- 1Department of Anesthesiology, Peking University Third Hospital, Beijing, China
- 2Neuroscience Research Institute, Peking University, Beijing, China
- 3Department of Neurobiology, School of Basic Medical Sciences, Peking University Health Science Center, Beijing, China
- 4Key Laboratory for Neuroscience, Ministry of Education of China and National Health Commission of China, Beijing, China
Diabetic peripheral neuropathy (DPN), a prevalent and debilitating complication of diabetes, involves complex interactions between peripheral nerve damage and central nervous system (CNS) dysfunction. While traditional research has focused on peripheral and spinal mechanisms, emerging evidence highlights that the brain plays a critical role in the development of painful DPN. This review synthesizes recent advances from neuroimaging, spectroscopy, and preclinical studies to delineate structural, functional, and neurochemical alterations in the central nervous system associated with DPN. Patients exhibit cortical thinning, subcortical atrophy, and disrupted connectivity in sensory, affective, and cognitive networks, accompanied by metabolic imbalances and excitatory–inhibitory neurotransmitter shifts. Preclinical models further implicate maladaptive plasticity, microglial activation, and region-specific astrocytic responses in amplifying central sensitization and pain chronicity. These mechanistic insights underscore the central nervous system as a therapeutic target. Non-invasive neuromodulation techniques, such as repetitive transcranial magnetic stimulation, and brain-directed pharmacological strategies show promising but preliminary benefits in alleviating neuropathic pain. Understanding the interplay between peripheral injury and brain dysfunction in DPN not only broadens the conceptual framework of its pathophysiology but also provides a foundation for developing novel interventions aimed at restoring central network balance and improving patient outcomes.
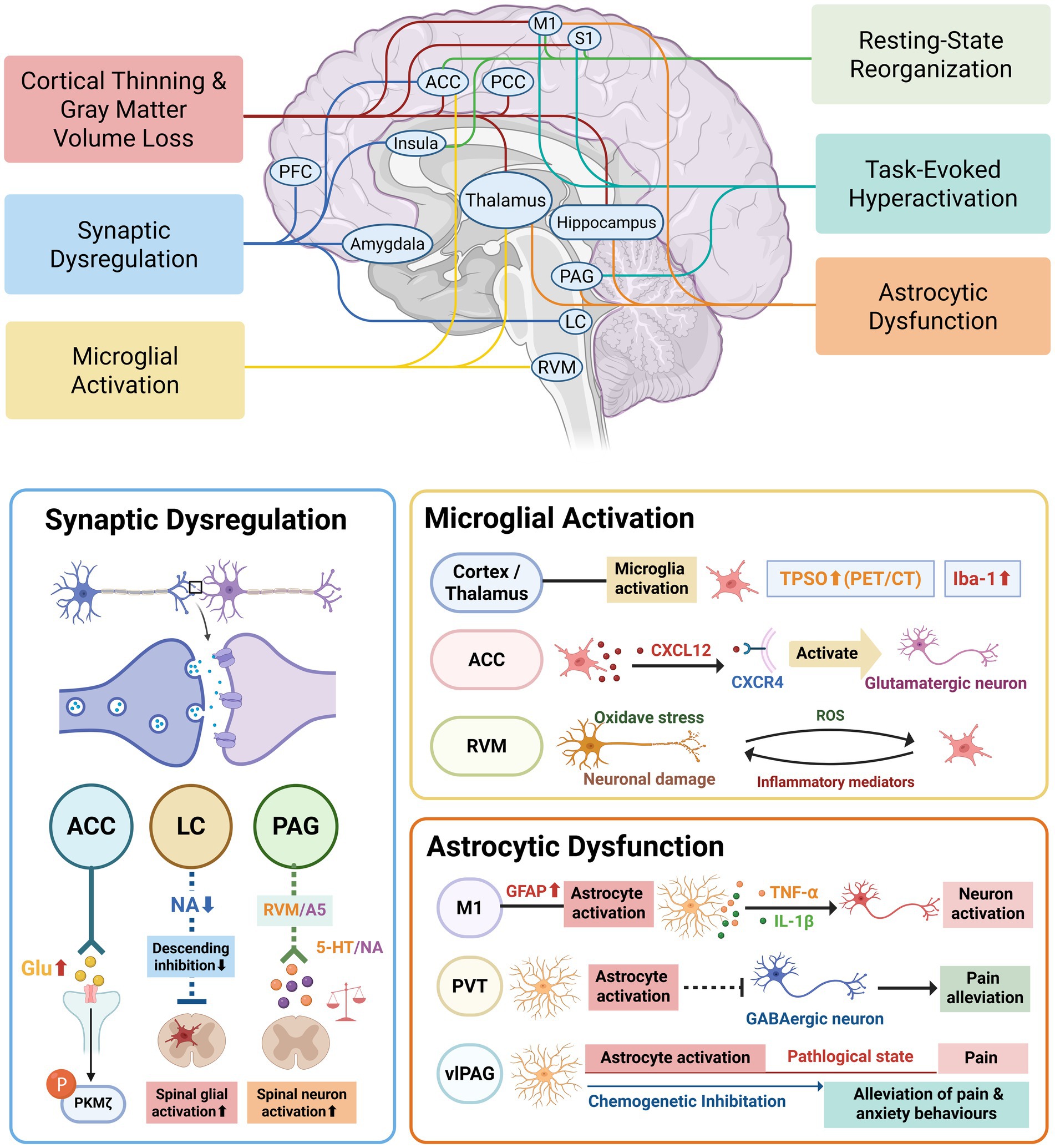
Graphical Abstract. ACC, anterior cingulate cortex; PCC, posterior cingulate cortex; PFC, prefrontal cortex; M1, primary motor cortex; S1, primary somatosensory cortex; PVT, paraventricular thalamus; vlPAG, ventrolateral periaqueductal gray; LC, locus coeruleus; RVM, rostral ventromedial medulla; A5, A5 noradrenergic cell group; Glu, glutamate; PKMζ, protein kinase M zeta; NA, noradrenaline; 5-HT, 5-hydroxytryptamine (serotonin); CXCL12, C-X-C motif chemokine ligand 12; CXCR4, C-X-C motif chemokine receptor 4; ROS, reactive oxygen species; TSPO, 18-kDa translocator protein; Iba-1, ionized calcium-binding adaptor molecule 1; GFAP, glial fibrillary acidic protein; TNF-α, tumor necrosis factor alpha; IL-1β, interleukin-1 beta.
1 Introduction
Diabetes mellitus is a chronic and complex metabolic disorder characterized by persistent hyperglycemia resulting from pancreatic β-cell dysfunction and insulin resistance. Over time, this condition leads to absolute or relative insulin deficiency, which contributes to a wide range of systemic complications. With rising global prevalence, diabetes has become one of the most pressing public health challenges. As of 2021, there were 529 million individuals diagnosed with diabetes, and this figure is projected to escalate to 1.3 billion by 2050, driven by an aging population, sedentary lifestyles, and dietary changes (Cho et al., 2018; Cole and Florez, 2020; Walker et al., 2023). The global economic and healthcare burden of diabetes is immense, requiring sustained efforts to develop effective prevention and management strategies (NCD Risk Factor Collaboration, 2016).
One of the most debilitating complications of diabetes is diabetic peripheral neuropathy (DPN), a progressive microvascular complication affecting approximately 50% of diabetic patients (Faselis et al., 2020). Among these patients, 15–25% experience painful DPN (Shillo et al., 2019), which is characterized by chronic and persistent pain that exacerbates the emotional and psychological challenges of managing diabetes (Dyck et al., 1993; Pouwer et al., 2024). Despite the profound clinical impact of DPN, effective treatment options remain limited, primarily due to an incomplete understanding of its pathogenesis.
Traditional research on DPN has predominantly focused on the peripheral nervous system and spinal pathways (Yang et al., 2025). These studies have identified key mechanisms such as peripheral nerve damage, microvascular complications, and oxidative stress. However, emerging evidence highlights the involvement of the brain in the integration and modulation of pain signals in DPN (Kapur, 2003). Pain perception is not solely determined at the peripheral or spinal level, but is shaped by a distributed brain network encompassing the cortex, thalamus, hippocampus, and brainstem nuclei (Yang et al., 2019; Latremoliere and Woolf, 2009). This constellation of brain regions, often referred to as the “pain matrix,” integrates sensory-discriminative, affective, and cognitive dimensions of pain processing (Garcia-Larrea and Peyron, 2013; Greig et al., 2014). Under diabetic conditions, structural and functional alterations in these brain areas can amplify pain sensitivity, disrupt descending inhibitory pathways, and contribute to the persistence of neuropathic pain (Segerdahl et al., 2018). While peripheral mechanisms initiate aberrant nociceptive signaling, central changes amplify and modulate these inputs, highlighting the complementary yet distinct contributions of peripheral and central processes to DPN pathophysiology (Schaible, 2007; Sloan et al., 2021).
Recognizing DPN as a condition that involves peripheral, spinal, and brain changes is essential for advancing our understanding and treatment of this complex disease. This review aims to provide a comprehensive overview of brain mechanisms implicated in painful DPN. Clarifying these brain-specific contributions may facilitate the development of novel neuromodulator or pharmacological interventions to better manage neuropathic pain in diabetic patients.
2 Structural changes in the brain induced by DPN
DPN is increasingly recognized as a condition involving not only peripheral nerve damage but also central nervous system (CNS) alterations (Tesfaye et al., 2016; Zang et al., 2023). Accumulating neuroimaging evidence indicates that DPN is associated with significant structural changes in the brain, including cortical thinning, gray matter atrophy, and regional volume loss (Selvarajah et al., 2023; Zhang et al., 2020; Selvarajah et al., 2014). These alterations are closely linked to the sensory and affective manifestations of neuropathic pain (Zang et al., 2023), highlighting the importance of brain involvement in the pathogenesis and clinical expression of DPN (Yang et al., 2025).
High-resolution structural magnetic resonance imaging (MRI), particularly surface-based morphometry (SBM) and voxel-based morphometry (VBM) have enabled accurate evaluation of cortical morphology in DPN patients (Zhang et al., 2020; Tae et al., 2025; Scheliga et al., 2024). Cortical thickness, typically assessed by SBM, measures the distance between the white matter and pial surfaces, whereas gray matter volume, quantified through VBM, incorporates both thickness and surface area (Clarkson et al., 2011; Tang et al., 2018). Although related, these markers are derived through distinct computational approaches and may reveal complementary, non-redundant aspects of cortical pathology (Clarkson et al., 2011; Schwarz et al., 2016).
2.1 Cortical alterations: thinning and volume loss
Cortical alterations in DPN primarily manifest as reductions in cortical thickness and volume, reflecting neuronal loss, dendritic retraction, or glial changes (Muhlau et al., 2007; Vidal-Pineiro et al., 2020). These structural deficits are commonly observed in brain regions involved in pain processing, sensorimotor integration, emotional regulation, and attentional modulation, and are more pronounced in painful DPN, suggesting a central contribution to pain chronification (Selvarajah et al., 2023; Davis and Moayedi, 2013; Tracey and Mantyh, 2007; Hostrup et al., 2025).
Cortical thinning in DPN reflects region-specific reductions in thickness, often seen as localized microstructural damage. Notably, cortical thinning has been reported in several key brain regions, including the primary somatosensory cortex (S1, postcentral gyrus) (Selvarajah et al., 2023; Zhang et al., 2020; Selvarajah et al., 2014; Hostrup et al., 2025; Selvarajah et al., 2019; Hansen et al., 2022a; Frokjaer et al., 2013), primary motor cortex (M1, precentral gyrus) (Selvarajah et al., 2023; Zhang et al., 2020; Hansen et al., 2022a), insular cortex (Selvarajah et al., 2023; Zhang et al., 2020), anterior cingulate cortex (ACC) (Selvarajah et al., 2023; Zhang et al., 2020), middle cingulate cortex (Zhang et al., 2020), superior parietal gyrus/lobule (Hansen et al., 2022b), and supramarginal gyrus (Selvarajah et al., 2014; Hostrup et al., 2025). These changes are typically more marked in painful DPN and are thought to reflect maladaptive plasticity triggered by chronic peripheral nerve injury (Li et al., 2016). Thinning in regions such as the insula and S1 has been associated with enhanced pain intensity and disrupted sensory-emotional integration, potentially contributing to central sensitization (Selvarajah et al., 2023; Zhang et al., 2020; Hansen et al., 2022b; He et al., 2025; Chao et al., 2022a). Cortical thinning is commonly associated with normal aging (Cao et al., 2017) and is often accelerated in neurodegenerative diseases such as Alzheimer’s disease (Wu et al., 2021). In the context of DPN, however, cortical thinning likely reflects a combination of diabetes-related systemic effects and central nervous system adaptations to chronic neuropathic pain (Hostrup et al., 2025).
Cortical volume loss, on the other hand, integrates cortical thickness with surface area and folding patterns, offering a broader perspective on atrophy (Winkler et al., 2010; Lemaitre et al., 2012). Reductions in gray matter volume have been identified in the S1 (Selvarajah et al., 2014; Hansen et al., 2022a), M1 (Hansen et al., 2022a), cingulate cortex (Selvarajah et al., 2023; Selvarajah et al., 2014), supramarginal gyrus (Selvarajah et al., 2014), and inferior/superior occipital gyrus (Hansen et al., 2022a). These volumetric losses often overlap with thinning regions but may also indicate more extensive neuronal compromise. In painful DPN, greater volume loss in areas such as the ACC has been linked to intensified affective symptoms like distress and catastrophizing (Penzo and Gao, 2021; Sifuentes-Franco et al., 2017), while atrophy in the posterior cingulate cortex and parietal regions may impair the sensory-discriminative components of pain perception. Mechanistically, chronic hyperglycemia, oxidative stress (Penzo and Gao, 2021; Sifuentes-Franco et al., 2017), and microvascular injury (Van Dam et al., 2013) may drive these structural alterations through neurodegenerative and neuroinflammatory pathways (see Table 1).
2.2 Subcortical alterations: volume loss
Subcortical volume loss, often quantified through voxel-based morphometry, has been consistently reported in DPN, particularly affecting deep gray matter nuclei integral to pain modulation and sensory processing (Selvarajah et al., 2014; Hansen et al., 2022b). Significant atrophy has been identified in the thalamus (Selvarajah et al., 2023; Zhang et al., 2020; Selvarajah et al., 2014; Selvarajah et al., 2019; Hansen et al., 2022b), putamen (Zhang et al., 2020; Hansen et al., 2022b; He et al., 2025), caudate nucleus (Zhang et al., 2020; Hansen et al., 2022b; He et al., 2025), pallidum (Zhang et al., 2020; He et al., 2025), hippocampus (Zhang et al., 2020), and amygdala (Zhang et al., 2020)—critical nodes in ascending and descending pain pathways that facilitate sensory processing, motor-sensory integration, autonomic regulation, and pain inhibition. Reductions in putamen and caudate nucleus volumes may disrupt basal ganglia-mediated modulation of sensorimotor and affective pain components (Chudler and Dong, 1995), potentially exacerbating movement-related symptoms and emotional distress in DPN (Zhang et al., 2020; Hansen et al., 2022b; He et al., 2025). Similarly, atrophy in the hippocampus and amygdala could impair descending inhibitory control, promoting pain persistence and contributing to associated emotional dysregulation, such as anxiety (Zhang et al., 2020). Phenotype-specific patterns have also emerged. For instance, thalamic volume appears more reduced in painless DPN than in painful DPN, particularly on the right side (Novo et al., 2022). Conversely, painful DPN may involve relatively preserved thalamic structure but dysfunctional thalamo-cortical signaling, contributing to abnormal nociceptive amplification (Hansen et al., 2022a; Novo et al., 2022).
3 Brain dysfunction induced by DPN
Advances in neuroimaging techniques, such as functional magnetic resonance imaging (fMRI), have facilitated the identification of microstructural and functional impairments within the central nervous system (Yen et al., 2023; Ugurbil et al., 2003). Using fMRI, functional disruptions in brain networks involved in the affective and cognitive modulation of pain can be revealed (Zhang L. B. et al., 2024; Martucci and Mackey, 2018).
3.1 Resting-state functional connectivity: disruptions in pain and sensory networks
Resting-state fMRI is a powerful tool used to evaluate spontaneous brain activity by measuring functional connectivity (Barkhof et al., 2014). RS-fMRI detects low-frequency blood-oxygen-level-dependent (BOLD) fluctuations, allowing for analysis of temporal correlations between spatially distinct brain regions—termed functional connectivity (FC)—and thus provides insights into brain network alterations without task-related stimulation (Baracchini et al., 2021; Allen et al., 2014).
One of the earliest studies applying RS-fMRI in DPN demonstrated significantly reduced thalamocortical functional connectivity in patients with painful DPN (PDN) (Cauda et al., 2009). Specifically, FC between the ventral posterior lateral (VPL) and mediodorsal thalamic nuclei and the S1 was diminished, supporting the notion that chronic pain disrupts thalamocortical feedback loops, a concept known as thalamocortical dysrhythmia (Cauda et al., 2009). Concurrently, modulation of the dorsolateral prefrontal cortex–anterior cingulate cortex–medial thalamus loop has been proposed in PDN, consistent with decreased anterior cingulate perfusion during rest.
A more recent study stratified DPN patients by nociceptor phenotype and found a double dissociation in thalamocortical FC: thalamus–insula FC was positively associated with neuropathic pain scores, while thalamus–somatosensory cortex FC was inversely correlated with the severity of peripheral nerve damage (Teh et al., 2021). The insula, implicated in affective and attentional pain processing, may be hyperactive in pain-promoting circuits among individuals with preserved nociceptor input.
In a diffusion MRI study assessing structural connectivity (SC), reduced thalamic and hypothalamic SC with the amygdala and insula has been reported in PDN, compared to both painless DPN and healthy controls (Chao et al., 2022b). Lower SC in the anterior cingulate cortex correlated with greater autonomic dysfunction, linking limbic disconnection to both pain and dysautonomia.
Furthermore, FC alterations appear phenotype-specific. A 2023 study found that, compared to painful DPN and controls, type 1 diabetes patients without neuropathy exhibited hyperconnectivity between the thalamus/postcentral gyrus and motor areas (Croosu et al., 2023). In contrast, PDN was associated with reduced FC in these pathways, with stronger associations observed between thalamic FC and both pain scores and nerve conduction deficits.
Another RS-fMRI study reported enhanced thalamic FC with the parietal and occipital cortices in patients with type 2 diabetes and PDN, implicating thalamoparietal overactivation in the pathophysiology of pain (Liu et al., 2021).
Complementing functional studies, graph theory analysis of structural networks constructed from diffusion tractography showed PDN-specific reductions in white matter connectivity within the insula, hippocampus, and temporal lobe, along with decreased global efficiency and betweenness centrality—indicative of widespread disintegration of integrative brain networks (Chao et al., 2022a).
Collectively, these studies demonstrate that PDN is associated with altered thalamocortical and limbic connectivity, involving both sensory-discriminative and affective-emotional components of pain. Such connectivity patterns are further modulated by disease phenotype and severity, suggesting potential for FC-based biomarkers in diagnosis and monitoring of DPN.
3.2 Task-based imaging: cortical reorganization and pain modulation
Task-based fMRI approach measures brain activity in response to specific stimuli or tasks, providing insights into the functional reorganization of neural circuits in response to sensory inputs or motor demands (Huang et al., 2024).
Studies using this technique have revealed how the brain responds to sensory stimuli in diabetic peripheral neuropathy. During thermal nociceptive stimulation, patients with severe diabetic distal symmetrical polyneuropathy exhibit expanded activation of the primary somatosensory cortex, with abnormal representations extending into non-somatotopic areas such as the facial and lip cortices (Selvarajah et al., 2019). This pattern reflects central plasticity resulting from peripheral deafferentation and suggests a cortical spread of nociceptive encoding.
Additionally, in response to noxious heat stimulation, patients with painful DPN show increased BOLD activation in the ACC, anterior insula, and supplementary motor areas—changes that positively correlate with pain intensity and affective distress (Tseng et al., 2013). In contrast, patients with painless DPN display reduced activation in the ACC and S1, highlighting distinct patterns of central reorganization between DPN subtypes.
Further supporting this, a study (Li et al., 2018) found that compared to healthy individuals and diabetic patients without neuropathy, those with DPN showed significantly stronger activation in somatosensory-related regions—including the right insula, left caudate nucleus, frontal gyrus, and cingulate cortex—in response to thermal stimuli. These findings underscore the potential of task-based fMRI as a sensitive tool for detecting early central nervous system involvement in DPN.
3.3 Neurochemical and metabolic alterations in the brain: insights from magnetic resonance spectroscopy (MRS)
MRS studies have been used to identify metabolic abnormalities in key brain regions to understand changes across chronic pain conditions (Cruz-Almeida and Porges, 2021). In addition to functional imaging insights, clinical metabolic and neurochemical assessments further implicate central involvement in DPN (Zhao et al., 2018).
Sloan et al. demonstrated that patients with painful DPN exhibit significantly reduced phosphocreatine-to-ATP (PCr: ATP) ratios in the primary somatosensory (S1) cortex compared with painless DPN, indicating higher cortical energy consumption in pain phenotypes (Sloan et al., 2023). Moreover, lower PCr: ATP ratios correlated with greater pain intensity, suggesting that altered cortical bioenergetics may serve as a biomarker of painful DPN.
Beyond high-energy phosphate changes, several MRS studies have identified alterations in metabolites reflecting neuronal integrity, glial activity, and membrane turnover. Selvarajah et al. (2008) found preserved S1 cortical metabolites but reduced thalamic N-acetylaspartate (NAA) -to-creatine (Cr) ratio in advanced painless DPN, with preservation in painful DPN, suggesting that intact thalamic neuronal function may be a prerequisite for pain perception. Similarly, Hansen et al. reported decreased NAA/Cr ratios and increased myo-inositol/Cr in parietal and cingulate regions in type 1 diabetes, with greater reductions linked to more severe DPN (Hansen et al., 2024). Painful DPN was further associated with increased glycerophosphocholine/Cr and elevated thalamic glutamate, indicating enhanced membrane turnover and heightened excitatory neurotransmission in pain phenotypes.
Altered neurotransmitter balance has also been reported in DPN. Petrou et al. found significantly higher glutamate/glutamine and lower γ-aminobutyric acid (GABA) levels in the posterior insula of patients with diabetic neuropathy and positive sensory symptoms compared with healthy controls, indicating an excitatory/inhibitory imbalance in key pain-processing areas (Petrou et al., 2012). Supporting this, Shillo et al. reported that painless DPN was characterized by the lowest thalamic GABA: H2O ratio compared with both healthy volunteers and diabetes patients without DPN, whereas painful DPN maintained partially preserved GABA levels, suggesting that central GABAergic pathways may be critical for neuropathic pain mechanisms (Shillo et al., 2024).
Taken together, clinical neuroimaging studies consistently support phenotype-specific central alterations in DPN, suggesting that painful and painless subtypes may follow partially distinct neurobiological trajectories. This distinction provides an important framework for interpreting mechanistic findings from preclinical models.
4 Brain-centered mechanistic findings from rodent models of DPN
To complement clinical imaging findings, preclinical studies in rodent models have been widely used to explore brain-specific mechanisms underlying diabetic neuropathic pain. These studies help elucidate cellular and molecular processes that are difficult to access in human subjects. As illustrated in Graphical abstract, the following sections summarize key brain mechanisms identified in animal models of DPN.
4.1 Synaptic and neurotransmitter dysregulation
Preclinical studies provide mechanistic validation for clinical findings and offer deeper insight into specific neural circuits involved in DPN.
In diabetic rodent models, elevated glutamatergic activity has been observed in ACC neurons (Li et al., 2014). This is driven by both increased presynaptic glutamate release and enhanced postsynaptic receptor responsiveness, accompanied by elevated PKMζ phosphorylation. Pharmacological blockade of PKMζ reversed thermal hyperalgesia and mechanical allodynia and normalized synaptic activity, underscoring its role in central sensitization (Li et al., 2014).
Further studies highlight dysfunction in descending pain modulation systems, particularly the locus coeruleus (LC) -spinal noradrenergic circuits. In DPN rats, reduced LC output correlates with diminished inhibition of spinal nociceptive transmission, poor analgesic efficacy, and persistent spinal glial activation (Li et al., 2022; Suehiro et al., 2013; Mesa-Lombardo et al., 2023). Moreover, LC dysfunction impairs regulation of emotional tone, exacerbating depressive and anxiety-like behaviors, consistent with clinical affective symptoms in DPN (Alba-Delgado et al., 2016; Espana et al., 2024).
Similarly, the periaqueductal gray (PAG)—a central hub for pain modulation—exhibits neurotransmitter dysregulation in DPN models (Morgado et al., 2011). Serotonin and noradrenaline imbalances impair descending inhibition, while insulin-like growth factor 1 (IGF-1) treatment has been shown to restore neurotransmitter balance within the PAG, leading to significant reductions in mechanical hyperalgesia (Morgado et al., 2011).
4.2 Microglial activation: linking neuroinflammation to neuronal injury
Microglia, the primary immune effector cells of the CNS, are central to neuroinflammation, and play a critical role in the central mechanisms underlying DPN (Wang et al., 2024).
In one study, positron emission tomography/computed tomography (PET/CT) imaging revealed increased translocator protein expression in the cortex and thalamus of diabetic rats, coupled with higher numbers of Iba-1-positive microglial cells (Zhang et al., 2022). These alterations are correlated with reduced mechanical and thermal pain thresholds, underscoring the role of microglia in pain hypersensitivity.
Further evidence from streptozotocin (STZ)-induced diabetic mouse models reveals marked microglial activation in the ACC, along with upregulated expression of the chemokine CXCL12 and its neuronal receptor CXCR4 (Song et al., 2023). This CXCL12/CXCR4 signaling enhances glutamatergic neuron excitability in the ACC, contributing to central sensitization and persistent mechanical pain in DPN.
The rostral ventromedial medulla (RVM), a brainstem center involved in descending pain facilitation, also shows early-stage microglial reactivity in DPN (Silva et al., 2016). Diabetic rodents demonstrate increased ON-cell activity, spinal 5-HT3 receptor expression, and TRPV1 upregulation, all of which facilitate nociceptive signal amplification (Silva et al., 2016). As DPN progresses, oxidative stress and microglial activation within the RVM further exacerbate neuroinflammation and neurodegeneration.
4.3 Region-specific astrocytic responses in central pain processing
Astrocytes, the most abundant glial cells in the CNS, play a crucial role in maintaining homeostasis, regulating neuronal activity, and mediating inflammatory responses (Giovannoni and Quintana, 2020). In DPN, astrocytic changes exhibit regional heterogeneity, with their activation contributing differentially to central pain amplification across pain-modulating brain structures (Cheng et al., 2022).
In STZ-induced diabetic models, the significantly increased expression of glial fibrillary acidic protein (GFAP)—a hallmark of astrocyte activation—has been detected in the motor cortex following the onset of DPN, indicating the involvement of motor cortex astrocytes in the pathogenesis of DPN (Lu et al., 2021). Functional inhibition of astrocytes in this region alleviated mechanical allodynia, alongside reduced expression of pro-inflammatory cytokine, including TNF-α and IL-1β.
The paraventricular thalamic nucleus (PVT), a midline thalamic structure crucial for sensory and nociceptive signal processing (Penzo and Gao, 2021), also exhibits significant astrocytic activation during DPN. In a study of DPN male rat, astrocytic activity within the PVT is markedly upregulated, accompanied by decreased neuronal activity at around 14 days following STZ administration (Chen et al., 2025). Chemogenetic inhibition of astrocytes in this region alleviates mechanical allodynia, whereas artificial activation in healthy rodents is sufficient to induce pain behavior.
Astrocyte activation is also evident in the ventrolateral periaqueductal gray (vlPAG), a core component of the descending pain inhibitory pathway (Tracey and Mantyh, 2007). Astrocytes in this region exhibit time-dependent activation and morphological changes, becoming significantly reactive after 14 days of STZ administration (Yang L. et al., 2022). Chemogenetic activation of vlPAG astrocytes in naive rats induces pain-like behaviors and aversion, while their inhibition in DPN model rats alleviates mechanical hypersensitivity and promotes preference behavior.
In vitro studies mimicking DPN with depression have shown that high glucose, substance P, and corticosterone exposure lead to astrocyte damage (Ge et al., 2020). This is marked by upregulated P2X7 receptor expression, elevated TNF-α and IL-1β levels, increased cytoplasmic Ca2+, and enhanced ERK1/2 phosphorylation. Notably, dihydromyricetin treatment protects primary hippocampal astrocytes from cytotoxicity and reduces inflammation, underscoring the importance of targeting astrocyte dysfunction to manage comorbidities in DPN.
5 Potential therapeutic strategies
The neuroimaging and mechanistic findings summarized above not only deepen our understanding of central alterations in DPN but also provide a critical basis for therapeutic development. Cortical reorganization and disrupted network activity revealed by neuroimaging point to neuromodulation of specific brain regions as a potential strategy (Chao et al., 2022a; Zeng et al., 2020; Li and Gao, 2025), while evidence of neuroinflammation and glial activation highlights molecular targets for pharmacological intervention (Kaur et al., 2025; Cheng et al., 2024). Building on these insights, the following section discusses emerging therapeutic approaches that exemplify how mechanistic discoveries can be translated into clinical strategies.
5.1 Transcranial non-invasive treatment of DPN
The following section summarizes findings primarily derived from clinical studies in human participants, focusing on non-invasive neuromodulatory approaches, particularly those targeting central pain processing pathways (Knotkova et al., 2021).
Transcranial magnetic stimulation (TMS), especially in the form of repetitive protocols (rTMS), utilizes pulsed magnetic fields to generate localized electric currents in targeted cortical areas (Davidson et al., 2024). This technique allows for precise modulation of neural circuits involved in pain perception and emotional regulation (Weise et al., 2023; Jayathilake et al., 2025).
A single-blinded randomized controlled trial investigated prolonged continuous theta burst stimulation (pcTBS) targeting both M1 and dorsolateral PFC in neuropathic pain patients (Thakkar et al., 2023). Neurophysiological assessments revealed modulation of motor corticospinal excitability and GABAergic activity, while no significant changes were observed in ascending/descending endogenous pain modulation systems. Although standardized pain scores remained unchanged, self-reported acute pain intensity showed a 13% improvement post-intervention, suggesting transient analgesic effects.
Similarly, another study evaluated the effect of a single-session pcTBS targeting the same cortical regions in patients with DPN (Thakkar et al., 2024). Findings indicated multidimensional analgesic effects, with improvements reported across sensory-discriminative, affective-motivational, and cognitive-evaluative domains of pain perception. Importantly, no adverse events were observed within 24 h post-intervention, supporting the safety and clinical feasibility of this non-invasive approach.
Further evidence was provided by a study that assessed the therapeutic efficacy of rTMS in DPN patients immediately after treatment and at a one-week follow-up (Yang S. et al., 2022). The results showed a sustained reduction in pain intensity along with improvements in overall quality of life. Specifically, both physical and mental component scores showed significant enhancements, underscoring the potential of rTMS not only to alleviate pain but also to improve psychosocial well-being.
These preliminary findings support transcranial non-invasive neuromodulation as a promising adjunctive strategy for the treatment of DPN. However, further research is needed to determine optimal stimulation parameters, treatment frequency, and patient selection criteria, which will be critical for maximizing clinical outcomes and individualizing therapy.
5.2 Brain-targeting compounds
Emerging research highlights the therapeutic potential of diverse compounds—ranging from natural phytochemicals to synthetic drugs—for the treatment of DPN (Qureshi et al., 2022; Arora et al., 2021; Zhang E. X. et al., 2024). However, the studies discussed in this section are derived entirely from preclinical/basic research in animal models. While they offer important mechanistic insights, their direct applicability to clinical practice remains to be established through rigorous translational and clinical studies.
Given the well-established role of neuroinflammation in DPN pathogenesis, strategies aimed at modulating central glial activation, particularly astrocytes and microglia, have garnered increasing interest (Llorian-Salvador et al., 2024). One promising example is Koumine, a bioactive alkaloid derived from Gelsemium elegans Benth., which has demonstrated anti-inflammatory and analgesic effects in preclinical studies (Que et al., 2021). Its therapeutic actions include the suppression of astrocyte activation in the basolateral amygdala and the subsequent reduction of proinflammatory cytokine release (Lu et al., 2023). These mechanisms are associated with attenuated mechanical hyperalgesia in rodent models of DPN.
In addition, an experimental study investigated fluorocitrate and neurotropin as potential therapies for DPN via central astrocyte modulation (Liu et al., 2022). Fluorocitrate, a glial-specific metabolic inhibitor that disrupts Krebs cycle activity (Zhuang et al., 2025), and neurotropin, a biologic agent derived from vaccinia virus-inoculated rabbit skin (Sprumont et al., 1995), were evaluated in diabetic rats. Both agents reduced mechanical hypersensitivity and normalized astrocyte activation markers in the vlPAG when administered via intrathecal (fluorocitrate) or systemic (neurotropin) routes (Liu et al., 2022). Critically, these analgesic effects occurred without altering blood glucose levels, suggesting a glucose-independent mechanism of action centered on astrocyte regulation. These findings highlight astrocytes as potential therapeutic targets for DPN management.
Beyond astrocytic modulation, other compounds targeting neuroinflammation through different mechanisms have also shown efficacy. Thalidomide, a derivative of glutamic acid, exhibits immunomodulatory and anti-inflammatory effects (Millrine and Kishimoto, 2017). RVM microinjections of thalidomide in Zucker diabetic fatty (ZDF) rats significantly reduced mechanical allodynia and thermal hyperalgesia (Yang et al., 2016). The analgesic effects were correlated with localized suppression of pro-inflammatory mediators (TNF-α, IL-1β) and NF-κB signaling within the RVM microenvironment. However, systemic cytokine levels remained unchanged, indicating region-specific anti-inflammatory action rather than global immunomodulation. It should be noted, nevertheless, that despite these mechanistic insights, the clinical application of thalidomide is limited by its well-documented toxicity concerns (Matthews and McCoy, 2003).
In addition to direct anti-inflammatory approaches, receptor-based interventions have emerged as another promising strategy. Among these, glucagon-like peptide-1 receptor agonists (GLP-1RA), commonly used for type 2 diabetes, have shown additional potential in DPN (Dhanapalaratnam et al., 2024). In animal models, intracerebroventricular administration of GLP-1RA has been shown to alleviate thermal and mechanical allodynia in DPN rats and suppress microglial activation in the cortex and thalamus, suggesting that GLP-1RA attenuates DPN, likely through inhibition of NLRP3 inflammasome activation in brain microglia (Zhang et al., 2022).
Beyond neuroinflammation and receptor modulation, mitochondrial dysfunction has been increasingly recognized as a shared pathological mechanism in neuropathic pain, including DPN (Yu et al., 2025; Espinoza and Papadopoulos, 2025). In the ZDF rat model, chronic oral administration of NSI-189, a neurogenic compound, ameliorated indices of neuropathy by improving mitochondrial bioenergetics (Jolivalt et al., 2022). Specifically, NSI-189 enhanced expression of mitochondrial respiratory complex subunits (III and V) and restored the activities of complexes I and IV in the brain cortex, changes that were accompanied by improved memory function and synaptic plasticity. These findings suggest that mitochondrial protection may represent an additional therapeutic avenue for targeting CNS dysfunction in DPN, though clinical translation remains to be established.
In summary, brain-targeting compounds primarily act by modulating central neuroinflammatory pathways, engaging specific receptor targets, or protecting mitochondrial function. While preclinical findings are encouraging, further translational research is required to clarify their safety, efficacy, and clinical applicability in DPN.
5.3 Clinical translation and future perspectives
Collectively, the integration of neuroimaging and preclinical findings provides a mechanistic foundation that can inform clinical interventions in DPN. For example, evidence of cortical and subcortical reorganization has guided the application of non-invasive brain stimulation techniques such as rTMS (Zeng et al., 2020), while the identification of neuroinflammatory and mitochondrial pathways has stimulated the search for brain-targeting pharmacological agents (Kaur et al., 2025; Zhu et al., 2023). Although most compounds remain at the preclinical stage, these mechanistic insights highlight promising therapeutic avenues that may complement existing symptomatic treatments. Importantly, future clinical trials should be designed to bridge these translational gaps, incorporating neuroimaging biomarkers to stratify patients and monitor treatment responses (Zhu et al., 2023; Hermann et al., 2025). Such an approach may accelerate the development of mechanism-based and personalized therapies for painful DPN (Atmaca et al., 2024).
While the present review highlights CNS alterations in DPN, it is equally important to consider how patient heterogeneity shapes these changes and modulates treatment responses, which is critical for advancing personalized therapy (Yang et al., 2019; Sloan et al., 2021; Teh et al., 2021; Croosu et al., 2023; Bonhof et al., 2019). Variability in pain phenotypes (e.g., painful vs. painless DPN), diabetes duration, and comorbidities such as anxiety or depression can influence the progression of CNS alterations (Shillo et al., 2019; Rosenberger et al., 2020; Gore et al., 2005; Lee and Won, 2025). For instance, patients with longer disease duration or genetic predispositions may show more pronounced thalamic atrophy or altered somatosensory connectivity, leading to greater central sensitization (Teh et al., 2021). Such heterogeneity contributes to differential CNS plasticity and variable responsiveness to interventions: neuromodulation (e.g., rTMS) may benefit certain phenotypes, whereas pharmacological therapies may be less effective in those with comorbid depression or genetic variants affecting drug metabolism (Sloan et al., 2021; Zeng et al., 2020; Zhu et al., 2023; Bonhof et al., 2019). To address these challenges, future studies should prioritize patient stratification based on phenotypes, CNS biomarkers, and complementary measures such as quantitative sensory testing or neuroimaging profiles, thereby facilitating more tailored and effective therapies (Yang et al., 2019; Sloan et al., 2021; Zhu et al., 2023; Atmaca et al., 2024; Bonhof et al., 2019; Lee and Won, 2025).
6 Conclusion
DPN is not solely a peripheral disorder; it involves profound changes in the brain’s pain-processing and modulation networks. Structural alterations, such as cortical thinning and subcortical atrophy, along with functional disruptions in connectivity and excitatory–inhibitory imbalance, contribute to central sensitization and pain persistence. Neuroinflammatory processes driven by microglial and astrocytic activation further amplify neuronal dysfunction and exacerbate chronic pain states.
These diverse alterations converge within the pain matrix, integrating sensory, emotional, and cognitive aspects of pain. Maladaptive reorganization of this matrix in DPN provides a unifying explanation for how peripheral injury, central sensitization, and higher-order processes interact to sustain chronic pain.
Understanding the role of the pain matrix in DPN provides a foundation for developing targeted therapies. Interventions aimed at restoring functional connectivity, reducing neuroinflammation, and enhancing descending inhibition hold promise for addressing the brain mechanisms of pain. Future research should prioritize longitudinal studies to elucidate the progression of central changes in DPN and explore multimodal approaches that integrate peripheral and central treatments for optimal pain management.
Author contributions
MW: Conceptualization, Writing – review & editing, Writing – original draft. YJ: Writing – review & editing, Writing – original draft, Visualization. JS: Writing – review & editing. GX: Supervision, Writing – review & editing. ML: Funding acquisition, Conceptualization, Writing – original draft, Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the National Natural Science Foundation of China (No. 82071411) and the Special Fund of the National Clinical Key Specialty Construction Program, China (2025).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alba-Delgado, C., Cebada-Aleu, A., Mico, J. A., and Berrocoso, E. (2016). Comorbid anxiety-like behavior and locus coeruleus impairment in diabetic peripheral neuropathy: a comparative study with the chronic constriction injury model. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 71, 45–56. doi: 10.1016/j.pnpbp.2016.06.007
Allen, E. A., Damaraju, E., Plis, S. M., Erhardt, E. B., Eichele, T., and Calhoun, V. D. (2014). Tracking whole-brain connectivity dynamics in the resting state. Cereb. Cortex 24, 663–676. doi: 10.1093/cercor/bhs352
Arora, K., Tomar, P. C., and Mohan, V. (2021). Diabetic neuropathy: an insight on the transition from synthetic drugs to herbal therapies. J. Diabetes Metab. Disord. 20, 1773–1784. doi: 10.1007/s40200-021-00830-2
Atmaca, A., Ketenci, A., Sahin, I., Sengun, I. S., Oner, R. I., Erdem Tilki, H., et al. (2024). Expert opinion on screening, diagnosis and management of diabetic peripheral neuropathy: a multidisciplinary approach. Front. Endocrinol. 15:1380929. doi: 10.3389/fendo.2024.1380929
Baracchini, G., Misic, B., Setton, R., Mwilambwe-Tshilobo, L., Girn, M., Nomi, J. S., et al. (2021). Inter-regional BOLD signal variability is an organizational feature of functional brain networks. NeuroImage 237:118149. doi: 10.1016/j.neuroimage.2021.118149
Barkhof, F., Haller, S., and Rombouts, S. A. (2014). Resting-state functional MR imaging: a new window to the brain. Radiology 272, 29–49. doi: 10.1148/radiol.14132388
Bonhof, G. J., Herder, C., Strom, A., Papanas, N., Roden, M., and Ziegler, D. (2019). Emerging biomarkers, tools, and treatments for diabetic polyneuropathy. Endocr. Rev. 40, 153–192. doi: 10.1210/er.2018-00107
Cao, B., Mwangi, B., Passos, I. C., Wu, M. J., Keser, Z., Zunta-Soares, G. B., et al. (2017). Lifespan gyrification trajectories of human brain in healthy individuals and patients with major psychiatric disorders. Sci. Rep. 7:511. doi: 10.1038/s41598-017-00582-1
Cauda, F., Sacco, K., D'Agata, F., Duca, S., Cocito, D., Geminiani, G., et al. (2009). Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in diabetic neuropathic pain. BMC Neurosci. 10:138. doi: 10.1186/1471-2202-10-138
Chao, C. C., Hsieh, P. C., Janice Lin, C. H., Huang, S. L., Hsieh, S. T., and Chiang, M. C. (2022a). Impaired brain network architecture as neuroimaging evidence of pain in diabetic neuropathy. Diabetes Res. Clin. Pract. 186:109833. doi: 10.1016/j.diabres.2022.109833
Chao, C. C., Tseng, M. T., Hsieh, P. C., Lin, C. J., Huang, S. L., Hsieh, S. T., et al. (2022b). Brain mechanisms of pain and dysautonomia in diabetic neuropathy: connectivity changes in thalamus and hypothalamus. J. Clin. Endocrinol. Metab. 107, e1167–e1180. doi: 10.1210/clinem/dgab754
Chen, J., Yang, L., Shen, J., Lu, J., Mo, X., Huang, L., et al. (2025). Distinct roles of astrocytes and GABAergic neurons in the paraventricular thalamic nucleus in modulating diabetic neuropathic pain. J. Neurosci. 45:e1013242024. doi: 10.1523/JNEUROSCI.1013-24.2024
Cheng, Y., Chen, Y., Li, K., Liu, S., Pang, C., Gao, L., et al. (2024). How inflammation dictates diabetic peripheral neuropathy: an enlightening review. CNS Neurosci. Ther. 30:e14477. doi: 10.1111/cns.14477
Cheng, T., Xu, Z., and Ma, X. (2022). The role of astrocytes in neuropathic pain. Front. Mol. Neurosci. 15:1007889. doi: 10.3389/fnmol.2022.1007889
Cho, N. H., Shaw, J. E., Karuranga, S., Huang, Y., da Rocha Fernandes, J. D., Ohlrogge, A. W., et al. (2018). IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 138, 271–281. doi: 10.1016/j.diabres.2018.02.023
Chudler, E. H., and Dong, W. K. (1995). The role of the basal ganglia in nociception and pain. Pain 60, 3–38. doi: 10.1016/0304-3959(94)00172-B
Clarkson, M. J., Cardoso, M. J., Ridgway, G. R., Modat, M., Leung, K. K., Rohrer, J. D., et al. (2011). A comparison of voxel and surface based cortical thickness estimation methods. NeuroImage 57, 856–865. doi: 10.1016/j.neuroimage.2011.05.053
Cole, J. B., and Florez, J. C. (2020). Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 16, 377–390. doi: 10.1038/s41581-020-0278-5
Croosu, S. S., Roikjer, J., Morch, C. D., Ejskjaer, N., Frokjaer, J. B., and Hansen, T. M. (2023). Alterations in functional connectivity of thalamus and primary somatosensory cortex in painful and painless diabetic peripheral neuropathy. Diabetes Care 46, 173–182. doi: 10.2337/dc22-0587
Cruz-Almeida, Y., and Porges, E. (2021). Additional considerations for studying brain metabolite levels across pain conditions using proton magnetic resonance spectroscopy. NeuroImage 224:117392. doi: 10.1016/j.neuroimage.2020.117392
Davidson, B., Bhattacharya, A., Sarica, C., Darmani, G., Raies, N., Chen, R., et al. (2024). Neuromodulation techniques - from non-invasive brain stimulation to deep brain stimulation. Neurotherapeutics 21:e00330. doi: 10.1016/j.neurot.2024.e00330
Davis, K. D., and Moayedi, M. (2013). Central mechanisms of pain revealed through functional and structural MRI. J. Neuroimmune Pharmacol. 8, 518–534. doi: 10.1007/s11481-012-9386-8
Dhanapalaratnam, R., Issar, T., Lee, A. T. K., Poynten, A. M., Milner, K. L., Kwai, N. C. G., et al. (2024). Glucagon-like peptide-1 receptor agonists reverse nerve morphological abnormalities in diabetic peripheral neuropathy. Diabetologia 67, 561–566. doi: 10.1007/s00125-023-06072-6
Dyck, P. J., Kratz, K. M., Karnes, J. L., Litchy, W. J., Klein, R., Pach, J. M., et al. (1993). The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester diabetic neuropathy study. Neurology 43, 817–824. doi: 10.1212/wnl.43.4.817
Espana, J. C., Yasoda-Mohan, A., and Vanneste, S. (2024). The locus Coeruleus in chronic pain. Int. J. Mol. Sci. 25:636. doi: 10.3390/ijms25168636
Espinoza, N., and Papadopoulos, V. (2025). Role of mitochondrial dysfunction in neuropathy. Int. J. Mol. Sci. 26:195. doi: 10.3390/ijms26073195
Faselis, C., Katsimardou, A., Imprialos, K., Deligkaris, P., Kallistratos, M., and Dimitriadis, K. (2020). Microvascular complications of type 2 diabetes mellitus. Curr. Vasc. Pharmacol. 18, 117–124. doi: 10.2174/1570161117666190502103733
Frokjaer, J. B., Brock, C., Softeland, E., Dimcevski, G., Gregersen, H., Simren, M., et al. (2013). A: macrostructural brain changes in patients with longstanding type 1 diabetes mellitus - a cortical thickness analysis study. Exp. Clin. Endocrinol. Diabetes 121, 354–360. doi: 10.1055/s-0033-1345120
Garcia-Larrea, L., and Peyron, R. (2013). Pain matrices and neuropathic pain matrices: a review. Pain 154 Suppl 1, S29–S43. doi: 10.1016/j.pain.2013.09.001
Ge, H., Sun, M., Wei, X., Zhang, M., Tu, H., Hao, Y., et al. (2020). Protective effects of dihydromyricetin on primary hippocampal astrocytes from cytotoxicity induced by comorbid diabetic neuropathic pain and depression. Purinergic Signal 16, 585–599. doi: 10.1007/s11302-020-09752-9
Giovannoni, F., and Quintana, F. J. (2020). The role of astrocytes in CNS inflammation. Trends Immunol. 41, 805–819. doi: 10.1016/j.it.2020.07.007
Gore, M., Brandenburg, N. A., Dukes, E., Hoffman, D. L., Tai, K. S., and Stacey, B. (2005). Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J. Pain Symptom Manag. 30, 374–385. doi: 10.1016/j.jpainsymman.2005.04.009
Greig, M., Tesfaye, S., Selvarajah, D., and Wilkinson, I. D. (2014). Insights into the pathogenesis and treatment of painful diabetic neuropathy. Handb. Clin. Neurol. 126, 559–578. doi: 10.1016/B978-0-444-53480-4.00037-0
Hansen, T. M., Croosu, S. S., Roikjer, J., Morch, C. D., Ejskjaer, N., and Frokjaer, J. B. (2024). Neuropathic phenotypes of type 1 diabetes are related to different signatures of magnetic resonance spectroscopy-assessed brain metabolites. Clin. Neurophysiol. 166, 11–19. doi: 10.1016/j.clinph.2024.06.017
Hansen, T. M., Frokjaer, J. B., Selvarajah, D., Muthulingam, J. A., Tesfaye, S., Juhl, A., et al. (2022a). Reduced thalamic volume and metabolites in type 1 diabetes with polyneuropathy. Exp. Clin. Endocrinol. Diabetes 130, 327–334. doi: 10.1055/a-1347-2579
Hansen, T. M., Muthulingam, J. A., Brock, B., Drewes, A. M., Juhl, A., Vorum, H., et al. (2022b). Reduced gray matter brain volume and cortical thickness in adults with type 1 diabetes and neuropathy. Neurosci. Res. 176, 66–72. doi: 10.1016/j.neures.2021.10.002
He, M., Yang, J., Liu, X., Zhou, J., Zhang, X., Li, J., et al. (2025). Brain morphological changes in type 2 diabetes patients with painful peripheral neuropathy. Metab. Brain Dis. 40:216. doi: 10.1007/s11011-025-01643-5
Hermann, D. M., Bacigaluppi, M., Bassetti, C. L., Bassotti, G., Boltze, J., Chan, A., et al. (2025). Most prominent challenges in translational neuroscience and strategic solutions to bridge the gaps: perspectives from an editorial board interrogation. Explor. Neurosci. 4:106. doi: 10.37349/en.2025.1006106
Hostrup, S. N., Croosu, S. S., Roikjer, J., Morch, C. D., Ejskjaer, N., Hansen, T. M., et al. (2025). Altered surface-based brain morphometry in type 1 diabetes and neuropathic pain. Neuroscience 566, 39–48. doi: 10.1016/j.neuroscience.2024.12.033
Huang, S., De Brigard, F., Cabeza, R., and Davis, S. W. (2024). Connectivity analyses for task-based fMRI. Phys Life Rev 49, 139–156. doi: 10.1016/j.plrev.2024.04.012
Jayathilake, N. J., Phan, T. T., Kim, J., Lee, K. P., and Park, J. M. (2025). Modulating neuroplasticity for chronic pain relief: noninvasive neuromodulation as a promising approach. Exp. Mol. Med. 57, 501–514. doi: 10.1038/s12276-025-01409-0
Jolivalt, C. G., Aghanoori, M. R., Navarro-Diaz, M. C., Han, M. M., Sanchez, G., Guernsey, L., et al. (2022). Enhancement of mitochondrial function by the neurogenic molecule NSI-189 accompanies reversal of peripheral neuropathy and memory impairment in a rat model of type 2 diabetes. J. Diabetes Res. 2022, 1–12. doi: 10.1155/2022/8566970
Kapur, D. (2003). Neuropathic pain and diabetes. Diabetes Metab. Res. Rev. 19, S9–S15. doi: 10.1002/dmrr.359
Kaur, G., Ali, S. O., Santos, A., Okdahl, T., Wegeberg, A. M., Ahluwalia, T. S., et al. (2025). Current advances in diabetic neuropathy proteins as therapeutic targets. Iscience 28:113466. doi: 10.1016/j.isci.2025.113466
Knotkova, H., Hamani, C., Sivanesan, E., Le Beuffe, M. F. E., Moon, J. Y., Cohen, S. P., et al. (2021). Neuromodulation for chronic pain. Lancet 397, 2111–2124. doi: 10.1016/S0140-6736(21)00794-7
Latremoliere, A., and Woolf, C. J. (2009). Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J. Pain 10, 895–926. doi: 10.1016/j.jpain.2009.06.012
Lee, J. E., and Won, J. C. (2025). Clinical phenotypes of diabetic peripheral neuropathy: implications for phenotypic-based therapeutics strategies. Diabetes Metab. J. 49, 542–564. doi: 10.4093/dmj.2025.0299
Lemaitre, H., Goldman, A. L., Sambataro, F., Verchinski, B. A., Meyer-Lindenberg, A., Weinberger, D. R., et al. (2012). Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiol. Aging 33:617.e1. doi: 10.1016/j.neurobiolaging.2010.07.013
Li, X., and Gao, L. (2025). Causal central network Remodeling in diabetic neuropathy: an integrated MR-fMRI study. Diabetes Metab. Syndr. Obes. 18, 2753–2765. doi: 10.2147/DMSO.S525219
Li, X. Y., Wan, Y., Tang, S. J., Guan, Y., Wei, F., and Ma, D. (2016). Maladaptive plasticity and neuropathic pain. Neural Plast. 2016, 1–2. doi: 10.1155/2016/4842159
Li, W., Wang, P., and Li, H. (2014). Upregulation of glutamatergic transmission in anterior cingulate cortex in the diabetic rats with neuropathic pain. Neurosci. Lett. 568, 29–34. doi: 10.1016/j.neulet.2014.03.038
Li, J., Wei, Y., Zhou, J., Zou, H., Ma, L., Liu, C., et al. (2022). Activation of locus coeruleus-spinal cord noradrenergic neurons alleviates neuropathic pain in mice via reducing neuroinflammation from astrocytes and microglia in spinal dorsal horn. J. Neuroinflammation 19:123. doi: 10.1186/s12974-022-02489-9
Li, J., Zhang, W., Wang, X., Yuan, T., Liu, P., Wang, T., et al. (2018). Functional magnetic resonance imaging reveals differences in brain activation in response to thermal stimuli in diabetic patients with and without diabetic peripheral neuropathy. PLoS One 13:e0190699. doi: 10.1371/journal.pone.0190699
Liu, X., He, J., Gao, J., and Xiao, Z. (2022). Fluorocitrate and neurotropin confer analgesic effects on neuropathic pain in diabetic rats via inhibition of astrocyte activation in the periaqueductal gray. Neurosci. Lett. 768:136378. doi: 10.1016/j.neulet.2021.136378
Liu, X., Xu, X., Mao, C., Zhang, P., Zhang, Q., Jiang, L., et al. (2021). Increased thalamo-cortical functional connectivity in patients with diabetic painful neuropathy: a resting-state functional MRI study. Exp. Ther. Med. 21:509. doi: 10.3892/etm.2021.9940
Llorian-Salvador, M., Cabeza-Fernandez, S., Gomez-Sanchez, J. A., and de la Fuente, A. G. (2024). Glial cell alterations in diabetes-induced neurodegeneration. Cell. Mol. Life Sci. 81:47. doi: 10.1007/s00018-023-05024-y
Lu, J. S., Yang, L., Chen, J., Xiong, F. F., Cai, P., Wang, X. Y., et al. (2023). Basolateral amygdala astrocytes modulate diabetic neuropathic pain and may be a potential therapeutic target for koumine. Br. J. Pharmacol. 180, 1408–1428. doi: 10.1111/bph.16011
Lu, J., Yang, L., Xu, Y., Ai, L., Chen, J., Xiong, F., et al. (2021). The modulatory effect of motor cortex astrocytes on diabetic neuropathic pain. J. Neurosci. 41, 5287–5302. doi: 10.1523/JNEUROSCI.2566-20.2021
Martucci, K. T., and Mackey, S. C. (2018). Neuroimaging of pain: human evidence and clinical relevance of central nervous system processes and modulation. Anesthesiology 128, 1241–1254. doi: 10.1097/ALN.0000000000002137
Matthews, S. J., and McCoy, C. (2003). Thalidomide: a review of approved and investigational uses. Clin. Ther. 25, 342–395. doi: 10.1016/s0149-2918(03)80085-1
Mesa-Lombardo, A., Garcia-Magro, N., Nunez, A., and Martin, Y. B. (2023). Locus coeruleus inhibition of vibrissal responses in the trigeminal subnucleus caudalis are reduced in a diabetic mouse model. Front. Cell. Neurosci. 17:1208121. doi: 10.3389/fncel.2023.1208121
Millrine, D., and Kishimoto, T. (2017). A brighter side to thalidomide: its potential use in immunological disorders. Trends Mol. Med. 23, 348–361. doi: 10.1016/j.molmed.2017.02.006
Morgado, C., Silva, L., Pereira-Terra, P., and Tavares, I. (2011). Changes in serotoninergic and noradrenergic descending pain pathways during painful diabetic neuropathy: the preventive action of IGF1. Neurobiol. Dis. 43, 275–284. doi: 10.1016/j.nbd.2011.04.001
Muhlau, M., Gaser, C., Ilg, R., Conrad, B., Leibl, C., Cebulla, M. H., et al. (2007). Gray matter decrease of the anterior cingulate cortex in anorexia nervosa. Am. J. Psychiatry 164, 1850–1857. doi: 10.1176/appi.ajp.2007.06111861
NCD Risk Factor Collaboration (2016). Collaboration: worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 387, 1513–1530. doi: 10.1016/S0140-6736(16)00618-8
Novo, J. L., Ruas, J. J., Ferreira, L. M., Carvalho, D., Barbosa, M., Brandao, S., et al. (2022). Thalamic volumetric abnormalities in type 1 diabetes mellitus and 'peripheral' neuropathy. Sci. Rep. 12:13053. doi: 10.1038/s41598-022-16699-x
Penzo, M. A., and Gao, C. (2021). The paraventricular nucleus of the thalamus: an integrative node underlying homeostatic behavior. Trends Neurosci. 44, 538–549. doi: 10.1016/j.tins.2021.03.001
Petrou, M., Pop-Busui, R., Foerster, B. R., Edden, R. A., Callaghan, B. C., Harte, S. E., et al. (2012). Altered excitation-inhibition balance in the brain of patients with diabetic neuropathy. Acad. Radiol. 19, 607–612. doi: 10.1016/j.acra.2012.02.004
Pouwer, F., Mizokami-Stout, K., Reeves, N. D., Pop-Busui, R., Tesfaye, S., Boulton, A. J. M., et al. (2024). Psychosocial Care for People with Diabetic Neuropathy: time for action. Diabetes Care 47, 17–25. doi: 10.2337/dci23-0033
Que, W., Wu, Z., Chen, M., Zhang, B., You, C., Lin, H., et al. (2021). Molecular mechanism of Gelsemium elegans (Gardner and Champ.) benth against neuropathic pain based on network pharmacology and experimental evidence. Front. Pharmacol. 12:792932. doi: 10.3389/fphar.2021.792932
Qureshi, Z., Ali, M. N., and Khalid, M. (2022). An insight into potential Pharmacotherapeutic agents for painful diabetic neuropathy. J. Diabetes Res. 2022, 1–19. doi: 10.1155/2022/9989272
Rosenberger, D. C., Blechschmidt, V., Timmerman, H., Wolff, A., and Treede, R. D. (2020). Challenges of neuropathic pain: focus on diabetic neuropathy. J. Neural Transm. 127, 589–624. doi: 10.1007/s00702-020-02145-7
Schaible, H. G. (2007). Peripheral and central mechanisms of pain generation. Handb. Exp. Pharmacol. 177, 3–28. doi: 10.1007/978-3-540-33823-9_1
Scheliga, S., Dohrn, M. F., Habel, U., Lampert, A., Rolke, R., Lischka, A., et al. (2024). Reduced Gray matter volume and cortical thickness in patients with small-Fiber neuropathy. J. Pain 25:104457. doi: 10.1016/j.jpain.2024.01.001
Schwarz, C. G., Gunter, J. L., Wiste, H. J., Przybelski, S. A., Weigand, S. D., Ward, C. P., et al. (2016). Alzheimer's Disease Neuroimaging: a large-scale comparison of cortical thickness and volume methods for measuring Alzheimer's disease severity. Neuroimage Clin. 11, 802–812. doi: 10.1016/j.nicl.2016.05.017
Segerdahl, A. R., Themistocleous, A. C., Fido, D., Bennett, D. L., and Tracey, I. (2018). A brain-based pain facilitation mechanism contributes to painful diabetic polyneuropathy. Brain 141, 357–364. doi: 10.1093/brain/awx337
Selvarajah, D., Sloan, G., Teh, K., Wilkinson, I. D., Heiberg-Gibbons, F., Awadh, M., et al. (2023). Structural brain alterations in key somatosensory and nociceptive regions in diabetic peripheral neuropathy. Diabetes Care 46, 777–785. doi: 10.2337/dc22-1123
Selvarajah, D., Wilkinson, I. D., Emery, C. J., Shaw, P. J., Griffiths, P. D., Gandhi, R., et al. (2008). Thalamic neuronal dysfunction and chronic sensorimotor distal symmetrical polyneuropathy in patients with type 1 diabetes mellitus. Diabetologia 51, 2088–2092. doi: 10.1007/s00125-008-1139-0
Selvarajah, D., Wilkinson, I. D., Fang, F., Sankar, A., Davies, J., Boland, E., et al. (2019). Structural and functional abnormalities of the primary somatosensory cortex in diabetic peripheral neuropathy: a multimodal MRI study. Diabetes 68, 796–806. doi: 10.2337/db18-0509
Selvarajah, D., Wilkinson, I. D., Maxwell, M., Davies, J., Sankar, A., Boland, E., et al. (2014). Magnetic resonance neuroimaging study of brain structural differences in diabetic peripheral neuropathy. Diabetes Care 37, 1681–1688. doi: 10.2337/dc13-2610
Shillo, P., Sloan, G., Greig, M., Hunt, L., Selvarajah, D., Elliott, J., et al. (2019). Painful and painless diabetic neuropathies: what is the difference? Curr. Diab. Rep. 19:32. doi: 10.1007/s11892-019-1150-5
Shillo, P., Sloan, G., Selvarajah, D., Greig, M., Gandhi, R., Anand, P., et al. (2024). Reduced thalamic gamma-aminobutyric acid (GABA) in painless but not painful diabetic peripheral neuropathy. Diabetes 73, 1317–1324. doi: 10.2337/db23-0921
Sifuentes-Franco, S., Pacheco-Moises, F. P., Rodriguez-Carrizalez, A. D., and Miranda-Diaz, A. G. (2017). The role of oxidative stress, mitochondrial function, and autophagy in diabetic polyneuropathy. J. Diabetes Res. 2017, 1–15. doi: 10.1155/2017/1673081
Silva, M., Costa-Pereira, J. T., Martins, D., and Tavares, I. (2016). Pain modulation from the brain during diabetic neuropathy: uncovering the role of the rostroventromedial medulla. Neurobiol. Dis. 96, 346–356. doi: 10.1016/j.nbd.2016.10.002
Sloan, G., Anton, A., Caunt, S., Wilkinson, I., Selvarajah, D., and Tesfaye, S. (2023). Higher sensory cortical energy metabolism in painful diabetic neuropathy: evidence from a cerebral magnetic resonance spectroscopy study. Diabetes 72, 1028–1034. doi: 10.2337/db23-0051
Sloan, G., Selvarajah, D., and Tesfaye, S. (2021). Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat. Rev. Endocrinol. 17, 400–420. doi: 10.1038/s41574-021-00496-z
Song, Z. H., Song, X. J., Yang, C. L., Cao, P., Mao, Y., Jin, Y., et al. (2023). Up-regulation of microglial chemokine CXCL12 in anterior cingulate cortex mediates neuropathic pain in diabetic mice. Acta Pharmacol. Sin. 44, 1337–1349. doi: 10.1038/s41401-022-01046-7
Sprumont, P., Kaelin-Lang, A., Van Lierde, S., Maenhaut, W., and De Reuck, J. (1995). Effect of neurotropin on cerebral edema, calcium and other elements in mice subarachnoidally injected with carrageenan. Eur. J. Pharmacol. 274, 95–99. doi: 10.1016/0014-2999(94)00724-l
Suehiro, K., Funao, T., Fujimoto, Y., Yamada, T., Mori, T., and Nishikawa, K. (2013). Relationship between noradrenaline release in the locus coeruleus and antiallodynic efficacy of analgesics in rats with painful diabetic neuropathy. Life Sci. 92, 1138–1144. doi: 10.1016/j.lfs.2013.04.015
Tae, W. S., Ham, B. J., Pyun, S. B., and Kim, B. J. (2025). Current clinical applications of structural MRI in neurological disorders. J. Clin. Neurol. 21, 277–293. doi: 10.3988/jcn.2025.0185
Tang, Y., Zhao, L., Lou, Y., Shi, Y., Fang, R., Lin, X., et al. (2018). Brain structure differences between Chinese and Caucasian cohorts: a comprehensive morphometry study. Hum. Brain Mapp. 39, 2147–2155. doi: 10.1002/hbm.23994
Teh, K., Wilkinson, I. D., Heiberg-Gibbons, F., Awadh, M., Kelsall, A., Pallai, S., et al. (2021). Somatosensory network functional connectivity differentiates clinical pain phenotypes in diabetic neuropathy. Diabetologia 64, 1412–1421. doi: 10.1007/s00125-021-05416-4
Tesfaye, S., Selvarajah, D., Gandhi, R., Greig, M., Shillo, P., Fang, F., et al. (2016). Diabetic peripheral neuropathy may not be as its name suggests: evidence from magnetic resonance imaging. Pain 157, S72–S80. doi: 10.1097/j.pain.0000000000000465
Thakkar, B., Peterson, C. L., and Acevedo, E. O. (2023). Prolonged continuous theta burst stimulation increases motor corticospinal excitability and intracortical inhibition in patients with neuropathic pain: an exploratory, single-blinded, randomized controlled trial. Neurophysiol. Clin. 53:102894. doi: 10.1016/j.neucli.2023.102894
Thakkar, B., Peterson, C. L., and Acevedo, E. O. (2024). Single session effects of prolonged continuous theta burst stimulation targeting two brain regions on pain perception in patients with painful diabetic neuropathy: a preliminary study. J. Integr. Neurosci. 23:54. doi: 10.31083/j.jin2303054
Tracey, I., and Mantyh, P. W. (2007). The cerebral signature for pain perception and its modulation. Neuron 55, 377–391. doi: 10.1016/j.neuron.2007.07.012
Tseng, M. T., Chiang, M. C., Chao, C. C., Tseng, W. Y., and Hsieh, S. T. (2013). fMRI evidence of degeneration-induced neuropathic pain in diabetes: enhanced limbic and striatal activations. Hum. Brain Mapp. 34, 2733–2746. doi: 10.1002/hbm.22105
Ugurbil, K., Toth, L., and Kim, D. S. (2003). How accurate is magnetic resonance imaging of brain function? Trends Neurosci. 26, 108–114. doi: 10.1016/S0166-2236(02)00039-5
Van Dam, P. S., Cotter, M. A., Bravenboer, B., and Cameron, N. E. (2013). Pathogenesis of diabetic neuropathy: focus on neurovascular mechanisms. Eur. J. Pharmacol. 719, 180–186. doi: 10.1016/j.ejphar.2013.07.017
Vidal-Pineiro, D., Parker, N., Shin, J., French, L., Grydeland, H., Jackowski, A. P., et al. (2020). The Australian imaging and a. lifestyle flagship study of: cellular correlates of cortical thinning throughout the lifespan. Sci. Rep. 10:21803. doi: 10.1038/s41598-020-78471-3
Walker, A. F., Graham, S., Maple-Brown, L., Egede, L. E., Campbell, J. A., Walker, R. J., et al. (2023). Interventions to address global inequity in diabetes: international progress. Lancet 402, 250–264. doi: 10.1016/S0140-6736(23)00914-5
Wang, Q., Xie, Y., Ma, S., Luo, H., and Qiu, Y. (2024). Role of microglia in diabetic neuropathic pain. Front. Cell Dev. Biol. 12:1421191. doi: 10.3389/fcell.2024.1421191
Weise, K., Numssen, O., Kalloch, B., Zier, A. L., Thielscher, A., Haueisen, J., et al. (2023). Precise motor mapping with transcranial magnetic stimulation. Nat. Protoc. 18, 293–318. doi: 10.1038/s41596-022-00776-6
Winkler, A. M., Kochunov, P., Blangero, J., Almasy, L., Zilles, K., Fox, P. T., et al. (2010). Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. NeuroImage 53, 1135–1146. doi: 10.1016/j.neuroimage.2009.12.028
Wu, Z., Peng, Y., Hong, M., and Zhang, Y. (2021). Gray matter deterioration pattern during Alzheimer's Disease progression: a regions-of-interest based surface morphometry study. Front. Aging Neurosci. 13:593898. doi: 10.3389/fnagi.2021.593898
Yang, S., Kwak, S. G., Choi, G. S., and Chang, M. C. (2022). Short-term effect of repetitive transcranial magnetic stimulation on diabetic peripheral neuropathic pain. Pain Physician 25, E203–E209.
Yang, L., Lu, J., Guo, J., Chen, J., Xiong, F., Wang, X., et al. (2022). Ventrolateral periaqueductal Gray astrocytes regulate nociceptive sensation and emotional motivation in diabetic neuropathic pain. J. Neurosci. 42, 8184–8199. Available at: https://www.painphysicianjournal.com/linkout?issn=&vol=25&page=E203
Yang, H., Sloan, G., Ye, Y., Wang, S., Duan, B., Tesfaye, S., et al. (2019). New perspective in diabetic neuropathy: from the periphery to the brain, a call for early detection, and precision medicine. Front. Endocrinol. 10:929. doi: 10.3389/fendo.2019.00929
Yang, Y., Zhang, Z., Guan, J., Liu, J., Ma, P., Gu, K., et al. (2016). Administrations of thalidomide into the rostral ventromedial medulla alleviates painful diabetic neuropathy in Zucker diabetic fatty rats. Brain Res. Bull. 125, 144–151. doi: 10.1016/j.brainresbull.2016.06.013
Yang, Y., Zhao, B., Wang, Y., Lan, H., Liu, X., Hu, Y., et al. (2025). Diabetic neuropathy: cutting-edge research and future directions. Signal Transduct. Target. Ther. 10:132. doi: 10.1038/s41392-025-02175-1
Yen, C., Lin, C. L., and Chiang, M. C. (2023). Exploring the Frontiers of Neuroimaging: a review of recent advances in understanding brain functioning and disorders. Life 13:472. doi: 10.3390/life13071472
Yu, H., Yang, C., Wang, G., and Wang, X. (2025). Mitochondrial dynamics in diabetic peripheral neuropathy: pathogenesis, progression, and therapeutic approaches. Medicine 104:e42748. doi: 10.1097/MD.0000000000042748
Zang, Y., Jiang, D., Zhuang, X., and Chen, S. (2023). Changes in the central nervous system in diabetic neuropathy. Heliyon 9:e18368. doi: 10.1016/j.heliyon.2023.e18368
Zeng, H., Pacheco-Barrios, K., Cao, Y., Li, Y., Zhang, J., Yang, C., et al. (2020). Non-invasive neuromodulation effects on painful diabetic peripheral neuropathy: a systematic review and meta-analysis. Sci. Rep. 10:19184. doi: 10.1038/s41598-020-75922-9
Zhang, L. B., Chen, Y. X., Li, Z. J., Geng, X. Y., Zhao, X. Y., Zhang, F. R., et al. (2024). Advances and challenges in neuroimaging-based pain biomarkers. Cell Rep. Med. 5:101784. doi: 10.1016/j.xcrm.2024.101784
Zhang, Q., Li, Q., Liu, S., Zheng, H., Ji, L., Yi, N., et al. (2022). Glucagon-like peptide-1 receptor agonist attenuates diabetic neuropathic pain via inhibition of NOD-like receptor protein 3 inflammasome in brain microglia. Diabetes Res. Clin. Pract. 186:109806. doi: 10.1016/j.diabres.2022.109806
Zhang, Y., Qu, M., Yi, X., Zhuo, P., Tang, J., Chen, X., et al. (2020). Sensorimotor and pain-related alterations of the gray matter and white matter in type 2 diabetic patients with peripheral neuropathy. Hum. Brain Mapp. 41, 710–725. doi: 10.1002/hbm.24834
Zhang, E. X., Yazdi, C., Islam, R. K., Anwar, A. I., Alvares-Amado, A., Townsend, H., et al. (2024). Diabetic neuropathy: a guide to pain management. Curr. Pain Headache Rep. 28, 1067–1072. doi: 10.1007/s11916-024-01293-9
Zhao, X., Han, Q., Gang, X., and Wang, G. (2018). Altered brain metabolites in patients with diabetes mellitus and related complications - evidence from (1)H MRS study. Biosci. Rep. 38:60. doi: 10.1042/BSR20180660
Zhu, J., Hu, Z., Luo, Y., Liu, Y., Luo, W., Du, X., et al. (2023). Diabetic peripheral neuropathy: pathogenetic mechanisms and treatment. Front. Endocrinol. 14:1265372. doi: 10.3389/fendo.2023.1265372
Keywords: diabetic peripheral neuropathy, brain mechanisms, neuroimaging, neuroinflammation, neuromodulation
Citation: Wei M, Jiang Y, Shou J, Xing G and Li M (2025) The role of brain mechanisms in diabetic peripheral neuropathy: recent advances and comprehensive analysis. Front. Cell. Neurosci. 19:1637357. doi: 10.3389/fncel.2025.1637357
Edited by:
Antonietta Bernardo, National Institute of Health (ISS), ItalyReviewed by:
Paramita Basu, University of Pittsburgh, United StatesAnkit Uniyal, Johns Hopkins University, United States
Copyright © 2025 Wei, Jiang, Shou, Xing and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Li, bGltaW5hbmVzdGhAYmptdS5lZHUuY24=; Guogang Xing, Z2d4aW5nQGJqbXUuZWR1LmNu
†These authors have contributed equally to this work
 Min Wei
Min Wei Ye Jiang
Ye Jiang Jiayin Shou
Jiayin Shou Guogang Xing
Guogang Xing Min Li
Min Li