., et al. (2024). A small-molecule TrkB ligand improves dendritic spine phenotypes and atypical behaviors in female Rett syndrome mice. Dis. Model. Mech. 17:dmm050612. doi: 10.1242/dmm.050612
Minichiello, L., Casagranda, F., Tatche, R. S., Stucky, C. L., Postigo, A., Lewin, G. R., et al. (1998). Point mutation in trkB causes loss of NT4-dependent neurons without major effects on diverse BDNF responses. Neuron 21, 335–345. doi: 10.1016/s0896-6273(00)80543-7
Miyata, T., Nakajima, K., Mikoshiba, K., and Ogawa, M. (1997). Regulation of Purkinje cell alignment by reelin as revealed with CR-50 antibody. J. Neurosci. 17, 3599–3609. doi: 10.1523/jneurosci.17-10-03599.1997
Moon, U. Y., Park, J. Y., Park, R., Cho, J. Y., Hughes, L. J., McKenna, J., et al. (2015). Impaired reelin-Dab1 signaling contributes to neuronal migration deficits of tuberous sclerosis complex. Cell Rep. 12, 965–978. doi: 10.1016/j.celrep.2015.07.013
Müller, C. C., Nguyen, T. H., Ahlemeyer, B., Meshram, M., Santrampurwala, N., Cao, S., et al. (2011). PEX13 deficiency in mouse brain as a model of Zellweger syndrome: abnormal cerebellum formation, reactive gliosis and oxidative stress. Dis. Model. Mech. 4, 104–119. doi: 10.1242/dmm.004622
Neale, B. M., Kou, Y., Liu, L., Ma'ayan, A., Samocha, K. E., Sabo, A., et al. (2012). Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485, 242–245. doi: 10.1038/nature11011
Neveu, I., and Arenas, E. (1996). Neurotrophins promote the survival and development of neurons in the cerebellum of hypothyroid rats in vivo. J. Cell Biol. 133, 631–646. doi: 10.1083/jcb.133.3.631
Ohgami, N., Iizuka, A., Hirai, H., Yajima, I., Iida, M., Shimada, A., et al. (2021). Loss-of-function mutation of c-ret causes cerebellar hypoplasia in mice with Hirschsprung disease and down's syndrome. J. Biol. Chem. 296:100389. doi: 10.1016/j.jbc.2021.100389
O'Roak, B. J., Vives, L., Girirajan, S., Karakoc, E., Krumm, N., Coe, B. P., et al. (2012). Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485, 246–250. doi: 10.1038/nature10989
Owens, D. F., and Kriegstein, A. R. (2002). Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 3, 715–727. doi: 10.1038/nrn919
Peeters, R. P., Hernandez, A., Ng, L., Ma, M., Sharlin, D. S., Pandey, M., et al. (2013). Cerebellar abnormalities in mice lacking type 3 deiodinase and partial reversal of phenotype by deletion of thyroid hormone receptor α1. Endocrinology 154, 550–561. doi: 10.1210/en.2012-1738
Pei, Y., Brun, S. N., Markant, S. L., Lento, W., Gibson, P., Taketo, M. M., et al. (2012). WNT signaling increases proliferation and impairs differentiation of stem cells in the developing cerebellum. Development 139, 1724–1733. doi: 10.1242/dev.050104
Portella, A. C., Carvalho, F., Faustino, L., Wondisford, F. E., Ortiga-Carvalho, T. M., and Gomes, F. C. (2010). Thyroid hormone receptor beta mutation causes severe impairment of cerebellar development. Mol. Cell. Neurosci. 44, 68–77. doi: 10.1016/j.mcn.2010.02.004
Qin, L., Wine-Lee, L., Ahn, K. J., and Crenshaw, E. B. (2006). Genetic analyses demonstrate that bone morphogenetic protein signaling is required for embryonic cerebellar development. J. Neurosci. 26, 1896–1905. doi: 10.1523/jneurosci.3202-05.2006
Quevedo, C., Sauzeau, V., Menacho-Márquez, M., Castro-Castro, A., and Bustelo, X. R. (2010). Vav3-deficient mice exhibit a transient delay in cerebellar development. Mol. Biol. Cell 21, 1125–1139. doi: 10.1091/mbc.e09-04-0292
Rahimi-Balaei, M., Bergen, H., Kong, J., and Marzban, H. (2018). Neuronal migration during development of the cerebellum. Front. Cell. Neurosci. 12:484. doi: 10.3389/fncel.2018.00484
Ramirez, M., Wu, J., Liu, M., Wu, D., Weeden, D., and Goldowitz, D. (2022). The cerebellar gene database: a collective database of genes critical for cerebellar development. Cerebellum 21, 606–614. doi: 10.1007/s12311-022-01445-w
Raymond, J. L., and Medina, J. F. (2018). Computational principles of supervised learning in the cerebellum. Annu. Rev. Neurosci. 41, 233–253. doi: 10.1146/annurev-neuro-080317-061948
Reddy, N. C., Majidi, S. P., Kong, L., Nemera, M., Ferguson, C. J., Moore, M., et al. (2021). CHARGE syndrome protein CHD7 regulates epigenomic activation of enhancers in granule cell precursors and gyrification of the cerebellum. Nat. Commun. 12:5702. doi: 10.1038/s41467-021-25846-3
Sadakata, T., Kakegawa, W., Mizoguchi, A., Washida, M., Katoh-Semba, R., Shutoh, F., et al. (2007). Impaired cerebellar development and function in mice lacking CAPS2, a protein involved in neurotrophin release. J. Neurosci. 27, 2472–2482. doi: 10.1523/jneurosci.2279-06.2007
Schwartz, P. M., Borghesani, P. R., Levy, R. L., Pomeroy, S. L., and Segal, R. A. (1997). Abnormal cerebellar development and foliation in BDNF−/− mice reveals a role for neurotrophins in CNS patterning. Neuron 19, 269–281. doi: 10.1016/s0896-6273(00)80938-1
Selvadurai, H. J., and Mason, J. O. (2011). Wnt/β-catenin signalling is active in a highly dynamic pattern during development of the mouse cerebellum. PLoS One 6:e23012. doi: 10.1371/journal.pone.0023012
Sepp, M., Leiss, K., Murat, F., Okonechnikov, K., Joshi, P., Leushkin, E., et al. (2024). Cellular development and evolution of the mammalian cerebellum. Nature 625, 788–796. doi: 10.1038/s41586-023-06884-x
Shen, C. L., Tsai, Y. Y., Chou, S. J., Chang, Y. M., and Tarn, W. Y. (2024). RBM4-mediated intron excision of Hsf1 induces BDNF for cerebellar foliation. Commun. Biol. 7:1712. doi: 10.1038/s42003-024-07328-6
Sheng, G., Xu, X., Lin, Y. F., Wang, C. E., Rong, J., Cheng, D., et al. (2008). Huntingtin-associated protein 1 interacts with Ahi1 to regulate cerebellar and brainstem development in mice. J. Clin. Invest. 118, 2785–2795. doi: 10.1172/jci35339
Shinoda, Y., Sadakata, T., Nakao, K., Katoh-Semba, R., Kinameri, E., Furuya, A., et al. (2011). Calcium-dependent activator protein for secretion 2 (CAPS2) promotes BDNF secretion and is critical for the development of GABAergic interneuron network. Proc. Natl. Acad. Sci. USA 108, 373–378. doi: 10.1073/pnas.1012220108
Sillitoe, R. V., and Joyner, A. L. (2007). Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu. Rev. Cell Dev. Biol. 23, 549–577. doi: 10.1146/annurev.cellbio.23.090506.123237
Sillitoe, R. V., Vogel, M. W., and Joyner, A. L. (2010). Engrailed homeobox genes regulate establishment of the cerebellar afferent circuit map. J. Neurosci. 30, 10015–10024. doi: 10.1523/jneurosci.0653-10.2010
Spassky, N., Han, Y. G., Aguilar, A., Strehl, L., Besse, L., Laclef, C., et al. (2008). Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev. Biol. 317, 246–259. doi: 10.1016/j.ydbio.2008.02.026
Stoodley, C. J., and Schmahmann, J. D. (2018). Functional topography of the human cerebellum. Handb. Clin. Neurol. 154, 59–70. doi: 10.1016/b978-0-444-63956-1.00004-7
Stoodley, C. J., Valera, E. M., and Schmahmann, J. D. (2012). Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage 59, 1560–1570. doi: 10.1016/j.neuroimage.2011.08.065
Stoodley, C. J., and Tsai, P. T. (2021). Adaptive prediction for social contexts: the cerebellar contribution to typical and atypical social behaviors. Annu. Rev. Neurosci. 44, 475–493. doi: 10.1146/annurev-neuro-100120-092143
Subashini, C., Dhanesh, S. B., Chen, C. M., Riya, P. A., Meera, V., Divya, T. S., et al. (2017). Wnt5a is a crucial regulator of neurogenesis during cerebellum development. Sci. Rep. 7:42523. doi: 10.1038/srep42523
Sudarov, A., and Joyner, A. L. (2007). Cerebellum morphogenesis: the foliation pattern is orchestrated by multi-cellular anchoring centers. Neural Dev. 2:26. doi: 10.1186/1749-8104-2-26
Sugihara, I. (2018). Crus I in the rodent cerebellum: its homology to crus I and II in the primate cerebellum and its anatomical uniqueness among neighboring lobules. Cerebellum 17, 49–55. doi: 10.1007/s12311-017-0911-4
Takahashi, M., Ishikawa, K., Sato, N., Obayashi, M., Niimi, Y., Ishiguro, T., et al. (2012). Reduced brain-derived neurotrophic factor (BDNF) mRNA expression and presence of BDNF-immunoreactive granules in the spinocerebellar ataxia type 6 (SCA6) cerebellum. Neuropathology 32, 595–603. doi: 10.1111/j.1440-1789.2012.01302.x
Talkowski, M. E., Rosenfeld, J. A., Blumenthal, I., Pillalamarri, V., Chiang, C., Heilbut, A., et al. (2012). Sequencing chromosomal abnormalities reveals neurodevelopmental loci that confer risk across diagnostic boundaries. Cell 149, 525–537. doi: 10.1016/j.cell.2012.03.028
Tong, K. K., and Kwan, K. M. (2013). Common partner Smad-independent canonical bone morphogenetic protein signaling in the specification process of the anterior rhombic lip during cerebellum development. Mol. Cell. Biol. 33, 1925–1937. doi: 10.1128/mcb.01143-12
Tong, K. K., Ma, T. C., and Kwan, K. M. (2015). BMP/Smad signaling and embryonic cerebellum development: stem cell specification and heterogeneity of anterior rhombic lip. Develop. Growth Differ. 57, 121–134. doi: 10.1111/dgd.12198
Trommsdorff, M., Gotthardt, M., Hiesberger, T., Shelton, J., Stockinger, W., Nimpf, J., et al. (1999). Reeler/disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 97, 689–701. doi: 10.1016/s0092-8674(00)80782-5
Tsai, P. T., Hull, C., Chu, Y., Greene-Colozzi, E., Sadowski, A. R., Leech, J. M., et al. (2012). Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature 488, 647–651. doi: 10.1038/nature11310
Tsai, Y. Y., Shen, C. L., Dhananjaya, D., Tsai, C. Y., and Tarn, W. Y. (2023). Activation of TrkB signaling mitigates cerebellar anomalies caused by Rbm4-Bdnf deficiency. Commun. Biol. 6:910. doi: 10.1038/s42003-023-05294-z
Valente, E. M., Marsh, S. E., Castori, M., Dixon-Salazar, T., Bertini, E., Al-Gazali, L., et al. (2005). Distinguishing the four genetic causes of Jouberts syndrome-related disorders. Ann. Neurol. 57, 513–519. doi: 10.1002/ana.20422
Valente, E. M., Silhavy, J. L., Brancati, F., Barrano, G., Krishnaswami, S. R., Castori, M., et al. (2006). Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat. Genet. 38, 623–625. doi: 10.1038/ng1805
Wagner, M. J., and Luo, L. (2020). Neocortex-cerebellum circuits for cognitive processing. Trends Neurosci. 43, 42–54. doi: 10.1016/j.tins.2019.11.002
Wallace, V. A. (1999). Purkinje-cell-derived sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr. Biol. 9, 445–448. doi: 10.1016/s0960-9822(99)80195-x
Wang, L., Nomura, M., Goto, Y., Tanaka, K., Sakamoto, R., Abe, I., et al. (2011). Smad2 protein disruption in the central nervous system leads to aberrant cerebellar development and early postnatal ataxia in mice. J. Biol. Chem. 286, 18766–18774. doi: 10.1074/jbc.M111.223271
Warming, S., Rachel, R. A., Jenkins, N. A., and Copeland, N. G. (2006). Zfp423 is required for normal cerebellar development. Mol. Cell. Biol. 26, 6913–6922. doi: 10.1128/mcb.02255-05
Wischhof, L., Maida, S., Piazzesi, A., Gioran, A., Barragan Sanz, K., Irsen, S., et al. (2017). The SWI/SNF subunit Bcl7a contributes to motor coordination and Purkinje cell function. Sci. Rep. 7:17055. doi: 10.1038/s41598-017-17284-3
Witter, L., and De Zeeuw, C. I. (2015). In vivo differences in inputs and spiking between neurons in lobules VI/VII of neocerebellum and lobule X of archaeocerebellum. Cerebellum 14, 506–515. doi: 10.1007/s12311-015-0654-z
Yang, H., Jensen, P., and Goldowitz, D. (2002). The community effect and Purkinje cell migration in the cerebellar cortex: analysis of scrambler chimeric mice. J. Neurosci. 22, 464–470. doi: 10.1523/jneurosci.22-02-00464.2002
Zentner, G. E., Layman, W. S., Martin, D. M., and Scacheri, P. C. (2010). Molecular and phenotypic aspects of CHD7 mutation in CHARGE syndrome. Am. J. Med. Genet. A 152a, 674–686. doi: 10.1002/ajmg.a.33323
Zhang, T., Liu, T., Mora, N., Guegan, J., Bertrand, M., Contreras, X., et al. (2021). Generation of excitatory and inhibitory neurons from common progenitors via notch signaling in the cerebellum. Cell Rep. 35:109208. doi: 10.1016/j.celrep.2021.109208
Keywords: cerebellar foliation, cerebellar fissure, cerebellar disorder, developmental signaling, BDNF
Citation: Shen C-L, Tsai Y-Y and Tarn W-Y (2025) Sculptors of cerebellar fissures and their potential as therapeutic targets for cerebellar dysfunction. Front. Cell. Neurosci. 19:1608185. doi: 10.3389/fncel.2025.1608185
Edited by:
Tatsuro Mutoh, Fujita Health University, JapanReviewed by:
Zhongjiao Jiang, University at Buffalo, United StatesYasuaki Mizutani, Fujita Health University, Japan
Copyright © 2025 Shen, Tsai and Tarn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Woan-Yuh Tarn, d3Rhcm5AaWJtcy5zaW5pY2EuZWR1LnR3
†These authors have contributed equally to this work
 Chiu-Lun Shen
Chiu-Lun Shen Yu-Young Tsai
Yu-Young Tsai Woan-Yuh Tarn
Woan-Yuh Tarn