- 1Department of Electronics and Information Engineering, Korea University, Sejong, Republic of Korea
- 2Interdisciplinary Graduate Program for Artificial Intelligence Smart Convergence Technology, Korea University, Sejong, Republic of Korea
- 3Neurive Co., Ltd., Gimhae, Republic of Korea
- 4Department of Medical Sciences, Graduate School of Medicine, Korea University, Seoul, Republic of Korea
- 5Department of Otorhinolaryngology-Head and Neck Surgery, Korea University Guro Hospital, Seoul, Republic of Korea
Introduction: Transcutaneous auricular vagus nerve stimulation (taVNS) has demonstrated efficacy in alleviating stress-related symptoms, yet its underlying neurophysiological mechanisms remain incompletely understood. In this study, we aimed to elucidate the effects of taVNS on stress regulation by employing self-report surveys and electroencephalography (EEG) measures.
Methods: Participants performed mental arithmetic (MA) and 2-back tasks to induce stress, receiving either taVNS or sham stimulation in a randomized, crossover design. Resting-state EEG was recorded at baseline and immediately after each stress-inducing task—thereby capturing persisting taVNS-induced changes in neural activity—and subjective stress levels were assessed immediately before and after stimulation.
Results: The survey results showed a significant increase in perceived stress following the tasks in the sham condition, whereas stress levels in the taVNS condition did not significantly change. Power spectral density (PSD) analyses revealed that theta and alpha band power in the frontal region significantly decreased only in the sham condition, suggesting elevated stress. Furthermore, whole-brain network analysis indicated a significant reduction in theta band path length in the taVNS condition following both tasks, pointing to enhanced global network efficiency that may help prevent stress escalation.
Discussion: By demonstrating distinctive EEG and behavioral differences between taVNS and sham stimulation, this study provides a neurophysiological basis for taVNS as a non-invasive intervention for stress regulation. Future research involving a more diverse participant pool and optimization of taVNS parameters will further clarify its therapeutic potential.
1 Introduction
Stress occurs continuously in daily life and can negatively impact mental and physical health. When stress is perceived, the autonomic nervous system (ANS) and the Locus coeruleus (LC) become activated, potentially disrupting homeostasis and leading to various physiological and psychological issues (1, 2). For example, stress increases the risk of developing depression (3) and can contribute to disorders such as post-traumatic stress disorder (PTSD) when individuals are exposed to severe stress (4). To treat disorders induced by stress, psychological counseling and pharmacological treatment methods are commonly used (5). However, due to factors such as drug resistance, these conventional treatment methods have shown meaningful efficacy in only about two-thirds of patients with stress-related disorders (6, 7).
Vagus nerve stimulation (VNS) has been proposed as an alternative therapeutic approach for alleviating symptoms in patients for whom standard treatments are ineffective, such as in cases involving drug resistance (8). VNS is a stimulation technique utilizing low-level electrical currents to stimulate the vagus nerve and has been shown to modulate both the ANS and LC, thereby influencing stress-related neural circuits (9, 10). VNS is a stimulation technique utilizing low-level electrical currents to stimulate the vagus nerve, and it has been applied effectively in treating disorders including depression (11, 12), epilepsy (13, 14), and stroke (15, 16). Additionally, VNS has shown efficacy in patients who respond poorly to conventional treatments (17–19). Nevertheless, VNS is generally used selectively in severe cases, as it requires surgical implantation of stimulation devices, such as electrodes and generators.
Recently, transauricular VNS (taVNS) has been proposed as a non-surgical alternative to VNS. taVNS does not require invasive procedures and has been shown to effectively alleviate symptoms associated with stress-related disorders, including depression and PTSD, similarly to conventional VNS (20–22). However, because neural mechanism underlying the beneficial effects of taVNS remain unclear, numerous researchers have explored its effects on brain activity using various neuroimaging techniques (23–25).
Among neuroimaging methods, electroencephalography (EEG) is extensively used in taVNS studies due to portability and high temporal resolution (25–29). For example, Ricci et al. (30) reported increased delta power during 60 min of taVNS in a resting state (30). Lee et al. (31) confirmed the neuromodulatory effects of taVNS using resting-state EEG and proposed optimal stimulation durations for achieving effective outcomes (31). In addition, EEG is frequently utilized to study changes in brain activity according to various mental states, particularly stress. Wen et al. (32) reported that theta and alpha frequency powers in the frontal region were inversely correlated with stress levels (32). Interestingly, theta and alpha band activity has also been shown to vary depending on sleep stages, with anti-correlation in REM sleep (associated with higher stress) and positive correlation in deep sleep (associated with lower stress), suggesting their relevance to the brain's stress regulation mechanisms during both wake and sleep states (33). Yan et al. reported that beta frequency power in the frontal region was higher under stress conditions compared to non-stress conditions (34). Additionally, Shim et al. reported that PTSD patients showed reduced theta network indices compared to healthy controls (35). Dimitrakopoulos et al. (36) found decreased network indices under high workload conditions related to low workload conditions (36).
Although some studies have investigated taVNS-related changes in brain activity using EEG or examined EEG changes associated with different stress levels, there is still a relative lack of research exploring the specific neural mechanisms and effects of taVNS across varying stress levels. In particular, most previous studies assessing the impact of taVNS on stress have relied on electrocardiogram (ECG) measurements (37–39), leading to an underrepresentation of EEG-based approaches in the literature. Furthermore, given the correlations between stress and brain activity, it is essential to investigate both the effects and the underlying neural mechanisms of taVNS on stress using EEG. Taken together, these findings suggest that EEG may be a more suitable modality for examining how taVNS modulates stress and brain activity.
In this study, we aimed to investigate the effects of taVNS on stress using both survey and EEG data. To achieve this, participants underwent either taVNS or sham stimulation during stress-inducing tasks. Resting-state EEG was recorded before and after each task, and self-report surveys were administered both before and after stimulation. Subsequently, power spectral density (PSD) and network indices were analyzed across three frequency bands—theta (4–7 Hz), alpha (8–13 Hz), and beta (14–30 Hz)—which have been consistently associated with stress in previous studies. In contrast, delta and gamma bands were not included in the analysis due to their inconsistent or limited relevance to stress-related EEG changes reported in the literature (40, 41).
2 Materials and methods
2.1 Participants
Fourteen healthy individuals initially participated in this study. However, due to technical issues during data collection, only 12 participants' data (7 males and 5 females; mean age = 24.83 ± 2.33 years) were ultimately included in the analysis. All participants had no history of neurological or psychiatric conditions that could have affected the study outcomes. Prior to the experiment, each participant was informed in detail about the experimental procedures and provided written informed consent. The study protocol was approved by the Institutional Review Board (IRB) of Korea University (KUIRB-2023-0281-05).
2.2 taVNS application
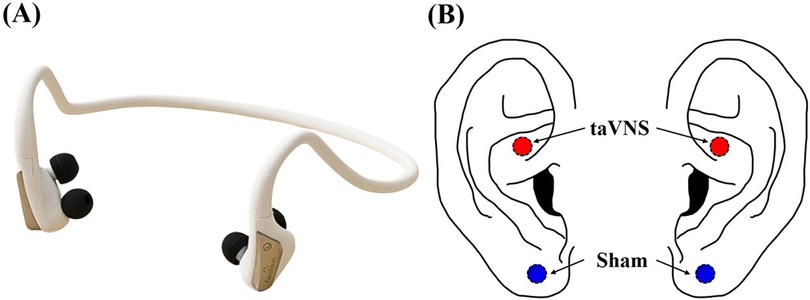
In this study, we employed a portable taVNS device equipped with two stimulation electrodes (Neurive, Co. Ltd., Gimhae, Republic of Korea) (Figure 1). Participants received either taVNS or sham stimulation in a randomized order, with a one-week interval between sessions. For taVNS, the electrodes were placed on the cymba conchae, where the vagus nerve is known to be present, whereas for sham stimulation, the electrodes were placed on the earlobe, which is considered devoid of vagus nerve innervation (Figure 1) (31, 42). During stimulation, the frequency was set to 30 Hz with a pulse width of 200 μs, and the protocol alternated 1-minute “on” periods of stimulation with 1-minute “off” (rest) periods (31, 43). Stimulation intensity was adjusted individually for each participant by gradually increasing from level 1 to level 10 until just below the participant's pain threshold (taVNS: 1.74 ± 0.06 mA, sham: 1.74 ± 0.04 mA). Our exclusion criterion was participants who reported pain even at the lowest intensity level (level 1), but no one met this condition.
2.3 Experimental protocol
To investigate the effects of taVNS on stress, we utilized two mental stress-inducing tasks: the 2-back task and the mental arithmetic (MA) task (44–46). The 2-back task included a total of 600 trials. In each trial, a number from 1 to 9 was displayed at the center of the screen for 500 ms, followed by a fixation cross for 1,500 ms (Figure 2B). Participants were instructed to press the right arrow key if the presented number matched the one shown two trials earlier, and the left arrow key if it did not. One-third of the trials were target trials. The MA task consisted of 40 trials, each involving repeated subtraction of a single-digit number from a three-digit number (Figure 2A). In each trial, the three-digit and single-digit numbers were displayed for 2 s, followed by a 10-second period for participants to perform repeated subtraction and enter their answer. A 0.5 s beep was provided at the start of every trial to maintain participants' focus. If no answer was submitted within 8 s, the system automatically proceeded to a rest period, marking the trial as failed and ensuring at least 8 s of rest. The order of the two stress-inducing tasks was counter-balanced across participants, and the same task order was maintained for each participant during two experimental sessions conducted one week apart. Participants received either taVNS or sham stimulation in a counter-balanced order to minimize potential sequence effects between the two tasks and stimulation conditions.
Prior to any stimulation, a 3 min resting-state EEG was recorded to capture baseline conditions (“baseline” in Figure 2C). After each stress-inducing task, another 3 min resting-state EEG was recorded to assess the modulatory effects of taVNS (“1st break” and “2nd break” in Figure 2C). Although EEG data were also collected during the two tasks (“1st task” and “2nd task” in Figure 2C), these data were excluded from analysis due to significant electrical interference introduced by the taVNS stimulation. Note that previous studies have indicated that taVNS-induced changes in EEG activity can persist from several minutes (47) up to approximately 30 min (48) post-stimulation, suggesting that the resting-state EEG immediately recorded after stimulation could still reflect taVNS effects. In addition, participants completed a self-report survey before and after stimulation to measure subjective stress levels. This survey used a visual analog scale ranging from 1 to 10, where higher numbers indicate greater stress.
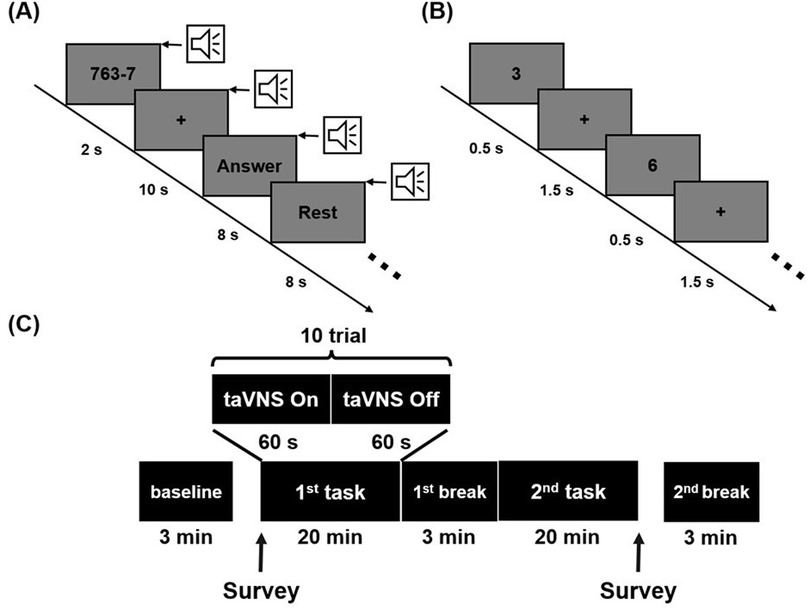
Figure 2. Experimental scheme illustrating (A) mental arithmetic task, (B) 2-back task and (C) the overall experimental paradigm. Stimulation was administered during each task, and resting-state EEG data were collected at baseline, the 1st break, and the 2nd break.
2.4 EEG recording and preprocessing
EEG data were recorded at a sampling rate of 1,000 Hz using 31 Ag/AgCl electrodes placed according to the international 10–20 system (Brain Products GmbH, Gilching, Germany). The reference and ground electrodes were positioned at FCz and AFz, respectively. Raw EEG signals were then downsampled to 200 Hz to reduce computational load. Common average referencing and 1–50 Hz band-pass filter were applied sequentially, and then independent component analysis (ICA) was performed to remove ocular and muscle artifacts. As baseline-based ICA evaluation was not applicable in our resting-state data (49), we instead applied a conservative component removal ratio, removing approximately one-third of the components on average (9.54 ± 0.89 out of 31), in line with previous research (50). The preprocessed EEG data were segmented into 5-second epochs, and any epoch exceeding ±75 μV in amplitude was excluded from power spectral density analysis (51). For the network analysis, segments identified as artifacts in any single channel were removed across all channels to maintain consistency in channel pairing. Consequently, artifact-free epochs were used for both PSD and network analyses. The total proportion of excluded epochs due to artifacts was approximately 0.45%.
2.5 PSD analysis
PSD was computed using short-time fast Fourier transform (FFT) with a 5 s window. PSD values were calculated for three frequency bands closely related to stress (52, 53): theta (4–7 Hz), alpha (8–13 Hz), and beta (14–30 Hz). To evaluate taVNS-driven stress modulation, the average PSD in each frequency band was computed specifically for the frontal electrodes (Fp1, Fp2, F3, F4, F7, F8), given their well-documented association with stress-related activity (54).
2.6 Network analysis
Network analysis was performed using imaginary coherence (iCoh) via the FieldTrip toolbox. iCoh was determined from spectral powers estimated by FFT with a single Hanning taper, ensuring a minimum of twenty cycles per frequency bin. While the PSD analysis focused on frontal channels closely linked to stress, the network analysis used all 31 electrodes to evaluate global brain network efficiency. Specifically, iCoh values were computed for each pair of the 31 electrodes across the theta, alpha, and beta frequency bands. From these iCoh matrices, we derived two global-level network indices—path length (PL) and clustering coefficient (CC)—using graph theoretical approaches. PL reflects the overall connectivity of the network, while CC indicates the clustering of neighboring nodes. All network computations were conducted using the Brain Connectivity Toolbox (BCT) in MATLAB.
2.7 Statistical analysis
As the data did not meet the assumptions of normality, non-parametric statistical methods were employed. To investigate taVNS-related changes in self-reported stress, Wilcoxon signed-rank tests were performed to compare pre- and post-stimulation survey scores. Additionally, the Friedman test was used to assess potential differences across baseline, the 1st break, and the 2nd break for both PSD and network indices. When significant effects were identified, post-hoc analyses were conducted using Wilcoxon signed-rank tests with false discovery rate (FDR) correction applied to account for multiple comparisons. Additionally, to complement the p-values and address concerns related to statistical power, we computed Wilcoxon effect sizes [r = |Z|/N; small: 0.1, medium: 0.3, large: 0.5] (55, 56).
3 Results
3.1 Stress level changes
Figure 3 illustrates both the individual and average changes in stress levels before and after stimulation for each condition, based on the self-report survey. In the sham condition, the mean stress level increased significantly after performing the task compared to before (r = 0.63, |Z| = 2.20, p < 0.05): seven participants reported increased stress levels, four showed no change, and one reported a decrease. In the taVNS condition, although the mean stress level also increased after the task, the change did not reach statistical significance (r = 0.52, |Z| = 1.80, p > 0.05): five participants reported increased stress, six reported no change, and one reported decreased stress. Note that there were no statistically significant differences in behavioral performance between taVNS and sham conditions for either task: MA accuracy (sham: 79.67 ± 3.11% vs. taVNS: 81.17 ± 3.72%) and average time required for one subtraction calculation (sham: 2.57 ± 0.22 s vs. taVNS: 2.61 ± 0.19 s); 2-back accuracy (sham: 83.36 ± 5.73% vs. taVNS: 83.26 ± 5.39%) and reaction time (sham: 658 ± 78 ms vs. taVNS: 648 ± 78 ms).
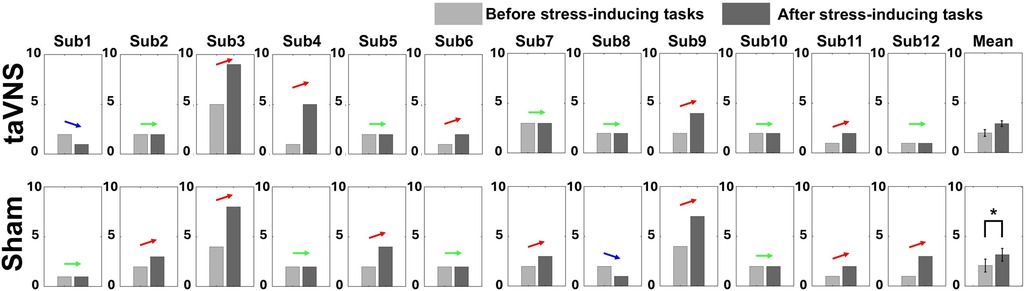
Figure 3. Changes in individual and averaged stress levels before and after two stress-inducing tasks for taVNS and sham conditions based on the self-report survey (*p < 0.05). Each bar graph represents the mean and standard error value.
3.2 PSD modulation
Figure 4 presents the average PSD changes for the three frequency bands (theta, alpha, and beta) in the frontal region, categorized by stimulation conditions. In the sham condition, theta and alpha PSDs decreased significantly from the 1st break to the 2nd break (theta: r = 0.86, |Z| = 2.98, p < 0.01, alpha: r = 0.79, |Z| = 2.75, p < 0.05), suggesting increased stress level, whereas no significant changes were observed in the taVNS condition. Neither the sham nor the taVNS condition showed significant changes in the beta band.
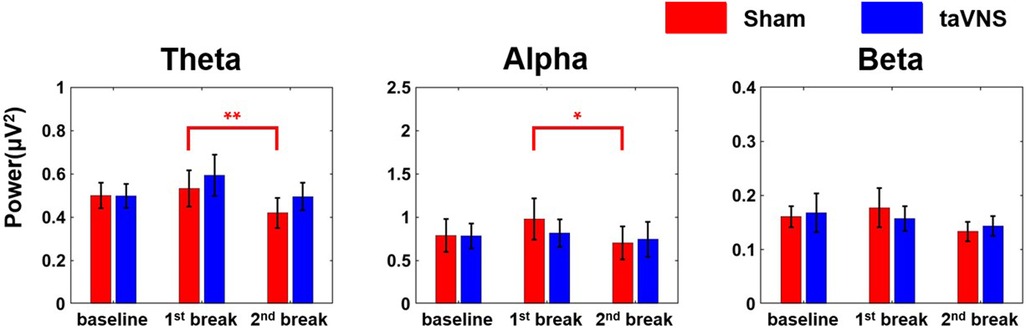
Figure 4. Changes in average PSD of each of the three frequency bands (theta, alpha, and beta) according to the stimulation condition in the frontal region (*p < 0.05, **p < 0.01). Each bar graph represents the mean and standard error value.
3.3 Network indices modulation
Figure 5 shows the average changes in path length (PL) and clustering coefficient (CC) for the three frequency bands across the whole brain under each stimulation condition. In the taVNS condition, PL in the theta band decreased significantly at the 2nd break compared to baseline (r = 0.77, |Z| = 2.67, p < 0.05), indicating enhanced global network efficiency. By contrast, no significant changes were found in the sham condition. Regarding CC, neither condition showed significant changes across any of the frequency bands.
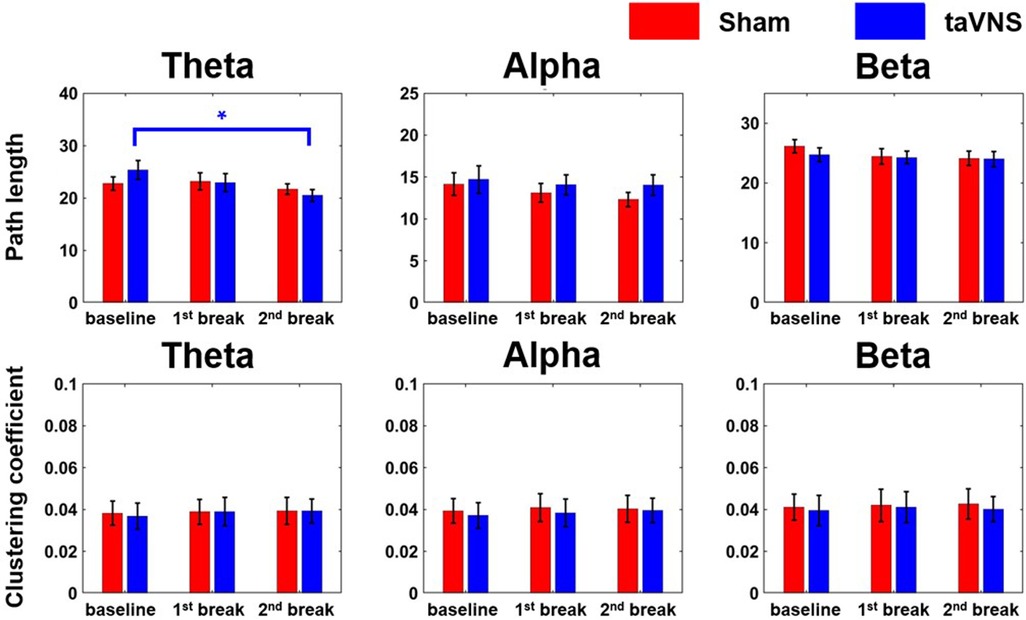
Figure 5. Changes in network indices (PL and CC) across the three frequency bands according to stimulation condition (*p < 0.05). Each bar graph represents the mean and standard error value.
4 Discussion
In this study, we investigated the effects of taVNS on stress using self-report surveys and resting-state EEG recordings immediately following stimulation, capturing taVNS-induced neural changes. The survey results indicated a significant increase in stress levels in the sham condition after the task, whereas no statistically significant difference was observed in the taVNS condition. This finding was further supported by the PSD results, which showed a significant reduction in theta and alpha power at the 2nd break compared to the 1st break only in the sham condition, indicating a significant in stress level. Additionally, path length (PL) in the theta band was significantly reduced at the 2nd break compared to baseline under the taVNS condition, meaning enhanced network efficiency. These findings are consistent with previous studies demonstrating the stress-regulating effects of taVNS through ECG-based measures (37, 38, 57, 58), suggesting its potential efficacy in stress modulation. Therefore, our results suggest neurological evidence that taVNS can help modulate or suppress stress responses.
Through PSD analysis, we found that theta and alpha power decreased significantly at the 2nd break compared to the 1st break in the sham condition only. Previous studies have reported reductions in theta and alpha PSD alongside increases in beta PSD under elevated stress (52, 54, 59). While our results are consistent with these findings in terms of theta and alpha bands, they do not exhibit the same pattern in the beta band. One possible explanation is the difference in data used for analysis: previous studies showing elevated beta PSD during higher stress typically analyzed data recorded during stress-inducing tasks (54), whereas our study focused on resting-state EEG recorded before and after stress tasks. Another reason may be the neurophysiological trait that theta and alpha band activity is more prominently observed in resting-state conditions (60, 61). Consequently, the selective changes seen in these bands can provide reasonable evidence of taVNS effect on stress, given the nature of our EEG data collection.
Moreover, we observed a significant decrease in theta PL under the taVNS condition in the 2nd break compared to baseline. For instance, Shim et al. reported lower theta PL in healthy subjects compared to patients with PTSD (35), implying that stress might reduce overall brain network efficiency. Consistent with this, other studies have found that CC and small-worldness were lower in high-workload conditions than in low-workload conditions (36), suggesting that stressful situations negatively impact brain network function. Consequently, our finding of reduced PL under taVNS during a stressed condition implies that taVNS may enhance network efficiency and potentially help prevent further increase in stress.
An important observation is that the most prominent changes in PSD and network indices were identified at the 2nd break. Because participants underwent two distinct tasks over 40 min by the time of the 2nd break—compared to only one 20-minute task at the 1st break—the 2nd break induced higher stress levels. In the PSD results, significant reductions in theta and alpha bands were found in the sham condition at the 2nd break compared to the 1st break. These findings suggest that effects of taVNS on stress may become more evident under higher stress levels.
While our study presents neurophysiological evidence supporting the stress-regulating effects of taVNS, the participant pool was primarily composed of individuals in their early to mid-20 s, which may limit the generalizability of our findings. Furthermore, the limited number of participants remains a concern even though we presented effect sizes to enhance the reliability of our results. Future research should include a larger and more diverse sample to validate the broader applicability of taVNS and to further optimize stimulation parameters (e.g., duration, frequency, and pulse width) as well as the design of the sham condition (e.g., sham target location) used as a control group for maximizing its impact on stress regulation. In addition, although the stress-regulating effect of taVNS observed in this study may contribute to stress management in patients with stress-related disorders, our findings were derived from healthy participants only. Given that the effect sizes of significant EEG outcomes were large, it is reasonable to assume potential clinical relevance. However, clinical significance was not directly evaluated in this study. Therefore, further studies involving more diverse populations—including different age groups and individuals with clinical conditions—are needed to confirm the generalizability and establish the clinical applicability of taVNS.
5 Conclusion
This study investigated the effects of transcutaneous auricular vagus nerve stimulation (taVNS) on stress regulation using EEG measures and self-report surveys. The self-report data revealed a significant increase in stress levels under the sham condition, but not under taVNS. Additionally, both power spectral density (PSD) and network-based EEG indices indicated that taVNS may effectively mitigate rising stress levels and enhance brain connectivity. Although these findings underscore the potential of taVNS for stress modulation and neuromodulation, further research is needed to refine our understanding. Future studies should include more diverse participant groups and optimization of taVNS parameters. Such efforts will provide deeper insight into the mechanisms underlying stress-regulating effects of taVNS and enhance its potential clinical or practical applications.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were approved by the Institutional Review Board (IRB) of Korea University (KUIRB-2023-0281-05). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
W-JK: Formal analysis, Conceptualization, Writing – review & editing, Writing – original draft. Y-SL: Conceptualization, Formal analysis, Writing – review & editing, Writing – original draft. KH: Resources, Conceptualization, Writing – review & editing. HC: Writing – review & editing, Conceptualization, Resources. J-JS: Conceptualization, Resources, Writing – original draft, Writing – review & editing, Supervision. H-JH: Writing – review & editing, Writing – original draft, Supervision, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the MSIT (Ministry of Science and ICT), Korea, under the ITRC (Information Technology Research Center) support program (IITP-2025-RS-2023-00258971, 50%) supervised by the IITP (Institute for Information & Communications Technology Planning & Evaluation) and the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. RS-2024-00455484, 50%).
Conflict of interest
KH, HC, J-JS were employed by Neurive Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ziegler MG. Chapter 61—psychological stress and the autonomic nervous system. In: Robertson D, Biaggioni I, Burnstock G, Low PA, Paton JFR, editors. in Primer on the Autonomic Nervous System. 3rd ed. San Diego: Academic Press (2012). p. 291–3. doi: 10.1016/B978-0-12-386525-0.00061-5
2. Sved AF, Cano G, Passerin AM, Rabin BS. The locus coeruleus, Barrington’s nucleus, and neural circuits of stress. Physiol Behav. (2002) 77:737–42. doi: 10.1016/S0031-9384(02)00927-7
3. Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, et al. The enduring effects of abuse and related adverse experiences in childhood. Eur Arch Psych Clin Neurosci. (2006) 256:174–86. doi: 10.1007/s00406-005-0624-4
4. Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB. Posttraumatic stress disorder: a state-of-the-science review. Eur Arch Psych Clin Neurosci. (2006) 40:1–21. doi: 10.1016/j.jpsychires.2005.07.005
5. Ballenger JC, Nutt DJ. Consensus statement on posttraumatic stress disorder from the international consensus group on depression and anxiety. J Clin Psychiatry. (2000) 61(Suppl 5):60–6. https://pubmed.ncbi.nlm.nih.gov/10761680/10761680
6. Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiat. (2006) 163:1905–17. doi: 10.1176/ajp.2006.163.11.1905
7. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. (2000) 342:314–9. doi: 10.1056/NEJM200002033420503
8. Bonaz B, Sinniger V, Pellissier S. The vagus nerve in the neuro-immune axis: implications in the pathology of the gastrointestinal tract. Front Immunol. (2017) 8:1452. doi: 10.3389/fimmu.2017.01452
9. Bonaz B, Sinniger V, Pellissier S. Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J Physiol. (2016) 594:5781–90. doi: 10.1113/JP271539
10. Wang W, Li R, Li C, Liang Q, Gao X. Advances in VNS efficiency and mechanisms of action on cognitive functions. Front Physiol. (2024) 15:1452490. doi: 10.3389/fphys.2024.1452490
11. Aaronson ST, Sears P, Ruvuna F, Bunker M, Conway CR, Dougherty DD, et al. A 5-year observational study of patients with treatment-resistant depression treated with vagus nerve stimulation or treatment as usual: comparison of response, remission, and suicidality. Am J Psychiatry. (2017) 174:640–8. doi: 10.1176/appi.ajp.2017.16010034
12. Rush AJ, George MS, Sackeim HA, Marangell LB, Husain MM, Giller C, et al. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study∗. Biol Psychiatry. (2000) 47:276–86. doi: 10.1016/S0006-3223(99)00304-2Asd
13. Ben-Menachem E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol. (2002) 1:477–82. doi: 10.1016/S1474-4422(02)00220-XAsd
14. Galbarriatu L, Pomposo I, Aurrecoechea J, Marinas A, Agúndez M, Gómez JC, et al. Vagus nerve stimulation therapy for treatment-resistant epilepsy: a 15-year experience at a single institution. Clin Neurol Neurosurg. (2015) 137:89–93. doi: 10.1016/j.clineuro.2015.06.023
15. Hays SA, Khodaparast N, Hulsey DR, Ruiz A, Sloan AM, Rennaker RL, et al. Vagus nerve stimulation during rehabilitative training improves functional recovery after intracerebral hemorrhage. Stroke. (2014) 45:3097–100. doi: 10.1161/STROKEAHA.114.006654
16. Pruitt DT, Danaphongse TT, Lutchman M, Patel N, Reddy P, Wang V, et al. Optimizing dosing of vagus nerve stimulation for stroke recovery. Transl Stroke Res. (2021) 12:65–71. doi: 10.1007/s12975-020-00829-6
17. Carreno FR, Frazer A. Vagal nerve stimulation for treatment-resistant depression. Neurotherapeutics. (2017) 14:716–27. doi: 10.1007/s13311-017-0537-8
18. Ben-Menachem E, Mañon-Espaillat R, Ristanovic R, Wilder BJ, Stefan H, Mirza W, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. Epilepsia. (1994) 35:616–26. doi: 10.1111/j.1528-1157.1994.tb02482.x
19. George MS, Rush AJ, Marangell LB, Sackeim HA, Brannan SK, Davis SM, et al. A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biol Psychiatry. (2005) 58:364–73. doi: 10.1016/j.biopsych.2005.07.028
20. Rong P, Liu J, Wang L, Liu R, Fang J, Zhao J, et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. J Affect Disord. (2016) 195:172–9. doi: 10.1016/j.jad.2016.02.031
21. Bauer S, Baier H, Baumgartner C, Bohlmann K, Fauser S, Graf W, et al. Transcutaneous vagus nerve stimulation (tVNS) for treatment of drug-resistant epilepsy: a randomized, double-blind clinical trial (cMPsE02). Brain Stimul. (2016) 9:356–63. doi: 10.1016/j.brs.2015.11.003
22. Gurel NZ, Wittbrodt MT, Jung H, Shandhi MMH, Driggers EG, Ladd SL, et al. Transcutaneous cervical vagal nerve stimulation reduces sympathetic responses to stress in posttraumatic stress disorder: a double-blind, randomized, sham controlled trial. Neurobiol Stress. (2020) 13:100264. doi: 10.1016/j.ynstr.2020.100264Asd
23. Borgmann D, Rigoux L, Kuzmanovic B, Edwin Thanarajah S, Münte TF, Fenselau H, et al. Technical note: modulation of fMRI brainstem responses by transcutaneous vagus nerve stimulation. Neuroimage. (2021) 244:118566. doi: 10.1016/j.neuroimage.2021.118566
24. Keatch C, Lambert E, Woods W, Kameneva T. Measuring brain response to transcutaneous vagus nerve stimulation (tVNS) using simultaneous magnetoencephalography (MEG). J Neural Eng. (2022) 19:026038. doi: 10.1088/1741-2552/ac620c
25. Höper S, Kaess M, Koenig J. Prefrontal cortex oxygenation and autonomic nervous system activity under transcutaneous auricular vagus nerve stimulation in adolescents. Auton Neurosci Basic Clin. (2022) 241:103008. doi: 10.1016/j.autneu.2022.103008
26. Keute M, Barth D, Liebrand M, Heinze H-J, Kraemer U, Zaehle T. Effects of transcutaneous vagus nerve stimulation (tVNS) on conflict-related behavioral performance and frontal midline theta activity. J Cogn Enhance. (2020) 4:121–30. doi: 10.1007/s41465-019-00152-5
27. Kang D, Choi Y, Lee J, Park E, Kim IY. Analysis of taVNS effects on autonomic and central nervous systems in healthy young adults based on HRV. EEG Parameters J Neural Eng. (2024) 21:046012. doi: 10.1088/1741-2552/ad5d16
28. Bömmer T, Schmidt LM, Meier K, Kricheldorff J, Stecher H, Herrmann CS, et al. Impact of stimulation duration in taVNS—exploring multiple physiological and cognitive outcomes. Brain Sci. (2024) 14:875. doi: 10.3390/brainsci14090875
29. Poppa T, Benschop L, Horczak P, Vanderhasselt M-A, Carrette E, Bechara A, et al. Auricular transcutaneous vagus nerve stimulation modulates the heart-evoked potential. Brain Stimul. (2022) 15:260–9. doi: 10.1016/j.brs.2021.12.004
30. Ricci L, Croce P, Lanzone J, Boscarino M, Zappasodi F, Tombini M, et al. Transcutaneous vagus nerve stimulation modulates EEG microstates and dlta activity in healthy subjects. Brain Sci. (2020) 10:668. doi: 10.3390/brainsci10100668
31. Lee Y-S, Kim W-J, Shim M, Hong KH, Choi H, Song J-J, et al. Investigating neuromodulatory effect of transauricular vagus nerve stimulation on resting-state electroencephalography. Biomed Eng Lett. (2024) 14:677–87. doi: 10.1007/s13534-024-00361-8
32. Yi Wen T, Mohd Aris SA. Electroencephalogram (EEG) stress analysis on alpha/beta ratio and theta/beta ratio. Indones J Electr Eng Comput Sci. (2020) 17:175. doi: 10.11591/ijeecs.v17.i1.pp175-182
33. Lin A, Liu KKL, Bartsch RP, Ivanov PC. Dynamic network interactions among distinct brain rhythms as a hallmark of physiologic state and function. Commun Biol. (2020) 3:197. doi: 10.1038/s42003-020-0878-4
34. Yan B, Wang Y, Yang Y, Wu D, Sun K, Xiao W. EEG Evidence of acute stress enhancing inhibition control by increasing attention. Brain Sci. (2024) 14:1013. doi: 10.3390/brainsci14101013
35. Shim M, Im C-H, Lee S-H. Disrupted cortical brain network in post-traumatic stress disorder patients: a resting-state electroencephalographic study. Transl Psychiatr. (2017) 7:e1231–e1231. doi: 10.1038/tp.2017.200
36. Dimitrakopoulos GN, Kakkos I, Anastasiou A, Bezerianos A, Sun Y, Matsopoulos GK. Cognitive reorganization due to mental workload: a functional connectivity analysis based on working memory paradigms. Appl Sci. (2023) 13:2129. doi: 10.3390/app13042129
37. Ylikoski J, Markkanen M, Pirvola U, Lehtimäki JA, Ylikoski M, Jing Z, et al. Stress and tinnitus; transcutaneous auricular vagal nerve stimulation attenuates tinnitus-triggered stress reaction. Front Psychol. (2020) 11:570196. doi: 10.3389/fpsyg.2020.570196
38. Tobaldini E, Toschi-Dias E, Appratto de Souza L, Rabello Casali K, Vicenzi M, Sandrone G, et al. Cardiac and peripheral autonomic responses to orthostatic stress during transcutaneous vagus nerve stimulation in healthy subjects. J Clin Med. (2019) 8:496. doi: 10.3390/jcm8040496
39. Sanchez-Perez JA, Gazi AH, Rahman FN, Seith A, Saks G, Sundararaj S, et al. Transcutaneous auricular vagus nerve stimulation and median nerve stimulation reduce acute stress in young healthy adults: a single-blind sham-controlled crossover study. Front Neurosci. (2023) 17:1213982. doi: 10.3389/fnins.2023.1213982
40. Tran Y, Thuraisingham RA, Wijesuriya N, Nguyen HT, Craig A. Detecting neural changes during stress and fatigue effectively: a comparison of spectral analysis and sample entropy. In: 2007 3rd International IEEE/EMBS Conference on Neural Engineering IEEE (2007). p. 350–53
41. Vaquero-Blasco MA, Perez-Valero E, Lopez-Gordo MA, Morillas C. Virtual reality as a portable alternative to chromotherapy rooms for stress relief: a preliminary study. Sensors. (2020) 20(21):6211. doi: 10.3390/s20216211
42. Yakunina N, Kim SS, Nam E-C. Optimization of transcutaneous vagus nerve stimulation using functional MRI. Neuromodulation. (2017) 20:290–300. doi: 10.1111/ner.12541
43. Gianlorenço AC, Pacheco-Barrios K, Daibes M, Camargo L, Choi H, Song J-J, et al. Age as an effect modifier of the effects of transcutaneous auricular vagus nerve stimulation (taVNS) on heart rate variability in healthy subjects. J Clin Med. (2024) 13:4267. doi: 10.3390/jcm13144267
44. Singh N, Aggarwal Y, Sinha RK. Heart rate variability analysis under varied task difficulties in mental arithmetic performance. Health Technol. (2019) 9:343–53. doi: 10.1007/s12553-018-0272-0
45. Wang X, Liu B, Xie L, Yu X, Li M, Zhang J. Cerebral and neural regulation of cardiovascular activity during mental stress. Biomed Eng Online. (2016) 15:160. doi: 10.1186/s12938-016-0255-1
46. Grässler B, Dordevic M, Darius S, Vogelmann L, Herold F, Langhans C, et al. Age-related differences in cardiac autonomic control at resting state and in response to mental stress. Diagnostics. (2021) 11:2218. doi: 10.3390/diagnostics11122218
47. Gianlorenco AC, Pacheco-Barrios K, Camargo L, Pichardo E, Choi H, Song J-J, et al. Understanding the effects of non-invasive transauricular vagus nerve stimulation on EEG and HRV. J Vis Exp. (2024) 203:e66309. doi: 10.3791/66309
48. Gurtubay IG, Perez-Rodriguez DR, Fernandez E, Librero-Lopez J, Calvo D, Bermejo P, et al. Immediate effects and duration of a short and single application of transcutaneous auricular vagus nerve stimulation on P300 event related potential. Front Neurosci. (2023) 17:1096865. doi: 10.3389/fnins.2023.1096865
49. Arad E, Bartsch RP, Kantelhardt JW, Plotnik M. Performance-based approach for movement artifact removal from electroencephalographic data recorded during locomotion. PLoS One. (2018) 13(5):e0197153. doi: 10.1371/journal.pone.0197153
50. Keding O, Alickovic E, Skoglund MA, Sandsten M. Novel bias-reduced coherence measure for EEG-based speech tracking in listeners with hearing impairment. Front Neurosci. (2024) 18:1415397. doi: 10.3389/fnins.2024.1415397
51. Perez-Valero E, Vaquero-Blasco MA, Lopez-Gordo MA, Morillas C. Quantitative assessment of stress through EEG during a virtual reality stress-relax session. Front Comput Neurosci. (2021) 15:684423. doi: 10.3389/fncom.2021.684423
52. Alonso JF, Romero S, Ballester MR, Antonijoan RM, Mañanas MA. Stress assessment based on EEG univariate features and functional connectivity measures. Physiol Meas. (2015) 36:1351. doi: 10.1088/0967-3334/36/7/1351
53. Sharon O, Fahoum F, Nir Y. Transcutaneous vagus nerve stimulation in humans induces pupil dilation and attenuates alpha oscillations. J Neurosci. (2021) 41:320–30. doi: 10.1523/JNEUROSCI.1361-20.2020
54. Al-Shargie F, Kiguchi M, Badruddin N, Dass SC, Hani AFM, Tang TB. Mental stress assessment using simultaneous measurement of EEG and fNIRS. Biomed Opt Express. (2016) 7:3882–98. doi: 10.1364/BOE.7.003882
55. Cooper H, Hedges LV. The Handbook of Research Synthesis. New York: Russell Sage Foundation (1993). p. 231–44.
56. Tomczak M, Tomczak E. The need to report effect size estimates revisited. An overview of some recommended measures of effect size. TRENDS Sport Sci. (2014) 1(21):19–25. https://www.wbc.poznan.pl/Content/325867/5_Trends_Vol21_2014_%20no1_20.pdf
57. Geng D, Liu X, Wang Y, Wang J. The effect of transcutaneous auricular vagus nerve stimulation on HRV in healthy young people. PLoS One. (2022) 17:e0263833. doi: 10.1371/journal.pone.0263833
58. Cuberos Paredes E, Goyes D, Mak S, Yardimian R, Ortiz N, McLaren A, et al. Transcutaneous auricular vagus nerve stimulation inhibits mental stress-induced cortisol release-potential implications for inflammatory conditions. Physiol Rep. (2025) 13:e70251. doi: 10.14814/phy2.70251
59. Gärtner M, Rohde-Liebenau L, Grimm S, Bajbouj M. Working memory-related frontal theta activity is decreased under acute stress. Psychoneuroendocrinology. (2014) 43:105–13. doi: 10.1016/j.psyneuen.2014.02.009
60. Palacios-García I, Silva J, Villena-González M, Campos-Arteaga G, Artigas-Vergara C, Luarte N, et al. Increase in beta power reflects attentional top-down modulation after psychosocial stress induction. Front Hum Neurosci. (2021) 15:630813. doi: 10.3389/fnhum.2021.630813
Keywords: transauricular vagus nerve stimulation (taVNS), stress, electroencephalography (EEG), power spectral density (PSD), brain network
Citation: Kim W-J, Lee Y-S, Hong KH, Choi H, Song J-J and Hwang H-J (2025) Effect of transcutaneous auricular vagus nerve stimulation on stress regulation: an EEG and questionnaire study. Front. Digit. Health 7:1593614. doi: 10.3389/fdgth.2025.1593614
Received: 21 March 2025; Accepted: 6 August 2025;
Published: 1 September 2025.
Edited by:
Oswald David Kothgassner, Medical University of Vienna, AustriaReviewed by:
Ronny P. Bartsch, Bar-Ilan University, IsraelGülşah Konakoğlu, Gelisim University, Türkiye
Copyright: © 2025 Kim, Lee, Hong, Choi, Song and Hwang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Han-Jeong Hwang, aHdhbmdoakBrb3JlYS5hYy5rcg==; Jae-Jun Song, ampzb25nMjNAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Woo-Jin Kim
Woo-Jin Kim Yun-Sung Lee
Yun-Sung Lee Ki Hwan Hong3
Ki Hwan Hong3 Hyuk Choi
Hyuk Choi Jae-Jun Song
Jae-Jun Song Han-Jeong Hwang
Han-Jeong Hwang