- 1School of Health Management and Information Sciences, Iran University of Medical Sciences, Tehran, Iran
- 2Gastrointestinal and Liver Diseases Research Center, Iran University of Medical Sciences, Tehran, Iran
Background: Cancer care increasingly emphasizes patient-centred approaches, leading to the adoption of electronic patient-reported outcome (ePRO) systems for essential patient data collection. Our systematic review investigates the landscape of electronic patient-reported outcome systems and their capability in cancer care, focusing on their potential to enhance patient-centred solutions.
Methods: We conducted a systematic review, encompassing studies on electronic patient-reported outcomes in cancer. We searched in Scopus, Web of Science, and PubMed using comprehensive Medical Subject Heading (MeSH) terms up to April 2024. Papers were categorized based on nine key aspects, including author, publication year, country/state, objective, participants, cancer type, system name, system capabilities, and type of platform. Eligible studies were appraised using a mixed-methods appraisal tool (MMAT).
Results: Analysis of 85 studies indicated a diverse range of electronic Patient-Reported Outcome systems and platforms in cancer care, Notably, PRO-CTCAE and CHES were frequently cited for their roles in data collection and analysis. Moreover, web-based platforms were predominant, followed by mobile-based and computer-based systems. In addition, Symptom assessment and management emerged as significant capabilities in the utilization of these systems for oncology care.
Conclusion: Our systematic review of electronic patient-reported outcome (ePRO) systems in cancer care focused on the capabilities of these systems for capturing patient data and improving cancer treatment outcomes. This study emphasized the potential of electronic systems to enhance patient-centred oncology practices and optimize cancer care delivery.
1 Introduction
Cancer, with its complex treatment regimens and multifaceted impact on patients, demands a comprehensive approach to care that centres on the individual's experience and needs (1). In recent years, there has been an increasing recognition of the significance of integrating patient-reported outcomes (PROs) into oncology care to gain a deeper understanding of patients’ experiences, symptoms, and quality of life. Electronic Patient-Reported Outcome (ePRO) systems have emerged as potent tools in this endeavour, providing tools to collect, monitor, and analyze patient-reported data in real-time (1, 2).
Notwithstanding the progress in oncology care, a central challenge persists the need to address patients’ needs effectively, promptly, and comprehensively. Traditional methods of data collection often lack timeliness and depth, hindering clinicians’ ability to intervene proactively. Herein lies the crucial role of ePRO systems: they serve as a conduit for timely, accurate, and comprehensive data collection from patients, enabling clinicians to tailor interventions and support services accordingly (3).
Key electronic systems such as the Computer-Based Health Evaluation System (CHES) and electronic patient self-Reporting of Adverse-events: Patient Information and aDvice (eRAPID) facilitate proactive monitoring of patients’ symptoms and adverse events, enabling timely interventions and support services to optimize patient outcomes. Moreover, these systems empower patients to actively engage in their care by providing them with tools and resources to self-report their symptoms and communicate with their healthcare providers. This collaborative approach not only enhances patient satisfaction but also improves treatment adherence and clinical outcomes (4, 5). CHES has been widely embraced for their capacity to streamline data collection processes and provide clinicians with actionable insights into patients’ symptoms and treatment responses (6). CHES enables the seamless capture of patient-reported data through user-friendly interfaces, empowering clinicians to remotely monitor patients’ progress and adjust treatment plans accordingly (4). Similarly, eRAPID has played a pivotal role in enhancing patient engagement and symptom management in oncology care (5). This web-based system allows patients to report their symptoms in real-time and receive personalized advice and support from healthcare providers, leading to enhanced patient outcomes and satisfaction (7).
The integration of electronic health systems and platforms in oncology care holds significant promise for transforming patient-centred solutions. By leveraging the power of ePRO systems and ePROMs, clinicians can gain valuable insights into patients’ experiences, preferences, and treatment outcomes, allowing for more personalized and holistic care delivery (8, 9). In addition to ePRO systems, electronic Patient-Reported Outcome Measures (ePROMs) play a crucial role in capturing patients’ perspectives on their health and well-being (10–12). Platforms like Noona have been designed to facilitate the collection of ePROMs through web-based surveys and questionnaires, enabling clinicians to assess patients’ symptoms, functional status, and quality of life (13, 14). Noona's intuitive interface and customizable reporting features make it a valuable tool for tracking patients’ progress over time and identifying areas for intervention or support (15). Similarly, the EPIC Electronic Health Record (EHR) system provides robust capabilities for incorporating ePROMs into clinical workflows, enabling seamless integration of patient-reported data with other electronic health records (16).
Despite the increasing adoption of electronic health systems in oncology care (17), there exists a need for a comprehensive review of their capabilities and impact on patient-centred solutions. Existing studies have predominantly focused on individual systems or specific aspects of electronic health technology, constraining our understanding of their collective potential in oncology care delivery (18, 19). Furthermore, given the rapid technological advancements and evolving healthcare landscape, there is an urgent requirement for up-to-date research that synthesizes the current state of electronic health systems and platforms in oncology care while identifying areas for future development and enhancement.
Our systematic review aims to explore the landscape of electronic patient-reported outcome systems and platforms utilized in oncology care, with a specific focus on their capability to transform patient-centred solutions. By synthesizing existing literature and evaluating the combined capabilities of these systems, this study seeks to pinpoint knowledge gaps, showcase best practices, and provide recommendations for future research endeavours and implementation strategies. Through this undertaking, we aspire to contribute significantly to the ongoing discourse on the role of electronic health technology in oncology care with the ultimate goal of enhancing the quality of care delivery and outcomes for cancer patients.
The research questions for our systematic review could be formulated as follows:
RQ1. What are the electronic Patient-Reported Outcome (ePRO) systems and how do they contribute to enhancing patient-centred solutions in oncology care?
RQ2. What are the capabilities of ePRO systems and how do they capture patient experiences, symptoms, and treatment outcomes in cancer care?
These research questions will aid in systematically evaluating the effectiveness of ePRO systems in cancer care and identifying optimal approaches for their successful implementation and widespread adoption.
2 Methods
We conducted a systematic review concentrating on electronic patient-reported outcome (ePRO) systems and their potential for patient-centred solutions in the context of cancer care. The final report follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting systematic reviews. Our study encompasses several key steps including search strategy, inclusion and exclusion criteria, study selection, quality appraisal, and data extraction and synthesis to ensure a comprehensive and rigorous analysis of the available literature (20).
2.1 Search strategy
We conducted our search by exploring academic articles across electronic databases such as Scopus, Web of Science, and PubMed. We searched Scopus, Web of Science, and PubMed using comprehensive Medical Subject Heading (MeSH) terms up to April 2024. The search strategy involved the use of specific keywords and medical subject heading (MeSH) terms focusing on two overarching themes: “electronic Patient Reported Outcomes” and “Cancer” to identify relevant studies. Additionally, a backward snowball search technique was employed to enhance the scope of our search. Following this process, all identified studies were imported into EndNote software to manage duplicates effectively. (See Supplementary Appendix A).
2.2 Inclusion and exclusion criteria
In this study, the inclusion and exclusion criteria are as follows:
Inclusion Criteria
1. Language: English language papers will be included.
2. Publication Type: Articles published in peer-reviewed journals or presented at reputable conferences that have full text will be included.
3. Study Designs: Quantitative, qualitative, and mixed-methods studies will be considered.
4. Topic Relevance: Studies focusing on electronic patient-reported outcomes (ePROs) will be included.
Exclusion Criteria
1. Technical Infrastructure Studies: Studies primarily focusing on technical infrastructure or software development unrelated to patient-reported outcomes will be excluded.
2. Paper-Based Assessments: Studies emphasizing paper-based patient-reported outcome measures will be excluded.
3. Irrelevant Topics: Studies not related to cancer or lacking clear indicators or outcomes pertinent to cancer care will be excluded.
2.3 Study selection
In our study selection process, two reviewers independently assessed the titles and abstracts of identified papers based on predefined criteria, resolving any disagreements with a third reviewer. After excluding irrelevant studies, one reviewer conducted data extraction, cross-verified by other team members for accuracy. This process occurred in two steps: initial screening of titles and abstracts, followed by full-text review. Both stages were independently carried out by two reviewers, with final inclusion contingent upon consensus to ensure the inclusion of only the most relevant research.
2.4 Quality assessment
The quality of the selected articles was evaluated using the Mixed Methods Appraisal Tool (MMAT), a comprehensive instrument designed to assess the methodological rigour of various types of research studies. The MMAT encompasses specific criteria tailored for qualitative, quantitative clinical trials, non-clinical trials, descriptive studies, and mixed methods research. Each article was categorized based on its study type, and the corresponding criteria were applied accordingly. During the assessment, reviewers responded to screening questions and rated the criteria within the chosen category. The “Can't tell” response category was utilized when the paper lacked sufficient information for evaluation. Articles were categorized based on the percentage of positive responses obtained during the quality assessment (21).
2.5 Data extraction
An initial data extraction form was developed to capture key information from the selected studies. Data elements were organized into general (author, year, country/state, objective, and participants) and specific items (cancer type, system name, system capabilities, type of platform) to facilitate a comprehensive analysis.
2.6 Data synthesis
Thematic analysis was employed to identify common themes across the literature, with a focus on systems and capabilities for patient-centred solutions associated with electronic patient-reported outcome systems in cancer care. To ensure validity, extracted themes were cross-checked and discussed by the study authors. The synthesized data, including frequencies and percentages of Electronic Systems and Platforms and capabilities, were presented in tables and figures, contributing to a better understanding of ePRO systems in cancer care. The selected papers were summarized in the final step of our methodology, and important factors were identified. (See Supplementary Appendix B).
3 Results
3.1 General findings
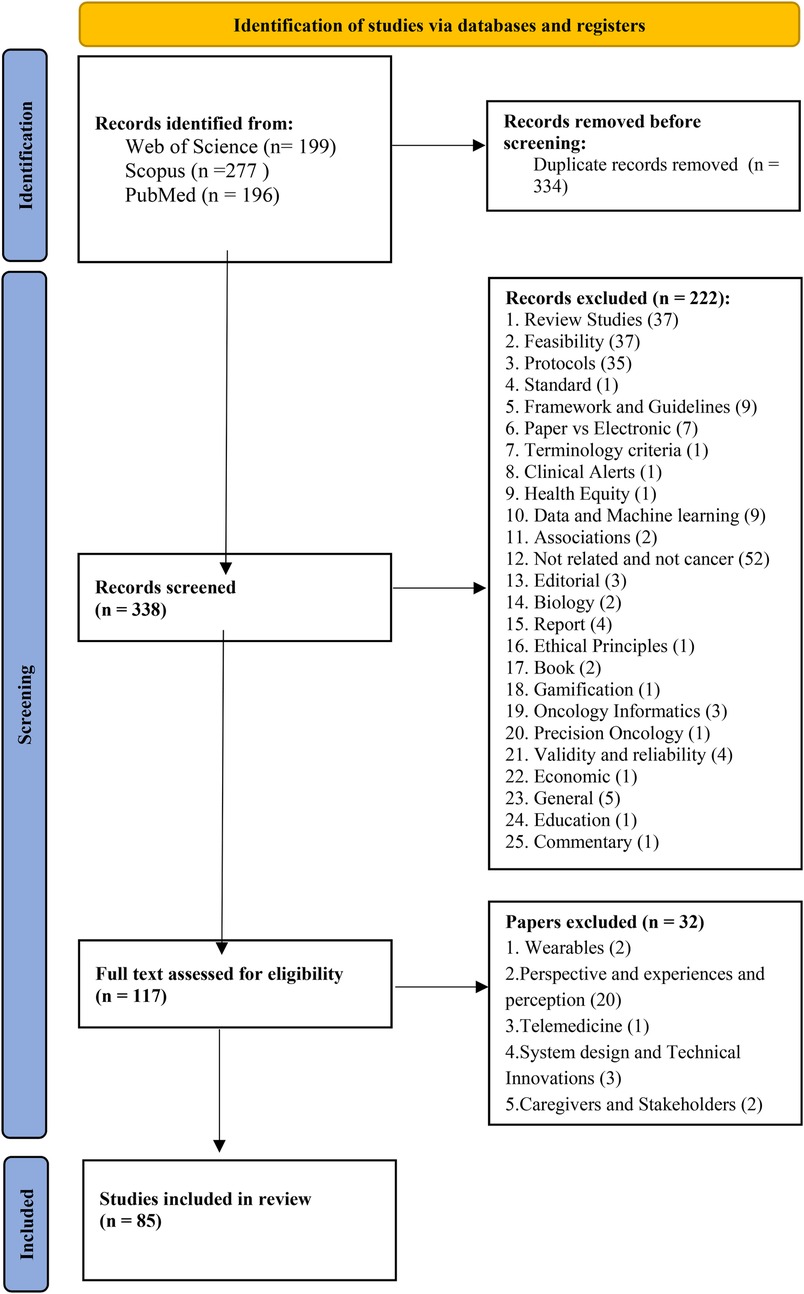
In our systematic review, we identified 672 papers, out of which 85 academic papers were included in our systematic review, providing a comprehensive exploration of electronic health platforms for cancer care. we present the key findings regarding the characteristics of the included studies, electronic systems and platforms, and their system capabilities as revealed in our systematic review. (See Figure 1).
3.2 Characteristics of included studies
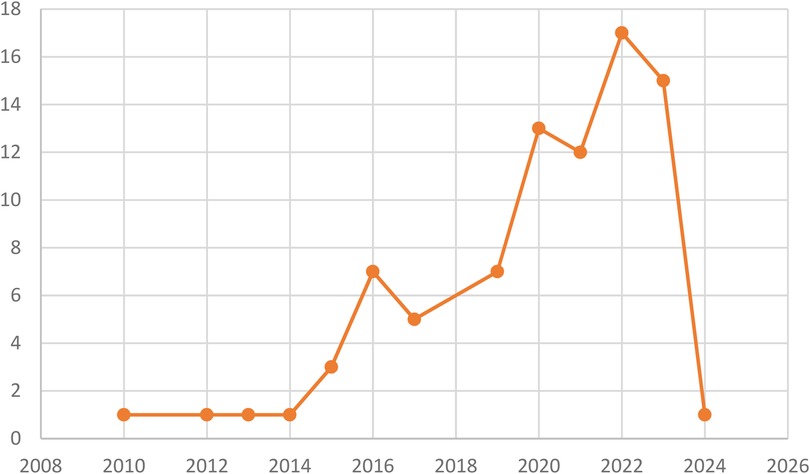
The distribution by year indicates that the majority of publications were from the years 2022 and 2023, with 17 and 15 contributions, respectively. Additionally, there were 12 publications from 2021, 13 from 2020, 7 from 2019, 7 from 2016, 5 from 2017, 3 from 2015, and 1 publication each from 2024, 2014, 2013, 2012, and 2010. (See Figure 2).
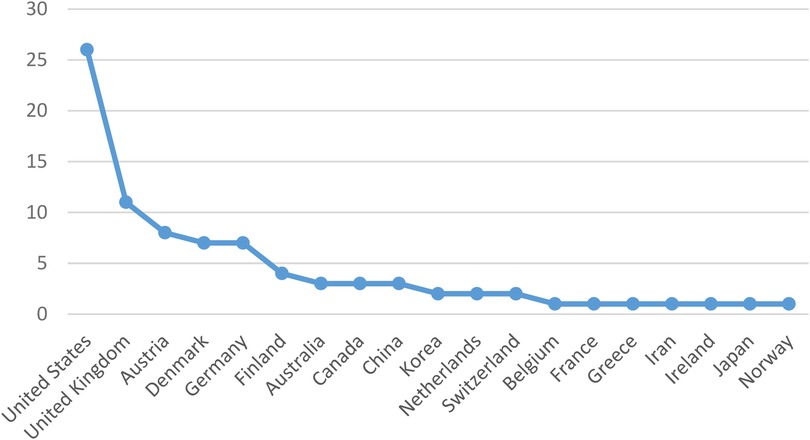
According to Figure 3, the United States emerged as the leading source of publications, followed by the United Kingdom in second place and Austria in third. Additionally, Belgium, France, Greece, Iran, Ireland, Japan, and Norway each made a single contribution.
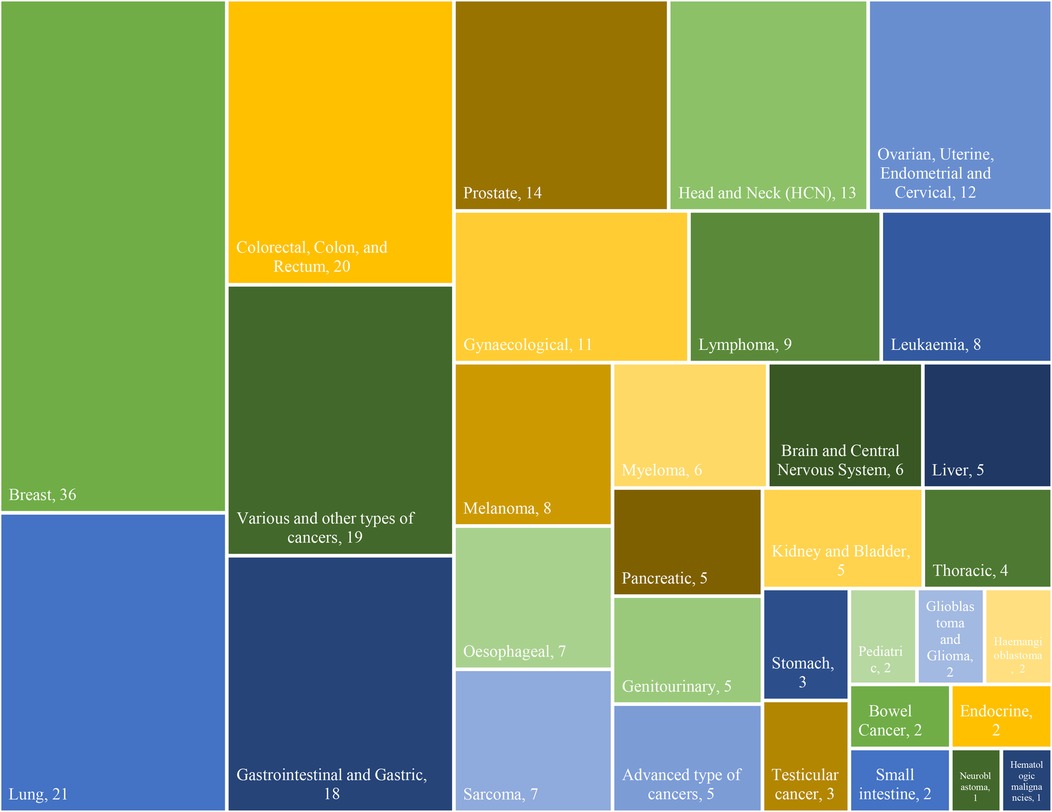
In terms of cancer types, a total of 32 different types of cancers, comprising 264 cases, were identified in the documents. The frequency distribution of cancer types is crucial as it highlights the prevalence of various malignancies across the studies. “Breast cancer” emerged as the most frequently studied, appearing in 36 articles, signifying its prominence in the literature (5, 7, 18, 22, 24, 25, 28, 33–35, 38, 39, 41, 43, 45, 47, 49, 52, 54–57, 60, 63–66, 72, 73, 76, 81–83, 87, 93, 96). “Lung” cancers (11, 14, 22, 24, 28, 34, 35, 38, 41, 44, 46–48, 54, 55, 60, 68, 72, 90, 93, 94) closely follow, sharing the second-highest frequency, represented by 21 studies. “Colorectal, (5, 7, 24, 28, 34, 35, 38, 41, 54, 60, 81, 83, 84, 86) colon, (22, 55, 66) and rectum” (18, 22, 55) cancers, also with a frequency of 20 cases, occupy the third-highest position. “Gastrointestinal (11, 15, 25, 29, 39, 45, 65, 72, 81, 90, 93, 96) and Gastric” (34, 35, 38, 41, 55, 65) cancers are shown as the fourth-highest, each with 18 occurrences. The Various and other cancer types collectively account for 16 instances, (4, 13, 16, 19, 22, 25, 35, 40, 41, 55, 58, 74, 88, 91) showcasing the diverse range of cancers and research focus. Furthermore, advanced types of cancers are represented by 5 studies. (15, 34, 36, 38, 53) Moreover, “neuroblastoma” (88) and “hematologic malignancies” (25) emerge as the least frequent cancer types, each documented in a single case. This analysis not only provides an overview of the distribution of cancer types but also highlights variations in research emphasis, emphasizing the need for a comprehensive understanding of different malignancies in the oncological landscape. The type and frequency of cancer within the study are indicated in Figure 4.
3.3 Electronic systems and platforms
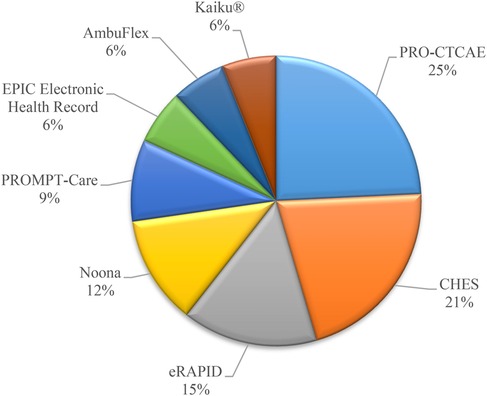
Based on the information gathered from various electronic Patient Reported Outcome Systems and platforms used in healthcare settings, it is evident that the frequency of utilization varies among different systems. Among the systems mentioned, PRO-CTCAE, a Patient Reported Outcome system, stands out with a frequency of 8. This system focuses on Patient-Reported Outcomes and is crucial for assessing treatment-related toxicity and therapeutic effectiveness (18, 19, 39, 51, 54, 57, 62, 74). Following closely is CHES (Computer-based Health Evaluation System) with a frequency of 7. CHES provides a comprehensive approach for electronic data capture and patient-reported outcome interpretation, making it a valuable tool in healthcare settings (4, 22, 55, 73, 90, 94, 95). In contrast, systems like eRAPID (N = 5) (5, 7, 29, 81, 83), Noona (N = 4) (13–15, 49), PROMPT-Care (N = 3) (44, 48, 69), EPIC Electronic Health Record (EHR) (N = 2) (16, 60), AmbuFlex (N = 2) (37, 84), Kaiku® (N = 2) (74, 85), and others have lower frequencies ranging from 5 to 1. Figure 5 shows the most popular repeated systems and platforms in studies.
In summary, while PRO-CTCAE and CHES emerge as more frequently mentioned systems in the sources, other platforms like eRAPID, Noona, and PROMPT-Care have lower frequencies but still play essential roles in electronic patient self-reporting and care management. Each system serves specific purposes within the healthcare landscape, contributing to improved patient care and outcomes. We provide the distribution of frequency of Electronic Systems and Platforms and their definition and usage of them in Supplementary Appendix C.
3.4 Types of ePRO platforms
Our study identified four types of platforms: Web-based, Mobile-based, Computer-based, and Web-based applications. Among the types of ePRO platforms in cancer, the most frequently mentioned category is Web-based platforms, with a total of 32 references (5, 7, 16, 25, 26, 29, 36–38, 42–44, 48, 51, 54, 56, 57, 59, 60, 62, 68, 69, 77–81, 83, 84, 89, 92, 93). Following closely is the Mobile-based category, cited 20 times. (15, 18, 19, 23, 27, 28, 30, 31, 33, 34, 41, 45, 50, 61, 66, 67, 71, 87, 88, 96) In contrast, Computer-based platforms are mentioned 16 times (4, 11, 22, 35, 39, 55, 64, 72, 73, 75, 76, 86, 90, 91, 94, 95). Interestingly, the least represented category among these ePRO platforms in cancer is the Web-based application type by 9 references (13, 14, 40, 49, 58, 65, 74, 82, 85). Moreover, 8 studies haven’t mentioned the system types and they only use ePRO systems (24, 32, 46, 47, 52, 53, 63, 70).
3.5 System capabilities
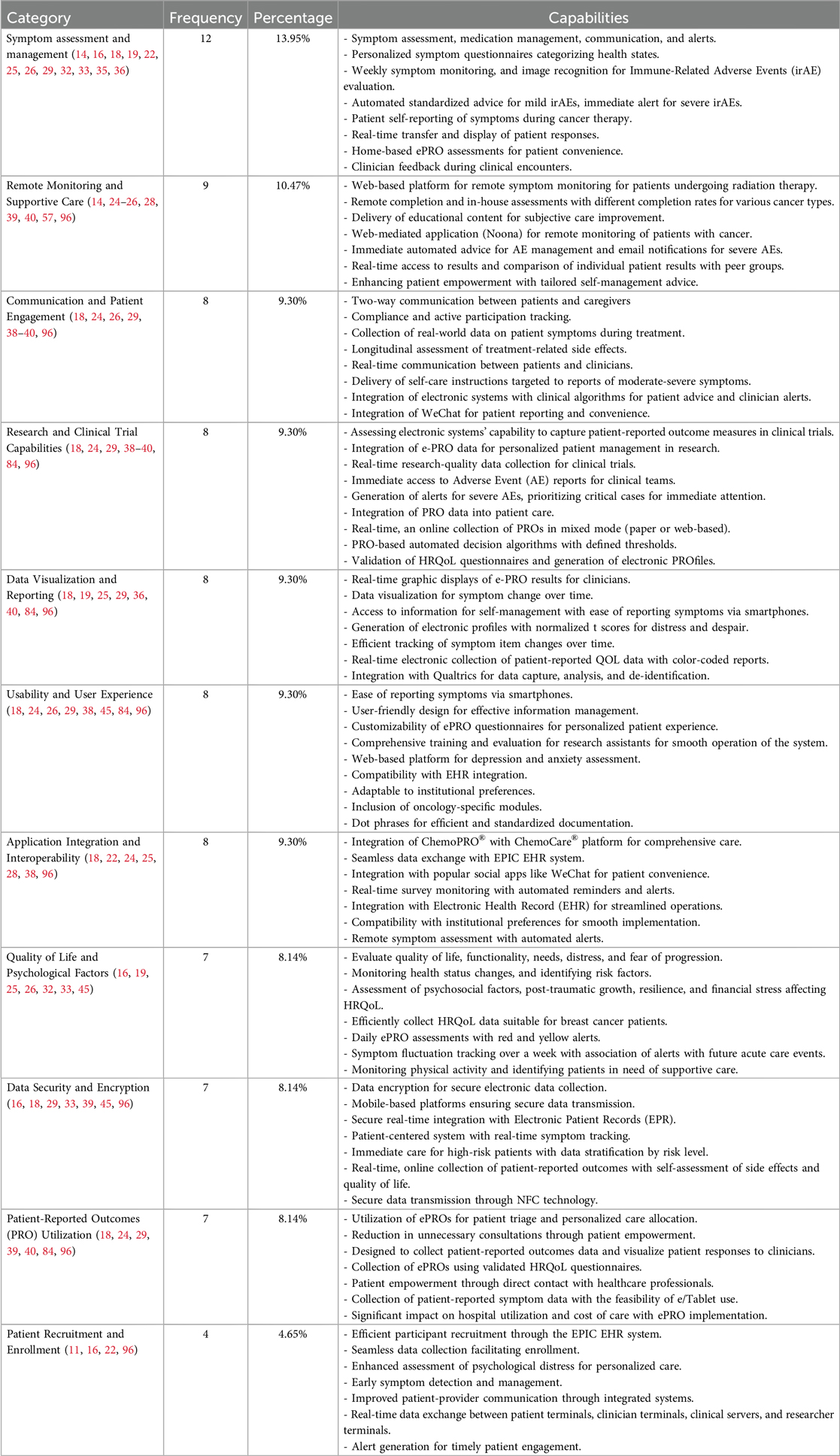
The evaluation of various system capabilities in oncology-focused electronic patient-reported outcome (ePRO) systems revealed a diverse range of functionalities and benefits across different domains. The study analyzed the frequency of different capabilities and features of electronic systems and platforms used in cancer care. To facilitate analysis and interpretation, the system capabilities were classified into distinct thematic categories based on their primary functions and roles in supporting patient care and research. These categories are defined as follows:
• Symptom Assessment and Management: Systems designed to detect, monitor, and manage patient symptoms, including the use of personalized symptom questionnaires and automated guidance for mild symptoms.
• Remote Monitoring and Supportive Care: Capabilities that enable continuous observation of patients’ health status remotely, allowing timely interventions and sustained supportive care.
• Communication and Patient Engagement: Features that promote interactive communication between patients and healthcare providers, enhance patient compliance, and support active participation in care.
• Research and Clinical Trial Capabilities: Functionalities supporting research data collection, patient eligibility screening, and integration into clinical trial workflows.
• Data Visualization and Reporting: Tools that transform patient-generated data into visual formats such as graphs or dashboards to support clinical interpretation and decision-making.
• Usability and User Experience: Assessments and features focused on ease of use, user satisfaction, and intuitive interface design for both patients and healthcare professionals.
• Application Integration and Interoperability: Capabilities enabling the exchange of data with Electronic Health Records (EHRs) and compatibility with third-party platforms, including mobile and social media applications.
• Quality of Life and Psychological Factors: Assessment modules that measure patient-reported quality of life, psychological well-being, distress levels, and related psychosocial indicators.
• Data Security and Encryption: Mechanisms for ensuring the secure collection, storage, and transfer of sensitive patient data in compliance with data protection regulations.
• Patient-Reported Outcomes (PRO) Utilization: Functions that facilitate the collection, analysis, and clinical use of patient-reported outcomes to inform care decisions.
• Patient Recruitment and Enrollment: Tools that assist in identifying, recruiting, and enrolling patients into clinical programs or research studies.
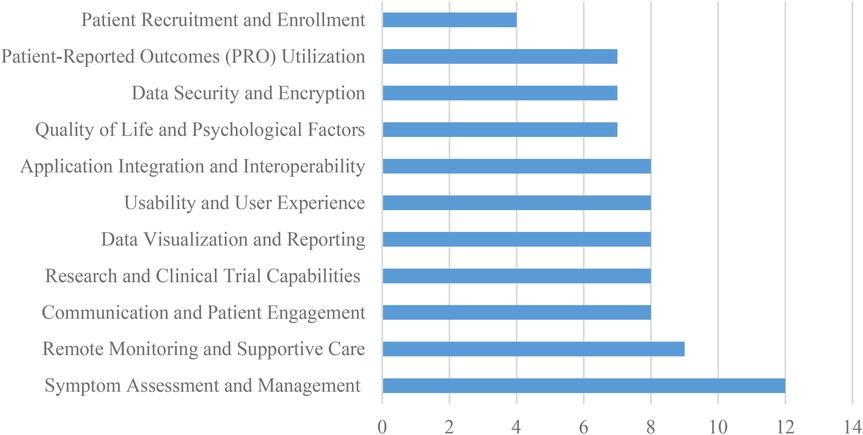
The most frequently studied capability was Symptom Assessment and Management, with 12 references (14, 16, 18, 19, 22, 25, 26, 29, 32, 33, 35, 36), followed by Remote Monitoring and Supportive Care, with a frequency of 9 references (14, 24–26, 28, 39, 40, 57, 96). This functionality enables early detection and management of symptoms, personalized symptom questionnaires, and automated standardized advice for mild symptoms.
Communication and Patient Engagement (18, 24, 26, 29, 38–40, 96), Research and Clinical Trial Capabilities (18, 24, 29, 38–40, 84, 96), Data Visualization and Reporting (18, 19, 25, 29, 36, 40, 84, 96), Usability and User Experience (18, 24, 26, 29, 38, 45, 84, 96), Application Integration and Interoperability (14, 18, 22, 24, 25, 28, 38, 96), all had 8 studies. Quality of Life and Psychological Factors (16, 19, 25, 26, 32, 33, 45), Data Security and Encryption (16, 18, 29, 33, 39, 45, 96), and Patient-Reported Outcomes (PRO) Utilization (18, 24, 29, 39, 40, 84, 96) all had 7 studies. These are also essential capabilities that can improve the quality of care and patient outcomes. The least studied capability was Patient Recruitment and Enrollment, with only 4 references (11, 16, 22, 96). For example, Communication and Patient Engagement can facilitate two-way communication between patients and caregivers, compliance and active participation tracking, and real-time communication between patients and clinicians (18), while Quality of Life and Psychological Factors can help evaluate quality of life, functionality, needs, distress, and fear of progression (11). Application Integration and Interoperability can facilitate seamless data exchange with Electronic Health Record (EHR) systems (14) and integration with popular social apps like WeChat for patient convenience (38). Data Security and Encryption ensures secure electronic data collection, while Patient-Reported Outcomes (PRO) Utilization can help collect patient-reported outcomes data and visualize patient responses to clinicians (40). Figure 6 shows the distribution of system capabilities in ePRO in Cancer.
In summary, the study found that Symptom Assessment and Management and Communication and Patient Engagement were the most frequently studied capabilities in electronic systems and platforms used in cancer care. Patient Recruitment and Enrollment was the least studied capability. Table 1 shows the overview of system capabilities in ePRO in cancer.
4 Discussion
In our study, we conducted a systematic review aimed at synthesizing literature concerning electronic systems and platforms in oncology care. Analysis of publication distribution by year revealed that the majority of articles were published in 2022 and 2023, with the United States being the primary source of publications. Breast cancer emerged as the most frequently studied cancer type. Our focus was to comprehend the diverse capabilities of these systems in improving patient outcomes and streamlining healthcare processes. Specifically, we investigated the contributions of electronic Patient-Reported Outcome (ePRO) Systems and platforms in data capture, storage, and real-time analysis, tailored to researchers, clinicians, and patients. Additionally, we explored how these systems facilitate patient engagement in their care through symptom reporting and monitoring.
4.1 Electronic systems and platforms
The development of electronic systems in oncology care reflects a clear temporal progression aligned with advances in digital health. Initial ePRO systems were largely desktop-based, focusing on basic symptom tracking through static interfaces, often utilizing dedicated computer programs or early personal digital assistants (PDAs) for data capture (97). Over time, a significant shift occurred toward web-based platforms that allowed for greater accessibility, remote data entry, and enhanced clinician-patient interaction beyond the clinical setting (98). The widespread proliferation of mobile devices led to the next major transition, mobile-enabled ePRO apps, which offered increased convenience, portability, and real-time data capture from patients in their daily environments, facilitating continuous monitoring and immediate feedback (99). More recent systems emphasize advanced interoperability with Electronic Health Records (EHRs), streamlining data flow and integration into clinical workflows. These modern platforms also incorporate sophisticated capabilities such as automated alerts for worsening symptoms, adaptive symptom questionnaires that adjust based on patient input, and seamless telehealth integration to support comprehensive remote care (100).
Furtheremore, Several electronic patient-reported outcomes (ePRO) systems play a crucial role in transforming oncology care by providing advanced functionalities tailored to patient needs. Among these systems, CHES (Computer-based Health Evaluation System) stands out for its comprehensive approach to PRO assessment and data interpretation. Developed by Evaluation Software Development (ESD), CHES provides a convenient software solution for electronic data capture, storage, and analysis, facilitating the interpretation of patient-reported outcomes with graphical real-time feedback. This system provides a multicomponent approach adaptable to the specific requirements of researchers, clinicians, patients, and organizational settings (4, 22, 55, 73, 90, 94, 95).
Another notable system is Noona, a web app and patient outcomes management solution designed to engage patients in their care through real-time symptom reporting and monitoring. Noona facilitates streamlined clinical workflows, evidence-based care promotion, and access to rich data insights for better management throughout the care continuum. With features like access to clinical care teams, structured data capture with actionable content, and rapid deployment capabilities for immediate patient impact, Noona enhances communication between patients and healthcare providers while collecting essential data on patients’ symptoms at different phases of cancer care (13–15, 49).
Additionally, the EPIC Electronic Health Record (EHR) system plays a vital role in enhancing patient engagement and facilitating remote care. Epic's cloud-based EHR solution caters to various specialities and provides a range of core EHR features with the flexibility to add speciality-specific modules. With a strong focus on patient engagement, Epic provides extensive patient portal features, and telehealth options, and supports video visits and post-surgical follow-ups. By evaluating these systems’ capabilities in patient recruitment, symptom assessment, quality of life evaluation, communication, data security, research capabilities, data visualization, usability, and remote monitoring, we gained insights into their collective impact on improving oncology care delivery. This system is widely used across different healthcare practices, from community hospitals to multi-speciality hospital groups (16, 60).
Furthermore, eRAPID (Electronic patient self-Reporting of Adverse-events: Patient Information and aDvice) is another significant ePRO system that focuses on patients’ self-reporting of adverse events. This web-based system allows patients to complete symptom reports from home or mobile devices and receive severity-based advice. eRAPID enhances patient engagement by enabling individuals to report their symptoms in real-time, providing relevant information and advice based on their reported experiences for proactive symptom management (5, 7, 29, 81, 83).
In the realm of personalized cancer treatment and care, PROMPT-Care (Patient Reported Outcome Measures for Personalized Treatment and Care) plays a crucial role in collecting patient-reported outcome measures. This system is instrumental in tailoring cancer treatment plans based on individual patient needs and experiences (44, 48, 69). Additionally, PRO-CTCAE (Patient Reported Outcome of Common Terminology Criteria for Adverse Events) focuses on capturing patient-reported adverse event data to enhance symptom management and personalized care in oncology settings (18, 19, 39, 51, 54, 57, 62, 74).
Each of these systems demonstrates the potential to transform oncology care, providing innovative approaches to enhance patient-centred solutions. In summary, ePRO systems have proven beneficial in enhancing patient-centred care, improving symptom assessment and management, and optimizing quality of life and psychological factors in oncology care. Briefly, these advanced electronic systems play a crucial role in transforming oncology care by improving patient outcomes, enhancing communication between patients and healthcare providers, ensuring data security, facilitating research capabilities, and providing user-friendly interfaces for efficient information management.
In terms of system and platform type, the distribution of mentions across different types of ePRO platforms in cancer sheds light on the prevalent utilization patterns within healthcare settings. The Web-based platforms, as evidenced are the frequent type of platforms that have been used, indicate the significance of online interfaces in facilitating patient-reported outcomes in oncology care. This emphasis on Web-based solutions aligns with the increasing trend towards digital health technologies and remote patient monitoring (5, 7, 16, 25, 26, 29, 36–38, 42–44, 48, 51, 54, 56, 57, 59, 60, 62, 68, 69, 77–81, 83, 84, 89, 92, 93).
In contrast, the Mobile-based category is the second and reflects the growing adoption of mobile applications to enhance patient engagement and data collection in cancer care. The portability and accessibility of mobile platforms provide unique advantages in capturing real-time patient-reported data and promoting continuous monitoring outside traditional healthcare settings (15, 18, 19, 23, 27, 28, 30, 31, 33, 34, 41, 45, 50, 61, 66, 67, 71, 87, 88, 96). Interestingly, Computer-based platforms, despite being foundational in healthcare technology, indicate a relatively lower prevalence compared to Web-based and Mobile-based solutions (4, 11, 22, 35, 39, 55, 64, 72, 73, 75, 76, 86, 90, 91, 94, 95).
The least represented category, Web-based application platforms, highlights a niche area within ePRO systems in cancer care. While these platforms may provide specific functionalities or targeted solutions, their lower frequency of mention suggests a narrower focus or limited adoption compared to other types of ePRO platforms (13, 40, 49, 58, 65, 74, 82, 85). Generally, the distribution of mentions among different ePRO platform categories indicates the dynamic landscape of electronic systems in oncology care, preferences. This observation may suggest a shift towards more agile and user-friendly electronic systems that align with modern healthcare delivery models.
4.2 System capabilities
The section on electronic systems and platforms in oncology care reveals several key themes. These include symptom assessment and management, emphasizing real-time feedback for improved care delivery; communication and patient engagement, facilitating active involvement in the care process; research and clinical trial capabilities, supporting efficient data collection and analysis; data visualization and reporting, aiding comprehensive decision-making; usability and user experience, ensuring ease of use for all stakeholders; remote monitoring and supportive care, enhancing accessibility and continuity of care; quality of life and psychological factors assessment, enabling holistic care approaches; application integration and interoperability, promoting seamless functionality across systems; data security and encryption, ensuring confidentiality and privacy; patient-reported outcomes utilization, aiding personalized care and treatment decisions; and patient recruitment and enrollment streamlining, improving efficiency in research participation.
The integration of electronic patient-reported outcome (ePRO) systems in oncology care has significantly enhanced patient-centred solutions through various capabilities. These systems have transformed patient recruitment and enrollment processes by efficiently utilizing Electronic Health Records (EHR) systems, enabling seamless data collection and early symptom detection (25). The ability to recruit and enrol patients in ePRO systems is crucial for their successful implementation (16, 22). Studies such as those conducted by Riedl et al. (22), Sprave et al. (23), and Patt et al. (24) highlighted the significance of understanding patient demographics and preferences. Moreover, the integration of ePRO systems with popular social apps like WeChat has facilitated real-time survey monitoring and automated reminders for enhanced patient convenience and engagement (38). Studies from Andrew Harper et al. (25), Oldenburger et al. (26), and others demonstrated the integration of ePROs into routine care. These studies indicated the potential of ePRO systems to integrate into existing healthcare infrastructure, enhancing communication and data exchange.
Symptom assessment and management have been greatly improved through personalized symptom questionnaires, weekly symptom monitoring, and immediate alerts for severe adverse events (25, 53, 54, 86, 87, 96). These advancements allow for timely intervention and personalized care plans based on patients’ health states and symptom severity levels (29). Studies such as those by Andrew Harper et al. (25), Eva Oldenburger et al. (26), and others explored the impact of ePROs on symptom severity. Harper et al. (25) evaluated the electronic collection of patient-reported outcomes data across ambulatory cancer centres, while Oldenburger et al. (26) described the symptom severity among adolescents and young adults. These findings reinforce the role of ePROs in enhancing symptom assessment, reducing hospitalizations, and improving patient outcomes.
Additionally, the assessment of quality of life and psychological factors has been optimized through the collection of patient-reported outcomes, enabling the evaluation of distress, fear of progression, and post-traumatic growth affecting Health-Related Quality of Life (HRQoL) (42) The impact of ePROs on the quality of life and psychological factors is evident in studies by David Riedl et al. (22), Tanja Sprave et al. (23), and Debra A. Patt et al. (24). Riedl et al. (22) explored the ability of adult patients to complete routine ePRO assessments, while Sprave et al. (23) investigated the study of integrating ePROs into the treatment surveillance pathway for Head and Neck Cancer patients. Patt et al. (24) emphasized the significant impact on hospital utilization and cost of care.
Communication and patient engagement have been strengthened by two-way communication channels between patients and caregivers, facilitating compliance tracking, real-time communication with clinicians, and the delivery of self-care instructions targeted to reported symptoms. The ability to effectively communicate with and engage patients is a cornerstone of successful ePRO system implementation (59, 67). David Riedl et al. (22) from Austria highlighted the importance of tailoring ePRO assessments to different age ranges, showcasing the need for personalized communication strategies. Moreover, Data security measures such as data encryption and secure transmission protocols ensure the confidentiality of patient information (28, 34), the integration of Electronic Patient-Reported Outcome (ePRO) systems into oncology care demands a steadfast commitment to data security and encryption. Authors like Silvia Hofer et al. (30) from Switzerland, assessing the usability of the ChemoPRO® app, emphasized the criticality of secure home-based ePRO assessments and electronic data collection while maintaining the confidentiality and privacy of patients’ health-related data. Similarly, Franziska Geese et al. (32) from Switzerland, evaluating the feasibility and acceptability of the eRAPID system, highlighted the need for secure Electronic Patient-Reported Outcome Measures (ePROMs). The assessment of quality of life, functionality, needs, distress, and fear of progression necessitated a robust infrastructure, ensuring the confidentiality of patient-reported information.
Numerous studies found the potential of ePRO systems in advancing research and clinical trials. Gvozdanovic et al. (33) from the United Kingdom leverage Vinehealth, a smartphone application, that uses behavioural science and machine learning for palliative STS treatment. Their work emphasizes the impact of treatment on health-related quality of life (HRQoL) and patient-reported outcomes, showcasing the potential for ePRO systems to contribute valuable data to clinical research. Zhang et al. (34) from China, in the Protecty study, delved into the effectiveness of an ePRO system for prostate cancer care. The digital telemonitoring platform not only monitors health status changes but also identifies risk factors, illustrating the multifaceted role ePROs can play in informing symptom management and supportive care within a clinical trial context.
The ability to visualize and report patient-reported data is crucial for healthcare providers. Authors like Patricia Holch et al. (29) from the United Kingdom, developing a smartphone-based app for prostate cancer patients, emphasize real-time transfer and display of patient responses. Their eRAPID system incorporates graphical and tabular summaries, facilitating efficient communication between patients and clinicians. Furthermore, James Convill et al. (46) from the United Kingdom, examining psychosocial factors affecting ovarian cancer survivors, through an ePRO platform, indicated the significance of collecting and visualizing patient data. By using Electronic Patient-Reported Outcome Measures (ePROMs), they not only gather valuable information on psychosocial factors but also identify patients for referral based on their smoking status.
Furthermore, the usability and user experience of ePRO systems have been optimized with user-friendly interfaces, customizable questionnaires, comprehensive training for research assistants, and compatibility with Electronic Health Record (EHR) integration (5) Usability and user experience are paramount for the successful implementation of ePRO systems. The study by Absolom et al. (5) from the United Kingdom, investigating the health-related quality of life in cancer survivors, places a spotlight on the eRAPID system's online symptom reporting, immediate severity-dependent advice, and real-time monitoring. These features contribute to a positive user experience, fostering patient engagement and adherence. Additionally, Sprave et al. (23) from Germany, assessing the feasibility of integrating ePROs for Head and Neck Cancer patients, highlight the improved reporting of symptom burden and increased patient satisfaction with the App-Controlled Treatment Monitoring and Support (APCOT) trial. The positive user experiences reported in this study pave the way for broader adoption of ePRO systems in oncology care.
Remote monitoring and supportive care are central to the patient-centred solutions provided by ePRO systems. Remote monitoring platforms like Noona provide web-based solutions for remote symptom monitoring, educational content delivery, and immediate automated advice for adverse event management, ultimately empowering patients in their care journey (13, 15, 49). Helissey et al. (31) from Helsinki and France, investigating the feasibility of remote patient monitoring, demonstrate the capabilities of the Cureety platform. Personalized symptom questionnaires, treatment advice, and medical assistance calls enable patients to actively engage in their care from a distance. Similarly, Bobby Daly et al. (45) from the United States, exploring factors associated with the adoption and compliance of ePROMs, reveal insights into InSight Care. Daily ePRO assessments with colour-coded alerts provide a mechanism for tracking symptom fluctuation and anticipating acute care events, providing a valuable avenue for remote supportive care.
The use of Patient-Reported Outcomes (PROs) in cancer care is a transformative aspect explored by various researchers. Andrew Harper et al. (25) in Canada assessed the impact of ePROs on adverse events and the total cost of care for metastatic cancer patients, emphasizing the utility of the ESAS-r tool. Eva Oldenburger et al. (26) from Belgium, through an online survey platform, focused on describing symptom severity among adolescents and young adults, highlighting the diverse applications of ePROs in capturing patient-reported outcomes. These studies highlight the versatility and applicability of PROs in oncology, demonstrating their potential to positively impact patient care. Riedl et al. (40) from Austria examined the impact of ePROs on the health-related quality of life of melanoma patients, highlighting the multifunctional web-based application - The Life App, which integrates rehabilitation interventions and showcases the potential of PROs in shaping comprehensive oncological treatment. Furthermore, Riis et al. (52) from Denmark detailed the development and implementation of integrated care pathways (ICPs) for the electronic collection of patient-reported outcomes (ePROs) in lung cancer patients.
Future research in electronic patient-reported outcome (ePRO) systems in cancer could delve into integrating artificial intelligence and machine learning algorithms for personalized symptom management, alongside wearable devices and mobile health applications for real-time monitoring. Additionally, evaluating the economic impact of ePRO implementation, conducting longitudinal studies on patient outcomes, integrating patient-reported outcomes into clinical trials, cross-cultural validation of ePRO systems, and investigating user experience and stakeholder engagement strategies are crucial. These research endeavours would contribute to enhancing patient-centred oncology care by optimizing treatment strategies, promoting continuous patient engagement, and ensuring the usability and acceptability of electronic solutions across diverse healthcare settings and patient populations.
5 Implications
Our study exhibited several strengths. Firstly, it provided a comprehensive analysis of various electronic health systems utilized in electronic patient-reported outcomes in cancer care, highlighting the widespread adoption of systems like CHES, Noona, and EPIC Electronic Health Record (EHR). This detailed examination provides valuable insights into the landscape of electronic systems in oncology care, setting a foundation for future studies to build upon. Secondly, by focusing on ePRO systems and ePROMs, our research sheds light on the pivotal role these technologies play in capturing patient experiences and enhancing patient-centred solutions. This emphasis highlights the importance of leveraging electronic systems to transform cancer care delivery.
6 Limitations
Despite its strengths, this review study had several limitations that provide valuable insights for future research. Firstly, due to time constraints, the study focused on published papers from three main databases, suggesting the need for a more extensive survey across diverse sources. Secondly, while ePRO interventions were examined, other technologies like artificial intelligence and wearable devices were not included in this research. Thirdly, the exclusion of various types of papers such as reports and editorials may have limited the scope of analysis. Fourthly, the study did not include systematic review studies, indicating a potential gap in synthesizing existing evidence. Lastly, the restriction to English publications implies a need for future studies to consider a more inclusive approach to analyzing ePRO systems in cancer care.
7 Conclusion
In this comprehensive study, we conducted a thorough analysis of electronic systems and platforms utilized in cancer care, focusing on their capabilities, frequencies of use, and contributions to patient-centred outcomes. Our findings indicate the diverse landscape of electronic patient-reported outcome (ePRO) systems, each providing unique functionalities tailored to address specific needs within oncology settings. Among the identified systems, PRO-CTCAE and CHES emerged as the most frequently mentioned platforms, highlighting their significance in capturing patient-reported outcomes and facilitating electronic data capture and interpretation. While these systems play pivotal roles in enhancing patient care and treatment monitoring, other platforms such as eRAPID, Noona, and PROMPT-Care also contribute significantly to patient engagement and symptom management.
Our analysis revealed a diverse range of capabilities within these systems, with Symptom Assessment and Management being the most frequently studied. These capabilities enable early symptom detection, personalized patient communication, and active patient participation in their care, thereby improving treatment outcomes and overall patient experience. Furthermore, our study provides information about the distribution of system types, with web-based platforms being the most prevalent followed by mobile-based and computer-based systems. Understanding the prevalence and functionalities of these systems provides valuable insights for healthcare providers and researchers aiming to implement or optimize electronic solutions in cancer care.
In general, this study enhances our understanding of the electronic systems and platforms available for cancer care, emphasizing their role in improving patient outcomes, enhancing communication between patients and healthcare providers, and facilitating personalized treatment approaches. Moving forward, continued research and innovation in electronic health solutions will be crucial for advancing patient-centred oncology practices and optimizing the delivery of cancer care.
8 Summary table
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
HS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing. SN: Conceptualization, Data curation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. MA: Conceptualization, Supervision, Validation, Writing – review & editing. MA: Conceptualization, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank Iran University of Medical Sciences for supporting this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdgth.2025.1560533/full#supplementary-material
Abbreviations
ePRO, electronic patient reported outcomes; ePROM, electronic patient reported outcome measure; PRO-CTCAE, patient reported outcome of common terminology criteria for adverse events; CHES, computer-based health evaluation system; eRAPID, electronic patient self-reporting of adverse-events: patient information and advice; PROMPT-Care, patient-reported outcome measures for personalized treatment and care; ePOS, electronic psycho-oncological and palliative screening; SAAD, serial assessment of anxiety and depressive symptoms in breast cancer; PAINReportIt®, pain reporting; REDCap, research electronic data capture; LASA, linear analog self-assessment; ePROCOM, electronic patient reported outcomes and compliance analysis; STAR, symptom tracking and reporting; PROMIS CATs, patient-reported outcomes measurement information system computer adaptive tests; APCOT, app-controlled treatment monitoring and support for head and neck cancer patients.
References
1. Elkefi S, Asan O. The impact of patient-centered care on cancer patients’ QOC, self-efficacy, and trust towards doctors: analysis of a national survey. J Patient Exp. (2023) 10:23743735231151533. doi: 10.1177/23743735231151533
2. Valsangkar N, Fernandez F, Khullar O. Patient reported outcomes: integration into clinical practices. J Thorac Dis. (2020) 12(11):6940–6. doi: 10.21037/jtd.2020.03.91
3. Brundage M, Bass B, Jolie R, Foley K, Tolomiczenko G, Evans WK, et al. A knowledge translation challenge: clinical use of quality of life data from cancer clinical trials. Qual Life Res. (2011) 20:979–85. doi: 10.1007/s11136-011-9848-0
4. Nordhausen T, Lampe K, Vordermark D, Holzner B, Al-Ali HK, Meyer G, et al. An implementation study of electronic assessment of patient-reported outcomes in inpatient radiation oncology. J Patient Rep Outcomes. (2022) 6:77. doi: 10.1186/s41687-022-00478-3
5. Absolom K, Warrington L, Hudson E, Hewison J, Morris C, Holch P, et al. Phase III randomized controlled trial of eRAPID: eHealth intervention during chemotherapy. J Clin Oncol. (2021) 39:734–47. doi: 10.1200/JCO.20.02015
6. Groen WG, Kuijpers W, Oldenburg HS, Wouters MWJ, Aaronson NK, van Harten WH. Empowerment of cancer survivors through information technology. An Integrative Review. J Med Internet Res. (2015) 17:e270. doi: 10.2196/jmir.4818
7. Warrington L, Absolom K, Holch P, Gibson A, Clayton B, Velikova G, et al. Online tool for monitoring adverse events in patients with cancer during treatment (eRAPID): field testing in a clinical setting. BMJ Open. (2019) 9:e025185. doi: 10.1136/bmjopen-2018-025185
8. Bennett AV, Jensen RE, Basch E. Electronic patient-reported outcome systems in oncology clinical practice. CA Cancer J Clin. (2012) 62:337–47. doi: 10.3322/caac.21150
9. Salmani H, Nasiri S, Ahmadi M. The advantages, disadvantages, threats, and opportunities of electronic patient-reported outcome systems in cancer: a systematic review. Digit Health. (2024) 10:20552076241257146. doi: 10.1177/20552076241257146
10. Meirte J, Hellemans N, Anthonissen M, Denteneer L, Maertens K, Moortgat P, et al. Benefits and disadvantages of electronic patient-reported outcome measures: systematic review. JMIR Perioper Med. (2020) 3:e15588. doi: 10.2196/15588
11. Warnecke E, Salvador Comino MR, Kocol D, Hosters B, Wiesweg M, Bauer S, et al. Electronic patient-reported outcome measures (ePROMs) improve the assessment of underrated physical and psychological symptom burden among oncological inpatients. Cancers (Basel). (2023) 15:329. doi: 10.3390/cancers15113029
12. Salmani H, Nasiri S, Alemrajabi M, Ahmadi M. Advancing patient-centered cancer care: a systematic review of electronic patient-reported outcome measures. Front Rehabil Sci. (2024) 5:1–16. doi: 10.3389/fresc.2024.1427712
13. Peltola MK, Poikonen-Saksela P, Mattson J, Virtanen T, Rantanen E, Parkkari T. A novel digital patient-reported outcome platform (noona) for clinical use in patients with cancer: pilot study assessing suitability. JMIR Form Res. (2021) 5:e16156. doi: 10.2196/16156
14. Dickson NR, Beauchamp KD, Perry TS, Roush A, Goldschmidt D, Edwards ML, et al. Real-world use and clinical impact of an electronic patient-reported outcome tool in patients with solid tumors treated with immuno-oncology therapy. J Patient Rep Outcomes. (2024) 8:23. doi: 10.1186/s41687-024-00700-4
15. Generalova O, Roy M, Hall E, Shah SA, Cunanan K, Fardeen T, et al. Implementation of a cloud-based electronic patient-reported outcome (ePRO) platform in patients with advanced cancer. J Patient Rep Outcomes. (2021) 5:91. doi: 10.1186/s41687-021-00358-2
16. Williams LA, Whisenant MS, Mendoza TR, Peek AE, Malveaux D, Griffin DK, et al. Measuring symptom burden in patients with cancer during a pandemic: the MD anderson symptom inventory for COVID-19 (MDASI-COVID). J Patient Rep Outcomes. (2023) 7:48. doi: 10.1186/s41687-023-00591-x
17. Kanas G, Morimoto L, Mowat F, Khan H. Use of electronic medical records in oncology outcomes research. Clinicoecon Outcomes Res. (2010) 2:1–14. doi: 10.2147/CEOR.S8411
18. McMullan C, Hughes SE, Aiyegbusi OL, Calvert M. Usability testing of an electronic patient-reported outcome system linked to an electronic chemotherapy prescribing and patient management system for patients with cancer. Heliyon. (2023) 9:e16453. doi: 10.1016/j.heliyon.2023.e16453
19. Moradian S, Ghasemi S, Boutorabi B, Sharifian Z, Dastjerdi F, Buick C, et al. Development of an eHealth tool for capturing and analyzing the immune-related adverse events (irAEs) in cancer treatment. Cancer Inform. (2023) 22:11769351231178587. doi: 10.1177/11769351231178587
20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
21. Hong QN, Pluye P, Fàbregues S, Bartlett G, Boardman F, Cargo M, et al. Improving the content validity of the mixed methods appraisal tool: a modified e-Delphi study. J Clin Epidemiol. (2019) 111:49–59.e1. doi: 10.1016/j.jclinepi.2019.03.008
22. Riedl D, Lehmann J, Rothmund M, Gauger U, Rothe F, Holzwarth U. Usability of electronic patient-reported outcome measures for older patients with cancer: secondary analysis of data from an observational single center study. J Med Internet Res. (2023) 25:e49476. doi: 10.2196/49476
23. Sprave T, Pfaffenlehner M, Stoian R, Seidlitz A, Nicolai N, Weber L. App-controlled treatment monitoring and support for patients with head and neck cancer undergoing radiotherapy: results from a prospective randomized controlled trial. J Med Internet Res. (2023) 25:e46189. doi: 10.2196/46189
24. Patt DA, Patel AM, Bhardwaj A, Gomez JI, Hingorani SR, Addeo P, et al. Impact of remote symptom monitoring with electronic patient-reported outcomes on hospitalization, survival, and cost in community oncology practice: the Texas two-step study. JCO Clin Cancer Inform. (2023) 7:e2300182. doi: 10.1200/CCI.23.00182
25. Harper A, Maseja N, Parkinson R, Amara S, Hull L, Davies E. Symptom severity and trajectories among adolescent and young adult patients with cancer. JNCI Cancer Spectr. (2023) 7:1–13. doi: 10.1093/jncics/pkad049
26. Oldenburger E, Isebaert S, Coolbrandt A, Van Den Wyngaert T, Pardon K, De Geest S. The use of electronic patient reported outcomes in follow-up after palliative radiotherapy: a survey study in Belgium. PEC Innovation. (2023) 3:100243. doi: 10.1016/j.pecinn.2023.100243
27. Mohseni M, Ayatollahi H, Arefpour AM. Electronic patient-reported outcome (ePRO) application for patients with prostate cancer. PLoS One. (2023) 18:e0289974. doi: 10.1371/journal.pone.0289974
28. Macanovic B, O’Reilly D, Harvey H, Walsh R, Murphy S, Jones C. A pilot project investigating the use of ONCOpatient®-an electronic patient-reported outcomes app for oncology patients. Digit Health. (2023) 9:20552076231185428. doi: 10.1177/20552076231185428
29. Holch P, Absolom KL, Henry AM, Follwell M, Locke S, Booth C. Online symptom monitoring during pelvic radiation therapy: randomized pilot trial of the eRAPID intervention. Int J Radiat Oncol Biol Phys. (2023) 115:664–76. doi: 10.1016/j.ijrobp.2022.09.078
30. Hofer S, Hentschel L, Richter S, Becker F, Müller A, Friedrich M. Electronic patient reported outcome (ePRO) measures in patients with soft tissue sarcoma (STS) receiving palliative treatment. Cancers (Basel). (2023) 15:1–8. doi: 10.3390/cancers15041233
31. Helissey C, Parnot C, Rivière C, Balavoine S, Borde J, Dourthe L. Effectiveness of electronic patient reporting outcomes, by a digital telemonitoring platform, for prostate cancer care: the protecty study. Front Digit Health. (2023) 5:1–9. doi: 10.3389/fdgth.2023.1104700
32. Geese F, Kaufmann S, Sivanathan M, Scheidhauer K, Hütter J, Becker G. Exploring the potential of electronic patient-reported outcome measures to inform and assess care in sarcoma centers: a longitudinal multicenter pilot study. Cancer Nurs. (2023) 47(6):E395–E403. doi: 10.1097/NCC.0000000000001248
33. Gvozdanovic A, Jozsa F, Fersht N, Johnson P, Newton J, O’Neill K. Integration of a personalised mobile health (mHealth) application into the care of patients with brain tumours: proof-of-concept study (IDEAL stage 1). BMJ Surg Interv Health Technol. (2022) 4:e000130. doi: 10.1136/bmjsit-2021-000130
34. Zhang Y, Li Z, Pang Y, Liu X, Chen Q, Wang P. Changes in patient-reported health Status in advanced cancer patients from a symptom management clinic: a longitudinal study conducted in China. J Oncol. (2022) 2022:7531545. doi: 10.1155/2022/7531545
35. Zhang L, Zhang X, Shen L, Li J, Wang F, Chen H. Efficiency of electronic health record assessment of patient-reported outcomes after cancer immunotherapy: a randomized clinical trial. JAMA Netw Open. (2022) 5:e224427. doi: 10.1001/jamanetworkopen.2022.4427
36. Wickline M, Wolpin S, Cho S, Vaughan L, Wang C, Speck RM. Usability and acceptability of the electronic self-assessment and care (eSAC) program in advanced ovarian cancer: a mixed methods study. Gynecol Oncol. (2022) 11:e66801. doi: 10.1016/j.ygyno.2022.09.010
37. Tolstrup LK, Pappot H, Bastholt L, Zwisler AD, Dieperink KB, Møller JE. Impact of patient-reported outcomes on symptom monitoring during treatment with checkpoint inhibitors: health-related quality of life among melanoma patients in a randomized controlled trial. J Patient Rep Outcomes. (2022) 6:8. doi: 10.1186/s41687-022-00414-5
38. Tang L, He Y, Pang Y, Xu J, Li Q, Wang Y. Implementing symptom management follow-up using an electronic patient-reported outcome platform in outpatients with advanced cancer: longitudinal single-center prospective study. JMIR Form Res. (2022) 6:e21458. doi: 10.2196/21458
39. Rocque GB, Dent DAN, Ingram SA, Lee R, Lally RM, McNaughton D, et al. Adaptation of remote symptom monitoring using electronic patient-reported outcomes for implementation in real-world settings. JCO Oncol Pract. (2022) 18:e1943–52. doi: 10.1200/OP.22.00360
40. Riedl D, Licht T, Nickels A, Reiter K, Löbl K, Kropshofer G. Large improvements in health-related quality of life and physical fitness during multidisciplinary inpatient rehabilitation for pediatric cancer survivors. Cancers (Basel). (2022) 14:1–16. doi: 10.3390/cancers14194855
41. Lee M, Kang D, Kim S, Park E, Yoon H, Choi Y. Who is more likely to adopt and comply with the electronic patient-reported outcome measure (ePROM) mobile application? A real-world study with cancer patients undergoing active treatment. Support Care Cancer. (2022) 30:659–68. doi: 10.1007/s00520-021-06473-6
42. Hlubocky FJ, Daugherty CK, Peppercorn J, Schreiber S, Malin J, Mooney K, et al. Utilization of an electronic patient-reported outcome platform to evaluate the psychosocial and quality-of-life experience among a community sample of ovarian cancer survivors. JCO Clin Cancer Inform. (2022) 6:e2200035. doi: 10.1200/CCI.22.00035
43. Graf J, Sickenberger N, Brusniak K, Oberste M, Gorner N, Kienle P. Implementation of an electronic patient-reported outcome app for health-related quality of life in breast cancer patients: evaluation and acceptability analysis in a two-center prospective trial. J Med Internet Res. (2022) 24:e16128. doi: 10.2196/16128
44. Girgis A, Bamgboje-Ayodele A, Rincones O, Levesque JF, Gerges M, Sandell T, et al. Stepping into the real world: a mixed-methods evaluation of the implementation of electronic patient reported outcomes in routine lung cancer care. J Patient Rep Outcomes. (2022) 6:70. doi: 10.1186/s41687-022-00475-6
45. Daly B, Nicholas K, Flynn J, Wood WA, Chang Y, Basch E. Analysis of a remote monitoring program for symptoms among adults with cancer receiving antineoplastic therapy. JAMA Netw Open. (2022) 5:e221078. doi: 10.1001/jamanetworkopen.2022.1078
46. Convill J, Blackhall F, Yorke J, Woolley T, White L, Essat M. The role of electronic patient-reported outcome measures in assessing smoking status and cessation for patients with lung cancer. Oncol Ther. (2022) 10:481–91. doi: 10.1007/s40487-022-00210-7
47. Boeke S, Hauth F, Fischer SG, Glatting G, Merz F, Nicolay NH. Acceptance of physical activity monitoring in cancer patients during radiotherapy, the GIROfit phase 2 pilot trial. Tech Innov Patient Support Radiat Oncol. (2022) 22:16–21. doi: 10.1016/j.tipsro.2022.03.004
48. Ayodele A, Avery S, Pearson J, Thompson M, Gerges M, Olver I. Adapting an integrated care pathway for implementing electronic patient reported outcomes assessment in routine oncology care: lessons learned from a case study. J Eval Clin Pract. (2022) 28:1072–83. doi: 10.1111/jep.13688
49. Takala L, Kuusinen T-E, Skyttä T, Karvonen S, Laaksonen L, Kellokumpu-Lehtinen P. Electronic patient-reported outcomes during breast cancer adjuvant radiotherapy. Clin Breast Cancer. (2021) 21:e252–70. doi: 10.1016/j.clbc.2020.10.004
50. Strachna O, Cohen MA, Allison MM, Genden E, Miles BA, Teng MS, et al. Case study of the integration of electronic patient-reported outcomes as standard of care in a head and neck oncology practice: obstacles and opportunities. Cancer. (2021) 127:359–71. doi: 10.1002/cncr.33272
51. Stormoen DR, Baeksted C, Taarnhøj GA, Hansen H, Lund M, Jensen H, et al. Patient reported outcomes interfering with daily activities in prostate cancer patients receiving antineoplastic treatment. Acta Oncol (Madr). (2021) 60:419–25. doi: 10.1080/0284186X.2021.1881818
52. Riis CL, Stie M, Bechmann T, Petersen AS, Møller TB, Johansen C, et al. ePRO-based individual follow-up care for women treated for early breast cancer: impact on service use and workflows. J Cancer Surviv. (2021) 15:485–96. doi: 10.1007/s11764-020-00942-3
53. Ravn S, Thaysen HV, Verwaal VJ, Østergren PB, Sørensen JB, Johansen C, et al. Cancer follow-up supported by patient-reported outcomes in patients undergoing intended curative complex surgery for advanced cancer. J Patient Rep Outcomes. (2021) 5:120. doi: 10.1186/s41687-021-00391-1
54. Patt D, Wilfong L, Hudson KE, Wilson SA, Baker T, Morris E, et al. Implementation of electronic patient-reported outcomes for symptom monitoring in a large multisite community oncology practice: dancing the Texas two-step through a pandemic. JCO Clin Cancer Inform. (2021) 12(12):615–21. doi: 10.1200/CCI.21.00063
55. Licht T, Nickels A, Rumpold G, Petzold MB, Holzhüter C, Heckmann J, et al. Evaluation by electronic patient-reported outcomes of cancer survivors’ needs and the efficacy of inpatient cancer rehabilitation in different tumor entities. Support Care Cancer. (2021) 29:5853–64. doi: 10.1007/s00520-021-06123-x
56. Lee J, Jung JH, Kim WW, Park SH, Yoon HJ, Cho WY, et al. Short-term serial assessment of electronic patient-reported outcome for depression and anxiety in breast cancer. BMC Cancer. (2021) 21:1065. doi: 10.1186/s12885-021-08771-y
57. Lapen K, Sabol C, Tin AL, Weinberg D, Jacobs A, Johnson D, et al. Development and pilot implementation of a remote monitoring system for acute toxicity using electronic patient-reported outcomes for patients undergoing radiation therapy for breast cancer. Int J Radiat Oncol Biol Phys. (2021) 111:979–91. doi: 10.1016/j.ijrobp.2021.07.1692
58. Judge MKM, Luedke R, Dyal BW, Williams EA, Fisher ES, Miller KF, et al. Clinical efficacy and implementation issues of an electronic pain reporting device among outpatients with cancer. Support Care Cancer. (2021) 29:5227–35. doi: 10.1007/s00520-021-06075-2
59. Doolin JW, Berry JL, Forbath NS, Patel RS, Smith AB, Johnson PB, et al. Implementing electronic patient-reported outcomes for patients with new oral chemotherapy prescriptions at an academic site and a community site. JCO Clin Cancer Inform. (2021) 5:631–40. doi: 10.1200/CCI.20.00191
60. Zylla DM, Gilmore GE, Steele GL, Mehta SS, Stevenson JN, Smith EM, et al. Collection of electronic patient-reported symptoms in patients with advanced cancer using epic MyChart surveys. Support Care Cancer. (2020) 28:3153–63. doi: 10.1007/s00520-019-05109-0
61. Tran C, Dicker A, Leiby B, Andersen R, Brooks J, Chen S, et al. Utilizing digital health to collect electronic patient-reported outcomes in prostate cancer: single-arm pilot trial. J Med Internet Res. (2020) 22:e12689. doi: 10.2196/12689
62. Tolstrup LK, Bastholt L, Dieperink KB, Zwisler AD, Pappot H, Møller JE, et al. The use of patient-reported outcomes to detect adverse events in metastatic melanoma patients receiving immunotherapy: a randomized controlled pilot trial. J Patient Rep Outcomes. (2020) 4:88. doi: 10.1186/s41687-020-00255-0
63. Sandhu S, King Z, Wong M, Bui A, Lee H, Liu S, et al. Implementation of electronic patient-reported outcomes in routine cancer care at an academic center: identifying opportunities and challenges. JCO Oncol Pract. (2020) 16:e1255–63. doi: 10.1200/OP.20.00357
64. Riis CL, Jensen PT, Bechmann T, Petersen AS, Johansen C, Stie M, et al. Satisfaction with care and adherence to treatment when using patient reported outcomes to individualize follow-up care for women with early breast cancer – a pilot randomized controlled trial. Acta Oncol (Madr). (2020) 59:444–52. doi: 10.1080/0284186X.2020.1717604
65. Richards HS, Blazeby JM, Portal A, Browne S, Guy R, Walters SJ, et al. A real-time electronic symptom monitoring system for patients after discharge following surgery: a pilot study in cancer-related surgery. BMC Cancer. (2020) 20:543. doi: 10.1186/s12885-020-07027-5
66. Mowlem FD, Sanderson B, Platko JV, Jefferson K, McDonald S, Thompson R, et al. Optimizing electronic capture of patient-reported outcome measures in oncology clinical trials: lessons learned from a qualitative study. J Comp Eff Res. (2020) 9:1195–204. doi: 10.2217/cer-2020-0143
67. Karamanidou C, Maramis C, Stamatopoulos K, Vassiliou V, Boumpas D, Maniatis A, et al. Development of a ePRO-based palliative care intervention for cancer patients: a participatory design approach. Stud Health Technol Inform. (2020) 270:941–5. doi: 10.3233/SHTI200300
68. Howell D, Rosberger Z, Mayer C, Hacker E, Broadhurst B, Kellan D, et al. Personalized symptom management: a quality improvement collaborative for implementation of patient reported outcomes (PROs) in “real-world” oncology multisite practices. J Patient Rep Outcomes. (2020) 4:47. doi: 10.1186/s41687-020-00212-x
69. Girgis A, Durcinoska I, Arnold A, Jefford M, Bermudez L, Cartwright C, et al. Web-based patient-reported outcome measures for personalized treatment and care (PROMPT-care): multicenter pragmatic nonrandomized trial. J Med Internet Res. (2020) 22:e19685. doi: 10.2196/19685
70. Dronkers EAC, Baatenburg de Jong RJ, van der Poel EF, Balm AJ, van der Wal JE, Takes RP, et al. Keys to successful implementation of routine symptom monitoring in head and neck oncology with “healthcare monitor” and patients’ perspectives of quality of care. Head Neck. (2020) 42:3590–600. doi: 10.1002/hed.26425
71. Biran N, Anthony Kouyaté R, Yucel E, Okonji S, Walker L, Taylor K, et al. Adaptation and evaluation of a symptom-monitoring digital health intervention for patients with relapsed and refractory multiple myeloma: pilot mixed-methods implementation study. JMIR Form Res. (2020) 4:e18982. doi: 10.2196/18982
72. Krogstad H, Sundt-Hansen SM, Hjermstad MJ, Rustøen T, Kristiansen T, Småstuen MC, et al. Usability testing of EirV3—a computer-based tool for patient-reported outcome measures in cancer. Support Care Cancer. (2019) 27:1835–44. doi: 10.1007/s00520-018-4435-3
73. Kikawa Y, Hatachi Y, Rumpold G, Kobayashi Y, Shimizu S, Takahashi T. Evaluation of health-related quality of life via the computer-based health evaluation system (CHES) for Japanese metastatic breast cancer patients: a single-center pilot study. Breast Cancer. (2019) 26:255–9. doi: 10.1007/s12282-018-0905-1
74. Iivanainen S, Alanko T, Peltola K, Räsänen P, Mussalo H, Kärkkäinen P. ePROs in the follow-up of cancer patients treated with immune checkpoint inhibitors: a retrospective study. J Cancer Res Clin Oncol. (2019) 145:765–74. doi: 10.1007/s00432-018-02835-6
75. Gressel GM, Dioun SM, Richley M, McNiff J, Jacobson C, Levinson KL, et al. Utilizing the patient reported outcomes measurement information system (PROMIS®) to increase referral to ancillary support services for severely symptomatic patients with gynecologic cancer. Gynecol Oncol. (2019) 152:509–13. doi: 10.1016/j.ygyno.2018.10.042
76. Brant JM, Hirschman KB, Keckler SL, Daly BJ, Ridgeway E, Allshouse AA, et al. Patient and provider use of electronic care plans generated from patient-reported outcomes. Oncol Nurs Forum. (2019) 46:715–26. doi: 10.1188/19.ONF.715-726
77. Avery KNL, Richards HS, Portal A, Blazeby JM, Williamson PR, Calvert M, et al. Developing a real-time electronic symptom monitoring system for patients after discharge following cancer-related surgery. BMC Cancer. (2019) 19:463. doi: 10.1186/s12885-019-5657-6
78. Schepers SA, Sint Nicolaas SM, Haverman L, Scheltinga M, Zadeh S, Grootenhuis MA, et al. Real-world implementation of electronic patient-reported outcomes in outpatient pediatric cancer care. Psychooncology. (2017) 26:951–9. doi: 10.1002/pon.4242
79. Niska JR, Halyard MY, Tan AD, Sloan JA, Garcia JJ, Khan H, et al. Electronic patient-reported outcomes and toxicities during radiotherapy for head-and-neck cancer. Qual Life Res. (2017) 26:1721–31. doi: 10.1007/s11136-017-1528-2
80. Lucas SM, Kim T-K, Ghani KR, Daignault S, Bertolet MH, Miller DC, et al. Establishment of a web-based system for collection of patient-reported outcomes after radical prostatectomy in a statewide quality improvement collaborative. Urology. (2017) 107:96–102. doi: 10.1016/j.urology.2017.04.058
81. Holch P, Warrington L, Bamforth LCA, Conner MT, Horne A, Avery KNL, et al. Development of an integrated electronic platform for patient self-report and management of adverse events during cancer treatment. Ann Oncol. (2017) 28:2305–11. doi: 10.1093/annonc/mdx317
82. Hartkopf AD, Graf J, Simoes E, Keilmann L, Grabe E, Wallwiener M, et al. Electronic-Based patient-reported outcomes: willingness, needs, and barriers in adjuvant and metastatic breast cancer patients. JMIR Cancer. (2017) 3:e11. doi: 10.2196/cancer.6996
83. Absolom K, Holch P, Warrington L, Gibson A, Clayton B, Velikova G, et al. Electronic patient self-reporting of adverse-events: patient information and aDvice (eRAPID): a randomised controlled trial in systemic cancer treatment. BMC Cancer. (2017) 17:318. doi: 10.1186/s12885-017-3303-8
84. Schougaard LM, Larsen LP, Jessen A, Sidenius P, Dorflinger L, de Thygesen CF, et al. Ambuflex: tele-patient-reported outcomes (telePRO) as the basis for follow-up in chronic and malignant diseases. Quality Life Res. (2016) 25:525–34. doi: 10.1007/s11136-015-1207-0
85. Peltola MK, Lehikoinen JS, Sippola LT, Mäkitie AA, Kinnunen I. A novel digital patient-reported outcome platform for head and neck oncology patients-A pilot study. Clin Med Insights Ear Nose Throat. (2016) 9:1–6. doi: 10.4137/CMENT.S40219
86. Mayrbäurl B, Giesinger JM, Burgstaller S, Waldmann A, Wintner LM, Meraner V, et al. Quality of life across chemotherapy lines in patients with advanced colorectal cancer: a prospective single-center observational study. Support Care Cancer. (2016) 24:667–74. doi: 10.1007/s00520-015-2828-0
87. Graf J, Simoes E, Wißlicen K, Keilmann L, Wallwiener M, Hartkopf AD, et al. Willingness of patients with breast cancer in the adjuvant and metastatic setting to use electronic surveys (ePRO) Depends on sociodemographic factors, health-related quality of life, disease Status and computer skills. Geburtshilfe Frauenheilkd. (2016) 76:535–41. doi: 10.1055/s-0042-105872
88. Duregger K, Hayn D, Nitzlnader M, Schreier G, Frühwald MC, Stary S, et al. Electronic patient reported outcomes in paediatric oncology—applying Mobile and near field communication technology. Stud Health Technol Inform. (2016) 223:281–8.27139415
89. Cowan RA, Suidan RS, Andikyan V, Rezk Y, Long R, Gardner GJ, et al. Electronic patient-reported outcomes from home in patients recovering from major gynecologic cancer surgery: a prospective study measuring symptoms and health-related quality of life. Gynecol Oncol. (2016) 143:362–6. doi: 10.1016/j.ygyno.2016.08.335
90. Wintner LM, Giesinger JM, Zabernigg A, Sztankay M, Oberguggenberger A, Sperner-Unterweger B, et al. Evaluation of electronic patient-reported outcome assessment with cancer patients in the hospital and at home. BMC Med Inform Decis Mak. (2015) 15:110. doi: 10.1186/s12911-015-0230-y
91. Wagner LI, Schink J, Bass M, Patel S, Diaz MV, Rothrock N, et al. Bringing PROMIS to practice: brief and precise symptom screening in ambulatory cancer care. Cancer. (2015) 121:927–34. doi: 10.1002/cncr.29104
92. Pollom EL, Wang E, Bui TT, Ognibene G, von Eyben R, Lavori P, et al. A prospective study of electronic quality of life assessment using tablet devices during and after treatment of head and neck cancers. Oral Oncol. (2015) 51:1132–7. doi: 10.1016/j.oraloncology.2015.10.003
93. Smith SK, Rowe K, Abernethy AP. Use of an electronic patient-reported outcome measurement system to improve distress management in oncology. Palliat Support Care. (2014) 12:69–73. doi: 10.1017/S1478951513000345
94. Wintner LM, Giesinger JM, Zabernigg A, Sztankay M, Meraner V, Pall G, et al. Quality of life during chemotherapy in lung cancer patients: results across different treatment lines. Br J Cancer. (2013) 109:2301–8. doi: 10.1038/bjc.2013.585
95. Zabernigg A, Giesinger JM, Pall G, Gamper EM, Gattringer K, Wintner LM, et al. Quality of life across chemotherapy lines in patients with cancers of the pancreas and biliary tract. BMC Cancer. (2012) 12:390. doi: 10.1186/1471-2407-12-390
96. Abernethy AP, Ahmad A, Zafar SY, Wheeler JL, Reese JB, Lyerly HK. Electronic patient-reported data capture as a foundation of rapid learning cancer care. Med Care. (2010) 48:S32–8. doi: 10.1097/MLR.0b013e3181db53a4
97. Coons SJ, Eremenco S, Lundy JJ, O’Donnell F, O‘Connor M, Condon DM, et al. Capturing patient-reported outcome (PRO) data electronically: the past, present, and promise of ePRO measurement in clinical trials. Patient. (2015) 8:301–9. doi: 10.1007/s40271-014-0090-z
98. Basch E, Pugh SL, Dueck AC, Smith ML, Rogak LJ, Kris MG, et al. Feasibility of patient reporting of symptomatic adverse events via the patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) in a chemoradiotherapy cooperative group multicenter clinical trial. Int J Radiat Oncol Biol Phys. (2017) 98:409–18. doi: 10.1016/j.ijrobp.2017.02.002
99. Basch E, Barbera L, Kerrigan CL, Novotny PJ, Basch CE, Williams GR, et al. Implementation of patient-reported outcomes in routine medical care. Am Soc Clin Oncol Educ Book. (2018) 38:122–34. doi: 10.1200/EDBK_200383
Keywords: electronic patient-reported outcome systems, ePRO, cancer, patient-centred solutions, capability
Citation: Salmani H, Nasiri S, Alemrajabi M and Ahmadi M (2025) Electronic patient-reported outcome systems and capabilities in cancer care: a systematic review. Front. Digit. Health 7:1560533. doi: 10.3389/fdgth.2025.1560533
Received: 6 February 2025; Accepted: 8 July 2025;
Published: 18 August 2025.
Edited by:
Stephen Gbenga Fashoto, Namibia University of Science and Technology, NamibiaReviewed by:
Kausik Basak, JIS Institute of Advanced Studies and Research, IndiaShunichi Toki, Shizuoka Cancer Center, Japan
Copyright: © 2025 Salmani, Nasiri, Alemrajabi and Ahmadi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Somayeh Nasiri, bmFzaXJpLnNvMjAwQGdtYWlsLmNvbQ==
 Hosna Salmani
Hosna Salmani Somayeh Nasiri1*
Somayeh Nasiri1*