- 1Graduate School, Guangxi University of Chinese Medicine, Nanning, Guangxi, China
- 2Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine, Nanning, Guangxi, China
Spinal cord injury (SCI) is a serious disorder that affects sensory, motor, and autonomic functions. Its pathological process is divided into two stages: primary and secondary injury. The secondary injury involves a variety of biological cascade reactions, leading to an imbalance in the spinal cord microenvironment. Non-coding RNAs (ncRNAs) play a crucial regulatory role in the pathophysiological process of spinal cord injury, including long non-coding RNAs (lncRNAs), circular RNAs (circRNAs), and microRNAs (miRNAs), all of which are involved in processes such as axonal regeneration, oxidative stress, inflammatory response, autophagy, and apoptosis. Although the pathophysiological process of spinal cord injury has been partially elucidated, its pathogenesis is not yet fully understood, and effective treatments are limited. This article reviews the regulatory role and molecular mechanisms of ncRNAs in the development and progression of spinal cord injury and proposes strategies for treating spinal cord injury by regulating ncRNAs.
1 Introduction
Spinal cord injury (SCI) is one of the most complex disorder, with pathological consequences that affect sensory, motor, and/or autonomic functions (Costăchescu et al., 2022; Li et al., 2020). The primary reason for this is that the spinal cord is the main communication system between the brain and the body, ensuring the exchange of information and signals for coordinated activities (Costăchescu et al., 2022). Its injury can lead to interruptions in neural circuits and connections, resulting in neural dysfunction (Yuan et al., 2021). Specifically, spinal cord injury can lead to damage to blood flow, respiration, body temperature, body pressure, and sensation, as well as permanent consequences such as paralysis, autonomic dysfunction, and neuropathic pain (Costăchescu et al., 2022).
The pathological process of spinal cord injury is divided into two stages. The primary injury is the first stage, which includes the death of neurons and glial cells, bleeding, foreign body invasion, and disruption of the axonal network (Ahuja et al., 2020). The second stage is secondary injury, which can last for several weeks and involves a series of biological cascade reactions, such as neuroexcitotoxicity, vascular dysfunction, inflammatory damage, apoptosis, free radical production, and lipid peroxidation (Ahuja et al., 2020; Anjum et al., 2020). At the same time, these factors significantly contribute to the imbalance of the spinal cord microenvironment. However, our understanding of the spinal cord microenvironment after spinal cord injury remains very limited (Seblani et al., 2023; Ortega et al., 2023; Chio et al., 2021; Peng et al., 2024). The “microenvironmental imbalance” after spinal cord injury is defined as the loss of homeostatic balance in tissues, cells, and molecules at different times and locations, which exacerbates and accelerates the progression of spinal cord injury (Peng et al., 2024; Fan et al., 2018). Studies have found that this process may involve abnormal gene expression, such as ncRNAs playing an important regulatory role in the pathophysiology of spinal cord injury.
Researchers have discovered that various non-coding RNAs (ncRNAs), such as long non-coding RNAs (lncRNAs), circular RNAs (circRNAs), and microRNAs (miRNAs), exhibit differential expression following central nervous system (CNS) injuries,such as spinal cord injury (Li et al., 2021). Furthermore, lncRNAs and circRNAs can function as competing endogenous RNAs (ceRNAs) to sponge and inhibit the expression of miRNAs, thereby creating complex regulatory networks (Guo et al., 2025). Existing studies have demonstrated that ncRNAs, including circRNAs (Xu et al., 2021; Yuan et al., 2020), lncRNAs (Zhou and Yu, 2021; Cai et al., 2023), and miRNAs (Baichurina et al., 2021; Xu et al., 2024), are involved in the pathophysiological processes of spinal cord injury, such as axonal regeneration, oxidative stress, inflammatory responses, autophagy, and apoptosis.
Although basic research has clarified the pathophysiological processes of spinal cord injury, the underlying pathogenic mechanisms remain incompletely understood, and effective treatment options are still limited. Therefore, this article aims to review and categorize the regulatory roles and molecular mechanisms of ncRNAs in the development and progression of spinal cord injury and proposes strategies for treating spinal cord injury by targeting the pathogenic mechanisms of ncRNAs.
2 NcRNA and spinal cord injury
ncRNAs refer to RNA molecules that do not have the potential to encode proteins, making up the vast majority of RNAs and accounting for approximately 98–99% of the RNA produced by the mammalian genome (Arraiano, 2021; Sun et al., 2022). This category includes RNAs with specific functions, such as rRNA, tRNA, snRNA, snoRNA, and miRNA. Additionally, lncRNA and circRNA are new members of the non-coding RNA family that can act as sponges for miRNAs, thereby reducing their expression levels (Yao X, et al., 2024). However, increasing evidence indicates that ncRNAs play a crucial role in spinal cord injury, suggesting their significant potential in the diagnosis, evaluation, and treatment of spinal cord injury.
MiRNAs are highly conserved single-stranded ncRNAs typically composed of 20–22 nucleotides (Yao X, et al., 2024). The typical function of miRNAs is to negatively regulate gene expression by binding to target mRNAs, leading to mRNA degradation or inhibition of translation (Yao X, et al., 2024). Studies have shown that each miRNA can target hundreds of genes and can regulate more than one-third of human genes (Sun et al., 2022), playing a role in the regulation of neurological disorders and disorders associated with nerve trauma (Arzhanov et al., 2022; Silvestro and Mazzon, 2022). For example, miR-7b-3p plays a dual role in supporting cortical plasticity and neuroprotection after spinal cord injury (Ghibaudi et al., 2021). The overexpression of miR-423-5p can act as a polarizing regulator of microglia, inhibiting the polarization of the M1 phenotype by suppressing the expression of NLRP3 (NOD-like receptor family pyrin domain-containing 3), and can be used for the treatment of spinal cord injury (Cheng et al., 2021).
LncRNAs are a class of RNA transcripts longer than 200 nucleotides that, despite lacking the ability to encode proteins, resemble mRNA (Salvatori et al., 2020). They possess various epigenetic regulatory forms, including DNA methylation, histone modification, and regulation of miRNAs (Yao X, et al., 2024). Additionally, numerous studies indicate that lncRNAs play significant roles in development, metabolism, as well as in the function of the nervous and immune systems (Chen and Kim, 2024). For instance, lncAirsci is significantly upregulated during the acute inflammatory phase of spinal cord injury. However, the inhibition of lncAirsci can alleviate the inflammatory response through NF-κB (Nuclear factor-κB) signaling pathway, promoting functional recovery (Zhang T, et al., 2021).
CircRNAs are generated from precursor mRNA through back-splicing of exons and are widely expressed in tissue-specific and developmental stage-specific patterns (Yao X, et al., 2024). Increasing evidence suggests that circRNAs regulate various cellular processes by acting as miRNA sponges, anchors for cRBPs (circRNA-binding proteins), transcriptional regulators, molecular scaffolds, and sources for the translation of small proteins/peptides (Misir et al., 2022). Unlike linear RNAs, circRNAs are circular molecules with covalently closed loop structures and are involved in a wide range of biological processes. Disruptions in their expression can lead to cellular dysfunction and disorder (Chen, 2016). A substantial body of evidence indicates that circRNAs are highly expressed in the spinal cord and play crucial roles in multiple processes of neurological disorders. For example, CircHIPK3 mitigates inflammation and neuronal apoptosis after spinal cord injury by regulating the miR-382-5p/DUSP1 (Dual-specificity phosphatase 1) axis (Yin et al., 2022). CircCDR1 as regulates scar formation, inflammation, and neural regeneration after spinal cord injury through the miR-7a-5p/TGF-β (Transforming growth factor-β) R2 axis (Wang et al., 2024).
3 NcRNA regulation of synaptic function after spinal cord injury
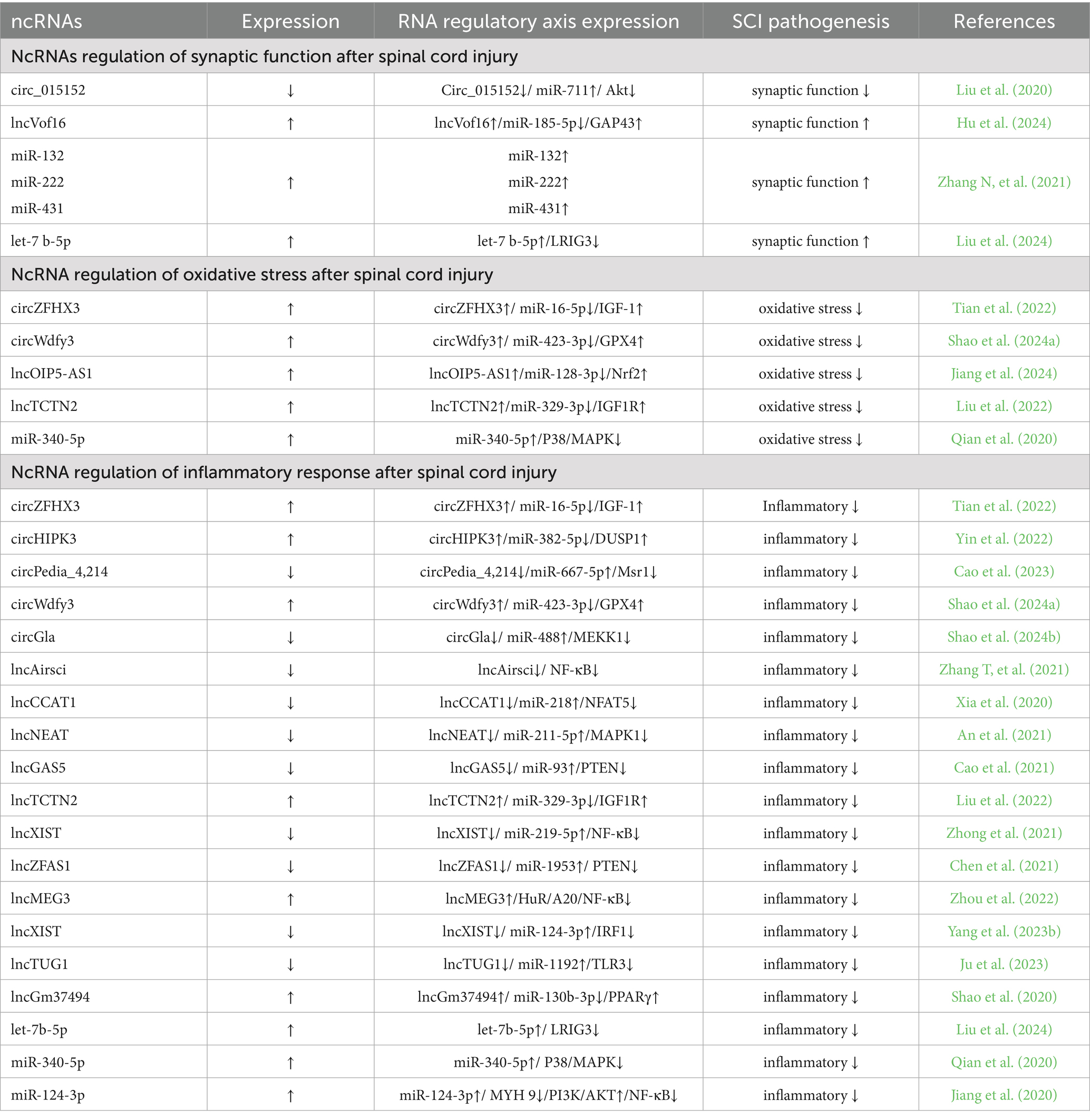
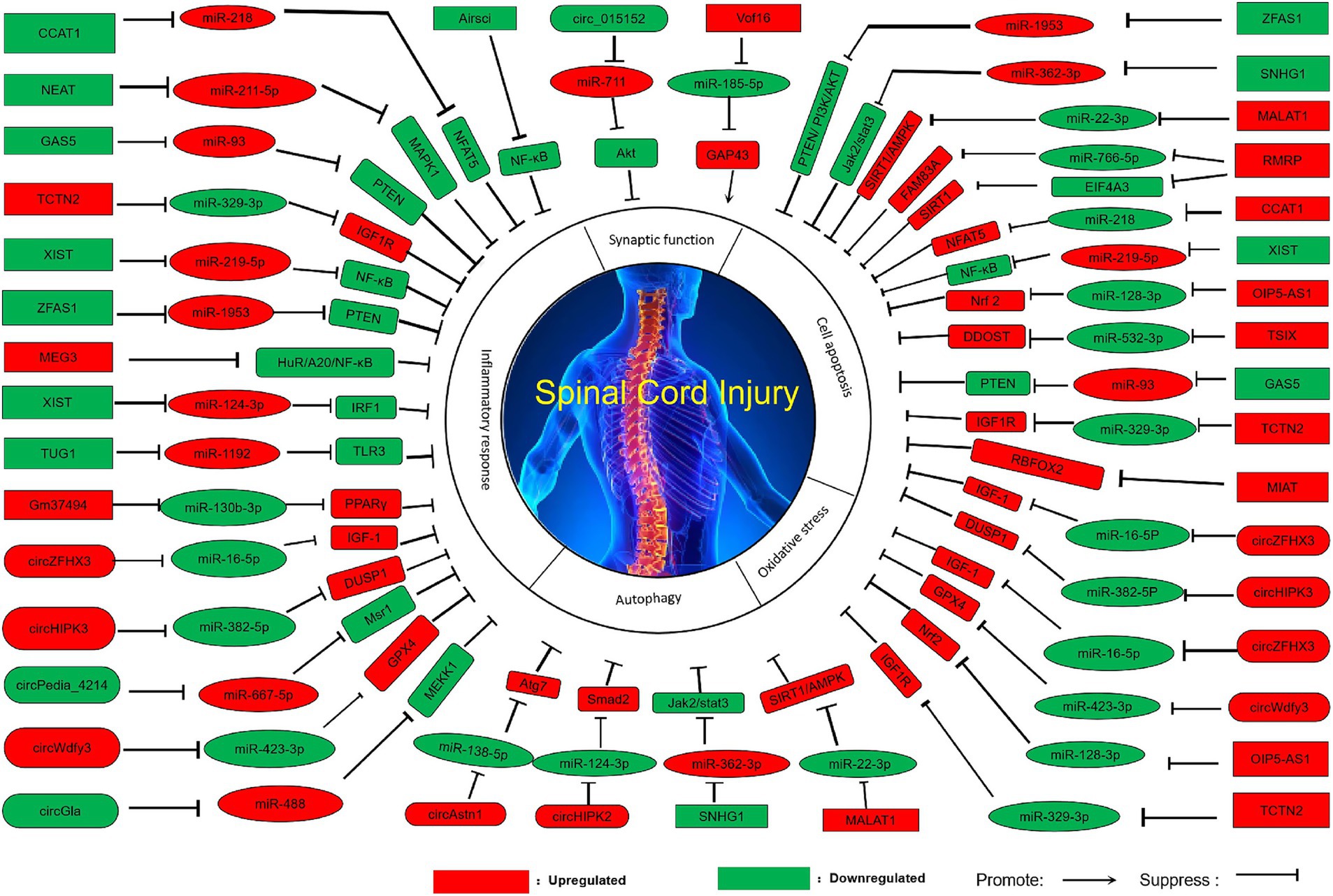
After spinal cord injury, inadequate axonal regeneration often leads to poor recovery, which is one of the most pressing challenges in the treatment of spinal cord injury (Bie et al., 2021). Developing successful regenerative strategies to reconnect axons within the central nervous system is crucial for spinal cord injury research (Shi et al., 2024). A large body of research results indicate that the biological functions of ncRNA are related to synaptic function (Table 1; Figure 1).
Circ_015152 can act as a sponge for miR-711. When its expression is reduced, the expression of miR-711 increases, inhibiting the activation of the Akt (Protein kinase B) pathway, thereby promoting axonal damage in the spinal cord (Liu et al., 2020). High expression of lncVof16 reduces the expression level of miR-185-5p through the miR-185-5p/GAP43 (Growth-associated protein 43) axis, thereby indirectly increasing the expression of GAP43, enhancing self-repair and promoting axonal growth to improve the prognosis after spinal cord injury (Hu et al., 2024). Overexpression of miR-132, miR-222, and miR-431 can significantly enhance axonal regeneration and functional recovery (Zhang N, et al., 2021).
Overexpression of let-7b-5p maintains the integrity of myelin by inhibiting its downstream target gene LRIG3 (Immunoglobulin domain-containing protein 3), and promotes axonal growth, ultimately restoring the functional ability of spinal cord injury mice (Liu et al., 2024). Perhaps by regulating the expression of ncRNAs, we can improve axonal regeneration function and thus achieve recovery and improvement of motor function.
Extensive studies have demonstrated that exosomes serve as critical intercellular communication tools for transferring ncRNAs between neurons and bodily fluids (Wang et al., 2022). Additionally, they possess the advantage of acting as drug delivery vehicles that can transport therapeutic agents to recipient cells without activating the immune system (Guo et al., 2025), suggesting that the future combination of exosomes and ncRNAs may become a key strategy for improving spinal cord injury treatment. Furthermore, different ncRNAs exhibit distinct functional roles, and future research might focus on coordinated multi-target regulation to achieve enhanced synaptic function repair, thereby optimizing therapeutic outcomes.
4 NcRNA regulates oxidative stress after spinal cord injury
Many experimental and clinical studies have found the key role of ROS (Reactive oxygen species) and lipid peroxidation in the development of spinal cord injury (Chio et al., 2022; Hou et al., 2021), the main reason is that the process of spinal cord injury development is accompanied by excessive production of free radicals, among which oxidative stress causes the spinal cord to be susceptible to oxidative damage, thereby triggering oxidative stress (Zhang H, et al., 2024; Yao H, et al., 2024). At the same time, a large number of studies have shown that ncRNAs play an important regulatory role in oxidative stress, and the abnormal expression of ncRNAs causes the occurrence of oxidative stress (Table 1; Figure 1).
Overexpression of circZFHX3 (Tian et al., 2022), circWdfy3 (Shao et al., 2024a), lncTCTN2 (Liu et al., 2022), miR-340-5p (Qian et al., 2020) increases their expression levels by directly or indirectly acting on downstream targets, thereby enhancing cell viability, reducing ROS accumulation, reducing oxidative stress, and promoting the recovery of motor function. In addition, overexpression of lncOIP5-AS1 improves mitochondrial function and reduces oxidative stress through the miR-128-3p/Nrf2 axis, and the specific mechanism is that overexpression of lncOIP5-AS1 indirectly leads to an increase in Nrf2 levels by increasing the spongy effect on miR-128-3p, thereby improving mitochondrial function, reducing oxidative stress, and promoting the recovery of spinal cord injury (Jiang et al., 2024).
Oxidative stress induced by spinal cord injury is caused by ROS accumulation and lipid peroxidation on the one hand, and mitochondrial function impairment on the other hand (Cui et al., 2025). Therefore, activation of antioxidant pathway cannot completely solve the damage caused by oxidative stress. Meanwhile, mitochondrial function should be repaired. By regulating the genes related to mitochondrial dynamics controlled by exosomes, mitochondrial membrane potential and ATP synthesis should be improved to alleviate energy metabolism disorders. Exosomal RNA has regulatory effects in both aspects. Therefore, the effect of exosomal RNA treatment is better than antioxidant treatment alone.
5 NcRNA regulation of inflammatory response after spinal cord injury
Inflammation is considered an important pathological process in the secondary injury phase, which can directly or indirectly determine the therapeutic effect of spinal cord injury. Inflammatory response can greatly trigger a series of secondary injuries, leading to neuronal death and ultimately resulting in neurological dysfunction after injury (Lu et al., 2024). Numerous studies have shown that ncRNAs play a crucial role in regulating the inflammatory response, which may become an important means of treating and improving spinal cord injury (Table 1; Figure 1).
The mechanism of action of low-expression circGla is that circGla, as a competitive endogenous RNA of miR-488, indirectly reduces the expression of MEKK1 by acting as a sponge, thereby reducing the inflammatory state of astrocytes (Shao et al., 2024b). Overexpression of circZFHX3 activates microglia, promotes cell viability, and inhibits inflammatory responses (Tian et al., 2022). Overexpression of circHIPK3 can increase DUSP1 expression through the miR-382-5p/DUSP1 axis, thereby reducing the cellular inflammatory response (Yin et al., 2022). Low expression circPedia-4214 promote macrophage M2 polarization and participate in the immuno-inflammatory response (Cao et al., 2023). Overexpression of circWdfy3 reduces the accumulation of inflammatory factors and improves the prognosis after spinal cord injury (Shao et al., 2024a).
Low-expression lncZFAS1 indirectly inhibits PTEN expression by binding to miR-1953, thereby indirectly activating the PI3K/AKT pathway to further inhibit the inflammatory response, thereby promoting spinal cord function recovery after spinal cord injury (Chen et al., 2021). In addition, low expression of lncAirsci (Zhang T, et al., 2021), lncNEAT1 (An et al., 2021), lncGAS5 (Cao et al., 2021), lncXIST (Zhong et al., 2021) can reduce the inflammatory response, thereby promoting functional recovery after spinal cord injury. Overexpression of lncCCAT1 (Xia et al., 2020) and lncTCTN2 indirectly increases the expression level of downstream proteins through spongy action on downstream miRNAs, thereby reducing the inflammatory response and promoting functional recovery of spinal cord injury (Liu et al., 2022).
Overexpression of lncMEG3 (Zhou et al., 2022) and lncGm37494 (Shao et al., 2020) and low expression of lncXIST (Yang et al., 2023b) and lncTUG1 (Ju et al., 2023) inhibit M1-type polarization of microglia through indirect regulation of downstream targets, promote M2-type polarization, and secrete anti-inflammatory factors to reduce inflammatory response. In addition, overexpression of let-7b-5p attenuated pyroptosis in microglia/macrophages by inhibiting its downstream target gene LRIG3, thereby reducing the secondary inflammatory response after spinal cord injury (Liu et al., 2024).
Overexpression of miR-124-3p reduces neuroinflammation through the MYH9/PI3K/AKT/NF-κB signaling pathway, and the main mechanism is that overexpression of miR-124-3p can inhibit MYH9, and inhibition of the MYH9 signaling pathway can activate the PI3K/AKT signaling pathway and inhibit the NF-κB signaling pathway, which in turn inhibits A1 astrocytes, thereby inhibiting the activation of M1 microglia and microglia-induced neuroinflammatory response (Jiang et al., 2020). Overexpression of miR-340-5p can reduce the inflammatory response by enhancing the inhibition of the downstream target P38-MAPK signaling pathway (Qian et al., 2020).
It can be seen that inflammation is the dominant factor in the pathological mechanism of spinal cord injury, and ncRNAs play an important role in the regulation of inflammatory response, which can achieve indirect regulation of inflammatory response by regulating the expression of ncRNAs, thereby improving spinal cord injury. However, for inflammatory response, anti-inflammatory alone cannot completely solve the progression of the disorder. Studies have shown that inflammation-oxidative stress is intermodulated (Xiong et al., 2023), so that the best therapeutic effect can be achieved through a dual antioxidant-anti-inflammatory targeting strategy.
6 NcRNA regulation of autophagy after spinal cord injury
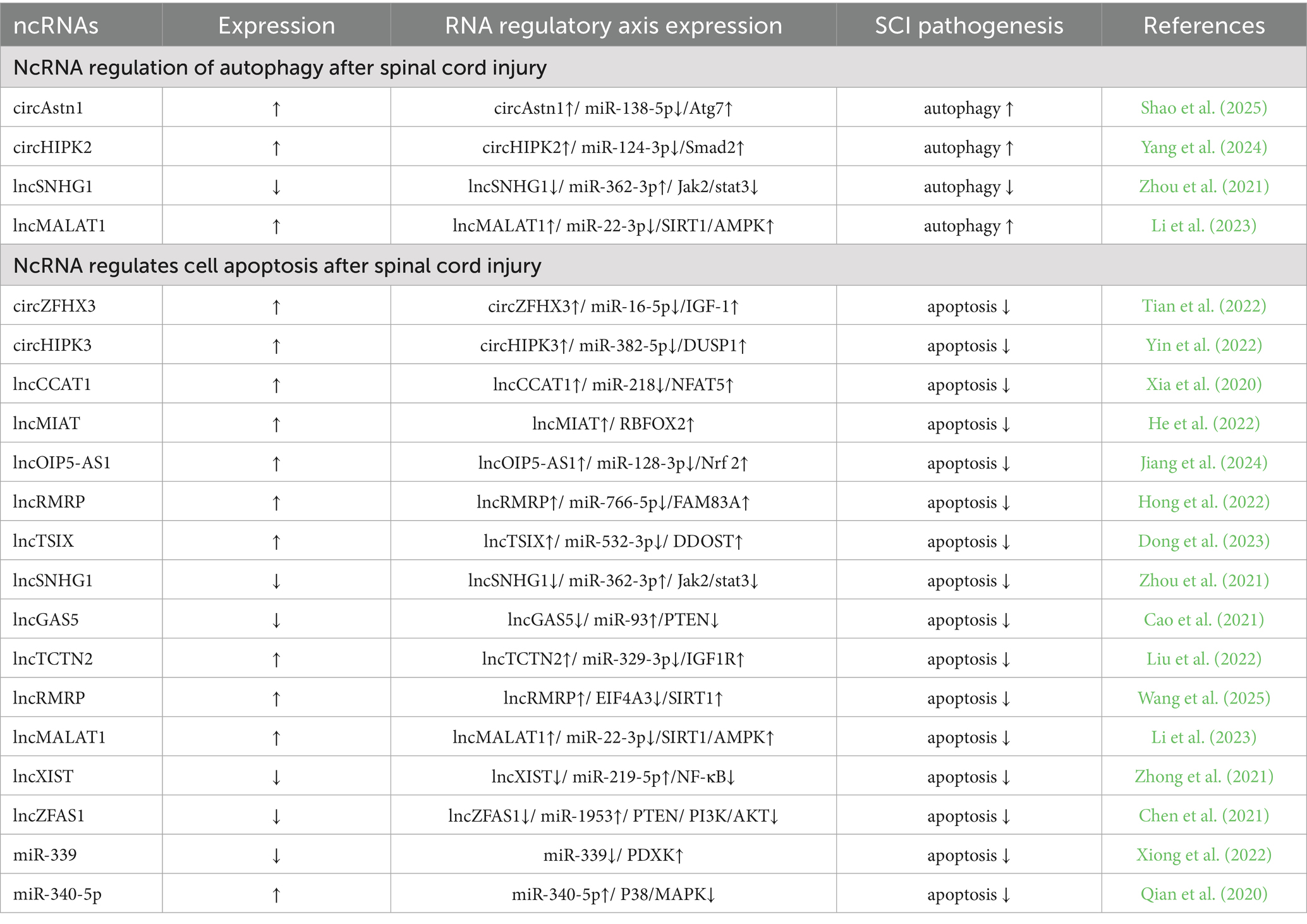
Autophagy is a lysosomal degradation pathway for cytoplasmic components and organelles, which is crucial for maintaining cellular homeostasis and defending against external stress (Yao H, et al., 2024; Li et al., 2022). Studies have found that autophagy plays an important role in improving spinal cord injury, as it alleviates nerve damage by regulating microtubule dynamics and mediating axonal regeneration, thereby exerting neuroprotective effects on spinal cord injury (Geng et al., 2024). Additionally, autophagy can inhibit systemic inflammatory responses, reduce tissue damage and neuronal cell death induced by inflammatory cascades, and promote the recovery of neurological function (Zhang H, et al., 2024). Through continuous exploration, it has been discovered that ncRNAs play an important role in the regulation of autophagy, and perhaps by indirectly regulating the expression of ncRNAs to achieve the regulation of autophagy, it can become a new treatment method for spinal cord injury (Table 2; Figure 1).
High expression of circAstn1 activates autophagy through the miR-138-5p/Atg7 (Autophagy related 7) pathway to promote spinal cord repair after injury. Both miR-138-5p and Atg7 are downstream targets of circAstn1, and high expression of circAstn1 enhances its sponge effect on miR-138-5p, reducing its expression and indirectly increasing Atg7 expression (Shao et al., 2025). Overexpression of circHIPK2 promotes autophagy and endoplasmic reticulum (ER) stress through the miR-124-3p/Smad2 pathway, further enhancing the activation of A1 astrocytes after spinal cord injury (Yang et al., 2024).
Low expression of lncSNHG1 reduces the sponge effect on miR-362-3p, indirectly inactivating the Jak2/Stat3 pathway and reducing cell autophagy (Zhou et al., 2021). Overexpression of lncMALAT1 promotes the SIRT1 (Sirtuin 1) /AMPK (AMP-activated protein kinase) pathway through the miR-22-3p/SIRT1/AMPK axis, activating autophagy and thereby exerting neuroprotective effects, promoting the recovery of neurological function in spinal cord injury (Li et al., 2023).
Autophagy reduces inflammation and oxidative stress by clearing damaged organelles in the early stages of spinal cord injury, but excessive activation can exacerbate neuronal death (Shen et al., 2023), so regulation of autophagy may become an important means to improve spinal cord injury in the future. Exosomes encapsulate ncRNAs (acting as autophagy inhibitors) and target them to the injury site, inhibiting neurodegeneration caused by excessive autophagy, thus achieving motor neural function recovery after spinal cord injury.
7 NcRNA regulates cell apoptosis after spinal cord injury
After spinal cord injury, the adverse microenvironment of the injury, such as ischemia and hypoxia, free radical release, and acute inflammation, leads to the death of neuronal cells (Ji et al., 2024), among which apoptosis is widely considered to be the key to secondary injury, which is the main reason for the deterioration of neurological function after spinal cord injury other than the primary mechanical injury (Yao et al., 2020). In addition, a large number of studies have found that in central nervous system disorders, inhibiting neuronal apoptosis will be beneficial to the recovery of motor function after spinal cord injury (Yin et al., 2022). At the same time, more and more evidence shows that ncRNAs are closely related to cell proliferation and apoptosis after spinal cord injury. Perhaps by regulating the expression of ncRNAs (Table 2; Figure 1), indirect regulation of cell proliferation and apoptosis can be achieved, which can become a new method for treating and improving spinal cord injury.
Overexpression of lncMALAT 1 promotes the SIRT1/AMPK pathway through the miR-22-3p/SIRT1/AMPK axis, inhibits apoptosis in nerve cells, and then exerts a neuroprotective role and promotes the recovery of nerve function after spinal cord injury (Li et al., 2023). In addition, overexpression of circZFHX3 (Tian et al., 2022), circHIPK3 (Yin et al., 2022), lncCCAT1 (Xia et al., 2020), lncMIAT (He et al., 2022), lncOIP5-AS1 (Jiang et al., 2024), lncRMRP (Hong et al., 2022; Wang et al., 2025), lncTSIX (Dong et al., 2023) and lnc TCTN2 (Liu et al., 2022) indirectly increased the expression of downstream proteins, thereby reducing apoptosis and promoting the recovery of motor function. Overexpression of miR-340-5p reduced apoptosis by inhibiting the P38/MAPK pathway, thereby promoting the recovery of neurological function after spinal cord injury (Qian et al., 2020).
Low expression of lncSNHG1 (Zhou et al., 2021), lncGAS5 (Cao et al., 2021), lncXIST (Zhong et al., 2021), and lncZFAS1 (Chen et al., 2021) inhibits apoptosis by inhibiting downstream proteins, thereby promoting spinal cord function recovery after spinal cord injury. Low expression of miR-339 can target PDXK, and PDXK overexpression can significantly improve motor function, increase neuronal activity, reduce neuronal apoptosis, and improve spinal cord injury (Xiong et al., 2022).
In recent years, significant progress has been made in the research on apoptosis caused by exosomal RNA regulation of spinal cord injury, the core of which lies in the regulation of apoptosis-related pathways by non-coding RNAs to inhibit secondary damage and promote nerve repair. At the same time, cell proliferation should also be promoted through exosomal RNA regulation. It can reduce apoptosis and promote cell proliferation, and achieve a better and more effective treatment strategy by inhibiting apoptosis and promoting proliferation.
8 Prospect
Spinal cord injury is a severe central nervous system disorder with complex pathogenesis that often leads to significant disability. However, despite advances in surgical techniques, there is still no effective treatment for this debilitating condition (Karsy and Hawryluk, 2019; Liu et al., 2025). After spinal cord injury, the blood-spinal cord barrier is disrupted, leading to immune microenvironmental disturbances and poor regeneration of the injured spinal cord (Valido et al., 2023; Al Mamun et al., 2021). Due to the decline in immune cell function, spinal cord injury patients exhibit a higher incidence of infections. Individuals experience a transition from the acute to the chronic phase, during which changes in gene expression are also time-dependent (Mun et al., 2022). Therefore, further exploration of the molecular mechanisms and changes in the microenvironment after spinal cord injury is crucial for developing better treatment strategies.
The blood-spinal cord barrier (BSCB), conceptually equivalent to the blood–brain barrier (BBB) in the spinal cord, provides a functional microenvironment similar to that of the BBB for spinal cord cellular components; therefore, the BSCB is considered a morphological extension of the BBB (Bartanusz et al., 2011), spinal cord injury also causes direct vascular damage and significant disruption of the BSCB (Sun et al., 2022; Whetstone et al., 2003). BBB damage has become an important factor in determining the progression and prognosis of central nervous system disorders, but currently, there are no clinical pharmacological treatments that directly address BBB dysfunction (Ihezie et al., 2021). Over the past decade, the involvement and regulatory functions of non-coding RNAs in BBB dysfunction in CNS disorders have been rapidly and extensively studied. A large body of evidence has demonstrated the effectiveness and capacity of miRNAs, lncRNAs, and circRNAs in protecting the BSCB in conditions such as spinal cord injury (Li et al., 2021).
Recent studies have shown that cell therapy plays an important role in the treatment of spinal cord injury. However, the therapeutic effects of cell transplantation in spinal cord injury models are still controversial, and their clinical application is limited by several factors, including potential tumorigenic risks (Zhang M, et al., 2024) and ethical concerns (Margiana et al., 2022). However, research indicates that exosomes derived from stem cells have anti-inflammatory effects and play an irreplaceable role in the treatment of spinal cord injury. As a new type of regenerative medicine therapeutic, they have advantages such as small size, low immunogenicity, and the ability to cross the blood-spinal cord barrier (Zhang et al., 2023).
However, despite the great potential of exosomal ncRNAs, its application as a therapeutic agent still faces significant challenges. One major obstacle is the effective monitoring and guidance of exosomes to reach their target receptor areas. Exosomes are small vesicles released by cells, making them difficult to track and control once administered (Guo et al., 2025; Wang et al., 2022), and there are also issues such as short duration of action. However, biomaterials are of great value in treating and repairing damaged tissues, as well as in assisting drug delivery and release. Emerging biomaterials not only aim to restore the structure and function of damaged tissues but also promote their regeneration through active and targeted interactions.
Compared with traditional drug interventions and surgical treatments, the use of biomaterial scaffolds can reduce some of the complex side effects of drugs and obstacles to functional recovery after surgery. However, biomaterials may be recognized as foreign objects by the patient’s immune system, triggering inflammatory reactions. This reaction may exacerbate the inflammatory microenvironment after spinal cord injury, further damaging neural tissue. Therefore, it is crucial to develop a treatment method that can target controlled drug delivery with minimal side effects.
Exosomes have strong biological activity but suffer from issues such as short duration of action, while biomaterials (such as hydrogels and scaffolds) can serve as sustained-release carriers for exosomes, prolonging their retention time at the injury site and improving the therapeutic effect (Yang et al., 2023a). The combination of the two can compensate for their respective shortcomings and improve the therapeutic effect. Perhaps in the future, through material engineering and gene editing techniques, the combination of exosomes and biomaterials can be further optimized, such as designing materials with specific degradation rates to match the release kinetics of exosomes, thereby improving the utilization efficiency of exosomes. At the same time, this can also reduce costs and be more conducive to clinical translation. On the premise of verifying the safety and effectiveness of the combination therapy, personalized biomaterial and exosome combination schemes can be designed based on the patient’s specific condition, which is expected to achieve more precise personalized treatment.
9 Conclusion
In recent years, the understanding of the pathological mechanisms of spinal cord injury has deepened, and at the same time, it has been discovered that the abnormal expression of ncRNA plays an increasingly important role in the regulation of the pathological mechanisms of spinal cord injury. ncRNA plays an important role in the pathophysiological process after spinal cord injury, including synaptic regeneration, oxidative stress, inflammatory response, autophagy, and cell proliferation and apoptosis. These processes are interwoven and collectively influence the outcome of spinal cord injury. Based on the critical role of ncRNA in the pathophysiology of spinal cord injury, ncRNA can be regarded as a potential therapeutic target. By regulating ncRNA, these processes can be intervened, providing new strategies for the treatment of spinal cord injury. For example, by designing specific ncRNA mimics or inhibitors, the expression and function of ncRNA can be targeted for regulation, thereby achieving targeted treatment of spinal cord injury.
Author contributions
JB: Formal analysis, Investigation, Methodology, Writing – original draft. WZ: Writing – original draft, Formal analysis, Supervision. SQ: Writing – original draft, Data curation, Supervision. HM: Writing – original draft, Data curation, Supervision. RL: Writing – review & editing, Supervision. CG: Data curation, Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahuja, C. S., Mothe, A., Khazaei, M., Badhiwala, J. H., Gilbert, E. A., van der Kooy, D., et al. (2020). The leading edge: emerging neuroprotective and neuroregenerative cell-based therapies for spinal cord injury. Stem Cells Transl. Med. 9, 1509–1530. doi: 10.1002/sctm.19-0135
Al Mamun, A., Monalisa, I., Tul Kubra, K., Akter, A., Akter, J., Sarker, T., et al. (2021). Advances in immunotherapy for the treatment of spinal cord injury. Immunobiology 226:152033. doi: 10.1016/j.imbio.2020.152033
An, Q., Zhou, Z., Xie, Y., Sun, Y., Zhang, H., and Cao, Y. (2021). Knockdown of long non-coding RNA NEAT1 relieves the inflammatory response of spinal cord injury through targeting miR-211-5p/MAPK1 axis. Bioengineered 12, 2702–2712. doi: 10.1080/21655979.2021.1930925
Anjum, A., Yazid, M. D., Fauzi Daud, M., Idris, J., Ng, A. M. H., Selvi Naicker, A., et al. (2020). Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int. J. Mol. Sci. 21:533. doi: 10.3390/ijms21207533
Arraiano, C. M. (2021). Regulatory noncoding RNAs: functions and applications in health and disease. FEBS J. 288, 6308–6309. doi: 10.1111/febs.16027
Arzhanov, I., Sintakova, K., and Romanyuk, N. (2022). The role of miR-20 in health and disease of the central nervous system. Cells 11:525. doi: 10.3390/cells11091525
Baichurina, I., Valiullin, V., James, V., Rizvanov, A., and Mukhamedshina, Y. (2021). The study of cerebrospinal fluid microRNAs in spinal cord injury and neurodegenerative diseases: methodological problems and possible solutions. Int. J. Mol. Sci. 23:114. doi: 10.3390/ijms23010114
Bartanusz, V., Jezova, D., Alajajian, B., and Digicaylioglu, M. (2011). The blood–spinal cord barrier: morphology and clinical implications. Ann. Neurol. 70, 194–206. doi: 10.1002/ana.22421
Bie, F., Wang, K., Xu, T., Yuan, J., Ding, H., Lv, B., et al. (2021). The potential roles of circular RNAs as modulators in traumatic spinal cord injury. Biomed. Pharmacother. 141:111826. doi: 10.1016/j.biopha.2021.111826
Cai, Z., Han, X., Li, R., Yu, T., Chen, L., Wu, X., et al. (2023). Research progress of long non-coding RNAs in spinal cord injury. Neurochem. Res. 48, 1–12. doi: 10.1007/s11064-022-03720-y
Cao, Y., Jiang, C., Lin, H., and Chen, Z. (2021). Silencing of long noncoding RNA growth arrest-specific 5 alleviates neuronal cell apoptosis and inflammatory responses through sponging microRNA-93 to repress PTEN expression in spinal cord injury. Front. Cell. Neurosci. 15:646788. doi: 10.3389/fncel.2021.646788
Cao, J., Pan, C., Zhang, J., Chen, Q., Li, T., He, D., et al. (2023). Analysis and verification of the circ RNA regulatory network RNO_CIRCpedia_ 4214/RNO-miR-667-5p/Msr 1 axis as a potential ce RNA promoting macrophage M2-like polarization in spinal cord injury. BMC Genomics 24:181. doi: 10.1186/s12864-023-09273-w
Chen, L. L. (2016). The biogenesis and emerging roles of circular RNAs. Nat. Rev. Mol. Cell Biol. 17, 205–211. doi: 10.1038/nrm.2015.32
Chen, L. L., and Kim, V. N. (2024). Small and long non-coding RNAs: Past, present, and future. Cell 187, 6451–6485. doi: 10.1016/j.cell.2024.10.024
Chen, Y., Wei, Z., Liu, J., Xie, H., Wang, B., Wu, J., et al. (2021). Long noncoding RNA ZFAS1 aggravates spinal cord injury by binding with miR-1953 and regulating the PTEN/PI3K/AKT pathway. Neurochem. Int. 147:104977. doi: 10.1016/j.neuint.2021.104977
Cheng, J., Hao, J., Jiang, X., Ji, J., Wu, T., Chen, X., et al. (2021). Ameliorative effects of miR-423-5p against polarization of microglial cells of the M1 phenotype by targeting a NLRP3 inflammasome signaling pathway. Int. Immunopharmacol. 99:108006. doi: 10.1016/j.intimp.2021.108006
Chio, J. C. T., Punjani, N., Hejrati, N., Zavvarian, M. M., Hong, J., and Fehlings, M. G. (2022). Extracellular matrix and oxidative stress following traumatic spinal cord injury: physiological and pathophysiological roles and opportunities for therapeutic intervention. Antioxid. Redox Signal. 37, 184–207. doi: 10.1089/ars.2021.0120
Chio, J. C. T., Xu, K. J., Popovich, P., David, S., and Fehlings, M. G. (2021). Neuroimmunological therapies for treating spinal cord injury: evidence and future perspectives. Exp. Neurol. 341:113704. doi: 10.1016/j.expneurol.2021.113704
Costăchescu, B., Niculescu, A. G., Dabija, M. G., Teleanu, R. I., Grumezescu, A. M., and Eva, L. (2022). Novel strategies for spinal cord regeneration. Int. J. Mol. Sci. 23:4552. doi: 10.3390/ijms23094552
Cui, J., Lin, S., and Zhang, M. (2025). Resveratrol loaded microglia-derived exosomes attenuate astrogliasis by restoring mitochondrial function to reduce spinal cord injury. Chem. Biol. Interact. 408:111407. doi: 10.1016/j.cbi.2025.111407
Dong, J., Wei, Z., and Zhu, Z. (2023). Lnc RNA TSIX aggravates spinal cord injury by regulating the PI3K/AKT pathway via the miR-532-3p/DDOST axis. J. Biochem. Mol. Toxicol. 37:e23384. doi: 10.1002/jbt.23384
Fan, B., Wei, Z., Yao, X., Shi, G., Cheng, X., Zhou, X., et al. (2018). Microenvironment imbalance of spinal cord injury. Cell Transplant. 27, 853–866. doi: 10.1177/0963689718755778
Geng, Y., Lou, J., Wu, J., Tao, Z., Yang, N., Kuang, J., et al. (2024). NEMO-Binding domain/IKKγ inhibitory peptide alleviates neuronal pyroptosis in spinal cord injury by inhibiting ASMase-induced lysosome membrane permeabilization. Adv Sci (Weinh) 11:e2405759. doi: 10.1002/advs.202405759
Ghibaudi, M., Boido, M., Green, D., Signorino, E., Berto, G. E., Pourshayesteh, S., et al. (2021). miR-7b-3p exerts a dual role after spinal cord injury, by supporting plasticity and neuroprotection at cortical level. Front. Mol. Biosci. 8:618869. doi: 10.3389/fmolb.2021.618869
Guo, C. H., You, Y. Q., Chen, J. B., Luo, N., and Li, F. J. (2025). Exosomes and non-coding RNAs: bridging the gap in Alzheimer's pathogenesis and therapeutics. Metab. Brain Dis. 40:84. doi: 10.1007/s11011-024-01520-7
He, X., Zhang, J., Guo, Y., Yang, X., Huang, Y., and Hao, D. (2022). Lnc RNA MIAT promotes spinal cord injury recovery in rats by regulating RBFOX2-mediated alternative splicing of MCL-1. Mol. Neurobiol. 59, 4854–4868. doi: 10.1007/s12035-022-02896-2
Hong, H., Xu, G., Chen, J., Zhang, J., Chen, C., Wu, C., et al. (2022). Lnc RNA RMRP contributes to the development and progression of spinal cord injury by regulating miR-766-5p/FAM83A axis. Mol. Neurobiol. 59, 6200–6210. doi: 10.1007/s12035-022-02968-3
Hou, Y., Luan, J., Huang, T., Deng, T., Li, X., Xiao, Z., et al. (2021). Tauroursodeoxycholic acid alleviates secondary injury in spinal cord injury mice by reducing oxidative stress, apoptosis, and inflammatory response. J. Neuroinflammation 18:216. doi: 10.1186/s12974-021-02248-2
Hu, Y., Sun, Y. F., Yuan, H., Liu, J., Chen, L., Liu, D. H., et al. (2024). Vof 16-miR-185-5p-GAP43 network improves the outcomes following spinal cord injury via enhancing self-repair and promoting axonal growth. CNS Neurosci. Ther. 30:e14535. doi: 10.1111/cns.14535
Ihezie, S. A., Mathew, I. E., McBride, D. W., Dienel, A., Blackburn, S. L., and Thankamani Pandit, P. K. (2021). Epigenetics in blood-brain barrier disruption. Fluids Barriers CNS 18:17. doi: 10.1186/s12987-021-00250-7
Ji, R., Hao, Z., Wang, H., Su, Y., Yang, W., Li, X., et al. (2024). Fisetin promotes functional recovery after spinal cord injury by inhibiting microglia/macrophage M1 polarization and JAK2/STAT3 signaling pathway. J. Agric. Food Chem. 72, 17964–17976. doi: 10.1021/acs.jafc.4c02985
Jiang, D., Gong, F., Ge, X., Lv, C., Huang, C., Feng, S., et al. (2020). Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J Nanobiotechnol. 18:105. doi: 10.1186/s12951-020-00665-8
Jiang, Z., Zhang, W., and Zhang, J. (2024). Lnc RNA OIP5-AS1 regulates ferroptosis and mitochondrial dysfunction-mediated apoptosis in spinal cord injury by targeting the miR-128-3p/Nrf 2 axis. Heliyon 10:e37704. doi: 10.1016/j.heliyon.2024.e37704
Ju, C., Ma, Y., Zuo, X., Wang, X., Song, Z., Zhang, Z., et al. (2023). Photobiomodulation promotes spinal cord injury repair by inhibiting macrophage polarization through lnc RNA TUG1-miR-1192/TLR3 axis. Cell. Mol. Biol. Lett. 28:5. doi: 10.1186/s11658-023-00417-0
Karsy, M., and Hawryluk, G. (2019). Modern medical management of spinal cord injury. Curr. Neurol. Neurosci. Rep. 19:65. doi: 10.1007/s11910-019-0984-1
Li, P., Jia, Y., Tang, W., Cui, Q., Liu, M., and Jiang, J. (2021). Roles of non-coding RNAs in central nervous system axon regeneration. Front. Neurosci. 15:630633. doi: 10.3389/fnins.2021.630633
Li, Y., Lei, Z., Ritzel, R. M., He, J., Li, H., Choi, H. M. C., et al. (2022). Impairment of autophagy after spinal cord injury potentiates neuroinflammation and motor function deficit in mice. Theranostics 12, 5364–5388. doi: 10.7150/thno.72713
Li, K., Liu, Z., Wu, P., Chen, S., Wang, M., Liu, W., et al. (2023). Micro electrical fields induced MSC-sEVs attenuate neuronal cell apoptosis by activating autophagy via lnc RNA MALAT1/miR-22-3p/SIRT1/AMPK axis in spinal cord injury. J Nanobiotechnol. 21:451. doi: 10.1186/s12951-023-02217-2
Li, L., Zhang, Y., Mu, J., Chen, J., Zhang, C., Cao, H., et al. (2020). Transplantation of human mesenchymal stem-cell-derived exosomes immobilized in an adhesive hydrogel for effective treatment of spinal cord injury. Nano Lett. 20, 4298–4305. doi: 10.1021/acs.nanolett.0c00929
Liu, J., Kong, G., Lu, C., Wang, J., Li, W., Lv, Z., et al. (2024). IPSC-NSCs-derived exosomal let-7b-5p improves motor function after spinal cord Injury by modulating microglial/macrophage pyroptosis. J Nanobiotechnol. 22:403. doi: 10.1186/s12951-024-02697-w
Liu, X., Li, Z., Tong, J., Wu, F., Jin, H., and Liu, K. (2025). Characterization of the expressions and m6A methylation modification patterns of mRNAs and lnc RNAs in a spinal cord injury rat model. Mol. Neurobiol. 62, 806–818. doi: 10.1007/s12035-024-04297-z
Liu, J., Lin, M., Qiao, F., and Zhang, C. (2022). Exosomes derived from lnc RNA TCTN2-modified mesenchymal stem cells improve spinal cord injury by miR-329-3p/IGF1R axis. J. Mol. Neurosci. 72, 482–495. doi: 10.1007/s12031-021-01914-7
Liu, Y., Liu, J., and Liu, B. (2020). Identification of circular RNA expression profiles and their implication in spinal cord injury rats at the immediate phase. J. Mol. Neurosci. 70, 1894–1905. doi: 10.1007/s12031-020-01586-9
Lu, Z. J., Pan, Q. L., and Lin, F. X. (2024). Epigenetic modifications of inflammation in spinal cord injury. Biomed. Pharmacother. 179:117306. doi: 10.1016/j.biopha.2024.117306
Margiana, R., Markov, A., Zekiy, A. O., Hamza, M. U., Al-Dabbagh, K. A., Al-Zubaidi, S. H., et al. (2022). Clinical application of mesenchymal stem cell in regenerative medicine: a narrative review. Stem Cell Res Ther 13:366. doi: 10.1186/s13287-022-03054-0
Misir, S., Wu, N., and Yang, B. B. (2022). Specific expression and functions of circular RNAs. Cell Death Differ. 29, 481–491. doi: 10.1038/s41418-022-00948-7
Mun, S., Han, K., and Hyun, J. K. (2022). The time sequence of gene expression changes after spinal cord injury. Cells 11:236. doi: 10.3390/cells11142236
Ortega, M. A., Fraile-Martinez, O., García-Montero, C., Haro, S., Álvarez-Mon, M., De Leon-Oliva, D., et al. (2023). A comprehensive look at the psychoneuroimmunoendocrinology of spinal cord injury and its progression: mechanisms and clinical opportunities. Mil. Med. Res. 10:26. doi: 10.1186/s40779-023-00461-z
Peng, R., Zhang, L., Xie, Y., Guo, S., Cao, X., and Yang, M. (2024). Spatial multi-omics analysis of the microenvironment in traumatic spinal cord injury: a narrative review. Front. Immunol. 15:1432841. doi: 10.3389/fimmu.2024.1432841
Qian, Z., Chang, J., Jiang, F., Ge, D., Yang, L., Li, Y., et al. (2020). Excess administration of miR-340-5p ameliorates spinal cord injury-induced neuroinflammation and apoptosis by modulating the P 38-MAPK signaling pathway. Brain Behav. Immun. 87, 531–542. doi: 10.1016/j.bbi.2020.01.025
Salvatori, B., Biscarini, S., and Morlando, M. (2020). Non-coding RNAs in nervous system development and disease. Front. Cell Dev. Biol. 8:273. doi: 10.3389/fcell.2020.00273
Seblani, M., Decherchi, P., and Brezun, J. M. (2023). Edema after CNS trauma: a focus on spinal cord injury. Int. J. Mol. Sci. 24:159. doi: 10.3390/ijms24087159
Shao, M., Jin, M., Feizhou, L., Ma, X., and Wei, Z. (2025). Administration of hypoxic pretreated adipose-derived mesenchymal stem cell exosomes promotes spinal cord repair after injury via delivery of circ-Astn 1 and activation of autophagy. Int. Immunopharmacol. 152:114324. doi: 10.1016/j.intimp.2025.114324
Shao, M., Jin, M., Xu, S., Zheng, C., Zhu, W., Ma, X., et al. (2020). Exosomes from long noncoding RNA-Gm37494-ADSCs repair spinal cord injury via shifting microglial M1/M2 polarization. Inflammation 43, 1536–1547. doi: 10.1007/s10753-020-01230-z
Shao, M., Ye, S., Chen, Y., Yu, C., and Zhu, W. (2024a). Exosomes from hypoxic ADSCs ameliorate neuronal damage post spinal cord injury through circ-Wdfy 3 delivery and inhibition of ferroptosis. Neurochem. Int. 177:105759. doi: 10.1016/j.neuint.2024.105759
Shao, Q., Zhang, Y., Zhang, Z., Jiang, W., Yin, Y., Fang, Y., et al. (2024b). Downregulation of circular RNA Gla reduced astrocyte inflammatory status by regulating miR-488/MEKK1 levels and promoted functional recovery after spinal cord injury. J. Inflamm. Res. 17, 7123–7139. doi: 10.2147/jir.S467940
Shen, Y., Wang, Y. P., Cheng, X., Yang, X., and Wang, G. (2023). Autophagy regulation combined with stem cell therapy for treatment of spinal cord injury. Neural Regen. Res. 18, 1629–1636. doi: 10.4103/1673-5374.363189
Shi, C., Xu, J., Ding, Y., Chen, X., Yuan, F., Zhu, F., et al. (2024). MCT1-mediated endothelial cell lactate shuttle as a target for promoting axon regeneration after spinal cord injury. Theranostics 14, 5662–5681. doi: 10.7150/thno.96374
Silvestro, S., and Mazzon, E. (2022). MiRNAs as promising translational strategies for neuronal repair and regeneration in spinal cord injury. Cells 11:177. doi: 10.3390/cells11142177
Sun, P., Hamblin, M. H., and Yin, K. J. (2022). Non-coding RNAs in the regulation of blood-brain barrier functions in central nervous system disorders. Fluids Barriers CNS 19:27. doi: 10.1186/s12987-022-00317-z
Tian, F., Yang, J., and Xia, R. (2022). Exosomes secreted from circ ZFHX3-modified mesenchymal stem cells repaired spinal cord injury through mir-16-5p/IGF-1 in mice. Neurochem. Res. 47, 2076–2089. doi: 10.1007/s11064-022-03607-y
Valido, E., Boehl, G., Krebs, J., Pannek, J., Stojic, S., Atanasov, A. G., et al. (2023). Immune status of individuals with traumatic spinal cord injury: a systematic review and meta-analysis. Int. J. Mol. Sci. 24:385. doi: 10.3390/ijms242216385
Wang, W., Liu, C., He, D., Shi, G., Song, P., Zhang, B., et al. (2024). Circ RNA CDR1as affects functional repair after spinal cord injury and regulates fibrosis through the SMAD pathway. Pharmacol. Res. 204:107189. doi: 10.1016/j.phrs.2024.107189
Wang, Z. Y., Wen, Z. J., Xu, H. M., Zhang, Y., and Zhang, Y. F. (2022). Exosomal noncoding RNAs in central nervous system diseases: biological functions and potential clinical applications. Front. Mol. Neurosci. 15:1004221. doi: 10.3389/fnmol.2022.1004221
Wang, C., Zhang, J., Chen, W., Gao, L., He, J., and Xia, Y. (2025). Exosomal lnc RNA RMRP-shuttled by olfactory mucosa-mesenchymal stem cells suppresses microglial pyroptosis to improve spinal cord injury via EIF4A3/SIRT1. Mol. Neurobiol. 23:4552. doi: 10.1007/s12035-025-04756-1
Whetstone, W. D., Hsu, J. Y., Eisenberg, M., Werb, Z., and Noble-Haeusslein, L. J. (2003). Blood-spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J. Neurosci. Res. 74, 227–239. doi: 10.1002/jnr.10759
Xia, X., Niu, H., Ma, Y., Qu, B., He, M., Yu, K., et al. (2020). Lnc RNA CCAT1 protects astrocytes against OGD/R-induced damage by targeting the miR-218/NFAT5-signaling axis. Cell. Mol. Neurobiol. 40, 1383–1393. doi: 10.1007/s10571-020-00824-3
Xiong, L. L., Qin, Y. X., Xiao, Q. X., Jin, Y., Al-Hawwas, M., Ma, Z., et al. (2022). Corrigendum: MicroRNA339 targeting PDXK improves motor dysfunction and promotes neurite growth in the remote cortex subjected to spinal cord transection. Front. Cell Dev. Biol. 10:877291. doi: 10.3389/fcell.2022.877291
Xiong, T., Yang, K., Zhao, T., Zhao, H., Gao, X., You, Z., et al. (2023). Multifunctional integrated Nanozymes facilitate spinal cord regeneration by remodeling the extrinsic neural environment. Adv Sci (Weinh) 10:e2205997. doi: 10.1002/advs.202205997
Xu, X., Liu, R., Li, Y., Zhang, C., Guo, C., Zhu, J., et al. (2024). Spinal cord injury: from MicroRNAs to exosomal MicroRNAs. Mol. Neurobiol. 61, 5974–5991. doi: 10.1007/s12035-024-03954-7
Xu, L., Ye, X., Zhong, J., Chen, Y. Y., and Wang, L. L. (2021). New insight of circular RNAs' roles in central nervous system post-traumatic injury. Front. Neurosci. 15:644239. doi: 10.3389/fnins.2021.644239
Yang, S., Chen, S., Zhang, C., Han, J., Lin, C., Zhao, X., et al. (2023a). Enhanced therapeutic effects of mesenchymal stem cell-derived extracellular vesicles within chitosan hydrogel in the treatment of diabetic foot ulcers. J. Mater. Sci. Mater. Med. 34:43. doi: 10.1007/s10856-023-06746-y
Yang, J., Dong, J., Li, H., Gong, Z., Wang, B., Du, K., et al. (2024). Circular RNA HIPK2 promotes A1 astrocyte activation after spinal cord injury through autophagy and endoplasmic reticulum stress by modulating miR-124-3p-mediated Smad 2 repression. ACS Omega 9, 781–797. doi: 10.1021/acsomega.3c06679
Yang, J., Gong, Z., Dong, J., Bi, H., Wang, B., Du, K., et al. (2023b). lnc RNA XIST inhibition promotes M2 polarization of microglial and aggravates the spinal cord injury via regulating miR-124-3p/IRF1 axis. Heliyon 9:e17852. doi: 10.1016/j.heliyon.2023.e17852
Yao, H., Cai, C., Huang, W., Zhong, C., Zhao, T., Di, J., et al. (2024). Enhancing mitophagy by ligustilide through BNIP3-LC3 interaction attenuates oxidative stress-induced neuronal apoptosis in spinal cord injury. Int. J. Biol. Sci. 20, 4382–4406. doi: 10.7150/ijbs.98051
Yao, X., Huang, X., Chen, J., Lin, W., and Tian, J. (2024). Roles of non-coding RNA in diabetic cardiomyopathy. Cardiovasc. Diabetol. 23:227. doi: 10.1186/s12933-024-02252-9
Yao, Y., Wang, J., He, T., Li, H., Hu, J., Zheng, M., et al. (2020). Microarray assay of circular RNAs reveals cic RNA. 7079 as a new anti-apoptotic molecule in spinal cord injury in mice. Brain Res. Bull. 164, 157–171. doi: 10.1016/j.brainresbull.2020.08.004
Yin, X., Zheng, W., He, L., Mu, S., Shen, Y., and Wang, J. (2022). Circ HIPK3 alleviates inflammatory response and neuronal apoptosis via regulating miR-382-5p/DUSP1 axis in spinal cord injury. Transpl. Immunol. 73:101612. doi: 10.1016/j.trim.2022.101612
Yuan, J., Botchway, B. O. A., Zhang, Y., Wang, X., and Liu, X. (2020). Role of circular ribonucleic acids in the treatment of traumatic brain and spinal cord injury. Mol. Neurobiol. 57, 4296–4304. doi: 10.1007/s12035-020-02027-9
Yuan, X., Yuan, W., Ding, L., Shi, M., Luo, L., Wan, Y., et al. (2021). Cell-adaptable dynamic hydrogel reinforced with stem cells improves the functional repair of spinal cord injury by alleviating neuroinflammation. Biomaterials 279:121190. doi: 10.1016/j.biomaterials.2021.121190
Zhang, X., Jiang, W., Lu, Y., Mao, T., Gu, Y., Ju, D., et al. (2023). Exosomes combined with biomaterials in the treatment of spinal cord injury. Front. Bioeng. Biotechnol. 11:1077825. doi: 10.3389/fbioe.2023.1077825
Zhang, T., Li, K., Zhang, Z. L., Gao, K., and Lv, C. L. (2021). Lnc RNA Airsci increases the inflammatory response after spinal cord injury in rats through the nuclear factor kappa B signaling pathway. Neural Regen. Res. 16, 772–777. doi: 10.4103/1673-5374.295335
Zhang, N., Lin, J., Lin, V. P. H., Milbreta, U., Chin, J. S., Chew, E. G. Y., et al. (2021). A 3D fiber-hydrogel based non-viral gene delivery platform reveals that microRNAs promote axon regeneration and enhance functional recovery following spinal cord injury. Adv Sci (Weinh) 8:e2100805. doi: 10.1002/advs.202100805
Zhang, H., Wang, W., Hu, X., Wang, Z., Lou, J., Cui, P., et al. (2024). Heterophyllin B enhances transcription factor EB-mediated autophagy and alleviates pyroptosis and oxidative stress after spinal cord injury. Int. J. Biol. Sci. 20, 5415–5435. doi: 10.7150/ijbs.97669
Zhang, M., Xing, J., Zhao, S., Lu, M., Liu, Y., Lin, L., et al. (2024). Exosomal YB-1 facilitates ovarian restoration by MALAT1/miR-211-5p/FOXO (3) axis. Cell Biol. Toxicol. 40:29. doi: 10.1007/s10565-024-09871-8
Zhong, X., Bao, Y., Wu, Q., Xi, X., Zhu, W., Chen, S., et al. (2021). Long noncoding RNA XIST knockdown relieves the injury of microglia cells after spinal cord injury by sponging miR-219-5p. Open Med (Wars) 16, 1090–1100. doi: 10.1515/med-2021-0292
Zhou, J., Li, Z., Zhao, Q., Wu, T., Zhao, Q., and Cao, Y. (2021). Knockdown of SNHG1 alleviates autophagy and apoptosis by regulating miR-362-3p/Jak 2/stat 3 pathway in LPS-injured PC12 cells. Neurochem. Res. 46, 945–956. doi: 10.1007/s11064-020-03224-7
Zhou, H. J., Wang, L. Q., Zhan, R. Y., Zheng, X. J., and Zheng, J. S. (2022). lnc RNA MEG3 restrained the M1 polarization of microglia in acute spinal cord injury through the HuR/A20/NF-κB axis. Brain Pathol. 32:e13070. doi: 10.1111/bpa.13070
Keywords: spinal cord injury, SCI, circular RNA, long non-coding RNA, microRNA
Citation: Bao J, Zhi W, Qi S, Mo H, Liu R and Guo C (2025) NcRNAs: a potential treatment for spinal cord injury. Front. Cell. Neurosci. 19:1645639. doi: 10.3389/fncel.2025.1645639
Edited by:
Stefania Dalise, Pisana University Hospital, ItalyReviewed by:
Igor Jakovcevski, Witten/Herdecke University, GermanyKazuya Kuboyama, Nagoya City University, Japan
Copyright © 2025 Bao, Zhi, Qi, Mo, Liu and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunhui Guo, MTA0MzUwNDgxNEBxcS5jb20=; Ruzhuan Liu, TGl1cnV6aHVhbjJAMTI2LmNvbQ==
†ORCID: Guo Chunhui, orcid.org/0009-0000-3751-6420
 Jie Bao1,2
Jie Bao1,2 Chunhui Guo
Chunhui Guo