- 1Cohen Center for Recovery from Complex Chronic Illness, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 2Visible Health Inc., Wilmington, DE, United States
- 3Polybio Research Foundation, Medford, MA, United States
Introduction: Complex chronic illnesses like Long Covid (LC) and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) are marked by fluctuating symptoms, often exacerbated by physical, cognitive, or emotional exertion in a phenomenon known as post-exertional malaise (PEM). Home monitoring technologies offer potential benefits by enabling individuals to track symptoms and biometrics, aiding in disease self-management. However, the general effectiveness of such tools is still unknown.
Methods: A random sample of users of the Visible mobile application (Visible Plus; requires both the armband and paid subscription), aged 18 or older and with self-identified complex chronic illnesses such as LC or ME/CFS, were invited to complete an online survey regarding the impact of the app on their chronic disease self-management. Descriptive statistics related to the responses were analyzed and reported.
Results: The survey was distributed to 2,636 people, with 1,301 participants responding (49.3% response rate). The average age was 46 years. 82% of respondents were female, 8% were male, 8% were non-binary, and 2% preferred not to say or preferred to self-describe. Participants self-identified as having ME/CFS only (n = 534, 42%), LC only (n = 396, 31%), ME/CFS and LC (n = 236, 18%), or another illness (n = 122, 10%). Of the n = 2,636 randomly selected subscribers, the mostly commonly listed “other illnesses” were Postural Orthostatic Tachycardia Syndrome (POTS, 6%), fibromyalgia (5.2%), Ehlers Danlos Syndrome (EDS; 1.7%) and Mast Cell Activation Syndrome (MCAS, 1.2%). Of those with at least 30 days of data, 77% reported seeing an improvements associated with app use, corresponding to 23% of all invited users, 85% (corresponding to 29% of all invited users) reported feeling somewhat (53%) or significantly (32%), and 94% (corresponding to 33% of all invited users) reported a better understanding of their energy budget.
Discussion: Home-monitoring based mobile applications are feasible and acceptable for a motivated subgroup of people with energy-limiting complex chronic illnesses, and are associated with self-reported benefits in energy management and participation in daily activities. The findings of this study should be interpreted as descriptive and hypothesis-generating and do not represent clinically significant effects, underscoring the need for randomized controlled trials to formally evaluate efficacy. Future studies should incorporate a comparison group to better differentiate intervention effects from improvements gained through lived experience.
Introduction
Complex chronic illnesses such as Long Covid (LC) and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) often involve symptoms that can fluctuate over time. For some people, this may mean feeling relatively well on one day, followed by a rapid onset or worsening of symptoms the next (e.g., fatigue, cognitive impairment, headache, shortness of breath), though patterns can vary between individuals. At times, the onset of these worsening symptoms may have a clear cause, for example, an increase in exertion, which is often referred to as post-exertional malaise (PEM) and is a feature of complex illnesses such as LC and ME/CFS. Furthermore, different types of exertion can trigger PEM, including but not limited to physical, cognitive, emotional, social forms of exertion (1, 2). At other times, the underlying cause behind the worsening of symptoms can be uncertain. Similarly, some people report a rapid onset of initial symptoms (e.g., at the start of the illness), whereas others note a more progressive increase in impairment (3).
The benefits of home-monitoring in health and disease are well understood (4). The efficacy of daily monitoring has been established in improving health in people living with chronic obstructive pulmonary disease (5), cystic fibrosis (6), cardiovascular disease (7), high health-risk older adults managing multiple pathologies (8), and healthy individuals (9). While there are few reports of home monitoring systems that allow people with energy-limiting complex chronic illnesses to track and report symptoms (10), self-monitoring and reporting of symptoms may provide this group with actionable feedback regarding various aspects of disease self-management. For example, heart rate (HR) monitoring may help people with ME/CFS understand and manage PEM symptoms and support participation in activities of daily living (11). The benefits of these self-monitoring strategies may include early identification of PEM that may impact on physical functioning (12), and access to data that can guide individualized care and inform treatment decisions in collaboration with a clinical team (11). Overall, applying home monitoring technology in the context of energy-limiting complex chronic illness has the potential to improve fatigue management, emotional wellbeing, and confidence in self-management (13).
The Visible application (Visible Health Inc., Delaware, USA; https://makevisible.com/) is a real-world mobile application that provides people with LC, ME/CFS and other energy-limiting conditions the ability to self-monitor symptoms and biometrics. The impact of using the application with a wearable heart monitor to record biometrics to manage illness, energy budgets, symptoms and activity has not been evaluated.
Aims
In people self-identifying with complex chronic illness using a mobile application connected to a wearable device to monitor biometrics and symptoms, we aimed to describe the participant characteristics and outcome results of an application-wide survey.
Methods
An online survey was distributed to users of the Visible mobile application (Visible Health Inc., Delaware, USA), who had been using the app in conjunction with a Polar Verity Sense™ HR monitor (Polar Electro, Inc., Kempele, Finland) in April 2024. The Polar Verity Sense™ armband is a wearable device that uses an optical sensor to continuously measure HR and was selected due to its comfort, ease of use and accuracy. Participants were using the Visible Plus version, which differs from the free version of the app in that it requires both the armband and an active subscription.
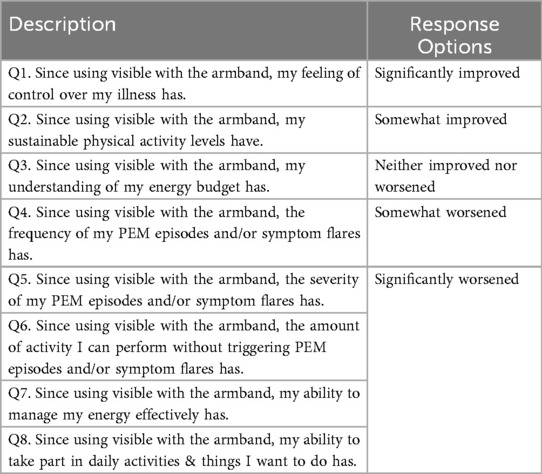
The survey was designed by the investigators to assess the participants’ experiences, specifically focusing on the perceived impact of their use of the Visible app and armband on PEM episodes. Survey questions covered multiple dimensions of PEM, including fatigue, pain, cognitive function, and other self-reported health outcomes, aiming to capture changes over time as perceived by the users (Table 1). Before distribution, the survey underwent pilot testing amongst the research team solely for technical validation of the survey.
Eligibility for survey participation required that subscribers of the Visible app had logged a minimum of 30 days of wearable HR data. This threshold was set to ensure that participants had ample data for assessing trends or changes in PEM symptoms. However, former subscribers who had stopped collecting wearable data were still eligible to participate, provided they met the criterion of 30 recorded days during their subscription period.
Participants were provided with clear information about the purpose of the survey, its voluntary nature, and the assurance of anonymity at the start of the survey by Visible Health. Participants provided electronic consent prior to enrollment. By clicking an “I accept” option, they agreed to allow Visible to collect and use their anonymous data for research purposes. Only anonymized, aggregate data were analyzed and reported in this study. Further information about the ethical processes is detailed in the ‘Ethical Approval and Consent to Participate’ section of this manuscript.
The survey was distributed electronically via a link embedded within the Visible mobile app and email invitations. To minimize response bias, the order of answer choices was randomized, with options presented in either ascending or descending order for each participant (Figure 1).

Figure 1. Participants were randomly presented with survey responses to the questions in Table 1 with more favorable responses in ascending (A) or descending (B) order. Screenshots from: Visible app, https://www.makevisible.com/.
Participants
People aged ≥18 years with self-identified complex chronic illness such as LC, ME/CFS, or people experiencing other causes of energy limitation who were using the Visible application and opted to share their data anonymously were eligible for inclusion.
Analysis
Descriptive statistics were used to summarize data.
Results
Participant demographics
The survey was circulated to n = 2,636 randomly selected subscribers from a total pool of n = 4,537 subscribers, yielding a total of n = 1,301 responses (49.3% response rate). The mean ± standard deviation age of participants was 46 ± 12 years. 82% of respondents indicated that their gender was female, 8% were male, 8% were non-binary, and 2% preferred not to say or preferred to self-describe. Participants self-identified as having ME/CFS only (n = 534, 42%), LC only (n = 396, 31%), ME/CFS and LC (n = 236, 18%), or another illness (n = 122, 10%). Of the n = 2,636 randomly selected subscribers, the mostly commonly listed “other illnesses” were Postural Orthostatic Tachycardia Syndrome (POTS, 6%), fibromyalgia (5.2%), Ehlers Danlos Syndrome (EDS; 1.7%) and Mast Cell Activation Syndrome (MCAS, 1.2%).
Survey responses
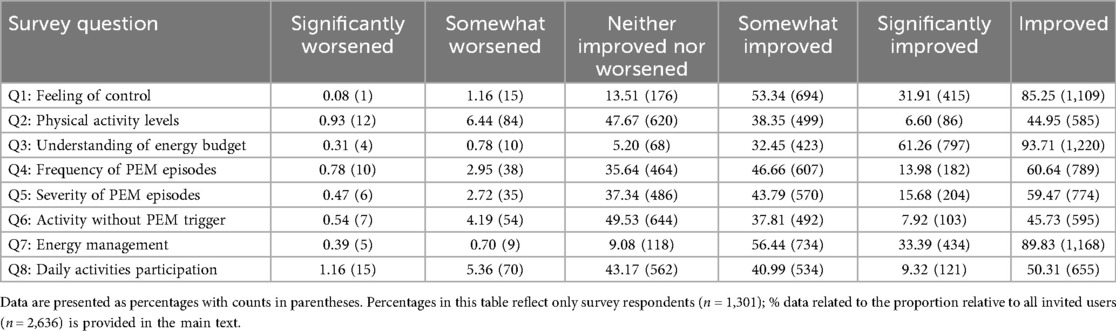
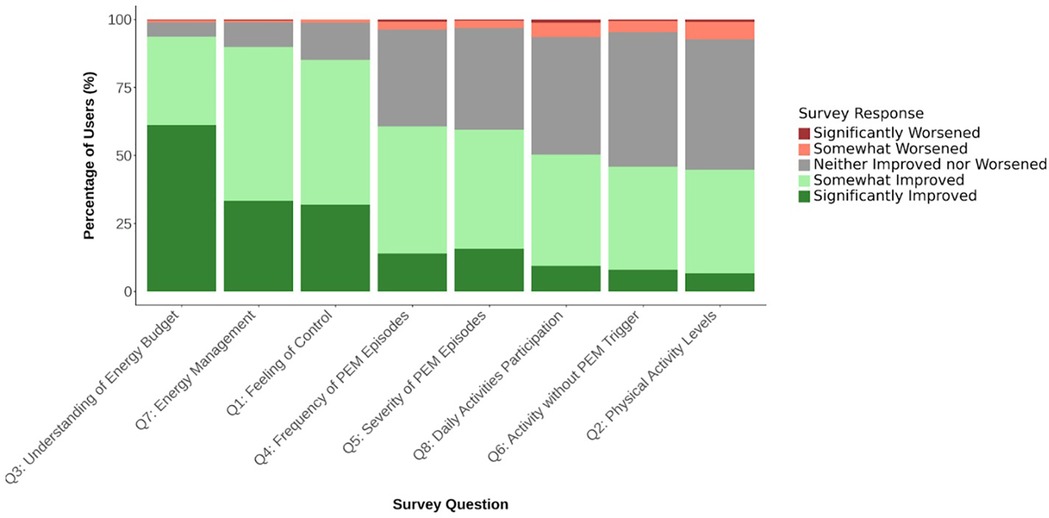
Table 2 highlights the distribution of user responses for users with at least 30 days of activity. In these users, 77% reported seeing an improvements associated with app use (corresponding to 23% all invited users), and 85% (corresponding to 29% of all invited users) reported feeling somewhat (53%) or significantly (32%) more in control of their illness since using Visible with the armband. 94% indicated a better understanding of their energy budget (corresponding to 33% of all invited users), and 90% reported experiencing enhanced energy management (corresponding to 31% of all invited users) (Figure 2). Reductions in the frequency and severity of PEM episodes were noted by 61% and 60% of respondents, respectively (corresponding to 15% of all invited users). Fewer reported substantial changes in physical activity levels (45%; corresponding to 12% of all invited users). There were no statistically significant differences between the distribution of data from 30 + days to 60 + or 90 + days (Supplementary Material 1).
Among users who connected their Armband in March 2024, 86% continued using it 30 days later. For those who connected their Armband in January 2024, 75% were still using it after 90 days.
Discussion
In a large sample of people with complex chronic illness, the majority of survey respondents reported substantial improvements across most categories. Almost all indicated a better understanding of their energy budget, enhanced energy management and improvements in the feeling of control. Reductions in the frequency and severity of at least one aspect of their PEM was reported by 77% of users, and over half of respondents reported increased participation in daily activities. In line with other work and the incidence of some complex chronic illness (3, 14), the survey respondents were predominantly female. These descriptive findings highlight self-reported benefits of using home-monitoring applications, including the Visible application and armband, and provide preliminary insights into how individuals managing energy-limiting complex chronic illness perceive their use of such tools.
Despite the potential benefits, in the present study, fewer users reported substantial increases in their tolerance of physical activity. A possible explanation is that using the Visible app may increase users’ awareness of activity thresholds that precipitate PEM. Prior to using the app, users may not have recognized that certain routine activities could trigger a response. Adopting a data-informed pacing strategy may help users identify these thresholds, which could contribute to the perception of reduced physical activity capacity in the survey responses. This possible explanation is speculative and not directly measured in the study and as such the findings should be interpreted as descriptive and hypothesis generating. In addition, the findings related to physical activity in the present study may reflect that while the app helps users pace themselves and avoid overexertion and PEM, these strategies do not directly influence the underlying physiological processes. In LC, local and systemic metabolic disturbances, severe exercise-induced myopathy, infiltration of amyloid-containing deposits, and immune cells in skeletal muscles are key characteristics of post-exertional malaise, which likely contribute to limited physical activity tolerance (15). Despite the demonstrated potential of mobile applications for symptom management in complex chronic illness, there still remains a critical need for clinical trials that address the physiological drivers of PEM.
Reduced fatigue following the use of home-monitoring and activity pacing have been reported elsewhere. In a systematic review of 14 studies of at least good quality [PEDro scoring (16)], activity pacing interventions were found to be an effective strategy to reduce fatigue and psychological distress, and to improve physical function in people with ME/CFS (17). In people with LC, simple pacing strategies such as the WHO Borg CR-10 pacing protocol has demonstrated significant reductions in PEM and improvement in activity levels over a 6-week period (18). The current work shows a high level of consistency with the benefits experienced by users of apps that target other chronic illnesses and conditions such as COPD (19), cystic fibrosis (6) and chronic pain (20). Although relatively little has been done to evaluate the impact of mobile applications on energy-limiting complex chronic illness, our results are consistent with others (11, 21). Clague-Baker et al. (11) surveyed 488 people with ME/CFS in 2023 and found that HR monitoring helped 72% of participants better understand their personalized PEM. Other benefits of HR monitoring were listed as having real-time feedback on current or prior activity (69%) and that this type of monitoring helped stop the boom/bust or push-crash cycle that is common to this condition (64%). Despite these perceived benefits, many (57%) still felt that support from medical professionals to utilize the pacing data was lacking (7), highlighting a persistent gap in integrating digital self-monitoring tools into clinical care. This gap may reflect clinicians’ limited familiarity with digital self-monitoring tools, competing demands during appointments, or uncertainty regarding how to incorporate individualized pacing data into care plans. As a result, even effective digital interventions may not achieve their full impact without integrated clinician support, underscoring the need for strategies that connect patient self-management with professional guidance. Although our work did not evaluate a single specific approach to chronic disease self-management, it is further validation that data-informed pacing can be highly beneficial to people living with energy-limiting complex chronic illness.
Limitations and future directions
The survey response rate suggests potential self-selection bias, as non-respondents may include users who discontinued due to lack of benefit, limiting the generalizability of results. Respondents in this study also self-reported their clinical conditions. As such, the authors have referred to the dataset as being derived from people with complex chronic illness, rather than a specific condition. Future work would benefit from focusing on specific diagnoses that have been confirmed clinically in order to further validate these findings. The study collected limited demographic data including age, gender, illness type, which restricts assessment of the sample's representativeness relative to the broader population. Future research should aim to include more comprehensive demographic information to enhance generalizability.
Approximately half (49.3%) of randomly selected survey recipients responded. While this is a high proportion of recipients for in-app surveys, it could still introduce bias toward users who had a positive experience with the app, highlighting the critical need for a controlled study of the utility of mobile applications for complex chronic illness that was beyond the scope of this initial preliminary analysis.
Understanding the natural progression of self-management skills in complex chronic illnesses is important for contextualizing the potential benefits of the intervention. However, this study was designed to explore feasibility and acceptability, rather than a comparative effectiveness trial. As such, we did not include a control group in the current study. Future studies should incorporate a comparison group to better differentiate intervention effects from improvements gained through lived experience.
Participants used the paid Visible Plus version, which requires purchase of the armband and a subscription (as opposed to the free app version). Subscription status may influence perceptions of value, engagement, and retention, introducing potential ascertainment bias, and the results may reflect a subgroup of highly motivated and engaged users. Furthermore, our analysis includes only participants with at least 30 days of use, potentially excluding early discontinuers and biasing results toward more engaged users. Participants who discontinued before 30 days were excluded and unmeasured factors, such as motivation, symptom severity, or type, may have influenced the likelihood of continued use. These factors may limit the generalizability of the findings.
For this initial exploratory survey, self-reported diagnoses were grouped together for pragmatic reasons. However, this approach limits the interpretability of the findings for any individual condition.
Finally, we acknowledge that relying on self-reported symptom and PEM changes without objective activity data is a limitation of the study.
Conclusion
The results of this descriptive study, conducted in the largest known group of people with energy-limiting complex chronic illness, highlights the feasibility and acceptability of the Visible app for insights regarding their energy-limiting complex chronic illness in a group of motivated users. These self-reported findings are preliminary and hypothesis generating; randomized controlled trials of app-based services for complex chronic illnesses could further validate the findings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Icahn School of Medicine at Mount Sinai. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the study involved minimal risk and was deemed exempt from full review according to DHHS regulations [45 CFR 46.104 (d)] (Icahn School of Medicine at Mount Sinai; STUDY#25-00263). Participants were provided with clear information about the study's purpose, voluntary nature, and anonymity at the start of the survey. Before submitting their responses, participants were asked for explicit consent to anonymous data collection for the purposes of the research. No identifiable personal information was collected, ensuring participants’ privacy in accordance with ethical and regulatory standards for minimal-risk research.
Author contributions
AS: Data curation, Formal analysis, Methodology, Project administration, Visualization, Writing – original draft. RP: Conceptualization, Investigation, Resources, Software, Supervision, Writing – review & editing. HL: Conceptualization, Investigation, Resources, Software, Supervision, Writing – review & editing. LM-F: Conceptualization, Investigation, Resources, Software, Writing – review & editing. AP: Conceptualization, Resources, Supervision, Writing – review & editing. DP: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research or publication of this article.
Acknowledgments
We are grateful to the Steven and Alexandra Cohen Foundation.
Conflict of interest
Authors RP, HL and LM-F were employed by Visible Health Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JMV declared a past co-authorship with the author(s) DP to the handling editor.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issue please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdgth.2025.1662255/full#supplementary-material
Abbreviations
EDS, Ehlers Danlos Syndrome; LC, long COVID; MCAS, mast cell activation syndrome; ME/CFS, myalgic encephalomyelitis/chronic fatigue syndrome; POTS, postural orthostatic tachycardia syndrome; WHO, World Health Organization.
References
1. Hartle M, Bateman L, Vernon SD. Dissecting the nature of post-exertional malaise. Fatigue: Biomed Health Behav. (2021) 9(1):33–44. doi: 10.1080/21641846.2021.1905415
2. Chu L, Valencia IJ, Garvert DW, Montoya JG. Deconstructing post-exertional malaise in myalgic encephalomyelitis/ chronic fatigue syndrome: a patient-centered, cross-sectional survey. PLoS One. (2018) 13(6):e0197811. doi: 10.1371/journal.pone.0197811
3. Tschopp R, König RS, Rejmer P, Paris DH. Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a preliminary survey among patients in Switzerland. Heliyon. (2023) 9(5):e15595. doi: 10.1016/j.heliyon.2023.e15595
4. Farias FAC, Dagostini CM, Bicca YA, Falavigna VF, Falavigna A. Remote patient monitoring: a systematic review. Telemed J E Health. (2020) 26:576–83. doi: 10.1089/tmj.2019.0066
5. Metting E, Dassen L, Aardoom J, Versluis A, Chavannes N. Effectiveness of telemonitoring for respiratory and systemic symptoms of asthma and COPD: a narrative review Life. Life (Basel). (2021) 11(11):1215. doi: 10.3390/life11111215
6. Wood J, Jenkins S, Putrino D, Mulrennan S, Morey S, Cecins N, et al. A smartphone application for reporting symptoms in adults with cystic fibrosis improves the detection of exacerbations: results of a randomised controlled trial. J Cyst Fibros. (2020) 19(2):271–6. doi: 10.1016/j.jcf.2019.09.002
7. Shimbo D, Artinian NT, Basile JN, Krakoff LR, Margolis KL, Rakotz MK, et al. Self-measured blood pressure monitoring at home: a joint policy statement from the American Heart Association and American Medical Association. Circulation. (2020) 142(4):e42–63. 32567342
8. Hamilton T, Johnson L, Quinn BT, Coppola J, Sachs D, Migliaccio J, et al. Telehealth intervention programs for seniors: an observational study of a community-embedded health monitoring initiative. Telemed J E Health. (2020) 26(4):438–45. doi: 10.1089/tmj.2018.0248
9. Schorr EN, Gepner AD, Dolansky MA, Forman DE, Park LG, Petersen KS, et al. Harnessing mobile health technology for secondary cardiovascular disease prevention in older adults: a scientific statement from the American Heart Association. Circ Cardiovasc Qual Outcomes. (2021) 14(5):e000103. doi: 10.1161/HCQ.0000000000000103
10. Mansoubi M, Dawes J, Bhatia A, Vashisht H, Collett J, Greenwood DC, et al. Digital home monitoring for capturing daily fluctuation of symptoms; a longitudinal repeated measures study: long COVID multi-disciplinary consortium to optimise treatments and services across the NHS (a LOCOMOTION study). BMJ Open. (2023) 13(8):e071428. doi: 10.1136/bmjopen-2022-071428
11. Clague-Baker N, Davenport TE, Madi M, Dickinson K, Leslie K, Bull M, et al. An international survey of experiences and attitudes towards pacing using a heart rate monitor for people with myalgic encephalomyelitis/chronic fatigue syndrome. Work. (2023) 74(4):1225–34. doi: 10.3233/WOR-220512
12. Jason LA, Holtzman CS, Sunnquist M, Cotler J. The development of an instrument to assess post-exertional malaise in patients with myalgic encephalomyelitis and chronic fatigue syndrome. J Health Psychol. (2018) 26(2):238–48. doi: 10.1177/1359105318805819
13. Busse M, Pallmann P, Riaz M, Potter C, Leggat FJ, Harris S, et al. Effectiveness of a personalised self-management intervention for people living with long COVID (listen trial): pragmatic, multicentre, parallel group, randomised controlled trial. BMJ Med. (2025) 4(1):e001068. doi: 10.1136/bmjmed-2024-001068
14. Carruthers BM, van de Sande MI, De Meirleir KL, Klimas DG, Broderick G, Mitchell T, et al. Myalgic encephalomyelitis: international consensus criteria. J Intern Med. (2011) 270:327–38. doi: 10.1111/j.1365-2796.2011.02428.x/pdf2
15. Appelman B, Charlton BT, Goulding RP, Kerkhoff TJ, Breedveld EA, Noort W, et al. Muscle abnormalities worsen after post-exertional malaise in long COVID. Nat Commun. (2024) 15(1):17. doi: 10.1038/s41467-023-44432-3
16. Physiotherapy Evidence Database. (1999). PEDro scale. Available online at: https://pedro.org.au/wp-content/uploads/PEDro_scale.pdf (Accessed April 10, 2025).
17. Casson S, Jones MD, Cassar J, Kwai N, Lloyd AR, Barry BK, et al. The effectiveness of activity pacing interventions for people with chronic fatigue syndrome: a systematic review and meta-analysis. Disabil Rehabil. (2023) 45(23):3788–802. doi: 10.1080/09638288.2022.2135776
18. Parker M, Sawant HB, Flannery T, Tarrant R, Shardha J, Bannister R, et al. Effect of using a structured pacing protocol on post-exertional symptom exacerbation and health status in a longitudinal cohort with the post-COVID-19 syndrome. J Med Virol. (2023) 95(1):e28373. doi: 10.1002/jmv.28373
19. Chmiel FP, Burns DK, Pickering JB, Blythin A, Wilkinson TM, Boniface MJ. Prediction of chronic obstructive pulmonary disease exacerbation events by using patient self-reported data in a digital health app: statistical evaluation and machine learning approach. JMIR Med Inform. (2022) 10(3):e26499. doi: 10.2196/26499
20. Delgado AD, Salazar SI, Rozaieski K, Putrino D, Tabacof L. Engagement in an mHealth-guided exercise therapy program is associated with reductions in chronic musculoskeletal pain. Am J Phys Med Rehabil. (2023) 102(11):984–9. doi: 10.1097/PHM.0000000000002257
Keywords: long covid, myalgic encephalitis, home monitoring, ME/CFS, survey
Citation: Sawyer A, Preston R, Leeming H, Martin-Fuller L, Proal A and Putrino D (2025) Wearable technology in the management of complex chronic illness: preliminary survey results on self-reported outcomes. Front. Digit. Health 7:1662255. doi: 10.3389/fdgth.2025.1662255
Received: 8 July 2025; Accepted: 17 September 2025;
Published: 8 October 2025.
Edited by:
Yang Gong, University of Texas Health Science Center at Houston, United StatesReviewed by:
Julia Moore Vogel, The Scripps Research Institute, United StatesMohsen Masoumian, Health International Inc, Canada
Copyright: © 2025 Sawyer, Preston, Leeming, Martin-Fuller, Proal and Putrino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Putrino, RGF2aWQucHV0cmlub0Btb3VudHNpbmFpLm9yZw==
 Abbey Sawyer
Abbey Sawyer Rory Preston2
Rory Preston2 Amy Proal
Amy Proal David Putrino
David Putrino