- 1Institute for Human Life Science, Ochanomizu University, Bunkyo, Japan
- 2Faculty of Medical Technology, Teikyo University, Itabashi, Japan
- 3Kitasato University School of Frontier Engineering, Kanagawa, Japan
Neurodegenerative diseases are frequently accompanied by inflammatory responses and alterations in lipid metabolism, both of which are believed to negatively affect neural regeneration in mammals. In addition to immune cells, glial cells such as astrocytes and microglia contribute significantly to these inflammatory processes, and it is now recognized that lipid droplet accumulation and cholesterol metabolism are dysregulated in these glial cells. Consequently, recent studies have examined inflammation and lipid metabolism from the standpoint of glial cell function; however, effective therapeutic strategies remain unestablished. By contrast, in zebrafish, a teleost species, robust neural regeneration occurs within a short period after injury to the telencephalon or spinal cord. In this review, we aimed to identify candidate functional factors by comparing mouse and zebrafish disease models and to explore molecules with potential therapeutic relevance for mammalian neurological disorders.
1 Introduction
In mammals, nerve regeneration after injury is generally limited. Therefore, brain and spinal cord injuries, along with many neurodegenerative diseases, remain incurable, and the replacement of lost neurons is extremely challenging. The activation of glial cells such as microglia and astrocytes, triggered by neuronal degeneration, is believed to induce inflammation and subsequently lead to the formation of glial scars, which inhibit neuronal regeneration (Gao et al., 2013; Lukacova et al., 2021). Moreover, recent studies have shown that lipid droplet accumulation and dysregulated cholesterol metabolism in glial cells further contribute to neuronal stress and hinder regeneration. By contrast, other studies suggest that astrocytes and microglia perform essential functions in forming glial scars, which act as protective barriers that limit the spread of inflammation following central nervous system (CNS) injury (Sofroniew, 2015; Sims and Yew, 2017; Adams and Gallo, 2018). Lipid droplet formation in astrocytes is likewise thought to play a protective role for neurons.
Furthermore, activated microglia have been reported to phagocytose dead neurons, thereby clearing cellular debris and creating space for potential tissue remodeling (Jurga et al., 2020).
Our previous studies have demonstrated that astrocytic and microglial activation is crucial for recovery from blood–brain barrier disruption after brain injury (Hashimoto et al., 2018; Ikeshima-Kataoka and Yasui, 2016) and have also revealed the neuroprotective roles of tenascin-C, one of the extracellular matrix glycoprotein, secreted by astrocytes in the mouse brain (Ikeshima-Kataoka et al., 2008; Nakashima et al., 2021). More recently, the neuroprotective effects of vitronectin, one of the extracellular matrix protein, have also been reported (Yamashita et al., 2025). Based on these findings, astrocytes and microglia may represent promising targets for promoting neuronal regeneration in mammals.
In contrast to mammals, zebrafish exhibit robust regenerative ability in the brain, including the telencephalon and spinal cord, without forming glial scars after injury (Marques et al., 2019; Diotel et al., 2020; Kolb et al., 2023). However, microglial activation and inflammation are indispensable for regeneration following telencephalic injury in zebrafish (Kyritsis et al., 2012; Palsamy et al., 2023).
In this review, we compare recent findings from mouse and zebrafish neurodegenerative disease models and focus on astrocytes and microglia to identify candidate factors that may provide clues for promoting neural regeneration in mammals.
2 Clinical features, genetics, and neuropathology of Alzheimer’s disease
The clinical manifestations of Alzheimer’s Disease (AD) are classified into early-onset and late-onset forms, distinguished primarily by the age at symptom onset. One widely accepted diagnostic standard is the guideline for AD diagnosis developed by the National Institute on Aging and the Alzheimer’s Association (McKhann et al., 2011).
Although familial AD has been documented, more than 90% of cases represent sporadic AD, with familial cases typically manifesting earlier in life. Two major genetic mutations associated with familial AD have provided key insights into disease mechanisms. The first involves dominant mutations in APP, which encodes the amyloid precursor protein (Aβ precursor peptide) (Li et al., 2019). The second involves missense mutations in PSEN1 and PSEN2, which encode subunits of the γ-secretase complex responsible for cleaving APP into Aβ (Sun et al., 2017). In addition, APOE ε4 has been identified as the strongest genetic risk allele for AD. Large-scale twin studies indicate that genetic factors influence the timing of AD onset, although non-genetic risk factors also play important roles (Gatz et al., 2006). Approximately 62% of people with sporadic AD carry the APOE ε4 allele (Rebeck et al., 1993). Furthermore, polygenic risk score analyses incorporating APOE have demonstrated that carriers of APOE ε4 develop AD approximately 4–5.5 years earlier than non-carriers (de Rojas et al., 2021). Less common genetic risk alleles include ABCA7, ABI3, BIN1, CR1, TREM2, and SORL1 (de Rojas et al., 2021). Recent mouse models of AD are summarized in Table 1.
The neuropathological hallmarks of AD include severe atrophy of the medial temporal lobes, particularly the hippocampus and entorhinal cortex, and the accumulation of extracellular senile plaques composed of Aβ, together with intracellular neurofibrillary tangles composed of phosphorylated tau (Whitwell, 2010; Tcw and Goate, 2017). Deposition levels of these aggregates are positively correlated, with Aβ deposition generally preceding tau pathology (Cras et al., 1991).
2.1 The neuroimmune system and microglia in AD patients and mouse models
A prominent feature of the AD brain is microglial activation, which has been actively investigated in recent years due to advances in transcriptomic technologies. Integration of AD genome-wide association studies (GWAS) with myeloid-specific epigenomic and transcriptomic datasets has shown that AD risk alleles are enriched in active enhancers of microglia (Novikova et al., 2021), suggesting that regulation of microglial function contributes to AD pathogenesis.
Microglial reactivation is essential for the phagocytosis of senile plaques, neurofibrillary tangles, and apoptotic neurons, thereby helping to maintain brain homeostasis in AD. The mechanisms underlying microglial dynamics have been characterized using various mouse models. In five familial Alzheimer’s disease (5 × FAD) and Center for Research in Neurodegenerative Diseases 8 (CRND8) mice, microglial processes have been shown to stably contact and envelop Aβ plaques. Interestingly, plaques covered by microglia tend to be more compact and exhibit lower Aβ42 affinity, leading to reduced dystrophic neurite formation (Condello et al., 2015). These findings indicate that microglia act as protective barriers against neurotoxicity.
Several mechanisms mediating microglial Aβ phagocytosis have been described. TAM receptors such as Axl and Mertk on microglia interact with Gas6 ligands surrounding Aβ plaques to promote phagocytosis in APP/PS1 mice (Huang et al., 2021). Consistent with this, Mertk deficiency in APP/PS1 mice results in hippocampal hyperexcitability due to impaired clearance of apoptotic neurons and defective synaptic pruning, which in turn leads to recurrent seizure activity and premature death (Huang and Lemke, 2022). Thus, microglial phagocytosis is crucial for eliminating damaged neurons and protecting brain function.
Tau pathology also induces microglial phagocytosis. In P301S-Tau mice, neurons containing tau inclusions expose phosphatidylserine, which serves as an “eat-me” signal to activate microglial phagocytosis and facilitate the removal of damaged neurons (Brelstaff et al., 2018). Moreover, tau secreted by neurons promotes microglial proliferation, which accelerates the clearance of damaged neurons in primary rat neuron–glia cultures (Pampuscenko et al., 2020). Collectively, these findings strongly suggest that microglia mitigate tau toxicity by removing compromised neurons.
Several lines of evidence indicate that microglial reactivation in AD is progressively regulated by TREM2. Large-scale exome sequencing of individuals with AD has identified rare damaging variants in TREM2, which encodes a receptor critical for microglial reactivation (Holstege et al., 2022). TREM2 governs the transition from homeostatic microglia to disease-associated microglia (DAM), a subset proposed to represent the reactivated state in neurodegenerative diseases. Specifically, the DAM subset was identified by single-cell RNA sequencing of 5 × FAD mouse brains and is characterized by elevated expression of Trem2, Cx3cr1, Apoe, Cd9, and Cst7, among other genes. DAM clusters around Aβ plaques and displays high phagocytic activity. Notably, microglial activation occurs through a two-step process: an initial TREM2-independent phase that initiates DAM activation, followed by a TREM2-dependent phase in which microglia acquire robust phagocytic activity (Keren-Shaul et al., 2017). Supporting the regulatory role of TREM2, loss-of-function mutations in TREM2 markedly impair microglia-mediated elimination of excitatory and inhibitory synapses, ultimately leading to attenuation of tau pathology and neurodegeneration (Gratuze et al., 2020; Dejanovic et al., 2022).
Several TREM2 variants, including R47H and R62H, alter the binding capacity of TREM2 to its ligands and are associated with an increased risk of AD (Song et al., 2017). APOE has been identified as a ligand for TREM2, and this interaction enhances the microglial phagocytic clearance of apoptotic neurons (Rebeck et al., 1993; Bailey et al., 2015). Consistent with these findings, the TREM2-APOE signaling pathway has been recognized as a major regulator of microglial phenotypic changes in AD. Phagocytic microglia demonstrate the most prominent upregulation of Apoe expression in APP/PS1 microglia. Furthermore, individuals with AD carrying TREM2 R47H and R62H variants exhibit reduced microglial clustering around Aβ plaques, which consequently promotes greater Aβ plaque deposition. In contrast, individuals with AD and TREM2 haplodeficiency display similar levels of microglial reactivation irrespective of APOE ε4 allele status (Krasemann et al., 2017), suggesting that APOE functions downstream of TREM2. Additionally, Trem2 deficiency-induced microglial quiescence in APP/PS1 models reduces ApoE localization to Aβ plaques, indicating that microglia serve as a pivotal source of plaque-associated ApoE (Parhizkar et al., 2019).
Given the preponderance of evidence supporting microglial reactivation as a gatekeeper of the neuroimmune system, we believe that a timely review of these studies will provide deeper insights into the physiological functions of microglia. However, the role of microglia in AD remains controversial. Single-cell RNA sequencing of human AD microglia has revealed a spectrum of subtypes: some subtypes show downregulation of AD pathology-related genes, others show upregulation of Aβ-related genes, and still others exhibit alterations in genes associated with neurofibrillary tangles (Olah et al., 2020). This transcriptional diversity suggests that individual AD microglia possess distinct molecular programs that contribute to barrier functions. It is also important to emphasize that there are marked species-specific differences in AD microglial biology. For example, comparison of Trem2−/−;5 × FAD mice with individuals carrying TREM2 mutations demonstrated that human AD exhibits upregulated expression in only part of the DAM signature, despite TREM2 being required for microglial reactivation (Zhou et al., 2020). Furthermore, RNA sequencing of microglia isolated from the human AD cortex revealed that most AD risk genes, termed human AD microglia (HAM) genes, which show microglia-specific preferential expression, overlap substantially with those of aged human microglia. Interestingly, the expression of DAM genes identified in the 5 × FAD mouse strain remains unchanged in human AD. By contrast, differentially expressed gene sets identified in aged microglia of PVM and PS2SPP mice are significantly enriched in HAM (Srinivasan et al., 2020). Therefore, it is necessary to interpret mouse and human microglial profiles separately. Moreover, even within mouse models, the functional properties of microglia may vary depending on the specific AD strain used.
2.2 Astrocyte function in AD patients and mouse models
Reactive astrocytes, referred to as disease-associated astrocytes (DAAs) by analogy to DAMs, also contribute to neuroinflammation in AD (Habib et al., 2020). However, their mechanisms of action are diverse and remain incompletely understood. Although RNA sequencing of fluorescence-activated cell sorting (FACS)–purified astrocytes derived from human AD samples shows no significant changes in the expression of genes associated with AD risk (Srinivasan et al., 2020), Single-cell RNA sequencing of multiple human brain regions—including the entorhinal cortex, inferior temporal gyrus, dorsolateral prefrontal cortex, secondary visual cortex, and primary visual cortex—spanning normal aging to severe AD progression shows that astrocytes can be classified into several subsets, with transcriptomic changes in synapse-related and stress response–related genes occurring along vulnerable neural networks and AD pathology stages, respectively (Serrano-Pozo et al., 2024). Astrocyte reactivation may be regulated by complex dynamics involving both spatial and temporal axes during the pathogenesis of AD.
Astrocytes play key roles in regulating synaptic plasticity in AD. They exhibit excitability through dynamic changes in intracellular Ca2+ concentration, acting as regulators of the brain environment. In the PS2APP mouse model of AD, Ca2+ signaling in astrocytes is markedly reduced due to the downregulation of the Ca2+ sensor protein STIM1, leading to astrocyte-dependent long-term synaptic plasticity decline and subsequent memory loss. Notably, STIM1 overexpression in astrocytes completely restores astrocytic Ca2+ signaling and synaptic plasticity in PS2APP mice (Lia et al., 2023).
Collectively, astrocytic activity contributes to memory decline through dysregulation of synaptic plasticity in AD. Consistent with this, astrocytes in TauP301S mice have been reported to engulf excitatory and inhibitory synapses in a C1q-dependent manner, compensating for microglial impairment of TREM2-related phagocytosis (Dejanovic et al., 2022). Similarly, in individuals with AD, astrocytes engulf synapses through recognition of MFG-E8 via αvβ5 integrin (Tzioras et al., 2023). Although it remains unclear how astrocytes coordinate synaptic connectivity and engulfment through Ca2+ signaling, astrocyte reactivity clearly contributes to memory decline in AD. Microglial dynamics are considered essential for astrocyte reactivation. Recent high-resolution spatial transcriptomics of APPNL-G-F mice revealed that astrocytic responses to Aβ plaques are heterogeneous. A neurotoxic astrocytic phenotype emerges in a microglial density–dependent manner, characterized by increased GABAergic signaling and decreased glutamatergic signaling, suggesting that reactive astrocytes contribute to the disruption of the excitatory–inhibitory balance in neuronal circuits (Mallach et al., 2024). Overall, these findings suggest that astrocytes display neurotoxic properties toward neuronal circuits while interacting with microglia and neurons in AD. Moreover, overexpression of tau in astrocytes has been reported to reduce neurogenesis and impair neuronal circuits, indicating that tau accumulation in astrocytes alone may be sufficient to induce AD-like symptoms (Richetin et al., 2020). Further elucidation of the mechanisms underlying astrocyte-related neuronal circuit coordination is warranted.
Here, to gain deeper insight into astrocyte function in AD, findings on astrocytic Aβ clearance are introduced. Astrocyte-released IL-3 has been reported to stimulate microglia to assemble around Aβ and tau aggregates and clear them through microglial phagocytosis in the 5 × FAD mouse brain (McAlpine et al., 2021). Moreover, exposure of Aβ to human astrocytes derived from stem cells of healthy donors promotes microglial Aβ phagocytosis, whereas astrocytes carrying the APOE4 allele and treated with Aβ show reduced microglial Aβ uptake, suggesting that astrocytes enhance microglial Aβ clearance in an APOE4-dependent manner (Lee et al., 2025). In addition to regulating microglial dynamics, astrocytes modulate Aβ accumulation through Nrf2 expression. Translating ribosome affinity purification sequencing of astrocytes from APP/PS1 and MAPTP301S mice revealed enriched expression of Nrf2, which encodes a stress-activated cytoprotective protein, in both models. Astrocytic Nrf2 expression reduces Aβ accumulation and tau phosphorylation (Jiwaji et al., 2022). These findings suggest that astrocytes act as neuroprotective regulators of Aβ and tau aggregation. Conversely, other evidence indicates that astrocytes can promote tau aggregation. It is well known that tau tangle accumulation is initiated by Aβ aggregation in AD. Interestingly, some Aβ-positive patients with AD do not develop tau pathology. Positron emission tomography analyses revealed that only patients classified as reactive astrocyte–positive, based on plasma GFAP detection, show tau tangle accumulation, suggesting that astrocyte reactivity is an important upstream regulator of early tau pathology induced by Aβ (Bellaver et al., 2023). Thus, astrocytes in AD regulate Aβ and tau proteinopathy in complex and multifaceted ways.
Astrocytes themselves secrete neurotoxic factors. Astrocytes play essential roles in maintaining various metabolic pathways, including glucose, lipid, and amino acid metabolism (Zhang et al., 2023), One report indicated that reactive astrocytes in both patients with AD and APP/PS1 mice display dual beneficial and toxic roles within the urea cycle. Reactive astrocytes uptake Aβ to detoxify it into urea; however, an excessive byproduct of this cycle, γ-aminobutyric acid (GABA), exerts toxic effects that impair memory (Ju et al., 2022). In addition, PS2APP and TauPS2APP mice show an increased number of reactive astrocytes expressing high levels of the complement factor C4b, a key mediator of synaptic degeneration (Lee et al., 2021; Zheng et al., 2025). Together, these findings indicate that astrocytes are sources of neurotoxic factors that accelerate AD progression.
3 Clinical features, neuropathology, and genetics of Parkinson’s disease
The clinical features of Parkinson’s Disease (PD) include motor symptoms such as tremor, rigidity, and bradykinesia, and diagnosis is made according to the criteria established by the International Parkinson and Movement Disorder Society (Postuma et al., 2015).
A defining neuropathological characteristic of PD is the presence of Lewy bodies, which are intracytoplasmic inclusions composed of full-length α-synuclein (α-Syn) in dot- or thread-like forms. Lewy bodies are primarily observed in the neuronal cytoplasm of the substantia nigra in individuals with PD (Spillantini et al., 1997, 1998).
Although familial PD has been documented, more than 90% of cases are idiopathic. Large-scale GWAS in PD have identified strong associations with SNCA, which encodes α-Syn, and MAPT, which encodes tau. In addition, LRRK2, PARK16, GBA, and TMEM175 have been reported as genetic risk alleles for PD (Simón-Sánchez et al., 2009; Nalls et al., 2014). These genes have been implicated as potential mediators of PD pathogenesis through proteogenomic network analysis (Doostparast Torshizi et al., 2024). Furthermore, higher expression of ApoE has been reported in melanized neurons of the substantia nigra (Wilhelmus et al., 2011). The major mouse models of PD are summarized in Table 2.
3.1 The neuroimmune system and microglia in PD patients and mouse models
Single-cell RNA sequencing of the substantia nigra from individuals with PD has revealed a significantly higher proportion of microglia (Martirosyan et al., 2024), suggesting that microglial function is altered in PD. A previous study reported that microglial depletion attenuates α-Syn accumulation and dopaminergic neuronal loss in the substantia nigra of PD model mice (Thi Lai et al., 2024). These findings support the idea that microglia exert neurotoxic effects more prominently in PD.
The role of microglia in PD is gradually being elucidated. Studies have shown that α-Syn–deficient microglia display higher cytokine production and reduced phagocytic activity, suggesting that α-Syn regulates microglial inflammatory responses (Austin et al., 2006). The uptake of α-Syn by microglia is regulated by leucine-rich repeat kinase 2 (LRRK2). Microglia carrying the LRRK2-G2019S mutation exhibit reduced motility and enhanced cytokine production due to inhibition of focal adhesion kinase activity and activation of the nuclear factor (NF)-κB pathway, respectively (Choi et al., 2015; Russo et al., 2018). On the other hand, α-Syn can also enhance the neurotoxic properties of microglia. Microglia-specific overexpression of α-Syn promotes their reactivation, leading to dopaminergic neuronal loss through increased production of reactive oxygen species (Bido et al., 2021). In addition, α-Syn interacts with microglia via the receptor for advanced glycation end products to promote cytokine secretion (Long et al., 2022). Collectively, these findings demonstrate that α-Syn can drive microglial neurotoxic activation.
In addition, it is possible that α-Syn contributes to neuronal cell death, but this effect can be mitigated through microglial interaction via tunneling nanotube formation. Specifically, microglia receive α-Syn from neurons through F-actin–dependent tunneling nanotubes and, in turn, donate intact mitochondria to neurons to reduce oxidative stress in vitro (Scheiblich et al., 2024). Interestingly, microglia containing α-Syn can transfer it and exchange mitochondria with neighboring microglia through F-actin–dependent intercellular connections, thereby escaping cell death induced by oxidative toxicity (Scheiblich et al., 2021). These findings suggest that microglia support the homeostasis of both neurons and themselves by inhibiting α-Syn exposure and supplying mitochondria. Notably, the above microglia–neuron communication system via tunneling nanotubes has been confirmed in vitro; therefore, further demonstration in vivo is anticipated.
Through a mechanism distinct from α-synuclein toxicity, DJ-1 also modulates microglial inflammation in PD. Knockdown of DJ-1 has been reported to enhance dopamine-induced cytokine production, oxidative stress, and phagocytosis in microglia via p65 nuclear translocation of the NFκB pathway, ultimately contributing to dopaminergic neuronal loss (Trudler et al., 2014; Lin et al., 2021). In contrast, under LPS-induced inflammatory conditions, DJ-1 deficiency suppresses microglial activation (Lind-Holm Mogensen et al., 2024), suggesting that DJ-1 represents a potential risk factor for PD.
3.2 Astrocytic function in PD patients and mouse models
Single-cell transcriptomic analysis of the substantia nigra from individuals with PD has also revealed a higher proportion of astrocytes (Martirosyan et al., 2024), suggesting that astrocytic as well as microglial functions play key roles in PD.
The crucial role of astrocytes in PD pathology is the clearance of α-synuclein (α-Syn). Interestingly, primary astrocytes from healthy rodent ventral midbrain and cortex have been shown to inhibit neuronal α-Syn aggregation. Specifically, administration of ventral-midbrain- or cortex-derived astrocytes into the midbrain of mice injected with α-Syn adeno-associated virus (AAV) and preformed fibrils to generate a PD model reduces α-Syn aggregation and neurodegeneration in the substantia nigra (Yang et al., 2022). Moreover, treatment with α-Syn preformed fibrils promotes α-Syn uptake in primary astrocyte cultures (Filippini et al., 2021). Therefore, healthy astrocytes exert therapeutic effects on PD progression. Conversely, PD astrocytes exhibit reduced α-Syn clearance ability, the molecular mechanisms of which are being elucidated. One mechanism of astrocytic α-Syn clearance involves regulation by extracellular chaperones. LRRK2 is known to regulate α-Syn uptake by astrocytes as well as microglia. LRRK2 G2019S knock-in mice show enhanced expression of the extracellular chaperone clusterin through the activity of microRNA miR-22-5p, leading to impaired α-Syn clearance in astrocytes. Notably, transfection of miR-22-5p improves astrocytic α-Syn clearance by reducing clusterin production (Filippini et al., 2021, 2025). Another mechanism of astrocytic α-Syn clearance involves the interaction between α-Syn and its membrane receptors. Injection of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) or α-Syn preformed fibrils into mice enhances expression of lipocalin-2 (LCN2) in astrocytes of the substantia nigra. LCN2 inhibits α-Syn binding to its membrane receptor 24p3R, thereby impairing astrocytic α-Syn uptake (Jiao et al., 2025). Collectively, astrocytic dynamics determine PD pathogenesis and progression through the regulation of α-Syn accumulation. It is possible that α-synuclein (α-Syn) is closely associated with astrocyte dynamics, although many aspects remain unknown. This possibility is supported by several studies reporting that expression of α-Syn with the A53T mutation in mice alters Ca2+ signaling in astrocytes, which may contribute to changes in both astrocytic and neuronal functions (Nanclares et al., 2023). Moreover, although α-Syn aggregation is well known to occur in neurons, astrocytes that internalize α-Syn can also accumulate it. During PD progression, astrocytic α-Syn first appears mainly in the amygdala at early stages, followed by the cingulate and entorhinal cortices, and finally affects the cerebellum and substantia nigra at advanced stages (Otero-Jimenez et al., 2025). Furthermore, astrocytes have been reported to acquire distinct forms of α-Syn truncated at the N-terminus between residues 21–33 and the C-terminus between residues 100–102, and phosphorylated at tyrosine 39 (Altay et al., 2022). Interestingly, α-Syn–laden astrocytes secrete abundant extracellular vesicles (EVs) as a result of lysosomal dysfunction in vitro. Consistent with this, plasma from PD patients contains more astrocyte-derived GLT-1+ EVs enriched in α-Syn (Wang et al., 2023), suggesting that astrocyte-derived EV content in plasma may serve as a potential biomarker for clinical diagnosis of PD.
Several lines of evidence demonstrate that PD astrocytes display a proinflammatory state. Single-cell RNA sequencing of the substantia nigra from PD patients indicates that CD44+ astrocytes are expanded, and CD44 promotes neuroinflammatory signaling through the JAK/STAT pathway in astrocytes (Ma et al., 2025), suggesting that astrocytes acquire neurotoxic properties in PD. It is thought that the neuroinflammatory state of astrocytes is induced by extracellular α-Syn. α-Syn fibrils are known to activate cytokine production in primary human astrocytes through receptor interacting protein kinase 1 (RIPK1)- and receptor interacting protein kinase 3 (RIPK3)-dependent NF-κB signaling, thereby promoting neuronal cell death (Chou et al., 2021). In addition, injection of α-Syn preformed fibrils into mice induces C4 secretion from neurons, leading to astrocyte-mediated, inflammation-related neuronal death (Zou et al., 2025). Furthermore, treatment with oligomeric α-Syn induces an inflammatory state in astrocytes derived from human stem cells, characterized by enhanced secretion of inflammatory factors, including IFNγ, IL-1β, and TNFα. Notably, IFNγ promotes expression of the A-to-I RNA-editing mediator ADAR1 p150 isoform, thereby sustaining the inflammatory state in astrocytes (D’Sa et al., 2025). Together, astrocyte-related inflammation contributes to neurodegeneration in PD.
Moreover, stem cells derived from PD patients demonstrate that enhanced TLR2 activity induces neurotoxic factor secretion in parallel with autophagy-dysfunction-related α-Syn aggregation in astrocytes (Weiss et al., 2024), suggesting that dysregulation of lysosomal degradation may promote neurotoxic factor release in PD astrocytes. Notably, a recent study reported that mice injected with α-Syn–AAV into the substantia nigra exhibit a proinflammatory astrocytic state in the midbrain but not in the striatum (Basurco et al., 2023). suggesting that astrocytic inflammation is activated in a brain-region-dependent manner.
It is also possible that PD astrocytes affect blood–brain barrier (BBB) dynamics as well as neuronal function. The substantia nigra in PD patients shows morphological BBB alterations, particularly a reduction in vessel area. RNA-sequencing data indicate that astrocytes derived from iPS cells carrying the PD-related LRRK2 G2019S mutation exhibit transcriptomic changes in angiogenesis-related and proinflammatory genes. Consistent with this, BBB coculture and histological analyses demonstrate that astrocytes with the LRRK2 G2019S mutation promote abnormal increases in BBB permeability, caused by morphological changes in brain microvascular endothelial cells (de Rus Jacquet et al., 2023). Insights into BBB dynamics in PD remain limited; therefore, further research is required.
4 Clinical features of traumatic brain injury
The severity of traumatic brain injury (TBI) is quantified using several evaluations, with the Glasgow Coma Scale being the most widely applied. This scale is scored based on eye, motor, and verbal responses (Teasdale and Jennett, 1974). TBI severity is influenced by the impact of both primary and secondary brain injuries. Primary brain injury refers to the immediate physical damage, including axonal loss and hemorrhage. Secondary brain injury, in contrast, arises from edema, ischemia, and inflammation-related gliosis and neuronal cell death, which develop minutes to days after the primary insult. Secondary brain injury is widely considered to exert a major impact on TBI outcomes. Consistent with this concept, individuals with severe TBI, including contusions, exhibit more pronounced gliosis and aggravated neuronal damage compared with those with mild TBI (Feichtenbiner et al., 2025). Furthermore, the degree of secondary injury depends on the severity of the primary injury. For example, macrophage interaction with fibrin, which accumulates near sites of extravasated blood components, activates inflammatory responses in TBI (Dean et al., 2024). Taken together, these findings highlight a strong correlation between the impact of primary brain injury and the extent of secondary brain injury. To mimic the characteristics of TBI, various murine models have been developed. Major murine models of TBI are summarized in Table 3.
4.1 The neuroimmune system with microglia in TBI patients and mouse models
Relieving secondary brain injury is crucial for improving the prognosis of TBI. Importantly, microglia act as critical regulators of secondary injury, demonstrating both neurotoxic and neuroprotective functions. Supporting this concept, microglial depletion has been reported to suppress proinflammatory factor production and neurodegeneration after brain injury (Henry et al., 2020; Packer et al., 2024), suggesting that the neurotoxic role of microglia is particularly influential in TBI.
Consistent with the importance of microglial activation in TBI, single-cell transcriptomic studies have identified a transformation of microglial phenotypes toward a proinflammatory state after injury. Single-cell RNA sequencing of human brain tissue from individuals with severe TBI revealed a higher proportion of microglia, representing the cell type with the most substantial population change (Garza et al., 2023). Notably, in mouse models, a distinct proinflammatory microglial cluster was expanded within 24 h of injury and persisted for up to 6 months (Jha et al., 2024), indicating that microglial activation occurs rapidly after trauma and sustains chronic inflammation. Together, these findings support the hypothesis that proinflammatory microglial activation contributes to the worsening of TBI prognosis.
Pertinent to the mechanism of microglial activation in TBI, several studies have reported that the stimulator of interferon genes (STING) pathway acts as a critical regulator of the transformation of microglia into a proinflammatory state (Figure 1). Activation of STING leads to the production of inflammatory factors (Abe and Barber, 2014; Yum et al., 2021). Consistent with these findings, deficiency of STING has been shown to inhibit microglial activation and morphological changes after brain injury (Packer et al., 2024). These data suggest that the STING pathway contributes to chronic inflammation in TBI. In addition, microglia-specific STING deficiency attenuates both microgliosis and proinflammatory factor expression (Packer et al., 2025). Taken together, the STING pathway plays a critical role in driving inflammatory responses through the sensing of damaged cell-derived DNA in microglia after TBI. Notably, although the STING pathway upregulates the transcription of interferon (IFN)- and NF-κB-mediated genes (Abe and Barber, 2014; Yum et al., 2021), deficiency of IFNAR1, the receptor for IFN, does not affect microglial activation after brain injury (Packer et al., 2025). Therefore, it is possible that STING pathway-induced chronic inflammation is mediated primarily through NF-κB-dependent production of interleukin (IL)-6, tumor necrosis factor (TNF)α, and IL-1β, rather than IFN signaling. Further studies are required to elucidate the detailed mechanisms of microglial activation after brain injury.
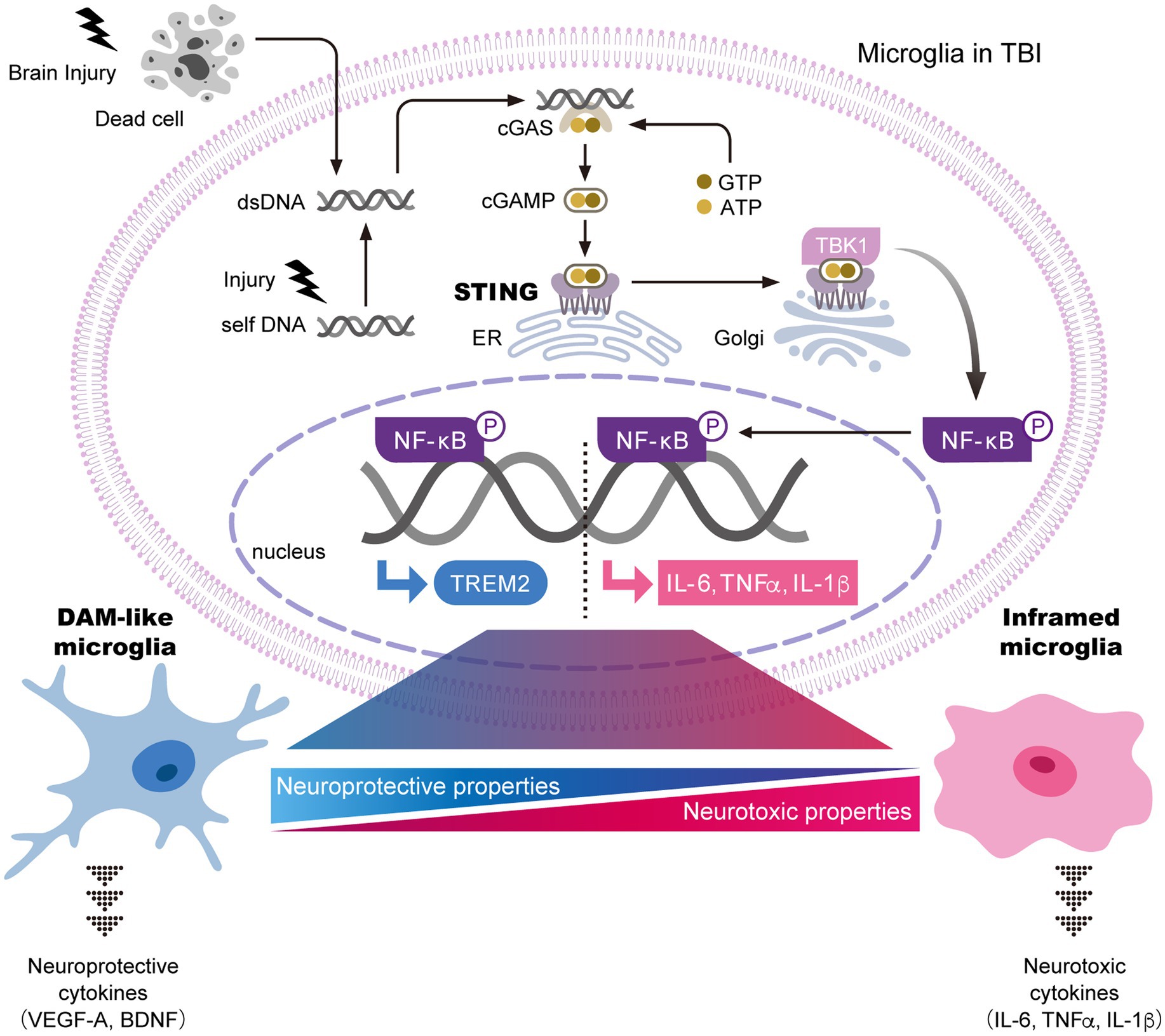
Figure 1. The STING pathway is a key regulator of microglial transformation into both neurotoxic and neuroprotective phenotypes. In general, the STING pathway is essential for the innate immune response and inflammatory signaling. In this pathway, damaged cell-derived exogenous or self-DNA activates cytoplasmic cGAS. The binding of cGAS to DNA triggers the production of cGAMP, which interacts with STING in the ER and activates it. Activated STING translocates to the Golgi apparatus, where it promotes the phosphorylation of NF-κB through TBK1. NF-κB then translocates into the nucleus to induce transcription of neurotoxic cytokines, including IL-6, TNFα, and IL-1β, thereby driving the transformation of microglia into an inflammatory phenotype with neurotoxic properties. In parallel, nuclear NF-κB promotes the transcription of TREM2, which induces the transformation of microglia into DAM-like microglia. DAM-like microglia secrete neuroprotective cytokines that support neuronal survival. Taken together, brain injury is thought to regulate the balance between neuroprotective and neurotoxic microglial properties through the STING pathway. Further identification of key factors that determine microglial states after brain injury is expected. The figure is a modified version of Hu et al. (2022). ATP; adenosine triphosphate, BDNF; brain-derived neurotrophic factor, cGAMP; cyclic GMP-AMP, cGAS; cyclic GMP-AMP synthase, DAM; disease-associated microglia, dsDNA; double stranded DNA, ER, endoplasmic reticulum; GTP; guanosine triphosphate, IL; interleukin, NF-κB; nuclear factor-κB, STING; stimulator of interferon genes, TBK1; tank-binding kinase 1, TREM2; triggering receptor expressed on myeloid cells 2, VEGFA; vascular endothelial growth factor A.
It is recognized that a subset of activated microglia demonstrate neuroprotective functions, not only neurotoxic properties, after brain injury. One such neuroprotective subset is referred to as DAM-like cells, which show marked upregulation of DAM markers such as Cd9, Cst7, Lpl, Csf, and Timp2. Interestingly, the severe neurodegeneration observed in Trem2−/− mice appears to result from the absence of DAM-like cells with neuroprotective properties, including the production of repair-related cytokines and the phagocytosis of cellular debris (Wang L. et al., 2025). In other words, DAM-like cells function to attenuate neurodegeneration in TBI. However, the boundary and transition between neuroprotective and neurotoxic microglia are difficult to define. The STING pathway has been shown to induce upregulation of TREM2 expression in addition to proinflammatory factor expression (Dabla et al., 2022), suggesting that STING may regulate transformation into both neurotoxic and neuroprotective microglial phenotypes (Figure 1). In contrast, STING deficiency in TBI models results in only a neuroprotective phenotype (Packer et al., 2024, 2025). This finding suggests either that TREM2-related DAM transformation does not depend on STING signaling or that neurotoxic microglia emerge before neuroprotective populations after brain injury. Therefore, it is possible that functional variations exist within the roles of activated microglia in TBI, which are now being more comprehensively characterized through transcriptomic and proteomic analyses.
Aside from inflamed and disease-associated microglia (DAM-like), the classifications of neurotoxic M1 and neuroprotective M2 phenotypes are the most well characterized. A marked increase in microglial numbers is observed within 7 days after brain injury, consisting of a mixture of M1 and M2 phenotypes. After this period, the population shifts toward a predominance of M2 microglia (Morganti et al., 2016). The mechanisms underlying M1-M2 polarization are becoming increasingly clear. Polarization toward the M1 type is mediated by the Fas–FasL pathway after brain injury (Xia et al., 2024). In addition, CD36, which is one of the molecules robustly upregulated after brain injury, promotes microglial polarization toward the M1 phenotype through the Traf5-MAPK pathway (Hou et al., 2024). Taken together, microglial polarization towards M1 is regulated by multiple pathways. It is important to note that the concept of a transitional microglial activation system between neurotoxic and neuroprotective states has become more widely accepted than the traditional M1–M2 polarization model, as comprehensive analyses have revealed the diversity of microglial phenotypes in TBI.
Activated microglia strongly influence neuronal dynamics in TBI. In individuals with severe TBI, a significant increase in microglia apposing neuronal somata has been observed, suggesting that brain injury enhances microglia–neuron communication (Feichtenbiner et al., 2025). A well-established form of microglia–neuron communication is mediated by microglia-derived proinflammatory cytokines, which promote neurodegeneration. M1 microglia secrete proinflammatory mediators such as IL-1 and TNF, thereby amplifying the neurotoxic environment (Xia et al., 2024; Packer et al., 2025). In addition to cytokine signaling, microglial phagocytic activity contributes to neurodegeneration through synaptic loss following brain injury. It has been demonstrated that brain injury induces neuronal C3 deposition around synapses, which triggers microglial phagocytosis of synaptic structures and consequently reduces neuronal activity (Borucki et al., 2024). These microglial properties collectively exacerbate neurodegeneration. By contrast, DAM-like cells exhibit neuroprotective functions. Specifically, DAM-like cells have been reported to secrete anti-inflammatory mediators that suppress neurodegeneration in controlled cortical impact models (Wang L. et al., 2025). Furthermore, sterol synthesis is activated by the liver X receptor (LXR) pathway in DAM-like cells, supplying essential lipids to oligodendrocytes to promote remyelination after brain injury (Li et al., 2024). Considering their diverse roles, including the production of pro- and anti-inflammatory mediators, phagocytosis of debris and synapses, and provision of essential trophic factors, microglia play a central role in regulating neuronal dynamics both directly and indirectly in TBI.
4.2 Astrocytic function in TBI patients and mouse models
Single-cell RNA sequencing of human TBI brain tissue indicates that astrocytes undergo transcriptomic alterations after brain injury, although their responses are less pronounced than those of microglia (Garza et al., 2023; Jha et al., 2024). Moreover, single-cell RNA sequencing of mouse brains with closed-head injury shows that the astrocyte population decreases immediately after injury, followed by expansion around the lesion over 6 months (Garza et al., 2023). Another study reports that cortical injury influences hippocampal neural stem cell dynamics, which regulate neurogenesis and astrogliogenesis. Controlled cortical impact promotes proliferation of neurons, astrocytes, and neural stem cells in the hippocampus, while single-cell RNA sequencing demonstrates that brain injury enhances neurogenesis derived from neural stem cells at the expense of astrogliogenesis (Bielefeld et al., 2024). In brief, astrocyte expansion after TBI is not derived from hippocampal neural stem cells. The expanded astrocyte population is thought to perform diverse functions, and the details of these functions in TBI are gradually being elucidated.
One critical function of astrocytes in TBI is glial scar formation, which limits damage caused by physical trauma and subsequent inflammation. It has been reported that stab wounds induce immediate upregulation of the actin-binding protein Drebrin in astrocytes, supporting glial scar formation through modulation of Rab8+ membrane tubule trafficking (Schiweck et al., 2021). However, glial scars have dual roles in TBI repair, as astrocytes expressing the water channel aquaporin-4 (AQP4) accelerate scar formation after TBI through cytotoxic swelling, consequently increasing post-traumatic seizure susceptibility (Lu et al., 2021). Collectively, astrocyte-associated glial scars can both minimize tissue damage and disturb neural activity in TBI.
Astrocytes have also been recognized as mediators of neuroinflammation through cytokine-dependent glia–neuron communication (Vincent et al., 2023). Although microglia exhibit more extensive transcriptomic alterations after brain injury than astrocytes, several genes—such as those related to interferon (IFN) signaling and major histocompatibility complex class I antigen presentation—show transcriptional changes in both cell types after injury (Todd et al., 2021). Therefore, astrocytes also undergo inflammatory state–related cellular changes after brain injury. While astrocytes are known to produce neurotoxic factors (Todd et al., 2021), they also possess anti-inflammatory properties. For example, astrocyte-derived exosomal noncoding RNA 4933431K23Rik suppresses injury-induced microglial activation and excessive microglial SMAD7 expression, thereby inhibiting NF-κB signaling and alleviating neuroinflammation (He et al., 2023). Furthermore, astrocytes modulate the neuroimmune response through the programmed cell death protein-1 (PD-1)/programmed cell death ligand-1 (PD-L1) immune checkpoint pathway. PD-L1+ reactive astrocytes accumulate around lesions and prevent excessive neuroimmune responses by suppressing infiltration of PD-1+ immune cells (Gao et al., 2022). Collectively, astrocytes propagate diverse molecular signals that influence both repair and injury processes after TBI, although the underlying mechanisms remain to be fully elucidated.
Astrocytes also affect neural functions in TBI. For instance, toll-like receptor 4 (TLR4)–expressing microglia interact with astrocytes to promote synaptic loss after brain injury, whereas astrocyte-derived thrombospondin-1 supports synaptic recovery, suggesting that reactive astrocytes contribute to synaptic remodeling in TBI (Rosa et al., 2021). Additionally, microRNAs (miRNAs) have emerged as another mediator of glia–neuron communication. Astrocyte-derived EVs deliver miR-382-5p to neurons, leading to mitochondrial dysfunction and neuronal apoptosis (Hu et al., 2024). Considering their diverse roles—including glial scar formation, pro- and anti-inflammatory signaling, synaptic remodeling, and regulation of neuronal survival—astrocytes play a crucial role in orchestrating brain repair after TBI.
5 Astrocyte-mediated lipid droplet accumulation and cholesterol metabolism in neuroinflammatory diseases
Advances in neuroscience technologies in the 2000s revealed that LDs, the storage organelles for energy-rich neutral lipids, are present in glial cells of the brain. Moreover, increased LD formation and accumulation have been associated with aging and several neuropathologies, including AD, PD, and multiple sclerosis (Ralhan et al., 2021; Smolič et al., 2022) and TBI (Li et al., 2023). Notably, Alois Alzheimer’s 1907 translated report already described one characteristic finding in the brain of a patient with AD as the presence of “adipose saccule” in glial cells (Stelzmann et al., 1995). Subsequent studies demonstrated that LD formation in astrocytes protects neurons from various cellular stresses, including oxidative, metabolic, and hypoxic stress (Sultana et al., 2013; Liu et al., 2017; Smolič et al., 2021), while also limiting lipid accumulation in neurons. However, when LD accumulation becomes excessive, astrocytes undergo phenotypic changes and become neurotoxic lipid-accumulating reactive astrocytes (LARAs) (Chen et al., 2023). At the same time, microglia exhibit reduced phagocytic function, increased production of reactive oxygen species, and enhanced secretion of inflammatory cytokines (Marschallinger et al., 2020). Oleic acid and palmitic acid induce LD accumulation in primary cultured astrocytes (Nakajima et al., 2019; Kwon et al., 2017). and these astrocytes subsequently secrete inflammatory cytokines that promote microglial migration and activation (Kwon et al., 2017). Furthermore, recent work has shown that microglia, which contribute to Aβ clearance in the early stages of AD, develop defects in Aβ phagocytosis when LDs accumulate, thereby accelerating amyloid pathology and leading to neuronal damage (Prakash et al., 2025). Thus, while LD formation in glial cells initially exerts protective functions for neurons, excessive LD accumulation in glial cells contributes to pathological processes in the brain. Importantly, LD accumulation precedes Aβ deposition. Because LD dysregulation is implicated in multiple brain diseases, investigating the mechanisms of LD accumulation and degradation is critical for understanding the underlying causes of these disorders.
Then, here we focus on the transport of triacylglycerols (TAGs) and cholesterol, the major components of LDs, between neurons and astrocytes (Figure 2). LDs contain TAGs and cholesterol esters in their core, surrounded by a phospholipid monolayer that incorporates free cholesterol and various proteins.

Figure 2. ApoE-regulated lipid transport between neurons and astrocytes and Aβ clearance. (A) Triacylglycerol (TAG) synthesized in neurons is transferred to astrocytes via ApoE-containing brain lipoproteins. (B) Cholesterol synthesized in astrocytes is transferred to neurons via ApoE-containing brain lipoproteins. (C) Aggregated Aβ binds to ApoE-containing brain lipoproteins, facilitating its clearance.
TAGs consist of a glycerol backbone esterified with three fatty acids (FAs). In astrocytes, FAs for TAG synthesis are supplied by neurons via ApoE-associated lipid particles (Ioannou et al., 2019) and from the bloodstream through the BBB. Neurons express ApoE during oxidative stress, and it has been suggested that ApoE facilitates lipid transfer (Ralhan et al., 2021) (Figure 2A). Additionally, astrocytes can de novo synthesize FAs from lactate via acetyl-CoA. FAs and diacylglycerol are then esterified to form TAGs by the enzyme diacylglycerol acyltransferase (DGAT), which is localized to the endoplasmic reticulum (Lan et al., 2023). The resulting TAGs are packaged into LDs in the astrocyte cytoplasm. These processes are especially active when neurons are under cellular stress.
Cholesterol exists in two principal forms: free cholesterol and cholesterol ester (CE). In the bloodstream, most cholesterol is esterified, with approximately two-thirds present as CE and one-third as free cholesterol. In contrast, in the brain, only about 1% of cholesterol is esterified (Hyuk Yoon et al., 2022), highlighting a fundamental difference in cholesterol biology between the CNS and peripheral tissues (Stubbs and Smith, 1984). Cholesterol in phospholipid bilayers influences both the fluidity and rigidity of lipid membranes. In the brain, many cells contain extensive phospholipid bilayer regions, including neurons with their long axons, astrocytes and microglia with their numerous processes, and oligodendrocytes with their myelin sheaths. Proper membrane fluidity and rigidity are essential for synaptic transmission mediated by vesicle fusion and budding, as well as for the activity of several transmembrane proteins, including receptors and ion channels. The brain requires 20–25% of the body’s total cholesterol, making it the tissue with the highest cholesterol concentration. However, because the BBB is impermeable to cholesterol, brain cholesterol levels are largely independent of peripheral cholesterol. Instead, most cholesterol in the brain is synthesized de novo by astrocytes (Shin et al., 2024). Once synthesized, cholesterol is secreted from astrocytes through ATP-binding cassette (ABC) transporters, esterified to CE by lecithin-cholesterol acyltransferase (LCAT), and transferred to neurons via ApoE-containing high-density lipoprotein (HDL)-like particles (i.e., brain lipoproteins) (Tsujita et al., 2024). These CE-containing ApoE particles are then taken up by neurons through LRP1 and LDLR (Jin et al., 2019) (Figure 2B). The cholesterol obtained by neurons supports neurogenesis and synaptogenesis, whereas excess cholesterol is converted into 24-hydroxycholesterol (24-OH), which can cross the BBB and be excreted. Thus, ABC transporters in astrocytes, LRP1/LDLR in neurons, and ApoE synthesized by astrocytes are all essential for intercellular cholesterol transport between astrocytes and neurons. Dysfunction of these proteins is linked to impaired lipid metabolism and LD accumulation in both neurons and astrocytes (Liu et al., 2010; Windham et al., 2024; Wang S. et al., 2025). Lipoproteins serve as vehicles to transport insoluble molecules, such as CE. Several neurodegenerative diseases, including AD and PD, are characterized by the accumulation of protein aggregates and the formation of insoluble plaques in the brain, ultimately leading to neuronal death. Aggregated Aβ is one of the key pathological factors in AD. Although insoluble, aggregated Aβ binds to ApoE-containing brain lipoproteins, and the resulting Aβ-ApoE complexes are eliminated from the CNS into systemic circulation via the BBB or the glymphatic system (Figure 2C) (Campos-Peña et al., 2022). Therefore, ApoE plays a critical role in Aβ clearance and is considered a promising therapeutic target for AD and other neurodegenerative disorders.
Recent advances in mass spectrometry have enabled detailed characterization of fatty acid chain composition. For example, studies have revealed that unsaturated FAs are decreased in the AD brain (Yin, 2023). As lipidomic research progresses and the components of brain lipids are identified with greater precision, our understanding of lipid contributions to neurodegeneration will become more comprehensive. Interestingly, it has also been reported that the composition of brain lipids in zebrafish is affected by environmental temperature (Maffioli et al., 2024). Moreover, studies using zebrafish have demonstrated that elimination of accumulated LDs is necessary to promote recovery during regeneration following TBI (Zambusi et al., 2022). Therefore, LD clearance facilitates neuroregeneration, a key finding from zebrafish studies that may provide translational insights for mammalian models. In the following chapters, we focus on zebrafish models of several neurodegenerative diseases.
6 Zebrafish as a model for glial cell research
Zebrafish, a teleost fish, offers several advantages compared to other animal models. They have a short life cycle, reaching sexual maturity within 3 months, and females can lay hundreds of eggs at a time. Furthermore, external development and the transparency of embryos and larvae allow for easy manipulation, and cellular processes can be imaged in vivo using fluorescent reporters (Dooley and Zon, 2000).
Comparative genomic analyses have shown that 76% of genes implicated in human diseases through GWAS are conserved in zebrafish (Howe et al., 2013). In addition, the zebrafish brain shares structural and functional similarities with the mammalian brain, including the presence of the same major cell types: neurons, astrocytes, oligodendrocytes, and microglia (Schmidt et al., 2013). A detailed review on the functional roles of immune cells and their responses in the zebrafish CNS has previously been published (Oosterhof et al., 2015).
As an introduction to this section, we first describe the glial cells and their primary functions in zebrafish, which are the focus of this review.
6.1 BBB in zebrafish
At the BBB, zebrafish BBB form tight junctions composed of ZO-1 and claudin-5 (CLDN5), similar to mammals, and maintain close contact with pericytes (O’Brown et al., 2018). In zebrafish, two paralogs, a CLDN-5a and -5b, are expressed in cerebrovascular endothelial cells; however, Li et al. demonstrated that CLDN-5a functions as the ortholog of mammalian CLDN5 (Li et al., 2022). Taken together, CLDN5 is highly conserved from fish to humans, making it an important factor in the BBB.
Key molecular mechanisms of BBB formation have also been elucidated using zebrafish. Notch3 signaling has been shown to be essential for pericyte proliferation, regulation of pericyte numbers, and BBB integrity in zebrafish (Wang et al., 2014). Furthermore, Hübner et al. employed high-resolution in vivo imaging and demonstrated that Wnt signaling is required for brain angiogenesis and BBB formation (Hübner et al., 2018). Inhibition of Wnt signaling with IWR-1 produced severe vascular defects and hemorrhages in the zebrafish larvae. In addition, America et al. revealed that GPR124/Reck function implicated in Wnt ligand-specific cellular responses in developing brain vasculature in both zebrafish and mice, providing evidence for functional conservation of these pathways across vertebrates (America et al., 2022). By contrast, double-transgenic zebrafish expressing glut1β:mcherry and plvap: EGFP clarified that VEGF signaling is critical for CNS angiogenesis but not required for Wnt/β-catenin-dependent BBB properties (Fetsko et al., 2023). They further demonstrated that activation of Wnt/β-catenin signaling occurs independently of VEGF in brain endothelial cells. Thus, Wnt signaling in zebrafish BBB formation remains controversial.
Neuronal contributions to BBB development have also been identified. Neurons secrete Spock1, which induces BBB integrity. Knockout of SPOCK1 in zebrafish and mice resulted in BBB leakage and altered pericyte-endothelial interactions (O’Brown et al., 2023). Remarkably, injection of recombinant human SPOCK1 protein rescued BBB leakage in mutants, suggesting conservation of SPOCK1 function from zebrafish to humans.
Finally, glial coverage of cerebral vasculature has been observed during zebrafish brain development (Gall et al., 2025). Thus, we next focus on the cellular contributors to BBB integrity in zebrafish.
6.2 Astrocytes in zebrafish
Radial glial cells (RGCs), the precursors of astrocytes in mammals, were previously classified in place of astrocytes in zebrafish (Jurisch-Yaksi et al., 2020). However, Chen et al. (2020) identified bona fide astrocytes in zebrafish that are highly similar to mammalian astrocytes. The authors demonstrated that astrocytes derived from RGCs in the larval brain and spinal cord could be visualized in vivo for the first time using advanced imaging systems (Chen et al., 2020). They further reported that the transparency of zebrafish larvae allowed tracking of single RGCs and astrocytes from birth through late larval stages. Moreover, their study revealed that FGFR3/4 are required for vertebrate astrocyte morphogenesis and that astrocytes become highly branched to establish distinct territories, as shown using a cell-specific CRISPR/Cas9 system.
In mammals, the water channel AQP4 is localized to astrocytic endfeet that contact the vasculature (Corbo et al., 2012; Ikeshima-Kataoka et al., 2013). By contrast, in zebrafish, AQP4 is distributed throughout the radial glial process and rarely contacts blood vessels (Grupp et al., 2010). The differences in AQP4 expression patterns between zebrafish and mammals are thought to result from species-specific adaptations to their respective environments during evolution. Thus, in vivo imaging analysis using transgenic zebrafish provides unique opportunities to elucidate the details of AQP4 localization in astrocytes.
Tripartite synapses, in which astrocytes are closely associated with pre- and postsynaptic elements, have been extensively studied in rodents, where astrocytes monitor synaptic activity and actively regulate synaptic transmission (Chung et al., 2015; del Río-Hortega Bereciartu, 2020). Recently, Koh et al. (2025) identified tripartite synapses in zebrafish using GCaMP6s imaging of dorsal root ganglion neurons, spinal neurons, and astrocytes. They showed that astrocytic processes directly contacted the synaptic cleft region in the spinal sensorimotor circuit, as confirmed by electron microscopy reconstruction. These findings indicate that astrocyte-neuron interactions at the synaptic level are evolutionarily conserved across vertebrates.
The glymphatic system differs from the lymphatic system, although its function is analogous to that of the lymphatic system, was first clearly reported using in vivo two-photon imaging in the mouse brain (Iliff et al., 2012). In this system, cerebrospinal fluid enters the parenchyma along perivascular spaces formed by astrocytic endfeet surrounding penetrating arteries, and interstitial fluid is cleared along perivenous drainage pathways. Interestingly, a similar system has also been identified in zebrafish. Li et al. (2025) demonstrated that radial glial astrocytes mediate brain lymphatic development in zebrafish, a process modulated by neural activity. Their findings highlighted the importance of the brain’s immune system via specific glial subpopulations in zebrafish. Thus, the glymphatic system appears to be conserved at least across vertebrates.
6.3 Pericytes in zebrafish
Pericytes display region-specific specialization within the brain. For example, cerebral pericytes are neural crest-derived, similar to mammalians, whereas pericytes in the hindbrain vasculature originate from the mesoderm (Bahrami and Childs, 2018). Thus, the developmental origins of pericytes in zebrafish differ across brain regions. Wang et al. (2014) demonstrated that Notch3 signaling establishes brain vascular integrity by regulating brain pericyte number using zebrafish embryos and larvae.
More recently, a pericyte-targeted knock-in zebrafish line was generated using a PDGFRβ promoter-driven P2A-Gal4-VP16 construct via CRISPR/Cas9 genome editing (Zi et al., 2025). Crossing this line with the transgenic 4x nrUAS: GFP zebrafish allows in vivo imaging of brain pericytes during development.
6.4 Microglia in zebrafish
To investigate the functional role of microglial cells in the developing brain, Wnt/β-catenin signaling was genetically reduced and compared between developing mouse and zebrafish brains (Van Steenwinckel et al., 2019). The authors demonstrated that the Wnt pathway regulates microglial activation and is critical in human brain injury. Thus, modulation of microglial activity may provide therapeutic strategies for developmental brain disorders.
Transgenic zebrafish lines carrying fluorescent reporters driven by the macrophage-expressed gene 1 (mpeg1) promoter revealed that microglia are more motile and phagocytic in larvae than in mature stages, as observed by real-time imaging (Svahn et al., 2013). Moreover, developing microglia frequently contact brain capillaries, and in the adult optic tectum (OT), microglia exhibit extensive branching, similar to mammalian microglia.
In vivo imaging of microglial cells in zebrafish embryos has been clearly demonstrated using apolipoprotein E locus–driven GFP (Apo-E-GFP) transgenic lines (Peri and Nüsslein-Volhard, 2008). Interestingly, the authors showed that microglia phagocytose injected Gram-negative E. coli bacteria in the brain and apoptotic neurons. Notably, they further demonstrated that the α1 subunit of the large vacuolar v-ATPase complex is required for the phagocytic and degradative activity of microglia in zebrafish. Because the authors observed “indigestion” of microglia in gene knockout zebrafish, investigating the functional consequences of microglial defects may provide critical insights into the development of degenerative diseases in humans.
A comprehensive single-cell transcriptomics analysis was recently reported for microglia, oligodendrocyte-lineage cells, and quiescent radial astrocytes (qRA) isolated from the zebrafish midbrain OT with or without stab wound injury at different developmental stages (Qin et al., 2025). The authors identified two centrally located qRA subtypes: qRA1, predominant at larval stages, and qRA2, enriched at juvenile and adult stages. Interestingly, TBI-induced qRA remained inactive following early larval TBI but showed proliferative responses at later stages. For microglial subtypes, larval-dominant MG1 cells mediated phagocytic clearance, whereas adult-enriched MG2 cells orchestrated synaptic refinement, as determined by gene ontology analysis. In addition, following TBI, three distinct reactive microglial states were identified: iMG-1, resembling activated macrophages; iMG-2, resembling regulatory macrophages; and iMG-3, corresponding to wound-healing macrophages. Unlike mammals, zebrafish possess the capacity for neuronal regeneration following TBI. Detailed investigation of these zebrafish microglial subtypes may pave the way for developing mammalian therapeutics for CNS degeneration.
6.5 Zebrafish models for neurodegenerative diseases
Zebrafish represent an excellent animal model for investigating drug discovery, therapeutic delivery, and treatment strategies for brain injury and neurodegenerative diseases.
In the following sections, we focus on recent findings regarding the involvement of glial cells in neurodegenerative diseases in zebrafish.
Moreover, we summarized several disease models using zebrafish and their results in Table 4.
6.5.1 Zebrafish models of AD
In one model, Aβ was microinjected into the cerebroventricular region of adult zebrafish, which produced clinically relevant AD-like symptoms (Javed et al., 2019). Interestingly, microinjection of casein-coated gold nanoparticles (βCas AuNPs) into AD model zebrafish alleviated these pathological symptoms. To clarify the molecular changes underlying BBB alterations in AD, single-nucleus RNA sequencing was performed on vascular and astrocytic cells in AD versus control brains (İş et al., 2024). The authors identified an inverse relationship between SMAD3 upregulation in AD pericytes and vascular endothelial growth factor downregulation in AD astrocytes in Ab42-treated zebrafish lines, which was further supported by pharmacological analyses. Because these altered molecular mechanisms of BBB integrity were conserved between zebrafish models and human patients, they represent potential therapeutic targets for AD pathology.
In another study, Meguro et al. demonstrated that cognitive impairment induced by excessive fat intake could be reproduced in zebrafish (Meguro et al., 2019). As described earlier in the “LD accumulation in brain” section, inadequate lipid regulation may contribute to neurodegeneration.
6.5.2 Zebrafish models of PD
Berberine, an alkaloid extracted from Berberis plants, has been applied in zebrafish PD models (Wang et al., 2021). Fluorescently labeled berberine demonstrated neuroprotective activity by alleviating PD-like behaviors induced by MPTP, a neurotoxin that induces PD-like pathology (Razali et al., 2022), and by reducing dopaminergic neuronal loss. The authors suggested that berberine exerts its protective effects partly through mitochondrial mechanisms and may represent a candidate anti-PD drug.
More recently, integrative analyses using PD neurons, organoids, zebrafish, and mouse models identified ATP-citrate lyase (ACLY), a key enzyme generating acetyl-CoA in the cytoplasm, as a risk factor for PD pathology (Son et al., 2025). The authors focused on the A53T mutation in SNCA, one of the most frequent missense mutations. Inhibition of ACLY restored autophagy and reduced pathological α-Syn levels in PD neurons and organoids. Furthermore, in A53T α-Syn transgenic zebrafish and mice, ACLY inhibition enhanced α-Syn degradation. These findings suggest that ACLY may represent a promising therapeutic target for PD.
6.5.3 Zebrafish models of epilepsy
Zebrafish models for seizures and epilepsy have been developed over the past two decades and are comprehensively summarized elsewhere (Yaksi et al., 2021). For instance, the ease of maintaining zebrafish across a wide range of aquarium temperatures allows hyperthermia to be used for inducing seizure models. Furthermore, genetic models of seizures and epilepsy are well established, and fluorescence calcium imaging of brain activity, combined with video recording of locomotor tail beats, takes advantage of zebrafish transparency. Adult zebrafish can also be used for epilepsy modeling (Cho et al., 2020a,b).
Fang et al. (2025) performed molecular analyses of transcriptional profiles from 15 patients to investigate mechanisms underlying epilepsy. They revealed that vascular malformations and abnormal SMCs generate ischemic–hypoxic (I/H) microenvironments, which subsequently disrupt neuronal and astrocytic activity, leading to neuronal loss. Using zebrafish models, they demonstrated that I/H microenvironments induced seizure activity, paralleling pathological features observed in human patients.
Recently, Necrostatin-1 (Nec-1), a potential anticonvulsant, was identified using the zebrafish pentylenetetrazol (PTZ)-induced seizure model (Ravikumar et al., 2025). Nec-1 inhibits receptor-interacting protein kinase 1 (RIPK1), a signaling molecule implicated in neuroinflammation and neurodegeneration. The authors showed that Nec-1 reduced seizure severity and reversed astrocytic activation in zebrafish. Given that abnormal astrocytic and microglial dysfunction has been reported in human epilepsy patients and animal models, these findings suggest that Nec-1 exerts anticonvulsant effects through its anti-inflammatory properties.
6.5.4 Zebrafish model of Alexander disease
Alexander disease (AxD) is a rare astrocytic neurodegenerative disorder classified as a leukodystrophy and caused by mutations in the GFAP gene (Brenner et al., 2001).
Recently, Bellitto et al. (2025) reported the generation of a stable transgenic zebrafish line (zAxD) carrying a human GFAP mutation associated with the severe phenotype of AxD type I patients. Transcriptomic and proteomic analyses of zAxD larvae confirmed alterations in cellular respiration and lipid metabolism, along with upregulation of oxidative stress (Bellitto et al., 2025). These alterations were newly identified and had not been reported in other animal models of AxD. Thus, this transgenic zebrafish model may contribute to clarifying the mechanisms of disease onset and to the development of therapeutic approaches for AxD.
In addition, Saito et al. (2024) revealed that microglia sense astrocytic dysfunction via P2Y12 signaling to protect against AxD pathology in AxD model mice. Therefore, microglial contributions to AxD pathogenesis may soon be elucidated in zebrafish models as well.
7 Comparison of brain regeneration mechanisms between zebrafish and mammals
Zebrafish are capable of regenerating their brains after injury, a capacity that distinguishes them from mammals.
Self-renewing neural stem cells have been identified in the adult zebrafish telencephalon (Adolf et al., 2006; Chapouton et al., 2007). These progenitors give rise to newborn neurons that reside in the ventricular zone. Previous studies demonstrated that inflammation is required and sufficient to induce proliferation of neural progenitors and to promote neurogenesis in the adult zebrafish brain (Kyritsis et al., 2012). In the spinal cord, Hui et al. (2015) identified key pluripotency-related factors, including upregulation of pou5f1 and sox2, during regeneration after spinal cord injury. The authors suggested that elucidating these molecular mechanisms may pave the way toward therapeutic targets for mammalian spinal cord injury. In addition, FGF signaling was shown to be a critical regulator of glial cell morphogenesis in axonal regeneration. For example, glial migration was observed 2 weeks after spinal cord injury, glial bridge formation required for axonal regeneration appeared at 3 weeks, and axonal remodeling was evident by 6 weeks (Goldshmit et al., 2012). Furthermore, Notch-1 signaling plays an important role in generating neural precursor cells that migrate to the injured region and subsequently differentiate into mature neurons (Kishimoto et al., 2012).
In contrast to mammals, zebrafish rapidly induce neurogenesis after a TBI (detailed in the “Clinical Features of TBI” section). In adult zebrafish, stab wound injury to the telencephalon results in wound closure within a few days without scar formation (Baumgart et al., 2012). Thus, the zebrafish telencephalon provides a useful system to explore the mechanisms of regeneration. Consistently, another study demonstrated that telencephalic stab wounds in zebrafish revealed constitutive neurogenic activity (Pellegrini et al., 2023). Recently, molecular mechanisms of regeneration have been clarified in zebrafish TBI models. Palsamy et al. (2023) showed that microglia are essential for regeneration in the zebrafish brain, using genetic mutant lines in which microglia were ablated after brain injury. They further revealed that phosphorylated Stat3 (signal transducer and activator of transcription 3) and β-catenin signaling in microglia are required for telencephalic regeneration. These findings suggest that targeting these pathways may be effective for promoting repair in the injured mammalian brain.
Chen et al. (2024) compared telencephalic regeneration in zebrafish and rodents and summarized the signaling pathways that regulate inflammation and the factors controlling regeneration in the adult zebrafish brain. They pointed out that BMP-Id3 upregulation in the SVZ promotes astrogenesis, leading to gliosis and glial scar formation. Because no reactive gliosis or scar formation is observed in the zebrafish brain after injury, modulating this signaling pathway may open new avenues for promoting regeneration in the mammalian brain.
8 Conclusion
In the first section, studies of astrocytic and microglial molecules have shown that these cells can serve as therapeutic targets for neurodegenerative diseases in mouse models (Figure 1 and Tables 1–3). In addition, the second section highlights the importance of lipid metabolism in glial cells, particularly in astrocytes, in the context of neurodegenerative disorders (Figure 2). In contrast, the third section underscores that relatively few studies have examined glial cells in relation to neurodegenerative diseases in zebrafish models (Table 4).
Taken together, we have introduced a range of recent studies targeting glial cells that utilize the advantages of different animal models to investigate gene functions in the brain. These studies collectively demonstrate that glial cells hold considerable promise for the development of therapeutic strategies against neurodegenerative diseases.
Author contributions
KH: Writing – review & editing, Writing – original draft. MG: Writing – review & editing, Funding acquisition, Writing – original draft. HI-K: Supervision, Conceptualization, Funding acquisition, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS, HI-K: 22K07375; MG: 23K04950).
Acknowledgments
We thank Ms. IKEHATA Kieko at Teikyo University for her support with scientific illustrations. We thank Editage (www.editage.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
LD, lipid droplet; DAM, disease-associated microglia; DAA, disease-associated astrocytes; LARA, lipid-accumulating reactive astrocytes; RGC, radial glial cells; qRA, quiescent radial astrocytes; MG1/MG2/iMG-1/iMG-2/iMG-3, microglial subtypes described in zebrafish models; AxD, Alexander disease.
References
Abe, T., and Barber, G. N. (2014). Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-κB activation through TBK1. J. Virol. 88, 5328–5341. doi: 10.1128/JVI.00037-14,
Abramova, V., Tomovic, E., Kysilov, B., Korinek, M., Dobrovolski, M., Hrcka Krausova, B., et al. (2025). Disruption of grin2A, an epilepsy-associated gene, produces altered spontaneous swim behavior in zebrafish. J. Neurosci. e0946252025. doi: 10.1523/jneurosci.0946-25.2025
Adams, K. L., and Gallo, V. (2018). The diversity and disparity of the glial scar. Nat. Neurosci. 21, 9–15. doi: 10.1038/s41593-017-0033-9,
Adolf, B., Chapouton, P., Lam, C. S., Topp, S., Tannhäuser, B., Strähle, U., et al. (2006). Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev. Biol. 295, 278–293. doi: 10.1016/j.ydbio.2006.03.023,
Altay, M. F., Liu, A. K. L., Holton, J. L., Parkkinen, L., and Lashuel, H. A. (2022). Prominent astrocytic alpha-synuclein pathology with unique post-translational modification signatures unveiled across Lewy body disorders. Acta Neuropathol. Commun. 10:163. doi: 10.1186/s40478-022-01468-8,
America, M., Bostaille, N., Eubelen, M., Martin, M., Stainier, D. Y. R., and Vanhollebeke, B. (2022). An integrated model for Gpr124 function in Wnt7a/b signaling among vertebrates. Cell Rep. 39:110902. doi: 10.1016/j.celrep.2022.110902,
Austin, S. A., Floden, A. M., Murphy, E. J., and Combs, C. K. (2006). Alpha-synuclein expression modulates microglial activation phenotype. J. Neurosci. 26, 10558–10563. doi: 10.1523/JNEUROSCI.1799-06.2006,
Bahrami, N., and Childs, S. J. (2018). “Pericyte biology in zebrafish” in Advances in experimental medicine and biology. A. Birbrair (ed.) (New York LLC: Springer), 33–51.
Bailey, C. C., DeVaux, L. B., and Farzan, M. (2015). The triggering receptor expressed on myeloid cells 2 binds apolipoprotein E. J. Biol. Chem. 290, 26033–26042. doi: 10.1074/jbc.M115.677286,
Balantzategi, U., Gaminde-Blasco, A., Kearns, C. A., Bayón-Cordero, L., Sánchez-Gómez, M. V., Zugaza, J. L., et al. (2025). Amyloid-β Dysregulates Oligodendroglial Lineage Cell Dynamics and Myelination via PKC in the Zebrafish Spinal Cord. Glia 73, 1437–1451. doi: 10.1002/glia.70015
Basurco, L., Abellanas, M. A., Ayerra, L., Conde, E., Vinueza-Gavilanes, R., Luquin, E., et al. (2023). Microglia and astrocyte activation is region-dependent in the α-synuclein mouse model of Parkinson’s disease. Glia 71, 571–587. doi: 10.1002/glia.24295,
Baumgart, E. V., Barbosa, J. S., Bally-cuif, L., Götz, M., and Ninkovic, J. (2012). Stab wound injury of the zebrafish telencephalon: a model for comparative analysis of reactive gliosis. Glia 60, 343–357. doi: 10.1002/glia.22269,
Bellaver, B., Povala, G., Ferreira, P. C. L., Ferrari-Souza, J. P., Leffa, D. T., Lussier, F. Z., et al. (2023). Astrocyte reactivity influences amyloid-β effects on tau pathology in preclinical Alzheimer’s disease. Nat. Med. 29, 1775–1781. doi: 10.1038/s41591-023-02380-x,
Bellitto, D., Bozzo, M., Ravera, S., Bertola, N., Rosamilia, F., Milia, J., et al. (2025). A multi-omics approach reveals impaired lipid metabolism and oxidative stress in a zebrafish model of Alexander disease. Redox Biol. 81:103544. doi: 10.1016/j.redox.2025.103544,
Bido, S., Muggeo, S., Massimino, L., Marzi, M. J., Giannelli, S. G., Melacini, E., et al. (2021). Microglia-specific overexpression of α-synuclein leads to severe dopaminergic neurodegeneration by phagocytic exhaustion and oxidative toxicity. Nat. Commun. 12:6237. doi: 10.1038/s41467-021-26519-x,
Bielefeld, P., Martirosyan, A., Martín-Suárez, S., Apresyan, A., Meerhoff, G. F., Pestana, F., et al. (2024). Traumatic brain injury promotes neurogenesis at the cost of astrogliogenesis in the adult hippocampus of male mice. Nat. Commun. 15:5222. doi: 10.1038/s41467-024-49299-6,
Borucki, D. M., Rohrer, B., and Tomlinson, S. (2024). Complement propagates visual system pathology following traumatic brain injury. J. Neuroinflammation 21:98. doi: 10.1186/s12974-024-03098-4,
Brelstaff, J., Tolkovsky, A. M., Ghetti, B., Goedert, M., and Spillantini, M. G. (2018). Living neurons with tau filaments aberrantly expose phosphatidylserine and are phagocytosed by microglia. Cell Rep. 24, 1939–1948.e4. doi: 10.1016/j.celrep.2018.07.072,
Brenner, M., Johnson, A. B., Boespflug-Tanguy, O., Rodriguez, D., Goldman, J. E., and Messing, A. (2001). Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat. Genet. 27, 117–120. doi: 10.1038/83679,
Campos-Peña, V., Pichardo-Rojas, P., Sánchez-Barbosa, T., Ortíz-Islas, E., Rodríguez-Pérez, C. E., Montes, P., et al. (2022). Amyloid β, lipid metabolism, basal cholinergic system, and therapeutics in Alzheimer’s disease. Int. J. Mol. Sci. 23:12092. doi: 10.3390/ijms232012092,
Chapouton, P., Jagasia, R., and Bally-Cuif, L. (2007). Adult neurogenesis in non-mammalian vertebrates. BioEssays 29, 745–757. doi: 10.1002/bies.20615,
Chen, J., Poskanzer, K. E., Freeman, M. R., and Monk, K. R. (2020). Live-imaging of astrocyte morphogenesis and function in zebrafish neural circuits. Nat. Neurosci. 23, 1297–1306. doi: 10.1038/s41593-020-0703-x,
Chen, J., Sanchez-Iranzo, H., Diotel, N., and Rastegar, S. (2024). Comparative insight into the regenerative mechanisms of the adult brain in zebrafish and mouse: highlighting the importance of the immune system and inflammation in successful regeneration. FEBS J. 291, 4193–4205. doi: 10.1111/febs.17231,
Chen, Z. P., Wang, S., Zhao, X., Fang, W., Wang, Z., Ye, H., et al. (2023). Lipid-accumulated reactive astrocytes promote disease progression in epilepsy. Nat. Neurosci. 26, 542–554. doi: 10.1038/s41593-023-01288-6,
Chinnappan, D., Krishnan, M., Kuppamuthu, A., and Elangovan, N. (2025). Developmental and Neuroprotective Effects of α-Terpineol in MPTP-Induced Parkinsonism in Zebrafish Larvae. Neurochem Res 50. doi: 10.1007/s11064-025-04407-w
Cho, S. J., Park, E., Baker, A., and Reid, A. (2020a). Age bias in zebrafish models of epilepsy: what can we learn from old fish? Front. Cell Dev. Biol. 8:573303. doi: 10.3389/fcell.2020.573303,
Cho, S. J., Park, E., Telliyan, T., Baker, A., and Reid, A. (2020b). Zebrafish model of posttraumatic epilepsy. Epilepsia 61, 1774–1785. doi: 10.1111/epi.16589,
Choi, I., Kim, B., Byun, J.-W., Baik, S. H., Huh, Y. H., Kim, J.-H., et al. (2015). LRRK2 G2019S mutation attenuates microglial motility by inhibiting focal adhesion kinase. Nat. Commun. 6:8255. doi: 10.1038/ncomms9255,
Chou, T.-W., Chang, N. P., Krishnagiri, M., Patel, A. P., Lindman, M., Angel, J. P., et al. (2021). Fibrillar α-synuclein induces neurotoxic astrocyte activation via RIP kinase signaling and NF-κB. Cell Death Dis. 12:756. doi: 10.1038/s41419-021-04049-0,
Chung, W. S., Allen, N. J., and Eroglu, C. (2015). Astrocytes control synapse formation, function, and elimination. Cold Spring Harb. Perspect. Biol. 7:a020370. doi: 10.1101/cshperspect.a020370,
Condello, C., Yuan, P., Schain, A., and Grutzendler, J. (2015). Microglia constitute a barrier that prevents neurotoxic protofibrillar Aβ42 hotspots around plaques. Nat. Commun. 6:6176. doi: 10.1038/ncomms7176,
Corbo, C. P., Othman, N. A., Gutkin, M. C., Alonso, A. D. C., and Fulop, Z. L. (2012). Use of different morphological techniques to analyze the cellular composition of the adult zebrafish optic tectum. Microsc. Res. Tech. 75, 325–333. doi: 10.1002/jemt.21061,
Cras, P., Kawai, M., Lowery, D., Gonzalez-DeWhitt, P., Greenberg, B., and Perry, G. (1991). Senile plaque neurites in Alzheimer disease accumulate amyloid precursor protein. Proc. Natl. Acad. Sci. USA 88, 7552–7556. doi: 10.1073/pnas.88.17.7552,
D’Sa, K., Choi, M. L., Wagen, A. Z., Setó-Salvia, N., Kopach, O., Evans, J. R., et al. (2025). Astrocytic RNA editing regulates the host immune response to alpha-synuclein. Sci. Adv. 11:eadp8504. doi: 10.1126/sciadv.adp8504,
Dabla, A., Liang, Y. C., Rajabalee, N., Irwin, C., Moonen, C. G. J., Willis, J. V., et al. (2022). TREM2 promotes immune evasion by mycobacterium tuberculosis in human macrophages. MBio 13:e0145622. doi: 10.1128/mbio.01456-22,
de Rojas, I., Moreno-Grau, S., Tesi, N., Grenier-Boley, B., Andrade, V., Jansen, I. E., et al. (2021). Common variants in Alzheimer’s disease and risk stratification by polygenic risk scores. Nat. Commun. 12:3417. doi: 10.1038/s41467-021-22491-8,
de Rus Jacquet, A., Alpaugh, M., Denis, H. L., Tancredi, J. L., Boutin, M., Decaestecker, J., et al. (2023). The contribution of inflammatory astrocytes to BBB impairments in a brain-chip model of Parkinson’s disease. Nat. Commun. 14:3651. doi: 10.1038/s41467-023-39038-8,
Dean, T., Mendiola, A. S., Yan, Z., Meza-Acevedo, R., Cabriga, B., Akassoglou, K., et al. (2024). Fibrin promotes oxidative stress and neuronal loss in traumatic brain injury via innate immune activation. J. Neuroinflammation 21:94. doi: 10.1186/s12974-024-03092-w,
Dejanovic, B., Wu, T., Tsai, M.-C., Graykowski, D., Gandham, V. D., Rose, C. M., et al. (2022). Complement C1q-dependent excitatory and inhibitory synapse elimination by astrocytes and microglia in Alzheimer’s disease mouse models. Nat. Aging 2, 837–850. doi: 10.1038/s43587-022-00281-1,
del Río-Hortega Bereciartu, J. (2020). Pío del Río-Hortega: the revolution of glia. Anat. Rec. 303, 1232–1241. doi: 10.1002/ar.24266,
Dharshan, S. S. R. M., Rao, S. M., Aswinanand, B., Ramamurthy, K., Muthuramamoorthy, M., et al. (2025). SG06, a Chalcone Derivative Targets PI3K/AKT1 Pathway for Neuroprotection and Cognitive Enhancement in an Alzheimer’s Disease-Like Zebrafish Model. Mol Neurobiol. doi: 10.1007/s12035-025-05023-z
Diotel, N., Lübke, L., Strähle, U., and Rastegar, S. (2020). Common and distinct features of adult neurogenesis and regeneration in the telencephalon of zebrafish and mammals. Front. Neurosci. 14:568930. doi: 10.3389/fnins.2020.568930,
Dooley, K., and Zon, L. I. (2000). Zebrafish: a model system for the study of human disease. Curr. Opin. Genet. Dev. 10, 252–256. doi: 10.1016/s0959-437x(00)00074-5,
Doostparast Torshizi, A., Truong, D. T., Hou, L., Smets, B., Whelan, C. D., and Li, S. (2024). Proteogenomic network analysis reveals dysregulated mechanisms and potential mediators in Parkinson’s disease. Nat. Commun. 15:6430. doi: 10.1038/s41467-024-50718-x,
Fang, C., Zhang, X., Yang, L., Sun, L., Lu, Y., Liu, Y., et al. (2025). Transcriptomic and morphologic vascular aberrations underlying FCDIIb etiology. Nat. Commun. 16:3320. doi: 10.1038/s41467-025-58535-6,
Feichtenbiner, A. B., Sytsma, K., O’Boyle, R. P., Mittenzwei, R., Maioli, H., Scherpelz, K. P., et al. (2025). Satellite microglia: marker of traumatic brain injury and regulator of neuronal excitability. J. Neuroinflammation 22:9. doi: 10.1186/s12974-024-03328-9,
Fetsko, A. R., Sebo, D. J., and Taylor, M. R. (2023). Brain endothelial cells acquire blood-brain barrier properties in the absence of Vegf-dependent CNS angiogenesis. Dev Biol. 494, 46–59. doi: 10.1016/j.ydbio.2022.11.007
Filippini, A., Carini, G., Barbon, A., Gennarelli, M., and Russo, I. (2025). Astrocytes carrying LRRK2 G2019S exhibit increased levels of clusterin chaperone via miR-22-5p and reduced ability to take up α-synuclein fibrils. Acta Neuropathol. Commun. 13:98. doi: 10.1186/s40478-025-02015-x,
Filippini, A., Mutti, V., Faustini, G., Longhena, F., Ramazzina, I., Rizzi, F., et al. (2021). Extracellular clusterin limits the uptake of α-synuclein fibrils by murine and human astrocytes. Glia 69, 681–696. doi: 10.1002/glia.23920,
Gall, L. G., Stains, C. M., Freitas-Andrade, M., Jia, B. Z., Patel, N., Megason, S. G., et al. (2025). Zebrafish glial-vascular interactions progressively expand over the course of brain development. iScience 28:111549. doi: 10.1016/j.isci.2024.111549,
Gao, L., Wang, B., Cui, X., Xia, L., Li, X., Figueredo, Y. N., et al. (2025). Neochlorogenic acid ameliorates Alzheimer’s disease-like pathology via scavenging oxidative stress and restoring blood-brain barrier function in zebrafish. Prog Neuropsychopharmacol Biol Psychiatry 138. doi: 10.1016/j.pnpbp.2025.111334
Gao, X., Li, W., Syed, F., Yuan, F., Li, P., and Yu, Q. (2022). PD-L1 signaling in reactive astrocytes counteracts neuroinflammation and ameliorates neuronal damage after traumatic brain injury. J. Neuroinflammation 19:43. doi: 10.1186/s12974-022-02398-x,
Gao, Z., Zhu, Q., Zhang, Y., Zhao, Y., Cai, L., Shields, C. B., et al. (2013). Reciprocal modulation between microglia and astrocyte in reactive gliosis following the CNS injury. Mol. Neurobiol. 48, 690–701. doi: 10.1007/s12035-013-8460-4,
Garza, R., Sharma, Y., Atacho, D. A. M., Thiruvalluvan, A., Abu Hamdeh, S., Jönsson, M. E., et al. (2023). Single-cell transcriptomics of human traumatic brain injury reveals activation of endogenous retroviruses in oligodendroglia. Cell Rep. 42:113395. doi: 10.1016/j.celrep.2023.113395,
Gatz, M., Reynolds, C. A., Fratiglioni, L., Johansson, B., Mortimer, J. A., Berg, S., et al. (2006). Role of genes and environments for explaining Alzheimer disease. Arch. Gen. Psychiatry 63, 168–174. doi: 10.1001/archpsyc.63.2.168,
Goldshmit, Y., Sztal, T. E., Jusuf, P. R., Hall, T. E., Nguyen-Chi, M., and Currie, P. D. (2012). Fgf-dependent glial cell bridges facilitate spinal cord regeneration in zebrafish. J. Neurosci. 32, 7477–7492. doi: 10.1523/JNEUROSCI.0758-12.2012,
Gratuze, M., Leyns, C. E., Sauerbeck, A. D., St-Pierre, M.-K., Xiong, M., Kim, N., et al. (2020). Impact of TREM2R47H variant on tau pathology-induced gliosis and neurodegeneration. J. Clin. Invest. 130, 4954–4968. doi: 10.1172/JCI138179,
Grupp, L., Wolburg, H., and Mack, A. F. (2010). Astroglial structures in the zebrafish brain. J. Comp. Neurol. 518, 4277–4287. doi: 10.1002/cne.22511,
Habib, N., McCabe, C., Medina, S., Varshavsky, M., Kitsberg, D., Dvir-Szternfeld, R., et al. (2020). Disease-associated astrocytes in Alzheimer’s disease and aging. Nat. Neurosci. 23, 701–706. doi: 10.1038/s41593-020-0624-8,
Hashimoto, K., Nakashima, M., Hamano, A., Gotoh, M., Ikeshima-Kataoka, H., Murakami-Murofushi, K., et al. (2018). 2-carba cyclic phosphatidic acid suppresses inflammation via regulation of microglial polarisation in the stab-wounded mouse cerebral cortex. Sci. Rep. 8:9715. doi: 10.1038/s41598-018-27990-1,
He, X., Huang, Y., Liu, Y., Zhang, X., Wang, Q., Liu, Y., et al. (2023). Astrocyte-derived exosomal lncRNA 4933431K23Rik modulates microglial phenotype and improves post-traumatic recovery via SMAD7 regulation. Mol. Ther. 31, 1313–1331. doi: 10.1016/j.ymthe.2023.01.031,
Henry, R. J., Ritzel, R. M., Barrett, J. P., Doran, S. J., Jiao, Y., Leach, J. B., et al. (2020). Microglial depletion with CSF1R inhibitor during chronic phase of experimental traumatic brain injury reduces neurodegeneration and neurological deficits. J. Neurosci. 40, 2960–2974. doi: 10.1523/JNEUROSCI.2402-19.2020,
Hogan, A. L., Kane, M., Chiu, P., Richter, G., Maurel, C., Wu, S., et al. (2025). Human TDP-43 overexpression in zebrafish motor neurons triggers MND-like phenotypes through gain-of-function mechanism. bioRxiv preprint. doi: 10.1101/2025.07.06.663393
Holstege, H., Hulsman, M., Charbonnier, C., Grenier-Boley, B., Quenez, O., Grozeva, D., et al. (2022). Exome sequencing identifies rare damaging variants in ATP8B4 and ABCA1 as risk factors for Alzheimer’s disease. Nat. Genet. 54, 1786–1794. doi: 10.1038/s41588-022-01208-7,
Hou, X., Qu, X., Chen, W., Sang, X., Ye, Y., Wang, C., et al. (2024). CD36 deletion prevents white matter injury by modulating microglia polarization through the Traf5-MAPK signal pathway. J. Neuroinflammation 21:148. doi: 10.1186/s12974-024-03143-2,
Howe, K., Clark, M. D., Torroja, C. F., Torrance, J., Berthelot, C., Muffato, M., et al. (2013). The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498–503. doi: 10.1038/nature12111,
Hu, Q., Wu, X., Guo, C., Wang, T., Guo, H., Wang, J., et al. (2024). Astrocyte-neuron crosstalk through extracellular vesicle-shuttled miRNA-382-5p promotes traumatic brain injury. Exp. Mol. Med. 56, 2642–2658. doi: 10.1038/s12276-024-01355-3,
Hu, X., Zhang, H., Zhang, Q., Yao, X., Ni, W., and Zhou, K. (2022). Emerging role of STING signalling in CNS injury: inflammation, autophagy, necroptosis, ferroptosis and pyroptosis. J. Neuroinflammation 19:242. doi: 10.1186/s12974-022-02602-y,
Huang, Y., Happonen, K. E., Burrola, P. G., O’Connor, C., Hah, N., Huang, L., et al. (2021). Microglia use TAM receptors to detect and engulf amyloid β plaques. Nat. Immunol. 22, 586–594. doi: 10.1038/s41590-021-00913-5,
Huang, Y., and Lemke, G. (2022). Early death in a mouse model of Alzheimer’s disease exacerbated by microglial loss of TAM receptor signaling. Proc. Natl. Acad. Sci. USA 119:e2204306119. doi: 10.1073/pnas.2204306119,
Hübner, K., Cabochette, P., Diéguez-Hurtado, R., Wiesner, C., Wakayama, Y., Grassme, K. S., et al. (2018). Wnt/β-catenin signaling regulates VE-cadherin-mediated anastomosis of brain capillaries by counteracting S1pr1 signaling. Nat. Commun. 9:4860. doi: 10.1038/s41467-018-07302-x,
Hui, S. P., Nag, T. C., and Ghosh, S. (2015). Characterization of proliferating neural progenitors after spinal cord injury in adult zebrafish. PLoS One 10:e0143595. doi: 10.1371/journal.pone.0143595,
Hyuk Yoon, J., Seo, Y., Suk Jo, Y., Lee, S., Cho, E., Cazenave-Gassiot, A., et al. (2022). Brain lipidomics: from functional landscape to clinical significance. Sci. Adv. 8:eadc9317. doi: 10.1126/sciadv.adc9317,
Ikeshima-Kataoka, H., Abe, Y., Abe, T., and Yasui, M. (2013). Immunological function of aquaporin-4 in stab-wounded mouse brain in concert with a pro-inflammatory cytokine inducer, osteopontin. Mol. Cell. Neurosci. 56, 65–75. doi: 10.1016/j.mcn.2013.02.002,
Ikeshima-Kataoka, H., Shen, J.-S., Eto, Y., Saito, S., and Yuasa, S. (2008). Alteration of inflammatory cytokine production in the injured central nervous system of tenascin-deficient mice. In Vivo 22, 409–413.
Ikeshima-Kataoka, H., and Yasui, M. (2016). Correlation between astrocyte activity and recovery from blood-brain barrier breakdown caused by brain injury. Neuroreport 27, 894–900. doi: 10.1097/WNR.0000000000000619,
Iliff, J. J., Wang, M., Liao, Y., Plogg, B. A., Peng, W., Gundersen, G. A., et al. (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4:147ra111. doi: 10.1126/scitranslmed.3003748,
Ioannou, M. S., Jackson, J., Sheu, S. H., Chang, C. L., Weigel, A. V., Liu, H., et al. (2019). Neuron-astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Cell 177, 1522–1535.e14. doi: 10.1016/j.cell.2019.04.001,
İş, Ö., Wang, X., Reddy, J. S., Min, Y., Yilmaz, E., Bhattarai, P., et al. (2024). Gliovascular transcriptional perturbations in Alzheimer’s disease reveal molecular mechanisms of blood brain barrier dysfunction. Nat. Commun. 15:4758. doi: 10.1038/s41467-024-48926-6,
Javed, I., Peng, G., Xing, Y., Yu, T., Zhao, M., Kakinen, A., et al. (2019). Inhibition of amyloid beta toxicity in zebrafish with a chaperone-gold nanoparticle dual strategy. Nat. Commun. 10:3780. doi: 10.1038/s41467-019-11762-0,
Jha, R. M., Rajasundaram, D., Sneiderman, C., Schlegel, B. T., O’Brien, C., Xiong, Z., et al. (2024). A single-cell atlas deconstructs heterogeneity across multiple models in murine traumatic brain injury and identifies novel cell-specific targets. Neuron 112, 3069–3088.e4. doi: 10.1016/j.neuron.2024.06.021,
Jiao, Y.-Y., Tian, T., Zhu, Z., Cao, L., Liu, Y., Wang, R.-A., et al. (2025). Extracellular LCN2 binding to 24p3R in astrocytes impedes α-synuclein endocytosis in Parkinson’s disease. Adv. Sci. 12:e01694. doi: 10.1002/advs.202501694,
Jin, U., Park, S. J., and Park, S. M. (2019). Cholesterol metabolism in the brain and its association with Parkinson’s disease. Exp. Neurobiol. 28, 554–567. doi: 10.5607/en.2019.28.5.554,
Jiwaji, Z., Tiwari, S. S., Avilés-Reyes, R. X., Hooley, M., Hampton, D., Torvell, M., et al. (2022). Reactive astrocytes acquire neuroprotective as well as deleterious signatures in response to tau and Aß pathology. Nat. Commun. 13:135. doi: 10.1038/s41467-021-27702-w,
Ju, Y. H., Bhalla, M., Hyeon, S. J., Oh, J. E., Yoo, S., Chae, U., et al. (2022). Astrocytic urea cycle detoxifies aβ-derived ammonia while impairing memory in Alzheimer’s disease. Cell Metab. 34, 1104–1120.e8. doi: 10.1016/j.cmet.2022.05.011
Jurga, A. M., Paleczna, M., and Kuter, K. Z. (2020). Overview of general and discriminating markers of differential microglia phenotypes. Front. Cell. Neurosci. 14:198. doi: 10.3389/fncel.2020.00198,
Jurisch-Yaksi, N., Yaksi, E., and Kizil, C. (2020). Radial glia in the zebrafish brain: functional, structural, and physiological comparison with the mammalian glia. Glia 68, 2451–2470. doi: 10.1002/glia.23849
Keren-Shaul, H., Spinrad, A., Weiner, A., Matcovitch-Natan, O., Dvir-Szternfeld, R., Ulland, T. K., et al. (2017). A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290.e17. doi: 10.1016/j.cell.2017.05.018
Kishimoto, N., Shimizu, K., and Sawamoto, K. (2012). Neuronal regeneration in a zebrafish model of adult brain injury. Dis. Model. Mech. 5, 200–209. doi: 10.1242/dmm.007336,
Koh, Z. M., Arceo, R. A., Hammer, J., Chau, K., Light, S. E. W., Dolojan, A., et al. (2025). An ultrastructural map of a spinal sensorimotor circuit reveals the potential of astroglial modulation. bioRxiv. doi: 10.1101/2025.03.05.641432,
Kolb, J., Tsata, V., John, N., Kim, K., Möckel, C., Rosso, G., et al. (2023). Small leucine-rich proteoglycans inhibit CNS regeneration by modifying the structural and mechanical properties of the lesion environment. Nat. Commun. 14:6814. doi: 10.1038/s41467-023-42339-7
Krasemann, S., Madore, C., Cialic, R., Baufeld, C., Calcagno, N., El Fatimy, R., et al. (2017). The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47, 566–581.e9. doi: 10.1016/j.immuni.2017.08.008,
Kwon, Y. H., Kim, J., Kim, C. S., Tu, T. H., Kim, M. S., Suk, K., et al. (2017). Hypothalamic lipid-laden astrocytes induce microglia migration and activation. FEBS Lett. 591, 1742–1751. doi: 10.1002/1873-3468.12691
Kyritsis, N., Kizil, C., Zocher, S., Kroehne, V., Kaslin, J., Freudenreich, D., et al. (2012). Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science 338, 1353–1356. doi: 10.1126/science.1228773,
Lan, Z. Q., Ge, Z. Y., Lv, S. K., Zhao, B., and Li, C. X. (2023). The regulatory role of lipophagy in central nervous system diseases. Cell Death Discov. 9:229. doi: 10.1038/s41420-023-01504-z,
Lee, S.-H., Rezzonico, M. G., Friedman, B. A., Huntley, M. H., Meilandt, W. J., Pandey, S., et al. (2021). TREM2-independent oligodendrocyte, astrocyte, and T cell responses to tau and amyloid pathology in mouse models of Alzheimer disease. Cell Rep. 37:110158. doi: 10.1016/j.celrep.2021.110158,
Lee, S.-I., Yu, J., Lee, H., Kim, B., Jang, M. J., Jo, H., et al. (2025). Astrocyte priming enhances microglial aβ clearance and is compromised by APOE4. Nat. Commun. 16:7551. doi: 10.1038/s41467-025-62995-1,
Li, H., Liu, P., Deng, S., Zhu, L., Cao, X., Bao, X., et al. (2023). Pharmacological upregulation of microglial lipid droplet alleviates neuroinflammation and acute ischemic brain injury. Inflammation 46, 1832–1848. doi: 10.1007/s10753-023-01844-z,
Li, J., Liu, M.-J., Du, W.-J., Peng, X.-L., Deng, H., Zi, H.-X., et al. (2025). Neural-activity-regulated and glia-mediated control of brain lymphatic development. Cell 188, 3274–3290. doi: 10.1016/j.cell.2025.04.008
Li, N.-M., Liu, K.-F., Qiu, Y.-J., Zhang, H.-H., Nakanishi, H., and Qing, H. (2019). Mutations of beta-amyloid precursor protein alter the consequence of Alzheimer’s disease pathogenesis. Neural Regen. Res. 14, 658–665. doi: 10.4103/1673-5374.247469,
Li, Y., Wang, C., Zhang, L., Chen, B., Mo, Y., and Zhang, J. (2022). Claudin-5a is essential for the functional formation of both zebrafish blood-brain barrier and blood-cerebrospinal fluid barrier. Fluids Barriers CNS 19:40. doi: 10.1186/s12987-022-00337-9,
Li, Z., Yu, S., Li, L., Zhou, C., Wang, L., Tang, S., et al. (2024). TREM2 alleviates white matter injury after traumatic brain injury in mice might be mediated by regulation of DHCR24/LXR pathway in microglia. Clin. Transl. Med. 14:e1665. doi: 10.1002/ctm2.1665,
Lia, A., Sansevero, G., Chiavegato, A., Sbrissa, M., Pendin, D., Mariotti, L., et al. (2023). Rescue of astrocyte activity by the calcium sensor STIM1 restores long-term synaptic plasticity in female mice modelling Alzheimer’s disease. Nat. Commun. 14:1590. doi: 10.1038/s41467-023-37240-2
Lin, Z., Chen, C., Yang, D., Ding, J., Wang, G., and Ren, H. (2021). DJ-1 inhibits microglial activation and protects dopaminergic neurons in vitro and in vivo through interacting with microglial p65. Cell Death Dis 12:715. doi: 10.1038/s41419-021-04002-1
Lind-Holm Mogensen, F., Sousa, C., Ameli, C., Badanjak, K., Pereira, S. L., Muller, A., et al. (2024). PARK7/DJ-1 deficiency impairs microglial activation in response to LPS-induced inflammation. J. Neuroinflammation 21:174. doi: 10.1186/s12974-024-03164-x,
Liu, L., MacKenzie, K. R., Putluri, N., Maletić-Savatić, M., and Bellen, H. J. (2017). The glia-neuron lactate shuttle and elevated ROS promote lipid synthesis in neurons and lipid droplet accumulation in glia via APOE/D. Cell Metab. 26, 719–737.e6. doi: 10.1016/j.cmet.2017.08.024,
Liu, Q., Trotter, J., Zhang, J., Peters, M. M., Cheng, H., Bao, J., et al. (2010). Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive, age-dependent synapse loss and neurodegeneration. J. Neurosci. 30, 17068–17078. doi: 10.1523/JNEUROSCI.4067-10.2010,
Long, H., Zhang, S., Zeng, S., Tong, Y., Liu, J., Liu, C., et al. (2022). Interaction of RAGE with α-synuclein fibrils mediates inflammatory response of microglia. Cell Rep. 40:111401. doi: 10.1016/j.celrep.2022.111401,
Lu, D. C., Zador, Z., Yao, J., Fazlollahi, F., and Manley, G. T. (2021). Aquaporin-4 reduces post-traumatic seizure susceptibility by promoting astrocytic glial scar formation in mice. J. Neurotrauma 38, 1193–1201. doi: 10.1089/neu.2011.2114,
Lukacova, N., Kisucka, A., Bimbova, K. K., Bacova, M., Ileninova, M., Kuruc, T., et al. (2021). Glial-neuronal interactions in pathogenesis and treatment of spinal cord injury. Int. J. Mol. Sci. 22:13577. doi: 10.3390/ijms222413577,
Ma, M., Paryani, F., Jakubiak, K., Xia, S., Antoku, S., Kannan, A., et al. (2025). The spatial landscape of glial pathology and T cell response in Parkinson’s disease substantia nigra. Nat. Commun. 16:7146. doi: 10.1038/s41467-025-62478-3
Maffioli, E., Nonnis, S., Negri, A., Fontana, M., Frabetti, F., Rossi, A. R., et al. (2024). Environmental temperature variation affects brain lipid composition in adult zebrafish (Danio rerio). Int. J. Mol. Sci. 25:9629. doi: 10.3390/ijms25179629,
Mallach, A., Zielonka, M., van Lieshout, V., An, Y., Khoo, J. H., Vanheusden, M., et al. (2024). Microglia-astrocyte crosstalk in the amyloid plaque niche of an Alzheimer’s disease mouse model, as revealed by spatial transcriptomics. Cell Rep. 43:114216. doi: 10.1016/j.celrep.2024.114216
Marques, I. J., Lupi, E., and Mercader, N. (2019). Model systems for regeneration: Zebrafish. Development 146:dev167692. doi: 10.1242/dev.167692,
Marschallinger, J., Iram, T., Zardeneta, M., Lee, S. E., Lehallier, B., Haney, M. S., et al. (2020). Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 23, 194–208. doi: 10.1038/s41593-019-0566-1
Martirosyan, A., Ansari, R., Pestana, F., Hebestreit, K., Gasparyan, H., Aleksanyan, R., et al. (2024). Unravelling cell type-specific responses to Parkinson’s disease at single cell resolution. Mol. Neurodegener. 19:7. doi: 10.1186/s13024-023-00699-0,
McAlpine, C. S., Park, J., Griciuc, A., Kim, E., Choi, S. H., Iwamoto, Y., et al. (2021). Astrocytic interleukin-3 programs microglia and limits Alzheimer’s disease. Nature 595, 701–706. doi: 10.1038/s41586-021-03734-6,
McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R., Kawas, C. H., et al. (2011). The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 263–269. doi: 10.1016/j.jalz.2011.03.005,
Meguro, S., Hosoi, S., and Hasumura, T. (2019). High-fat diet impairs cognitive function of zebrafish. Sci Rep 9. doi: 10.1038/s41598-019-53634-z
Morganti, J. M., Riparip, L.-K., and Rosi, S. (2016). Call Off the Dog(ma): M1/M2 Polarization Is Concurrent following Traumatic Brain Injury. PLoS One 11:e0148001. doi: 10.1371/journal.pone.0148001
Murthy, H., Hoang, N., Stark, J. C., Cui, S., Pannia, E., Tsoi, C. T., et al. (2025). Variants in DENND2B are associated with vulnerability for neurodevelopmental impairment, psychosis and catatonia. Brain. doi: 10.1093/brain/awaf273
Nakajima, S., Gotoh, M., Fukasawa, K., Murakami-Murofushi, K., and Kunugi, H. (2019). Oleic acid is a potent inducer for lipid droplet accumulation through its esterification to glycerol by diacylglycerol acyltransferase in primary cortical astrocytes. Brain Res. 1725:146484. doi: 10.1016/j.brainres.2019.146484,
Nakashima, M., Gotoh, M., Hashimoto, K., Endo, M., Murakami-Murofushi, K., Ikeshima-Kataoka, H., et al. (2021). The neuroprotective function of 2-carba-cyclic phosphatidic acid: implications for tenascin-C via astrocytes in traumatic brain injury. J. Neuroimmunol. 361:577749. doi: 10.1016/j.jneuroim.2021.577749,
Nalls, M. A., Pankratz, N., Lill, C. M., Do, C. B., Hernandez, D. G., Saad, M., et al. (2014). Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet. 46, 989–993. doi: 10.1038/ng.3043
Nanclares, C., Poynter, J., Martell-Martinez, H. A., Vermilyea, S., Araque, A., Kofuji, P., et al. (2023). Dysregulation of astrocytic Ca2+ signaling and gliotransmitter release in mouse models of α-synucleinopathies. Acta Neuropathol. 145, 597–610. doi: 10.1007/s00401-023-02547-3,
Novikova, G., Kapoor, M., Tcw, J., Abud, E. M., Efthymiou, A. G., Chen, S. X., et al. (2021). Integration of Alzheimer’s disease genetics and myeloid genomics identifies disease risk regulatory elements and genes. Nat. Commun. 12:1610. doi: 10.1038/s41467-021-21823-y
O’Brown, N., Patel, N., Hartmann, U., Klein, A., Gu, C., and Megason, S. (2023). The secreted neuronal signal Spock1 promotes blood-brain barrier development. Dev. Cell 58, 1534–1547.e6. doi: 10.1016/j.devcel.2023.06.005,
O’Brown, N. M., Pfau, S. J., and Gu, C. (2018). Bridging barriers: a comparative look at the blood-brain barrier across organisms. Genes Dev. 32, 466–478. doi: 10.1101/gad.309823,
Olah, M., Menon, V., Habib, N., Taga, M. F., Ma, Y., Yung, C. J., et al. (2020). Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer’s disease. Nat. Commun. 11:6129. doi: 10.1038/s41467-020-19737-2,
Oosterhof, N., Boddeke, E., and van Ham, T. J. (2015). Immune cell dynamics in the CNS: learning from the zebrafish. Glia 63, 719–735. doi: 10.1002/glia.22780,
Otero-Jimenez, M., Wojewska, M. J., Binding, L. P., Jogaudaite, S., Gray-Rodriguez, S., Young, A. L., et al. (2025). Neuropathological stages of neuronal, astrocytic and oligodendrocytic alpha-synuclein pathology in Parkinson’s disease. Acta Neuropathol. Commun. 13:25. doi: 10.1186/s40478-025-01944-x,
Packer, J. M., Bray, C. E., Beckman, N. B., Wangler, L. M., Davis, A. C., Goodman, E. J., et al. (2024). Impaired cortical neuronal homeostasis and cognition after diffuse traumatic brain injury are dependent on microglia and type I interferon responses. Glia 72, 300–321. doi: 10.1002/glia.24475,
Packer, J. M., Giammo, S. G., Wangler, L. M., Davis, A. C., Bray, C. E., and Godbout, J. P. (2025). Diffuse traumatic brain injury induced stimulator of interferons (STING) signaling in microglia drives cortical neuroinflammation, neuronal dysfunction, and impaired cognition. J. Neuroinflammation 22:128. doi: 10.1186/s12974-025-03451-1,
Palsamy, K., Chen, J. Y., Skaggs, K., Qadeer, Y., Connors, M., Cutler, N., et al. (2023). Microglial depletion after brain injury prolongs inflammation and impairs brain repair, adult neurogenesis and pro-regenerative signaling. Glia 71, 2642–2663. doi: 10.1002/glia.24444,
Pampuscenko, K., Morkuniene, R., Sneideris, T., Smirnovas, V., Budvytyte, R., Valincius, G., et al. (2020). Extracellular tau induces microglial phagocytosis of living neurons in cell cultures. J. Neurochem. 154, 316–329. doi: 10.1111/jnc.14940
Parhizkar, S., Arzberger, T., Brendel, M., Kleinberger, G., Deussing, M., Focke, C., et al. (2019). Loss of TREM2 function increases amyloid seeding but reduces plaque-associated ApoE. Nat. Neurosci. 22, 191–204. doi: 10.1038/s41593-018-0296-9,
Pellegrini, E., Fernezelian, D., Malleret, C., Gueguen, M. M., Patche-Firmin, J., Rastegar, S., et al. (2023). Estrogenic regulation of claudin 5 and tight junction protein 1 gene expression in zebrafish: a role on blood–brain barrier? J. Comp. Neurol. 531, 1828–1845. doi: 10.1002/cne.25543,
Peri, F., and Nüsslein-Volhard, C. (2008). Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell 133, 916–927. doi: 10.1016/j.cell.2008.04.037
Postuma, R. B., Berg, D., Stern, M., Poewe, W., Olanow, C. W., Oertel, W., et al. (2015). MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601. doi: 10.1002/mds.26424,
Prakash, P., Manchanda, P., Paouri, E., Bisht, K., Sharma, K., Rajpoot, J., et al. (2025). Amyloid-β induces lipid droplet-mediated microglial dysfunction via the enzyme DGAT2 in Alzheimer’s disease. Immunity. doi: 10.1016/j.immuni.2025.04.029
Qin, H., Yu, S., Han, R., and He, J. (2025). Age-dependent glial heterogeneity and traumatic injury responses in a vertebrate brain structure. Cell Rep. 44:115508. doi: 10.1016/j.celrep.2025.115508,
Rahimmi, A., and Khademerfan, M. (2025). Selenium and resveratrol attenuate rotenone-induced Parkinson’s disease in zebrafish model. Mol Biol Rep 52. doi: 10.1007/s11033-025-10925-1
Ralhan, I., Chang, C. L., Lippincott-Schwartz, J., and Ioannou, M. S. (2021). Lipid droplets in the nervous system. J. Cell Biol. 220:e202102136. doi: 10.1083/jcb.202102136
Ravikumar, M., Durairaj, B., and Uvarajan, D. (2025). Necrostatin-1 as a potential anticonvulsant: insights from zebrafish larvae model of PTZ-induced seizures. Mol. Neurobiol. 62, 4534–4544. doi: 10.1007/s12035-024-04571-0,
Razali, K., Mohd Nasir, M. H., Othman, N., Doolaanea, A. A., Kumar, J., Ibrahim, W. N., et al. (2022). Characterization of neurobehavioral pattern in a zebrafish 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced model: a 96-hour behavioral study. PLoS One 17:e0274844. doi: 10.1371/journal.pone.0274844
Rebeck, G. W., Reiter, J. S., Strickland, D. K., and Hyman, B. T. (1993). Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron 11, 575–580. doi: 10.1016/0896-6273(93)90070-8,
Richetin, K., Steullet, P., Pachoud, M., Perbet, R., Parietti, E., Maheswaran, M., et al. (2020). Tau accumulation in astrocytes of the dentate gyrus induces neuronal dysfunction and memory deficits in Alzheimer’s disease. Nat. Neurosci. 23, 1567–1579. doi: 10.1038/s41593-020-00728-x
Rieser, N. N., Ronchetti, M., Hotz, A. L. L., and Neuhauss, S. C. (2025). Multifaceted role of galanin in brain excitability. Elife 13. doi: 10.7554/elife.98634
Rosa, J. M., Farré-Alins, V., Ortega, M. C., Navarrete, M., Lopez-Rodriguez, A. B., Palomino-Antolín, A., et al. (2021). TLR4 pathway impairs synaptic number and cerebrovascular functions through astrocyte activation following traumatic brain injury. Br. J. Pharmacol. 178, 3395–3413. doi: 10.1111/bph.15488,
Russo, I., Di Benedetto, G., Kaganovich, A., Ding, J., Mercatelli, D., Morari, M., et al. (2018). Leucine-rich repeat kinase 2 controls protein kinase a activation state through phosphodiesterase 4. J. Neuroinflammation 15:297. doi: 10.1186/s12974-018-1337-8,
Saito, K., Shigetomi, E., Shinozaki, Y., Kobayashi, K., Parajuli, B., Kubota, Y., et al. (2024). Microglia sense astrocyte dysfunction and prevent disease progression in an Alexander disease model. Brain 147, 698–716. doi: 10.1093/brain/awad358,
Scheiblich, H., Dansokho, C., Mercan, D., Schmidt, S. V., Bousset, L., Wischhof, L., et al. (2021). Microglia jointly degrade fibrillar alpha-synuclein cargo by distribution through tunneling nanotubes. Cell 184, 5089–5106.e21. doi: 10.1016/j.cell.2021.09.007,
Scheiblich, H., Eikens, F., Wischhof, L., Opitz, S., Jüngling, K., Cserép, C., et al. (2024). Microglia rescue neurons from aggregate-induced neuronal dysfunction and death through tunneling nanotubes. Neuron 112, 3106–3125.e8. doi: 10.1016/j.neuron.2024.06.029
Schiweck, J., Murk, K., Ledderose, J., Münster-Wandowski, A., Ornaghi, M., Vida, I., et al. (2021). Drebrin controls scar formation and astrocyte reactivity upon traumatic brain injury by regulating membrane trafficking. Nat. Commun. 12:1490. doi: 10.1038/s41467-021-21662-x,
Schmidt, R., Strähle, U., and Scholpp, S. (2013). Neurogenesis in zebrafish-from embryo to adult. Neural Dev. 8:3. doi: 10.1186/1749-8104-8-3,
Serrano-Pozo, A., Li, H., Li, Z., Muñoz-Castro, C., Jaisa-Aad, M., Healey, M. A., et al. (2024). Astrocyte transcriptomic changes along the spatiotemporal progression of Alzheimer’s disease. Nat. Neurosci. 27, 2384–2400. doi: 10.1038/s41593-024-01791-4
Shin, K. C., Ali Moussa, H. Y., and Park, Y. (2024). Cholesterol imbalance and neurotransmission defects in neurodegeneration. Exp. Mol. Med. 56, 1685–1690. doi: 10.1038/s12276-024-01273-4
Simón-Sánchez, J., Schulte, C., Bras, J. M., Sharma, M., Gibbs, J. R., Berg, D., et al. (2009). Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat. Genet. 41, 1308–1312. doi: 10.1038/ng.487,
Sims, N. R., and Yew, W. P. (2017). Reactive astrogliosis in stroke: contributions of astrocytes to recovery of neurological function. Neurochem. Int. 107, 88–103. doi: 10.1016/j.neuint.2016.12.016
Smolič, T., Tavčar, P., Horvat, A., Černe, U., Halužan Vasle, A., Tratnjek, L., et al. (2021). Astrocytes in stress accumulate lipid droplets. Glia 69, 1540–1562. doi: 10.1002/glia.23978,
Smolič, T., Zorec, R., and Vardjan, N. (2022). Pathophysiology of lipid droplets in neuroglia. Antioxidants 11:22. doi: 10.3390/antiox11010022,
Sofroniew, M. V. (2015). Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 16, 249–263. doi: 10.1038/nrn3898
Son, S. M., Siddiqi, F. H., Lopez, A., Ansari, R., Tyrkalska, S. D., Park, S. J., et al. (2025). Alpha-synuclein mutations mislocalize cytoplasmic p300 compromising autophagy, which is rescued by ACLY inhibition. Neuron 113, 1908–1924.e13. doi: 10.1016/j.neuron.2025.03.028
Song, W., Hooli, B., Mullin, K., Jin, S. C., Cella, M., Ulland, T. K., et al. (2017). Alzheimer’s disease-associated TREM2 variants exhibit either decreased or increased ligand-dependent activation. Alzheimers Dement. 13, 381–387. doi: 10.1016/j.jalz.2016.07.004,
Spillantini, M. G., Crowther, R. A., Jakes, R., Hasegawa, M., and Goedert, M. (1998). Alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc. Natl. Acad. Sci. USA 95, 6469–6473. doi: 10.1073/pnas.95.11.6469,
Spillantini, M. G., Schmidt, M. L., Lee, V. M., Trojanowski, J. Q., Jakes, R., and Goedert, M. (1997). Alpha-synuclein in lewy bodies. Nature 388, 839–840. doi: 10.1038/42166,
Srinivasan, K., Friedman, B. A., Etxeberria, A., Huntley, M. A., van der Brug, M. P., Foreman, O., et al. (2020). Alzheimer’s patient microglia exhibit enhanced aging and unique transcriptional activation. Cell Rep. 31:107843. doi: 10.1016/j.celrep.2020.107843
Stelzmann, R. A., Norman Schnitzlein, H., and Reed Murtagh, F. (1995). An english translation of alzheimer’s 1907 paper, “über eine eigenartige erkankung der hirnrinde”. Clin. Anat. 8, 429–431. doi: 10.1002/ca.980080612
Stubbs, C. D., and Smith, A. D. (1984). The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim. Biophys. Acta 779, 89–137. doi: 10.1016/0304-4157(84)90005-4,
Sultana, R., Perluigi, M., and Butterfield, D. A. (2013). Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic. Biol. Med. 62, 157–169. doi: 10.1016/j.freeradbiomed.2012.09.027
Sun, L., Zhou, R., Yang, G., and Shi, Y. (2017). Analysis of 138 pathogenic mutations in presenilin-1 on the in vitro production of Aβ42 and Aβ40 peptides by γ-secretase. Proc. Natl. Acad. Sci. USA 114, E476–E485. doi: 10.1073/pnas.1618657114,
Svahn, A. J., Graeber, M. B., Ellett, F., Lieschke, G. J., Rinkwitz, S., Bennett, M. R., et al. (2013). Development of ramified microglia from early macrophages in the zebrafish optic tectum. Dev. Neurobiol. 73, 60–71. doi: 10.1002/dneu.22039
Tcw, J., and Goate, A. M. (2017). Genetics of β-amyloid precursor protein in Alzheimer’s disease. Cold Spring Harb. Perspect. Med. 7:a024539. doi: 10.1101/cshperspect.a024539
Teasdale, G., and Jennett, B. (1974). Assessment of coma and impaired consciousness. A practical scale. Lancet 304, 81–84. doi: 10.1016/s0140-6736(74)91639-0,
Thi Lai, T., Kim, Y. E., Nguyen, L. T. N., Thi Nguyen, T., Kwak, I. H., Richter, F., et al. (2024). Microglial inhibition alleviates alpha-synuclein propagation and neurodegeneration in Parkinson’s disease mouse model. NPJ Parkinsons Dis. 10:32. doi: 10.1038/s41531-024-00640-2,
Todd, B. P., Chimenti, M. S., Luo, Z., Ferguson, P. J., Bassuk, A. G., and Newell, E. A. (2021). Traumatic brain injury results in unique microglial and astrocyte transcriptomes enriched for type I interferon response. J. Neuroinflammation 18:151. doi: 10.1186/s12974-021-02197-w,
Trudler, D., Weinreb, O., Mandel, S. A., Youdim, M. B. H., and Frenkel, D. (2014). DJ-1 deficiency triggers microglia sensitivity to dopamine toward a pro-inflammatory phenotype that is attenuated by rasagiline. J Neurochem 129, 434–447. doi: 10.1111/jnc.12633
Tsujita, M., Melchior, J. T., and Yokoyama, S. (2024). Lipoprotein particles in cerebrospinal fluid. Arterioscler. Thromb. Vasc. Biol. 44, 1042–1052. doi: 10.1161/ATVBAHA.123.318284,
Tzioras, M., Daniels, M. J. D., Davies, C., Baxter, P., King, D., McKay, S., et al. (2023). Human astrocytes and microglia show augmented ingestion of synapses in Alzheimer’s disease via MFG-E8. Cell Rep. Med. 4:101175. doi: 10.1016/j.xcrm.2023.101175,
Uvarajan, D., Gnanarajan, R., Karuppusamy, P., Ravichandran, N., Govindasamy, C., Vellingiri, B., et al. (2025). Neuroprotective Effects of Berberine Chloride Against the Aluminium Chloride-Induced Alzheimer’s Disease in Zebra Fish Larvae. Mol Biotechnol. doi: 10.1007/s12033-025-01392-x
Van Steenwinckel, J., Schang, A. L., Krishnan, M. L., Degos, V., Delahaye-Duriez, A., Bokobza, C., et al. (2019). Decreased microglial Wnt/β-catenin signalling drives microglial pro-inflammatory activation in the developing brain. Brain 142, 3806–3833. doi: 10.1093/brain/awz319,
Vincent, J. C., Garnett, C. N., Watson, J. B., Higgins, E. K., Macheda, T., Sanders, L., et al. (2023). IL-1R1 signaling in TBI: assessing chronic impacts and neuroinflammatory dynamics in a mouse model of mild closed-head injury. J. Neuroinflammation 20:248. doi: 10.1186/s12974-023-02934-3,
Wang, P., Lan, G., Xu, B., Yu, Z., Tian, C., Lei, X., et al. (2023). α-Synuclein-carrying astrocytic extracellular vesicles in Parkinson pathogenesis and diagnosis. Transl. Neurodegener. 12:40. doi: 10.1186/s40035-023-00372-y,
Wang, S., Li, B., Li, J., Cai, Z., Hugo, C., Sun, Y., et al. (2025). Cellular senescence induced by cholesterol accumulation is mediated by lysosomal ABCA1 in APOE4 and AD. Mol. Neurodegener. 20:15. doi: 10.1186/s13024-025-00802-7,
Wang, T., Niu, X., Ouyang, S., Cheng, M., Yang, H., Liu, C., et al. (2025). A zebrafish model unravels the role of PHF21A in neurodevelopment and epilepsy. Neuroscience 584, 138–147. doi: 10.1016/j.neuroscience.2025.08.007
Wang, L., Ouyang, D., Li, L., Cao, Y., Wang, Y., Gu, N., et al. (2025). TREM2 affects DAM-like cell transformation in the acute phase of TBI in mice by regulating microglial glycolysis. J. Neuroinflammation 22:6. doi: 10.1186/s12974-025-03337-2
Wang, Y., Pan, P., Moens, C., and Appel, B. (2014). Notch3 establishes brain vascular integrity by regulating pericyte number. Development 141, 307–317. doi: 10.1242/dev.096107,
Wang, L., Sheng, W., Tan, Z., Ren, Q., Wang, R., Stoika, R., et al. (2021). Treatment of Parkinson’s disease in zebrafish model with a berberine derivative capable of crossing blood brain barrier, targeting mitochondria, and convenient for bioimaging experiments. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 249:109151. doi: 10.1016/j.cbpc.2021.109151,
Weiss, F., Hughes, L., Fu, Y., Bardy, C., Halliday, G. M., and Dzamko, N. (2024). Astrocytes contribute to toll-like receptor 2-mediated neurodegeneration and alpha-synuclein pathology in a human midbrain Parkinson’s model. Transl. Neurodegener. 13:62. doi: 10.1186/s40035-024-00448-3,
Whitwell, J. L. (2010). Progression of atrophy in Alzheimer’s disease and related disorders. Neurotox. Res. 18, 339–346. doi: 10.1007/s12640-010-9175-1,
Wilhelmus, M. M. M., Bol, J. G. J. M., Van Haastert, E. S., Rozemuller, A. J. M., Bu, G., Drukarch, B., et al. (2011). Apolipoprotein E and LRP1 increase early in Parkinson’s disease pathogenesis. Am. J. Pathol. 179, 2152–2156. doi: 10.1016/j.ajpath.2011.07.021,
Windham, I. A., Powers, A. E., Ragusa, J. V., Wallace, E. D., Zanellati, M. C., Williams, V. H., et al. (2024). APOE traffics to astrocyte lipid droplets and modulates triglyceride saturation and droplet size. J. Cell Biol. 223:e202305003. doi: 10.1083/jcb.202305003,
Xia, M., Yi, M., Guo, C., Xie, Y., Yu, W., Wang, D., et al. (2024). β-Asarone regulates microglia polarization to alleviate TBI-induced nerve damage via Fas/FasL signaling axis. Hum. Cell 38:33. doi: 10.1007/s13577-024-01161-z,
Yaksi, E., Jamali, A., Diaz Verdugo, C., and Jurisch-Yaksi, N. (2021). Past, present and future of zebrafish in epilepsy research. FEBS J. 288, 7243–7255. doi: 10.1111/febs.15694
Yamashita, M., Nakahira, N., Hashimoto, K., Kobayashi, H., Nakashima, M., Ikeshima-Kataoka, H., et al. (2025). Reactive astrocyte-derived neurotoxicity is mitigated by vitronectin in traumatic brain injury mouse model. IBRO Neurosci. Rep. 19, 300–306. doi: 10.1016/j.ibneur.2025.07.009,
Yang, Y., Song, J.-J., Choi, Y. R., Kim, S.-H., Seok, M.-J., Wulansari, N., et al. (2022). Therapeutic functions of astrocytes to treat α-synuclein pathology in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 119:e2110746119. doi: 10.1073/pnas.2110746119
Yin, F. (2023). Lipid metabolism and Alzheimer’s disease: clinical evidence, mechanistic link and therapeutic promise. FEBS J. 290, 1420–1453. doi: 10.1111/febs.16344,
Yum, S., Li, M., Fang, Y., and Chen, Z. J. (2021). TBK1 recruitment to STING activates both IRF3 and NF-κB that mediate immune defense against tumors and viral infections. Proc. Natl. Acad. Sci. USA 118:e2100225118. doi: 10.1073/pnas.2100225118,
Zambusi, A., Novoselc, K. T., Hutten, S., Kalpazidou, S., Koupourtidou, C., Schieweck, R., et al. (2022). TDP-43 condensates and lipid droplets regulate the reactivity of microglia and regeneration after traumatic brain injury. Nat. Neurosci. 25, 1608–1625. doi: 10.1038/s41593-022-01199-y,
Zhang, Y.-M., Qi, Y.-B., Gao, Y.-N., Chen, W.-G., Zhou, T., Zang, Y., et al. (2023). Astrocyte metabolism and signaling pathways in the CNS. Front. Neurosci. 17:1217451. doi: 10.3389/fnins.2023.1217451,
Zheng, Y., Liu, T., Luo, L., Li, F., Tang, C., Xu, C., et al. (2025). Complement C4b as a key mediator of synaptic loss and cognitive decline in brain aging. Mol. Ther. doi: 10.1016/j.ymthe.2025.09.005,
Zhou, Y., Song, W. M., Andhey, P. S., Swain, A., Levy, T., Miller, K. R., et al. (2020). Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat. Med. 26, 131–142. doi: 10.1038/s41591-019-0695-9,
Zi, H., Peng, X., Du, J., and Li, J. (2025). Protocol for generating a pericyte reporter zebrafish line Ki(pdgfrb-P2A-GAL4-VP16) using a CRISPR-Cas9-mediated knockin technique. STAR Protoc. 6:103490. doi: 10.1016/j.xpro.2024.103490,
Zou, W., Kou, L., Wang, Y., Jin, Z., Xiong, N., Wang, T., et al. (2025). Complement C4 exacerbates astrocyte-mediated neuroinflammation and promotes α-synuclein pathology in Parkinson’s disease. NPJ Parkinsons Dis. 11:141. doi: 10.1038/s41531-025-01005-z,
Keywords: Alzheimer’s disease, astrocyte, lipid droplet, microglia, mouse, Parkinson’s disease, TBI, zebrafish
Citation: Hashimoto K, Gotoh M and Ikeshima-Kataoka H (2025) Astrocytic and microglial cell functions in neuroinflammatory diseases and their animal models. Front. Cell. Neurosci. 19:1708775. doi: 10.3389/fncel.2025.1708775
Edited by:
Maja Potokar, University of Ljubljana, SloveniaReviewed by:
Jernej Jorgačevski, University of Ljubljana, SloveniaSimona D'Aprile, University of Catania, Italy
Copyright © 2025 Hashimoto, Gotoh and Ikeshima-Kataoka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroko Ikeshima-Kataoka, aWtlc2hpbWEuaGlyb2tvQGtpdGFzYXRvLXUuYWMuanA=
 Kei Hashimoto
Kei Hashimoto Mari Gotoh
Mari Gotoh Hiroko Ikeshima-Kataoka
Hiroko Ikeshima-Kataoka