- 1The Graduate School, Heilongjiang University of Chinese Medicine, Harbin, Heilongjiang, China
- 2The First Affiliated Hospital of Heilongjiang University of Chinese Medicine, Harbin, China
Background: Major depressive disorder (MDD) is a common chronic psychiatric disorder that affects individuals of all ages worldwide, causing significant impairment to patients’ physical and mental health as well as social functioning. Vascular endothelial growth factor (VEGF), traditionally recognized as a regulator of angiogenesis and vascular permeability, has been identified in recent studies to possess neurotrophic and neuroprotective potential in the central nervous system (CNS) and is implicated in the pathological processes of MDD.
Aim: To systematically elaborate on the role of VEGF in the pathological mechanisms of MDD and its potential as a target for antidepressant therapy.
Key findings: Through interactions with its receptors (VEGFR1, VEGFR2, and VEGFR3), VEGF regulates critical pathways such as gene expression, blood-brain barrier (BBB) function, and brain-derived neurotrophic factor (BDNF), thereby establishing physiological and pathological associations with MDD. Its signaling pathway serves as a core target for various antidepressant treatments, including conventional antidepressants, ketamine, electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation (rTMS), and resolvins. Short-term upregulation of central VEGF may exert antidepressant effects by promoting the benign remodeling of neurovascular networks, and its subsequent return to baseline levels during treatment can avoid BBB damage, providing novel insights for the management of rapid-onset and treatment-resistant depression.
Conclusion: Vascular endothelial growth factor holds significant importance in the pathology and treatment of MDD. In-depth exploration of its regulatory mechanisms may provide a basis for the development of novel antidepressant therapies.
1 Introduction
Major depressive disorder (MDD) is a common mental disorder with a chronic course, characterized primarily by persistent and uncontrollable mood disturbances and cognitive impairments. It is often accompanied by fatigue, diminished interest, loss of pleasure, and suicidal ideation, affecting over 300 million people worldwide (GBD 2021 Diseases and Injuries Collaborators, 2024; Meng and Wang, 2023). The World Health Organization notes that MDD is one of the leading causes of mental and physical disability globally. Over the past half-century, the number of people with depression worldwide has increased by nearly 50% (Liu et al., 2020); notably, 28% of new cases globally emerged in 2020 alone, with a marked rise in prevalence in countries severely affected by COVID-19, where females and young people are the most impacted groups (COVID-19 Mental Disorders Collaborators, 2021). According to statistics published in 2023, the economic costs attributed to MDD in the United States alone have increased by 48% compared to previous periods (Greenberg et al., 2023). MDD has thus become a major threat to global health, with its economic and societal impacts continuing to intensify (Monroe and Harkness, 2022).
Many complex pathological processes are involved in the development and progression of MDD, such as genes, inflammation, mitochondrial dysfunction, oxidative stress, and the HPA axis (Dean and Keshavan, 2017; Fries et al., 2023). Neurotrophic factors play a key role in normal brain development and function, and the neurotrophic hypothesis of MDD focuses on fluctuations in neurotrophic factors within the center with changes in depressive symptoms, suggesting that impaired neurotrophic support is a key mechanism for MDD-associated neurological activity and brain-related changes (Kishi et al., 2017). Recent data suggest that levels of the key protein Vascular Endothelial Growth Factor (VEGF) may be an important marker of MDD and that its alteration is necessary to promote the pathologic progression of MDD (Annam et al., 2024). In turn, traditional antidepressant drugs are required to alleviate depressive symptoms with the help of VEGF signaling to exert their pharmacological effects (Warner-Schmidt and Duman, 2008). Thus, VEGF is a key player in the pathogenesis, amelioration, and treatment of depression, and the detection and targeting of VEGF may provide new ideas for the clinical prevention and treatment of MDD.
2 Mechanism of action of VEGF
2.1 VEGF and the receptor family
Vascular endothelial growth factor was initially discovered and named for its roles in regulating vascular permeability and promoting angiogenesis (Dvorak, 2006). As a key signaling molecule, VEGF is widely expressed throughout the body; it is not only present in neurons, astrocytes, and vascular endothelial cells within the central nervous system (Jais et al., 2016) but also produced by various peripheral cell types, including epithelial cells, smooth muscle cells, fibroblasts, macrophages, tumor cells, and retinal pigment epithelial cells (Preusser et al., 2012; Wang et al., 2025; Wartiovaara et al., 1998). This broad cellular source underpins VEGF’s central role in systemic physiological and pathological processes. Its classical functions include significantly increasing vascular permeability, facilitating extracellular matrix remodeling, stimulating the migration and proliferation of vascular endothelial cells, and ultimately driving the formation of new blood vessels (Connolly et al., 1989; Ferrara and Henzel, 1989; Senger et al., 1983).
Within the central nervous system (CNS), in addition to its involvement in regulating blood-brain barrier (BBB) function and angiogenesis, VEGF exhibits important neurotrophic and neuroprotective properties (Rosenstein et al., 2003). Through interactions with specific receptors on endothelial cells, neurons, and glial cells, it activates downstream signaling pathways and influences gene expression. These actions collectively promote neuronal survival, the proliferation of neural progenitor cells, and the self-renewal of embryonic and adult neural stem cells, thereby exerting a significant impact on neuronal plasticity and neurogenesis (Shen et al., 2004; Tillo et al., 2012).
The secreted glycoprotein family to which VEGF belongs contains six members: VEGF-B, -C, -D, -E, and placental-derived growth factor (PlGF). VEGF-A is the prototype member of this family. Among the four identified splicing variants of VEGF-A, VEGF120, which is early-active, and VEGF164, which is upregulated in adulthood, are the primary brain subtypes. The receptors that can specifically bind to VEGF are called vascular endothelial growth factor receptors (VEGFRs), including VEGFR-1 (Flt-1), VEGFR-2 (Flk-1), and VEGFR-3 (Flt-4). VEGFRs have expression specificity at different developmental stages and in different brain regions (de Vries et al., 1992; Licht and Keshet, 2013). In addition, NRP-1 and NRP-2 of the neuropilin family (NRP) can also bind to VEGF as co-receptors. Different members of VEGF bind to their specific receptors to exert various functions (Dakowicz et al., 2022). Binding VEGF-A, VEGF-B, and PlGF to VEGFR-1 can promote angiogenesis, hematopoiesis, and inflammatory cell recruitment. VEGFR-2 binds to VEGF-A with low affinity and can bind to proteolytically processed VEGF-C and VEGF-D, playing important roles in maintaining endothelial cell proliferation, blood-brain barrier permeability, neuronal cell survival and metastasis, and axon guidance. The binding of VEGFR-3 to VEGF-C and VEGF-D is a key regulatory factor for lymphatic endothelial function (Koch and Claesson-Welsh, 2012; Lange et al., 2016; Figure 1).
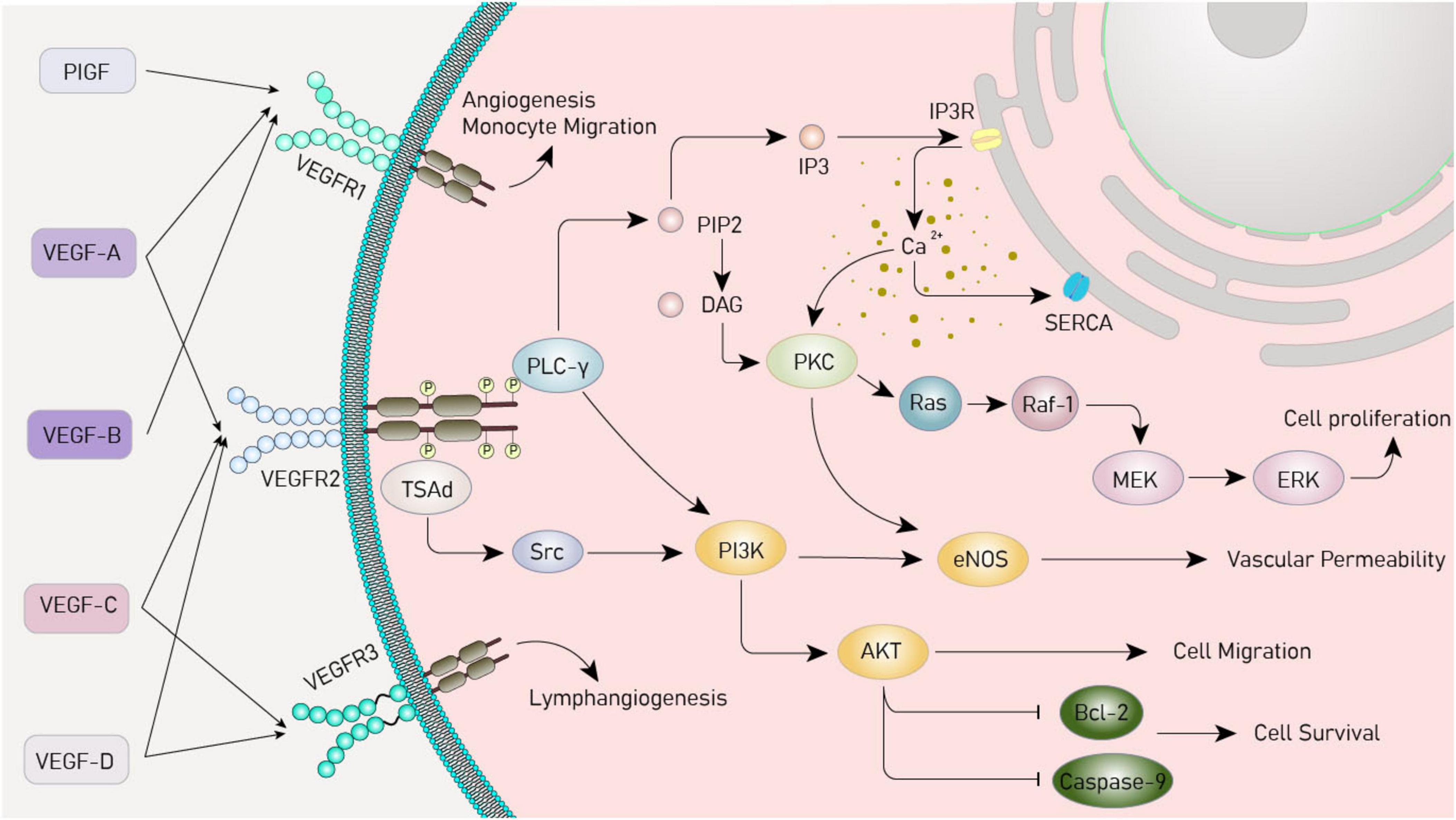
Figure 1. Vascular endothelial growth factor (VEGF) signaling pathway. VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; PIGF, placental growth factor; PI3K, phosphoinositide 3-kinases; Akt, protein kinase B; MAPK, mitogen-activated protein kinase; PLC-γ, phospholipase C-γ; PIP2, phosphatidylinositol 4,5-bisphosphate; DAG, diacylglycerol; IP3, inositol trisphosphate; IP3R, inositol trisphosphate receptor; SERCA, sarcoplasmic/endoplasmic reticulum calcium ATPase; PKC, protein kinase C; Ras, rat sarcoma virus oncogene homolog; Raf-1, rapidly accelerated fibrosarcoma 1; ERK, extracellular signal - regulated kinase; TsAd, T cell-specific adapter protein; Src, sarcoma gene; eNOS, endothelial nitric oxide synthase; Bcl-2, B-cell lymphoma/leukemia -2.
2.2 Signaling mechanisms of VEGF
Vascular endothelial growth factor plays an important physiological role by activating important downstream signaling pathways. The pathways include the PI3K/Akt, MAPK, and PLCγ. Activation of phosphatidylinositol 3-kinase (PI3K) by the VEGF receptor upregulates protein kinase B (Akt) protein, which inhibits apoptosis and promotes cell growth and survival (Jiang and Liu, 2008; Zachary, 2003) and the mitogen-activated protein kinase (MAPK) pathway involves a series of cascade reactions, which ultimately regulate the process of cell proliferation, migration, and survival and promote angiogenesis (Breslin et al., 2003; Estrada et al., 2019; Simons and Eichmann, 2015). In the phospholipase C-γ (PLCγ) pathway, activated PLCγ hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) on the cell membrane, generating the secondary messenger molecules diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), and elevating the intracellular calcium ion concentration (Chen and Simons, 2021; Sjöberg et al., 2023; Figure 1).
In the human brain, VEGF promotes neurogenesis, and its roles in protecting neural function and synaptic plasticity also rely on the aforementioned mechanisms. Studies have demonstrated that catalpol, an extract of traditional Chinese medicine, exerts effects such as promoting cortical axonal growth, improving the neuronal microenvironment, and inhibiting neuronal apoptosis by enhancing VEGF-PI3K/AKT and VEGF-MAPK/ERK signaling (Wang et al., 2019, 2022). Similarly, the neuroprotective effects of danshensu, including improving cerebral blood flow in the peri-infarct region and alleviating behavioral and cognitive deficits in mice after middle cerebral artery infarction, have been confirmed to be achieved by upregulating VEGF levels through the regulation of the PI3K/AKT/mTOR pathway. Furthermore, VEGF deficiency may induce progressive neuronal degeneration, and reduced neurovascular perfusion accelerates the progression of neurodegeneration. In vitro experiments have verified that even under conditions of stress and susceptibility genes, VEGF can prevent the downregulation of B-cell lymphoma/leukemia-2 (Bcl-2), inhibit neuronal apoptosis, delay motor neuron degeneration, and improve neuronal survival by increasing PI3K and MAPK activity (Li et al., 2003; Lunn et al., 2009; Oosthuyse et al., 2001).
It can be seen from this that the functions of VEGF are not merely confined to traditional angiogenesis and the regulation of vascular permeability. It is also of great significance in neuronal protection. However, assessing VEGF content remains a research topic that needs further exploration. Since the human body is susceptible to VEGF, the constancy of its level is necessary for maintaining the functions of endothelial cells and the BBB. Pathologically, increased VEGF can lead to enhanced vascular permeability and leakage, damage the integrity of the BBB, and trigger the onset of various neurological diseases (Ruiz de Almodovar et al., 2009).
2.3 The relationship between VEGF and MDD
Numerous studies, through animal experiments and clinical trials, have confirmed from multiple perspectives that VEGF level deficiency is associated with the pathogenesis of depression. A plasma comparison study involving 40 MDD patients and 40 healthy controls showed that peripheral VEGF levels were negatively correlated with the severity of MDD (Kotan et al., 2012). A recent study covering 469 MDD cases further revealed that plasma VEGF levels in patients during the episode were significantly lower than those in healthy controls, and remained lower even after antidepressant treatment (Rigal et al., 2020). Additionally, other studies have confirmed that reduced VEGF levels also exist in the CNS of MDD patients (Kranaster et al., 2019). Suicide, as the most severe consequence of MDD, has an incidence of suicidal ideation as high as 37% among patients (Navarro et al., 2023). Clinical data indicated that cerebrospinal fluid VEGF levels in suicide attempters were significantly lower than those in the control group, and such levels were significantly negatively correlated with the severity of depression assessed by the MADRS scale, which is consistent with previous research conclusions (Isung et al., 2012a,2012b). Furthermore, chronic stress is an important factor in inducing and exacerbating MDD. An animal experiment measured the surface area covered by the vasculature, the proportion of vessel-associated newborn cells, and analyzed the expression of VEGF and Flk-1 proteins in the hippocampus of rats in the control group, chronic stress group, and recovery group. The results showed that 32% of proliferating cells in the rat hippocampus were associated with blood vessels. After chronic stress, the levels of VEGF and Flk-1 proteins in the granular cell layer were significantly decreased (Heine et al., 2005). Low VEGF levels may reflect insufficient neurotrophic support and reduced neurogenesis in the CNS, leading to atrophy of limbic structures that regulate emotions, impaired synaptic plasticity, and subsequent depressive symptoms (Clark-Raymond et al., 2014; Vaseghi et al., 2023). Peripheral and central VEGF are both relatively independent and interact through complex mechanisms such as the HPA axis. Therefore, reduced peripheral VEGF levels may be a consequence of decreased central VEGF, which to some extent reflects abnormal central neuroplasticity in MDD patients (Cao et al., 2005; Mikulska et al., 2021).
The restoration of VEGF levels is closely associated with the improvement of MDD symptoms. The VEGF and FIk-1 signaling pathways are important routes for antidepressant treatment. The latest animal experiments show that encapsulated mesenchymal stem cells (eMSCs) can effectively alleviate the depressive symptoms of treatment-resistant depressed rats. The antidepressant effect of eMSCs is related to its up-regulation of VEGF and Flk-1 expression in the hippocampus (Kin et al., 2020). Moreover, knocking out VEGF in the dentate gyrus of the hippocampus of mice using specific viruses can reduce hippocampal neurogenesis and induce depressive-like behaviors in specific behavioral tests (forced swimming, novel feeding), and these changes are partially blocked by ketamine (Choi et al., 2016). Different types of antidepressants have the effect of promoting hippocampal cell proliferation and neurogenesis, which is considered to be one of the bases of their antidepressant effects (Kodama et al., 2004; Malberg et al., 2000). VEGF is an important mediator of the neurogenic and behavioral effects of multiple types of antidepressants. Four experimental models of depressive behaviors have demonstrated that traditional antidepressants such as selective serotonin reuptake inhibitors (SSRIs) like fluoxetine, tricyclic antidepressants (TCAs) like desipramine, and even electroconvulsive therapy (ECT) can increase VEGF mRNA in the granule cell layer of the hippocampus. Selective, potent inhibitor experiments further clarify that VEGF/Flk-1 signal transduction is indispensable for antidepressant-induced cell proliferation (Deyama and Kaneda, 2023; Warner-Schmidt and Duman, 2007).
Interestingly, in recent years, multiple studies have examined the association between VEGF levels in humans and the severity of MDD from various perspectives, with results showing heterogeneity compared to previous data. For instance, a study investigating changes in plasma VEGF protein concentrations in adolescents with MDD after short-term antidepressant treatment revealed that VEGF protein levels were abnormally elevated in these adolescents, and antidepressant treatment could downregulate such levels (Krivosova et al., 2023). Another study, which compared serum VEGF levels between MDD patients and healthy subjects, observed significantly increased VEGF mRNA levels and serum protein concentrations in the MDD group (Berent et al., 2014; Maes et al., 2024). Additionally, clinical studies have confirmed that plasma VEGF levels in MDD patients rise during acute episodes (Lee and Kim, 2012). Research exploring changes in VEGF levels from the perspective of astrocyte-derived extracellular vesicles (ADEVs) also indicated that baseline VEGF levels in plasma ADEVs of MDD patients were elevated (Li et al., 2025). Maintaining a constant low level of VEGF is necessary: insufficient VEGF levels can affect the integrity of the brain, particularly the mesolimbic structures, while abnormally elevated VEGF levels can promote BBB permeability and excessive formation of new leaky blood vessels (Wu et al., 2022; Zhang et al., 2000). Both scenarios may occur in MDD patients, contributing to the pathogenesis and progression of the disease. The reasons for such discrepancies may be multifaceted. For example, in patients with acute MDD, stress triggers inflammatory responses and transient activation of the HPA axis, leading to a compensatory increase in VEGF to maintain neurovascular stability (Furtado and Katzman, 2015). Furthermore, obese patients may exhibit elevated peripheral VEGF due to leptin resistance (Caroleo et al., 2019). Patient-specific factors (age, gender), sample types (plasma, serum), and disease states (acute phase, medication status, specific subtypes, comorbidities) may all be important factors mediating such deviations (Homorogan et al., 2021; Kirschner et al., 2011). Therefore, in formulating VEGF-targeted therapeutic regimens for depression, clarifying the extent to which confounding factors affect VEGF levels in MDD patients may be a key direction for future research.
3 The mechanism of the association between VEGF and MDD
3.1 Gene
Major depressive disorder is a familial disease with a heritability of approximately 30%–40% (Sullivan et al., 2000). The genetic traits of MDD are complex and subtle, influenced by multiple variations (Ripke et al., 2013). A meta-analysis of data from 807,553 individuals across multiple MDD genome-wide association studies (GWAS) identified 102 independent variants associated with MDD, involving 269 genes and 15 gene sets (Howard et al., 2019). Genetic associations with MDD have also been observed for gender, height, weight, and comorbidities (Badamasi et al., 2019; Labonté et al., 2017; Lango Allen et al., 2010; Speliotes et al., 2010). The VEGF-related single-nucleotide polymorphism (SNP) rs69994 is associated with treatment-resistant depression (Viikki et al., 2010). As the role of VEGF in the pathological mechanism of MDD is gradually revealed, how SNPs in its gene and receptors affect depression susceptibility, symptom severity, and treatment response by regulating VEGF expression or function has become a research focus.
Firstly, VEGF-related SNPs are involved in MDD pathological mechanisms by regulating expression or function. For example, a variant at the rs4416670 locus of the VEGF gene has been confirmed to reduce VEGF transcriptional activity, leading to decreased expression levels, and individuals carrying this risk allele have a significantly increased risk of developing MDD (Xie et al., 2017). This finding is consistent with the core role of VEGF in maintaining neurovascular homeostasis–insufficient VEGF expression may disrupt vascular integrity in mesolimbic systems (such as the hippocampus), exacerbate neuronal damage, and thereby increase depression susceptibility. Notably, the serum heritability of the VEGF gene is as high as 50%, suggesting that such SNPs have a broad impact on the overall regulation of VEGF levels in the population (Debette et al., 2011). Another case-control study focusing on SNPs of VEGF, Flt1, and kinase insert domain receptor (KDR) showed that the Flt1-related SNP rs7993418 (a key VEGF receptor) was significantly associated with lower MDD symptom intensity (p = 0.003). At the same time, homozygous AA carriers of the rs699947 polymorphism had higher VEGF concentrations (p = 0.002) and an association with an increased number of suicide attempts (p = 0.041) (Nunes et al., 2022), indicating that SNPs may be involved in pathology by affecting VEGF downstream signaling or receptor function.
Furthermore, SNP-mediated abnormal VEGF function is associated with brain structural changes in MDD. Abnormal hippocampal structure (especially volume reduction) is one of the core pathological features of MDD. A genome-wide association study showed that drug-naive MDD patients carrying the VEGF gene rs6921438 locus had significantly smaller left subiculum volumes in the hippocampus compared to healthy controls (p = 0.039), and a multiple regression model confirmed a “Genotype–diagnosis interaction” at this locus (Nguyen et al., 2019). This finding is highly consistent with clinical and basic research results on MDD: as a key region for emotional regulation and memory processing, structural abnormalities of the hippocampal subiculum have been repeatedly confirmed to be closely related to depressive episodes in clinical trials, animal models, and autopsy studies (Rosoklija et al., 2000; Stockmeier et al., 2004; Watanabe et al., 2017; Willard et al., 2013). Therefore, VEGF-related SNPs may influence hippocampal development or repair by regulating VEGF expression, thereby promoting disease progression.
Interestingly, the function of VEGF is not independent; it interacts with SNPs of genes in classic depression-related pathways such as the serotonin (5-HT) system, collectively participating in the pathological regulation of MDD. 5-HT1A is one of the most abundant serotonin receptors in the human brain and has long been proven to act as a key factor in MDD involved in emotional regulation (Kaufman et al., 2016). Studies have shown that activation of the 5-HT1A gene can induce upregulated VEGF expression in the hippocampus, suggesting a genetic link between 5-HT1A and VEGF (Samaddar et al., 2015). A recent study analyzing 528 samples of Han Chinese MDD patients from northern China demonstrated that the interaction effect between VEGF (rs699947, rs833061, rs2010963) and 5-HT1A (rs6295, rs1364043, rs878567) genes has a clear pathological correlation with MDD (CV consistency = 10/10, P = 0.0107) (Han et al., 2019).
In addition, VEGF-related SNPs may also affect the therapeutic efficacy of MDD. Electroconvulsive therapy (ECT), which can increase hippocampal volume, promote neurogenesis, and enhance synaptic plasticity, has been proven to be the most effective biological therapy for MDD (Gbyl and Videbech, 2018; Kho et al., 2003; Olesen et al., 2017). A study based on elderly MDD patients combining MRI and genotyping observed that the rs699947 genotype could influence hippocampal volume after ECT, particularly in the right hippocampus (Van Den Bossche et al., 2019). Another study showed that alleles associated with lower VEGF concentrations blocked the therapeutic effect of ECT in MDD patients (p = 0.01), and this result was mediated by SNPs at the 6p21.1 gene locus (Maffioletti et al., 2020; Minelli et al., 2014). Additionally, the rapid antidepressant effect of ketamine has been shown to be associated with the VEGF rs79568085 genotype (Tsai et al., 2023).
Thus, VEGF-related SNPs are involved in MDD pathology by regulating VEGF expression (e.g., rs4416670, rs699947) or function (e.g., rs7993418). They promote disease occurrence by affecting hippocampal structure (e.g., rs6921438), amplify pathological effects through interactions with genes such as 5-HT1A, and can serve as biomarkers for predicting treatment responses to ECT, ketamine, etc. Future studies need to verify specific molecular mechanisms through functional experiments and expand sample sizes to clarify their roles in different populations, thereby promoting the translation from genetic associations to clinical applications.
3.2 Blood-brain barrier
The BBB is a semi-permeable and highly selective system that regulates the transport of substances in the brain and maintains the stability of the CNS environment. It separates the blood from the brain’s extracellular fluid and prevents neurotoxic substances, blood cells, and pathogens from entering the brain (Alahmari, 2021). Angiogenesis and blood vessel pattern formation driven by VEGF/Flk-1 signal transduction are significant for forming and fine-tuning the BBB (Wälchli et al., 2015). Abnormalities in the structure and function of the BBB can disrupt the brain’s environmental homeostasis and induce various risk factors such as inflammation, endothelial degeneration, capillary leakage, and protein deposition, thus establishing pathological connections with multiple neurological diseases (Sweeney et al., 2019).
A growing body of evidence indicates structural and functional impairments of the BBB in patients with MDD. First, S100B is a calcium-binding protein produced by glial cells and is widely used as a molecular marker for BBB disruption and brain injury (Gayger-Dias et al., 2023). A meta-analysis incorporating the results of 93 studies revealed that the blood of MDD patients contained elevated levels of S100B. Additionally, alterations were observed in other BBB protein markers, including fibrinogen, Aβ, and MMPs. These changes indicated an augmentation in BBB leakage (Futtrup et al., 2020). The tight-junction protein claudin-5 related to the BBB has also been proven to have decreased expression in the hippocampus of MDD individuals. Other tight-junction mRNA transcripts (claudin-12 and ZO-1) are associated with the age of onset and duration of MDD (Greene et al., 2020). Moreover, a recent study recruited 23 MDD patients and 18 healthy subjects. It calculated the mean volume transfer constant (Ktrans) through dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) to assess the BBB leakage status. The results showed that the Ktrans in the local brain regions of MDD patients was elevated and decreased after treatment. Moreover, the Hamilton Anxiety Scale (HAMA) score was positively correlated with Ktrans, suggesting that BBB leakage is significantly correlated with MDD and depressive symptoms (Shang et al., 2024). Finally, stress is a recognized key factor leading to MDD. Animal experiments have confirmed that chronic stress can disrupt the BBB structure in the emotion-related brain regions of female mice and induce anxiety and depressive-like behaviors (Dion-Albert et al., 2022).
Vascular endothelial growth factor may mediate the link between MDD and BBB dysfunction. Firstly, astrocytes have a unique structure that enwraps endothelial cells and releases neurotrophic factors to maintain the BBB (Zidarič et al., 2022). Consequently, VEGF released by astrocytes regulates BBB permeability (Manu et al., 2023). Further research has confirmed that the up-regulation of VEGF levels induced by the activation of astrocytes in MDD patients significantly increases the permeability of the BBB, which enables more pro-inflammatory cytokines to enter the brain, fuel neuroinflammation in the human brain, and leads to depressive-like behaviors (Argaw et al., 2009; Dudek et al., 2020). Moreover, the VEGF/Flk-1 pathway may be an important molecular route through which BBB breakdown induces depressive behaviors. Research shows that microwave radiation can induce changes in BBB permeability by activating the VEGF/Flk-1 - ERK pathway and down-regulating the expression of BBB - related proteins occludin and zonula occludens-1 (ZO-1) (Wang et al., 2015). Conversely, inhibiting the expression of Flk-1 using specific monoclonal antibodies can effectively restore the BBB permeability in the chronic restraint stress (RS) animal model and alleviate various depressive behaviors (Matsuno et al., 2022; Figure 2).
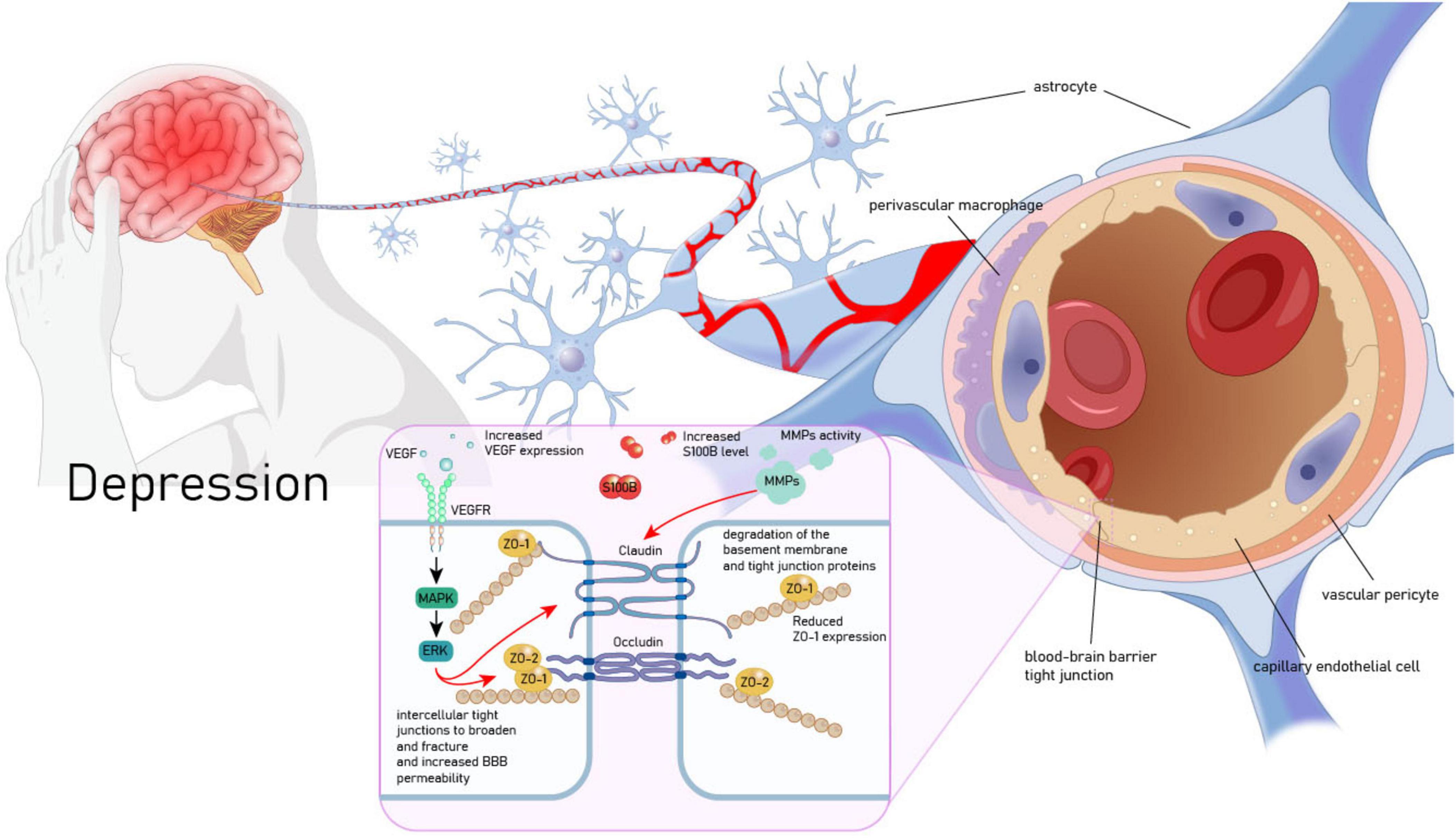
Figure 2. Vascular endothelial growth factor (VEGF) mediates the pathological association between MDD and BBB. VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; MAPK, mitogen-activated protein kinase; ERK, extracellular signal - regulated kinase; ZO-1, zonula occludens - 1; ZO-2, zonula occludens - 2; MMPs, matrix metalloproteinases; S100B, S100 calcium - binding protein B.
All these findings indicate a key pathological link between MDD and BBB dysfunction, with VEGF potentially mediating this relationship. The contradiction between its vascular permeability-disrupting effects and neuroprotective roles can be explained by temporal dynamics and receptor selectivity: transient elevation of VEGF during acute stress exerts protective effects by promoting angiogenesis and supporting neurotrophy, whereas sustained high expression during the chronic depressive phase leads to BBB leakage, enabling peripheral inflammatory factors to infiltrate and damage neurons (Kaya et al., 2005; Proescholdt et al., 1999). Furthermore, the VEGF receptors Flt-1 and Flk-1 exert antagonistic functions in MDD: Flt-1 disrupts BBB tight junctions (e.g., ZO-1, occludin) via the PLCγ/NO pathway, mediating pathological permeability; in contrast, Flk-1 promotes neuronal survival and synaptic plasticity through the PI3K/Akt pathway (Mesquita-Britto et al., 2020; Wang et al., 2024). Such differences in receptor selectivity determine the disease trajectory. Thus, exploring individual variations and identifying universal principles for maintaining the low-permeability homeostasis of the BBB may represent a potential direction for MDD treatment, though more direct evidence is still required to support this.
3.3 Brain-derived neurotrophic factor
The neurotrophic hypothesis of MDD posits that reduced neurotrophic factors, especially brain-derived neurotrophic factor (BDNF) and VEGF, can induce neuronal atrophy in brain regions associated with MDD, an important mechanism underlying depressive symptoms (Duman et al., 2016; Rana et al., 2021; Uslu and Yildiz, 2024). BDNF is the most highly expressed member of the nerve growth factor family. It contributes to the survival, growth, and maintenance of neurons and is involved in various learning and memory-related functions, playing a crucial role in regulating activity-dependent neuronal plasticity (Ateaque et al., 2023). The specific receptor tyrosine kinase receptor (TrkB) is an important high-affinity receptor for BDNF. BDNF must bind to it to activate different downstream signaling pathways (such as PI3K/Akt, Raf/ERK) and exert various neurotrophic effects (Zeng et al., 2024). For example, recent results have shown that by up-regulating the BDNF/TrkB - mediated PI3K/Akt/mTOR signaling pathway, the expression of postsynaptic density 95 and synapsin in the hippocampus of MDD rats can be increased, and the depressive behaviors of the lipopolysaccharide (LPS) -induced MDD rat model can be improved (Wu et al., 2024).
However, there is an interrelated effect between BDNF and VEGF. On the one hand, VEGF has the capacity to upregulate the production of BDNF. Research has found that the knockout of Flk - 1 accelerates the reduction of BDNF (Le et al., 2021). Moreover, single-nucleus RNA sequencing has revealed that VEGF exerts a neuro-supportive function by upregulating the BDNF signaling pathway in brain cells and reducing ischemic stroke damage (Simoes Braga Boisserand et al., 2023). On the other hand, BDNF can also promote an increase in VEGF secretion. The VEGF mRNA levels in neuroblastoma cells rise 8–16 h after exposure to BDNF. BDNF induces a 2- to 4-fold increase in VEGF promoter activity (Nakamura et al., 2006). Another study has confirmed that BDNF can stimulate the expression and secretion of VEGF in osteoblasts through the TrkB/ERK signaling pathway (Zhang et al., 2017).
In the pathological process of MDD, there is also an interdependence between BDNF and VEGF. The neuroprotective and antidepressant-like effects of BDNF and VEGF necessitate close collaboration between them (Dattilo et al., 2020). On the one hand, the antidepressant - effects of BDNF in three different behavioral paradigms (behavioral despair model, motivation and rewards model, and anxiety model) rely on neuron-derived extracellular VEGF support. Moreover, the influence of BDNF-induced neurotrophic effects on dendritic complexity requires VEGF/Flk-1 signaling in primary cortical neurons. On the other hand, BDNF/TrkB signal transduction promotes the release of VEGF in neurons. In the medial prefrontal cortex (mPFC), the co-infusion of BDNF-neutralizing antibodies can block the antidepressant behavior induced by VEGF infusion (Deyama et al., 2019; Figure 3).
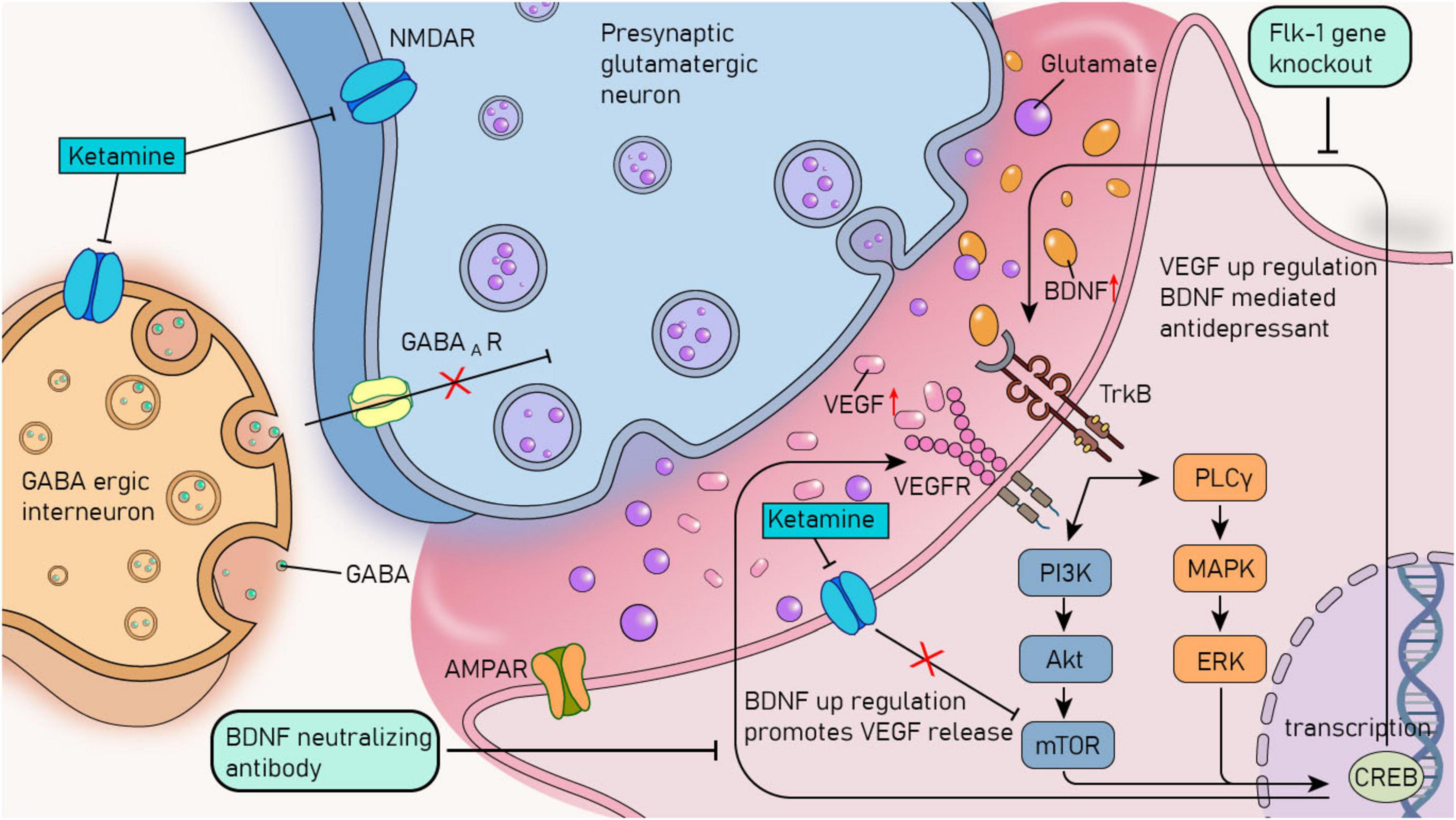
Figure 3. The crosstalk mechanism between BDNF and VEGF and the rapid antidepressant mechanism of ketamine. VEGF, vascular endothelial growth factor; BDNF, brain - derived neurotrophic factor; TrkB, tyrosine kinase receptor B; VEGFR, vascular endothelial growth factor receptor; PI3K, phosphoinositide 3-kinases; Akt, protein kinase B; MAPK, mitogen-activated protein kinase; PLCγ, phospholipase Cγ; ERK, extracellular signal - regulated kinase; mTOR, mammalian target of rapamycin; GABA, γ - aminobutyric acid; GABAAR, γ - aminobutyric acid type A receptor; CREB, cAMP - responsive element binding protein; NMDAR, N - methyl - D - aspartate receptor; AMPAR, α - amino - 3 - hydroxy - 5 - methyl - 4 - isoxazolepropionic acid receptor.
The crosstalk between neurotrophic factors also plays a crucial role in treating MDD. For example, research has found that the increased expression of these factors can restore the brain’s structural and functional impairments caused by stress and depression. It provides trophic support and protection, thereby reversing the atrophy and synaptic loss that lead to the pathophysiology of MDD, and mediates the rapid antidepressant effect of ketamine (Duman et al., 2021; Joshi and Salton, 2022; Figure 3). Moreover, the latest research has confirmed that local infusion of resolvin E1 (RvE1) into the mPFC or the dorsal hippocampal dentate gyrus (DG) through intracerebroventricular infusion can upregulate mTOR by activating the activity-dependent BDNF/VEGF pathway, thus producing antidepressant-like effects (Deyama et al., 2023).
These findings highlight the synergistic effect of BDNF and VEGF in the physiological and pathological processes of MDD. The inhibition of BDNF and VEGF can block the antidepressant effect of a single neurotrophic factor. Targeted treatment of MDD by targeting BDNF and VEGF may be a new research direction in the future.
4 Antidepressant treatment with VEGF as the therapeutic target
4.1 Traditional antidepressants
Classic antidepressants, such as tricyclic antidepressants (TCAs) and selective serotonin (5 - HT) reuptake inhibitors (SSRIs), have been shown to be capable of regulating VEGF and its related pathways. Preclinical data have shown that treatment with duloxetine at 20 mg/kg can increase plasma 5-HT levels and activate the PI3K/AKT/VEGF signaling pathway, thereby exerting a protective effect against indomethacin-induced gastric ulcers (Ding et al., 2024). Moreover, fluoxetine has also been proven to increase the protein expression of VEGF and its receptor VEGFR in the brain of rats with the middle cerebral artery occlusion model, promote angiogenesis, and play a protective role in neurological impairment after ischemic stroke (Hu et al., 2020).
Hippocampal neurogenesis plays a vital role in the antidepressant effects of traditional antidepressants, in which VEGF may play an important role. Early animal experiments demonstrated that long-term use of fluoxetine could increase VEGF mRNA in hippocampal neurons and endothelial cells. Meanwhile, the pharmacological inhibition of the VEGF/Flk-1 pathway blocked the improvement effect of fluoxetine in three behavioral tests (sucrose preference, forced swimming, and novelty suppression) in rats of the chronic unpredictable mild stress (CUMS) model (Deyama and Duman, 2020; Greene et al., 2009). A recent rodent experiment also proved that the antidepressant-like effects of repeated desipramine and chronic fluoxetine treatment could be entirely blocked by neuron-specific VEGF or Flk-1 knockout (Deyama et al., 2024a). However, these results are also controversial. For example, Chen’s research showed that vortioxetine could upregulate the protein level of VEGF in the hippocampus and improve the hippocampal microvasculature. In contrast, fluoxetine failed to improve these indicators (Chen et al., 2021).
4.2 Ketamine
Traditional antidepressants can rapidly increase extracellular monoamine levels, yet their clinical efficacy is slow to manifest, often taking weeks to months. This delay in effectiveness increases the risk of suicidal behavior during the first month of antidepressant treatment (Jick et al., 2004). Meanwhile, the efficacy of monoaminergic antidepressants is limited, with approximately one-third of patients showing no response to the drugs (Trivedi et al., 2006). Therefore, there is an urgent need to break through the mechanisms of traditional antidepressants and pursue more efficient and rapid-acting antidepressant treatments.
Ketamine is a non-competitive N - methyl - D - aspartate receptor (NMDAR) antagonist that has been used as a clinical anesthetic for over half a century (Lavender et al., 2020). Subsequently, a series of studies have uncovered that ketamine has rapid (within a few hours) and long-lasting (up to a week) antidepressant effects, making it a promising new - type of antidepressant for treating treatment-resistant depression and various mixed mood disorders (Berman et al., 2000; Zarate et al., 2006). The molecular mechanisms of ketamine’s antidepressant effects are extensive. Increasing central neurogenesis and improving synaptic plasticity are among its core features, which are closely related to its ability to promote the release of neurotrophic factors (BDNF, VEGF) (Kim et al., 2024; Figure 3). Rodent experiments have confirmed that VEGF and its signaling pathway influence the antidepressant effect of ketamine. Deyama et al. (2024a) have fully elucidated the potential mechanism of the relationship between VEGF/Flk-1 signal transduction and the rapid antidepressant effect of ketamine through a series of co - infusion experiments of neutralizing antibodies and behavioral methods. Neuron-specific deletion of VEGF or Flk-1 in the mPFC and hippocampus can effectively block the antidepressant effect of ketamine. Infusing anti-VEGF neutralizing antibodies into the mPFC 30 min before ketamine administration blocks the antidepressant effect of ketamine, and in contrast, infusing the same antibody 2 h after ketamine administration does not affect the drug’s efficacy (Kenwood et al., 2019; Figure 3). A clinical study involving 25 patients with MDD examined plasma samples collected 1 h before and 4 h after ketamine infusion at a dosage of 0.5 mg/kg. The results of this study confirmed that a single infusion of ketamine can lead to an increase in the level of VEGF. Moreover, a significant group × time interaction was observed in the mRNA level of VEGF, with a p-value of 0.029 (McGrory et al., 2020).
However, the research findings regarding the association between VEGF and the antidepressant effect of ketamine are inconsistent. A study conducted six infusions of ketamine (0.5 mg/kg) on 78 MDD patients and found that, compared with the baseline, there was no difference in the change of plasma VEGF concentration between anhedonia responders and non-responders on the 13th and 26th days of treatment (p > 0.05) (Zheng et al., 2021). Another clinical study involving 48 MDD patients also confirmed that a single infusion of 0.5 mg/kg ketamine did not alter the VEGF level in MDD patients (Chen et al., 2023).
This discrepancy may arise from multiple factors. Firstly, VEGF in peripheral plasma may not timely reflect its expression in the brain. Secondly, various factors in clinical studies (such as age, gender, treatment course, and administration time) may affect the detection results. Further research is required to explore the impact of VEGF on the rapid and long-term antidepressant effects of ketamine.
4.3 Electroconvulsive therapy
The efficacy of ECT in treating MDD is closely associated with its induction of neuroplastic changes, in which VEGF plays a critical mediating role (Maffioletti et al., 2021). Accumulating evidence indicates that ECT can rapidly and transiently increase VEGF levels in both the brain and peripheral circulation, and this dynamic change is considered an initiating mechanism for ECT-triggered adaptive neuroplastic remodeling.
Clinical studies have demonstrated the responsive pattern of VEGF to ECT stimulation. A study involving 110 MDD patients confirmed that 8-weeks treatment with combined ECT and medication significantly upregulated serum VEGF levels. In contrast, no such effect was observed in the medication-only group, reflecting the specific regulatory role of ECT on VEGF (Zhang et al., 2023). Dynamic monitoring at specific time points after single treatments further revealed that plasma VEGF concentrations in patients increased significantly at 2 and 4 h after the first and fifth ECT sessions. Notably, despite the acute elevation of VEGF induced by single treatments, VEGF levels typically return to baseline after the completion of the entire ECT course, indicating the prominently short-term and transient nature of its upregulation (Sorri et al., 2021).
Animal experimental models provide deeper insights into the temporal profile of VEGF and its causal relationship with neuroplasticity. Rodent studies have confirmed that hippocampal VEGF levels increase rapidly within 3 h after ECT intervention and begin to decrease after 6 h, which is highly consistent with the rapid and transient pattern observed in clinical settings (Elfving and Wegener, 2012). More importantly, within 24 h after ECT treatment, significant increases in VEGF levels in the CA1, CA2, CA3, and DG regions of the hippocampus in MDD model rats were observed, accompanied by rapid improvements in hippocampal structural plasticity and synaptic ultrastructural plasticity (Chen et al., 2020). This suggests a temporal correlation between the acute elevation of VEGF and early beneficial remodeling of neuroplasticity.
Although VEGF levels may eventually return to baseline after repeated ECT sessions (Chen et al., 2020), the beneficial neuroplastic changes it triggers (such as a significant increase in hippocampal volume) can persist for at least 3 months (Nordanskog et al., 2010; Takamiya et al., 2023). This phenomenon suggests that the short-term significant rise in VEGF constitutes a critical initiating event for adaptive neuroplastic remodeling. Its role may lie in rapidly activating downstream signaling pathways, inducing processes such as synaptic protein synthesis, dendritic spine formation, or neurogenesis, thereby laying the foundation for long-term functional and structural changes in neural circuits (Madsen et al., 2000). Even after VEGF concentrations subsequently decline, the molecular and cellular cascades it initiates can persist, ultimately leading to a relatively stable neuroplastic state associated with symptom remission.
In summary, ECT induces a rapid and transient significant increase in VEGF, which serves as the initial driving force for initiating beneficial neuroplastic remodeling in the hippocampus and related brain regions. Although the acute changes in VEGF are time-limited, the biological effects it triggers are sufficient to promote long-term structural and functional optimization of neural circuits, providing an important molecular biological basis for understanding the antidepressant mechanism of ECT.
4.4 Repetitive transcranial electrical stimulation
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive brain stimulation technique that can serve as an adjunctive treatment for patients with MDD who respond poorly to antidepressant medications. Compared with ECT, rTMS does not require general anesthesia, has higher safety, fewer side effects, and better clinical acceptance (Kaster and Blumberger, 2024). Recent clinical studies have further indicated that rTMS may improve neuroplasticity by upregulating VEGF levels in the CNS, providing important clues for its antidepressant mechanism. For example, a randomized controlled trial by Xu B. et al. (2024) on patients with post-stroke cognitive impairment found that 4-weeks dual-target rTMS stimulation significantly increased serum VEGF levels, and the degree of VEGF elevation was positively correlated with improvements in cognitive function. Furthermore, a mechanistic study by Xing et al.’s (2023) in an animal model of ischemic stroke further revealed that rTMS treatment could significantly enhance VEGF expression in brain tissues, activate vasculogenesis and angiogenesis pathways, and help reverse neuronal death and synaptic structural damage associated with post-stroke dysfunction. Inspired by the findings of ECT studies, in recent years, many researchers have begun to explore whether VEGF is involved in the antidepressant mechanism of rTMS (Fukuda et al., 2020).
A large number of studies have confirmed the effectiveness of rTMS in improving neuroplasticity in MDD patients. The latest clinical study, using measurements of neurophysiological parameters of transcranial magnetic stimulation, confirmed that the baseline level of Long-Term Potentiation-like (LTP-like) plasticity in the motor cortex of MDD patients is low, and the improvement of clinical symptoms after rTMS intervention is positively correlated with the increase in LTP-like plasticity (Scho et al., 2024). A clinical study based on resting-state functional connectivity further showed that the enhancement of neural functional connectivity between the rTMS stimulation site and the limbic network is a key mechanism mediating antidepressant efficacy (Long et al., 2024). Several recent clinical and preclinical studies have suggested that VEGF may be involved in the mechanism by which rTMS induces neurogenesis and remodels synaptic plasticity to exert antidepressant effects. A study by Elemery et al. (2022) recruited 17 patients with clinically refractory depression who received 10 sessions of medication combined with rTMS treatment, and found that serum VEGF is sensitive to changes in anhedonia during rTMS treatment, and baseline VEGF levels may be an important predictor of rTMS treatment efficacy. Xu Q. et al. (2024) found that mice exposed to a simulated spatially complex environment (SSCE) for 7 days exhibited depression-like behaviors, while 14-days rTMS treatment could significantly improve SSCE-induced emotional and social dysfunction by regulating VEGF signaling in the mPFC.
These findings suggest that rTMS may exert neuroprotective effects by increasing VEGF levels, thereby playing a role in the treatment of depression. Meanwhile, baseline VEGF levels may predict the therapeutic effect of rTMS. However, the sample sizes of the studies and the number of conducted studies are small, requiring further data for confirmation.
4.5 Resolvins
The resolvin D series (RvD1, RvD2) and E series (RvE1, RvE2, RvE3) are bioactive lipid mediators derived from docosahexaenoic acid and eicosapentaenoic acid, respectively, named for their critical role in the resolution of inflammation. As early as 2014, studies confirmed that RvD1 and RvD2 could ameliorate depressive-like behaviors in experimental animals (Gilbert et al., 2014; Klein et al., 2014). Deyama (2020), through a series of experiments, demonstrated that exogenously administered resolvin could accelerate spontaneous recovery and mitigate depressive symptoms. For instance, the quantitative infusion of RvD1, RvD2, RvE1, RvE2, and RvE3 into the DG and mPFC could effectively improve the depressive behaviors of mice induced by LPS and CUMS, significantly reducing the immobility time in the tail-suspension test and forced-swimming test (Deyama et al., 2017, 2018a,Deyama et al., 2018b; Ishikawa et al., 2017). Secondly, large-scale clinical data analysis showed that treatment with synthetic glucocorticoids (e.g., prednisolone) increases the risk of MDD by 3-fold and the risk of suicide by 7-fold in patients (Fardet et al., 2012; Patten, 2000). A recent experiment on rodents has proven that a single dose of RvE1 can induce rapid and sustained neuroplastic changes in brain regions like the mPFC, reversing the increased immobility in the tail-suspension test induced by prednisolone (Aoki et al., 2022).
Animal studies have confirmed that the rapid antidepressant effect of RvE1 is closely associated with VEGF. At the molecular level, subcutaneous injection of resolvins can significantly upregulate VEGF gene expression in the rat brain and promote neovascularization (Jiang et al., 2022). Behavioral experiments further confirmed that intranasal administration of RvE1 exerts rapid and sustained antidepressant effects similar to those of ketamine (Deyama, 2020). Intranasal administration of RvE1 in LPS-induced depressive mice effectively reduced immobility in the tail suspension test. It forced a swimming test, a phenomenon that was entirely blocked by quantitative intracerebroventricular injection of VEGF-neutralizing antibodies (Deyama et al., 2024b). Thus, the antidepressant effect of RvE1 depends on VEGF released from the mPFC, similar to the mechanism of ketamine.
Resolvin has the potential to be a highly promising candidate drug for rapid-acting antidepressants in clinical practice. However, more research is needed to confirm its mechanism, efficacy, and safety.
5 Conclusion
The studies described in this review indicate that VEGF holds significant importance in the pathological process of MDD. VEGF may be pathologically intertwined with MDD through pathways involving genes, the BBB, and BDNF. At the same time, VEGF and its related pathways have also been shown to mediate numerous antidepressant treatments, such as traditional antidepressants, ketamine, ECT, rTMS, and resolvins. VEGF therapy may confer benefits in MDD by reducing depression susceptibility through enhancing vascular and neuroplasticity, as well as exerting direct neuroprotective effects. However, upregulating VEGF levels may trigger BBB disruption and vascular leakage. Existing evidence suggests that short-term rapid upregulation of VEGF expression in the CNS may trigger long-term molecular cascades, promoting progressive and beneficial remodeling of the neurovascular network, and the subsequent return of VEGF to baseline levels during treatment can avoid BBB damage. This mechanism is expected to become a core breakthrough point in the treatment of rapid-acting and treatment-resistant depression, with excellent research value.
Future research should focus on the differential mechanisms in specific populations (age/gender/ethnicity), disease phases (acute/chronic), brain region structures, and specific receptors (Flk-1/Flt-1). It is necessary to develop brain region-targeted delivery systems combining Flt-1 inhibitors and neuroprotective VEGF subtypes to promote angiogenesis and the recovery of neuroplasticity in the CNS while preventing BBB leakage. In-depth clarification of the physiological functions of VEGF in brain development will provide important support for developing novel antidepressant strategies and advancing the field of nervous system repair.
Author contributions
JW: Methodology, Writing – review & editing, Validation, Investigation, Data curation, Writing – original draft, Formal analysis. FM: Writing – review & editing, Methodology, Writing – original draft, Data curation. LW: Writing – review & editing, Supervision, Formal analysis. ZL: Formal analysis, Conceptualization, Supervision, Data curation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation (81303044), the Natural Science Foundation of Heilongjiang Province (LH2022H082), the Postdoctoral Scientific Research Developmental Fund of Heilongjiang Province (LBH-Q19185), the Heilongjiang Provincial Administration of Traditional Chinese Medicine Project (ZHY2020-120), and the Graduate Research and Innovation Project in Heilongjiang University Of Chinese Medicine (2020yjscx016), China.
Acknowledgments
We would like to thank the sponsors, National Natural Science Foundation of China. We would also like to thank the professors from the First Affiliated Hospital of Heilongjiang University of Traditional Chinese Medicine for their assistance in the literature search of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alahmari, A. (2021). Blood-brain barrier overview: Structural and functional correlation. Neural Plast. 2021:6564585. doi: 10.1155/2021/6564585
Annam, J., Galfalvy, H., Keilp, J., Simpson, N., Huang, Y., Nandakumar, R., et al. (2024). Plasma cytokine and growth factor response to acute psychosocial stress in major depressive disorder. J. Psychiatr. Res. 169, 224–230. doi: 10.1016/j.jpsychires.2023.11.029
Aoki, S., Deyama, S., Sugie, R., Ishimura, K., Fukuda, H., Shuto, S., et al. (2022). The antidepressant-like effect of resolvin E1 in repeated prednisolone-induced depression model mice. Behav. Brain Res. 418, 113676. doi: 10.1016/j.bbr.2021.113676
Argaw, A., Gurfein, B., Zhang, Y., Zameer, A., and John, G. R. (2009). VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc. Natl. Acad. Sci. U S A. 106, 1977–1982. doi: 10.1073/pnas.0808698106
Ateaque, S., Merkouris, S., and Barde, Y. (2023). Neurotrophin signalling in the human nervous system. Front. Mol. Neurosci. 16:1225373. doi: 10.3389/fnmol.2023.1225373
Badamasi, I., Lye, M., Ibrahim, N., and Stanslas, J. (2019). Genetic endophenotypes for insomnia of major depressive disorder and treatment-induced insomnia. J. Neural Transm. 126, 711–722. doi: 10.1007/s00702-019-02014-y
Berent, D., Macander, M., Szemraj, J., Orzechowska, A., and Galecki, P. (2014). Vascular endothelial growth factor A gene expression level is higher in patients with major depressive disorder and not affected by cigarette smoking, hyperlipidemia or treatment with statins. Acta Neurobiol. Exp. 74, 82–90. doi: 10.55782/ane-2014-1974
Berman, R., Cappiello, A., Anand, A., Oren, D., Heninger, G., Charney, D., et al. (2000). Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 47, 351–354. doi: 10.1016/s0006-3223(99)00230-9
Breslin, J., Pappas, P., Cerveira, J., Hobson, R., and Durán, W. N. (2003). VEGF increases endothelial permeability by separate signaling pathways involving ERK-1/2 and nitric oxide. Am. J. Physiol. Heart Circ. Physiol. 284, H92–H100. doi: 10.1152/ajpheart.00330.2002
Cao, J., Papadopoulou, N., Kempuraj, D., Boucher, W., Sugimoto, K., Cetrulo, C., et al. (2005). Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J. Immunol. 174, 7665–7675. doi: 10.4049/jimmunol.174.12.7665
Caroleo, M., Carbone, E., Greco, M., Corigliano, D., Arcidiacono, B., Fazia, G., et al. (2019). Brain-behavior-immune interaction: Serum cytokines and growth factors in patients with eating disorders at extremes of the Body mass index (BMI) spectrum. Nutrients 11:1995. doi: 10.3390/nu11091995
Chen, D., and Simons, M. (2021). Emerging roles of PLCγ1 in endothelial biology. Sci. Signal. 14:eabc6612. doi: 10.1126/scisignal.abc6612
Chen, F., Danladi, J., Ardalan, M., Nyengaard, J., Sanchez, C., and Wegener, G. (2021). The rat hippocampal gliovascular system following one week vortioxetine and fluoxetine. Eur. Neuropsychopharmacol. 42, 45–56. doi: 10.1016/j.euroneuro.2020.11.008
Chen, F., Danladi, J., Wegener, G., Madsen, T., and Nyengaard, J. (2020). Sustained ultrastructural changes in rat hippocampal formation after repeated electroconvulsive seizures. Int. J. Neuropsychopharmacol. 23, 446–458. doi: 10.1093/ijnp/pyaa021
Chen, M., Lin, W., Li, C., Wu, H., Bai, Y., Tsai, S., et al. (2023). Effects of low-dose ketamine infusion on vascular endothelial growth factor and matrix metalloproteinase-9 among patients with treatment-resistant depression and suicidal ideation. J. Psychiatr. Res. 165, 91–95. doi: 10.1016/j.jpsychires.2023.07.022
Choi, M., Lee, S., Chang, H., and Son, H. (2016). Hippocampal VEGF is necessary for antidepressant-like behaviors but not sufficient for antidepressant-like effects of ketamine in rats. Biochim. Biophys. Acta 1862, 1247–1254. doi: 10.1016/j.bbadis.2016.04.001
Clark-Raymond, A., Meresh, E., Hoppensteadt, D., Fareed, J., Sinacore, J., and Halaris, A. (2014). Vascular endothelial growth factor: A potential diagnostic biomarker for major depression. J. Psychiatr. Res. 59, 22–27. doi: 10.1016/j.jpsychires.2014.08.005
Connolly, D., Heuvelman, D., Nelson, R., Olander, J., Eppley, B., Delfino, J., et al. (1989). Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J. Clin. Invest. 84, 1470–1478. doi: 10.1172/JCI114322
COVID-19 Mental Disorders Collaborators. (2021). Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 398, 1700–1712. doi: 10.1016/S0140-6736(21)02143-7
Dakowicz, D., Zajkowska, M., and Mroczko, B. (2022). Relationship between VEGF family members, their receptors and cell death in the neoplastic transformation of colorectal cancer. Int. J. Mol. Sci. 23:3375. doi: 10.3390/ijms23063375
Dattilo, V., Amato, R., Perrotti, N., and Gennarelli, M. (2020). The emerging role of SGK1 (Serum- and glucocorticoid-regulated kinase 1) in major depressive disorder: Hypothesis and mechanisms. Front. Genet. 11:826. doi: 10.3389/fgene.2020.00826
de Vries, C., Escobedo, J., Ueno, H., Houck, K., Ferrara, N., and Williams, L. (1992). The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 255, 989–991. doi: 10.1126/science.1312256
Dean, J., and Keshavan, M. (2017). The neurobiology of depression: An integrated view. Asian J. Psychiatr. 27, 101–111. doi: 10.1016/j.ajp.2017.01.025
Debette, S., Visvikis-Siest, S., Chen, M., Ndiaye, N., Song, C., Destefano, A., et al. (2011). Identification of cis- and trans-acting genetic variants explaining up to half the variation in circulating vascular endothelial growth factor levels. Circ. Res. 109, 554–563. doi: 10.1161/CIRCRESAHA.111.243790
Deyama, S. (2020). [Resolvins as novel targets for rapid-acting antidepressants]. Nihon Yakurigaku Zasshi 155, 381–385. doi: 10.1254/fpj.20044
Deyama, S., and Duman, R. (2020). Neurotrophic mechanisms underlying the rapid and sustained antidepressant actions of ketamine. Pharmacol. Biochem. Behav. 188:172837. doi: 10.1016/j.pbb.2019.172837
Deyama, S., and Kaneda, K. (2023). Role of neurotrophic and growth factors in the rapid and sustained antidepressant actions of ketamine. Neuropharmacology 224:109335. doi: 10.1016/j.neuropharm.2022.109335
Deyama, S., Aoki, S., Sugie, R., Fukuda, H., Shuto, S., Minami, M., et al. (2023). Intranasal administration of resolvin E1 produces antidepressant-Like effects via BDNF/VEGF-mTORC1 signaling in the medial prefrontal cortex. Neurotherapeutics 20, 484–501. doi: 10.1007/s13311-022-01337-1
Deyama, S., Bang, E., Kato, T., Li, X., and Duman, R. (2019). Neurotrophic and antidepressant actions of brain-derived neurotrophic factor require vascular endothelial growth factor. Biol. Psychiatry 86, 143–152. doi: 10.1016/j.biopsych.2018.12.014
Deyama, S., Ishikawa, Y., Yoshikawa, K., Shimoda, K., Ide, S., Satoh, M., et al. (2017). Resolvin D1 and D2 reverse lipopolysaccharide-induced depression-like behaviors through the mTORC1 signaling pathway. Int. J. Neuropsychopharmacol. 20, 575–584. doi: 10.1093/ijnp/pyx023
Deyama, S., Li, X., and Duman, R. (2024a). Neuron-specific deletion of VEGF or its receptor Flk-1 occludes the antidepressant-like effects of desipramine and fluoxetine in mice. Neuropsychopharmacol. Rep. 44, 246–249. doi: 10.1002/npr2.12393
Deyama, S., Minami, M., and Kaneda, K. (2024b). [Resolvin E1 as a potential lead for the treatment of depression]. Nihon Yakurigaku Zasshi 159, 210–213. doi: 10.1254/fpj.23008
Deyama, S., Shimoda, K., Ikeda, H., Fukuda, H., Shuto, S., and Minami, M. (2018a). Resolvin E3 attenuates lipopolysaccharide-induced depression-like behavior in mice. J. Pharmacol. Sci. 138, 86–88. doi: 10.1016/j.jphs.2018.09.006
Deyama, S., Shimoda, K., Suzuki, H., Ishikawa, Y., Ishimura, K., Fukuda, H., et al. (2018b). Resolvin E1/E2 ameliorate lipopolysaccharide-induced depression-like behaviors via ChemR23. Psychopharmacology 235, 329–336. doi: 10.1007/s00213-017-4774-7
Ding, H., Wang, Y., Gao, Y., Ye, F., Yao, K., Cao, L., et al. (2024). Duloxetine protected indomethacin-induced gastric mucosal injury by increasing serotonin-dependent RANTES expression and activating PI3K-AKT-VEGF pathway. Toxicol. Appl. Pharmacol. 486:116950. doi: 10.1016/j.taap.2024.116950
Dion-Albert, L., Cadoret, A., Doney, E., Kaufmann, F., Dudek, K., Daigle, B., et al. (2022). Vascular and blood-brain barrier-related changes underlie stress responses and resilience in female mice and depression in human tissue. Nat. Commun. 13:164. doi: 10.1038/s41467-021-27604-x
Dudek, K., Dion-Albert, L., Lebel, M., LeClair, K., Labrecque, S., Tuck, E., et al. (2020). Molecular adaptations of the blood-brain barrier promote stress resilience vs. depression. Proc. Natl. Acad. Sci. U S A. 117, 3326–3336. doi: 10.1073/pnas.1914655117
Duman, R., Aghajanian, G., Sanacora, G., and Krystal, J. (2016). Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med. 22, 238–249. doi: 10.1038/nm.4050
Duman, R., Deyama, S., and Fogaça, M. (2021). Role of BDNF in the pathophysiology and treatment of depression: Activity-dependent effects distinguish rapid-acting antidepressants. Eur. J. Neurosci. 53, 126–139. doi: 10.1111/ejn.14630
Dvorak, H. (2006). Discovery of vascular permeability factor (VPF). Exp. Cell. Res. 312, 522–526. doi: 10.1016/j.yexcr.2005.11.026
Elemery, M., Kiss, S., Dome, P., Pogany, L., Faludi, G., and Lazary, J. (2022). Change of circulating vascular endothelial growth factor level and reduction of anhedonia are associated in patients with major depressive disorder treated with repetitive transcranial magnetic stimulation. Front. Psychiatry 13:806731. doi: 10.3389/fpsyt.2022.806731
Elfving, B., and Wegener, G. (2012). Electroconvulsive seizures stimulate the vegf pathway via mTORC1. Synapse 66, 340–345. doi: 10.1002/syn.21518
Estrada, C., Maldonado, A., and Mallipattu, S. (2019). Therapeutic inhibition of VEGF signaling and associated nephrotoxicities. J. Am. Soc. Nephrol. 30, 187–200. doi: 10.1681/ASN.2018080853
Fardet, L., Petersen, I., and Nazareth, I. (2012). Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care. Am. J. Psychiatry 169, 491–497. doi: 10.1176/appi.ajp.2011.11071009
Ferrara, N., and Henzel, W. (1989). Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Commun. 161, 851–858. doi: 10.1016/0006-291x(89)92678-8
Fries, G., Saldana, V., Finnstein, J., and Rein, T. (2023). Molecular pathways of major depressive disorder converge on the synapse. Mol. Psychiatry 28, 284–297. doi: 10.1038/s41380-022-01806-1
Fukuda, A., Hindley, L., Kang, J., Tirrell, E., Tyrka, A., Ayala, A., et al. (2020). Peripheral vascular endothelial growth factor changes after transcranial magnetic stimulation in treatment-resistant depression. Neuroreport 31, 1121–1127. doi: 10.1097/WNR.0000000000001523
Furtado, M., and Katzman, M. (2015). Examining the role of neuroinflammation in major depression. Psychiatry Res. 229, 27–36. doi: 10.1016/j.psychres.2015.06.009
Futtrup, J., Margolinsky, R., Benros, M., Moos, T., Routhe, L., Rungby, J., et al. (2020). Blood-brain barrier pathology in patients with severe mental disorders: A systematic review and meta-analysis of biomarkers in case-control studies. Brain Behav. Immun. Health 6:100102. doi: 10.1016/j.bbih.2020.100102
Gayger-Dias, V., Vizuete, A., Rodrigues, L., Wartchow, K., Bobermin, L., Leite, M., et al. (2023). How S100B crosses brain barriers and why it is considered a peripheral marker of brain injury. Exp. Biol. Med. 248, 2109–2119. doi: 10.1177/15353702231214260
GBD 2021 Diseases and Injuries Collaborators. (2024). Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 403, 2133–2161. doi: 10.1016/S0140-6736(24)00757-8
Gbyl, K., and Videbech, P. (2018). Electroconvulsive therapy increases brain volume in major depression: A systematic review and meta-analysis. Acta Psychiatr. Scand. 138, 180–195. doi: 10.1111/acps.12884
Gilbert, K., Bernier, J., Godbout, R., and Rousseau, G. (2014). Resolvin D1, a metabolite of omega-3 polyunsaturated fatty acid, decreases post-myocardial infarct depression. Mar. Drugs 12, 5396–5407. doi: 10.3390/md12115396
Greenberg, P., Chitnis, A., Louie, D., Suthoff, E., Chen, S., Maitland, J., et al. (2023). The economic burden of adults with major depressive disorder in the United States (2019). Adv. Ther. 40, 4460–4479. doi: 10.1007/s12325-023-02622-x
Greene, C., Hanley, N., and Campbell, M. (2020). Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl. Psychiatry 10:373. doi: 10.1038/s41398-020-01054-3
Greene, J., Banasr, M., Lee, B., Warner-Schmidt, J., and Duman, R. (2009). Vascular endothelial growth factor signaling is required for the behavioral actions of antidepressant treatment: Pharmacological and cellular characterization. Neuropsychopharmacology 34, 2459–2468. doi: 10.1038/npp.2009.68
Han, D., Qiao, Z., Qi, D., Yang, J., Yang, X., Ma, J., et al. (2019). Epistatic interaction between 5-HT1A and vascular endothelial growth factor gene polymorphisms in the northern chinese han population with major depressive disorder. Front. Psychiatry 10:218. doi: 10.3389/fpsyt.2019.00218
Heine, V., Zareno, J., Maslam, S., Joëls, M., and Lucassen, P. (2005). Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur. J. Neurosci. 21, 1304–1314. doi: 10.1111/j.1460-9568.2005.03951.x
Homorogan, C., Enatescu, V., Nitusca, D., Marcu, A., Seclaman, E., and Marian, C. (2021). Distribution of microRNAs associated with major depressive disorder among blood compartments. J. Int. Med. Res. 49:3000605211006633. doi: 10.1177/03000605211006633
Howard, D., Adams, M., Clarke, T., Hafferty, J., Gibson, J., Shirali, M., et al. (2019). Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 22, 343–352. doi: 10.1038/s41593-018-0326-7
Hu, Q., Liu, L., Zhou, L., Lu, H., Wang, J., Chen, X., et al. (2020). Effect of fluoxetine on HIF-1α- Netrin/VEGF cascade, angiogenesis and neuroprotection in a rat model of transient middle cerebral artery occlusion. Exp. Neurol. 329:113312. doi: 10.1016/j.expneurol.2020.113312
Ishikawa, Y., Deyama, S., Shimoda, K., Yoshikawa, K., Ide, S., Satoh, M., et al. (2017). Rapid and sustained antidepressant effects of resolvin D1 and D2 in a chronic unpredictable stress model. Behav. Brain Res. 332, 233–236. doi: 10.1016/j.bbr.2017.06.010
Isung, J., Aeinehband, S., Mobarrez, F., Mårtensson, B., Nordström, P., Asberg, M., et al. (2012a). Low vascular endothelial growth factor and interleukin-8 in cerebrospinal fluid of suicide attempters. Transl. Psychiatry 2:e196. doi: 10.1038/tp.2012.123
Isung, J., Mobarrez, F., Nordström, P., Asberg, M., and Jokinen, J. (2012b). Low plasma vascular endothelial growth factor (VEGF) associated with completed suicide. World J. Biol. Psychiatry 13, 468–473. doi: 10.3109/15622975.2011.624549
Jais, A., Solas, M., Backes, H., Chaurasia, B., Kleinridders, A., Theurich, S., et al. (2016). Myeloid-derived VEGF maintains brain glucose uptake and limits cognitive impairment in obesity. Cell 165, 882–895. doi: 10.1016/j.cell.2016.03.033
Jiang, B., and Liu, L. (2008). PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochim. Biophys. Acta 1784, 150–158. doi: 10.1016/j.bbapap.2007.09.008
Jiang, X., Liu, J., Li, S., Qiu, Y., Wang, X., He, X., et al. (2022). The effect of resolvin D1 on bone regeneration in a rat calvarial defect model. J. Tissue Eng. Regen. Med. 16, 987–997. doi: 10.1002/term.3345
Jick, H., Kaye, J., and Jick, S. (2004). Antidepressants and the risk of suicidal behaviors. JAMA 292, 338–343. doi: 10.1001/jama.292.3.338
Joshi, R., and Salton, S. R. J. (2022). Neurotrophin Crosstalk in the Etiology and Treatment of Neuropsychiatric and Neurodegenerative Disease. Front. Mol. Neurosci. 15:932497. doi: 10.3389/fnmol.2022.932497
Kaster, T., and Blumberger, D. (2024). Positioning rTMS within a sequential treatment algorithm of depression. Am. J. Psychiatry 181, 781–783. doi: 10.1176/appi.ajp.20240604
Kaufman, J., DeLorenzo, C., Choudhury, S., and Parsey, R. (2016). The 5-HT1A receptor in major depressive disorder. Eur. Neuropsychopharmacol. 26, 397–410. doi: 10.1016/j.euroneuro.2015.12.039
Kaya, D., Gürsoy-Ozdemir, Y., Yemisci, M., Tuncer, N., Aktan, S., and Dalkara, T. (2005). VEGF protects brain against focal ischemia without increasing blood–brain permeability when administered intracerebroventricularly. J. Cereb. Blood Flow Metab. 25, 1111–1118. doi: 10.1038/sj.jcbfm.9600109
Kenwood, M., Roseboom, P., Oler, J., and Kalin, N. (2019). New insights into the mechanisms of Ketamine’s antidepressant effects: Understanding the role of VEGF in mediating plasticity processes. Am. J. Psychiatry 176, 333–335. doi: 10.1176/appi.ajp.2019.19030282
Kho, K., van Vreeswijk, M., Simpson, S., and Zwinderman, A. H. (2003). A meta-analysis of electroconvulsive therapy efficacy in depression. J. ECT 19, 139–147. doi: 10.1097/00124509-200309000-00005
Kim, J., Suzuki, K., Kavalali, E., and Monteggia, L. (2024). Ketamine: Mechanisms and relevance to treatment of depression. Annu. Rev. Med. 75, 129–143. doi: 10.1146/annurev-med-051322-120608
Kin, K., Yasuhara, T., Kameda, M., Tomita, Y., Umakoshi, M., Kuwahara, K., et al. (2020). Cell encapsulation enhances antidepressant effect of the mesenchymal stem cells and counteracts depressive-like behavior of treatment-resistant depressed rats. Mol. Psychiatry 25, 1202–1214. doi: 10.1038/s41380-018-0208-0
Kirschner, M., Kao, S., Edelman, J., Armstrong, N., Vallely, M., van Zandwijk, N., et al. (2011). Haemolysis during sample preparation alters microRNA content of plasma. PLoS One 6:e24145. doi: 10.1371/journal.pone.0024145
Kishi, T., Yoshimura, R., Ikuta, T., and Iwata, N. (2017). Brain-derived neurotrophic factor and major depressive disorder: Evidence from meta-analyses. Front. Psychiatry 8:308. doi: 10.3389/fpsyt.2017.00308
Klein, C., Sperotto, N., Maciel, I., Leite, C., Souza, A., and Campos, M. (2014). Effects of D-series resolvins on behavioral and neurochemical changes in a fibromyalgia-like model in mice. Neuropharmacology 86, 57–66. doi: 10.1016/j.neuropharm.2014.05.043
Koch, S., and Claesson-Welsh, L. (2012). Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb. Perspect. Med. 2:a006502. doi: 10.1101/cshperspect.a006502
Kodama, M., Fujioka, T., and Duman, R. (2004). Chronic olanzapine or fluoxetine administration increases cell proliferation in hippocampus and prefrontal cortex of adult rat. Biol. Psychiatry 56, 570–580. doi: 10.1016/j.biopsych.2004.07.008
Kotan, Z., Sarandöl, E., Kırhan, E., Ozkaya, G., and Kırlı, S. (2012). Serum brain-derived neurotrophic factor, vascular endothelial growth factor and leptin levels in patients with a diagnosis of severe major depressive disorder with melancholic features. Ther. Adv. Psychopharmacol. 2, 65–74. doi: 10.1177/2045125312436572
Kranaster, L., Blennow, K., Zetterberg, H., and Sartorius, A. (2019). Reduced vascular endothelial growth factor levels in the cerebrospinal fluid in patients with treatment resistant major depression and the effects of electroconvulsive therapy-A pilot study. J. Affect. Disord. 253, 449–453. doi: 10.1016/j.jad.2019.04.080
Krivosova, M., Adamcakova, J., Kaadt, E., Mumm, B., Dvorska, D., Brany, D., et al. (2023). The VEGF protein levels, miR-101-3p, and miR-122-5p are dysregulated in plasma from adolescents with major depression. J. Affect. Disord. 334, 60–68. doi: 10.1016/j.jad.2023.04.094
Labonté, B., Engmann, O., Purushothaman, I., Menard, C., Wang, J., Tan, C., et al. (2017). Sex-specific transcriptional signatures in human depression. Nat. Med. 23, 1102–1111. doi: 10.1038/nm.4386
Lange, C., Storkebaum, E., de Almodóvar, C., Dewerchin, M., and Carmeliet, P. (2016). Vascular endothelial growth factor: A neurovascular target in neurological diseases. Nat. Rev. Neurol. 12, 439–454. doi: 10.1038/nrneurol.2016.88
Lango Allen, H., Estrada, K., Lettre, G., Berndt, S., Weedon, M., Rivadeneira, F., et al. (2010). Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 467, 832–838. doi: 10.1038/nature09410
Lavender, E., Hirasawa-Fujita, M., and Domino, E. (2020). Ketamine’s dose related multiple mechanisms of actions: Dissociative anesthetic to rapid antidepressant. Behav. Brain Res. 390:112631. doi: 10.1016/j.bbr.2020.112631
Le, Y., Xu, B., Chucair-Elliott, A., Zhang, H., and Zhu, M. (2021). VEGF mediates retinal müller cell viability and neuroprotection through BDNF in diabetes. Biomolecules 11:712. doi: 10.3390/Biom11050712
Lee, B., and Kim, Y. (2012). Increased plasma VEGF levels in major depressive or manic episodes in patients with mood disorders. J. Affect. Disord. 136, 181–184. doi: 10.1016/j.jad.2011.07.021
Li, B., Xu, W., Luo, C., Gozal, D., and Liu, R. (2003). VEGF-induced activation of the PI3-K/Akt pathway reduces mutant SOD1-mediated motor neuron cell death. Brain Res. Mol. Brain Res. 111, 155–164. doi: 10.1016/s0169-328x(03)00025-1
Li, K., Wang, K., Xu, S., Xie, X., Tang, Y., Zhang, L., et al. (2025). In vivo evidence of increased vascular endothelial growth factor in patients with major depressive disorder. J. Affect. Disord. 368, 151–159. doi: 10.1016/j.jad.2024.09.073
Licht, T., and Keshet, E. (2013). Delineating multiple functions of VEGF-A in the adult brain. Cell. Mol. Life Sci. 70, 1727–1737. doi: 10.1007/s00018-013-1280-x
Liu, Q., He, H., Yang, J., Feng, X., Zhao, F., and Lyu, J. (2020). Changes in the global burden of depression from 1990 to 2017: Findings from the Global burden of disease study. J. Psychiatr. Res. 126, 134–140. doi: 10.1016/j.jpsychires.2019.08.002
Long, Z., Du, L., and Marino, M. (2024). Individual resting-state network functional connectivity predicts treatment improvement of repetitive transcranial magnetic stimulation in major depressive disorder: A pilot study. Psychiatry Res. 331:115616. doi: 10.1016/j.psychres.2023.115616
Lunn, J., Sakowski, S., Kim, B., Rosenberg, A., and Feldman, E. (2009). Vascular endothelial growth factor prevents G93A-SOD1-induced motor neuron degeneration. Dev. Neurobiol. 69, 871–884. doi: 10.1002/dneu.20747
Madsen, T., Treschow, A., Bengzon, J., Bolwig, T., Lindvall, O., and Tingström, A. (2000). Increased neurogenesis in a model of electroconvulsive therapy. Biol. Psychiatry 47, 1043–1049. doi: 10.1016/s0006-3223(00)00228-6
Maes, M., Zhou, B., Vasupanrajit, A., Jirakran, K., Klomkliew, P., Chanchaem, P., et al. (2024). A further examination of growth factors, T helper 1 polarization, and the gut microbiome in major depression: Associations with reoccurrence of illness, cognitive functions, suicidal behaviors, and quality of life. J. Psychiatr. Res. 176, 430–441. doi: 10.1016/j.jpsychires.2024.06.037
Maffioletti, E., Carvalho Silva, R., Bortolomasi, M., Baune, B., Gennarelli, M., and Minelli, A. (2021). Molecular biomarkers of electroconvulsive therapy effects and clinical response: Understanding the present to shape the future. Brain Sci. 11, 1120. doi: 10.3390/brainsci11091120
Maffioletti, E., Gennarelli, M., Magri, C., Bocchio-Chiavetto, L., Bortolomasi, M., Bonvicini, C., et al. (2020). Genetic determinants of circulating VEGF levels in major depressive disorder and electroconvulsive therapy response. Drug Dev. Res. 81, 593–599. doi: 10.1002/ddr.21658
Malberg, J., Eisch, A., Nestler, E., and Duman, R. (2000). Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 20, 9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000
Manu, D., Slevin, M., Barcutean, L., Forro, T., Boghitoiu, T., and Balasa, R. (2023). Astrocyte involvement in blood-brain barrier function: A critical update highlighting novel, complex, neurovascular interactions. Int. J. Mol. Sci. 24:17146. doi: 10.3390/ijms242417146
Matsuno, H., Tsuchimine, S., O’Hashi, K., Sakai, K., Hattori, K., Hidese, S., et al. (2022). Association between vascular endothelial growth factor-mediated blood-brain barrier dysfunction and stress-induced depression. Mol. Psychiatry 27, 3822–3832. doi: 10.1038/s41380-022-01618-3
McGrory, C., Ryan, K., Gallagher, B., and McLoughlin, D. (2020). Vascular endothelial growth factor and pigment epithelial-derived factor in the peripheral response to ketamine. J. Affect. Disord. 273, 380–383. doi: 10.1016/j.jad.2020.04.013
Meng, F., and Wang, L. (2023). Bidirectional mechanism of comorbidity of depression and insomnia based on synaptic plasticity. Zhong Nan Da Xue Xue Bao Yi Xue Ban 48, 1518–1528. doi: 10.11817/j.issn.1672-7347.2023.230082
Mesquita-Britto, M., Mendonça, M., Soares, E., de Oliveira, G., Solon, C., Velloso, L., et al. (2020). VEGF/VEGFR-2 system exerts neuroprotection against Phoneutria nigriventer spider envenomation through PI3K-AKT-dependent pathway. Toxicon 185, 76–90. doi: 10.1016/j.toxicon.2020.06.019
Mikulska, J., Juszczyk, G., Gawrońska-Grzywacz, M., and Herbet, M. (2021). HPA axis in the pathomechanism of depression and schizophrenia: New therapeutic strategies based on its participation. Brain Sci. 11:1298. doi: 10.3390/brainsci11101298
Minelli, A., Maffioletti, E., Bortolomasi, M., Conca, A., Zanardini, R., Rillosi, L., et al. (2014). Association between baseline serum vascular endothelial growth factor levels and response to electroconvulsive therapy. Acta Psychiatr. Scand. 129, 461–466. doi: 10.1111/acps.12187
Monroe, S., and Harkness, K. (2022). Major depression and its recurrences: Life course matters. Annu. Rev. Clin. Psychol. 18, 329–357. doi: 10.1146/annurev-clinpsy-072220-021440
Nakamura, K., Martin, K., Jackson, J., Beppu, K., Woo, C., and Thiele, C. (2006). Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer Res. 66, 4249–4255. doi: 10.1158/0008-5472.CAN-05-2789
Navarro, D., Marín-Mayor, M., Gasparyan, A., García-Gutiérrez, M., Rubio, G., and Manzanares, J. (2023). Molecular changes associated with suicide. Int. J. Mol. Sci. 24:16726. doi: 10.3390/ijms242316726
Nguyen, L., Kakeda, S., Katsuki, A., Sugimoto, K., Otsuka, Y., Ueda, I., et al. (2019). Relationship between VEGF-related gene polymorphisms and brain morphology in treatment-naïve patients with first-episode major depressive disorder. Eur. Arch. Psychiatry Clin. Neurosci. 269, 785–794. doi: 10.1007/s00406-018-0953-8
Nordanskog, P., Dahlstrand, U., Larsson, M., Larsson, E., Knutsson, L., and Johanson, A. (2010). Increase in hippocampal volume after electroconvulsive therapy in patients with depression: A volumetric magnetic resonance imaging study. J. ECT 26, 62–67. doi: 10.1097/YCT.0b013e3181a95da8
Nunes, F., Ferezin, L., Pereira, S., Figaro-Drumond, F., Pinheiro, L., Menezes, I., et al. (2022). The association of biochemical and genetic biomarkers in VEGF pathway with depression. Pharmaceutics 14:2757. doi: 10.3390/pharmaceutics14122757
Olesen, M., Wörtwein, G., Folke, J., and Pakkenberg, B. (2017). Electroconvulsive stimulation results in long-term survival of newly generated hippocampal neurons in rats. Hippocampus 27, 52–60. doi: 10.1002/hipo.22670
Oosthuyse, B., Moons, L., Storkebaum, E., Beck, H., Nuyens, D., Brusselmans, K., et al. (2001). Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat. Genet. 28, 131–138. doi: 10.1038/88842
Patten, S. (2000). Exogenous corticosteroids and major depression in the general population. J. Psychosom. Res. 49, 447–449. doi: 10.1016/s0022-3999(00)00187-2
Preusser, M., Hassler, M., Birner, P., Rudas, M., Acker, T., Plate, K., et al. (2012). Microvascularization and expression of VEGF and its receptors in recurring meningiomas: Pathobiological data in favor of anti-angiogenic therapy approaches. Clin. Neuropathol. 31, 352–360. doi: 10.5414/NP300488
Proescholdt, M., Heiss, J., Walbridge, S., Mühlhauser, J., Capogrossi, M., Oldfield, E., et al. (1999). Vascular endothelial growth factor (VEGF) modulates vascular permeability and inflammation in rat brain. J. Neuropathol. Exp. Neurol. 58, 613–627. doi: 10.1097/00005072-199906000-00006
Rana, T., Behl, T., Sehgal, A., Srivastava, P., and Bungau, S. (2021). Unfolding the Role of BDNF as a Biomarker for Treatment of Depression. J. Mol. Neurosci. 71, 2008–2021. doi: 10.1007/s12031-020-01754-x
Rigal, A., Colle, R., Asmar, K., Trabado, S., Loeb, E., Martin, S., et al. (2020). Lower plasma vascular endothelial growth factor A in major depressive disorder not normalized after antidepressant treatment: A case control study. Aust. N. Z. J. Psychiatry 54, 402–408. doi: 10.1177/0004867419893433
Ripke, S., Wray, N., Lewis, C., Hamilton, S., Weissman, M., et al. (2013). A mega-analysis of genome-wide association studies for major depressive disorder. Mol. Psychiatry 18, 497–511. doi: 10.1038/mp.2012.21
Rosenstein, J., Mani, N., Khaibullina, A., and Krum, J. (2003). Neurotrophic effects of vascular endothelial growth factor on organotypic cortical explants and primary cortical neurons. J. Neurosci. 23, 11036–11044. doi: 10.1523/JNEUROSCI.23-35-11036.2003
Rosoklija, G., Toomayan, G., Ellis, S., Keilp, J., Mann, J., Latov, N., et al. (2000). Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: Preliminary findings. Arch. Gen. Psychiatry 57, 349–356. doi: 10.1001/archpsyc.57.4.349
Ruiz de Almodovar, C., Lambrechts, D., Mazzone, M., and Carmeliet, P. (2009). Role and therapeutic potential of VEGF in the nervous system. Physiol. Rev. 89, 607–648. doi: 10.1152/physrev.00031.2008
Samaddar, S., Ranasinghe, B., Tantry, S., Debata, P., and Banerjee, P. (2015). Involvement of vascular endothelial growth factor in serotonin 1A receptor-mediated neuroproliferation in neonatal mouse hippocampus. Adv. Exp. Med. Biol. 842, 375–388. doi: 10.1007/978-3-319-11280-0_23
Scho, S., Brüchle, W., Schneefeld, J., and Rosenkranz, K. (2024). Enhancing neuroplasticity in major depression: A novel 10 Hz-rTMS protocol is more effective than iTBS. J. Affect. Disord. 367, 109–117. doi: 10.1016/j.jad.2024.08.166
Senger, D., Galli, S., Dvorak, A., Perruzzi, C., Harvey, V., and Dvorak, H. (1983). Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219, 983–985. doi: 10.1126/science.6823562
Shang, B., Wang, T., Zhao, S., Yi, S., Zhang, T., Yang, Y., et al. (2024). Higher Blood-brain barrier permeability in patients with major depressive disorder identified by DCE-MRI imaging. Psychiatry Res. Neuroimaging 337:111761. doi: 10.1016/j.pscychresns.2023.111761
Shen, Q., Goderie, S., Jin, L., Karanth, N., Sun, Y., Abramova, N., et al. (2004). Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 304, 1338–1340. doi: 10.1126/science.1095505
Simoes Braga Boisserand, L., Bouchart, J., Geraldo, L. H., Lee, S., Sanganahalli, B. G., Parent, M., et al. (2023). VEGF-C promotes brain-derived fluid drainage, confers neuroprotection, and improves stroke outcomes. bioRxiv [Preprint] doi: 10.1101/2023.05.30.542708
Simons, M., and Eichmann, A. (2015). Molecular controls of arterial morphogenesis. Circ. Res. 116, 1712–1724. doi: 10.1161/CIRCRESAHA.116.302953
Sjöberg, E., Melssen, M., Richards, M., Ding, Y., Chanoca, C., Chen, D., et al. (2023). Endothelial VEGFR2-PLCγ signaling regulates vascular permeability and antitumor immunity through eNOS/Src. J. Clin. Invest. 133:e161366. doi: 10.1172/JCI161366
Sorri, A., Järventausta, K., Kampman, O., Lehtimäki, K., Björkqvist, M., Tuohimaa, K., et al. (2021). Electroconvulsive therapy increases temporarily plasma vascular endothelial growth factor in patients with major depressive disorder. Brain Behav. 11:e02001. doi: 10.1002/brb3.2001
Speliotes, E., Willer, C., Berndt, S., Monda, K., Thorleifsson, G., Jackson, A., et al. (2010). Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 42, 937–948. doi: 10.1038/ng.686
Stockmeier, C., Mahajan, G., Konick, L., Overholser, J., Jurjus, G., Meltzer, H., et al. (2004). Cellular changes in the postmortem hippocampus in major depression. Biol. Psychiatry 56, 640–650. doi: 10.1016/j.biopsych.2004.08.022
Sullivan, P., Neale, M., and Kendler, K. (2000). Genetic epidemiology of major depression: Review and meta-analysis. Am. J. Psychiatry 157, 1552–1562. doi: 10.1176/appi.ajp.157.10.1552
Sweeney, M., Zhao, Z., Montagne, A., Nelson, A., and Zlokovic, B. (2019). Blood-brain barrier: From physiology to disease and back. Physiol. Rev. 99, 21–78. doi: 10.1152/physrev.00050.2017
Takamiya, A., Kishimoto, T., and Mimura, M. (2023). What can we tell about the effect of electroconvulsive therapy on the human hippocampus? Clin. EEG Neurosci. 54, 584–593. doi: 10.1177/15500594211044066
Tillo, M., Ruhrberg, C., and Mackenzie, F. (2012). Emerging roles for semaphorins and VEGFs in synaptogenesis and synaptic plasticity. Cell. Adh. Migr. 6, 541–546. doi: 10.4161/cam.22408
Trivedi, M., Rush, A., Wisniewski, S., Nierenberg, A., Warden, D., Ritz, L., et al. (2006). Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. Am. J. Psychiatry 163, 28–40. doi: 10.1176/appi.ajp.163.1.28
Tsai, S., Kao, C., Su, T., Li, C., Lin, W., Hong, C., et al. (2023). Cytokine- and vascular endothelial growth factor-related gene-based genome-wide association study of low-dose ketamine infusion in patients with treatment-resistant depression. CNS Drugs 37, 243–253. doi: 10.1007/s40263-023-00989-7
Uslu, E., and Yildiz, S. (2024). Is serum VEGF-a level an indicator of early-onset poststroke depression? Medicina 60:1828. doi: 10.3390/medicina60111828
Van Den Bossche, M., Emsell, L., Dols, A., Vansteelandt, K., De Winter, F., Van den Stock, J., et al. (2019). Hippocampal volume change following ECT is mediated by rs699947 in the promotor region of VEGF. Transl. Psychiatry 9:191. doi: 10.1038/s41398-019-0530-6
Vaseghi, S., Mostafavijabbari, A., Alizadeh, M., Ghaffarzadegan, R., Kholghi, G., and Zarrindast, M. (2023). Intricate role of sleep deprivation in modulating depression: Focusing on BDNF, VEGF, serotonin, cortisol, and TNF-α. Metab. Brain Dis. 38, 195–219. doi: 10.1007/s11011-022-01124-z
Viikki, M., Anttila, S., Kampman, O., Illi, A., Huuhka, M., Setälä-Soikkeli, E., et al. (2010). Vascular endothelial growth factor (VEGF) polymorphism is associated with treatment resistant depression. Neurosci. Lett. 477, 105–108. doi: 10.1016/j.neulet.2010.04.039
Wälchli, T., Wacker, A., Frei, K., Regli, L., Schwab, M., Hoerstrup, S., et al. (2015). Wiring the vascular network with neural cues: A CNS perspective. Neuron 87, 271–296. doi: 10.1016/j.neuron.2015.06.038
Wang, H., Ding, R., Jiang, W., Li, S., Wu, Y., Mao, J., et al. (2025). Effects of anti-VEGF on peripapillary retinal nerve fiber layer and papillary/peripapillary blood circulation in retinopathies (Review). Int. J. Mol. Med. 56:133. doi: 10.3892/ijmm.2025.5574
Wang, H., Ran, H., Yin, Y., Xu, X., Jiang, B., Yu, S., et al. (2022). Catalpol improves impaired neurovascular unit in ischemic stroke rats via enhancing VEGF-PI3K/AKT and VEGF-MEK1/2/ERK1/2 signaling. Acta Pharmacol. Sin. 43, 1670–1685. doi: 10.1038/s41401-021-00803-4
Wang, J., Wan, D., Wan, G., Wang, J., Zhang, J., and Zhu, H. (2019). Catalpol induces cell activity to promote axonal regeneration via the PI3K/AKT/mTOR pathway in vivo and in vitro stroke model. Ann. Transl. Med. 7:756. doi: 10.21037/atm.2019.11.101
Wang, J., Wu, X., Fang, J., and Li, Q. (2024). Intervention of exogenous VEGF protect brain microvascular endothelial cells from hypoxia-induced injury by regulating PLCγ/RAS/ERK and PI3K/AKT pathways. Exp. Gerontol. 192:112452. doi: 10.1016/j.exger.2024.112452
Wang, L., Li, X., Gao, Y., Wang, S., Zhao, L., Dong, J., et al. (2015). Activation of VEGF/Flk-1-ERK pathway induced blood-brain barrier injury after microwave exposure. Mol. Neurobiol. 52, 478–491. doi: 10.1007/s12035-014-8848-9
Warner-Schmidt, J., and Duman, R. S. (2007). VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc. Natl. Acad. Sci. U S A. 104, 4647–4652. doi: 10.1073/pnas.0610282104
Warner-Schmidt, J., and Duman, R. S. (2008). VEGF as a potential target for therapeutic intervention in depression. Curr. Opin. Pharmacol. 8, 14–19. doi: 10.1016/j.coph.2007.10.013
Wartiovaara, U., Salven, P., Mikkola, H., Lassila, R., Kaukonen, J., Joukov, V., et al. (1998). Peripheral blood platelets express VEGF-C and VEGF which are released during platelet activation. Thromb. Haemost. 80, 171–175.
Watanabe, R., Kakeda, S., Watanabe, K., Liu, X., Katsuki, A., Umeno-Nakano, W., et al. (2017). Relationship between the hippocampal shape abnormality and serum cortisol levels in first-episode and drug-naïve major depressive disorder patients. Depress Anxiety 34, 401–409. doi: 10.1002/da.22604
Willard, S., Riddle, D., Forbes, M., and Shively, C. (2013). Cell number and neuropil alterations in subregions of the anterior hippocampus in a female monkey model of depression. Biol. Psychiatry 74, 890–897. doi: 10.1016/j.biopsych.2013.03.013
Wu, M., Gong, Y., Jiang, L., Zhang, M., Gu, H., Shen, H., et al. (2022). VEGF regulates the blood-brain barrier through MMP-9 in a rat model of traumatic brain injury. Exp. Ther. Med. 24:728. doi: 10.3892/etm.2022.11664
Wu, Y., Zhu, Z., Lan, T., Li, S., Li, Y., Wang, C., et al. (2024). Levomilnacipran improves lipopolysaccharide-induced dysregulation of synaptic plasticity and depression-like behaviors via activating BDNF/TrkB Mediated PI3K/Akt/mTOR signaling pathway. Mol. Neurobiol. 61, 4102–4115. doi: 10.1007/s12035-023-03832-8
Xie, T., Stathopoulou, M., de Andrés, F., Siest, G., Murray, H., Martin, M., et al. (2017). VEGF-related polymorphisms identified by GWAS and risk for major depression. Transl. Psychiatry 7:e1055. doi: 10.1038/tp.2017.36
Xing, Y., Zhang, Y., Li, C., Luo, L., Hua, Y., Hu, J., et al. (2023). Repetitive transcranial magnetic stimulation of the brain after ischemic stroke: Mechanisms from animal models. Cell. Mol. Neurobiol. 43, 1487–1497. doi: 10.1007/s10571-022-01264-x
Xu, B., Lin, C., Wang, Y., Wang, H., Liu, Y., and Wang, X. (2024). Using dual-target rTMS, Single-target rTMS, or sham rTMS on post-stroke cognitive impairment. J. Integr. Neurosci. 23:161. doi: 10.31083/j.jin2308161
Xu, Q., Liang, R., Gao, J., Fan, Y., Dong, J., Wang, L., et al. (2024). rTMS Ameliorates time-varying depression and social behaviors in stimulated space complex environment associated with VEGF signaling. Life Sci. Space Res. 42, 17–26. doi: 10.1016/j.lssr.2024.04.001
Zachary, I. (2003). VEGF signalling: Integration and multi-tasking in endothelial cell biology. Biochem. Soc. Trans. 31, 1171–1177. doi: 10.1042/bst0311171
Zarate, C., Singh, J., Carlson, P., Brutsche, N., Ameli, R., Luckenbaugh, D., et al. (2006). A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 63, 856–864. doi: 10.1001/archpsyc.63.8.856
Zeng, J., Xie, Z., Chen, L., Peng, X., Luan, F., Hu, J., et al. (2024). Rosmarinic acid alleviate CORT-induced depressive-like behavior by promoting neurogenesis and regulating BDNF/TrkB/PI3K signaling axis. Biomed. Pharmacother. 170:115994. doi: 10.1016/j.biopha.2023.115994
Zhang, T., Tang, X., Wei, Y., Xu, L., Hu, Y., Cui, H., et al. (2023). Serum angioneurin levels following electroconvulsive therapy for mood disorders. Bipolar Disord. 25, 671–682. doi: 10.1111/bdi.13317
Zhang, Z., Zhang, L., Jiang, Q., Zhang, R., Davies, K., Powers, C., et al. (2000). VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J. Clin. Invest. 106, 829–838. doi: 10.1172/JCI9369
Zhang, Z., Zhang, Y., Zhou, Z., Shi, H., Qiu, X., Xiong, J., et al. (2017). BDNF regulates the expression and secretion of VEGF from osteoblasts via the TrkB/ERK1/2 signaling pathway during fracture healing. Mol. Med. Rep. 15, 1362–1367. doi: 10.3892/mmr.2017.6110
Zheng, W., Gu, L., Zhou, Y., Wang, C., Lan, X., Zhang, B., et al. (2021). Association of VEGF with antianhedonic effects of repeated-dose intravenous ketamine in treatment-refractory depression. Front. Psychiatry 12:780975. doi: 10.3389/fpsyt.2021.780975
Keywords: vascular endothelial growth factor, brain-derived neurotrophic factor, depression, blood-brain barrier, ketamine
Citation: Wang J, Meng F, Wang L and Li Z (2025) Vascular endothelial growth factor: a key factor in the onset and treatment of depression. Front. Cell. Neurosci. 19:1645437. doi: 10.3389/fncel.2025.1645437
Received: 11 June 2025; Accepted: 14 August 2025;
Published: 02 September 2025.
Edited by:
Shingo Miyata, Kindai University, JapanReviewed by:
Yasuyuki Ishikawa, Maebashi Institute of Technology, JapanJosé Luis Castañeda-Cabral, University of Guadalajara, Mexico
Copyright © 2025 Wang, Meng, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zeguang Li, d2ppbmdreUAxNjMuY29t
 Jing Wang1
Jing Wang1 Zeguang Li
Zeguang Li