- 1Department of Orthopaedics, The Second Affiliated Hospital of Anhui Medical University, Hefei, China
- 2Institute of Orthopaedics, Research Center for Translational Medicine, The Second Affiliated Hospital of Anhui Medical University, Hefei, China
Cognitive impairment is a frequent but underrecognized complication of neurodegenerative and traumatic central nervous system disorders. Although research on Alzheimer’s disease (AD) revealed that microglial triggering receptor expressed on myeloid cells 2 (TREM2) plays a critical role in inhibiting neuroinflammation and improving cognition, its contribution to cognitive impairment following spinal cord injury (SCI) is unclear. Evidence from AD shows that TREM2 drives microglial activation, promotes pathological protein clearance, and disease-associated microglia (DAM) formation. SCI patients also experience declines in attention, memory, and other functions, yet the specific mechanism of these processes remains unclear. In SCI, microglia and TREM2 are involved in inflammation and repair, but their relationship with higher cognitive functions has not been systematically examined. We infer that TREM2 might connect injury-induced neuroinflammation in the SCI with cognitive deficits, providing a new treatment target. Artificial intelligence (AI) offers an opportunity to accelerate this endeavor by incorporating single-cell transcriptomics, neuroimaging, and clinical data for the identification of TREM2-related disorders, prediction of cognitive trajectories, and applications to precision medicine. Novel approaches or modalities of AI-driven drug discovery and personalized rehabilitation (e.g., VR, brain–computer interface) can more precisely steer these interventions. The interface between lessons learned from AD and SCI for generating new hypotheses and opportunities for translation.
1 Introduction
Cognitive impairment is a common complication in neurodegenerative and traumatic CNS (CNS) disorders (Jessen et al., 2014; Wang C. et al., 2022; Mathys et al., 2023). Memory, attention, and executive impairments in Alzheimer’s disease (AD) are well-known phenomena strongly contributing to the overall disease process (Leuzy et al., 2024; Nasb et al., 2024; Peña-Bautista et al., 2024; Reyes et al., 2025). Also, spinal cord injury (SCI) patients often suffer from problems with attention, learning, and memory, which affect the ability to rehabilitate and life quality (Pasipanodya et al., 2021; Shabany et al., 2022; Vaccaro et al., 2022; Yang et al., 2023; Li Y. et al., 2024; Welkamp et al., 2024). These findings indicate cognition to have a critical impact on neurological outcome in various situations.
As the resident immune cells of the CNS, microglia are important in shaping cognitive functions (Haure-Mirande et al., 2022; Shi K. et al., 2022; Shi Q. et al., 2022). Besides immune surveillance, they are involved in synaptic pruning, neurogenesis, and circuit remodeling (Bellver-Landete et al., 2019; Zhou et al., 2020; Brennan et al., 2022; Choi et al., 2023). Impaired microglial activity has been proposed to be linked to impairment of cognitive function, indicating that immune–neural interaction may be a shared mechanism common in different diseases (Zhang et al., 2022).
One major advance is the identification of TREM2 (triggering receptor expressed on myeloid cells 2) as a key regulator of microglial functions (Haure-Mirande et al., 2022; Shi K. et al., 2022; Shi Q. et al., 2022). In AD, genetic variants in TREM2 increase disease risk, and functional studies show that TREM2 signaling promotes microglial responses, enhances clearance of pathological proteins, and influences cognitive outcomes (Jiang et al., 2014; Fracassi et al., 2023; Huang et al., 2023; Li et al., 2023). These findings highlight TREM2 as both a mechanistic driver and a therapeutic target.
Cognitive impairment in SCI gradually receives more attention, although the mechanisms of the process are not well understood (Craig et al., 2017; Sachdeva et al., 2018; Li et al., 2020; Alcántar-Garibay et al., 2022). Research has mostly focused on systemic inflammation, chronic pain, and mood disorders (Jure and Labombarda, 2017; Molina-Gallego et al., 2024), and the role of unique immune pathway proteins such as TREM2 has been less well researched (Craig et al., 2017; Sachdeva et al., 2018; Li et al., 2020; Alcántar-Garibay et al., 2022). This gap restricts our mechanistic knowledge and therapy development.
The question at the core of this review is whether mechanistic insights from AD, including those addressing microglial TREM2, may inform pathways leading to cognitive dysfunction post-SCI. Cognitive impairment following SCI has been stably demonstrated in both animal and human studies (Craig et al., 2017; Sachdeva et al., 2018; Li et al., 2020; Alcántar-Garibay et al., 2022), with activation of microglia in the hippocampus and prefrontal cortex implicated in the induction of a chronic, low-grade neuroinflammatory state that impairs synaptic homeostasis and neuronal plasticity (Jure et al., 2017; Yu et al., 2024). TREM2 mutations clearly lead to cognitive decline in AD patients (Jiang et al., 2014; Fracassi et al., 2023; Li et al., 2023). Yet again, sensitizing evidence directly connecting TREM2 mutations with post-SCI cognitive impairments is still absent; such linkage currently constitutes an inferred hypothesis based on the mechanistic overlap of AD and SCI. In line with this, the present review will (1) bring together consolidated knowledge from AD and SCI concerning microglial TREM2 and cognition, and (2) comment on the role of artificial intelligence (AI) in assisting hypothesis generation and translational breakthroughs in this nascent field.
Here, we propose that the empirical knowledge in AD can provide important clues for exploring the role of the TREM2 signaling pathway-mediated microglial cell response in cognitive impairment following SCI. We also review the potential role of AI to expedite progress by integrating multimodal datasets, discovering therapeutic targets, and guiding individualized rehabilitation. Collectively, this framework can integrate neuroimmunology with cognition and computation for the development of translational strategies.
2 Cognitive dysfunction and microglial TREM2 in Alzheimer’s disease
2.1 Cognitive dysfunction in AD
To understand the potential contribution of TREM2 in SCI-related cognitive deficits, it is necessary to first summarize its established role in AD. Cognitive impairment is a typical symptom of AD, consisting most prominently of amnesia and executive deficits (Collij et al., 2024). Patients with AD commonly experience declines in encoding and retrieving information, problem-solving, as well as monitoring goal-directed behavior (Bäckman et al., 2004; Moguilner et al., 2024). These impairments are caused by neuropathological features such as tau pathology, synaptic loss, and network dysfunction, which mainly affect the integrity of cognitive circuits (Lin et al., 2022, 2025; Hu et al., 2024).
2.2 TREM2 as a regulator of microglial function in AD
TREM2 is a transmembrane receptor that is mainly expressed on microglia of the CNS, where it has an essential role in the regulation of microglial activation, phagocytosis, as well as inflammatory responses (Li et al., 2022; Li Z. et al., 2024; Yan et al., 2022). Through these functions, TREM2 promotes the ability of microglia to respond to neuronal injury and maintain CNS homeostasis (Kobayashi et al., 2016; Nugent et al., 2020).
In AD, TREM2 has been identified as a critical regulator in neuroimmune processes that maintain cognitive function (Li et al., 2022). Functionally (Figure 1), TREM2 promotes the clearance of deposited amyloid-β (Aβ) plaques and relieves neuronal toxicity (Nugent et al., 2020). Additionally, TREM2 can drive the transformation of baseline microglia into “disease-associated microglia” (DAM) phenotype, which upregulates the phagocytosis of Aβ and other microglial responses (Nugent et al., 2020). These microglial responses are associated with maintenance of synaptic integrity and cognition in experimental models (Wang S. et al., 2022).
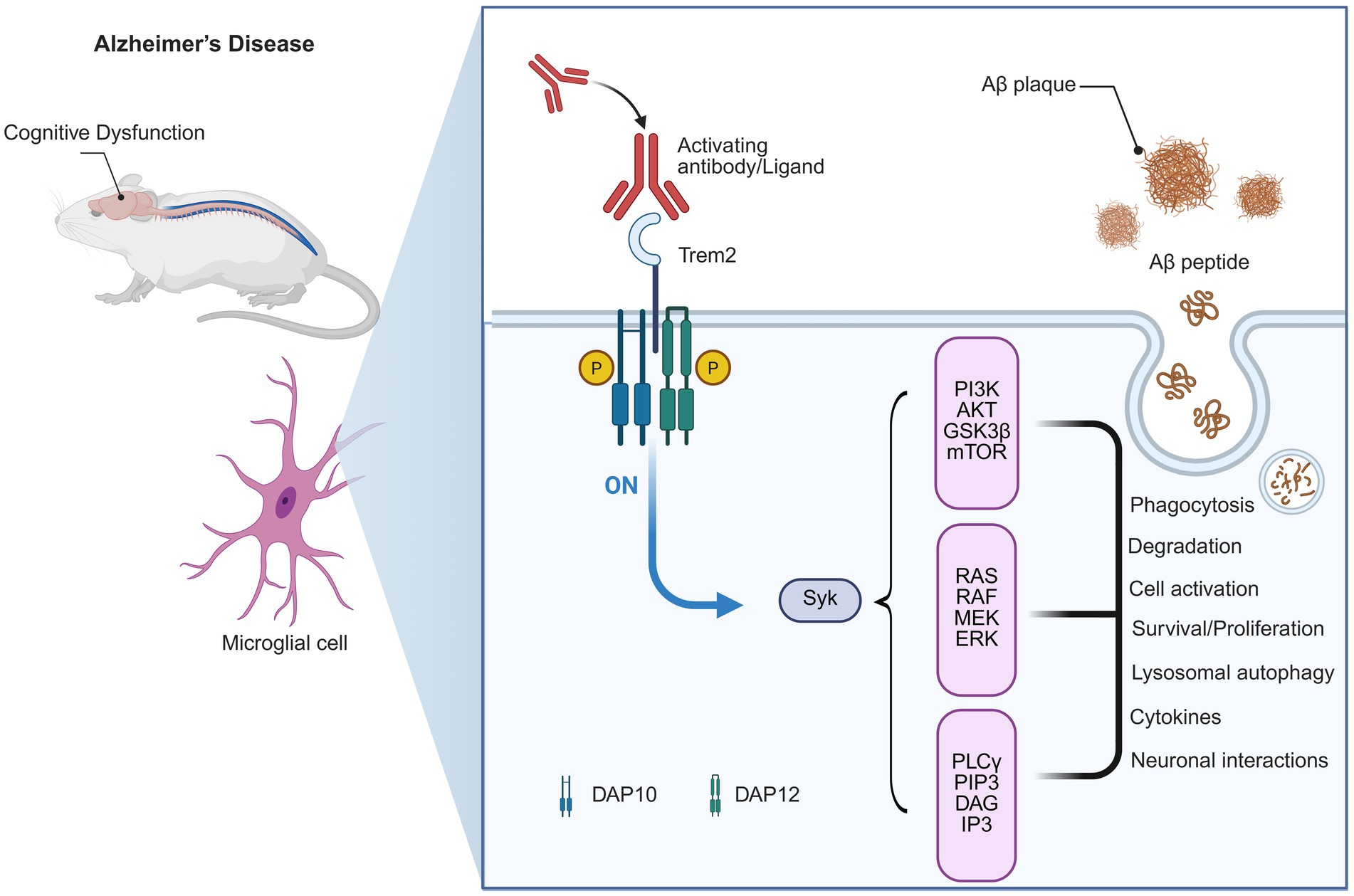
Figure 1. TREM2 signaling pathway in Alzheimer’s Disease and its link to cognitive dysfunction. This diagram illustrates the role of TREM2 in Alzheimer’s disease (AD) pathology and its association with cognitive dysfunction. In AD, activating antibodies or ligands (including amyloid-beta plaques) can activate TREM2. This activation triggers downstream signaling pathways, including the PI3K-AKT, RAS-RAF-MEK-ERK, and PLCγ pathways. These pathways are involved in critical cellular processes, such as Aβ plaque phagocytosis, degradation, and the modulation of neuroinflammation, all of which influence the progression of cognitive decline in AD. The activation of TREM2 and its signaling also plays a role in microglial cell activation and neuronal interactions, ultimately impacting the neurodegenerative process.
Genetic investigations further emphasize the importance of TREM2 for cognitive functions (Rachmian et al., 2024). Rare TREM2 variants are strongly associated with increased risk for AD, and individuals who carry these mutations show an earlier age of onset or more severe cognitive decline than noncarriers (Peng et al., 2023). Taken all together, these observations place TREM2 as both a key regulator of microglial activity and as a potential drug target to prevent cognitive impairment in AD (Jia et al., 2025).
3 Cognitive dysfunction and microglial TREM2 in spinal cord injury
3.1 Overlooked cognitive dysfunction after SCI
Based on AD findings, we next consider whether the analogous mechanisms may apply to SCI, while noting the limited direct evidence. Although SCI has conventionally been regarded as a disorder characterized by motor and sensory dysfunction, there is now accumulating literature to indicate that cognitive impairment is an important but under-recognized consequence (Craig et al., 2017; Sachdeva et al., 2018; Li et al., 2020; Alcántar-Garibay et al., 2022). Attention, working memory, and processing speed are often impaired in patients with SCI, which can interfere with rehabilitation and daily life quality (Craig et al., 2017; Sachdeva et al., 2018; Li et al., 2020; Alcántar-Garibay et al., 2022). In addition to the cognitive decline, such components as chronic pain, sleep disturbances, and mood disorders often worsen cognitive load and function outcome (Widerström-Noga, 2017; Eller et al., 2022; Wu et al., 2023).
The specific mechanisms of cognitive impairment after SCI remain poorly understood (Welkamp et al., 2024). Previous research in SCI has largely focused on systemic inflammation, secondary injury cascades, and psychosocial factors, and ignored molecular drivers of cognitive impairment (Brennan et al., 2022, 2024). Nevertheless, clinical neuroimaging evidence indicates that SCI may produce structural and functional changes in the brain, such as disrupted connections within prefrontal and hippocampal networks, which are important for attention, memory, and executive function (Jure and Labombarda, 2017; Welkamp et al., 2024). These data suggest that SCI has consequences beyond the damage in the spinal cord, affecting the wider networks that participate in cognitive processes.
Considering the dependence of microglia for modulating cognitive function in other neurologic diseases, it is possible that microglial pathways also participate in the impaired cognition process after SCI (Jure et al., 2017). In particular, molecules such as TREM2, which regulate microglial activation and synaptic remodeling in AD, may play a similar role in SCI (Gao et al., 2023; Zhao C. et al., 2025). Investigating TREM2-mediated pathological processes in the context of SCI could provide effective strategies for exploring the pathophysiology of cognitive impairment and identifying therapeutic targets as well as developing corresponding drugs for mitigating these often-overlooked deficits.
Clinical and neuroimaging studies suggest that SCI can bring substantial changes to brain network connectivity and functional activation patterns, specifically within regions implicated in attention and memory (Jure et al., 2022; Qin et al., 2025). Regardless of the causes, the mechanisms of cognitive impairment in AD and SCI may partially overlap (Jure et al., 2017; Oveisgharan et al., 2018). Substantial neuroinflammation can disrupt neural circuit remodeling and synaptic plasticity due to the prolonged activation of brain microglia (Jure et al., 2017; Oveisgharan et al., 2018; Doorduijn et al., 2019; Deng et al., 2024). Changes in neurotransmitter signaling, dendritic spine density, and synaptic connectivity also continue to hinder the ease of information transfer (Bäckman et al., 2004; Moguilner et al., 2024). Altogether, these findings suggest that immune-mediated synaptopathies and circuit changes may represent a common pathological substrate for impaired cognition in both neurodegenerative and traumatic CNS disorders.
3.2 TREM2 as a potential regulator in SCI-induced neuroinflammation and dysfunction
Microglial TREM2 was identified as a critical modulator for neuroinflammation and cognitive function in AD, whereas its involvement in SCI remains largely unknown (Gao et al., 2023; Zhao T. et al., 2025). SCI can induce microglial activation not only at the lesion site but also in supraspinal regions, which may have a dual effect in chronic inflammation and neuronal damage (Milich et al., 2021; Gong et al., 2023; Skinnider et al., 2024). Given TREM2’s role to modulate microglial phagocytic activity, inflammatory cytokine release, and synaptic remodeling (Kobayashi et al., 2016; Li et al., 2022; Li Z. et al., 2024; Yan et al., 2022), it is reasonable to speculate that the cognitive performance following SCI may also be modulated by TREM2 (Figure 2).
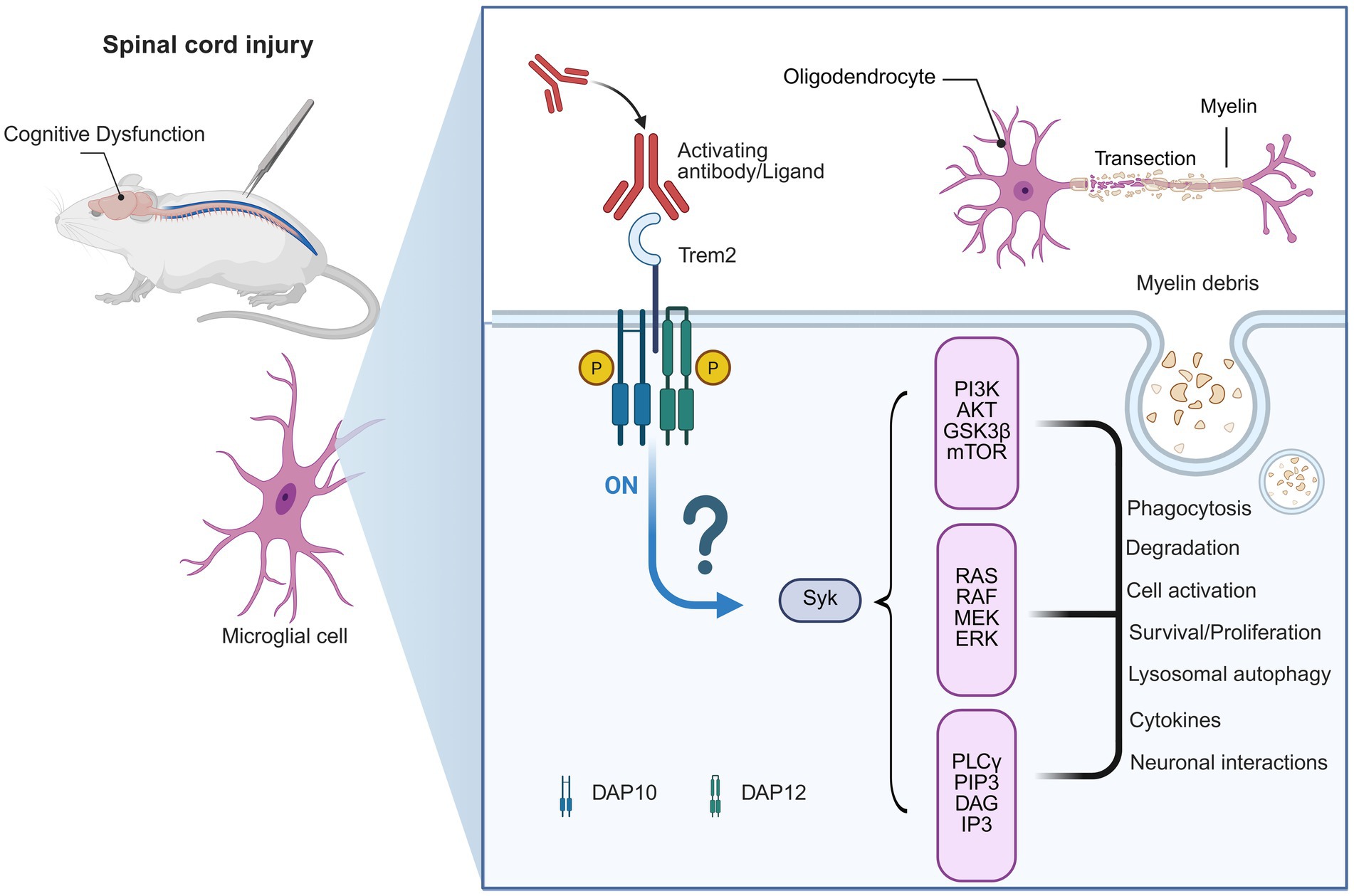
Figure 2. Hypothetical mechanism of TREM2 in spinal cord injury-induced cognitive dysfunction. This figure illustrates a hypothetical model. Although microglial activation after spinal cord injury (SCI) is well established, the specific pathways through which these cells exert their functions remain unclear. This diagram shows a potential mechanism for cognitive dysfunction following SCI. While research on TREM2’s role in SCI is limited, we hypothesize that TREM2 may play a role in SCI-related cognitive impairment. SCI leads to myelin debris produced and microglial activation. TREM2 could be involved in clearing myelin debris at the injury site, and may also contribute to other changes in the brain following SCI, potentially influencing neuroinflammation, neuronal survival, and cognitive function, similar to its role in Alzheimer’s disease.
Data from AD support the idea that TREM2 has been shown to drive the conversion of microglia into DAM that are involved in the clearance of cellular debris and maintenance of the synaptic integrity, which are critical for improving cognitive capacity (Nugent et al., 2020; Wu et al., 2022; Parhizkar et al., 2023; John et al., 2025; Zhu et al., 2025; Wu et al., 2022; Parhizkar et al., 2023; John et al., 2025; Zhu et al., 2025). It should be stressed that these are hypothesis-driven extrapolations, as there is little experimental or clinical SCI-specific cognition-related data on TREM2. Therefore, the discussion below should be read as a conceptual comparison rather than evidence-based. If we extend these findings to SCI, it can be speculated that TREM2-related microglial responses may reduce maladaptive inflammation, ameliorate synaptic plasticity in cortical and hippocampal circuits, and ultimately prevent attention and memory deficits. Such a mechanistic model offers a new insight into the cellular substrates of SCI-induced cognitive deficiency.
4 Therapeutic implications of targeting TREM2 in SCI
Investigating TREM2 in the context of SCI also opens avenues for therapeutic innovation (Gao et al., 2023; Zhao T. et al., 2025). By targeting TREM2, it may be possible to modulate microglial activity in a way that both reduces chronic neuroinflammation and enhances cognitive resilience (Yan et al., 2022; Li Z. et al., 2024). These approaches can potentially complement current rehabilitation paradigms, providing precision medicine guidelines. In addition, when AI is used for analyzing multimodal datasets (single cell transcriptomics, neuroimaging, and clinical cognitive outcomes), it will help speed up the discovery of these TREM2-related mechanisms/facilitate the potential intervention points that could open vistas towards next-generation therapies (Kalaga and Ray, 2025; Liu X. et al., 2025). While most research stresses protective roles of TREM2, other studies also indicate that TREM2 activation may potentially exacerbate inflammation or impair recovery after SCI (Zhao T. et al., 2025). These findings highlight the need for context-specific investigations.
5 AI-assisted strategies for discovery and translation
5.1 Computational approaches for mechanism discovery and therapy design
With these mechanistic understandings in hand, we now consider AI as a potential catalyst for discovery and translation (Liu et al., 2024b, 2025c). AI offers great potential to expedite the discovery of microglial targets in cognitive impairment from SCI (Kalaga and Ray, 2025). Leveraging on big-data resources derived from single-cell transcriptomics, proteomics, brain imaging, and cognitive clinical data can connect TREM2-high expressed microglial subpopulations with inferred functional states and predictions of their potential consequence for synapse plasticity and neural circuit performance (Liu X. et al., 2025). Such analyses may reveal novel cellular and molecular pathways connecting SCI-induced neuroinflammation with cognitive impairment (Table 1).
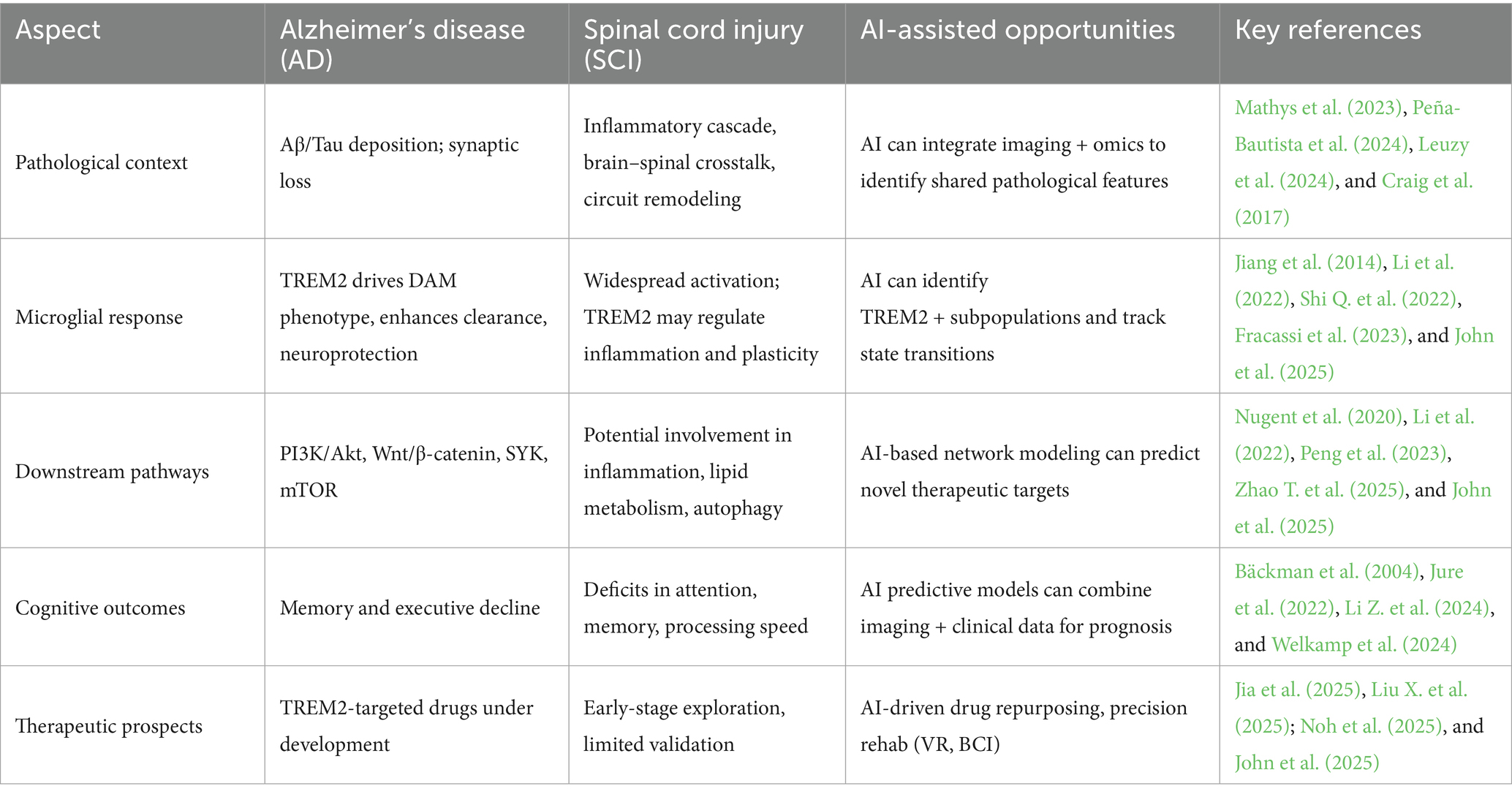
Table 1. TREM2’s role in Alzheimer’s disease (AD)/spinal cord injury (SCI) and AI-assisted opportunities.
In addition to mechanism discovery, AI has potential in guiding the direction for therapeutic development (Liu et al., 2020; Fang et al., 2022). Deep learning and computational modeling can promote high-throughput virtual screening of small molecules or biologicals targeting TREM2 activity for prioritizing candidates with desirable efficacy and safety profiles (Fang et al., 2022; Liu X. et al., 2025). Furthermore, AI-based predictive models can predict patient-specific cognitive trajectories to design precision interventions for each SCI patient according to molecular and clinical biomarkers (Möhle et al., 2021; Trivedi et al., 2023; Calderone et al., 2024; Kale et al., 2024; Bitsch et al., 2025; Noh et al., 2025).
AI can also strengthen cognitive rehabilitation approaches (Lee et al., 2023; Liu et al., 2025a). Brain–computer interfaces, virtual reality platforms, and adaptive neurofeedback systems can be integrated with AI models to dynamically adjust training programs according to real-time cognitive performance and microglial biomarker profiles, leading to adaptive neurofeedback systems modifying training programs (Lee et al., 2023; Kishikawa et al., 2024; Yoo et al., 2024; Liu et al., 2025b; Noh et al., 2025). These approaches could enable tailored interventions to the enhancement or suppression of neural plasticity and immune-mediated responses that may be dovetailed in an attempt to best optimize individual recovery therapies (Kale et al., 2024). For example, a group proposed an AI-based motion analysis for rehabilitation in patients with SCI (Lee et al., 2023). Another group verified the machine learning models for prediction after cervical SCI (Kishikawa et al., 2024), whereas some researchers used neural networks to detect neuropathic pain signatures following SCI (Deulofeu et al., 2024). In TREM2-related biology, some studies applied interpretable deep learning to represent microglial activation states in AD and is a methodological blueprint for SCI (Trivedi et al., 2023). In contrast to classical statistics, these methods enable to combine high-dimensional data and to make prediction for individual-patient, that constitutes an exceptional feature.
Collectively, AI-augmented methods provide a complementary environment to connect basic mechanistic insights with translational and clinical practices (Deng et al., 2020; Perosa et al., 2021; Pancholi et al., 2024; Tao et al., 2024). By combining both computational resources and neuroimmunological experience, these approaches could hasten the discovery of new TREM2-directed interventions and enhance cognitive recovery in SCI patients.
5.2 Challenges and future directions in AI-driven neuroimmunology
Although research on TREM2 has revealed how microglia are regulated, some obvious challenges still exist when applying these findings to understand cognitive impairment after SCI (Gao et al., 2023; Zhao T. et al., 2025). First, direct experimental evidence of a role for TREM2 in cognition following SCI is lacking (Zhao T. et al., 2025), and knowledge remains scarce and needs to be filled by targeted preclinical studies. Second, the heterogeneity of SCI patients (e.g., level and severity of lesion, age, and comorbidities) leads to a lack of common biomarkers and therapeutic targets (Welkamp et al., 2024). Third, current bio-verification of computerized AI-based predictions is only available in the context of standardized (multi-)modal data types and more complicated computational processing pipelines that have not been broadly validated across the SCI research community (Deulofeu et al., 2024; Habibi et al., 2024; Daungsupawong and Wiwanitkit, 2025). Fourth, AI also faces barriers, including limited availability of high-quality multimodal datasets, lack of reproducibility across cohorts, and difficulties in regulatory validation for clinical use (Liu et al., 2024a).
More work remains for modest investigation of the TREM2 role with post-SCI cognitive outcomes, which may be carried out in animal models, single cells at the molecular level, as well as patients by means of longitudinal clinical studies (Španić Popovački et al., 2023; Zhang et al., 2023, 2025). Furthermore, interdisciplinary approaches fusing neuroimmunology with cognitive neuroscience and computational modelling are required to help design predictive personalized treatments (Pereira et al., 2022; Kale et al., 2024). For instance, AI-driven drug discovery and patient stratification, as well as adaptive rehabilitation programs, have emerged as very promising options to translate mechanistic insights into clinical interventions (Fang et al., 2022; Liu X. et al., 2025). The field can thus progress toward more specific approaches to treat cognitive dysfunction in SCI patients.
6 Summary and outlook
Cognitive impairment is an important but relatively unappreciated complication of SCI and has far-reaching effects on patient outcome and quality of life. Given that microglial TREM2 is a known modulator of neuroinflammation and cognition in AD, it provides an attractive target for mechanistic studies relevant to SCI. Merging insights from AD and SCI research conceptualizes how TREM2-dependent microglial responses can affect attention, memory, and executive function following CNS injury.
AI could further improve this framework by helping to define TREM2-related pathways, drug discovery, and personalized cognitive rehabilitation. Connecting neuroimmunology, the cognitive sciences, and AI-led therapeutics, we provide a perspective on new avenues for both mechanistic knowledge and translational impact. Prospective investigations in this category will be able to improve cognitive status and the general recovery process for SCI by focusing on TREM2.
Author contributions
ZW: Resources, Visualization, Writing – original draft. SY: Conceptualization, Funding acquisition, Writing – review & editing. DT: Conceptualization, Funding acquisition, Writing – review & editing. LC: Conceptualization, Funding acquisition, Writing – review & editing. JJ: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (Grant numbers 82271413, 82401616), Anhui Provincial Clinical Research Transformation Project (Grant numbers 202304295107020013, 202304295107020009, 202304295107020011), Natural Science Foundation of Anhui Province (2408085QH262), and Natural Science Research Key Project of Colleges and Universities of Anhui Province (Grant number: KJ2021A0310).
Acknowledgments
We acknowledge the experimental platform provided by the Scientific Research and Experiment Center of the Second Hospital of Anhui Medical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AD, Alzheimer’s disease; AI, Artificial intelligence; Aβ, amyloid-β; CNS, central nervous system; DAM, disease-associated microglia; SCI, Spinal cord injury; TREM2, triggering receptor expressed on myeloid cells 2.
References
Alcántar-Garibay, O., Incontri-Abraham, D., and Ibarra, A. (2022). Spinal cord injury-induced cognitive impairment: a narrative review. Neural Regen. Res. 17:2649. doi: 10.4103/1673-5374.339475
Bäckman, L., Jones, S., Berger, A. -K., Laukka, E. J., and Small, B. J. (2004). Multiple cognitive deficits during the transition to Alzheimer’s disease. J. Intern. Med. 256, 195–204. doi: 10.1111/j.1365-2796.2004.01386.x
Bellver-Landete, V., Bretheau, F., Mailhot, B., Vallières, N., Lessard, M., Janelle, M.-E., et al. (2019). Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun. 10:518. doi: 10.1038/s41467-019-08446-0
Bitsch, A., Henrich, M., Körber, S. S. E., Büttner, K., and Herden, C. (2025). Establishment of an AI-supported scoring system for neuroglial cells. Front. Cell. Neurosci. 19:1584422. doi: 10.3389/fncel.2025.1584422
Brennan, F. H., Li, Y., Wang, C., Ma, A., Guo, Q., Li, Y., et al. (2022). Microglia coordinate cellular interactions during spinal cord repair in mice. Nat. Commun. 13:4096. doi: 10.1038/s41467-022-31797-0
Brennan, F. H., Swarts, E. A., Kigerl, K. A., Mifflin, K. A., Guan, Z., Noble, B. T., et al. (2024). Microglia promote maladaptive plasticity in autonomic circuitry after spinal cord injury in mice. Sci. Transl. Med. 16:eadi3259. doi: 10.1126/scitranslmed.adi3259
Calderone, A., Latella, D., Bonanno, M., Quartarone, A., Mojdehdehbaher, S., Celesti, A., et al. (2024). Towards transforming neurorehabilitation: the impact of artificial intelligence on diagnosis and treatment of neurological disorders. Biomedicine 12:2415. doi: 10.3390/biomedicines12102415
Choi, B.-R., Johnson, K. R., Maric, D., and McGavern, D. B. (2023). Monocyte-derived IL-6 programs microglia to rebuild damaged brain vasculature. Nat. Immunol. 24, 1110–1123. doi: 10.1038/s41590-023-01521-1
Collij, L. E., Mastenbroek, S. E., Mattsson-Carlgren, N., Strandberg, O., Smith, R., Janelidze, S., et al. (2024). Lewy body pathology exacerbates brain hypometabolism and cognitive decline in Alzheimer’s disease. Nat. Commun. 15:8061. doi: 10.1038/s41467-024-52299-1
Craig, A., Guest, R., Tran, Y., and Middleton, J. (2017). Cognitive impairment and mood states after spinal cord injury. J. Neurotrauma 34, 1156–1163. doi: 10.1089/neu.2016.4632
Daungsupawong, H., and Wiwanitkit, V. (2025). Artificial intelligence in spinal cord injury management: comment. Global Spine J. :21925682251328679. doi: 10.1177/21925682251328679
Deng, Q., Wu, C., Parker, E., Liu, T. C.-Y., Duan, R., and Yang, L. (2024). Microglia and astrocytes in Alzheimer’s disease: significance and summary of recent advances. Aging Dis. 15, 1537–1564. doi: 10.14336/AD.2023.0907
Deng, L., Zhong, W., Zhao, L., He, X., Lian, Z., Jiang, S., et al. (2020). Artificial intelligence-based application to explore inhibitors of neurodegenerative diseases. Front. Neurorobot. 14:617327. doi: 10.3389/fnbot.2020.617327
Deulofeu, M., Peña-Méndez, E. M., Vaňhara, P., Havel, J., Moráň, L., Pečinka, L., et al. (2024). Discriminating fingerprints of chronic neuropathic pain following spinal cord injury using artificial neural networks and mass spectrometry analysis of female mice serum. Neurochem. Int. 181:105890. doi: 10.1016/j.neuint.2024.105890
Doorduijn, A. S., Visser, M., Van De Rest, O., Kester, M. I., De Leeuw, F. A., Boesveldt, S., et al. (2019). Associations of AD biomarkers and cognitive performance with nutritional status: the NUDAD project. Nutrients 11:1161. doi: 10.3390/nu11051161
Eller, O. C., Willits, A. B., Young, E. E., and Baumbauer, K. M. (2022). Pharmacological and non-pharmacological therapeutic interventions for the treatment of spinal cord injury-induced pain. Front. Pain Res. 3:991736. doi: 10.3389/fpain.2022.991736
Fang, J., Zhang, P., Wang, Q., Chiang, C.-W., Zhou, Y., Hou, Y., et al. (2022). Artificial intelligence framework identifies candidate targets for drug repurposing in Alzheimer’s disease. Alzheimers Res. Ther. 14:7. doi: 10.1186/s13195-021-00951-z
Fracassi, A., Marcatti, M., Tumurbaatar, B., Woltjer, R., Moreno, S., and Taglialatela, G. (2023). TREM2 -induced activation of microglia contributes to synaptic integrity in cognitively intact aged individuals with Alzheimer’s neuropathology. Brain Pathol. 33:e13108. doi: 10.1111/bpa.13108
Gao, H., Di, J., Clausen, B. H., Wang, N., Zhu, X., Zhao, T., et al. (2023). Distinct myeloid population phenotypes dependent on TREM2 expression levels shape the pathology of traumatic versus demyelinating CNS disorders. Cell Rep. 42:112629. doi: 10.1016/j.celrep.2023.112629
Gong, L., Gu, Y., Han, X., Luan, C., Liu, C., Wang, X., et al. (2023). Spatiotemporal dynamics of the molecular expression pattern and intercellular interactions in the glial scar response to spinal cord injury. Neurosci. Bull. 39, 213–244. doi: 10.1007/s12264-022-00897-8
Habibi, M. A., Naseri Alavi, S. A., Soltani Farsani, A., Mousavi Nasab, M. M., Tajabadi, Z., and Kobets, A. J. (2024). Predicting the outcome and survival of patients with spinal cord injury using machine learning algorithms: a systematic review. World Neurosurg. 188, 150–160. doi: 10.1016/j.wneu.2024.05.103
Haure-Mirande, J.-V., Audrain, M., Ehrlich, M. E., and Gandy, S. (2022). Microglial TYROBP/DAP12 in Alzheimer’s disease: transduction of physiological and pathological signals across TREM2. Mol. Neurodegener. 17:55. doi: 10.1186/s13024-022-00552-w
Hu, J., Zhang, M., Zhang, Y., Zhuang, H., Zhao, Y., Li, Y., et al. (2024). Neurometabolic topography and associations with cognition in Alzheimer’s disease: a whole-brain high-resolution 3D MRSI study. Alzheimer’s Dement. 20, 6407–6422. doi: 10.1002/alz.14137
Huang, Q., Wang, Y., Chen, S., and Liang, F. (2023). Glycometabolic reprogramming of microglia in neurodegenerative diseases: insights from neuroinflammation. Aging Dis. 15, 1155–1175. doi: 10.14336/AD.2023.0807
Jessen, F., Amariglio, R. E., Van Boxtel, M., Breteler, M., Ceccaldi, M., Chételat, G., et al. (2014). A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 10, 844–852. doi: 10.1016/j.jalz.2014.01.001
Jia, B., Xu, Y., and Zhu, X. (2025). Cognitive resilience in Alzheimer’s disease: mechanism and potential clinical intervention. Ageing Res. Rev. 106:102711. doi: 10.1016/j.arr.2025.102711
Jiang, T., Tan, L., Zhu, X.-C., Zhang, Q.-Q., Cao, L., Tan, M.-S., et al. (2014). Upregulation of TREM2 ameliorates neuropathology and rescues spatial cognitive impairment in a transgenic mouse model of Alzheimer’s disease. Neuropsychopharmacology 39, 2949–2962. doi: 10.1038/npp.2014.164
John, R. K., Vogel, S. P., Zia, S., Lee, K. V., Nguyen, A. T., Torres-Espin, A., et al. (2025). Reawakening inflammation in the chronically injured spinal cord using lipopolysaccharide induces diverse microglial states. J. Neuroinflammation 22:56. doi: 10.1186/s12974-025-03379-6
Jure, I., De Nicola, A. F., Encinas, J. M., and Labombarda, F. (2022). Spinal cord injury leads to hippocampal glial alterations and neural stem cell inactivation. Cell. Mol. Neurobiol. 42, 197–215. doi: 10.1007/s10571-020-00900-8
Jure, I., and Labombarda, F. (2017). Spinal cord injury drives chronic brain changes. Neural Regen. Res. 12:1044. doi: 10.4103/1673-5374.211177
Jure, I., Pietranera, L., De Nicola, A. F., and Labombarda, F. (2017). Spinal cord injury impairs neurogenesis and induces glial reactivity in the Hippocampus. Neurochem. Res. 42, 2178–2190. doi: 10.1007/s11064-017-2225-9
Kalaga, P., and Ray, S. K. (2025). Mental health disorders due to gut microbiome alteration and NLRP3 inflammasome activation after spinal cord injury: molecular mechanisms, promising treatments, and aids from artificial intelligence. Brain Sci. 15:197. doi: 10.3390/brainsci15020197
Kale, M., Wankhede, N., Pawar, R., Ballal, S., Kumawat, R., Goswami, M., et al. (2024). AI-driven innovations in Alzheimer’s disease: integrating early diagnosis, personalized treatment, and prognostic modelling. Ageing Res. Rev. 101:102497. doi: 10.1016/j.arr.2024.102497
Kishikawa, J., Kobayakawa, K., Saiwai, H., Yokota, K., Kubota, K., Hayashi, T., et al. (2024). Verification of the accuracy of cervical spinal cord injury prognosis prediction using clinical data-based artificial neural networks. J. Clin. Med. 13:253. doi: 10.3390/jcm13010253
Kobayashi, M., Konishi, H., Sayo, A., Takai, T., and Kiyama, H. (2016). TREM2/DAP12 signal elicits proinflammatory response in microglia and exacerbates neuropathic pain. J. Neurosci. 36, 11138–11150. doi: 10.1523/JNEUROSCI.1238-16.2016
Lee, H. J., Jin, S. M., Kim, S. J., Kim, J. H., Kim, H., Bae, E., et al. (2023). Development and validation of an artificial intelligence-based motion analysis system for upper extremity rehabilitation exercises in patients with spinal cord injury: a randomized controlled trial. Healthcare 12:7. doi: 10.3390/healthcare12010007
Leuzy, A., Heeman, F., Bosch, I., Lenér, F., Dottori, M., Quitz, K., et al. (2024). REAL AD—validation of a realistic screening approach for early Alzheimer’s disease. Alzheimer’s Dement. 20, 8172–8182. doi: 10.1002/alz.14219
Li, Y., Cao, T., Ritzel, R. M., He, J., Faden, A. I., and Wu, J. (2020). Dementia, depression, and associated brain inflammatory mechanisms after spinal cord injury. Cells 9:1420. doi: 10.3390/cells9061420
Li, Y., Hu, Y., Pozzato, I., Arora, M., Schoffl, J., McBain, C., et al. (2024). Efficacy of interventions to improve cognitive function in adults with spinal cord injury: a systematic review. J. Neurotrauma 41, 2075–2088. doi: 10.1089/neu.2024.0032
Li, R.-Y., Qin, Q., Yang, H.-C., Wang, Y.-Y., Mi, Y.-X., Yin, Y.-S., et al. (2022). TREM2 in the pathogenesis of AD: a lipid metabolism regulator and potential metabolic therapeutic target. Mol. Neurodegener. 17:40. doi: 10.1186/s13024-022-00542-y
Li, Y., Xu, H., Wang, H., Yang, K., Luan, J., and Wang, S. (2023). TREM2: potential therapeutic targeting of microglia for Alzheimer’s disease. Biomed. Pharmacother. 165:115218. doi: 10.1016/j.biopha.2023.115218
Li, Z., Yu, S., Li, L., Zhou, C., Wang, L., Tang, S., et al. (2024). TREM2 alleviates white matter injury after traumatic brain injury in mice might be mediated by regulation of DHCR24/LXR pathway in microglia. Clin. Transl. Med. 14:e1665. doi: 10.1002/ctm2.1665
Lin, L., Ni, L., Wang, X., and Sheng, C. (2022). Longitudinal cognitive change and duration of Alzheimer’s disease stages in relation to cognitive reserve. Neuroscience 504, 47–55. doi: 10.1016/j.neuroscience.2022.09.017
Lin, Y., Shi, X., Mu, J., Ren, H., Jiang, X., Zhu, L., et al. (2025). Uncovering stage-specific neural and molecular progression in Alzheimer’s disease: implications for early screening. Alzheimers Dement. 21:e70182. doi: 10.1002/alz.70182
Liu, G., Ren, S., Wang, J., and Zhou, W. (2025a). Efficient group cosine convolutional neural network for EEG-based seizure identification. IEEE Trans. Instrum. Meas. 74, 1–14. doi: 10.1109/TIM.2025.3569362
Liu, G., Wen, Y., Hsiao, J. H., Zhang, D., Tian, L., and Zhou, W. (2024a). EEG-based familiar and unfamiliar face classification using filter-Bank differential entropy features. IEEE Trans. Human-Mach. Syst. 54, 44–55. doi: 10.1109/THMS.2023.3332209
Liu, X., Xu, J., Zheng, S., Yang, Y., Xie, Y., Liu, J., et al. (2025). AI-driven discovery of brain-penetrant Galectin-3 inhibitors for Alzheimer’s disease therapy. Pharmacol. Res. 218:107834. doi: 10.1016/j.phrs.2025.107834
Liu, G., Zhang, J., Chan, A. B., and Hsiao, J. H. (2024b). Human attention guided explainable artificial intelligence for computer vision models. Neural Netw. 177:106392. doi: 10.1016/j.neunet.2024.106392
Liu, G., Zhang, R., Tian, L., and Zhou, W. (2025b). Fine-grained spatial-frequency-time framework for motor imagery brain–computer Interface. IEEE J. Biomed. Health Inform. 29, 4121–4133. doi: 10.1109/JBHI.2025.3536212
Liu, G., Zheng, Y., Tsang, M. H. L., Zhao, Y., and Hsiao, J. H. (2025c). Understanding the role of eye movement pattern and consistency during face recognition through EEG decoding. NPJ Sci. Learn. 10:28. doi: 10.1038/s41539-025-00316-3
Liu, G., Zhou, W., and Geng, M. (2020). Automatic seizure detection based on S-transform and deep convolutional neural network. Int. J. Neural Syst. 30:1950024. doi: 10.1142/S0129065719500242
Mathys, H., Peng, Z., Boix, C. A., Victor, M. B., Leary, N., Babu, S., et al. (2023). Single-cell atlas reveals correlates of high cognitive function, dementia, and resilience to Alzheimer’s disease pathology. Cell 186, 4365–4385.e27. doi: 10.1016/j.cell.2023.08.039
Milich, L. M., Choi, J. S., Ryan, C., Cerqueira, S. R., Benavides, S., Yahn, S. L., et al. (2021). Single-cell analysis of the cellular heterogeneity and interactions in the injured mouse spinal cord. J. Exp. Med. 218:e20210040. doi: 10.1084/jem.20210040
Moguilner, S. G., Berezuk, C., Bender, A. C., Pellerin, K. R., Gomperts, S. N., Cash, S. S., et al. (2024). Sleep functional connectivity, hyperexcitability, and cognition in Alzheimer’s disease. Alzheimer’s Dement. 20, 4234–4249. doi: 10.1002/alz.13861
Möhle, L., Bascuñana, P., Brackhan, M., and Pahnke, J. (2021). Development of deep learning models for microglia analyses in brain tissue using DeePathology™ STUDIO. J. Neurosci. Methods 364:109371. doi: 10.1016/j.jneumeth.2021.109371
Molina-Gallego, B., Ugarte-Gurrutxaga, M. I., Molina-Gallego, L., Plaza Del Pino, F. J., Carmona-Torres, J. M., and Santacruz-Salas, E. (2024). Anxiety and depression after spinal cord injury: a cross-sectional study. Healthcare 12:1759. doi: 10.3390/healthcare12171759
Nasb, M., Tao, W., and Chen, N. (2024). Alzheimer’s disease puzzle: delving into pathogenesis hypotheses. Aging Dis. 15, 43–73. doi: 10.14336/AD.2023.0608
Noh, M.-Y., Kwon, H. S., Kwon, M.-S., Nahm, M., Jin, H. K., Bae, J., et al. (2025). Biomarkers and therapeutic strategies targeting microglia in neurodegenerative diseases: current status and future directions. Mol. Neurodegener. 20:82. doi: 10.1186/s13024-025-00867-4
Nugent, A. A., Lin, K., Van Lengerich, B., Lianoglou, S., Przybyla, L., Davis, S. S., et al. (2020). TREM2 regulates microglial cholesterol metabolism upon chronic phagocytic challenge. Neuron 105, 837–854.e9. doi: 10.1016/j.neuron.2019.12.007
Oveisgharan, S., Buchman, A. S., Yu, L., Farfel, J., Hachinski, V., Gaiteri, C., et al. (2018). APOE ε2ε4 genotype, incident AD and MCI, cognitive decline, and AD pathology in older adults. Neurology 90, e2127–e2134. doi: 10.1212/WNL.0000000000005677
Pancholi, S., Everett, T. H., and Duerstock, B. S. (2024). Advancing spinal cord injury care through non-invasive autonomic dysreflexia detection with AI. Sci. Rep. 14:3439. doi: 10.1038/s41598-024-53718-5
Parhizkar, S., Gent, G., Chen, Y., Rensing, N., Gratuze, M., Strout, G., et al. (2023). Sleep deprivation exacerbates microglial reactivity and aβ deposition in a TREM2 -dependent manner in mice. Sci. Transl. Med. 15:eade6285. doi: 10.1126/scitranslmed.ade6285
Pasipanodya, E. C., Dirlikov, B., Castillo, K., and Shem, K. L. (2021). Cognitive profiles among individuals with spinal cord injuries: predictors and relations with psychological well-being. Arch. Phys. Med. Rehabil. 102, 431–439. doi: 10.1016/j.apmr.2020.06.022
Peña-Bautista, C., Álvarez-Sánchez, L., García-Lluch, G., Raga, L., Quevedo, P., Peretó, M., et al. (2024). Relationship between plasma lipid profile and cognitive status in early Alzheimer disease. Int. J. Mol. Sci. 25:5317. doi: 10.3390/ijms25105317
Peng, X., Guo, H., Zhang, X., Yang, Z., Ruganzu, J. B., Yang, Z., et al. (2023). TREM2 inhibits tau hyperphosphorylation and neuronal apoptosis via the PI3K/Akt/GSK-3β Signaling pathway in vivo and in vitro. Mol. Neurobiol. 60, 2470–2485. doi: 10.1007/s12035-023-03217-x
Pereira, J. B., Janelidze, S., Strandberg, O., Whelan, C. D., Zetterberg, H., Blennow, K., et al. (2022). Microglial activation protects against accumulation of tau aggregates in nondemented individuals with underlying Alzheimer’s disease pathology. Nat. Aging 2, 1138–1144. doi: 10.1038/s43587-022-00310-z
Perosa, V., Scherlek, A. A., Kozberg, M. G., Smith, L., Westerling-Bui, T., Auger, C. A., et al. (2021). Deep learning assisted quantitative assessment of histopathological markers of Alzheimer’s disease and cerebral amyloid angiopathy. Acta Neuropathol. Commun. 9:141. doi: 10.1186/s40478-021-01235-1
Qin, T., Qin, Y., Jin, Y., Liang, X., Sun, Y., Liu, B., et al. (2025). Extracellular vesicle-mediated spinal cord-brain crosstalk induces hippocampal neurogenesis impairment and cognitive deficits post-spinal cord injury. Theranostics 15, 7584–7606. doi: 10.7150/thno.110560
Rachmian, N., Medina, S., Cherqui, U., Akiva, H., Deitch, D., Edilbi, D., et al. (2024). Identification of senescent, TREM2-expressing microglia in aging and Alzheimer’s disease model mouse brain. Nat. Neurosci. 27, 1116–1124. doi: 10.1038/s41593-024-01620-8
Reyes, A., Zawar, I., Punia, V., Sarkis, R. A., Kapur, J., Busch, R. M., et al. (2025). Clinical and cognitive profiles of individuals with Alzheimer’s disease and comorbid seizures. Ann. Neurol. 98, 533–546. doi: 10.1002/ana.27284
Sachdeva, R., Gao, F., Chan, C. C. H., and Krassioukov, A. V. (2018). Cognitive function after spinal cord injury: a systematic review. Neurology 91, 611–621. doi: 10.1212/WNL.0000000000006244
Shabany, M., Ghodsi, S. M., Arejan, R. H., Baigi, V., Ghodsi, Z., Rakhshani, F., et al. (2022). Cognitive appraisals of disability in persons with traumatic spinal cord injury: a scoping review. Spinal Cord 60, 954–962. doi: 10.1038/s41393-022-00756-3
Shi, Q., Chang, C., Saliba, A., and Bhat, M. A. (2022). Microglial mTOR activation upregulates Trem2 and enhances β-amyloid plaque clearance in the 5XFAD Alzheimer’s disease model. J. Neurosci. 42, 5294–5313. doi: 10.1523/JNEUROSCI.2427-21.2022
Shi, K., Chen, L., Chen, L., Tan, A., Xie, G., Long, Q., et al. (2022). Epimedii folium and Curculiginis Rhizoma ameliorate lipopolysaccharides-induced cognitive impairment by regulating the TREM2 signaling pathway. J. Ethnopharmacol. 284:114766. doi: 10.1016/j.jep.2021.114766
Skinnider, M. A., Gautier, M., Teo, A. Y. Y., Kathe, C., Hutson, T. H., Laskaratos, A., et al. (2024). Single-cell and spatial atlases of spinal cord injury in the tabulae paralytica. Nature 631, 150–163. doi: 10.1038/s41586-024-07504-y
Španić Popovački, E., Babić Leko, M., Langer Horvat, L., Brgić, K., Vogrinc, Ž., Boban, M., et al. (2023). Soluble TREM2 concentrations in the cerebrospinal fluid correlate with the severity of neurofibrillary degeneration, cognitive impairment, and inflammasome activation in Alzheimer’s disease. Neurol. Int. 15, 842–856. doi: 10.3390/neurolint15030053
Tao, G., Yang, S., Xu, J., Wang, L., and Yang, B. (2024). Global research trends and hotspots of artificial intelligence research in spinal cord neural injury and restoration—a bibliometrics and visualization analysis. Front. Neurol. 15:1361235. doi: 10.3389/fneur.2024.1361235
Trivedi, M. R., Joshi, A. M., Shah, J., Readhead, B. P., Wilson, M. A., Su, Y., et al. (2023). Interpretable deep learning framework for understanding molecular changes in human brains with Alzheimer’s disease: implications for microglia activation and sex differences. NPJ Aging 11:66. doi: 10.1101/2023.12.18.572226
Vaccaro, D. H., Weir, J. P., Noonavath, M., Bryce, T. N., Escalon, M. X., Huang, V., et al. (2022). Orthostatic systemic and cerebral hemodynamics in newly injured patients with spinal cord injury. Auton. Neurosci. 240:102973. doi: 10.1016/j.autneu.2022.102973
Wang, S., Sudan, R., Peng, V., Zhou, Y., Du, S., Yuede, C. M., et al. (2022). TREM2 drives microglia response to amyloid-β via SYK-dependent and -independent pathways. Cell 185, 4153–4169.e19. doi: 10.1016/j.cell.2022.09.033
Wang, C., Wang, Z., Xie, B., Shi, X., Yang, P., Liu, L., et al. (2022). Binaural processing deficit and cognitive impairment in Alzheimer’s disease. Alzheimer’s Dement. 18, 1085–1099. doi: 10.1002/alz.12464
Welkamp, A. A. W., Leeuwen, V., Post, C., W, M, M., and Stolwijk-Swüste, J. M. (2024). Cognitive assessment during inpatient rehabilitation after spinal cord injury, a retrospective cross-sectional study. Spinal Cord 62, 683–689. doi: 10.1038/s41393-024-01035-z
Widerström-Noga, E. (2017). Neuropathic pain and spinal cord injury: phenotypes and pharmacological management. Drugs 77, 967–984. doi: 10.1007/s40265-017-0747-8
Wu, M., Liao, M., Huang, R., Chen, C., Tian, T., Wang, H., et al. (2022). Hippocampal overexpression of TREM2 ameliorates high fat diet induced cognitive impairment and modulates phenotypic polarization of the microglia. Genes Dis. 9, 401–414. doi: 10.1016/j.gendis.2020.05.005
Wu, Z., Zhu, R., Yu, Y., Wang, J., Hu, X., Xu, W., et al. (2023). Spinal cord injury-activated C/EBPβ-AEP axis mediates cognitive impairment through APP C586/tau N368 fragments spreading. Prog. Neurobiol. 227:102467. doi: 10.1016/j.pneurobio.2023.102467
Yan, J., Zhang, Y., Wang, L., Li, Z., Tang, S., Wang, Y., et al. (2022). TREM2 activation alleviates neural damage via Akt/CREB/BDNF signalling after traumatic brain injury in mice. J. Neuroinflammation 19:289. doi: 10.1186/s12974-022-02651-3
Yang, S., Mu, P., and Huang, W. (2023). Cognitive behaviour therapy in adults with spinal cord injury: a scoping review. Int. J. Nurs. Pract. 29:e13078. doi: 10.1111/ijn.13078
Yoo, H.-J., Lee, K.-S., Koo, B., Yong, C.-W., and Kim, C.-W. (2024). Deep learning-based prediction model for gait recovery after a spinal cord injury. Diagnostics 14:579. doi: 10.3390/diagnostics14060579
Yu, Y., Chen, R., Mao, K., Deng, M., and Li, Z. (2024). The role of glial cells in synaptic dysfunction: insights into Alzheimer’s disease mechanisms. Aging Dis. 15, 459–479. doi: 10.14336/AD.2023.0718
Zhang, L., Liu, Y., Wang, X., Wu, H., Xie, J., and Liu, Y. (2025). Treadmill exercise ameliorates hippocampal synaptic injury and recognition memory deficits by TREM2 in AD rat model. Brain Res. Bull. 223:111280. doi: 10.1016/j.brainresbull.2025.111280
Zhang, X., Tang, L., Yang, J., Meng, L., Chen, J., Zhou, L., et al. (2023). Soluble TREM2 ameliorates tau phosphorylation and cognitive deficits through activating transgelin-2 in Alzheimer’s disease. Nat. Commun. 14:6670. doi: 10.1038/s41467-023-42505-x
Zhang, S., Zhu, L., Peng, Y., Zhang, L., Chao, F., Jiang, L., et al. (2022). Long-term running exercise improves cognitive function and promotes microglial glucose metabolism and morphological plasticity in the hippocampus of APP/PS1 mice. J. Neuroinflammation 19:34. doi: 10.1186/s12974-022-02401-5
Zhao, T., Di, J., Kang, Y., Zhang, H., Yao, S., Liu, B., et al. (2025). TREM2 impedes recovery after spinal cord injury by regulating microglial lysosomal membrane permeabilization-mediated autophagy. Cell Prolif. 58:e70047. doi: 10.1111/cpr.70047
Zhao, C., Qi, W., Lv, X., Gao, X., Liu, C., and Zheng, S. (2025). Elucidating the role of Trem2 in lipid metabolism and neuroinflammation. CNS Neurosci. Ther. 31:e70338. doi: 10.1111/cns.70338
Zhou, X., Wahane, S., Friedl, M.-S., Kluge, M., Friedel, C. C., Avrampou, K., et al. (2020). Microglia and macrophages promote corralling, wound compaction and recovery after spinal cord injury via plexin-B2. Nat. Neurosci. 23, 337–350. doi: 10.1038/s41593-020-0597-7
Keywords: Alzheimer’s disease, artificial intelligence, cognitive impairment, microglia, spinal cord injury, TREM2
Citation: Wu Z, Yu S, Tian D, Cheng L and Jing J (2025) Microglial TREM2 and cognitive impairment: insights from Alzheimer’s disease with implications for spinal cord injury and AI-assisted therapeutics. Front. Cell. Neurosci. 19:1705069. doi: 10.3389/fncel.2025.1705069
Edited by:
Guoyang Liu, Shandong University, ChinaReviewed by:
Chen Zheng, University of Science and Technology of China, ChinaJunjie Wei, Netherlands Institute for Neuroscience (KNAW), Netherlands
Copyright © 2025 Wu, Yu, Tian, Cheng and Jing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juehua Jing, ampoX2h1QDE2My5jb20=; Li Cheng, Y2hlbmdsaTc3ODgyMDI1QDEyNi5jb20=
 Zhonghan Wu
Zhonghan Wu Shuisheng Yu1,2
Shuisheng Yu1,2 Juehua Jing
Juehua Jing