- 1Department of Pathology and Laboratory Medicine, Western University, London, ON, Canada
- 2School of Biomedical Engineering, Western University, London, ON, Canada
- 3Department of Ophthalmology, Western University, London, ON, Canada
With increasing incidence of diabetes worldwide, there is an ever-expanding number of patients with chronic diabetic complications such as diabetic retinopathy (DR), one of the leading causes of blindness in the working age population. Early screening for the onset and severity of DR is essential for timely intervention. With recent advancements in genomic technologies, epigenetic alterations in DR are beginning to unravel. Long non-coding RNAs (lncRNAs), which are key epigenetic mediators, have demonstrated implications in several (DR) related processes. Based on the previous research, we have developed a serum-based, multi-panel PCR test using 9 lncRNAs (ANRIL, MALAT1, WISPER, ZFAS1, H19, HOTAIR, HULC, MEG3, and MIAT) to identify and validate whether this panel could be used as a diagnostic and prognostic tool for DR. We initially used a cell culture model (human retinal endothelial cells) and confirmed that 25 mM glucose induces upregulations of ANRIL, HOTAIR, HULC, MALAT1, and ZFAS1, and downregulation of H19 compared to 5 mM glucose controls. Then as an initial proof-of-concept, we tested vitreous humor and serum samples from a small cohort of non-diabetic (N=10) and diabetic patients with proliferative retinopathy (PDR, N=11) and measured the levels of the 9 lncRNAs. Differential expressions of lncRNAs were found in the vitreous and serum of patients and showed significant correlations. We expanded our approach and assessed the same lncRNAs using samples from a larger cohort of diabetic (n= 59; M/F:44/15) and non-diabetic patients (n= 11; M/F:4/7). Significant increased lncRNA expressions of ANRIL, H19, HOTAIR, HULC, MIAT, WISPER and ZFAS1 were observed in the serum of diabetic patients (with varying stages of DR) compared to non-diabetics. No significant correlations were demonstrated between lncRNA expressions and creatinine or glycated hemoglobin (HbA1C) levels. Using ROC and further analyses, we identified distinct lncRNA phenotype combinations, which may be used to identify patients with DR. Data from this study indicate that a panel of serum lncRNAs may be used for a potential screening test for DR. Further large-scale studies are needed to validate this notion.
Introduction
Diabetes mellitus (DM) continues to wreak havoc across the world, where 463 million of the global population are estimated to have DM, while 700 million are projected to have DM by 2045 (1). DM is characterized by chronic hyperglycemia and the sustained elevation of blood glucose levels promotes cellular and tissue damage, which consequently leads to the development of diabetic complications. Among these complications, diabetic retinopathy (DR) is a prevalent microvascular complication of DM and one of the leading causes of preventable blindness in the working age population (2). To prevent irreversible visual loss from DR, screening for the onset and severity of DR has become critical for timely and necessary ophthalmic examination and treatment (3). To bridge this gap in healthcare delivery, here we describe a novel lncRNA-based serum test which may facilitate effective screening and identification of DR.
According to the International Council of Ophthalmology classification, DR can be categorized into five progressive stages of retinopathy: no retinopathy, mild non-proliferative, moderate non-proliferative, severe non-proliferative, and proliferative retinopathy (4). Each stage of DR is categorized by distinct clinical observations, which can include retinal vascular-related abnormalities (microaneurysms, intraretinal hemorrhages, and venous beading), retinal thickening (edema), accumulation of lipid deposits, presence of neovascularization, and/or vitreous/preretinal hemorrhage (4). According to one study in the US involving 4617 patients, 6.6% of patients with non-proliferative DR (NPDR) progressed to proliferative DR (PDR). Proliferative DR is considered the more advanced stage of DR and is characterized by the onset of neovascularization at various retinal regions (including new vessels at the inner surface of the retina, on or near the optic disc and new vessels elsewhere in the retina) compared to non-proliferative stages of DR (4, 5). These vessels are prone to bleeding and may undergo fibrosis and contraction, which can ultimately lead to blindness and a profound impact on the quality of life for patients (6).
Presently, there are several relevant risk factors that are used to monitor potential development of DR, including the duration of diabetes, poor glycemic control (high glycated hemoglobin [HbA1c levels]), presence of hypertension, dyslipidemia, and a higher body mass index (7). However, based on several large population-based clinical studies, substantial variation in the onset and severity of DR have been documented in patients living with DM that is not completely explained by the known risk factors. For example, total glycemic exposure (duration of diabetes and HbA1c values) explained only ~11% of the variation in DR risk for the entire study population in the Diabetes Control and Complications Trial, which suggests that the remaining 89% of the variation risk is due to other factors (i.e., genetic determinants and/or epigenetic factors) in the diabetic milieu that are independent of HbA1c (8). As a result, to effectively monitor glycemic control and implement timely treatment protocols, it becomes imperative that research efforts are invested towards identifying these unexplained factors and novel molecular players that continue to contribute to the development and progression of microvascular complications such as DR. Furthermore, implementation of a test to effectively identify and treat patients early in the disease process will result in considerable cost savings, since the cost of caring for patients with PDR is much greater than the costs of managing patients with NPDR (9, 10).
Given the advent of novel genomic technologies within the last decade, recent studies examining the epigenetic landscape in DR have shed light on the ‘dark matter’ of the epi/genome and have opened the door for the selective targeting of promising molecular candidates in the diagnosis and/or therapy of DR. Among these molecules, long non-coding RNAs (lncRNAs), which do not have protein-coding potential and are greater than 200 nucleotides in length, have demonstrated critical implications in a number of DR-related processes including angiogenesis (11–13), inflammation (14), and even fibrosis-related mechanisms (i.e., endothelial-to-mesenchymal transition) (15).
In this study, based on the collective findings from our previous lncRNA microarray involving human retinal endothelial cells (HRECs) subjected to basal and high glucose conditions (GEO ID: 122189) and existing mechanistic-based research performed by us and others (11–19), we selected 9 prominent lncRNAs (ANRIL, MALAT1, WISPER, ZFAS1, H19, HOTAIR, HULC, MEG3, and MIAT) and developed a multi-lncRNA PCR panel in order to investigate and validate whether a diagnostic biomarker panel could be used to distinguish between patients with non-DR and DR. We then carried out additional statistical analyses to determine the potential discriminative ability of this lncRNA-panel to diagnose and identify the various sub-stages of DR.
Materials and Methods
Development of the Multi-lncRNA PCR Panel
Customized human lncRNA primers were developed (Table 1) and subsequently aliquoted and air dried into a 96-well plastic qPCR plate. Custom qPCR plates were stored in -20°C prior to use. A single panel (consisting of 10 wells) in the lncRNA PCR plate examined 9 distinct lncRNAs (MALAT1, HOTAIR, H19, MEG3, ANRIL, MIAT, WISPER, ZFAS1, and HULC) and one house-keeping gene (β-actin). Synthesized cDNA (following the protocol below) was diluted and combined with SYBR-green master mix (Takara Bio, Mountain View, CA, USA), and then aliquoted into the 96-well PCR plate containing the pre-aliquoted PCR panel. The panel was then inserted into the LightCycler 96 System (Roche Diagnostics, Laval, QC, CAN) for amplification.
RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
As described by us previously (12–15), total RNA was extracted using the TRIzol reagent (Invitrogen). RNA concentrations were quantified using a spectrophotometer (260 nm; Gene Quant, Pharmacia Biotech, USA) and 1-2 μg of total RNA was reverse transcribed to complementary DNA (cDNA) using a high-capacity cDNA reverse-transcription kit (Applied Biosystems/Thermo Fisher Scientific). cDNA was then amplified in the LightCycler 96 System (Roche Diagnostics, Laval, QC, CAN) using the multi-lncRNA PCR panel. RT-qPCR results were analyzed using the LightCycler 96 SW 1.1 software (Roche) and expression levels were calculated by the relative standard curve method using β-actin as an internal control for sample normalization.
Cell Culture
Prior to the clinical validation of our panel, we first validated our assay in vitro. Human retinal microvascular endothelial cells (HRECs; Cell Systems, Kirkland, WA, USA; catalog number ACBRI 181) were cultured in endothelial basal media-2 (EBM-2, Lonza, Walkersville, MD, USA) containing endothelial growth media-2 (EGM-2) SingleQuots (Lonza). All cells were grown in 75 cm2 culture flasks and maintained in a humidified incubator containing 5% CO2 at 37°C. As described previously (12, 14), to reduce variability for experimentation, cells were used between passages three and six and the cellular densities were determined accordingly based on the type of culture plates used for each experiment. Generally, once 80% confluence was obtained post-seeding, ECs were cultured in serum and growth factor-free medium overnight before exposure to different D-glucose levels (final glucose concentrations of 5 mmol/L, mimicking normoglycemia [NG], and 25 mmol/L, mimicking hyperglycemia [HG]) for 48 hours; the selected glucose level is based on a large volume of previous experiments (12–15). Twenty-five mmol/L L-glucose was used as osmotic control. All in vitro experiments were independently repeated at least three times and performed with six replicates, unless specified.
Collection and Analyses of Vitreous and Serum Samples
The study was approved by the Western Research Ethics Board and Lawson Health Research Institute at the University of Western Ontario (London, ON, CAN). Informed consent was received from patients prior to obtaining specimens and the research was conducted following the Declaration of Helsinki. As an initial test to identify whether serum lncRNAs appropriately reflect the vitreous lncRNAs, we collected both serum and undiluted vitreous humor (VH) samples from patients, undergoing a pars plana vitrectomy by an experienced vitreoretinal surgeon (12). These samples were categorized into two groups: control and DR.
In the initial cohort, the DR group comprised of patients diagnosed with advanced stages of DR, including proliferative DR (PDR; n= 11; mean age ± SD= 60.9 ± 10.4 years; 10 males and 1 female, 1 type I diabetic and 10 type 2 diabetics), while the control group consisted of patients that had no previous history of DR and were diagnosed with idiopathic macular hole or a separate non-diabetic ocular condition (n= 10; mean age ± SD= 69.2 ± 8.9 years; 2 males and 8 females). PDR was defined as the presence of neovascularization or fibrous proliferation of the disc or elsewhere on the retina. Total RNA was extracted from 500 μL of VH and 200 μL of serum samples using a serum RNA extraction kit (Bio Basic Inc., Markham, ON, CAN) and was subsequently examined by RT-qPCR.
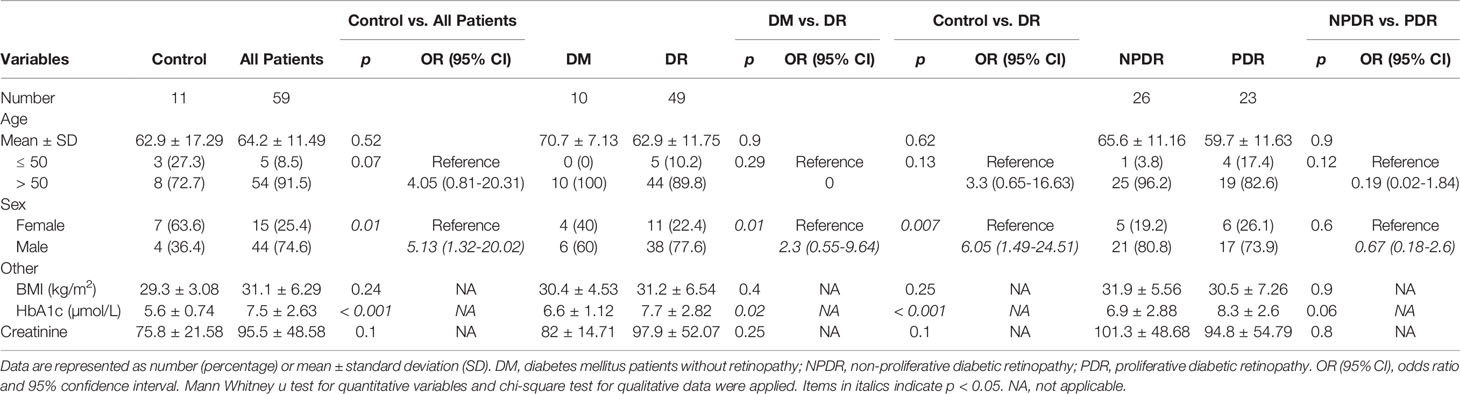
Following the results of our initial experiment, we expanded our test using only serum samples from a larger new cohort of patients. Briefly, undiluted serum samples were collected in BD gold-top serum separator tubes. Serum specimens were submitted to the research laboratory, where total RNA was extracted from 200 μL of serum samples using TRIzol and the serum RNA extraction kit mentioned above. The expression levels of the 9-specific lncRNAs (ANRIL, H19, HOTAIR, HULC, MALAT1, MEG3, MIAT, WISPER and ZFAS1) were assessed using RT-qPCR, where a research technician was masked to the sample type. The subjects include 7 type I diabetic and 52 type 2 diabetics. Specimens were categorized into two groups: control (‘C’) and diabetic retinopathy (‘P’; DR). The ‘P’ group comprised of diabetic patients diagnosed with varying stages of DR (indicated as ‘0’= diabetic patients with no retinopathy, ‘1’ = diabetic patients with non-proliferative DR [NPDR], and ‘2’= diabetic patients with proliferative DR [PDR]) (n= 59; mean age ± SD= 64.2 ± 11.5 years; 44 males and 15 females), while the control group consisted of non-diabetic patients who were seen at the eye clinic for conditions unrelated to diabetes (n= 11; mean age ± SD= 62.9 ± 17.3 years; 4 males and 7 females).
Statistical Analyses
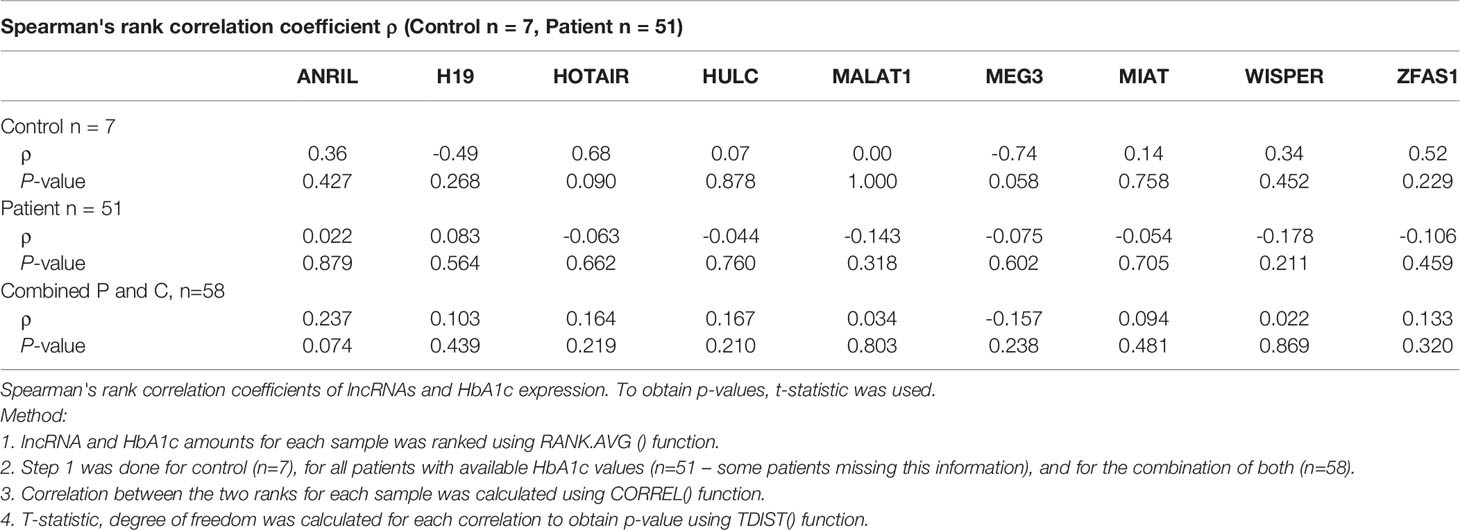
Statistical differences were evaluated between groups using GraphPad Prism 7 (La Jolla, CA, USA) and Microsoft Excel (Washington, USA). Data were considered statistically significant if the p value was less than 0.05. Statistical significance for the clinical samples (with non-normal distribution) were identified using the Mann-Whitney U test (when comparing two conditions) or Kruskal-Wallis one-way ANOVA (for multiple group comparisons) and linear regression analyses wherever appropriate. ROC curves were used to elucidate clinical significance of lncRNA molecules in control versus diabetic patients with no retinopathy, NPDR and PDR by comparing sensitivities and false positive rates. AUC numbers were approximated simply by sum of the areas of rectangles in the ROC curves. Creatinine and HbA1c levels were compared between control and patient groups using Spearman’s rank correlation coefficient. Two outliers from control samples I6 and A1 for MEG3 (86096.52 and 2022.94, respectively) were removed for better graphical representation of the results.
Data Availability
All data generated or analyzed during this study are included in this published article.
Results
Glucose Causes Differential Expressions of lncRNAs in Retinal Endothelial Cells
Using our established cell culture model and RT-qPCR (12–15, 17), we confirmed the lncRNA expressions of ANRIL, H19, HOTAIR, HULC, MALAT1, MEG3, MIAT, WISPER, and ZFAS1 in HRECs exposed to 25 mM (HG) or 5 mM (NG) glucose over 48 hours from our array (Figure 1). As depicted in Figure 1, HG induced significant upregulations of ANRIL, HOTAIR, HULC, MALAT1, and ZFAS1, and a significant downregulation of H19 in HRECs compared to NG controls. Although not statistically significant, in comparison to NG controls, general trends of upregulation were observed for MIAT and WISPER, while MEG3 appeared to demonstrate decreased expressions following HG stimulation. No alterations of such lncRNA expressions were seen when the cells were exposed to 25mM L-glucose (not shown).
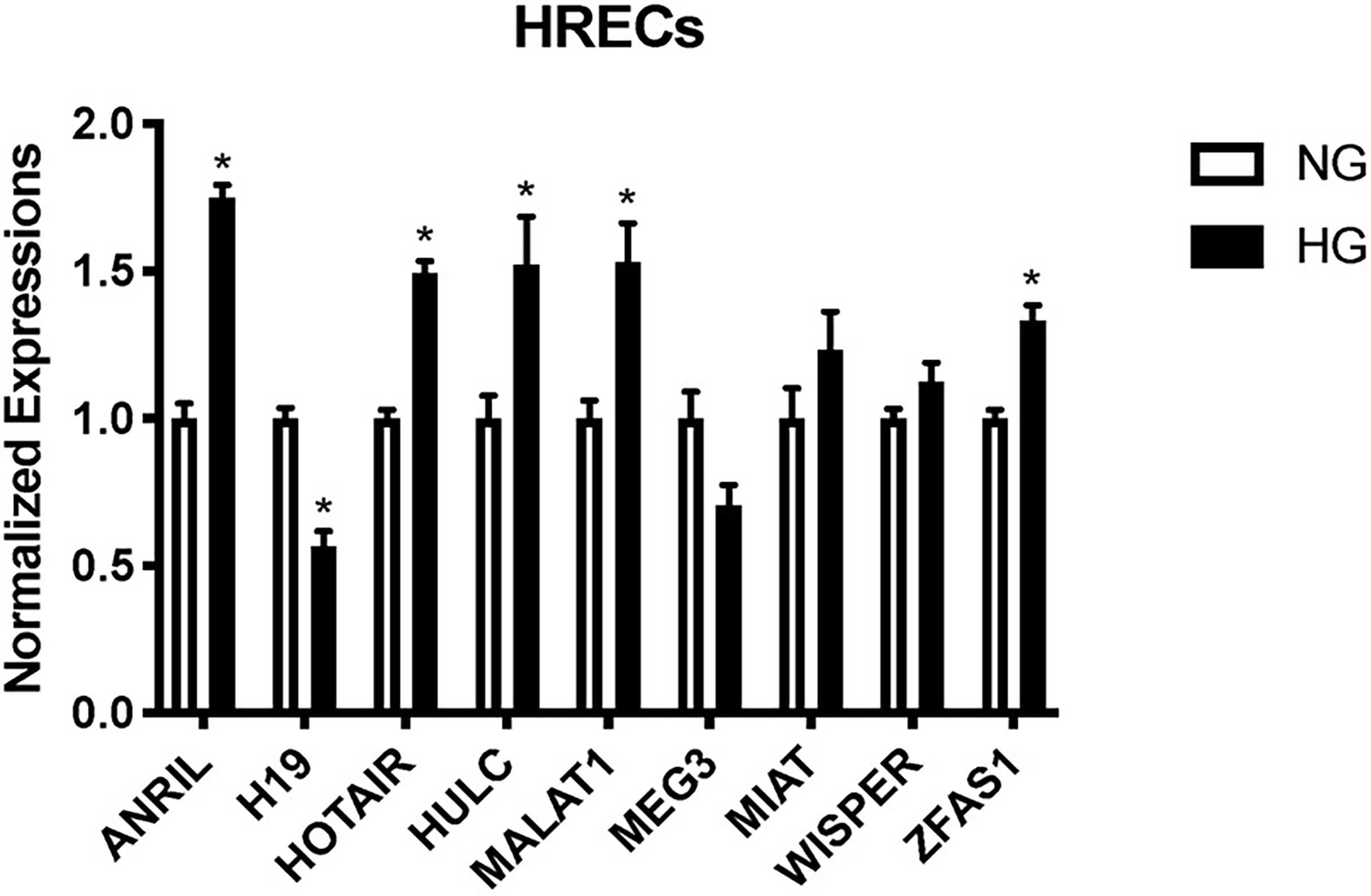
Figure 1 Glucose-treated human retinal endothelial cells (HRECs) demonstrate differential expressions of lncRNAs at 48 hours. RT-qPCR analysis of ANRIL, H19, HOTAIR, HULC, MALAT1, MEG3, MIAT, WISPER and ZFAS1 expressions in HRECs exposed to 25 mM (HG) or 5 mM (NG) glucose over 48 hours [data (mean ± SEM); N=6 per group; normalized to β-actin and expressed as a fold change of NG; *p<0.05 compared to NG].
Expressions of lncRNAs in the Serum Correlates With Vitreous Levels in Diabetic Retinopathy
As an initial proof-of-concept experiment, we wanted to determine whether the selected lncRNAs could be detected in human DR. So, we analyzed both vitreous humor and serum samples from an initial small cohort of patients as outlined above using the customized qPCR-based panel. Similar to the trends observed in vitro, differential expressions of lncRNAs were found in the vitreous and serum of patients with PDR compared to non-PDR patients—further suggesting the biological significance of lncRNAs in human DR (Figure 2). Specifically, alterations were significantly pronounced in the serum expression levels of ANRIL (Figure 2A), H19 (Figure 2B), HOTAIR (Figure 2C), MALAT1 (Figure 2E), WISPER (Figure 2H) and ZFAS1 (Figure 2I) in PDR. However, we did not find significant alterations with respect to the serum expressions of HULC (Figure 2D), MEG3 (Figure 2F) and MIAT (Figure 2G) in PDR, and such findings may be related to the relatively small sample size. Moreover, as shown in Figure 3, significant expressions of ANRIL (Figure 3A), HOTAIR (Figure 3B), H19 (Figure 3C), MALAT1 (Figure 3E), MIAT (Figure 3G), WISPER (Figure 3H) and ZFAS1 (Figure 3I) were observed in the vitreous of PDR patients, while statistical significances were not observed for HULC (Figure 3D) and MEG3 expressions (Figure 3F).
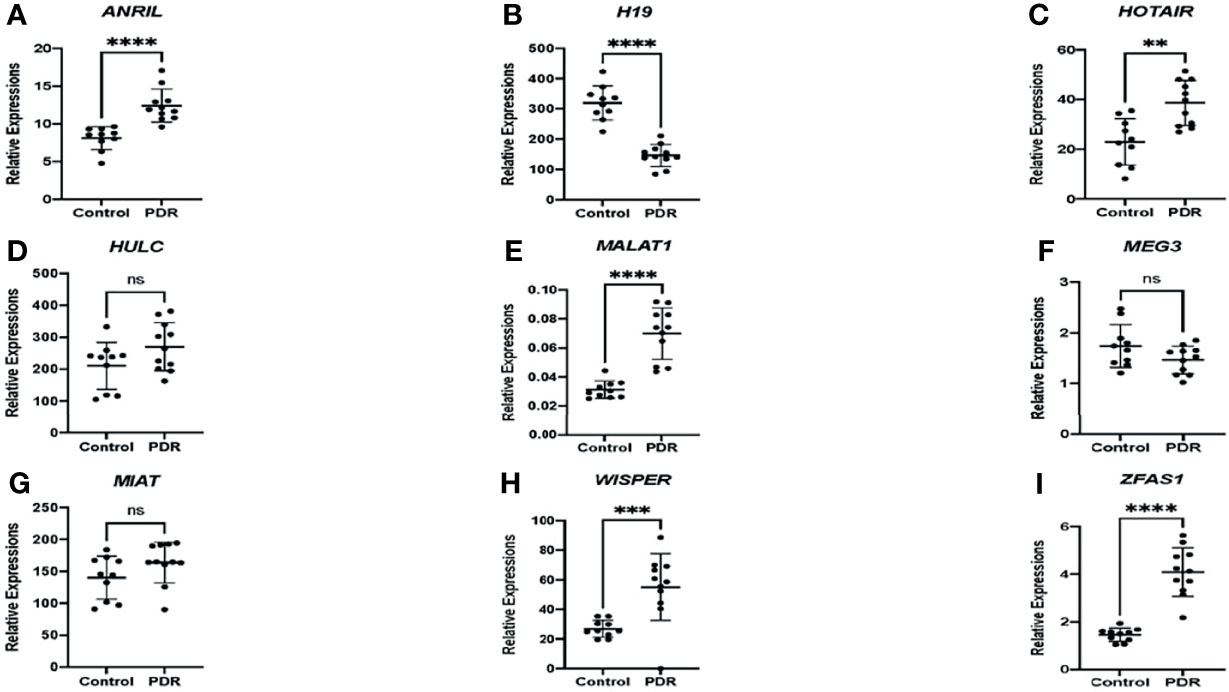
Figure 2 Differential expressions of lncRNAs in the serum of patients. RT-qPCR analyses of (A) ANRIL, (B) H19, (C) HOTAIR, (D) HULC, (E) MALAT1, (F) MEG3, (G) MIAT, (H) WISPER, and (I) ZFAS1 expressions in the serum of patients with proliferative diabetic retinopathy (PDR) compared to controls (non-diabetic patients). Data is expressed as a ratio to β-actin. Statistical significance was assessed using the Mann-Whitney U test. Data represents the mean ± SD (N=10 per control group or N=11 per PDR group; ns, not significant, **p<0.01, ***p<0.001, or ****p<0.0001).
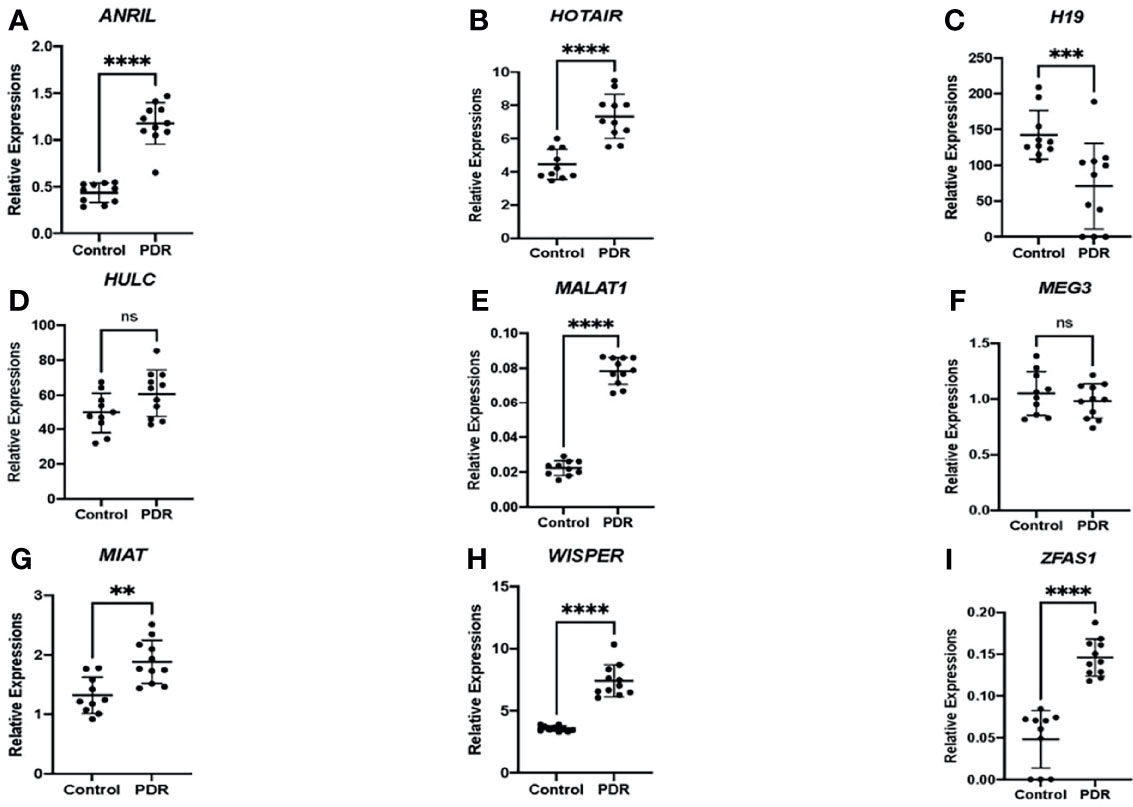
Figure 3 Differential expressions of lncRNAs in the vitreous fluid of patients. RT-qPCR analyses of (A) ANRIL, (B) HOTAIR, (C) H19, (D) HULC, (E) MALAT1, (F) MEG3, (G) MIAT, (H) WISPER, and (I) ZFAS1 expressions in the vitreous of patients with proliferative diabetic retinopathy (PDR) compared to controls (non-diabetic patients). Data is expressed as a ratio to β-actin. Statistical significance was assessed using the Mann-Whitney U test. Data represents the mean ± SD (N=10 per control group or N=11 per PDR group; ns, not significant, **p<0.01, ***p<0.001, or ****p<0.0001).
When comparing the level of lncRNA expressions between serum and vitreous samples, significant correlations were observed for HOTAIR (Figure 4A), ANRIL (Figure 4B), H19 (Figure 4C), MALAT1 (Figure 4E), WISPER (Figure 4H) and ZFAS1 (Figure 4I), which suggests that the level of expression for these lncRNAs in the serum parallels that of the lncRNA expression levels in the vitreous. We did not find significant correlations between serum and vitreous concentrations of HULC (Figure 4D), MEG3 (Figure 4F), and MIAT (Figure 4G).
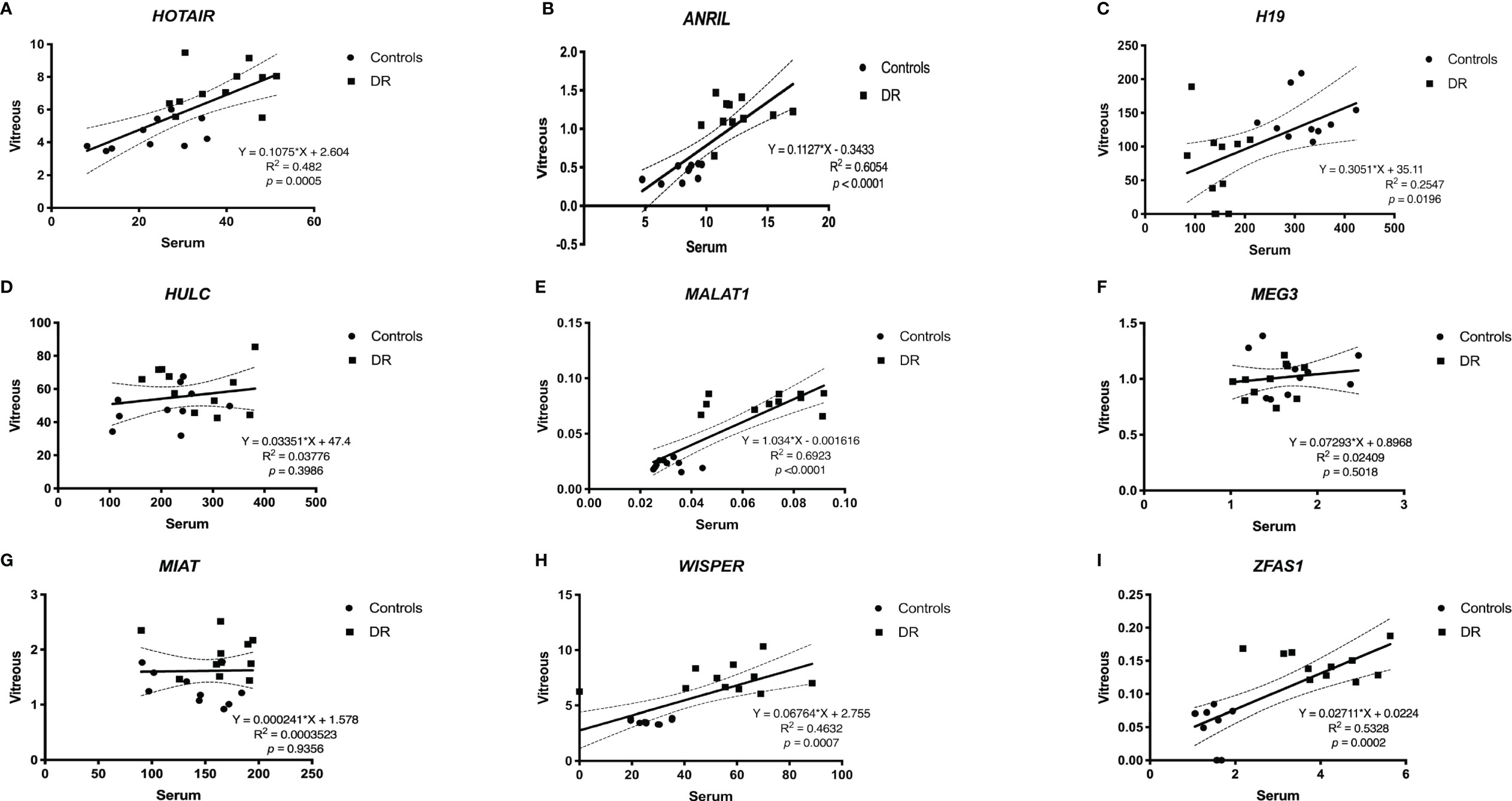
Figure 4 Pearson correlation analyses between serum and vitreous samples. When comparing between serum and vitreous samples, significant correlations were observed for (A) ANRIL, (B) H19, (C) HOTAIR, (E) MALAT1, (H) WISPER and (I) ZFAS1, which suggests that the expressions of these lncRNAs can be reflected from the serum and vitreous of patients with DR. Although we did find significant correlations between serum and vitreous concentrations of (D) HULC, (F) MEG3, and (G) MIAT, including a larger sample size may further help confirm the relationships between these markers and sample types [p<0.05 was considered significant; dotted lines represent 95% confidence intervals. N= 10 for control group and N= 11 for diabetic group; and normalized to β-actin]. DR, diabetic retinopathy.
Demographic Data and Disease Characteristics of the Study Population
Following the results from our proof-of-concept experiment, we expanded our study by examining the expression of the above lncRNAs in the serum of a new cohort of non-diabetic patients and diabetic patients with various stages of DR. As shown in Table 2, males were more predominant in the diabetic patient group, while females were more prevalent in the control group. As expected, the diabetic patient group also had a slightly higher means of BMI, hemoglobin A1c [HbA1c], and creatinine levels compared to the control group. However, when examining the diabetic patient groups according to the presence of retinopathy, no significant differences were observed for age, BMI, or creatinine levels, while significances were observed for HbA1c and gender distribution between diabetic patients with retinopathy and those without. Furthermore, when comparing between NPDR and PDR patients, no significant differences were observed across age, type of diabetes, BMI, creatinine, HbA1c, and gender distribution.
A Pattern of lncRNA Alterations Were Observed in the Serum in Diabetes
As shown in Figure 5, significant increased lncRNA expressions of ANRIL, H19, HOTAIR, HULC, MIAT, WISPER and ZFAS1 were observed in the serum of diabetic patients (with varying stages of DR; group ‘P’) compared to non-diabetic patients (without DR; group ‘C’). Interestingly, although not significant, MEG3 lncRNA levels demonstrated decreasing trends in diabetic patients compared to control patients (p = 0.67), while MALAT1 levels exhibited increasing trends in the diabetic patient group (p = 0.09). No significant correlations were demonstrated between the lncRNA expression profiles and the creatinine (Table 3) or HbA1C levels (Table 4) in the non-diabetic and diabetic patient groups.

Figure 5 Expression levels of lncRNAs in patients and controls. Boxplots represent comparison between expression level of 9 specific lncRNAs (ANRIL, H19, HOTAIR, HULC, MALAT1, MEG3, MIAT, WISPER and ZFAS1) detected by RT-qPCR in patient and control groups. The median and interquartile range (25th -75th percentile), are shown as solid horizontal lines. Two outliers from control samples I6 and A1 for MEG3 (86096.52 and 2022.94, respectively) were removed for better graphical representation of the results. Statistical significance was assessed using Mann-Whitney U test (*p<0.05, **p<0.005, or ***p<0.0005; N=11 for control patients and N= 59 for diabetic patients). Statistical significance was considered at p<0.05. Legend: Control = Non-diabetic patients without any diabetic retinopathy; Patient = Diabetic patients with or without diabetic retinopathy.
Stage Dependent Alterations of lncRNAs in DR
When the data of various patient subgroups were analyzed, the average expression levels for 8 lncRNAs were generally increased across all diabetic patient sub-groups (‘DM’, ‘NPDR’, and ‘PDR’) when compared to controls (Figure 6). Such alterations were not observed with MEG3. Interestingly, when comparing between control and ‘DM’ groups, significances were observed for the lncRNAs ANRIL, H19, HULC, MIAT, WISPER, and ZFAS1. In addition, when the control group was compared with the ‘NPDR’ sub-group, significances were observed for 8 out of the 9 lncRNAs, apart from MEG3. Additionally, comparing the lncRNA expression levels between control and PDR groups also demonstrated statistical significance for ANRIL, H19, HOTAIR, HULC, MIAT, ZFAS1, and WISPER, while MEG3 and MALAT1 did not show significance. These observations suggest a duration-dependent change in the lncRNA expression profiles in the serum, where the duration of diabetes may reflect distinct lncRNA expression patterns. Collectively, the results suggest that these 9 lncRNAs can be used as biomarkers of DR.
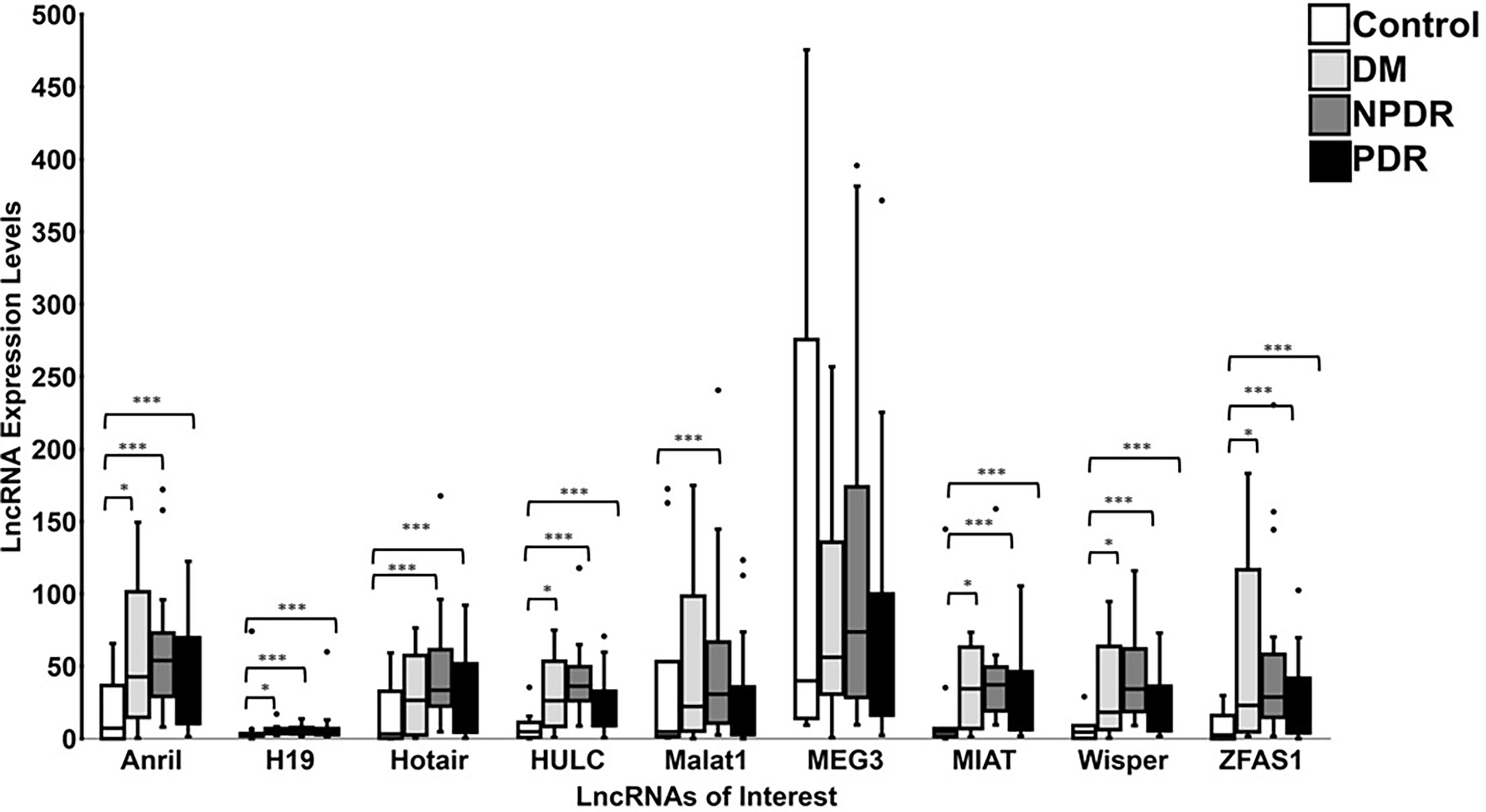
Figure 6 Expression levels of lncRNAs in controls and patients diagnosed with varying stages of DR. Boxplots represent comparison between expression level of 9 specific lncRNAs (ANRIL, H19, HOTAIR, HULC, MALAT1, MEG3, MIAT, WISPER and ZFAS1) detected by RT-qPCR in control and patient groups (DM = diabetic patients with no retinopathy, NPDR = diabetic patients with non-proliferative DR, and PDR = diabetic patients with proliferative DR). The median and interquartile range (25th -75th percentile), are shown as solid horizontal lines. Two outliers from control samples I6 and A1 for MEG3 (86096.52 and 2022.94, respectively) were removed for better graphical representation of the results. Statistical significance was assessed using Mann-Whitney U test (*p<0.05, ***p<0.0005; N=11 for control patients, N=10 for DM patients, N=26 for NPDR patients, and N=23 for PDR patients). Statistical significance was considered at p<0.05.
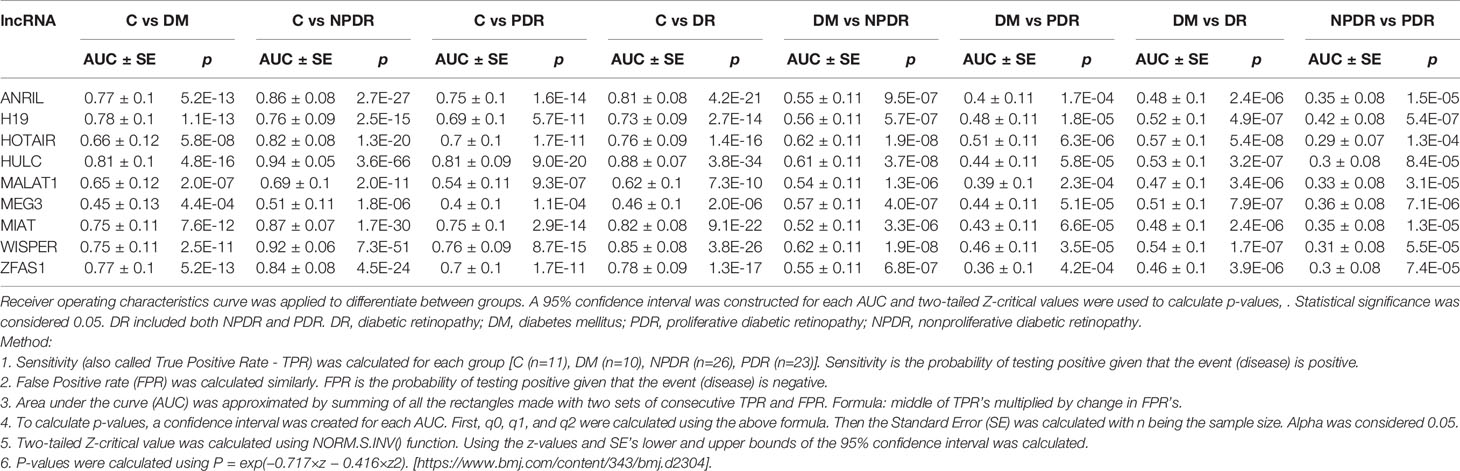
To further examine significance of the serum levels of the analytes, area under the curve (AUC) was calculated. AUC analyses demonstrated that the lncRNAs of interest have potential diagnostic value in discriminating diabetic patient sub-groups (diabetes, NPDR, and PDR) from control non-diabetic patients. (95% CI, two-tailed Z-critical values were used to calculate p-values) (Table 5). Specifically, using AUC, comparison between control patients and diabetic patients with no DR demonstrated a significant difference in 9 lncRNAs, where HULC had the highest AUC (p < 0.05) and MEG3 had the lowest AUC (p < 0.05). Furthermore, when analyzing the AUC values for control and NPDR patients, all 9 lncRNAs demonstrated statistical significance and 7 out of 9 lncRNAs had AUC values greater than 0.7, except for MEG3 and MALAT1. Additionally, comparison of AUC values between control patients and PDR patients demonstrated significance for all the lncRNA markers and 6 out of the 9 lncRNAs had AUC values greater than or equal to 0.7, apart from H19, MEG3 and MALAT1. Interestingly, when comparing between diabetic patients with no retinopathy and diabetic patients with DR (NPDR or PDR), all 9 lncRNAs demonstrated AUC values ranging between 0.460 to 0.570 with significant statistical differences. Furthermore, although significant, AUC values for the 9 lncRNAs ranged between 0.290 to 0.420 when comparing between NPDR and PDR patients. Collectively, these results demonstrate that the 9 examined lncRNAs may be used as a prognostic tool to discriminate between non-diabetic and diabetic patients with DR, as well as potentially discriminate various stages of DR (mild NPDR to severe PDR) from diabetic patients without DR.
Alteration of Specific lncRNAs Phenotypes May Be Predictive of DR
Given the various lncRNA expression profiles, we wanted to further determine whether combinations of specific lncRNA phenotypes existed across control and diabetic patient sub-groups. Through Z-score calculations and by comparing the mean and standard deviations for each lncRNA expression levels in diabetic patients against controls, we found that the ANRIL+/HOTAIR+/HULC+/(H19), (MIAT)/WISPER+/ZFAS1+/(H19), and ANRIL+/HOTAIR+/HULC+/WISPER+/ZFAS1+/(H19) phenotypes were prevalent in 33.9%, 35.6%, and 30.5% of all diabetic patients (n = 59 patients), respectively (Table 6). Interestingly, when examining both diabetic patients with either NPDR or PDR against control patients (n = 49 patients), 34.7% of these patients presented with the ANRIL+/HOTAIR+/HULC+/(H19) phenotype, 38.8% exhibited the (MIAT)/WISPER+/ZFAS1+/(H19) phenotype, and 30.6% of these patients demonstrated the ANRIL+/HOTAIR+/HULC+/WISPER+/ZFAS1+/(H19) phenotype. Specifically, in just the NPDR group (n = 26 patients), the most prevalent phenotypes included ANRIL+/HOTAIR+/HULC+/(H19) (46.2%), (MIAT)/WISPER+/ZFAS1+/(H19) (53.8%), and ANRIL+/HOTAIR+/HULC+/WISPER+/ZFAS1+/(H19) (38.5%). While the prevalent lncRNA phenotypes in the PDR group (n = 23 patients) included ANRIL+/HOTAIR+/HULC+/(H19) (21.7%), (MIAT)/WISPER+/ZFAS1+/(H19) (21.7%) and ANRIL+/HOTAIR+/HULC+/WISPER+/ZFAS1+/(H19) (21.7%). These findings suggest that unique lncRNA phenotypes could exist for specific diabetic patients, particularly those presenting at a certain stage of DR. We further applied regression model in this analysis (Supplementary Table 1). However, no further improvement in the diagnostic accuracy was seen.
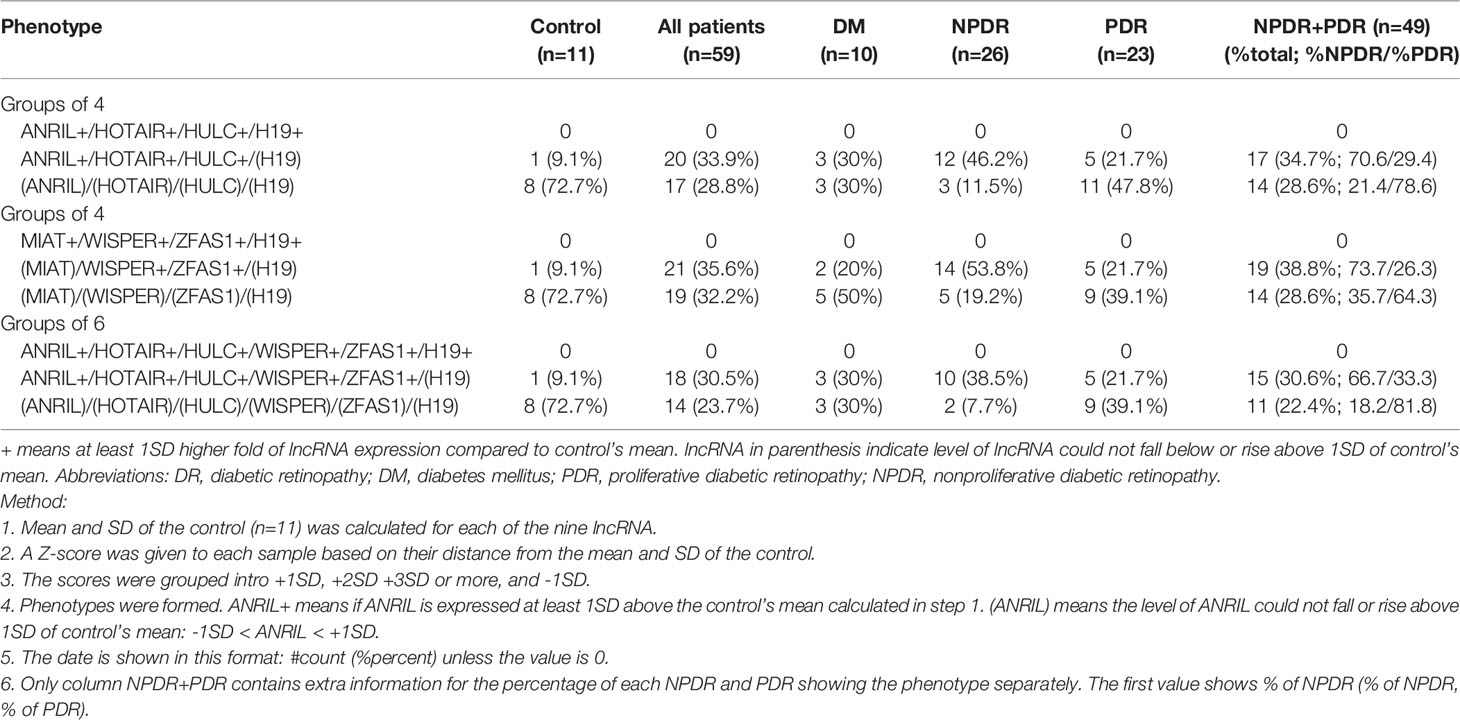
Table 6 Diagnostic performance of lncRNA of various potential combinations of lncRNAs in the Control and Patient groups.
Discussion
The emergence of diabetes as a global epidemic is a major challenge to human health in the 21st century, with a global prevalence of 8.8% of the global adult population in 2017 (20). Among the diabetic population, a recent meta-analysis in 2020 estimated that the global prevalence for DR was 22.27%, 6.17% for vision-threatening DR, and 4.07% for clinically significant macular edema (21). If the current rate of DR prevalence remains constant over the next 25 years, it is estimated that the global number of adults with DR will further increase by 25.9% (129.84 million) in 2030 and by 55.6% (160.50 million) in 2045 (21). Although the likelihood of DR-induced vision impairment can be greatly reduced if DR is diagnosed in its early stages and treated accordingly, nearly one-third of patients with diabetes fail to follow vision care guidelines in the US and non-adherence has reached more than 60% in developing countries, which is largely attributable to the limited accessibility of ophthalmic services and elevated costs (22). Furthermore, the current gold standard for DR screening is based on clinical examinations (with various diagnostic tools), and DR is clinically diagnosed with the onset of ophthalmoscopic signs. However, since biochemical and functional defects in the eye often precede the development of vascular lesions and the onset of clinical signs (23), it is critical that novel, accurate, low-cost, and predictive screening tools are developed that can identify the best therapeutic window for patients who have not yet developed clinically evident retinopathy and still have their visual function intact—since this sub-population of diabetic patients comprise the majority and respond better to regimented therapies early on (23). Nevertheless, the current approaches for diagnostic evaluation can be labor-intensive, time-consuming, and susceptible to diagnostic variation due to clinical subjectivity (24) and the advent of novel diagnostic technologies, which provide effective clinical utility, is greatly required, as the prevalence of DR continues to rise exponentially.
Long non-coding RNAs (lncRNAs) represent a promising class of RNA molecules that can be targeted and exploited for therapeutic and diagnostic purposes. Given their limited protein-coding capacities, research over the last decade have demonstrated the versatile and critical roles of lncRNAs in cancer progression (25), neurodegenerative diseases (26), diabetes (27), and even diabetic complications such as DR (12, 14, 15, 28). To further demonstrate the importance of lncRNAs in the context of DR screening, the present study examines the diagnostic and prognostic potential of 9 circulating lncRNAs in diabetic patients with or without DR compared to non-diabetic controls. Serum samples of diabetic patients (with varying stages of DR) exhibited significant increases in the expressions of ANRIL, H19, HOTAIR, HULC, MIAT, WISPER and ZFAS1, while expression levels of MALAT1 and MEG3 did not demonstrate statistical significance when compared to controls. However, when further stratifying the diabetic patients into distinct subsets of DR and comparing the lncRNA expression levels against controls, significant differential expressions were observed for 8 out of the 9 lncRNAs except for MEG3. The differential expression profiles observed for the lncRNAs in this study are consistent with previous reports that have documented the de-regulated nature of lncRNAs in DR (12, 14, 15, 29–31), which further supports the diagnostic and prognostic potential of lncRNAs for DR screening. It is to be noted that in this exploratory study, we didn’t use a synthetic spike-in RNA as control. We used β-actin as internal control along with several positive controls (cell culture) and negative controls for our assays.
Although the mechanisms of action for lncRNAs continue to be elucidated, a large body of literature supports the notion that lncRNAs can govern gene expressions through various mechanisms: 1) guiding molecular complexes (e.g., chromatin-modifying enzymes) to target genomic regions (32), 2) functioning as a decoy for transcription factors (33), 3) serving as a scaffold to assist in the assembly of protein complexes (34), 4) directly enhancing the activation of neighboring genes (35), and 5) acting as a sponge that sequesters critical microRNAs (36). Although lncRNAs may exert more than one of the above mechanisms, the subcellular localization of lncRNAs can further provide insights into the structural features and distinct functionalities of lncRNAs, with nuclear-retained lncRNAs playing important roles in transcriptional regulation and cytoplasmic-retained lncRNAs being implicated in post-transcriptional regulation (37, 38). Furthermore, in the context of diabetes, chronic hyperglycemia can initiate various cellular events, such as angiogenesis, inflammation, oxidative damage, and fibrosis, which can further stimulate the transcription of various lncRNAs (28).
The lncRNAs used in the PCR panel are well-documented in literature. Specifically, HOTAIR is a trans-acting lncRNA that can directly interact with several epigenetic enzymes (e.g., polycomb repressive complex 2; PRC2) to regulate the expressions of multiple genes involved in various disease processes, including cancer progression, ischemic stroke, diabetes, and recently angiogenesis in DR (12, 29, 39, 40). As for the lncRNA MALAT1 (also known as NEAT2), aberrant MALAT1 expressions have been documented in the retinas of diabetic rodents and various retinal cells cultured in high glucose environments (31). Several studies have also demonstrated MALAT1’s inflammatory functionalities in various diabetic complications (14, 41, 42). Interestingly, in alignment with our findings, Shaker et al. have demonstrated that significant increases in serum HOTAIR and MALAT1 levels were evident in NPDR and PDR patients compared to healthy controls and through ROC analyses, these lncRNAs could discriminate NPDR and PDR from non-DR controls, further supporting the diagnostic and prognostic utility of lncRNAs as potential non-invasive biomarkers for DR screening and early diagnosis of PDR (29).
H19, a conserved and maternally imprinted lncRNA, is implicated in several pathophysiological processes (43). Few studies have examined H19 in DR, however we have previously confirmed H19’s role in preventing the glucose-induced phenotypic switch of endothelial cells in the diabetic retina (known as endothelial-to-mesenchymal transition; EndMT) (15). Hyperglycemia was shown to promote the upregulation of mesenchymal markers and the downregulation of both H19 and endothelial cell markers, and subsequent overexpression of H19 in HG-treated HRECs dramatically reversed the trends evoked by hyperglycemia, suggesting a potential protective role for H19 in preventing EndMT in DR. Interestingly, in our current findings, H19 expression levels were relatively increased across all diabetic patient sub-groups with or without DR compared to controls, which may be attributed to patient-specific and/or biological differences (e.g., absence of EndMT). Similar to H19, maternally expressed gene 3 (MEG3) is a maternally imprinted gene that exerts critical developmental properties (44). Several lines of evidence also suggest that the inactivation of this gene and the subsequent loss of the MEG3 lncRNA (along with its diseases-suppressive properties) are frequently documented in numerous cancers and diabetic environments (19, 45–47). In parallel with our findings, reduced serum levels of MEG3 were observed in patients with DR compared to controls, which may suggest a reduction in the protective mechanisms exerted by MEG3 (46), however additional studies are warranted to confirm this notion.
Although the lncRNAs WISPER (Wisp2 super-enhancer-associated RNA), HULC (Highly upregulated in liver cancer), and ZFAS1 (ZNFX Antisense RNA 1) have not been extensively examined in the context of DR, these critical lncRNAs are pathologically involved in the development of cardiac fibrosis (16), metastatic progression of hepatocellular carcinoma (48), and diabetic cardiomyopathy (17), respectively. Given the increased expressions of these lncRNAs in HG-treated HRECs and the serum and vitreous specimens of DR patients observed from our study, it may be reasonable to speculate that WISPER, ZFAS1 and HULC may have similar pathogenetic phenotypes in patients with DR, however additional mechanistic studies are warranted to further delineate their mechanisms in DR progression.
The antisense RNA to INK4 locus (ANRIL; also known as CDKN2B-AS1) gene gives rise to a 3.8-kb lncRNA that is prominently deregulated in many diseases, including diabetic cardiomyopathy and nephropathy (49–51). Whether ANRIL exerts similar functional and mechanistic capabilities in DR remains limited. However, a recent study, demonstrated for the first time by us, alludes to the angiogenic capabilities of this lncRNA in advancing DR. As evident by the findings from both in vitro and in vivo experiments, hyperglycemia can significantly induce the upregulation of ANRIL in HG-treated HRECs and in the retinas of diabetic mice, and subsequent knockdown of ANRIL can greatly hamper glucose-induced retinal angiogenesis (13). Furthermore, in a separate study by Toraih et al., ANRIL and 3 additional circulating lncRNAs (RNCR2, MALAT1, and PVT1) did not show an association with DR progression or anti-VEGF therapy response, but the expression patterns of these lncRNAs demonstrated good diagnostic performance in differentiating DM from controls and DR (30). Another prominent lncRNA involved in the pathogenesis of DR is myocardial infarction-associated transcript (MIAT; also referred to as RNCR2, Gomafu, or AK028326) (11, 52). MIAT is significantly upregulated in the retinas of diabetic rodents, and in the fibrovascular membranes of diabetic patients compared to non-diabetic controls (11). In vitro experiments also indicated that expressions of MIAT are upregulated in several HG-treated retinal cell lines, while increased plasma levels of MIAT have been found to be significantly associated with the presence of DR (53). Taken together, the increased MIAT and ANRIL expressions observed in the present study confirms the pathogenetic nature of these lncRNAs documented in previous studies, and long-term clinical studies are required to further discern the diagnostic performance of these lncRNAs.
Undoubtedly, the results from the current study provide unique insights into the potential discriminative abilities of lncRNAs for identifying patients with DR from patients without DR. However, given the small sample size and cross-sectional nature of the study, additional modelling, and extrapolation of long-term outcomes between lncRNA expression levels, diabetes mellitus, and the various subsets of DR could not be determined. As well, due to the exploratory nature of this study, selection bias was inevitably introduced with respect to specimen selection. Future prospective screening studies, with larger sample sizes, should be conducted in order to increase the sensitivity of the assay and help determine whether certain sub-stages of DR can be detected, through lncRNA expression levels and/or combination of lncRNA phenotypes, before clinical diagnosis.
Nevertheless, the on-going research work on lncRNAs continues to evolve our understanding of various pathologies and their respective molecular mechanisms. Given the critical nature of lncRNAs and their involvement in disease progression, exploiting these RNA transcripts for diagnostic and/or therapeutic approaches may guide optimal treatment strategies and long-term benefits for patients. As such, the collective findings from this study highlight unique expression patterns for a subset of 9 well-studied lncRNAs (MALAT1, HOTAIR, ANRIL, ZFAS1, HULC, H19, WISPER, MIAT, and MEG3) for patients with and without DR, and subsequent analysis of these lncRNA signatures may provide important clinical utility for the prediction and diagnosis of DR severity. However, additional long-term and larger prospective studies are warranted in order to determine such utility of lncRNA biomarkers.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Western Research Ethics Board and Lawson Health Research Institute at the University of Western Ontario (London, ON, CAN). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SC conceived, designed, and directed the study. ShC and SB performed the in vitro experiments. SB, AC, MG, ShC, and SC collected clinical samples and performed data analyses. SC and JG provided reagents, materials, and analysis tools. SB, AC, and SC wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The Canadian Institutes of Health Research funded and supported this research.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.851967/full#supplementary-material
Abbreviations
DR, diabetic retinopathy; lncRNA, long non-coding RNA; DM, diabetes mellitus; ECs, endothelial cells; NPDR, non-proliferative DR; PDR, proliferative DR; VEGF, vascular endothelial growth factor; MALAT1, metastasis associated lung adenocarcinoma transcript 1; HOTAIR, HOX transcript anti-sense RNA; H19, H19 imprinted maternally expressed transcript; WISPER, Wisp2 super-enhancer-associated RNA; ZFAS1, ZNFX1 antisense RNA 1; MIAT, myocardial infarction-associated transcript; HULC, highly up-regulated in liver cancer; ANRIL, antisense non-coding RNA in the INK4 locus; MEG3, maternally expressed gene 3; NG, normoglycemia; HG, hyperglycemia or high glucose; HRECs, human retinal microvascular endothelial cells.
References
1. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results From the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res Clin Pract (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
2. Cheung N, Mitchell P, Wong TY. Diabetic Retinopathy. Lancet (London England) (2010) 376(9735):124–36. doi: 10.1016/S0140-6736(09)62124-3
3. Bascaran C, Mwangi N, D’Esposito F, Gordon I, Alberto Lopez Ulloa J, Mdala S, et al. Effectiveness of Task-Shifting for the Detection of Diabetic Retinopathy in Low- and Middle-Income Countries: A Rapid Review Protocol. Syst Rev (2021) 10:4. doi: 10.1186/s13643-020-01553-w
4. American Academy of Ophthalmology Retina/Vitreous Panel. Preferred Practice Pattern Guidelines. In: Diabetic Retinopathy. San Francisco, CA: American Academy of Ophthalmology (2016). Available at: www.aao.org/ppp.
5. Harris Nwanyanwu K, Talwar N, Gardner TW, Wrobel JS, Herman WH, Stein JD. Predicting Development of Proliferative Diabetic Retinopathy. Diabetes Care (2013) 36(6):1562–8. doi: 10.2337/dc12-0790
6. Gabrielian A, Hariprasad SM, Jager RD, Green JL, Mieler WF. The Utility of Visual Function Questionnaire in the Assessment of the Impact of Diabetic Retinopathy on Vision-Related Quality of Life. Eye (London England) (2010) 24(1):29–35. doi: 10.1038/eye.2009.56
7. Wong TY, Cheung CM, Larsen M, Sharma S, Simó R. Diabetic Retinopathy. Nat Rev Dis Primers (2016) 2:16012. doi: 10.1038/nrdp.2016.12
8. Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN. & DCCT/EDIC Research Group. Effect of Glycemic Exposure on the Risk of Microvascular Complications in the Diabetes Control and Complications Trial–Revisited. Diabetes (2008) 57(4):995–1001. doi: 10.2337/db07-1618
9. Woung LC, Tsai CY, Chou HK, Tsai MT, Tsai WH, Chou P, et al. Healthcare Costs Associated With Progressive Diabetic Retinopathy Among National Health Insurance Enrollees in Taiwan, 2000-2004. BMC Health Serv Res (2010) 10:136. doi: 10.1186/1472-6963-10-136
10. Schmier JK, Covert DW, Lau EC, Matthews GP. Medicare Expenditures Associated With Diabetes and Diabetic Retinopathy. Retina (Philadelphia Pa.) (2009) 29(2):199–206. doi: 10.1097/IAE.0b013e3181884f2d
11. Yan B, Yao J, Liu JY, Li XM, Wang XQ, Li YJ, et al. lncRNA-MIAT Regulates Microvascular Dysfunction by Functioning as a Competing Endogenous RNA. Circ Res (2015) 116(7):1143–56. doi: 10.1161/CIRCRESAHA.116.305510
12. Biswas S, Feng B, Chen S, Liu J, Aref-Eshghi E, Gonder J, et al. The Long Non-Coding RNA HOTAIR Is a Critical Epigenetic Mediator of Angiogenesis in Diabetic Retinopathy. Invest Ophthalmol Visual Sci (2021) 62(3):20. doi: 10.1167/iovs.62.3.20
13. Thomas AA, Feng B, Chakrabarti S. ANRIL: A Regulator of VEGF in Diabetic Retinopathy. Invest Ophthalmol Visual Sci (2017) 58(1):470–80. doi: 10.1167/iovs.16-20569
14. Biswas S, Thomas AA, Chen S, Aref-Eshghi E, Feng B, Gonder J, et al. MALAT1: An Epigenetic Regulator of Inflammation in Diabetic Retinopathy. Sci Rep (2018) 8(1):6526. doi: 10.1038/s41598-018-24907-w
15. Thomas AA, Biswas S, Feng B, Chen S, Gonder J, Chakrabarti S. lncRNA H19 Prevents Endothelial-Mesenchymal Transition in Diabetic Retinopathy. Diabetologia (2019) 62(3):517–30. doi: 10.1007/s00125-018-4797-6
16. Micheletti R, Plaisance I, Abraham BJ, Sarre A, Ting CC, Alexanian M, et al. The Long Noncoding RNA Wisper Controls Cardiac Fibrosis and Remodeling. Sci Trans Med (2017) 9(395):eaai9118. doi: 10.1126/scitranslmed.aai9118
17. Feng B, Chen S, Chakrabarti S. Long Noncoding RNA Zfas1 in Diabetic Cardiomyopathy. Diabetes (2018) 67(Supplement 1):473–P. doi: 10.2337/db18-473-P
18. Jin C, Shi W, Wang F, Shen X, Qi J, Cong H, et al. Long Non-Coding RNA HULC as a Novel Serum Biomarker for Diagnosis and Prognosis Prediction of Gastric Cancer. Oncotarget (2016) 7(32):51763–72. doi: 10.18632/oncotarget.10107
19. Qiu GZ, Tian W, Fu HT, Li CP, Liu B. Long Noncoding RNA-MEG3 Is Involved in Diabetes Mellitus-Related Microvascular Dysfunction. Biochem Biophys Res Commun (2016) 471(1):135–41. doi: 10.1016/j.bbrc.2016.01.164
20. Standl E, Khunti K, Hansen TB, Schnell O. The Global Epidemics of Diabetes in the 21st Century: Current Situation and Perspectives. Eur J Prev Cardiol (2019) 26(2_suppl):7–14. doi: 10.1177/2047487319881021
21. Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden Through 2045: Systematic Review and Meta-Analysis. Ophthalmology (2021) 128(11):1580–91. doi: 10.1016/j.ophtha.2021.04.027
22. Zheng Y, He M, Congdon N. The Worldwide Epidemic of Diabetic Retinopathy. Indian J Ophthalmol (2012) 60(5):428–31. doi: 10.4103/0301-4738.100542
23. Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, et al. & JDRF Diabetic Retinopathy Center Group. Diabetic Retinopathy: Seeing Beyond Glucose-Induced Microvascular Disease. Diabetes (2006) 55(9):2401–11. doi: 10.2337/db05-1635
24. Heitmar R, Kalitzeos AA, Patel SR, Prabhu-Das D, Cubbidge RP. Comparison of Subjective and Objective Methods to Determine the Retinal Arterio-Venous Ratio Using Fundus Photography. J Optomet (2015) 8(4):252–7. doi: 10.1016/j.optom.2014.07.002
25. Jiang MC, Ni JJ, Cui WY, Wang BY, Zhuo W. Emerging Roles of lncRNA in Cancer and Therapeutic Opportunities. Am J Cancer Res (2019) 9(7):1354–66. doi: 10.1038/nm.3981
26. Riva P, Ratti A, Venturin M. The Long Non-Coding RNAs in Neurodegenerative Diseases: Novel Mechanisms of Pathogenesis. Curr Alzheimer Res (2016) 13(11):1219–31. doi: 10.2174/1567205013666160622112234
27. Leung A, Natarajan R. Long Noncoding RNAs in Diabetes and Diabetic Complications. Antioxid Redox Signaling (2018) 29(11):1064–73. doi: 10.1089/ars.2017.7315
28. Biswas S, Chakrabarti S. The Multifaceted Roles of lncRNAs in Diabetic Complications: A Promising, Yet Perplexing Paradigm. In: Jurga S, Barciszewski J, editors. The Chemical Biology of Long Noncoding RNAs. Cham: Springer Series, RNA Technologies (2020). p. 491–521. doi: 10.1007/978-3-030-44743-4_19
29. Shaker OG, Abdelaleem OO, Mahmoud RH, Abdelghaffar NK, Ahmed TI, Said OM, et al. Diagnostic and Prognostic Role of Serum miR-20b, miR-17-3p, HOTAIR, and MALAT1 in Diabetic Retinopathy. IUBMB Life (2019) 71(3):310–20. doi: 10.1002/iub.1970
30. Toraih EA, Abdelghany AA, Abd El Fadeal NM, Al Ageeli E, Fawzy MS. Deciphering the Role of Circulating lncRNAs: RNCR2, NEAT2, CDKN2B-AS1, and PVT1 and the Possible Prediction of Anti-VEGF Treatment Outcomes in Diabetic Retinopathy Patients. Graefe’s Arch Clin Exp Ophthalmol = Albrecht Von Graefes Archiv Fur Klinische Und Experimentelle Ophthalmol (2019) 257(9):1897–913. doi: 10.1007/s00417-019-04409-9
31. Yan B, Tao ZF, Li XM, Zhang H, Yao J, Jiang Q. Aberrant Expression of Long Noncoding RNAs in Early Diabetic Retinopathy. Invest Ophthalmol Visual Sci (2014) 55(2):941–51. doi: 10.1167/iovs.13-13221
32. Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, et al. Functional Demarcation of Active and Silent Chromatin Domains in Human HOX Loci by Noncoding RNAs. Cell (2007) 129(7):1311–23. doi: 10.1016/j.cell.2007.05.022
33. Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP. Noncoding RNA Gas5 Is a Growth Arrest- and Starvation-Associated Repressor of the Glucocorticoid Receptor. Sci Signaling (2010) 3(107):ra8. doi: 10.1126/scisignal.2000568
34. Zappulla DC, Cech TR. RNA as a Flexible Scaffold for Proteins: Yeast Telomerase and Beyond. Cold Spring Harbor Symp Quant Biol (2006) 71:217–24. doi: 10.1101/sqb.2006.71.011
35. Ørom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, et al. Long Noncoding RNAs With Enhancer-Like Function in Human Cells. Cell (2010) 143(1):46–58. doi: 10.1016/j.cell.2010.09.001
36. Wang Y, Sun L, Wang L, Liu Z, Li Q, Yao B, et al. Long Non-Coding RNA DSCR8 Acts as a Molecular Sponge for miR-485-5p to Activate Wnt/β-Catenin Signal Pathway in Hepatocellular Carcinoma. Cell Death Dis (2018) 9(9):851. doi: 10.1038/s41419-018-0937-7
37. Vance KW, Ponting CP. Transcriptional Regulatory Functions of Nuclear Long Noncoding RNAs. Trends Genet: TIG (2014) 30(8):348–55. doi: 10.1016/j.tig.2014.06.001
38. Yoon JH, Abdelmohsen K, Gorospe M. Posttranscriptional Gene Regulation by Long Noncoding RNA. J Mol Biol (2013) 425(19):3723–30. doi: 10.1016/j.jmb.2012.11.024
39. Hajjari M, Salavaty A. HOTAIR: An Oncogenic Long Non-Coding RNA in Different Cancers. Cancer Biol Med (2015) 12(1):1–9. doi: 10.7497/j.issn.2095-3941.2015.0006
40. Wang H, Xia Y, Zhang Y. Diagnostic Significance of Serum lncRNA HOTAIR and Its Predictive Value for the Development of Chronic Complications in Patients With Type 2 Diabetes Mellitus. Diabetol Metab Syndrome (2021) 13(1):97. doi: 10.1186/s13098-021-00719-3
41. Gordon AD, Biswas S, Feng B, Chakrabarti S. MALAT1: A Regulator of Inflammatory Cytokines in Diabetic Complications. Endocrinol Diabetes Metab (2018) 1(2):e00010. doi: 10.1002/edm2.10
42. Puthanveetil P, Chen S, Feng B, Gautam A, Chakrabarti S. Long non-Coding RNA MALAT1 Regulates Hyperglycaemia Induced Inflammatory Process in the Endothelial Cells. J Cell Mol Med (2015) 19(6):1418–25. doi: 10.1111/jcmm.12576
43. Wang B, Suen CW, Ma H, Wang Y, Kong L, Qin D, et al. The Roles of H19 in Regulating Inflammation and Aging. Front Immunol (2020) 11:579687:579687. doi: 10.3389/fimmu.2020.579687
44. da Rocha ST, Edwards CA, Ito M, Ogata T, Ferguson-Smith AC. Genomic Imprinting at the Mammalian Dlk1-Dio3 Domain. Trends Genet: TIG (2008) 24(6):306–16. doi: 10.1016/j.tig.2008.03.011
45. Zhou Y, Zhang X, Klibanski A. MEG3 Noncoding RNA: A Tumor Suppressor. J Mol Endocrinol (2012) 48(3):R45–53. doi: 10.1530/JME-12-0008
46. Zhang D, Qin H, Leng Y, Li X, Zhang L, Bai D, et al. LncRNA MEG3 Overexpression Inhibits the Development of Diabetic Retinopathy by Regulating TGF-β1 and VEGF. Exp Ther Med (2018) 16(3):2337–42. doi: 10.3892/etm.2018.6451
47. Tong P, Peng QH, Gu LM, Xie WW, Li WJ. LncRNA-MEG3 Alleviates High Glucose Induced Inflammation and Apoptosis of Retina Epithelial Cells via Regulating miR-34a/SIRT1 Axis. Exp Mol Pathol (2019) 107:102–9. doi: 10.1016/j.yexmp.2018.12.003
48. Li T, Xie J, Shen C, Cheng D, Shi Y, Wu Z, et al. Amplification of Long Noncoding RNA ZFAS1 Promotes Metastasis in Hepatocellular Carcinoma. Cancer Res (2015) 75(15):3181–91. doi: 10.1158/0008-5472.CAN-14-3721
49. Broadbent HM, Peden JF, Lorkowski S, Goel A, Ongen H, Green F, et al. & PROCARDIS Consortium. Susceptibility to Coronary Artery Disease and Diabetes Is Encoded by Distinct, Tightly Linked SNPs in the ANRIL Locus on Chromosome 9p. Hum Mol Genet (2008) 17(6):806–14. doi: 10.1093/hmg/ddm352
50. Li Z, Yu X, Shen J. ANRIL: A Pivotal Tumor Suppressor Long Non-Coding RNA in Human Cancers. Tumour Biol (2016) 37(5):5657–61. doi: 10.1007/s13277-016-4808-5
51. Thomas AA, Feng B, Chakrabarti S. ANRIL Regulates Production of Extracellular Matrix Proteins and Vasoactive Factors in Diabetic Complications. Am J Physiol Endocrinol Metab (2018) 314(3):E191–200. doi: 10.1152/ajpendo.00268.2017
52. Ishii N, Ozaki K, Sato H, Mizuno H, Susumu S, Takahashi A, et al. Identification of a Novel Non-Coding RNA, MIAT, That Confers Risk of Myocardial Infarction. J Hum Genet (2006) 51(12):1087–99. doi: 10.1007/s10038-006-0070-9
Keywords: lncRNA, epigenetics, diabetic retinopathy, biomarker, diagnosis
Citation: Biswas S, Coyle A, Chen S, Gostimir M, Gonder J and Chakrabarti S (2022) Expressions of Serum lncRNAs in Diabetic Retinopathy – A Potential Diagnostic Tool. Front. Endocrinol. 13:851967. doi: 10.3389/fendo.2022.851967
Received: 11 January 2022; Accepted: 03 March 2022;
Published: 07 April 2022.
Edited by:
Ping Wang, Michigan State University, United StatesReviewed by:
Xuan Shi, Peking University People’s Hospital, ChinaDonato Santovito, National Research Council (CNR), Italy
Aixia Sun, Michigan State University, United States
Copyright © 2022 Biswas, Coyle, Chen, Gostimir, Gonder and Chakrabarti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Subrata Chakrabarti, subrata.chakrabarti@lhsc.on.ca
†These authors have contributed equally to this work
 Saumik Biswas
Saumik Biswas Ali Coyle
Ali Coyle Shali Chen1
Shali Chen1 Miso Gostimir
Miso Gostimir John Gonder
John Gonder Subrata Chakrabarti
Subrata Chakrabarti