- 1Department of Pharmacology, Wayne State University School of Medicine, Detroit, MI, United States
- 2Department of Neurology, Wayne State University School of Medicine, Detroit, MI, United States
- 3Translational Neuroscience Program, Wayne State University School of Medicine, Detroit, MI, United States
The discovery of neuronal activity-dependent calcium transients in astrocytes has driven the conceptualization of these cells as computational units in the nervous system. Tripartite synapses, consisting of pre- and postsynaptic terminals along with an adjacent astrocyte, enable astrocytes to communicate with and shape the activity of local synapses. In the hippocampus, astrocytes encode and modulate information through a variety of mechanisms, including tuning the gating of single synapses in their domains, coordinating oscillatory activity across neuronal circuits through astrocytic networks, and providing a foundation for long-term memory formation through intracellular signaling and metabolic coupling. The bidirectional and complementary activities of astrocytes and neurons can be situated in a framework that positions astrocytes as integrators and modulators of neuronal activity, both locally and globally. In this review, we focus on hippocampal astrocytes and discuss recent progress toward understanding astrocytic function in concert with neurons to mediate circuit function and, ultimately, behavior.
1 Introduction
The hippocampus plays a crucial role in various brain functions, including learning and memory. By integrating poly-sensory and mnemonic information with cell-intrinsic properties into abstract, plastic representations of behavioral experience, it provides a scaffold for episodic, spatial, and contextual memories. Synaptic plasticity of hippocampal neurons is a critical mechanism by which learning is facilitated (Huganir and Nicoll, 2013; Whitlock et al., 2006). This plasticity, expressed through molecular rearrangements in neuronal compartments, is modulated by the activity of adjacent astrocytes.
Beyond their long-established supporting role, accumulating evidence suggests that astrocytes play an active role in encoding and processing information in the brain. Neuronal activity-dependent calcium signaling in astrocytes has long been observed (Dani et al., 1992), the significance of which has been demonstrated in hippocampus-dependent learning and memory (Adamsky et al., 2018; Kol et al., 2020; Ma et al., 2023; Refaeli et al., 2024; Suthard et al., 2024) and sleep (Foley et al., 2017; Ingiosi et al., 2020; Tsunematsu et al., 2021). Recent investigations have identified diverse forms of hippocampal astrocytic activity in response to synaptic transmission that shape circuit function and behavior, suggesting that these cells are integral for the essential functions of this region (Armbruster et al., 2022; Ioannou et al., 2019; Suzuki et al., 2011; Theparambil et al., 2024). In this review, we discuss recent research that has advanced understanding of the hippocampus by investigating the complementary actions of neurons and astrocytes underlying circuit function and behavior.
2 Astrocyte–synapse contacts in domains and across networks
The hippocampus consists of two parallel circuits. The cornu ammonis 1 (CA1) region, the principal output source of the hippocampus, receives inputs directly from the entorhinal cortex (the direct pathway) as well as from the indirect, or trisynaptic, pathway (Basu and Siegelbaum, 2015). The trisynaptic pathway consists of three synaptic connections: first, the perforant pathway links the entorhinal cortex to dentate gyrus (DG) granule cells, which then connect to CA3 pyramidal neurons through the mossy fiber pathway; these in turn synapse onto CA1 pyramidal neurons via the Schaffer collateral (SC) pathway (Basu and Siegelbaum, 2015).
Neuronal terminals are contacted by adjacent astrocytes to form tripartite synapses, which serve to process and transmit information in the hippocampus. Astrocytes have a stellate morphology with fine processes extending from the soma. These processes are highly plastic—for instance, environmental enrichment (Viola et al., 2009), memory encoding (Choi et al., 2016), and aerobic exercise (Saur et al., 2014) enhance the ramification of astrocytic processes in mice. The endfeet of these fine processes ensheathe synapses as perisynaptic astrocytic processes (PAPs) to form tripartite synapses (Aten et al., 2022; Haber et al., 2006) and facilitate bidirectional communication to regulate the structure and activity of astrocytes and neurons. This plasticity supports various hippocampal functions, including learning and memory.
The hippocampus exhibits diverse forms of synaptic plasticity. In addition to the classically established roles of long-term potentiation (LTP) and long-term depression (LTD) in facilitating memory formation (Bliss and Lømo, 1973; Tsien et al., 1996; Whitlock et al., 2006), paradigms such as spike timing-dependent plasticity (STDP) and behavioral timescale plasticity have been developed to further explore the breadth of synaptic plasticity under different physiological and behavioral conditions (Bittner et al., 2017; Magee and Johnston, 1997). LTP occurs at excitatory connections in hippocampal pathways through various mechanisms (Morgan and Teyler, 2001; Zalutsky and Nicoll, 1990). One of the most extensively studied forms depends on N-methyl-D-aspartate receptors (NMDARs) and occurs in response to high-frequency stimulation of synapses (Collingridge et al., 1983; Dudek and Bear, 1992; Tsien et al., 1996). NMDARs are ionotropic glutamate receptor complexes that respond to the coincidence of presynaptic glutamate release and postsynaptic depolarization with calcium influx at the postsynaptic membrane. This activates a calcium/calmodulin-dependent protein kinase II-dependent pathway by which α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs)—another class of ionotropic glutamate receptors—are trafficked to the postsynaptic membrane, thereby augmenting synaptic strength (Hayashi et al., 2000; Malenka et al., 1989; Malinow et al., 1989). LTD, in contrast, occurs in response to low-frequency stimulation of a synapse; NMDAR-dependent LTD mediates the endocytosis of AMPARs, thereby diminishing synaptic strength (Beattie et al., 2000; Dudek and Bear, 1992; Mulkey et al., 1994). This process is critical for a variety of hippocampal processes, including novelty acquisition (Dong et al., 2012; Kemp and Manahan-Vaughan, 2004; Kim et al., 2011; Manahan-Vaughan and Braunewell, 1999) and cognitive flexibility (Nicholls et al., 2008).
The established view of synaptic plasticity has been expanded by evidence suggesting a role for astrocytes in modulating neuronal activity to shape hippocampal function. Across brain regions, astrocytes support neuronal function through a variety of mechanisms, including the buffering of ions (Bellot-Saez et al., 2017; Nowak et al., 1987; Rose and Ransom, 1996), uptake of neurotransmitters (Bergles and Jahr, 1997; Gadea and López-Colomé, 2001), coordination of metabolism (Rouach et al., 2008), and regulation of synaptogenesis (Christopherson et al., 2005; Eroglu et al., 2009). Beyond these homeostatic functions, astrocytes are critical in facilitating the transmission of information through neuronal circuits. PAPs, as the astrocytic component of the tripartite synapse, are regulated by neuronal activity, both morphologically (Bernardinelli et al., 2014; Cornell-Bell et al., 1990) and translationally (Sapkota et al., 2022). In turn, astrocytes can phagocytose synapses in the CA1 region in response to neuronal activity throughout adulthood, a process critical to sustaining LTP and memory formation by eliminating superfluous excitatory connections (Lee et al., 2021). These changes are highly circuit-dependent; accordingly, distinct astrocytic transcriptional programs emerge across different brain regions during development to govern the circuit specificity of tripartite synapses (Huang et al., 2020).
Astrocytes are involved in modulating various forms of synaptic plasticity. STDP modulates the strength of a synapse based on the temporal dynamics of pre- and postsynaptic activity (Magee and Johnston, 1997). If a presynaptic neuron fires a short time before the postsynaptic neuron does, the synapse strengthens; by contrast, if postsynaptic activity precedes presynaptic activity, the synapse weakens. During postnatal development, SC–CA1 synapses transition from spike timing-dependent LTD to spike timing-dependent LTP, a process dependent on downregulating NMDAR expression and upregulating metabotropic glutamate receptor (mGluR) expression, as well as astrocytic calcium activity and glutamate release (Falcón-Moya et al., 2020). This astrocytic activity and subsequent vesicular release are also critical for the induction of spike timing-dependent LTD at perforant pathway–DG synapses (Martínez-Gallego et al., 2024).
As a paradigm, it may be useful to consider two complementary scopes by which astrocytes regulate synaptic function. First, astrocytes form structural and functional domains that tile the central nervous system, intermingling with the fine processes of adjacent astrocytes only at the periphery of the cell (Grosche et al., 2013; Ogata and Kosaka, 2002; Refaeli et al., 2021; Viana et al., 2023). This potentially allows the modular regulation of synapses in the domain of an astrocyte (Figure 1). Second, astrocytes form networks through gap junction coupling, which mediates the broad coordination of neuronal circuits as well as the intercellular flow of nutrients to sustain synaptic transmission (Figure 2). Both astrocytic domains and networks emerge early in postnatal development (Bushong et al., 2004; Zhong et al., 2023), and their functions are critical for learning and memory in the hippocampus (Rouach et al., 2008; Suzuki et al., 2011). These two perspectives of astrocytic function are not mutually exclusive but are often interwoven by molecular crosstalk between neurons and astrocytes (Bonvento and Bolaños, 2021). By facilitating both the discretized and integrated regulation of synaptic transmission, astrocytes are able to dynamically and precisely modulate hippocampal circuitry.

Figure 1. Hippocampal astrocytes exhibit diverse forms of calcium signaling in response to neuronal activity, both in the hippocampus and in connections to and from the hippocampus, to shape behavior. (A) Astrocytic activation in the Schaffer collateral (SC)–CA1 pathway induces LTP through the activation of postsynaptic NMDARs (Adamsky et al., 2018). (B) Sustained glutamatergic activity in the mossy fiber–CA3 pathway elicits broad calcium activity in nearby astrocytes (Haustein et al., 2014). (C) Single synaptic activity in the perforant pathway–dentate gyrus (DG) elicits calcium transients in adjacent astrocytes and potentiates synapses through the astrocytic release of ATP/adenosine (Di Castro et al., 2011). (D) CA1 astrocytic activation promotes functional connectivity between the CA1 region and the anterior cingulate cortex (ACC) to promote long-term contextual and spatial memory (Adamsky et al., 2018; Kol et al., 2020; Refaeli et al., 2024). (E) The activation of CA1 astrocytic calcium signaling promotes the induction of LTP in the direct pathway–CA1 by eliciting D-serine release downstream of nicotinic cholinergic activity, facilitating the formation of episodic memory by modulating neuronal NMDAR activity (Ma et al., 2023). (F) Calcium activity in the CA1 astrocytes can be elicited spontaneously or by the binding of a ligand (often a neurotransmitter or neuromodulator) to an astrocytic receptor, and is modulated by locomotion and sleep/wakefulness (Bindocci et al., 2017; Dani et al., 1992; Doron et al., 2022; Duffy and MacVicar, 1995; Haustein et al., 2014; Ingiosi et al., 2020; Nett et al., 2002; Rupprecht et al., 2024; Sharma and Vijayaraghavan, 2001; Tang et al., 2015; Tsunematsu et al., 2021; Wu et al., 2014).
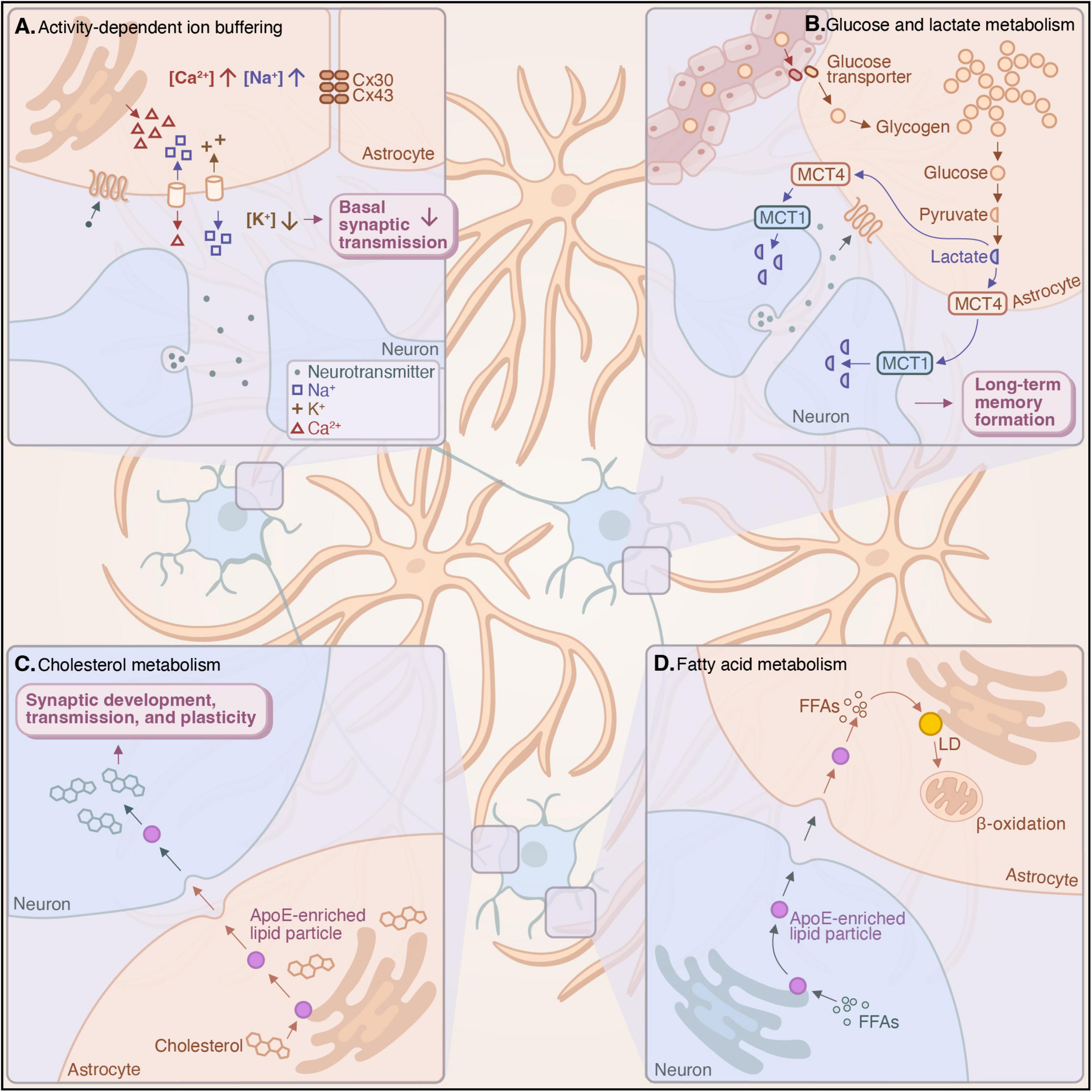
Figure 2. Hippocampal astrocytes form networks through gap-junction coupling, which play a crucial role in buffering ions and neurotransmitters and facilitating metabolic coupling with neurons. (A) Astrocytic networks mediate the uptake and release of ions to buffer extracellular potassium concentrations, thereby modulating synaptic transmission and enhancing synaptic fidelity (Chever et al., 2016; Dossi et al., 2024; Wang et al., 2012; Zhong et al., 2023). (B) Astrocytes shuttle glucose across the blood-brain barrier (BBB) and produce lactate through aerobic glycolysis, the delivery of which to hippocampal neurons is critical for the formation of long-term memory (Gao et al., 2016; Suzuki et al., 2011). (C) Astrocytic cholesterol biosynthesis and its delivery to neurons via lipoprotein particles are necessary for synaptic development, transmission, and plasticity (Ferris et al., 2017; Fünfschilling et al., 2007; van Deijk et al., 2017). (D) Astrocytes take up fatty acids from neurons in response to neuronal activation via ApoE-enriched particles for storage in lipid droplets (LDs), which can subsequently be mobilized to provide energy to neurons through β-oxidation (Chen et al., 2023; Ioannou et al., 2019; Qi et al., 2021).
3 Astrocytic signaling during behavior
Investigations into hippocampal astrocytic activity, particularly calcium activity, have been demonstrated to be important for memory processing. The initial discovery that glutamate elicits transient changes in intracellular calcium concentrations in hippocampal astrocytes (Dani et al., 1992) spurred the conceptualization of these cells as actively encoding and transmitting information. Indeed, later investigations demonstrated that astrocytic intracellular calcium activity, when coincident with neuronal activation, is sufficient to induce LTP at single hippocampal synapses and facilitate memory formation (Adamsky et al., 2018; Perea and Araque, 2007; Figure 1A).
Much of the research to date has examined astrocytic signaling through calcium dynamics. While other forms of neuronal activity-dependent signaling have been demonstrated in astrocytes (Armbruster et al., 2022; Theparambil et al., 2024), their behavioral significance is still emerging. For instance, genetically encoded voltage indicators have revealed changes in astrocytic membrane potential during neuronal activity. Microdomain depolarizations in PAPs occur during synaptic activity, mediated by potassium uptake as well as the electrogenic uptake of glutamate (Armbruster et al., 2022). This cellular response to neuronal activity suggests that molecular mechanisms beyond those of calcium may contribute to astrocytic encoding and regulation of synaptic transmission. Nevertheless, investigations into astrocytic calcium dynamics have revealed their profound role in shaping hippocampal circuit activity.
Advances in molecular tools, such as the development of genetically encoded calcium indicators (e.g., GCaMP), have allowed investigators to finely elucidate calcium activity in astrocytes at rest and in response to neuronal activity. This cellular activity has been demonstrated to be indispensable for mediating information flow across synapses and for shaping hippocampus-dependent behavior. Investigating the dynamics and significance of astrocytic calcium signaling in response to neuronal activity will continue to provide a more comprehensive understanding of hippocampal function.
3.1 Calcium dynamics in astrocytes
Hippocampal astrocytes exhibit distinct spatiotemporal calcium activity both at rest and in response to synaptic transmission. At rest, intracellular calcium concentrations in astrocytes are unevenly distributed throughout the cell (Zheng et al., 2015), influencing the peak level and amplitude of calcium signals (King et al., 2020). This heterogeneity suggests the existence of functional microdomains in astrocytes, through which these cells may modulate the activity of individual synapses. Astrocytes can respond to synaptic transmission with calcium activity but also exhibit spontaneous calcium transients independent of neuronal activity (Haustein et al., 2014; Nett et al., 2002). Evoked astrocytic calcium activity can arise from either extracellular or intracellular sources, such as the endoplasmic reticulum through inositol trisphosphate (IP3) signaling (Agarwal et al., 2017; Glaum et al., 1990; Perea and Araque, 2005). By contrast, spontaneous astrocytic activity occurs independently of IP3 signaling (Srinivasan et al., 2015), instead relying on the influx of extracellular calcium (Hjukse et al., 2023; Rungta et al., 2016) and calcium efflux from mitochondria (Agarwal et al., 2017). This spontaneous activity exhibits distinct temporal dynamics, in which the duration of a signal is generally logarithmically proportional to its spatial distribution (Wu et al., 2014). In the hippocampus, PAPs form calcium microdomains; that is, astrocytic fine processes apposing synapses exhibit distinct calcium signaling generally restricted to the region in which it originated (Arizono et al., 2020). This suggests that a single astrocyte may be structurally and functionally capable of differentially communicating with many synapses in parallel in its domain.
Calcium activity in an astrocyte varies depending on the synapses in its domain. For instance, in the mossy fiber–CA3 pathway, sustained glutamate release elicits broad calcium activity throughout surrounding astrocytes, dependent on mGluR activation (Haustein et al., 2014; Figure 1B). By contrast, astrocytes in the perforant pathway–DG (Di Castro et al., 2011) and SC–CA1 pathway can respond to single synaptic activity with calcium transients, a process dependent on astrocytic mGluR5 and purinergic signaling (Bernardinelli et al., 2011; Panatier et al., 2011; Figure 1C). Astrocytes in the perforant pathway–DG can also encode coincident transmission across synapses through IP3-mediated calcium activity (Di Castro et al., 2011). Moreover, calcium activity in hippocampal astrocytes is modulated by behavior. For instance, locomotion tends to be accompanied by neuronal signaling from the locus coeruleus, which activates α1-adrenergic receptors on CA1 astrocytes to trigger broad, synchronized calcium events in hippocampal astrocytes, during which calcium transients originating from fine processes intensify and propagate toward the soma simultaneously across a population of astrocytes (Bindocci et al., 2017; Rupprecht et al., 2024). This circuit- and behavior-dependent signaling suggests a role for astrocytes in encoding and integrating synaptic transmission. The specific effectors upstream of this signaling that confer the consistent calcium signatures often observed in response to neuronal activity, as well as the downstream effectors that promote its behavioral effects, remain open questions. Nevertheless, experimental manipulation of astrocytic calcium signaling has provided insights into its behavioral relevance, highlighting its essential roles in hippocampal function.
3.2 Learning and memory
The use of chemogenetic and optogenetic approaches to activate astrocytes in vivo has provided valuable insights into the role of these cells in mediating hippocampus-dependent memory. The hippocampus plays an important role in processing declarative, or explicit, memory (Squire, 2004), spatial information and memory (O’Keefe and Burgess, 1996; Tsien et al., 1996), and episodic memory (Kraus et al., 2013; MacDonald et al., 2013; Pastalkova et al., 2008). Both neurons and astrocytes in the ventral CA1 region exhibit distinct patterns of calcium activity during memory acquisition. Optogenetic reactivation of this population of DG neurons elicits calcium activity in both cell types in the CA1 region resembling that of natural recall, concomitant with the artificial recall of the memory (Suthard et al., 2024). The functional significance of this signaling in CA1 astrocytes has been elucidated through genetic manipulations in these cells, which demonstrate its sufficiency in facilitating the long-term consolidation of memory by promoting cortico-hippocampal synaptic connectivity (Adamsky et al., 2018; Kol et al., 2020; Refaeli et al., 2024). Calcium signaling occurs in astrocytes, at least in part, through G-protein-coupled receptors (GPCRs), including mGluRs, γ-aminobutyric acid B (GABAB) receptors, adenosine receptors, adrenergic receptors, and cannabinoid receptors, which elicit the intracellular release of calcium from the endoplasmic reticulum through IP3 signaling (Agulhon et al., 2010; Araque et al., 2002; Coiret et al., 2012; Duffy and MacVicar, 1995; Panatier et al., 2011; Pascual et al., 2005; Petravicz et al., 2008; Serrano et al., 2006). In neurons, GPCRs coupled to Gq proteins elicit intracellular calcium activity, while GPCRs coupled to Gi proteins diminish this activity. By contrast, experimental stimulation of both Gq and Gi signaling activates calcium signaling in astrocytes (Durkee et al., 2019). These forms of G protein signaling have differing effects in different cell types, as CA1 astrocytic Gq signaling enhances the recall of hippocampus-dependent memory, while neuronal Gq signaling and astrocytic Gi signaling impair this recall (Adamsky et al., 2018; Kol et al., 2020; Refaeli et al., 2024).
A robust paradigm for studying hippocampus-dependent memory is contextual fear memory, wherein an animal’s fear response to a spatial environment previously coupled with an aversive stimulus is recorded as a correlate of memory. Chemogenetic stimulation of CA1 astrocytic Gq signaling during fear memory acquisition enhances recent recall of contextual memory in mice by eliciting the release of D-serine, activating neuronal NMDARs, and strengthening functional connectivity between the CA1 region and anterior cingulate cortex (ACC) (Adamsky et al., 2018; Refaeli et al., 2024; Figure 1D). By contrast, chemogenetic Gi-GPCR activation in CA1 astrocytes during memory acquisition impairs remote memory recall, concomitant with reduced functional connectivity between CA1 and ACC (Kol et al., 2020; Refaeli et al., 2024). These findings suggest that the Gq pathway in hippocampal astrocytes facilitates memory consolidation by strengthening the synaptic connections between CA1 and ACC, while the Gi pathway exerts the opposite effect, thereby diminishing recall. The temporal differences between the effects of Gq and Gi signaling may be due to the progressive increase in CA1–ACC functional connectivity during memory consolidation; therefore, Gq activation has the greatest impact at an earlier time point, when connectivity is generally lower, and Gi activation has the greatest impact at a later time point, when connectivity is generally higher (Refaeli et al., 2024). Overall, these results suggest that CA1 astrocytes play a crucial role in the long-term consolidation of memories by mediating the transfer of hippocampus-dependent memories into cortical areas.
Astrocytes play a role in regulating memory engrams in the hippocampus. Engrams have been proposed as the structural and functional changes in neuronal ensembles associated with memory, serving as the cellular substrate of memory (Josselyn et al., 2017). In the hippocampus, memory acquisition is considered the strengthening of synaptic connections among a constellation of neurons. After memory consolidation, their reactivation—induced either artificially or through the natural retrieval of the memory—reliably elicits recall of the memory from which they were formed (Liu et al., 2012; Ramirez et al., 2013). Engram neurons are often identified by their upregulated expression of immediate early genes (e.g., c-Fos) shortly after memory acquisition (Liu et al., 2012). Intriguingly, learning also induces c-Fos expression in a subset of astrocytes in the CA1 and DG. Engram neurons are located in the domains of these c-Fos-expressing astrocytes (Williamson et al., 2025). Astrocyte-specific knockout of this expression reduces hippocampal LTP and contextual memory formation (Williamson et al., 2025), while chemogenetic activation of these peri-engram astrocytes promotes LTP at SC–CA1 synapses and evokes the artificial recall of a contextual memory outside of the context in which it was formed, suggesting that this subset of hippocampal astrocytes is specifically crucial for mediating memory recall (Williamson et al., 2025).
Hippocampal astrocytic calcium activity also contributes to the acquisition and maintenance of spatial memory, as chemogenetic activation of Gq-GPCR signaling in CA1 astrocytes enhances spatial memory in mice (Adamsky et al., 2018). The discovery of place cells in the hippocampus, which exhibit activity when the animal is in a certain location (O’Keefe and Dostrovsky, 1971), was a pivotal breakthrough in understanding how this region encodes spatial information. A population of place cells, known as a place field, forms an abstract representation of space in the hippocampus (Kjelstrup et al., 2008). The activity of these neurons can be decoded to determine the location of an animal moving through a familiar environment. While the classical view of place fields in the hippocampus involves only neurons, calcium imaging in freely behaving mice has identified topographically organized astrocyte activity in the hippocampus, to the extent that decoding the location of the mouse is more accurate when astrocytic signals are considered alongside neuronal ones (Curreli et al., 2022). Furthermore, in CA1 astrocytes, intracellular calcium activity increases as a mouse approaches a reward (Doron et al., 2022), suggesting that spatially dependent activity in hippocampal astrocytes may aid in recollecting a behaviorally salient feature of the environment. However, this calcium activity could also reflect past events, such as changes in neuronal activity, locomotion, or pupil dilation (Rupprecht et al., 2024).
Astrocytic activity has also been shown to be critical for episodic memory formation (Fortin et al., 2002). Time cells in the CA1 region are a critical feature of hippocampal temporal encoding (Kraus et al., 2013; MacDonald et al., 2011; MacDonald et al., 2013). These cells exhibit temporally specific firing patterns, analogous to the spatial specificity of place cells. In fact, the same population of CA1 neurons can function as either time cells or place cells (Cabral et al., 2014; Kraus et al., 2013). These neurons replay their spatial or temporal sequences during quiet wake to facilitate consolidation (Carr et al., 2011). CA1 astrocytes appear to contribute to episodic memory: chemogenetic or optogenetic activation of CA1 astrocytes enhances LTP at synapses in the direct pathway–CA1 and promotes episodic memory formation, while disruption of this signaling through astrocyte-specific expression of a plasma membrane calcium pump (hPMCA2w/b) diminishes this form of memory (Ma et al., 2023; Figure 1E).
Overall, astrocytic calcium signaling in the hippocampus, particularly in the CA1 region, has been demonstrated to be sufficient for coordinating neuronal activity toward the formation and long-term consolidation of hippocampus-dependent memory, including contextual, spatial, and episodic memory. This calcium activity occurs both spontaneously and in response to neuronal or behavioral changes (Figure 1F), suggesting a capacity for CA1 astrocytes to dynamically tune hippocampal neuronal activity to the context from which it arises. However, fundamental questions remain, such as the potential role of astrocytes in allocating neurons to an engram, as well as the extent to which astrocytes contribute to the spatiotemporal properties of behavioral experience through their proximity to place and time cells. Exploring these questions will further elucidate the cellular mechanisms underlying memory, as well as the emergent properties of experience constructed by the hippocampus in relation to learning and memory.
3.3 Sleep and wakefulness
Sleep is critical for maintaining hippocampal function and promoting memory consolidation (Karni et al., 1994; Walker and Stickgold, 2004). The four stages of sleep include three non-rapid eye movement (NREM) stages and one rapid eye movement (REM) stage. Stage 3 of NREM sleep, also known as slow-wave sleep, is characterized by neuronal delta (0.5–4 Hz) oscillations. During slow-wave sleep, hippocampal neuronal ensembles are reactivated to promote memory consolidation (Lee and Wilson, 2002; Wilson and McNaughton, 1994). During REM sleep, cholinergic projections to the hippocampus mediate theta (4–7 Hz) oscillations, which are also crucial for memory consolidation (Griffin et al., 2004; Marrosu et al., 1995).
The activities of neurons and astrocytes vary across sleep stages. Hippocampal synaptic transmission becomes synchronized during sleep, reaching its peak during NREM sleep and exhibiting its lowest levels of synchronization during wakefulness (Bódizs et al., 2001; Moroni et al., 2007). By contrast, astrocytic calcium signaling desynchronizes during NREM sleep and synchronizes during wakefulness, a state in which increased vigilance promotes broad, coordinated calcium activity across astrocytes (Bindocci et al., 2017; Ingiosi et al., 2020; Rupprecht et al., 2024; Tsunematsu et al., 2021). Calcium concentrations in hippocampal astrocytes are highest during wakefulness and lowest during REM sleep, a phenomenon reliable enough that astrocytic calcium activity in the hippocampus can be decoded to predict sleep and wakefulness states (Tsunematsu et al., 2021).
Furthermore, the manipulation of calcium activity in hippocampal astrocytes has revealed their role in mediating sleep behavior. A study in which astrocytic calcium signaling was experimentally diminished through knockout of stromal interaction molecule 1 revealed its role in regulating sleep; this manipulation reduced both sleep time and sleep pressure in mice (Ingiosi et al., 2020). In another study, mice with disrupted astrocytic calcium signaling, achieved by expression of IP3 5’-phosphatase, entered REM sleep more frequently, concomitant with increased hippocampal theta oscillations (Foley et al., 2017). Together, these studies suggest a role for hippocampal astrocytic calcium signaling in sleep homeostasis.
3.4 Tripartite synapses in neurodegeneration
Investigations of astrocytes in various mouse models of Alzheimer’s disease (AD) have revealed profound changes in neuron–astrocyte interactions in the hippocampus that mediate the pathogenesis of neurodegeneration. AD is a progressive neurodegenerative disorder characterized by the deposition of β-amyloid (Aβ) plaques and neurofibrillary tangles containing hyperphosphorylated tau (Long and Holtzman, 2019).
Various AD mouse models have revealed changes in tripartite synaptic structure and function that may contribute to AD pathologies. The APP/PS1 mouse model, which carries a mutant amyloid precursor protein (APP) and a mutant presenilin 1 (PS1), exhibits Aβ pathology, gliosis, and memory deficits by 6 months of age (Jankowsky et al., 2004). Transcriptomic studies have revealed that APP/PS1 astrocytes in the hippocampus are deficient in genes critical for synaptic function and neurotransmitter homeostasis (Jiwaji et al., 2022; Endo et al., 2022). Furthermore, APP/PS1 astrocytes in the hippocampus aberrantly phagocytose synapses for lysosomal degradation (Li et al., 2024; St-Pierre et al., 2023) and exhibit deficits in their support of the axon initial segment early in development (Benitez et al., 2024). These changes may contribute to neurodegeneration by compromising the integrity of hippocampal tripartite synapses and reducing their capacity for learning-induced plasticity. For instance, the structural changes observed in tripartite synapses after memory acquisition, dependent on morphological plasticity of PAPs, are altered in APP/PS1 mice, concomitant with impaired memory formation (Kater et al., 2023).
Neuronal hyperactivity has been considered a crucial event in the pathogenesis of AD, which contributes to aberrant neural network changes and excitotoxicity. Both Aβ and tau pathology can contribute to changes in astrocyte–neuron coupling that promote hyperactivity. For instance, tau pathology downregulates glutamate transporter 1 expression in astrocytes, which diminishes the capacity of astrocytes to buffer this excitatory neurotransmitter (Puma et al., 2022). Changes in astrocytic calcium activity, downstream of Aβ pathology, may be a triggering event for neuronal hyperactivity in the hippocampus in neurodegenerative contexts. Aβ production upregulates mGluR5 and IP3 receptor expression in hippocampal astrocytes (Grolla et al., 2013a; Grolla et al., 2013b; Ronco et al., 2014). This upregulated expression elicits calcium hyperactivity in astrocytes (Ronco et al., 2014). Aβ-induced calcium hyperactivity in astrocytes, which is observed in multiple mouse models of AD (Huffels et al., 2022; Ronco et al., 2014), can increase glutamatergic neuronal activity in the CA1 region (Bosson et al., 2017), which is dependent on astrocytic calcium-dependent glutamate release and neuronal NMDAR activation (Paumier et al., 2022; Pirttimaki et al., 2013), and trigger synaptic spine loss (Talantova et al., 2013). This increased spontaneous astrocytic calcium activity is correlated with vascular tone instability, which promotes neuronal death (Takano et al., 2007). Furthermore, high concentrations of calcium in the astrocytic endoplasmic reticulum may contribute to endoplasmic reticulum stress as well as a reduction in protein synthesis (Dematteis et al., 2020; Dematteis et al., 2025; Tapella et al., 2022). This cellular stress induces aberrant changes in mitochondrial morphology and increases reactive oxygen species production from astrocytes. Metabolic adaptations downstream of these changes increase the cellular use of glutamine, which elicits the excessive release of glutamate (Carvalho et al., 2023), further contributing to neuronal hyperactivity.
Overall, astrocytic calcium signaling plays a crucial role in facilitating information flow and mediating both the homeostatic and behavioral functions of the hippocampus in health and disease. Astrocytes can modulate the activity of synapses in their domains through precise and localized signaling in their fine processes. This discretized, specific modulation of information flow is complemented by the activity of extensive networks formed by adjacent astrocytes that allow the regulation of neuronal circuits. Both levels of astrocytic organization are indispensable for hippocampal function, positioning astrocytes to play an active role in processing information both locally and broadly.
4 Neuron–astrocyte metabolic coupling and astrocytic networks
Interconnected networks of astrocytes play an important role in buffering ions and shuttling metabolites to shape neuronal activity. These networks enable neuron–astrocyte electrical coupling through the buffering of ions and neurotransmitters (Dossi et al., 2024; Zhong et al., 2023), which is critical for maintaining basal synaptic transmission (Wang et al., 2012) as well as tuning the signal-to-noise ratio of synaptic transmission to promote the synchronization of neuronal ensembles (Chever et al., 2016). They also facilitate the intercellular trafficking of nutrients for neuronal uptake after their transport across the blood-brain barrier (BBB), a structure formed by contacts between astrocytic endfeet and vascular endothelial cells (Abbott, 2002; Nortley and Attwell, 2017). This metabolic coupling between neurons and astrocytes is necessary to deliver essential metabolites to hippocampal neurons (Rouach et al., 2008), thereby supporting long-term memory formation (Suzuki et al., 2011).
Astrocytic networks are formed through gap junction coupling among adjacent astrocytes. The gap junction proteins that form these networks are connexin-30 and connexin-43 (Dermietzel, 1974; Masa and Mugnaini, 1982). Memory consolidation upregulates connexin-43 expression in the hippocampus (Choi et al., 2016), suggesting that these networks dynamically respond to neuronal activity and behavioral changes. Conditional knockout of one or both of these gap junction proteins specifically in astrocytes has revealed their role in maintaining basal synaptic transmission and facilitating learning and memory. Loss of gap junction coupling leads to extracellular accumulation of potassium ions and glutamate and abolishes the activity-dependent shuttling of glucose and its metabolites through astrocytic networks. These knockout models show invasion of astrocytic processes into synaptic clefts, alterations in basal synaptic transmission and LTP, and a reduction in memory formation (Hösli et al., 2022; Pannasch et al., 2014; Rouach et al., 2008; Theis et al., 2003; Wallraff et al., 2006). These results highlight the necessity of astrocytic networks in buffering ions and neurotransmitters and in trafficking metabolites to ensure the fidelity and metabolic support of hippocampal synaptic transmission toward learning and memory.
The development of astrocytic networks coincides with the coordinated buffering of sodium and potassium ions by astrocytes and neurons that characterizes syncytial isopotentiality (Zhong et al., 2023). These networks regulate neuronal synchronization and oscillations by modulating the balance between excitatory synaptic transmission and release probability. Generally, there is an inverse relationship between basal excitatory activity and synaptic release probability, a relationship that can be likened to a signal-to-noise ratio of synaptic transmission. Astrocytic networks help maintain this balance (Wang et al., 2012), promoting neuronal synchronization by reducing basal synaptic activity and thereby enhancing release probability (Chever et al., 2016). Mechanistically, this may be achieved through the buffering of extracellular potassium (Dossi et al., 2024). Together, these findings suggest that astrocytic networks are critical for maintaining and modulating the electrochemical gradient across neuronal membranes to shape the basal activity of neuronal circuits (Figure 2A).
Astrocytic networks also play a crucial role in brain metabolism. Metabolic coordination between neurons and astrocytes is essential to brain function. The coupling of glucose and lipid metabolism between these cell types allows astrocytes to sustain synaptic transmission and promote long-term memory formation through the shuttling of glucose and its metabolites, the buffering of potentially toxic lipid species generated by neuronal activity, and the provision of cholesterol to support synaptic plasticity (Ferris et al., 2017; Ioannou et al., 2019; Rouach et al., 2008; Suzuki et al., 2011). These neuronal activity-dependent metabolic functions are especially critical in the hippocampus; for instance, the delivery of glucose and its metabolites by astrocytic networks sustains neuronal synchronization and epileptiform events (Rouach et al., 2008), the transfer of the glycolytic metabolite lactate from hippocampal astrocytes to neurons is required for the formation of long-term memories (Gao et al., 2016; Suzuki et al., 2011), and defects in hippocampal astrocytic lipid metabolism are implicated in neurodegeneration (Qi et al., 2021).
4.1 Glucose metabolism and memory
Astrocytes are responsible for the uptake and transport of glucose and its metabolites from central circulation to neurons throughout the brain. Perivascular astrocytes contact blood vessels through their endfeet to form the BBB. These astrocytes are positioned for glucose uptake, which can subsequently be metabolized and trafficked intercellularly through astrocytic networks. Glucose transporters in astrocytic foot processes shuttle glucose across the BBB (Koepsell, 2020; Morgello et al., 1995), whereupon it can be released for neuronal uptake, transported through astrocytic networks to meet the energetic needs of distal neurons (Rouach et al., 2008), or stored intracellularly as glycogen (Figure 2B). In transit, glucose may be metabolized through aerobic glycolysis, the oxygen-dependent conversion of glucose into lactate. This mechanism depends on oxygen availability as well as astrocytic calcium concentration, which in turn regulates vasodilation (Gordon et al., 2008), indicating the intricacy with which astrocytes coordinate metabolism across the BBB. Glycolytic pathways can also metabolize glucose into L-serine, the precursor of D-serine, a critical transmitter released by astrocytes to regulate neuronal activity (Henneberger et al., 2010; Suzuki et al., 2015).
The astrocyte–neuron lactate shuttle hypothesis suggests that astrocytes take up glutamate in response to neuronal activity, which in turn stimulates aerobic glycolysis to sustain synaptic activity through the provision of lactate to neurons (Pellerin and Magistretti, 1994). While studies have suggested that this metabolic coupling underlies synaptic activity (Bittner et al., 2011; Pellerin and Magistretti, 1994; Pellerin et al., 2007), other evidence indicates that sustained neuronal activity relies on neuronal glycolysis rather than lactate uptake, as neurons exhibit a net export of lactate under stimulation (Díaz-García et al., 2017). Although the precise role of coordinated lactate metabolism among astrocytes and neurons in supporting basal synaptic activity remains unresolved, the transport of glucose and its metabolites through astrocytic networks in response to neuronal activity appears to be necessary to locally support synaptic transmission in the hippocampus (Rouach et al., 2008).
Beyond basal activity, evidence suggests that astrocyte–neuron lactate metabolism is critical for LTP and memory formation (Gao et al., 2016; Suzuki et al., 2011; Figure 2B). Astrocytic aerobic glycolysis can be elicited by neuronal activity, such as the release of glutamate (Pellerin and Magistretti, 1994), norepinephrine (Gao et al., 2016), or adenosine (Theparambil et al., 2024), promoting the transport of lactate from astrocytes to neurons to support LTP. The breakdown of astrocytic glycogen into lactate through glycogenolysis and its transport via monocarboxylate transporters (MCTs) for neuronal uptake is required for long-term memory formation in rats (Suzuki et al., 2011). MCT1 is primarily expressed by neurons, while MCT4 is enriched in astrocytes (Pierre and Pellerin, 2005). The expression of MCT1 is upregulated during memory encoding (Suzuki et al., 2011), indicating an increased capacity for neuronal lactate uptake. MCT1- or MCT4-deficient rodents exhibit impaired memory of aversive stimuli (Suzuki et al., 2011; Tadi et al., 2015), which is rescued by lactate administration when neuronal uptake is not disrupted (Suzuki et al., 2011). Furthermore, AD mouse models exhibit defects in hippocampal astrocytic glycolysis, which is restored by upregulating astrocytic glycolysis or administering glycolytic metabolites (Andersen et al., 2021; Dematteis et al., 2020; Le Douce et al., 2020; Minhas et al., 2024). This suggests that astrocytic glucose metabolism and the provision of lactate from astrocytes to neurons are specifically necessary for the formation of hippocampus-dependent long-term memory. Whether this lactate transport simply supports neurons metabolically or has an additional signaling function in the hippocampus remains to be determined.
4.2 Lipid metabolism and neurodegeneration
Astrocytes are critical in mediating brain cholesterol metabolism (Figure 2C). Developing neurons are especially rich in cholesterol (Zhang and Liu, 2015), which is essential for synaptogenesis (Fester et al., 2009; Goritz et al., 2005; Mauch et al., 2001), axonal guidance (de Chaves et al., 1997), and synaptic transmission (Linetti et al., 2010; Liu et al., 2010). Because it cannot cross the BBB, cholesterol is synthesized de novo in the brain, primarily in astrocytes by adulthood (Ferris et al., 2017; Fünfschilling et al., 2007; van Deijk et al., 2017). Disrupting astrocytic cholesterol biosynthesis results in significant reductions in hippocampal volume and deficits in spatial memory in wild-type mice and aggravates Aβ pathology in AD mice (Ferris et al., 2017; Wang et al., 2021). APP/PS1 mice also display disrupted cholesterol homeostasis in hippocampal astrocytes (Endo et al., 2022; Habib et al., 2020), suggesting that this metabolic coupling is essential for both the homeostatic and behavioral functions of the hippocampus.
Apolipoprotein E (ApoE), a principal cholesterol carrier, is expressed by astrocytes to facilitate cholesterol delivery to neurons (Wang and Eckel, 2014), where it plays a critical role in synapse formation, plasticity, and transmission (Hayashi et al., 2004; Holtzman et al., 1995; Mauch et al., 2001). Astrocytic ApoE also regulates cholesterol metabolism and epigenetic modifications in hippocampal neurons to facilitate memory formation (Li et al., 2021). ApoE4 expression in astrocytes disrupts cholesterol metabolism, resulting in reduced astrocytic cholesterol efflux. This suggests that the provision of cholesterol from astrocytes to neurons throughout life is essential to their function, and that deficits in astrocytic cholesterol metabolism contribute to the pathological consequences of ApoE4 expression (TCW et al., 2022).
Recent studies have proposed a model by which astrocytes are able to buffer reactive oxygen species in the central nervous system as well as metabolically support neurons. Because neurons are vulnerable to reactive oxygen species damage from fatty acid oxidation (Schönfeld and Reiser, 2017), neuronal fatty acids are often taken up by surrounding astrocytes in ApoE-enriched particles and incorporated into lipid droplets (LDs), organelles that store neutral lipids within a phospholipid monolayer. LDs provide intracellular energy stores and often form in response to oxidative stress to prevent the toxic peroxidation of cellular lipids (Ralhan et al., 2021). The capacity of astrocytes to buffer neuronal fatty acids protects neurons from oxidative stress during periods of high activity (Chen et al., 2023), a state in which lipids are more prone to peroxidation, which is reinforced by the initiation of a transcriptional program in LD-accumulated astrocytes to detoxify and metabolize these lipids. In turn, astrocytic LDs can be mobilized through lipolysis and β-oxidation to provide fuel to sustain synaptic transmission (Ioannou et al., 2019; Figure 2D).
Defects in astrocytic lipid metabolism may also contribute to neurodegeneration. Lipid accumulation in the central nervous system has been associated with neurodegeneration since the first characterization of AD over a century ago (Stelzmann et al., 1995). Analysis of both postmortem human AD brains and mouse models has revealed that lipid accumulation is a pathological feature of AD and precedes Aβ aggregation and neurofibrillary tangle formation (Hamilton et al., 2015). The transfer of potentially toxic lipids from neurons to surrounding glia can be mediated by ApoE (Ioannou et al., 2019; Liu et al., 2017). The ApoE4 allele represents the strongest genetic risk factor for AD (Corder et al., 1993; Strittmatter et al., 1993) and is associated with hippocampal atrophy (Mishra et al., 2018; Shi et al., 2017). Astrocytes expressing ApoE4 exhibit various cellular dysfunctions, including disrupted fatty acid and sterol metabolism and efflux, triglyceride and reactive oxygen species accumulation, impaired mitochondrial function, reduced autophagic flux, impaired blood-brain barrier maintenance, and reduced support of hippocampal neurite growth (de Leeuw et al., 2022; Farmer et al., 2019; Jackson et al., 2022; Lee et al., 2023; Rawat et al., 2019; Sienski et al., 2021; Sun et al., 1998). Mechanistically, the pathological effects conferred by ApoE4 may be due, at least in part, to disrupted lipid transfer between neurons and glia (Ioannou et al., 2019; Liu et al., 2017; Qi et al., 2021), resulting in reduced neuronal lipid sequestration and astrocytic lipid utilization (Qi et al., 2021), as well as defective astrocytic cholesterol metabolism (TCW et al., 2022).
Overall, astrocytic glucose and lipid metabolism are critical for supporting the essential functions of the hippocampus. Glucose metabolism in the brain is profoundly reliant on astrocytic networks, which facilitate the neuronal activity-dependent flow of nutrients to support synaptic transmission. The sequestration of potentially toxic fatty acids and the efflux of cholesterol are critical mechanisms by which astrocytes support neuronal activity and have broad implications for the molecular basis of neurodegeneration.
5 Astrocytic encoding of neurotransmitters and gliotransmission
The metabolic and signaling functions of astrocytes are often complementary. For instance, the chemogenetic activation of CA1 astrocytes increases glucose metabolism specifically in the hippocampus (Ardanaz et al., 2024). Moreover, D-serine can be derived from glycolysis (Suzuki et al., 2015) and exhibits calcium-dependent release from astrocytes (Henneberger et al., 2010), thereby regulating neuronal activity. These essential astrocytic functions are closely interwoven by complex molecular crosstalk between astrocytes and neurons. Exploring the nature of this bidirectional communication is necessary to gain a unified view of astrocytic function and a comprehensive understanding of the hippocampus.
Astrocytes play a crucial role in supporting synaptic transmission by transporting and metabolizing neurotransmitters. These cells support both glutamatergic and GABAergic neuronal activity through the glutamate–glutamine cycle, a process essential for maintaining synaptic transmission. In this cycle, astrocytes produce glutamine from glucose, which is transported to neurons and converted into glutamate or GABA for presynaptic exocytosis. Following neurotransmission, glutamate and GABA are subsequently taken up by astrocytes from the synaptic cleft and metabolized to glutamine, allowing it to re-enter the cycle (Rothman et al., 2011). This process is necessary to maintain both excitatory (Tani et al., 2014) and inhibitory (Liang et al., 2006) synaptic transmission.
Beyond supporting neuronal activity through the metabolic flux of transmitters, astrocytes also express receptors for a variety of neurotransmitters and neuromodulators, including glutamate, GABA, acetylcholine, norepinephrine, adenosine triphosphate (ATP)/adenosine, and endocannabinoids. In turn, they are also able to release transmitters such as glutamate, GABA, ATP/adenosine, and D-serine through gliotransmission—the process by which astrocytes release transmitters to regulate synaptic activity (Araque et al., 2014)—to modulate synaptic transmission.
The specific expression of a dominant-negative form of soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) in astrocytes to disrupt SNARE-mediated exocytosis—and thus gliotransmission—has demonstrated the importance of this process in maintaining hippocampal function. These studies have revealed the critical role that gliotransmission plays in astrocytic morphology (Viana et al., 2023), dendritic spine maturation (Sultan et al., 2015), hippocampal LTP (Abreu et al., 2023), neuronal coordination (Pascual et al., 2005; Sardinha et al., 2017), memory formation (Lee et al., 2014; Sardinha et al., 2017), and sleep homeostasis (Florian et al., 2011; Halassa et al., 2009). Here, we review some of the key transmitters encoded and released by astrocytes to facilitate the homeostatic and behavioral functions of the hippocampus.
5.1 Glutamate
Glutamate is the major excitatory neurotransmitter in the central nervous system. Hippocampal astrocytes express glutamate transporters (Huang et al., 2004) to modulate synaptic transmission through the electrogenic uptake of glutamate (Bergles and Jahr, 1997). This prevents excitotoxicity as well as neurotransmitter spillover, ensuring the specificity of synaptic transmission (Rothstein et al., 1996). Hippocampal astrocytes also express both ionotropic glutamate receptors (e.g., AMPARs, NMDARs, and kainic acid receptors) and metabotropic receptors, including mGluR1 and mGluR5 (Fan et al., 1999; Porter and McCarthy, 1995; Porter and McCarthy, 1996; Shelton and McCarthy, 1999). In the SC–CA1 region, astrocytic AMPAR activation inhibits potassium channels (Schröder et al., 2002) and facilitates the intercellular trafficking of glutamate across astrocytic networks to refine neuronal activity (Hösli et al., 2022; Rouach et al., 2008). NMDAR expression allows astrocytes to modulate presynaptic inputs to the CA1 (Chipman et al., 2021; Letellier et al., 2016). Furthermore, neuronal activity elicits astrocytic calcium activity through mGluR activation, which is required for the ensheathment of synapses by PAPs following LTP induction (Bernardinelli et al., 2014; Bowser and Khakh, 2004; Panatier et al., 2011; Porter and McCarthy, 1996; Rungta et al., 2016; Tang et al., 2015). Astrocytes are also capable of releasing glutamate downstream of calcium signaling as a gliotransmitter in response to neuronal activity. Recent evidence indicates that this ability to release glutamate is limited to a subset of astrocytes. In the hippocampus, these glutamate-releasing astrocytes are differentially distributed along the dorsoventral axis, with the highest proportion in the DG molecular layer, indicating regional diversity of gliotransmission (de Ceglia et al., 2023). This glutamate release can enhance LTP at SC–CA1 synapses (Adamsky et al., 2018; Perea and Araque, 2007). Furthermore, the specific deletion of vesicular glutamate transporter 1 in astrocytes diminishes contextual fear memory by disrupting glutamatergic gliotransmission in cortico-hippocampal circuitry (de Ceglia et al., 2023). These results suggest that glutamate release from astrocytes is sufficient to facilitate LTP in the hippocampus and that vesicular glutamate transporter 1-expressing astrocytes are specifically necessary for the consolidation of memories into extra-hippocampal areas.
5.2 GABA
GABA is the major inhibitory neurotransmitter in the central nervous system. GABAergic inputs impinge on nearly all excitatory synapses in the hippocampus. Astrocytes express both ionotropic (GABAA) and metabotropic (GABAB) receptors (Liu et al., 2022). GABAB receptors allow these cells to respond to GABAergic neuronal activity to promote oscillatory hippocampal activity via glutamate release (Perea et al., 2016) or synaptic depression at SC–CA1 synapses via ATP/adenosine release (Serrano et al., 2006). Astrocytes are also capable of releasing GABA as a gliotransmitter (Gaidin et al., 2020; Le Meur et al., 2012) to diminish neuronal excitability (Gaidin et al., 2020). This process may be relevant in neurodegeneration, as multiple AD mouse models exhibit excessive GABA production and release from hippocampal astrocytes, which promotes tonic inhibition in the hippocampus through presynaptic GABA receptors, likely to compensate for synaptic hyperactivity. This GABA release impairs synaptic plasticity and memory formation, and suppressing this signaling restores memory deficits in these mice (Aldabbagh et al., 2022; Bhalla et al., 2025; Jo et al., 2014; Portal et al., 2024).
5.3 ATP/adenosine
ATP and its metabolite adenosine are neuromodulators closely linked to brain metabolism. Adenosine, the hydrolytic product of ATP, accumulates extracellularly during neuronal activity (Blutstein and Haydon, 2013; Dunwiddie et al., 1997). ATP released from SC–CA1 synapses drives astrocytic calcium signals through the activation of P2 purinergic receptors (Tang et al., 2015) as well as the cyclic AMP–protein kinase A signaling pathway (Theparambil et al., 2024). This latter pathway, downstream of adenosine A2B receptor activation, elicits lactate release from astrocytes to facilitate neuronal activity and LTP. The knockdown of this receptor in astrocytes downregulates aerobic glycolysis and diminishes LTP induction, which is only partially rescued by lactate administration, indicating that the signaling pathway likely employs additional mechanisms beyond lactate release to promote LTP. This A2B receptor activation in astrocytes is necessary for hippocampus-dependent memory formation and maintains sleep homeostasis by promoting delta-range neuronal activity during slow-wave sleep (Theparambil et al., 2024). This astrocytic encoding of purinergic neurotransmission may be critical in neurodegeneration, as APP/PS1 astrocytes aberrantly express adenosine A2A receptors, impairing memory formation (Orr et al., 2015). ATP/adenosine also acts as a gliotransmitter to regulate both glutamatergic and GABAergic synaptic transmission. In the CA1 region, astrocytic ATP/adenosine release and presynaptic A2A receptor activation modulate basal glutamate release (Panatier et al., 2011), while presynaptic A1 receptor activity facilitates the GABAergic induction of hetero-synaptic depression (Pascual et al., 2005; Serrano et al., 2006) and NMDAR-dependent spike timing-dependent LTD (Falcón-Moya et al., 2020; Pérez-Rodríguez et al., 2019). This signaling may be critical to maintaining sleep homeostasis, as ATP/adenosine release by astrocytes and its action on neuronal adenosine A1 receptors is necessary for both the accumulation of sleep pressure and the cognitive deficits associated with sleep deprivation in mice (Florian et al., 2011; Halassa et al., 2009).
5.4 D-serine
D-serine is a gliotransmitter and an NMDAR co-agonist; ligand binding to the NMDAR co-agonist site is critical for its function (Henneberger et al., 2010; Panatier et al., 2006; Papouin et al., 2012; Yang et al., 2003), as well as for LTP (Abreu et al., 2023; Henneberger et al., 2010; Yang et al., 2003) and LTD (Koh et al., 2022) induction. Studies in mice expressing a dominant-negative form of SNARE, which disrupts gliotransmitter release from astrocytes, have elucidated the significance of this astrocytic signaling. In the hippocampus, this abolishment leads to deficits in neuronal dendritic spine maturation (Sultan et al., 2015; Viana et al., 2023), diminished LTP at SC–CA1 synapses (Abreu et al., 2023), and disrupted coordination of cortico-hippocampal circuitry toward the formation of spatial memory (Sardinha et al., 2017). These effects are rescued by the administration of D-serine, which promotes the morphological development (Sultan et al., 2015; Viana et al., 2023) and integration (Sultan et al., 2015) of neuronal dendrites, reduces basal synaptic transmission to enhance LTP (Abreu et al., 2023), and promotes theta-phase oscillatory activity between the hippocampus and prefrontal cortex to facilitate spatial memory (Sardinha et al., 2017). These results suggest that D-serine, as a gliotransmitter, is essential for hippocampal functions from the molecular to the behavioral level. D-serine release can also be indirectly dependent on astrocytic glycolysis (Fernández-Moncada et al., 2024; Le Douce et al., 2020), a metabolic pathway likely relevant in neurodegeneration, as impaired glycolysis in AD mouse models leads to a reduction in D-serine levels concomitant with spatial memory deficits, which are rescued by D-serine administration (Le Douce et al., 2020).
5.5 Acetylcholine
Acetylcholine is a neuromodulator crucial for the acquisition of hippocampus-dependent memory. Cholinergic afferents from the basal forebrain form part of the reticular activating system, modulating arousal and acting as a neurochemical correlate of wakefulness and vigilance (Zaborszky et al., 2008). Acetylcholine can elicit calcium activity in hippocampal astrocytes through binding nicotinic (Sharma and Vijayaraghavan, 2001) or muscarinic (Araque et al., 2002; Navarrete et al., 2012) cholinergic receptors. This signaling from the basal forebrain through hippocampal astrocytes can induce LTP at SC–CA1 synapses, dependent on presynaptic mGluR activity (Navarrete et al., 2012). Studies have elucidated the behavioral consequences of this signaling, suggesting that D-serine acts as the downstream mechanism through which cholinergic afferents mediate hippocampal function through astrocytes (Ma et al., 2023; Pabst et al., 2016; Papouin et al., 2017). Septal cholinergic signaling elicits nicotinic cholinergic receptor activation and calcium-dependent D-serine release from hippocampal astrocytes to regulate NMDAR activation, which activates hilar interneurons in the perforant pathway–DG to inhibit dentate gyrus granule cells (Pabst et al., 2016). In the direct pathway–CA1, this signaling promotes the formation of temporally-associated memory (Ma et al., 2023; Figure 1E). At SC–CA1 synapses, this cholinergic-mediated astrocytic activity modulates hippocampus-dependent memory formation across the circadian cycle (Papouin et al., 2017).
5.6 Norepinephrine
Norepinephrine is a catecholamine primarily released by the locus coeruleus and modulates the vigilance state of the animal (Levitt and Moore, 1978). Projections from the locus coeruleus reach many areas of the brain, including the hippocampus. Hippocampal astrocytes respond to norepinephrine with calcium transients (Duffy and MacVicar, 1995) through their expression of β2-adrenergic receptors (Gao et al., 2016) and α1-adrenergic receptors (Duffy and MacVicar, 1995). The binding of norepinephrine to β2-adrenergic receptors allows astrocytes to respond to noradrenergic activity with the release of lactate to support neurons during hippocampus-dependent memory formation (Gao et al., 2016). Astrocytes thus regulate synaptic activity according to vigilance state and mediate the noradrenergic modulation of hippocampal function (Gao et al., 2016; Koh et al., 2022). This mechanism highlights the close relationship between the signaling and metabolic functions of astrocytes in facilitating neuronal activity during memory formation. In addition, astrocytic α1-adrenergic receptor activation elicits calcium activity and ATP/adenosine release at SC–CA1 synapses, which suppresses synaptic activity through the activation of presynaptic adenosine A1 receptors (Lefton et al., 2025), suggesting that noradrenergic modulation of hippocampal synapses is mediated by astrocytic purinergic signaling. Astrocytes may also play a role in integrating noradrenergic afferents to the hippocampus. Noradrenergic projections from the locus coeruleus activate α1-adrenergic receptors on astrocytes to elicit global astrocytic calcium activity that propagates toward the soma as a mouse approaches a reward. This calcium activity likely reflects past events, such as changes in neuronal activity, locomotion, and pupil dilation stimulated by noradrenergic activity. Furthermore, this somatic calcium activity occurs across a longer timescale than the neuronal activity it may encode (Rupprecht et al., 2024). The implications of this encoding remain obscure, but it positions hippocampal astrocytes as slow integrators of noradrenergic afferents.
5.7 Endocannabinoids
Endocannabinoids are a class of neuromodulators that mediate synaptic plasticity in the hippocampus (Izumi and Zorumski, 2012; Ohno-Shosaku et al., 2007; Zhu and Lovinger, 2007). The expression of cannabinoid receptor 1 (CB1R) in astrocytes is necessary to maintain synchronized neuronal activity in the hippocampus during epileptiform events (Coiret et al., 2012), indicating that this signaling underlies a mechanism by which astrocytes coordinate neuronal circuits. Furthermore, the activation of astrocytic CB1Rs also upregulates lactate metabolism, thereby promoting D-serine synthesis. Mice lacking astrocytic CB1R exhibit impaired hippocampus-dependent memory, which is rescued by D-serine administration or upregulation of the glycolytic pathway producing this gliotransmitter (Fernández-Moncada et al., 2024). Dendritic spiking—an action potential generated in a dendrite—of CA1 pyramidal neurons is critical in encoding spatial information. This activity is elicited by theta-range pyramidal neuronal activity in the CA1 region, the co-occurrence of which is critical for the formation of spatial memory (Epsztein et al., 2011; Harvey et al., 2009). Pyramidal cell activity in the theta range occurs during spatial exploration and activates CB1Rs on astrocytes, which exhibit calcium transients and release D-serine to lower the threshold of dendritic spiking; this astrocytic signaling is necessary for the formation of spatial memory (Bohmbach et al., 2022). In this manner, astrocytes may provide the mechanistic link between theta-range activity in pyramidal cells and the potentiation of dendritic spiking to facilitate the formation of spatial memory.
6 Discussion
To fully understand hippocampal function, it is increasingly apparent that astrocytes must be considered for their role in the active encoding, integration, and modulation of information across synapses in this region. Here, we have summarized the circuit-specific roles of astrocytes, which depend both on the fine-tuning of single synapses in their cellular domains and on the coordination and metabolic support of neuronal circuits through astrocytic networks. These dual perspectives are intricately coordinated by complex molecular crosstalk between astrocytes and neurons through the processes of neurotransmission and gliotransmission.
Hippocampal astrocytes respond dynamically to the spatiotemporal encoding of experience by hippocampal neurons, the energetic needs of local circuitry, and the broader contexts of brain state, such as vigilance state, to shape behavioral outcomes. These processes converge on the precise, plastic control of synaptic transmission to support the functions of the hippocampus as a scaffold for memory, underpinning synaptic plasticity and memory formation in this region, while also facilitating cortico-hippocampal communication to mediate the long-term consolidation and generalization of memory.
Understanding the roles of astrocytes in the acquisition and consolidation of hippocampus-dependent memory is expected to deepen our understanding of the behavioral functions of this brain region. Determining the implications of the diverse array of cellular signaling and its integration with metabolism, both centrally and peripherally, in hippocampal astrocytes will be key to elucidating how these cells encode and modulate the flow of information across synapses. This aim may yield invaluable insights into the cellular mechanisms of memory formation.
Overall, astrocytes are indispensable for hippocampal function, both locally and globally. We hope that the conceptual framework of their circuit-dependent roles provided here will foster clarity for future research, wherein a more comprehensive understanding of the hippocampus may be achieved through investigations into the dynamics of astrocytic activity in this brain area. For instance, identifying the effectors downstream of calcium signaling that facilitate sleep homeostasis and memory formation, clarifying the implications of cellular signaling beyond calcium, defining the precise role of neuron–astrocyte metabolic coupling in learning and memory, and establishing whether astrocytes contribute to the allocation of sparse neuronal populations to an engram all represent frontier areas. Extending the lines of investigation outlined in this review holds promise for advancing a deeper understanding of the behavioral functions of the hippocampus.
Author contributions
AS: Writing – original draft, Writing – review & editing. JP: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by National Institutes of Health (NIH) R01 AG089566, NIH R21 AG083760, Chan Zuckerberg Initiative Neurodegeneration Challenge Network Collaborative Pairs Pilot Grant, Alzheimer’s Association Research Grant 23AARG-1026776, and NARSAD Young Investigator Grant (Frederick and Alice Coles and Thomas and Nancy Coles Investigator).
Acknowledgments
We thank Rodrigo Andrade and Jeeyun Chung for providing valuable input. We also thank members of the Park lab for helpful discussions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CA1, cornu ammonis 1; DG, dentate gyrus; SC, Schaffer collateral; PAP, perisynaptic astrocytic process; LTP, long-term potentiation; LTD, long-term depression; STDP, spike timing-dependent plasticity; NMDAR, N-methyl-D-aspartate receptor; AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; mGluR, metabotropic glutamate receptor; IP3, inositol trisphosphate; GPCR, G protein-coupled receptor; GABA, γ-aminobutyric acid; ACC, anterior cingulate cortex; NREM, non-rapid eye movement; REM, rapid eye movement; AD, Alzheimer’s disease; Aβ, β-amyloid; APP, amyloid precursor protein; PS1, presenilin 1; BBB, blood-brain barrier; MCT, monocarboxylate transporter; ApoE, apolipoprotein E; LD, lipid droplet; ATP, adenosine triphosphate; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor; CB1R, cannabinoid receptor 1.
References
Abbott, N. J. (2002). Astrocyte–endothelial interactions and blood–brain barrier permeability. J. Anat. 200, 629–638. doi: 10.1046/j.1469-7580.2002.00064.x
Abreu, D. S., Gomes, J. I., Ribeiro, F. F., Diógenes, M. J., Sebastião, A. M., and Vaz, S. H. (2023). Astrocytes control hippocampal synaptic plasticity through the vesicular-dependent release of D-serine. Front. Cell Neurosci. 17:1282841. doi: 10.3389/fncel.2023.1282841
Adamsky, A., Kol, A., Kreisel, T., Doron, A., Ozeri-Engelhard, N., Melcer, T., et al. (2018). Astrocytic activation generates de novo neuronal potentiation and memory enhancement. Cell 174, 59–71.e14. doi: 10.1016/j.cell.2018.05.002.
Agarwal, A., Wu, P.-H., Hughes, E. G., Fukaya, M., Tischfield, M. A., Langseth, A. J., et al. (2017). Transient opening of the mitochondrial permeability transition pore induces micorodomain calcium transients in astrocyte processes. Neuron 92, 587–605.e7. doi: 10.1016/j.neuron.2016.12.034.
Agulhon, C., Fiacco, T. A., and McCarthy, K. D. (2010). Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 327, 1250–1254. doi: 10.1126/science.1184821
Aldabbagh, Y., Islam, A., Zhang, W., Whiting, P., and Ali, A. B. (2022). Alzheimer’s disease enhanced tonic inhibition is correlated with upregulated astrocyte GABA transporter-3/4 in a knock-in APP mouse model. Front. Pharmacol. 13:822499. doi: 10.3389/fphar.2022.822499
Andersen, J. V., Skotte, N. H., Christensen, S. K., Polli, F. S., Shabani, M., Markussen, K. H., et al. (2021). Hippocampal disruptions of synaptic and astrocyte metabolism are primary events of early amyloid pathology in the 5xFAD mouse model of Alzheimer’s disease. Cell Death Dis. 12:954. doi: 10.1038/s41419-021-04237-y
Araque, A., Carmignoto, G., Haydon, P. G., Oliet, S. H. R., Robitaille, R., and Volterra, A. (2014). Gliotransmitters travel in time and space. Neuron 81, 728–739. doi: 10.1016/j.neuron.2014.02.007
Araque, A., Martín, E. D., Perea, G., Arellano, J. I., and Buño, W. (2002). Synaptically released acetylcholine evokes Ca2+ elevations in astrocytes in hippocampal slices. J. Neurosci. 22, 2443–2450. doi: 10.1523/JNEUROSCI.22-07-02443.2002
Ardanaz, C. G., De La Cruz, A., Minhas, P. S., Hernández-Martín, N., Pozo, M. Á,Valdecantos, M. P., et al. (2024). Astrocytic GLUT1 reduction paradoxically improves central and peripheral glucose homeostasis. Sci. Adv. 10:ead1115. doi: 10.1126/sciadv.adp1115
Arizono, M., Inavalli, V. V. G. K., Panatier, A., Pfeiffer, T., Angibaud, J., Levet, F., et al. (2020). Structural basis of astrocytic Ca2+ signals at tripartite synapses. Nat. Commun. 11:1906. doi: 10.1038/s41467-020-15648-4
Armbruster, M., Naskar, S., Garcia, J. P., Sommer, M., Kim, E., Adam, Y., et al. (2022). Neuronal activity drives pathway-specific depolarization of peripheral astrocyte processes. Nat. Neurosci. 25, 607–616. doi: 10.1038/s41593-022-01049-x
Aten, S., Kiyoshi, C. M., Arzola, E. P., Patterson, J. A., Taylor, A. T., Du, Y., et al. (2022). Ultrastructural view of astrocyte arborization, astrocyte-astrocyte and astrtrocyte-synapse contacts, intracellular vesicle-like structures, and mitochondrial network. Prog. Neurobiol. 213:102264. doi: 10.1016/j.pneurobio.2022.102264
Basu, J., and Siegelbaum, S. A. (2015). The corticohippocampal circuit, synaptic plasticity, and memory. Cold Spring Harb. Perspect. Biol. 7:a021733. doi: 10.1101/cshperspect.a021733
Beattie, E. C., Carroll, R. C., Yu, X., Morishita, W., Yasuda, H., Von Zastrow, M., et al. (2000). Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat. Neurosci. 3, 1291–1300. doi: 10.1038/81823
Bellot-Saez, A., Kékesi, O., Morley, J. W., and Buskila, Y. (2017). Astrocytic modulation of neuronal excitability through K+ spatial buffering. Neurosci. Biobehav. Rev. 77, 87–97. doi: 10.1016/j.neubiorev.2017.03.002
Benitez, M. J., Retana, D., Ordoñez-Gutiérrez, L., Colmena, I., Goméz, M. J., Álvarez, R., et al. (2024). Transcriptomic alterations in APP/PS1 mice astrocytes lead to early postnatal axon initial segment structural changes. Cell Mol. Life Sci. 81:444. doi: 10.1007/s00018-024-05485-9
Bergles, D. E., and Jahr, C. E. (1997). Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron 19, 1297–1308. doi: 10.1016/S0896-6273(00)80420-1
Bernardinelli, Y., Randall, J., Janett, E., Nikonenko, I., König, S., Jones, E. V., et al. (2014). Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr. Biol. 24, 1679–1688. doi: 10.1016/j.cub.2014.06.025
Bernardinelli, Y., Salmon, C., Jones, E. V., Farmer, W. T., Stellwagen, D., and Murai, K. K. (2011). Astrocytes display complex and localized calcium responses to single-neuron stimulation in the hippocampus. J. Neurosci. 31, 8905–8919. doi: 10.1523/JNEUROSCI.6341-10.2011
Bhalla, M., Joo, J., Kim, D., Shin, J. I., Park, Y. M., Ju, Y. H., et al. (2025). SIRT2 and ALDH1A1 as critical enzymes for astrocytic GABA production and Alzheimer’s disease. Mol. Neurodegeneration 20:6. doi: 10.1186/s13024-024-00788-8
Bindocci, E., Savtchouk, I., Liaudet, N., Becker, D., Carriero, G., and Volterra, A. (2017). Three-dimensional Ca2+ imaging advances understanding of astrocyte biology. Science 356:eaai8185. doi: 10.1126/science.aai8185
Bittner, C. X., Valdebenito, R., Ruminot, I., Loaiza, A., Larenas, V., Sotelo-Hitschfeld, T., et al. (2011). Fast and reversible stimulation of astrocytic glycolysis by K+ and a delayed and persistent effect of glutamate. J. Neurosci. 31, 4709–4713. doi: 10.1523/JNEUROSCI.5311-10.2011
Bittner, K. C., Milstein, A. D., Sandro-Romani, C. G., and Magee, J. C. (2017). Behavioral time scale synaptic plasticity underlies CA1 place fields. Science 357, 1033–1036. doi: 10.1126/science.aan3846
Bliss, T. V., and Lømo, T. (1973). Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 232, 331–356. doi: 10.1113/jphysiol.1973.sp010273
Blutstein, T., and Haydon, P. G. (2013). The importance of astrocyte-derived purines in the modulation of sleep. Glia 61, 129–139. doi: 10.1002/glia.22422
Bódizs, R., Kántor, S., Szabó, G., Szûcs, A., Erõss, L., and Halász, P. (2001). Rhythmic hippocampal slow oscillation characterizes REM sleep in humans. Hippocampus 11, 747–753. doi: 10.1002/hipo.1090
Bohmbach, K., Masala, N., Schönhense, E. M., Hill, K., Haubrich, A. N., Zimmer, A., et al. (2022). An astrocytic signaling loop for frequency-dependent control of dendritic integration and spatial learning. Nat. Commun. 13:7932. doi: 10.1038/s41467-022-35620-8
Bonvento, G., and Bolaños, J. P. (2021). Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metabol. 33, 1546–1564. doi: 10.1016/j.cmet.2021.07.006
Bosson, A., Paumier, A., Boisseau, S., Jacquier-Sarlin, M., Buisson, A., and Albrieux, M. (2017). TRPA1 channels promote astrocytic Ca2+ hyperactivity and synaptic dysfunction mediated by oligomeric forms of amyloid-β peptide. Mol. Neurodegenerat. 12:53. doi: 10.1186/s13024-017-0194-8
Bowser, D. N., and Khakh, B. S. (2004). ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks. J. Neurosci. 24, 8606–8620. doi: 10.1523/JNEUROSCI.2660-04.2004
Bushong, E. A., Martone, M. E., and Ellisman, M. H. (2004). Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. Int. J. Dev. Neurosci. 22, 73–86. doi: 10.1016/j.ijdevneu.2003.12.008
Cabral, H. O., Vinck, M., Fouquet, C., Pennartz, C. M. A., Rondi-Reig, L., and Battaglia, F. P. (2014). Oscillatory dynamics and place field maps reflect hippocampal ensemble processing of sequence and place memory under NMDA receptor control. Neuron 81, 402–415. doi: 10.1016/j.neuron.2013.11.010
Carr, M. F., Jadhav, S. P., and Frank, L. M. (2011). Hippocampal replay in the awake state: A potential substrate for memory consolidation and retrieval. Nat. Neurosci. 14, 147–153. doi: 10.1038/nn.2732
Carvalho, D., Diaz-Amarilla, P., Dapueto, R., Santi, M. D., Duarte, P., Savio, E., et al. (2023). Transcriptomic analyses of neurotoxic astrocytes derived from adult triple transgenic Alzheimer’s disease mice. J. Mol. Neurosci. 73, 487–515. doi: 10.1007/s12031-023-02105-2
Chen, Z.-P., Wang, S., Zhao, X., Fang, W., Wang, Z., Ye, H., et al. (2023). Lipid-accumulated reactive astrocytes promote disease progression in epilepsy. Nat. Neurosci. 26, 542–554. doi: 10.1038/s41593-023-01288-6
Chever, O., Dossi, E., Pannasch, U., Derangeon, M., and Rouach, N. (2016). Astroglial networks promote neuronal coordination. Sci. Signal. 9:ra6. doi: 10.1126/scisignal.aad3066
Chipman, P. H., Fung, C. C. A., Fernandez, A. P., Sawant, A., Tedoldi, A., Kawai, A., et al. (2021). Astrocyte GluN2C NMDA receptors control basal synaptic strengths of hippocampal CA1 pyramidal neurons in the stratum radiatum. eLife 10:e70818. doi: 10.7554/eLife.70818
Choi, M., Ahn, S., Yang, E.-J., Kim, H., Chong, Y. H., and Kim, H.-S. (2016). Hippocampus-based contextual memory alters the morphological characteristics of astrocytes in the dentate gyrus. Mol. Brain 9:72. doi: 10.1186/s13041-016-0253-z
Christopherson, K. S., Ullian, E. M., Stokes, C. C. A., Mullowney, C. E., Hell, J. W., Agah, A., et al. (2005). Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120, 421–433. doi: 10.1016/j.cell.2004.12.020
Coiret, G., Ster, J., Grewe, B., Wendling, F., Helmchen, F., Gerber, U., et al. (2012). Neuron to astrocyte communication via cannabinoid receptors is necessary for sustained epileptiform activity in rat hippocampus. PLoS One 7:e37320. doi: 10.1371/journal.pone.0037320
Collingridge, G. L., Kehl, S. J., and McLennan, H. (1983). Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J. Physiol. 334, 33–46. doi: 10.1113/jphysiol.1983.sp014478
Corder, E. H., Saunders, A. M., Strittmatter, W. J., Schmechel, D. E., Gaskell, P. C., Small, G. W., et al. (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923. doi: 10.1126/science.8346443
Cornell-Bell, A. H., Thomas, P. G., and Smith, S. J. (1990). The excitatory neurotransmitter glutamate causes filopodia formation in cultured hippocampal astrocytes. Glia 3, 322–334. doi: 10.1002/glia.440030503
Curreli, S., Bonato, J., Romanzi, S., Panzeri, S., and Fellin, T. (2022). Complementary encoding of spatial information in hippocampal astrocytes. PLoS Biol. 20:e3001530. doi: 10.1371/journal.pbio.3001530
Dani, J. W., Chernjavsky, A., and Smith, S. J. (1992). Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron 8, 429–440. doi: 10.1016/0896-6273(92)90271-E
de Ceglia, R., Ledonne, A., Litvin, D. G., Lind, B. L., Carriero, G., Latagliata, E. C., et al. (2023). Specialized astrocytes mediate glutamatergic gliotransmission in the CNS. Nature 622, 120–129. doi: 10.1038/s41586-023-06502-w
de Chaves, E. I. P., Rusiñol, A. E., Vance, D. E., Campenot, R. B., and Vance, J. E. (1997). Role of lipoproteins in the delivery of lipids to axons during axonal regeneration. J. Biol. Chem. 272, 30766–30773. doi: 10.1074/jbc.272.49.30766
de Leeuw, S. M., Kirschner, A. W. T., Lindner, K., Rust, R., Budny, V., Wolski, W. E., et al. (2022). APOE2, E3, and E4 differentially modulate cellular homeostasis, cholesterol metabolism, and inflammatory response in isogenic iPSC-derived astrocytes. Stem Cell Rep. 17, 110–126. doi: 10.1016/j.stemcr.2021.11.007
Dematteis, G., Gong, C., Malecka, J., Tonelli, E., Genazzani, A., Tapella, L., et al. (2025). Rescue of protein dyshomeostasis in hippocampal astrocytes from an Alzheimer’s disease mouse model by stabilizing ER-mitochondrial interactions at a 20 nm distance. Alz. Res. Therapy 17:148. doi: 10.1186/s13195-025-01793-9
Dematteis, G., Vydmantaité, G., Ruffinatti, F. A., Chahin, M., Farruggio, S., Barberis, E., et al. (2020). Proteomic analysis links alterations of bioenergetics, mitochondria-ER interactions and proteostasis in hippocampal astrocytes from 3xTg-AD mice. Cell Death Dis. 11:645. doi: 10.1038/s41419-020-02911-1
Dermietzel, R. (1974). Junctions in the central nervous system of the cat: III. Gap junctions and membrane-associated orthogonal particle complexes (MOPC) in astrocytic membranes. Cell Tissue Res. 149, 121–135. doi: 10.1007/BF00209055
Di Castro, M. A., Chuquet, J., Liaudet, N., Bhaukaurally, K., Santello, M., Bouvier, D., et al. (2011). Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat. Neurosci. 14, 1276–1284. doi: 10.1038/nn.2929
Díaz-García, C. M., Mongeon, R., Lahmann, C., Koveal, D., Zucker, H., and Yellen, G. (2017). Neuronal stimulation triggers neuronal glycolysis and not lactate uptake. Cell Metabol. 26, 361–374.e4. doi: 10.1016/j.cmet.2017.06.021.
Dong, Z., Gong, B., Li, H., Bai, Y., Wu, X., Huang, Y., et al. (2012). Mechanisms of hippocampal long-term depression are required for memory enhancement by novelty exploration. J. Neurosci. 32, 11980–11990. doi: 10.1523/JNEUROSCI.0984-12.2012
Doron, A., Rubin, A., Benmelech-Chovav, A., Benaim, N., Carmi, T., Refaeli, R., et al. (2022). Hippocampal astrocytes encode reward location. Nature 609, 772–778. doi: 10.1038/s41586-022-05146-6
Dossi, E., Zonca, L., Pivonkova, H., Milior, G., Moulard, J., Vargova, L., et al. (2024). Astroglial gap junctions strengthen hippocampal network activity by sustaining afterhyperpolarization via KCNQ channels. Cell Rep. 43:114158. doi: 10.1016/j.celrep.2024.114158
Dudek, S. M., and Bear, M. F. (1992). Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc. Natl. Acad. Sci. U. S. A. 89, 4363–4367. doi: 10.1073/pnas.89.10.4363
Duffy, S., and MacVicar, B. (1995). Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. J. Neurosci. 15, 5535–5550. doi: 10.1523/JNEUROSCI.15-08-05535.1995
Dunwiddie, T. V., Diao, L., and Proctor, W. R. (1997). Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J. Neurosci. 17, 7673–7682. doi: 10.1523/JNEUROSCI.17-20-07673.1997
Durkee, C. A., Covelo, A., Lines, J., Kofuji, P., Aguilar, J., and Araque, A. (2019). Gi/o protein-coupled receptors inhibit neurons but activate astrocytes and stimulate gliotransmission. Glia 67, 1076–1093. doi: 10.1002/glia.23589
Endo, F., Kasai, A., Soto, J. S., Yu, X., Qu, Z., Hashimoto, H., et al. (2022). Molecular basis of astrocyte diversity and morphology across the CNS in health and disease. Science 378:eadc9020. doi: 10.1126/science.adc9020
Epsztein, J., Brecht, M., and Lee, A. K. (2011). Intracellular determinants of hippocampal CA1 place and silent cell activity in a novel environment. Neuron 70, 109–120. doi: 10.1016/j.neuron.2011.03.006
Eroglu, Ç,Allen, N. J., Susman, M. W., O’Rourke, N. A., Park, C. Y., Özkan, E., et al. (2009). Gabapentin receptor α2δ-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 139, 380–392. doi: 10.1016/j.cell.2009.09.025
Falcón-Moya, R., Pérez-Rodríguez, M., Prius-Mengual, J., Andrade-Talavera, Y., Arroyo-García, L. E., Pérez-Artés, R., et al. (2020). Astrocyte-mediated switch in spike timing-dependent plasticity during hippocampal development. Nat. Commun. 11:4388. doi: 10.1038/s41467-020-18024-4
Fan, D., Grooms, S. Y., Araneda, R. C., Johnson, A. B., Dobrenis, K., Kessler, J. A., et al. (1999). AMPA receptor protein expression and function in astrocytes cultured from hippocampus. J. Neurosci. Res. 57, 557–571. doi: 10.1002/(SICI)1097-4547(19990815)57:4<557::AID-JNR16<3.0.CO;2-I
Farmer, B. C., Kluemper, J., and Johnson, L. A. (2019). Apolipoprotein E4 alters astrocyte fatty acid metabolism and lipid droplet formation. Cells 8:182. doi: 10.3390/cells8020182
Fernández-Moncada, I., Lavanco, G., Fundazuri, U. B., Bollmohr, N., Mountadem, S., Dalla Tor, T., et al. (2024). A lactate-dependent shift of glycolysis mediates synaptic and cognitive processes in male mice. Nat. Commun. 15:6842. doi: 10.1038/s41467-024-51008-2
Ferris, H. A., Perry, R. J., Moreira, G. V., Shulman, G. I., Horton, J. D., and Kahn, C. R. (2017). Loss of astrocyte cholesterol synthesis disrupts neuronal function and alters whole-body metabolism. Proc. Natl. Acad. Sci. U. S. A. 114, 1189–1194. doi: 10.1073/pnas.1620506114
Fester, L., Zhou, L., Bütow, A., Huber, C., Von Lossow, R., Prange-Kiel, J., et al. (2009). Cholesterol-promoted synaptogenesis requires the conversion of cholesterol to estradiol in the hippocampus. Hippocampus 19, 692–705. doi: 10.1002/hipo.20548
Florian, C., Vecsey, C. G., Halassa, M. M., Haydon, P. G., and Abel, T. (2011). Astrocyte-derived adenosine and A1 receptor activity contribute to sleep loss-induced deficits in hippocampal synaptic plasticity and memory in mice. J. Neurosci. 31, 6956–6962. doi: 10.1523/JNEUROSCI.5761-10.2011
Foley, J., Blutstein, T., Lee, S., Erneux, C., Halassa, M. M., and Haydon, P. (2017). Astrocytic IP3/Ca2+ signaling modulates theta rhythm and REM sleep. Front. Neural Circuits 11:3. doi: 10.3389/fncir.2017.00003
Fortin, N. J., Agster, K. L., and Eichenbaum, H. B. (2002). Critical role of the hippocampus in memory for sequences of events. Nat. Neurosci. 5, 458–462. doi: 10.1038/nn834
Fünfschilling, U., Saher, G., Xiao, L., Möbius, W., and Nave, K.-A. (2007). Survival of adult neurons lacking cholesterol synthesis in vivo. BMC Neurosci. 8:1. doi: 10.1186/1471-2202-8-1
Gadea, A., and López-Colomé, A. M. (2001). Glial transporters for glutamate, glycine, and GABA: II. GABA transporters. J. Neurosci. Res. 63, 461–468. doi: 10.1002/jnr.1040
Gaidin, S. G., Zinchenko, V. P., Sergeev, A. I., Teplov, I. Y., Mal’tseva, V. N., and Kosenkov, A. M. (2020). Activation of alpha-2 adrenergic receptors stimulates GABA release by astrocytes. Glia 68, 1114–1130. doi: 10.1002/glia.23763
Gao, V., Suzuki, A., Magistretti, P. J., Lengacher, S., Pollonini, G., Steinman, M. Q., et al. (2016). Astrocytic β2-adrenergic receptors mediate hippocampal long-term memory consolidation. Proc. Natl. Acad. Sci. U. S. A. 113, 8526–8531. doi: 10.1073/pnas.1605063113
Glaum, S. R., Holzwarth, J. A., and Miller, R. J. (1990). Glutamate receptors activate Ca2+ mobilization and Ca2+ influx into astrocytes. Proc. Nat. Acad. Sci. U. S. A. 87, 3454–3458. doi: 10.1073/pnas.87.9.3454
Gordon, G. R. J., Choi, H. B., Rungta, R. L., Ellis-Davies, G. C. R., and MacVicar, B. A. (2008). Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 456, 745–749. doi: 10.1038/nature07525
Goritz, C., Mauch, D. H., and Pfrieger, F. W. (2005). Multiple mechanisms mediate cholesterol-induced synaptogenesis in a CNS neuron. Mol. Cell. Neurosci. 29, 190–201. doi: 10.1016/j.mcn.2005.02.006
Griffin, A. L., Asaka, Y., Darling, R. D., and Berry, S. D. (2004). Theta-contingent trial presentation accelerates learning rate and enhances hippocampal plasticity during trace eyeblink conditioning. Behav. Neurosci. 118, 403–411. doi: 10.1037/0735-7044.118.2.403
Grolla, A. A., Fakhfouri, G., Balzaretti, G., Marcello, E., Gardoni, F., Canonico, P. L., et al. (2013a). Aβ leads to Ca2+ signaling alterations and transcriptional changes in glial cells. Neurobiol. Aging 34, 511–522. doi: 10.1016/j.neurobiolaging.2012.05.005
Grolla, A. A., Sim, J. A., Dim, D., Rodriguez, J. J., Genazzani, A. A., and Verkhratsky, A. (2013b). Amyloid-β and Alzheimer’s disease type pathology differentially affects the calcium signalling toolkit in astrocytes from different brain regions. Cell Death Dis. 4:e623. doi: 10.1038/cddis.2013.145
Grosche, A., Grosche, J., Tackenberg, M., Scheller, D., Gerstner, G., Gumprecht, A., et al. (2013). Versatile and simple approach to determine astrocyte territories in mouse neocortex and hippocampus. PLoS One 8:e69143. doi: 10.1371/journal.pone.0069143
Haber, M., Zhou, L., and Murai, K. K. (2006). Cooperative astrocyte and dendritic spine dynamics at hippocampal excitatory synapses. J. Neurosci. 26, 8881–8891. doi: 10.1523/JNEUROSCI.1302-06.2006
Habib, N., McCabe, C., Medina, S., Varshavsky, M., Kitsberg, D., Dvir-Szternfeld, R., et al. (2020). Disease-associated astrocytes in Alzheimer’s disease and aging. Nat. Neurosci. 23, 701–706. doi: 10.1038/s41593-020-0624-8
Halassa, M. M., Florian, C., Fellin, T., Munoz, J. R., Lee, S.-Y., Abel, T., et al. (2009). Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 61, 213–219. doi: 10.1016/j.neuron.2008.11.024
Hamilton, L. K., Dufresne, M., Joppé, S. E., Petryszyn, S., Aumont, A., Calon, F., et al. (2015). Aberrant lipid metabolism in the forebrain niche suppresses adult neural stem cell proliferation in an animal model of Alzheimer’s Disease. Cell Stem Cell 17, 397–411. doi: 10.1016/j.stem.2015.08.001
Harvey, C. D., Collman, F., Dombeck, D. A., and Tank, D. W. (2009). Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 461, 941–946. doi: 10.1038/nature08499
Haustein, M. D., Kracun, S., Lu, X.-H., Shih, T., Jackson-Weaver, O., Tong, X., et al. (2014). Conditions and constraints for astrocyte calcium signaling in the hippocampal mossy fiber pathway. Neuron 82, 413–429. doi: 10.1016/j.neuron.2014.02.041
Hayashi, H., Campenot, R. B., Vance, D. E., and Vance, J. E. (2004). Glial lipoproteins stimulate axon growth of central nervous system neurons in compartmented cultures. J. Biol. Chem. 279, 14009–14015. doi: 10.1074/jbc.M313828200
Hayashi, Y., Shi, S.-H., Esteban, J. A., Piccini, A., Poncer, J.-C., and Malinow, R. (2000). Driving AMPA receptors into synapses by LTP and CaMKII: Requirement for GluR1 and PDZ domain interaction. Science 287, 2262–2267. doi: 10.1126/science.287.5461.2262
Henneberger, C., Papouin, T., Oliet, S. H. R., and Rusakov, D. A. (2010). Long-term potentiation depends on release of D-serine from astrocytes. Nature 463, 232–236. doi: 10.1038/nature08673
Hjukse, J. B., Puebla, M. F. D. L., Vindedal, G. F., Sprengel, R., and Jensen, V. (2023). Increased membrane Ca2+ permeability drives astrocytic Ca2+ dynamics during neuronal stimulation at excitatory synapses. Glia 71, 2770–2781. doi: 10.1002/glia.24450
Holtzman, D. M., Pitas, R. E., Kilbridge, J., Nathan, B., Mahley, R. W., Bu, G., et al. (1995). Low density lipoprotein receptor-related protein mediates apolipoprotein E-dependent neurite outgrowth in a central nervous system-derived neuronal cell line. Proc. Natl. Acad. Sci. U. S. A. 92, 9480–9484. doi: 10.1073/pnas.92.21.9480
Hösli, L., Binini, N., Ferrari, K. D., Thieren, L., Looser, Z. J., Zuend, M., et al. (2022). Decoupling astrocytes in adult mice impairs synaptic plasticity and spatial learning. Cell Rep. 38:110484. doi: 10.1016/j.celrep.2022.110484
Huang, A. Y.-S., Woo, J., Sardar, D., Lozzi, B., Bosquez Huerta, N. A., Lin, C.-C. J., et al. (2020). Region-specific transcriptional control of astrocyte function oversees local circuit activities. Neuron 106, 992–1008.e9. doi: 10.1016/j.neuron.2020.03.025.
Huang, Y. H., Sinha, S. R., Tanaka, K., Rothstein, J. D., and Bergles, D. E. (2004). Astrocyte glutamate transporters regulate metabotropic glutamate receptor-mediated excitation of hippocampal interneurons. J. Neurosci. 24, 4551–4559. doi: 10.1523/JNEUROSCI.5217-03.2004
Huffels, C. F. M., Osborn, L. M., Cappaert, N. L. M., and Hol, E. M. (2022). Calcium signaling in individual APP/PS1 mouse dentate gyrus astrocytes increases ex vivo with Aβ pathology and age without affecting astrocyte network activity. J. Neurosci. Res. 100, 1281–1296. doi: 10.1002/jnr.25042
Huganir, R. L., and Nicoll, R. A. (2013). AMPARs and synaptic plasticity: The last 25 years. Neuron 80, 704–717. doi: 10.1016/j.neuron.2013.10.025
Ingiosi, A. M., Hayworth, C. R., Harvey, D. O., Singletary, K. G., Rempe, M. J., Wisor, J. P., et al. (2020). A role for astroglial calcium in mammalian sleep and sleep regulation. Curr. Biol. 30, 4373–4383.e7. doi: 10.1016/j.cub.2020.08.052.
Ioannou, M. S., Jackson, J., Sheu, S.-H., Chang, C.-L., Weigel, A. V., Liu, H., et al. (2019). Neuron-astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Cell 177, 1522–1535.e14. doi: 10.1016/j.cell.2019.04.001.
Izumi, Y., and Zorumski, C. F. (2012). NMDA receptors, mGluR5, and endocannabinoids are involved in a cascade leading to hippocampal long-term depression. Neuropsychopharmacology 37, 609–617. doi: 10.1038/npp.2011.243
Jackson, R. J., Meltzer, J. C., Nguyen, H., Commins, C., Bennett, R. E., Hudry, E., et al. (2022). APOE4 derived from astrocytes leads to blood-brain barrier impairment. Brain 145, 3582–3593. doi: 10.1093/brain/awab478
Jankowsky, J. L., Slunt, H. H., Gonzales, V., Jenkins, N. A., Copeland, N. G., and Borchelt, D. R. (2004). APP processing and amyloid deposition in mice haplo-insufficient for presenilin 1. Neurobiol. Aging 25, 885–892. doi: 10.1016/j.neurobiolaging.2003.09.008
Jiwaji, Z., Tiwari, S. S., Avilés-Reyes, R. X., Hooley, M., Hampton, D., Torvell, M., et al. (2022). Reactive astrocytes acquire neuroprotective as well as deleterious signatures in response to Tau and Aβ pathology. Nat. Commun. 13:135. doi: 10.1038/s41467-021-27702-w
Jo, S., Yarishkin, O., Hwang, Y. J., Chun, Y. E., Park, M., Woo, D. H., et al. (2014). GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat. Med. 20, 886–896. doi: 10.1038/nm.3639
Josselyn, S. A., Köhler, S., and Frankland, P. W. (2017). Heroes of the engram. J. Neurosci. 37, 4647–4657. doi: 10.1523/JNEUROSCI.0056-17.2017
Karni, A., Tanne, D., Rubenstein, B. S., Askenasy, J. J. M., and Sagi, D. (1994). Dependence on REM sleep of overnight improvement of a perceptual skill. Science 265, 679–682. doi: 10.1126/science.8036518
Kater, M. S. J., Badia-Soteras, A., van Weering, J. R. T., Smit, A. B., and Verheijen, M. H. G. (2023). Electron microscopy analysis of astrocyte-synapse interactions shows altered dynamics in an Alzheimer’s disease mouse model. Front. Cell. Neurosci. 17:1085690. doi: 10.3389/fncel.2023.1085690
Kemp, A., and Manahan-Vaughan, D. (2004). Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc. Nat. Acad. Sci. U. S. A. 101, 8192–8197. doi: 10.1073/pnas.0402650101
Kim, J.-I., Lee, H.-R., Sim, S.-E., Baek, J., Yu, N.-K., Choi, J.-H., et al. (2011). PI3Kγ is required for NMDA receptor-dependent long-term depression and behavioral flexibility. Nat. Neurosci. 14, 1447–1454. doi: 10.1038/nn.2937
King, C. M., Bohmbach, K., Minge, D., Delekate, A., Zheng, K., Reynolds, J., et al. (2020). Local resting Ca2+ controls the scale of astroglial Ca2+ signals. Cell Rep. 30, 3466–3477.e4. doi: 10.1016/j.celrep.2020.02.043.
Kjelstrup, K. B., Solstad, T., Brun, V. H., Hafting, T., Leutgeb, S., Witter, M. P., et al. (2008). Finite scale of spatial representation in the hippocampus. Science 321, 140–143. doi: 10.1126/science.1157086
Koepsell, H. (2020). Glucose transporters in the brain in health and disease. Pflügers Arch – Eur J Physiol. 472, 1299–1343. doi: 10.1007/s00424-020-02441-x
Koh, W., Park, M., Chun, Y. E., Lee, J., Shim, H. S., Park, M. G., et al. (2022). Astrocytes render memory flexible by releasing D-serine and regulating NMDA receptor tone in the hippocampus. Biol. Psychiatr. 91, 740–752. doi: 10.1016/j.biopsych.2021.10.012
Kol, A., Adamsky, A., Groysman, M., Kreisel, T., London, M., and Goshen, I. (2020). Astrocytes contribute to remote memory formation by modulating hippocampal–cortical communication during learning. Nat. Neurosci. 23, 1229–1239. doi: 10.1038/s41593-020-0679-6
Kraus, B. J., Robinson, R. J., White, J. A., Eichenbaum, H., and Hasselmo, M. E. (2013). Hippocampal “time cells”: Time versus path integration. Neuron 78, 1090–1101. doi: 10.1016/j.neuron.2013.04.015
Le Douce, J., Maugard, M., Veran, J., Matos, M., Jégo, P., Vigneron, P.-A., et al. (2020). Impairment of glycolysis-derived L-serine production in astrocytes contributes to cognitive deficits in Alzheimer’s disease. Cell Metabol. 31, 503–517.e8. doi: 10.1016/j.cmet.2020.02.004.
Le Meur, K., Mendizabal-Zubiaga, J., Grandes, P., and Audinat, E. (2012). GABA release by hippocampal astrocytes. Front. Comput. Neurosci. 6:59. doi: 10.3389/fncom.2012.00059
Lee, A. K., and Wilson, M. A. (2002). Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36, 1183–1194. doi: 10.1016/S0896-6273(02)01096-6
Lee, H. S., Ghetti, A., Pinto-Duarte, A., Wang, X., Dziewczapolski, G., Galimi, F., et al. (2014). Astrocytes contribute to gamma oscillations and recognition memory. Proc. Natl. Acad. Sci. U. S. A. 111, E3343–E3352. doi: 10.1073/pnas.1410893111
Lee, H., Cho, S., Kim, M.-J., Park, Y. J., Cho, E., Jo, Y. S., et al. (2023). ApoE4-dependent lysosomal cholesterol accumulation impairs mitochondrial homeostasis and oxidative phosphorylation in human astrocytes. Cell Rep. 42:113183. doi: 10.1016/j.celrep.2023.113183
Lee, J.-H., Kim, J., Noh, S., Lee, H., Lee, S. Y., Mun, J. Y., et al. (2021). Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis. Nature 590, 612–617. doi: 10.1038/s41586-020-03060-3
Lefton, K. B., Wu, Y., Dai, Y., Okuda, T., Zhang, Y., Yen, A., et al. (2025). Norepinephrine signals through astrocytes to modulate synapses. Science 388, 776–783. doi: 10.1126/science.adq5480
Letellier, M., Park, Y. K., Chater, T. E., Chipman, P. H., Gautam, S. G., Oshima-Takago, T., et al. (2016). Astrocytes regulate heterogeneity of presynaptic strengths in hippocampal networks. Proc. Nat. Acad. Sci. U. S. A. 113, E2685–E2694. doi: 10.1073/pnas.1523717113
Levitt, P., and Moore, R. Y. (1978). Noradrenaline neuron innervation of the neocortex in the rat. Brain Res. 139, 219–231. doi: 10.1016/0006-8993(78)90925-3
Li, L., Lu, S., Zhu, J., Yu, X., Hou, S., Huang, Y., et al. (2024). Astrocytes excessively engulf synapses in a mouse model of Alzheimer’s disease. Int. J. Mol. Sci. 25:1160. doi: 10.3390/ijms25021160
Li, X., Zhang, J., Li, D., He, C., He, K., Xue, T., et al. (2021). Astrocytic ApoE reprograms neuronal cholesterol metabolism and histone-acetylation-mediated memory. Neuron 109, 957–970.e8. doi: 10.1016/j.neuron.2021.01.005.
Liang, S.-L., Carlson, G. C., and Coulter, D. A. (2006). Dynamic regulation of synaptic GABA release by the glutamate-glutamine cycle in hippocampal area CA1. J. Neurosci. 26, 8537–8548. doi: 10.1523/JNEUROSCI.0329-06.2006
Linetti, A., Fratangeli, A., Taverna, E., Valnegri, P., Francolini, M., Cappello, V., et al. (2010). Cholesterol reduction impairs exocytosis of synaptic vesicles. J. Cell Sci. 123, 595–605. doi: 10.1242/jcs.060681
Liu, J., Feng, X., Wang, Y., Xia, X., and Zheng, J. C. (2022). Astrocytes: GABAceptive and GABAergic cells in the brain. Front. Cell. Neurosci. 16:892497. doi: 10.3389/fncel.2022.892497
Liu, L., MacKenzie, K. R., Putluri, N., Maletić-Savatić, M., and Bellen, H. J. (2017). The glia-neuron lactate shuttle and elevated ROS promote lipid synthesis in neurons and lipid droplet accumulation in glia via APOE/D. Cell Metabol. 26, 719–737.e6. doi: 10.1016/j.cmet.2017.08.024.
Liu, Q., Trotter, J., Zhang, J., Peters, M. M., Cheng, H., Bao, J., et al. (2010). Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive, age-dependent synapse loss and neurodegeneration. J. Neurosci. 30, 17068–17078. doi: 10.1523/JNEUROSCI.4067-10.2010
Liu, X., Ramirez, S., Pang, P. T., Puryear, C. B., Govindarajan, A., Deisseroth, K., et al. (2012). Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385. doi: 10.1038/nature11028
Long, J. M., and Holtzman, D. M. (2019). Alzheimer Disease: An update on pathobiology and treatment strategies. Cell 179, 312–339. doi: 10.1016/j.cell.2019.09.001
Ma, W., Si, T., Wang, Z., Wen, P., Zhu, Z., Liu, Q., et al. (2023). Astrocytic α4-containing nAChR signaling in the hippocampus governs the formation of temporal association memory. Cell Rep. 42:112674. doi: 10.1016/j.celrep.2023.112674
MacDonald, C. J., Carrow, S., Place, R., and Eichenbaum, H. (2013). Distinct hippocampal time cell sequences represent odor memories in immobilized rats. J. Neurosci. 33, 14607–14616. doi: 10.1523/JNEUROSCI.1537-13.2013
MacDonald, C. J., Lepage, K. Q., Eden, U. T., and Eichenbaum, H. (2011). Hippocampal “time cells” bridge the gap in memory for discontiguous Events. Neuron 71, 737–749. doi: 10.1016/j.neuron.2011.07.012
Magee, J. C., and Johnston, D. (1997). A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science 1997, 209–213. doi: 10.1126/science.275.5297.209
Malenka, R. C., Kauer, J. A., Perkel, D. J., Mauk, M. D., Kelly, P. T., Nicoll, R. A., et al. (1989). An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature 340, 554–557. doi: 10.1038/340554a0
Malinow, R., Schulman, H., and Tsien, R. W. (1989). Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science 245, 862–866. doi: 10.1126/science.2549638
Manahan-Vaughan, D., and Braunewell, K.-H. (1999). Novelty acquisition is associated with induction of hippocampal long-term depression. Proc. Nat. Acad. Sci. U. S. A. 96, 8739–8744. doi: 10.1073/pnas.96.15.8739
Marrosu, F., Portas, C., Mascia, M. S., Casu, M. A., Fà, M., Giagheddu, M., et al. (1995). Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 671, 329–332. doi: 10.1016/0006-8993(94)01399-3
Martínez-Gallego, I., Coatl-Cuaya, H., and Rodriguez-Moreno, A. (2024). Astrocytes mediate two forms of spike timing-dependent depression at entorhinal cortex-hippocampal synapses. eLife 13:R98031. doi: 10.7554/eLife.98031.3
Masa, P. T., and Mugnaini, E. (1982). Cell junctions and intramembrane particles of astrocytes and oligodendrocytes: A freeze-fracture study. Neuroscience 7, 523–538. doi: 10.1016/0306-4522(82)90285-8
Mauch, D. H., Nägler, K., Schumacher, S., Göritz, C., Müller, E.-C., Otto, A., et al. (2001). CNS synaptogenesis promoted by glia-derived cholesterol. Science 294, 1354–1357. doi: 10.1126/science.294.5545.1354
Minhas, P. S., Jones, J. R., Latif-Hernandez, A., Sugiura, Y., Durairaj, A. S., Wang, Q., et al. (2024). Restoring hippocampal glucose metabolism rescues cognition across Alzheimer’s disease pathologies. Science 385:eabm6131. doi: 10.1126/science.abm6131
Mishra, S., Blazey, T. M., Holtzman, D. M., Cruchaga, C., Su, Y., Morris, J. C., et al. (2018). Longitudinal brain imaging in preclinical Alzheimer disease: Impact of APOE ε4 genotype. Brain 141, 1828–1839. doi: 10.1093/brain/awy103
Morgan, S. L., and Teyler, T. J. (2001). Electrical stimuli patterned after the theta-rhythm induce multiple forms of LTP. J. Neurophysiol. 86, 1289–1296. doi: 10.1152/jn.2001.86.3.1289
Morgello, S., Uson, R. R., Schwartz, E. J., and Haber, R. S. (1995). The human blood-brain barrier glucose transporter (GLUT1) is a glucose transporter of gray matter astrocytes. Glia 14, 43–54. doi: 10.1002/glia.440140107
Moroni, F., Nobili, L., Curcio, G., De Carli, F., Fratello, F., Marzano, C., et al. (2007). Sleep in the human hippocampus: A stereo-EEG study. PLoS One 2:e867. doi: 10.1371/journal.pone.0000867
Mulkey, R. M., Endo, S., Shenolikar, S., and Malenka, R. C. (1994). Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature 369, 486–488. doi: 10.1038/369486a0
Navarrete, M., Perea, G., De Sevilla, D. F., Gómez-Gonzalo, M., Núñez, A., Martín, E. D., et al. (2012). Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Biol. 10:e1001259. doi: 10.1371/journal.pbio.1001259
Nett, W. J., Oloff, S. H., and McCarthy, K. D. (2002). Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J. Neurophysiol. 87, 528–537. doi: 10.1152/jn.00268.2001
Nicholls, R. E., Alarcon, J. M., Malleret, G., Carroll, R. C., Grody, M., Vronskaya, S., et al. (2008). Transgenic mice lacking NMDAR-dependent LTD exhibit deficits in behavioral flexibility. Neuron 58, 104–117. doi: 10.1016/j.neuron.2008.01.039
Nortley, R., and Attwell, D. (2017). Control of brain energy supply by astrocytes. Curr. Opin. Neurobiol. 47, 80–85. doi: 10.1016/j.conb.2017.09.012
Nowak, L., Ascher, P., and Berwald-Netter, Y. (1987). Ionic channels in mouse astrocytes in culture. J. Neurosci. 7, 101–109. doi: 10.1523/JNEUROSCI.07-01-00101.1987
O’Keefe, J., and Burgess, N. (1996). Geometric determinants of the place fields of hippocampal neurons. Nature 381, 425–428. doi: 10.1038/381425a0
O’Keefe, J., and Dostrovsky, J. (1971). The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175. doi: 10.1016/0006-8993(71)90358-1
Ogata, K., and Kosaka, T. (2002). Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience 113, 221–233. doi: 10.1016/S0306-4522(02)00041-6
Ohno-Shosaku, T., Hashimotodani, Y., Ano, M., Takeda, S., Tsubokawa, H., and Kano, M. (2007). Endocannabinoid signalling triggered by NMDA receptor-mediated calcium entry into rat hippocampal neurons. J. Physiol. 584, 407–418. doi: 10.1113/jphysiol.2007.137505
Orr, A. G., Hsiao, E. C., Wang, M. M., Ho, K., Kim, D. H., Wang, X., et al. (2015). Astrocytic adenosine receptor A2A and GS-coupled signaling regulate memory. Nat. Neurosci. 18, 423–434. doi: 10.1038/nn.3930
Pabst, M., Braganza, O., Dannenberg, H., Hu, W., Pothmann, L., Rosen, J., et al. (2016). Astrocyte intermediaries of septal cholinergic modulation in the hippocampus. Neuron 90, 853–865. doi: 10.1016/j.neuron.2016.04.003
Panatier, A., Theodosis, D. T., Mothet, J.-P., Touquet, B., Pollegioni, L., Poulain, D. A., et al. (2006). Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 125, 775–784. doi: 10.1016/j.cell.2006.02.051
Panatier, A., Vallée, J., Haber, M., Murai, K. K., Lacaille, J.-C., and Robitaille, R. (2011). Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146, 785–798. doi: 10.1016/j.cell.2011.07.022
Pannasch, U., Freche, D., Dallérac, G., Ghézali, G., Escartin, C., Ezan, P., et al. (2014). Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nat. Neurosci. 17, 549–558. doi: 10.1038/nn.3662
Papouin, T., Dunphy, J. M., Tolman, M., Dineley, K. T., and Haydon, P. G. (2017). Septal cholinergic neuromodulation tunes the astrocyte-dependent gating of hippocampal NMDA receptors to wakefulness. Neuron 94, 840–854.e7. doi: 10.1016/j.neuron.2017.04.021.
Papouin, T., Ladépêche, L., Ruel, J., Sacchi, S., Labasque, M., Hanini, M., et al. (2012). Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 150, 633–646. doi: 10.1016/j.cell.2012.06.029
Pascual, O., Casper, K. B., Kubera, C., Zhang, J., Revilla-Sanchez, R., Sul, J.-Y., et al. (2005). Astrocytic purinergic signaling coordinates synaptic networks. Science 310, 113–116. doi: 10.1126/science.1116916
Pastalkova, E., Itskov, V., Amarasingham, A., and Buzsaìki, G. (2008). Internally generated cell assembly sequences in the rat hippocampus. Science 321, 1322–1327. doi: 10.1126/science.1159775
Paumier, A., Boisseau, S., Jacquier-Sarlin, M., Pernet-Gallay, K., Buisson, A., and Albrieux, M. (2022). Astrocyte–neuron interplay is critical for Alzheimer’s disease pathogenesis and is rescued by TRPA1 channel blockade. Brain 145, 388–405. doi: 10.1093/brain/awab281
Pellerin, L., and Magistretti, P. J. (1994). Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. U. S. A. 91, 10625–10629. doi: 10.1073/pnas.91.22.10625
Pellerin, L., Bouzier-Sore, A.-K., Aubert, A., Serres, S., Merle, M., Costalat, R., et al. (2007). Activity-dependent regulation of energy metabolism by astrocytes: An update. Glia 55, 1251–1262. doi: 10.1002/glia.20528
Perea, G., and Araque, A. (2005). Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J. Neurosci. 25, 2192–2203. doi: 10.1523/JNEUROSCI.3965-04.2005
Perea, G., and Araque, A. (2007). Astrocytes potentiate transmitter release at single hippocampal synapses. Science 317, 1083–1086. doi: 10.1126/science.1144640
Perea, G., Gómez, R., Mederos, S., Covelo, A., Ballesteros, J. J., Schlosser, L., et al. (2016). Activity-dependent switch of GABAergic inhibition into glutamatergic excitation in astrocyte-neuron networks. eLife 5:e20362. doi: 10.7554/eLife.20362
Pérez-Rodríguez, M., Arroyo-García, L. E., Prius-Mengual, J., Andrade-Talavera, Y., Armengol, J. A., Pérez-Villegas, E. M., et al. (2019). Adenosine receptor-mediated development loss of spike timing-dependent depression in the hippocampus. Cereb. Cortex 29, 3266–3281. doi: 10.1093/cercor/bhy194
Petravicz, J., Fiacco, T. A., and McCarthy, K. D. (2008). Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J. Neurosci. 28, 4967–4973. doi: 10.1523/JNEUROSCI.5572-07.2008
Pierre, K., and Pellerin, L. (2005). Monocarboxylate transporters in the central nervous system: Distribution, regulation and function. J. Neurochem. 94, 1–14. doi: 10.1111/j.1471-4159.2005.03168.x
Pirttimaki, T. M., Codadu, N. K., Awni, A., Pratik, P., Nagel, D. A., Hill, E. J., et al. (2013). α7 nicotonic receptor-mediated astrocytic gliotransmitter release: Aβ effects in a preclinical Alzheimer’s mouse model. PLoS One 8:e81828. doi: 10.1371/journal.pone.0081828
Portal, B., Södergren, M., Parés I Borrell, T., Giraud, R., Metzendorf, N. G., Hultqvist, G., et al. (2024). Early astrocytic dysfunction is associated with mistuned synapses as well as anxiety and depressive-like behavior in the APPNL-F mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 100, 1017–1037. doi: 10.3233/JAD-231461
Porter, J. T., and McCarthy, K. D. (1995). GFAP-positive hippocampal astrocytes in situ respond to glutamatergic neuroligands with increases in [Ca2+]i. Glia 13, 101–112. doi: 10.1002/glia.440130204
Porter, J. T., and McCarthy, K. D. (1996). Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J. Neurosci. 16, 5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996
Puma, D. D. L., Ripoli, C., Puliatti, G., Pastore, F., Lazzarino, G., Tavazzi, B., et al. (2022). Extracellular tau oligomers affect extracellular glutamate handling by astrocytes through downregulation of GLT-1 expression and impairment of NKA1A2 function. Neuropathol. Appl. Neurobiol. 48, e12811. doi: 10.1111/nan.12811
Qi, G., Mi, Y., Shi, X., Gu, H., Brinton, R. D., and Yin, F. (2021). ApoE4 impairs neuron-astrocyte coupling of fatty acid metabolism. Cell Rep. 34:108572. doi: 10.1016/j.celrep.2020.108572
Ralhan, I., Chang, C.-L., Lippincott-Schwartz, J., and Ioannou, M. S. (2021). Lipid droplets in the nervous system. J. Cell Biol. 220:e202102136. doi: 10.1083/jcb.202102136
Ramirez, S., Liu, X., Lin, P.-A., Suh, J., Pignatelli, M., Redondo, R. L., et al. (2013). Creating a false memory in the hippocampus. Science 341, 387–391. doi: 10.1126/science.1239073
Rawat, V., Wang, S., Sima, J., Bar, R., Liraz, O., Gundimeda, U., et al. (2019). ApoE4 alters ABCA1 membrane trafficking in astrocytes. J. Neurosci. 39, 9611–9622. doi: 10.1523/JNEUROSCI.1400-19.2019
Refaeli, R., Doron, A., Benmelech-Chovav, A., Groysman, M., Kreisel, T., Loewenstein, Y., et al. (2021). Features of hippocampal astrocytic domains and their spatial relation to excitatory and inhibitory neurons. Glia 69, 2378–2390. doi: 10.1002/glia.24044
Refaeli, R., Kreisel, T., Yaish, T. R., Groysman, M., and Goshen, I. (2024). Astrocytes control recent and remote memory strength by affecting the recruitment of the CA1→ACC projection to engrams. Cell Rep. 43:113943. doi: 10.1016/j.celrep.2024.113943
Ronco, V., Grolla, A. A., Glasnov, T. N., Canonico, P. L., Verkhratsky, A., Genazzani, A. A., et al. (2014). Differential deregulation of astrocytic calcium signalling by amyloid-β, TNF-α, IL-1β and LPS. Cell Calcium 55, 219–229. doi: 10.1016/j.ceca.2014.02.016
Rose, C. R., and Ransom, B. R. (1996). Intracellular sodium homeostasis in rat hippocampal astrocytes. J. Physiol. 491, 291–305. doi: 10.1113/jphysiol.1996.sp021216
Rothman, D. L., De Feyter, H. M., De Graaf, R. A., Mason, G. F., and Behar, K. L. (2011). 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR Biomed. 24, 943–957. doi: 10.1002/nbm.1772
Rothstein, J. D., Dykes-Hoberg, M., Pardo, C. A., Bristol, L. A., Jin, L., Kuncl, R. W., et al. (1996). Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16, 675–686. doi: 10.1016/S0896-6273(00)80086-0
Rouach, N., Koulakoff, A., Abudara, V., Willecke, K., and Giaume, C. (2008). Astroglial metabolic networks sustain hippocampal synaptic transmission. Science 322, 1551–1555. doi: 10.1126/science.1164022
Rungta, R. L., Bernier, L.-P., Dissing-Olesen, L., Groten, C. J., LeDue, J. M., Ko, R., et al. (2016). Ca2+ transients in astrocyte fine processes occur via Ca2+ influx in the adult mouse hippocampus. Glia 64, 2093–2103. doi: 10.1002/glia.23042
Rupprecht, P., Duss, S. N., Becker, D., Lewis, C. M., Bohacek, J., and Helmchen, F. (2024). Centripetal integration of past events in hippocampal astrocytes regulated by locus coeruleus. Nat. Neurosci. 27, 927–939. doi: 10.1038/s41593-024-01612-8
Sapkota, D., Kater, M. S. J., Sakers, K., Nygaard, K. R., Liu, Y., Koester, S. K., et al. (2022). Activity-dependent translation dynamically alters the proteome of the perisynaptic astrocyte process. Cell Rep. 41:111474. doi: 10.1016/j.celrep.2022.111474
Sardinha, V. M., Guerra-Gomes, S., Caetano, I., Tavares, G., Martins, M., Reis, J. S., et al. (2017). Astrocytic signaling supports hippocampal–prefrontal theta synchronization and cognitive function. Glia 65, 1944–1960. doi: 10.1002/glia.23205
Saur, L., Baptista, P. P. A., De Senna, P. N., Paim, M. F., Nascimento, P. D., Ilha, J., et al. (2014). Physical exercise increases GFAP expression and induces morphological changes in hippocampal astrocytes. Brain Struct. Funct. 219, 293–302. doi: 10.1007/s00429-012-0500-8
Schönfeld, P., and Reiser, G. (2017). Brain energy metabolism spurns fatty acids as fuel due to their inherent mitotoxicity and potential capacity to unleash neurodegeneration. Neurochem. Int. 109, 68–77. doi: 10.1016/j.neuint.2017.03.018
Schröder, W., Seifert, G., Hüttmann, K., Hinterkeuser, S., and Steinhäuser, C. (2002). AMPA receptor-mediated modulation of inward rectifier K+ channels in astrocytes of mouse hippocampus. Mol. Cell. Neurosci. 19, 447–458. doi: 10.1006/mcne.2001.1080
Serrano, A., Haddjeri, N., Lacaille, J.-C., and Robitaille, R. (2006). GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J. Neurosci. 26, 5370–5382. doi: 10.1523/JNEUROSCI.5255-05.2006
Sharma, G., and Vijayaraghavan, S. (2001). Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proc. Natl. Acad. Sci. U. S. A. 98, 4148–4153. doi: 10.1073/pnas.071540198
Shelton, M. K., and McCarthy, K. D. (1999). Mature hippocampal astrocytes exhibit functional metabotropic and ionotropic glutamate receptors in situ. Glia 26, 1–11. doi: 10.1002/(SICI)1098-1136(199903)26:1<1::AID-GLIA1<3.0.CO;2-Z
Shi, Y., Yamada, K., Liddelow, S. A., Smith, S. T., Zhao, L., Luo, W., et al. (2017). ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549, 523–527. doi: 10.1038/nature24016
Sienski, G., Narayan, P., Bonner, J. M., Kory, N., Boland, S., Arczewska, A. A., et al. (2021). APOE4 disrupts intracellular lipid homeostasis in human iPSC-derived glia. Sci. Transl. Med. 13:eaaz4564. doi: 10.1126/scitranslmed.aaz4564
Squire, L. R. (2004). Memory systems of the brain: A brief history and current perspective. Neurobiol. Learn. Mem. 82, 171–177. doi: 10.1016/j.nlm.2004.06.005
Srinivasan, R., Huang, B., Venugopal, S., Johnston, A. D., Chai, H., Zeng, H., et al. (2015). Ca2+ signaling in astrocytes from Ip3r2–/– mice in brain slices and during startle responses in vivo. Nat. Neurosci. 18, 708–717. doi: 10.1038/nn.4001
Stelzmann, R. A., Norman Schnitzlein, H., and Reed Murtagh, F. (1995). An English translation of Alzheimer’s 1907 paper, “Über eine eigenartige Erkankung der Hirnrinde.”. Clin. Anat. 8, 429–431. doi: 10.1002/ca.980080612
St-Pierre, M.-K., Carrier, M., Ibáñez, F. G., Khakpour, M., Wallman, M.-J., Parent, M., et al. (2023). Astrocytes display ultrastructural alterations and heterogeneity in the hippocampus of aged APP-PS1 mice and human post-mortem brain samples. J. Neuroinflamm. 20:73. doi: 10.1186/s12974-023-02752-7
Strittmatter, W. J., Saunders, A. M., Schmechel, D., Pericak-Vance, M., Enghild, J., Salvesen, G. S., et al. (1993). Apolipoprotein E: High-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A. 90, 1977–1981. doi: 10.1073/pnas.90.5.1977
Sultan, S., Li, L., Moss, J., Petrelli, F., Cassé, F., Gebara, E., et al. (2015). Synaptic integration of adult-born hippocampal neurons is locally controlled by astrocytes. Neuron 88, 957–972. doi: 10.1016/j.neuron.2015.10.037
Sun, Y., Wu, S., Bu, G., Onifade, M. K., Patel, S. N., LaDu, M. J., et al. (1998). Glial fibrillary acidic protein–apolipoprotein E (apoE) transgenic mice: Astrocyte-specific expression and differing biological effects of astrocyte-secreted apoE3 and apoE4 lipoproteins. J. Neurosci. 18, 3261–3272. doi: 10.1523/JNEUROSCI.18-09-03261.1998
Suthard, R. L., Senne, R. A., Buzharsky, M. D., Diep, A. H., Pyo, A. Y., and Ramirez, S. (2024). Engram reactivation mimics cellular signatures of fear. Cell Rep. 43:113850. doi: 10.1016/j.celrep.2024.113850
Suzuki, A., Stern, S. A., Bozdagi, O., Huntley, G. W., Walker, R. H., Magistretti, P. J., et al. (2011). Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144, 810–823. doi: 10.1016/j.cell.2011.02.018
Suzuki, M., Sasabe, J., Miyoshi, Y., Kuwasako, K., Muto, Y., Hamase, K., et al. (2015). Glycolytic flux controls D-serine synthesis through glyceraldehyde-3-phosphate dehydrogenase in astrocytes. Proc. Natl. Acad. Sci. U. S. A. 112, E2217–E2224. doi: 10.1073/pnas.1416117112
Tadi, M., Allaman, I., Lengacher, S., Grenningloh, G., and Magistretti, P. J. (2015). Learning-induced gene expression in the hippocampus reveals a role of neuron-astrocyte metabolic coupling in long term memory. PLoS One 10:e0141568. doi: 10.1371/journal.pone.0141568
Takano, T., Han, X., Deane, R., Zlokovic, B., and Nedergaard, M. (2007). Two-photon imaging of astrocytic Ca2+ signaling and the microvasculature in experimental mice models of Alzheimer’s disease. Ann. N. Y. Acad. Sci. 1097, 40–50. doi: 10.1196/annals.1379.004
Talantova, M., Sanz-Blasco, S., Zhang, X., Xia, P., Akhtar, M. W., Okamoto, S.-I., et al. (2013). Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc. Natl. Acad. Sci. U. S. A. 110, E2518–E2527. doi: 10.1073/pnas.1306832110
Tang, W., Szokol, K., Jensen, V., Enger, R., Trivedi, C. A., and Hvalby, Ø, et al. (2015). Stimulation-evoked Ca2+ signals in astrocytic processes at hippocampal CA3–CA1 synapses of adult mice are modulated by glutamate and ATP. J. Neurosci. 35, 3016–3021. doi: 10.1523/JNEUROSCI.3319-14.2015
Tani, H., Dulla, C. G., Farzampour, Z., Taylor-Weiner, A., Huguenard, J. R., and Reimer, R. J. (2014). A local glutamate-glutamine cycle sustains synaptic excitatory transmitter release. Neuron 81, 888–900. doi: 10.1016/j.neuron.2013.12.026
Tapella, L., Dematteis, G., Moro, M., Pistolato, B., Tonelli, E., Vanella, V. V., et al. (2022). Protein synthesis inhibition and loss of homeostatic functions in astrocytes from an Alzheimer’s disease mouse model: A role for ER-mitochondria interaction. Cell Death Dis. 13:878. doi: 10.1038/s41419-022-05324-4
TCW, J., Qian, L., Pipalia, N. H., Chao, M. J., Liang, S. A., Shi, Y., et al. (2022). Cholesterol and matrisome pathways dysregulated in astrocytes and microglia. Cell 185, 2213–2233.e25. doi: 10.1016/j.cell.2022.05.017.
Theis, M., Jauch, R., Zhuo, L., Speidel, D., Wallraff, A., Döring, B., et al. (2003). Accelerated hippocampal spreading depression and enhanced locomotory activity in mice with astrocyte-directed inactivation of connexin43. J. Neurosci. 23, 766–776. doi: 10.1523/JNEUROSCI.23-03-00766.2003
Theparambil, S. M., Kopach, O., Braga, A., Nizari, S., Hosford, P. S., Sagi-Kiss, V., et al. (2024). Adenosine signalling to astrocytes coordinates brain metabolism and function. Nature 632, 139–146. doi: 10.1038/s41586-024-07611-w
Tsien, J. Z., Huerta, P. T., and Tonegawa, S. (1996). The essential role of hippocampal CA1 NMDA receptor–dependent synaptic plasticity in spatial memory. Cell 87, 1327–1338. doi: 10.1016/S0092-8674(00)81827-9
Tsunematsu, T., Sakata, S., Sanagi, T., Tanaka, K. F., and Matsui, K. (2021). Region-specific and state-dependent astrocyte Ca2+ dynamics during the sleep-wake cycle in mice. J. Neurosci. 41, 5440–5452. doi: 10.1523/JNEUROSCI.2912-20.2021
van Deijk, A. F., Camargo, N., Timmerman, J., Heistek, T., Brouwers, J. F., Mogavero, F., et al. (2017). Astrocyte lipid metabolism is critical for synapse development and function in vivo. Glia 65, 670–682. doi: 10.1002/glia.23120
Viana, J. F., Machado, J. L., Abreu, D. S., Veiga, A., Barsanti, S., Tavares, G., et al. (2023). Astrocyte structural heterogeneity in the mouse hippocampus. Glia 71, 1667–1682. doi: 10.1002/glia.24362
Viola, G. G., Rodrigues, L., Américo, J. C., Hansel, G., Vargas, R. S., Biasibetti, R., et al. (2009). Morphological changes in hippocampal astrocytes induced by environmental enrichment in mice. Brain Res. 1274, 47–54. doi: 10.1016/j.brainres.2009.04.007
Walker, M. P., and Stickgold, R. (2004). Sleep-dependent learning and memory consolidation. Neuron 44, 121–133. doi: 10.1016/j.neuron.2004.08.031
Wallraff, A., Köhling, R., Heinemann, U., Theis, M., Willecke, K., and Steinhäuser, C. (2006). The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J. Neurosci. 26, 5438–5447. doi: 10.1523/JNEUROSCI.0037-06.2006
Wang, F., Smith, N. A., Xu, Q., Fujita, T., Baba, A., Matsuda, T., et al. (2012). Astrocytes modulate neural network activity by Ca2+-dependent uptake of extracellular K+. Sci. Signal. 5:ra26. doi: 10.1126/scisignal.2002334
Wang, H., and Eckel, R. H. (2014). What are lipoproteins doing in the brain? Trends Endocrinol. Metab. 25, 8–14. doi: 10.1016/j.tem.2013.10.003
Wang, H., Kulas, J. A., Wang, C., Holtzman, D. M., Ferris, H. A., and Hansen, S. B. (2021). Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol. Proc. Natl. Acad. Sci. U. S. A. 118:e2102191118. doi: 10.1073/pnas.2102191118
Whitlock, J. R., Heynen, A. J., Shuler, M. G., and Bear, M. F. (2006). Learning induces long-term potentiation in the hippocampus. Science 313, 1093–1097. doi: 10.1126/science.1128134
Williamson, M. R., Kwon, W., Woo, J., Ko, Y., Maleki, E., Yu, K., et al. (2025). Learning-associated astrocyte ensembles regulate memory recall. Nature 637, 478–486. doi: 10.1038/s41586-024-08170-w
Wilson, M. A., and McNaughton, B. L. (1994). Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679. doi: 10.1126/science.8036517
Wu, Y.-W., Tang, X., Arizono, M., Bannai, H., Shih, P.-Y., Dembitskaya, Y., et al. (2014). Spatiotemporal calcium dynamics in single astrocytes and its modulation by neuronal activity. Cell Calcium 55, 119–129. doi: 10.1016/j.ceca.2013.12.006
Yang, Y., Ge, W., Chen, Y., Zhang, Z., Shen, W., Wu, C., et al. (2003). Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc. Natl. Acad. Sci. U. S. A. 100, 15194–15199. doi: 10.1073/pnas.2431073100
Zaborszky, L., Hoemke, L., Mohlberg, H., Schleicher, A., Amunts, K., and Zilles, K. (2008). Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. NeuroImage 42, 1127–1141. doi: 10.1016/j.neuroimage.2008.05.055
Zalutsky, R. A., and Nicoll, R. A. (1990). Comparison of two forms of long-term potentiation in single hippocampal neurons. Science 248, 1619–1624. doi: 10.1126/science.2114039
Zhang, J., and Liu, Q. (2015). Cholesterol metabolism and homeostasis in the brain. Protein Cell 6, 254–264. doi: 10.1007/s13238-014-0131-3
Zheng, K., Bard, L., Reynolds, J. P., King, C., Jensen, T. P., Gourine, A. V., et al. (2015). Time-resolved imaging reveals heterogeneous landscapes of nanomolar Ca2+ in neurons and astroglia. Neuron 88, 277–288. doi: 10.1016/j.neuron.2015.09.043
Zhong, S., Kiyoshi, C. M., Du, Y., Wang, W., Luo, Y., Wu, X., et al. (2023). Genesis of a functional astrocyte syncytium in the developing mouse hippocampus. Glia 71, 1081–1098. doi: 10.1002/glia.24327
Keywords: astrocyte, hippocampus, calcium signaling, metabolism, synaptic transmission, learning and memory
Citation: Squires A and Park J (2025) Emerging roles of astrocytes in hippocampal circuitry and behavior. Front. Cell. Neurosci. 19:1694643. doi: 10.3389/fncel.2025.1694643
Received: 28 August 2025; Revised: 30 October 2025; Accepted: 06 November 2025;
Published: 21 November 2025.
Edited by:
Bo Hu, Houston Methodist Research Institute, United StatesReviewed by:
Mathieu Letellier, UMR5297 Institut Interdisciplinaire de Neurosciences (IINS), FranceAres Orlando Cuellar-Santoyo, Instituto Potosino de Investigación Científica y Tecnológica (IPICYT), Mexico
Copyright © 2025 Squires and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joongkyu Park, am9vbmdreXUucGFya0B3YXluZS5lZHU=
 Ada Squires
Ada Squires Joongkyu Park
Joongkyu Park