- Department of Medical Cell Biology, Uppsala University, Uppsala, Sweden
Glucagon-like peptide-1 (GLP-1) is a metabolic hormone secreted by L-cells in the gut and it stimulates insulin secretion in the pancreatic islets by activating GLP-1 receptors (GLP-1Rs). In the brain, the GLP-1Rs are expressed in many regions including the hippocampus. We examined whether GLP-1 modulation of GABA-activated currents in the mouse hippocampus varied along the hippocampal dorsal-ventral axis. We recorded spontaneous inhibitory postsynaptic (sIPSCs) and tonic extrasynaptic currents in dorsal and ventral hippocampal dentate gyrus (DG) granule cells in brain slices from 2-month-old mice. GLP-1 (100 pM) did not modulate the GABA-activated fast or slow phasic postsynaptic currents in either the dorsal or the ventral hippocampal slices. In contrast, the tonic extrasynaptic current was potentiated by GLP-1 but, only consistently in the DG granule cells of the ventral hippocampus. Thus, GLP-1 modulation of the DG neurons depends on the dorso-ventral longitudinal hippocampal axis and further, with the subcellular location (synaptic vs. extrasynaptic) of the GABAA receptors (GABAAR) in the DG granule cells. The results are consistent with GLP-1 enhancing the tonic inhibitory extrasynaptic current by a postsynaptic mechanism.
Introduction
The incretins are secreted in response to ingestion of food, and it is well established that they regulate insulin secretion in a glucose-dependent manner (Astrup, 2024; Muller et al., 2019). GLP-1 is one of the incretins hormones that are secreted by cells in the gut. Moreover, GLP-1 and GLP-1Rs are also found in the brain where they have been ascribed various functions including neuroprotection, modulating food intake and enhancing memory and learning in the hippocampus (Astrup, 2024; Gupta et al., 2023; Holscher, 2022; Kanoski and Grill, 2017). Nucleus of the solitary tract (NTS) is the primary neuronal source of the endogenous GLP-1 in the brain (Larsen et al., 1997) where the preproglucagon is processed to GLP-1 (Ugleholdt et al., 2004). However, axons from NTS do not project to the hippocampus. How GLP-1 reaches the hippocampus is still under investigation, but it has been suggested that GLP-1 may reach the hippocampus by simple diffusion or through volume transmission from the ventricular system (Gupta et al., 2023; Buller and Blouet, 2024; Hsu et al., 2015; Kanoski et al., 2016; Kastin et al., 2002). Both the dorsal and the ventral DG regions of the mouse hippocampus express GLP-1R (Holst, 2024). In recent years glucagon-like peptide-1 receptor agonists (GLP-1RAs) have been shown in clinical studies to be neuroprotective, to have metabolic benefits and have emerged as effective treatments for both type 2 diabetes and obesity (Astrup, 2024; Dash, 2024), while effects related to decreasing the rate of cognitive decline are not as clear and are still being explored (Dash, 2024; Liang et al., 2024).
The hippocampal longitudinal axis ranges from dorsal (septal) to ventral (temporal) in rodents and corresponds to posterior-to-anterior hippocampus in humans (Papatheodoropoulos, 2018; Strange et al., 2014). Information from sensory cortices is received by the dorsal hippocampus whereas the ventral hippocampus has more connectivity with the amygdala, prefrontal cortex and hypothalamus (Strange et al., 2014; Swanson and Cowan, 1977). Hippocampal activity is modulated, at least in part, by hormones, as the expression of many hormone receptors has been detected (Lathe, 2001). The role of the hippocampus in formation of spatial memories, navigation and emotional responses is well established (Strange et al., 2014) but what is less well known is that the hippocampus participates in regulating physiological homeostasis in a topographical manner (Risold and Swanson, 1996). The ventral hippocampal neurons, for instance, via a synapse in the septum, inhibit hypothalamic neurons (Risold and Swanson, 1996; Decarie-Spain et al., 2022). It is, therefore, not surprising that metabolic hormones like insulin and GLP-1 have been shown to modulate synaptic transmission in hippocampal neurons (Ferrario and Reagan, 2018; Hammoud et al., 2021; Korol et al., 2015).
γ-Aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the central nervous system (Sieghart and Savic, 2018). It activates GABAA and GABAB receptors that are ion channels and G-protein coupled receptors, respectively. When GABA is released from presynaptic terminals it activates synaptic GABAA receptors (GABAARs) on postsynaptic neurons generating phasic spontaneous inhibitory postsynaptic currents (sIPSCs) (Otis et al., 1991). These phasic currents comprise both fast and slow sIPSCs (Figure 1A), which are distinguished not only by their kinetics but also by their distinct presynaptic GABAergic neurons that evoke them, and play different roles in neuronal circuits (Armstrong et al., 2012; Capogna and Pearce, 2011). GABAARs located outside of synapses are termed extrasynaptic receptors and are activated by ambient GABA concentrations and mediate extrasynaptic tonic current (Figure 1A) (Semyanov et al., 2003). GABAARs containing α4β2/3δ mainly mediate tonic inhibition in mouse DG granule cells (Chandra et al., 2006; Stell et al., 2003). We have previously shown in rat dorsal hippocampal CA3 neurons that GLP-1 and its mimetics enhanced both synaptic and extrasynaptic GABA-activated currents (Korol et al., 2015; Babateen et al., 2017; Korol et al., 2015). Here, we examine whether GLP-1 differentially modulates GABAergic inhibition in mouse dorsal and ventral hippocampal DG granule cells. In line with common simplification of the endogenous diversification of the hippocampus, we divided the structure along the dorsoventral axis into dorsal, intermediate and ventral domains (Strange et al., 2014; Paxinos and Watson, 1986). Our previous work has shown that GABAergic inhibition in the mouse hippocampus varies and is dependent on the dorsoventral axis and cell type (DG granule cells and CA3 pyramidal neurons) (Netsyk et al., 2020). We then studied the effects of GLP-1 (100 pM) on GABAergic signaling in dorsal and ventral dentate gyrus (DG) granule cells. The results show that GLP-1 modulates the GABAergic currents mediated via extrasynaptic GABAA receptors in DG granule cells only in the ventral part of the mouse hippocampus.
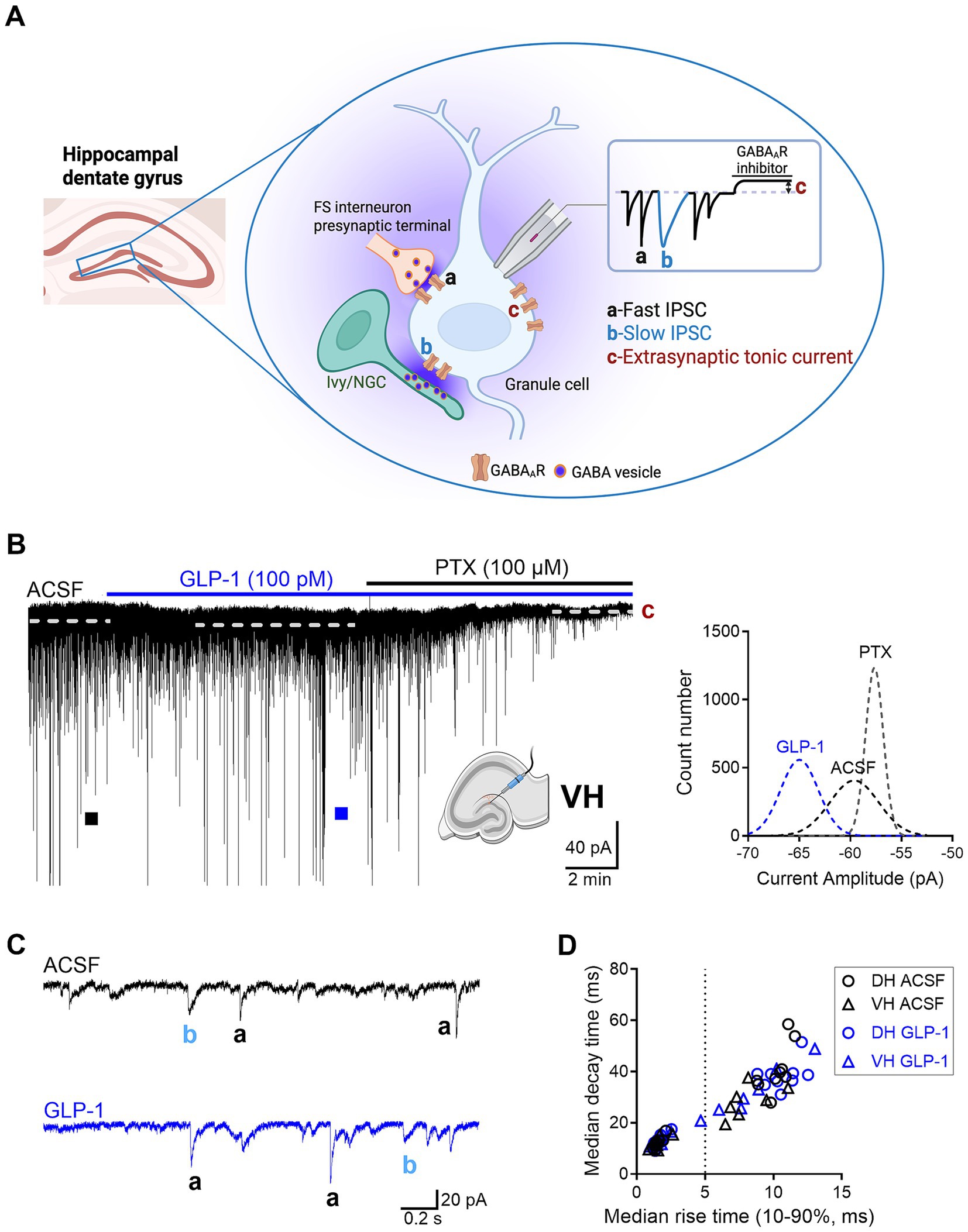
Figure 1. The effect of GLP-1 on GABAAR-mediated currents in dentate gyrus granule cells of the mouse hippocampus. (A) A schematic illustration of three forms of GABAAR-mediated currents recorded in dentate gyrus (DG) granule cells: a, fast inhibitory postsynaptic current (IPSC); b, slow IPSC; c, extrasynaptic tonic current. FS interneuron, fast-spiking interneuron; Ivy/NGC, Ivy/neurogliaform cell. Created in BioRender. Jin (2025), https://BioRender.com/g5wydyv. (B) A representative current trace recorded from a DG granule cell in the ventral hippocampus (VH) before and after GLP-1 (100 pM) application. The difference between the dashed lines represents the extrasynaptic tonic current amplitude (c), estimated from Gaussian fits to all-points histograms derived from sIPSC-free baseline segments (right panel). (C) Fast IPSC (a) and slow IPSC (b) from segments marked with filled squares are shown on an expanded scale below. ACSF, artificial cerebrospinal fluid; PTX, picrotoxin. (D) A scatter plot illustrating the median 63% decay time plotted against the median 10–90% rise time of sIPSCs measured in individual DG granule cells (DH, n = 9 from 6 mice; VH, n = 7 from 5 mice). sIPSCs were classified as fast or slow based on a 5-ms rise-time cutoff.
Materials and methods
Animals
All experiments were conducted in accordance with the local ethical guidelines and protocols approved by Uppsala Animal Ethical Committee, Swedish law and regulations based on the Directive 2010/63/EU and C129/14. C57BL/6 J male mice (Taconic M&B, Denmark), aged 8–10 weeks, were used in all experiments. Recordings were made from DG granule cells in hippocampal dorsal and ventral brain slices.
Hippocampal slice preparation
Mice were euthanized by cervical dislocation followed by decapitation. Brain slices were prepared as previously described (Netsyk et al., 2020; Jin et al., 2011; Ting et al., 2014). Briefly, the brain was removed and placed into ice-cold N-methyl D-glucamine (NMDG)-based solution containing (mM): 93 NMDG, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES), 25 D-glucose, 10 MgSO4, 0.5 CaCl2, 5 Na ascorbate, 2 thiourea, 3 Na pyruvate, pH 7.3–7.4 (adjusted with HCl), saturated with 95% O2 and 5% CO2, osmolarity 300–305 mOsm (adjusted with sucrose). Hippocampal slices (350 μm thick) were cut using a microtome (Leica VT1200 S, Leica Microsystems AB, Germany). Dorsal and ventral DG were defined in coronal and horizontal slices, respectively, according to Paxinos and Watson (1986). The slices were incubated in NMDG-based solution at 32 °C for 12–15 min, then transferred to a HEPES-based holding solution (in mM): 92 NaCl, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 D-glucose, 2 MgSO4, 2 CaCl2, 5 Na ascorbate, 2 thiourea, 3 Na pyruvate, pH 7.3–7.4 (adjusted with NaOH), saturated with 95% O2 and 5% CO2; osmolarity 300–305 mOsm (adjusted with sucrose). The slices were kept at room temperature (20–22 °C) for at least 1 h before use.
Electrophysiology
Whole-cell patch-clamp recordings were performed on DG granule cells from the dorsal and ventral regions of the hippocampus (Netsyk et al., 2020). All experiments were conducted at room temperature. The slice was transferred to the recording chamber and perfused (1.5–2 mL/min) with artificial cerebrospinal fluid (ACSF) containing (mM): 119 NaCl, 2.5 KCl, 1.3 MgSO4, 1 NaH2PO4, 26.2 NaHCO3, 2.5 CaCl2, 11 D-glucose and 3 kynurenic acid, pH 7.3–7.4, equilibrated with 95% O2 and 5% CO2, osmolarity 300–303 mOsm (adjusted with sucrose). Borosilicate glass patch pipettes (4–5 MΩ in resistance) were filled with an intracellular solution containing (mM): 140 CsCl, 8 NaCl, 2 EGTA, 0.2 MgCl2, 10 HEPES, 2 MgATP, 0.3 Na3GTP, 5 QX314Br, pH 7.2 (adjusted with CsOH), osmolarity 285–290 mOsm. The order of DH and VH recordings was randomized. The experimenter was not blinded to treatment due to the pre−/post-application design. Data collection began approximately 7–10 min after achieving whole-cell configuration. sIPSCs were recorded for ≥ 5 min after baseline stabilization and ≥ 8 min during GLP-1 application. Picrotoxin (100 μM) was applied to block GABAAR and reveal extrasynaptic tonic currents. Kynurenic acid (3 mM) was added to block glutamatergic synaptic transmission. Voltage-clamp current recordings were made at −60 mV holding potential, filtered at 2 kHz using a Multipatch 700B amplifier and Axon Digidata board 1550A, controlled by pCLAMP 10.5 software (Axon Instruments, Molecular Devices, CA, USA).
Drugs
GLP-1 (7-36) amide, human was purchased from Anaspec (AS-22462, Anaspec Europe, Belgium); other chemicals were from Sigma-Aldrich (Steinheim, Germany).
GLP-1 lyophilized powder was reconstituted in distilled water as a stock solution, aliquoted, and stored at −20 °C. Each aliquot was then thawed once and diluted in ACSF immediately before use.
Data analysis
The currents were analyzed as described previously (Netsyk et al., 2020; Netsyk et al., 2025). The membrane capacitance of DG granule cells in the DH was significantly lower than in the VH (DH, 54.1 ± 3.6 pF, n = 9; VH, 68.9 ± 4.9 pF, n = 7; unpaired Student’s t test, p = 0.026), consistent with our previous study (Netsyk et al., 2020). The average access resistance (Ra) did not differ in DG granule cells between DH and VH (DH, 42.33 ± 3 0.84 MΩ, n = 9; VH, 38.94 ± 6.45 MΩ, n = 7; unpaired Student’s t test, p = 0.64). Ra was monitored throughout each recording, and recordings with >25% change in Ra were excluded from analysis. Briefly, sIPSCs were analyzed using MiniAnalysis software 6.0 (Synaptosoft, Decatur, GA, USA). sIPSC events were detected if larger than a threshold value set as 5xRMS (root-mean-square of the baseline noise) and visually inspected. RMS baseline noise was similar in DG granule cell recordings from both DH and VH (DH, 1.85 ± 0.14 pA, n = 9; VH, 1.99 ± 0.076 pA, n = 7, unpaired Student’s t test, p = 0.41). A 3–5 min segment was used for analysis. sIPSC parameters (frequency, median amplitude, 10–90% median rise time, 63% median decay time and median charge transfer) were automatically analyzed by the MiniAnalysis software. sIPSC with 10–90% rise times ≤ 5 ms were classified as fast; > 5 ms as slow (Figure 1D and Supplementary Figures 1A,B) (Netsyk et al., 2025). Tonic currents were analyzed using pCLAMP 10.5 software (Axon Instruments, Molecular Devices, San Jose, CA, USA). To determine baseline current amplitude, Gaussian fits were performed on all-points histograms derived from baseline current segments that were free of sIPSC (Figures 1B, 3D). The extrasynaptic tonic current amplitude was quantified as the shift of the baseline current after application of picrotoxin (Jin et al., 2011).
Statistics
Data were analyzed using GraphPad Prism 10 (GraphPad Software La Jolla, CA, USA). Normality was assessed with the Shapiro–Wilk test. Paired comparisons used Student’s t-test (normal data) or Wilcoxon signed-rank test (non-normal data). p-value <0.05 was considered statistically significant.
Results
GABA activates GABAARs to mediate various forms of inhibitory currents with specialized functional roles, including phasic currents (fast and slow sIPSCs), and extrasynaptic tonic currents. In the hippocampus, fast and slow sIPSCs are mainly evoked by GABA release from presynaptic fast-spiking interneurons and neurogliaform/Ivy cells (via volume transmission), respectively (Figure 1A) (Armstrong et al., 2012; Capogna and Pearce, 2011; Netsyk et al., 2025). Slow sIPSCs can also result from distal inputs targeting granule-cell dendrites (e.g., somatostatin-expressing interneurons, such as hilar perforant path-associated cells), where electrotonic filtering and spatial attenuation prolong rise and decay time. Outside the synapses, ambient GABA can activate high affinity extrasynaptic GABAARs, which generates persistent tonic currents (Figure 1A) (Bai et al., 2001).
Here, we investigated the effects of GLP-1 on the three types of GABAAR-mediated currents in mouse DG granule cells from the ventral and dorsal hippocampus. We used a low, physiologically relevant concentration of GLP-1 (100 pM), which we had previously shown to effectively modulate GABA signaling (Korol et al., 2015). Figures 1B,C illustrate typical GABA-activated currents and the effect of GLP-1 on DG granule cells from ventral mouse hippocampus. The characteristic sIPSCs were abolished by picrotoxin (100 μM), a GABAAR open-channel blocker, and the holding current shifted, revealing the extrasynaptic, tonic GABA-activated current present in the DG granule cells. In ventral hippocampal DG granule cells, analysis of phasic currents (fast and slow sIPSCs) revealed no changes in the frequency, median amplitude, 10–90% rise time, 63% decay time, charge transfer or total current following GLP-1 application (Figures 2A–C; Table 1). However, GLP-1 consistently enhanced the extrasynaptic tonic current (Figures 1B, 2D) in these cells (Paired Student’s t test, n = 6, t = 3.885, df = 5, 95% CI 0.01134 to 0.05569, p = 0.0116). In contrast, neither phasic currents (fast and slow sIPSCs) (Figures 3A–C; Table 1) nor tonic currents (Figures 3D,E) were affected by GLP-1 in dorsal hippocampal DG granule cells. These findings demonstrate that GLP-1 can enhance GABA-activated currents in the hippocampus, but this effect is dependent on the subcellular location and hippocampal axis location.
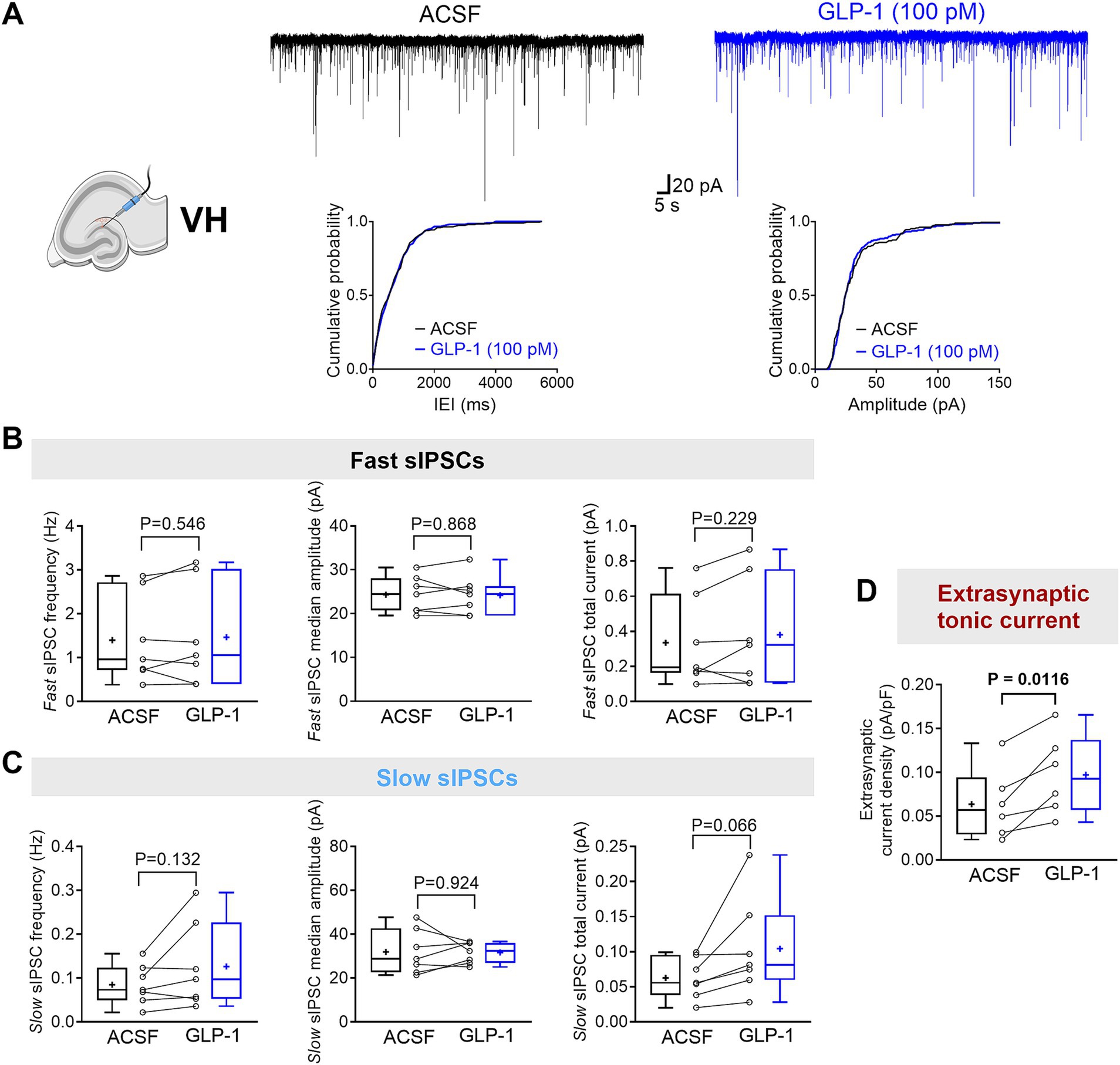
Figure 2. GLP-1 selectively potentiates GABAAR-mediated extrasynaptic tonic currents in dentate gyrus granule cells of the mouse ventral hippocampus. (A) The segments of representative current traces recorded from a DG granule cell in the ventral hippocampus (VH) before and after GLP-1 (100 pM) application. Cumulative probability plots for the inter-event interval (IEI) and median amplitude of fast IPSCs are shown below. ACSF, artificial cerebrospinal fluid. (B,C) Summary statistics for frequency, median amplitude, and total current of fast IPSC (B) and slow IPSC (C) (n = 7 from 5 mice). (D) The GABAAR-mediated extrasynaptic tonic current density was significantly increased after GLP-1 application (n = 6 from 5 mice). Data are presented as individual values with paired lines (before and after GLP-1 application), and box and whisker plots (whiskers defined by Tukey’s method). Mean values are denoted by “+.” All datasets passed the Shapiro–Wilk normality test. Statistical analysis used paired Student’s t-test, with p < 0.05 considered statistically significant.
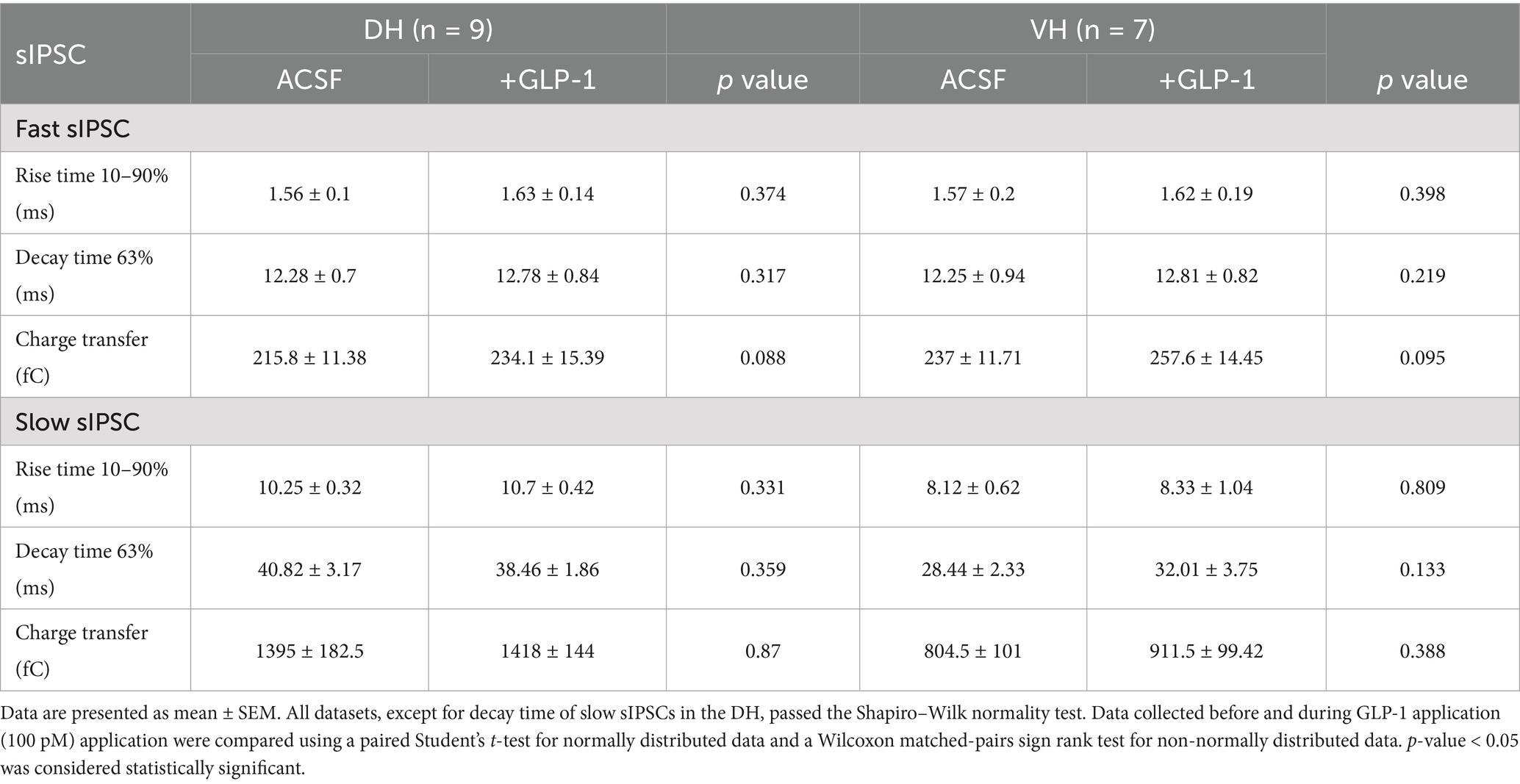
Table 1. GLP-1 effect on GABA-mediated fast and slow IPSC parameters in the dorsal and ventral hippocampal DG granule cells.
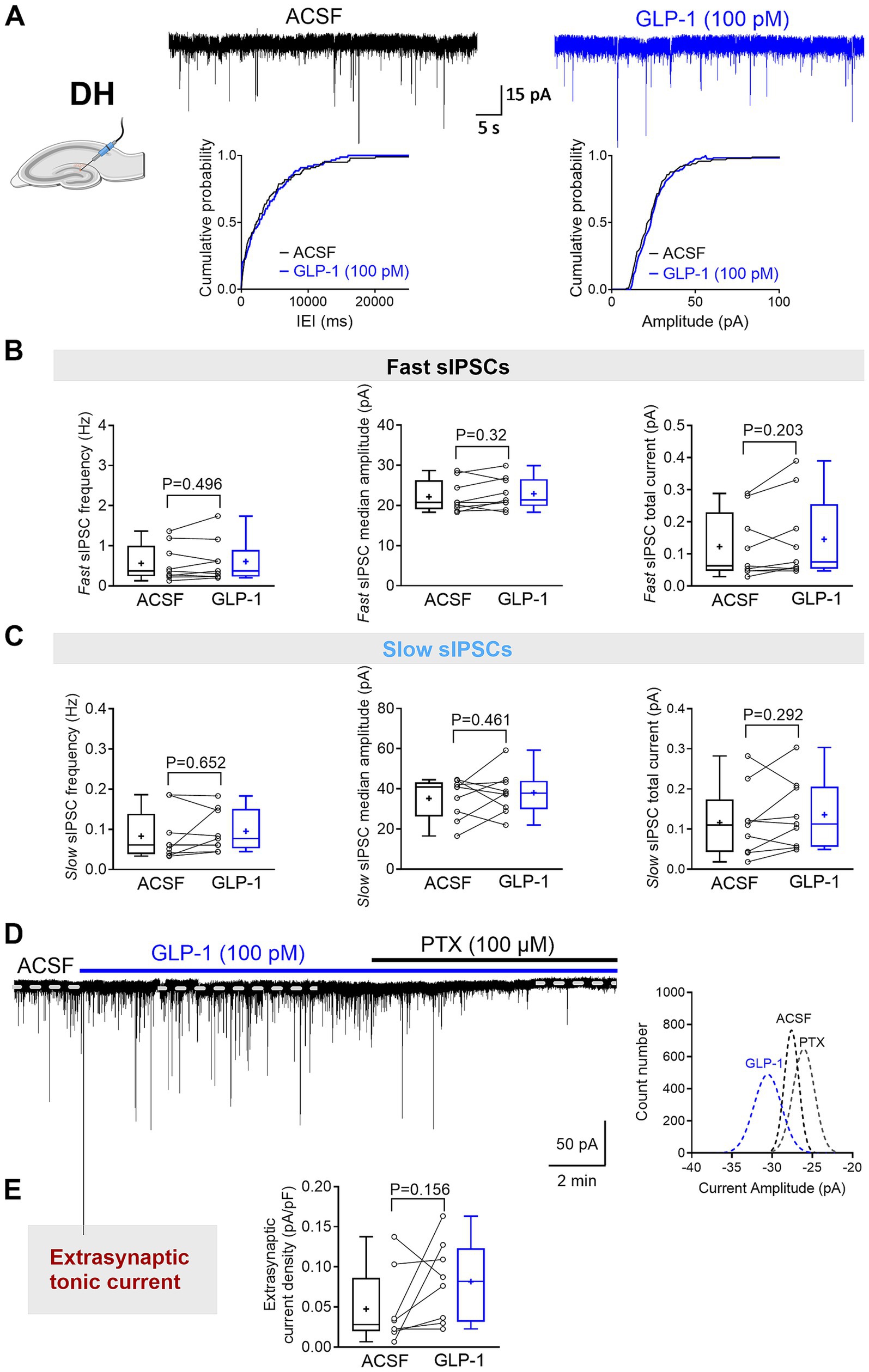
Figure 3. GLP-1 does not affect GABAAR-mediated currents in dentate gyrus granule cells of the mouse dorsal hippocampus. (A) The segments of representative current traces recorded from a DG granule cell in the dorsal hippocampus (DH) before and after GLP-1 (100 pM) application. Cumulative probability plots for the inter-event interval (IEI) and median amplitude of fast IPSCs are shown below. ACSF, artificial cerebrospinal fluid. (B,C) Summary statistics for frequency, median amplitude, and total current of fast IPSC (B) and slow IPSC (C) (n = 9 from 6 mice). (D) A representative current trace recorded from a DG granule cell in the DH before and after GLP-1 (100 pM) application. The difference between the dashed lines represents the extrasynaptic tonic current amplitude, estimated from Gaussian fits to all-points histograms derived from sIPSC-free baseline segments (right panel). (E) The GABAAR-mediated extrasynaptic tonic current density was not changed during GLP-1 application (n = 8 from 5 mice). Data are presented as individual values with paired lines (before and after GLP-1 application), and box and whisker plots (whiskers defined by Tukey’s method). Mean values are denoted by “+.” Only the median amplitude and total current of slow sIPSCs passed the Shapiro–Wilk normality test; all other datasets failed. For statistical analysis, a paired Student’s t-test was applied for normally distributed data, while a Wilcoxon matched-pairs sign rank test was used for non-normally distributed data. p < 0.05 was considered statistically significant.
Discussion
The hippocampus is a well-known brain structure required for learning and memory, with a particularly critical role in spatial navigation (Strange et al., 2014; Decarie-Spain et al., 2022). Importantly, the hippocampus is increasingly recognized to participate in modulation of metabolic regulation and homeostasis (Gupta et al., 2023; Lathe, 2001; Ferrario and Reagan, 2018; Hammoud et al., 2021; Netsyk et al., 2025). In DG granule cells from 2-month-old mouse hippocampus, GLP-1 only consistently enhanced the extrasynaptic GABA-activated currents in the ventral hippocampus and did not modulate the GABAergic phasic currents recorded in these cells, neither in the dorsal nor in the ventral hippocampus. Our results demonstrate selected effects of GLP-1 on mouse hippocampal GABA-activated signal transmission.
The hippocampus is a lamellar structure that is organized along the longitudinal, dorsal-ventral axis into functional domains (Papatheodoropoulos, 2018; Strange et al., 2014; Papatheodoropoulos, 2015). A variety of hormone receptors are expressed in the hippocampus (Lathe, 2001) but, receptors associated with feeding are in higher density in the ventral as compared to the dorsal hippocampus (Kanoski and Grill, 2017), including the GLP-1 receptors. The precise distribution pattern of the GLP-1 receptors varies somewhat between different species (Gupta et al., 2023; Cork et al., 2015; Graham et al., 2020; Jensen et al., 2018; Merchenthaler et al., 1999). GLP-1 releasing neurons from the nucleus of the solitary tract (NTS) do not directly innervate the hippocampus, raising the question of GLP-1’s origins in the hippocampus. But although, the hippocampus lacks GLP-1-containing axon terminals, GLP-1 has been detected in the hippocampus both in humans (Gupta et al., 2023) and in rodents (Hsu et al., 2015; Kastin et al., 2002). The GLP-1 presumably enters the hippocampal parenchyma by volume transmission from the cerebrospinal fluid or from the circulation (Gupta et al., 2023; Buller and Blouet, 2024; Hsu et al., 2015; Kastin et al., 2002). GLP-1 has been shown to enhance release of the neurotransmitters GABA or glutamate by presynaptic mechanism, but also, potentiate the GABA-activated currents in dorsal rat hippocampal neurons by a postsynaptic mechanism (Korol et al., 2015; Korol et al., 2015; Mietlicki-Baase et al., 2014; Rebosio et al., 2018; Shao et al., 2026; Wang et al., 2023). Although the GLP-1 receptor is not detected in interneurons of mouse DG (Jensen et al., 2018), it is enriched in glutamatergic mossy cells of the ventral DG, which innervate interneurons (Steiner et al., 2022). Activation of the GLP-1 receptor increases the action potential firing frequency of mossy cells, potentially leading to an indirect enhancement of GABA release from interneurons (Steiner et al., 2022). However, GLP-1 does not alter the frequency and amplitude of phasic inhibitory currents (fast and slow sIPSCs), which reflect presynaptic GABA release. This suggests that GLP-1 is unlikely to change the ambient GABA levels through spillover. Therefore, in the current study, only postsynaptic mechanism and only in the ventral DG granule cells were activated by GLP-1. This is in accordance with a study on mouse brains where the GLP-1 receptor was expressed in mature granule neurons (Graham et al., 2020). Enhanced tonic inhibition by GLP-1 in the ventral hippocampus decreases the excitability of the DG granule cells at this location.
Metabolic hormones have emerged as significant biological regulators of hippocampal functions. Hippocampal neuronal outputs map onto the hypothalamus in a topographical manner via neurons in the septum and commonly result in inhibition of hypothalamic activity (Risold and Swanson, 1996; Decarie-Spain et al., 2022; Arszovszki et al., 2014). Recent studies have identified the importance of the ventral hippocampus in regulating feeding behavior, food intake and food-directed memory (Hsu et al., 2015; Decarie-Spain et al., 2022; Hsu et al., 2018). The current results add to the mounting evidence of the functional variation between the dorsal and the ventral hippocampus. The differential effects of GLP-1 in the dorsal and ventral DG granule neurons indicates a distinct role of GLP-1 in these hippocampal regions.
This study has several limitations. First, the use of specific GLP-1R antagonists or conditional, region-specific GLP-1R knockout mouse models is needed to confirm that the observed effects on GABAergic transmission are mediated by GLP-1Rs rather than off-target actions. Second, sample sizes were relatively small and should be increased in future studies. Third, only male mice were used; including female mice will be important to assess potential sex-dependent difference. Finally, all experiments were performed at room temperature, whereas repeating them at physiological temperature (32–37 °C) would provide a more accurate reflection of in vivo conditions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Uppsala Animal Ethical Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
ON: Conceptualization, Writing – review & editing, Investigation, Formal analysis, Methodology, Visualization. SVK: Visualization, Formal analysis, Writing – review & editing, Supervision. BB: Formal analysis, Project administration, Conceptualization, Supervision, Visualization, Writing – original draft, Funding acquisition, Resources, Writing – review & editing. ZJ: Visualization, Conceptualization, Funding acquisition, Writing – original draft, Resources, Formal analysis, Supervision, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by Swedish Research Council grants 2018-02952 and 2015-02417 to BB, Excellence of Diabetes Research in Sweden (EXODIAB) to BB and ZJ, and Gun och Bertil Stohnes Stiftelse (2024) and O.E. och Edla Johanssons vetenskapliga Stiftelse (2024) to ZJ.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fncel.2025.1638550/full#supplementary-material
References
Armstrong, C., Krook-Magnuson, E., and Soltesz, I. (2012). Neurogliaform and ivy cells: a major family of nNOS expressing GABAergic neurons. Front Neural Circuits 6:23. doi: 10.3389/fncir.2012.00023
Arszovszki, A., Borhegyi, Z., and Klausberger, T. (2014). Three axonal projection routes of individual pyramidal cells in the ventral CA1 hippocampus. Front. Neuroanat. 8:53. doi: 10.3389/fnana.2014.00053
Astrup, A. (2024). Reflections on the discovery GLP-1 as a satiety hormone: implications for obesity therapy and future directions. Eur. J. Clin. Nutr. 78, 551–556. doi: 10.1038/s41430-024-01460-6
Babateen, O., Korol, S. V., Jin, Z., Bhandage, A. K., Ahemaiti, A., and Birnir, B. (2017). Liraglutide modulates GABAergic signaling in rat hippocampal CA3 pyramidal neurons predominantly by presynaptic mechanism. BMC Pharmacol. Toxicol. 18:83. doi: 10.1186/s40360-017-0191-0
Bai, D., Zhu, G., Pennefather, P., Jackson, M. F., MacDonald, J. F., and Orser, B. A. (2001). Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(a) receptors in hippocampal neurons. Mol. Pharmacol. 59, 814–824. doi: 10.1124/mol.59.4.814
Buller, S., and Blouet, C. (2024). Brain access of incretins and incretin receptor agonists to their central targets relevant for appetite suppression and weight loss. Am. J. Physiol. Endocrinol. Metab. 326, E472–E480. doi: 10.1152/ajpendo.00250.2023
Capogna, M., and Pearce, R. A. (2011). GABA a,slow: causes and consequences. Trends Neurosci. 34, 101–112. doi: 10.1016/j.tins.2010.10.005
Chandra, D., Jia, F., Liang, J., Peng, Z., Suryanarayanan, A., Werner, D. F., et al. (2006). GABAA receptor alpha 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc. Natl. Acad. Sci. USA 103, 15230–15235. doi: 10.1073/pnas.0604304103
Cork, S. C., Richards, J. E., Holt, M. K., Gribble, F. M., Reimann, F., and Trapp, S. (2015). Distribution and characterisation of glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol. Metab. 4, 718–731. doi: 10.1016/j.molmet.2015.07.008
Dash, S. (2024). Opportunities to optimize lifestyle interventions in combination with glucagon-like peptide-1-based therapy. Diabetes Obes. Metab. 26, 3–15. doi: 10.1111/dom.15829
Decarie-Spain, L., Liu, C. M., Lauer, L. T., Subramanian, K., Bashaw, A. G., Klug, M. E., et al. (2022). Ventral hippocampus-lateral septum circuitry promotes foraging-related memory. Cell Rep. 40:111402. doi: 10.1016/j.celrep.2022.111402
Ferrario, C. R., and Reagan, L. P. (2018). Insulin-mediated synaptic plasticity in the CNS: anatomical, functional and temporal contexts. Neuropharmacology 136, 182–191. doi: 10.1016/j.neuropharm.2017.12.001
Graham, D. L., Durai, H. H., Trammell, T. S., Noble, B. L., Mortlock, D. P., Galli, A., et al. (2020). A novel mouse model of glucagon-like peptide-1 receptor expression: a look at the brain. J. Comp. Neurol. 528, 2445–2470. doi: 10.1002/cne.24905
Gupta, T., Kaur, M., Shekhawat, D., Aggarwal, R., Nanda, N., and Sahni, D. (2023). Investigating the glucagon-like Peptide-1 and its receptor in human brain: distribution of expression, functional implications, age-related changes and species specific characteristics. Basic Clin. Neurosci. 14, 341–354. doi: 10.32598/bcn.2021.2554.2
Hammoud, H., Netsyk, O., Tafreshiha, A. S., Korol, S. V., Jin, Z., Li, J. P., et al. (2021). Insulin differentially modulates GABA signalling in hippocampal neurons and, in an age-dependent manner, normalizes GABA-activated currents in the tg-APPSwe mouse model of Alzheimer's disease. Acta Physiol (Oxf.) 232:e13623. doi: 10.1111/apha.13623
Holscher, C. (2022). Glucagon-like peptide 1 and glucose-dependent insulinotropic peptide hormones and novel receptor agonists protect synapses in Alzheimer's and Parkinson's diseases. Front Synaptic Neurosci. 14:955258. doi: 10.3389/fnsyn.2022.955258
Holst, J. J. (2024). GLP-1 physiology in obesity and development of incretin-based drugs for chronic weight management. Nat. Metab. 6, 1866–1885. doi: 10.1038/s42255-024-01113-9
Hsu, T. M., Hahn, J. D., Konanur, V. R., Lam, A., and Kanoski, S. E. (2015). Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology 40, 327–337. doi: 10.1038/npp.2014.175
Hsu, T. M., Noble, E. E., Liu, C. M., Cortella, A. M., Konanur, V. R., Suarez, A. N., et al. (2018). A hippocampus to prefrontal cortex neural pathway inhibits food motivation through glucagon-like peptide-1 signaling. Mol. Psychiatry 23, 1555–1565. doi: 10.1038/mp.2017.91
Jensen, C. B., Pyke, C., Rasch, M. G., Dahl, A. B., Knudsen, L. B., and Secher, A. (2018). Characterization of the glucagonlike Peptide-1 receptor in male mouse brain using a novel antibody and in situ hybridization. Endocrinology 159, 665–675. doi: 10.1210/en.2017-00812
Jin, Z., Jin, Y., and Birnir, B. (2011). GABA-activated single-channel and tonic currents in rat brain slices. J. Vis. Exp. 53:2858. doi: 10.3791/2858-v
Kanoski, S. E., and Grill, H. J. (2017). Hippocampus contributions to food intake control: mnemonic, neuroanatomical, and endocrine mechanisms. Biol. Psychiatry 81, 748–756. doi: 10.1016/j.biopsych.2015.09.011
Kanoski, S. E., Hayes, M. R., and Skibicka, K. P. (2016). GLP-1 and weight loss: unraveling the diverse neural circuitry. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R885–R895. doi: 10.1152/ajpregu.00520.2015
Kastin, A. J., Akerstrom, V., and Pan, W. (2002). Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J. Mol. Neurosci. 18, 07–13. doi: 10.1385/JMN:18:1-2:07
Korol, S. V., Jin, Z., Babateen, O., and Birnir, B. (2015). GLP-1 and exendin-4 transiently enhance GABAA receptor-mediated synaptic and tonic currents in rat hippocampal CA3 pyramidal neurons. Diabetes 64, 79–89. doi: 10.2337/db14-0668
Korol, S. V., Jin, Z., and Birnir, B. (2015). The GLP-1 receptor agonist Exendin-4 and diazepam differentially regulate GABAA receptor-mediated tonic currents in rat hippocampal CA3 pyramidal neurons. PLoS One 10:e0124765. doi: 10.1371/journal.pone.0124765
Larsen, P. J., Tang-Christensen, M., Holst, J. J., and Orskov, C. (1997). Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77, 257–270. doi: 10.1016/S0306-4522(96)00434-4
Lathe, R. (2001). Hormones and the hippocampus. J. Endocrinol. 169, 205–231. doi: 10.1677/joe.0.1690205
Liang, Y., Dore, V., Rowe, C. C., and Krishnadas, N. (2024). Clinical evidence for GLP-1 receptor agonists in Alzheimer's disease: a systematic review. J. Alzheimers Dis. Rep. 8, 777–789. doi: 10.3233/ADR-230181
Merchenthaler, I., Lane, M., and Shughrue, P. (1999). Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J. Comp. Neurol. 403, 261–280. doi: 10.1002/(SICI)1096-9861(19990111)403:2<261::AID-CNE8>3.0.CO;2-5
Mietlicki-Baase, E. G., Ortinski, P. I., Reiner, D. J., Sinon, C. G., McCutcheon, J. E., Pierce, R. C., et al. (2014). Glucagon-like peptide-1 receptor activation in the nucleus accumbens core suppresses feeding by increasing glutamatergic AMPA/kainate signaling. J. Neurosci. 34, 6985–6992. doi: 10.1523/JNEUROSCI.0115-14.2014
Muller, T. D., Finan, B., Bloom, S. R., D'Alessio, D., Drucker, D. J., Flatt, P. R., et al. (2019). Glucagon-like peptide 1 (GLP-1). Mol. Metab. 30, 72–130. doi: 10.1016/j.molmet.2019.09.010
Netsyk, O., Hammoud, H., Korol, S. V., Jin, Z., Tafreshiha, A. S., and Birnir, B. (2020). Tonic GABA-activated synaptic and extrasynaptic currents in dentate gyrus granule cells and CA3 pyramidal neurons along the mouse hippocampal dorsoventral axis. Hippocampus 30, 1146–1157. doi: 10.1002/hipo.23245
Netsyk, O., Korol, S. V., Li, J. P., Birnir, B., and Jin, Z. (2025). GABA-activated slow spontaneous inhibitory postsynaptic currents are decreased in dorsal hippocampal dentate gyrus granule cells in an aged mouse model of Alzheimer's disease. J Alzheimer's Dis 104, 420–428. doi: 10.1177/13872877251317608
Otis, T. S., Staley, K. J., and Mody, I. (1991). Perpetual inhibitory activity in mammalian brain slices generated by spontaneous GABA release. Brain Res. 545, 142–150. doi: 10.1016/0006-8993(91)91280-E
Papatheodoropoulos, C. (2015). Striking differences in synaptic facilitation along the dorsoventral axis of the hippocampus. Neuroscience 301, 454–470. doi: 10.1016/j.neuroscience.2015.06.029
Papatheodoropoulos, C. (2018). Electrophysiological evidence for long-axis intrinsic diversification of the hippocampus. Front. Biosci. (Landmark Ed). 23, 109–145. doi: 10.2741/4584
Paxinos, G., and Watson, C. (1986). The rat brain in stereotaxic coordinates. 2nd Edn. San Diego, CA: Academic Press.
Rebosio, C., Balbi, M., Passalacqua, M., Ricciarelli, R., and Fedele, E. (2018). Presynaptic GLP-1 receptors enhance the depolarization-evoked release of glutamate and GABA in the mouse cortex and hippocampus. Biofactors 44, 148–157. doi: 10.1002/biof.1406
Risold, P. Y., and Swanson, L. W. (1996). Structural evidence for functional domains in the rat hippocampus. Science 272, 1484–1486. doi: 10.1126/science.272.5267.1484
Semyanov, A., Walker, M. C., and Kullmann, D. M. (2003). GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat. Neurosci. 6, 484–490. doi: 10.1038/nn1043
Shao, Y. Q., Wang, Y. C., Wang, L., Ruan, H. Z., Liu, Y. F., Zhang, T. H., et al. (2026). Topical administration of GLP-1 eyedrops improves retinal ganglion cell function by facilitating presynaptic GABA release in early experimental diabetes. Neural Regen. Res. 21, 800–810. doi: 10.4103/NRR.NRR-D-24-00001
Sieghart, W., and Savic, M. M. (2018). International Union of Basic and Clinical Pharmacology. CVI: GABA(a) receptor subtype- and function-selective ligands: key issues in translation to humans. Pharmacol. Rev. 70, 836–878. doi: 10.1124/pr.117.014449
Steiner, A., Owen, B. M., Bauer, J. P., Seanez, L., Kwon, S., Biddinger, J. E., et al. (2022). Glucagon-like peptide-1 receptor differentially controls mossy cell activity across the dentate gyrus longitudinal axis. Hippocampus 32, 797–807. doi: 10.1002/hipo.23469
Stell, B. M., Brickley, S. G., Tang, C. Y., Farrant, M., and Mody, I. (2003). Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc. Natl. Acad. Sci. USA 100, 14439–14444. doi: 10.1073/pnas.2435457100
Strange, B. A., Witter, M. P., Lein, E. S., and Moser, E. I. (2014). Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci. 15, 655–669. doi: 10.1038/nrn3785
Swanson, L. W., and Cowan, W. M. (1977). An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J. Comp. Neurol. 172, 49–84. doi: 10.1002/cne.901720104
Ting, J. T., Daigle, T. L., Chen, Q., and Feng, G. (2014). Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol. Biol. 1183, 221–242. doi: 10.1007/978-1-4939-1096-0_14
Ugleholdt, R., Zhu, X., Deacon, C. F., Orskov, C., Steiner, D. F., and Holst, J. J. (2004). Impaired intestinal proglucagon processing in mice lacking prohormone convertase 1. Endocrinology 145, 1349–1355. doi: 10.1210/en.2003-0801
Keywords: GABA, inhibition, GABAA receptor, glucagon-like peptide-1, hormone
Citation: Netsyk O, Korol SV, Birnir B and Jin Z (2025) GLP-1 selectively enhances tonic GABAA receptor-mediated currents in mouse dentate gyrus granule cells of the ventral hippocampus. Front. Cell. Neurosci. 19:1638550. doi: 10.3389/fncel.2025.1638550
Edited by:
Grzegorz Hess, Jagiellonian University, PolandReviewed by:
Joanna Urban-Ciecko, Polish Academy of Sciences, PolandXinyan Li, Huazhong University of Science and Technology, China
Copyright © 2025 Netsyk, Korol, Birnir and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhe Jin, emhlLmppbkBtY2IudXUuc2U=
†Present address: Olga Netsyk, Department of Biochemistry and Biophysics, Stockholm University, Stockholm, Sweden
 Olga Netsyk
Olga Netsyk Sergiy V. Korol
Sergiy V. Korol Bryndis Birnir
Bryndis Birnir Zhe Jin
Zhe Jin