- 1Department of Otolaryngology, UMass Memorial Medical Center, Worcester, MA, United States
- 2University of Massachusetts Chan Medical School, Worcester, MA, United States
- 3The Eaton-Peabody Laboratories, The Massachusetts Eye and Ear Department of Otolaryngology - Head and Neck Surgery, Boston, MA, United States
- 4Harvard Medical School Department of Otolaryngology - Head and Neck Surgery, Boston, MA, United States
Schwann cells are classically known as the constituent supporting cells of the peripheral nervous system. Beyond the scope of merely myelinating axons of the more saliently known neurons, Schwann cells comprise the majority of peripheral nervous system tissue. Through the lens of the inner ear, additional properties of Schwann cells are becoming elucidated. Therein, the process of myelin formation in development is more aptly understood as a homeostatic oscillation of differentiation status. Perpetual interaction between neural and non-neural cells of the inner ear maintains an intricate balance of guidance, growth, and maturation during development. In disease, aberration to Schwann cell myelination contributes to sensorineural hearing loss in conditions such as Guillain-Barre Syndrome and Charcot-Marie-Tooth disease, and tumorigenic over proliferation of Schwann cells defines vestibular schwannomas seen in neurofibromatosis type 2. Schwann cells demonstrate plasticity during oscillations between differentiation and dedifferentiation, a property that is now being leveraged in efforts to regenerate lost neurons. Emerging strategies of reprogramming, small molecule modulation, and gene therapy suggest that Schwann cells could serve as progenitor cells for regenerated neurons. Understanding the duality of Schwann cells in pathology and repair could transform the approach to treating sensorineural hearing loss.
1 Introduction
Schwann cells are the fundamental glia of the peripheral nervous system, PNS. In the PNS, almost 80% of the cells surrounding the neurons are of glial origin (Rowitch and Kriegstein, 2010; Zuchero and Barres, 2015). Schwann cells are the supporting glial cells in the PNS and can be divided into myelinating and non-myelinating glial cells. Furthermore, satellite glia are a specialized subtype of peripheral glia, surrounding the neuronal cell bodies in the ganglia (Birren et al., 2025). Myelination by Schwann cells results in saltatory conduction, resulting in increased conduction velocity and temporal precision as compared to non-myelinated axons (Waxman, 1980; Salzer, 2015).
Schwann cells are vital within the inner ear, an intricate sensory organ responsible for maintaining hearing and balance. Schwann cells within the cochlea provide structural and trophic support, supplementation of nutrients, and neurotransmitter recycling for auditory neurons that reside within the spiral ganglion, SGN, and vestibular neurons that reside with the vestibular ganglion, VN (also known as Scarpa's ganglia; Varon and Bunge, 1978). Schwann cells also play a role in the regeneration of damaged axons, first by clearing debris and by maintaining spatial arrangement guiding the regrowing axon to innervation (Jessen and Mirsky, 2019a). Auditory nerve fibers that span from inner hair cells in the organ of Corti all the way to the cochlear nucleus of the brainstem rely on Schwann cell myelination for temporally precise signal transmission to properly function (Figure 1).
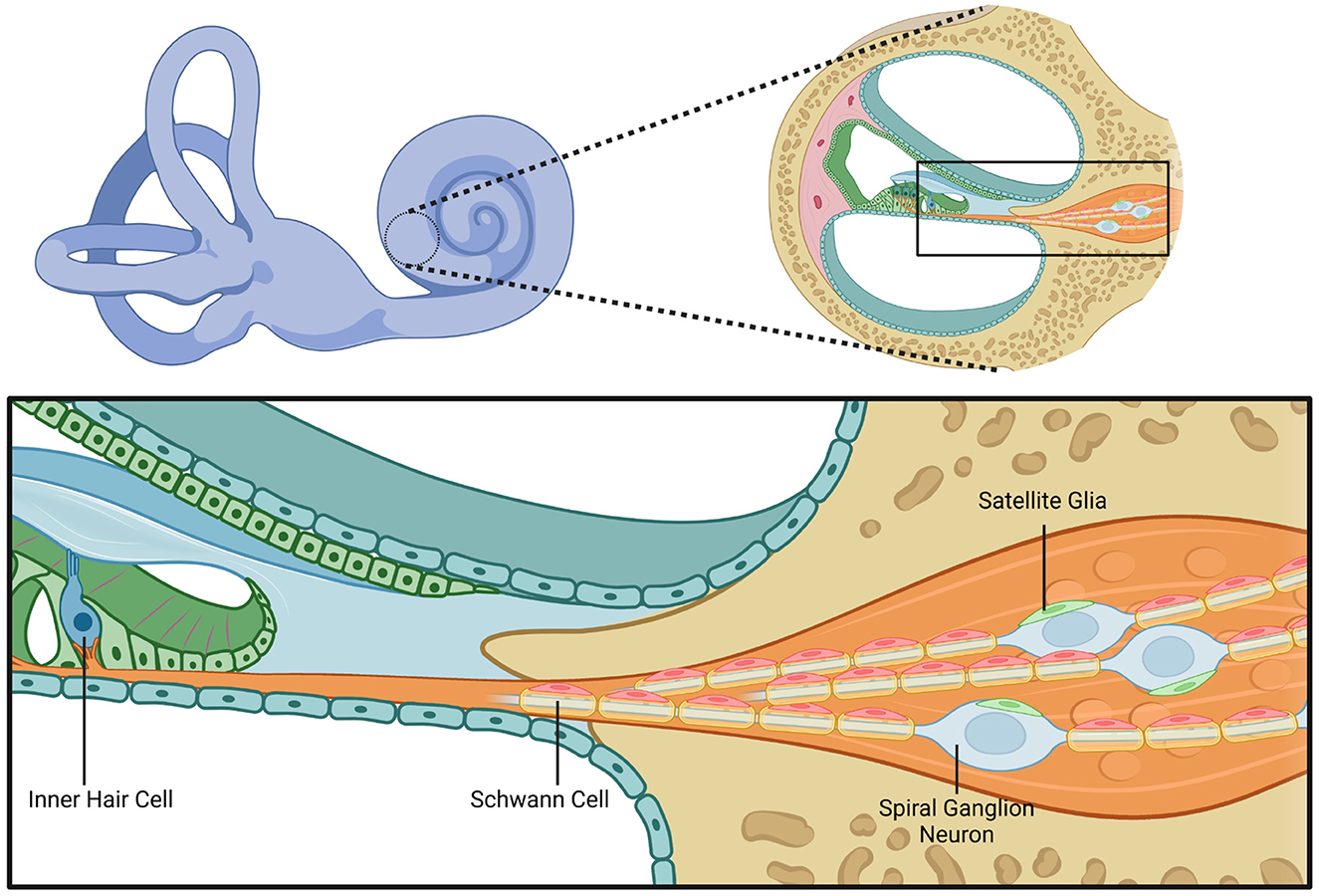
Figure 1. Cochlear structure and glial cell anatomy. This figure illustrates the anatomical structure of the cochlea with a focus on the cellular components involved in hair cell innervation by spiral ganglion neurons and the associated glial interactions. The left upper panel shows a schematic of the inner ear, highlighting the cochlea. The right upper panel shows a magnified cross-sectional view of the cochlear duct. The bottom panel provides a depiction of an inner hair cell within the organ of Corti and adjacent structures. The inner hair cell is innervated by the peripheral processes of spiral ganglion neurons. The peripheral processes are myelinated by Schwann cells. Within the spiral ganglion, neuronal cell bodies are surrounded by satellite glial cells. This figure highlights the spatial relationships between the inner hair cells that compose the sensory epithelium, spiral ganglion neurons, Schwann cells, and satellite glia. Created in BioRender. Montigny, D. (2025) https://BioRender.com/3b16cek.
2 Development
Schwann cells and neurons originate from a common progenitor in the ectoderm, indicating an intricate relationship. Understanding the developmental pathways of Schwann cells and neurons in the inner ear helps guide efforts to regenerate neurons of the SGN.
During the third week of embryonic development, the ectoderm thickens and begins to invaginate to form the otic placode. The otic placode further envelops into the otic pit, later developing into the otic vesicle. A portion of the epithelial layer of the otic vesicle, composed of neuroblasts, delaminates and migrates out of the otic vesicle and into the mesenchyme to later make up the SGN and VN whose peripheral processes innervate cochlear and vestibular hair cells, respectively, and whose central processes will make up the vestibular-cochlear nerve (Fritzsch et al., 2015; Alsina et al., 2009).
During the third week of human embryonic development, neurulation occurs in the ectoderm, which gives rise to the neural tube. The rostral portion of the neural tube is divided into six sections called rhombomeres (Bok et al., 2007). Ventral neural crest cells that migrate from the fourth rhombomere and differentiate into glial progenitor cells, also known as Schwann cell precursors, later differentiate into either myelinating Schwann cells that will myelinate the axons of SGN neurons, non-myelinating satellite glia that will reside within the SGN, or presynaptic Schwann cells (Alsina et al., 2009).
These neural crest-derived glial progenitors will move toward the recently migrated neuroblasts in the SGN and VN. Schwann cells migrate distally from SGN toward what will become the organ of Corti where they will myelinate the peripheral processes of SGN neurons (Alsina et al., 2009). Satellite glia will reside in their final location in the ganglia and will not migrate further (Alsina et al., 2009).
A distinct population of neural crest-derived glia form a passage to the cochlear duct to allow for the SGN neurons to innervate cochlear hair cells (Romand and Romand, 1990).
Immature Schwann cell precursors exist in proximity to both neuronal cell bodies and their processes. They both influence axonal pathfinding for peripheral axons and are influenced by their presence (Tylstedt et al., 1997).
Axonal activity and signaling influences Schwann cell differentiation (Moore and Linthicum, 2001). In the absence of Schwann cells, axons will still form and extend their processes, but they will extend past their destination and in various disordered directions (Mao et al., 2014). Schwann cell precursors act as intermediate targets for growing neurites (Sepp et al., 2001).
Neuronal processes and Schwann cell precursors create a scaffolding for which peripheral and central processes of these bipolar neurons that reside in the SGN can extend. Scaffolding is essential, and the glial precursors play a role in signaling for the axons to extend through this heterogeneous environment. Processes grow faster in the hind of the extending processes and the processes at the nerve front grow faster when in proximity to glia (Druckenbrod et al., 2020).
Sox10 is a known SRY-related HMG box family transcription factor expressed in differentiating and mature glia. In the mice lacking expression of Sox10, the peripheral processes of type 1 SGNs would extend past their peripheral target destination, the inner hair cells (Mao et al., 2014). This supports the findings of other studies that propose this mechanism where SCs guide and inhibit peripheral extension. Targeted conditional deletion of Sox10 caused peripheral extensions to extend past the inner hair cells and even to the lateral parts of the cochlea (Mao et al., 2014). However, central processes were not affected in the same way. Central processes were still successfully synapsing on their targets in the cochlear nucleus. This indicates central modulation that is not Sox10-dependent as it is in the periphery.
At gestational week 9, where the cochlea consists of one full turn, which will ultimately comprise the basilar turn, mature myelinating glia were located along central processes, within the ganglia around cell bodies and along peripheral processes (Locher et al., 2014). Sox10-positive glia were more densely located along the border of the SGN and not as prevalent throughout the center of the ganglia and the density of maturated myelinating glial cells was more pronounced centrally and decreased peripherally, indicative of a developmental wavefront. As this wavefront moves peripherally, Sox10-positive cells that remain in the SGN will most likely differentiate into satellite glia. Distinct from the glia that myelinate the central processes, the glia around the peripheral processes also expressed NGFR, nerve growth factor receptor. The presence of NGFR further implicates the existence of a developmental wavefront, implying that contact inhibition guides growing wavefront from the central aspect toward the periphery (Locher et al., 2014).
3 Myelination
Schwann and satellite cells, as they exist within the PNS, provide structural, metabolic, and trophic support to neurons through the mechanism of myelination (Varon and Bunge, 1978). Neurons depend on this support not only during development but also throughout the lifespan of the neuron.
Just as neurons depend on glia for survival, Schwann cell myelination is dependent on signaling pathways, some of which come from detecting normal neural signaling and temporally regular action potentials (Bouçanova and Chrast, 2020).
During promyelination, the stage immediately preceding full myelination, a temporally regulated epigenetic switch leads to the activation state of NF-kB, nuclear factor kappa-light-chain-enhancer beta subsequently leading to the expression of transcription factors including: Krox-20, also known as EGR2, Early Growth Response 2, Sox10, and Pou3F1, POU Class 3 Homeobox 1 (formerly Oct6; Chen et al., 2011). These transcription factors, induced by the activation state of NF-kB, lead to the production and promotion of myelinating proteins (Ghislain and Charnay, 2006). In response to upregulation of these transcription factors, Schwann cells begin to express myelin proteins such as P0, myelin protein zero, MAG, myelin-associated glycoprotein, and proteolipid protein 1 (Plp1; Fields, 2009). The promotion in the production of these proteins leads to molecular attraction both between the axon and myelin and between myelin and itself. Cellular adhesion molecules allow for the ensheathing of axons by myelin from Schwann cells in the PNS (Takeda et al., 2001; Garcia and Zuchero, 2019; Sukhanov et al., 2021). These extracellular signaling pathways play a role in tissue development. They are similar to the mechanisms discussed earlier that give rise to the anatomical structure of the ear during embryonic development.
Because myelination is inherently tied to normal and regular neural signaling, myelinating Schwann cells downregulate myelin-associated gene expression in the absence of neural activity and dedifferentiate into a promyelinating or non-myelinating phenotype (Momenzadeh and Jami, 2021). In the case of the inner ear of ototoxic drug-deafened mice, Schwann cells myelinating peripheral and central processes of SGNs downregulated P0 (Hurley et al., 2007).
The myelination status of Schwann cells in the PNS is most commonly understood in terms of progression from undifferentiated to differentiated, whereas it could best be understood as an oscillation between differentiated (myelinating), dedifferentiation (non-myelinating), and redifferentiation (myelinating) depending both on developmental processes and pathological processes (Salzer, 2015). Neuregulins are a family of epidermal growth factors that provide trophic support in neuron–glia interactions in the CNS and PNS (Falls, 2003). In the periphery, sensory neurons in the ganglia express Neuregulin1 (Nrg1; Rio et al., 1997). Nrg1, expressed by neurons, binds ErbB2, v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2, on Schwann cells (Rio et al., 1997). A transgenic mouse model expressing double negative ErbB2 receptors led to significant neuronal loss without loss of sensory hair cells, suggestive of a feedback loop between SGN neurons and Schwann cells (Stankovic et al., 2004). In this model, Schwann cells significantly downregulated neurotrophic factor 3 (NT3), while significantly upregulating glial-derived neurotrophic factor (GDNF; Sugawara et al., 2007; Stankovic et al., 2004). A recent in vitro study that deprived Schwann cells of Nrg1 significantly upregulated ErbB2 receptors (Chiasson-MacKenzie et al., 2023). NT3, expressed by Schwann cells under homeostatic conditions, binds to TrkC receptors on SGNs and plays a key role in neuronal survival, neurite outgrowth, and maintenance. The loss of NT3 following ErbB2 inhibition suggests that Nrg1/ErbB2 signaling is required to maintain NT3 expression. In contrast, upregulation of GDNF may represent a response by the Schwann cells to preserve remaining SGNs. These findings demonstrate that the Nrg1/ErbB2 axis may not only be implicated for structural myelination but also shift the environment from homeostatic to reactive (Gómez-Casati et al., 2010; Kohrman et al., 2021). This interplay between sensory neurons and Schwann cells is one feedback method they use to remain in homeostasis (Newbern and Birchmeier, 2010; Figure 2).
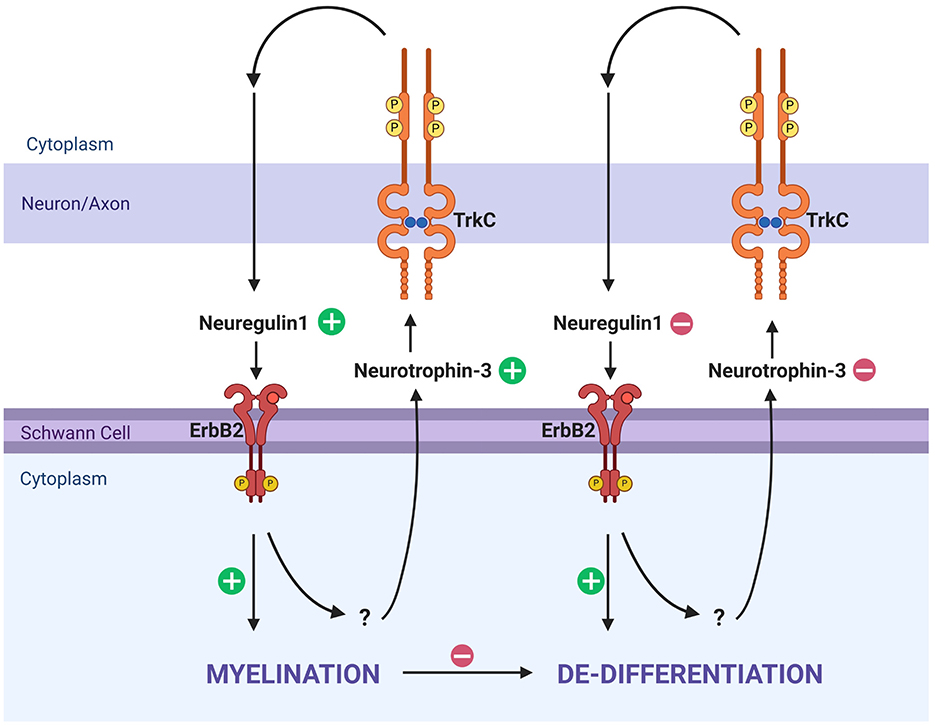
Figure 2. Myelination and dedifferentiation via ErbB2 and TrkC signaling. This schematic illustrates molecular signaling pathways that influence Schwann cell myelination and dedifferentiation in response to axonal feedback. Neuregulin1 and Neurotrophin-3 are key ligands interacting with ErbB2 on Schwann cells and TrkC on axons, respectively. On the left side, Neurotrophin-3 from Schwann cells binds TrkC on axons, leading to Neuregulin1 signaling from axons which will then bind ErbB2, reinforcing myelination in a homeostatic manner. When this process is disrupted, the Schwann cell will trend toward dedifferentiation, and no longer produce Neurotrophin-3. TrkC is likewise not bound, and the axon does not produce Neuregulin1, subsequently not binding ErbB2, reinforcing the de-differentiated state. Created in BioRender. Montigny, D. (2025) https://BioRender.com/gdz3ihk.
Axonally expressed N-cadherin and beta-catenin play a role in Schwann cell proliferation and differentiation. N-cadherin is a cell adhesion molecule that is present on the surface of the axon. When N-cadherin was bound by an inhibitor, Schwann cells decreased association with the axon and reduced proliferation. In addition, reduction of beta-catenin, the binding partner of N-cadherin, significantly decreased Schwann cell proliferation despite the addition of mitogenic Heregulin-beta1 (a synthetic homolog to Nrg1), suggestive of a relationship between Nrg1/ErbB2 and beta-catenin dependent WNT proliferation (Gess et al., 2008).
The extracellular matrix also plays a role in the myelination status of Schwann cells in the PNS. Early in the differentiation of Schwann cells, laminin binds beta1 integrins on the cell surface, leading to the assembly of basement membranes necessitated prior to the development of myelination. Laminin/integrin interactions lead to scaffolding formation and cell adhesion secondary to the basement membrane formation (McKee et al., 2012).
Secondary messenger systems also influence the myelination of Schwann cells via G-protein coupled receptor 126 (Gpr126). To date, the ligand that binds to Gpr126 is unknown, although it is likely signaling from the axon (Glenn and Talbot, 2013a; Lemke and Chao, 1988). During the promyelination stage, Schwann cells are activated via the Gpr126 cAMP pathway that leads to expression of transcription factor Pou3F1 and Pou3F2, POU Class 3 Homeobox 2 (Oct6 and Brn-2; Jaegle et al., 2003). Gpr126 is essential for the initial expression of these transcription factors, leading to the expression of Egr2, yet Gpr126 is not needed for sustained myelination (Glenn and Talbot, 2013a).
Both Oct6 and Nrg1 are needed for remyelination following injury (Fricker and Bennett, 2011; Fricker et al., 2011). In the post-injury environment, mammalian target of rapamycin complex 1 (mTORC1) activation is needed to drive dedifferentiation in Schwann cells through increasing translation of cellular Jun proto-oncogene (c-Jun) mRNA. Prolonged mTORC1 activation led to prolonged time to remyelination (Norrmén et al., 2018). Similarly, the Schwann cells remained dedifferentiated so long as mTORC1 was activated, allowing redifferentiation and remyelination upon mTORC1 downregulation (Norrmén et al., 2018).
4 Non-myelinating peripheral glia of the inner ear
The major constituent non-myelinating peripheral glia of the inner ear are satellite glia. These non-myelinating glia play a role in the inner ear similar to that of glia in the CNS in terms of metabolic and trophic support. Satellite glia around SGN neurons have membrane proteins that allow a slight inward leak of ions around the time of hearing onset, which may indicate an alternative role for these glia (Smith et al., 2021). Potentially, these glia foster a cellular environment that will maintain homeostasis while potentiating neural pathways during the onset of hearing. Satellite glia that ensheathe the soma of peripheral neurons uphold intracellular neural pathways that lead to neurite regeneration (Avraham et al., 2020). The satellite glia within the inner ear, specifically a particular population of Sox2, sex-determining region Y (SRY)-box 2, non-myelinating satellite glia, can serve as progenitors for neural regeneration (Chen et al., 2021).
Myelination of neuronal cell bodies is uncommon across the whole PNS in human. In neonatal humans, myelination of the soma of SGN neurons has not been observed. Some myelination within the SGN has been reported in humans, with an age-associated increase in ganglionic myelination, albeit, at most only a few lamellae are observed to surround the SGNs in human. However, at its maximum in the elderly, a mere 2% of SGN somas are associated with myelination by satellite glia (Arnold, 1987; Nadol et al., 1990). To date, there is no accepted consensus on why the human cochlea lacks neuronal soma myelination by satellite glia. When comparing myelination within the SGN between humans and non-human mammals, expression levels of myelin-basic protein (MBP) demonstrated a lack of organized myelination in humans (Liu et al., 2012). In contrast, regularly patterned myelination by satellite glia has been observed in mice, rats, and guinea pigs (Nadol et al., 1990). Schwann cells that myelinate the axon provide axonal trophic support while satellite glia that myelinate the soma provide somatic trophic support. The SGN's fortitude depends on the presence of presynaptic peripheral innervation of the hair cells and postsynaptic transmission through the cochlear nerve (Alam et al., 2007; Spoendlin, 1975). Normal signaling necessitates receiving and transmitting signals through both peripheral processes myelinated by Schwann cells and central processes myelinated by oligodendrocytes. In non-human mammals, satellite glia within the ganglia provide the necessary trophic support and maintain electrical signaling necessary for the survival of the neurons. There are cross-species associative differences between SGNs; in non-myelinating human SGN, neurons closely associate into a patterned formation, whereas in non-human mammals, the structure appears to be provided by myelinating satellite glia within the ganglia (Liu et al., 2012). There must be some advantage that the lack of myelination provides to these neurons. One possible explanation suggests that SGNs have evolved to associate closely with each other intentionally (Jyothi et al., 2010). The lack of myelination and close association may confer an advantage during afferent apoptosis, or hair cell death (Jyothi et al., 2010).
This increases robustness of SGN survival secondary to hair cell loss in the human SGN as compared to other non-human mammals (McFadden et al., 2004; Suzuka and Schuknecht, 1988; Zilberstein et al., 2012). The somas of human SGNs lose the trophic and metabolic support from the myelination of glia within the ganglia. However, they gain potential for electrical coupling and avoid apoptotic pathways due to insufficient signaling. If it is true that there are no myelinating satellite glia in the human SGN, then there may be alternative pathways and mechanisms unique to humans that are not seen in other mammalian models (McFadden et al., 2004; Suzuka and Schuknecht, 1988; Zilberstein et al., 2012).
Within the spiral ganglia, there are two types of SGNs. Type 1 SGNs have large myelinated fibers that relay sensory information from inner hair cells and compose approximately 95% of SGNs (Spoendlin, 1971). Type 2 SGNs have small unmyelinated fibers that relay output to outer hair cells to modulate and attenuate cochlear amplification and compose about 5% of SGNs (Spoendlin, 1971). The difference in myelination is likely necessitated for temporal precision of incoming auditory information by Type 1 SGNs. Interestingly, following transection of the cochlear nerve, Type 1 SGNs exhibit retrograde degeneration while Type 2 SGNs do not degenerate. This evidences that Type 1 SGNs are dependent on target mediated trophic support and continual synaptic activity (Spoendlin, 1971).
5 Sensorineural hearing loss and myelination related to aging
Sensorineural hearing loss, SNHL, is hearing loss related to damage or degeneration of inner ear starting anywhere from inner hair cells onto pathways connecting the inner ear to the brain, as juxtaposed to conductive hearing loss related to the middle and outer ear. Presbycusis, age-related hearing loss, was traditionally defined by the loss of SGN neurons secondary to hair cell loss (Elliott et al., 2022). Reversible threshold shifts with no hair cell loss still cause a substantial permanent loss of synapses.
Schwann cell pathology, as seen in aging, is due to a reduction in myelin maintenance and thinning of the myelin sheath that imparts a loss of regenerative and communicative capacity to their neural counterparts, as well as an increased vulnerability to environmental stressors (Glenn and Talbot, 2013b; Stassart et al., 2013).
Schwann cells myelinating auditory nerve fibers also demonstrate age-related deterioration. Schwann cells and satellite glia provide trophic support for neurons. Age-related aberration and loss of myelination, represented by depleted expression levels of MBP, myelin basic protein, in aging human and mouse cochlea has been observed (Lang et al., 2011). Even a transient bout of demyelination can elicit a lasting hidden hearing loss (HHL) phenotype (Wan and Corfas). HHL refers to hearing loss and abnormalities that are difficult to diagnose with typical audiogram style hearing tests and usually requires testing of extended high frequencies, distortion product otoacoustic emissions and auditory brainstem responses (DPOAEs and ABRs), respectively. HHL presents with normal pure tone audiogram thresholds but abnormal ABR data (Wan and Corfas, 2017). ABRs also known as auditory brainstem response are an electrical waveform representative of neural conduction encoding sound as it passes from the cochlea to the brain. Peak one of the ABR represents the specific activity of the SGNs (Jewett and Williston, 1971). Peak one amplitude represents the summative potential of the SGNs action potentials, where selective ablation of SGNs with ouabain decreases peak one amplitude (Yuan et al., Lang et al., 2008). Peak one latency is inversely proportional to conduction velocity and by logic the health of myelinating Schwann cells (Jewett and Williston, 1971; Salzer, 2015). There is a decrease in peak one amplitude in aging humans and mice as compared to their younger counterparts (Lang et al., 2011; Xing et al., 2012). It remains unclear whether this decrease in peak one amplitude occurs prior to the neural loss and is mutually exclusive of hair cell loss. Age-aberrated myelination offers a potentially explanatory pathway in which myelin sheath loss occurs prior to neural degeneration and is an area of future exploration. Based on ultrastructural study finding that the vast majority of human SGN neurons lack myelination at the soma, the fact that age-associated loss of neurons in all mammals is suggestive that neural degeneration may occur primary to secondary myelination aberration (Nadol et al., 1990; Lang et al., 2011). Age-related myelin deficits offer an explanation for some cases of HHL wherein loss of myelination and conductance velocity confers perturbed temporal processing and appraisal (Kohrman et al., 2021).
6 Schwann cell disorders
Schwann cells play a significant role in the pathology of the inner ear. On one end of the spectrum, over proliferation of Schwann cells in the vestibulo-cochlear nerve forming vestibular schwannomas leads to hearing loss in diseases such as neurofibromatosis type 2-schwannomatosis (NF2-SWN), while demyelinating disorders, such as in Charcot-Marie-Tooth Disease Type 1A, CMT1A, or Guillain-Barre Syndrome (GBS), are associated with hidden hearing loss (Wan and Corfas, 2017; Cassinotti et al., 2024). Schwann cells uphold the delicate balance within the inner ear; their range of functionalities discussed thus far carries the converse during dysregulation.
6.1 Myelinopathies
When discussing the importance and intricate balance Schwann cells maintain in the inner ear, it would be remiss not to mention demyelinating disorders and their role in inner ear pathology. GBS is an acute autoimmune demyelinating disorder. Onset typically occurs after respiratory or digestive illness; although the exact pathophysiology remains unknown, but for functional purposes, T lymphocytes lose autoimmune tolerance and attack peripheral nerve components, including Schwann cells and their myelin sheaths (Dalakas, 2013).
Transient loss of myelin in the periphery, specifically the Schwann cells that myelinate the SGN peripheral processes, is associated with HHL characteristic symptoms such as the drastic increase in peak one latency and decrease in peak one amplitude despite otherwise normal ABR/DPOAE (Wan and Corfas, 2017). These changes were permanent despite the Schwann cells remyelinating the peripheral processes.
Patients with Charcot-Marie-Tooth Disease Type 1A have similar pure tone audiograms as compared to age matched controls; however, speech in-noise testing revealed a significant difference wherein the CMT1A patients had much more difficulty understanding speech in noise. The difference was accounted to a reduction in temporal resolution associated with an aberration in the Schwann cell myelination (Cassinotti et al., 2024; Choi et al., 2018).
A mouse model of CMT1A confirmed little to no loss of auditory nerve fibers yet disruptions in myelination at the heminodes were associated with the HHL phenotype observed in patients with CMT1A (Cassinotti et al., 2024).
It was discerned that this mechanism was independent of synaptopathy as the effects from hair cell ablation were additive to the changes from acute demyelination. This, in addition to findings from Charcot-Marie-Tooth Disease, system lupus erythematosus and other episodes of acute demyelination both support and necessitate the need for homeostatic Schwann cell myelination in the inner ear. In the cases of Schwann cell demyelination due to GBS and systemic lupus erythematosus, the immune system, specifically the quiescent macrophage phenotype, is the purported culprit (Choi et al., 2018; Berger et al., 2006; Griffin and Sheikh, 1999; Di Stadio and Ralli, 2017; Budak et al., 2021, 2022). There is some evidence that this is analogous to multiple sclerosis by way of oligodendrocytes in the CNS regarding abnormal latencies on auditory brainstem responses (Furst and Levine, 2015).
6.2 NF2 and schwannomatosis
Schwannomas that frequently present in patients with NF2-SWN are tumors of the nerve sheath known as vestibular schwannomas, or formerly acoustic neuromas. Pertinent to the inner ear, the presence of vestibular schwannomas can lead to hearing loss, loss of balance, tinnitus, and vertigo. Patients with vestibular schwannomas present with hearing loss as the first symptom in approximately 90% of cases, with high frequency SNHL being the most prevalent (Harner et al., 2000). As the vestibular schwannoma progresses, it will put pressure on surrounding areas. Outgrowth that compresses the brainstem is fatal. Both natural growth and surgical treatment can also damage the facial nerve which lies in the surrounding area. In many cases, this leads to facial paralysis, numbness, or paresthesia (MacCollin et al., 2005; Vakharia et al., 2012). NF2-SWN is due to an autosomal-dominant germline mutation in the NF2 gene. The NF2 gene, a tumor suppressor gene, normally codes for a protein known as Merlin, also known as moesin-ezrin-radixin-like-protein, schwannomin or neurofibromin 2. As a normally functional tumor suppressor protein, Merlin inhibits several key proliferative and expansive pathways in Schwann cells. Merlin was traditionally considered a cytoplasmic scaffolding protein that provided mechanistic information from the extracellular environment to intracellular cytoplasmic pathways. There is varying utilization and intracellular signaling with regard to these growth factors (McClatchey and Giovannini, 2005). In addition, the loss of Merlin in NF2 leads to a lack of growth inhibition and tumorigenesis. Similarly, Merlin does, in fact, function to inhibit mitogenic pathways. In the functional pathway, Merlin migrates to the nucleus to elicit tumor-suppressive gene expression (Li et al., 2012; Beltrami et al., 2013; Ren et al., 2021).
Schwannomatosis as a disease is characterized by uninhibited proliferation of Schwann cells, inevitably giving rise to a tumor known as schwannoma. Schwannomatosis not related to NF2 can result from mutations in tumor suppressor genes on chromosome 22 including but not limited to the SWI/SNF-related BAF chromatin remodeling complex subunit B1 (SMARCB1) and leucine zipper like post-translational regulator 1 (LZTR1 genes; Tamura, 2021). As a disease, it is classified by at least one schwannoma confirmed pathologically and a common variant of SMARCB1, LZTR1, or loss of heterozygosity at chromosome 22q in two anatomically separate schwannomas and without the presence of bilateral vestibular schwannomas. However, the current criteria do not exclude unilateral vestibular schwannomas or meningiomas (Plotkin et al., 2022). The presence of bilateral vestibular schwannomas is rather indicative of NF2-SWN. Patients with unilateral vestibular schwannomas have ipsilateral SNHL in approximately 20% of cases and are at an increased risk for developing moderate hearing loss in the contralateral ear, suggestive of underlying genetic predisposition and may serve as evidence of tumor secretions although these mechanisms remain ill defined (Sauvaget et al., 2005).
Histologically, peripheral nerve sheath tumors such as schwannomas are described to maintain two distinct extracellular architectures, which can be seen as recognizable patterning: Antoni A and Antoni B. Antoni A is characterized by hypercellularity. Antoni A regions of schwannomas are organized in a stacked wave-like fashion known as a palisade. Antoni A patterning is uncommon in vestibular schwannomas. Antoni B, in contrast, is hypocellular and is most common in VS. Extracellular architecture includes regions of acellularity called Verocay bodies and microcysts. Unlike Antoni A, the extracellular environment of Antoni B supports the activation of lymphocytes and microglia. Antoni B areas foster an environment susceptible to immune cell infiltration (Labit-Bouvier et al., 2000). Immune cells, specifically tumor-associated macrophages, foster a pro-inflammatory cascade that drives tumorigenesis via angiogenesis as well as chemokine/cytokine signaling (Hannan et al., 2020). A study comparing the proliferative potential of schwannomas identified that MIB-1-defined proliferation mostly occurred at the transition zone of Antoni A and B areas alongside the presence of macrophages. However, mitosis was only noted as positive in Antoni A areas (Abe et al., 2000). In an earlier study, Antoni B areas were ridden with signs of degeneration, including fragmented and detached basal lamina, disrupted tissue, and acellularity (Sian and Ryan, 1981). Schwann cell-to-neuron feedback loops of contact and contact inhibition are integral in maintaining healthy myelination and homeostasis (Bouçanova and Chrast, 2020; Cristobal and Lee, 2022).
Schwann cells secrete TNF-alpha, a pro-inflammatory cytokine (Wagner and Myers, 1996). The Schwann cell release of TNF-alpha related to a NF-kB-derived pathway (Qin et al., 2012). NF-kB signaling stimulates the release of pro-inflammatory cytokines and chemokines, and NF-kB pathways promote cell proliferation while inhibiting apoptosis (Liu et al., 2017). NF-kB promotes the growth of schwannomas (Elmaci et al., 2018). Merlin inhibits the NF-kB signaling pathway (Kim et al., 2002). NF-kB-mediated tumorigenesis is evidenced through results focused on attenuating cyclooxygenase 1 pathways. Tumor growth was reduced in patients taking acetylsalicylic acid (Kandathil et al., 2014; Dilwali et al., 2015). The NF-kB pathway may be the driving force of schwannoma proliferation.
Vestibular schwannomas almost always arise on the vestibular portion of the vestibulo-cochlear nerve in the internal auditory canal or cerebellar pontine angle (Sataloff et al., 1988; Gosselin et al., 2016; Zhang et al., 2022; Choayb et al., 2023; Khrais et al., 2008). Cases of intralabyrinthine schwannomas have been reported, but the origination of these Schwannomas remains for debate (Zhang et al., 2022). Hearing loss associated with vestibular schwannomas was historically understood as mechanical compression of the cochlear nerve leading to aberration in neural transduction which was realized as hearing loss. However, hearing loss develops regardless of tumor size or tumor growth (Brown et al., 2022). Some degree of hearing loss seen with vestibular Schwannomas may be due to mass effect as seen with meningiomas and other masses in close proximity to auditory pathway (Ruiz-Garcia et al., 2024).
Patients with NF2-SWN present with peak one latency and decrease of peak one amplitude in ABR (Thomsen et al., 1978; Musiek et al., 1980). The audiological abnormalities observed in the ABRs of NF2-SWN patients parallel changes in ABRs seen in cases of auditory nerve demyelination (Wan and Corfas, 2017). Given that vestibular schwannomas arise from Schwann cell origins, aberration of Schwann cell myelination could serve as the etiological basis for hearing loss in NF2 (Figure 3). These findings suggest that hearing loss with vestibular Schwannoma may not only involve neural compression but a reprogramming of Schwann cells that may coincide with tumor onset and progression (Mohamed et al., 2023).
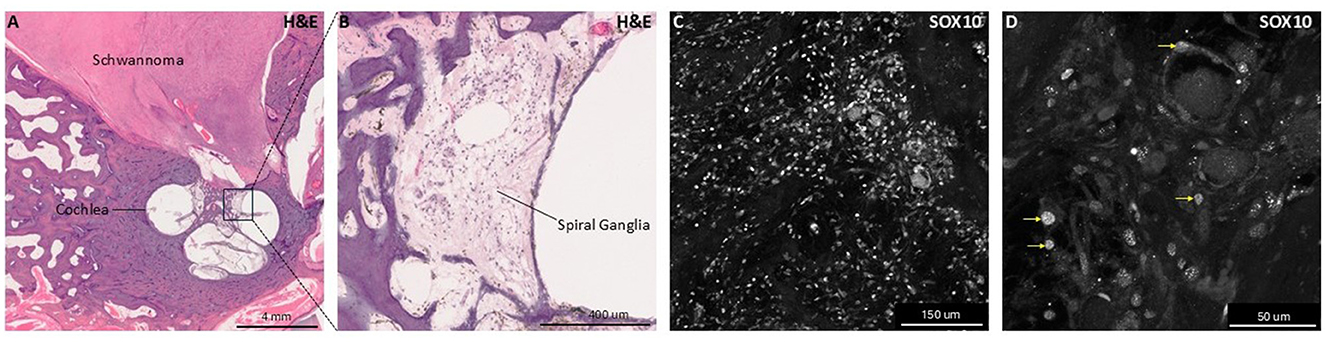
Figure 3. Histologic and immunohistochemical human temporal bone samples with Schwannoma involvement. This figure presents hematoxylin and eosin (H&E) stained and SOX10 immunolabeled images for human temporal bone sections highlighting glial pathology and anatomy. Panel (A) shows a low-magnification H&E-stained section of a human temporal one containing a vestibular schwannoma from a patient with neurofibromatosis type 2-associated schwannomatosis (NF2-SWN) occupying the internal auditory canal, illustrating schwannoma localization to inner ear structures. Panel (B) shows higher magnification view focusing on the spiral ganglion, where spiral ganglion neurons and glia reside. Panels (C, D) show immunofluorescent labeling of SOX10, a nuclear glial cell marker, within the spiral ganglia. Panel (D) includes yellow arrows indicating examples of SOX10+ nuclei, emphasizing the presence and distribution of glial cells within the ganglia. These images demonstrate both histological architecture of glial cell populations and pathological human cochlear tissue.
7 Inner ear Schwann cells and regeneration
SNHL in humans and mammals has historically been viewed as irreversible due to the lack of spontaneous repair or renewal of damaged cellular structures, including hair cells, synapses, and neurons. However, two decades ago, the discovery of progenitor-like cells within the cochlea opened up new possibilities for hearing restoration. While these dormant cells initially claimed limited regenerative potential in vivo, they demonstrated promise for regeneration when stimulated in vitro (Li et al., 2003).
The inner ear regeneration field has since focused on three main goals: identifying and characterizing inner ear progenitors, identifying manipulators to initiate cell fate switches, and increasing the yield of newly generated hair cells.
SNHL is linked to the loss of hair cells and/or neurons, which has been linked to age- and noise-related hearing loss, as well as ototoxic and genetic factors. Unlike mammals, non-mammalian species such as amphibians and birds can spontaneously regenerate hair cells through the continuous division and transdifferentiation of supporting cells. Researchers have aimed to understand the molecular mechanisms underlying hair cell regeneration in these species to apply similar principles to the mammalian inner ear. Supporting cells are developmentally related to hair cells and provide trophic support during development and throughout life. After damage to hair cells, supporting cells can be stimulated and coaxed to differentiate into hair cells.
Inner ear Schwann and satellite cells have many similarities to supporting cells; these glia and inner ear neurons share a distant common progenitor, and inner ear glial cells provide trophic support for normal neuronal function during development, aging, and disease (Delaunay et al., 2008; Doetsch, 2003; Rowitch and Kriegstein, 2010). Glial cells of the CNS can be coaxed into becoming neurons upon forced overexpression of pro-neural genes, i.e., Achaete-scute homolog 1 (Ascl1) and Neurogenin 1 (Ngn1; Jessen and Mirsky, 2019a; Davis and Temple, 1994). Astrocytes are implicated to modulate neurogenesis, synaptic reorganization, and neural plasticity overall in the CNS.
Overexpression of transcription factors Paired Box Gene 6 (Pax6) and Neurogenin 1 and 2 induce astrocytic conversion to glutamatergic neurons, while Ascl1 and Dix1/2 were able to convert astrocytes to GABAergic neurons (Guillemot, 2005; Schuurmans et al., 2004; Casarosa et al., 1999; Parras et al., 2002). Albeit there was CNS region-specific ability to convert to particular neuronal subtypes and lack of evidence of a functional readout, epigenetic modulation of the CNS was able to reprogram astrocytes to neural phenotypes.
In the post-injury environment in the CNS, there is a period known as reactive gliosis, which includes a proliferation of reactive astrocytes (Robel et al., 2011). During this proliferation, reactive astrocytes upregulate Sox2, a neural stem cell marker, and eventually differentiate into fully functional neurons (Niu et al., 2015). After this proliferation, the glia form what is known as a glial scar in the nervous tissue, which functions to prevent the spread of inflammation through microglial invasion and creates a border to limit the bounds of fibrotic tissue (Yang et al., 2020). Glial scarring, however, inhibits the outgrowth of reforming neural processes both through the formation of a fibrous scar and cytokine release (Kimura-Kuroda et al., 2010).
After neuronal damage or loss in the PNS, Schwann cells and satellite cells remain within the cochlea and usually survive long term, but rather than regenerating into neurons, only a glial scar is initiated (Yuan et al., 2014). Both in spinal cord injury and facial nerve injury, Schwann cells contribute to the regenerative process by migrating toward the site of damage, proliferating, and guiding damaged axon's regrowth by providing cellular scaffolding (Jessen and Mirsky, 2019b; Wang et al., 2022), although maladaptive Schwann cell proliferation and gliosis during the post-injury stage may ultimately hinder functional recovery due to glial scarring (Jessen and Mirsky, 2019b; Wang et al., 2022).
Following loss of sensory epithelium in deafened rat cochlea, Schwann cells dedifferentiated to non-myelinating phenotypes (Hurley et al., 2007). Recent work indicates that, if properly stimulated, these glial cells may also exhibit regenerative potential (Lang et al., 2011). Immediately after primary degeneration of the inner ear, Schwann cells upregulate the transcription factor Sox2 (Lang et al., 2011). Similarly, the Sox2-positive phenotype of these Schwann cells mimics the Sox2-positive neural progenitor phenotype of astrocytes in the CNS. Furthermore, the Sox2-positive population of Schwann cells that resides in the SGN, in vitro, can be reprogrammed to become phenotypical neurons (Chen et al., 2021).
During the development of the inner ear, expression of pro-neural transcription factor Ngn1 and basic helix-loop-helix, bHLH, transcription factor neuronal differentiation 1, NeuroD1 drive neural differentiation (Jeon et al., 2011; Evsen et al., 2013). In the CNS, expression of NeuroD1 and Ngn1 is sufficient to drive neural conversion from glia, while expression of NeuoD1 and Ascl1 drove neural conversion from astroglia. In an injury model, overexpression of pro-neural genes in glia was necessary for glia-to-neuron conversion (Heinrich et al., 2014; Su et al., 2014).
Subsequently in the same fashion, explanted Schwann cells from the SGN were grown in cell culture and, when treated with specific substrates, would develop into what is now referred to as neurospheres (Diensthuber et al., 2014). Ensuing ideas based on hair cell regeneration from supporting cells looked to see if the same could be accomplished for turning Schwann cells toward a neurogenic fate. In vitro, explanted Plp1-positive glia spontaneously differentiated into neurons and began the outgrowth of neurites, even forming synapses (Martinez-Monedero et al., 2007; Guo et al., 2009; Le Bras et al., 2005; McLean et al., 2017; Shi and Edge, 2013; Denton et al., 2016).
Within the SGN of the inner ear, there is a population of early neural crest progenitor cells that express Plp1 (Li et al., 2003; McLean et al., 2016; Breuskin et al., 2010; Harlow et al., 2014;; Kempfle et al., 2020).
However, within the bounds of the post-injury environment, Sox2-positive, or more broadly Plp1-positive, Schwann cells within the SGN can be reprogrammed into neurons through upregulation of key transcriptional factors and a variety of small molecules. Sox2 and c-Jun coregulate myelination negatively in human schwannomas, pushing Schwann cells toward a dedifferentiated state (Shivane et al., 2013). Unlike Schwann cells in the somatic PNS, where injury induced dedifferentiation supports axonal regeneration over long distances, Schwann cells in the inner ear rarely support axonal regrowth in vivo despite transcriptional changes. While c-Jun upregulation is common to both environments, the outcome of regenerated axons is far more limited in the cochlea. There may be differences in extracellular matrix, absence of permissive structural scaffolds, or suppression of inflammatory cascades needed for axonal regrowth or remyelination.
The pertinent Sox2-positive cells upregulate key neuronal factors such as Ascl1 or high mobility group A2 (Hmg2a). As they convert to a more neural phenotype, Sox2 expression is downregulated while Ascl1 remains upregulated.
Similarly, an RNA-binding protein Lin28 promotes the proliferation of neural precursors during early murine development. At later time points, Lin28 interacts with Let7 microRNA to promote differentiation of these proliferating progenitor cells. In addition, Lin28 upregulated Hmg2a, which was upregulated in Sox2-positive cells. Similarly, Lin28 maintained Plp1 progenitor cells in the inner ear and promoted neuronal differentiation (Cimadamore et al., 2013; Newman et al., 2008; Viswanathan and Daley, 2010; Heo et al., 2008). Ngn1 drives the expression of NeuroD1 when activated by RNA binding protein Lin28 (Kempfle et al., 2020). In conjunction, Let7 induction via the Lin28/Let7 axis decreases the Hmg2a neural stemness and allows the cell to express and upregulate neural-derived bHLH transcription factors Ngn1 and Ascl1 (Cimadamore et al., 2013; Kumar et al., 2022), pushing the cells to neural fate through facilitating cell cycle exit via upregulation of post-mitotic pro-neural genes. To this day, it remains unclear, which subpopulation of glial cells in the inner ear truly harbors the most regenerative potential for neuronal regeneration. The search for “dormant” glial progenitor cells in the inner ear continues, with the hope of further enhancing the understanding and potential for inner ear neuron regeneration (Kempfle et al., 2020).
8 Potential gene and cell therapy approaches for SNHL
Gene therapy is gaining traction for regeneration of neurons. Current strategies involve viral vectors, i.e., adeno-associated viruses (AAVs), to deliver reprogramming factors and modulate gene expression mainly targeted at the transcription factors Ascl1, Sox2, and NeuroD1.
Various small molecule drugs have been used in combination with viral vectors to increase neural yield in conversion in both the PNS and CNS that may serve as a more clinically approachable solution to reprogramming neurons in the inner ear of humans (Pfisterer et al., 2016; Wapinski et al., 2013; Treutlein et al., 2016; Mall and Wernig, 2017; Velasco et al., 2017; Ma et al., 2019). Histone modifiers: molecules such as Trichostatin A and Valproic acid alter histone acetylation increasing chromatin accessibility lending toward neural reprograming (Smith et al., 2016). Compounds such as forskolin, CHIR99021(a GSK-3 inhibitor), and ISX-9 leverage signaling pathways toward neuronal differentiation through transcriptional level changes, i.e., the ability to modulate transcription factor binding (Wapinski et al., 2013; Pfisterer et al., 2016; Yin et al., 2019; Figure 4).
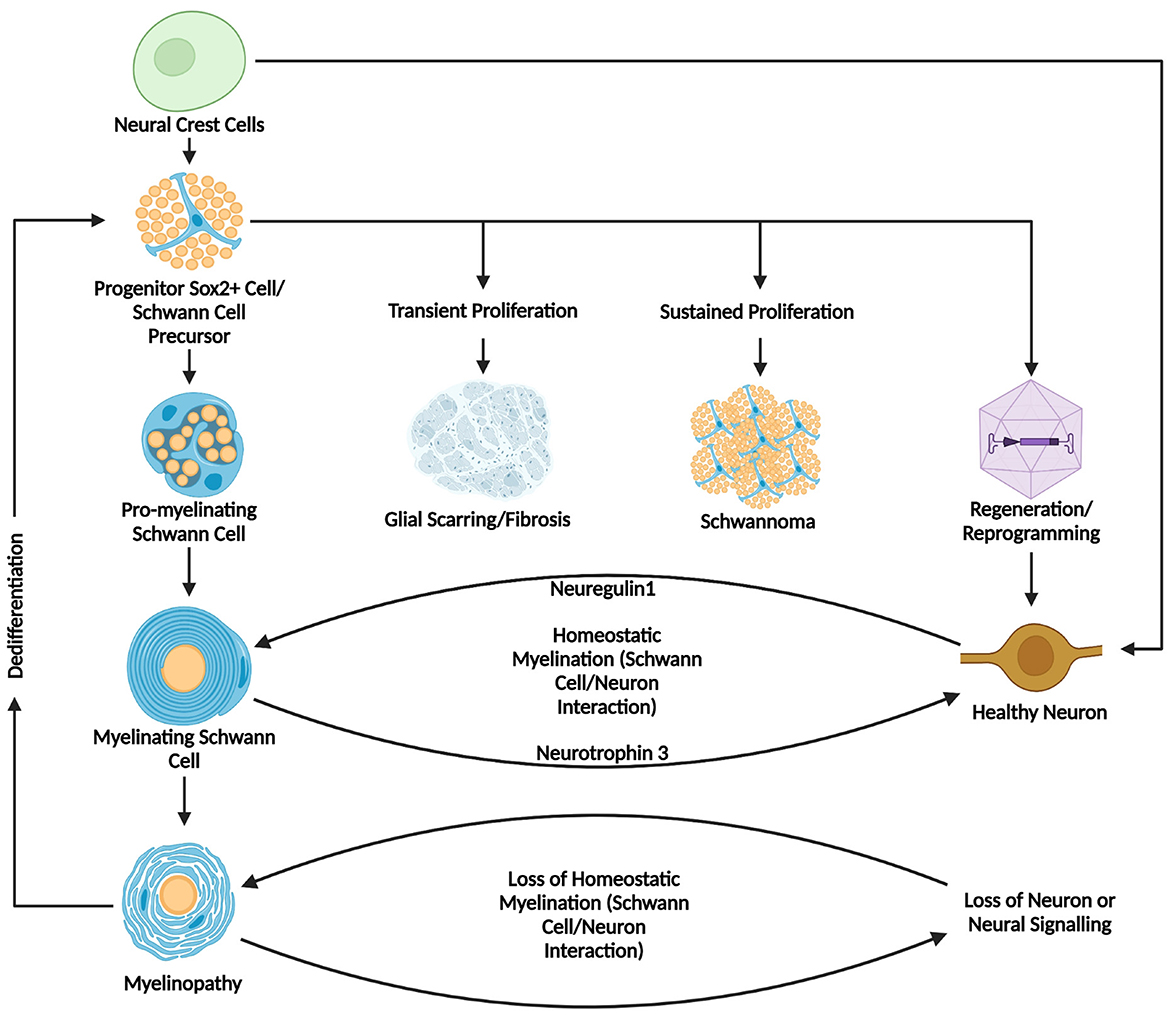
Figure 4. Summary diagram of Schwann cell development, myelination, and pathology in homeostasis and disease. This figure summarizes the developmental origins, pathways of differentiation, and potential outcomes of Schwann cells, highlighting their dynamic roles in homeostasis and pathology. Starting from neural crest cells and later progressing to Schwann cell precursors before developing further to pro-myelinating and mature myelinating Schwann cells. Healthy myelinating Schwann cells interact with neurons through a Neuregulin1/Neurotrophin-3 mediated axis, while myelinopathy or loss of healthy neural tissue can disrupt this feedback loop. Downstream outcomes in pathological states can lead to myelinopathy and subsequent de-differentiation leading to a less differentiated state. It is in this state that Schwann cells can proliferate transiently, leading to formation of a glial scar, or proliferate continuously leading to a schwannoma, or alternatively, be prompted toward a neural fate through cellular reprogramming or transdifferentiation. Created in BioRender. Montigny, D. (2025) https://BioRender.com/s9fe79f.
The potential for Schwann cells in the inner ear to serve as a source of regenerative neural progenitors represents a novel conceptual shift in the field. Traditionally known as purely supportive, emerging evidence suggests that Schwann cells may be harnessed for neural reprogramming and transdifferentiation. Schwann cells in the inner ear exhibit the capacity for plasticity and position themselves to not only contribute to disease but to be the target for the future of therapeutic strategy in SNHL (Meas et al., 2018).
9 Conclusion
In all the harm that can come from Schwann cell degeneration, there is a far greater potential for what they can create. Schwann cells play a significant role in the pathology of the inner ear. Proliferation of Schwann cells in the vestibulo-cochlear nerve defines the etiology of hearing loss in diseases such as neurofibromatosis type 2 (NF2), while a brief period of demyelination such as in Guillain-Barre Syndrome could also lead to hearing loss. Schwann cells uphold the delicate balance within the inner ear; their range of functionalities carries the converse during dysregulation. Schwann cell and neuron interactions implicated in normal function and disease are rooted in early development where they share a common progenitor. This pliability of Schwann cells makes them a valuable therapeutic target for inducing trans-differentiation or cellular reprogramming to restore neurons within the spiral ganglia.
Author contributions
DM: Conceptualization, Data curation, Visualization, Writing – original draft, Writing – review & editing. JK: Conceptualization, Supervision, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abe, M., Kawase, T., Urano, M., Mizoguchi, Y., Kuroda, M., Kasahara, M., et al. (2000). Analyses of proliferative potential in schwannomas. Brain Tumor Pathol. 17, 35–40. doi: 10.1007/BF02478916
Alam, S. A., Robinson, B. K., Huang, J., and Green, S. H. (2007). Prosurvival and proapoptotic intracellular signaling in rat spiral ganglion neurons in vivo after the loss of hair cells. J. Comp. Neurol. 503, 832–852. doi: 10.1002/cne.21430
Alsina, B., Giraldez, F., and Pujades, C. (2009). Patterning and cell fate in ear development. Int. J. Dev. Biol. 53, 1503–1513. doi: 10.1387/ijdb.072422ba
Arnold, W. (1987). Myelination of the human spiral ganglion. Acta Otolaryngol. Suppl. 436, 76–84. doi: 10.3109/00016488709124979
Avraham, O., Deng, P. Y., Jones, S., Kuruvilla, R., Semenkovich, C. F., Klyachko, V. A., et al. (2020). Satellite glial cells promote regenerative growth in sensory neurons. Nat. Commun. 11:4891. doi: 10.1038/s41467-020-18642-y
Beltrami, S., Kim, R., and Gordon, J. (2013). Neurofibromatosis type 2 protein, NF2: an uncoventional cell cycle regulator. Anticancer Res. 33, 1–11.
Berger, P., Niemann, A., and Suter, U. (2006). Schwann cells and the pathogenesis of inherited motor and sensory neuropathies (Charcot-Marie-Tooth disease). Glia 54, 243–257. doi: 10.1002/glia.20386
Birren, S. J., Goodrich, L. V., and Segal, R. A. (2025). Satellite glial cells: no longer the most overlooked glia. Cold Spring Harb. Perspect. Biol. 17:a041367. doi: 10.1101/cshperspect.a041367
Bok, J., Brunet, L. J., Howard, O., Burton, Q., and Wu, D. K. (2007). Role of hindbrain in inner ear morphogenesis: analysis of Noggin knockout mice. Dev. Biol. 311, 69–78. doi: 10.1016/j.ydbio.2007.08.013
Bouçanova, F., and Chrast, R. (2020). Metabolic interaction between schwann cells and axons under physiological and disease conditions. Front. Cell. Neurosci. 14:148. doi: 10.3389/fncel.2020.00148
Breuskin, I., Bodson, M., Thelen, N., Thiry, M., Borgs, L., Nguyen, L., et al. (2010). Glial but not neuronal development in the cochleo-vestibular ganglion requires Sox10. J. Neurochem. 114, 1827–1839. doi: 10.1111/j.1471-4159.2010.06897.x
Brown, A., Early, S., Vasilijic, S., and Stankovic, K. M. (2022). Sporadic vestibular schwannoma size and location do not correlate with the severity of hearing loss at initial presentation. Front. Oncol. 12:836504. doi: 10.3389/fonc.2022.836504
Budak, M., Grosh, K., Sasmal, A., Corfas, G., Zochowski, M., and Booth, V. (2021). Contrasting mechanisms for hidden hearing loss: synaptopathy vs myelin defects. PLoS Comput. Biol. 17:e1008499. doi: 10.1371/journal.pcbi.1008499
Budak, M., Roberts, M. T., Grosh, K., Corfas, G., Booth, V., and Zochowski, M. (2022). Binaural processing deficits due to synaptopathy and myelin defects. Front. Neural Circuits 16:856926. doi: 10.3389/fncir.2022.856926
Casarosa, S., Fode, C., and Guillemot, F. (1999). Mash1 regulates neurogenesis in the ventral telencephalon. Development 126, 525–534. doi: 10.1242/dev.126.3.525
Cassinotti, L. R., Ji, L., Yuk, M. C., Desai, A. S., Cass, N. D., Amir, Z. A., et al. (2024). Hidden hearing loss in a Charcot-Marie-Tooth type 1A mouse model. JCI Insight 9:e180315. doi: 10.1172/jci.insight.180315
Chen, Y., Wang, H., Yoon, S. O., Xu, X., Hottiger, M. O., Svaren, J., et al. (2011). HDAC-mediated deacetylation of NF-kappaB is critical for Schwann cell myelination. Nat. Neurosci. 14, 437–441. doi: 10.1038/nn.2780
Chen, Z., Huang, Y., Yu, C., Liu, Q., Qiu, C., and Wan, G. (2021). Cochlear Sox2(+) glial cells are potent progenitors for spiral ganglion neuron reprogramming induced by small molecules. Front. Cell Dev. Biol. 9:728352. doi: 10.3389/fcell.2021.728352
Chiasson-MacKenzie, C., Vitte, J., Liu, C. H., Wright, E. A., Flynn, E. A., Stott, S. L., et al. (2023). Cellular mechanisms of heterogeneity in NF2-mutant schwannoma. Nat. Commun. 14:1559. doi: 10.1038/s41467-023-37226-0
Choayb, S., El Harras, Y., Fikri, M., Ech-Cherif El Kettani, N., Jiddane, M., and Touarsa, F. (2023). Intracochlear schwannoma: imaging diagnosis. J. Otol. 18, 101–103. doi: 10.1016/j.joto.2023.03.001
Choi, J. E., Seok, J. M., Ahn, J., Ji, Y. S., Lee, K. M., Hong, S. H., et al. (2018). Hidden hearing loss in patients with Charcot-Marie-Tooth disease type 1A. Sci. Rep. 8:10335. doi: 10.1038/s41598-018-28501-y
Cimadamore, F., Amador-Arjona, A., Chen, C., Huang, C. T., and Terskikh, A. V. (2013). SOX2-LIN28/let-7 pathway regulates proliferation and neurogenesis in neural precursors. Proc. Natl. Acad. Sci. U.S.A. 110, E3017–E3026. doi: 10.1073/pnas.1220176110
Cristobal, C. D., and Lee, H. K. (2022). Development of myelinating glia: an overview. Glia 70, 2237–2259. doi: 10.1002/glia.24238
Dalakas, M. C. (2013). Pathophysiology of autoimmune polyneuropathies. Presse Med. 42, e181–e192. doi: 10.1016/j.lpm.2013.01.058
Davis, A. A., and Temple, S. (1994). A self-renewing multipotential stem cell in embryonic rat cerebral cortex. Nature 372, 263–266. doi: 10.1038/372263a0
Delaunay, D., Heydon, K., Cumano, A., Schwab, M. H., Thomas, J. L., Suter, U., et al. (2008). Early neuronal and glial fate restriction of embryonic neural stem cells. J. Neurosci. 28, 2551–2562. doi: 10.1523/JNEUROSCI.5497-07.2008
Denton, K. R., Xu, C. C., and Li, X. J. (2016). Modeling axonal phenotypes with human pluripotent stem cells. Methods Mol. Biol. 1353, 309–321. doi: 10.1007/7651_2014_167
Di Stadio, A., and Ralli, M. (2017). Systemic lupus erythematosus and hearing disorders: literature review and meta-analysis of clinical and temporal bone findings. J. Int. Med. Res. 45, 1470–1480. doi: 10.1177/0300060516688600
Diensthuber, M., Zecha, V., Wagenblast, J., Arnhold, S., Edge, A. S., and Stover, T. (2014). Spiral ganglion stem cells can be propagated and differentiated into neurons and glia. Biores. Open Access 3, 88–97. doi: 10.1089/biores.2014.0016
Dilwali, S., Kao, S. Y., Fujita, T., Landegger, L. D., and Stankovic, K. M. (2015). Nonsteroidal anti-inflammatory medications are cytostatic against human vestibular schwannomas. Transl. Res. 166, 1–11. doi: 10.1016/j.trsl.2014.12.007
Doetsch, F. (2003). A niche for adult neural stem cells. Curr. Opin. Genet. Dev. 13, 543–550. doi: 10.1016/j.gde.2003.08.012
Druckenbrod, N. R., Hale, E. B., Olukoya, O. O., Shatzer, W. E., and Goodrich, L. V. (2020). Neuronal processes and glial precursors form a scaffold for wiring the developing mouse cochlea. Nat. Commun. 11:5866. doi: 10.1038/s41467-020-19521-2
Elliott, K. L., Fritzsch, B., Yamoah, E. N., and Zine, A. (2022). Age-related hearing loss: sensory and neural etiology and their interdependence. Front. Aging Neurosci. 14:814528. doi: 10.3389/fnagi.2022.814528
Elmaci, I., Altinoz, M. A., and Sari, R. (2018). Immune pathobiology of schwannomas: a concise review. J. Neurol. Surg. A Cent. Eur. Neurosurg. 79, 159–162. doi: 10.1055/s-0037-1603949
Evsen, L., Sugahara, S., Uchikawa, M., Kondoh, H., and Wu, D. K. (2013). Progression of neurogenesis in the inner ear requires inhibition of Sox2 transcription by neurogenin1 and neurod1. J. Neurosci. 33, 3879–3890. doi: 10.1523/JNEUROSCI.4030-12.2013
Falls, D. L. (2003). Neuregulins: functions, forms, and signaling strategies. Exp. Cell Res. 284, 14–30. doi: 10.1016/S0014-4827(02)00102-7
Fields, R. D. (2009). “Schwann cells and axon relationship,” in Encyclopedia of Neuroscience, ed. L Squire (Oxford: Elsevier; Academic Press), 485–489. doi: 10.1016/B978-008045046-9.00698-7
Fricker, F. R., and Bennett, D. L. (2011). The role of neuregulin-1 in the response to nerve injury. Future Neurol. 6, 809–822. doi: 10.2217/fnl.11.45
Fricker, F. R., Lago, N., Balarajah, S., Tsantoulas, C., Tanna, S., Zhu, N., et al. (2011). Axonally derived neuregulin-1 is required for remyelination and regeneration after nerve injury in adulthood. J. Neurosci. 31, 3225–3233. doi: 10.1523/JNEUROSCI.2568-10.2011
Fritzsch, B., Pan, N., Jahan, I., and Elliott, K. L. (2015). Inner ear development: building a spiral ganglion and an organ of Corti out of unspecified ectoderm. Cell Tissue Res. 361, 7–24. doi: 10.1007/s00441-014-2031-5
Furst, M., and Levine, R. A. (2015). Hearing disorders in multiple sclerosis. Handb. Clin. Neurol. 129, 649–665. doi: 10.1016/B978-0-444-62630-1.00036-6
Garcia, M. A., and Zuchero, J. B. (2019). Anchors away: glia-neuron adhesion regulates myelin targeting and growth. Dev. Cell 51, 659–661. doi: 10.1016/j.devcel.2019.11.018
Gess, B., Halfter, H., Kleffner, I., Monje, P., Athauda, G., Wood, P. M., et al. (2008). Inhibition of N-cadherin and beta-catenin function reduces axon-induced Schwann cell proliferation. J. Neurosci. Res. 86, 797–812. doi: 10.1002/jnr.21528
Ghislain, J., and Charnay, P. (2006). Control of myelination in Schwann cells: a Krox20 cis-regulatory element integrates Oct6, Brn2 and Sox10 activities. EMBO Rep. 7, 52–58. doi: 10.1038/sj.embor.7400573
Glenn, T. D., and Talbot, W. S. (2013a). Analysis of Gpr126 function defines distinct mechanisms controlling the initiation and maturation of myelin. Development 140, 3167–3175. doi: 10.1242/dev.093401
Glenn, T. D., and Talbot, W. S. (2013b). Signals regulating myelination in peripheral nerves and the Schwann cell response to injury. Curr. Opin. Neurobiol. 23, 1041–1048. doi: 10.1016/j.conb.2013.06.010
Gómez-Casati, M. E., Murtie, J. C., Rio, C., Stankovic, K., Liberman, M. C., and Corfas, G. (2010). Nonneuronal cells regulate synapse formation in the vestibular sensory epithelium via erbB-dependent BDNF expression. Proc. Natl. Acad. Sci. U.S.A. 107, 17005–17010. doi: 10.1073/pnas.1008938107
Gosselin, É., Maniakas, A., and Saliba, I. (2016). Meta-analysis on the clinical outcomes in patients with intralabyrinthine schwannomas: conservative management vs. microsurgery. Eur. Arch. Otorhinolaryngol. 273, 1357–1367. doi: 10.1007/s00405-015-3548-2
Griffin, J. W., and Sheikh, K. (1999). Schwann cell-axon interactions in Charcot-Marie-Tooth disease. Ann. N. Y. Acad. Sci. 883, 77–90. doi: 10.1111/j.1749-6632.1999.tb08571.x
Guillemot, F. (2005). Cellular and molecular control of neurogenesis in the mammalian telencephalon. Curr. Opin. Cell Biol. 17, 639–647. doi: 10.1016/j.ceb.2005.09.006
Guo, F., Ma, J., Mccauley, E., Bannerman, P., and Pleasure, D. (2009). Early postnatal proteolipid promoter-expressing progenitors produce multilineage cells in vivo. J. Neurosci. 29, 7256–7270. doi: 10.1523/JNEUROSCI.5653-08.2009
Hannan, C. J., Lewis, D., O'leary, C., Donofrio, C. A., Evans, D. G., Roncaroli, F., et al. (2020). The inflammatory microenvironment in vestibular schwannoma. Neurooncol. Adv. 2:vdaa023. doi: 10.1093/noajnl/vdaa023
Harlow, D. E., Saul, K. E., Culp, C. M., Vesely, E. M., and Macklin, W. B. (2014). Expression of proteolipid protein gene in spinal cord stem cells and early oligodendrocyte progenitor cells is dispensable for normal cell migration and myelination. J. Neurosci. 34, 1333–1343. doi: 10.1523/JNEUROSCI.2477-13.2014
Harner, S. G., Fabry, D. A., and Beatty, C. W. (2000). Audiometric findings in patients with acoustic neuroma. Am. J. Otol. 21, 405–411. doi: 10.1016/S0196-0709(00)80052-6
Heinrich, C., Bergami, M., Gascón, S., Lepier, A., Viganò, F., Dimou, L., et al. (2014). Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Rep. 3, 1000–1014. doi: 10.1016/j.stemcr.2014.10.007
Heo, I., Joo, C., Cho, J., Ha, M., Han, J., and Kim, V. N. (2008). Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Mol. Cell 32, 276–284. doi: 10.1016/j.molcel.2008.09.014
Hurley, P. A., Crook, J. M., and Shepherd, R. K. (2007). Schwann cells revert to non-myelinating phenotypes in the deafened rat cochlea. Eur. J. Neurosci. 26, 1813–1821. doi: 10.1111/j.1460-9568.2007.05811.x
Jaegle, M., Ghazvini, M., Mandemakers, W., Piirsoo, M., Driegen, S., Levavasseur, F., et al. (2003). The POU proteins Brn-2 and Oct-6 share important functions in Schwann cell development. Genes Dev. 17, 1380–1391. doi: 10.1101/gad.258203
Jeon, E. J., Xu, N., Xu, L., and Hansen, M. R. (2011). Influence of central glia on spiral ganglion neuron neurite growth. Neuroscience 177, 321–334. doi: 10.1016/j.neuroscience.2011.01.014
Jessen, K. R., and Mirsky, R. (2019a). Schwann cell precursors; multipotent glial cells in embryonic nerves. Front. Mol. Neurosci. 12:69. doi: 10.3389/fnmol.2019.00069
Jessen, K. R., and Mirsky, R. (2019b). The success and failure of the schwann cell response to nerve injury. Front. Cell. Neurosci. 13:33. doi: 10.3389/fncel.2019.00033
Jewett, D. L., and Williston, J. S. (1971). Auditory-evoked far fields averaged from the scalp of humans. Brain 94, 681–696. doi: 10.1093/brain/94.4.681
Jyothi, V., Li, M., Kilpatrick, L. A., Smythe, N., Larue, A. C., Zhou, D., et al. (2010). Unmyelinated auditory type I spiral ganglion neurons in congenic Ly5.1 mice. J. Comp. Neurol. 518, 3254–3271. doi: 10.1002/cne.22398
Kandathil, C. K., Dilwali, S., Wu, C. C., Ibrahimov, M., Mckenna, M. J., Lee, H., et al. (2014). Aspirin intake correlates with halted growth of sporadic vestibular schwannoma in vivo. Otol. Neurotol. 35, 353–357. doi: 10.1097/MAO.0000000000000189
Kempfle, J. S., Luu, N. C., Petrillo, M., Al-Asad, R., Zhang, A., and Edge, A. S. B. (2020). Lin28 reprograms inner ear glia to a neuronal fate. Stem Cells 38, 890–903. doi: 10.1002/stem.3181
Khrais, T., Romano, G., and Sanna, M. (2008). Nerve origin of vestibular schwannoma: a prospective study. J. Laryngol. Otol. 122, 128–131. doi: 10.1017/S0022215107001028
Kim, J. Y., Kim, H., Jeun, S. S., Rha, S. J., Kim, Y. H., Ko, Y. J., et al. (2002). Inhibition of NF-kappaB activation by merlin. Biochem. Biophys. Res. Commun. 296, 1295–1302. doi: 10.1016/S0006-291X(02)02077-6
Kimura-Kuroda, J., Teng, X., Komuta, Y., Yoshioka, N., Sango, K., Kawamura, K., et al. (2010). An in vitro model of the inhibition of axon growth in the lesion scar formed after central nervous system injury. Mol. Cell. Neurosci. 43, 177–187. doi: 10.1016/j.mcn.2009.10.008
Kohrman, D. C., Borges, B. C., Cassinotti, L. R., Ji, L., and Corfas, G. (2021). Axon-glia interactions in the ascending auditory system. Dev. Neurobiol. 81, 546–567. doi: 10.1002/dneu.22813
Kumar, P., Sharma, S., Kaur, C., Pal, I., Bhardwaj, D. N., Vanamail, P., et al. (2022). The ultrastructural study of human cochlear nerve at different ages. Hear. Res. 416:108443. doi: 10.1016/j.heares.2022.108443
Labit-Bouvier, C., Crebassa, B., Bouvier, C., Andrac-Meyer, L., Magnan, J., and Charpin, C. (2000). Clinicopathologic growth factors in vestibular schwannomas: a morphological and immunohistochemical study of 69 tumours. Acta Otolaryngol. 120, 950–954. doi: 10.1080/00016480050218681
Lang, H., Li, M., Kilpatrick, L. A., Zhu, J., Samuvel, D. J., Krug, E. L., et al. (2011). Sox2 up-regulation and glial cell proliferation following degeneration of spiral ganglion neurons in the adult mouse inner ear. J. Assoc. Res. Otolaryngol. 12, 151–171. doi: 10.1007/s10162-010-0244-1
Lang, H., Schulte, B. A., Goddard, J. C., Hedrick, M., Schulte, J. B., Wei, L., et al. (2008). Transplantation of mouse embryonic stem cells into the cochlea of an auditory-neuropathy animal model: effects of timing after injury. J. Assoc. Res. Otolaryngol. 9, 225–240. doi: 10.1007/s10162-008-0119-x
Le Bras, B., Chatzopoulou, E., Heydon, K., Martínez, S., Ikenaka, K., Prestoz, L., et al. (2005). Oligodendrocyte development in the embryonic brain: the contribution of the plp lineage. Int. J. Dev. Biol. 49, 209–220. doi: 10.1387/ijdb.041963bl
Lemke, G., and Chao, M. (1988). Axons regulate Schwann cell expression of the major myelin and NGF receptor genes. Development 102, 499–504. doi: 10.1242/dev.102.3.499
Li, H., Liu, H., and Heller, S. (2003). Pluripotent stem cells from the adult mouse inner ear. Nat. Med. 9, 1293–1299. doi: 10.1038/nm925
Li, W., Cooper, J., Karajannis, M. A., and Giancotti, F. G. (2012). Merlin: a tumour suppressor with functions at the cell cortex and in the nucleus. EMBO Rep. 13, 204–215. doi: 10.1038/embor.2012.11
Liu, T., Zhang, L., Joo, D., and Sun, S. C. (2017). NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2:17023. doi: 10.1038/sigtrans.2017.23
Liu, W., Boström, M., Kinnefors, A., Linthicum, F., and Rask-Andersen, H. (2012). Expression of myelin basic protein in the human auditory nerve - an immunohistochemical and comparative study. Auris Nasus Larynx 39, 18–24. doi: 10.1016/j.anl.2011.04.007
Locher, H., De Groot, J. C., Van Iperen, L., Huisman, M. A., Frijns, J. H., and Chuva De Sousa Lopes, S. M. (2014). Distribution and development of peripheral glial cells in the human fetal cochlea. PLoS ONE 9:e88066. doi: 10.1371/journal.pone.0088066
Ma, N. X., Yin, J. C., and Chen, G. (2019). Transcriptome analysis of small molecule-mediated astrocyte-to-neuron reprogramming. Front. Cell Dev. Biol. 7:82. doi: 10.3389/fcell.2019.00082
MacCollin, M., Chiocca, E. A., Evans, D. G., Friedman, J. M., Horvitz, R., Jaramillo, D., et al. (2005). Diagnostic criteria for schwannomatosis. Neurology 64, 1838–1845. doi: 10.1212/01.WNL.0000163982.78900.AD
Mall, M., and Wernig, M. (2017). The novel tool of cell reprogramming for applications in molecular medicine. J. Mol. Med. 95, 695–703. doi: 10.1007/s00109-017-1550-4
Mao, Y., Reiprich, S., Wegner, M., and Fritzsch, B. (2014). Targeted deletion of Sox10 by Wnt1-cre defects neuronal migration and projection in the mouse inner ear. PLoS ONE 9:e94580. doi: 10.1371/journal.pone.0094580
Martinez-Monedero, R., Oshima, K., Heller, S., and Edge, A. S. (2007). The potential role of endogenous stem cells in regeneration of the inner ear. Hear. Res. 227, 48–52. doi: 10.1016/j.heares.2006.12.015
McClatchey, A. I., and Giovannini, M. (2005). Membrane organization and tumorigenesis–the NF2 tumor suppressor, Merlin. Genes Dev. 19, 2265–2277. doi: 10.1101/gad.1335605
McFadden, S. L., Ding, D., Jiang, H., and Salvi, R. J. (2004). Time course of efferent fiber and spiral ganglion cell degeneration following complete hair cell loss in the chinchilla. Brain Res. 997, 40–51. doi: 10.1016/j.brainres.2003.10.031
McKee, K. K., Yang, D. H., Patel, R., Chen, Z. L., Strickland, S., Takagi, J., et al. (2012). Schwann cell myelination requires integration of laminin activities. J. Cell Sci. 125, 4609–4619. doi: 10.1242/jcs.107995
McLean, W. J., Mclean, D. T., Eatock, R. A., and Edge, A. S. (2016). Distinct capacity for differentiation to inner ear cell types by progenitor cells of the cochlea and vestibular organs. Development 143, 4381–4393. doi: 10.1242/dev.139840
McLean, W. J., Yin, X., Lu, L., Lenz, D. R., Mclean, D., Langer, R., et al. (2017). Clonal expansion of Lgr5-positive cells from mammalian cochlea and high-purity generation of sensory hair cells. Cell Rep. 18, 1917–1929. doi: 10.1016/j.celrep.2017.01.066
Meas, S. J., Zhang, C. L., and Dabdoub, A. (2018). Reprogramming glia into neurons in the peripheral auditory system as a solution for sensorineural hearing loss: lessons from the central nervous system. Front. Mol. Neurosci. 11:77. doi: 10.3389/fnmol.2018.00077
Mohamed, T., Melfi, V., Colciago, A., and Magnaghi, V. (2023). Hearing loss and vestibular schwannoma: new insights into Schwann cells implication. Cell Death Dis. 14:629. doi: 10.1038/s41419-023-06141-z
Momenzadeh, S., and Jami, M. S. (2021). Remyelination in PNS and CNS: current and upcoming cellular and molecular strategies to treat disabling neuropathies. Mol. Biol. Rep. 48, 8097–8110. doi: 10.1007/s11033-021-06755-6
Moore, J. K., and Linthicum, F. H. (2001). Myelination of the human auditory nerve: different time courses for Schwann cell and glial myelin. Ann. Otol. Rhinol. Laryngol. 110, 655–661. doi: 10.1177/000348940111000711
Musiek, F. E., Sachs, E., Geurkink, N. A., and Weider, D. J. (1980). Auditory brainstem response and eighth nerve lesions: a review and presentation of cases. Ear Hear. 1, 297–301. doi: 10.1097/00003446-198011000-00001
Nadol, J. B., Burgess, B. J., and Reisser, C. (1990). Morphometric analysis of normal human spiral ganglion cells. Ann. Otol. Rhinol. Laryngol. 99, 340–348. doi: 10.1177/000348949009900505
Newbern, J., and Birchmeier, C. (2010). Nrg1/ErbB signaling networks in Schwann cell development and myelination. Semin. Cell Dev. Biol. 21, 922–928. doi: 10.1016/j.semcdb.2010.08.008
Newman, M. A., Thomson, J. M., and Hammond, S. M. (2008). Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. RNA 14, 1539–1549. doi: 10.1261/rna.1155108
Niu, W., Zang, T., Smith, D. K., Vue, T. Y., Zou, Y., Bachoo, R., et al. (2015). SOX2 reprograms resident astrocytes into neural progenitors in the adult brain. Stem Cell Rep. 4, 780–794. doi: 10.1016/j.stemcr.2015.03.006
Norrmén, C., Figlia, G., Pfistner, P., Pereira, J. A., Bachofner, S., and Suter, U. (2018). mTORC1 Is transiently reactivated in injured nerves to promote c-Jun elevation and Schwann cell dedifferentiation. J. Neurosci. 38, 4811–4828. doi: 10.1523/JNEUROSCI.3619-17.2018
Parras, C. M., Schuurmans, C., Scardigli, R., Kim, J., Anderson, D. J., and Guillemot, F. (2002). Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity. Genes Dev. 16, 324–338. doi: 10.1101/gad.940902
Pfisterer, U., Ek, F., Lang, S., Soneji, S., Olsson, R., and Parmar, M. (2016). Small molecules increase direct neural conversion of human fibroblasts. Sci. Rep. 6:38290. doi: 10.1038/srep38290
Plotkin, S. R., Messiaen, L., Legius, E., Pancza, P., Avery, R. A., Blakeley, J. O., et al. (2022). Updated diagnostic criteria and nomenclature for neurofibromatosis type 2 and schwannomatosis: an international consensus recommendation. Genet. Med. 24, 1967–1977. doi: 10.1016/j.gim.2022.05.007
Qin, Y., Hua, M., Duan, Y., Gao, Y., Shao, X., Wang, H., et al. (2012). TNF-α expression in Schwann cells is induced by LPS and NF-κB-dependent pathways. Neurochem. Res. 37, 722–731. doi: 10.1007/s11064-011-0664-2
Ren, Y., Chari, D. A., Vasilijic, S., Welling, D. B., and Stankovic, K. M. (2021). New developments in neurofibromatosis type 2 and vestibular schwannoma. Neurooncol. Adv. 3:vdaa153. doi: 10.1093/noajnl/vdaa153
Rio, C., Rieff, H. I., Qi, P., Khurana, T. S., and Corfas, G. (1997). Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron 19, 39–50. doi: 10.1016/S0896-6273(00)80346-3
Robel, S., Berninger, B., and Götz, M. (2011). The stem cell potential of glia: lessons from reactive gliosis. Nat. Rev. Neurosci. 12, 88–104. doi: 10.1038/nrn2978
Romand, M. R., and Romand, R. (1990). Development of spiral ganglion cells in mammalian cochlea. J. Electron Microsc. Tech. 15, 144–154. doi: 10.1002/jemt.1060150206
Rowitch, D. H., and Kriegstein, A. R. (2010). Developmental genetics of vertebrate glial-cell specification. Nature 468, 214–222. doi: 10.1038/nature09611
Ruiz-Garcia, C., Lassaletta, L., Lopez-Larrubia, P., Varela-Nieto, I., and Murillo-Cuesta, S. (2024). Tumors of the nervous system and hearing loss: beyond vestibular schwannomas. Hear. Res. 447:109012. doi: 10.1016/j.heares.2024.109012
Salzer, J. L. (2015). Schwann cell myelination. Cold Spring Harb. Perspect. Biol. 7:a020529. doi: 10.1101/cshperspect.a020529
Sataloff, R. T., Roberts, B. R., and Feldman, M. (1988). Intralabyrinthine schwannoma. Am. J. Otol. 9, 323–326.
Sauvaget, E., Kici, S., Kania, R., Herman, P., and Tran Ba Huy, P. (2005). Sudden sensorineural hearing loss as a revealing symptom of vestibular schwannoma. Acta Otolaryngol. 125, 592–595. doi: 10.1080/00016480510030246
Schuurmans, C., Armant, O., Nieto, M., Stenman, J. M., Britz, O., Klenin, N., et al. (2004). Sequential phases of cortical specification involve Neurogenin-dependent and -independent pathways. EMBO J. 23, 2892–2902. doi: 10.1038/sj.emboj.7600278
Sepp, K. J., Schulte, J., and Auld, V. J. (2001). Peripheral glia direct axon guidance across the CNS/PNS transition zone. Dev. Biol. 238, 47–63. doi: 10.1006/dbio.2001.0411
Shi, F., and Edge, A. S. (2013). Prospects for replacement of auditory neurons by stem cells. Hear. Res. 297, 106–112. doi: 10.1016/j.heares.2013.01.017
Shivane, A., Parkinson, D. B., Ammoun, S., and Hanemann, C. O. (2013). Expression of c-Jun and Sox-2 in human schwannomas and traumatic neuromas. Histopathology 62, 651–656. doi: 10.1111/his.12062
Sian, C. S., and Ryan, S. F. (1981). The ultrastructure of neurilemoma with emphasis on Antoni B tissue. Hum. Pathol. 12, 145–160. doi: 10.1016/S0046-8177(81)80102-5
Smith, D. K., Yang, J., Liu, M. L., and Zhang, C. L. (2016). Small molecules modulate chromatin accessibility to promote NEUROG2-mediated fibroblast-to-neuron reprogramming. Stem Cell Rep. 7, 955–969. doi: 10.1016/j.stemcr.2016.09.013
Smith, K. E., Murphy, P., and Jagger, D. J. (2021). Divergent membrane properties of mouse cochlear glial cells around hearing onset. J. Neurosci. Res. 99, 679–698. doi: 10.1002/jnr.24744
Spoendlin, H. (1971). Degeneration behaviour of the cochlear nerve. Arch. Klin. Exp. Ohren Nasen Kehlkopfheilkd. 200, 275–291. doi: 10.1007/BF00373310
Spoendlin, H. (1975). Retrograde degeneration of the cochlear nerve. Acta Otolaryngol. 79, 266–275. doi: 10.3109/00016487509124683
Stankovic, K., Rio, C., Xia, A., Sugawara, M., Adams, J. C., Liberman, M. C., et al. (2004). Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J. Neurosci. 24, 8651–8661. doi: 10.1523/JNEUROSCI.0733-04.2004
Stassart, R. M., Fledrich, R., Velanac, V., Brinkmann, B. G., Schwab, M. H., Meijer, D., et al. (2013). A role for Schwann cell-derived neuregulin-1 in remyelination. Nat. Neurosci. 16, 48–54. doi: 10.1038/nn.3281
Su, Z., Niu, W., Liu, M. L., Zou, Y., and Zhang, C. L. (2014). In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat. Commun. 5:3338. doi: 10.1038/ncomms4338
Sugawara, M., Murtie, J. C., Stankovic, K. M., Liberman, M. C., and Corfas, G. (2007). Dynamic patterns of neurotrophin 3 expression in the postnatal mouse inner ear. J. Comp. Neurol. 501, 30–37. doi: 10.1002/cne.21227
Sukhanov, N., Vainshtein, A., Eshed-Eisenbach, Y., and Peles, E. (2021). Differential contribution of Cadm1-Cadm3 cell adhesion molecules to peripheral myelinated axons. J. Neurosci. 41, 1393–1400. doi: 10.1523/JNEUROSCI.2736-20.2020
Suzuka, Y., and Schuknecht, H. F. (1988). Retrograde cochlear neuronal degeneration in human subjects. Acta Otolaryngol. Suppl. 450, 1–20. doi: 10.3109/00016488809098973
Takeda, Y., Murakami, Y., Asou, H., and Uyemura, K. (2001). The roles of cell adhesion molecules on the formation of peripheral myelin. Keio J. Med. 50, 240–248. doi: 10.2302/kjm.50.240
Tamura, R. (2021). Current understanding of neurofibromatosis type 1, 2, and schwannomatosis. Int. J. Mol. Sci. 22:5850. doi: 10.3390/ijms22115850
Thomsen, J., Terkildsen, K., and Osterhammel, P. (1978). Auditory brain stem responses in patients with acoustic neuromas. Scand. Audiol. 7, 179–183. doi: 10.3109/01050397809076285
Treutlein, B., Lee, Q. Y., Camp, J. G., Mall, M., Koh, W., Shariati, S. A., et al. (2016). Dissecting direct reprogramming from fibroblast to neuron using single-cell RNA-seq. Nature 534, 391–395. doi: 10.1038/nature18323
Tylstedt, S., Kinnefors, A., and Rask-Andersen, H. (1997). Neural interaction in the human spiral ganglion: a TEM study. Acta Otolaryngol. 117, 505–512. doi: 10.3109/00016489709113429
Vakharia, K. T., Henstrom, D., Plotkin, S. R., Cheney, M., and Hadlock, T. A. (2012). Facial reanimation of patients with neurofibromatosis type 2. Neurosurgery 70, 237–243. doi: 10.1227/NEU.0b013e31823a819f
Varon, S. S., and Bunge, R. P. (1978). Trophic mechanisms in the peripheral nervous system. Annu. Rev. Neurosci. 1, 327–361. doi: 10.1146/annurev.ne.01.030178.001551
Velasco, S., Ibrahim, M. M., Kakumanu, A., Garipler, G., Aydin, B., Al-Sayegh, M. A., et al. (2017). A multi-step transcriptional and chromatin state cascade underlies motor neuron programming from embryonic stem cells. Cell Stem Cell 20, 205–217.e8. doi: 10.1016/j.stem.2016.11.006
Viswanathan, S. R., and Daley, G. Q. (2010). Lin28: a microRNA regulator with a macro role. Cell 140, 445–449. doi: 10.1016/j.cell.2010.02.007
Wagner, R., and Myers, R. R. (1996). Schwann cells produce tumor necrosis factor alpha: expression in injured and non-injured nerves. Neuroscience 73, 625–629. doi: 10.1016/0306-4522(96)00127-3
Wan, G., and Corfas, G. (2017). Transient auditory nerve demyelination as a new mechanism for hidden hearing loss. Nat. Commun. 8:14487. doi: 10.1038/ncomms14487
Wang, R., Zhou, R., Chen, Z., Gao, S., and Zhou, F. (2022). The Glial Cells Respond to Spinal Cord Injury. Front. Neurol. 13:844497. doi: 10.3389/fneur.2022.844497
Wapinski, O. L., Vierbuchen, T., Qu, K., Lee, Q. Y., Chanda, S., Fuentes, D. R., et al. (2013). Hierarchical mechanisms for direct reprogramming of fibroblasts to neurons. Cell 155, 621–635. doi: 10.1016/j.cell.2013.09.028
Waxman, S. G. (1980). Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve 3, 141–150. doi: 10.1002/mus.880030207
Xing, Y., Samuvel, D. J., Stevens, S. M., Dubno, J. R., Schulte, B. A., and Lang, H. (2012). Age-related changes of myelin basic protein in mouse and human auditory nerve. PLoS ONE 7:e34500. doi: 10.1371/journal.pone.0034500
Yang, T., Dai, Y., Chen, G., and Cui, S. (2020). Dissecting the dual role of the glial scar and scar-forming astrocytes in spinal cord injury. Front. Cell. Neurosci. 14:78. doi: 10.3389/fncel.2020.00270
Yin, J. C., Zhang, L., Ma, N. X., Wang, Y., Lee, G., Hou, X. Y., et al. (2019). Chemical conversion of human fetal astrocytes into neurons through modulation of multiple signaling pathways. Stem Cell Rep. 12, 488–501. doi: 10.1016/j.stemcr.2019.01.003
Yuan, Y., Shi, F., Yin, Y., Tong, M., Lang, H., Polley, D. B., et al. (2014). Ouabain-induced cochlear nerve degeneration: synaptic loss and plasticity in a mouse model of auditory neuropathy. J. Assoc. Res. Otolaryngol. 15, 31–43. doi: 10.1007/s10162-013-0419-7
Zhang, D., Fu, Z., Han, S., Guo, F., Tang, N., Zhao, Y., et al. (2022). Cochlear implantation in a patient with neurofibromatosis type 2 and an intracochlear schwannoma: a case report and literature review. J. Otorhinolaryngol. Relat. Spec. 84, 425–428. doi: 10.1159/000522473
Zilberstein, Y., Liberman, M. C., and Corfas, G. (2012). Inner hair cells are not required for survival of spiral ganglion neurons in the adult cochlea. J. Neurosci. 32, 405–410. doi: 10.1523/JNEUROSCI.4678-11.2012
Zuchero, J. B., and Barres, B. A. (2015). Glia in mammalian development and disease. Development 142, 3805–3809. doi: 10.1242/dev.129304
Nomenclature
ABR, Auditory Brainstem Response; ASCL1, Achaete-scute homolog 1; bHLH, Basic helix-loop-helix; CMT1A, Charcot-Marie-Tooth Disease Type 1A; CNS, Central Nervous System; c-Jun, Cellular Jun proto-oncogene; DPOAE, Distortion Product Otoacoustic Emissions; ERBB2/HER2, v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2; GDNF, Glial-Derived Neurotrophic Factor; GBS, Guillain-Barre Syndrome; GPCR, G Protein-Coupled Receptor; GSK, Glycogen Synthase Kinase; HDAC, Histone Deacetylase; HHL, Hidden Hearing Loss; HMG, High Mobility Group; HMG2A, High Mobility Group A2; KROX20/EGR2, Early Growth Response 2; Let7, Lethal-7-microRNA; Lin28, Lin-28 Homolog A; LZTR1, Leucine Zipper Like Post-Translational Regulator 1; MAG, Myelin-Associated Glycoprotein; MBP, Myelin Basic Protein; mTORC1, Mammalian Target of Rapamycin Complex 1; NEUROD1, Neuronal Differentiation 1; NGFR, Nerve Growth Factor Receptor; NGN1, Neurogenin1; NF-κB, Nuclear Factor kappa B; NF2-SWN, Neurofibromatosis Type 2 associated Schwannomatosis; NRG1, Neuregulin 1; NT3, Neurotrophic Factor 3; PAX6, Paired Box Gene 6; P0, myelin protein zero; Plp1, Proteolipid Protein 1; PNS, Peripheral Nervous System; POU3F1/OCT6, POU Class 3 Homeobox 1; POU3F2/BRN2, POU Class 3 Homeobox 2; SGN, Spiral Ganglion Neuron; SMARCB1, SWI/SNF-related BAF chromatin remodeling complex subunit B1; SNHL, Sensorineural Hearing Loss; SOX10, SRY-related HMG Box 10; SOX2, SRY-related HMG Box 2; SRY, Sex-determining Region Y; TNF, Tumor Necrosis Factor; VN, Vestibular Neuron; VS, Vestibular Schwannoma; WNT, Wingless-Related Integration Site (Wnt Signaling Pathway).
Keywords: Schwann cells, glia, inner ear, cochlea, schwannoma, NF2, regeneration, myelin
Citation: Montigny DJ and Kempfle JS (2025) Schwann cells in the inner ear: development, disease, and regeneration. Front. Cell. Neurosci. 19:1662274. doi: 10.3389/fncel.2025.1662274
Received: 08 July 2025; Accepted: 25 August 2025;
Published: 10 September 2025.
Edited by:
Francisco M. Nadal-Nicolás, National Eye Institute (NIH), United StatesReviewed by:
Valerio Magnaghi, University of Milan, ItalyKirsten Haastert-Talini, Hannover Medical School, Germany
David Lutz, Ruhr-University Bochum, Germany
Copyright © 2025 Montigny and Kempfle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Judith S. Kempfle, anVkaXRoLmtlbXBmbGVAdW1hc3NtZW1vcmlhbC5vcmc=
 Drew J. Montigny
Drew J. Montigny Judith S. Kempfle
Judith S. Kempfle