- 1Department of Cellular and Systems Pharmacology, College of Pharmacy, University of Florida, Gainesville, FL, United States
- 2Department of Neuroscience, College of Medicine, University of Florida, Gainesville, FL, United States
- 3McKnight Brain Institute, University of Florida, Gainesville, FL, United States
- 4Department of Psychiatry, College of Medicine, University of Florida, Gainesville, FL, United States
The basolateral amygdala (BLA) plays a crucial role in context-specific learning and memory by integrating valence-specific stimuli with internal physiological states. Cholinergic signaling systems modulate neural excitability to influence information processing in the BLA. Muscarinic acetylcholine receptors (mAChRs) are of particular interest because aberrant mAChR signaling in BLA circuits is associated with neuropsychiatric disorders, cognitive impairment, substance use, and age-related cognitive decline. This study evaluates mAChR activation in BLA principal neurons (PNs) in juvenile rat brain slices using whole-cell patch-clamp recordings. We found that bath application of carbachol (CCh) produces a pirenzepine sensitive excitatory response in BLA PNs voltage clamped near the resting potential, which depends on an underlying biphasic change in membrane resistance, indicating an involvement of multiple effectors. More specifically, we observed that CCh excites BLA PNs by inhibiting the afterhyperpolarization (AHP), by reducing a steady state inhibitory current, and by promoting an afterdepolarization (ADP). We further identify and characterize a CCh-induced and calcium-activated non-selective cation current (ICAN) that underlies the ADP in voltage clamp. Overall, our findings provide new insights into specific effectors modulated by activation of pirenzepine sensitive mAChRs expressed by BLA PNs. We also reveal new details about the time- and voltage-dependence of current carried by the CCh -activated ICAN like current in BLA PNs, and highlight its ability to promote a suprathreshold ADP capable of generating sustained firing after a brief excitatory stimulus. Improved understanding of these effectors will provide potentially valuable new insights on the wide range of mechanisms through which cholinergic system dysfunction can lead to impaired executive function.
1 Introduction
Neural processing of information traveling through BLA circuitry impacts the balance of fear, stress, motivation, decision-making, and reward processing, which underlie complex behaviors that facilitate survival. Aberrant signaling in the BLA is associated with clinical manifestations such as post-traumatic stress disorder, substance use disorders, anxiety disorders, aggression, and impulsivity, but despite its important role in health and disease the mechanisms that mediate information processing through the BLA remain incompletely understood (Sharp, 2017; Buades-Rotger et al., 2019; Patel et al., 2018; Hosie et al., 2018; Winstanley et al., 2004). The BLA receives heterogeneous synaptic inputs from numerous brain centers including the lateral amygdala, hippocampus, prefrontal cortex, and the bed nucleus of the stria terminals, and the BLA also sends projections to many locations including the central amygdala, medial amygdala, prefrontal cortex, thalamus, hypothalamus, and nucleus accumbens (Huang et al., 2021). Glutamatergic inputs to the BLA are the primary source of fast excitatory neurotransmission, but BLA neurons are sensitive to a wide variety of neurotransmitters and neuromodulators including acetylcholine, norepinephrine, serotonin, cholecystokinin, corticotropin releasing hormone, endocannabinoids, and more, which modify their excitability to fine-tune how information is processed as it travels through the BLA (Pliota et al., 2020; Li et al., 2023; Hajisoltani and Meftahi, 2024; Valipour et al., 2023; Meis et al., 2007).
Cholinergic signaling and its ability to modulate information flow through the BLA is of particular interest because pharmacological interventions that target acetylcholine receptors (AChRs) have the potential to improve anxiety, schizophrenia, mood disorders, substance use, and age-related cognitive decline (Walker and Lawrence, 2020; Mei et al., 2020; Karami et al., 2019; Kruse et al., 2014; Bubser et al., 2011; Crimmins et al., 2023). The BLA has a high density of cholinergic terminals originating from the basal forebrain and brainstem. BLA PNs express muscarinic acetylcholine receptors (mAChRs) which can be studied experimentally using carbachol (CCh), a non-selective cholinergic agonist (Eglen et al., 1987). CCh sensitive mAChRs expressed by BLA PNs ultimately activate several distinct effector systems (Washburn and Moises, 1992; Womble and Moises, 1992). A major excitatory action of CCh on BLA PNs is caused by the closing of potassium channels that are open near the resting membrane potential, which depolarizes neurons, and also increases their sensitivity to excitatory synaptic inputs due to increased membrane resistance. Activation of mAChRs also influences complex firing behavior of BLA PNs. Strong firing activates a milieu of voltage-gated and/or calcium-activated currents to produce an inhibitory afterhyperpolarization (AHP) and an excitatory afterdepolarization (ADP), which combine in complex ways to influence synaptic integration by modulating the probability and timing of subsequent action potentials. Unal et al. (2015) demonstrated that optogenetic release of ACh in the BLA results in a large activity-dependent ADP that is sensitive to flufenamic acid (FFA), suggesting it may be caused by a calcium-activated non-selective cation current (ICAN). Activation of mAChRs also reduces calcium-activated potassium currents underlying the slow AHP (sIAHP), and the voltage-dependent potassium current (IM), which collectively further contributes to a net excitatory effect (Womble and Moises, 1992; Womble and Moises, 1993).
In this study we used whole-cell patch-clamp techniques to further characterize how mAChR activation influences excitability of BLA PNs in acute rat brain slices. Consistent with previous reports, we found that mAChR activation at subthreshold potentials produces a depolarizing current through the combined action of closing potassium channels and opening calcium-dependent and likely non-selective cation channels. In addition, we find that CCh produces a calcium-dependent and FFA-sensitive ADP, and we extend previous reports by isolating this current (IADP) in voltage-clamp configuration to evaluate its time dynamics and voltage dependence. In addition to these findings, the method we describe to isolate and characterize the IADP can be applied to future studies that probe alterations in cholinergic signaling associated with various physiological and pathological states that influence BLA-dependent behaviors.
2 Methods
2.1 Acute brain slice preparation
All animal procedures were reviewed and approved by the University of Florida Institutional Animal Care and Use Committee (IACUC). Juvenile male Sprague–Dawley rats (P16-28) were used for this study. Acute brain slices were prepared as previously described (Harden and Frazier, 2016). Briefly, rats were administered a ketamine/xylazine cocktail (100 mg/kg ketamine, 10 mg/kg xylazine, i.p.) and after adequate anesthesia was achieved (no response to hind-paw pinch) rats were rapidly decapitated and brains were rapidly extracted and submerged in ice-cold sucrose-laden artificial cerebrospinal solution (ACSF) containing (in mM) 205 sucrose, 10 dextrose, 1 MgSO4, 2 KCl, 1.25 NaH2PO4, 1 CaCl2, and 25 NaHCO3, oxygenated with carbogen (95% O2/5% CO2). Brains were coronally sectioned at 300 microns using a Leica VT1200S vibratome, then transferred to an incubation solution containing (in mM) 124 NaCl, 10 dextrose, 3 MgSO4, 2.5 KCl, 1.23 NaH2PO4, 1 CaCl2, 25 NaHCO3, oxygenated with carbogen and maintained at 35°C for 30 min. Brain slices were then permitted to passively equilibrate to room temperature for at least 30 min prior to recording. Brain slices were transferred to a perfusion chamber and submersed in ACSF containing (in mM) 126 NaCl, 11 dextrose, 1.5 MgSO4, 3 KCl, 1.2 NaH2PO4, 2.4 CaCl2, 25 NaHCO3, continuously oxygenated with carbogen, perfused at 2 mL/min, and maintained at 28°C. ACSF for all experiments was supplemented with glutamate- and GABA receptor antagonists (20 μM DNQX, 40 μM DL-AP5, 100 μM PTX, and 10 μM CGP-55845) to minimize influence of synaptic signaling (on AMPA, NMDA, GABAA, and GABAB receptors, respectively) and enhance measurement of isolated somatic currents.
2.2 Electrophysiology
Brain slices were visualized using an Olympus BX-WI upright stereomicroscope equipped for infrared differential interference contrast microscopy, a 12-bit CCD camera (QImaging Rolera-XR), and micro-manager software (Edelstein et al., 2014). Patch-clamp experiments were performed using a Multiclamp 700B patch-clamp amplifier with a CV-7B headstage, a DigiData 1440A digitizer (Molecular Devices), and pClamp 11 software. Data were obtained at 20 kHz and voltage-clamp data were filtered using a 7-pole Bessel low-pass filter with a 2 kHz − 3 dB cutoff. On-line analysis was performed with custom software written using Python 3.11 and the pyABF package. Off-line analysis was performed with OriginPro (OriginLab, Northampton, MA) using custom software written in OriginC by CJF.
2.3 Intracellular solutions
Patch pipettes were pulled from borosilicate glass (Sutter Instrument BF150-86-10) using a Flaming/Brown pipette puller (Sutter Instrument SU-P97) and had an open-tip resistance of 4–6 MΩ when filled with a K-gluconate based internal solution containing (in mM) 115 K-gluconate, 10 di-tris phosphocreatine, 10 HEPES, 0.5 EGTA, 2 MgCl2, 4 Na2ATP, 0.4 Na3GTP, 5 KCl. This solution was adjusted to pH 7.25 and 295 mOsm. In some experiments K-gluconate was replaced with an equimolar concentration of Cs-gluconate. For experiments requiring intracellular delivery of BAPTA, a modified internal solution was created substituting 10 mM of the primary cation with BAPTA. The liquid junction potential (LJP) was calculated according to the stationary Nernst–Planck equation (Marino et al., 2014) using LJPcalc (RRID: SCR_025044) and estimated to be 14.8 mV and 15.3 mV for the potassium and cesium based internal solutions, respectively. Results presented are based on calculations that used raw data uncorrected for the LJP.
2.4 Protocols
Following establishment of a whole-cell recording, passive membrane properties were evaluated in all neurons using a membrane test protocol that delivered a 200 ms 10 mV hyperpolarizing pulse from a holding potential of −70 mV. Principal neurons were identified by their large soma and regular firing pattern with a maximum firing frequency of <40 Hz, consistent with previous studies (Hernandez et al., 2019; Ryan et al., 2018). Time course experiments in which access resistance drifted >30% were also excluded from this study. Whole-cell capacitance was measured using a bidirectional ramp protocol described by Golowasch et al. (2009). Experiments in which membrane test properties were compared before vs. after CCh report mean values from 2.5–4.5 and 8–10 min after CCh application relative to the mean value over a 5-min baseline period before CCh application. Current-clamp experiments evaluating the AHP and/or ADP were performed using a protocol that applied a constant current to hold cells near −70 mV, and a 500 ms depolarizing pulse to cause robust action potential firing (at > = 66% of the maximum observed rate). AHP and/or ADP measurements were obtained over a 2 s window beginning 100 ms after termination of the that depolarizing current pulse and are reported as area under the curve (AUC) relative to the mean voltage over a 1 s period immediately before the depolarizing step. Voltage-clamp experiments evaluating the currents underlying the ADP and/or AHP used a protocol in which neurons were continuously held at −60 mV and stepped to +50 mV for 500 ms. Variations of this protocol recorded multiple sweeps with stimulation duration (0–500 ms in 50 ms steps) or post-stimulation voltage commands of −90 to +20 mV (in 10 mV steps).
2.5 Statistics
Population values are presented as mean ± the SEM. Electrophysiological changes before vs. after application of a drug were evaluated for significance in using paired t-tests on raw data, or one-sample t-tests on baseline subtracted data (null hypothesis mean = 0). Curve fitting was performed with OriginPro (Originlab, Northampton, MA).
3 Results
3.1 Carbachol produces a net excitatory effect on BLA PNs via activation of multiple effectors
Somatic effects of CCh were evaluated in BLA PNs targeted for whole-cell recording in acute brain slices according to their unique morphological and electrophysiological features (see Methods). Passive membrane properties evaluated in control conditions (membrane resistance: 78.71 ± 4.64 MΩ, whole-cell capacitance: 192.31 ± 7.97 pF, n = 34) were consistent with previous reports (Hernandez et al., 2019). Holding current at −70 mV was continuously measured as CCh was delivered using a syringe pump to achieve a bath concentration of 10 μM. We found that CCh reliably produced an excitatory shift in holding current (−45.65 ± 3.90 pA, n = 34, p = 2.59e-13) that plateaued approximately 8 min after perfusion began (Figure 1A).
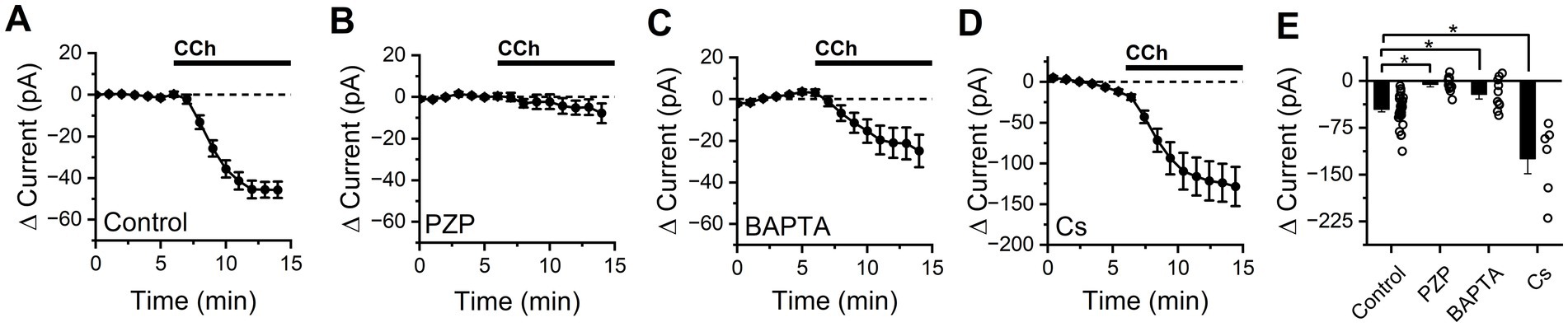
Figure 1. CCh produces an M1/M4 mAChR dependent excitatory current in BLA PNs at subthreshold potentials that is partially blocked by intracellular BAPTA, but not by intracellular cesium. (A) Bath application of CCh causes a negative shift in holding current in BLA PNs voltage clamped at −70 mV, consistent with activation of net excitatory conductances (n = 34). (B) Application of the M1/M4 mAChR antagonist pirenzepine (PZP) prevents changes in holding current following CCh exposure (n = 10). (C) Intracellular application of BAPTA (10 mM) reduces but does not eliminate the negative shift in holding current produced by CCh (n = 10). (D) Intracellular cesium fails to block (in fact increases, see results) overall negative shift in holding current produced by bath application of CCh (n = 6). (E) Summary data highlighting change in holding current observed in BLA PNs voltage clamped at −70 mV after 10 min of CCh exposure, for each condition described in panels (A–D) (open circles represent individual cells, bar graphs represent mean ± SE, asterisks indicate p < 0.05, unpaired Student’s t-tests).
To gain insight into which mAChR subtype mediates the response to CCh, experiments were repeated in the presence of M1/M4-selective mAChR antagonist pirenzepine (PZP, 1 μM). We found that PZP prevented CCh from causing an excitatory shift in holding current (−5.72 ± 4.03 pA, n = 10, p = 0.19, Figure 1B) suggesting that the CCh-induced excitatory current was mediated primarily by M1/M4 mAChRs. When the intracellular solution was supplemented with 10 mM BAPTA (a strong calcium chelator), CCh still produced a negative shift in holding current (−21.29 ± 7.65 pA, n = 10, p = 0.021, Figure 1C) but it was significantly smaller than the effect observed in control conditions (p = 5.35e-03), suggesting that excitation by CCh is at least partially dependent on an effector that is modulated by intracellular calcium. Cesium-based pipette solutions are commonly used to block some types of potassium channels, but when experiments were repeated using a cesium-based intracellular solution we found CCh still produced a large excitatory shift in holding current (−124.5 ± 20.1 pA, n = 6, p = 3.41e-3, Figure 1D). In fact, this effect was even larger than in observed with a potassium based internal (p = 0.02), likely due to the significantly higher basal membrane resistance observed with a Cs-based internal solution (107.8 ± 13.0 MΩ, p = 0.02 vs. control conditions).
Interestingly, despite observing an expected strong net excitatory effect of CCh on BLA PNs, we found that CCh reliably produced a biphasic change in membrane resistance (Figure 2A). Specifically, an early decrease in membrane resistance was observed (−9.4 ± 1.1%, p = 4.7e-6, n = 34, measured 2.5–4.5 min after CCh application), followed by an increase in Rm which was apparent by 8–10 min after the onset of CCh application (10.3 ± 2.4%, p = 1.8e-4, n = 34). This finding suggests that CCh may activate at least two distinct types of effectors: one that opens ion channels to lower membrane resistance, and another that closes ion channels to raise it. Consistent with our findings that PZP prevents CCh from changing holding current, we also found that PZP effectively prevents both the observed CCh-mediated decrease and later CCh-mediated increase in Rm (early: −0.66 ± 1.0%, p = 0.56, n = 10, late: −1.4 ± 2.0%, p = 0.48, n = 10, Figure 2B).
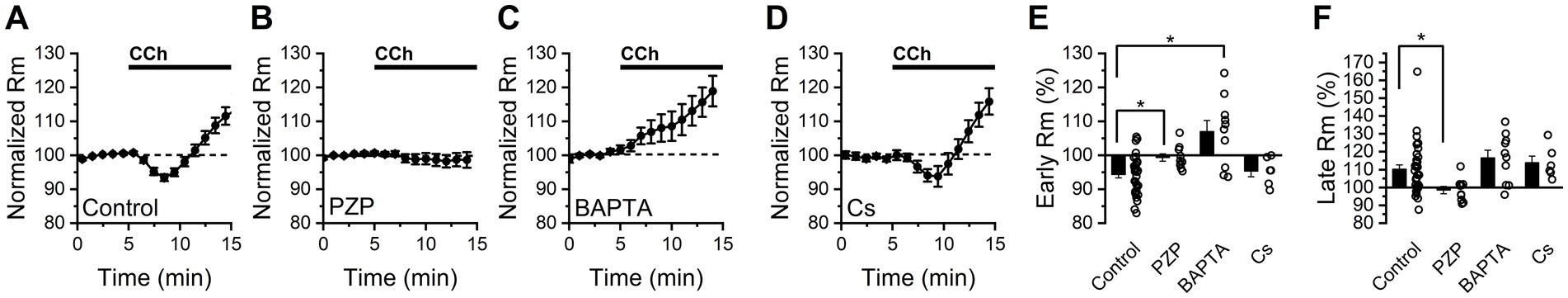
Figure 2. Bath application of CCh has a complex effect of membrane resistance in BLA PNs voltage clamped at −70 mV. (A) Bath application of CCh (10 mM) produces an initial decrease in membrane resistance followed by a later increase (n = 34). (B) Continuous application of the M1/M4 mAChR antagonist pirenzepine prevents both the early decrease and late increase in membrane resistance produced by CCh (n = 10). (C) Intracellular BAPTA (10 mM) prevents the early CCh-induced decrease in membrane resistance but does not block the later CCh-induced increase in membrane resistance (n = 10). (D) Intracellular cesium does not block either the early or late effects of CCh on membrane resistance (n = 6). (E,F) Group comparison of the change in membrane resistance at early and late time points (2.5–4.5 min and 8–10 min following CCh exposure, respectively, open circles represent individual cells, bar graphs represent mean ± SE, asterisks indicate p < 0.05, unpaired Student’s t-tests).
Next, we noted that when 10 mM BAPTA was included in the internal solution, CCh increased membrane resistance at the early time point (by 7.02 ± 3.20%, p = 0.057, n = 10), and continued to do so at the later time point (16.5 ± 4.3%, p = 0.004, n = 10, Figure 2C). In contrast, when a cesium-based internal solution was used we found that membrane resistance changed similarly to control conditions at both early and late time points (early: −5.59 ± 1.68%, n = 6, p = 0.039, late: +13.9 ± 3.7%, n = 6, p = 0.013, Figure 2D), with no statistically significant difference between cesium and control groups at either time point (p = 0.66 early, 0.54 late, vs. control conditions). Figures 2E,F provide summary data across all conditions at both the early and late time points, respectively. Collectively, these data in combination with those presented in Figure 1, suggest that CCh drives depolarization of BLA PNs from near the resting potential in part by activating a cesium-insensitive and calcium-dependent current that drives the membrane potential positive of rest, and also by inhibiting a calcium-independent and cesium-insensitive current that drives the membrane potential negative of rest.
3.2 Carbachol enhances an activity dependent afterdepolarization observed in current clamp
The CCh has been reported to act on calcium- and voltage-sensitive ion channels to influence excitability of BLA PNs in response to strong firing (Unal et al., 2015). To evaluate how mAChR activation influences the AHP and ADP in BLA PNs, a 500 ms current pulse was delivered from a membrane potential near −70 mV to produce action potential firing at > = 66% of the maximum observed rate. The AHP or ADP was then measured during a 2 s window shortly after the depolarizing current pulse (see Methods). In control conditions, strong firing was followed by an AHP in most cells tested (11 of 14, Figure 3A), with a population mean of −1.42 ± 0.49 mV * sec (p = 0.012, n = 14). Following 10 min of exposure to 10 μM CCh, strong firing resulted in a large ADP in all cells tested (13.9 ± 0.24 mV * sec, p = 2.99e-4, n = 10, Figure 3B), which was significantly more positive than the pre-stimulus baseline (p = 1.22e-04). In a subpopulation of cells (2 of 10) the ADP was large enough to reach suprathreshold potentials and cause continuous firing (Figure 3C), a phenomenon that was never observed in the absence of CCh.
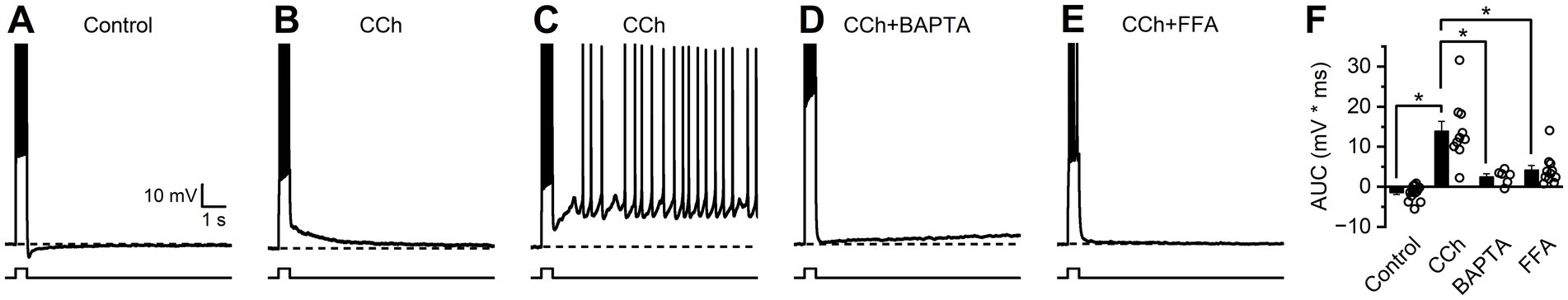
Figure 3. Strong firing in the presence of CCh produces a large ADP. Current-clamp recordings were obtained from neurons held at −70 mV and stimulated with a 500 ms depolarizing current pulse to cause strong firing. (A) Representative trace demonstrating an afterhyperpolarization (AHP) in control conditions. (B) Representative trace demonstrating a large afterdepolarization (ADP) following CCh. (C) Representative trace demonstrating behavior presented by a subset of cells (2 of 10) where CCh resulted in suprathreshold ADPs that caused continuous firing. (D,E) Representative traces demonstrating absence of a large ADP when CCh is applied in the presence of BAPTA or FFA. (F) Group comparison of change in membrane potential after strong firing (Control n = 14, CCh n = 10, BAPTA n = 6, FFA n = 11, open circles represent individual cells, bar graphs represent mean ± SE, asterisks indicate p < 0.05, unpaired Student’s t-tests).
Current-clamp experiments evaluating the CCh-induced enhancement of ADP were repeated using modified extracellular or intracellular solutions to gain insight into the signaling pathways that underlie this behavior. When experiments were repeated using a pipette solution containing 10 mM BAPTA, CCh still produced a small ADP (2.55 ± 0.73 mV, p = 0.018, n = 6, Figure 3D) but it was significantly smaller than the ADP observed without BAPTA (p = 2.87e-04) suggesting that much of the ADP is the result of a calcium-dependent intracellular signaling pathway. Similarly, experiments repeated in the presence of ICAN antagonist flufenamic acid (FFA, 100 μM) demonstrated that CCh produces an ADP (4.20 ± 1.12 mV, p = 3.75e-3, n = 6, Figure 3E) significantly smaller than the ADP observed without FFA (p = 4.20e-04). Together these findings suggest that CCh acts on BLA PNs activate an FFA sensitive and calcium-dependent non-selective cation channel, which is primarily responsible for driving the ADP.
3.3 Voltage clamp experiments can reveal the detailed kinetics and voltage dependence of the current that underlies the CCh enhanced afterdepolarization
The excitatory current underlying the ADP observed in the presence of CCh (IADP) was further characterized in voltage-clamp configuration using a 500 ms step to +50 mV to simulate strong firing from a −60 mV subthreshold potential. IADP was measured as area under the curve (AUC) of the voltage-clamp trace for a 3 s period after the depolarizing voltage step relative to the current before the step (see Methods). In the absence of CCh, this protocol allowed the current underlying the AHP (Figure 3A, dashed line) to be measured as an outward current following stimulation (97.4 ± 27.8 pA * sec, p = 0.07, n = 3, Figures 4A,B). Following 10 min of exposure to 10 μM CCh, the depolarizing voltage step resulted in a large inward current, the IADP (−47.6 ± 11.4 pA * sec, p = 0.05, n = 3, Figures 4A,B). In order to better isolate and evaluate the IADP, these experiments were repeated with a cesium-based pipette solution, which blocked the potassium channels underlying the AHP that was observed in the absence of CCh. Using this pipette solution, we found a small inward rather than outward current was present in baseline conditions, and the size of this current was significantly increased by bath application of 10 μM CCh (see Figure 4C for representative example). The IADP activated by CCh can then be isolated by calculating the difference between the CCh trace and baseline trace, as illustrated in Figure 4D.
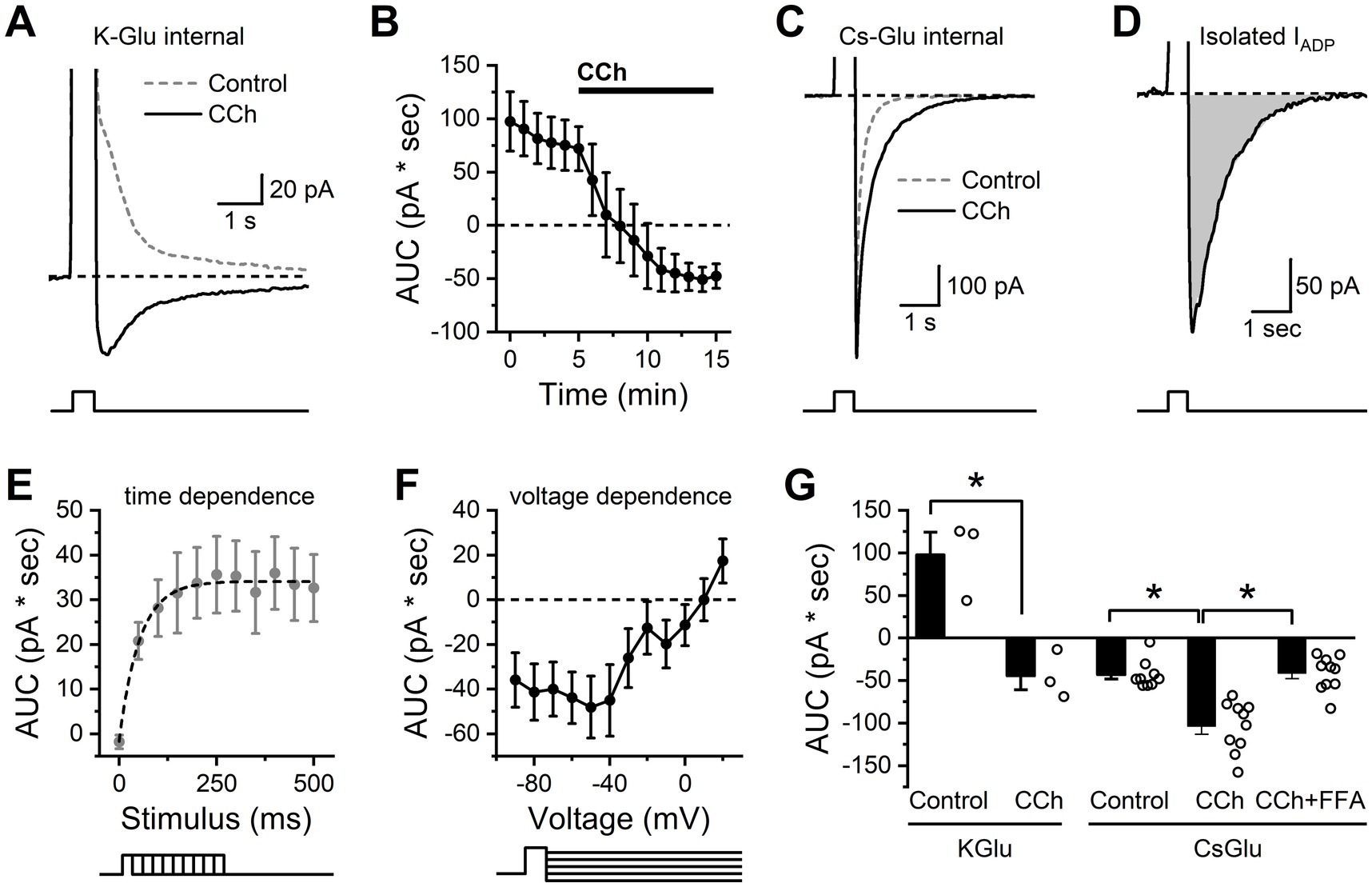
Figure 4. Isolation and characterization of the CCh-activated ADP in BLA PNs. (A) Representative traces indicating that, in control conditions, a brief but strong depolarization is followed by an outward current (dashed gray line), while in the presence of CCh, the same depolarization is followed by an inward current (IADP, solid black line). (B) Repeated measurement of the stimulation-induced current over time further demonstrates that bath application of CCh causes a shift from an outward current to an inward current (n = 3). (C) The same stimulation using a cesium-based pipette solution prevents the outward current in baseline conditions (dashed gray line) but preserves the ability of CCh to enhance the inward current (IADP, solid black line). (D) The isolated CCh-induced IADP, presented as the difference between CCh and control curves from panel (C). (E) Voltage steps of varying duration were used to reveal that depolarizing steps of 250 ms or greater were required to produce maximal activation of the CCh-induced IADP as observed with a cesium-based pipette solution (n = 11). (F) Voltage dependence of the excitation-activated current (also obtained using a cesium-based pipette solution) demonstrates reversal potential near +10 mV (n = 12). (G) Summary data for each cell indicating area of the post stimulus current as observed in voltage clamp using both K-gluconate and Cs-gluconate internal solutions after a 250 ms depolarization (open circles represent individual cells, bar graphs represent mean ± SE, asterisks indicate p < 0.05, unpaired Student’s t-tests, n values for all groups are specified in the main text). Note that the Cs-gluconate internal blocks the outward current observed in control conditions, but CCh still enhances the inward current. Also note that the inward IADP as isolated here with the Cs-gluconate internal is largely blocked by FFA.
Using this method, we proceeded to evaluate the time and voltage dependence of the isolated CCh-activated IADP. Specifically, experiments that varied the stimulus duration revealed that the maximum IADP could be produced with a stimulus duration of ~250 ms (Figure 4E). The mean amplitude of the maximum IADP was 35.3 pA, and the time constant of the activation curve was 52.3 ms. To evaluate voltage dependence, we used a constant stimulus duration of 500 ms, but varied the post-stimulation voltage command. This experiment revealed that the IADP demonstrated little voltage dependence at subthreshold potentials but had a linear slope at suprathreshold potentials with a reversal potential near +10 mV (Figure 4F).
To more clearly summarize data across these experiments, in Figure 4G we present area under the curve of poststimulation currents (recorded at −60 mV after a brief step to +50 mV), both in control conditions and in the presence of CCh. Specifically, the data obtained with a K-gluconate internal (left side of Figure 4G) further emphasizes that CCh converts the post-depolarization current from outward to inward (n = 3, p = 0.017), consistent with the current clamp data in Figure 3 that revealed a CCh-induced shift from an AHP to an ADP. By contrast, in cells filled with Cs-gluconate (right side of Figure 4G), we observed a post-stimulus inward current in control conditions that was significantly larger when observed in the presence of CCh (n = 9, 10 for control vs. CCh, p = 5.58e-4). Finally, still in cells with Cs-gluconate internal, we noted that if both CCh and FFA were present in the bath, then the post-stimulus current was no larger than observed in control conditions (n = 9, 10, respectively, p = 0.86), and was also significantly smaller than observed in the presence of CCh without FFA (n = 10,10, respectively, p = 4.74e-5). These data qualitatively mirror the effects of FFA on post-stimulus responses in current clamp, but importantly represent measurements of a much better isolated cholinergic response. We expect this method to be useful in future studies for making detailed measurements of cholinergic function in a variety of pathological conditions.
4 Discussion
This study evaluates the effects of CCh on BLA PNs. In voltage-clamp experiments in which neurons were continuously held at a subthreshold voltage, CCh induced an excitatory shift in holding current with a concomitant biphasic change in membrane resistance (an initial decrease followed by a slower but sustained increase). All these effects of CCh were blocked by 1 mM PZP, suggesting they result from activation of M1/M4 mAChRs. That said, the complex effect on membrane resistance suggests that multiple effectors contribute to the overall excitatory effect of CCh that can be observed in BLA PNs voltage clamped near the resting potential. Indeed, BAPTA partially reduced the overall CCh-induced excitatory response and prevented the early CCh-induced decrease in membrane resistance, without blocking the later increase in membrane resistance. By contrast, cesium failed to reduce the effect of CCh on holding current and did not block either phase of the biphasic effect on membrane resistance. Collectively, these results suggest that CCh can excite BLA PNs in part by opening a calcium-dependent and cesium-insensitive non-selective cation channel, and in part by closing a population of cesium insensitive potassium channels that are open near the resting potential.
The CCh was also observed to promote a clear ADP in current clamp, which could sometimes result in sustained firing after a brief stimulus. This effect was similarly prevented by BAPTA, and notably was also blocked by FFA, suggesting that the mechanism of action involves calcium-dependent activation of FFA sensitive non-selective cation channels. We then used voltage clamp protocols to isolate currents after a brief depolarization in both the presence and absence of CCh. In cells filled with a K-gluconate internal, CCh effectively converted a post-depolarization outward current observed in control conditions, to a post-depolarization inward current observed in CCh (that we have referred to as the IADP). These results match the CCh-mediated conversion from an AHP to an ADP after brief depolarization observed in current clamp. In cells filled with cesium gluconate, we noted there was no post-stimulus outward current in control conditions, and that the post-stimulus inward current was both robustly enhanced by CCh, and strongly blocked by FFA. These data strongly suggest that these voltage clamp protocols are enabling us to isolate the current which drives the ADP observed in the presence of CCh in current clamp. Having effectively isolated this current in voltage clamp, we then carefully evaluated both the time and voltage dependence of its activation. In brief, we found a 250 ms depolarization to +50 mV was sufficient to produce near maximal activation of the CCh-induced ADP, and that current carrying the ADP reversed around +10 mV.
Overall, these data are generally in agreement with prior work indicating that BLA PNs have a voltage- and time-dependent potassium current (IM) and a calcium-activated K+ current (sIAHP), both of which are inhibited by CCh, resulting in depolarization (Womble and Moises, 1992; Womble and Moises, 1993). Our results also largely align with prior work revealing an FFA sensitive activity dependent current in these neurons (Unal et al., 2015). That said, the present study goes to greater lengths to both pharmacologically and mathematically isolate the specific CCh-induced and activity-dependent current that promotes an ADP in current clamp and a post-excitatory inward current in voltage clamp. In broad terms, this current is similar to a calcium- and FFA-sensitive current previously characterized in other brain areas (Schiller, 2004; Hofmann and Frazier, 2010; Anderson and Strowbridge, 2014; Ghamari-Langroudi and Bourque, 2002), although further research may reveal intriguing differences in receptor coupling and in detailed biophysical properties including the voltage and time dependence of activation and of inactivation.
It is worth highlighting that within the BLA there are clearly multiple types of both ionotropic and metabotropic cholinergic receptors, a number of which can be activated by CCh (Unal et al., 2015; Muller et al., 2016; Klein and Yakel, 2006; Power and Sah, 2008). In our view the clear effectiveness of PZP against multiple effects of CCh observed in this study in BLA PNs voltage clamped at −60 mV leads us to conclude that the vast majority of the responses apparent throughout this study are very likely mediated by activation of M1 and/or M4 AChRs (Greig et al., 2013). Nevertheless, it seems likely nicotinic AChRs and or M2 containing mAChRs impact neuronal excitability in BLA in other contexts. Interestingly, Yajeya et al. (1999) reported that BLA PNs also have a calcium-independent non-selective cation conductance that is activated by CCh. If this current were present in our experiments, it could plausibly contribute to the change in holding current observed in the presence of BAPTA in Figure 1C. Similarly, although FFA acts on a few different ion channels (Guinamard et al., 2013), collectively our data strongly suggest that an FFA-sensitive and calcium-activated non-selective cation channel is being engaged by direct excitation of BLA PNs in the presence of CCh. It would likely be interesting for further studies to determine whether transient receptor potential (TRP) cation channels contribute directly to the CCh-induced ADP observed in these neurons. Interestingly, Meis et al. (2007) provide compelling evidence for the presence of a non-selective cation channel in mouse BLA projection neurons that is activated downstream of CCK receptors, is sensitive to TRP channel blockers, and is not dependent on increases in intracellular calcium.
Overall, this work provides additional insight into the multiple cholinergic effectors that modulate the intrinsic excitability of BLA PNs, and particularly demonstrates methods that can be used to isolate the current underlying classic ADPs and plateau potentials, so that their biophysical properties can be more precisely measured, and ultimately compared across healthy and pathophysiological conditions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committee of the University of Florida. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
TS: Writing – review & editing, Conceptualization, Investigation, Formal analysis, Writing – original draft, Visualization. SH: Investigation, Software, Writing – original draft, Conceptualization, Formal analysis. JB: Writing – review & editing, Funding acquisition, Supervision, Conceptualization. BS: Writing – review & editing, Supervision, Funding acquisition, Conceptualization. CF: Project administration, Supervision, Conceptualization, Writing – review & editing, Formal analysis, Software, Funding acquisition, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by NIA RF1AG060778 and NIA R01AG067429.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anderson, R. W., and Strowbridge, B. W. (2014). Regulation of persistent activity in hippocampal mossy cells by inhibitory synaptic potentials. Learn. Mem. 21, 263–271. doi: 10.1101/lm.033829.113
Buades-Rotger, M., Engelke, C., and Krämer, U. M. (2019). Trait and state patterns of basolateral amygdala connectivity at rest are related to endogenous testosterone and aggression in healthy young women. Brain Imaging Behav. 13, 564–576. doi: 10.1007/s11682-018-9884-2
Bubser, M., Byun, N., Wood, M. R., and Jones, C. K. (2011). Muscarinic Receptors. Handb. Exp. Pharmacol. 208, 121–166. doi: 10.1007/978-3-642-23274-9_7
Crimmins, B. E., Lingawi, N. W., Chieng, B. C., Leung, B. K., Maren, S., and Laurent, V. (2023). Basal forebrain cholinergic signaling in the basolateral amygdala promotes strength and durability of fear memories. Neuropsychopharmacology 48, 605–614. doi: 10.1038/s41386-022-01427-w
Edelstein, A. D., Tsuchida, M. A., Amodaj, N., Pinkard, H., Vale, R. D., and Stuurman, N. (2014). Advanced methods of microscope control using μManager software. J Biological Methods. 1:10. doi: 10.14440/jbm.2014.36
Eglen, R. M., Kenny, B. A., Michel, A. D., and Whiting, R. L. (1987). Muscarinic activity of McN-A-343 and its value in muscarinic receptor classification. Br. J. Pharmacol. 90, 693–700. doi: 10.1111/j.1476-5381.1987.tb11222.x
Ghamari-Langroudi, M., and Bourque, C. W. (2002). Flufenamic acid blocks depolarizing afterpotentials and phasic firing in rat supraoptic neurones. J. Physiol. 545, 537–542. doi: 10.1113/jphysiol.2002.033589
Golowasch, J., Thomas, G., Taylor, A. L., Patel, A., Pineda, A., Khalil, C., et al. (2009). Membrane capacitance measurements revisited: dependence of capacitance value on measurement method in nonisopotential neurons. J. Neurophysiol. 102, 2161–2175. doi: 10.1152/jn.00160.2009
Greig, N., Reale, M., and Tata, A. (2013). New pharmacological approaches to the cholinergic system: an overview on muscarinic receptor ligands and cholinesterase inhibitors. Recent Pat. CNS Drug Discov. 8, 123–141. doi: 10.2174/1574889811308020003
Guinamard, R., Simard, C., and Negro, C. D. (2013). Flufenamic acid as an ion channel modulator. Pharmacol Therapeut. 138, 272–284. doi: 10.1016/j.pharmthera.2013.01.012
Hajisoltani, R., and Meftahi, G. H. (2024). Epinephrine injected into the basolateral amygdala affects anxiety-like behavior and memory performance in stressed rats. Neurosci. Lett. 819:137590. doi: 10.1016/j.neulet.2023.137590
Harden, S. W., and Frazier, C. J. (2016). Oxytocin depolarizes fast-spiking hilar interneurons and induces GABA release onto mossy cells of the rat dentate gyrus. Hippocampus 26, 1124–1139. doi: 10.1002/hipo.22595
Hernandez, C. M., Orsini, C. A., Labiste, C. C., Wheeler, A. R., Eyck, T. W. T., Bruner, M. M., et al. (2019). Optogenetic dissection of basolateral amygdala contributions to intertemporal choice in young and aged rats. eLife 8:e46174. doi: 10.7554/eLife.46174
Hofmann, M. E., and Frazier, C. J. (2010). Muscarinic receptor activation modulates the excitability of hilar mossy cells through the induction of an afterdepolarization. Brain Res. 1318, 42–51. doi: 10.1016/j.brainres.2010.01.011
Hosie, S., Malone, D. T., Liu, S., Glass, M., Adlard, P. A., Hannan, A. J., et al. (2018). Altered amygdala excitation and CB1 receptor modulation of aggressive behavior in the Neuroligin-3R451C mouse model of autism. Front. Cell. Neurosci. 12:234. doi: 10.3389/fncel.2018.00234
Huang, L., Chen, Y., Jin, S., Lin, L., Duan, S., Si, K., et al. (2021). Organizational principles of amygdalar input-output neuronal circuits. Mol. Psychiatry 26, 7118–7129. doi: 10.1038/s41380-021-01262-3
Karami, A., Eriksdotter, M., Kadir, A., Almkvist, O., Nordberg, A., and Darreh-Shori, T. (2019). CSF cholinergic index, a new biomeasure of treatment effect in patients with Alzheimer’s disease. Front. Mol. Neurosci. 12:239. doi: 10.3389/fnmol.2019.00239
Klein, R. C., and Yakel, J. L. (2006). Functional somato-dendritic α7-containing nicotinic acetylcholine receptors in the rat basolateral amygdala complex. J. Physiol. 576, 865–872. doi: 10.1113/jphysiol.2006.118232
Kruse, A. C., Kobilka, B. K., Gautam, D., Sexton, P. M., Christopoulos, A., and Wess, J. (2014). Muscarinic acetylcholine receptors: novel opportunities for drug development. Nat. Rev. Drug Discov. 13, 549–560. doi: 10.1038/nrd4295
Li, M., Lv, X., Li, T., Cui, C., Yang, X., Peng, X., et al. (2023). Basolateral amygdala cannabinoid CB1 receptor controls formation and elimination of social fear memory. ACS Chem. Neurosci. 14, 3674–3685. doi: 10.1021/acschemneuro.3c00297
Marino, M., Misuir, L., and Brogioli, D. A new open source software for the calculation of the liquid junction potential between two solutions according to the stationary Nernst-Planck equation. (2014). Available online at: https://arxiv.org/abs/1403.3640 (Accessed March 8, 2024)
Mei, L., Zhou, Y., Sun, Y., Liu, H., Zhang, D., Liu, P., et al. (2020). Acetylcholine muscarinic receptors in ventral hippocampus modulate stress-induced anxiety-like Behaviors in mice. Front. Mol. Neurosci. 13:598811. doi: 10.3389/fnmol.2020.598811
Meis, S., Munsch, T., Sosulina, L., and Pape, H. C. (2007). Postsynaptic mechanisms underlying responsiveness of amygdaloid neurons to cholecystokinin are mediated by a transient receptor potential-like current. Mol. Cell. Neurosci. 35, 356–367. doi: 10.1016/j.mcn.2007.03.012
Muller, J. F., Mascagni, F., Zaric, V., Mott, D. D., and McDonald, A. J. (2016). Localization of the M2 muscarinic cholinergic receptor in dendrites, cholinergic terminals, and noncholinergic terminals in the rat basolateral amygdala: an ultrastructural analysis. J. Comp. Neurol. 524, 2400–2417. doi: 10.1002/cne.23959
Patel, D., Anilkumar, S., Chattarji, S., and Buwalda, B. (2018). Repeated social stress leads to contrasting patterns of structural plasticity in the amygdala and hippocampus. Behav. Brain Res. 347, 314–324. doi: 10.1016/j.bbr.2018.03.034
Pliota, P., Böhm, V., Grössl, F., Griessner, J., Valenti, O., Kraitsy, K., et al. (2020). Stress peptides sensitize fear circuitry to promote passive coping. Mol. Psychiatry 25, 428–441. doi: 10.1038/s41380-018-0089-2
Power, J. M., and Sah, P. (2008). Competition between calcium-activated K+ channels determines cholinergic action on firing properties of basolateral amygdala projection neurons. J. Neurosci. 28, 3209–3220. doi: 10.1523/JNEUROSCI.4310-07.2008
Ryan, S., Li, C., Menigoz, A., Hazra, R., Dabrowska, J., Ehrlich, D., et al. (2018). Repeated shock stress facilitates basolateral amygdala synaptic plasticity through decreased cAMP-specific phosphodiesterase type IV (PDE4) expression. Brain Struct. Funct. 223, 1731–1745. doi: 10.1007/s00429-017-1575-z
Schiller, Y. (2004). Activation of a calcium-activated cation current during epileptiform discharges and its possible role in sustaining seizure-like events in neocortical slices. J. Neurophysiol. 92, 862–872. doi: 10.1152/jn.00972.2003
Sharp, B. M. (2017). Basolateral amygdala and stress-induced hyperexcitability affect motivated behaviors and addiction. Transl. Psychiatry 7:e 1194. doi: 10.1038/tp.2017.161
Unal, C. T., Pare, D., and Zaborszky, L. (2015). Impact of basal forebrain cholinergic inputs on basolateral amygdala neurons. J. Neurosci. 35, 853–863. doi: 10.1523/JNEUROSCI.2706-14.2015
Valipour, H., Jahromi, G. P., Mohammadi, A., and Meftahi, G. H. (2023). Effects of the suppression of 5-HT1A receptors in the left, right, or bilateral basolateral amygdala on memory consolidation in chronic stress in male rats. Naunyn Schmiedeberg's Arch. Pharmacol. 307, 1–16. doi: 10.1007/s00210-023-02790-1
Walker, L. C., and Lawrence, A. J. (2020). Allosteric modulation of muscarinic receptors in alcohol and substance use disorders. Adv. Pharmacol. 88, 233–275. doi: 10.1016/bs.apha.2020.01.003
Washburn, M. S., and Moises, H. C. (1992). Muscarinic responses of rat basolateral amygdaloid neurons recorded in vitro. J Physiology. 449, 121–154. doi: 10.1113/jphysiol.1992.sp019078
Winstanley, C. A., Theobald, D. E., Cardinal, R. N., and Robbins, T. W. (2004). Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J. Neurosci. 24, 4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004
Womble, M. D., and Moises, H. C. (1992). Muscarinic inhibition of M-current and a potassium leak conductance in neurones of the rat basolateral amygdala. J. Physiol. 457, 93–114. doi: 10.1113/jphysiol.1992.sp019366
Womble, M. D., and Moises, H. C. (1993). Muscarinic modulation of conductances underlying the afterhyperpolarization in neurons of the rat basolateral amygdala. Brain Res. 621, 87–96. doi: 10.1016/0006-8993(93)90301-3
Keywords: basolateral amygdala, muscarinic acetylcholine receptor, afterdepolarization, afterhyperpolarization, ICAN
Citation: Sahagian TJ, Harden SW, Bizon JL, Setlow B and Frazier CJ (2025) Muscarinic acetylcholine receptor activated effectors in principal neurons of the rat basolateral amygdala. Front. Cell. Neurosci. 19:1634594. doi: 10.3389/fncel.2025.1634594
Edited by:
Dirk Feldmeyer, Helmholtz Association of German Research Centres (HZ), GermanyReviewed by:
Zhongjiao Jiang, University at Buffalo, United StatesM. McLean Bolton, Max Planck Florida Institute for Neuroscience (MPFI), United States
Copyright © 2025 Sahagian, Harden, Bizon, Setlow and Frazier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charles J. Frazier, Y2pmcmF6QHVmbC5lZHU=
 Todd J. Sahagian1
Todd J. Sahagian1 Barry Setlow
Barry Setlow Charles J. Frazier
Charles J. Frazier