- 1UQ Centre for Clinical Research, Faculty of Medicine, The University of Queensland, Brisbane, QLD, Australia
- 2School of Electrical Engineering and Computer Science, The University of Queensland, Brisbane, QLD, Australia
- 3School of Health and Rehabilitation Sciences, The University of Queensland, Brisbane, QLD, Australia
- 4Queensland Aphasia Research Centre, The University of Queensland, Herston, QLD, Australia
- 5Department of Occupational Therapy, Princess Alexandra Hospital, Brisbane, QLD, Australia
- 6School of Psychology, The University of Queensland, Brisbane, QLD, Australia
Background: Routine administration of neuropsychological assessments to evaluate cognitive decline in Parkinson's disease (PD) may not be feasible in current clinical services. This is due to lengthy administration time, lack of specialised neuropsychologists and other limitations in resources. While technology integration could improve efficiency, understanding the existing assessment journey is crucial for successful implementation in clinical services. This preliminary study from the PDCogniCare project aims to explore current practice in neuropsychological assessments for people with PD and identify opportunities for technological integration.
Methods: A qualitative study using semi-structured interviews was conducted with 15 clinical experts across two public health services in Australia. Data were analysed using inductive coding and journey mapping approaches to develop a comprehensive map of neuropsychological assessment journey.
Results: Analysis revealed a four-phase assessment journey: initiation, brief cognitive screening, detailed neuropsychological assessment, and feedback, with distinct variations between clinical pathways. Key challenges included long waiting times, assessment duration, complex reporting, and limited awareness of cognitive assessments. While technology integration could begin to address some of these challenges through streamlined processes and improved access, barriers such as system integration, user adoption, and assessment methodology constraints require consideration.
Conclusion: This study revealed the complexity of neuropsychological assessment pathways and identified potential areas for technological enhancement. Future research from the PDCogniCare project will aim to address these areas by employing appropriate methodologies and theoretical models to guide the design and development of technologies for neuropsychological assessments in PD.
Introduction
People living with Parkinson's disease (PD) are at elevated risk of developing dementia (1). This risk increases as the disease progresses (2). Neuropsychological assessment is a standard approach for evaluating cognitive decline associated with the onset of dementia in PD (2–4). These assessments provide standardised metrics for examining brain-behaviour relationships, evaluating cognitive deficits, and identifying patterns in cognition linked to brain disorders (5). While neuropsychological assessments are useful, they take several hours to conduct and are not always feasible within routine clinical practice (6). These assessments are also impacted by costs and limited access to neuropsychology services (7). Consequently, shorter cognitive assessments, including brief instruments such as the Mini-Mental State Examination (MMSE) or the Montreal Cognitive Assessment (MoCA), are often used as an initial screening step (8). However, there is limited clarity regarding the selection and timing of assessments, as well as the standardisation of clinical processes (9–11). Further research is therefore needed to understand these diverse clinical pathways for assessing cognitive impairment and dementia risk in people with PD. This need is also driven by people with PD who require prioritised psychological research on cognitive functioning to understand how the condition influences their cognitive abilities and to obtain practical information for managing cognitive symptoms (12).
Clinical pathways, grounded in evidence and clinical guidelines, have demonstrated effectiveness in healthcare delivery (13). This effectiveness could be enhanced through the integration of health information technology (13). Health information technology, particularly telehealth, has shown significant potential in improving access to healthcare services regardless of geographic location, while also reducing healthcare costs (14, 15). Additionally, it showed good reliability and agreement compared to face-to-face assessments. For instance, Hernandez et al. (16) found good reliability (ICC = 0.80–0.82) between face-to-face and remote cognitive testing in older adults, though remote scores were significantly higher for Abbreviated Mental Test (AMT) and Chinese Mini-Mental State Examination (mCMMSE). Another study by Zadik et al. (17) found excellent agreement (ICC = 0.89) in total MOCA score between face-to-face and via videoconference using a mobile phone. Beyond reliability and agreement, the study by Bălăeţ et al. (18) discussed the various benefits of technology-based cognitive assessments over traditional pen-and-paper scales. These benefits included reducing administrative costs and travel burdens while enabling repeated testing, gamification, and large-scale longitudinal monitoring from home (18). Furthermore, such technologies provide automated scoring, streamlined data management, and detailed performance modelling that can isolate specific cognitive processing components such as visuomotor slowing (18). This potential offers advantages in neuropsychological assessment processes, where there is a growing interest (19, 20). PDCogniCare is an Australian project that aims to address this need by improving the delivery of neuropsychological services for people with PD through technology. Understanding how novel technologies integrate within existing clinical pathways is crucial when implementing new solutions (21), and hence a key tenant in the PDCogniCare project.
In the past, journey mapping approaches have shown significant potential in identifying and analysing clinical pathways (22). Journey mapping looks to create a visual timeline that illustrates the multidimensional relationship between the individual and the health service (23). This visual representation helps identify gaps in health service, allowing for improvements to the overall patient experience and health outcomes (24). Journey mapping in medical research remains an emerging field (23), with a notable lack of research specifically addressing neuropsychological assessments and opportunities for technological integration. This study aims to explore how journey mapping applies to understand clinical pathways in neuropsychological assessments in PD and identify potential areas where technology could be integrated to improve overall clinical workflows.
Methods
Study design
The study used a qualitative descriptive design based on semi-structured interviews. The semi-structured qualitative interviews were conducted with clinical experts from two public outpatient clinical services. Interviews aimed to understand the current practices in conducting neuropsychological assessments and potential for technology integration. A journey map of their experiences in conducting and/or utilising results of neuropsychological assessments in their roles within public health services in Queensland, Australia was created. The study was approved as part of the PDCogniCare project by the Metro North Health Human Research Ethics Committee (Project ID: 100098), and the University of Queensland Human Research Ethics Committee (Project Number: 2023/HE002029).
Participant recruitment
Clinical experts, including neurologists, geriatricians, neuropsychologists, movement disorders nurses, psychiatrists, and allied health professionals, were recruited from each public health service. Clinical experts were purposefully selected based on their experience working with people with PD. Purposeful sampling was employed to select participants who could provide detailed and insightful information on the phenomenon being studied (25). In this case, the phenomenon focused on experience with conducting or referring for neuropsychological assessments in PD. Participants were recruited through a snowballing approach, including personal contacts and contacts of research participants.
Data collection
Semi-structured interviews were conducted via video conferencing platforms such as Zoom and Microsoft Teams by five researchers (DB, JY, KS, LM and PW). An interview guide was developed based on two frameworks of the Theoretical Domains Framework (26) and the Consolidated Framework for Implementation Research (27) to determine potential opportunities for the implementation of a technology within the clinical pathways. It covered topics such as (i) standard practices for conducting cognitive assessments, (ii) needs related to routine cognitive assessments, and (iii) the potential of technology to address existing limitations. Each interview took approximately 60 min and was audio and/or video recorded with the participants' consent, and transcribed verbatim.
Data analysis
Transcripts were transferred to NVivo 12 software for analysis. After familiarisation, qualitative content analysis was performed (28). The coded text was combined by identifying key similarities and differences in the journey and altering the pathways to ensure comprehensiveness. This was conducted in consultation with the wider author group to create a preliminary journey map. This map helped to understand the structure of the overall assessment pathway. It also highlighted potential areas of weakness and informed approaches for integrating technology. These insights contributed to the development of a comprehensive assessment journey.
Initially, eight transcripts from clinician stakeholders were extracted and coded. These codes were mapped to the pilot map, resulting in eight distinct journeys. These journeys were grouped by public health service. The journey maps within each group were compared to identify similarities and differences, which were merged to form a preliminary assessment map. This map was expanded by incorporating seven additional clinician interview transcripts. After multiple iterations, two pathways were identified, one for each public health service. The pathways were simplified and condensed into a single comprehensive assessment journey representing neuropsychological assessments at a public health setting in Queensland, Australia. Further, this map outlined potential approaches for technology integration.
Results
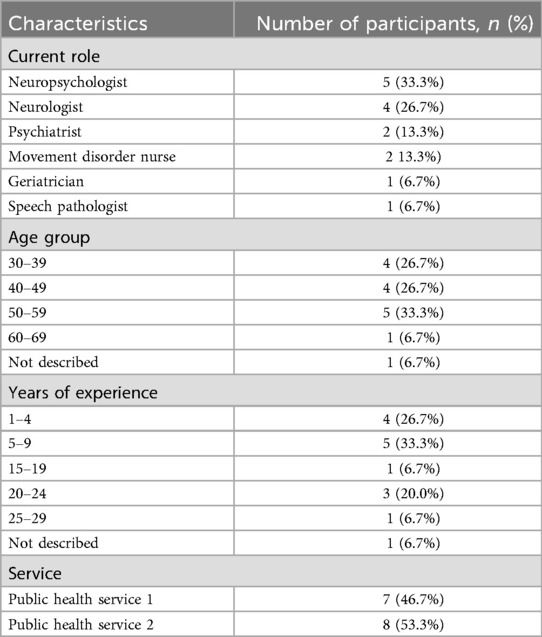
In total 15 clinical experts (8 men and 7 women), aged between 30 and 69, were interviewed. Clinical experts included 5 neuropsychologists, 4 consultant neurologists, 2 consultant psychiatrists, 2 movement disorder nurses, a geriatrician, and 1 speech pathologist. Their combined experience ranged from 1 to 29 years in their current roles. One participant did not disclose their age group, and another did not provide their years of experience. The demographic data of each participant is presented in Table 1.
Neuropsychological assessment journey
The neuropsychological assessment process was categorised into four distinct phases: initiation, brief cognitive screening, detailed neuropsychological assessment and feedback. Each phase involves the participation of people with PD, their support person, and either a nurse or physician, as illustrated in Figure 1. It is important to acknowledge the existence of two distinct clinical pathways (CP1 and CP2) that were constructed from data from two different public health service, each operating with their own established procedures.
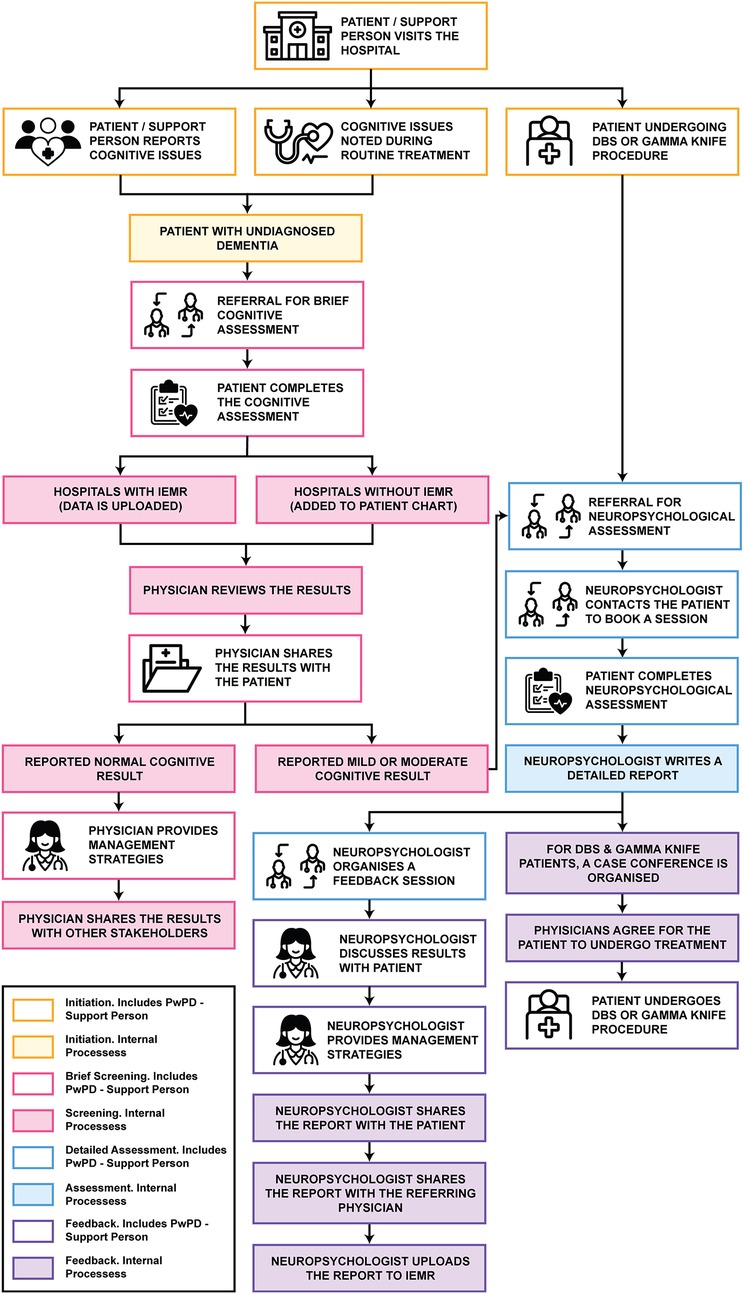
Figure 1. Assessment journey illustrating the typical stages of neuropsychological assessment. Icons reproduced from Noun Project.
Phase 1. Initiation
The initiation phase marks the point at which the need for cognitive assessment is identified. This can occur when cognitive issues are reported by the person with PD or their support person, or when cognitive concerns are detected during routine assessments. Additionally, this phase may be triggered when the person with PD is being considered for deep brain stimulation (DBS) or gamma knife procedures. For people with PD with undiagnosed dementia that have reported cognitive issues or if cognitive issues are identified during routine assessments, referrals for cognitive assessment are made by the neurologist (CP1 and CP2) or by the geriatrician (CP2). However, for PD-Dementia patients, they would undergo discussions with the neurologists (CP1).
For individuals being considered for DBS or gamma knife procedures, referral to a neuropsychologist for a comprehensive neuropsychological assessment is standard across both clinical pathways. In CP1, this referral is facilitated by the neurologist, while in CP2, either a neurologist or a geriatrician may initiate the referral process.
Phase 2. Brief cognitive screening
The brief cognitive screening phase involves conducting an assessment to confirm the presence of cognitive impairment in the person living with PD. In both clinical pathways, the individual undergoes a brief cognitive assessment, which may be administered by a trained neurologist (CP1 and CP2), geriatrician (CP2), psychiatrist (CP1 and CP2), registrar (CP1 and CP2), or nurse (CP2). While these assessments are generally performed within an outpatient setting in both pathways, CP2 also included the provision for trained nurses to conduct cognitive assessments at the individual's home. The data collected from these assessments in both CP1 and CP2 were uploaded to the integrated electronic medical record (iEMR) where available, or alternatively, recorded in the patient's chart (CP1).
Once added to the iEMR or patient chart, the primary physician, either a neurologist (CP1 and CP2) or a geriatrician (CP2), reviews the results of the brief cognitive assessment. In certain cases, particularly in CP2, the primary physician may refer the person with PD to a speech pathologist if their cognitive assessment results are borderline, to evaluate the impact of communication on cognition. The speech pathologist's findings are then verbally communicated to the primary physician. The physician subsequently shares the results with the person living with PD, their support person, and, in CP1, their general practitioner (GP). For individuals with normal cognitive finding, the primary physician provides preventive management strategies and symptom control. However, in cases of mild or moderate cognitive functioning, a referral is sometimes made to a neuropsychologist for a comprehensive neuropsychological assessment. This referral typically includes the referral letter, patient notes and brief cognitive assessment results, which in CP1 are sent directly by the primary physician to the neuropsychologist, while in CP2, the nurse facilitates the referral process.
Phase 3. Detailed neuropsychological assessment
While both CP1 and CP2 followed a similar process during the in-depth neuropsychological assessment phase, CP1 includes an additional preliminary step in which the neuropsychologist contacts the person with PD for a 30-minute call to review their case and schedule the appointment. During the visit to the neuropsychologist, the person with PD undergoes a comprehensive battery of assessments, conducted over a 2–5-h appointment. Upon completion of the assessment, the neuropsychologist schedules a feedback session with the person with PD and their support person to discuss the findings of the assessment.
Phase 4. Feedback
Following the detailed neuropsychological assessment, the neuropsychologist reviews the results and prepares a detailed report. This report is scanned and uploaded into the iEMR and is subsequently shared with the referring physician or nurse. For individuals following the DBS or gamma knife procedure track, a case conference is convened in CP2, involving relevant physicians and nurses, to assess eligibility for the procedure. If deemed eligible, the primary physician proceeds with the referral for the procedure. For persons with PD on the alternate journey, the results are communicated during the feedback session, and appropriate management strategies are provided by the neuropsychologist. Moreover, in CP2, the referring physician or nurse typically shares the results with the person with PD's general practitioner to facilitate ongoing support within the community.
Opportunities for technology integration
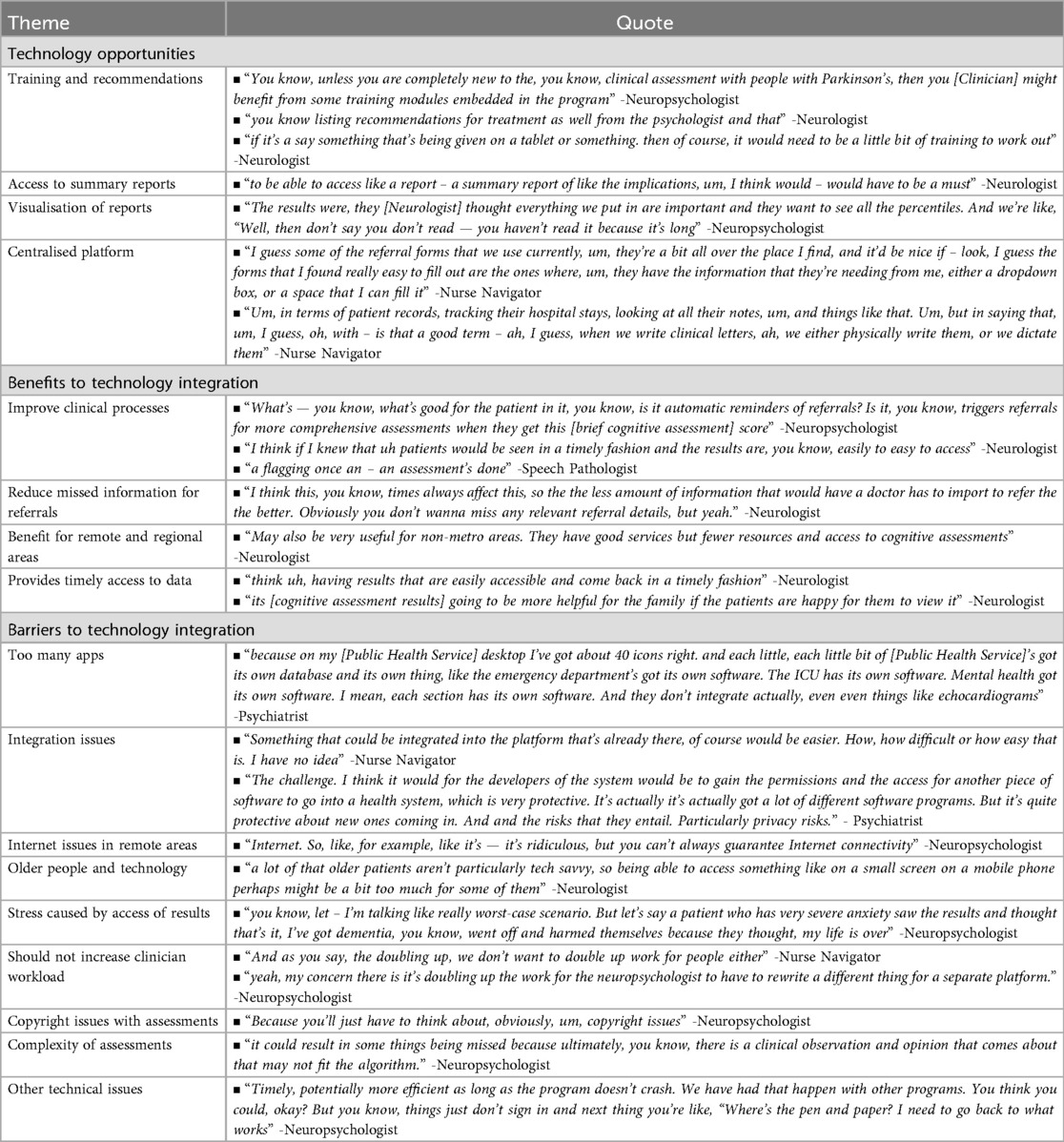
During the neuropsychological assessment process for people with PD, participants identified several technological opportunities to enhance clinical care delivery (Table 2). Among these opportunities, technology-enabled training support emerged as a promising solution towards delivering specialised training across multiple roles in the assessment pathway. For instance, at the brief cognitive screening phase, nurse navigators in CP2 conducted screenings but required additional training for this role. Neurologists needed enhanced knowledge regarding referral pathways after receiving cognitive assessment results. At the detailed neuropsychological assessment and feedback phases, neuropsychologists across both clinical pathways required training in PD-specific practices.
Beyond training support, participants suggested specific technological solutions to enhance the assessment process. The findings emphasised a need for technology that enabled neurologists to view neuropsychologist availability before making referrals, streamlining the referral process. The participants also highlighted the importance of developing technology capable of providing concise assessment summaries. Visualisations could be incorporated to improve interpretation, while search functionality would enable quick access to specific clinical information. Given these various requirements for managing both referrals and clinical information, strong support emerged for developing a centralised platform. This platform would not only manage referrals throughout the neuropsychological assessment process but gather necessary clinical information and facilitate sharing between various healthcare providers.
Benefits and barriers to technology integration
Participants described the integration of technology within clinical pathways could provide numerous benefits (Table 2). The primary benefit is that technology could support the clinical processes, while reducing the risk of missed referrals. The technology could offer significant advantages to clinicians in remote or regional areas, where face-to-face services are limited. It would promote collaboration among stakeholders and enable monitoring of cognition over time, while providing patients access to their results.
Despite these benefits, several challenges were identified related to (1) technology, (2) user adoption and accessibility, (3) patient experience and concerns, and (4) assessment methodologies and constraints. Participants expressed concerns about the number of applications already in use within their workstations and preferred integrating the platform into existing systems. This would avoid the added burden of maintaining multiple systems. However, they acknowledged the integration challenges due to security and privacy concerns within healthcare infrastructure. Furthermore, given technology would benefit rural and remote regions, participants raised concerns about internet access in these areas (Barrier 1).
Challenges regarding the use of technology by people with PD and their support persons, particularly considering the older age of this population (Barrier 2) and the potential stress caused by sharing results before a feedback session (Barrier 3), were described. Additionally, the implemented technology needs to be easy to use for the physician, requiring minimal learning or training (Barrier 2).
Finally, several concerns emerged about conducting neuropsychological assessments online. Technical problems included program crashes during assessments, poor internet connectivity, and sign-in difficulties. These technical challenges often forced neuropsychologists to return to traditional methods. Copyright issues with assessment materials and challenges translating clinical observations used in neuropsychological assessment into an algorithm were reported as significant concerns (Barrier 4).
Discussion
This research examined the complex landscape of neuropsychological assessments for people with PD across two healthcare service models. Through systematic analysis, we mapped a four-phase progression: the initial recognition of cognitive assessment needs, preliminary screening protocols, comprehensive assessment procedures, and post-feedback management. Our findings revealed variations between clinical pathways, particularly in their approach to healthcare delivery and stakeholder involvement. The study highlighted several challenges, including limited awareness, time constraints, long waiting periods for assessments, lengthy appointment times, and concerns regarding report length. The literature has also highlighted these challenges, which impact service access among people with PD and contribute to poor understanding of cognitive impairment and limited support and treatment (29).
According to the study finding, health technologies could support some of the healthcare processes. The implementation of technology in healthcare settings has been driven by goals of enhancing service efficiency, care quality, cost effectiveness, accessibility, and minimising wait times (30, 31). This broader movement toward technology integration in neuropsychology is exemplified by Singh et al. (32) hybrid neuropsychology model, which proposes developing technology-based practices, integrating data science, and collaborating with innovators from other field. Consequently, many health service improvement studies have adopted this approach when investigating technological interventions (33–37), including those focused on cognitive assessments (38, 39). Of these technologies, telemedicine for remote neuropsychological assessment was predominantly studied (40–42), as most studies demonstrated strong agreement between telehealth and face-to-face neuropsychological assessments (42). For instance, Carotenuto et al. (40) highlighted that Mini-Mental State Examination (MMSE) showed comparable results between traditional cognitive screening and telehealth administration.
This raises the question of which technological solutions could enhance these clinical pathways. Findings present design considerations for such technology. Integration emerged as a primary consideration, with stakeholders emphasising the need for solutions that seamlessly connect with existing Electronic Medical Records to avoid the burden of managing multiple platforms. This integration requirement becomes crucial given the complex nature of neuropsychological assessments and the need to maintain comprehensive patient records across different healthcare providers. Accessibility formed the second consideration, particularly given the diverse needs of remote and regional areas, as well as the varying technical proficiencies of different user groups. The study found that technology solutions must balance sophisticated functionality with user-friendly interfaces to accommodate both healthcare providers and individuals who may have limited technology experience. The third consideration centred on workflow support, emphasising streamlined referral management, summarised report presentation and improved result visualisation. This includes tools for scheduling, assessment tracking, simplified report generation, and lengthy reporting processes. A final consideration is the need for the technology to increase awareness of the role of cognitive assessment in PD.
However, the findings revealed several potential barriers to technology adoption, including security and privacy concerns, age-related challenges, usability issues, and copyright restrictions. Germine et al. (43) also identified major implementation barriers related to digital neuropsychology such as the impact on test interpretation and normative data, varying cognitive and motor demands across devices, and potential test obsolescence due to rapid technological advancement. These barriers can be identified and mitigated through appropriate design and development methodologies, as described by previous studies focusing on older populations (44) and technical-organisational implementation (45). Co-design represents one such methodology that has been recognised as important for conducting research with people who have PD and cognitive impairments (46, 47). This approach is particularly well-suited to PD populations, as recent research demonstrates that people with early to mid-stage PD maintain accurate awareness of their cognitive status and can reliably report on their difficulties (48), making them valuable partners in identifying authentic user needs. The co-design process involves actively involving people with lived experience into the design process, by treating them as individuals with equal creative input as designers in the development process (49). Several approaches can be implemented to facilitate involvement including the use of drawings, photographs and prototypes to explore and develop solutions (49). These co-design methodologies provide a pathway for developing technology solutions that are both technically robust and genuinely responsive to the complex needs identified in the clinical pathways.
Strengths and limitations
Our study provides the first comprehensive mapping of neuropsychological assessment pathways for people with PD across two Australian public health services, incorporating perspectives from clinical experts. The use of journey mapping enabled detailed visualisation and analysis of complex clinical workflows. We achieved strong participation across various clinical roles, with fifteen participants representing different specialties and experience levels.
Several limitations should be noted. Firstly, our study focused on two public health services in Queensland, Australia, limiting generalisability to other healthcare settings or regions. Secondly, while we captured clinician perspectives, we did not include people with PD or their support persons, whose lived experience could provide valuable insights into the assessment process. Finally, we included clinical experts in the public health system, excluding perspectives from private practice and other healthcare providers who may have different experiences and approaches.
Conclusion
This study provides insights into the current landscape of neuropsychological assessments for people with PD from the perspective of healthcare professionals across two public health services in Queensland, revealing a complex four-phase process with variations between clinical pathways. Our findings highlight challenges in the assessment process, including limited clinician awareness, time constraints, and lengthy waiting periods, while identifying opportunities for technology integration. The journey mapping approach successfully visualised these clinical pathways and identified areas for improvement through technological solutions. However, successful implementation of technologies must consider key barriers including system integration requirements, accessibility needs, and copyright restrictions for assessment tools. These findings suggest that while technology offers potential to enhance neuropsychological assessment, implementation must be designed to address barriers while maintaining assessment quality and accommodating the diverse needs of healthcare providers and patients. The PDCogniCare project will build upon this foundation to advance the development of technology-enabled neuropsychological assessments.
Data availability statement
The datasets presented in this article are not readily available because given the sample size it may be able to identify individuals participants, which is against what was approved in our ethics approval. Requests to access the datasets should be directed to Elton Lobo,ZWx0b24ubG9ib0B1cS5lZHUuYXU=.
Ethics statement
The studies involving humans were approved by Metro North Health Human Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
EL: Conceptualization, Formal analysis, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. PW: Conceptualization, Investigation, Methodology, Writing – review & editing. DB: Data curation, Formal analysis, Investigation, Project administration, Validation, Writing – review & editing. KS: Investigation, Writing – review & editing. DP: Resources, Writing – original draft, Writing – review & editing. DS: Data curation, Formal analysis, Validation, Writing – review & editing. RF: Resources, Writing – original draft. JY: Data curation, Investigation, Writing – review & editing. JL: Writing – review & editing. LM: Investigation, Writing – review & editing. ND: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the 2022 MRFF Dementia Ageing and Aged Care Mission Grant. ND is funded by the University of Queensland Amplify Fellowship.
Acknowledgments
The authors sincerely appreciate the feedback on the study procedures provided by the members of the Consumer and Community Involvement Group. We acknowledge the PDCogniCare team members: Tiffany Au, Prof Elizabeth Beattie, A/Prof Annette Broome, Prof Gerard Byrne, A/Prof Mark Chatfield, Anna Kelder, Dr Syed Afroz Keramat, Prof Sharon Naismith, Prof Peter Nestor, Stuart Robertson, Kumar Sivakumaran, Dr Donna Spooner, Helen Tinson, Professor Martie-Louise Verreynne. We also acknowledge PDCogniCare partners: Parkinson's Queensland Inc., Dementia Australia, Royal Brisbane & Women's Hospital, Princess Alexandra Hospital, OPN365 Pty Ltd., ADNeT, Lions Club of Brisbane Inner North, and Lions District 201Q3.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gibson LL, Weintraub D, Lemmen R, Perera G, Chaudhuri KR, Svenningsson P, et al. Risk of dementia in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. (2024) 39(10):1697–709. doi: 10.1002/mds.29918
2. Gallagher J, Gochanour C, Caspell-Garcia C, Dobkin RD, Aarsland D, Alcalay RN, et al. Long-term dementia risk in Parkinson disease. Neurology. (2024) 103(5):e209699. doi: 10.1212/WNL.0000000000209699
3. Pigott K, Rick J, Xie SX, Hurtig H, Chen-Plotkin A, Duda JE, et al. Longitudinal study of normal cognition in Parkinson disease. Neurology. (2015) 85(15):1276–82. doi: 10.1212/WNL.0000000000002001
4. Zucchella C, Federico A, Martini A, Tinazzi M, Bartolo M, Tamburin S. Neuropsychological testing. Pract Neurol. (2018) 18(3):227. doi: 10.1136/practneurol-2017-001743
5. Howieson D. Current limitations of neuropsychological tests and assessment procedures. Clin Neuropsychol. (2019) 33(2):200–8. doi: 10.1080/13854046.2018.1552762
6. Rascovsky K. A primer in neuropsychological assessment for dementia. Pract Neurol. (2016) 15(6):20–5.
7. Allahham R, Wong D, Pike KE. What do private practice referrers value about neuropsychology assessment? Aust Psychol. (2023) 58(6):411–31. doi: 10.1080/00050067.2023.2239436
8. Scheffels JF, Fröhlich L, Kalbe E, Kessler J. Concordance of mini-mental state examination, Montreal cognitive assessment and Parkinson neuropsychometric dementia assessment in the classification of cognitive performance in Parkinson’s disease. J Neurol Sci. (2020) 412:116735. doi: 10.1016/j.jns.2020.116735
9. Mccombe N, Ding X, Prasad G, Gillespie P, Finn DP, Todd S, et al. Alzheimer’s disease assessments optimized for diagnostic accuracy and administration time. IEEE J Transl Eng Health Med. (2022) 10:1–9. doi: 10.1109/JTEHM.2022.3164806
10. Roitsch J, Prebor J, Raymer Anastasia M. Cognitive assessments for patients with neurological conditions: a preliminary survey of speech-language pathology practice patterns. Am J Speech-Lang Pathol. (2021) 30(5):2263–74. doi: 10.1044/2021_AJSLP-20-00187
11. Creavin ST, Fish M, Lawton M, Cullum S, Bayer A, Purdy S, et al. A diagnostic test accuracy study investigating general practitioner clinical impression and brief cognitive assessments for dementia in primary care, compared to specialized assessment. J Alzheimers Dis. (2023) 95(3):1189–200. doi: 10.3233/JAD-230320
12. Schipper K, Dauwerse L, Hendrikx A, Leedekerken JW, Abma TA. Living with Parkinson’s disease: priorities for research suggested by patients. Parkinsonism Relat Disord. (2014) 20(8):862–6. doi: 10.1016/j.parkreldis.2014.04.025
13. Zhang Y, Padman R, Patel N. Paving the COWpath: learning and visualizing clinical pathways from electronic health record data. J Biomed Inform. (2015) 58:186–97. doi: 10.1016/j.jbi.2015.09.009
14. Snoswell CL, Taylor ML, Comans TA, Smith AC, Gray LC, Caffery LJ. Determining if telehealth can reduce health system costs: scoping review. J Med Internet Res. (2020) 22(10):e17298. doi: 10.2196/17298
15. Stillerova T, Liddle J, Gustafsson L, Lamont R, Silburn P. Could everyday technology improve access to assessments? A pilot study on the feasibility of screening cognition in people with Parkinson’s disease using the Montreal cognitive assessment via internet videoconferencing. Aust Occup Ther J. (2016) 63(6):373–80. doi: 10.1111/1440-1630.12288
16. Hernandez HHC, Ong PL, Anthony P, Ang SL, Salim NBM, Yew PYS, et al. Cognitive assessment by telemedicine: reliability and agreement between face-to-face and remote videoconference-based cognitive tests in older adults attending a memory clinic. Ann Geriatr Med Res. (2022) 26(1):42–8. doi: 10.4235/agmr.22.0005
17. Zadik L, Perlman S, Barak O, Ziv-Baran T. Evaluation of Montreal cognitive assessment (MoCA) administered via videoconference. J Am Med Dir Assoc. (2023) 24(12):1942–7.e3. doi: 10.1016/j.jamda.2023.08.015
18. Bălăeţ M, Alhajraf F, Zerenner T, Welch J, Razzaque J, Lo C, et al. Online cognitive monitoring technology for people with Parkinson’s disease and REM sleep behavioural disorder. NPJ Digit Med. (2024) 7(1):118. doi: 10.1038/s41746-024-01124-6
19. Marques-Costa C, Simões MR, Almiro PA, Prieto G, Salomé Pinho M. Integrating technology in neuropsychological assessment: a narrative review of the main obstacles, new developments, and potential issues in assessment through tablets. Eur Psychol. (2023) 28(1):1–11. doi: 10.1027/1016-9040/a000484
20. Parsey CM, Schmitter-Edgecombe M. Applications of technology in neuropsychological assessment. Clin Neuropsychol. (2013) 27(8):1328–61. doi: 10.1080/13854046.2013.834971
21. Lenz R, Blaser R, Beyer M, Heger O, Biber C, Bäumlein M, et al. IT support for clinical pathways—lessons learned. Int J Med Inf. (2007) 76:S397–402. doi: 10.1016/j.ijmedinf.2007.04.012
22. Joseph AL, Kushniruk AW, Borycki EM. Patient journey mapping: current practices, challenges and future opportunities in healthcare. Knowl Manag E-Learn. (2020) 12(4):387–404. English. doi: 10.34105/j.kmel.2020.12.021
23. Ly S, Runacres F, Poon P. Journey mapping as a novel approach to healthcare: a qualitative mixed methods study in palliative care. BMC Health Serv Res. (2021) 21(1):915. doi: 10.1186/s12913-021-06934-y
24. Kushniruk AW, Borycki EM, Parush A. A case study of patient journey mapping to identify gaps in healthcare: learning from experience with cancer diagnosis and treatment. Knowl Manag E-Learn. (2020) 12(4):405. doi: 10.34105/j.kmel.2020.12.022
25. Ayton D, Tsindos T, Berkovic D. Qualitative Research: A Practical Guide for Health and Social Care Researchers and Practitioners. Melbourne, VIC: Council of Australian University Librarians, Open Educational Resources (2023).
26. Atkins L, Francis J, Islam R, O'Connor D, Patey A, Ivers N, et al. A guide to using the theoretical domains framework of behaviour change to investigate implementation problems. Implement Sci. (2017) 12(1):77. doi: 10.1186/s13012-017-0605-9
27. Breimaier HE, Heckemann B, Halfens RJG, Lohrmann C. The consolidated framework for implementation research (CFIR): a useful theoretical framework for guiding and evaluating a guideline implementation process in a hospital-based nursing practice. BMC Nurs. (2015) 14(1):43. doi: 10.1186/s12912-015-0088-4
28. Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. (2008) 62(1):107–15. doi: 10.1111/j.1365-2648.2007.04569.x
29. Pigott JS, Davies N, Chesterman E, Read J, Nimmons D, Walters K, et al. Delivering optimal care to people with cognitive impairment in Parkinson’s disease: a qualitative study of patient, caregiver, and professional perspectives. Park Dis. (2023) 2023(1):9732217. doi: 10.1155/2023/9732217
30. McCarthy S, O’Raghallaigh P, Woodworth S, Lim YL, Kenny LC, Adam F. An integrated patient journey mapping tool for embedding quality in healthcare service reform. J Decis Syst. (2016) 25(sup1):354–68. doi: 10.1080/12460125.2016.1187394
31. Weizenbaum EL, Fulford D, Torous J, Pinsky E, Kolachalama VB, Cronin-Golomb A. Smartphone-based neuropsychological assessment in Parkinson’s disease: feasibility, validity, and contextually driven variability in cognition. J Int Neuropsychol Soc. (2022) 28(4):401–13. doi: 10.1017/S1355617721000503
32. Singh S, Germine L. Technology meets tradition: a hybrid model for implementing digital tools in neuropsychology. Int Rev Psychiatry. (2021) 33(4):382–93. doi: 10.1080/09540261.2020.1835839
33. Das A, Bøthun S, Reitan J, Dahl Y, Marcus A. The Use of Generative Techniques in Co-design of MHealth Technology and Healthcare Services for COPD Patients. Design, User Experience, and Usability: Interactive Experience Design. Cham: Springer International Publishing (2015). p. 587–95.
34. Lavallee DC, Lee JR, Semple JL, Lober WB, Evans HL. Engaging patients in co-design of Mobile health tools for surgical site infection surveillance: implications for research and implementation. Surg Infect (Larchmt). (2019) 20(7):535–40. doi: 10.1089/sur.2019.148
35. Leite H, Hodgkinson IR. Telemedicine co-design and value co-creation in public health care. Aust J Public Admin. (2021) 80(2):300–23. doi: 10.1111/1467-8500.12473
36. Song T, Yu P, Bliokas V, Probst Y, Peoples GE, Qian S, et al. A clinician-led, experience-based co-design approach for developing mHealth services to support the patient self-management of chronic conditions: development study and design case. JMIR Mhealth Uhealth. (2021) 9(7):e20650. doi: 10.2196/20650
37. Ospina-Pinillos L, Davenport T, Mendoza Diaz A, Navarro-Mancilla A, Scott EM, Hickie IB. Using participatory design methodologies to co-design and culturally adapt the Spanish version of the mental health eClinic: qualitative study. J Med Internet Res. (2019) 21(8):e14127. doi: 10.2196/14127
38. Casaletto KB, Heaton RK. Neuropsychological assessment: past and future. J Int Neuropsychol Soc. (2017) 23(9–10):778–90. doi: 10.1017/S1355617717001060
39. Spreij LA, Gosselt IK, Visser-Meily JMA, Nijboer TCW. Digital neuropsychological assessment: feasibility and applicability in patients with acquired brain injury. J Clin Exp Neuropsychol. (2020) 42(8):781–93. doi: 10.1080/13803395.2020.1808595
40. Carotenuto A, Traini E, Fasanaro AM, Battineni G, Amenta F. Tele-neuropsychological assessment of Alzheimer’s disease. J Pers Med. (2021) 11(8):688. doi: 10.3390/jpm11080688
41. Walker EJ, Kirkham FJ, Stotesbury H, Dimitriou D, Hood AM. Tele-neuropsychological assessment of children and young people: a systematic review. J Pediatr Neuropsychol. (2023) 9(3):113–26. doi: 10.1007/s40817-023-00144-6
42. Zeghari R, Guerchouche R, Tran-Duc M, Bremond F, Langel K, Ramakers I, et al. Feasibility study of an internet-based platform for tele-neuropsychological assessment of elderly in remote areas. Diagnostics. (2022) 12(4):925. doi: 10.3390/diagnostics12040925
43. Germine L, Reinecke K, Chaytor NS. Digital neuropsychology: challenges and opportunities at the intersection of science and software. Clin Neuropsychol. (2019) 33(2):271–86. doi: 10.1080/13854046.2018.1535662
44. Ostrowski AK, Harrington CN, Breazeal C, Park HW. Personal narratives in technology design: the value of sharing older Adults’ stories in the design of social robots. Front Robot AI. (2021) 8:716581. English. doi: 10.3389/frobt.2021.716581
45. Lobo EH, Frølich A, Rasmussen LJ, Livingston PM, Grundy J, Abdelrazek M, et al. Understanding the methodological issues and solutions in the research design of stroke caregiving technology. Front Public Health. (2021) 9:647249. English. doi: 10.3389/fpubh.2021.647249
46. Wang G, Marradi C, Albayrak A, van der Cammen TJM. Co-designing with people with dementia: a scoping review of involving people with dementia in design research. Maturitas. (2019) 127:55–63. doi: 10.1016/j.maturitas.2019.06.003
47. McNaney R, Vines J, Dow A, Robinson H, Robinson H, McDonald K, et al. Enabling the participation of people with Parkinson’s and their caregivers in co-inquiry around collectivist health technologies. Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems; 2018; Montreal QC, Canada: Association for Computing Machinery (2018). p. Paper 491. doi: 10.1145/3173574.3174065
48. Bălăeţ M, Alhajraf F, Bourke NJ, Welch J, Razzaque J, Malhotra P, et al. Metacognitive accuracy differences in Parkinson’s disease and REM sleep behavioral disorder relative to healthy controls [original research]. Front Neurol. (2024) 15:1399313. English. doi: 10.3389/fneur.2024.1399313
Keywords: cognition, journey map, neuropsychological assessment, Parkinson's disease, technology
Citation: Lobo EH, Worthy P, Brooks D, Shrubsole K, Pourzinal D, Sriram D, Fels R, Yang JH, Liddle J, Mitchell LK and Dissanayaka NN (2025) Enhancing neuropsychological assessment clinical pathways in Parkinson's disease through the use of technology. Front. Digit. Health 7:1681221. doi: 10.3389/fdgth.2025.1681221
Received: 7 August 2025; Accepted: 24 September 2025;
Published: 23 October 2025.
Edited by:
Hosna Salmani, Iran University of Medical Sciences, IranReviewed by:
Julia Das, Northumbria University, United KingdomMaria Balaet, Imperial College London, United Kingdom
Copyright: © 2025 Lobo, Worthy, Brooks, Shrubsole, Pourzinal, Sriram, Fels, Yang, Liddle, Mitchell and Dissanayaka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elton H. Lobo, ZWx0b24ubG9ib0B1cS5lZHUuYXU=
†ORCID:
Elton H. Lobo
orcid.org/0000-0003-0096-6318
 Elton H. Lobo
Elton H. Lobo Peter Worthy
Peter Worthy Deborah Brooks1
Deborah Brooks1 Jacki Liddle
Jacki Liddle Leander K. Mitchell
Leander K. Mitchell Nadeeka N. Dissanayaka
Nadeeka N. Dissanayaka