- 1School of Medicine, Tsinghua Medicine, Tsinghua University, Beijing, China
- 2Translational Neuroscience Program, Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States
- 3Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States
- 4Department of Neurology, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States
Microtubule-associated protein 2 (MAP2) is a key regulator of cytoskeletal dynamics and neuronal function. It stabilizes microtubules, shapes dendrites, influences synaptic plasticity, and regulates transportation and protein synthesis through its interactions with other proteins. MAP2 undergoes extensive phosphorylation, which dynamically modulates these interactions and alters MAP2 functions. This review provides a comprehensive overview of MAP2 structure, its diverse functional roles in neurons, the kinases that regulate its phosphorylation. We highlight how phosphorylation by Src family kinases, proline-directed kinases, MARK, PKA, PKC, and CAMKII governs MAP2’s role in cytoskeletal organization, protein chaperone activity, and dendrite outgrowth.
1 Introduction
The regulation of neuronal structure and function is critical for understanding the mechanisms underlying neurodevelopmental and neurodegenerative disorders. Microtubules are a key component of the neuronal cytoskeleton that provide structural support and facilitate intracellular trafficking, both of which are essential for maintaining neuronal architecture and synaptic function (Goodson and Jonasson, 2018). The stability and organization of the microtubule network are regulated by microtubule-associated proteins (MAPs), which play an integral role in modulating microtubule dynamics (Dehmelt and Halpain, 2004). Among these, microtubule-associated protein 2 (MAP2) is the primary dendritic MAP, playing a pivotal role in dendrite formation and organization by cross-linking and stabilizing microtubules (Dehmelt and Halpain, 2004). The specific localization of MAP2 in dendrites has underscored its importance in synaptic plasticity and neuronal signaling, though new functions of MAP2 continue to be identified (Abel et al., 1997; Gumy et al., 2017; Ribeiro and de Wit, 2017; Harada et al., 2002; Ainsztein and Purich, 1994; Grubisha et al., 2021; Kim et al., 2020).
MAP2 is highly phosphorylated in vivo, and its phosphorylation state is tightly regulated during neuronal development by multiple signaling pathways (Yang et al., 2023; Riederer et al., 1995). Phosphorylation influences MAP2’s interactions with other proteins, especially its interaction with microtubules and its ability to stabilize them (Grubisha et al., 2021; Brugg and Matus, 1991; Komulainen et al., 2014; Liu et al., 2019; Zamora-Leon et al., 2001; DeGiosio et al., 2023). Dysregulated MAP2 phosphorylation has been implicated in a variety of neurological and psychiatric disorders, including Alzheimer’s disease and schizophrenia, highlighting the importance of understanding how phosphorylation modulates MAP2’s function (Grubisha et al., 2021; DeGiosio et al., 2019; Zhang and Dong, 2012; Rudrabhatla et al., 2011). Despite significant advances in research, the precise mechanisms by which phosphorylation alters MAP2 activity, and the extent to which these changes contribute to disease progression, remain incompletely understood.
This review aims to provide a comprehensive view of current knowledge of MAP2 phosphorylation and its impact on MAP2 affinity to its interactors. We will discuss the functional consequences of phosphorylation at key sites. Additionally, we will highlight recent findings on the kinases that regulate MAP2 phosphorylation.
2 MAP2 structure
MAP2 is produced in several isoforms through alternative splicing, which can be divided into two major groups: high-molecular-weight (HMW) isoforms, MAP2A (280 kDa) and MAP2b (270 kDa), and low-molecular-weight (LMW) isoforms, MAP2c (70 kDa) and MAP2d (75 kDa) (Figures 1, 2) (Neve et al., 1986; Kalcheva et al., 1995). Both HMW and LMW isoforms share four core domains: a PKA-binding domain, a proline-rich domain, a microtubule-binding domain, and a C-terminal domain. The HMW variants are distinguished by the presence of an additional projection domain located between the PKA-binding and proline-rich domain, which is absent in the LMW variants (Figure 2) (Kindler et al., 1990).
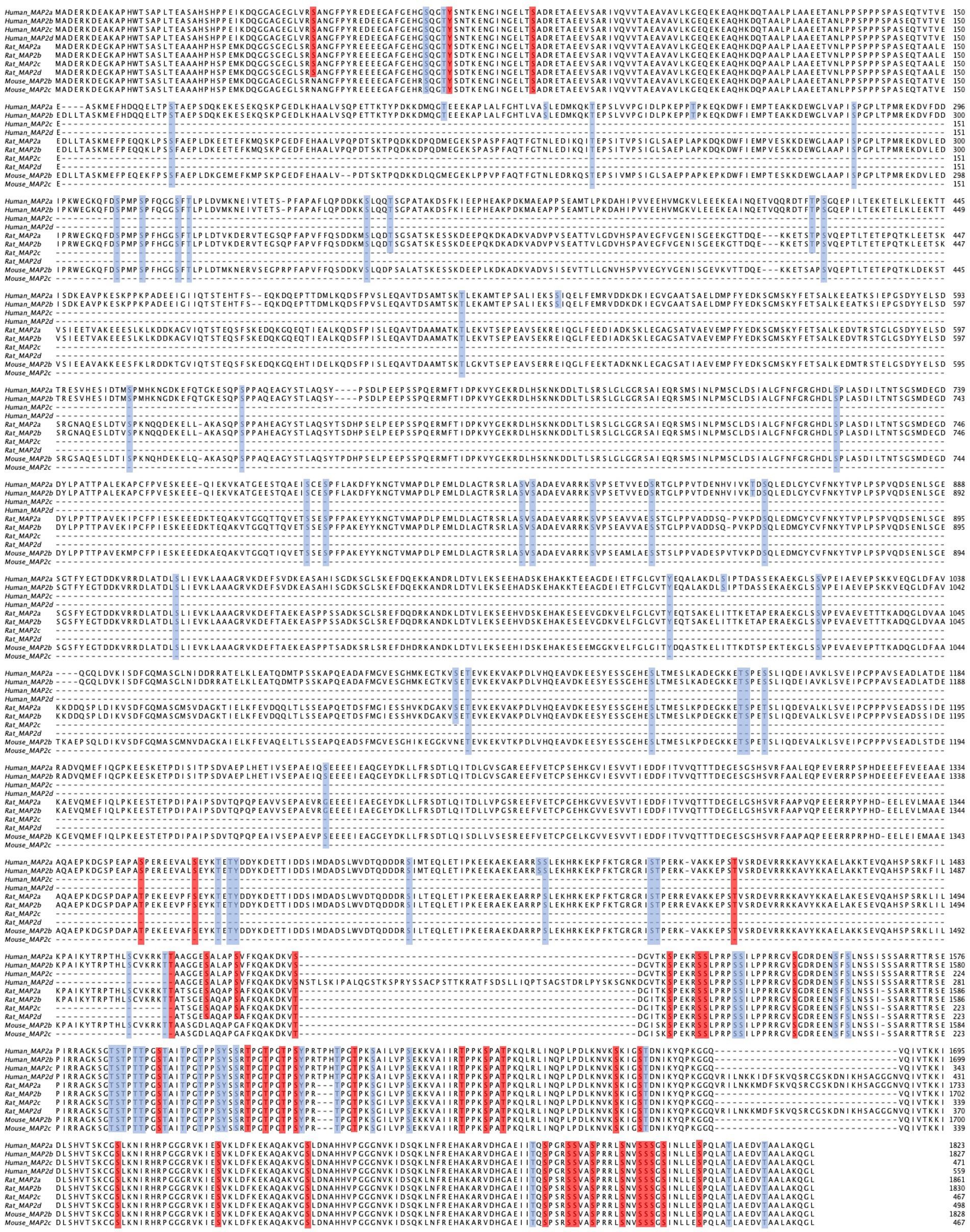
Figure 1. Multiple sequence alignment of MAP2 isoforms from human, mouse, and rat. Conserved regions are evident at the N-and C-termini, while notable differences are seen in the central region corresponding to the presence or absence of the projection domain. Residues highlighted in blue represent high-throughput screened phosphorylation sites, whereas those in red indicate low-throughput validated phosphorylation sites. Note, however, that many residues conserved across species are nevertheless identified by slightly different amino acid positions e.g., (see S1782 in human MAP2b, S1785 in rat MAP2b, and S1783 in mouse MAP2b). The alignment was performed using MAFFT and visualized with Jalview (Katoh and Standley, 2013; Waterhouse et al., 2009).
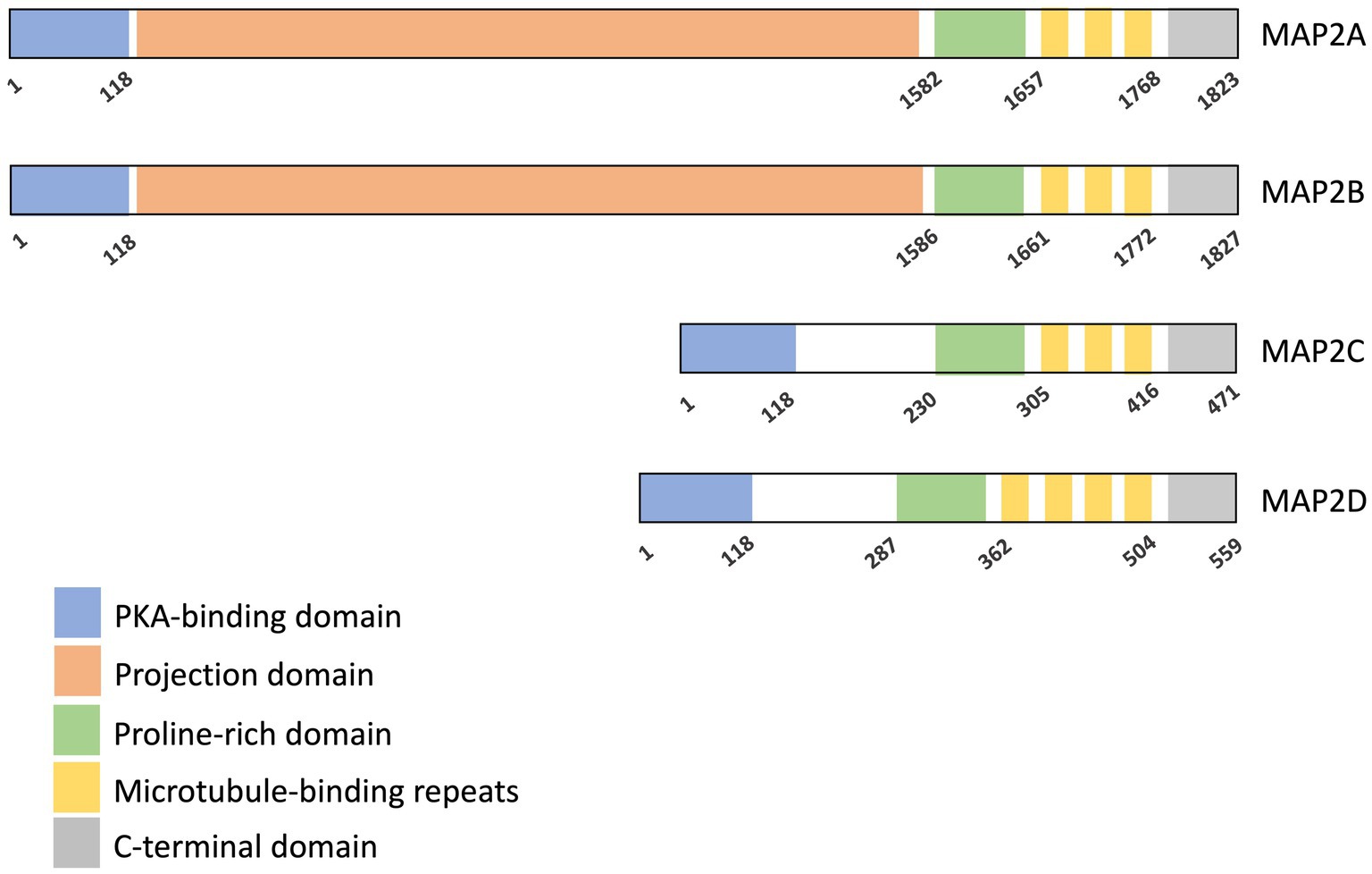
Figure 2. Domain organization of MAP2 isoforms. Four isoforms of human MAP2 are shown here. All isoforms share a PKA-binding domain, a proline-rich domain, a microtubule-binding domain (MTBD), and a C-terminal region. MAP2A and MAP2B additionally contain a projection domain. Start and end amino acid positions for each domain are indicated (Melkova et al., 2019). Microtubule interaction is primarily mediated by the microtubule-binding repeats, with contributions from the proline-rich and C-terminal domains. The projection domain is known to modulate the affinity of MAP2 for microtubules.
The microtubule-binding domain contains three or four conserved binding repeats, each 18 amino acids in length. It is critical for MAP2’s primary function of binding and stabilizing microtubules (Doll et al., 1993; Goode et al., 1997). Other regions, including the proline-rich and the C-terminal domain, also contribute to this binding activity (Chen et al., 1992; Ferralli et al., 1994). The projection domain, exclusive to HMW isoforms, acts as a regulator of microtubule interactions and also a spacer to ensure adequate spacing between microtubules and nearby cellular components (Chen et al., 1992). Furthermore, it prevents the entry of MAP2A and MAP2B into axons, thereby directing their localization to dendrites (Kanai and Hirokawa, 1995).
MAP2 isoforms are intrinsically disordered in their unbound state, characterized by a lack of secondary structure and a high degree of conformational flexibility. This disordered nature allows MAP2 to interact dynamically with the complex cytoskeleton (Kosciolek et al., 2017; Novacek et al., 2013). Importantly, this intrinsic disorder can be modulated by post-translational modifications, such as phosphorylation, which can alter MAP2’s interactions and functional properties within the cell (Grubisha et al., 2021; Jansen et al., 2017). Despite this disordered nature, MAP2 also contains rigid motifs, including the PKA-binding and microtubule-binding motifs (Melkova et al., 2019).
3 MAP2 functions
Knowledge of MAP2 functions provides the context for understanding the significance of its phosphorylation. As a key regulator of cytoskeletal dynamics, cargo transport, and dendritic signaling, MAP2’s functions are intricately linked to its structural and interaction properties. Phosphorylation plays a pivotal role in modulating these functions by altering MAP2’s interactions with cytoskeletal components and other proteins.
3.1 Cytoskeleton structure
MAP2 was identified as a microtubule-associated protein due to its ability to bind to and stabilize microtubules. It exerts this stabilization effect by reducing the frequency of catastrophes and slowing down the shortening rate of microtubules (Gamblin et al., 1996; Itoh and Hotani, 1994). Furthermore, MAP2 can facilitate microtubule bundling, acting as a spacer within the microtubule bundle (Chen et al., 1992; Weisshaar and Matus, 1993). MAP2 has been shown to promote tubulin polymerization through its microtubulin-binding region, which can be regulated by phosphorylation (Ainsztein and Purich, 1994). MAP2, via its microtubulin-binding region can also bind to other cytoskeleton components, such as actin, acting as the crosslinker between microtubule and actin. MAP2 can also interact with Plectin (an intermediate filament-associated cytolinker protein), which can antagonize the microtubule stabilization ability of MAP2 (Valencia et al., 2013).
3.2 Dendritic morphology
Neurite initiation depends on the rapid reorganization of the cytoskeleton, achieved through the coordination of microtubules and actin filaments. Given its ability to influence both microtubule and actin organization, it creates a conducive environment for triggering neurite initiation (L. Dehmelt et al., 2003). MAP2 has been shown to be shifted and concentrated in the outgrowth and branching areas of dendrites in several cell lines (Matus et al., 1986; Ferreira et al., 1989; Chamak et al., 1987). Evidence indicates that the expression of MAP2c can induce the rapid formation of dense and stable microtubule bundles at the distal plus end. Conversely, knocking down MAP2 in primary cortical neurons and hippocampal neurons results in a reduction in both the number and length of dendrites, as well as a decrease in microtubule presence within dendrites (Sharma et al., 1994; Harada et al., 2002; LeClerc et al., 1993; Dehmelt et al., 2003). MAP2 serves as the receptor for neurosteroid pregnenolone (PREG) to enhance neurite outgrowth by stimulating microtubule polymerization (Fontaine-Lenoir et al., 2006).
3.3 Synaptic plasticity
MAP2 is essential for long-term potentiation (LTP) and the dendritic spine changes induced by LTP. Kim et al. have demonstrated that MAP2 is translocated to the spines in response to LTP stimulation and the knockdown of HMW MAP2 causes a deficit of LTP induction and abolishes LTP-induced surface delivery of AMPA receptors and spine enlargement. However, the knockdown of LMW MAP2 does not show the same effect (Kim et al., 2020). Also, MAP2 contributes to the inhibition of microtubule entry into dendritic spines induced by long-term depression (LTD) by mediating the prolonged redistribution of EB3 (End-binding protein 3) along MAP2-positive microtubule bundles in the dendritic shaft, thereby suppressing microtubule dynamics (Kapitein et al., 2011). EB3 is a member of microtubule plus-end binding proteins, which regulate microtubule dynamics (Jaworski et al., 2009).
3.4 Protein folding
MAP2 has been reported to show a chaperone-like activity, preventing protein aggregation under thermal or chemical induction and assisting in enzyme refolding (Sarkar et al., 2004). Specifically, MAP2c plays a role in preventing tau aggregation, which is implicated in Alzheimer’s disease. Electron microscopy studies show that MAP2c inhibits arachidonic acid-induced tau aggregation in vitro, suggesting a role in maintaining tau homeostasis. Despite the high homology between the C-terminal regions of tau and MAP2c, the N-terminal region of MAP2c alone does not mediate its chaperone activity (Mitra et al., 2015).
3.5 Cargo transportation
MAP2 is responsible for regulating kinesin/dynein-dependent microtubule transporting in neurons (Heins et al., 1991; Lopez and Sheetz, 1993; Seitz et al., 2002; Hagiwara et al., 1994). This regulation is achieved in two ways: (1) MAP2 and motor proteins both bind to the C-terminus of tubulin, competing for the same binding sites on microtubules; (2) HMW MAP2 can interact with motor proteins, like KIF5B, preventing its binding to microtubules through the steric inhibition caused by MAP2B’s projection domain. By coordinating with motor proteins, MAP2B forms a filtering zone at the axon initial segment that controls cargo entrance to the axon in sensory neurons (Hagiwara et al., 1994; Gumy et al., 2017). The regulation by MAP2 of cargo transportation thus enables the polarized distribution of neuronal proteins and organelles, which is vital to normal neuronal function.
3.6 Signal transduction
MAP2 serves as the dominant anchoring protein of cAMP-dependent protein kinase (PKA) in dendrites, whose kinase activity is known to be involved in various biological functions in neurons (Abel et al., 1997; Malleret et al., 2001). MAP2 binds to the regulatory subunit II (RII) of PKA through its N-terminus and determines the localization of PKA in neurons (Vallee et al., 1981; Theurkauf and Vallee, 1982). MAP2 serves as a reservoir for PKA. A deficit in MAP2 impairs the ability of PKA to phosphorylate key substrates, such as CREB and AMPA receptors, following signal stimulation. This disruption ultimately blocks the downstream signal transduction pathway (Zhong et al., 2009; Harada et al., 2002). Furthermore, the RII subunit of PKA, but not RI, binds specifically to MAP2 and is shielded from calpain degradation when associated with MAP2 (Alexa et al., 1996).
Additionally, MAP2 comprises 11 PXXP motifs which are the potential binding ligands of Src homology 3 (SH3) domains. MAP2 interacts with several SH3 domain-containing proteins, for example, the non-receptor protein tyrosine kinase Src and Fyn and the adaptor protein Grb2 (see 4.1 below). Src family kinases are a group of non-receptor kinases containing conserved SH3 and SH2 domains, which mediate substrate recognition and protein–protein interactions (Parsons and Parsons, 2004). These kinases play critical roles in neuronal signaling and cytoskeletal regulation. Also, the MAP2 Co-IP experiment identified several SH-domain-containing proteins within the MAP2 interactome, including guanine nucleotide exchange factors and members of the Rho family of GTPases, both of which are involved in signal transduction (Lyu et al., 2024). The interaction between MAP2 and proteins mentioned above may indicate the role of MAP2 as a scaffold protein.
3.7 mRNA binding and protein synthesis
MAP2 has been implicated in the regulation of protein synthesis. Overexpression of the MAP2c isoform has been shown to inhibit protein synthesis in HEK cells, suggesting a direct role in translational control, though the mechanism is still unclear (Grubisha et al., 2021). This inhibitory role is further supported by the composition of the MAP2 interactome, which includes multiple RNA-binding proteins known to modulate translation, many of which are associated with suppressing protein synthesis. A key interaction underlying this regulatory function is between MAP2 and insulin-like growth factor 2 mRNA-binding protein 1 (IMP1). MAP2 binds directly to IMP1 via its K-homology (KH) domains, which are critical for nucleic acid binding (Nielsen et al., 2002). Furthermore, proteomic analyses of the MAP2 interactome have identified numerous RNA-binding proteins involved in the regulation of translation, many of which are associated with inhibitory effects on protein synthesis (Grubisha et al., 2021). Together, these findings suggest that MAP2 contributes to the modulation of protein synthesis, potentially through interactions with RNA-binding proteins and direct effects on translational machinery.
4 Key kinases regulating MAP2 phosphorylation
MAP2 undergoes extensive phosphorylation in vivo, which, as mentioned earlier, is crucial due to its intrinsically disordered nature. This phosphorylation can induce significant changes in its structure and thereby influencing its function primarily by regulating its interactions with other proteins (Tsuyama et al., 1987; Newcombe et al., 2022; Melkova et al., 2019). Phosphorylation plays a crucial role in regulating MAP2 function, primarily by modulating its interactions with other proteins. Over the years, considerable research has focused on identifying the specific phosphorylation sites on MAP2, as well as elucidating how phosphorylation modulates its activities. These modifications, catalyzed by a range of kinases, influence MAP2’s role in cytoskeletal dynamics, transport, and chaperone activity. Understanding how these kinases regulate MAP2 phosphorylation is essential for elucidating MAP2’s involvement in neuronal development and disease.
4.1 Src family kinase
MAP2 contains a proline-rich RTPPKSP motif which specifically interacts with the SH3 domain of Src family kinases. Among the Src family kinases, MAP2 has been reported to interact with two key members of the Src family kinase, Fyn and Src (Zamora-Leon et al., 2001; Sontag et al., 2012). Phosphorylation of MAP2 by Fyn was initially demonstrated in vitro (Zamora-Leon et al., 2001), with mutagenesis studies of MAP2c later identifying tyrosine 67 (Y67) as the primary phosphorylation site on the MAP2c isoform (Zamora-Leon et al., 2005). Phosphorylation at this site has also been confirmed in the human fetal brain, and it assists in recruiting the SH2 domain of Grb2 (Zamora-Leon et al., 2005). The binding of Fyn to MAP2 also inhibits the interaction between MAP2 and protein phosphatase 2A (PP2A), thereby suppressing PP2A’s dephosphorylation activity, which may stabilize MAP2 phosphorylation states (Sontag et al., 2012).
Interestingly, although MAP2 interacts with Src via the SH3 domain, Src does not phosphorylate MAP2 in vivo (Lim and Halpain, 2000).
4.2 Proline-directed protein kinases (PDPK)
PDPKs are a class of serine/threonine kinases that specifically phosphorylate substrates at serine or threonine residues followed by a proline (Ser/Thr-Pro motifs). These kinases are crucial regulators of cell signaling pathways. The MAPK (mitogen-activated protein kinase) family, cyclin-dependent kinases (CDKs), and glycogen synthase kinase 3 (GSK-3) are among the well-known PDPKs. MAP2’s proline-rich domain is abundant in Ser/Thr-Pro motifs, making it a target for PDPKs.
4.2.1 Mitogen-activated protein kinases (MAPKs)
The family of mitogen-activated protein kinases (MAPKs) includes extracellular signal-regulated kinase (ERK), p38, and c-Jun NH (2)-terminal kinase (JNK). Among them, ERK and JNK are known kinases of MAP2. ERK1 and ERK2 are coded by two distinct genes, MAPK3 and MAPK1,and are highly expressed in the brain. They are associated with microtubules and levels increase during brain development (Busca et al., 2016; Boulton et al., 1991; Reszka et al., 1995). ERK is a key component of the Raf–MEK–ERK signaling pathway, contributing to cellular processes like adhesion, migration, and neuronal differentiation (Busca et al., 2016). The phosphorylation sites of ERK in MAP2c have been screened by NMR spectrometry with single residue resolution (Plucarova et al., 2022), identifying 12 Pro-Xxx-Ser/Thr-Pro sequences and 7 Ser/Thr-Pro motifs of MAP2c as the targets of ERK2 (Plucarova et al., 2022). Whether, or to what extent, these sites may also be targets for regulation by ERK1 is not established. Phosphorylation of MAP2 by ERK happens primarily in the proline-rich domain and microtubule-binding domain in MAP2, and it disrupts the interaction of MAP2 with various proteins, namely regulatory subunit RIIα of cAMP-dependent PKA, Grb2, Fyn, Src, and even ERK itself (Lim and Halpain, 2000; Zamora-Leon et al., 2001; Plucarova et al., 2022; Hoshi et al., 1992). Also, the phosphorylation by ERK abolishes the ability to induce tubulin nucleation and polymerization of MAP2 in vitro (Hoshi et al., 1992). During dendrite formation induced by neuronal depolarization, ERK and CAMKII activation enhances MAP2 phosphorylation and expression, highlighting ERK’s role in activity-dependent neuronal remodeling (Vaillant et al., 2002). Also, phosphorylation of MAP2 by ERK is crucial for its translocation to dendritic spines in response to LTP stimulation (Kim et al., 2020).
The function of phosphorylation by ERK2 at specific sites has also been studied. Specific phosphorylation events, such as those at T197 and T293 of MAP2c, further impair microtubule assembly and inhibit MAP2’s actin-binding capacity, linking ERK signaling to cytoskeletal reorganization (DeGiosio et al., 2023). It should be pointed out that ERK2 phosphorylates S422 of rat MAP2c (homologous to S426 of human MAP2c and S1782 of human MAP2b). Notably, S426/S1782 is abnormally hyperphosphorylated in schizophrenia. Phosphomimetic mutation at this site has been shown to reduce dendritic length, complexity, and spine density in mouse brains. Co-immunoprecipitation studies reveal that phosphorylation at S1782 disrupts interactions with a significant portion of the MAP2 interactome, including tubulin and actin, while promoting binding to a smaller subset of proteins, including protein phosphatase 1 L (PPM1L) and Kelch-Like Family Member 8 (KLHL8) (Grubisha et al., 2021; Lyu et al., 2024; DeGiosio et al., 2023).
JNKs, are critical regulators of cellular responses to stress, inflammation, and synaptic plasticity (Schellino et al., 2019). JNK activation is a hallmark of pathological cell death in conditions such as Alzheimer’s disease, emphasizing its role in neuronal dysfunction (Yarza et al., 2015). MAP2 was first identified as a downstream effector of JNK in neurons in 2005 (Bjorkblom et al., 2005). It’s reported that JNK can phosphorylate MAP2 in intact neurons and the phosphorylation sites accumulate in the proline-rich domain of MAP2 (Bjorkblom et al., 2005). Phosphorylation by JNK promotes MAP2-dependent process elongation, facilitating dendrite shape regulation in neurons. Notably, JNK-mediated regulation of dendrite morphology is dominant over ERK in this context (Bjorkblom et al., 2005). Mechanistically, JNK1 phosphorylates high-molecular-weight MAP2 (HMW-MAP2) at T1619, T1622, and T1625, enhancing MAP2’s interaction with and stabilization of microtubules (Komulainen et al., 2014).
4.2.2 Glycogen synthase kinase 3 (GSK3)
GSK is a serine/threonine kinase implicated in diverse cellular processes, including metabolism, cytoskeletal regulation, and neuronal function. In mammals, GSK3 exists in two highly homologous isoforms, GSK3α and GSK3β, encoded by two different genes (Woodgett, 1990). GSK3 plays a broad role in neurodevelopment, particularly through its regulation of a wide range of transcription factors and cytoskeletal dynamics, including microtubules (Hur and Zhou, 2010). The regulation of the cytoskeleton by GSK3 is in part by GSK3-mediated phosphorylation of MAPs, like tau, MAP1B, and MAP2 (Barnes et al., 2007; Trivedi et al., 2005; Sanchez et al., 2000). As with many other PDPKs, GSK3 phosphorylates MAP2 within its proline-rich domain, targeting specific threonine residues (Sanchez et al., 1996). Initial studies identified phosphorylation of GSK3α/β at the threonine residues within the sequence “RTPGTPGTPSY,” which was also detected in vivo in rat brain (Sanchez et al., 1996). Further investigations localized these phosphorylation events to T1620 and T1623 in the MAP2B isoform (Sanchez et al., 1996). Functional studies in COS-1 cells revealed that GSK3β-mediated phosphorylation of MAP2C reduced its binding affinity for microtubules, disrupting the formation of microtubule bundles and thereby altering cytoskeletal organization (Sanchez et al., 2000).
The phosphorylation activity of GSK3 is tightly regulated and can be reversed by protein phosphatases, specifically protein phosphatase 1 (PP1) and 2A (PP2A) (Sanchez et al., 1996). Pathologically, MAP2 phosphorylated at T1620 and T1623 has been observed within granules formed during the early stages of neurofibrillary tangle formation, a hallmark of neurodegenerative diseases. Notably, these granules also contain hyperphosphorylated GSK3β (Andres-Benito et al., 2023). Additionally, evidence suggests that activation of the Akt/GSK3 pathway is associated with increased MAP2 expression, though the underlying mechanisms remain unclear (Chang et al., 2013; Repar et al., 2018; Zhang et al., 2024).
4.2.3 Cyclin-dependent kinases (CDKs)
Cyclin-dependent kinases (CDKs) are a family of serine/threonine kinases crucial for cell cycle regulation. Beyond their well-established roles in cell cycle progression, CDKs are also implicated in transcriptional control and neuronal functions (Lim and Kaldis, 2013; Dhariwala and Rajadhyaksha, 2008). CDKs require association with cyclins or other regulatory proteins for activation, and their activity is tightly controlled by phosphorylation (Lim and Kaldis, 2013; Dhariwala and Rajadhyaksha, 2008). In the nervous system, CDK5 is the most abundant CDK in neurons. CDK5 does not regulate the cell cycle like other CDKs but instead plays a pivotal role in neuron development and functions (Dhariwala and Rajadhyaksha, 2008). It modulates cytoskeletal proteins like MAP2 and tau, influencing neuronal structure and connectivity (Shah and Lahiri, 2017).
The relation of MAP2 and CDK5 was first observed in mice with NPC-1 gene mutation, where MAP2 was found to be hyperphosphorylated and accumulated in the brain. This coincided with increased CDK5 activity and elevated levels of its activators, p25 and p35 (Bu et al., 2002). Further studies revealed that CDK5 phosphorylates HMW MAP2 at multiple sites, including threonine 1613TPGTPGTP1620 and S1782 (homologous to S426 in LMW MAP2, discussed earlier). The phosphorylation within the 1613TPGTPGTP1620 motif, which contains two PXXP sequences, is predicted to interfere with interactions involving SH3 domains (Tseng et al., 2005). Phosphoproteomic studies using cdk5−/− mouse brains further identified hypophosphorylation of MAP2 at T1650, suggesting CDK5’s site-specificity in regulating MAP2 phosphorylation (Contreras-Vallejos et al., 2014).
Other than CDK5, MAP2 can be phosphorylated by CDK1 (cdc2 kinase) and CDK2. 60% of CDK1 phosphorylation events are localized to the microtubule-binding domain (Itoh et al., 1997). This includes phosphorylation of the “RTPGTPGTPSY” sequence, which is known to impair MAP2’s microtubule nucleation and stabilization activity (Sanchez et al., 1996; Itoh et al., 1997). CDK2, on the other hand, phosphorylates MAP2C at S264, S178, and other ERK2-targeted sites except for S274 and S448, with enhanced efficiency at S178 (Plucarova et al., 2022).
4.3 Microtubule affinity-regulating kinases (MARKs)
Microtubule affinity-regulating kinases (MARKs), also known as Par-1 kinases, are serine/threonine kinases that regulate microtubule dynamics by phosphorylating microtubule-associated proteins (MAPs), including MAP2, MAP4, and tau (Drewes et al., 1997). MARKs are involved in maintaining cytoskeletal stability, neuronal polarity, and intracellular transport, making them essential for proper neuronal function (Drewes et al., 1997).
Microtubule affinity-regulating kinases (MARKs) were first identified for their ability to phosphorylate microtubule-associated proteins (MAPs) and disrupt their interaction with microtubules (MTs), profoundly altering cytoskeletal dynamics (Drewes et al., 1995). The first discovered MARK isoform, p110MARK, was initially characterized for phosphorylating tau and later shown to phosphorylate MAP2 at conserved KXGS motifs in the MAP2 MT binding domain (Drewes et al., 1995). Two major phosphorylation sites in MAP2 targeted by p110MARK are Ser1713 (KCGS motif) and Ser1682 (KIGS motif). This phosphorylation dissociates MAP2 from MTs, leading to increased dynamic instability of the microtubule network (Illenberger et al., 1996).
Further studies demonstrated that MARK1 and MARK2, primarily expressed in brain, share this ability to efficiently block MAP2-MT interactions (Drewes et al., 1997). Overexpression of MARK1 or MARK2 in CHO cells results in the destruction of the microtubule network without affecting the microfilament network, highlighting their specific regulatory role. Conversely, co-transfection with a MAP2c mutant (S319A/S350A) resistant to MARK phosphorylation effectively counteracted this microtubule destabilization, underscoring the direct correlation between KXGS motif phosphorylation and cytoskeletal disassembly (Drewes et al., 1997).
4.4 cAMP-dependent protein kinase (PKA)
PKA, a serine/threonine kinase activated by cyclic AMP (cAMP), plays a critical role in regulating cytoskeletal dynamics and synaptic plasticity (Huang et al., 2013). MAP2 serves as a major substrate of PKA in neurons (Theurkauf and Vallee, 1982, 1983). The regulatory subunit RII of PKA interacts with a conserved 83-amino-acid sequence at the distal N-terminus of MAP2 isoforms (MAP2A, MAP2B, and MAP2C) (Obar et al., 1989). In vitro studies demonstrate that PKA can phosphorylate MAP2B at up to 15 moles of phosphate per molecule, with 70% of these phosphorylation events distributed across the proline-rich and microtubule-binding domains (Itoh et al., 1997). Detailed analyses using tryptic digestion and two-dimensional phosphopeptide mapping identified 11 major PKA-specific phosphorylation sites, all localized to serine residues (Goldenring et al., 1985). These phosphopeptides were also present in the rat brain and GH3 cells (Diaz-Nido et al., 1990; Jefferson and Schulman, 1991). NMR spectrometry has further resolved these phosphorylation sites on MAP2C with single-residue precision (Jansen et al., 2017). Notably, four PKA phosphorylation sites overlap with those targeted by calcium/calmodulin-dependent protein kinase II (CaMKII) (Walaas and Nairn, 1989). During development, in cytosol, PKA phosphorylation of MAP2 increased from 2 days to adult in proportion to the increase in the concentration of MAP2 in chicken brains (Koszka et al., 1991).
Phosphorylation of MAP2 by PKA alters its interactions with microtubules and other cellular structures. PKA-mediated phosphorylation of MAP2 reduces its ability to bind to and nucleate microtubules, although its ability to stabilize microtubules remains unaffected (Itoh et al., 1997). Within the microtubule-binding domain of MAP2C, PKA phosphorylates serine residues at the KXGS motifs (S319, S350, and S382). These phosphorylations result in MAP2C dissociation from microtubules and redistribution to the peripheral actin cytoskeleton (Ozer and Halpain, 2000). Additionally, phosphorylation by PKA enhances MAP2C’s affinity for 14–3-3ζ, a regulatory protein that competes with tubulin for MAP2 binding, although this interaction does not disrupt MAP2C-microtubule binding (Jansen et al., 2017). PKA-mediated phosphorylation also abolished the chaperone-like ability of MAP2c for preventing tau fibril formation induced by arachidonic acid in vitro (Mitra et al., 2015).
The functional implications of PKA-mediated phosphorylation extend beyond microtubule dynamics. For example, phosphorylation at T220 significantly inhibits the calpain-induced hydrolysis of MAP2, a protection not observed with CaMKII-mediated phosphorylation (Johnson and Foley, 1993; Alexa et al., 1996; Alexa et al., 2002). Also, phosphorylation by PKA reduced binding to SH3 domain containing proteins, like Src and Grb2 (Lim and Halpain, 2000).
MAP2 phosphorylated by PKA from the brain can be dephosphorylated by calcineurin and protein phosphatase with Km values in the range of 1–3 μM and 1.6–2.7 μM, respectively (Goto et al., 1985; Yamamoto et al., 1988). PKA signaling also mediates dendritic remodeling. Activation of D1 dopamine receptors (D1Rs), which stimulate the cAMP-PKA pathway, increases MAP2 phosphorylation and correlates with decreased dendritic extension (Song et al., 2002).
4.5 Calcium/phospholipid-dependent protein kinase (PKC)
Protein kinase C (PKC) is a serine/threonine kinase widely distributed across various brain regions. It is activated by Ca2+ and phospholipids (Saito et al., 1988). PKC in brain tissue can be classified into three main subtypes α, β (I and II) and γ, with primary structures highly homologous and conserved. The γ subtype is localized in the brain and spinal cord uniquely (Domanska-Janik, 1996).
PKC was first identified as a kinase capable of phosphorylating MAP2 in rat brains, using two-dimensional gel electrophoresis (Rodnight et al., 1985). Subsequent studies revealed that PKC specifically phosphorylates serine residues on MAP2 and is capable of incorporating at least 15 moles of phosphate into MAP2 (Walaas and Nairn, 1989; Akiyama et al., 1986). The phosphorylation sites targeted by PKC are located within the tubulin-binding domain (S1703, S1711, and S1728 in mouse Map2b, homologous to S1702, S1710, and S1727 in human MAP2b) and the projection domain. The homologous phosphorylation sites have been validated in rat brain tissues (S1705, S1713, and S1730 in rat MAP2b) (Tsuyama et al., 1986; Ainsztein and Purich, 1994).
Functionally, PKC-mediated phosphorylation modulates the ability of MAP2 to interact with cytoskeletal components. Overall phosphorylation by PKC reduces both microtubule polymerization and actin cross-linking induced by MAP2, while phosphorylation at S1728 completely abolishes MAP2’s microtubule-binding ability (Hoshi et al., 1988; Ainsztein and Purich, 1994; Diaz-Nido et al., 1990). Furthermore, under pathological conditions such as hyperammonemia, PKC activity on MAP2 is significantly reduced, highlighting its regulatory importance in both normal and diseased states (Felipo et al., 1993).
4.6 Calcium/calmodulin-dependent protein kinase II (CAMKII)
CAMKII is a serine/threonine kinase activated upon binding of Ca2+/calmodulin. It is the most abundant post-synaptic domain protein. The three isoforms of CAMKII, CaMKIIβ, CAMKIIγ and CAMKIIδ, are more broadly distributed across the brain regions and cell types, whereas CaMKIIα is predominantly expressed in excitatory neurons and certain inhibitory neurons, such as Purkinje cells (Bayer et al., 1999). CAMKII is essential to synaptic plasticity, learning, and memory (Silva et al., 1992; Yasuda et al., 2022).
CaMKII phosphorylates MAP2 in the brain, incorporating up to 5 moles of phosphate into the protein (Vallano et al., 1986; Yamauchi and Fujisawa, 1982). Tryptic digestion and two-dimensional phosphopeptide mapping identified five major phosphopeptides targeted by CaMKII, four of which involve threonine residues. The remaining serine residue is shared as a phosphorylation site with PKA (Goldenring et al., 1985; Jefferson and Schulman, 1991). Interestingly, different studies using varied phosphorylation conditions and protease digestion have reported four overlapping phosphorylation sites between CaMKII and PKA (Walaas and Nairn, 1989; Jefferson and Schulman, 1991; Schulman, 1984). These phosphorylation sites identified in vitro have also been detected in rat brains and pancreatic cells (Yamamoto et al., 1983; Yamamoto et al., 1985; Krueger et al., 1997).
The functional consequences of MAP2 phosphorylation by CaMKII include inhibition of MAP2-induced microtubule assembly (Yamamoto et al., 1983). During development, MAP2 phosphorylation level decreases in proportion to the declining concentration of CaMKII in chicken brains, indicating a developmental regulation of MAP2 function by this kinase (Koszka et al., 1991). Additionally, the reversal of CaMKII-mediated phosphorylation by calcineurin and protein phosphatase C restores MAP2’s ability to promote microtubule assembly, underscoring the dynamic regulation of MAP2 phosphorylation and dephosphorylation in cellular processes (Goto et al., 1985; Yamamoto et al., 1988).
5 Mapping MAP2 phosphorylations
Whereas the above section focused on cataloging the effects of MAP2 phosphorylations by the upstream kinase tested, an alternative way to comprehend the effects of phosphorylation on MAP2 interactions is at the level of the individual site. In Supplementary Table 1 and Figure 1, we present a list of all currently identified MAP2 phosphosites, and where known, their impact on MAP2 function.
6 Conclusion
This review has summarized the effects of phosphorylation at various sites on MAP2 function, and looked to link these to the kinases that have been identified to date as able to phosphorylate MAP2 at those sites. As the predominant microtubule-associated protein in dendrites, MAP2 plays a crucial role in regulating cytoskeletal dynamics, serving as a scaffold protein for signaling pathways, and facilitating cargo transport within dendrites. MAP2 is highly phosphorylated in vivo, and this phosphorylation is tightly regulated during development. Dysregulation of MAP2 phosphorylation is associated with several neuropsychiatric and neurodegenerative disorders (for further discussion, please see DeGiosio et al., 2022).
Due to the intrinsically disordered nature of MAP2, phosphorylation flexibly influences MAP2 structure and thus its function, modulating its interactions with its many interacting proteins. However, existing studies of the effect of MAP2 phosphorylation have had several limitations. Most studies have focused on MAP2 binding to microtubules and actin, leaving effects on additional MAP2 protein interactions and resultant functions, such as regulation of trafficking, synaptic plasticity, or protein synthesis, largely unexplored. Advanced tools, such as phosphomimetic mutations in combination with proteomic techniques able to concurrently assess MAP2 interactions with multiple binding partners, provide valuable opportunities to address these gaps and identify the functional consequences of specific phosphorylation events in MAP2 in greater depth.
Additionally, the phosphorylation of MAP2 by kinases such as ERK2, CDK2, and PKA has been investigated extensively. However, systematic screening of MAP2 phosphorylations by individual kinases, for example by combining kinase exposure with phosphoenrichment and mass spectrometry, has not been widely employed, resulting in many phosphorylation sites potentially remaining unidentified. Moreover, approaches to identify kinase-specific phosphorylation sites in vitro, while valuable, do not fully replicate cellular phosphorylation dynamics. Future research should aim to overcome these limitations by combining in vivo approaches with high-resolution analytical techniques to achieve a comprehensive understanding of MAP2 phosphorylation and its functional implications.
A third limitation is the limited number of kinases studied in relation to MAP2 phosphorylation. While it is not feasible to experimentally investigate all potential kinases, this gap is being partially addressed through advances in bioinformatic methods that predict kinase-substrate interactions. Bioinformatic prediction of kinase activity is predicated on two components: (1) accurately and conclusively associating a kinase with a specific phosphosite, and (2) quality of assignment of the phosphosite to a specific amino acid within the protein. Within the last 20 years, advances in mass spectrometry-based proteomics have greatly improved both the specificity of phosphosite mapping and associating kinases with their substrates (DeMarco and Hall, 2023; Shuken, 2023). Several labs have endeavored to collate known kinase-phosphosite relationships across multiple species, and kinase substrate recognition sequences from the literature into publicly available databases, most notably PhosphoSite Plus (Supplementary Table 2) (Linding et al., 2008; Krug et al., 2019; Dinkel et al., 2011; Diella et al., 2004; Diella et al., 2008; Huang et al., 2018; Johnson et al., 2023; Poll et al., 2024; Yaron-Barir et al., 2024; Hornbeck et al., 2015). This has spurred the development of bioinformatics tools for inferring kinase activity based on the level of phosphorylation of its substrate or substrate recognition motif (Lai et al., 2012; Terfve et al., 2015; Wiredja et al., 2017; Casado et al., 2013).
Author contributions
JL: Writing – original draft. AD: Writing – review & editing. RS: Writing – review & editing. MG: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported in part by: MH116046 (RS), R01 MH132586 (MG), and MH118513 (MG). JL received support from the Tsinghua University Education Foundation and the Tsinghua Education Foundation North America.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fncel.2025.1610371/full#supplementary-material
References
Abel, T., Nguyen, P. V., Barad, M., Deuel, T. A., Kandel, E. R., and Bourtchouladze, R. (1997). Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88, 615–626. doi: 10.1016/s0092-8674(00)81904-2
Ainsztein, A. M., and Purich, D. L. (1994). Stimulation of tubulin polymerization by MAP-2. Control by protein kinase C-mediated phosphorylation at specific sites in the microtubule-binding region. J. Biol. Chem. 269, 28465–28471. doi: 10.1016/S0021-9258(18)46950-5
Akiyama, T., Nishida, E., Ishida, J., Saji, N., Ogawara, H., Hoshi, M., et al. (1986). Purified protein kinase C phosphorylates microtubule-associated protein 2. J. Biol. Chem. 261, 15648–15651. doi: 10.1016/S0021-9258(18)66765-1
Alexa, A., Schmidt, G., Tompa, P., Ogueta, S., Vazquez, J., Kulcsar, P., et al. (2002). The phosphorylation state of threonine-220, a uniquely phosphatase-sensitive protein kinase a site in microtubule-associated protein MAP2c, regulates microtubule binding and stability. Biochemistry 41, 12427–12435. doi: 10.1021/bi025916s
Alexa, A., Tompa, P., Baki, A., Vereb, G., and Friedrich, P. (1996). Mutual protection of microtubule-associated protein 2 (MAP2) and cyclic AMP-dependent protein kinase II against mu-calpain. J. Neurosci. Res. 44, 438–445. doi: 10.1002/(SICI)1097-4547(19960601)44:5<438::AID-JNR4>3.0.CO;2-G
Andres-Benito, P., Carmona, M., Pirla, M. J., Torrejon-Escribano, B., Del Rio, J. A., and Ferrer, I. (2023). Dysregulated protein phosphorylation as main contributor of granulovacuolar degeneration at the first stages of neurofibrillary tangles pathology. Neuroscience 518, 119–140. doi: 10.1016/j.neuroscience.2021.10.023
Barnes, A. P., Lilley, B. N., Pan, Y. A., Plummer, L. J., Powell, A. W., Raines, A. N., et al. (2007). LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell 129, 549–563. doi: 10.1016/j.cell.2007.03.025
Bayer, K. U., Lohler, J., Schulman, H., and Harbers, K. (1999). Developmental expression of the CaM kinase II isoforms: ubiquitous gamma-and delta-CaM kinase II are the early isoforms and most abundant in the developing nervous system. Brain Res. Mol. Brain Res. 70, 147–154. doi: 10.1016/s0169-328x(99)00131-x
Bjorkblom, B., Ostman, N., Hongisto, V., Komarovski, V., Filen, J. J., Nyman, T. A., et al. (2005). Constitutively active cytoplasmic c-Jun N-terminal kinase 1 is a dominant regulator of dendritic architecture: role of microtubule-associated protein 2 as an effector. J. Neurosci. 25, 6350–6361. doi: 10.1523/JNEUROSCI.1517-05.2005
Boulton, T. G., Nye, S. H., Robbins, D. J., Ip, N. Y., Radziejewska, E., Morgenbesser, S. D., et al. (1991). ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell 65, 663–675. doi: 10.1016/0092-8674(91)90098-j
Brugg, B., and Matus, A. (1991). Phosphorylation determines the binding of microtubule-associated protein 2 (MAP2) to microtubules in living cells. J. Cell Biol. 114, 735–743. doi: 10.1083/jcb.114.4.735
Bu, B., Li, J., Davies, P., and Vincent, I. (2002). Deregulation of cdk5, hyperphosphorylation, and cytoskeletal pathology in the Niemann-pick type C murine model. J. Neurosci. 22, 6515–6525. doi: 10.1523/JNEUROSCI.22-15-06515.2002
Busca, R., Pouyssegur, J., and Lenormand, P. (2016). ERK1 and ERK2 map kinases: specific roles or functional redundancy? Front. Cell Dev. Biol. 4:53. doi: 10.3389/fcell.2016.00053
Casado, P., Rodriguez-Prados, J.-C., Cosulich, S. C., Guichard, S., Vanhaesebroeck, B., Joel, S., et al. (2013). Kinase-substrate enrichment analysis provides insights into the heterogeneity of signaling pathway activation in leukemia cells. Sci. Signal. 6:rs6. doi: 10.1126/scisignal.2003573
Chamak, B., Fellous, A., Glowinski, J., and Prochiantz, A. (1987). MAP2 expression and neuritic outgrowth and branching are coregulated through region-specific neuro-astroglial interactions. J. Neurosci. 7, 3163–3170. doi: 10.1523/JNEUROSCI.07-10-03163.1987
Chang, S. L., Chou, R. H., Zeng, H. J., Lin, Y. H., Chiu, T. Y., Yang, D. M., et al. (2013). Downregulation of DAB2IP promotes mesenchymal-to-neuroepithelial transition and neuronal differentiation of human mesenchymal stem cells. PLoS One 8:e75884. doi: 10.1371/journal.pone.0075884
Chen, J., Kanai, Y., Cowan, N. J., and Hirokawa, N. (1992). Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature 360, 674–677. doi: 10.1038/360674a0
Contreras-Vallejos, E., Utreras, E., Borquez, D. A., Prochazkova, M., Terse, A., Jaffe, H., et al. (2014). Searching for novel Cdk5 substrates in brain by comparative phosphoproteomics of wild type and Cdk5−/− mice. PLoS One 9:e90363. doi: 10.1371/journal.pone.0090363
DeGiosio, R. A., Grubisha, M. J., MacDonald, M. L., McKinney, B. C., Camacho, C. J., and Sweet, R. A. (2022). More than a marker: potential pathogenic functions of MAP2. Front. Mol. Neurosci. 15:974890. doi: 10.3389/fnmol.2022.974890
DeGiosio, R., Kelly, R. M., DeDionisio, A. M., Newman, J. T., Fish, K. N., Sampson, A. R., et al. (2019). Map2 immunoreactivity deficit is conserved across the cerebral cortex within individuals with schizophrenia. NPJ Schizophr. 5:13. doi: 10.1038/s41537-019-0081-0
DeGiosio, R. A., Needham, P. G., Andrews, O. A., Tristan, H., Grubisha, M. J., Brodsky, J. L., et al. (2023). Differential regulation of MAP2 by phosphorylation events in proline-rich versus C-terminal domains. FASEB J. 37:e23194. doi: 10.1096/fj.202300486R
Dehmelt, L., and Halpain, S. (2004). The MAP2/tau family of microtubule-associated proteins. Genome Biol. 6:204. doi: 10.1186/gb-2004-6-1-204
Dehmelt, L., Smart, F. M., Ozer, R. S., and Halpain, S. (2003). The role of microtubule-associated protein 2c in the reorganization of microtubules and lamellipodia during neurite initiation. J. Neurosci. 23, 9479–9490. doi: 10.1523/JNEUROSCI.23-29-09479.2003
DeMarco, A. G., and Hall, M. C. (2023). Phosphoproteomic approaches for identifying phosphatase and kinase substrates. Molecules 28:675. doi: 10.3390/molecules28093675
Dhariwala, F. A., and Rajadhyaksha, M. S. (2008). An unusual member of the Cdk family: Cdk5. Cell. Mol. Neurobiol. 28, 351–369. doi: 10.1007/s10571-007-9242-1
Diaz-Nido, J., Serrano, L., Hernandez, M. A., and Avila, J. (1990). Phosphorylation of microtubule proteins in rat brain at different developmental stages: comparison with that found in neuronal cultures. J. Neurochem. 54, 211–222. doi: 10.1111/j.1471-4159.1990.tb13303.x
Diella, F., Cameron, S., Gemünd, C., Linding, R., Via, A., Kuster, B., et al. (2004). Phospho.ELM: a database of experimentally verified phosphorylation sites in eukaryotic proteins. BMC Bioinformatics 5:79. doi: 10.1186/1471-2105-5-79
Diella, F., Gould, C. M., Chica, C., Via, A., and Gibson, T. J. (2008). Phospho.ELM: a database of phosphorylation sites—update 2008. Nucleic Acids Res. 36, D240–D244. doi: 10.1093/nar/gkm772
Dinkel, H., Chica, C., Via, A., Gould, C. M., Jensen, L. J., Gibson, T. J., et al. (2011). Phospho.ELM: a database of phosphorylation sites--update 2011. Nucleic Acids Res. 39, D261–D267. doi: 10.1093/nar/gkq1104
Doll, T., Meichsner, M., Riederer, B. M., Honegger, P., and Matus, A. (1993). An isoform of microtubule-associated protein 2 (MAP2) containing four repeats of the tubulin-binding motif. J. Cell Sci. 106, 633–639. doi: 10.1242/jcs.106.2.633
Domanska-Janik, K. (1996). Protein serine/threonine kinases (PKA, PKC and CaMKII) involved in ischemic brain pathology. Acta Neurobiol. Exp. (Wars) 56, 579–585. doi: 10.55782/ane-1996-1163
Drewes, G., Ebneth, A., Preuss, U., Mandelkow, E. M., and Mandelkow, E. (1997). MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell 89, 297–308. doi: 10.1016/s0092-8674(00)80208-1
Drewes, G., Trinczek, B., Illenberger, S., Biernat, J., Schmitt-Ulms, G., Meyer, H. E., et al. (1995). Microtubule-associated protein/microtubule affinity-regulating kinase (p110mark). A novel protein kinase that regulates tau-microtubule interactions and dynamic instability by phosphorylation at the Alzheimer-specific site serine 262. J. Biol. Chem. 270, 7679–7688. doi: 10.1074/jbc.270.13.7679
Felipo, V., Grau, E., Minana, M. D., and Grisolia, S. (1993). Hyperammonemia decreases protein-kinase-C-dependent phosphorylation of microtubule-associated protein 2 and increases its binding to tubulin. Eur. J. Biochem. 214, 243–249. doi: 10.1111/j.1432-1033.1993.tb17917.x
Ferralli, J., Doll, T., and Matus, A. (1994). Sequence analysis of MAP2 function in living cells. J. Cell Sci. 107, 3115–3125. doi: 10.1242/jcs.107.11.3115
Ferreira, A., Busciglio, J., and Caceres, A. (1989). Microtubule formation and neurite growth in cerebellar macroneurons which develop in vitro: evidence for the involvement of the microtubule-associated proteins, MAP-1a, HMW-MAP2 and tau. Brain Res. Dev. Brain Res. 49, 215–228. doi: 10.1016/0165-3806(89)90023-0
Fontaine-Lenoir, V., Chambraud, B., Fellous, A., David, S., Duchossoy, Y., Baulieu, E. E., et al. (2006). Microtubule-associated protein 2 (MAP2) is a neurosteroid receptor. Proc. Natl. Acad. Sci. USA 103, 4711–4716. doi: 10.1073/pnas.0600113103
Gamblin, T. C., Nachmanoff, K., Halpain, S., and Williams, R. C. (1996). Recombinant microtubule-associated protein 2c reduces the dynamic instability of individual microtubules. Biochemistry 35, 12576–12586. doi: 10.1021/bi961135d
Goldenring, J. R., Vallano, M. L., and DeLorenzo, R. J. (1985). Phosphorylation of microtubule-associated protein 2 at distinct sites by calmodulin-dependent and cyclic-AMP-dependent kinases. J. Neurochem. 45, 900–905. doi: 10.1111/j.1471-4159.1985.tb04078.x
Goode, B. L., Denis, P. E., Panda, D., Radeke, M. J., Miller, H. P., Wilson, L., et al. (1997). Functional interactions between the proline-rich and repeat regions of tau enhance microtubule binding and assembly. Mol. Biol. Cell 8, 353–365. doi: 10.1091/mbc.8.2.353
Goodson, H. V., and Jonasson, E. M. (2018). Microtubules and microtubule-associated proteins. Cold Spring Harb. Perspect. Biol. 10:608. doi: 10.1101/cshperspect.a022608
Goto, S., Yamamoto, H., Fukunaga, K., Iwasa, T., Matsukado, Y., and Miyamoto, E. (1985). Dephosphorylation of microtubule-associated protein 2, tau factor, and tubulin by calcineurin. J. Neurochem. 45, 276–283. doi: 10.1111/j.1471-4159.1985.tb05504.x
Grubisha, M. J., Sun, X., MacDonald, M. L., Garver, M., Sun, Z., Paris, K. A., et al. (2021). MAP2 is differentially phosphorylated in schizophrenia, altering its function. Mol. Psychiatry 26, 5371–5388. doi: 10.1038/s41380-021-01034-z
Gumy, L. F., Katrukha, E. A., Grigoriev, I., Jaarsma, D., Kapitein, L. C., Akhmanova, A., et al. (2017). MAP2 defines a pre-axonal filtering zone to regulate KIF1-versus KIF5-dependent cargo transport in sensory neurons. Neuron 94, 347–362. doi: 10.1016/j.neuron.2017.03.046
Hagiwara, H., Yorifuji, H., Sato-Yoshitake, R., and Hirokawa, N. (1994). Competition between motor molecules (kinesin and cytoplasmic dynein) and fibrous microtubule-associated proteins in binding to microtubules. J. Biol. Chem. 269, 3581–3589. doi: 10.1016/S0021-9258(17)41903-X
Harada, A., Teng, J., Takei, Y., Oguchi, K., and Hirokawa, N. (2002). MAP2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J. Cell Biol. 158, 541–549. doi: 10.1083/jcb.200110134
Heins, S., Song, Y. H., Wille, H., Mandelkow, E., and Mandelkow, E. M. (1991). Effect of MAP2, MAP2c, and tau on kinesin-dependent microtubule motility. J. Cell Sci. Suppl. 14, 121–124. doi: 10.1242/jcs.1991.supplement_14.24
Hornbeck, P. V., Zhang, B., Murray, B., Kornhauser, J. M., Latham, V., and Skrzypek, E. (2015). Phosphositeplus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 43, D512–D520. doi: 10.1093/nar/gku1267
Hoshi, M., Akiyama, T., Shinohara, Y., Miyata, Y., Ogawara, H., Nishida, E., et al. (1988). Protein-kinase-C-catalyzed phosphorylation of the microtubule-binding domain of microtubule-associated protein 2 inhibits its ability to induce tubulin polymerization. Eur. J. Biochem. 174, 225–230. doi: 10.1111/j.1432-1033.1988.tb14086.x
Hoshi, M., Ohta, K., Gotoh, Y., Mori, A., Murofushi, H., Sakai, H., et al. (1992). Mitogen-activated-protein-kinase-catalyzed phosphorylation of microtubule-associated proteins, microtubule-associated protein 2 and microtubule-associated protein 4, induces an alteration in their function. Eur. J. Biochem. 203, 43–52. doi: 10.1111/j.1432-1033.1992.tb19825.x
Huang, H., Arighi, C. N., Ross, K. E., Ren, J., Li, G., Chen, S. C., et al. (2018). iPTMnet: an integrated resource for protein post-translational modification network discovery. Nucleic Acids Res. 46, D542–d550. doi: 10.1093/nar/gkx1104
Huang, Y. A., Kao, J. W., Tseng, D. T., Chen, W. S., Chiang, M. H., and Hwang, E. (2013). Microtubule-associated type II protein kinase a is important for neurite elongation. PLoS One 8:e73890. doi: 10.1371/journal.pone.0073890
Hur, E. M., and Zhou, F. Q. (2010). GSK3 signalling in neural development. Nat. Rev. Neurosci. 11, 539–551. doi: 10.1038/nrn2870
Illenberger, S., Drewes, G., Trinczek, B., Biernat, J., Meyer, H. E., Olmsted, J. B., et al. (1996). Phosphorylation of microtubule-associated proteins MAP2 and MAP4 by the protein kinase p110mark. Phosphorylation sites and regulation of microtubule dynamics. J. Biol. Chem. 271, 10834–10843. doi: 10.1074/jbc.271.18.10834
Itoh, T. J., Hisanaga, S., Hosoi, T., Kishimoto, T., and Hotani, H. (1997). Phosphorylation states of microtubule-associated protein 2 (MAP2) determine the regulatory role of MAP2 in microtubule dynamics. Biochemistry 36, 12574–12582. doi: 10.1021/bi962606z
Itoh, T. J., and Hotani, H. (1994). Microtubule-stabilizing activity of microtubule-associated proteins (MAPs) is due to increase in frequency of rescue in dynamic instability: shortening length decreases with binding of MAPs onto microtubules. Cell Struct. Funct. 19, 279–290. doi: 10.1247/csf.19.279
Jansen, S., Melkova, K., Trosanova, Z., Hanakova, K., Zachrdla, M., Novacek, J., et al. (2017). Quantitative mapping of microtubule-associated protein 2c (MAP2c) phosphorylation and regulatory protein 14-3-3zeta-binding sites reveals key differences between MAP2c and its homolog tau. J. Biol. Chem. 292:10316. doi: 10.1074/jbc.A116.771097
Jaworski, J., Kapitein, L. C., Gouveia, S. M., Dortland, B. R., Wulf, P. S., Grigoriev, I., et al. (2009). Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron 61, 85–100. doi: 10.1016/j.neuron.2008.11.013
Jefferson, A. B., and Schulman, H. (1991). Phosphorylation of microtubule-associated protein-2 in GH3 cells. Regulation by cAMP and by calcium. J. Biol. Chem. 266, 346–354. doi: 10.1016/S0021-9258(18)52441-8
Johnson, G. V., and Foley, V. G. (1993). Calpain-mediated proteolysis of microtubule-associated protein 2 (MAP-2) is inhibited by phosphorylation by cAMP-dependent protein kinase, but not by Ca2+/calmodulin-dependent protein kinase II. J. Neurosci. Res. 34, 642–647. doi: 10.1002/jnr.490340607
Johnson, J. L., Yaron, T. M., Huntsman, E. M., Kerelsky, A., Song, J., Regev, A., et al. (2023). An atlas of substrate specificities for the human serine/threonine kinome. Nature 613, 759–766. doi: 10.1038/s41586-022-05575-3
Kalcheva, N., Albala, J., O'Guin, K., Rubino, H., Garner, C., and Shafit-Zagardo, B. (1995). Genomic structure of human microtubule-associated protein 2 (MAP-2) and characterization of additional MAP-2 isoforms. Proc. Natl. Acad. Sci. USA 92, 10894–10898. doi: 10.1073/pnas.92.24.10894
Kanai, Y., and Hirokawa, N. (1995). Sorting mechanisms of tau and MAP2 in neurons: suppressed axonal transit of MAP2 and locally regulated microtubule binding. Neuron 14, 421–432. doi: 10.1016/0896-6273(95)90298-8
Kapitein, L. C., Yau, K. W., Gouveia, S. M., van der Zwan, W. A., Wulf, P. S., Keijzer, N., et al. (2011). NMDA receptor activation suppresses microtubule growth and spine entry. J. Neurosci. 31, 8194–8209. doi: 10.1523/JNEUROSCI.6215-10.2011
Katoh, K., and Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
Kim, Y., Jang, Y. N., Kim, J. Y., Kim, N., Noh, S., Kim, H., et al. (2020). Microtubule-associated protein 2 mediates induction of long-term potentiation in hippocampal neurons. FASEB J. 34, 6965–6983. doi: 10.1096/fj.201902122RR
Kindler, S., Schulz, B., Goedert, M., and Garner, C. C. (1990). Molecular structure of microtubule-associated protein 2b and 2c from rat brain. J. Biol. Chem. 265, 19679–19684. doi: 10.1016/S0021-9258(17)45425-1
Komulainen, E., Zdrojewska, J., Freemantle, E., Mohammad, H., Kulesskaya, N., Deshpande, P., et al. (2014). JNK1 controls dendritic field size in L2/3 and L5 of the motor cortex, constrains soma size, and influences fine motor coordination. Front. Cell. Neurosci. 8:272. doi: 10.3389/fncel.2014.00272
Kosciolek, T., Buchan, D. W. A., and Jones, D. T. (2017). Predictions of backbone dynamics in intrinsically disordered proteins using de novo fragment-based protein structure predictions. Sci. Rep. 7:6999. doi: 10.1038/s41598-017-07156-1
Koszka, C., Brent, V. A., and Rostas, J. A. (1991). Developmental changes in phosphorylation of MAP-2 and synapsin I in cytosol and taxol polymerised microtubules from chicken brain. Neurochem. Res. 16, 637–644. doi: 10.1007/BF00965549
Krueger, K. A., Bhatt, H., Landt, M., and Easom, R. A. (1997). Calcium-stimulated phosphorylation of MAP-2 in pancreatic betaTC3-cells is mediated by Ca2+/calmodulin-dependent kinase II. J. Biol. Chem. 272, 27464–27469. doi: 10.1074/jbc.272.43.27464
Krug, K., Mertins, P., Zhang, B., Hornbeck, P., Raju, R., Ahmad, R., et al. (2019). A curated resource for Phosphosite-specific signature analysis. Mol. Cell. Proteomics 18, 576–593. doi: 10.1074/mcp.TIR118.000943
Lai, A. C. W., Nguyen Ba, A. N., and Moses, A. M. (2012). Predicting kinase substrates using conservation of local motif density. Bioinformatics 28, 962–969. doi: 10.1093/bioinformatics/bts060
LeClerc, N., Kosik, K. S., Cowan, N., Pienkowski, T. P., and Baas, P. W. (1993). Process formation in Sf9 cells induced by the expression of a microtubule-associated protein 2C-like construct. Proc. Natl. Acad. Sci. USA 90, 6223–6227. doi: 10.1073/pnas.90.13.6223
Lim, R. W., and Halpain, S. (2000). Regulated association of microtubule-associated protein 2 (MAP2) with Src and Grb2: evidence for MAP2 as a scaffolding protein. J. Biol. Chem. 275, 20578–20587. doi: 10.1074/jbc.M001887200
Lim, S., and Kaldis, P. (2013). Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development 140, 3079–3093. doi: 10.1242/dev.091744
Linding, R., Jensen, L. J., Pasculescu, A., Olhovsky, M., Colwill, K., Bork, P., et al. (2008). NetworKIN: a resource for exploring cellular phosphorylation networks. Nucleic Acids Res. 36, D695–D699. doi: 10.1093/nar/gkm902
Liu, G., Thangavel, R., Rysted, J., Kim, Y., Francis, M. B., Adams, E., et al. (2019). Loss of tau and Fyn reduces compensatory effects of MAP2 for tau and reveals a Fyn-independent effect of tau on calcium. J. Neurosci. Res. 97, 1393–1413. doi: 10.1002/jnr.24517
Lopez, L. A., and Sheetz, M. P. (1993). Steric inhibition of cytoplasmic dynein and kinesin motility by MAP2. Cell Motil. Cytoskeleton 24, 1–16. doi: 10.1002/cm.970240102
Lyu, J., MacDonald, M. L., Ruiz, S., Chou, S., Gilardi, J., Buchwald, S. C., et al. (2024). Deciphering the alteration of MAP2 interactome caused by a schizophrenia-associated phosphorylation. Neurobiol. Dis. 203:106731. doi: 10.1016/j.nbd.2024.106731
Malleret, G., Haditsch, U., Genoux, D., Jones, M. W., Bliss, T. V., Vanhoose, A. M., et al. (2001). Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell 104, 675–686. doi: 10.1016/s0092-8674(01)00264-1
Matus, A., Bernhardt, R., Bodmer, R., and Alaimo, D. (1986). Microtubule-associated protein 2 and tubulin are differently distributed in the dendrites of developing neurons. Neuroscience 17, 371–389. doi: 10.1016/0306-4522(86)90253-8
Melkova, K., Zapletal, V., Narasimhan, S., Jansen, S., Hritz, J., Skrabana, R., et al. (2019). Structure and functions of microtubule associated proteins tau and MAP2c: similarities and differences. Biomol. Ther. 9:105. doi: 10.3390/biom9030105
Mitra, G., Gupta, S., Poddar, A., and Bhattacharyya, B. (2015). MAP2c prevents arachidonic acid-induced fibril formation of tau: role of chaperone activity and phosphorylation. Biophys. Chem. 205, 16–23. doi: 10.1016/j.bpc.2015.06.003
Neve, R. L., Harris, P., Kosik, K. S., Kurnit, D. M., and Donlon, T. A. (1986). Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Brain Res. 387, 271–280. doi: 10.1016/0169-328x(86)90033-1
Newcombe, E. A., Delaforge, E., Hartmann-Petersen, R., Skriver, K., and Kragelund, B. B. (2022). How phosphorylation impacts intrinsically disordered proteins and their function. Essays Biochem. 66, 901–913. doi: 10.1042/EBC20220060
Nielsen, F. C., Nielsen, J., Kristensen, M. A., Koch, G., and Christiansen, J. (2002). Cytoplasmic trafficking of IGF-II mRNA-binding protein by conserved KH domains. J. Cell Sci. 115, 2087–2097. doi: 10.1242/jcs.115.10.2087
Novacek, J., Janda, L., Dopitova, R., Zidek, L., and Sklenar, V. (2013). Efficient protocol for backbone and side-chain assignments of large, intrinsically disordered proteins: transient secondary structure analysis of 49.2 kDa microtubule associated protein 2c. J. Biomol. NMR 56, 291–301. doi: 10.1007/s10858-013-9761-7
Obar, R. A., Dingus, J., Bayley, H., and Vallee, R. B. (1989). The RII subunit of cAMP-dependent protein kinase binds to a common amino-terminal domain in microtubule-associated proteins 2A, 2B, and 2C. Neuron 3, 639–645. doi: 10.1016/0896-6273(89)90274-2
Ozer, R. S., and Halpain, S. (2000). Phosphorylation-dependent localization of microtubule-associated protein MAP2c to the actin cytoskeleton. Mol. Biol. Cell 11, 3573–3587. doi: 10.1091/mbc.11.10.3573
Parsons, S. J., and Parsons, J. T. (2004). Src family kinases, key regulators of signal transduction. Oncogene 23, 7906–7909. doi: 10.1038/sj.onc.1208160
Plucarova, J., Jansen, S., Narasimhan, S., Lanikova, A., Lewitzky, M., Feller, S. M., et al. (2022). Specific phosphorylation of microtubule-associated protein 2c by extracellular signal-regulated kinase reduces interactions at its pro-rich regions. J. Biol. Chem. 298:102384. doi: 10.1016/j.jbc.2022.102384
Poll, B. G., Leo, K. T., Deshpande, V., Jayatissa, N., Pisitkun, T., Park, E., et al. (2024). A resource database for protein kinase substrate sequence-preference motifs based on large-scale mass spectrometry data. Cell Commun. Signal 22:137. doi: 10.1186/s12964-023-01436-2
Repar, N., Li, H., Aguilar, J. S., Li, Q. Q., Drobne, D., and Hong, Y. (2018). Silver nanoparticles induce neurotoxicity in a human embryonic stem cell-derived neuron and astrocyte network. Nanotoxicology 12, 104–116. doi: 10.1080/17435390.2018.1425497
Reszka, A. A., Seger, R., Diltz, C. D., Krebs, E. G., and Fischer, E. H. (1995). Association of mitogen-activated protein kinase with the microtubule cytoskeleton. Proc. Natl. Acad. Sci. USA 92, 8881–8885. doi: 10.1073/pnas.92.19.8881
Ribeiro, L. F., and de Wit, J. (2017). Neuronal polarity: MAP2 shifts secretory vesicles into high gear for long-haul transport down the axon. Neuron 94, 223–225. doi: 10.1016/j.neuron.2017.04.002
Riederer, B. M., Draberova, E., Viklicky, V., and Draber, P. (1995). Changes of MAP2 phosphorylation during brain development. J. Histochem. Cytochem. 43, 1269–1284. doi: 10.1177/43.12.8537643
Rodnight, R., Trotta, E. E., and Perrett, C. (1985). A simple and economical method for studying protein phosphorylation in vivo in the rat brain. J. Neurosci. Methods 13, 87–95. doi: 10.1016/0165-0270(85)90021-4
Rudrabhatla, P., Jaffe, H., and Pant, H. C. (2011). Direct evidence of phosphorylated neuronal intermediate filament proteins in neurofibrillary tangles (NFTs): phosphoproteomics of Alzheimer's NFTs. FASEB J. 25, 3896–3905. doi: 10.1096/fj.11-181297
Saito, N., Kikkawa, U., Nishizuka, Y., and Tanaka, C. (1988). Distribution of protein kinase C-like immunoreactive neurons in rat brain. J. Neurosci. 8, 369–382. doi: 10.1523/JNEUROSCI.08-02-00369.1988
Sanchez, C., Perez, M., and Avila, J. (2000). GSK3beta-mediated phosphorylation of the microtubule-associated protein 2C (MAP2C) prevents microtubule bundling. Eur. J. Cell Biol. 79, 252–260. doi: 10.1078/s0171-9335(04)70028-x
Sanchez, C., Tompa, P., Szucs, K., Friedrich, P., and Avila, J. (1996). Phosphorylation and dephosphorylation in the proline-rich C-terminal domain of microtubule-associated protein 2. Eur. J. Biochem. 241, 765–771. doi: 10.1111/j.1432-1033.1996.00765.x
Sarkar, T., Mitra, G., Gupta, S., Manna, T., Poddar, A., Panda, D., et al. (2004). MAP2 prevents protein aggregation and facilitates reactivation of unfolded enzymes. Eur. J. Biochem. 271, 1488–1496. doi: 10.1111/j.1432-1033.2004.04053.x
Schellino, R., Boido, M., and Vercelli, A. (2019). JNK signaling pathway involvement in spinal cord neuron development and death. Cells 8:576. doi: 10.3390/cells8121576
Schulman, H. (1984). Differential phosphorylation of MAP-2 stimulated by calcium-calmodulin and cyclic AMP. Mol. Cell. Biol. 4, 1175–1178. doi: 10.1128/mcb.4.6.1175-1178
Seitz, A., Kojima, H., Oiwa, K., Mandelkow, E. M., Song, Y. H., and Mandelkow, E. (2002). Single-molecule investigation of the interference between kinesin, tau and MAP2c. EMBO J. 21, 4896–4905. doi: 10.1093/emboj/cdf503
Shah, K., and Lahiri, D. K. (2017). A tale of the good and bad: remodeling of the microtubule network in the brain by Cdk5. Mol. Neurobiol. 54, 2255–2268. doi: 10.1007/s12035-016-9792-7
Sharma, N., Kress, Y., and Shafit-Zagardo, B. (1994). Antisense MAP-2 oligonucleotides induce changes in microtubule assembly and neuritic elongation in pre-existing neurites of rat cortical neurons. Cell Motil. Cytoskeleton 27, 234–247. doi: 10.1002/cm.970270305
Shuken, S. R. (2023). An introduction to mass spectrometry-based proteomics. J. Proteome Res. 22, 2151–2171. doi: 10.1021/acs.jproteome.2c00838
Silva, A. J., Paylor, R., Wehner, J. M., and Tonegawa, S. (1992). Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science 257, 206–211. doi: 10.1126/science.1321493
Song, Z. M., Undie, A. S., Koh, P. O., Fang, Y. Y., Zhang, L., Dracheva, S., et al. (2002). D1 dopamine receptor regulation of microtubule-associated protein-2 phosphorylation in developing cerebral cortical neurons. J. Neurosci. 22, 6092–6105. doi: 10.1523/JNEUROSCI.22-14-06092.2002
Sontag, J. M., Nunbhakdi-Craig, V., White, C. L., Halpain, S., and Sontag, E. (2012). The protein phosphatase PP2A/bα binds to the microtubule-associated proteins tau and MAP2 at a motif also recognized by the kinase Fyn. J. Biol. Chem. 287, 14984–14993. doi: 10.1074/jbc.M111.338681
Terfve, C. D. A., Wilkes, E. H., Casado, P., Cutillas, P. R., and Saez-Rodriguez, J. (2015). Large-scale models of signal propagation in human cells derived from discovery phosphoproteomic data. Nat. Commun. 6:8033. doi: 10.1038/ncomms9033
Theurkauf, W. E., and Vallee, R. B. (1982). Molecular characterization of the cAMP-dependent protein kinase bound to microtubule-associated protein 2. J. Biol. Chem. 257, 3284–3290. doi: 10.1016/S0021-9258(19)81107-9
Theurkauf, W. E., and Vallee, R. B. (1983). Extensive cAMP-dependent and cAMP-independent phosphorylation of microtubule-associated protein 2. J. Biol. Chem. 258, 7883–7886. doi: 10.1016/S0021-9258(18)32261-0
Trivedi, N., Marsh, P., Goold, R. G., Wood-Kaczmar, A., and Gordon-Weeks, P. R. (2005). Glycogen synthase kinase-3beta phosphorylation of MAP1B at Ser1260 and Thr1265 is spatially restricted to growing axons. J. Cell Sci. 118, 993–1005. doi: 10.1242/jcs.01697
Tseng, H. C., Ovaa, H., Wei, N. J., Ploegh, H., and Tsai, L. H. (2005). Phosphoproteomic analysis with a solid-phase capture-release-tag approach. Chem. Biol. 12, 769–777. doi: 10.1016/j.chembiol.2005.05.012
Tsuyama, S., Bramblett, G. T., Huang, K. P., and Flavin, M. (1986). Calcium/phospholipid-dependent kinase recognizes sites in microtubule-associated protein 2 which are phosphorylated in living brain and are not accessible to other kinases. J. Biol. Chem. 261, 4110–4116. doi: 10.1016/S0021-9258(17)35631-4
Tsuyama, S., Terayama, Y., and Matsuyama, S. (1987). Numerous phosphates of microtubule-associated protein 2 in living rat brain. J. Biol. Chem. 262, 10886–10892. doi: 10.1016/S0021-9258(18)61047-6
Vaillant, A. R., Zanassi, P., Walsh, G. S., Aumont, A., Alonso, A., and Miller, F. D. (2002). Signaling mechanisms underlying reversible, activity-dependent dendrite formation. Neuron 34, 985–998. doi: 10.1016/s0896-6273(02)00717-1
Valencia, R. G., Walko, G., Janda, L., Novacek, J., Mihailovska, E., Reipert, S., et al. (2013). Intermediate filament-associated cytolinker plectin 1c destabilizes microtubules in keratinocytes. Mol. Biol. Cell 24, 768–784. doi: 10.1091/mbc.E12-06-0488
Vallano, M. L., Goldenring, J. R., Lasher, R. S., and Delorenzo, R. J. (1986). Association of calcium/calmodulin-dependent kinase with cytoskeletal preparations: phosphorylation of tubulin, neurofilament, and microtubule-associated proteins. Ann. N. Y. Acad. Sci. 466, 357–374. doi: 10.1111/j.1749-6632.1986.tb38406.x
Vallee, R. B., DiBartolomeis, M. J., and Theurkauf, W. E. (1981). A protein kinase bound to the projection portion of MAP 2 (microtubule-associated protein 2). J. Cell Biol. 90, 568–576. doi: 10.1083/jcb.90.3.568
Walaas, S. I., and Nairn, A. C. (1989). Multisite phosphorylation of microtubule-associated protein 2 (MAP-2) in rat brain: peptide mapping distinguishes between cyclic AMP-, calcium/calmodulin-, and calcium/phospholipid-regulated phosphorylation mechanisms. J. Mol. Neurosci. 1, 117–127. doi: 10.1007/BF02896895
Waterhouse, A. M., Procter, J. B., Martin, D. M., Clamp, M., and Barton, G. J. (2009). Jalview version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191. doi: 10.1093/bioinformatics/btp033
Weisshaar, B., and Matus, A. (1993). Microtubule-associated protein 2 and the organization of cellular microtubules. J. Neurocytol. 22, 727–734. doi: 10.1007/BF01181318
Wiredja, D. D., Koyutürk, M., and Chance, M. R. (2017). The KSEA app: a web-based tool for kinase activity inference from quantitative phosphoproteomics. Bioinformatics 33, 3489–3491. doi: 10.1093/bioinformatics/btx415
Woodgett, J. R. (1990). Molecular cloning and expression of glycogen synthase kinase-3/factor a. EMBO J. 9, 2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x
Yamamoto, H., Fukunaga, K., Goto, S., Tanaka, E., and Miyamoto, E. (1985). Ca2+, calmodulin-dependent regulation of microtubule formation via phosphorylation of microtubule-associated protein 2, tau factor, and tubulin, and comparison with the cyclic AMP-dependent phosphorylation. J. Neurochem. 44, 759–768. doi: 10.1111/j.1471-4159.1985.tb12880.x
Yamamoto, H., Fukunaga, K., Tanaka, E., and Miyamoto, E. (1983). Ca2+− and calmodulin-dependent phosphorylation of microtubule-associated protein 2 and tau factor, and inhibition of microtubule assembly. J. Neurochem. 41, 1119–1125. doi: 10.1111/j.1471-4159.1983.tb09060.x
Yamamoto, H., Saitoh, Y., Fukunaga, K., Nishimura, H., and Miyamoto, E. (1988). Dephosphorylation of microtubule proteins by brain protein phosphatases 1 and 2A, and its effect on microtubule assembly. J. Neurochem. 50, 1614–1623. doi: 10.1111/j.1471-4159.1988.tb03051.x
Yamauchi, T., and Fujisawa, H. (1982). Phosphorylation of microtubule-associated protein 2 by calmodulin-dependent protein kinase (kinase II) which occurs only in the brain tissues. Biochem. Biophys. Res. Commun. 109, 975–981. doi: 10.1016/0006-291x(82)92035-6
Yang, G., Zuo, C., Lin, Y., Zhou, X., Wen, P., Zhang, C., et al. (2023). Comprehensive proteome, phosphoproteome and kinome characterization of luminal a breast cancer. Front. Oncol. 13:1127446. doi: 10.3389/fonc.2023.1127446
Yaron-Barir, T. M., Joughin, B. A., Huntsman, E. M., Kerelsky, A., Cizin, D. M., Cohen, B. M., et al. (2024). The intrinsic substrate specificity of the human tyrosine kinome. Nature 629, 1174–1181. doi: 10.1038/s41586-024-07407-y
Yarza, R., Vela, S., Solas, M., and Ramirez, M. J. (2015). C-Jun N-terminal kinase (JNK) signaling as a therapeutic target for Alzheimer's disease. Front. Pharmacol. 6:321. doi: 10.3389/fphar.2015.00321
Yasuda, R., Hayashi, Y., and Hell, J. W. (2022). CaMKII: a central molecular organizer of synaptic plasticity, learning and memory. Nat. Rev. Neurosci. 23, 666–682. doi: 10.1038/s41583-022-00624-2
Zamora-Leon, S. P., Bresnick, A., Backer, J. M., and Shafit-Zagardo, B. (2005). Fyn phosphorylates human MAP-2c on tyrosine 67. J. Biol. Chem. 280, 1962–1970. doi: 10.1074/jbc.M411380200
Zamora-Leon, S. P., Lee, G., Davies, P., and Shafit-Zagardo, B. (2001). Binding of Fyn to MAP-2c through an SH3 binding domain. Regulation of the interaction by ERK2. J. Biol. Chem. 276, 39950–39958. doi: 10.1074/jbc.M107807200
Zhang, J., and Dong, X. P. (2012). Dysfunction of microtubule-associated proteins of MAP2/tau family in prion disease. Prion 6, 334–338. doi: 10.4161/pri.20677
Zhang, L., Yang, M., Wang, Z., Fan, D., Shen, F., Zou, X., et al. (2024). Sevoflurane postconditioning ameliorates cerebral hypoxia/reoxygenation injury in zebrafish involving the Akt/GSK-3β pathway activation and the microtubule-associated protein 2 promotion. Biomed. Pharmacother. 175:116693. doi: 10.1016/j.biopha.2024.116693
Keywords: microtubule-associated protein 2 (MAP2), phosphorylation, kinase, cytoskeleton, microtubule, dendrite
Citation: Lyu J, DeMarco AG, Sweet RA and Grubisha MJ (2025) MAP2 phosphorylation: mechanisms, functional consequences, and emerging insights. Front. Cell. Neurosci. 19:1610371. doi: 10.3389/fncel.2025.1610371
Edited by:
Hyong Kyu Kim, Chungbuk National University, Republic of KoreaReviewed by:
Swati Banerjee, The University of Texas Health Science Center at San Antonio, United StatesPiotr Michaluk, Nencki Institute of Experimental Biology (PAS), Poland
Copyright © 2025 Lyu, DeMarco, Sweet and Grubisha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Melanie J. Grubisha, Z3J1YmlzaGFtQHVwbWMuZWR1
 Jiali Lyu
Jiali Lyu Andrew G. DeMarco
Andrew G. DeMarco Robert A. Sweet
Robert A. Sweet Melanie J. Grubisha
Melanie J. Grubisha