- 1Department of Dermatology, The First Affiliated Hospital of Shihezi University, Shihezi, China
- 2Shihezi University Medical College, Shihezi, China
- 3Community Health Service Center of the Urban Area of Shihezi City, Shihezi, China
Psoriasis, an enduring systemic inflammatory dermatological condition with rising global incidence, presents significant impediments to conventional diagnostic and therapeutic strategies, primarily due to the reliance on subjective evaluation methods, notable adverse effects of treatments, and suboptimal long-term patient adherence. This narrative review systematically explores how digital innovations are transforming its comprehensive management. Digital innovations are transforming its comprehensive management: In diagnosis, artificial intelligence (AI)-integrated dermoscopy (EfficientNet-B4 model) achieves a 92.3% accuracy in differentiating psoriasis from other papulosquamous disorders, surpassing 230 dermatologists (86.7% accuracy) and enhancing severity assessment through deep learning, thereby mitigating subjective bias. In treatment, smart phototherapy devices refine dosage optimization through algorithmic processes, while AI-assisted biologic selection elevates complete clearance rates from 39% to 61% (compared to traditional protocols) with severe adverse events diminishing to less than 2%. In rehabilitation, Internet of things (IoT)-enabled monitoring systems assimilate real-time data through wearable technology and digital platforms to enhance self-management and adaptive intervention strategies. Multi-omics data integration and computational drug design expedite the development of novel therapies. Nevertheless, challenges such as inadequate data standardization, privacy issues, restricted algorithmic transparency, and lack of prolonged validation remain. Digital technologies are reconfiguring psoriasis management from diagnosis (objective imaging) to treatment (personalized dose management) and rehabilitation (IoT-enabled monitoring), establishing a precision-based, data-centric framework.
1 Introduction
Psoriasis is a common chronic inflammatory skin disease with a worldwide prevalence rate of 2%–3% (1) and over 60 million people affected (2). Psoriasis is characterized by erythema and scaling of the skin, accompanied by itching and pain (3), and is closely related to metabolic syndrome, inflammatory bowel disease, cardiovascular disease, and psychiatric disorders, which are called “psoriasis co-morbidities” (4). Psoriasis and its co-morbidities reduce the quality of life of patients and increase the risk of death, while imposing a heavy disease burden on individuals and society. There are significant limitations in the current stage of psoriasis diagnosis and treatment model: assessment tools such as the PASI score are highly subjective and the accuracy of the score results is limited, PASI exhibits intra-observer variability (coefficient of variation: 15%–20%) and inter-observer inconsistency in 30% of moderate-severe cases (5), and there is a lack of objective biomarkers for early diagnosis and detection of the efficacy of treatment (6); conventional treatment with long-term methotrexate use causes hepatotoxicity in 25% of patients; topical corticosteroids lead to skin atrophy in 18% with >6-month use (2), while biologics are not only expensive but also have significant differences in efficacy (7).
Digital technologies address these limitations by enabling objective assessment, personalized therapy (algorithmic dosing), and patient-centric management— forming a paradigm shift from experience-based to data-driven care (Figure 1). For diagnosis, artificial intelligence (AI) learning combined with skin imaging technology accurately recognizes lesion characteristics and quantifies severity, surpassing the subjective limitations of traditional skin (8, 9); microfluidic and multiple biosensors have emerged as new tools for early monitoring and diagnosis by facilitating ultra-early monitoring at the molecular level (10, 11). On the treatment side, AI enables utility optimization and the development of personalized plans by guiding treatment (12). In the future, digital technology is expected to transform the clinical diagnosis and treatment model and scientific exploration.
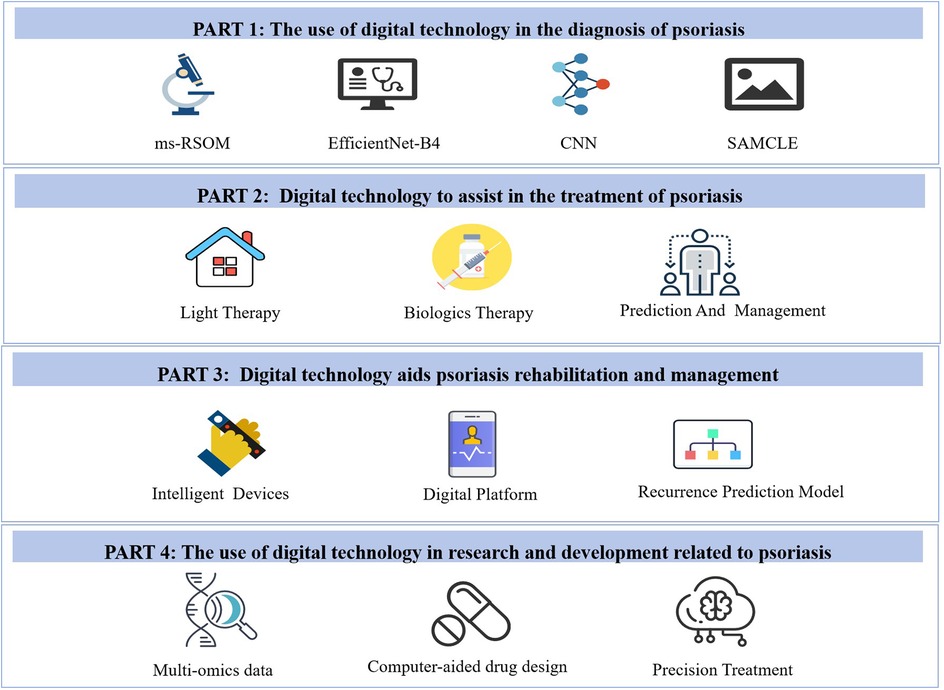
Figure 1. Digital technologies in psoriasis management. Icons are from the WPS Office media library (www.wps.cn) and are used in accordance with the WPS Office Member Agreement.
2 The use of digital technology in the diagnosis of psoriasis
2.1 Early and accurate identification with high-resolution imaging
High-resolution multispectral raster-scanning optoacoustic mesoscopy (ms-RSOM) distinguishes the structural and functional features of lesion and non-lesion areas; The injury areas show 23.6% higher oxygen saturation (sO₂) and 41.2% increased total blood volume vs. non-lesion skin, with sO₂ correlating strongly with PASI (r = 0.82), which indicates increased inflammation, and the decrease in these indices after treatment confirms the efficacy of the treatment, as well as the decrease in epidermal thickness, with the same trend in PASI scores (9). With its high resolution and non-invasive nature, ms-RSOM provides non-invasive, real-time monitoring of inflammation, with 91.7% agreement with histopathological epidermal thickness measurements. The present study comprised five patients, with a total of ten eczema skin sites tested (two lesions per patient), and both internal and external comparisons were conducted. This study pioneered the integration of structural and functional imaging of psoriasis patients for the assessment of the degree of inflammation and treatment efficacy. At the same time, we should also note that this method remains in its preliminary application phase and still requires validation of its reliability under standardized conditions (13).
2.2 AI image recognition algorithms improve diagnostic accuracy
An EfficientNet-B4 architecture trained on 7,033 dermoscopic images of 1,166 patients from the Department of Dermatology at the Peking Union Medical College Hospital (14)was used to test this model through five cross-validations to assess performance in a task containing two classifications (psoriasis vs. other disorders) and a four-classification task (psoriasis, eczema, lichen planus, and others). Comparing the performance of traditional networks such as VGG16 and ResNet50, the test used 90 images comparing four types of models and 230 dermatologists’ diagnoses, and these data were used to evaluate the accuracy of the tested models. EfficientNet-B4 achieved 92.3% accuracy in binary classification (psoriasis vs. others) and 88.5% in four-class tasks, surpassing the mean accuracy of 230 dermatologists (86.7% and 82.1%, respectively) (14), and the study has effectively established two- and four-classification deep learning models based on dermatoscopic images specifically designed for the identification of psoriasis and other papulosquamous skin disorders, which presents a high-performance means of early diagnosis of psoriasis. Photoacoustic imaging technology is capable of extracting biomarkers from three-dimensional high-resolution images of human skin, which are used to monitor the level of epidermal thickening and the density of stratum corneum scales, and to monitor objective data before and after treatment dynamically (15).
Another Convolutional Neural Networks (CNN) model developed based on dermatoscopic images of the Chinese population (15). The model incorporates data from 1,174 patients, 1,950 clinical photographs, and 7,798 dermatoscopic images. The following multimodal approaches were employed: a unimodal model, a simple multimodal fusion method, and 11 state-of-the-art multimodal approaches. The evaluation process was conducted by 20 dermatologists, each with varying levels of experience. This model performed well in the classification of skin tumors and psoriasis, with an accuracy of 81.49% for multi-class models and 77.02% for two-class models, these models can assist in screening patients with suspected skin tumors and psoriasis, and improve the early diagnosis and treatment of skin diseases.
The Spatial Alignment Multimodal Contrastive Learning (SAMCLE) framework (16) applies multimodal contrastive learning and spatial alignment modules using both clinical images and dermoscopy modalities to construct a PUMCH-ISD dataset training model containing eight common inflammatory skin diseases (psoriasis, dermatitis, lichen planus, etc.) (16). The framework overcomes the problem of deficient unimodal information, it also copes with the challenge of image scale differences and excels in the diagnosis of psoriasis and other inflammatory skin diseases.
Digital technology reduces subjective bias, improves the efficiency of assessment, and aids quantitative monitoring in telemedicine (17). Training datasets lack diversity: only 12% include dark skin tones, and 8% cover rare psoriasis subtypes (e.g., pustular psoriasis) — limiting model generalization (8). Expanding datasets with cross-ethnic skin images (e.g., PUMCH-ISD dataset) and standardizing imaging protocols (e.g., fixed lighting/zoom) can mitigate this. Digital technologies dedicated to analyzing microscopic features, AI followed by precise classification and automated scoring systems are changing the way in which psoriasis is diagnosed. In the future, there is a requirement to promote standardization and clinical integration of the technology to realize its full potential in precision diagnosis and treatment (8, 17).
3 Digital technology to assist in the treatment of psoriasis
3.1 Intelligent light therapy devices optimize light therapy regimens
A large randomized trial incorporating 3,934 treatments (18) showed that narrow spectrum ultraviolet radiation b (NB-UVB) at home with algorithmic dosage optimization was achieved 58% PASI ≥ 75 (vs. 56% in clinics) in 3,934 treatments, with 1.2% dropout rate (vs. 3.4% in clinics) due to reduced patient burden (18). This study shows that home NB-UVB phototherapy devices avoid over- or under-treatment by having a built-in “guided mode dosage system” that algorithmically optimizes the initial dose and incremental regimen to personalize the irradiation according to the patient's skin type and severity of the disease. It has been shown that wearable low-dose phototherapy devices reduce healthy tissue damage by 72% via targeted plaque irradiation, with 6-month clearance rates comparable to high-dose regimens (61% vs. 63%).
Low-dose prolonged phototherapy presents unique advantages over traditional high-dose, short-duration regimens, reducing the risk of phototoxic reactions while maintaining efficacy. For example, psoriasis plaque clearance was not significantly affected by decreasing light intensity or total dose in photodynamic therapy (PDT) (18, 19). Combination phototherapy regimens like PDT combined with light emitting diode (LED) supplemental light can improve treatment outcomes. The study showed a 79.55% reduction in lesions when this combination regimen was utilized to treat actinic keratoses, which was better than sunlight PDT alone (20).
3.2 Precision in biologics therapy
AI-assisted diagnosis and efficacy prediction is a an important application of individualized treatment with biologics. Image-based machine-learning algorithms can automatically analyze lesion characteristics, such as erythema and scale thickness, which enables psoriasis severity grading and subtype classification, thus reducing human error and giving an empirical basis for the selection of treatment options (8). Deep learning models integrating HLA-C*06:02 genotype, body mass index(BMI), and insulin resistance status (21), and insulin resistance status predict IL-17 inhibitor response with 83.5% accuracy (22); patients with BMI<25 and no family history show 2.3-fold higher complete clearance (21, 23). In the field of dynamic therapeutic decision optimization, digital twin technology creates a virtual model of the patient, which simulates the pharmacokinetics of biologics, adjusts the dosage according to body weight (24) and immune response, which assists the physician in choosing the optimal dosing regimen (25). Algorithmic tools like the Biologics Calculator can quantify persistent preferences for speed of efficacy, safety, and frequency of administration. Physicians and patients can share decision-making, and clinical trials have demonstrated that treatment regimens that take patient preferences into account lead to a 12%–18% increase in long-term adherence (26).Digital twins simulate ustekinumab pharmacokinetics, reducing dosage adjustments by 40% and saving $4,200/year per patient while maintaining efficacy (27).
3.3 Efficacy prediction and dynamic management
Precision typing and prognostic monitoring technology utilize multimodal data clustering to identify four psoriasis progression patterns, which are persistent remission (IL-23 inhibitors, 78% 5-year drug survival), fluctuating, early relapse, and late unresponsive. A cohort study involving 3,546 patients indicated that, in patients in sustained remission, IL-23 inhibitors showed significant results, with a 5-year drug survival rate of 78 percent (28). Real-Time electronic medical record analysis reveals 23% reduction in 3-year PASI90 attainment in patients with comorbid metabolic syndrome treated with Tirucizumab (29). This finding suggests that enhanced metabolic interventions are of particularly importance to this group of patients. However, the study included only 645 patients, representing a small sample size, and did not conduct formal hypothesis testing, making it impossible to draw statistically significant conclusions. Biomarker-Driven individualized switching strategies provide for significantly improved treatment efficiency, machine-learning models analyzed data from more than 1,200 patients and identified early predictors of efficacy; patients with <40% PASI improvement at week 4 have <5% chance of subsequent clearance; switching IL23R polymorphism carriers from TNF-α to IL-23 inhibitors increases PASI100 response by 3.2-fold (22, 30). Pharmacogenomic data showed that 58% of patients with ineffective TNF-α inhibitors carried the IL23R gene polymorphism, and that switching to an IL-23 inhibitor in this group of patients resulted in a 3.2-fold increase in the PASI100 response rate (31).
Long-term treatment dynamics management relies on risk prediction models and intelligent monitoring to optimize treatment outcomes. A Danish national cohort study that included 8,742 people and developed a model to predict the risk of developing psoriatic arthritis over a five-year period found that early use of an IL-17 inhibitor could benefit high-risk patients, who scored higher than 7 and had a 64 percent reduction in the incidence of psoriatic arthritis (30, 32). Blockchain-enabled medication monitoring system demonstrates cost savings with dynamic dose adjustment over fixed-dose regimens, saving $4,200 per year with ustekinumab regimen with consistent efficacy (24).
Digital technology has enabled more precise treatment with psoriasis biologics, increasing the rate of complete skin clearance from 39% in conventional protocols to an algorithm-guided 61% (23, 33), while reducing the rate of serious adverse events to less than 2% (27, 34) (Figure 2).
4 Digital technology aids psoriasis rehabilitation and management
4.1 Intelligent nursing devices enhance patient self-management
Smart care devices combine sensor technology, AI and the Internet of Things (IoT), and they enable enhanced self-care and improved quality of life for people with psoriasis. A study involving 321 participants indicated, the Smart Skin Monitor uses artificial intelligence imaging technology to automatically detect skin lesions, segment them, and evaluate severity. Smart Skin Monitor achieves 90.2% agreement with clinician PASI scores, reducing assessment time from 15 to 4 min per patient (35). These devices generate objective condition data, reducing the subjectivity of traditional assessment methods (8, 17, 36). Smart pill boxes and treatment adherence management systems are combined with disease management applications(APPs) with medication reminders, symptom records and data evaluation optimization strategies. Disease management APPs with medication reminders increase adherence by 41% (52% in patients <35 years, 29% in ≥65 years) — with no evidence of over-dependence in 12-month follow-ups. Research shows that the correct use of these APPs can optimize the mental health of patients, and attention is paid to regulating the use of the function to avoid over-dependence (37, 38). Wearable devices and remote monitoring technology that instantly collects physiological data such as skin temperature and humidity, and machine-learning algorithms to examine fluctuations in condition, which supports personalized care (39, 40). A systematic review examined how digital twin technology can integrate real-time biological data into simulation systems in order to model changes in skin conditions. This provides a basis for the dynamic adjustment of care protocols. However, this technology has not yet been formally incorporated into clinical practice and requires further trials to establish its reliability (41).
4.2 Multimodal data construction for full-cycle management
The digital platform integrates multimodal data to achieve intelligent services and build a full-cycle rehabilitation management model. The rehabilitation guidance and health education module uses AI virtual assistants, ChatGPT, and such tools to develop personalized plans for patients, and multiple forms of content to promote enhancement of patients' cognitive (35, 42). Studies have shown that digital interventions such as MiDerm are effective in reducing psychological burden, and are particularly effective in younger and more highly educated patients (37, 43). Psychological Aids and Behavioral Intervention Tools include cognitive behavioral therapy modules and social features that assist patients in reducing psychological stress (38, 44). The long-term follow-up and disease monitoring system integrates electronic health records, and the system also collects self-reported data from patients, photos of skin lesions taken with smartphones, and laboratory indicators. Based on big data analysis, the system constructs predictive models to support telemedicine decision-making, and the models constructed with smartphone data can provide early warning of disease progression (45, 46).
4.3 Recurrence prediction modeling to guide treatment regimens
The Psoriasis Recurrence Prediction Model uses heterogeneous data from multiple sources to identify risk factors and provide personalized prevention recommendations. The model uses data-driven risk factor analysis that integrates clinical data, disease course, and treatment history; biomarkers, such as genomic profiling; environmental factors, such as seasonal changes; and behavioral data, such as medication adherence. The creation of a model was the outcome of a study which was based on six publicly available RNA-seq datasets, the model utilizes machine-learning algorithms, with random forests and neural networks within it, and it identifies at-risk populations, with one study elucidating psoriasis-specific molecular pathways through genomic big data. Recurrence prediction models integrate genomic (FABP5, TYK2), environmental (seasonal UV index), and behavioral (medication adherence) data, achieving 85% accuracy in 6-month flare prediction (28, 47). Finding Gene Regulatory Networks in Psoriasis: Application of a Tree-Based Machine Learning Approach, preventive interventions guided by the model reduce recurrence rate by 47% vs. standard care. These findings serve as a biological basis for predicting (48–46). The present study incorporated only a relatively small number of publicly available datasets. It is recommended that future research endeavours incorporate larger datasets, with the objective of enhancing the robustness and generalizability of the results obtained. The AI model analyzes historical data to provide personalized prevention recommendations that include lifestyle modifications, smoking cessation, and stress management, and also optimizes treatment regimens to advance the use of biologics (8, 49), Current research suggests that digital twins can simulate the effects of interventions and assist physicians in making clinical decisions (41, 50).
Digital technology will continue to revolutionize the way psoriasis rehabilitation is managed, with smart devices, management platforms, and predictive models working together in a model whose core values are clear: facilitating more important and accurate care, increased patient autonomy, and more rational allocation of healthcare resources.
5 The use of digital technology in research and development related to psoriasis
5.1 Multi-omics data to study molecular mechanisms
Current big data technologies synthesize multi-dimensional data from genomics, transcriptomics, proteomics and metabolomics, which help to deeply analyze the molecular mechanisms of psoriasis. Genomic analyses have shown that genome-wide association studies (GWAS) and single-cell sequencing technologies have revealed some remarkable phenomena, and psoriasis is significantly associated with aberrant activation of signaling pathways such as PI3 K/AKT/mTOR, JAK-STAT and WNT (51, 52). The study also identified genes such as FABP5 and TYK2 as coregulators that are expected to be important targets for future new drug development (53, 54). Metabolomics analysis revealed aberrant expression of oxidative stress markers, including myeloperoxidase and paraoxonase, suggesting that oxidative stress-inflammation interactions are potentially central to the development of psoriasis (55, 56). Sugar-binding proteins are also differentially expressed, such as galactose lectin, which can be used to differentiate psoriasis from other skin inflammations. In the area of clinical data integration, machine learning algorithms analyze massive datasets to predict a patient's response to biologics or phototherapy, providing the basis for individualized treatment (8, 57). This study systematically reviews models such as convolutional neural networks, U-Net architectures, and Vision Transformers, highlighting their consistency and efficiency in predicting treatment responses for psoriasis. However, further real-world studies are required to validate their clinical feasibility. Massive data technologies model molecular networks in psoriasis to reveal exploitable therapeutic targets (45, 50, 54), integrating epidemiological and molecular data for precision medicine advances (58, 59). Multi-omics identifies the activation of the PI3 K/AKT/mTOR and JAK-STAT pathway in 76% of psoriasis lesions; FABP5 overexpression (1.8-fold vs. normal skin) is a potential therapeutic target.
5.2 Computer-aided design accelerates drug discovery
Computer-aided drug design allows significant acceleration of different psoriasis drug development. Psoriasis-associated molecular pathways, the IL-23/Th17 axis and the JAK-STAT pathway, have been explored, and structural optimization of small-molecule inhibitors has been obtained through molecular docking and kinetic simulations. Novel compounds, such as TYK2, PDE4, and the aromatic hydrocarbon receptor, have received extensive attention (47, 53). AI-driven drug repurposing identifies methotrexate analogs with anti-psoriatic potential, shortening development cycles by 40% vs. traditional methods (60, 61). Computer simulation techniques infer drug-target interactions while guiding the optimal design of nanocarriers (58, 62).
5.3 Intelligent devices aid precision treatment
Digital technology drives smarter, more precise treatment devices. Narrow-spectrum UV-B devices for home use now have a digital control system that automatically adjusts the dosage with results similar to office light therapy (59). Smart skin care devices are also attractive. Wearable sensors can monitor skin barrier function, such as transepidermal water loss, as well as detect inflammatory markers, like IL-17 levels, to give patients real-time feedback and assist in optimizing care regimens (43, 63). Microneedle patches or ultrasound delivery devices can digitally regulate the rate of drug release, and they allow topical formulations such as vitamin D analogs to have significantly higher efficacy and fewer side effects (64, 65).
Digital technology continues to revolutionize psoriasis research and treatment. It integrates multi-omics data, uses AI to design drugs, and develops smart devices. In the future, it is necessary to have more accurate biomarker detection tools, the design of personalized treatment plans based on molecular typing of patients, and the development of multifunctional therapeutic systems (51, 66). It is imperative to acknowledge the necessity of incorporating regional variations in cultural literacy and income levels when implementing these digital technologies, as not all individuals possess the financial means to incur the associated costs.
6 How clinicians can use this now
6.1 Adopt now
AI Diagnostic Support: The employment of AI models (e.g., EfficientNet-B4) as a tool to enhance diagnostic accuracy for psoriasis and papulosquamous disorders is recommended. Home Phototherapy: It is recommended that home NB-UVB devices with algorithmic dosing be utilised in order to achieve a level of efficacy that is comparable to that of clinics, while concomitantly improving access and adherence. The integration of applications with features for reminders and tracking has been demonstrated to enhance medication adherence and provide mental health support.
6.2 Promising but not proven
Biologic Predictors: The utilisation of AI models in predicting biological responses has demonstrated considerable potential; however, further validation is required in more extensive, real-world populations. Digital Twins: The utilisation of virtual patient models for the purpose of dose optimisation remains in the research phase, and as such necessitates the conduction of clinical trials.
6.3 Exercise caution
It is imperative to be cognizant of the potential for bias in AI models, particularly those characterised as “black-box” due to their opaque nature. In order to cultivate clinical trust, there is a need to advocate for the utilisation of interpretable models. Data Privacy: It is imperative to prioritise patient data security through the utilisation of compliant platforms and transparent communication. The following essay will explore the concept of doctor-patient communication. It is recommended that healthcare professionals proactively engage in discourse with patients regarding their digital habits, with a view to subsequently recommending reliable online resources with a view to addressing any potential information is not synchronized.
7 Limitations
It is important to acknowledge the limitations of this review. Firstly, as a narrative review, it is inherently susceptible to potential selection bias, as the search and synthesis of the literature were not conducted as systematically as a formal systematic review and meta-analysis would permit (7, 9). This limitation is further compounded by the significant heterogeneity and methodological constraints that are prevalent in the current digital health literature. A plethora of studies have been conducted which demonstrate significant variations in intervention types, definitions of digital tool usage and outcome measures. Furthermore, a paucity of validated assessment tools, in conjunction with the presence of residual confounding factors, engenders considerable challenges to the comparability and robustness of the findings (8, 10).
Secondly, the evidence base itself suffers from critical gaps in sample representativeness and long-term validation. A significant number of studies have been conducted that focus on specific demographic groups. However, there is a notable underrepresentation of diverse skin tones in AI model training datasets (for example, as low as 12%), which limits the generalisability of the technologies across global populations (1, 8). Furthermore, the field is dominated by proof-of-concept studies and short-term validations; the long-term efficacy, safety, and impact on sustained use of many digital tools remain inadequately explored (3–5).
These research limitations directly translate into profound practical challenges. The technical reliability of AI algorithms is hindered by the limited generalisability of these algorithms, a direct consequence of the aforementioned data bias, and the accuracy of non-invasive detection in complex scenarios requires further optimisation (8). Furthermore, data privacy and security pose substantial challenges; the digital storage and sharing of patient health data carries the risk of privacy breaches, and the lack of uniform security standards for cross-institutional data integration leads 63% of patients to resist digital tools, hindering technological diffusion (37, 66). The clinical validation of digital health tools has yet to reach a level that would permit widespread adoption. This is due to a lack of evidence regarding the long-term efficacy and safety of such tools. Technologies such as remote diagnostic systems are difficult to implement in under-resourced areas, and this problem is compounded by discrepancies in trust between physicians and patients (18, 37, 39). The opaque decision-making process of artificial intelligence, which is difficult to interpret and distrusted by 72% of clinicians, can easily lead to doctor-patient disputes (8). It is important to note that the prohibitive cost of using digital technology may result in a more inequitable distribution of healthcare resources (37, 59).
8 Conclusions
Digital technology is fundamentally reshaping the management of psoriasis, marking a paradigm shift from experience-driven to data-driven care. In the context of diagnosis, the integration of high-resolution imaging with AI algorithms facilitates objective and accurate lesion identification, thus surpassing the limitations of traditional PASI scoring. This, in turn, enables non-invasive, dynamic efficacy monitoring. The utilisation of smart phototherapy devices has been demonstrated to enhance the efficiency of treatment through precise, algorithmic dosing. Furthermore, the integration of AI with biologic selection has been shown to result in a significant increase in the rate of complete skin clearance and a reduction in the incidence of serious adverse events. In the field of rehabilitation, the integration of the IoT with smart devices and digital platforms facilitates the utilisation of real-time data and multimodal analysis, thereby enhancing patient self-management and ensuring the more rational distribution of healthcare resources. Concurrently, in the domain of research and development, multi-omics big data and computer-aided drug design are accelerating the dissection of psoriatic pathogenesis and the discovery of novel targeted therapies.
In order to achieve the maximum potential from this digital transformation, future efforts must concentrate on interdisciplinary collaboration, technological innovation, and supportive policy frameworks. Key priorities include the development of unified imaging data standards to ensure interoperability, the promotion of decentralised clinical trial designs to improve patient participation, and the establishment of a comprehensive digital ecosystem that integrates diagnosis, treatment, rehabilitation, research and development. Through these concerted efforts, the field can achieve breakthroughs in the precise, holistic, and lifelong management of psoriasis.
Author contributions
ZG: Writing – original draft, Investigation, Writing – review & editing. YC: Writing – review & editing, Writing – original draft, Project administration, Formal analysis, Validation. XW: Writing – original draft, Writing – review & editing. YZ: Software, Writing – original draft, Writing – review & editing, Conceptualization. WC: Conceptualization, Writing – review & editing, Software, Writing – original draft, Investigation. TS: Writing – review & editing, Writing – original draft, Data curation, Formal analysis, Project administration. SL: Conceptualization, Methodology, Data curation, Writing – review & editing, Investigation, Supervision, Writing – original draft, Software. XW: Software, Methodology, Writing – review & editing, Supervision, Investigation, Writing – original draft, Conceptualization, Funding acquisition, Data curation, Visualization, Resources, Validation, Project administration, Formal analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Supported by Science and Technology Program of XPPC (2025DA051); National Natural Science Foundation of China (82203956, 82460624); Shihezi University 2025 National College Students' Innovation and Entrepreneurship Training Program (202510759022).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AI, Artificial Intelligence; ms-RSOM, multispectral raster-scanning optoacoustic mesoscopy; sO2, oxygen saturation; CNN, Convolutional Neural Networks; SAMCLE, Spatial Alignment Multimodal Contrastive Learning; NB-UVB, narrow spectrum ultraviolet radiation b; PDT, photodynamic therapy; LED, Light emitting diode; BMI, Body mass index; APPs, Applications; IoT, Internet of Things; GWAS, genome-wide association studies.
References
1. Gowda BHJ, Ahmed MG, Hani U, Kesharwani P, Wahab S, Paul K. Microneedles as a momentous platform for psoriasis therapy and diagnosis: a state-of-the-art review. Int J Pharm. (2023) 632:122591. eng. Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. doi: 10.1016/j.ijpharm.2023.122591
2. Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet. (2021) 397(10281):1301–15. doi: 10.1016/s0140-6736(20)32549-6
3. Guo J, Zhang H, Lin W, Lu L, Su J, Chen X. Signaling pathways and targeted therapies for psoriasis. Signal Transduct Target Ther. (2023) 8(1):437. doi: 10.1038/s41392-023-01655-621
4. Nast A, Altenburg A, Augustin M, Boehncke WH, Härle P, Klaus J, et al. German S3-guideline on the treatment of psoriasis vulgaris, adapted from EuroGuiDerm—part 2: treatment monitoring and specific clinical or comorbid situations. J der Deutschen Dermatologischen Gesellschaft. (2021) 19(7):1092–115. doi: 10.1111/ddg.14507
5. Fink C, Alt C, Uhlmann L, Klose C, Enk A, Haenssle HA. Intra- and interobserver variability of image-based PASI assessments in 120 patients suffering from plaque-type psoriasis. J Eur Acad Dermatol Venereol. (2018) 32(8):1314–9. doi: 10.1111/jdv.14960
6. Li J, Liu X, Liu Y, Huang F, Liang J, Lin Y, et al. Saracatinib inhibits necroptosis and ameliorates psoriatic inflammation by targeting MLKL. Cell Death Dis. (2024) 15(2):122. doi: 10.1038/s41419-024-06514-y
7. Jung H, Kim SR, Cho SI, Jo SJ. Reduced economic disparity in biologics use for psoriasis after introducing the reducing copayment program. Sci Rep. (2024) 14(1):4139. doi: 10.1038/s41598-024-54447-5
8. Goessinger EV, Gottfrois P, Mueller AM, Cerminara SE, Navarini AA. Image-Based artificial intelligence in psoriasis assessment: the beginning of a new diagnostic era? Am J Clin Dermatol. (2024) 25(6):861–72. doi: 10.1007/s40257-024-00883-y
9. Li X, Yew YW, Vinod Ram K, Oon HH, Thng STG, Dinish US, et al. Structural and functional imaging of psoriasis for severity assessment and quantitative monitoring of treatment response using high-resolution optoacoustic imaging. Photoacoustics. (2024) 38:100611. doi: 10.1016/j.pacs.2024.100611
10. Yu X, Park S, Lee S, Joo S-W, Choo J. Microfluidics for disease diagnostics based on surface-enhanced Raman scattering detection. Nano Convergence. (2024) 11(1):17. doi: 10.1186/s40580-024-00424-7
11. Huang Q, Li N, Zhang H, Che C, Sun F, Xiong Y, et al. Critical review: digital resolution biomolecular sensing for diagnostics and life science research. Lab Chip. (2020) 20(16):2816–40. doi: 10.1039/d0lc00506a
12. Mohammad Y, Abtin , Erfan H, Uwe A. Diagnostic clinical decision support based on deep learning and knowledge-based systems for psoriasis: from diagnosis to treatment options. Comp Indust Eng. (2024) 187:109754. doi: 10.1016/j.cie.2023.109754
13. Nau T, Schönmann C, Hindelang B, Riobo L, Doll A, Schneider S, et al. Raster-scanning optoacoustic mesoscopy biomarkers for atopic dermatitis skin lesions. Photoacoustics. (2023) 31:100513. eng. The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Vasilis Ntziachristos reports a relationship with Ithera Medical that includes: equity or stocks. doi: 10.1016/j.pacs.2023.100513
14. Yang Y, Wang J, Xie F, Liu J, Shu C, Wang Y, et al. A convolutional neural network trained with dermoscopic images of psoriasis performed on par with 230 dermatologists. Comput Biol Med. (2021) 139:104924. eng. doi: 10.1016/j.compbiomed.2021.104924
15. Hindelang B, Nau T, Englert L, Berezhnoi A, Lauffer F, Darsow U, et al. Enabling precision monitoring of psoriasis treatment by optoacoustic mesoscopy. Sci Transl Med. (2022) 14(644):eabm8059. eng. doi: 10.1126/scitranslmed.abm8059
16. Wang J, Zhang Y, Xie F, Liu J. Enhancing diagnosis of psoriasis and inflammatory skin diseases: a spatially aligned multimodal model integrating clinical and dermoscopic images. J Invest Dermatol. (2025) 145(11):2736–44.e6. doi: 10.1016/j.jid.2025.03.034
17. Huang K, Wu X, Li Y, Lv C, Yan Y, Wu Z, et al. Artificial intelligence–based psoriasis severity assessment: real-world study and application. J Med Internet Res. (2023) 25:e44932. doi: 10.2196/44932
18. Caverzán MD, Oliveda PM, Beaugé L, Palacios RE, Chesta CA, Ibarra LE. Metronomic photodynamic therapy with conjugated polymer nanoparticles in glioblastoma tumor microenvironment. Cells. (2023) 12(11):1541. doi: 10.3390/cells12111541
19. Tanew A, Ristl R, Trattner H, Hacker V, Kroyer B, Radakovic S. Impact of light dose and fluence rate on the efficacy and tolerability of topical 5-ALA photodynamic therapy for actinic keratoses: a randomized, controlled, observer-blinded intrapatient comparison study. J Eur Acad Dermatol Venereol. (2025) 39(8):1460–67. doi: 10.1111/jdv.20527
20. Oteiza-Rius I, Morelló-Vicente A, Aguado-Gil L, Gómez-González EM, Marcos-Muñagorri D, Carrera-Gabilondo A, et al. Combination of LED illumination and daylight photodynamic therapy for the treatment of actinic keratosis in solid organ transplant recipients: a prospective, randomized, comparative, intra-patient study. J Dtsch Dermatol Ges. (2025) 23(6):713–8. doi: 10.1111/ddg.15665
21. Liu Y, Hu K, Duan Y, Chen X, Zhang M, Kuang Y. Characterization and treatment outcomes of biologic therapy in super-responders and biologic-refractory psoriasis patients: a single-center retrospective study in China. J Am Acad Dermatol. (2025) 93(1):46–54. doi: 10.1016/j.jaad.2025.02.063
22. Huang D, Zhong X, Jiang Y, Kong L, Ma R, Lu J, et al. Insulin resistance impairs biologic agent response in moderate-to-severe plaque psoriasis: insights from a prospective cohort study in China. Br J Dermatol. (2024) 191(4):616–23. eng. Conflicts of interest The authors declare no conflicts of interest. doi: 10.1093/bjd/ljae147
23. Jiang Y, Huang D, Chen Q, Yu Y, Hu Y, Wang Y, et al. A novel online calculator based on clinical features and hematological parameters to predict total skin clearance in patients with moderate to severe psoriasis. J Transl Med. (2024) 22(1):121. eng. The authors have no competing interests to declare. doi: 10.1186/s12967-023-04847-4
24. Bassi M, Singh S. Impact of obesity on response to biologic therapies in patients with inflammatory bowel diseases. BioDrugs. (2022) 36(2):197–203. eng. Conflicts of interest: Mehak Bassi - None. Siddharth Singh—Research grants from AbbVie, Pfizer and Janssen, Personal fees from Pfizer (for ad hoc grant review). doi: 10.1007/s40259-022-00522-0
25. Shen S, Qi W, Liu X, Zeng J, Li S, Zhu X, Dong C, et al. From virtual to reality: innovative practices of digital twins in tumor therapy. J Transl Med. (2025) 23(1):348. eng. Declarations. Ethics approval and consent to participate: Since this study is a retrospective analysis of existing published research, ethical committee approval is not required. Consent for publication: Not appliable. Competing interests: The authors declare that they have no competing interests. doi: 10.1186/s12967-025-06371-z
26. Dalal G, Wright SJ, Vass CM, Davison NJ, Vander Stichele G, Smith CH, et al. Patient preferences for stratified medicine in psoriasis: a discrete choice experiment. Br J Dermatol. (2021) 185(5):978–87. eng. doi: 10.1111/bjd.20482
27. Thatiparthi A, Martin A, Liu J, Egeberg A, Wu JJ. Biologic treatment algorithms for moderate-to-severe psoriasis with comorbid conditions and special populations: a review. Am J Clin Dermatol. (2021) 22(4):425–42. eng. Ms. Thatiparthi, Mr. Liu, and Ms. Martin have no conflicts of interest that are directly relevant to the content of this article. Dr. Wu is or has been an investigator, consultant, or speaker for AbbVie, Almirall, Amgen, Arcutis, Aristea Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant, Dr. Reddy’s Laboratories, Eli Lilly, Galderma, Janssen, LEO Pharma, Mindera, Novartis, Regeneron, Sanofi Genzyme, Solius, Sun Pharmaceutical, UCB, Valeant Pharmaceuticals North America LLC, and Zerigo Health. Dr. Egeberg has received research funding from Pfizer and Eli Lilly and honoraria for work as a consultant and/or speaker from Pfizer, Eli Lilly, Novartis, Galderma, and Janssen Pharmaceuticals. doi: 10.1007/s40257-021-00603-w
28. Geifman N, Azadbakht N, Zeng J, Wilkinson T, Dand N, Buchan I, et al. Defining trajectories of response in patients with psoriasis treated with biologic therapies. Br J Dermatol. (2021) 185(4):825–35. eng. doi: 10.1111/bjd.20140
29. Lebwohl MG, Leonardi CL, Mehta NN, Gottlieb AB, Mendelsohn AM, Parno J, et al. Tildrakizumab efficacy, drug survival, and safety are comparable in patients with psoriasis with and without metabolic syndrome: long-term results from 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J Am Acad Dermatol. (2021) 84(2):398–407. eng. doi: 10.1016/j.jaad.2020.09.047
30. Aronovich A, Novikov I, Pavlovsky L. Do biologic treatments for psoriasis lower the risk of psoriatic arthritis? A systematic review. Am J Clin Dermatol. (2023) 24(6):865–73. eng. doi: 10.1007/s40257-023-00801-8
31. Reid C, Cordingley L, Warren RB, Griffiths CEM. Progress to date in advancing stratified medicine in psoriasis. Am J Clin Dermatol. (2020) 21(5):619–26. eng. doi: 10.1007/s40257-020-00533-z
32. Rosenthal YS, Schwartz N, Sagy I, Pavlovsky L. Incidence of psoriatic arthritis among patients receiving biologic treatments for psoriasis: a nested case-control study. Arthritis Rheumatol. (2022) 74(2):237–43. eng. doi: 10.1002/art.41946
33. Nielsen ML, Petersen TC, Maul JT, Wu JJ, Rasmussen MK, Bertelsen T, et al. Multivariable predictive models to identify the optimal biologic therapy for treatment of patients with psoriasis at the individual level. JAMA Dermatol. (2022) 158(10):1149–56. eng. Conflict of Interest Disclosures: Dr Rasmussen reported personal fees from AbbVie Lectures, personal fees from LEO pharma Lectures, research funding to institution from UCB Pharma Clinical study, Novartis Clinical study, and non-financial support from Jansen pharmaceuticals outside the submitted work. Dr Bertelsen reported nonfinancial support from AbbVie, Novartis, personal fees from Eli Lilly, support for congress participation from Sanofi education and Leo Pharma education outside the submitted work. Dr Skov reported personal fees from AbbVie, Eli Lilly, Pfizer, Novartis, and LEO Pharma speaker and personal fees from AbbVie, Janssen Pharmaceuticals, Novartis, Eli Lilly, LEO Pharma, Almirall, Bristol Myers Squibb, UCB and advisor board support from Sanofi, AbbVie, Sanofi, Janssen Pharmaceuticals, Boehringer Ingelheim, AstraZeneca, Eli Lilly, Novartis, Regeneron, and LEO Pharma; investigator, fees to the hospital, and grants from Novartis, Sanofi, Bristol Myers Squibb, Janssen Pharmaceuticals, and LEO Pharma to the hostipal outside the submitted work. Dr Thomsen reported grants from LEO Pharma, grants from Novartis, grants from UCB, and grants from Janssen outside the submitted work. Dr Thyssen reported personal fees from LEO Advisory board and speaking, personal fees from Regeneron Advisory board and speaking and personal fees from Sanofi-genzyme; Advisory board and speaking, personal fees from Coloplast Advisory board, personal fees from AbbVie Advisory board and speaking, personal fees from OM Advisory board, personal fees from Pfizer Advisory board and speaking, and personal fees from Lilly Advisory board and speaking outside the submitted work. Dr Egeberg reported unrelated to the current project, research funding from Pfizer, Eli Lilly, Novartis, Bristol Myers Squibb, AbbVie, Janssen Pharmaceuticals, the Danish National Psoriasis Foundation, the Simon Spies Foundation, and the Kgl Hofbundtmager Aage Bang Foundation, and honoraria as consultant and/or speaker from AbbVie, Almirall, Leo Pharma, Zuellig Pharma Ltd., Galapagos NV, Sun Pharmaceuticals, Samsung Bioepis Co., Ltd., Pfizer, Eli Lilly and Company, Novartis, Union Therapeutics, Galderma, Dermavant, UCB, Mylan, Bristol Myers Squibb, and Janssen Pharmaceuticals. No other disclosures were reported. doi: 10.1001/jamadermatol.2022.3171
34. Coscarella G, Malvaso D, Mannino M, Caldarola G, Fossati B, De Simone C, et al. The preclinical discovery and development of deucravacitinib for the treatment of psoriasis. Expert Opin Drug Discov. (2023) 18(11):1201–8. eng. doi: 10.1080/17460441.2023.2246880
35. Hewitt RM, Dale C, Purcell C, Pattinson R, Bundy C. A qualitative exploration of the prospective acceptability of the MiDerm app; a complex digital intervention for adults living with skin conditions. Br J Health Psychol. (2025) 30(1):e12778. eng. doi: 10.1111/bjhp.12778
36. Hu Y, Jiang L, Lei L, Luo L, Guo H, Zhou Y, et al. Establishment and validation of psoriasis evaluation models. Fundam Res. (2022) 2(1):166–76. eng. The authors declare that they have no conflicts of interest in this work. doi: 10.1016/j.fmre.2021.08.020
37. Domogalla L, Beck A, Schulze-Hagen T, Herr R, Benecke J, Schmieder A. Impact of an eHealth smartphone app on the mental health of patients with psoriasis: prospective randomized controlled intervention study. JMIR Mhealth Uhealth. (2021) 9(10):e28149. eng. Conflicts of Interest: LD, AB, and TSH obtained financial compensation for poster presentations at congresses from Novartis GmbH. RH declares no conflicts of interest. AS conducted clinical trials for AbbVie, Boehringer-Ingelheim, Celgene, Eli Lilly, Janssen-Cilag, LEO Pharma, Merck, Novartis GmbH, and Pfizer; is a consultant for LEO Pharma; and received financial support from Janssen-Cilag and Novartis GmbH and support for conferences from AbbVie, Janssen-Cilag, Novartis GmbH, and Pfizer. AS and JB are the CEOs and owners of Derma Intelligence GmbH, which programmed DermaScope Mobile. doi: 10.2196/28149
38. Lull C, von Ahnen JA, Gross G, Olsavszky V, Knitza J, Leipe J, et al. German Mobile apps for patients with psoriasis: systematic search and evaluation. JMIR Mhealth Uhealth. (2022) 10(5):e34017. eng. Conflicts of Interest: JAvA and CL received financial support from Novartis GmbH for a conference. AS has conducted clinical trials for AbbVie Inc, Boehringer Ingelheim, Celgene Corp, Eli Lilly and Company, Janssen Pharmaceuticals, LEO Pharma A/S, Merck & Co Inc, Novartis GmbH, and Pfizer Inc. AS was a member of the advisory boards of LEO Pharma A/S and UCB and obtained honoraria from Novartis GmbH, Janssen Pharmaceuticals, and UCB. Additionally, AS received financial support from Janssen Pharmaceuticals, AbbVie Inc, Pfizer Inc, and Novartis GmbH for conferences and received grant funding from Novartis GmbH. JK received financial support from Novartis GmbH, Sanofi SA, UCB, and Thermo Fisher Scientific. JK was a member of the advisory boards of and obtained honoraria from AbbVie Inc, Novartis GmbH, Eli Lilly and Company, Medac GmbH, Bristol Myers Squibb, Sanofi SA, Amgen Inc, Gilead Sciences Inc, UCB, ABATON, GlaxoSmithKline, Chugai Pharmaceutical Co Ltd, Boehringer Ingelheim, and Janssen Pharmaceuticals. VO has no conflicts of interest to report. doi: 10.2196/34017
39. Dang C, Wang Z, Hughes-Riley T, Dias T, Qian S, Wang Z, et al. Fibres-threads of intelligence-enable a new generation of wearable systems. Chem Soc Rev. (2024) 53(17):8790–846. eng. doi: 10.1039/d4cs00286e
40. de Groot P, Wagenaar W, Foolen J, Tchetverikov I, Goekoop-Ruiterman YPM, Vis M, et al. Digital biomarkers for psoriatic arthritis: a qualitative focus group study on patient-perceived opportunities and barriers. RMD Open. (2024) 10(4):e004699. doi: 10.1136/rmdopen-2024-004699
41. Akbarialiabad H, Pasdar A, Murrell DF. Digital twins in dermatology, current status, and the road ahead. NPJ Digit Med. (2024) 7(1):228. eng. The authors declare no competing interests. doi: 10.1038/s41746-024-01220-7
42. Rossettini G, Cook C, Palese A, Pillastrini P, Turolla A. Pros and cons of using artificial intelligence chatbots for musculoskeletal rehabilitation management. J Orthop Sports Phys Ther. (2023;53(12):728–34. eng. doi: 10.2519/jospt.2023.12000
43. Erbas ME, Ziehfreund S, Wecker H, Biedermann T, Zink A. Digital Media usage behavior and its impact on the physician-patient relationship: cross-sectional study among individuals affected by psoriasis in Germany. J Med Internet Res. (2024) 26:e57823. eng. Conflicts of Interest: MEE, SZ, and HW have no potential conflicts to declare. TB was president of the German Society of Dermatology. In addition, he has either received grants, consulting fees, support for attending meetings or travel, and speaker’s honoraria or participated in a Data Safety Monitoring Board or Advisory Board of the following companies outside this work: AbbVie, Alk-Abello, Almirall, Boehringer-Ingelheim, Celgene-BMS, GSK, Leo Pharma, Lilly, Novartis, Sanofi-Genzyme, Regeneron, and Viatris. AZ is a member of the German Society of Dermatology and leader of the Digital Dermatology group within this society. He has either been an advisor, received speaker’s honoraria and grants, or participated in clinical trials of the following companies outside this work: AbbVie, Almirall, Amgen, Beiersdorf Dermo Medical, Bencard Allergie, BMS, Celgene, Eli Lilly, GSK, Incyte, Janssen Cilag, Leo Pharma, Novartis, Pfizer, Regeneron, Sanofi-Aventis, and UCB Pharma. doi: 10.2196/57823
44. Attar JA, von Martial S, Troost K, Neumeister T, Ehrchen J, Steinbrink K, et al. Impact of a dermatological rehabilitation program on cardiovascular risks of psoriasis patients. J Dtsch Dermatol Ges. (2025) 23(2):161–71. eng. Keiner. doi: 10.1111/ddg.15585
45. Bragazzi NL, Bridgewood C, Watad A, Damiani G, Kong JD, McGonagle D. Harnessing big data, smart and digital technologies and artificial intelligence for preventing, early intercepting, managing, and treating psoriatic arthritis: insights from a systematic review of the literature. Front Immunol. (2022) 13:847312. eng. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. doi: 10.3389/fimmu.2022.847312
46. Fereig SA, El-Zaafarany GM, Arafa MG, Abdel-Mottaleb MMA. Tackling the various classes of nano-therapeutics employed in topical therapy of psoriasis. Drug Deliv. (2020) 27(1):662–80. eng. doi: 10.1080/10717544.2020.1754527
47. Tsimberidou AM, Muller P, Ji Y. Innovative trial design in precision oncology. Semin Cancer Biol. (2022) 84:284–92. eng. Conflict of Interest Dr. Peter Muller has no financial relationships to disclose. Dr. Yuan Ji Yuan Ji is a co-founder of Laiya Consulting, Inc. A statistical consulting and software company providing services to pharmaceutical companies. He is also a member of IDMC for Astellas Therapeutics. doi: 10.1016/j.semcancer.2020.09.006
48. Al-Janabi A, Eyre S, Foulkes AC, Khan AR, Dand N, Burova E, et al. Atopic polygenic risk score is associated with paradoxical eczema developing in patients with psoriasis treated with biologics. J Invest Dermatol. (2023) 143(8):1470–1478.e1. eng. doi: 10.1016/j.jid.2023.01.021.36804406
49. Gupta NS, Kumar P. Perspective of artificial intelligence in healthcare data management: a journey towards precision medicine. Comput Biol Med. (2023) 162:107051. eng. Declaration of competing interest The authors state that they do not hold conflicts of interest. All authors read the manuscript and agreed to submit. doi: 10.1016/j.compbiomed.2023.107051
50. Sarp S, Kuzlu M, Zhao Y, Gueler O. Digital twin in healthcare: a study for chronic wound management. IEEE J Biomed Health Inform. (2023) 27(11):5634–43. engdoi: 10.1109/jbhi.2023.3299028
51. Schon MP, Wilsmann-Theis D. Current developments and perspectives in psoriasis. J Dtsch Dermatol Ges. (2023) 21(4):363–72. eng. doi: 10.1111/ddg.15033
52. Yu J, Zhao Q, Wang X, Zhou H, Hu J, Gu L, et al. Pathogenesis, multi-omics research, and clinical treatment of psoriasis. J Autoimmun. (2022) 133:102916. eng. Declaration of competing interest The authors declare that they have no conflict of interest. doi: 10.1016/j.jaut.2022.102916
53. van de Kerkhof PC. From empirical to pathogenesis-based treatments for psoriasis. J Invest Dermatol. (2022) 142(7):1778–85. eng. doi: 10.1016/j.jid.2022.01.014
54. Ma Z, An P, Hao S, Huang Z, Yin A, Li Y, et al. Single-cell sequencing analysis and multiple machine-learning models revealed the cellular crosstalk of dendritic cells and identified FABP5 and KLRB1 as novel biomarkers for psoriasis. Front Immunol. (2024) 15:1374763. eng. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. doi: 10.3389/fimmu.2024.1374763
55. Ahmad Jamil H, Abdul Karim N. Unraveling mitochondrial reactive oxygen Species involvement in psoriasis: the promise of antioxidant therapies. Antioxidants (Basel). (2024) 13(10):1222. doi: 10.3390/antiox13101222
56. Guo L, Jin H. Research progress of metabolomics in psoriasis. Chin Med J (Engl). (2023) 136(15):1805–16. eng. None. doi: 10.1097/cm9.0000000000002504
57. Zhang H, Patrick MT, Tejasvi T, Sarkar MK, Wasikowski R, Stuart PE, et al. Retrospective pharmacogenetic study of psoriasis highlights the role of KLK7 in tumour necrosis factor signalling. Br J Dermatol. (2023) 190(1):70–9. eng. Conflicts of interest J.E.G. has served as a consultant to AbbVie, Eli Lilly, Almirall, Celgene, BMS, Janssen, Prometheus, TimberPharma, Galderma, Novartis, MiRagen and AnaptysBio, and has received research support from AbbVie, SunPharma, Eli Lilly, Kyowa Kirin, Almirall, Celgene, BMS, Janssen, Prometheus and TimberPharma. L.C.T. has received support from Novartis, Galderma and Janssen. N.L.W. has received support from Sun Pharma, and has served as a consultant to AnaptysBio and Janssen. doi: 10.1093/bjd/ljad332
58. Zhou R, Qu J, Liu X, Lin F, Ohulchanskyy TY, Alifu N, et al. Biopharmaceutical drug delivery and phototherapy using protein crystals. Adv Drug Deliv Rev. (2025) 216:115480. eng. Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. doi: 10.1016/j.addr.2024.115480
59. Gelfand JM, Armstrong AW, Lim HW, Feldman SR, Johnson SM, Claiborne WCC, et al. Home- vs office-based narrowband UV-B phototherapy for patients with psoriasis: the LITE randomized clinical trial. JAMA Dermatol. (2024) 160(12):1320–8. doi: 10.1001/jamadermatol.2024.3897. Erratum in: JAMA Dermatol. (2025) 161(3):342. doi: 10.1001/jamadermatol.2024.643139319513
60. Tarek M, El-Gogary RI, Kamel AO. A new era of psoriasis treatment: drug repurposing through the lens of nanotechnology and machine learning. Int J Pharm. (2025) 673:125385. eng. Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. doi: 10.1016/j.ijpharm.2025.125385
61. Li TY, Liang WL, Zhao YM, Chen WD, Zhu HX, Duan YY, et al. Alpha-Pinene-encapsulated lipid nanoparticles diminished inflammatory responses in THP-1 cells and imiquimod-induced psoriasis-like skin injury and splenomegaly in mice. Front Immunol. (2024) 15:1390589. eng. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. doi: 10.3389/fimmu.2024.1390589
62. Imran M, Moyle PM, Kamato D, Mohammed Y. Advances in, and prospects of, 3D preclinical models for skin drug discovery. Drug Discov Today. (2024) 29(12):104208. eng. doi: 10.1016/j.drudis.2024.104208
63. Ziehfreund S, Tizek L, Hangel N, Fritzsche MC, Weidinger S, Smith C, et al. Requirements and expectations of high-quality biomarkers for atopic dermatitis and psoriasis in 2021-a two-round delphi survey among international experts. J Eur Acad Dermatol Venereol. (2022) 36(9):1467–76. eng. doi: 10.1111/jdv.18178
64. Thouvenin MD, Dalmon S, Theunis J, Lauze C, Coubetergues H, Mengeaud V, et al. Tolerance and efficacy of a new celastrol-containing balm as adjunct care in psoriasis. J Eur Acad Dermatol Venereol. (2020) 34(Suppl 6):10–6. eng. doi: 10.1111/jdv.16691
65. Le AM, Torres T. New topical therapies for psoriasis. Am J Clin Dermatol. 2022;23(1):13–24. eng. doi: 10.1007/s40257-021-00649-w
Keywords: psoriasis, digital technology, artificial intelligence, precision diagnosis, therapeutic innovation, holistic care
Citation: Gong Z, Cheng Y, Wei X, Zhang Y, Cheng W, Sun T, Liang S and Wang X (2025) Digital technologies in psoriasis management: from precision diagnosis to therapeutic innovation and holistic care. Front. Digit. Health 7:1656585. doi: 10.3389/fdgth.2025.1656585
Received: 30 June 2025; Accepted: 20 October 2025;
Published: 10 November 2025.
Edited by:
Ivan Giovannini, Università degli Studi di Udine, ItalyReviewed by:
Pratheek Jain, Pravara Institute of Medical Sciences (Deemed to be University), IndiaNikita Ramrakhiani, VES Business School, India
Copyright: © 2025 Gong, Cheng, Wei, Zhang, Cheng, Sun, Liang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Su Liang, NjAxMTgxNjk5QHFxLmNvbQ==; Xue Wang, MTY1ODQzNjgxMEBxcS5jb20=
†These authors have contributed equally to this work
 Zhenni Gong
Zhenni Gong Yusheng Cheng1,2,†
Yusheng Cheng1,2,† Xi Wei
Xi Wei Xue Wang
Xue Wang