- Institute of Physiology, University Medical Center Mainz, Johannes Gutenberg University of Mainz, Mainz, Germany
Neuronal activity in the cerebral cortex comes in surprisingly early and influences or even controls a number of important developmental process like neurogenesis, neuronal migration, myelination, formation of cortical maps and local circuits, and programmed cell death. During the late prenatal and early postnatal period, the neocortical network shows a developmental transition from sparse, synchronized, low activity patterns to continuous, desynchronized, high activity patterns. This developmental sequence has been demonstrated in various neocortical areas of different mammalian species. This review article aims to provide a comprehensive overview of the early development of neuronal network activity in the cerebral cortex. We mainly focus on the rodent barrel cortex and a developmental period when the cortex gains mature functional properties at the cellular and network level. After briefly summarizing the developmental processes underlying the construction, reconstruction, and deconstruction of neocortical circuits, we describe the age-dependent changes in spontaneous and sensory driven network activity. Next we discuss the functional role of transient cortical structures and cell types in the generation of early activity patterns and in the activity-dependent maturation of local and large-scale cortical networks. Finally, we present an outlook on the models and techniques to study the cellular and network mechanisms underlying neuronal activity in the developing cerebral cortex.
Introduction
Under unnatural in vitro conditions, in a dish with supply of artificial cerebrospinal fluid and nutrients, dissociated neurons from embryonic or neonatal mammalian brains have the surprising capability to generate spontaneous neuronal activity (action potentials) and to make synaptic connections to neighboring neurons. Within a few days in vitro, passive and active membrane properties (e.g., resting membrane potential and discharge pattern) reach more mature values similar to those obtained under in vivo conditions. With the functional maturation of inhibitory and excitatory synaptic connections, spontaneous (ongoing) activity becomes more complex and synchronized network activity emerges, recognizable as burst discharges, which can be local or propagate in a wave-like pattern across the cell culture network (Opitz et al., 2002; Sun et al., 2010). To some extent the development of intrinsic and synaptic activity recorded in various in vitro preparations, from dissociated cell cultures to brain organoids, resembles the developmental sequences described in vivo (for review Wu et al., 2024; Yuste et al., 2024). This is surprising since in vitro preparations (i) receive no sensory inputs, (ii) lack sensory-motor interactions, and (iii) are not influenced by neuromodulatory systems – factors that play important, if not essential roles in the physiological development of neuronal networks (for review Khazipov et al., 2008; Luhmann et al., 2016; Kilb and Fukuda, 2017; Martini et al., 2021; Cossart and Garel, 2022). In particular, they contribute as follows: (i) Already during perinatal development, the activity of thalamic or sensory cortical areas is driven by the sensory periphery, which plays a central role in the formation of cortical networks (Hanganu et al., 2006; Colonnese et al., 2010; Yang et al., 2013; Kersbergen et al., 2023; Aníbal-Martínez et al., 2025). (ii) Central pattern generators (CPGs) in spinal cord and brainstem produce spontaneous activity evoking motor commands and movements (Kreider and Blumberg, 2000; Khazipov et al., 2004), which subsequently activate sensory networks (An et al., 2014) and form early sensory-motor circuits. (iii) Neuromodulators have a fast and strong impact on cellular function and control the development of the neuronal network already at early stages (Crépel et al., 2007; Hanganu et al., 2007; Maldonado et al., 2021; Wong et al., 2022; for review Wu et al., 2024).
Distinct cortical activity patterns have been not only demonstrated during specific developmental stages in different mammalian species (for review Khazipov and Luhmann, 2006; Wu et al., 2024; Yuste et al., 2024), but also represent clinically important biomarkers of early physiological development in humans (Pavlidis et al., 2017; Routier et al., 2023; Kota et al., 2024). Cortical EEG patterns, spontaneous movements, and sensory-motor interactions provide important information on the functional state of the brain in newborns and can predict future clinical outcome (Iyer et al., 2015; Airaksinen et al., 2020; Lloyd et al., 2021). Therefore, a better understanding of the properties, underlying mechanisms, functions and dysfunctions of early network activity will form the basis for improvements in clinical diagnosis and therapies in neuropediatric (Luhmann et al., 2022).
The aim of this review article is to provide a comprehensive overview of the early development of spontaneous and sensory driven activity in the cerebral cortex. We focus on the developmental period when the cortical network gains mature functional properties at the cellular and network level, a process which in rodents mostly happens during the first postnatal month.
Construction, reconstruction, and deconstruction of neocortical circuits during early development
During early development the cerebral cortex is characterized by highly dynamic changes in its molecular, anatomical and physiological properties. The initial cortical network consists of three layers: (i) The marginal zone, which beside other cell types, contains transiently expressed Cajal–Retzius neurons (CRNs) and later becomes cortical layer (L) 1 (for review Kirischuk et al., 2014; Causeret et al., 2021). (ii) The cortical plate, which is progressively populated by excitatory and inhibitory neurons. These neurons are generated in distinct brain regions and migrate along vertical and horizontal paths into the cortical plate to form the characteristic six-layered structure in an inside first – outside last sequence (for review Huttner, 2023). In the perinatal rodent cortex, one cortical layer is generated each day. (iii) The subplate, which contains a variety of transient neurons, and either disappears or later transforms into L6b (for review Hoerder-Suabedissen and Molnár, 2015; Kostović, 2020). During this dynamic postnatal phase of reorganization, CRNs, subplate cells, and a smaller number of neurons in L2–L6 are eventually eliminated by programmed cell death (apoptosis) (for review Causeret et al., 2018). These contrasting developmental processes – neurogenesis versus cell death and formation of new cortical layers versus loss of existing ones – are accompanied by the formation, stabilization and pruning of synaptic connections, developmental changes in synaptic function (e.g., number, affinity and subunit composition of receptors, location of synapses, and properties of transmitter release), and the increasing influence of neuromodulatory systems (e.g., cholinergic, serotonergic, and noradrenergic innervation). As a result, in rodents, the perinatal cerebral cortex profoundly changes its structure and function on a daily basis. This raises several important questions: How can the cortical network maintain its functional integrity under such dynamic conditions of construction, reconstruction and deconstruction? Is there functional stability in network activity, or do we see (abrupt) changes in network function during early development? Can we observe consistent patterns or gradual transitions of network activity during this dynamic period of development? What is the role of early activity patterns for the maturation of the cortical network? These questions will be addressed in the following subsections.
Age-dependent changes in spontaneous network activity
It is the destiny of developing (cortical) neurons to generate spontaneous action potentials and to form synaptic connections. And it is the destiny of developing (cortical) networks to generate spontaneous synchronized burst discharges. These processes can be observed in simple cell cultures of dissociated neocortical neurons from newborn rodents (Opitz et al., 2002; Sun et al., 2010), in organotypic mouse neocortical slices (Bak et al., 2024), in human brain organoids (Trujillo et al., 2019; Landry et al., 2023), in rodent cerebral cortex in vivo (Minlebaev et al., 2009; Yang et al., 2009), and in EEG recordings from preterm human infants (Milh et al., 2007; Tolonen et al., 2007). While this list could be extended to other species and other brain structures, it remains unclear whether the network activity recorded under different conditions, in different models and in different species represents the same type of activity. Therefore, in the next sections we mostly focus on data obtained from the barrel cortex of rodents, which will be discussed in comparison to in vitro and in vivo observations from other neocortical areas and species.
Sparse spontaneous activity can be observed in vivo with calcium imaging and patch-clamp recordings in embryonic mouse neocortex as early as embryonic day (E) 14 (Yuryev et al., 2016; Munz et al., 2023). This is the period when genetic programs (e.g., spatio-temporal expression of specific transcription factors) and activity-dependent mechanisms interact (for review Simi and Studer, 2018). Between E14.5 and P2 spontaneous waves of activity can be monitored ex vivo with calcium imaging in sensory and higher-order thalamic nuclei of the mouse (Moreno-Juan et al., 2017). These calcium waves propagate via gap junctions across sensory-modality thalamic nuclei and regulate the formation of sensory neocortical areas (for review Antón-Bolaños et al., 2018). At E18, thalamic activity elicits a local, column-like activation of the subplate and the overlying cortical plate, suggesting that spontaneous thalamic activity instructs the columnar architecture and cortical maps before the arrival of sensory inputs (Antón-Bolaños et al., 2019; for review Martini et al., 2021). Interestingly, already during this developmental period, between E14.5 and E17.5, L5 pyramidal neurons form transient, multi-layered synaptic connections and participate in two different circuit motifs (Munz et al., 2023).
At P0, the day of birth, spontaneous neocortical activity patterns become more complex. Local, columnar domains of spontaneously coactive neurons as well as large-scale propagating activity can be observed in vitro and in vivo with calcium imaging, electrophysiological recordings and voltage-sensitive dye imaging (Yuste et al., 1995; Garaschuk et al., 2000; Minlebaev et al., 2007; Yang et al., 2009; Warm et al., 2025; for review Luhmann, 2017). Intracortical multi-electrode recordings in vivo have demonstrated that two distinct patterns dominate the spontaneous network activity in the neonatal rodent barrel cortex in vivo: local spindle-shaped oscillations (spindle bursts) and faster events in the gamma frequency range (gamma oscillations) (Minlebaev et al., 2007; Yang et al., 2009; Figure 1). Both patterns of spontaneous activity synchronize local, column-like networks (Figure 2A) and can be elicited by the thalamus (Minlebaev et al., 2011; Yang et al., 2013; for review Khazipov et al., 2013; Luhmann et al., 2016; Yang et al., 2016). In fact, in contrast to the activity in embryonic cortex, synchronized network activity in newborn rodent somatosensory, auditory and visual cortex can be triggered at this age by endogenous activity in the sensory periphery, e.g., retinal waves (for review Kirkby et al., 2013; Martini et al., 2021; Kersbergen and Bergles, 2024). A pivotal role in relaying these thalamic inputs is played by the subplate (Yang et al., 2009; Viswanathan et al., 2012), which is crucial in early circuit formation (for review Kanold and Luhmann, 2010; Luhmann et al., 2018; Molnár et al., 2020).
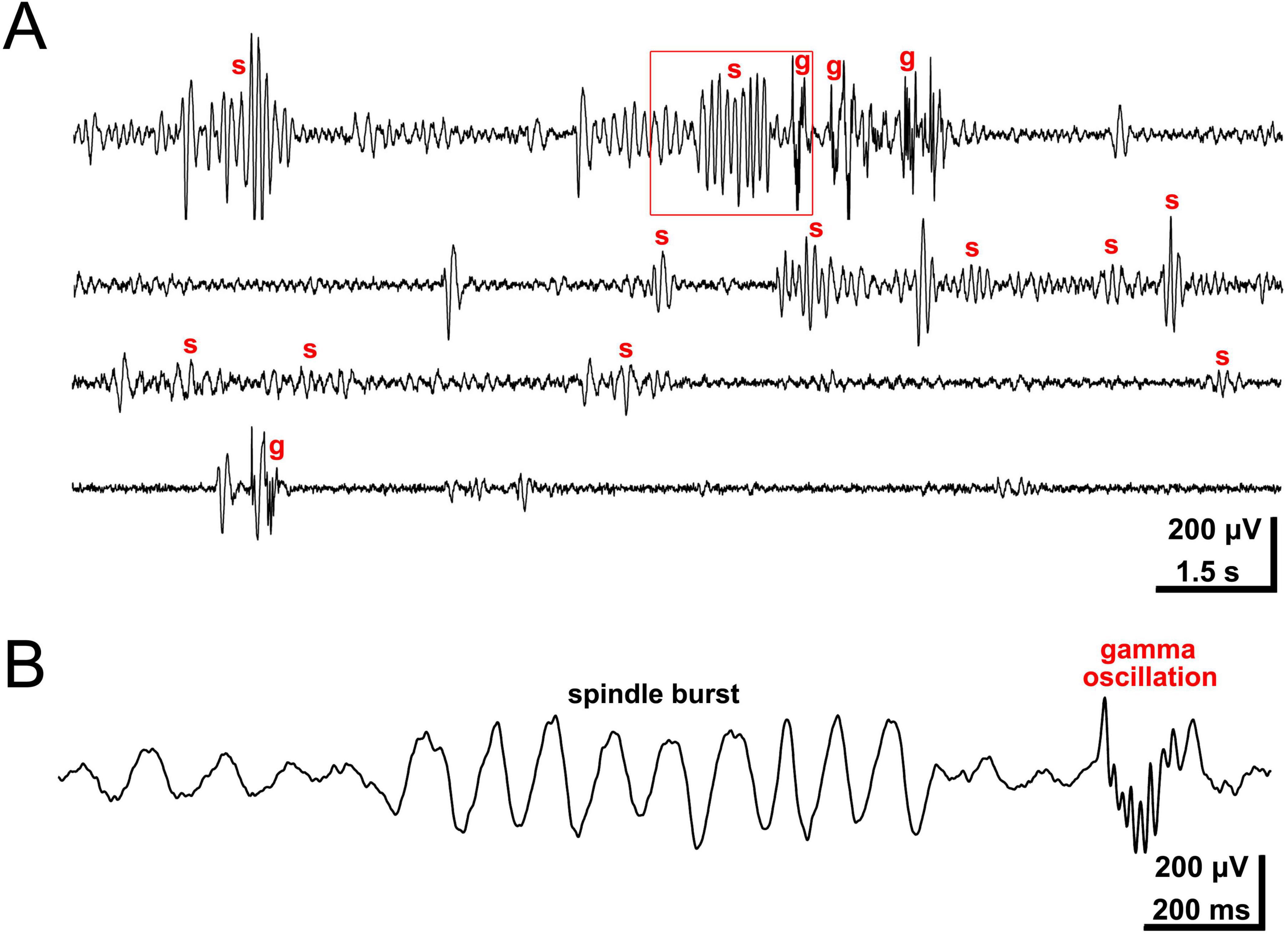
Figure 1. Intracortical recording of local field potential in barrel cortex of lightly anesthetized P3 rat. (A) Network activity consists of spindle bursts (s) and gamma oscillations (g). (B) Spindle burst and gamma oscillation marked in panel (A) by red rectangle at higher temporal resolution. Spontaneous and sensory-driven spindle bursts present with a dominant frequency in the alpha band (maximal frequency around 10 Hz), while intermittent gamma oscillations range around 30–40 Hz in frequency. Reproduced with permission from Yang et al. (2009).
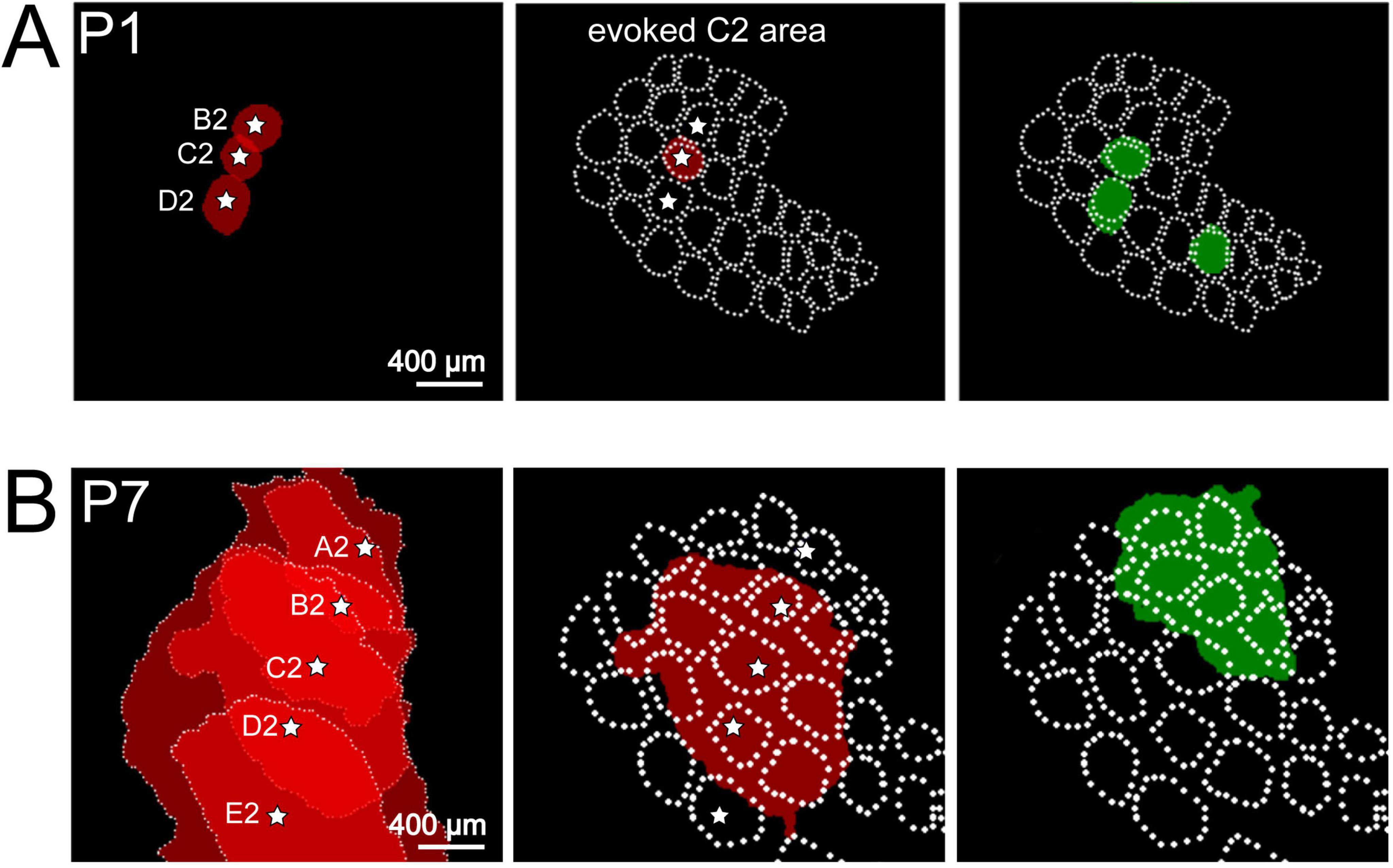
Figure 2. Spontaneous and evoked activity recorded with voltage-sensitive dye imaging (VSDI) in barrel cortex of P1 and P7 rat. (A) At P1, mechanical stimulation of single whisker B2, C2, or D2 elicits a local columnar response in the barrel field map (red circle-like areas). Local spontaneous events (green circle-like areas) overlap with the topographic representation of single whiskers indicating that spontaneous activity unmasks the functional barrel field map already shortly after birth. (B) At P7, single whisker (A2 to E2) stimulation elicits a wider response covering several columns. Spontaneous activity also covers several whisker-related columns. Reproduced with permission from Yang et al. (2013). White star marks the center of each activated barrel.
Beside activity from the sensory periphery activating the subplate-cortical plate network via thalamocortical connections, spindle bursts in newborn rat cortex are also modulated by interhemispheric connections via the corpus callosum (Marcano-Reik and Blumberg, 2008). These observations suggest that the subplate may serve as a transient hub station fulfilling important roles in cortical map and circuit formation. Selective removal of the subplate in newborn rat somatosensory cortex does not only abolish spindle burst activity, but disturbs the development of the characteristic barrel field pattern (Tolner et al., 2012). Thus, spontaneous burst activity transmitted via the subplate to the developing network in the cortical plate influences the formation of topographic maps and regulates intracortical connectivity (for review Kanold, 2009). Patch-clamp recordings in acute brain slices from postmortem human fetal cerebral cortex have demonstrated that during the second trimester of gestation subplate neurons spontaneously discharge in bursts and exhibit the highest level of functional differentiation when compared to other neocortical neurons (Moore et al., 2009; Moore et al., 2011). Gap junctions contribute to this spontaneous burst activity both in fetal human cortex (Moore et al., 2014) as well as in newborn rodent cortex (Hanganu et al., 2009; Singh et al., 2018). Conditional deletion of the gap junction protein Connexin 26 reduces spontaneous synchronized activity in newborn mouse neocortical slices and causes long-term behavioral deficits (Su et al., 2017), providing additional support that spontaneous burst activity at this developmental stage fulfills an important functional role.
With further development, spontaneous cortical activity becomes more complex, appears more often and frequently activates neighboring columns within the barrel cortex (Figure 2B) or propagates in a wave-like pattern across the cortex (Shen and Colonnese, 2016; Mojtahedi et al., 2021; Warm et al., 2025). At P4/5, when all neocortical layers have been formed, a patchwork-type of spontaneous activity pattern can be demonstrated by two-photon calcium imaging in L4 of the mouse barrel cortex (Golshani et al., 2009; Mizuno et al., 2018), indicating that the thalamus-driven columnar barrel field activation observed in P0/1 rodents (Yang et al., 2013) has shifted from the subplate to L4. During the first postnatal week, this transformation process in spontaneous activity patterns is accompanied by changes in intrinsic excitability (e.g., decrease in gap junctional coupling, age-dependent alterations in voltage-dependent currents) and an increasingly stronger participation of glutamate and GABA mediated synaptic transmission (Minlebaev et al., 2007, 2009; Mizuno et al., 2021; for review Moody and Bosma, 2005; Allene and Cossart, 2010; Luhmann et al., 2016; Yuste et al., 2024).
At the end of the second postnatal week (∼P14) spontaneous network activity in the mouse barrel cortex reveals a fundamental transition from high-frequency, synchronized activity to low-frequency, desynchronized activity (Golshani et al., 2009). A developmental desynchronization can be also observed in specific cell types, like the 5HT3a-receptor expressing interneurons, as demonstrated by longitudinal calcium imaging in barrel cortex of non-anesthetized mice (Che et al., 2018). Sequential maturation of GABAergic inhibition was recently shown to be critical for this network state transition in the barrel cortex (Modol et al., 2020; Mòdol et al., 2024). Further, a very similar development has been demonstrated in primary visual cortex of non-anesthetized rats (Colonnese et al., 2010) and mice (Shen and Colonnese, 2016). During the first postnatal week spontaneous activity is sparse with long (>10 s) periods of network silence. At the end of the second week, the time point of eye opening, network activity in visual cortex accelerates in frequency, becomes more continuous and resembles the adult pattern (Shen and Colonnese, 2016). Similar as in the barrel cortex, at this age spontaneous multi-unit activity (MUA) in the visual cortex increases, both in the awake state as well as during quiet sleep (Colonnese et al., 2010).
Moreover, spontaneous synchronized activity patterns in pre- and neonatal human cerebral cortex are remarkably similar to those observed in other mammalian species (for review Khazipov and Luhmann, 2006; Molnár et al., 2020; Chini and Hanganu-Opatz, 2021; Yuste et al., 2024). EEG recordings from preterm babies and in utero MEG recordings during the third trimester of pregnancy, demonstrated different physiological patterns of spontaneous activity (Vanhatalo et al., 2002; Rose and Eswaran, 2004; Vanhatalo and Kaila, 2006; for review Wallois et al., 2021). The spindle bursts described in rodents and other species resemble the so-called delta brushes recorded in preterm human babies (Milh et al., 2007; Kidokoro, 2021). As in other mammals, the cortical network activity in humans also shows a transition from sparse to discontinuous, synchronized and finally to continuous, desynchronized activity during late prenatal and early postnatal development (for review Wu et al., 2024).
Although the neocortical activity in this section was termed “spontaneous,” it has often not been clarified whether the spindle and gamma bursts are generated intrinsically within the cortical network or whether they arise from subcortical and non-cortical networks. Therefore, in the following section we focus on the role of the sensory periphery in triggering cortical network activity.
Developmental changes in sensory driven cortical network activity
Spontaneous activity in sensory neocortical areas does not necessarily have to originate solely within intracortical circuits, but rather reflects the interaction of endogenous cortical activity with incoming thalamocortical, short- and long-range cortico-cortical inputs and the influence of neuromodulatory systems. In this section the role of the sensory periphery in triggering or modulating cortical network activity during early stages will be discussed.
The sensory periphery can trigger cortical network activity by two different mechanisms: through endogenous activity generated spontaneously within the sensory organs or by adequate physiological stimulation of the sensory cells. Both mechanisms occur surprisingly early – well before birth – in various sensory systems and across many mammalian species.
In the rodents’ visual system, spontaneous activity in the retina, called retinal waves, undergoes maturation in three developmental stages and plays an essential role for the refinement of visual maps in retinofugal targets in the thalamus and the superior colliculus (for review Blumberg et al., 2015). Retinal waves also trigger spindle bursts in the visual cortex of newborn rats in vivo (Hanganu et al., 2006). During the first postnatal week the visual cortex of non-anesthetized mice still cannot be activated by visual stimulation, but spindle bursts in response to retinal waves endure (Shen and Colonnese, 2016). Visual responses emerge as evoked spindle bursts at P8. In a laborious study Colonnese et al. (2010) investigated the development of spontaneous and evoked activity in the visual cortex of non-anesthetized rats (between P5 and P19) and preterm human infants (between gestational week GW27 and GW42). Their observations in the rat are almost identical to those obtained in the mouse. During the first postnatal week, sparse retinal wave-driven spontaneous spindle bursts could be observed in the visual cortex. Starting at P8, light flashes evoked visual cortical responses. In vivo patch-clamp recordings at this age demonstrated that the sensory evoked responses entail glutamatergic and GABAergic synaptic currents, which correlate with local field potential activity. Between P11 and P12 a rapid (<12 h) transition (“switch”) from light-evoked bursting to the more mature activity pattern could be observed. This developmental switch occurs shortly before eye opening at P13/14 and is accompanied by a sudden increase in mobility and explorative behavior (van der Bourg et al., 2017). A switch to the adult-like visual evoked activity pattern was also observed in EEG recordings from preterm human infants after GW36, which is shortly before term at GW37-40 (Colonnese et al., 2010). This transition from bursting to acuity is accompanied by the loss of gamma bursts organized in a columnar manner in rats and by the loss of delta brushes in preterms (Colonnese et al., 2010).
In the auditory system as well, the sensory periphery drives cortical activity during earliest developmental stages and spontaneous activity shapes the formation of the tonotopic map in primary auditory cortex (for review Kersbergen and Bergles, 2024). The mechanisms underlying spontaneous activity generation in the pre-hearing cochlea have been the focus of recent detailed studies: spontaneous calcium-dependent activity in glia-like supporting cells synchronizes the activity of nearby inner hair cells, which in turn increases the probability of afferent terminal recruitment (Wang et al., 2015; De Faveri et al., 2025). The network mechanisms causing sensory-driven activation of the auditory cortex during early developmental stages have been elegantly studied in ferrets, which are born at a very immature stage. Before the opening of the ears, sound-evoked activity in ferret auditory cortex emerges first in the subplate (Wess et al., 2017). At this age, abolishing peripheral function or presenting complex sound stimuli causes changes in the wiring pattern of subplate neurons (Meng et al., 2021; Mukherjee et al., 2022). Stimulus evoked responses can also be observed with standard EEG recordings in temporal cortical areas of preterm human infants (Chipaux et al., 2013), where the auditory responses consist of slow wave activity mixed with rapid oscillations. Thus, this delta-brush activity pattern is present in all cortical areas studied and resembles spindle bursts demonstrated in the perinatal cerebral cortex of other mammals (for review Khazipov and Luhmann, 2006).
The role of the sensory periphery in triggering neuronal activity in the immature somatosensory cortex is particularly complex, as its diverse receptors (mechano-, proprio-, thermo-, and nociceptors) are distributed throughout the body and can activate various cortical networks. In this section we will mostly focus on the rodent barrel cortex, which is stimulated by the whiskers on the contralateral snout of the animal (for review Feldmeyer et al., 2013; Staiger and Petersen, 2021). Mechanical stimulation of a single whisker elicits a reliable response in the rat barrel cortex as early as P0/1 (Figure 3). Recordings with 8-shanks, 32-electrodes arrays covering a horizontal range of 1.4 mm and a depth of >700 μm allow the simultaneous recording of activity over several barrel-related columns and cortical layers in the barrel cortex of the neonatal rodent (Figure 3A). In the neonatal rat (Yang et al., 2013) the evoked whisker response consists of a short early component and a longer lasting late response. The early response shows the characteristic gamma oscillation frequency of 40–60 Hz and the late response oscillates in the spindle burst frequency of 10–20 Hz (Figure 3B). Both components are tightly coupled to the MUA (Yang et al., 2013). These intracortical multi-electrode recordings and voltage-sensitive dye imaging (VSDI) in barrel cortex of P0/1 rats (Yang et al., 2009; Colonnese et al., 2010; Yang et al., 2013) demonstrated single whisker stimulation elicited responses that were mostly restricted to a single cortical column (Figures 2A, 3C). Thus, sensory evoked responses as well as spontaneous activity in neonatal barrel cortex are organized in functional columns before structural columns can be firstly identified with anatomical and immunocytochemical methods at ∼P5 (for review Erzurumlu and Gaspar, 2012).
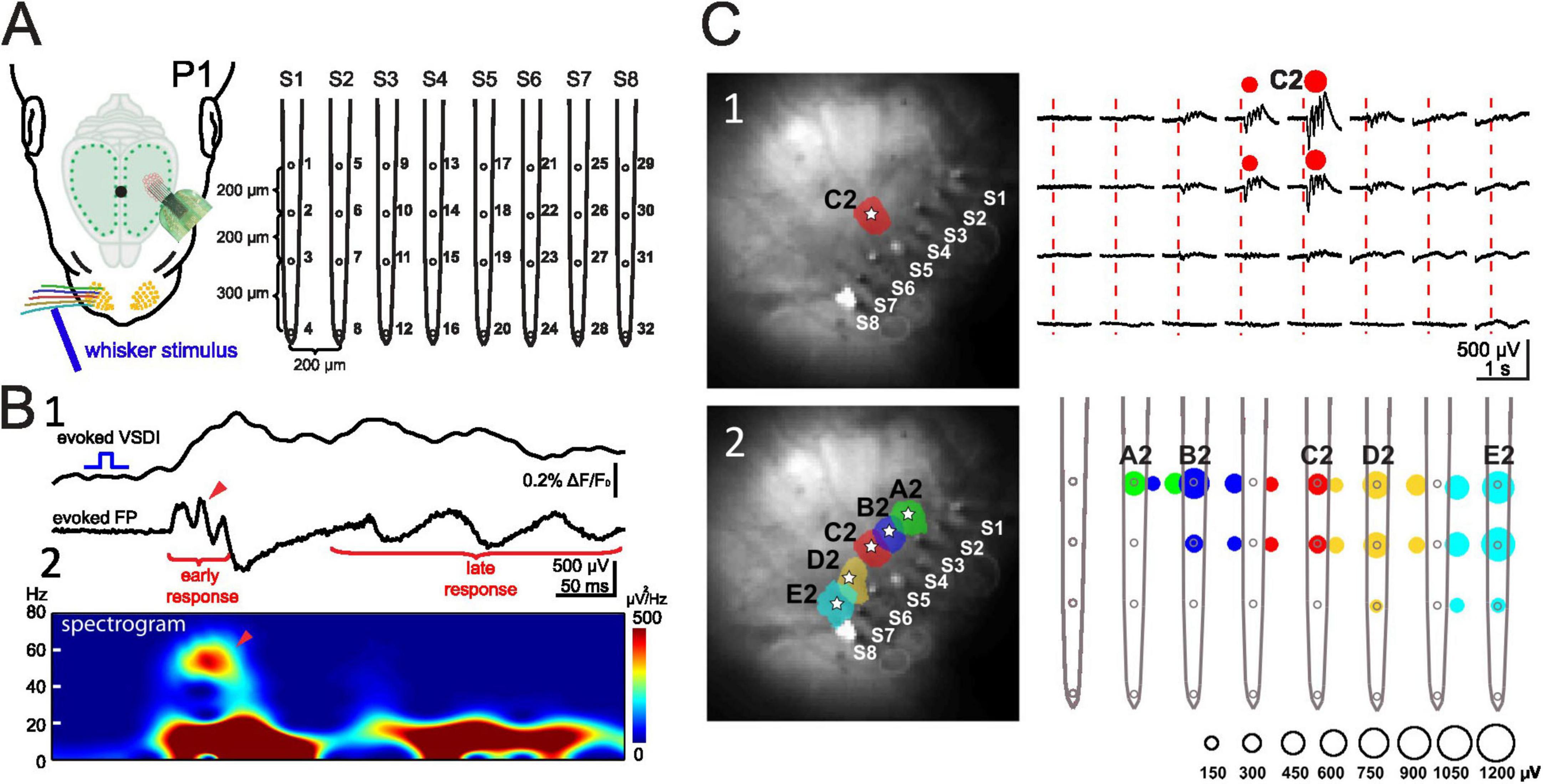
Figure 3. Evoked network activity recorded with VSDI and multi-electrode array in P1 rat. (A) Experimental setup showing the 8-shanks, 32-electrodes array. (B) VSDI and FP response recorded simultaneously in the cortical E2 barrel following mechanical stimulation of the E2 whisker. (B1) Simultaneous VSDI (upper trace) and local field potential (LFP) response (lower trace) to single whisker stimulation reveals early gamma oscillation and late spindle burst. (B2) Spectogram of the LFP shown in panel (B1) shows faster early gamma response followed by late spindle burst component. (C) Mechanical single whisker stimulation evokes a local columnar LFP response. (C1) Stimulation of whisker C2 elicits a local VSDI (left) and local electrophysiological (right) response in the C2 barrel. (C2) Color-coded localization of the evoked cortical VSDI (left) and electrophysiological (right) response amplitude to single whisker A2 to E2 stimulation. The LFP response amplitude corresponds to the size of the color-coded circles as shown below the graph. Reproduced with permission from Yang et al. (2013). White star marks the center of each activated barrel.
The question arises how and under which natural conditions the whiskers may be mechanically activated in a newborn rodent. Using a combination of multi-electrode recordings and video monitoring of the whiskers’ and head movements in non-anesthetized newborn rats, Akhmetshina et al. (2016) could demonstrate that whiskers move spontaneously and are also mechanically stimulated by tactile signals arising from its littermates’ movements. Both, endogenous self-generated whisker movements as well as exogenous stimulation by the littermates, efficiently evoke the typical early and late cortical response. What are the mechanisms underlying the spontaneous whisker movements? Rapid and asynchronous whisker movements have been demonstrated in P3–P6 rats during active sleep. These twitches are tightly coupled to bursts of activity in whisker thalamus and triggered cortical activity (Tiriac et al., 2012). However, this study does not fully explain the mechanisms of spontaneous whisker movements because a motor command has not been identified. Such a motor command may arise from the spinal cord, brainstem or motor cortex (for review Luhmann et al., 2016). In neonatal rats local spindle bursts in primary somatosensory cortex are triggered in a somatotopic manner by spontaneous muscle twitches (Khazipov et al., 2004) and the motor command for these twitches may arise from CPGs in the spinal cord. Sensory and motor zones of the spinal cord show synchronized activity, and twitches are correlated with movement-generating bursts in motor zones, which are followed by bursts in sensory zones (Inácio et al., 2016). The brainstem is another potential CPG for spontaneous motor activity during early development, since facial motor neurons in the brainstem elicit rhythmic whisker movements (Hattox et al., 2003). A whisking CPG may be also located in the primary motor cortex (M1). In awake adult rats, intracortical electrical stimulation of the M1 whisker representation elicits natural-like, rhythmic whisking (Haiss and Schwarz, 2005; Cramer and Keller, 2006). Notably, M1 is capable to trigger movements already in the neonatal rat, since focal electrical stimulation of the M1 forepaw representation in the physiologically relevant frequencies of spindle bursts (10 Hz) or gamma activity (40 Hz) reliably elicited movements of the contralateral forepaw (An et al., 2014). Approximately one quarter of the spontaneous bursts in M1 triggered forepaw movements and spindle bursts in M1 were tightly synchronized with spindle bursts in S1 (An et al., 2014). Carbocyanine dye (DiI) tracing experiments have shown reciprocal axonal connections between S1 and M1 in the P0 mouse and in vitro multi-electrode recordings demonstrated functional connectivity between these areas in the subplate and lower cortical layers at this age (Gellért et al., 2023). These data indicate that synchronized spontaneous activity in M1 and S1 mediated via corticocortical connections can influence maturation of sensorimotor interactions as early as P0 in rodents. The close interactions between the motor and the somatosensory system have been also nicely demonstrated with EEG recordings in preterm human infants. Prominent delta-brush activity can be observed in the somatosensory cortex, not only following a light touch of the baby’s shoulder, but also after spontaneous movements of the hand (Milh et al., 2007). Taken together this evidence suggests that these processes play a general role in the very early development of the sensorimotor system in mammals.
Using a combination of multi-electrode recordings, 2-photon calcium imaging and complex axial and/or lateral single whisker stimulation, van der Bourg et al. (2017) investigated evoked responses across all barrel cortical layers in anesthetized P10 to P28 mice. They found rapid functional changes at P13/14 marked by increased response selectivity and sharpened temporal spiking profiles. This developmental time point coincides with the onset of active whisking and exploratory behavior. In another study the same authors investigated in anesthetized P11 to P27 mice the responses to complex single- or dual-whisker stimulation (van der Bourg et al., 2019). Their results demonstrate a developmental decrease in the onset latency of the early response and duration of the late response, and an increase in the trans-columnar spread of the early activity. Herewith, these studies provide further evidence for a developmental sharpening of the temporal and spatial processing of whisker-evoked activity with the onset of active whisking.
The data discussed so far were mostly obtained in primary sensory cortical areas, such as the barrel cortex. Functional developmental changes in higher-order cortical areas have been less studied and remain largely unknown. Cai et al. (2022) used voltage-sensitive dye imaging, wide-field calcium imaging and intracortical multi-electrode recordings in anesthetized P0 to P56 mice to investigate the developmental engagement of the secondary whisker somatosensory area (wS2) and the emergence of cortico-cortical interactions from barrel cortex to wS2. They report that the thalamocortical input to wS2 is functionally delayed as compared to the thalamic input to the barrel cortex. Furthermore, cortico-cortical connections from barrel cortex begin to provide excitatory inputs to wS2 at P6–P8 and additional inhibitory inputs at P14–P16 (Cai et al., 2022). This developmental time point coincides with the sharpening of sensory evoked activity as exemplified by the shortening of evoked MUA. With the developmental transition of spontaneous activity from discontinuous and synchronized to continuous and desynchronized, sensory evoked activity interacts with an increasingly active cortex during network maturation. Thus, whereas sensory and thalamocortical activity encounter a relatively “silent” cortex during the first 10 postnatal days, patterned sensory inputs from the periphery reach a more “active” cortex in the succeeding stage. This developmental transition coincides with the period of eye opening, active hearing and voluntary movements (for review Martini et al., 2021), e.g., active exploratory whisking behavior (Arakawa and Erzurumlu, 2015).
Role of early network activity in neocortical development
The developmental progression of functional properties in both spontaneous and stimulus evoked cortical activity patterns appears to be highly similar across the different sensory systems and all mammalian species studied to date (for review Wu et al., 2024). This suggests that early network activity plays an instrumental role in the development of the cerebral cortex during late prenatal and early postnatal stages.
Neuronal activity begins to influence cortical development at a surprisingly early stage. Spontaneous retinal waves at E15/16 control neurogenesis (cell cycle withdrawal), and pharmacological inhibition of retinal waves induces cortical layer malformations at later stages (Bonetti and Surace, 2010). Although the exact mechanisms underlying this activity-dependence and periphery-drive of neurogenesis are not yet clear, retinal waves may influence spontaneous calcium waves, which propagate through radial glial cells in the embryonic cortical ventricular zone (VZ) (Weissman et al., 2004; Yuryev et al., 2016). These waves are most prominent during the peak of neurogenesis in the VZ, and disrupting the calcium wave signaling pathway reduces VZ proliferation (Weissman et al., 2004).
The next step in corticogenesis, the migration of newborn cortical neurons to their final layer position, is also influenced by neuronal activity (Komuro and Rakic, 1996; De Marco García et al., 2011). NMDA receptors (Reiprich et al., 2005) as well as GABA-A receptors (Heck et al., 2007) are involved in this activity-dependent control of neuronal migration in the developing cerebral cortex. Subplate neurons participate in this process by forming transient NMDA receptor mediated synapses to multipolar neurons and inducing a change in the migration mode from slow multipolar migration to faster radial glial-guided locomotion (Ohtaka-Maruyama et al., 2018).
Recently it became clear that neuronal activity also influences the myelination process (for review Fields, 2015; Krämer-Albers and Werner, 2023). Release of glutamate from synaptic vesicles along active axons induces a local rise in cytoplasmic calcium in oligodendrocytes. This calcium signal stimulates local translation of myelin basic protein to initiate myelination (Wake et al., 2011; Wake et al., 2015). Furthermore, the activity-dependent rise in the extracellular potassium concentration also increases myelin basic protein synthesis (Friess et al., 2016; Hammann et al., 2018). Whether specific early activity patterns in the typical spindle and gamma burst frequency induce myelination in developing cortical axons is currently unknown.
During embryonic stages spontaneous activity influences the cortical patterning at the macroscopic scale (cortical arealization) followed by the patterning at the microscopic scale (cortical columns) (for review Martini et al., 2021). The impact of cell-intrinsic genetic programs (e.g., transcription factors) gradually diminishes during embryonic development (for review Simi and Studer, 2018). At late prenatal and early postnatal stages the subplate functions as an important relay and amplifier of thalamocortical inputs (for review Luhmann et al., 2018; Martini et al., 2018; Molnár et al., 2020). Subplate neurons are key elements in the generation of spindle bursts, and selective elimination of the subplate causes a disorganization of topographic cortical maps, such as the barrel field pattern (Tolner et al., 2012). In addition to subplate neurons, CRNs in the marginal zone/L1 are also activated by spontaneous thalamic inputs during prenatal stages. Thalamocortical activity regulates the density of CRNs, the distribution of upper layer interneurons and the dendritic activation of pyramidal neurons (Genescu et al., 2022). During the first postnatal week, reelin-positive interneurons in L1 of the barrel cortex, which co-express the serotonin 5-HT3a receptor, participate in a transient thalamocortical circuit and display spontaneous calcium transients synchronous with other superficial neurons (Che et al., 2018). Knockdown of NMDA receptors in these interneurons induces changes in whisker responses, barrel map formation and whisker-related behavior. Interestingly, this specific population of cortical interneurons also shows the typical developmental desynchronization from the first to the second postnatal week (Che et al., 2018).
Beside neurogenesis, migration, myelination, circuit and cortical map formation, early neuronal activity also influences programmed cell death (apoptosis) (Figure 4). In the mouse cerebral cortex, 30%–40% of glutamatergic and GABAergic neurons are removed between P4 and P11 with a peak at ∼P7 (for review Blanquie et al., 2017; Causeret et al., 2018). The impact of spontaneous network activity on the E/I ratio by regulation of neuronal apoptosis has been elegantly studied with longitudinal calcium imaging in the somatosensory cortex of non-anesthetized mouse pups. During the first postnatal week, cortical interneurons deriving from the medial ganglionic eminence play a crucial role in the generation of GABA-driven neuronal dynamics underlying developmental apoptosis (Duan et al., 2020). The apoptosis process is regulated by specific activity patterns, which resemble the spindle burst activity observed during perinatal stage (Golbs et al., 2011; Wong Fong Sang et al., 2021). In a longitudinal in vitro study combining multi-electrode recordings and calcium imaging, Warm et al. (2022) could demonstrate that specific spontaneous activity features predict the survival or death of developing cortical neurons. High spontaneous firing rates, discharges in bursts, synchronized activity of close neighbors and large somatic calcium increases exerted a pro-survival effect. This activity pattern controls the downstream balance between mitochondrial pro- and anti-apoptotic factors, in particular BAX and BCL-2, which determines whether a cell survives or dies (Schroer et al., 2023). Further, the maturation of specific intracortical circuits is also regulated by activity-dependent apoptosis. GABAergic interneurons in L1 provide a major synaptic input to CRNs before the latter die (Kilb and Luhmann, 2001). The death of CRNs in the barrel cortex is crucial for the proper development of inhibitory projections from L1 interneurons to L2/3 pyramidal cells, the maturation of stimulus-evoked cortical responses and for the emergence of whisker-dependent behavior (Damilou et al., 2024).
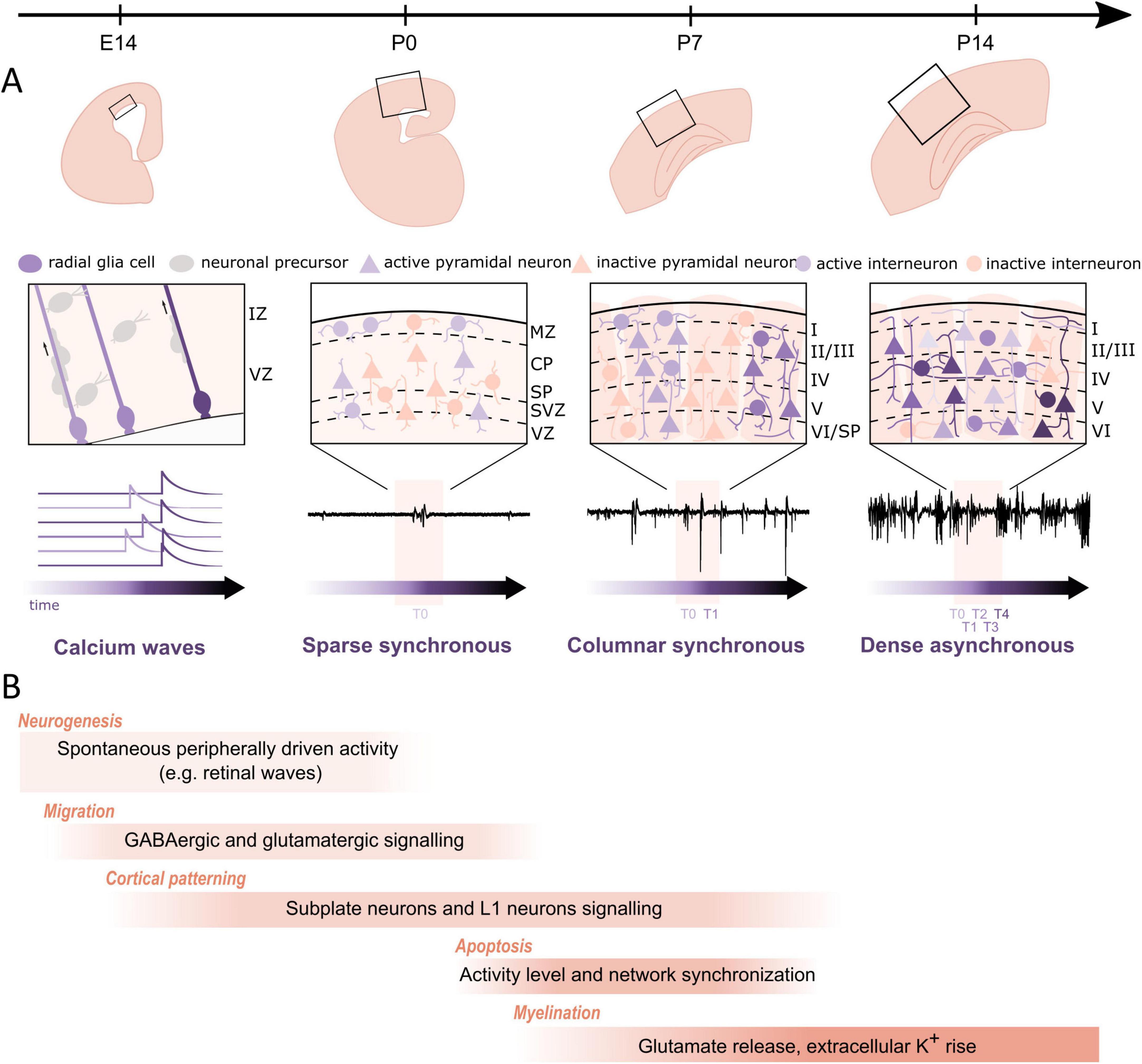
Figure 4. Regulation of cortical developmental processes by neuronal activity during late embryonic stage and the first two postnatal weeks in rodents. (A) Structural and functional maturation of the barrel cortex across the different perinatal stages. Top row: structural maturation at the macroscopic level. Middle row: close-up of the local circuitry. Bottom row: typical activity patterns (calcium signals at E14 and electrophysiological traces from P0 on) across the different developmental stages. At E14, slow calcium transients are observed in radial glial cells of the ventricular zone. At P0, sparse synchronous network activation underlies the sporadic and intermittent extracellular events. At P7, canonical synchronous columnar events drive more frequent bursts. At P14, the transition to dense and desynchronized activity generates the adult-like pattern. (B) Developmental processes that are regulated by spontaneous and/or evoked activity with illustrative mechanisms. During late embryonic stages, spontaneous calcium waves propagate through radial glia cells in the ventricular zone and promote proliferation. Next, activity drives radial and tangential migration of cortical neurons, e.g., through GABA-A receptor and NMDA-receptor mediated signaling. Subplate and layer 1 neurons serve as transient, intermediate relays and amplifiers of thalamocortical inputs and thereby facilitate the formation of cortical maps. During the first postnatal week a substantial subset of neurons is eliminated through apoptotic death, which is also regulated by intrinsic cellular and network activities. As neuronal activity increases, myelination of neurons is also triggered and remodeled, e.g., through synaptic glutamate release or an increase in extracellular potassium.
It is not surprising that disturbances in the early activity patterns can have long-term consequences (for review Kirischuk et al., 2017; Hanganu-Opatz et al., 2021). In rodents, impaired gamma oscillations in the immature prefrontal cortex may induce neuronal and behavioral dysfunction resembling psychiatric disorders in humans (for review Chini and Hanganu-Opatz, 2021; Bitzenhofer, 2023). Disturbances in the activity patterns present in rodent cortex during the first postnatal week are typically characterized by aberrant neuronal synchrony and disrupted inhibitory interneuron activity, which is associated to neurodevelopmental disorders (for review Iannone and De Marco García, 2021). Early pathophysiological changes in spontaneous activity patterns may be of genetic origin. Perturbations of genes associated with schizophrenia and/or autism spectrum disorder (ASD) cause developmental disturbances in cellular and network function of the cerebral cortex. Tuberous sclerosis complex (TSC) is an autosomal dominant genetic disorder that induces brain tumors, intellectual decline, epileptic seizures, and ASD (for review Bassetti et al., 2021a). A mutation in TSC genes (Tsc1 and Tsc2) causes alterations in cortical E/I balance, which may be related to a dysfunction in glutamatergic or GABAergic synaptic function. Pyramidal neurons in the medial prefrontal cortex of Tsc2+/ mice reveal a weakening of tonic GABA-B receptor mediated tonic inhibition that increases neuronal intrinsic excitability and network excitability (Bassetti et al., 2020). Interestingly, the GABA-B agonist baclofen shifts the E/I ratio toward excitation only in Tsc2+/ neurons, but not in wildtype (Bassetti et al., 2021b). In another mouse model the early developmental switch between two distinct network states is disturbed by mutations in ASD associated genes (Munz et al., 2023). Non-genetic factors also influence spontaneous activity patterns in the developing cortex. Pro-inflammatory cytokines, including tumor necrosis factor (TNF) alpha, are released in response to maternal infection or perinatal hypoxia and induce a variety of neuronal and non-neuronal cascades (for review Deverman and Patterson, 2009). Experimental induction of inflammation by the application of the endotoxin lipopolysaccharide (LPS) to the newborn rat somatosensory cortex in vivo causes a rapid change in the pattern of spontaneous spindle bursts and gamma oscillations, which subsequently leads to an increase in apoptosis (Nimmervoll et al., 2013). This pathophysiological response to LPS-induced inflammation is mediated by a selective activation of microglial cells via the toll-like receptor-4 receptor. Activated microglia release TNF alpha and other pro-inflammatory factors, which modify the spontaneous activity patterns (Nimmervoll et al., 2013). Recently, Pöpplau et al. (2024) demonstrated with chronic extracellular recordings and optogenetic manipulations that microglia plays a central role in the developmental profile of early spontaneous network activity in mouse prefrontal cortex and that microglia ablation causes long-lasting disruption of cognitive prefrontal function.
In summary, synchronized neuronal network activity plays an important role in various important processes of early cortical development, from neurogenesis to formation of cortical maps and columnar modules. Disturbances of early activity by endogenous or exogenous noxae may have immediate, but also long-term consequences on cortical structure and function.
Discussion
Using a wide repertoire of molecular biological, neuroanatomical, electrophysiological, and imaging techniques we gained a very good understanding of the early activity-dependent development of the rodent cerebral cortex. Comparative studies in other mammalian species demonstrate that the basic developmental sequences are very similar from mouse to human (for review Khazipov and Luhmann, 2006; Cossart and Garel, 2022; Yuste et al., 2024). We also learned that isolated in vitro models are rather limited in their relevance to comprehend activity-dependent developmental processes beyond the molecular and cellular scale. A lesson, which neurophysiologists have learned some decades ago (for review Steriade, 2001) and that already holds true at early embryonic stages, when development of the cerebral cortex depends on specific network input from subcortical regions and sensory periphery. Thus, an in vitro brain model without sensory organs, spinal cord and without a body, can only partly model the development of the brain (for review Nikolakopoulou et al., 2020; Luhmann, 2022).
The question arises, what would be currently the best model and technique to study the role of spontaneous and sensory evoked neuronal activity during early development. Obviously a whole animal model is required, preferentially in non-anesthetized recording conditions. Such recordings are technically less demanding in newborn rodents, which mostly sleep and exhibit only minor movements. However, the weight of the imaging or electrophysiological monitoring device is clearly a limiting factor, yet two-photon calcium imaging of the mouse embryonic cortex has been already successfully performed (Yuryev et al., 2016). Another requirement would be the monitoring of the same neurons over many days in the newborn mouse. Such a demanding experimental approach has been recently developed by Majnik et al. (2025) in the mouse barrel cortex. Their Track2p termed method allows the longitudinal cell tracking of several hundreds of individual neurons from daily two-photon calcium imaging over one postnatal week. With this powerful method the authors observed the sharp developmental transition from highly synchronized activity to multidimensional, behavior-state dependent neural dynamics centered on P11 (Majnik et al., 2025). Combining this experimental approach with acute or chronic optogenetic stimulation of genetically defined cell types, will provide further insights into the cellular mechanisms underlying spontaneous and evoked network activity in the developing cerebral cortex.
Author contributions
EN: Writing – original draft, Writing – review & editing, Visualization. J-WY: Data curation, Methodology, Visualization, Investigation, Software, Writing – review & editing, Formal Analysis, Writing – original draft. HL: Validation, Resources, Writing – review & editing, Project administration, Conceptualization, Supervision, Writing – original draft, Funding acquisition. AS: Investigation, Writing – review & editing, Project administration, Funding acquisition, Supervision, Writing – original draft, Conceptualization, Visualization, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by a grant of the Deutsche Forschungsgemeinschaft (LU 375/15-1).
Acknowledgments
We are most thankful to the former and current lab members who contributed to the topic of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Airaksinen, M., Räsänen, O., Ilén, E., Häyrinen, T., Kivi, A., Marchi, V., et al. (2020). Automatic posture and movement tracking of infants with wearable movement sensors. Sci. Rep. 10:169. doi: 10.1038/s41598-019-56862-5
Akhmetshina, D., Nasretdinov, A., Zakharov, A., Valeeva, G., and Khazipov, R. (2016). The nature of the sensory input to the neonatal rat barrel cortex. J. Neurosci. 36, 9922–9932. doi: 10.1523/JNEUROSCI.1781-16.2016
Allene, C., and Cossart, R. (2010). Early NMDA receptor-driven waves of activity in the developing neocortex: Physiological or pathological network oscillations? J. Physiol. 588, 83–91. doi: 10.1113/jphysiol.2009.178798
An, S., Kilb, W., and Luhmann, H. (2014). Sensory-evoked and spontaneous gamma and spindle bursts in neonatal rat motor cortex. J. Neurosci. 34, 10870–10883. doi: 10.1523/JNEUROSCI.4539-13.2014
Aníbal-Martínez, M., Puche-Aroca, L., Pérez-Montoyo, E., Pumo, G., Madrigal, M., Rodríguez-Malmierca, L., et al. (2025). A prenatal window for enhancing spatial resolution of cortical barrel maps. Nat. Commun. 16:1955. doi: 10.1038/s41467-025-57052-w
Antón-Bolaños, N., Espinosa, A., and López-Bendito, G. (2018). Developmental interactions between thalamus and cortex: A true love reciprocal story. Curr. Opin. Neurobiol. 52, 33–41. doi: 10.1016/j.conb.2018.04.018
Antón-Bolaños, N., Sempere-Ferràndez, A., Guillamón-Vivancos, T., Martini, F., Pérez-Saiz, L., Gezelius, H., et al. (2019). Prenatal activity from thalamic neurons governs the emergence of functional cortical maps in mice. Science 364, 987–990. doi: 10.1126/science.aav7617
Arakawa, H., and Erzurumlu, R. (2015). Role of whiskers in sensorimotor development of C57BL/6 mice. Behav. Brain Res. 287, 146–155. doi: 10.1016/j.bbr.2015.03.040
Bak, A., Schmied, K., Jakob, M., Bedogni, F., Squire, O., Gittel, B., et al. (2024). Temporal dynamics of neocortical development in organotypic mouse brain cultures: A comprehensive analysis. J. Neurophysiol. 132, 1038–1055. doi: 10.1152/jn.00178.2024
Bassetti, D., Lombardi, A., Kirischuk, S., and Luhmann, H. (2020). Haploinsufficiency of Tsc2 leads to hyperexcitability of medial prefrontal cortex via weakening of tonic gabab receptor-mediated inhibition. Cereb. Cortex 30, 6313–6324. doi: 10.1093/cercor/bhaa187
Bassetti, D., Luhmann, H., and Kirischuk, S. (2021a). Effects of mutations in TSC genes on neurodevelopment and synaptic transmission. Int. J. Mol. Sci. 22:7273. doi: 10.3390/ijms22147273
Bassetti, D., Luhmann, H., and Kirischuk, S. (2021b). Presynaptic GABAB receptor-mediated network excitation in the medial prefrontal cortex of Tsc2+/- mice. Pflugers Arch. 473, 1261–1271. doi: 10.1007/s00424-021-02576-5
Bitzenhofer, S. (2023). Gamma oscillations provide insights into cortical circuit development. Pflugers Arch. 475, 561–568. doi: 10.1007/s00424-023-02801-3
Blanquie, O., Kilb, W., Sinning, A., and Luhmann, H. (2017). Homeostatic interplay between electrical activity and neuronal apoptosis in the developing neocortex. Neuroscience 358, 190–200. doi: 10.1016/j.neuroscience.2017.06.030
Blumberg, M., Sokoloff, G., Tiriac, A., and Del Rio-Bermudez, C. (2015). A valuable and promising method for recording brain activity in behaving newborn rodents. Dev. Psychobiol. 57, 506–517. doi: 10.1002/dev.21305
Bonetti, C., and Surace, E. (2010). Mouse embryonic retina delivers information controlling cortical neurogenesis. PLoS One 5:e15211. doi: 10.1371/journal.pone.0015211
Cai, L., Yang, J., Wang, C., Chou, S., Luhmann, H., and Karayannis, T. (2022). Identification of a developmental switch in information transfer between whisker S1 and S2 cortex in mice. J. Neurosci. 42, 4435–4448. doi: 10.1523/JNEUROSCI.2246-21.2022
Causeret, F., Coppola, E., and Pierani, A. (2018). Cortical developmental death: Selected to survive or fated to die. Curr. Opin. Neurobiol. 53, 35–42. doi: 10.1016/j.conb.2018.04.022
Causeret, F., Moreau, M., Pierani, A., and Blanquie, O. (2021). The multiple facets of Cajal-Retzius neurons. Development 148:dev199409. doi: 10.1242/dev.199409
Che, A., Babij, R., Iannone, A., Fetcho, R., Ferrer, M., Liston, C., et al. (2018). Layer I interneurons sharpen sensory maps during neonatal development. Neuron 99:98–116.e7. doi: 10.1016/j.neuron.2018.06.002
Chini, M., and Hanganu-Opatz, I. (2021). Prefrontal cortex development in health and disease: Lessons from rodents and humans. Trends Neurosci. 44, 227–240. doi: 10.1016/j.tins.2020.10.017
Chipaux, M., Colonnese, M., Mauguen, A., Fellous, L., Mokhtari, M., Lezcano, O., et al. (2013). Auditory stimuli mimicking ambient sounds drive temporal “delta-brushes” in premature infants. PLoS One 8:e79028. doi: 10.1371/journal.pone.0079028
Colonnese, M., Kaminska, A., Minlebaev, M., Milh, M., Bloem, B., Lescure, S., et al. (2010). A conserved switch in sensory processing prepares developing neocortex for vision. Neuron 67, 480–498. doi: 10.1016/j.neuron.2010.07.015
Cossart, R., and Garel, S. (2022). Step by step: Cells with multiple functions in cortical circuit assembly. Nat. Rev. Neurosci. 23, 395–410. doi: 10.1038/s41583-022-00585-6
Cramer, N., and Keller, A. (2006). Cortical control of a whisking central pattern generator. J. Neurophysiol. 96, 209–217. doi: 10.1152/jn.00071.2006
Crépel, V., Aronov, D., Jorquera, I., Represa, A., Ben-Ari, Y., and Cossart, R. (2007). A parturition-associated nonsynaptic coherent activity pattern in the developing hippocampus. Neuron 54, 105–120. doi: 10.1016/j.neuron.2007.03.007
Damilou, A., Cai, L., Argunşah, A. Ö, Han, S., Kanatouris, G., Karatsoli, M., et al. (2024). Developmental Cajal-Retzius cell death contributes to the maturation of layer 1 cortical inhibition and somatosensory processing. Nat. Commun. 15:6501. doi: 10.1038/s41467-024-50658-6
De Faveri, F., Ceriani, F., and Marcotti, W. (2025). In vivo spontaneous Ca2+ activity in the pre-hearing mammalian cochlea. Nat. Commun. 16:29. doi: 10.1038/s41467-024-55519-w
De Marco García, N., Karayannis, T., and Fishell, G. (2011). Neuronal activity is required for the development of specific cortical interneuron subtypes. Nature 472, 351–355. doi: 10.1038/nature09865
Deverman, B., and Patterson, P. (2009). Cytokines and CNS development. Neuron 64, 61–78. doi: 10.1016/j.neuron.2009.09.002
Duan, Z., Che, A., Chu, P., Modol, L., Bollmann, Y., Babij, R., et al. (2020). GABAergic restriction of network dynamics regulates interneuron survival in the developing cortex. Neuron 105:75–92.e5. doi: 10.1016/j.neuron.2019.10.008
Erzurumlu, R., and Gaspar, P. (2012). Development and critical period plasticity of the barrel cortex. Eur. J. Neurosci. 35, 1540–1553. doi: 10.1111/j.1460-9568.2012.08075.x
Feldmeyer, D., Brecht, M., Helmchen, F., Petersen, C., Poulet, J., Staiger, J., et al. (2013). Barrel cortex function. Prog. Neurobiol. 103, 3–27. doi: 10.1016/j.pneurobio.2012.11.002
Fields, R. D. (2015). A new mechanism of nervous system plasticity: Activity-dependent myelination. Nat. Rev. Neurosci. 16, 756–767. doi: 10.1038/nrn4023
Friess, M., Hammann, J., Unichenko, P., Luhmann, H., White, R., and Kirischuk, S. (2016). Intracellular ion signaling influences myelin basic protein synthesis in oligodendrocyte precursor cells. Cell Calcium 60, 322–330. doi: 10.1016/j.ceca.2016.06.009
Garaschuk, O., Linn, J., Eilers, J., and Konnerth, A. (2000). Large-scale oscillatory calcium waves in the immature cortex. Nat. Neurosci. 3, 452–459. doi: 10.1038/74823
Gellért, L., Luhmann, H., and Kilb, W. (2023). Axonal connections between S1 barrel, M1, and S2 cortex in the newborn mouse. Front. Neuroanat. 17:1105998. doi: 10.3389/fnana.2023.1105998
Genescu, I., Aníbal-Martínez, M., Kouskoff, V., Chenouard, N., Mailhes-Hamon, C., Cartonnet, H., et al. (2022). Dynamic interplay between thalamic activity and Cajal-Retzius cells regulates the wiring of cortical layer 1. Cell Rep. 39:110667. doi: 10.1016/j.celrep.2022.110667
Golbs, A., Nimmervoll, B., Sun, J., Sava, I., and Luhmann, H. (2011). Control of programmed cell death by distinct electrical activity patterns. Cereb. Cortex 21, 1192–1202. doi: 10.1093/cercor/bhq200
Golshani, P., Gonçalves, J., Khoshkhoo, S., Mostany, R., Smirnakis, S., and Portera-Cailliau, C. (2009). Internally mediated developmental desynchronization of neocortical network activity. J. Neurosci. 29, 10890–10899. doi: 10.1523/JNEUROSCI.2012-09.2009
Haiss, F., and Schwarz, C. (2005). Spatial segregation of different modes of movement control in the whisker representation of rat primary motor cortex. J. Neurosci. 25, 1579–1587. doi: 10.1523/JNEUROSCI.3760-04.2005
Hammann, J., Bassetti, D., White, R., Luhmann, H., and Kirischuk, S. (2018). α2 isoform of Na+,K+-ATPase via Na+,Ca2+ exchanger modulates myelin basic protein synthesis in oligodendrocyte lineage cells in vitro. Cell Calcium 73, 1–10. doi: 10.1016/j.ceca.2018.03.003
Hanganu, I., Ben-Ari, Y., and Khazipov, R. (2006). Retinal waves trigger spindle bursts in the neonatal rat visual cortex. J. Neurosci. 26, 6728–6736. doi: 10.1523/JNEUROSCI.0752-06.2006
Hanganu, I., Okabe, A., Lessmann, V., and Luhmann, H. (2009). Cellular mechanisms of subplate-driven and cholinergic input-dependent network activity in the neonatal rat somatosensory cortex. Cereb. Cortex 19, 89–105. doi: 10.1093/cercor/bhn061
Hanganu, I., Staiger, J., Ben-Ari, Y., and Khazipov, R. (2007). Cholinergic modulation of spindle bursts in the neonatal rat visual cortex in vivo. J. Neurosci. 27, 5694–5705. doi: 10.1523/JNEUROSCI.5233-06.2007
Hanganu-Opatz, I., Butt, S., Hippenmeyer, S., De Marco García, N., Cardin, J., Voytek, B., et al. (2021). The logic of developing neocortical circuits in health and disease. J. Neurosci. 41, 813–822. doi: 10.1523/JNEUROSCI.1655-20.2020
Hattox, A., Li, Y., and Keller, A. (2003). Serotonin regulates rhythmic whisking. Neuron 39, 343–352. doi: 10.1016/s0896-6273(03)00391-x
Heck, N., Kilb, W., Reiprich, P., Kubota, H., Furukawa, T., Fukuda, A., et al. (2007). GABA-A receptors regulate neocortical neuronal migration in vitro and in vivo. Cereb. Cortex 17, 138–148. doi: 10.1093/cercor/bhj135
Hoerder-Suabedissen, A., and Molnár, Z. (2015). Development, evolution and pathology of neocortical subplate neurons. Nat. Rev. Neurosci. 16, 133–146. doi: 10.1038/nrn3915
Iannone, A., and De Marco García, N. (2021). The emergence of network activity patterns in the somatosensory cortex –an early window to autism spectrum disorders. Neuroscience 466, 298–309. doi: 10.1016/j.neuroscience.2021.04.005
Inácio, A., Nasretdinov, A., Lebedeva, J., and Khazipov, R. (2016). Sensory feedback synchronizes motor and sensory neuronal networks in the neonatal rat spinal cord. Nat. Commun. 7:13060. doi: 10.1038/ncomms13060
Iyer, K., Roberts, J., Hellström-Westas, L., Wikström, S., Hansen Pupp, I., Ley, D., et al. (2015). Cortical burst dynamics predict clinical outcome early in extremely preterm infants. Brain 138, 2206–2218. doi: 10.1093/brain/awv129
Kanold, P. (2009). Subplate neurons: Crucial regulators of cortical development and plasticity. Front. Neuroanat. 3:16. doi: 10.3389/neuro.05.016.2009
Kanold, P., and Luhmann, H. (2010). The subplate and early cortical circuits. Annu. Rev. Neurosci. 33, 23–48. doi: 10.1146/annurev-neuro-060909-153244
Kersbergen, C., and Bergles, D. (2024). Priming central sound processing circuits through induction of spontaneous activity in the cochlea before hearing onset. Trends Neurosci. 47, 522–537. doi: 10.1016/j.tins.2024.04.007
Kersbergen, C., Babola, T., Kanold, P., and Bergles, D. (2023). Preservation of developmental spontaneous activity enables early auditory system maturation in deaf mice. PLoS Biol. 21:e3002160. doi: 10.1371/journal.pbio.3002160
Khazipov, R., and Luhmann, H. (2006). Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 29, 414–418. doi: 10.1016/j.tins.2006.05.007
Khazipov, R., Minlebaev, M., and Valeeva, G. (2013). Early gamma oscillations. Neuroscience 250, 240–252. doi: 10.1016/j.neuroscience.2013.07.019
Khazipov, R., Sirota, A., Leinekugel, X., Holmes, G., Ben-Ari, Y., and Buzsáki, G. (2004). Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature 432, 758–761. doi: 10.1038/nature03132
Khazipov, R., Tyzio, R., and Ben-Ari, Y. (2008). Effects of oxytocin on GABA signalling in the foetal brain during delivery. Prog. Brain Res. 170, 243–257. doi: 10.1016/S0079-6123(08)00421-4
Kidokoro, H. (2021). Delta brushes are not just a hallmark of EEG in human preterm infants. Pediatr. Int. 63, 130–136. doi: 10.1111/ped.14420
Kilb, W., and Fukuda, A. (2017). Taurine as an essential neuromodulator during perinatal cortical development. Front. Cell. Neurosci. 11:328. doi: 10.3389/fncel.2017.00328
Kilb, W., and Luhmann, H. (2001). Spontaneous GABAergic postsynaptic currents in Cajal-Retzius cells in neonatal rat cerebral cortex. Eur. J. Neurosci. 13, 1387–1390. doi: 10.1046/j.0953-816x.2001.01514.x
Kirischuk, S., Luhmann, H., and Kilb, W. (2014). Cajal-Retzius cells: Update on structural and functional properties of these mystic neurons that bridged the 20th century. Neuroscience 275, 33–46. doi: 10.1016/j.neuroscience.2014.06.009
Kirischuk, S., Sinning, A., Blanquie, O., Yang, J., Luhmann, H., and Kilb, W. (2017). Modulation of neocortical development by early neuronal activity: Physiology and pathophysiology. Front. Cell. Neurosci. 11:379. doi: 10.3389/fncel.2017.00379
Kirkby, L., Sack, G., Firl, A., and Feller, M. B. (2013). A role for correlated spontaneous activity in the assembly of neural circuits. Neuron 80, 1129–1144. doi: 10.1016/j.neuron.2013.10.030
Komuro, H., and Rakic, P. (1996). Intracellular Ca2+ fluctuations modulate the rate of neuronal migration. Neuron 17, 275–285. doi: 10.1016/s0896-6273(00)80159-2
Kostović, I. (2020). The enigmatic fetal subplate compartment forms an early tangential cortical nexus and provides the framework for construction of cortical connectivity. Prog. Neurobiol. 194:101883. doi: 10.1016/j.pneurobio.2020.101883
Kota, S., Kang, S., Liu, Y., Liu, H., Montazeri, S., Vanhatalo, S., et al. (2024). Prognostic value of quantitative EEG in early hours of life for neonatal encephalopathy and neurodevelopmental outcomes. Pediatr. Res. 96, 685–694. doi: 10.1038/s41390-024-03255-8
Krämer-Albers, E., and Werner, H. (2023). Mechanisms of axonal support by oligodendrocyte-derived extracellular vesicles. Nat. Rev. Neurosci. 24, 474–486. doi: 10.1038/s41583-023-00711-y
Kreider, J., and Blumberg, M. (2000). Mesopontine contribution to the expression of active ‘twitch’ sleep in decerebrate week-old rats. Brain Res. 872, 149–159. doi: 10.1016/s0006-8993(00)02518-x
Landry, C., Yip, M., Zhou, Y., Niu, W., Wang, Y., Yang, B., et al. (2023). Electrophysiological and morphological characterization of single neurons in intact human brain organoids. J. Neurosci. Methods 394:109898. doi: 10.1016/j.jneumeth.2023.109898
Lloyd, R., O’Toole, J., Livingstone, V., Filan, P., and Boylan, G. (2021). Can EEG accurately predict 2-year neurodevelopmental outcome for preterm infants? Arch. Dis. Child Fetal Neonatal Ed. 106, 535–541. doi: 10.1136/archdischild-2020-319825
Luhmann, H. (2017). Review of imaging network activities in developing rodent cerebral cortex in vivo. Neurophotonics 4:031202. doi: 10.1117/1.NPh.4.3.031202
Luhmann, H. (2022). Neurophysiology of the developing cerebral cortex: What we have learned and what we need to know. Front. Cell. Neurosci. 15:814012. doi: 10.3389/fncel.2021.814012
Luhmann, H., Kanold, P., Molnár, Z., and Vanhatalo, S. (2022). Early brain activity: Translations between bedside and laboratory. Prog. Neurobiol. 213:102268. doi: 10.1016/j.pneurobio.2022.102268
Luhmann, H., Kirischuk, S., and Kilb, W. (2018). The superior function of the subplate in early neocortical development. Front. Neuroanat. 12:97. doi: 10.3389/fnana.2018.00097
Luhmann, H., Sinning, A., Yang, J., Reyes-Puerta, V., Stüttgen, M., Kirischuk, S., et al. (2016). Spontaneous neuronal activity in developing neocortical networks: From single cells to large-scale interactions. Front. Neural Circuits 10:40. doi: 10.3389/fncir.2016.00040
Majnik, J., Mantez, M., Zangila, S., Bugeon, S., Guignard, L., Platel, J. C., et al. (2025). Longitudinal tracking of neuronal activity from the same cells in the developing brain using Track2p. bioRxiv [Preprint]. doi: 10.1101/2025.02.26.640367 bioRxiv: 2025.02.26.640367.
Maldonado, P., Nuno-Perez, A., Kirchner, J., Hammock, E., Gjorgjieva, J., and Lohmann, C. (2021). Oxytocin shapes spontaneous activity patterns in the developing visual cortex by activating somatostatin interneurons. Curr. Biol. 31:322–333.e5. doi: 10.1016/j.cub.2020.10.028
Marcano-Reik, A., and Blumberg, M. (2008). The corpus callosum modulates spindle-burst activity within homotopic regions of somatosensory cortex in newborn rats. Eur. J. Neurosci. 28, 1457–1466. doi: 10.1111/j.1460-9568.2008.06461.x
Martini, F., Guillamón-Vivancos, T., Moreno-Juan, V., Valdeolmillos, M., and López-Bendito, G. (2021). Spontaneous activity in developing thalamic and cortical sensory networks. Neuron 109, 2519–2534. doi: 10.1016/j.neuron.2021.06.026
Martini, F., Moreno-Juan, V., Filipchuk, A., Valdeolmillos, M., and López-Bendito, G. (2018). Impact of thalamocortical input on barrel cortex development. Neuroscience 368, 246–255. doi: 10.1016/j.neuroscience.2017.04.005
Meng, X., Mukherjee, D., Kao, J., and Kanold, P. (2021). Early peripheral activity alters nascent subplate circuits in the auditory cortex. Sci. Adv. 7:eabc9155. doi: 10.1126/sciadv.abc9155
Milh, M., Kaminska, A., Huon, C., Lapillonne, A., Ben-Ari, Y., and Khazipov, R. (2007). Rapid cortical oscillations and early motor activity in premature human neonate. Cereb. Cortex 17, 1582–1594. doi: 10.1093/cercor/bhl069
Minlebaev, M., Ben-Ari, Y., and Khazipov, R. (2007). Network mechanisms of spindle-burst oscillations in the neonatal rat barrel cortex in vivo. J. Neurophysiol. 97, 692–700. doi: 10.1152/jn.00759.2006
Minlebaev, M., Ben-Ari, Y., and Khazipov, R. (2009). NMDA receptors pattern early activity in the developing barrel cortex in vivo. Cereb. Cortex 19, 688–696. doi: 10.1093/cercor/bhn115
Minlebaev, M., Colonnese, M., Tsintsadze, T., Sirota, A., and Khazipov, R. (2011). Early γ oscillations synchronize developing thalamus and cortex. Science 334, 226–229. doi: 10.1126/science.1210574
Mizuno, H., Ikezoe, K., Nakazawa, S., Sato, T., Kitamura, K., and Iwasato, T. (2018). Patchwork-type spontaneous activity in neonatal barrel cortex layer 4 transmitted via thalamocortical projections. Cell Rep. 22, 123–135. doi: 10.1016/j.celrep.2017.12.012
Mizuno, H., Rao, M., Mizuno, H., Sato, T., Nakazawa, S., and Iwasato, T. (2021). NMDA receptor enhances correlation of spontaneous activity in neonatal barrel cortex. J. Neurosci. 41, 1207–1217. doi: 10.1523/JNEUROSCI.0527-20.2020
Modol, L., Bollmann, Y., Tressard, T., Baude, A., Che, A., Duan, Z., et al. (2020). Assemblies of perisomatic GABAergic neurons in the developing barrel cortex. Neuron 105:93–105.e4. doi: 10.1016/j.neuron.2019.10.007
Mòdol, L., Moissidis, M., Selten, M., Oozeer, F., and Marín, O. (2024). Somatostatin interneurons control the timing of developmental desynchronization in cortical networks. Neuron 112:2015–2030.e5. doi: 10.1016/j.neuron.2024.03.014
Mojtahedi, N., Kovalchuk, Y., Böttcher, A., and Garaschuk, O. (2021). Stable behavioral state-specific large scale activity patterns in the developing cortex of neonates. Cell Calcium 98:102448. doi: 10.1016/j.ceca.2021.102448
Molnár, Z., Luhmann, H., and Kanold, P. (2020). Transient cortical circuits match spontaneous and sensory-driven activity during development. Science 370:eabb2153. doi: 10.1126/science.abb2153
Moody, W., and Bosma, M. (2005). Ion channel development, spontaneous activity, and activity-dependent development in nerve and muscle cells. Physiol. Rev. 85, 883–941. doi: 10.1152/physrev.00017.2004
Moore, A., Filipovic, R., Mo, Z., Rasband, M., Zecevic, N., and Antic, S. (2009). Electrical excitability of early neurons in the human cerebral cortex during the second trimester of gestation. Cereb. Cortex 19, 1795–1805. doi: 10.1093/cercor/bhn206
Moore, A., Zhou, W., Jakovcevski, I., Zecevic, N., and Antic, S. (2011). Spontaneous electrical activity in the human fetal cortex in vitro. J. Neurosci. 31, 2391–2398. doi: 10.1523/JNEUROSCI.3886-10.2011
Moore, A., Zhou, W., Sirois, C., Belinsky, G., Zecevic, N., and Antic, S. (2014). Connexin hemichannels contribute to spontaneous electrical activity in the human fetal cortex. Proc. Natl. Acad. Sci. U.S.A. 111, E3919–E3928. doi: 10.1073/pnas.1405253111
Moreno-Juan, V., Filipchuk, A., Antón-Bolaños, N., Mezzera, C., Gezelius, H., Andrés, B., et al. (2017). Prenatal thalamic waves regulate cortical area size prior to sensory processing. Nat. Commun. 8:14172. doi: 10.1038/ncomms14172
Mukherjee, D., Meng, X., Kao, J., and Kanold, P. (2022). Impaired hearing and altered subplate circuits during the first and second postnatal weeks of otoferlin-deficient mice. Cereb. Cortex 32, 2816–2830. doi: 10.1093/cercor/bhab383
Munz, M., Bharioke, A., Kosche, G., Moreno-Juan, V., Brignall, A., Rodrigues, T., et al. (2023). Pyramidal neurons form active, transient, multilayered circuits perturbed by autism-associated mutations at the inception of neocortex. Cell 186:1930–1949.e31. doi: 10.1016/j.cell.2023.03.025
Nikolakopoulou, P., Rauti, R., Voulgaris, D., Shlomy, I., Maoz, B., and Herland, A. (2020). Recent progress in translational engineered in vitro models of the central nervous system. Brain 143, 3181–3213. doi: 10.1093/brain/awaa268
Nimmervoll, B., White, R., Yang, J., An, S., Henn, C., Sun, J., et al. (2013). LPS-induced microglial secretion of TNFα increases activity-dependent neuronal apoptosis in the neonatal cerebral cortex. Cereb. Cortex 23, 1742–1755. doi: 10.1093/cercor/bhs156
Ohtaka-Maruyama, C., Okamoto, M., Endo, K., Oshima, M., Kaneko, N., Yura, K., et al. (2018). Synaptic transmission from subplate neurons controls radial migration of neocortical neurons. Science 360, 313–317. doi: 10.1126/science.aar2866
Opitz, T., De Lima, A., and Voigt, T. (2002). Spontaneous development of synchronous oscillatory activity during maturation of cortical networks in vitro. J. Neurophysiol. 88, 2196–2196. doi: 10.1152/jn.00316.2002
Pavlidis, E., Lloyd, R., and Boylan, G. B. (2017). EEG –a valuable biomarker of brain injury in preterm infants. Dev. Neurosci. 39, 23–35. doi: 10.1159/000456659
Pöpplau, J., Schwarze, T., Dorofeikova, M., Pochinok, I., Günther, A., Marquardt, A., et al. (2024). Reorganization of adolescent prefrontal cortex circuitry is required for mouse cognitive maturation. Neuron 112:421–440.e7. doi: 10.1016/j.neuron.2023.10.024
Reiprich, P., Kilb, W., and Luhmann, H. (2005). Neonatal NMDA receptor blockade disturbs neuronal migration in rat somatosensory cortex in vivo. Cereb. Cortex 15, 349–358. doi: 10.1093/cercor/bhh137
Rose, D., and Eswaran, H. (2004). Spontaneous neuronal activity in fetuses and newborns. Exp. Neurol. 190, S37–S43. doi: 10.1016/j.expneurol.2004.06.026
Routier, L., Mahmoudzadeh, M., Panzani, M., Saadatmehr, B., Gondry, J., Bourel-Ponchel, E., et al. (2023). The frontal sharp transient in newborns: An endogenous neurobiomarker concomitant to the physiological and critical transitional period around delivery? Cereb. Cortex 33, 4026–4039. doi: 10.1093/cercor/bhac324
Schroer, J., Warm, D., De Rosa, F., Luhmann, H., and Sinning, A. (2023). Activity-dependent regulation of the BAX/BCL-2 pathway protects cortical neurons from apoptotic death during early development. Cell Mol. Life Sci. 80:175. doi: 10.1007/s00018-023-04824-6
Shen, J., and Colonnese, M. (2016). Development of activity in the mouse visual cortex. J. Neurosci. 36, 12259–12275. doi: 10.1523/JNEUROSCI.1903-16.2016
Simi, A., and Studer, M. (2018). Developmental genetic programs and activity-dependent mechanisms instruct neocortical area mapping. Curr. Opin. Neurobiol. 53, 96–102. doi: 10.1016/j.conb.2018.06.007
Singh, M., White, J., McKimm, E., Milosevic, M., and Antic, S. (2018). Mechanisms of spontaneous electrical activity in the developing cerebral cortex-mouse subplate zone. Cereb. Cortex 29, 3363–3379. doi: 10.1093/cercor/bhy205
Staiger, J., and Petersen, C. (2021). Neuronal circuits in barrel cortex for whisker sensory perception. Physiol. Rev. 101, 353–415. doi: 10.1152/physrev.00019.2019
Su, X., Chen, J., Liu, L., Huang, Q., Zhang, L., Li, X., et al. (2017). Neonatal CX26 removal impairs neocortical development and leads to elevated anxiety. Proc. Natl. Acad. Sci. U.S.A. 114, 3228–3233. doi: 10.1073/pnas.1613237114
Sun, J., Kilb, W., and Luhmann, H. (2010). Self-organization of repetitive spike patterns in developing neuronal networks in vitro. Eur. J. Neurosci. 32, 1289–1299. doi: 10.1111/j.1460-9568.2010.07383.x
Tiriac, A., Uitermarkt, B., Fanning, A., Sokoloff, G., and Blumberg, M. (2012). Rapid whisker movements in sleeping newborn rats. Curr. Biol. 22, 2075–2080. doi: 10.1016/j.cub.2012.09.009
Tolner, E., Sheikh, A., Yukin, A., Kaila, K., and Kanold, P. (2012). Subplate neurons promote spindle bursts and thalamocortical patterning in the neonatal rat somatosensory cortex. J. Neurosci. 32, 692–702. doi: 10.1523/JNEUROSCI.1538-11.2012
Tolonen, M., Palva, J., Andersson, S., and Vanhatalo, S. (2007). Development of the spontaneous activity transients and ongoing cortical activity in human preterm babies. Neuroscience 145, 997–1006. doi: 10.1016/j.neuroscience.2006.12.070
Trujillo, C., Gao, R., Negraes, P., Gu, J., Buchanan, J., Preissl, S., et al. (2019). Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell 25:558–569.e7. doi: 10.1016/j.stem.2019.08.002
van der Bourg, A., Yang, J., Reyes-Puerta, V., Laurenczy, B., Wieckhorst, M., Stüttgen, M., et al. (2017). Layer-specific refinement of sensory coding in developing mouse barrel cortex. Cereb. Cortex 27, 4835–4850. doi: 10.1093/cercor/bhw280
van der Bourg, A., Yang, J., Stüttgen, M., Reyes-Puerta, V., Helmchen, F., and Luhmann, H. (2019). Temporal refinement of sensory-evoked activity across layers in developing mouse barrel cortex. Eur. J. Neurosci. 50, 2955–2969. doi: 10.1111/ejn.14413
Vanhatalo, S., and Kaila, K. (2006). Development of neonatal EEG activity: From phenomenology to physiology. Semin. Fetal Neonatal Med. 11, 471–478. doi: 10.1016/j.siny.2006.07.008
Vanhatalo, S., Tallgren, P., Andersson, S., Sainio, K., Voipio, J., and Kaila, K. (2002). DC-EEG discloses prominent, very slow activity patterns during sleep in preterm infants. Clin. Neurophysiol. 113, 1822–1825. doi: 10.1016/s1388-2457(02)00292-4
Viswanathan, S., Bandyopadhyay, S., Kao, J., and Kanold, P. (2012). Changing microcircuits in the subplate of the developing cortex. J. Neurosci. 32, 1589–1601. doi: 10.1523/JNEUROSCI.4748-11.2012
Wake, H., Lee, P., and Fields, R. (2011). Control of local protein synthesis and initial events in myelination by action potentials. Science 333, 1647–1651. doi: 10.1126/science.1206998
Wake, H., Ortiz, F., Woo, D., Lee, P., Angulo, M., and Fields, R. (2015). Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nat. Commun. 6:7844. doi: 10.1038/ncomms8844
Wallois, F., Routier, L., Heberlé, C., Mahmoudzadeh, M., Bourel-Ponchel, E., and Moghimi, S. (2021). Back to basics: The neuronal substrates and mechanisms that underlie the electroencephalogram in premature neonates. Neurophysiol. Clin. 51, 5–33. doi: 10.1016/j.neucli.2020.10.006
Wang, H., Lin, C., Chong, R., Zhang-Hooks, Y., Agarwal, A., Ellis-Davies, G., et al. (2015). Spontaneous activity of cochlear hair cells triggered by fluid secretion mechanism in adjacent support cells. Cell 163, 1348–1359. doi: 10.1016/j.cell.2015.10.070
Warm, D., Bassetti, D., Gellèrt, L., Yang, J., Luhmann, H., and Sinning, A. (2025). Spontaneous mesoscale calcium dynamics reflect the development of the modular functional architecture of the mouse cerebral cortex. Neuroimage 309:121088. doi: 10.1016/j.neuroimage.2025.121088
Warm, D., Bassetti, D., Schroer, J., Luhmann, H., and Sinning, A. (2022). Spontaneous activity predicts survival of developing cortical neurons. Front. Cell Dev. Biol. 10:937761. doi: 10.3389/fcell.2022.937761
Weissman, T., Riquelme, P., Ivic, L., Flint, A., and Kriegstein, A. (2004). Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron 43, 647–661. doi: 10.1016/j.neuron.2004.08.015
Wess, J., Isaiah, A., Watkins, P., and Kanold, P. (2017). Subplate neurons are the first cortical neurons to respond to sensory stimuli. Proc. Natl. Acad. Sci. U.S.A. 114, 12602–12607. doi: 10.1073/pnas.1710793114
Wong Fong Sang, I. E., Schroer, J., Halbhuber, L., Warm, D., Yang, J. W., Luhmann, H. J., et al. (2021). Optogenetically controlled activity pattern determines survival rate of developing neocortical neurons. Int. J. Mol. Sci. 22:6575. doi: 10.3390/ijms22126575
Wong, F., Selten, M., Rosés-Novella, C., Sreenivasan, V., Pallas-Bazarra, N., Serafeimidou-Pouliou, E., et al. (2022). Serotonergic regulation of bipolar cell survival in the developing cerebral cortex. Cell Rep. 40:111037. doi: 10.1016/j.celrep.2022.111037
Wu, M., Kourdougli, N., and Portera-Cailliau, C. (2024). Network state transitions during cortical development. Nat. Rev. Neurosci. 25, 535–552. doi: 10.1038/s41583-024-00824-y
Yang, J., An, S., Sun, J., Reyes-Puerta, V., Kindler, J., Berger, T., et al. (2013). Thalamic network oscillations synchronize ontogenetic columns in the newborn rat barrel cortex. Cereb. Cortex 23, 1299–1316. doi: 10.1093/cercor/bhs103
Yang, J., Hanganu-Opatz, I., Sun, J., and Luhmann, H. (2009). Three patterns of oscillatory activity differentially synchronize developing neocortical networks in vivo. J. Neurosci. 29, 9011–9025. doi: 10.1523/JNEUROSCI.5646-08.2009
Yang, J., Reyes-Puerta, V., Kilb, W., and Luhmann, H. (2016). Spindle bursts in neonatal rat cerebral cortex. Neural Plast. 2016:3467832. doi: 10.1155/2016/3467832
Yuryev, M., Pellegrino, C., Jokinen, V., Andriichuk, L., Khirug, S., Khiroug, L., et al. (2016). In vivo calcium imaging of evoked calcium waves in the embryonic cortex. Front. Cell. Neurosci. 9:500. doi: 10.3389/fncel.2015.00500
Yuste, R., Cossart, R., and Yaksi, E. (2024). Neuronal ensembles: Building blocks of neural circuits. Neuron 112, 875–892. doi: 10.1016/j.neuron.2023.12.008
Keywords: development, mouse, rat, neocortex, network activity, spontaneous activity, sensory evoked activity
Citation: Nigi E, Yang J-W, Luhmann HJ and Sinning A (2025) Development of spontaneous and sensory evoked network activity in rodent cerebral cortex in vivo. Front. Cell. Neurosci. 19:1648685. doi: 10.3389/fncel.2025.1648685
Received: 17 June 2025; Accepted: 24 July 2025;
Published: 06 August 2025.
Edited by:
Giusi Manassero, Neuroscience institute Cavalieri Ottolenghi (NICO), ItalyReviewed by:
Rocco Pizzarelli, European Brain Research Institute, ItalyJui-Yen Huang, Indiana University Bloomington, United States
Copyright © 2025 Nigi, Yang, Luhmann and Sinning. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heiko J. Luhmann, bHVobWFubkB1bmktbWFpbnouZGU=
†Present address: Jenq-Wei Yang, BioPro Scientific, Hsinchu, Taiwan
‡These authors share first authorship
 Elena Nigi
Elena Nigi Jenq-Wei Yang†‡
Jenq-Wei Yang†‡ Heiko J. Luhmann
Heiko J. Luhmann Anne Sinning
Anne Sinning