- 1Department of Psychiatry and Behavioral Sciences, Texas A&M University, Bryan, TX, United States
- 2Department of Medical Physiology, Texas A&M University, Bryan, TX, United States
- 3Matica Biotechnology, College Station, TX, United States
- 4Department of Anesthesiology, Perioperative, and Pain Medicine, Brigham and Women's Hospital, Boston, MA, United States
Exposure to traumatic stress can lead to psychopathology, including post-traumatic stress disorder (PTSD), but may also cause inflammation and cardiovascular dysfunction. Clinical evidence suggests that exposure to traumatic stress, independent of psychopathology development, may be sufficient to induce pathophysiological sequelae, but this has not been thoroughly investigated. Using a novel model of repeated social defeat stress (RSDS) that allows for both sexes, we explored links between the behavioral and physiological consequences of this paradigm. RSDS was sufficiently able to elevate systemic inflammation in both male and female mice, with no observed sex differences. RSDS also induced a heightened blood pressure sensitization response to low dose exogenous angiotensin II (AngII), suggesting the model was also sufficient in generating cardiovascular pathology. Interestingly, the RSDS-induced sensitization to AngII was completely abrogated in mice lacking T-lymphocytes (i.e., Rag2−/− mice). Of note, Rag2−/− mice demonstrated similar pro-social and anxiety-like behavior to wild-type mice, inferring that (1) differences in these behavioral outcomes do not explain the loss of RSDS-induced AngII sensitization in Rag2−/− mice and (2) T-lymphocytes do not appear to impact these specific RSDS-induced behaviors. Indeed, intra-animal correlations demonstrate a tight association between the inflammatory and cardiovascular consequences post-RSDS, but no associations between these parameters with behavior. Together, our data suggest that exposure to traumatic stress, independent of psychopathology, is sufficient to induce pathophysiology. These findings have significant clinical implications, specifically for individuals who have experienced traumatic stress without the development of psychopathology, as this overlooked population may have similar risks of developing somatic diseases.
1 Introduction
It is estimated that 70-90% adults in the United States will have experienced one or more traumatic stressors, as defined by the Diagnostic and Statistical Manual, in their lifetime (Kessler et al., 2005; Kessler et al., 1995; Kilpatrick et al., 2013). The most common psychopathology to develop from these traumatic stressors is post-traumatic stress disorder (PTSD), which arises in approximately 10-40% of those exposed to a traumatic stressor (Kessler et al., 1995; Santiago et al., 2013; Breslau et al., 1998; North et al., 2012). This striking statistic suggests that >50% of individuals exposed to a traumatic stressor never develop PTSD (or possibly any other psychopathology), which would classify these individuals as more “resilient” to those who develop behavioral pathology. Indeed, a significant amount of research over the recent decades in both humans and animals has been devoted to identifying the underlying mechanisms that cause resiliency and susceptibility to psychopathology after traumatic stress (Colucci et al., 2020; Krishnan et al., 2007; Hodes et al., 2014; Shimo et al., 2022; Elkhatib et al., 2020; Shansky, 2015; Maul et al., 2020). However, given that psychopathology is currently diagnosed exclusively by patient reported or observed behavioral changes (Battle, 2013), by definition, the characterization of resiliency and susceptibility has been defined only by the behavioral spectrum. In doing so, the impact of traumatic stressors on physiological changes throughout the body is often overlooked, not recorded, and it remains unknown if these somatic changes are coupled in any way to behavioral pathology.
A significant amount of research has demonstrated that PTSD increases the risk of developing comorbid pathologies such cardiovascular diseases (Bookwalter et al., 2020; O'Donovan et al., 2015; O'Donnell et al., 2021; Wilson et al., 2019; Burg et al., 2017; Burg and Soufer, 2016). While many PTSD patients partake in activities that independently increase their risk for cardiovascular disease (i.e., tobacco use, poor diet, and alcohol consumption), epidemiologic assessment has unequivocally demonstrated these confounding variables do not explain the increased risk for cardiovascular diseases in this patient population (Kubzansky et al., 2007; Dong et al., 2004; Dube et al., 2009; Felitti et al., 1998). This indicates a pathophysiological change is occurring in these patients with PTSD that predisposes them to altered cardiovascular function. Indeed, several recent studies suggest that various psychopathologies, including PTSD, can alter endogenous cardiovascular regulatory systems such as the sympathetic nervous system or the renin-angiotensin system (Seligowski et al., 2021; Ali et al., 2024; Gong and Deng, 2022). These studies suggest that the modification of these systems resets an endogenous threshold, thus increasing the sensitivity to cardiovascular disease with psychopathology like PTSD (Johnson and Xue, 2018), but this hypothesis has not been thoroughly explored.
However, by focusing specifically on PTSD, most of these studies have overlooked the majority population of individuals who experienced traumatic stress but did not develop overt symptoms to be classified with PTSD. The concept that psychological trauma alone, independent of the development of PTSD, is the underlying cause for the development of secondary pathophysiology is recently (albeit slowly) gaining traction. For example, both psychological trauma and PTSD share known biological effects that elevate the risk for inflammatory disorders, such as elevated sympathetic nervous system activity, immune dysregulation, redox imbalance, mitochondrial dysfunction, and alterations in the renin-angiotensin system (Sumner et al., 2023). Given this, it is possible that the reason PTSD induces a high risk for inflammatory comorbidities is simply due to the underlying exposure to a traumatic stressor, as opposed to the development of behavioral abnormalities characteristic of PTSD. Therefore, studies specifically exploring outcomes and relationships between behavior and pathophysiology following psychological trauma are highly needed to address this hypothesis.
Immune cells, particularly T-lymphocytes, are overly sensitive to the psychobiological changes of traumatic stress, and T-lymphocytes are primary contributors to cardiovascular diseases including hypertension (Svendsen, 1973; Svendsen, 1978; Guzik et al., 2007; Harrison et al., 2011). For example, patients with PTSD have decreased numbers of naïve and regulatory (anti-inflammatory) T-lymphocytes with concurrent increases in pro-inflammatory memory T-lymphocytes (Sommershof et al., 2009; Wilson et al., 2012). Furthermore, circulating cytokines produced from T-lymphocytes, including interleukin 17A (IL-17A), are also elevated with PTSD (Zhou et al., 2014; Imai et al., 2018; von Känel et al., 2007; Maloley et al., 2019). IL-17A is highly significant, as it has been shown to directly contribute to the pathogenesis of cardiovascular diseases including hypertension (Kamat et al., 2015; Madhur et al., 2010; Madhur et al., 2011; Saleh et al., 2016; Kuwabara et al., 2017; Nguyen et al., 2013). However, studies investigating if traumatic stress (with or without behavioral changes) leads to cardiovascular disease via T-lymphocyte dysregulation are non-existent.
Herein, using an adapted novel model of chronic and severe traumatic stress in both male and female mice (i.e., repeated social defeat stress, RSDS), we investigated the impact of psychological trauma on the development of cardiovascular dysfunction, particularly hypertension. RSDS has been hampered in the past by the limitation of male-only studies, so the use of this sex-inclusive model allowed for some of the first examinations of these parameters across both males and females. We found that while RSDS significantly elevated blood pressure during the active stress paradigm, blood pressure returned to unstressed control levels immediately after the traumatic stressor was removed. However, RSDS elevated sensitization to a mild cardiovascular challenge of low dose angiotensin II (AngII), where previously stressed animals demonstrated significantly higher blood pressure response to the AngII compared to unstressed controls. Moreover, this blood pressure sensitization to AngII appeared to be dependent upon IL-17A and T-lymphocytes. Importantly, while RSDS-induced blood pressure sensitization appeared tightly linked with systemic IL-17A levels, it was not linked to two major behavioral outputs of the RSDS paradigm (i.e., pro-social and anxiety-like behavior). Together, our findings demonstrate that exposure to traumatic stress appears to lead to enhanced sensitization to cardiovascular dysfunction via immune dysregulation, and that these pathophysiological consequences of traumatic stress are not coupled with psychopathology. These results may have significant implications regarding the treatment of psychological trauma, as they suggest the absence of chronic behavioral pathology does not rule out potential for life-threatening somatic diseases as a result of the trauma alone.
2 Methods
2.1 Mice
Wild-type C57BL/6J (#000664; shorthand WT), Rag2 knock-out mice (#008449; shorthand Rag2−/−), and estrogen receptor 1 alpha cre (#017913; shorthand Esr1-cre) mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). CD1 mice were purchased from Charles River (#022, Wilmington, MA, USA). Esr1-cre mice were backcrossed to CD1 mice >10 generations to create a congenic CD1 background strain. All mice were bred in house to eliminate shipping stress and microbiome shifts, as well as co-housed with their littermates (≤5 mice per cage) prior to the start of experimentation to eliminate social isolation stress. Mice were housed with standard pine chip bedding, paper nesting material, and given access to standard chow (#8604 Teklad rodent diet, Inotiv, West Lafayette, IN, USA) and water ad libitum. Male and female experimental mice between the ages of 8-12 weeks were utilized in all experiments. Experimental mice were randomized, and when possible, experimenters were blinded to the respective cohorts until the completion of the study. Mice were sacrificed by pentobarbital overdose (150 mg/kg, Fatal Plus, Vortech Pharmaceuticals, Dearborn, MI, USA) administered intraperitoneally. All mice were sacrificed between 7:00 and 9:00 Central Time to eliminate circadian rhythm effects on T-lymphocyte function. The total number of animals used for all experiments is as follows: 35 WT controls (18 male, 17 female), 40 WT RSDS (20 male, 20 female), 30 Rag2−/− controls (15 male, 15 female), and 40 Rag2−/− RSDS (20 male, 20 female). All procedures were reviewed and approved by Texas A&M University Institutional Animal Care and Use Committee.
2.2 RSDS
To overcome the limitation of using males only in RSDS, we have adapted a novel model of RSDS that utilizes chemogenetically altered aggressive mice that indiscriminately show aggression to both male and female experimental mice as previously described (Lauten et al., 2024; Takahashi et al., 2017). Briefly, male Esr1-cre mice are injected with Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) viral constructs into the aggression center of the ventromedial hypothalamus (AP, −1.5; ML, ± 0.7; DV, −5.7 mm from bregma). Four weeks post-injection, male Esr1-cre mice were screened for aggression after administration of clozapine-N-oxide (CNO). To screen the aggressors, Esr1-cre mice were injected intraperitoneally with 1 mg/kg CNO and allowed to incubate for approximately 20 min until they became aggressive. Esr1-cre mice were placed into a novel cage with a screener C57BL/6 J WT mouse (either male or female, between 8 and 12 weeks of age) for 60 s. This was followed by replacement of the screener mouse with a new screener mouse of the opposite sex. Exposure to two screener mice comprised a session, and two sessions were performed 1 week apart using new screener mice in each session. During each of the sessions, latency to first aggressive encounter and the number of aggressive encounters were recorded. Aggressive mice were positively screened if the latency to initial aggression was <30 s and >2 aggressive encounters occurred with each screener mouse. Mice that demonstrated indiscriminate aggression toward both male and female experimental mice were utilized in subsequent RSDS experiments. For RSDS, experimental mice were placed in the home cage of a male Esr1-cre mouse (pre-injected with CNO; 30 min prior to experimentation) to induce a physical confrontation and fear induction for 1 min given the highly aggressive nature of the chemogenetically-modified male Esr1-cre mice. Following the aggressive interactions, a clear perforated divider was placed into the cage and both mice remained co-housed for 24 h. This process was repeated with a new aggressive Esr1-cre mouse daily for 10 consecutive days. Control mice were pair housed in the absence of an Esr1-cre mouse for the duration of the protocol. Mice were continuously monitored for visual wounding or lameness; None of the mice utilized herein met the threshold for exclusion (wounds >1 cm or any lameness).
2.3 Behavior
For this study, pro-social and anxiety-like behaviors were assessed on Day 1 of the recovery period after RSDS. First, pro-social behaviors were evaluated using the social interaction test as previously described (Moshfegh et al., 2019). Briefly, experimental mice were allowed to explore a standard open field chamber with an empty mesh cage at one end for 2.5 min. Following this, mice were tested again in the open field chamber in the presence of a novel aggressive Esr1-cre mouse in the mesh cage for an additional 2.5 min. Sessions were recorded and digitally analyzed by blinded reviewers using Noldus Ethovision XT 13 software (Leesburg, VA, USA). The social interaction zone ratio was calculated by dividing the time the mouse spent in the interaction zone close to the mesh cage in the presence versus the absence of a novel Esr1-cre mouse. Second, anxiety-like behaviors were evaluated using the elevated zero maze as previously described (Moshfegh et al., 2019). Experimental mice were individually run on the maze for a period of 5 min. Total distance moved along the maze and time spent in the open arms of the maze were assessed by blinded reviewers using recorded and digitally analyzed footage via Noldus Ethovision XT 13 software (Leesburg, VA, USA).
2.4 Hypertension induction and mean arterial pressure recording
Following a 10-day recovery period after RSDS, induction of hypertension was performed by implantation of subcutaneous osmotic minipumps (Alzet #1002, Durect Corporation, Cupertino, CA) delivering AngII (200 ng/kg/min; Sigma #A9525, St. Louis, MO) for 2 weeks (Case and Zimmerman, 2015). The dose of AngII was chosen as a low or sub-pressor dose in control animals (Kawada et al., 2002), which allowed for the assessment of sensitization after RSDS. Telemetric recording of mouse hemodynamics was performed as previously described (Case and Zimmerman, 2015). Briefly, blood pressure recordings were performed using intra-carotid arterial catheters (HD-X11, Data Sciences International, Minneapolis, MN) attached to radio telemeters for direct measurement of mean arterial pressure and heart rate in conscious unrestrained animals. Hemodynamic recordings were performed for 20 s every minute for the same 2 h time periods daily (i.e., 8-10 a.m., 12-2 p.m., 2-4 p.m.) for the duration of the experiment. Averages of mean arterial pressure were calculated daily over the total 6 h time periods when the mice displayed minimal activity and accurate resting blood pressures.
2.5 IL-17A quantification
At the completion of the AngII infusion, mice were sacrificed and blood collected via cardiac puncture. Plasma was isolated via centrifugation, and circulating levels of IL-17A were measured using a custom Mesoscale Discovery U-Plex kits (Mesoscale Discovery, Rockville, MD, USA) per manufacturer’s instructions. Analysis was performed using a Mesoscale QuickPlex SQ 120 and analyzed using Mesoscale Discovery software.
2.6 Statistical analyses
All data are presented as mean ± standard error of the mean (SEM) with sample numbers displayed as individual data points and N values are included within figure legends where appropriate. Data were first assessed for normality utilizing the Shapiro–Wilk test. Following this, data were analyzed using 2-way ANOVA and Pearson correlation where appropriate (specific tests identified in the figure legend). All statistics were calculated using GraphPad Prism (V10, GraphPad).
3 Results
3.1 T-lymphocytes do not impact pro-social or anxiety-like behavior
To assess the effectiveness of the RSDS paradigm, mice were evaluated for pro-social and anxiety-like behaviors. As we have previously demonstrated (Elkhatib et al., 2020; Moshfegh et al., 2019; Moshfegh et al., 2022; Elkhatib et al., 2022; Elkhatib et al., 2021), RSDS effectively reduced both total distanced moved and time spent in the open arms on the elevated zero maze (indicative of increased anxiety-like behavior) as well as decreased the social interaction ratio on the social interaction test (indicative of decreased pro-social behavior) in male wild-type mice (Figures 1A–C). Female wild-type mice demonstrated identical behavioral parameters after RSDS, demonstrating the model successfully is able to socially defeat females (Figures 1A–C). Intriguingly, while we have shown that T-lymphocytes are highly impacted by the effects of RSDS (Elkhatib et al., 2020; Moshfegh et al., 2019; Moshfegh et al., 2022; Elkhatib et al., 2022; Elkhatib et al., 2021), Rag2−/− mice that lack T-lymphocytes demonstrated no differences in behavior compared to wild-type mice (either male or female) after RSDS (Figures 1D–F). Together, these data demonstrate the RSDS paradigm is highly effective in inducing anxiety-like behavior while minimizing pro-social behavior in mice independent of T-lymphocytes.
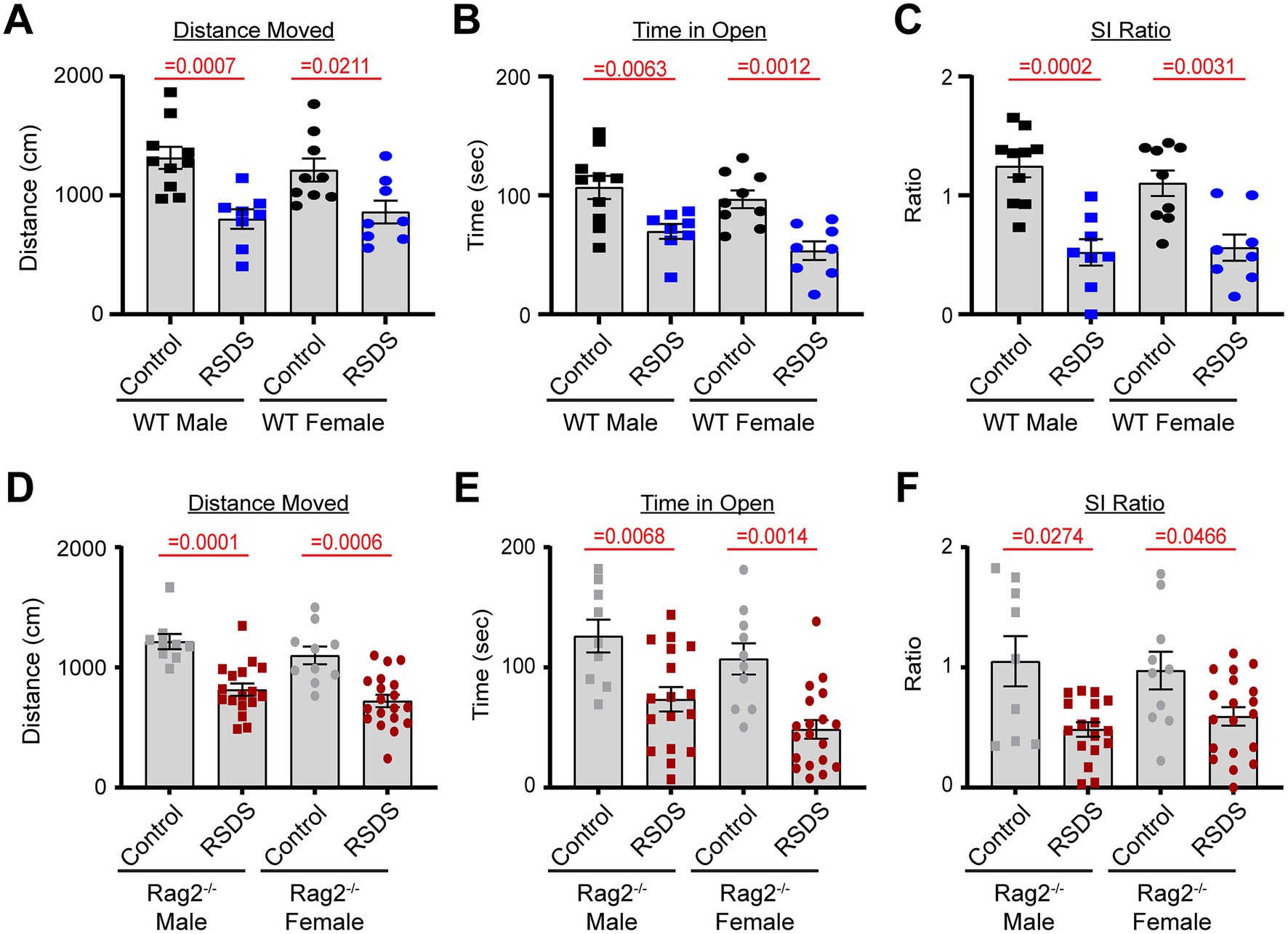
Figure 1. T-lymphocytes do not impact pro-social or anxiety-like behavior. RSDS was induced in male and female wild-type (WT) and Rag2−/− mice followed by the assessment of pro-social behavior by the social interaction test and anxiety-like behavior by the elevated zero maze. (A) Total distance moved on the elevated zero maze for WT mice (pValues: Stress = <0.0001, Sex = 0.9598, Interaction = 0.5542). (B) Time spent in the open arms of the elevated zero maze for WT mice (pValues: Stress = <0.0001, Sex = 0.0925, Interaction = 0.7460). (C) Social interaction (SI) ratio of WT mice in the social interaction test (pValues: Stress = <0.0001, Sex = 0.5176, Interaction = 0.5933). (D) Total distance moved on the elevated zero maze for Rag2−/− mice (pValues: Stress = <0.0001, Sex = 0.3513, Interaction = 0.5355). (E) Time spent in the open arms of the elevated zero maze for Rag2−/− mice (pValues: Stress = <0.0001, Sex = 0.1279, Interaction = 0.3467). (F) Social interaction (SI) ratio of Rag2−/− mice in the social interaction test (pValues: Stress = <0.0001, Sex = 0.3907, Interaction = 0.3585). Statistics by 2-way ANOVA with Bonferroni post-hoc.
3.2 RSDS sensitizes mice to AngII via T-lymphocytes
Implanted radiotelemetry allows for the accurate measurement of hemodynamic parameters in conscious unrestrained mice, which limits unnecessary stress and makes it the ideal method for assessing cardiovascular pathology as a result of RSDS. As expected, wild-type mice (males and females pooled due to similar responses) showed a robust and significant elevation in mean arterial pressure during the RSDS paradigm (Figure 2A). However, after beginning the recovery period following RSDS, mean arterial pressure immediately returned back to baseline (Figure 2A). Given that we have previously demonstrated that at this same time point wild-type RSDS mice possess significantly elevated levels of systemic pro-inflammatory cytokines (Elkhatib et al., 2020; Moshfegh et al., 2019; Elkhatib et al., 2021), we postulated that while not sufficient to raise basal blood pressure, this inflammation may sensitize mice to a hypertensive challenge such as AngII. Indeed, by infusing a subpressor dose of AngII into mice 10 days following RSDS, wild-type mice that were previously stressed demonstrated a more significant elevation in blood pressure compared to wild-type controls (Figure 2A). Conversely, Rag2−/− mice showed no sensitization to AngII after RSDS (Figure 2B), even though they experienced the initial blood pressure increase during the RSDS paradigm (data not shown), which suggests T-lymphocytes mediate the RSDS-induced blood pressure sensitization to AngII.
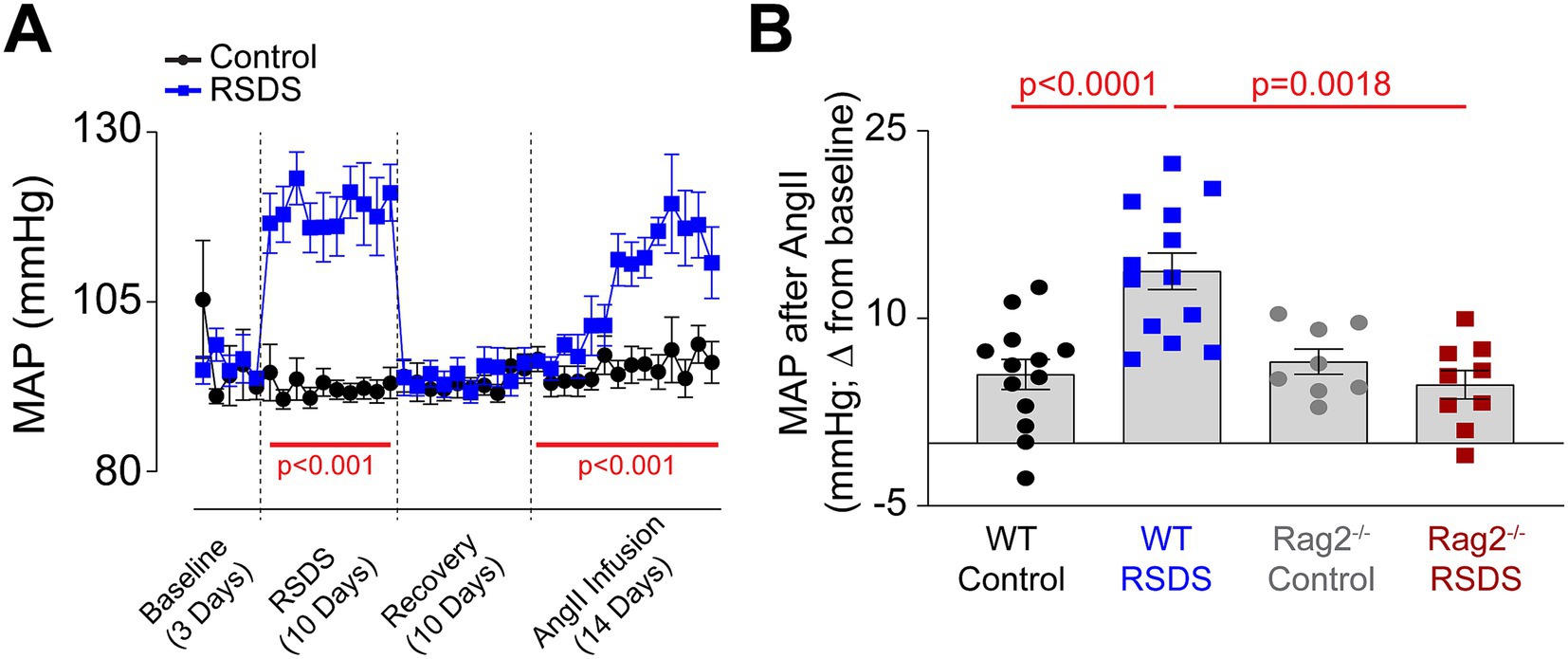
Figure 2. RSDS sensitizes mice to AngII via T-lymphocytes. RSDS was induced in male and female wild-type (WT) and Rag2−/− mice with implanted radiotelemetry transmitters, and mean arterial pressure (MAP) was assessed daily throughout the duration of the experiment. (A) MAP over time for the duration of the experimental paradigm in WT mice. (B) Quantification of maximum blood pressure change from baseline to maximum level during AngII infusion in WT and Rag2−/− mice. (pValues: Stress = 0.0202, Genotype = 0.0043, Interaction = 0.0005). No sex differences were noted between males and females, so data are shown as pooled (N = 7 male, 6 female WT Control; 7 male, 6 female WT RSDS; 4 male, 4 female Rag2−/− Control; 5 male, 4 female Rag2−/− RSDS). Statistics by mixed-effects analysis with repeated measures (A) or 2-way ANOVA with Bonferroni post-hoc (B).
3.3 IL-17A is elevated after RSDS, but not in Rag2−/− mice
The lack of an AngII sensitization response in T-lymphocyte deficient Rag2−/− mice suggested an inflammatory effector molecule from these cells may be the intermediate linking the psychological trauma to the cardiovascular pathology. We have previously performed a large scale screen of inflammatory proteins that are elevated after RSDS in wild-type mice, and out of more than 40 investigated, only 8 were consistently and significantly elevated systemically after RSDS (Elkhatib et al., 2020). However, when comparing these inflammatory proteins from wild-type mice to Rag2−/− mice after RSDS, only 1 differed between the two genotypes: IL-17A (Figure 3A). All other 7 inflammatory proteins in Rag2−/− mice showed similar levels of elevation to wild-type mice after RSDS (data not shown), suggesting they do not explain the absence of an AngII sensitization response in the Rag2−/− mice. Further, both male and female wild-type mice demonstrate similarly elevated levels of IL-17A after RSDS (Figure 3B), which aligns with the earlier observation of a similar AngII sensitization between the sexes (Figure 2A). Given that IL-17A has been demonstrated to be tightly linked to the development of cardiovascular diseases (Kamat et al., 2015; Madhur et al., 2010; Madhur et al., 2011; Saleh et al., 2016; Kuwabara et al., 2017; Nguyen et al., 2013), these data imply the possibility of IL-17A mediating the cardiovascular sequelae of RSDS.
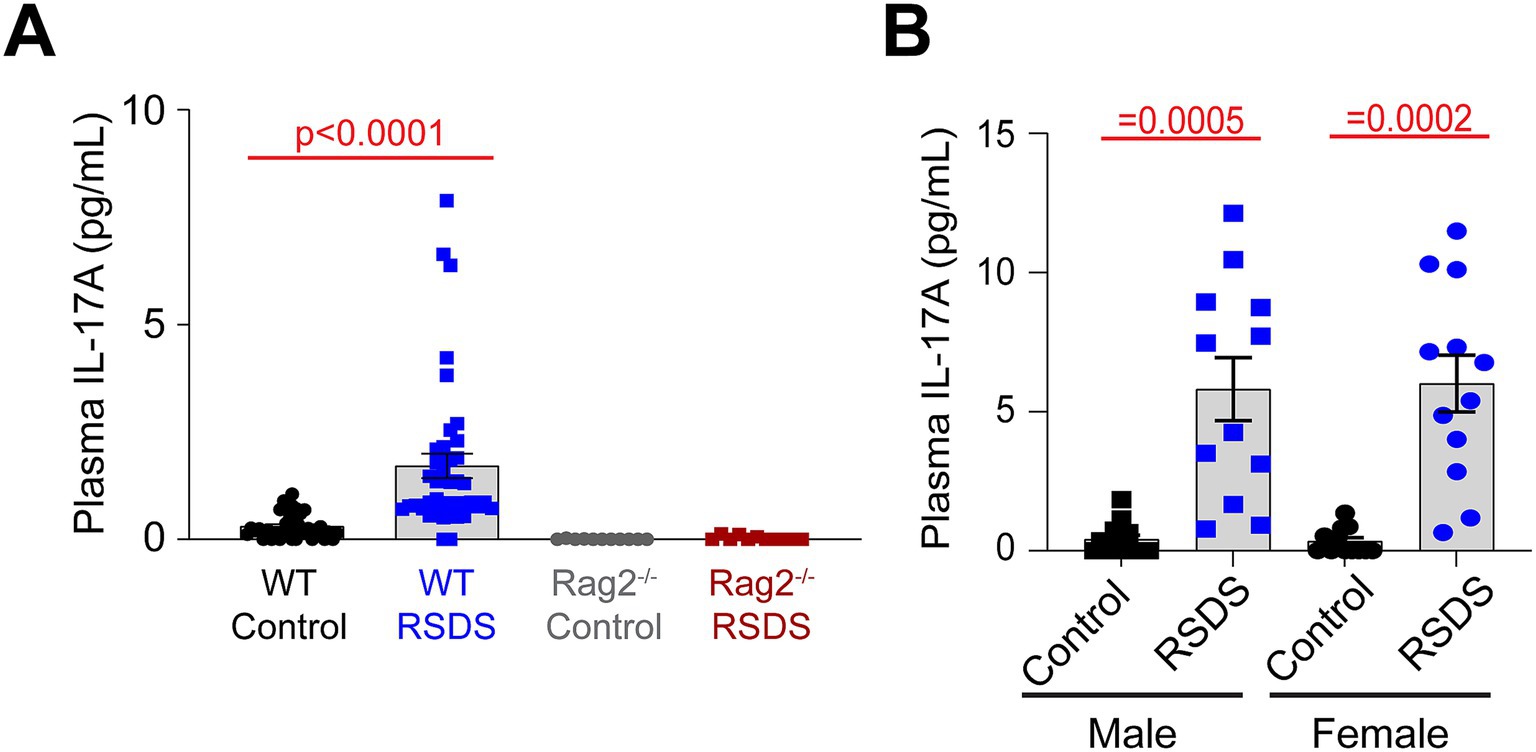
Figure 3. IL-17A is elevated after RSDS, but not in Rag2−/− mice. RSDS was induced in male and female wild-type (WT) and Rag2−/− mice followed by the extraction of peripheral blood and plasma isolation. (A) IL-17A levels assessed by Mesoscale Discovery assay in WT and Rag2−/− mice. Males and females pooled among genotypes. (pValues: Stress = 0.0129, Genotype = 0.0007, Interaction = 0.0160). (N = 19 male, 16 female WT Control; 20 male, 20 female WT RSDS; 6 male, 5 female Rag2−/− Control; 6 male, 5 female Rag2−/− RSDS). (B) IL-17A levels assessed by Mesoscale Discovery assay in WT male and female animals (pValues: Stress = <0.0001, Sex = 0.9286, Interaction = 0.8632). Statistics by 2-way ANOVA with Bonferroni post-hoc.
3.4 AngII sensitization is linked to IL-17A, but not behavior after RSDS
To assess how the cardiovascular, inflammatory, and behavioral consequences of RSDS are related, we performed intra-animal correlations of these specific parameters. First, we found both IL-17A and AngII sensitization were positively correlated (Figure 4A), which aligned with previous data demonstrating the necessity of T-lymphocytes to the RSDS-induced AngII sensitization (Figure 2B). However, AngII sensitization showed no correlation with pro-social or anxiety-like behaviors (Figures 4B,C). Given the tight relationship between IL-17A and AngII sensitization, IL-17A also did not correlate with pro-social or anxiety-like behaviors (data not shown). Together, these data are highly suggestive that RSDS-induced behavioral phenotypes are not tightly coupled with pathophysiological outcomes, and that separate mechanisms may be at play within individual animals regulating separate pathways.
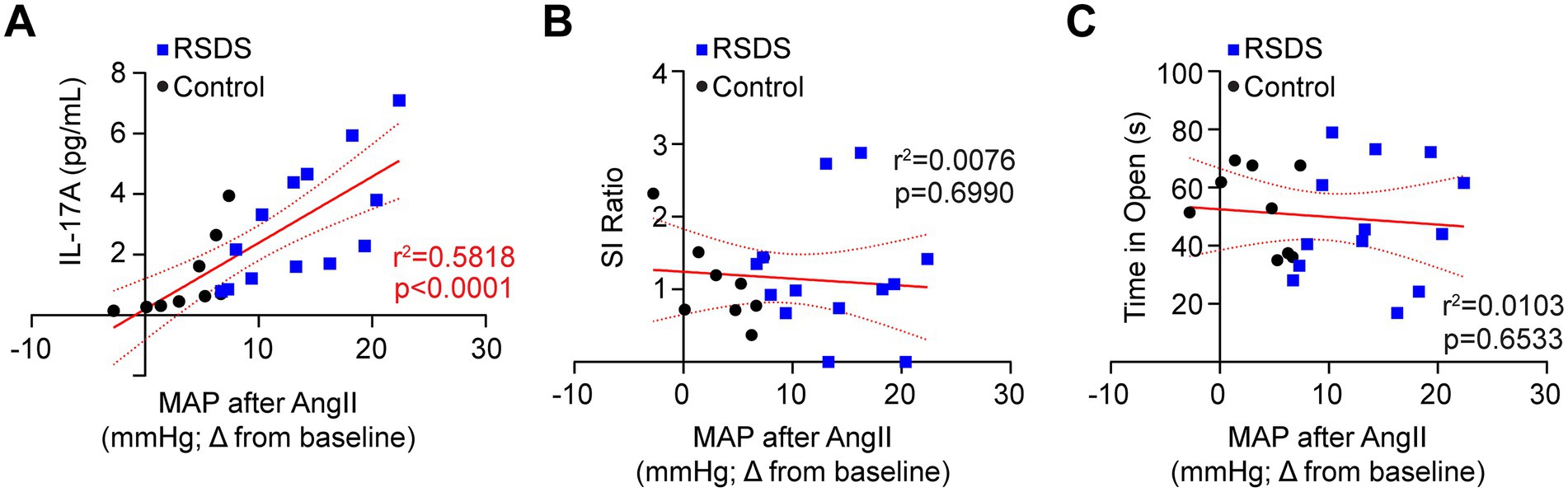
Figure 4. AngII sensitization is linked to IL-17A, but not behavior after RSDS. RSDS was induced in male and female wild-type (WT) with implanted radiotelemetry followed by the assessment of behavior and circulating IL-17A levels. (A) Correlation between IL-17A and maximum mean arterial pressure (MAP) after AngII infusion. (B) Correlation between social interaction (SI) ratio and maximum mean arterial pressure (MAP) after AngII infusion. (C) Correlation between time spent in the open arms of the elevated zero maze and maximum mean arterial pressure (MAP) after AngII infusion. Males and females pooled (N = 5 male, 4 female WT Control; 7 male, 6 female WT RSDS). Statistics by Pearson correlation analysis.
4 Discussion
Herein, we explored the relationship between psychological trauma, immune dysregulation, and cardiovascular dysfunction. First, we found that using chemogenetically-altered aggressive mice was sufficient to induce social defeat behavior in both males and females with no apparent sex differences observed in the parameters assessed. While this may seem at first to be a negative result, it actually confirms the ability of this system to sufficiently generate stress-induced psychopathology and pathophysiology in both sexes, which will allow for future investigations to further tease out any sex-specific responses. Second, we detected that T-lymphocytes do not appear directly involved in the behavioral consequences of RSDS, as T-lymphocyte deficient Rag2−/− mice displayed identical behavioral patterns to wild-type mice in both males and females. Third, RSDS is sufficient to produce a blood pressure sensitization to exogenous AngII, which is dependent upon T-lymphocytes and tightly linked to systemic IL-17A levels. Last, RSDS-induced AngII sensitization was not associated with the behavioral consequences of RSDS, suggesting the potential for disparate mechanisms underlying psychopathology versus pathophysiology. Together, our results demonstrate a new brain-immune-cardiovascular axis that is impacted by psychological trauma.
Over the last few decades, numerous meta-analyses have demonstrated tight associations between PTSD and the development of cardiovascular disease (Bookwalter et al., 2020; O'Donovan et al., 2015; O'Donnell et al., 2021; Wilson et al., 2019; Burg et al., 2017; Burg and Soufer, 2016). While incredibly informative in elucidating these links, these studies have also raised many questions. For example, given the results of the studies, many investigators often presume that PTSD causes cardiovascular disease, but it is just as likely that cardiovascular disease (or at least some underlying pathophysiology related to cardiovascular disease, such as inflammation) elevates the risk of developing PTSD after psychological trauma. While this directional quandary has been excellently discussed and evaluated (Sumner et al., 2019), the take home is that the majority of clinical studies have not been designed to address this specific question, though some have provided evidence suggesting a bidirectionality exists. For example, by using a longitudinal study design of pre- versus post-deployment military personnel, several studies have uncovered certain inflammatory signatures that existed prior to the development of PTSD after psychological trauma (Eraly et al., 2014; Breen et al., 2015; Glatt et al., 2013; Tylee et al., 2015; van Zuiden et al., 2011; Sumner et al., 2018). These again do not specifically delineate cause and effect, but suggest that the presence of specific elevated inflammatory markers may predict those who develop PTSD. Conversely, several studies have identified that PTSD does appear to increase inflammation over time (Glaus et al., 2018; Solomon et al., 2017; Jergović et al., 2015; Sumner et al., 2017), which may underlie the elevated risk for cardiovascular disease. While these conclusions may seem at odds, it is highly possible that both situations are occurring simultaneously. In other words, individuals with elevated inflammation possibly have a lower threshold or decreased resilience to psychological trauma, which predisposes them to PTSD development, but trauma and PTSD further elevate the inflammation pushing the body into somatic pathophysiology, such as cardiovascular disease. Our work using rodents supports the notion that psychological trauma elevates inflammation (Elkhatib et al., 2020; Lauten et al., 2024; Moshfegh et al., 2019; Elkhatib et al., 2022; Elkhatib et al., 2021; Moshfegh et al., 2022; Yadav et al., 2023), though we have not comprehensively explored inflammatory parameters pre-RSDS and how they impact chronic outcomes. Importantly, given our data presented herein which suggest behavior and physiology may not be tightly coupled, it would be imperative to explore not only how inflammation pre-RSDS shapes behavioral outcomes after psychological trauma, but also consequences that are physiological in nature.
While it may be intuitive to assume that psychopathology and pathophysiology are tightly linked following psychological trauma, this concept appears much more nuanced than a simple binary relationship. For example, if PTSD patients develop inflammation or cardiovascular disease, then by definition psychopathology and pathophysiology will be coupled in these individuals given that a diagnosis of PTSD requires a specific subset of behavioral pathology. However, not all PTSD patients develop inflammation or cardiovascular diseases, and emerging evidence also suggests that exposure to psychological trauma alone may be sufficient to generate pathophysiology in the absence of psychopathology (Sumner et al., 2023). These investigations have demonstrated that traumatic stress alone was sufficient to increase circulating inflammation (Tursich et al., 2014; Baumeister et al., 2016), elevate mitochondrial dysfunction (Boeck et al., 2016; Tyrka et al., 2016), and alter the function of the renin-angiotensin system (Terock et al., 2019; Terock et al., 2019). Our data presented herein, as well as our previously published observations (Elkhatib et al., 2020), also demonstrate that exposure to traumatic stress elevates inflammation and cardiovascular pathology independently of behavioral manifestations. Together, these findings suggest that traumatic stress can produce multiple phenotypes: individuals with psychopathology and pathophysiology, those with one or the other, or those with neither. These phenotypes may be even more subcategorized when taking into consideration the specific dimensions of behavioral changes as well as the particular pathophysiology. Importantly, these findings have significant clinical implications in that currently exposure to psychological trauma is not an actively treated “disease.” Only once that exposure develops into a form of chronic psychopathology is the patient therapeutically managed, which implies if no over behavioral pathology develops, the patient is essentially considered “healthy” with no additional consideration of their potential underlying pathophysiology. These current unknowns warrant longitudinal studies exploring these specific outcomes of psychological trauma and how behavior and physiology are related.
The study presented here does possess certain limitations. First, while we have identified an association between IL-17A and the blood pressure sensitization to AngII, we have not yet mechanistically defined this relationship. In future studies, we intend on using both pharmacological (i.e., neutralizing antibodies) and genetic methods of reducing IL-17A levels in RSDS to assess the chronic cardiovascular outcomes. Along these same lines, while the use of Rag2−/− animals showed no detectable IL-17A and a blunted blood pressure sensitization to AngII, we cannot fully conclude this is due specifically to T-lymphocytes. Rag2−/− mice lack all adaptive immune cells, which include both T-lymphocytes and B-lymphocytes. It is possible the lack of B-lymphocytes, which can act as antigen presenting cells, also plays a role in the observed RSDS-induced phenotype. In previous work, we have performed adoptive transfer of only T-lymphocytes into Rag2−/− mice and demonstrated this was sufficient to restore RSDS-induced IL-17A levels, but we will need to confirm this also restores their blood pressure sensitivity to AngII. Adoptive transfer of B-lymphocytes in a similar manner would also confirm the role of specific adaptive immune cells in these proceses. Second, this study only explored two dimensions of behavior: pro-social and anxiety-like. While there were no direct associations with these behaviors and the RSDS-induced pathophysiology, it is highly possible that these specific psychopathologies are not coupled with these particular pathophysiologies. The exploration of more behaviors such as anhedonia, depression-like behavior, cognition, and many others are highly warranted to truly uncover any nuance linking behavior to physiology. Third, while we have uncovered that RSDS-induces an increased pressor response to AngII, it remains unknown if other cardiovascular challenges (e.g., high salt, nitric oxide sequestration, coronary ligation, etc.) also demonstrate sensitization. Last, while RSDS is an accepted as a model of psychological trauma, it does possess reports of non-PTSD outcomes such as decreased fear extinction and increased HPA feedback (Deslauriers et al., 2018). Validating the results presented herein using additional models of psychological trauma will aid in the understanding of the broader applicability of our findings.
In summary, this investigation has uncovered an apparent pathway linking psychological trauma to cardiovascular dysfunction via T-lymphocyte-mediated inflammation. Given the lack of a correlation between the psychopathology and pathophysiology in this model, our data suggests that psychological trauma alone is sufficient to induce pathophysiology, which warrants deeper exploration into patients exposed to traumatic stress independent of their development of psychopathology. In doing so, clinical practice may shift to a model of intervening as soon as possible to the traumatic event in hopes of preventing downstream negative behavioral and physiological consequences.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Texas A&M University Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
AC: Funding acquisition, Writing – original draft, Data curation, Methodology, Resources, Formal analysis, Investigation, Visualization, Supervision, Conceptualization, Writing – review & editing, Project administration. TN: Investigation, Formal analysis, Writing – review & editing. LP: Formal analysis, Writing – review & editing, Investigation. TL: Investigation, Formal analysis, Writing – review & editing. ER: Investigation, Writing – review & editing, Formal analysis. CM: Investigation, Writing – review & editing, Formal analysis. SE: Investigation, Writing – review & editing, Formal analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Institutes of Health (NIH) R01HL158521 (AC), T32GM135115 (ER), F31HL176172 (TL), and F30HL154535 (SE).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ali, N. H., Al-Kuraishy, H. M., Al-Gareeb, A. I., Albuhadily, A. K., Hamad, R. S., Alexiou, A., et al. (2024). Role of brain renin-angiotensin system in depression: a new perspective. CNS Neurosci. Ther. 30:e14525. doi: 10.1111/cns.14525
Battle, D. E. (2013). Diagnostic and statistical manual of mental disorders (DSM). Codas 25, 191–192. doi: 10.1590/s2317-17822013000200017
Baumeister, D., Akhtar, R., Ciufolini, S., Pariante, C. M., and Mondelli, V. (2016). Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol. Psychiatry 21, 642–649. doi: 10.1038/mp.2015.67
Boeck, C., Koenig, A. M., Schury, K., Geiger, M. L., Karabatsiakis, A., Wilker, S., et al. (2016). Inflammation in adult women with a history of child maltreatment: the involvement of mitochondrial alterations and oxidative stress. Mitochondrion 30, 197–207. doi: 10.1016/j.mito.2016.08.006
Bookwalter, D. B., Roenfeldt, K. A., LeardMann, C. A., Kong, S. Y., Riddle, M. S., and Rull, R. P. (2020). Posttraumatic stress disorder and risk of selected autoimmune diseases among US military personnel. BMC Psychiatry 20:23. doi: 10.1186/s12888-020-2432-9
Breen, M. S., Maihofer, A. X., Glatt, S. J., Tylee, D. S., Chandler, S. D., Tsuang, M. T., et al. (2015). Gene networks specific for innate immunity define post-traumatic stress disorder. Mol. Psychiatry 20, 1538–1545. doi: 10.1038/mp.2015.9
Breslau, N., Kessler, R. C., Chilcoat, H. D., Schultz, L. R., Davis, G. C., and Andreski, P. (1998). Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch. Gen. Psychiatry 55, 626–632. doi: 10.1001/archpsyc.55.7.626
Burg, M. M., Brandt, C., Buta, E., Schwartz, J., Bathulapalli, H., Dziura, J., et al. (2017). Risk for incident hypertension associated with PTSD in military veterans, and the effect of PTSD treatment. Psychosom. Med. 79, 181–188. doi: 10.1097/PSY.0000000000000376
Burg, M. M., and Soufer, R. (2016). Post-traumatic stress disorder and cardiovascular disease. Curr. Cardiol. Rep. 18, 9–5. doi: 10.1007/s11886-016-0770-5
Case, A. J., and Zimmerman, M. C. (2015). Redox-regulated suppression of splenic T-lymphocyte activation in a model of sympathoexcitation. Hypertension 65, 916–923. doi: 10.1161/HYPERTENSIONAHA.114.05075
Colucci, P., Marchetta, E., Mancini, G. F., Alva, P., Chiarotti, F., Hasan, M. T., et al. (2020). Predicting susceptibility and resilience in an animal model of post-traumatic stress disorder (PTSD). Transl. Psychiatry 10:243. doi: 10.1038/s41398-020-00929-9
Deslauriers, J., Toth, M., Der-Avakian, A., and Risbrough, V. B. (2018). Current status of animal models of posttraumatic stress disorder: behavioral and biological phenotypes, and future challenges in improving translation. Biol. Psychiatry 83, 895–907. doi: 10.1016/j.biopsych.2017.11.019
Dong, M., Giles, W. H., Felitti, V. J., Dube, S. R., Williams, J. E., Chapman, D. P., et al. (2004). Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation 110, 1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F
Dube, S. R., Fairweather, D., Pearson, W. S., Felitti, V. J., Anda, R. F., and Croft, J. B. (2009). Cumulative childhood stress and autoimmune diseases in adults. Psychosom. Med. 71, 243–250. doi: 10.1097/PSY.0b013e3181907888
Elkhatib, S. K., Moshfegh, C. M., Watson, G. F., and Case, A. J. (2020). Peripheral inflammation is strongly linked to elevated zero maze behavior in repeated social defeat stress. Brain Behav. Immun. 90, 279–285. doi: 10.1016/j.bbi.2020.08.031
Elkhatib, S. K., Moshfegh, C. M., Watson, G. F., and Case, A. J. (2022). T-lymphocyte tyrosine hydroxylase regulates T H 17 T-lymphocytes during repeated social defeat stress. Brain Behav. Immun. 104, 18–28. doi: 10.1016/j.bbi.2022.05.007
Elkhatib, S. K., Moshfegh, C. M., Watson, G. F., Schwab, A. D., Katsurada, K., Patel, K. P., et al. (2021). Splenic denervation attenuates repeated social defeat stress-induced T-lymphocyte inflammation. Biol. Psychiatry Glob. Open Sci. 1, 190–200. doi: 10.1016/j.bpsgos.2021.05.004
Eraly, S. A., Nievergelt, C. M., Maihofer, A. X., Barkauskas, D. A., Biswas, N., Agorastos, A., et al. (2014). Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry 71, 423–431. doi: 10.1001/jamapsychiatry.2013.4374
Felitti, V. J., Anda, R. F., Nordenberg, D., Williamson, D. F., Spitz, A. M., Edwards, V., et al. (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) study. Am. J. Prev. Med. 14, 245–258. doi: 10.1016/S0749-3797(98)00017-8
Glatt, S. J., Tylee, D. S., Chandler, S. D., Pazol, J., Nievergelt, C. M., Woelk, C. H., et al. (2013). Blood-based gene-expression predictors of PTSD risk and resilience among deployed marines: a pilot study. Am. J. Med. Genet. B Neuropsychiatr. Genet. 162B, 313–326. doi: 10.1002/ajmg.b.32167
Glaus, J., von Känel, R., Lasserre, A. M., Strippoli, M. F., Vandeleur, C. L., Castelao, E., et al. (2018). The bidirectional relationship between anxiety disorders and circulating levels of inflammatory markers: Results from a large longitudinal population-based study. Depress. Anxiety 35, 360–371. doi: 10.1002/da.22710
Gong, S., and Deng, F. (2022). Renin-angiotensin system: The underlying mechanisms and promising therapeutical target for depression and anxiety. Front. Immunol. 13:1053136. doi: 10.3389/fimmu.2022.1053136
Guzik, T. J., Hoch, N. E., Brown, K. A., McCann, L. A., Rahman, A., Dikalov, S., et al. (2007). Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 204, 2449–2460. doi: 10.1084/jem.20070657
Harrison, D. G., Guzik, T. J., Lob, H. E., Madhur, M. S., Marvar, P. J., Thabet, S. R., et al. (2011). Inflammation, immunity, and hypertension. Hypertension 57, 132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576
Hodes, G. E., Pfau, M. L., Leboeuf, M., Golden, S. A., Christoffel, D. J., Bregman, D., et al. (2014). Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl. Acad. Sci. USA 111, 16136–16141. doi: 10.1073/pnas.1415191111
Imai, R., Hori, H., Itoh, M., Lin, M., Niwa, M., Ino, K., et al. (2018). Inflammatory markers and their possible effects on cognitive function in women with posttraumatic stress disorder. J. Psychiatr. Res. 102, 192–200. doi: 10.1016/j.jpsychires.2018.04.009
Jergović, M., Bendelja, K., Savić Mlakar, A., Vojvoda, V., Aberle, N., Jovanovic, T., et al. (2015). Circulating levels of hormones, lipids, and immune mediators in post-traumatic stress disorder - a 3-month follow-up study. Front. Psychiatry 6:49. doi: 10.3389/fpsyt.2015.00049
Johnson, A. K., and Xue, B. (2018). Central nervous system neuroplasticity and the sensitization of hypertension. Nat. Rev. Nephrol. 14, 750–766. doi: 10.1038/s41581-018-0068-5
Kamat, N. V., Thabet, S. R., Xiao, L., Saleh, M. A., Kirabo, A., Madhur, M. S., et al. (2015). Renal transporter activation during angiotensin-II hypertension is blunted in interferon-gamma−/− and interleukin-17A−/− mice. Hypertension 65, 569–576. doi: 10.1161/HYPERTENSIONAHA.114.04975
Kawada, N., Imai, E., Karber, A., Welch, W. J., and Wilcox, C. S. (2002). A mouse model of angiotensin II slow pressor response: role of oxidative stress. J Am Soc Nephrol 13, 2860–2868. doi: 10.1097/01.ASN.0000035087.11758.ED
Kessler, R. C., Berglund, P., Demler, O., Jin, R., Merikangas, K. R., and Walters, E. E. (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 593–602. doi: 10.1001/archpsyc.62.6.593
Kessler, R. C., Sonnega, A., Bromet, E., Hughes, M., and Nelson, C. B. (1995). Posttraumatic stress disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 52, 1048–1060. doi: 10.1001/archpsyc.1995.03950240066012
Kilpatrick, D. G., Resnick, H. S., Milanak, M. E., Miller, M. W., Keyes, K. M., and Friedman, M. J. (2013). National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J. Trauma. Stress. 26, 537–547. doi: 10.1002/jts.21848
Krishnan, V., Han, M. H., Graham, D. L., Berton, O., Renthal, W., Russo, S. J., et al. (2007). Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404. doi: 10.1016/j.cell.2007.09.018
Kubzansky, L. D., Koenen, K. C., Spiro, A. 3rd, Vokonas, P. S., and Sparrow, D. (2007). Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Arch. Gen. Psychiatry 64, 109–116. doi: 10.1001/archpsyc.64.1.109
Kuwabara, T., Ishikawa, F., Kondo, M., and Kakiuchi, T. (2017). The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediat. Inflamm. 2017, 1–11. doi: 10.1155/2017/3908061
Lauten, T. H., Elkhatib, S. K., Natour, T., Reed, E. C., Jojo, C. N., and Case, A. J. (2024). T H 17/Treg lymphocyte balance is regulated by beta adrenergic and cAMP signaling. Brain Behav. Immun. 123, 1061–1070. doi: 10.1016/j.bbi.2024.11.013
Madhur, M. S., Funt, S. A., Li, L., Vinh, A., Chen, W., Lob, H. E., et al. (2011). Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler. Thromb. Vasc. Biol. 31, 1565–1572. doi: 10.1161/ATVBAHA.111.227629
Madhur, M. S., Lob, H. E., McCann, L. A., Iwakura, Y., Blinder, Y., Guzik, T. J., et al. (2010). Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55, 500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094
Maloley, P. M., England, B. R., Sayles, H., Thiele, G. M., Michaud, K., Sokolove, J., et al. (2019). Post-traumatic stress disorder and serum cytokine and chemokine concentrations in patients with rheumatoid arthritis. Semin. Arthritis Rheum. 49, 229–235. doi: 10.1016/j.semarthrit.2019.02.002
Maul, S., Giegling, I., Fabbri, C., Corponi, F., Serretti, A., and Rujescu, D. (2020). Genetics of resilience: Implications from genome-wide association studies and candidate genes of the stress response system in posttraumatic stress disorder and depression. Am. J. Med. Genet. B Neuropsychiatr. Genet. 183, 77–94. doi: 10.1002/ajmg.b.32763
Moshfegh, C. M., Elkhatib, S. K., Collins, C. W., Kohl, A. J., and Case, A. J. (2019). Autonomic and Redox Imbalance Correlates With T-Lymphocyte Inflammation in a Model of Chronic Social Defeat Stress. Front. Behav. Neurosci. 13:103. doi: 10.3389/fnbeh.2019.00103
Moshfegh, C. M., Elkhatib, S. K., Watson, G. F., Drake, J., Taylor, Z. N., Reed, E. C., et al. (2022). S100a9 protects against the effects of repeated social defeat stress. Biol. Psychiatry Glob. Open Sci. 3, 919–929. doi: 10.1016/j.bpsgos.2022.12.002
Nguyen, H., Chiasson, V. L., Chatterjee, P., Kopriva, S. E., Young, K. J., and Mitchell, B. M. (2013). Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc. Res. 97, 696–704. doi: 10.1093/cvr/cvs422
North, C. S., Oliver, J., and Pandya, A. (2012). Examining a comprehensive model of disaster-related posttraumatic stress disorder in systematically studied survivors of 10 disasters. Am. J. Public Health 102, e40–e48. doi: 10.2105/AJPH.2012.300689
O'Donnell, C. J., Schwartz Longacre, L., Cohen, B. E., Fayad, Z. A., Gillespie, C. F., Liberzon, I., et al. (2021). Stein: Posttraumatic Stress Disorder and Cardiovascular Disease: State of the Science, Knowledge Gaps, and Research Opportunities. JAMA Cardiol. 6, 1207–1216. doi: 10.1001/jamacardio.2021.2530
O'Donovan, A., Cohen, B. E., Seal, K. H., Bertenthal, D., Margaretten, M., Nishimi, K., et al. (2015). Elevated risk for autoimmune disorders in iraq and afghanistan veterans with posttraumatic stress disorder. Biol. Psychiatry 77, 365–374. doi: 10.1016/j.biopsych.2014.06.015
Saleh, M. A., Norlander, A. E., and Madhur, M. S. (2016). Inhibition of Interleukin 17-A but not Interleukin-17F Signaling Lowers Blood Pressure and Reduces End-organ Inflammation in Angiotensin II-induced Hypertension. JACC Basic Transl. Sci. 1, 606–616. doi: 10.1016/j.jacbts.2016.07.009
Santiago, P. N., Ursano, R. J., Gray, C. L., Pynoos, R. S., Spiegel, D., Lewis-Fernandez, R., et al. (2013). A systematic review of PTSD prevalence and trajectories in DSM-5 defined trauma exposed populations: intentional and non-intentional traumatic events. PLoS One 8:e59236. doi: 10.1371/journal.pone.0059236
Seligowski, A. V., Duffy, L. A., Merker, J. B., Michopoulos, V., Gillespie, C. F., Marvar, P. J., et al. (2021). The renin-angiotensin system in PTSD: a replication and extension. Neuropsychopharmacology 46, 750–755. doi: 10.1038/s41386-020-00923-1
Shansky, R. M. (2015). Sex differences in PTSD resilience and susceptibility: Challenges for animal models of fear learning. Neurobiol. Stress 1, 60–65. doi: 10.1016/j.ynstr.2014.09.005
Shimo, Y., Cathomas, F., Chan, K. L., Parise, L. F., Li, L., Ferrer-Pérez, C., et al. (2022) Social stress induces autoimmune responses against the brain to promote stress susceptibility. Proc. Natl. Acad. Sci. USA 1:e2305778120. doi: 10.1073/pnas.2305778120
Solomon, Z., Levin, Y., Assayag, E. B., Furman, O., Shenhar-Tsarfaty, S., Berliner, S., et al. (2017). The Implication of Combat Stress and PTSD Trajectories in Metabolic Syndrome and Elevated C-Reactive Protein Levels: A Longitudinal Study. J. Clin. Psychiatry 78, e1180–e1186. doi: 10.4088/JCP.16m11344
Sommershof, A., Aichinger, H., Engler, H., Adenauer, H., Catani, C., Boneberg, E. M., et al. (2009). Substantial reduction of naive and regulatory T cells following traumatic stress. Brain Behav. Immun. 23, 1117–1124. doi: 10.1016/j.bbi.2009.07.003
Sumner, J. A., Chen, Q., Roberts, A. L., Winning, A., Rimm, E. B., Gilsanz, P., et al. (2017). Cross-Sectional and Longitudinal Associations of Chronic Posttraumatic Stress Disorder With Inflammatory and Endothelial Function Markers in Women. Biol. Psychiatry 82, 875–884. doi: 10.1016/j.biopsych.2017.06.020
Sumner, J. A., Chen, Q., Roberts, A. L., Winning, A., Rimm, E. B., Gilsanz, P., et al. (2018). Posttraumatic stress disorder onset and inflammatory and endothelial function biomarkers in women. Brain Behav. Immun. 69, 203–209. doi: 10.1016/j.bbi.2017.11.013
Sumner, J. A., Cleveland, S., Chen, T., and Gradus, J. L. (2023). Psychological and biological mechanisms linking trauma with cardiovascular disease risk. Transl. Psychiatry 13:25. doi: 10.1038/s41398-023-02330-8
Sumner, J. A., Nishimi, K. M., Koenen, K. C., Roberts, A. L., and Kubzansky, L. D. (2019). Posttraumatic Stress Disorder and Inflammation: Untangling Issues of Bidirectionality. Biol. Psychiatry 87, 885–897. doi: 10.1016/j.biopsych.2019.11.005
Svendsen, U. G. (1973). Increased cellular reaction to damage caused by angiotensin in arterioles of normal recipient rats after transfer of lymphocytes from hypertensive rats. Acta Pathol. Microbiol. Scand. A 81, 241–246.
Svendsen, U. G. (1978). The importance of thymus for hypertension and hypertensive vascular disease in rats and mice. Acta Pathol. Microbiol. Scand. Suppl. 1978, 1–15.
Takahashi, A., Chung, J. R., Zhang, S., Zhang, H., Grossman, Y., Aleyasin, H., et al. (2017). Establishment of a repeated social defeat stress model in female mice. Sci. Rep. 7, 1283–1288. doi: 10.1038/s41598-017-12811-8
Terock, J., Hannemann, A., Janowitz, D., Freyberger, H. J., Felix, S. B., Dörr, M., et al. (2019). Associations of trauma exposure and post-traumatic stress disorder with the activity of the renin-angiotensin-aldosterone-system in the general population. Psychol. Med. 49, 843–851. doi: 10.1017/S0033291718001496
Terock, J., Hannemann, A., Janowitz, D., Van der Auwera, S., Bahls, M., Völzke, H., et al. (2019). Differential activation of the renin-angiotensin-aldosterone-system in response to childhood and adulthood trauma. Psychoneuroendocrinology 107, 232–240. doi: 10.1016/j.psyneuen.2019.05.026
Tursich, M., Neufeld, R. W., Frewen, P. A., Harricharan, S., Kibler, J. L., Rhind, S. G., et al. (2014). Association of trauma exposure with proinflammatory activity: a transdiagnostic meta-analysis. Transl. Psychiatry 4:e413. doi: 10.1038/tp.2014.56
Tylee, D. S., Chandler, S. D., Nievergelt, C. M., Liu, X., Pazol, J., Woelk, C. H., et al. (2015). Blood-based gene-expression biomarkers of post-traumatic stress disorder among deployed marines: A pilot study. Psychoneuroendocrinology 51, 472–494. doi: 10.1016/j.psyneuen.2014.09.024
Tyrka, A. R., Parade, S. H., Price, L. H., Kao, H. T., Porton, B., Philip, N. S., et al. (2016). Alterations of Mitochondrial DNA Copy Number and Telomere Length With Early Adversity and Psychopathology. Biol. Psychiatry 79, 78–86. doi: 10.1016/j.biopsych.2014.12.025
van Zuiden, M., Heijnen, C. J., van de Schoot, R., Amarouchi, K., Maas, M., Vermetten, E., et al. (2011). Cytokine production by leukocytes of military personnel with depressive symptoms after deployment to a combat-zone: a prospective, longitudinal study. PLoS One 6:e29142. doi: 10.1371/journal.pone.0029142
von Känel, R., Hepp, U., Kraemer, B., Traber, R., Keel, M., Mica, L., et al. (2007). Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J. Psychiatr. Res. 41, 744–752. doi: 10.1016/j.jpsychires.2006.06.009
Wilson, R. S., Boyle, P. A., Levine, S. R., Yu, L., Anagnos, S. E., Buchman, A. S., et al. (2012). Emotional neglect in childhood and cerebral infarction in older age. Neurology 79, 1534–1539. doi: 10.1212/WNL.0b013e31826e25bd
Wilson, M. A., Liberzon, I., Lindsey, M. L., Lokshina, Y., Risbrough, V. B., Sah, R., et al. (2019). Common pathways and communication between the brain and heart: connecting post-traumatic stress disorder and heart failure. Stress 22, 530–547. doi: 10.1080/10253890.2019.1621283
Yadav, S. K., Ahmad, R., Moshfegh, C. M., Sankarasubramanian, J., Joshi, V., Elkhatib, S. K., et al. (2023). Repeated Social Defeat Stress Induces an Inflammatory Gut Milieu by Altering the Mucosal Barrier Integrity and Gut Microbiota Homeostasis. Biol. Psychiatry Glob. Open Sci. 3, 824–836. doi: 10.1016/j.bpsgos.2023.03.005
Keywords: repeated social defeat stress, interleukin 17A, T cells, behavior, stress
Citation: Case AJ, Natour T, Pitts LJ, Lauten TH, Reed EC, Moshfegh CM and Elkhatib SK (2025) Psychological trauma increases blood pressure sensitivity to angiotensin II via T-lymphocytes independent of psychopathology. Front. Cell. Neurosci. 19:1691047. doi: 10.3389/fncel.2025.1691047
Edited by:
Renu Sah, University of Cincinnati, United StatesReviewed by:
Jian Wang, General Hospital of Western Theater Command, ChinaEmily Allgire, Northern Kentucky University, United States
Copyright © 2025 Case, Natour, Pitts, Lauten, Reed, Moshfegh and Elkhatib. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adam J. Case, YWNhc2VAdGFtdS5lZHU=
 Adam J. Case
Adam J. Case Tamara Natour
Tamara Natour Lauren J. Pitts
Lauren J. Pitts Tatlock H. Lauten
Tatlock H. Lauten Emily C. Reed
Emily C. Reed Cassandra M. Moshfegh
Cassandra M. Moshfegh Safwan K. Elkhatib
Safwan K. Elkhatib