- 1Oncological Endocrinology Unit, Scientific Institute for Research, Hospitalization and Healthcare (IRCCS) Regina Elena National Cancer Institute, Rome, Italy
- 2Department of Experimental Medicine, Sapienza University of Rome, Rome, Italy
Peptide receptor radionuclide therapy (PRRT) using radiolabeled somatostatin analogs has been used for over two decades for the treatment of well-differentiated neuroendocrine tumors (NETs), and the publication of the NETTER-1 trials has further strengthened its clinical use. However, many aspects of this treatment are still under discussion. The purpose of this review is to collect and discuss the new available evidence, published in 2021, on the use of 177Lu-Oxodotreotide (DOTATATE) or 90Y-Edotreotide (DOTATOC) in adult patients with NETs focusing on the following hot topics: 1) PRRT use in new clinical settings, broaden its indications; 2) the short- and long-term safety; and 3) the identification of prognostic and predictive factors. The review suggests a possible future increase of PRRT applications, using it in other NETs, as a neoadjuvant treatment, or for rechallenge. Regarding safety, available studies, even those with long follow-up, supported the low rates of adverse events, even though 1.8% of treated patients developed a second malignancy. Finally, there is a lack of prognostic and predictive factors for PRRT, with the exception of the crucial role of nuclear imaging for both patient selection and treatment response estimation.
1 Introduction
Peptide receptor radionuclide therapy (PRRT) with radiolabeled somatostatin analogs has been used for two decades for the treatment of well-differentiated neuroendocrine tumors (NETs) expressing somatostatin receptor (SSTR) type 2. The two most commonly used peptides are 177Lutetium (177Lu)-DOTATATE and 90Yttrium (90Y)-DOTATOC, respectively, beta- or gamma-emitting radionuclides (1). The publication of the NETTER-1 trial in 2017 has further confirmed the efficacy of this kind of therapy in NETs, also considering that the control arm of the study was represented by above label doses of somatostatin analogs, a very effective treatment (2). This study led to the approval of 177Lu-oxodotreotide (®Lutathera) by the European Medicines Agency and thus facilitated access to this treatment. However, this treatment is actually recommended only for progressive grade 1–2 gastroenteropancreatic (GEP) NETs. European Society for Medical Oncology (ESMO) guidelines recommend considering PRRT also in carcinoid syndrome (CS) and functional pancreatic (Pan) NETs refractory to somatostatin analogs and in selected cases of NET G3 (3).
Although PRRT represents a major cornerstone of treatment of well-differentiated low-grade GEP-NETs, some important aspects such as additional clinical indications, long-term safety, and predictive markers are not well established, and great attention is paid to these topics in recent literature.
This review aims to collect the evidence published in 2021 on the use of 177Lu-DOTATATE or 90Y-DOTATOC in adult patients with NETs in order to summarize the new evidence in 3 main research fields: 1) the use of PRRT in new settings, to broaden clinical indications; 2) short- and long-term safety assessment; and 3) the identification of prognostic and predictive factors.
2 Methods
We searched the PubMed database for articles in English on PRRT published in 2021. The search strategies used were “peptide receptor radionuclide therapy” and “radioligand therapy.” The latest search was carried out on November 18, 2021. We have selected all the articles concerning the use of PRRT in human patients affected by neuroendocrine neoplasms of any origin regarding one of three reported topics. Articles not on humans, not using 177Lu or 90Y compounds, or on children were excluded from this review. Excluding duplicate articles, from the original number of 531 articles, 453 were excluded after abstract screening and 33 after full-text evaluation. Ultimately, 45 studies were included, as reported in Supplementary Figure S1.
3 Results
3.1 New Indications and Settings for Peptide Receptor Radionuclide Therapy
3.1.1 Expanding the Clinical Indication
The overexpression of SSTRs in NETs other than GEP has led PRRT to be used in these neoplasms even if not actually approved.
A retrospective study evaluated the long-term outcome of 177Lu-DOTATATE in patients with paragangliomas (PGLs), demonstrating a disease control rate (DCR) of 67%. At 40 months, the observed progression-free survival (PFS) rate was 63% (95% CI: 30–96) and the overall survival (OS) rate was 65% (95% CI: 32–97) (4). These data were confirmed in a prospective phase II clinical trial (5), in which an overall DCR of 80% was observed (95% CI: 68.9–91.9) after a mean of five cycles of PRRT. Patients treated with 177Lu-DOTATATE showed a better OS compared with those treated with 90Y-DOTATOC (143 vs. 92 months). No high-grade renal and hematological toxicities occurred in both studies. Regarding the risk of PRRT-induced acute catecholamine crisis, premedication combining alpha- and beta-adrenergic blocking agents was effective in preventing this complication in a series of 5 patients (6).
A retrospective multicenter study aimed at evaluating the role of 68Ga-DOTATATE PET/CT in metastatic medullary thyroid cancer (MTC) and for patients’ selection for PRRT. Twenty-one of 71 patients, with tumor expressing SSTR, were treated with PRRT, with 177Lu-DOTATATE or 90Y-DOTATOC or both (median number of treatment cycles, 3; range, 1–4). At baseline, 10 patients had radiological and 3 had biochemical progression. After a median follow-up of 12 months, 12 patients had radiological progression, 1 had biochemical progression, and 3 patients died. The median time to treatment failure (including radiological or biochemical progression or death) was 14 months (95% CI: 8–25) without difference in terms of age, type of radionuclide, calcitonin serum level, or gallium avidity (7).
Bronchopulmonary NETs expressing SSTR may also benefit from PRRT. A retrospective study evaluated the role of combined 68Ga-DOTATATE and 18F-FDG PET/CT imaging to guide the choice of PRRT treatment in patients with typical and atypical carcinoids (TC and AC). About half of the patients (46% TC, 53% AC) were unsuitable for PRRT. In 16 patients who were treated with PRRT, DCR at 3 months was 85% with an OS of 54.6 months (95% CI 44–70). Patients with all lesions 68Ga-DODATATE positive and 18FDG PET/CT negative were less likely to develop disease progression (8).
Finally, patients with functioning tumors can benefit from PRRT. A retrospective cohort study that included patients with refractory CS, without evidence of disease progression, demonstrated a reduction in bowel movement frequency of more than 30% with PRRT in 47% of patients, also with a benefit on flushing. Importantly, no carcinoid crisis occurred with the use of short-acting octreotide subcutaneously between cycles (9).
3.1.2 Peptide Receptor Radionuclide Therapy in the Neoadjuvant Setting
Promising evidence is emerging on the use of PRRT as a neoadjuvant treatment. In an open-label retrospective study, enrolling patients with unresectable GEP-NET, 177Lu-DOTATATE resulted in a significant tumor shrinkage, allowing primary tumor resection in 26.3% of the patients. Baseline significant response predictors were primary duodenal tumor site, the size of the primary tumor (<5 cm), absence of regional lymph node involvement, the size (≤1.5 cm) and number (≤3) of liver metastases, and 18F-FDG uptake (SUVmax <5) in the primary tumor (10).
3.1.3 Retreatment With Peptide Receptor Radionuclide Therapy
Several uncontrolled studies have evaluated the outcome and feasibility of retreatment after an initial response to the first single course of PRRT followed by later disease progression (rechallenge with PRRT).
A retrospective study on 40 patients with advanced GEP-NETs with progressive disease after the first PRRT course demonstrated that the second PRRT course determined partial remission in 5% of patients, stable disease in 52.5% of patients, and disease progression in 42.5% of patients. The median OS was 122.1 months and was significantly longer in patients without uptake at 18F-FDG-PET CT (145.50 vs. 95.06 months, respectively) (11).
In a large Danish retrospective study, progression after the first PRRT course was seen in 62% of patients. Thirty-two patients were submitted to a second series of PRRT, and progression was observed in 64% of patients. The median PFS was 19 (range, 10–32) months. Interestingly, this study also included 8 patients who underwent a third PRRT series, with a PFS of 12 (range, 8–15) months (12).
Two meta-analyses have been published on the rechallenge with PRRT (the term “salvage treatment” was used in some articles included in the meta-analyses). A meta-analysis on 13 studies involving 560 patients evaluated the efficacy and safety of PRRT retreatment in patients with GEP-NETs, with encouraging results. Median pooled PFS was 12.52 months (95% CI: 9.82–15.22), and pooled DCR was 71% (95% CI: 66–75). The safety profile for retreatment was comparable to the initial PRRT, with grade 3–4 adverse events occurring in 5% (95% CI: 2–8) of patients (13).
Similar results emerged from another meta-analysis, including 9 studies on 426 patients. After PRRT retreatment, pooled DCR was 76.9% (95% CI: 72.3–81.0) months, pooled PFS was 14.1 (95% CI: 12.2–15.9) months, and pooled median OS of 26.8 (95% CI: 18.8–34.9) months. As expected, PRRT showed a significantly lower DCR and shorter PFS compared to initial PRRT, without significant differences in hematologic and renal toxicities (14).
3.1.4 Positioning Peptide Receptor Radionuclide Therapy in the Treatment Sequence
Until now, the optimal treatment sequence for NETs is not well established. A retrospective study in patients with metastatic G2 Pan-NETs, treated with more than one systemic therapy, showed that patients who received PRRT in the treatment sequence (most frequently as third or fourth line) had significantly prolonged survival compared with those who did not receive PRRT [median, 84 vs. 56 months; hazard ratio (HR), 0.55; 95% CI: 0.31–0.98] (15).
Parghane et al. (16) evaluated the long-term outcome of a combined chemotherapy and PRRT protocol with a “sandwich” regimen in the treatment of metastatic progressive NETs with both 18F-FDG and 68Ga-DODATOC avid lesions. In 38 patients analyzed, DCR of 84%, PFS of 72.5%, and OS of 80.4% at 36 months were observed. A longer PFS and higher DCR were noted in patients without metastatic bone involvement. Only low-grade and transient toxicities were registered, without renal toxicities of any grade (16).
The features of the main articles on the new indications and settings and safety of PRRT are summarized in Table 1.
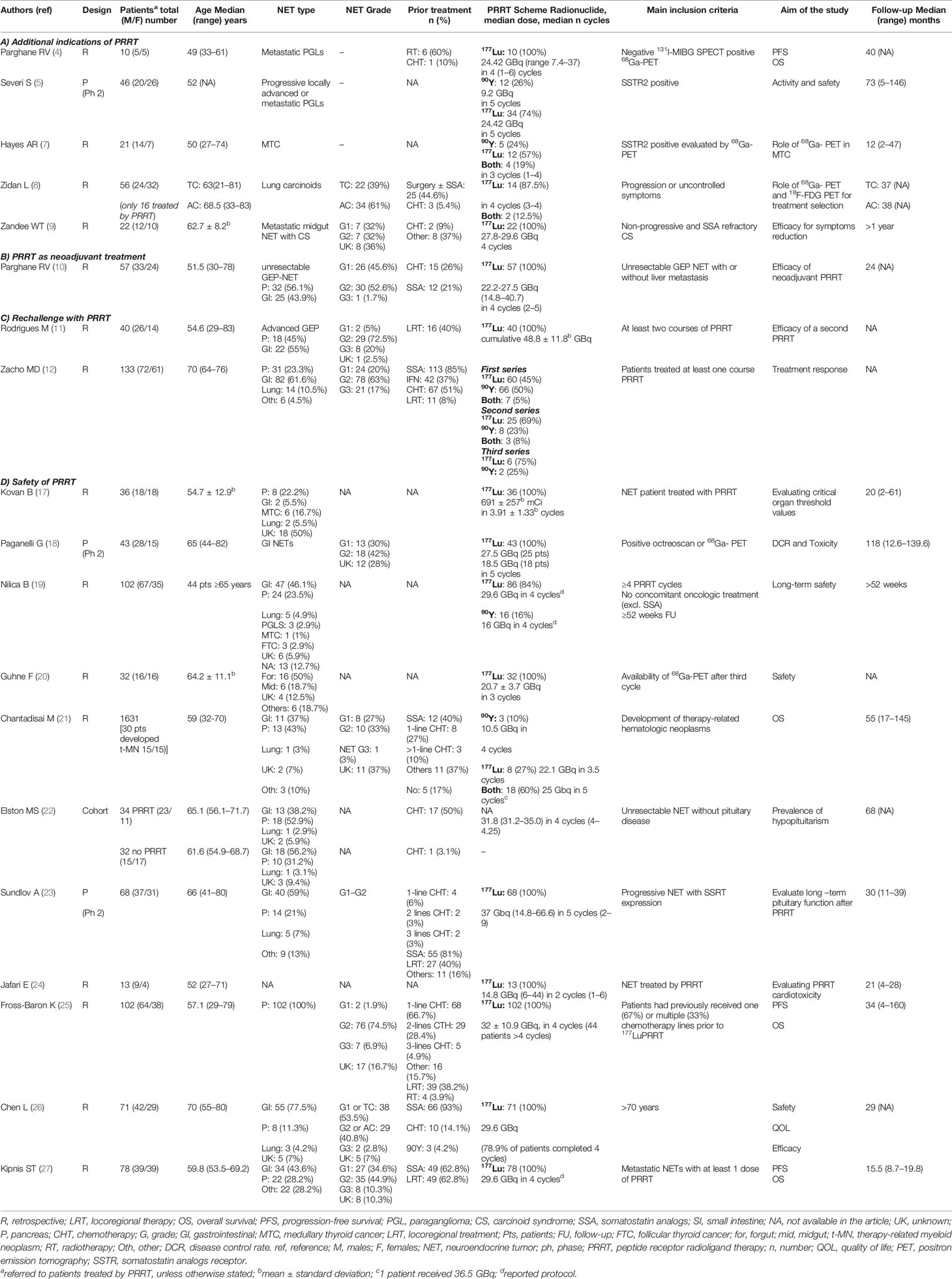
Table 1 List of the main studies published in 2021 on (A) additional indications, (B) neoadjuvant role, (C) rechallenge, and (D) safety of PRRT treatment in patients affected by NETs.
3.2 Short- and Long-Term Safety of Peptide Receptor Radionuclide Therapy
The critical organs to consider before PRRT are the kidney and bone marrow. Until now, the accepted upper limit doses have been adapted by external radiotherapy (23 Gy for kidneys and 2 Gy for bone marrow) (28). Data from a retrospective study including 37 patients receiving 177Lu-DOTATATE showed that only 5.5% reached 2 Gy to the bone marrow and the threshold value of 23 Gy for kidney was reached in 21% of patients receiving 4 cycles and in 37.5% in case of more than 4 cycles. However, no long-term renal dysfunction occurred with a kidney dose of 23–29 Gy, suggesting a possible increase of kidney threshold levels (17). Accordingly, an open-label, prospective, phase II study showed the absence of grade 3–4 hematological toxicities and renal impairment using 177Lu-DOTATATE at two different doses (18.5 and 27.5 GBq in 5 cycles) (18). Globally, PRRT was safe, with a low incidence of severe nephrotoxicity and hematotoxicity. Notably, in the majority of the studies, a protocol of amino acid infusion was used in order to reduce renal injury. A large study described an impairment in kidney function and hemoglobin in 20.6% of patients 1 year after the start of the treatment. Age over 65 years seems to be a risk factor for the development of anemia. Leukocyte and platelet count reduction was 14.7% and 10.8% of patients, respectively (19). Another retrospective study did not confirm any significant change in glomerular filtrate after PRRT (20). Considering the late effects of PRRT, in a large series of 1,631 treated patients, only 1.8% developed therapy-related myeloid neoplasm, including myelodysplastic syndrome and acute myeloid leukemia, after a median time of 43 months (range, 6–123) (21). A case series on 5 patients with bone marrow infiltration of NETs and myelosuppression demonstrated that PRRT could be safe also in these patients when prophylactic peripheral blood stem cell collection was performed before PRRT (29). In another study, grade 1–2 hematological toxicities were observed in 60.3% of patients and grade 3–4 toxicities were observed in 25 patients (32.1%), without the development of myelodysplasia or the need for dialysis or liver failure (27).
Two studies have evaluated the effect of PRRT on pituitary function, as normal pituitary tissue expresses SSTRs. Comparing patients treated or not with PRRT, after a long follow-up (68 months), the prevalence of hypopituitarism was the same in the two groups (22). Another study evaluated pituitary function at baseline and 1 year after high-dose PRRT. The study demonstrated a significant decrease in insulin like growth factor 1 (IGF1) levels, which was related to the number of cycles and the absorbed radiation dose, without changes in the adrenal and thyroid axes (23).
Strosberg et al. (30) reported a 3% incidence of risk of bowel obstruction within 3 months in patients receiving PRRT. All patients had a mesenteric or peritoneal disease and responded to high doses of corticosteroid (30). PRRT-related cardiotoxicity has been investigated in 13 patients affected by NETs. No significant change in serum troponin I was demonstrated after PRRT (24).
The safety of 177Lu-DOTATATE was also confirmed in patients with advanced PanNET heavily pretreated with chemotherapy. Grade 3–4 bone marrow toxicities occurred in 10.8% and were unrelated to the type and duration of previous chemotherapy, amount of activity administered, and dose absorbed from the bone marrow. One patient (1.0%) developed acute myeloid leukemia (25). In older patients (≥70 years) treated with PRRT, the most common adverse events were fatigue and grade 1–2 gastrointestinal disturbances, occurring in 98.3% of patients. The most common hematological adverse events were grade 1–2 lymphocytopenia and anemia. An increase in creatinine values after PRRT occurred in 12.7% of patients (grade 1–2) (26). In a small study evaluating the combination of 177Lu-DOTATATE and 90Y-DOTATOC therapy in 9 patients affected by NETs with a large bulky lesion (≥5 cm), posttreatment imaging showed excellent uptake of the radionuclides in the lesions in almost all patients, and only mild-grade adverse events were observed (31).
The frequencies of adverse events described in the main studies are summarized in Supplementary Table S1.
3.3 Prognostic and Predictive Factors
Many studies have focused on the role of factors that could predict prognosis or response to PRRT, including circulating biomarkers, clinical parameters, and imaging.
Starting from the role of inflammation in NET progression, Ohlendorf et al. (32), in a study on 33 patients with advanced GEP-NETs treated with PRRT, evaluated the predictive role of inflammatory markers. C-reactive protein (CRP), composite index as Platelet × CRP multiplier (PCM), CPR/albumin ratio, and absolute neutrophil count were all significantly higher in patients who were non-responders to PRRT. Interestingly, in this study, the first 68Ga-DOTATATE PET/CT was performed early (after two cycles of treatment); at this time point, CRP and neutrophil-to-lymphocyte ratio were predictors of change in tumor burden (32). Another inflammatory biomarker, platelet-to-lymphocyte ratio (PLR), was evaluated in a retrospective study on a heterogeneous population of 42 patients affected by NET (all grades and sites) and treated by 177Lu-DOTATATE. Patients with PLR greater than 173.1 had significantly reduced PFS, with a univariate HR for progression or death of 3.82 (95% CI: 1.21–12.03) (33).
The predictive role of the classical neuroendocrine markers is debated. A study by Papantoniou et al. (34) demonstrated that changes in chromogranin A and 5-hydroxyindoleacetic acid during treatment were not predictors of PRRT response (34), although baseline values correlated to PFS (34, 35).
In the field of biochemical markers, growing attention is paid to NETest, an application of liquid biopsy in the field of NET, which has also demonstrated a prognostic role (36). In a larger study on the personalized approach to patients affected by neuroendocrine neoplasms, Frilling et al. (37) described that NETest scores decreased after 6 months in 9/9 patients with metastatic small bowel NETs treated with a combination of surgery and PRRT, and NETest values directly correlated with tumor volume. On the contrary, cell-free DNA levels, although higher in patients with NETs than those in healthy controls, were unable to predict OS and response to PRRT (38).
Many clinical parameters have been proposed as prognostic markers. Factors associated with a reduction in PFS and OS in PRRT-treated patients were ascites (35), marked liver metastasis burden (18, 25, 35), unusual metastatic sites (35), and age >65 years at the time of PRRT (18). Other factors such as interim ascites, the presence of ≥5 bone metastases, and NETs other than GEP were predictors of worse OS (35). The importance of bone metastasis is also confirmed by the evidence that an increase in baseline alkaline phosphatase is associated with poorer PFS and OS (25, 26). Das et al. (39) developed an interesting clinical score that included 5 elements, availability of treatments other than 177Lu-DOTATATE, prior systemic treatments, symptoms, tumor burden of critical organs, and peritoneal carcinomatosis, that was able to predict PFS only in patients treated with PRRT. One study failed in demonstrating the role of sarcopenia and myosteatosis in predicting PFS in 49 patients with NET (any grade) treated by PRRT (40). Finally, one study confirmed that resection of the primary tumor had a beneficial effect in increasing OS after PRRT (41).
Morphological and functional imaging has been proposed for treatment response prediction. In a study on 66 patients with PanNET undergoing PRRT, the authors evaluated the tumor growth rate (TGR), expressed as change/month. TGR decreases significantly during PRRT with 177Lu-DOTATATE, and patients with TGR ≥0.5%/month had shorter PFS (HR, 2.82; 95% CI: 1.05–7.57) (42). Many studies focused on the prognostic role of 18F-FDG PET/CT status even in the setting of PRRT-treated patients (18, 43, 44). An interesting prospective 10-year follow-up study of 166 patients demonstrated that 18F-FDG PET/CT is more effective than grading in predicting OS and PFS. In the subgroup of 78 patients who received PRRT, 18F-FDG PET/CT negative cases had significantly longer survival. Interestingly, PRRT increased OS in patients with positive 18F-FDG PET/CT when compared with non-treated patients, while no difference in OS was found between treated and not-treated subgroup of patients with negative 18F-FDG PET/CT (43). A recent meta-analysis on 12 studies and 1,492 patients evaluated the prognostic role of pretreatment 18F-FDG PET/CT in patients affected by any grade NETs treated with PRRT. Positive uptake at 18F-FDG PET/CT was associated with a higher risk of worse outcome [odds ratio (OR), 4.85; 95% CI: 2.27–10.36]. Regarding PFS, the pooled HR for progression was higher in case of positive 18F-FDG PET/CT (HR, 2.45; 95% CI: 1.48–4.04), and likewise, OS was lower (HR, 2.25; 95% CI: 1.55–3.28) (44). SSTR2 expression assessment by nuclear imaging is mandatory for selecting patients for PRRT. However, its prognostic value is less clear. Two studies evaluated the role of standardized uptake value (SUV) parameters at 68Ga-DOTATATE PET/CT in predicting PFS and response to treatment (45, 46). The mean SUVmax was significantly higher in responders than that in non-responders (45, 46) and was higher in patients with PFS >18 months (46). In a subset of 36 patients, another 68Ga-DOTATATE PET/CT scan was performed before the second cycle of PRRT, and SUVmax correlated to therapy response (45). Accordingly, another study demonstrated that the evaluation after two cycles can predict further response. With stable disease after 2 cycles, patients with PanNET were more likely than patients with other NETs to achieve a response (0.60 vs. 0.11) after 4 cycles. In patients with a response after two cycles, all PanNETs demonstrated a continuous response after 4 cycles compared with only 66% of other NETs (47).
PRRT absorbed dose may play a role in predicting the response. Both for small intestine and PanNETs, a dose–response relationship was found between the absorbed dose and tumor shrinkage, which was more pronounced in PanNET (48). Histological parameters have also been proposed as predictors of treatment response. The expression of SSTR2, assessed by immunohistochemistry in tumor samples, was not a predictive factor for PRRT response in a study on 42 patients with small intestine NETs (49). It has also been proposed that in unresponsive patients, PRRT may result in a clonal selection of resistant cells. In a case series on 7 patients with metastatic PanNET treated by PRRT and with evidence of progressive disease within 6 months from treatment, 3 patients underwent a new biopsy. In 2 cases, Ki 67 labeling index increased significantly, and in one patient morphology changed to poorly differentiated. The hypothesis of initial tumor heterogeneity was also supported by the positivity of both gallium and 18F-FDG PET/CT (50).
4 Conclusions
In 2021, many articles have been published on three hot topics of PRRT treatment in NETs: new clinical indications, safety, and prognostic and predictive markers. The main findings are summarized in Figure 1. Considering the evidence that this treatment has been used in PGLs, MTC, pulmonary carcinoids, and uncontrolled CS and in the neoadjuvant or salvage settings, PRRT indications are likely to increase in the near future. Despite the concern of the kidney and bone marrow toxicities of PRRT, available studies, including long follow-up studies, demonstrated the safety of this treatment, with the worse complication, the development of second neoplasia, appearing in 1.8% of treated patients. Finally, as in other aspects of NETs, prognostic and predictive factors are also lacking for PRRT. New evidence confirmed the crucial role of nuclear imaging not only for the selection of the patients but also for estimating treatment response.
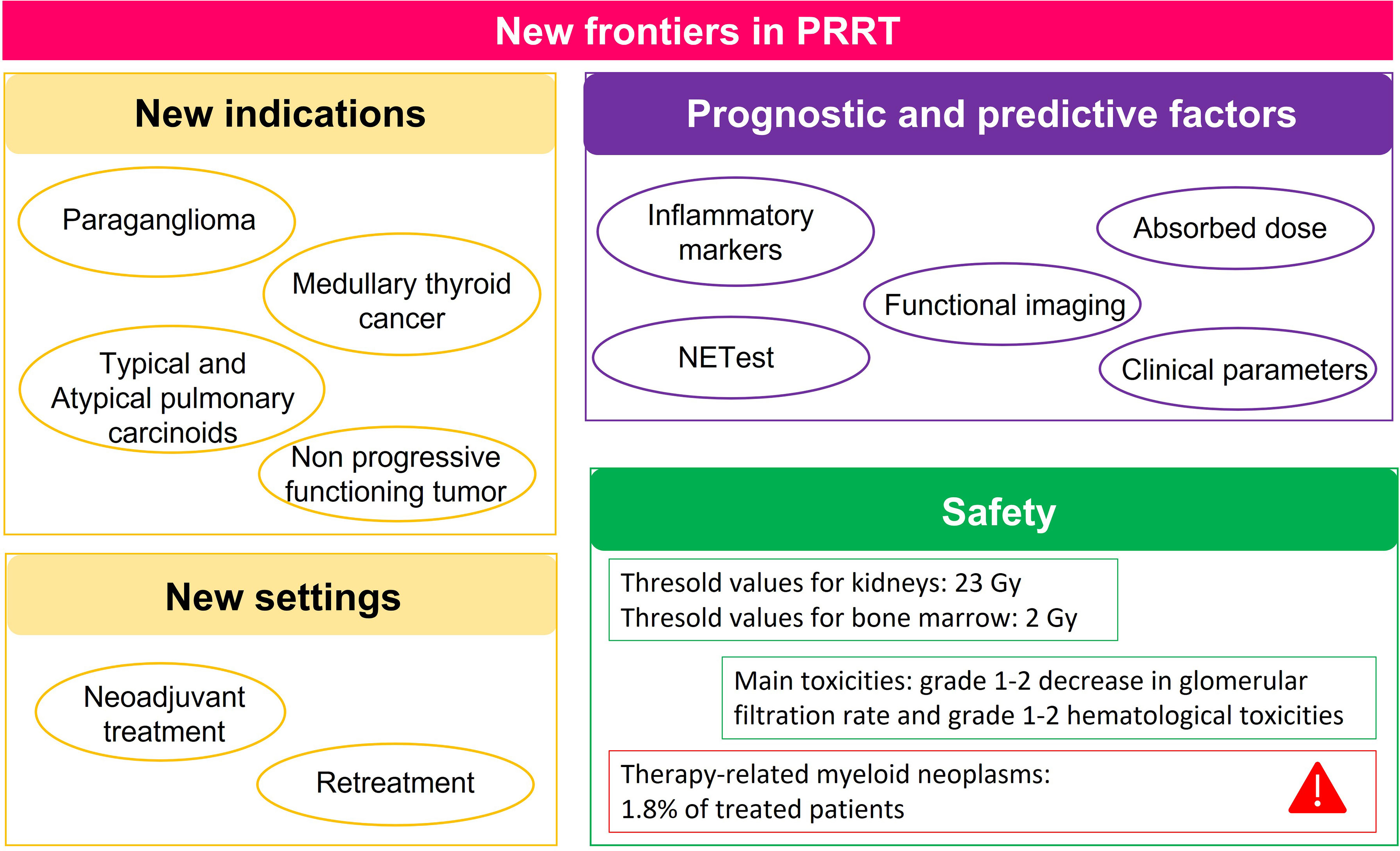
Figure 1 Summary of the main findings of articles published in 2021 on the 3 hot topics of peptide receptor radionuclide therapy (PRRT) in neuroendocrine tumors (NETs): new indications and settings, prognostic and predictive factors, and safety.
Author Contributions
Conceptualization: GP and MA. Data curation: GP, AC, MM, MB, and RL. Methodology and validation: GP. Writing—original draft: GP, AC, MM, MB, and RL. Writing—review and editing and supervision: MA. All authors contributed to the article and approved the submitted version.
Conflict of Interest
MA does consultancy and has received research grants from Bayer, Eisai, and Eli-Lilly.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.861434/full#supplementary-material
Supplementary Table 1 | Frequency of adverse events of PRRT reported in main studies.
Supplementary Figure 1 | PRISMA flow diagram of the search strategy.
References
1. Kwekkeboom DJ, Krenning EP, Lebtahi R, Komminoth P, Kos-Kudla B, de Herder WW, et al. Enets Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Peptide Receptor Radionuclide Therapy With Radiolabeled Somatostatin Analogs. Neuroendocrinology (2009) 90(2):220–6. doi: 10.1159/000225951
2. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med (2017) 376(2):125–35. doi: 10.1056/NEJMoa1607427
3. Pavel M, Oberg K, Falconi M, Krenning EP, Sundin A, Perren A, et al. Gastroenteropancreatic Neuroendocrine Neoplasms: Esmo Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2020) 31(7):844–60. doi: 10.1016/j.annonc.2020.03.304
4. Parghane RV, Talole S, Basu S. (131)I-Mibg Negative Progressive Symptomatic Metastatic Paraganglioma: Response and Outcome With (177)Lu-Dotatate Peptide Receptor Radionuclide Therapy. Ann Nucl Med (2021) 35(1):92–101. doi: 10.1007/s12149-020-01541-z
5. Severi S, Bongiovanni A, Ferrara M, Nicolini S, Di Mauro F, Sansovini M, et al. Peptide Receptor Radionuclide Therapy in Patients With Metastatic Progressive Pheochromocytoma and Paraganglioma: Long-Term Toxicity, Efficacy and Prognostic Biomarker Data of Phase Ii Clinical Trials. ESMO Open (2021) 6(4):100171. doi: 10.1016/j.esmoop.2021.100171
6. Tsang ES, Funk G, Leung J, Kalish G, Kennecke HF. Supportive Management of Patients With Advanced Pheochromocytomas and Paragangliomas Receiving Prrt. Curr Oncol (2021) 28(4):2823–9. doi: 10.3390/curroncol28040247
7. Hayes AR, Crawford A, Al Riyami K, Tang C, Bomanji J, Baldeweg SE, et al. Metastatic Medullary Thyroid Cancer: The Role of 68gallium-Dota-Somatostatin Analogue Pet/Ct and Peptide Receptor Radionuclide Therapy. J Clin Endocrinol Metab (2021) 106(12):e4903–e16. doi: 10.1210/clinem/dgab588
8. Zidan L, Iravani A, Kong G, Akhurst T, Michael M, Hicks RJ. Theranostic Implications of Molecular Imaging Phenotype of Well-Differentiated Pulmonary Carcinoid Based on (68)Ga-Dotatate Pet/Ct and (18)F-Fdg Pet/Ct. Eur J Nucl Med Mol Imaging (2021) 48(1):204–16. doi: 10.1007/s00259-020-04915-7
9. Zandee WT, Brabander T, Blazevic A, Minczeles NS, Feelders RA, de Herder WW, et al. Peptide Receptor Radionuclide Therapy With 177lu-Dotatate for Symptomatic Control of Refractory Carcinoid Syndrome. J Clin Endocrinol Metab (2021) 106(9):e3665–e72. doi: 10.1210/clinem/dgab289
10. Parghane RV, Bhandare M, Chaudhari V, Ostwal V, Ramaswamy A, Talole S, et al. Surgical Feasibility, Determinants, and Overall Efficacy of Neoadjuvant (177)Lu-Dotatate Prrt for Locally Advanced Unresectable Gastroenteropancreatic Neuroendocrine Tumors. J Nucl Med (2021) 62(11):1558–63. doi: 10.2967/jnumed.120.258772
11. Rodrigues M, Winkler KK, Svirydenka H, Nilica B, Uprimny C, Virgolini I. Long-Term Survival and Value of (18)F-Fdg Pet/Ct in Patients With Gastroenteropancreatic Neuroendocrine Tumors Treated With Second Peptide Receptor Radionuclide Therapy Course With (177)Lu-Dotatate. Life (Basel) (2021) 11(3). doi: 10.3390/life11030198
12. Zacho MD, Iversen P, Villadsen GE, Baunwall SMD, Arveschoug AK, Gronbaek H, et al. Clinical Efficacy of First and Second Series of Peptide Receptor Radionuclide Therapy in Patients With Neuroendocrine Neoplasm: A Cohort Study. Scand J Gastroenterol (2021) 56(3):289–97. doi: 10.1080/00365521.2021.1872095
13. Strosberg J, Leeuwenkamp O, Siddiqui MK. Peptide Receptor Radiotherapy Re-Treatment in Patients With Progressive Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. Cancer Treat Rev (2021) 93:102141. doi: 10.1016/j.ctrv.2020.102141
14. Kim YI. Salvage Peptide Receptor Radionuclide Therapy in Patients With Progressive Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. Nucl Med Commun (2021) 42(4):451–8. doi: 10.1097/MNM.0000000000001350
15. Hayes AR, Mak IYF, Evans N, Naik R, Crawford A, Khoo B, et al. Understanding the Treatment Algorithm of Patients With Metastatic Pancreatic Neuroendocrine Neoplasms: A Single-Institution Retrospective Analysis Comparing Outcomes of Chemotherapy, Molecular Targeted Therapy, and Peptide Receptor Radionuclide Therapy in 255 Patients. Neuroendocrinology (2021) 111(9):863–75. doi: 10.1159/000511662
16. Parghane RV, Ostwal V, Ramaswamy A, Bhandare M, Chaudhari V, Talole S, et al. Long-Term Outcome of “Sandwich” Chemo-Prrt: A Novel Treatment Strategy for Metastatic Neuroendocrine Tumors With Both Fdg- and Sstr-Avid Aggressive Disease. Eur J Nucl Med Mol Imaging (2021) 48(3):913–23. doi: 10.1007/s00259-020-05004-5
17. Kovan B, Ozkan ZG, Demir B, Tuncman D, Isik EG, Simsek DH, et al. An Analysis for Therapeutic Doses of Patients With Neuroendocrine Tumor Treated With Lutetium-177-Dotatate. Cancer Biother Radiopharm (2021) 37(1):17–22. doi: 10.1089/cbr.2021.0071
18. Paganelli G, Sansovini M, Nicolini S, Grassi I, Ibrahim T, Amadori E, et al. (177)Lu-Prrt in Advanced Gastrointestinal Neuroendocrine Tumors: 10-Year Follow-Up of the Irst Phase Ii Prospective Study. Eur J Nucl Med Mol Imaging (2021) 48(1):152–60. doi: 10.1007/s00259-020-04873-0
19. Nilica B, Svirydenka A, Fritz J, Bayerschmidt S, Kroiss AS, Gruber L, et al. Nephrotoxicity and Hematotoxicity One Year After Four Cycles of Peptide Receptor Radionuclide Therapy (Prrt) and Its Impact on Future Treatment Planning. A Retrospective Analysis. Rev Esp Med Nucl Imagen Mol (Engl Ed) (2021). doi: 10.1016/j.remn.2021.03.004
20. Guhne F, Heinzig A, Seifert P, Drescher R, Freesmeyer M. The Dependence of Renal (68)Ga[Ga]-Dotatoc Uptake on Kidney Function and Its Relevance for Peptide Receptor Radionuclide Therapy With (177)Lu[Lu]-Dotatoc. Diagnostics (Basel) (2021) 11(7):1216–27. doi: 10.3390/diagnostics11071216
21. Chantadisai M, Kulkarni HR, Baum RP. Therapy-Related Myeloid Neoplasm After Peptide Receptor Radionuclide Therapy (Prrt) in 1631 Patients From Our 20 Years of Experiences: Prognostic Parameters and Overall Survival. Eur J Nucl Med Mol Imaging (2021) 48(5):1390–8. doi: 10.1007/s00259-020-05127-9
22. Elston MS, Love A, Kevat D, Carroll R, Siow ZR, Pattison S, et al. Pituitary Function Following Peptide Receptor Radionuclide Therapy for Neuroendocrine Tumours. Cancer Med (2021) 10(23):8405–11. doi: 10.1002/cam4.4345
23. Sundlov A, Sjogreen-Gleisner K, Tennvall J, Dahl L, Svensson J, Akesson A, et al. Pituitary Function After High-Dose 177lu-Dotatate Therapy and Long-Term Follow-Up. Neuroendocrinology (2021) 111(4):344–53. doi: 10.1159/000507761
24. Jafari E, Amini AL, Ahmadzadehfar H, Bagheri D, Assadi M. Cardiotoxicity and Cardiac Monitoring Following the Use of Radiotheranostics Agents Including 177lu-Psma for Prostate Cancer and 177lu-Dotatate for Neuroendocrine Tumors. Nuklearmedizin (2021) 60(2):99–105. doi: 10.1055/a-1332-8230
25. Fross-Baron K, Garske-Roman U, Welin S, Granberg D, Eriksson B, Khan T, et al. 177lu-Dotatate Therapy of Advanced Pancreatic Neuroendocrine Tumors Heavily Pretreated With Chemotherapy: Analysis of Outcome, Safety, and Their Determinants. Neuroendocrinology (2021) 111(4):330–43. doi: 10.1159/000506746
26. Chen L, Navalkissoor S, Quigley AM, Gnanasegaran G, Mandair D, Toumpanakis C, et al. (177)Lu-Dotatate in Older Patients With Metastatic Neuroendocrine Tumours: Safety, Efficacy and Health-Related Quality of Life. Eur J Nucl Med Mol Imaging (2021) 48(11):3582–94. doi: 10.1007/s00259-021-05332-0
27. Kipnis ST, Hung M, Kumar S, Heckert JM, Lee H, Bennett B, et al. Laboratory, Clinical, and Survival Outcomes Associated With Peptide Receptor Radionuclide Therapy in Patients With Gastroenteropancreatic Neuroendocrine Tumors. JAMA Netw Open (2021) 4(3):e212274. doi: 10.1001/jamanetworkopen.2021.2274
28. Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of Normal Tissue to Therapeutic Irradiation. Int J Radiat Oncol Biol Phys (1991) 21(1):109–22. doi: 10.1016/0360-3016(91)90171-y
29. Sabet A, Mader N, Bittenbring JT, Khreish F, Grunwald F, Biersack HJ, et al. Prophylactic Peripheral Blood Stem Cell Collection in Patients With Extensive Bone-Marrow Infiltration of Neuroendocrine Tumours Prior to Peptide Receptor Radionuclide Therapy With (177)Lu-Dotatate. Pharmaceuticals (Basel) (2021) 14(10):1022–8. doi: 10.3390/ph14101022
30. Strosberg JR, Al-Toubah T, Pelle E, Smith J, Haider M, Hutchinson T, et al. Risk of Bowel Obstruction in Patients With Mesenteric or Peritoneal Disease Receiving Peptide Receptor Radionuclide Therapy. J Nucl Med (2021) 62(1):69–72. doi: 10.2967/jnumed.120.242875
31. Parghane RV, Mitra A, Bannore TU, Rakshit S, Banerjee S, Basu S. Initial Clinical Evaluation of Indigenous (90)Y-Dotatate in Sequential Duo-Prrt Approach ((177)Lu-Dotatate and (90)Y-Dotatate) in Neuroendocrine Tumors With Large Bulky Disease: Observation on Tolerability, (90)Y-Dotatate Post- Prrt Imaging Characteristics (Bremsstrahlung and Petct) and Early Adverse Effects. World J Nucl Med (2021) 20(1):73–81. doi: 10.4103/wjnm.WJNM_52_20
32. Ohlendorf F, Werner RA, Henkenberens C, Ross TL, Christiansen H, Bengel FM, et al. Predictive and Prognostic Impact of Blood-Based Inflammatory Biomarkers in Patients With Gastroenteropancreatic Neuroendocrine Tumors Commencing Peptide Receptor Radionuclide Therapy. Diagnostics (Basel) (2021) 11(3):504–15. doi: 10.3390/diagnostics11030504
33. Satapathy S, Bhattacharya A, Sood A, Kapoor R, Gupta R, Sood A, et al. Hematological Markers as Predictors of Treatment Outcomes With Lu-177 Dotatate in Patients With Advanced Neuroendocrine Tumors. Cancer Biother Radiopharm (2021) 37(1):23–9. doi: 10.1089/cbr.2021.0053
34. Papantoniou D, Gronberg M, Landerholm K, Welin S, Ziolkowska B, Nordvall D, et al. Assessment of Hormonal Levels as Prognostic Markers and of Their Optimal Cut-Offs in Small Intestinal Neuroendocrine Tumours Grade 2. Endocrine (2021) 72(3):893–904. doi: 10.1007/s12020-020-02534-8
35. Swiha MM, Sutherland DEK, Sistani G, Khatami A, Abazid RM, Mujoomdar A, et al. Survival Predictors of (177)Lu-Dotatate Peptide Receptor Radionuclide Therapy (Prrt) in Patients With Progressive Well-Differentiated Neuroendocrine Tumors (Nets). J Cancer Res Clin Oncol (2022) 148(1):225–36. doi: 10.1007/s00432-021-03672-w
36. Puliani G, Di Vito V, Feola T, Sesti F, Centello R, Pandozzi C, et al. Netest: A Systematic Review Focusing on the Prognostic and Predictive Role. Neuroendocrinology (2021). doi: 10.1159/000518873
37. Frilling A, Clift AK, Frampton AE, Bomanji J, Kaemmerer D, Al-Nahhas A, et al. A Combination of Surgery, Theranostics, and Liquid Biopsy - a Personalised Oncologic Approach to Treatment of Patients With Advanced Metastatic Neuroendocrine Neoplasms. Int J Med Sci (2021) 18(10):2166–75. doi: 10.7150/ijms.51740
38. Oversoe SK, Sorensen BS, Tabaksblat EM, Gronbaek H, Kelsen J. Cell-Free DNA and Clinical Characteristics in Patients With Small Intestinal or Pancreatic Neuroendocrine Tumors. Neuroendocrinology (2022) 112(1):43–50. doi: 10.1159/000514457
39. Das S, Du L, Schad A, Jain S, Jessop A, Shah C, et al. A Clinical Score for Neuroendocrine Tumor Patients Under Consideration for Lu-177-Dotatate Therapy. Endocr Relat Cancer (2021) 28(3):203–12. doi: 10.1530/ERC-20-0482
40. Chan DL, Clarke SJ, Engel A, Diakos CI, Pavlakis N, Roach PJ, et al. Computed Tomography (Ct)-Defined Sarcopenia and Myosteatosis Are Prevalent in Patients With Neuroendocrine Neoplasms (Nens) Treated With Peptide Receptor Radionuclide Therapy (Prrt). Eur J Clin Nutr (2022) 76(1):143–9. doi: 10.1038/s41430-021-00915-4
41. Kaemmerer D, Twrznik M, Kulkarni HR, Horsch D, Sehner S, Baum RP, et al. Prior Resection of the Primary Tumor Prolongs Survival After Peptide Receptor Radionuclide Therapy of Advanced Neuroendocrine Neoplasms. Ann Surg (2021) 274(1):e45–53. doi: 10.1097/SLA.0000000000003237
42. Pettersson OJ, Fross-Baron K, Crona J, Sundin A. Tumor Growth Rate in Pancreatic Neuroendocrine Tumor Patients Undergoing Prrt With 177lu-Dotatate. Endocr Connect (2021) 10(4):422–31. doi: 10.1530/EC-21-0027
43. Binderup T, Knigge U, Johnbeck CB, Loft A, Berthelsen AK, Oturai P, et al. (18)F-Fdg Pet Is Superior to Who Grading as a Prognostic Tool in Neuroendocrine Neoplasms and Useful in Guiding Prrt: A Prospective 10-Year Follow-Up Study. J Nucl Med (2021) 62(6):808–15. doi: 10.2967/jnumed.120.244798
44. Alevroudis E, Spei ME, Chatziioannou SN, Tsoli M, Wallin G, Kaltsas G, et al. Clinical Utility of (18)F-Fdg Pet in Neuroendocrine Tumors Prior to Peptide Receptor Radionuclide Therapy: A Systematic Review and Meta-Analysis. Cancers (Basel) (2021) 13(8):1813–27. doi: 10.3390/cancers13081813
45. Ortega C, Wong RKS, Schaefferkoetter J, Veit-Haibach P, Myrehaug S, Juergens R, et al. Quantitative (68)Ga-Dotatate Pet/Ct Parameters for the Prediction of Therapy Response in Patients With Progressive Metastatic Neuroendocrine Tumors Treated With (177)Lu-Dotatate. J Nucl Med (2021) 62(10):1406–14. doi: 10.2967/jnumed.120.256727
46. Teker F, Elboga U. Is Suvmax a Useful Marker for Progression-Free Survival in Patients With Metastatic Gep-Net Receiving (177)Lu-Dotatate Therapy? Hell J Nucl Med (2021) 24(2):122–31. doi: 10.1967/s002449912352
47. Vaghaiwalla T, Ruhle B, Memeh K, Angelos P, Kaplan E, Liao CY, et al. Response Rates in Metastatic Neuroendocrine Tumors Receiving Peptide Receptor Radionuclide Therapy and Implications for Future Treatment Strategies. Surgery (2021) 169(1):162–7. doi: 10.1016/j.surg.2020.04.001
48. Jahn U, Ilan E, Sandstrom M, Lubberink M, Garske-Roman U, Sundin A. Peptide Receptor Radionuclide Therapy (Prrt) With (177)Lu-Dotatate; Differences in Tumor Dosimetry, Vascularity and Lesion Metrics in Pancreatic and Small Intestinal Neuroendocrine Neoplasms. Cancers (Basel) (2021) 13(5):962–76. doi: 10.3390/cancers13050962
49. Elf AK, Johanson V, Marin I, Bergstrom A, Nilsson O, Svensson J, et al. Evaluation of Sstr2 Expression in Si-Nets and Relation to Overall Survival After Prrt. Cancers (Basel) (2021) 13(9):2035–47. doi: 10.3390/cancers13092035
Keywords: peptide receptor radionuclide therapy, radioligand therapy, predictive factors, prognostic factors, neuroendocrine tumors, neuroendocrine neoplasms, safety
Citation: Puliani G, Chiefari A, Mormando M, Bianchini M, Lauretta R and Appetecchia M (2022) New Insights in PRRT: Lessons From 2021. Front. Endocrinol. 13:861434. doi: 10.3389/fendo.2022.861434
Received: 24 January 2022; Accepted: 07 March 2022;
Published: 05 April 2022.
Edited by:
Antonino Belfiore, University of Catania, ItalyReviewed by:
Krystallenia I. Alexandraki, National and Kapodistrian University of Athens, GreeceCopyright © 2022 Puliani, Chiefari, Mormando, Bianchini, Lauretta and Appetecchia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marialuisa Appetecchia, marialuisa.appetecchia@ifo.it
 Giulia Puliani
Giulia Puliani Alfonsina Chiefari
Alfonsina Chiefari Marilda Mormando
Marilda Mormando Marta Bianchini
Marta Bianchini Rosa Lauretta
Rosa Lauretta Marialuisa Appetecchia
Marialuisa Appetecchia