- 1UK Dementia Research Institute, The University of Edinburgh, Edinburgh, United Kingdom
- 2British Heart Foundation - UK Dementia Research Institute Centre for Vascular Dementia Research, The University of Edinburgh, Edinburgh, United Kingdom
- 3Centre for Clinical Brain Sciences, The University of Edinburgh, Edinburgh, United Kingdom
The vasculature of the central nervous system (CNS) is a highly specialized structure that delivers oxygen and nutrients to energy-demanding neural cells while protecting them from the toxicity of blood-borne substances. Pericytes, located alongside microvessels, coordinate with endothelial cells to maintain the integrity of the blood-CNS barriers and to regulate vascular responses to neural activity. Pericytes extend processes that typically wrap around or align the endothelial cells, remaining embedded within the vascular basement membrane. Occasionally, however, some of these processes detach and form bridges between separate capillaries. These bridging structures are the focus of ongoing debate. While some studies propose they serve as tunneling nanotubes mediating neurovascular coupling, others argue they may be remnants of vascular regression or involved in the process of pericyte migration. In this review, we aim to clarify these varying interpretations of bridging pericyte processes and provide a unified understanding to guide future research. We discuss their reported roles in both CNS health and disease, highlighting their potential significance in vascular aging and rejuvenation.
1 Introduction
1.1 The elusive definition of pericytes
Pericytes are mural cells found along microvessels, lining the abluminal side of the endothelium, and are present in all vascularized tissues of the body. The earliest references to cells wrapping around capillaries, characterized by contractile activity and a nucleus bulging on the outside, can be traced back to independent publications by Rouget and Eberth in the late 19th century (Eberth, 1871; Rouget, 1874). These early descriptions gave rise to the now-classic “bump-on-a-log” pericyte morphotype. This foundational work was greatly expanded 50 years later by the German anatomist Zimmermann, whose landmark paper (Zimmermann, 1923) offered a comprehensive characterization of these cells.
In his extensive study, Zimmermann observed silver-stained cells lining capillaries, venules, and arterioles across various vertebrate tissues, which he named “pericytes” (Pericyten). Unlike smooth muscle cells (SMCs) found on larger vessels, pericytes present a range of morphologies related to their position, or zonation, along the vascular tree, whether on pre-capillary arterioles, capillaries, or post-capillary venules (Zimmermann, 1923).
As might be expected for cells described as existing along a continuum rather than fitting into a fixed category, the definition of pericytes has fluctuated over time. While multiple pericyte markers have been used, such as CD13 (Aminopeptidase N) (Kunz et al., 1994), NG2 (Neural/glial antigen 2), PDGFRβ (Platelet-derived growth factor beta) (Franklin et al., 1981), and Atp13a5 (ATPase 13a5) (Guo et al., 2024), they may also be expressed by other cells like SMCs and fibroblasts. As a result, pericytes are often defined using a combination of zonation, morphology, and marker expression (Attwell et al., 2016; Krueger and Bechmann, 2010).
These inconsistencies in definition become particularly important when they influence conclusions about the functional roles and pathophysiological relevance of pericytes. One such point of contention is their contractile capacity, which has long been, and remains, debated (Attwell et al., 2016; Hall et al., 2014; Hartmann et al., 2022, 2021; Hill et al., 2015; Hørlyck et al., 2021; Krueger and Bechmann, 2010). While we will not delve further into this debate in the present review, it is worth noting that, for example, the conflicting view on pericyte expression of actin, a part of the contractile machinery, may stem from experimental differences (Alarcon-Martinez et al., 2018; Bandopadhyay et al., 2001; Nehls and Drenckhahn, 1991).
1.2 Pericytes and endothelial cells—an intimate story
Traits that are unambiguously shared by all pericytes and which are crucial part of their functional roles are their embedding within the vascular wall and their close association with endothelial cells (Krueger and Bechmann, 2010). This intimate relationship can be observed in the so-called “peg-and-socket” junctions (Leeson, 1979), interdigitations of the plasma membranes of the two cell types, which not only reinforce mechanical attachment but increase contact surface area. These structures are mostly located beneath the pericyte soma but can also be found along the borders of pericyte processes (Abdelazim et al., 2022; Ornelas et al., 2021).
Further reinforcing this tight association, numerous junctional complexes, such as gap and dense/cadherin junctions, interlink pericytes and endothelial cells (Sims, 1986). While pericyte-to-pericyte junctions do exist, endothelial cells remain their primary coupling partners (Kovacs-Oller et al., 2020). This critical cellular axis appears early during development (Payne et al., 2021) and plays key roles in both neurovascular coupling (Grubb et al., 2021) and endothelial stabilization (Ayloo et al., 2022; Park et al., 2017).
Because of this close interaction, pericyte coverage of the endothelium is often used as an indicator of microvascular health. A reduction in pericyte coverage has been associated with aging (Berthiaume et al., 2022), diabetes mellitus (Corliss et al., 2020; Pfister et al., 2008), glaucoma (Alarcon-Martinez et al., 2022), hypertension (Baggeroer et al., 2024), inflammation (Medina-Flores et al., 2023) and various neurovascular diseases, including Alzheimer’s disease (Ding et al., 2020; Halliday et al., 2016; Montagne et al., 2015).
However, when comparing pericyte coverage across studies, methodological differences must be considered. In most electron microscopy (EM)-based studies, pericyte coverage is quantified as the ratio of the pericyte-to-endothelial cell surface contact relative to the total external surface of the endothelial cell. Compared to immunofluorescence-based methods, the use of ultrathin sections in EM allows for a more precise measurement of this contact. However, due to EM’s inherently limited field of view, such analyzes are generally confined to a small number of vessels. In contrast, immunofluorescence studies typically estimate coverage by calculating the area of pericyte marker expression relative to that of vascular markers. While this latter approach allows for quick estimations, it has notable limitations, particularly the loss of spatial information from analyzing Z-projections of 3D stacks. To improve reproducibility, we developed a FIJI macro code that provides users greater control over pericyte coverage measurements (Chagnot and Montagne, 2024; Munro et al., 2024).
Reported pericyte-to-endothelial cell ratios depict the CNS as one of the richest organs, with often cited values ranging from 1:1 in retina to 1:3 in the cortex. In contrast, ratios in the lung and skeletal muscle are commonly cited as low as 1:10 and 1:100, respectively. However, it is worth noting that these extreme figures arose from isolated observations rather than aggregated data (Frank et al., 1987; Shepro and Morel, 1993), and may even be inaccurate, as some studies suggest that the number of pericytes in muscle are comparable to, or even exceed, those in the retina (Tilton et al., 1985; Williamson et al., 1980). Advanced methodologies pairing immunohistochemistry with single-cell omics allow more precise evaluations of cell numbers in tissues (Mäe et al., 2021).
1.3 Pericyte subtypes—a question of processes?
Most of the morphological diversity observed in pericytes is related to their processes (Figure 1A). As early as 1923, Zimmerman described primary processes, which emanate directly from the cell’s ovoid soma and extending longitudinally along the vessel, and secondary processes, which enwrap the underlying capillary (Zimmermann, 1923).
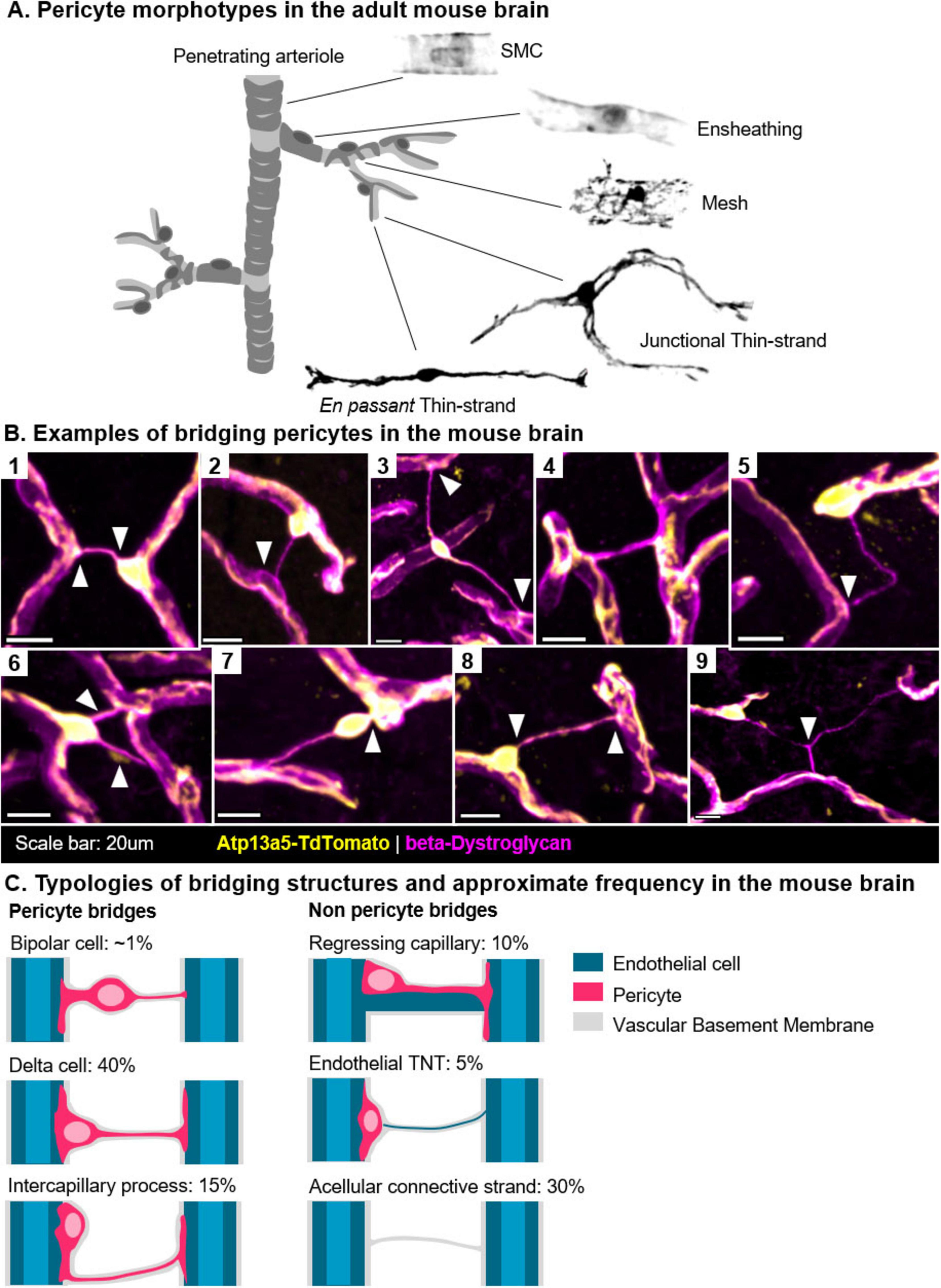
Figure 1. Bridging pericytes in the adult mouse brain. (A) Pericytes exhibit a variety of morphologies that reflect their zonation along the vascular tree, here presented by Atp13a5 + cells. In contrast, smooth muscle cells (SMCs) are primarily contractile, lack an ovoid soma, and are located along the penetrating arterioles. (B) Visualization of bridging pericytes in the mouse brain (Atp13a5: yellow, β-Dystroglycan: magenta). (B1) A classic “delta” morphology, with an endfoot extending to a neighboring capillary (arrow). (B2) Interaction between an Atp13a5 + cell and an Atp13a5- cell, potentially representing a pericyte-to-endothelial tunneling nanotube (TNT). (B3) A bipolar cell located midway along a long bridge. (B4) A bridge containing an Atp13a5 + process, though distant from the originating soma. (B5) A tortuous Atp13a5- bridge connected to a cell soma. (B6) A rare occurrence showing two bridges emerging from a single soma. (B7) A bipolar cell with a thickened process anchoring it to the nearest capillary. (B8) A delta-shaped cell. (B9) A rare occurrence of a Y-shaped bridge, with a delta cell positioned at its left extremity. (C) Summary of the different bridge morphologies observed in the adult (6-month-old) mouse brain. In over half of the bridges, pericytes were the dominant cellular component. In approximately one-third of cases, cellular processes could not be clearly identified.
Zonation along the vascular tree has been proposed as a way to identify pericyte subtypes (Grant et al., 2019). Branch order 0 starts at the penetrating arteriole, increasing with each new branch. Pericytes are considered absent from penetrating arterioles and larger vessels, where they are replaced by SMCs. While this classification is convenient when the entire vascular tree is accessible for annotation, it may not be the case in thin immunohistochemistry or electron microscopy sections. Advances in omics technologies offer promising avenues for exploring the vascular landscape; however, translating these molecular insights into conventional pericyte morphologies remains an open challenge (Sewell et al., 2025).
Pericytes found on precapillary arterioles (branch orders 1–4) are referred to as “ensheathing pericytes” due to their formation of a continuous, uniform sleeve completely wrapping the arteriole (Hartmann et al., 2015; Grant et al., 2019). The identification of these cells as bona fide pericytes has been contested, given their similarities to SMCs located upstream on arterioles and arteries (Attwell et al., 2016; Hill et al., 2015). Their contractile nature and expression of α-SMA (alpha smooth muscle actin) are not debated. However, their protruding ovoid cell body, absent in SMCs, has led some researchers to classify them as pericytes in keeping with Zimmermann’s historical definition (Grant et al., 2019). While the distinct morphology of ensheathing pericyte is almost certainly a consequence of differential gene expression relative to SMCs, no molecular marker has yet been identified that reliably discriminates between these two cell types.
At the junction between penetrating arterioles and their downstream branches, contractile “precapillary sphincters” have been described. These are formed and supported by ensheathing pericytes. By reducing the lumen size at this interface, these sphincters help to reduce the pressure load onto the downstream microvessels (Grubb et al., 2020).
Capillaries of the lowest order, those closest to arterioles, are typically covered by “mesh pericytes”, which extend a network of apparently disorganized processes over their vascular territory, creating a mesh-like appearance (Hartmann et al., 2015; Grant et al., 2019). These cells are thoughts to represent the transition toward the most common pericyte morphotype: the “thin-strand pericyte”.
Thin-strand pericytes are predominant along the capillary bed. They are characterized by long, thin primary processes that run along the vessel. Often spanning multiple capillary branches, their soma are frequently located at vascular bifurcations (Hartmann et al., 2015; Berthiaume et al., 2018). Tiny secondary processes extend tangentially and regularly from the primary ones, giving them a caterpillar-like appearance in electronic microscopy (Sims, 1986). These processes are dynamic, especially at their terminal segments, and can rapidly expand to cover exposed endothelial cells following the loss of a neighboring pericyte (Berthiaume et al., 2018). Subclassifications have also been proposed, such as “junctional pericytes”, with bodies at bifurcations, and “en passant (passing by) pericytes”, located along capillaries (Ornelas et al., 2021).
Post-capillary venules are also covered by mesh pericytes morphologically similar to those found upstream. These cells appear to represent a transitional type toward venular SMCs, which adopt a more stellate morphology at this level (Smyth et al., 2018).
Given the intimate interactions between pericytes and endothelial cells, the existence of pericyte processes and structures detached from the endothelium raises question about their nature and function. In the following section, we will explore the historical background of these so-called bridging cells and the various ways they have been described in the literature.
2 Bridging cells in the central nervous system
2.1 Early reports: comparative anatomy and structural characterization
In his seminal 1923 paper, Zimmermann described various “pericytes” morphologies on capillaries across a wide range of vertebrate tissues. He noted that these cells occasionally extend primary processes between neighboring vessels, a phenomenon he observed in all examined tissues. While he hypothesized that these extensions might transmit signals between capillaries, he also speculated that they could originate from regressing endothelial cells (Zimmermann, 1923).
Forty years later, Cammermeyer introduced the term “delta cells” to describe triangular-shaped somas found at the base of intervascular strands in the CNS. Occasionally, he observed somas located midway along the strands (Figure 1B). He described a variety of shapes including Y-shaped structures and noted that these formations were most prevalent in the cerebellum, midbrain, and medulla, while frontal regions such as the cortex contained relatively few. Across species, birds exhibited significantly more bridges than mammals, a difference he attributed to a structural function of the bridges, suggesting they might help organize and stabilize capillary loops. He speculated that in a context of edema, these bridges might become deleterious, compressing capillaries and causing ischemia (Cammermeyer, 1960).
In a follow-up study, Cammermeyer reported the presence of “granules” and “vacuoles” within these bridges, proposing they might serve as routes for the transmission of signals and substances between vascular territories. He also described an orchestrated presence of various cell types around the bridges, including microglia, oligodendrocytes, and, in the peripheral nervous system, mast cells (Cammermeyer, 1965).
As the morphological diversity of these “bridges”, “intervascular strands”, and “string capillaries” became apparent, Guseo and Gallyas proposed a classification based on brightfield observations of silver-stained human brains. They distinguished three types: (1) endothelial protrusion giving rise to new capillaries, (2) collapsed or regressing capillaries, and (3) pericyte-based structures. Type (1) was common in newborn brains, particularly in myelinating areas, while types (2) and (3) were more prevalent in adult and pathological tissues (Guseo and Gallyas, 1974).
As originally reported by Zimmermann and Cammermeyer, these bridges are not exclusive to the CNS (Cammermeyer, 1965, 1960; Zimmermann, 1923). Similar structures were observed in the skin (Imayama and Urabe, 1984) and muscle (Williamson et al., 1980), typically interpreted as playing structural roles.
In 1988, Leibnitz and Bär published a comprehensive report on “bridging cells in the brain”, proposing that they represent a subtype of pericytes. They highlighted the inherent asymmetry of these structures, with a spindle or bell-shaped cell, with a process ending on a neighboring capillary in a disc- or pyramid-shaped thickening. Even “bipolar cells” located within strands displayed a process significantly thicker than the other. They also observed that apparent bending of capillaries near bridges, which they interpreted either as mechanical tension or as endothelial cell proliferation. Though cautious in their conclusions, they proposed that bridges could represent pericyte detachment as well as serve as guides for angiogenesis. Nonetheless, they argued that the relative rarity of these structures made them unlikely contributors to neurovascular coupling (Leibnitz and Bär, 1988).
2.2 Bridging cells as migrating pericytes
In the retina, bridging cells are often observed in the context of diabetes mellitus. Experimental hyperglycemia is sufficient to trigger pericyte detachment and migration from capillaries via pathways involving angiotensin-2 and PDGFRβ. This process can be rescued with insulin or the PDGFRβ inhibitor imatinib (Corliss et al., 2020; Pfister et al., 2008). Similar phenomena have been observed in the cochlea following acoustic trauma (Hou et al., 2018). In these settings, bridges are interpreted as hallmarks of a pathological process marked by pericyte loss and microvascular destabilization.
2.3 Bridges as tunneling nanotubes
Intercellular communications through protrusions of the plasma membrane were first reported 20 years ago by Rustom et al. (2004). These so-called “tunneling nanotubes” or TNTs were originally observed in cultured cells and defined by their sub-micron diameters and considerable lengths, often exceeding the size of the originating cell. Their lifecycle ranged from a few minutes to several hours and featured an actin-rich, tubulin-poor cytoskeleton. However, as TNT-like structures were reported across multiple models, it became apparent that a continuum of superficially similar structures exists (Korenkova et al., 2020), often termed cytoplasmic bridges (Haglund et al., 2011) or cytonemes (Sowinski et al., 2011). TNTs form in virtually all animal tissues and are diverse in nature and functions, encompassing bundled actin/tubulin hybrids (Sartori-Rupp et al., 2019) and involved in organelle transfer (Önfelt et al., 2006), electrical coupling (Wang et al., 2010), and even in the spread of misfolded proteins in neurodegenerative disorders (Zhang et al., 2021; Zhang, 2011).
A recent interpretation suggests that the bridges observed in the CNS may be TNTs extending between pericytes. Alarcon-Martinez et al. (2020) described these inter-pericytes TNTs in the mouse retina as exhibiting hybrid characteristics, sharing features of both pericyte processes and the TNTs originally described by Alarcon-Martinez et al. (2020), Rustom et al. (2004), Zurzolo (2021). Their study demonstrated mitochondrial transport through these structures (Alarcon-Martinez et al., 2020), reminiscent of the “granules” observed by Cammermeyer (1965), a phenomenon also reported in some TNTs (Kempf et al., 2024; Wang and Gerdes, 2015). However, unlike classical TNTs that connect two distinct cells, these bridges appear more akin to extensions of a singles pericyte. Specifically, Alarcon-Martinez et al. (2020) showed that the “TNTs” they identified established cytoplasmic continuity between two parts of the same pericyte spanning different capillaries. This was confirmed through electroporation of a high molecular weight tracer. In their study, bridging pericytes were described as comprising a soma containing the nucleus and most of cell mass, an “endfoot” in contact with another capillary, and a connecting structure, the so-called “TNT”. Rather than linking two separate cells, as bona fide TNTs do, these bridges resemble elongated pericyte processes that span adjacent capillaries, consistent with early observations by Zimmermann (1923).
A more surprising connection made in the same study suggests a potential role for pericyte bridges in neurovascular coupling. Using a Ca2+ activity reporter, the authors found synchronized calcium transients between pericytes connected by bridges, with opposite capillary diameter changes upon light stimulation. Bridge disruption during ischemia led to desynchronization (Alarcon-Martinez et al., 2020). Follow-up work showed that glaucoma-induced elevations in intracellular calcium also led to bridges ruptures (Alarcon-Martinez et al., 2022). It is plausible that the nature of communication occurring along bridging structures resembles that of pericyte processes, although this has yet to be confirmed. The opposing coupling response reported by Alarcon-Martinez et al. (2020) 2022 may represent a further refinement of the neurovascular response, but the underlying mechanisms remain to be elucidated. One possibility is that contraction of the pericyte bridge results in a form of capillary constriction or “strangulation”, as originally proposed by Guseo and Gallyas (1974).
While the bridges presented in the works of Alarcon-Martinez et al. are more likely detached processes, true TNTs can form between brain types. Kempf et al. (2024) hypothesized that some of the observed bridges might connect pericytes to endothelial cells rather than other pericytes. In diabetic mouse retina, the authors observed an increased number in bridging structures but did not attributed this response to pericyte detachment and migration (as others had; Corliss et al., 2020; Pfister et al., 2008). In vitro experiments showed however that pericytes could emit TNTs toward stressed endothelial cells, transferring mitochondria, and restoring metabolic activity (Kempf et al., 2024). It is however unclear whether the structure presented on tissue sections are genuine TNT and not pericytes processes.
2.4 The non-pericyte candidates for bridging cells
In their 2012 study, Mendes-Jorge et al. (2012) investigated the morphology and marker expression of bridging cells in the mouse retina, uncovering several intriguing features. They found that these cells express markers shared by both endothelial cells and pericytes, such as CD34, poly-N-lactosamine, and CD13, while lacking expression of CD31, a key marker of endothelial cells, and actin. However, the limited co-staining combinations presented in this study do not allow for a clear determination of whether these markers are specific to bridging cells or are expressed separately in distinct cell types. Interestingly, the presence of condensed chromatin (indicative of a transcriptionally inactive state) and a higher prevalence of bridging cells in young animals evoke similarities with mesenchymal progenitor cells (Mendes-Jorge et al., 2012).
Telocytes, formerly known as interstitial Cajal-like cells, also share striking structural similarities with bridging cells (Popescu and Faussone-Pellegrini, 2010; Zhang et al., 2023). Present in the interstitial tissue of multiple organs, they extend long, thin processes, spanning hundreds of micrometers, from a small soma. However, the novelty of telocytes has been heavily debated, given their similarities with fibroblasts. Their lack of specific association with capillaries and their often convoluted, branching morphology make them unlikely candidates for the bridging cells observed in the CNS (Varga et al., 2019).
Fibroblasts, on the other hand, can play stabilizing roles in blood vessels and appear earlier than SMCs or pericytes during development. They help lay down basement membranes that guide new vessel growth (Rajan et al., 2020). It is interesting to note that nearly all reports of bridging cells describe their association with vascular basement membrane (Alarcon-Martinez et al., 2022, 2020; Ando et al., 1999; Cammermeyer, 1965, 1960; Corliss et al., 2020; Hou et al., 2018; Kempf et al., 2024; Leibnitz and Bär, 1988; Mendes-Jorge et al., 2012; Guseo and Gallyas, 1974; Pfister et al., 2008), an intricate relationship also shared by fibroblasts.
2.5 The lead of regressing vessels
Long, thin strands of conjunctive tissue extending between capillaries are not unfamiliar to neuroanatomists. Reports date back to the 19th century, with prominent figures such as Henle and Ramón y Cajal describing such structures in the nervous systems of various vertebrates, including humans (Cammermeyer, 1960). In a comprehensive 2010 review, Brown detailed the morphology and dynamics of “string vessels” (Brown, 2010), empty tubes of basement membrane resulting from vascular regression. These structures are frequently found in the aging brain, more prominently in Alzheimer’s disease, but also in the developing CNS, where they likely reflect the intense vascular remodeling of early life (Coelho−Santos and Shih, 2020).
In a 2025, Gao et al. (2025) extensively mapped regressing vessels across the mouse brain. They found that most of these strands contained components, and to a lesser extent, endothelial elements, embedded within basement membrane tubes. In fewer than 20% of cases, they observed bulging somas reminiscent of Cammermeyer’s delta cells, while fully embedded bipolar cells were seen in only ∼2% of string vessels. In human brains, these structures were slightly more frequent and appeared longer (Gao et al., 2025). Following experimental ischemia in mice, the number of regressing vessels increased and correlated with neuronal dysfunction, suggesting a potential link between vascular regression and impaired brain function (Gao et al., 2025).
3 Discussion
The varying descriptions (Table 1) and proposed functional roles of bridging cells and intercapillary strands are reminiscent of the historical ambiguities surrounding the definition of pericytes. In the absence of clear definitions, structures that appear morphologically identical (Figure 1C) can be subject to widely divergent interpretations. Indeed, bridging cells have been interpreted as signs of vascular regression (Gao et al., 2025; Guseo and Gallyas, 1974; Zimmermann, 1923), elements structuring capillary loops (Ando et al., 1999; Cammermeyer, 1960; Mendes-Jorge et al., 2012; Williamson et al., 1980), sites of angiogenesis (Leibnitz and Bär, 1988; Guseo and Gallyas, 1974), progenitor niches (Mendes-Jorge et al., 2012), indicators of pericyte migration or detachment (Corliss et al., 2020; Gao et al., 2025; Hou et al., 2018; Kempf et al., 2024; Leibnitz and Bär, 1988; Pfister et al., 2008), or even tunneling nanotubes (Alarcon-Martinez et al., 2022, 2020; Cammermeyer, 1965; Figure 2A). These interpretations are not mutually exclusive if we consider them as part of the same framework.
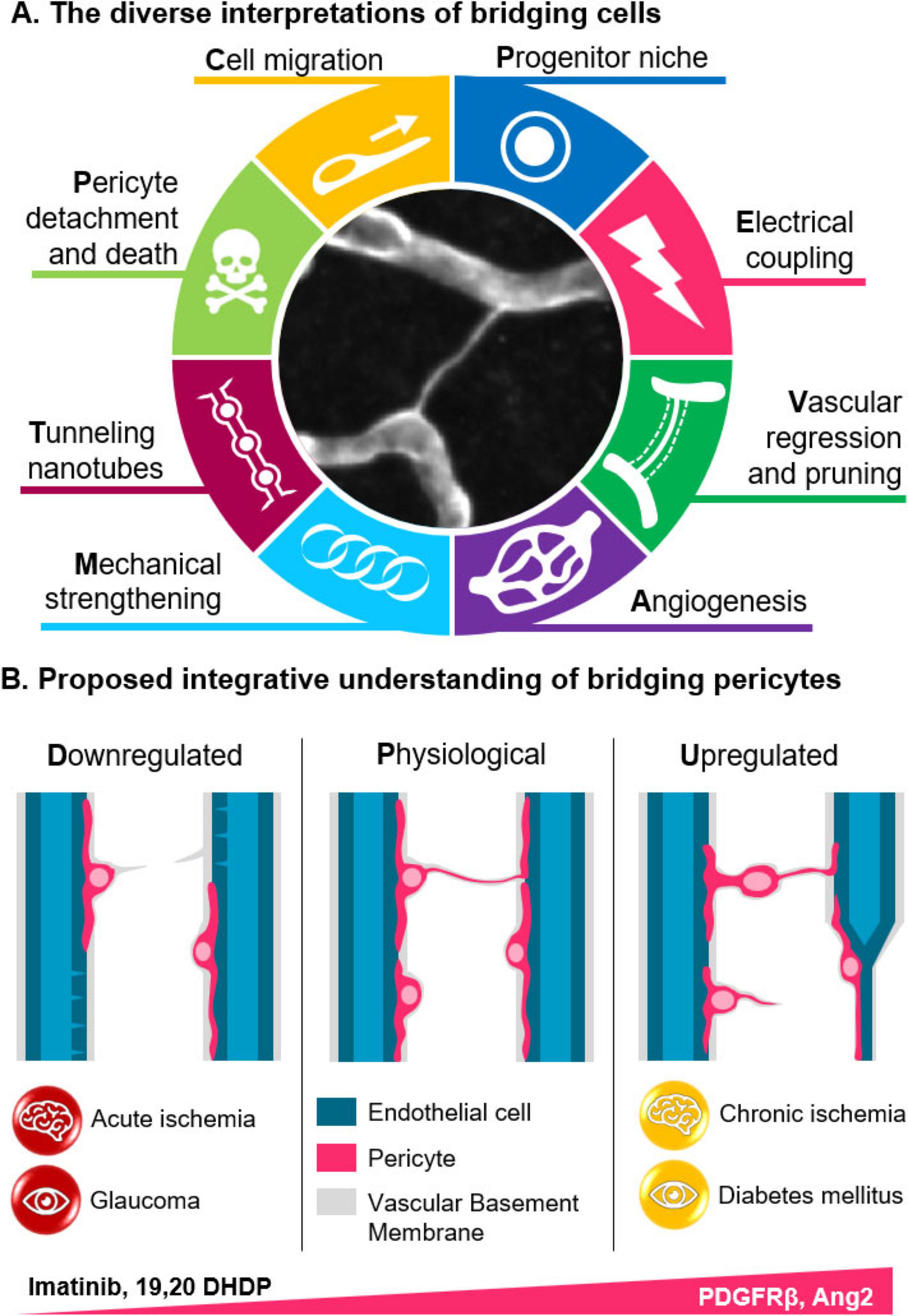
Figure 2. Significance and roles of bridging cells. (A) Bridging cells have been proposed to serve various roles, including functioning as progenitor cells, remnants of pruned capillaries, guides for angiogenesis, structural components, tunneling nanotubes involved in neurovascular coupling, or as migrating and detaching pericytes. (B) While bridging cells are presentr in healthy brains, their numbers increase significantly when pericyte migration is enhanced, such as in diabetes or following vascular regression after ischemia. In contrast, there bridges are disrupted in the acute phase of ischemia and in glaucoma. (PDGFRβ: Platelet-derived growth factor beta; 19,20-DHDP: 19,20-dihydroxydocosapentaenoic acid).
First, while some authors have identified bridges as TNTs (Alarcon-Martinez et al., 2022, 2020), these structures do not appear to differ fundamentally from conventional cellular processes. Rather than connecting pericytes to each other, they maintain cellular continuity by linking a single pericyte’s extensions across neighboring capillaries. The observation of electrical coupling between pericytes separated by these bridges is not unexpected, given the well-established intimate coupling between pericytes and endothelial cells (Kovacs-Oller et al., 2020). It is therefore more plausible that the observed pericyte-pericyte coupling is indirectly mediated via endothelial cells, rather than through a direct TNT-like structure. Regarding the functional significance of these bridges in neurovascular coupling, it remains to be demonstrated whether they play an active role or simply represent incidental anatomical arrangement. As suggested by Leibnitz and Bär (1988), the low frequency of these bridges makes it unlikely that they contribute substantially to neurovascular dynamics.
The presence of bona fide TNTs within connective strands remains conceivable, although they are typically described as lacking association with basement membranes (Zhang, 2011). While Kempf et al. (2024) demonstrated that pericytes can transfer mitochondria to damaged endothelial cells via TNTs, yet the relevance of intercapillary connections remains puzzling, as most endothelial cells are already covered by pericytes, offering a more immediate opportunity for support.
The hypothesis for pericyte migration is better established and supported by different studies (Corliss et al., 2020; Hou et al., 2018; Pfister et al., 2008). Angiotensin-2 and PDGFRβ upregulation appear to increase pericyte motility and detachment, potentially resulting in the presence of bipolar cells transiting across these bridges. The fate of detached pericytes remains to be determined; however, rather than undergoing cell death, some may transdifferentiate into other cell types such as microglia (Sakuma et al., 2016; Nirwane and Yao, 2022; Morris et al., 2023) or myofibroblasts (Zhao et al., 2022). Interestingly, migrating pericytes sometimes extend processes toward other vessels, a behavior that contrasts with the typical detachment and migration patterns observed during development (Payne et al., 2021). It is conceivable that some pericytes migrate within the basement membrane, only to become irreversibly trapped within the fibrotic remnants of regressed capillaries, where they may ultimately die.
Vascular regression and pruning are well known sources of connective strands in the CNS (Brown, 2010; Ouarné et al., 2021; Reissenweber and Pessacq, 1971). As endothelial cells retract, they can leave behind pericytes encased within empty sleeves of basement membrane, structures that likely give rise to bridging cells (Gao et al., 2025; Ouarné et al., 2021). Structural features support this mechanism: for example, the bending of capillaries near the origin of a bridge may reflect a residual angle from a former bifurcation rather than mechanical tension (Leibnitz and Bär, 1988). Rare occurrences of Y- or V-shaped bridges can be explained by the pruning of several vessels. Future studies could help identify bridges resulting specifically from vascular regression by detecting endothelial-derived components of the basement membrane. However, the factors determining pericyte fate during vascular regression remain unclear. Notably, Gao et al. (2025) claim that bridging cells can persist for months after endothelium loss.
Several factors have been reported to influence the number of bridging cells. In the retina, hypometabolic conditions such as glaucoma or acute ischemia are associated with a reduction in bridges numbers (Alarcon-Martinez et al., 2022, 2020), whereas hypermetabolic states like diabetes promote pericyte migration and an increase in bridges formation (Corliss et al., 2020; Pfister et al., 2008). This effect can be counteracted by the PDGFRβ inhibitor imatinib (Hou et al., 2018; Figure 2B). Although observations in the brain remain limited, they support the idea that vascular regression is a major source of bridging structure, as seen during capillary pruning in development (Brown, 2010; Leibnitz and Bär, 1988) and after ischemic events (Gao et al., 2025). Moreover, substances that disrupt plasma membrane dynamics and the formation of TNTs, such as 19,20-dihydroxydocosapentaenoic acid (19,20-DHDP), also reduce bridge numbers (Kempf et al., 2024) – albeit through a distinct mechanism.
Intriguingly, some bridging cells express markers indicative of endothelial-pericyte hybrids or even progenitor cells (Mendes-Jorge et al., 2012). The involvement of pericytes in vascular growth is well established (von Tell et al., 2006; Payne et al., 2021), and endothelial cells are known to proliferate within empty basement membrane sleeves (Archer et al., 1991). Bridging cells have been proposed to serve as guides for angiogenesis (Leibnitz and Bär, 1988), though this mechanism differs from the classical role of endothelial tip cells in forming new vascular connections (Payne et al., 2021). Rather than promoting regrowth, pericytes may act as stabilizers, potentially inhibiting endothelial regeneration after pruning (Gao et al., 2025). While this function is essential during development, it could hinder vascular repair in the aging brain. Such antagonistic pleiotropic mechanisms are not unprecedented. Targeting bridging cells, while preserving the basement membrane sleeve, might offer a strategy to promote endothelial regrowth. It is also worth noting that the condensed chromatin observed in bridging cells (Mendes-Jorge et al., 2012) may be a sign of cellular senescence.
Far from being mere anatomical curiosities, bridging cells and connective strands raise important questions about cerebrovascular dynamics. While pericyte loss has been reported across a wide range of conditions, including Alzheimer’s (Ding et al., 2020), stroke (Hall et al., 2014), and even healthy aging (Soto et al., 2015), the mechanisms underlying pericyte detachment remain poorly understood. Studies such as those by Corliss et al. (2020) and Hou et al. (2018) suggest that bridging pericytes may arise from dysregulated cell migration and recruitment; however, how these processes ultimately lead to pericyte death remains unclear.
Conversely, the recovery or replacement of pericytes following injury has been scarcely documented. It is plausible that such recovery would require cells to migrate along the vascular tree, potentially leading to the formation of new bridging structures. Interpreting the presence of bridging cells as indicators of vascular remodeling, whether regenerative or degenerative, is compelling, particularly given that similar structures may emerge during vascular pruning (Brown, 2010). Assessing whether such remodeling originates from pericytes or endothelial cells might be addressed by further investigation of the matrix components within these bridges.
Bridging pericytes may also play an active role in the pruning process by stabilizing endothelial cells and thereby inhibiting vascular regrowth (von Tell et al., 2006). While this stabilizing function facilitates pruning during development, it may conversely hinder vascular recovery in aging or disease, making these cells a potential therapeutic target for restoring brain perfusion in conditions of hypoperfusion.
Interestingly, some researchers suggest that the bridging cells may serve functions beyond being transient by-products of vascular remodeling. Although observations of the temporal dynamics of these structures are limited, available evidence suggests that a significant proportion of bridges persist for months (Gao et al., 2025). This longevity raises the possibility of functional roles for bridging cells, an idea beginning to be explored in dedicated studies (Alarcon-Martinez et al., 2020; Kempf et al., 2024). Nevertheless, in vivo functional investigations remain scarce and are currently limited to the retina, which is more accessible than other regions of the CNS
4 Conclusion
Since their discovery alongside pericytes, bridging cells have been examined from a wide range of perspectives. Despite the diverse interpretations they have attracted, these elusive cells are now generally recognized as pericytes whose processes extend between capillaries. As research has progressed from descriptive studies to functional analyzes, new hypotheses have emerged regarding their origin and relevance within the central nervous system. Although pericyte migration and vascular regression are considered the two primary sources of these capillary bridges, the underlying mechanisms remain to be fully elucidated. Unraveling these pathways holds promises for the identification of therapeutic targets in vascular diseases of the CNS and beyond.
Author contributions
AC: writing – review and editing, writing – original draft. AM: writing – review and editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The work of AM is supported by the UK Dementia Research Institute (award number UKDRI-4209) through UK DRI Ltd, principally funded by the UK Medical Research Council, and additional funding partners Alzheimer’s Society UK (ASUK), Alzheimer’s Research UK (ARUK), and British Heart Foundation (BHF). AM also holds a UKRI MRC fellowship (Career Development Award MR/V032488/1) and a UK DRI Theme Funding Program Award (DRI-TFP-2024-7).
Acknowledgments
We thank Dorota Stefancova for sharing the microscopy images that were assembled in Figure 1B.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelazim, H., Payne, L. B., Nolan, K., Paralkar, K., Bradley, V., Kanodia, R., et al. (2022). Pericyte heterogeneity identified by 3D ultrastructural analysis of the microvessel wall. Front. Physiol. 13:1016382. doi: 10.3389/fphys.2022.1016382
Alarcon-Martinez, L., Shiga, Y., Villafranca-Baughman, D., Belforte, N., Quintero, H., Dotigny, F., et al. (2022). Pericyte dysfunction and loss of interpericyte tunneling nanotubes promote neurovascular deficits in glaucoma. Proc. Natl. Acad. Sci. U S A. 119:e2110329119. doi: 10.1073/pnas.2110329119
Alarcon-Martinez, L., Villafranca-Baughman, D., Quintero, H., Kacerovsky, J. B., Dotigny, F., Murai, K. K., et al. (2020). Interpericyte tunnelling nanotubes regulate neurovascular coupling. Nature 585, 91–95. doi: 10.1038/s41586-020-2589-x
Alarcon-Martinez, L., Yilmaz-Ozcan, S., Yemisci, M., Schallek, J., Kılıç, K., Can, A., Di Polo, A., et al. (2018). Capillary pericytes express α-smooth muscle actin, which requires prevention of filamentous-actin depolymerization for detection. eLife 7:e34861. doi: 10.7554/eLife.34861
Ando, M., Kakigi, A., and Takeuchi, S. (1999). Elongated pericyte-like cells connect discrete capillaries in the cochlear stria vascularis of gerbils and rats. Cell. Tissue Res. 296, 673–676. doi: 10.1007/s004410051327
Archer, D. B., Amoaku, W. M. K., and Gardiner, T. A. (1991). Radiation retinopathy—Clinical, histopathological, ultrastructural and experimental correlations. Eye 5, 239–251. doi: 10.1038/eye.1991.39
Attwell, D., Mishra, A., Hall, C. N., O’Farrell, F. M., and Dalkara, T. (2016). What is a pericyte? J. Cereb. Blood Flow Metab. 36, 451–455. doi: 10.1177/0271678X15610340
Ayloo, S., Lazo, C. G., Sun, S., Zhang, W., Cui, B., and Gu, C. (2022). Pericyte-to-endothelial cell signaling via vitronectin-integrin regulates blood-CNS barrier. Neuron 110:1641–1655.e15. doi: 10.1016/j.neuron.2022.02.017
Baggeroer, C. E., Cambronero, F. E., Savan, N. A., Jefferson, A. L., and Santisteban, M.M. (2024). Basic mechanisms of brain injury and cognitive decline in hypertension. Hypertension 81, 34–44. doi: 10.1161/HYPERTENSIONAHA.123.19939
Bandopadhyay, R., Orte, C., Lawrenson, J. G., Reid, A. R., De Silva, S., and Allt, G. (2001). Contractile proteins in pericytes at the blood-brain and blood-retinal barriers. J. Neurocytol. 30, 35–44. doi: 10.1023/A:1011965307612
Berthiaume, A.-A., Grant, R. I., McDowell, K. P., Underly, R. G., Hartmann, D. A., Levy, M., et al. (2018). Dynamic remodelling of pericytes in vivo maintains capillary coverage in the adult mouse brain. Cell. Rep. 22, 8–16. doi: 10.1016/j.celrep.2017.12.016
Berthiaume, A.-A., Schmid, F., Stamenkovic, S., Coelho-Santos, V., Nielson, C. D., Weber, B., et al. (2022). Pericyte remodelling is deficient in the aged brain and contributes to impaired capillary flow and structure. Nat. Commun. 13:5912. doi: 10.1038/s41467-022-33464-w
Brown, W. R. (2010). A review of string vessels or collapsed, empty basement membrane tubes. J. Alzheimers Dis. 21, 725–739. doi: 10.3233/JAD-2010-100219
Cammermeyer, J. (1960). A comparative study of intervascular connective tissue strands in the central nervous system. J. Comp. Neurol. 114, 189–208. doi: 10.1002/cne.901140206
Cammermeyer, J. (1965). Cerebral intervascular strands of connective tissue as routes of transportation. Anatomical Rec. 151, 251–259. doi: 10.1002/ar.1091510306
Coelho-Santos, V., and Shih, A. Y. (2020). Postnatal development of cerebrovascular structure and the neurogliovascular unit. Wiley Interdiscip. Rev. Dev. Biol. 9:e363. doi: 10.1002/wdev.363
Corliss, B. A., Ray, H. C., Doty, R. W., Mathews, C., Sheybani, N., Fitzgerald, K., et al. (2020). Pericyte bridges in homeostasis and hyperglycemia. Diabetes 69, 1503–1517. doi: 10.2337/db19-0471
Ding, R., Hase, Y., Ameen-Ali, K. E., Ndung’u, M., Stevenson, W., Barsby, J., et al. (2020). Loss of capillary pericytes and the blood–brain barrier in white matter in poststroke and vascular dementias and Alzheimer’s disease. Brain Pathol. 30, 1087–1101. doi: 10.1111/bpa.12888
Eberth, C. J. (1871). Handbuch der Lehre von den Geweben des Menschen und der Thiere (Leipzig: Engelmann) 191–214.
Frank, R. N., Dutta, S., and Mancini, M. A. (1987). Pericyte coverage is greater in the retinal than in the cerebral capillaries of the rat. Invest. Ophthalmol. Visual Sci. 28, 1086–1091.
Franklin, W. A., Christison, W. H., Colley, M., Montag, A. G., Stephens, J. K., and Hart, C. E. (1981). In situ distribution of the β-subunit of platelet-derived growth factor receptor in nonneoplastic tissue and in soft tissue tumors. Cancer Res. 50, 6344–6348.
Gao, X., Chen, X., Ye, M., and Li, J.-L., and Lu, N. (2025). Reduction of neuronal activity mediated by blood-vessel regression in the adult brain. Nat. Commun. 16:5840. doi: 10.1038/s41467-025-60308-0
Grant, R. I., Hartmann, D. A., Underly, R. G., Berthiaume, A.-A., Bhat, N. R., and Shih, A. Y. (2019). Organizational hierarchy and structural diversity of microvascular pericytes in adult mouse cortex. J. Cereb. Blood Flow Metab. 39, 411–425. doi: 10.1177/0271678X17732229
Grubb, S., Cai, C., Hald, B. O., Khennouf, L., Murmu, R. P., Jensen, A. G. K., et al. (2020). Precapillary sphincters maintain perfusion in the cerebral cortex. Nat. Commun. 11:395. doi: 10.1038/s41467-020-14330-z
Grubb, S., Lauritzen, M., and Aalkjær, C. (2021). Brain capillary pericytes and neurovascular coupling. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 254:110893. doi: 10.1016/j.cbpa.2020.110893
Guo, X., Xia, S., Ge, T., Lin, Y., Hu, S., Wu, H., et al. (2024). Atp13a5 marker reveals pericyte specification in the mouse central nervous system. J. Neurosci. 44:e0727242024. doi: 10.1523/JNEUROSCI.0727-24.2024
Guseo, A., and Gallyas, F. (1974). Intercapillary bridges and the development of brain capillaries, in pathology of cerebral microcirculation. (Berlin: De Gruyter Brill) 448-453
Haglund, K., Nezis, I. P., and Stenmark, H. (2011). Structure and functions of stable intercellular bridges formed by incomplete cytokinesis during development. Commun. Integr. Biol. 4, 1–9. doi: 10.4161/cib.4.1.13550
Hall, C. N., Reynell, C., Gesslein, B., Hamilton, N. B., Mishra, A., Sutherland, B. A., et al. (2014). Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508, 55–60. doi: 10.1038/nature13165
Halliday, M. R., Rege, S. V., Ma, Q., Zhao, Z., Miller, C. A., Winkler, E. A., et al. (2016). Accelerated pericyte degeneration and blood–brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J. Cereb. Blood Flow Metab. 36, 216–227. doi: 10.1038/jcbfm.2015.44
Hartmann, D. A., Berthiaume, A.-A., Grant, R. I., Harrill, S. A., Koski, T., Tieu, T., et al. (2021). Brain capillary pericytes exert a substantial but slow influence on blood flow. Nat. Neurosci. 24, 633–645. doi: 10.1038/s41593-020-00793-2
Hartmann, D. A., Coelho-Santos, V., and Shih, A. Y. (2022). Pericyte control of blood flow across microvascular zones in the central nervous system. Annu. Rev. Physiol. 84, 331–354. doi: 10.1146/annurev-physiol-061121-040127
Hartmann, D. A., Underly, R. G., Grant, R. I., Watson, A. N., Lindner, V., and Shih, A. Y. (2015). Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. NPh 2:041402. doi: 10.1117/1.NPh.2.4.041402
Hill, R. A., Tong, L., Yuan, P., Murikinati, S., Gupta, S., and Grutzendler, J. (2015). Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 87, 95–110. doi: 10.1016/j.neuron.2015.06.001
Hørlyck, S., Cai, C., Helms, H. C. C., Lauritzen, M., and Brodin, B. (2021). ATP induces contraction of cultured brain capillary pericytes via activation of P2Y-type purinergic receptors. Am. J. Physiol. Heart Circulatory Physiol. 320, H699–H712. doi: 10.1152/ajpheart.00560.2020
Hou, Z., Wang, X., Cai, J., Zhang, J., Hassan, A., Auer, M., et al. (2018). Platelet-derived growth factor subunit b signaling promotes pericyte migration in response to loud sound in the cochlear stria vascularis. J. Assoc. Res. Otolaryngol. 19, 363–379. doi: 10.1007/s10162-018-0670-z
Imayama, S., and Urabe, H. (1984). Pericytes on the dermal microvasculature of the rat skin. Anat. Embryol. 169, 271–274. doi: 10.1007/BF00315632
Kempf, S., Popp, R., Naeem, Z., Frömel, T., Wittig, I., Klatt, S., et al. (2024). Pericyte-to-endothelial cell communication via tunneling nanotubes is disrupted by a diol of docosahexaenoic acid. Cells 13:1429. doi: 10.3390/cells13171429
Korenkova, O., Pepe, A., and Zurzolo, C. (2020). Fine intercellular connections in development: TNTs, cytonemes, or intercellular bridges? Cell Stress 4, 30–43. doi: 10.15698/cst2020.02.212
Kovacs-Oller, T., Ivanova, E., Bianchimano, P., and Sagdullaev, B. T. (2020). The pericyte connectome: Spatial precision of neurovascular coupling is driven by selective connectivity maps of pericytes and endothelial cells and is disrupted in diabetes. Cell Discov. 6, 1–18. doi: 10.1038/s41421-020-0180-0
Krueger, M., and Bechmann, I. (2010). CNS pericytes: Concepts, misconceptions, and a way out. Glia 58, 1–10. doi: 10.1002/glia.20898
Kunz, J., Krause, D., Kremer, M., and Dermietzel, R. (1994). The 140-kDa protein of blood-brain barrier-associated pericytes is identical to aminopeptidase N. J. Neurochem. 62, 2375–2386. doi: 10.1046/j.1471-4159.1994.62062375.x
Leibnitz, L., and Bär, B. (1988). A blood capillaries-bridging cell type in adult mammalian brains. J. Hirnforsch. 29, 367–375.
Mäe, M. A., He, L., Nordling, S., Vazquez-Liebanas, E., Nahar, K., Jung, B., et al. (2021). Single-cell analysis of blood-brain barrier response to pericyte loss. Circulation Res. 128, e46–e62. doi: 10.1161/CIRCRESAHA.120.317473
Medina-Flores, F., Hurtado-Alvarado, G., Deli, M. A., and Gómez-González, B. (2023). The active role of pericytes during neuroinflammation in the adult brain. Cell. Mol. Neurobiol. 43, 525–541. doi: 10.1007/s10571-022-01208-5
Mendes-Jorge, L., Llombart, C., Ramos, D., López-Luppo, M., Valença, A., Nacher, V., et al. (2012). Intercapillary bridging cells: Immunocytochemical characteristics of cells that connect blood vessels in the retina. Exp. Eye Res. 98, 79–87. doi: 10.1016/j.exer.2012.03.010
Montagne, A., Barnes, S. R., Sweeney, M. D., Halliday, M. R., Sagare, A. P., Zhao, Z., et al. (2015). Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302. doi: 10.1016/j.neuron.2014.12.032
Morris, G. P., Foster, C. G., Courtney, J., Collins, J. M., Cashion, J. M., Brown, L. S., et al. (2023). Microglia directly associate with pericytes in the central nervous system. Glia 71, 1847–1869. doi: 10.1002/glia.24371
Munro, D. A. D., Bestard-Cuche, N., McQuaid, C., Chagnot, A., Shabestari, S. K., Chadarevian, J. P., et al. (2024). Microglia protect against age-associated brain pathologies. Neuron 112, 2732–2748.e1. doi: 10.1016/j.neuron.2024.05.018
Nehls, V., and Drenckhahn, D. (1991). Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J. Cell Biol. 113, 147–154. doi: 10.1083/jcb.113.1.147
Nirwane, A., and Yao, Y. (2022). SMAlow/undetectable pericytes differentiate into microglia- and macrophage-like cells in ischemic brain. Cell. Mol. Life Sci. 79:264. doi: 10.1007/s00018-022-04322-1
Önfelt, B., Nedvetzki, S., Benninger, R. K. P., Purbhoo, M. A., Sowinski, S., Hume, A. N., et al. (2006). Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria1. J. Immunol. 177, 8476–8483. doi: 10.4049/jimmunol.177.12.8476
Ornelas, S., Berthiaume, A.-A., Bonney, S. K., Coelho-Santos, V., Underly, R. G., Kremer, A., et al. (2021). Three-dimensional ultrastructure of the brain pericyte-endothelial interface. J. Cereb. Blood Flow Metab. 41, 2185–2200. doi: 10.1177/0271678X211012836
Ouarné, M., Pena, A., and Franco, C. A. (2021). From remodeling to quiescence: The transformation of the vascular network. Cells Dev. Quant. Cell Dev. Biol. 168:203735. doi: 10.1016/j.cdev.2021.203735
Park, D. Y., Lee, J., Kim, J., Kim, K., Hong, S., Han, S., et al. (2017). Plastic roles of pericytes in the blood–retinal barrier. Nat. Commun. 8:15296. doi: 10.1038/ncomms15296
Payne, L. B., Darden, J., Suarez-Martinez, A. D., Zhao, H., Hendricks, A., Hartland, C., et al. (2021). Pericyte migration and proliferation are tightly synchronized to endothelial cell sprouting dynamics. Integr Biol. 13, 31–43. doi: 10.1093/intbio/zyaa027
Pfister, F., Feng, Y., Vom Hagen, F., Hoffmann, S., Molema, G., Hillebrands, J.-L., et al. (2008). Pericyte migration. Diabetes 57, 2495–2502. doi: 10.2337/db08-0325
Popescu, L. M., and Faussone-Pellegrini, M.-S. (2010). Telocytes – a case of serendipity: The winding way from Interstitial cells of Cajal, via Interstitial Cajal-Like Cells to Telocytes. J. Cell. Mol. Med. 14, 729–740. doi: 10.1111/j.1582-4934.2010.01059.x
Rajan, A. M., Ma, R. C., Kocha, K. M., Zhang, D. J., and Huang, P. (2020). Dual function of perivascular fibroblasts in vascular stabilization in zebrafish. PLoS Genet. 16:e1008800. doi: 10.1371/journal.pgen.1008800
Reissenweber, N. J., and Pessacq, T. (1971). Intervascular strands in the central nervous system. A histochemical approach. Acta Anat. 78, 51–57. doi: 10.1159/000143574
Rouget, C. (1874). Note sur le développement de la tunique contractile des vaisseaux, in Comptes rendus hebdomadaires des séances de l’Académie des sciences (Académie des Sciences: Paris) 559–562.
Rustom, A., Saffrich, R., Markovic, I., Walther, P., and Gerdes, H.-H. (2004). Nanotubular highways for intercellular organelle transport. Science 303, 1007–1010. doi: 10.1126/science.1093133
Sakuma, R., Kawahara, M., Nakano-Doi, A., Takahashi, A., Tanaka, Y., and Narita, A. et al. (2016). Brain pericytes serve as microglia-generating multipotent vascular stem cells following ischemic stroke. J. Neuroinflammation 13:57. doi: 10.1186/s12974-016-0523-9
Sartori-Rupp, A., Cordero Cervantes, D., Pepe, A., Gousset, K., Delage, E., Corroyer-Dulmont, S., et al. (2019). Correlative cryo-electron microscopy reveals the structure of TNTs in neuronal cells. Nat. Commun. 10:342. doi: 10.1038/s41467-018-08178-7
Sewell, M., Fialova, N., and Montagne, A. (2025). Unraveling the transcriptomic landscape of brain vascular cells in dementia: A systematic review. Alzheimers Dement. 21:e14512. doi: 10.1002/alz.14512
Shepro, D., and Morel, N. M. (1993). Pericyte physiology. FASEB J. 7, 1031–1038. doi: 10.1096/fasebj.7.11.8370472
Sims, D. E. (1986). The pericyte - A review. Tissue Cell 18, 153–174. doi: 10.1016/0040-8166(86)90026-1
Smyth, L. C. D., Rustenhoven, J., Scotter, E. L., Schweder, P., Faull, R. L. M., and Park, T. I. H. et al. (2018). Markers for human brain pericytes and smooth muscle cells. J. Chem. Neuroanat. 92, 48–60. doi: 10.1016/j.jchemneu.2018.06.001
Soto, I., Graham, L. C., Richter, H. J., Simeone, S. N., Radell, J. E., Grabowska, J., et al. (2015). APOE stabilization by exercise prevents aging neurovascular dysfunction and complement induction. PLoS Biol. 13:e1002279. doi: 10.1371/journal.pbio.1002279
Sowinski, S., Alakoskela, J.-M., Jolly, C., and Davis, D. M. (2011). Optimized methods for imaging membrane nanotubes between T cells and trafficking of HIV-1. Methods 53, 27–33. doi: 10.1016/j.ymeth.2010.04.002
Tilton, R. G., Miller, E. J., Kilo, C., and Williamson, J. R. (1985). Pericyte form and distribution in rat retinal and uveal capillaries. Invest. Ophthalmol. Vis. Sci. 26, 68–73.
Varga, I., Kyselovič, J., Danišovič, L’., Gálfiová, P., Kachlík, D., Polák, Š., et al. (2019). Recently discovered interstitial cells termed telocytes: Distinguishing cell-biological and histological facts from fictions. Biologia 74, 195–203. doi: 10.2478/s11756-018-0162-y
von Tell, D., Armulik, A., and Betsholtz, C. (2006). Pericytes and vascular stability. Exp. Cell Res. Special Issue Angiogenesis 312, 623–629. doi: 10.1016/j.yexcr.2005.10.019
Wang, X., and Gerdes, H.-H. (2015). Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 22, 1181–1191. doi: 10.1038/cdd.2014.211
Wang, X., Veruki, M. L., Bukoreshtliev, N. V., Hartveit, E., and Gerdes, H.-H. (2010). Animal cells connected by nanotubes can be electrically coupled through interposed gap-junction channels. Proc. Natl. Acad. Sci. U S A. 107, 17194–17199.
Williamson, J. R., Tilton, R. G., Kilo, C., and Yu, S. (1980). Immunofluorescent imaging of capillaries and pericytes in human skeletal muscle and retina. Microvasc. Res. 20, 233–241. doi: 10.1016/0026-2862(80)90010-2
Zhang, K., Sun, Z., Chen, X., Zhang, Y., and Guo, A. (2021). Intercellular transport of Tau protein and β-amyloid mediated by tunneling nanotubes. Am. J. Transl. Res. 13, 12509–12522. doi: 10.1002/ctd2.186
Zhang, X., Lu, P., and Shen, X. (2023). Morphologies and potential roles of telocytes in nervous tissue. Clin. Transl. Discov. 3:e186. doi: 10.1002/ctd2.186
Zhang, Y. (2011). Tunneling-nanotube: A new way of cell-cell communication. Commun. Integr. Biol. 4, 324–325. doi: 10.4161/cib.4.3.14855
Zhao, Z., Zhang, Y., Zhang, C., Zhang, J., Luo, X., Qiu, Q., et al. (2022). TGF-β promotes pericyte-myofibroblast transition in subretinal fibrosis through the Smad2/3 and Akt/mTOR pathways. Exp. Mol. Med. 54, 673–684.
Zimmermann, K. W. (1923). Der feinere Bau der Blutcapillaren. Z. Anat. Entwickl. Gesch. 68, 29–109. doi: 10.1007/BF02593544
Keywords: pericytes, brain, capillaries, bridging cells, tunneling nanotubes, pericyte migration, vascular regression
Citation: Chagnot A and Montagne A (2025) Walking on the tightrope: the shared roles of the bridging pericytes in the brain. Front. Cell. Neurosci. 19:1615579. doi: 10.3389/fncel.2025.1615579
Received: 23 April 2025; Accepted: 10 July 2025;
Published: 22 July 2025.
Edited by:
Stephanie Bonney, Seattle Children’s Research Institute, United StatesReviewed by:
Ravichand Palakurti, University of Pittsburgh, United StatesAnnika Keller, University Hospital Zürich, Switzerland
Albert L. Gonzales, University of Nevada, Reno, United States
Copyright © 2025 Chagnot and Montagne. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Axel Montagne, YXhlbC5tb250YWduZUBlZC5hYy51aw==; Audrey Chagnot, YXVkcmV5LmNoYWdub3RAZWQuYWMudWs=
 Audrey Chagnot
Audrey Chagnot Axel Montagne
Axel Montagne