- 1Department of GI Oncology, H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL, United States
- 2Department of Head and Neck - Endocrine Oncology, H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL, United States
- 3Department of Radiology, H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL, United States
- 4Department of Nuclear Medicine, H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL, United States
Targeted radionuclide therapy plays an increasingly important role in managing endocrine-related tumors and significantly advances the therapeutic landscape for patients with these diseases. With increasing FDA-approved therapies and advances in the field, come an increased knowledge of the potential for long-term toxicities associated with these therapies and the field must develop new strategies to increase potency and efficacy while individualizing the selection of patients to those most likely to respond to treatment. Novel agents and modalities of therapy are also being explored. This review will discuss the current landscape and describe the avenues for growth in the field currently being explored.
Introduction
Targeted radionuclide therapy plays an increasingly important role in management of neuroendocrine tumors. Currently available treatments include Lu-177 DOTATATE which is approved for advanced gastroenteropancreatic neuroendocrine tumors (GEP-NETs), and I-131 meta-iodobenzylguanidine (MIBG) which is approved for treatment of advanced pheochromocytomas/paragangliomas (1, 2). Lu-177 DOTATATE is a form of peptide receptor radionuclide therapy (PRRT) which targets somatostatin receptor (SSTR) subtypes overexpressed by most well-differentiated NETs. I-131 MIBG relies on the norepinephrine transporter mechanism which takes up amines in tissues derived from the neural crest such as the adrenal medulla and sympathetic nervous system (3).
Peptide receptor radionuclide therapy (PRRT) is a targeted systemic therapy that uses radiolabeled peptides to deliver cytotoxic radiation levels directly to tumors that overexpress specific receptors (4). This systemic administration of targeted radiopharmaceutical delivers therapeutic doses of radiation to specific disease sites while minimizing the radiation effect on healthy tissue.
In addition, several new therapies are currently being developed preclinically, and currently approved therapies are now being explored in other indications to see whether they can benefit patient populations with unmet needs. The changing treatment landscape of neuroendocrine tumors raises questions regarding optimal dosing, patient selection, role of combination therapy and sequencing of treatments.
Neuroendocrine neoplasms
Neuroendocrine neoplasms (NEN) represent a heterogeneous family of cancers that can arise from various organ systems, primarily in the gastroenteropancreatic tract and lungs (5). The incidence of diagnoses has increased significantly in the last three decades (6). NEN biology, primarily defined by tumor grade and differentiation, dictates available therapies. Several treatment options are available for well-differentiated NETs, including somatostatin analogs, targeted therapies with mTOR inhibitors (everolimus), angiogenesis inhibitors (sunitinib), and oral chemotherapy agents (capecitabine/temozolomide) (7–9). In addition, clinical trials have explored several other therapies, including pazopanib, lenvatinib, surufatinib, and immunotherapy with checkpoint inhibitors, among other treatments (10–22). Poorly differentiated neuroendocrine carcinomas represent a different biology and are treated more aggressively with cytotoxic chemotherapy, primarily with platinum-based regimens (23–26). Therapy with radiolabeled somatostatin analogs, broadly encompassed under the class of PRRT, represents the newest treatment that has been approved for the GEP-NET patient population.
Data on therapeutic radionuclides in neuroendocrine neoplasms began with In-111-labeled octreotide, a weakly cytotoxic radiolabeled somatostatin analog (SSA). β-emitting isotopes were subsequently developed with the potential to elicit a more significant tumor response in patients with well-differentiated GEP-NETs that express SSTRs (27–29). Y-90ttrium (Y-90) and Lu-177-based radiolabeled SSAs (e.g., Y-90 DOTATOC and Lu-177 DOTATATE) have shown evidence of safety and efficacy in multiple studies with objective response rates ranging from approximately 15% to 40% and long median durations of progression-free survival (PFS), typically exceeding 2 years (30–38). The NETTER-1 trial was the first prospective phase III trial evaluating Lu-177 DOTATATE versus high-dose octreotide in patients with advanced small intestinal (midgut) NETs (34). The primary endpoint of the study was met with a 79% improvement in PFS. On final overall survival (OS) analysis, a secondary endpoint, median overall survival (OS) improved from 36.3 months on the high dose octreotide arm to 48 months on the Lu-177 DOTATATE arm (39). However, this result was not statistically significant, likely due to lack of power to detect OS differences and the effects of crossover after progression on high-dose octreotide. Toxicity with Lu-177 DOTATATE arm has generally been mild, with most side effects limited to minor nausea, fatigue, and reversible myelosuppression (38, 40–44). The risk of treatment-related myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) is estimated to be 2-3% and typically develops more than 2 years after completion of therapy (34, 39, 40). Sequential or combination therapy with alkylating-based chemotherapy agents may result in a higher risk of developing a long-term hematologic toxicity, with some series reporting up to a 10-20% risk (43–50). Renal toxicity has been negligible, likely due to the nephroprotective amino-acid infusion administered alongside the PRRT (42, 51–53). Recent data point to risk of severe, and often irreversible bowel obstruction among patients with extensive peritoneal or mesenteric disease receiving Lu-177 DOTATATE (54, 55). Data from the NETTER-1 study as well as a large Dutch cohort analysis of 610 NET patients led to approval of Lu-177 DOTATATE for patients with progressive, SSTR positive GEP-NETs.
Limited data is available in patients with lung NENs, partly due to typically heterogeneous uptake on SSTR imaging in this patient population (30, 31, 56–60). Data are available from small studies and phase II studies, showing mildly inferior PFS results compared to patients with GEP-NETs.
Medullary thyroid cancer
Medullary thyroid cancer (MTC) is a neuroendocrine tumor of the parafollicular thyroid cells that can occur sporadically or as a component of multiple endocrine neoplasia (MEN) type 2 (61). MTCs secrete calcitonin and carcinoembryonic antigen (CEA). The primary systemic therapies approved for advanced MTC include tyrosine kinase inhibitors (TKIs), primarily targeting RET mutations, including selepercatinib, and pralsetinib, and multi-kinase inhibitors such as vandetanib and cabozantinib (62–78). Several others, sorafenib, sunitinib, and lenvatinib have also been studied (79–81). Due to the expression of CEA on MTC cells, radiolabeled anti-CEA monoclonal antibodies were developed. In an initial phase I trial of an anti-CEA hMN-14 x m734 bispecific antibody (BsMAb) and I-131 di-diethylenetriamine pentaacetic acid (DTPA)-indium hapten, 9 MTC patients were enrolled, and another 29 in a phase II trial later on (82). Ultimately, the drug has not been further developed in this patient population, likely due to the significant hematologic toxicities and lack of improvement in overall survival, despite its ability to induce long-term disease stabilization.
A retrospective study of 21 MTC patients who received treatment with Y-90 DOTATOC reported a radiographic disease control rate (DCR) of 67% (10% complete response (CR), 57% stable disease (SD)) (83). Biochemically (calcitonin and CEA levels), the disease control rate was 43% and the duration of response ranged from 3-40 months. Another retrospective analysis of 10 MTC patients treated with Lu-177 DOTATATE showed only 4 patients with disease stabilization at first follow-up (84). This analysis evaluated the percentage of patients at their institution with positive In-111 pentetreotide (OctreoScan) and found that most patients (89%) had low uptake on scans, and the remaining patients had only intermediate uptake (Krenning score grade 2). The patients in this study who had disease stabilization were characterized as those with uptake ≥ grade 3 on OctreoScan. Both studies have shown that PRRT in this patient population is unlikely to be effective in most patients and should be limited to those with high uptake on SSTR imaging (Cu-64 or Ga-68 PET DOTATATE) and without any other treatment options (85).
Pheochromocytomas and paraganglioma
Pheochromocytomas and paragangliomas are catecholamine-secreting neuroendocrine tumors that arise from chromaffin cells of either the adrenal medulla (pheochromocytomas) or neuroendocrine cells of the extra-adrenal autonomic paraganglioma (paragangliomas) (86). Most are benign, however approximately 10% of pheochromocytomas and 25% of paragangliomas are malignant.
For patients with distant metastases, systemic therapies can include somatostatin analogs such as octreotide or lanreotide (although benefit is unproven), systemic chemotherapy with cyclophosphamide, vincristine and dacarbazine (CVD) or temozolomide, sunitinib, or radionuclide therapy with beta-emitting isotopes iobenguane I-131 MIBG (for patients with MIBG positive scans) or PRRT with Lu-177 DOTATATE (for patients with positive SSTR imaging) (87–94).
Iobenguane I-131 MIBG is a I-131 labeled radiopharmaceutical that is similar in structure to the neurotransmitter norepinephrine that is taken up by the norepinephrine transporter in adrenergic nerve terminals and accumulates in innervated adrenergic tissues as well as tumors of neural crest origin. Pheochromocytomas and paragangliomas express high norepinephrine transporter levels on cell surfaces. Patients must have positive metaiobenzeneguanidine (MIBG) scans to be eligible for treatment.
I-131 MIBG is the first FDA approved therapy for this patient population and is typically administered as a total of 2 doses, minimum of 90 days apart, at a weight-based dose of 8 mCi/kg for patients who weigh less than 62.5kg and a flat dose of 500 mCi for patients who weigh >62.5kg. Several small case series showed objective response rates of around 30% and stability in 40% of patients receiving treatment. A large phase II trial of 81 patients led to FDA approval of the drug (95). Patients were assigned to receive 2 dosimetric doses of MIBG, followed by up to 2 therapeutic doses, then followed for 12 months for efficacy and 4 years for overall survival. The study’s primary endpoint was at least 50% reduction in all antihypertensive medications lasting ≥ 6 months from the beginning of the efficacy period. Secondary endpoints included objective response rate (ORR) and OS. 68 patients received at least one therapeutic dose and 50 received both doses. Primary endpoint was met by 25% of those who received one therapeutic dose and 32% of those who received both doses, meeting pre-specified protocol criteria for a positive study. In addition, 23% of patients who received one dose and 30% of those who received two doses achieved a partial response (PR) per RECIST 1.1. Median OS was 36.7 months, and five-year OS was 64%. Most common treatment-related toxicities included significant myelosuppression, with ≥ grade 3 neutropenia and thrombocytopenia in 87% of patients. 4 patients required autologous hematopoietic cell rescue and 2 patients developed MDS. 2 patients each developed grade 4 acute respiratory distress and cryptogenic organizing pneumonia.
Several retrospective studies of I-131 MIBG have also been reported (96). For example, one study of 125 patients with metastatic pheochromocytoma/paraganglioma reported a median survival post-treatment of 4 years, an ORR of 34%, and DCR of 86%; the median PFS was two years. In addition, 59% of patients achieved a biochemical response, with the median time to laboratory progression of 2.8 years.
PRRT with Lu-177 DOTATATE or Y-90 DOTATOC has been evaluated in retrospective studies and small case reports/case series in patients with SSTR-expressing pheochromocytomas/paragangliomas (92, 97). One study evaluated the outcomes of 28 patients who received Y-90 DOTATOC alone or with Lu-177 DOTATATE (88). Two patients experienced a PR; overall DCR was 71%, of whom 50% maintained their response at 19 months mean follow-up. Another study reported the outcomes of 30 patients who received 4 cycles of Lu-177 DOTATATE. 23% of patients achieved a PR per RECIST 1.1, and stable disease in 67%. Median PFS for patients with parasympathetic paragangliomas was 91 months, 13 months in patients with sympathetic paragangliomas, and 10 months in patients with pheochromocytoma. 20% of patients experienced ≥ grade 3 hematologic toxicities, and 2 patients had reversible cardiac failure following catecholamine release. Patients must be monitored and pre-treated with alpha blockade therapy if functional tumors are present to prevent hypertensive crises after treatment with any of these types of treatment (98).
Future of PRRT and novel approaches to treatment
Dosimetry and predictive biomarkers
Individualized dosimetry strategies have been proposed and primarily focus on minimizing renal and bone marrow exposure while maximizing the potential for anti-tumor activity (42, 51, 99–103). Unfortunately, these approaches have been limited by the lack of standardization in dosimetric methodology, lack of clear thresholds for renal exposure, and difficulties in accurately measuring bone marrow exposure.
The European Association of Nuclear Medicine (EANM) recommends dosimetry, where feasible, in patients receiving radiolabeled somatostatin-receptor ligands and radiolabeled MIBG therapy, particularly for patients with larger tumor volume, due to the relatively limited spatial resolution of MIBG imaging (104).
Biomarkers to predict responders to PRRT are being investigated. Ki67% has been noted to significantly impact OS in both GEP NETs and pheochromocytoma/paraganglioma but is likely more of a prognostic than predictive factor (105–107). Tumor imaging with baseline MIBG scans, PET DOTATATE, and FDG-PET scans can help delineate who is likely to respond (or be eligible). Obtaining baseline FDG-PET scans, particularly in patients with the higher-grade disease, can help delineate the avidity of disease, and for patients with higher avidity on FDG-PET, PRRT is likely to be less effective (108). It is also essential to confirm that all lesions express SSTRs, particularly in patients with higher-grade disease. A genomic signature (blood and tumor-based NET transcript assay – PRRT Predictive Quotient or PPQ) to identify NET responders to PRRT with Lu-177 DOTATATE has been developed and is currently being evaluated in a large, multi-center, clinical trial (109–111).
A blood-based RNA assay was developed to identify gene expression differences in patients receiving I-131 MIBG and demonstrated the ability to use biodosimetric gene expression panels as predictive biomarkers of internal exposure and differentiate exposed individuals up to 15 days after exposure to treatment (112). However, data on predictive biomarkers for all radioisotopes are limited, and further research is necessary to advance this field.
PRRT retreatment
Retreatment with Lu-177 DOTATATE beyond the standard 4 cycles is often recommended for patients who benefit from initial treatment (52, 113, 114). The lifetime maximum of standard dose Lu-177 DOTATATE (200mCi per treatment) is roughly 6-8 cycles. Retreatment is typically reserved for patients who initially experience at least 12 months of disease response or stability following completion of initial treatment. In one cohort of 168 patients retreated with 2 additional cycles of Lu-177 DOTATATE, the median PFS was 14.6 months (115). Similarly, 131I-MIBG is typically given as a set of 2 cycles, 3 months apart, but retreatment with additional cycles, usually at 6-month intervals after treatment with the initial regimen has been reported; however, the optimal dosimetry is not yet established (116). Reports have described cumulative doses of 557 – 2322 mCi with individual doses between 100 – 200 mCi.
Intra-arterial and liver directed therapy
For patients with liver dominant disease, intra-hepatic administration of PRRT provides a unique opportunity to target progressing disease with higher concentrations of the therapy and reduce systemic toxicity. This approach has been evaluated in patients with GEP-NETs retrospectively, showing that patients with liver-limited disease had longer median PFS and OS than those across the entire cohort of patients (33.4 and 75.8 months vs. 29 and 70 months, respectively) (37, 117).
Combination therapy
Several studies have explored Lu-177 DOTATATE and Y-90 DOTATOC combined with different cytotoxic agents: primarily capecitabine and temozolomide (49, 50, 118, 119). Patients were either treated concurrently, sequentially, or in one instance, in a “sandwich” fashion, where patients received 2 cycles of PRRT followed by cycles of chemotherapy before they received the remaining PRRT doses. Response rates tended to be higher in these patient populations, with ORR as high as 57%, however, long-term follow-up of these cohorts revealed higher rates of treatment-related MDS or acute leukemia and no significant improvement in PFS or OS compared to PRRT alone. A recent study of 49 patients who received PRRT and chemotherapy with capecitabine/temozolomide reported a 10% risk of developing t-AML/AL, a significantly higher risk than PRRT alone (120).
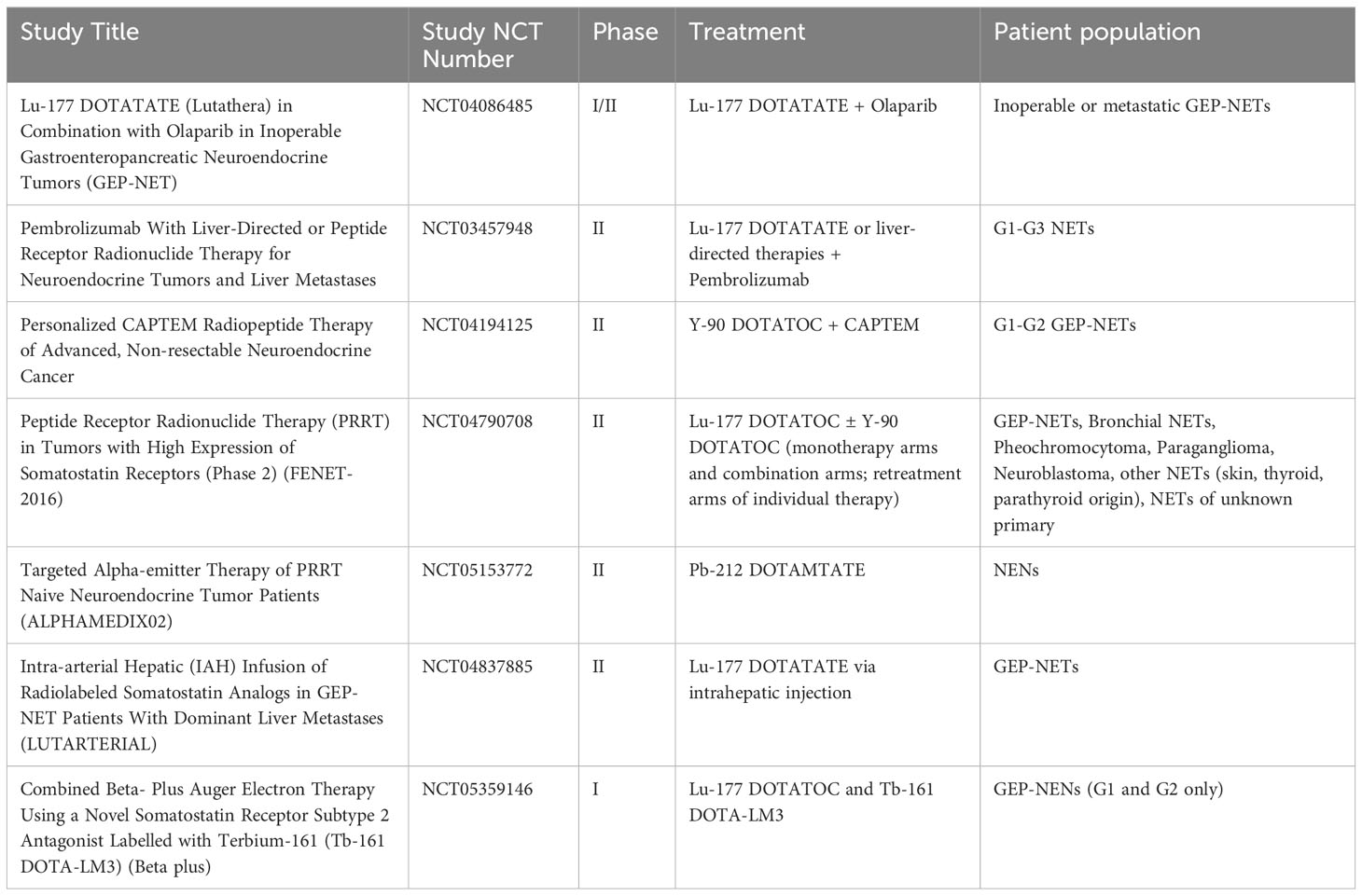
Other avenues being explored in primarily the GEP-NET population are combinations with mTOR inhibitors, radiation sensitizers (such as cell signaling inhibitors, DNA damage repair inhibitors, and DNA damage inducers), and tandem PRRT with multiple radioisotopes (121–127). The majority of research thus far has been preclinical in mouse models and xenografts, however a few clinical trials have recently begun enrollment and are exploring the combinations of radiosensitizers and DNA-damage-repair inhibitors in combination with PRRT. Table 1 summarizes ongoing and currently recruiting clinical trials.
Alpha-emitting isotopes and somatostatin-receptor antagonists
Alpha-emitters allow for more precise targeted therapy due to their shorter penetration range and higher linear energy (102, 128). A phase I study of Pb-212 DOTAMTATE reported a response rate of 80% among PRRT naïve patients on the highest dose of the drug (129). In addition, a cohort study with Ac-225 DOTATATE reported a >44% ORR in GEP-NET patients who had previously received Lu-177 DOTATATE (130). These data are promising, and further development is ongoing, with several clinical trials currently recruiting patients.
SSTR antagonists can occupy more binding sites and have lower dissociation rates than somatostatin analogs, which can lead to higher tumor uptake and a lower risk of radiation to healthy surrounding tissue (131). Several studies are ongoing in this arena in patients with NETs; however, no final data has been published. Lu-177 OPS201 (also known as Lu-177 DOTA-JR11 or Satoreotide tetraxetan) is one of the antagonists that is now being tested in clinical trials NCT02592707, NCT03773133 (132).
Conclusions
Radionuclide therapies represent a significant advancement in the therapeutic landscape for patients with endocrine-related cancers with limited availability of FDA-approved systemic therapies for advanced, progressive disease. The field has seen significantly more advances in patients with GEP-NETs and pheochromocytoma/paraganglioma, with the approval of I-131 MIBG and Lu-177 DOTATATE. Despite these advances, there remains a great deal of research to be done on other cancer types and indications. With increased knowledge of potential long-term toxicities associated with these therapies, we must develop strategies to increase the potency and efficacy while also individualizing the selection of patients who will most likely respond to treatment by employing biomarkers and imaging studies. Novel agents with α-emitters and SSTR antagonists, combination treatments (whether with other radionuclides or systemic targeted/chemotherapies), radiosensitizers, or unique modalities of treatment administration such as intra-arterial therapies are all promising advances in the field, however, most of the effort is currently focused on GEP-NETs. Clinical trials focusing on refining this therapy in other endocrine cancers will be important to expand impact of radiopharmaceuticals.
Author contributions
TA-T conducted the literature search and wrote the first draft of the manuscript. TA-T, GE-H contributed to conception and design of the manuscript. JS, J-JH, and GE-H provided expert feedback and edits to content. All authors contributed to the article and approved the submitted version.
Conflict of interest
GE-H: Novartis, Bayer, Boston Scientific, Terumo Consult; JH-J: HRA Pharma Consult.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of (177)Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med (2017) 376:125–35. doi: 10.1056/NEJMoa1607427
2. Shah MH, Goldner WS, Benson AB, Bergsland E, Blaszkowsky LS, Brock P, et al. Neuroendocrine and adrenal tumors, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2021) 19:839–68. doi: 10.6004/jnccn.2021.0032
3. Van Der Harst E, De Herder WW, Bruining HA, Bonjer HJ, De Krijger RR, Lamberts SW, et al. [(123)I]metaiodobenzylguanidine and [(111)In]octreotide uptake in begnign and Malignant pheochromocytomas. J Clin Endocrinol Metab (2001) 86:685–93. doi: 10.1210/jcem.86.2.7238
4. Hennrich U, Kopka K. Lutathera((R)): the first FDA- and EMA-approved radiopharmaceutical for peptide receptor radionuclide therapy. Pharm (Basel) (2019) 12. doi: 10.3390/ph12030114
5. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology (2020) 76:182–8. doi: 10.1111/his.13975
6. Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol (2017) 3:1335–42. doi: 10.1001/jamaoncol.2017.0589
7. Kulke MH, Lenz HJ, Meropol NJ, Posey J, Ryan DP, Picus J, et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol (2008) 26:3403–10. doi: 10.1200/JCO.2007.15.9020
8. Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet (2016) 387:968–77. doi: 10.1016/S0140-6736(15)00817-X
9. Kunz PL, Graham NT, Catalano PJ, Nimeiri HS, Fisher GA, Longacre TA, et al. A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors (ECOG-ACRIN E2211). J Clin Oncol (2023) 41(7):1359–69. doi: 10.1200/JCO.22.01013
10. Phan AT, Halperin DM, Chan JA, Fogelman DR, Hess KR, Malinowski P, et al. Pazopanib and depot octreotide in advanced, well-differentiated neuroendocrine tumours: a multicentre, single-group, phase 2 study. Lancet Oncol (2015) 16:695–703. doi: 10.1016/S1470-2045(15)70136-1
11. Chan JA, Faris JE, Murphy JE, Blaszkowsky LS, Kwak EL, Mccleary NJ, et al. Phase II trial of cabozantinib in patients with carcinoid and pancreatic neuroendocrine tumors (pNET). J Clin Oncol (2017) 35:228–8. doi: 10.1200/JCO.2017.35.4_suppl.228
12. Bergsland EK, Mahoney MR, Asmis TR, Hall N, Kumthekar P, Maitland ML, et al. Prospective randomized phase II trial of pazopanib versus placebo in patients with progressive carcinoid tumors (CARC) (Alliance A021202). J Clin Oncol (2019) 37:4005–5. doi: 10.1200/JCO.2019.37.15_suppl.4005
13. Strosberg J, Mizuno N, Doi T, Grande E, Delord JP, Shapira-Frommer R, et al. Efficacy and safety of pembrolizumab in previously treated advanced neuroendocrine tumors: results from the phase II KEYNOTE-158 study. Clin Cancer Res (2020) 26:2124–30. doi: 10.1158/1078-0432.CCR-19-3014
14. Vijayvergia N, Dasari A, Deng M, Litwin S, Al-Toubah T, Alpaugh RK, et al. Pembrolizumab monotherapy in patients with previously treated metastatic high-grade neuroendocrine neoplasms: joint analysis of two prospective, non-randomised trials. Br J Cancer (2020) 122:1309–14. doi: 10.1038/s41416-020-0775-0
15. Xu J, Shen L, Bai C, Wang W, Li J, Yu X, et al. Surufatinib in advanced pancreatic neuroendocrine tumours (SANET-p): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol (2020) 21:1489–99. doi: 10.1016/S1470-2045(20)30493-9
16. Xu J, Shen L, Zhou Z, Li J, Bai C, Chi Y, et al. Surufatinib in advanced extrapancreatic neuroendocrine tumours (SANET-ep): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol (2020) 21:1500–12. doi: 10.1016/S1470-2045(20)30496-4
17. Capdevila J, Fazio N, Lopez C, Teule A, Valle JW, Tafuto S, et al. Lenvatinib in patients with advanced grade 1/2 pancreatic and gastrointestinal neuroendocrine tumors: results of the phase II TALENT trial (GETNE1509). J Clin Oncol (2021) 39:2304–12. doi: 10.1200/JCO.20.03368
18. Patel SP, Mayerson E, Chae YK, Strosberg J, Wang J, Konda B, et al. A phase II basket trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART) SWOG S1609: High-grade neuroendocrine neoplasm cohort. Cancer (2021) 127:3194–201. doi: 10.1002/cncr.33591
19. Xu J. Current treatments and future potential of surufatinib in neuroendocrine tumors (NETs). Ther Adv Med Oncol (2021) 13:17588359211042689. doi: 10.1177/17588359211042689
20. Xu J, Bai Y, Sun H, Bai C, Jia R, Li Y, et al. A single-arm, multicenter, open-label phase 2 trial of surufatinib in patients with unresectable or metastatic biliary tract cancer. Cancer (2021) 127:3975–84. doi: 10.1002/cncr.33803
21. Yao JC, Strosberg J, Fazio N, Pavel ME, Bergsland E, Ruszniewski P, et al. Spartalizumab in metastatic, well/poorly-differentiated neuroendocrine neoplasms. Endocr Relat Cancer. (2021). doi: 10.1530/ERC-20-0382
22. Al-Toubah T, Pelle E, Strosberg J. Risk of myelodysplastic syndrome/acute leukemia with sequential capecitabine/temozolomide and 177Lu-dotatate. (2022). doi: 1530/endoabs.89.C7
23. Das S, Al-Toubah T, Strosberg J. Chemotherapy in neuroendocrine tumors. Cancers (Basel) (2021) 13. doi: 10.3390/cancers13194872
24. Dasari A, Shen C, Devabhaktuni A, Nighot R, Sorbye H. Survival according to primary tumor location, stage, and treatment patterns in locoregional gastroenteropancreatic high-grade neuroendocrine carcinomas. Oncologist (2022) 27:299–306. doi: 10.1093/oncolo/oyab039
25. Erstad DJ, Dasari A, Taggart MW, Kaur H, Konishi T, Bednarski BK, et al. Prognosis for poorly differentiated, high-grade rectal neuroendocrine carcinomas. Ann Surg Oncol (2022) 29:2539–48. doi: 10.1245/s10434-021-11016-8
26. Liang Y, Yu XJ, Chen J. [The diagnosis and treatment of G3 neuroendocrine tumors according to the new NCCN guideline]. Zhonghua Yi Xue Za Zhi (2022) 102:982–7. doi: 10.3760/cma.j.cn112137-20210826-01943
27. Anthony LB, Woltering EA, Espenan GD, Cronin MD, Maloney TJ, Mccarthy KE. Indium-111-pentetreotide prolongs survival in gastroenteropancreatic Malignancies. Semin Nucl Med (2002) 32:123–32. doi: 10.1053/snuc.2002.31769
28. Buscombe JR, Caplin ME, Hilson AJ. Long-term efficacy of high-activity 111in-pentetreotide therapy in patients with disseminated neuroendocrine tumors. J Nucl Med (2003) 44:1–6.
29. Castaldi P, Rufini V, Treglia G, Bruno I, Perotti G, Stifano G, et al. Impact of 111In-DTPA-octreotide SPECT/CT fusion images in the management of neuroendocrine tumours. Radiol Med (2008) 113:1056–67. doi: 10.1007/s11547-008-0319-9
30. Waldherr C, Haldemann A, Maecke HR, Crazzolara A, Mueller-Brand J. Exceptional results in neuroendocrine-metastases-caused paraplegia treated with [90Y-DOTA]-D-Phe1-Tyr3-octreotide (90Y-DOTATOC), a radiolabelled somatostatin analogue. Clin Oncol (R Coll Radiol) (2000) 12:121–3.
31. Waldherr C, Pless M, Maecke HR, Haldemann A, Mueller-Brand J. The clinical value of [90Y-DOTA]-D-Phe1-Tyr3-octreotide (90Y-DOTATOC) in the treatment of neuroendocrine tumours: a clinical phase II study. Ann Oncol (2001) 12:941–5. doi: 10.1023/A:1011160913619
32. Forrer F, Waldherr C, Maecke HR, Mueller-Brand J. Targeted radionuclide therapy with 90Y-DOTATOC in patients with neuroendocrine tumors. Anticancer Res (2006) 26:703–7.
33. Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, Feelders RA, De Herder WW, et al. Long-term efficacy, survival, and safety of [(177)Lu-DOTA(0),Tyr(3)]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res (2017) 23:4617–24. doi: 10.1158/1078-0432.CCR-16-2743
34. Strosberg J, Krenning E. 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med (2017) 376:1391–2. doi: 10.1056/NEJMoa1607427
35. Thang SP, Lung MS, Kong G, Hofman MS, Callahan J, Michael M, et al. Peptide receptor radionuclide therapy (PRRT) in European Neuroendocrine Tumour Society (ENETS) grade 3 (G3) neuroendocrine neoplasia (NEN) - a single-institution retrospective analysis. Eur J Nucl Med Mol Imaging (2018) 45:262–77. doi: 10.1007/s00259-017-3821-2
36. Sorbye H, Kong G, Grozinsky-Glasberg S. PRRT in high-grade gastroenteropancreatic neuroendocrine neoplasms (WHO G3). Endocr Relat Cancer (2020) 27:R67–77. doi: 10.1530/ERC-19-0400
37. Haider M, Das S, Al-Toubah T, Pelle E, El-Haddad G, Strosberg J. Somatostatin receptor radionuclide therapy in neuroendocrine tumors. Endocr Relat Cancer (2021) 28:R81–93. doi: 10.1530/ERC-20-0360
38. Sitani K, Parghane RV, Talole S, Basu S. Long-term outcome of indigenous (177)Lu-DOTATATE PRRT in patients with Metastatic Advanced Neuroendocrine Tumours: a single institutional observation in a large tertiary care setting. Br J Radiol (2021) 94:20201041. doi: 10.1259/bjr.20201041
39. Strosberg JR, Caplin ME, Kunz PL, Ruszniewski PB, Bodei L, Hendifar A, et al. (177)Lu-Dotatate plus long-acting octreotide versus high−dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol (2021) 22:1752–63. doi: 10.1016/S1470-2045(21)00572-6
40. Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging (2015) 42:5–19. doi: 10.1007/s00259-014-2893-5
41. Bergsma H, Konijnenberg MW, Kam BL, Teunissen JJ, Kooij PP, De Herder WW, et al. Subacute haematotoxicity after PRRT with (177)Lu-DOTA-octreotate: prognostic factors, incidence and course. Eur J Nucl Med Mol Imaging (2016) 43:453–63. doi: 10.1007/s00259-015-3193-4
42. Bergsma H, Konijnenberg MW, van der Zwan WA, Kam BL, Teunissen JJ, Kooij PP, et al. Nephrotoxicity after PRRT with (177)Lu-DOTA-octreotate. Eur J Nucl Med Mol Imaging (2016) 43:1802–11. doi: 10.1007/s00259-016-3382-9
43. Bergsma H, Van Lom K, Raaijmakers M, Konijnenberg M, Kam B, Teunissen JJM, et al. Persistent hematologic dysfunction after peptide receptor radionuclide therapy with (177)Lu-DOTATATE: incidence, course, and predicting factors in patients with gastroenteropancreatic neuroendocrine tumors. J Nucl Med (2018) 59:452–8. doi: 10.2967/jnumed.117.189712
44. Alkassis S, Ali M, Awadelkarim AM, Saad E, Halboni A, Alhusain R, et al. Lutetium-177 dotatate-induced hemolytic anemia and myelodysplastic syndrome. Cureus (2022) 14:e22392. doi: 10.7759/cureus.22392
45. Bhatia S. Therapy-related myelodysplasia and acute myeloid leukemia. Semin Oncol (2013) 40:666–75. doi: 10.1053/j.seminoncol.2013.09.013
46. Brieau B, Hentic O, Lebtahi R, Palazzo M, Ben Reguiga M, Rebours V, et al. High risk of myelodysplastic syndrome and acute myeloid leukemia after 177Lu-octreotate PRRT in NET patients heavily pretreated with alkylating chemotherapy. Endocr Relat Cancer (2016) 23:L17–23. doi: 10.1530/ERC-15-0543
47. Shaheen S, Moradi F, Gamino G, Kunz PL. Patient selection and toxicities of PRRT for metastatic neuroendocrine tumors and research opportunities. Curr Treat Options Oncol (2020) 21:25. doi: 10.1007/s11864-020-0711-9
48. Fröss-Baron K, Garske-Roman U, Welin S, Granberg D, Eriksson B, Khan T, et al. 177Lu-DOTATATE therapy of advanced pancreatic neuroendocrine tumors heavily pretreated with chemotherapy: analysis of outcome, safety, and their determinants. Neuroendocrinology (2021) 111:330–43. doi: 10.1159/000506746
49. Kesavan M, Grover P, Lam WS, Claringbold PG, Turner JH. Long-term hematologic toxicity of 177Lu-octreotate-capecitabine-temozolomide therapy of GEPNET. Endocr Relat Cancer (2021) 28:521–7. doi: 10.1530/ERC-21-0082
50. Parghane RV, Ostwal V, Ramaswamy A, Bhandare M, Chaudhari V, Talole S, et al. Long-term outcome of "Sandwich" chemo-PRRT: a novel treatment strategy for metastatic neuroendocrine tumors with both FDG- and SSTR-avid aggressive disease. Eur J Nucl Med Mol Imaging (2021) 48:913–23. doi: 10.1007/s00259-020-05004-5
51. Matthews LF, Jones IW. The effect of kidney volume estimation on dosimetry in lutetium-177 DOTATATE therapy. Nucl Med Commun (2018) 39:527–32. doi: 10.1097/MNM.0000000000000821
52. Rudisile S, Gosewisch A, Wenter V, Unterrainer M, Boning G, Gildehaus FJ, et al. Salvage PRRT with (177)Lu-DOTA-octreotate in extensively pretreated patients with metastatic neuroendocrine tumor (NET): dosimetry, toxicity, efficacy, and survival. BMC Cancer (2019) 19:788. doi: 10.1186/s12885-019-6000-y
53. Kovan B, Ozkan ZG, Demir B, Tuncman D, Isik EG, Simsek DH, et al. An analysis for therapeutic doses of patients with neuroendocrine tumor treated with lutetium 177 ((177)Lu)-DOTATATE. Cancer Biother Radiopharm (2022) 37:17–22. doi: 10.1089/cbr.2021.0071
54. Strosberg JR, Al-Toubah T, El-Haddad G. Reply: bowel obstruction as a complication of peptide receptor radionuclide therapy. J Nucl Med (2021) 62 1321. doi: 10.2967/jnumed.121.262340
55. Strosberg JR, Al-Toubah T, Pelle E, Smith J, Haider M, Hutchinson T, et al. Risk of bowel obstruction in patients with mesenteric or peritoneal disease receiving peptide receptor radionuclide therapy. J Nucl Med (2021) 62:69–72. doi: 10.2967/jnumed.120.242875
56. Imhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol (2011) 29:2416–23. doi: 10.1200/JCO.2010.33.7873
57. Filice A, Fraternali A, Frasoldati A, Asti M, Grassi E, Massi L, et al. Radiolabeled somatostatin analogues therapy in advanced neuroendocrine tumors: a single centre experience. J Oncol (2012) 2012:320198. doi: 10.1155/2012/320198
58. Parghane RV, Talole S, Prabhash K, Basu S. Clinical response profile of metastatic/advanced pulmonary neuroendocrine tumors to peptide receptor radionuclide therapy with 177Lu-DOTATATE. Clin Nucl Med (2017) 42:428–35. doi: 10.1097/RLU.0000000000001639
59. Sabet A, Haug AR, Eiden C, Auernhammer CJ, Simon B, Bartenstein P, et al. Efficacy of peptide receptor radionuclide therapy with (177)Lu-octreotate in metastatic pulmonary neuroendocrine tumors: a dual-centre analysis. Am J Nucl Med Mol Imaging (2017) 7:74–83.
60. Mirvis E, Toumpanakis C, Mandair D, Gnanasegaran G, Caplin M, Navalkissoor S. Efficacy and tolerability of peptide receptor radionuclide therapy (PRRT) in advanced metastatic bronchial neuroendocrine tumours (NETs). Lung Cancer (2020) 150:70–5. doi: 10.1016/j.lungcan.2020.10.005
61. Wells SA Jr., Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid (2015) 25:567–610. doi: 10.1089/thy.2014.0335
62. Wells SA Jr., Gosnell JE, Gagel RF, Moley J, Pfister D, Sosa JA, et al. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol (2010) 28:767–72. doi: 10.1200/JCO.2009.23.6604
63. Kurzrock R, Sherman SI, Ball DW, Forastiere AA, Cohen RB, Mehra R, et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol (2011) 29:2660–6. doi: 10.1200/JCO.2010.32.4145
64. Nagilla M, Brown RL, Cohen EE. Cabozantinib for the treatment of advanced medullary thyroid cancer. Adv Ther (2012) 29:925–34. doi: 10.1007/s12325-012-0060-6
65. Elisei R, Schlumberger MJ, Muller SP, Schoffski P, Brose MS, Shah MH, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol (2013) 31:3639–46. doi: 10.1200/JCO.2012.48.4659
66. Viola D, Cappagli V, Elisei R. Cabozantinib (XL184) for the treatment of locally advanced or metastatic progressive medullary thyroid cancer. Future Oncol (2013) 9:1083–92. doi: 10.2217/fon.13.128
67. Cabanillas ME, Brose MS, Holland J, Ferguson KC, Sherman SI. A phase I study of cabozantinib (XL184) in patients with differentiated thyroid cancer. Thyroid (2014) 24:1508–14. doi: 10.1089/thy.2014.0125
68. Fallahi P, Ferrari SM, Di Bari F, Materazzi G, Benvenga S, Miccoli P, et al. Cabozantinib in thyroid cancer. Recent Pat Anticancer Drug Discovery (2015) 10:259–69. doi: 10.2174/1574892810666150708110816
69. Krajewska J, Olczyk T, Jarzab B. Cabozantinib for the treatment of progressive metastatic medullary thyroid cancer. Expert Rev Clin Pharmacol (2016) 9:69–79. doi: 10.1586/17512433.2016.1102052
70. Starenki D, Hong SK, Wu PK, Park JI. Vandetanib and cabozantinib potentiate mitochondria-targeted agents to suppress medullary thyroid carcinoma cells. Cancer Biol Ther (2017) 18:473–83. doi: 10.1080/15384047.2017.1323594
71. Subbiah V, Velcheti V, Tuch BB, Ebata K, Busaidy NL, Cabanillas ME, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol (2018) 29:1869–76. doi: 10.1093/annonc/mdy137
72. Bim LV, Navarro FCP, Valente FOF, Lima-Junior JV, Delcelo R, Dias-Da-Silva MR, et al. Retroposed copies of RET gene: a somatically acquired event in medullary thyroid carcinoma. BMC Med Genomics (2019) 12:104. doi: 10.1186/s12920-019-0552-1
73. Ceolin L, Duval M, Benini AF, Ferreira CV, Maia AL. Medullary thyroid carcinoma beyond surgery: advances, challenges, and perspectives. Endocr Relat Cancer (2019) 26:R499–518. doi: 10.1530/ERC-18-0574
74. Joo LJS, Weiss J, Gill AJ, Clifton-Bligh R, Brahmbhatt H, Macdiarmid JA, et al. RET kinase-regulated microRNA-153-3p improves therapeutic efficacy in medullary thyroid carcinoma. Thyroid (2019) 29:830–44. doi: 10.1089/thy.2018.0525
75. FDA. FDA approves Selpercatinib; Pralsetinib may soon follow. Cancer Discovery (2020) 10:OF1. doi: 10.1158/2159-8290.CD-NB2020-052
76. Alqahtani T, Kumarasamy VM, Huczynski A, Sun D. Salinomycin and its derivatives as potent RET transcriptional inhibitors for the treatment of medullary thyroid carcinoma. Int J Oncol (2020) 56:348–58. doi: 10.1096/fasebj.2020.34.s1.05420
77. Markham A. Pralsetinib: first approval. Drugs (2020) 80:1865–70. doi: 10.1007/s40265-020-01427-4
78. Carra S, Gaudenzi G, Dicitore A, Saronni D, Cantone MC, Plebani A, et al. Vandetanib versus cabozantinib in medullary thyroid carcinoma: A focus on anti-angiogenic effects in Zebrafish model. Int J Mol Sci (2021) 22. doi: 10.3390/ijms22063031
79. Kim DW, Jo YS, Jung HS, Chung HK, Song JH, Park KC, et al. An orally administered multitarget tyrosine kinase inhibitor, SU11248, is a novel potent inhibitor of thyroid oncogenic RET/papillary thyroid cancer kinases. J Clin Endocrinol Metab (2006) 91:4070–6. doi: 10.1210/jc.2005-2845
80. Hong DS, Sebti SM, Newman RA, Blaskovich MA, Ye L, Gagel RF, et al. Phase I trial of a combination of the multikinase inhibitor sorafenib and the farnesyltransferase inhibitor tipifarnib in advanced Malignancies. Clin Cancer Res (2009) 15:7061–8. doi: 10.1158/1078-0432.CCR-09-1241
81. Schlumberger M, Jarzab B, Cabanillas ME, Robinson B, Pacini F, Ball DW, et al. A phase II trial of the multitargeted tyrosine kinase inhibitor lenvatinib (E7080) in advanced medullary thyroid cancer. Clin Cancer Res (2016) 22:44–53. doi: 10.1158/1078-0432.CCR-15-1127
82. Kraeber-Bodere F, Rousseau C, Bodet-Milin C, Ferrer L, Faivre-Chauvet A, Campion L, et al. Targeting, toxicity, and efficacy of 2-step, pretargeted radioimmunotherapy using a chimeric bispecific antibody and 131I-labeled bivalent hapten in a phase I optimization clinical trial. J Nucl Med (2006) 47:247–55.
83. Iten F, Muller B, Schindler C, Rochlitz C, Oertli D, Macke HR, et al. Response to [90Yttrium-DOTA]-TOC treatment is associated with long-term survival benefit in metastasized medullary thyroid cancer: a phase II clinical trial. Clin Cancer Res (2007) 13:6696–702. doi: 10.1158/1078-0432.CCR-07-0935
84. Beukhof CM, Brabander T, Van Nederveen FH, Van Velthuysen MF, De Rijke YB, Hofland LJ, et al. Peptide receptor radionuclide therapy in patients with medullary thyroid carcinoma: predictors and pitfalls. BMC Cancer (2019) 19:325. doi: 10.1186/s12885-019-5540-5
85. Parghane RV, Naik C, Talole S, Desmukh A, Chaukar D, Banerjee S, et al. Clinical utility of (177) Lu-DOTATATE PRRT in somatostatin receptor-positive metastatic medullary carcinoma of thyroid patients with assessment of efficacy, survival analysis, prognostic variables, and toxicity. Head Neck (2020) 42:401–16. doi: 10.1002/hed.26024
86. Mete O, Asa SL, Gill AJ, Kimura N, De Krijger RR, Tischler A. Overview of the 2022 WHO classification of paragangliomas and pheochromocytomas. Endocr Pathol (2022) 33:90–114. doi: 10.1007/s12022-022-09704-6
87. Baudin E, Goichot B, Berruti A, Hadoux J, Moalla S, Laboureau S, et al. First International Randomized Study in Malignant Progressive Pheochromocytoma and Paragangliomas (FIRSTMAPPP): An academic double-blind trial investigating sunitinib. Ann Oncol.
88. Forrer F, Riedweg I, Maecke HR, Mueller-Brand J. Radiolabeled DOTATOC in patients with advanced paraganglioma and pheochromocytoma. Q J Nucl Med Mol Imaging (2008) 52:334–40.
89. Huang H, Abraham J, Hung E, Averbuch S, Merino M, Steinberg SM, et al. Treatment of Malignant pheochromocytoma/paraganglioma with cyclophosphamide, vincristine, and dacarbazine: recommendation from a 22-year follow-up of 18 patients. Cancer (2008) 113:2020–8. doi: 10.1002/cncr.23812
90. Bravo EL, Kalmadi SR, Gill I. Clinical utility of temozolomide in the treatment of Malignant paraganglioma: a preliminary report. Horm Metab Res (2009) 41:703–6. doi: 10.1055/s-0029-1224135
91. Inaki A, Yoshimura K, Murayama T, Imai Y, Kuribayashi Y, Higuchi T, et al. A phase I clinical trial for [(131)I]meta-iodobenzylguanidine therapy in patients with refractory pheochromocytoma and paraganglioma: a study protocol. J Med Invest (2017) 64:205–9. doi: 10.2152/jmi.64.205
92. Lenders JWM, Eisenhofer G. Update on modern management of pheochromocytoma and paraganglioma. Endocrinol Metab (Seoul) (2017) 32:152–61. doi: 10.3803/EnM.2017.32.2.152
93. Prades CA, Atassi B, Nazeer H. Metastatic Malignant paraganglioma: A case report and review of literature. World J Oncol (2017) 8:92–5. doi: 10.14740/wjon1033w
94. Fishbein L, Del Rivero J, Else T, Howe JR, Asa SL, Cohen DL, et al. The North American neuroendocrine tumor society consensus guidelines for surveillance and management of metastatic and/or unresectable pheochromocytoma and paraganglioma. Pancreas (2021) 50:469–93. doi: 10.1097/MPA.0000000000001792
95. Pryma DA, Chin BB, Noto RB, Dillon JS, Perkins S, Solnes L, et al. Efficacy and safety of high-specific-activity 131I-MIBG therapy in patients with advanced pheochromocytoma or paraganglioma. J Nucl Med (2019) 60:623–30. doi: 10.2967/jnumed.118.217463
96. Thorpe MP, Kane A, Zhu J, Morse MA, Wong T, Borges-Neto S. Long-term outcomes of 125 patients with metastatic pheochromocytoma or paraganglioma treated with 131-I MIBG. J Clin Endocrinol Metab (2020) 105. doi: 10.1210/clinem/dgz074
97. Jha A, Taieb D, Carrasquillo JA, Pryma DA, Patel M, Millo C, et al. High-specific-activity-(131)I-MIBG versus (177)Lu-DOTATATE targeted radionuclide therapy for metastatic pheochromocytoma and paraganglioma. Clin Cancer Res (2021) 27:2989–95. doi: 10.1158/1078-0432.CCR-20-3703
98. Garg MK, Kharb S, Brar KS, Gundgurthi A, Mittal R. Medical management of pheochromocytoma: Role of the endocrinologist. Indian J Endocrinol Metab (2011) 15 Suppl 4:S329–336. doi: 10.4103/2230-8210.86976
99. Zhang J, Wang H, Jacobson O, Cheng Y, Niu G, Li F, et al. Safety, pharmacokinetics, and dosimetry of a long-acting radiolabeled somatostatin analog (177)Lu-DOTA-EB-TATE in patients with advanced metastatic neuroendocrine tumors. J Nucl Med (2018) 59:1699–705. doi: 10.2967/jnumed.118.209841
100. Del Prete M, Buteau FA, Arsenault F, Saighi N, Bouchard LO, Beaulieu A, et al. Personalized (177)Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumours: initial results from the P-PRRT trial. Eur J Nucl Med Mol Imaging (2019) 46:728–42. doi: 10.1007/s00259-018-4209-7
101. Jahn U, Ilan E, Sandstrom M, Lubberink M, Garske-Roman U, Sundin A. Peptide receptor radionuclide therapy (PRRT) with (177)Lu-DOTATATE; differences in tumor dosimetry, vascularity and lesion metrics in pancreatic and small intestinal neuroendocrine neoplasms. Cancers (Basel) (2021) 13. doi: 10.3390/cancers13050962
102. Harris PE, Zhernosekov K. The evolution of PRRT for the treatment of neuroendocrine tumors; What comes next? Front Endocrinol (Lausanne) (2022) 13:941832. doi: 10.3389/fendo.2022.941832
103. Kamaldeep, Loharkar S, Das T, Basu S, Banerjee S. Estimation of absorbed doses of indigenously produced "Direct-route" Lutetium-177-[(177)Lu]Lu-DOTA-TATE PRRT in normal organs and tumor lesions in patients of metastatic neuroendocrine tumors: comparison with no-carrier-added [(177)Lu]Lu-DOTA-TATE and the trend with multiple cycles. Cancer Biother Radiopharm (2022) 37:214–25. doi: 10.1089/cbr.2021.0340
104. Ambrosini V, Kunikowska J, Baudin E, Bodei L, Bouvier C, Capdevila J, et al. Consensus on molecular imaging and theranostics in neuroendocrine neoplasms. Eur J Cancer (2021) 146:56–73. doi: 10.1016/j.ejca.2021.01.008
105. Ezziddin S, Attassi M, Yong-Hing CJ, Ahmadzadehfar H, Willinek W, Grünwald F, et al. Predictors of long-term outcome in patients with well-differentiated gastroenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med (2014) 55:183–90. doi: 10.2967/jnumed.113.125336
106. Durmo R, Filice A, Fioroni F, Cervati V, Finocchiaro D, Coruzzi C, et al. Predictive and prognostic role of pre-therapy and interim 68Ga-DOTATOC PET/CT parameters in metastatic advanced neuroendocrine tumor patients treated with PRRT. Cancers (Basel) (2022) 14. doi: 10.3390/cancers14030592
107. Swiha MM, Sutherland DEK, Sistani G, Khatami A, Abazid RM, Mujoomdar A, et al. Survival predictors of (177)Lu-Dotatate peptide receptor radionuclide therapy (PRRT) in patients with progressive well-differentiated neuroendocrine tumors (NETS). J Cancer Res Clin Oncol (2022) 148:225–36. doi: 10.1007/s00432-021-03672-w
108. Zhang J, Kulkarni HR, Singh A, Niepsch K, Müller D, Baum RP. Peptide receptor radionuclide therapy in grade 3 neuroendocrine neoplasms: safety and survival analysis in 69 patients. J Nucl Med (2019) 60:377–85. doi: 10.2967/jnumed.118.215848
109. Bodei L, Kidd MS, Singh A, van der Zwan WA, Severi S, Drozdov IA, et al. PRRT genomic signature in blood for prediction of (177)Lu-octreotate efficacy. Eur J Nucl Med Mol Imaging (2018) 45:1155–69. doi: 10.1007/s00259-018-3967-6
110. Bodei L, Kidd MS, Singh A, van der Zwan WA, Severi S, Drozdov IA, et al. PRRT neuroendocrine tumor response monitored using circulating transcript analysis: the NETest. Eur J Nucl Med Mol Imaging (2020) 47:895–906. doi: 10.1007/s00259-019-04601-3
111. Bodei L, Schoder H, Baum RP, Herrmann K, Strosberg J, Caplin M, et al. Molecular profiling of neuroendocrine tumours to predict response and toxicity to peptide receptor radionuclide therapy. Lancet Oncol (2020) 21:e431–43. doi: 10.1016/S1470-2045(20)30323-5
112. Evans AC, Setzkorn T, Edmondson DA, Segelke H, Wilson PF, Matthay KK, et al. Peripheral blood transcript signatures after internal 131I-mIBG therapy in relapsed and refractory neuroblastoma patients identifies early and late biomarkers of internal 131I exposures. Radiat Res (2022) 197:101–12. doi: 10.1667/RADE-20-00173.1
113. Vaughan E, Machta J, Walker M, Toumpanakis C, Caplin M, Navalkissoor S. Retreatment with peptide receptor radionuclide therapy in patients with progressing neuroendocrine tumours: efficacy and prognostic factors for response. Br J Radiol (2018) 91:20180041. doi: 10.1259/bjr.20180041
114. Strosberg J, Leeuwenkamp O, Siddiqui MK. Peptide receptor radiotherapy re-treatment in patients with progressive neuroendocrine tumors: A systematic review and meta-analysis. Cancer Treat Rev (2021) 93:102141. doi: 10.1016/j.ctrv.2020.102141
115. Van Der Zwan WA, Brabander T, Kam BLR, Teunissen JJM, Feelders RA, Hofland J, et al. Salvage peptide receptor radionuclide therapy with [(177)Lu-DOTA,Tyr(3)]octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging (2019) 46:704–17. doi: 10.1007/s00259-018-4158-1
116. Kayano D, Kinuya S. Current consensus on I-131 MIBG therapy. Nucl Med Mol Imaging (2018) 52:254–65. doi: 10.1007/s13139-018-0523-z
117. Bajwa R, Madoff DC, Kishore SA. Embolotherapy for hepatic oncology: current perspectives and future directions. Dig Dis Interv (2020) 4:134–47. doi: 10.1055/s-0040-1712146
118. Kong G, Grozinsky-Glasberg S, Hofman MS, Akhurst T, Meirovitz A, Maimon O, et al. Highly favourable outcomes with peptide receptor radionuclide therapy (PRRT) for metastatic rectal neuroendocrine neoplasia (NEN). Eur J Nucl Med Mol Imaging (2019) 46:718–27. doi: 10.1007/s00259-018-4196-8
119. Al-Toubah T, Pelle E, Valone T, Haider M, Strosberg JR. Efficacy and toxicity analysis of capecitabine and temozolomide in neuroendocrine neoplasms. J Natl Compr Canc Netw (2021) 20:29–36. doi: 10.6004/jnccn.2021.7017
120. Al-Toubah T, Halfdanarson T, Gile J, Morse B, Sommerer K, Strosberg J. Efficacy of ipilimumab and nivolumab in patients with high-grade neuroendocrine neoplasms. ESMO Open (2022) 7:100364. doi: 10.1016/j.esmoop.2021.100364
121. Pach D, Sowa-Staszczak A, Kunikowska J, Królicki L, Trofimiuk M, Stefańska A, et al. Repeated cycles of peptide receptor radionuclide therapy (PRRT)–results and side-effects of the radioisotope 90Y-DOTA TATE, 177Lu-DOTA TATE or 90Y/177Lu-DOTA TATE therapy in patients with disseminated NET. Radiother Oncol (2012) 102:45–50. doi: 10.1016/j.radonc.2011.08.006
122. Seregni E, Maccauro M, Chiesa C, Mariani L, Pascali C, Mazzaferro V, et al. Treatment with tandem [90Y]DOTA-TATE and [177Lu]DOTA-TATE of neuroendocrine tumours refractory to conventional therapy. Eur J Nucl Med Mol Imaging (2014) 41:223–30. doi: 10.1007/s00259-013-2578-5
123. Kunikowska J, Pawlak D, Bąk MI, Kos-Kudła B, Mikołajczak R, Królicki L. Long-term results and tolerability of tandem peptide receptor radionuclide therapy with (90)Y/(177)Lu-DOTATATE in neuroendocrine tumors with respect to the primary location: a 10-year study. Ann Nucl Med (2017) 31:347–56. doi: 10.1007/s12149-017-1163-6
124. Spetz J, Langen B, Rudqvist N, Parris TZ, Helou K, Nilsson O, et al. Hedgehog inhibitor sonidegib potentiates (177)Lu-octreotate therapy of GOT1 human small intestine neuroendocrine tumors in nude mice. BMC Cancer (2017) 17:528. doi: 10.1186/s12885-017-3524-x
125. Hofving T, Arvidsson Y, Almobarak B, Inge L, Pfragner R, Persson M, et al. The neuroendocrine phenotype, genomic profile and therapeutic sensitivity of GEPNET cell lines. Endocr Relat Cancer (2018) 25:X1–x2. doi: 10.1530/ERC-17-0445e
126. Purohit NK, Shah RG, Adant S, Hoepfner M, Shah GM, Beauregard JM. Potentiation of (177)Lu-octreotate peptide receptor radionuclide therapy of human neuroendocrine tumor cells by PARP inhibitor. Oncotarget (2018) 9:24693–706. doi: 10.18632/oncotarget.25266
127. Aljubran AH, Alrowaili M, Raef H, Bazarbashi S, Alzahrani AM, Almuhaideb A, et al. Combination of everolimus and lu-177 PRRT in treatment of G1-2 neuroendocrine tumors (NET): Phase 1-2 study. J Clin Oncol (2019) 37:386–6. doi: 10.1200/JCO.2019.37.4_suppl.386
128. Cives M, Pelle E, Strosberg J. Emerging treatment options for gastroenteropancreatic neuroendocrine tumors. J Clin Med (2020) 9:2–4. doi: 10.3390/jcm9113655
129. Delpassand ES, Tworowska I, Esfandiari R, Torgue J, Hurt J, Shafie A, et al. Targeted α-emitter therapy with (212)Pb-DOTAMTATE for the treatment of metastatic SSTR-expressing neuroendocrine tumors: first-in-humans dose-escalation clinical trial. J Nucl Med (2022) 63:1326–33. doi: 10.2967/jnumed.121.263230
130. Ballal S, Yadav MP, Tripathi M, Sahoo RK, Bal C. Survival outcomes in metastatic gastroenteropancreatic neuroendocrine tumor patients receiving concomitant 225Ac-DOTATATE targeted alpha therapy and capecitabine: A real-world scenario management based long-term outcome study. J Nucl Med (2022). doi: 10.2967/jnumed.122.264043
131. Fani M, Nicolas GP, Wild D. Somatostatin receptor antagonists for imaging and therapy. J Nucl Med (2017) 58:61s–6s. doi: 10.2967/jnumed.116.186783
Keywords: neuroendocrine, endocrine, pheochromocytoma, paraganglioma, radiopharmaceutical
Citation: Al-Toubah T, Strosberg J, Hallanger-Johnson J and El-Haddad G (2023) Targeted radionuclide therapy in endocrine-related cancers: advances in the last decade. Front. Endocrinol. 14:1187870. doi: 10.3389/fendo.2023.1187870
Received: 16 March 2023; Accepted: 26 October 2023;
Published: 20 November 2023.
Edited by:
Elcin Zan, New York University, United StatesReviewed by:
Timon Vandamme, Antwerp University Hospital, BelgiumMurat Fani Bozkurt, Hacettepe University, Türkiye
Mark Kidd, Wren Laboratories, United States
Copyright © 2023 Al-Toubah, Strosberg, Hallanger-Johnson and El-Haddad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ghassan El-Haddad, ghassan.elhaddad@moffitt.org
 Taymeyah Al-Toubah
Taymeyah Al-Toubah Jonathan Strosberg
Jonathan Strosberg Julie Hallanger-Johnson2
Julie Hallanger-Johnson2 Ghassan El-Haddad
Ghassan El-Haddad