- Department of Crop and Soil Sciences, University of Georgia, Griffin, GA, United States
Enzyme-Catalyzed Oxidative Humification Reactions (ECOHRs) are primarily recognized for their involvement in the degradation of lignin. Lignolytic fungi produce extracellular enzymes under nutrient-deficient conditions, which can act directly or indirectly through small-molecule mediators to modify a range of compounds in the environment. The enzymes mediating ECOHRs mainly include laccases, lignin peroxidases (LiP), and manganese peroxidases (MnP), whose properties and catalysis mechanisms are summarized and compared in this review. As an example showcasing the possible environmental application of ECOHRs, the effects of ECOHRs in mediating the transformation of two key per- and polyfluoroalkyl substances (PFAS), perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS), were discussed. Reports have shown their significant concentration reduction (40%–59%) in systems with ECOHRs induced by amendments with laccases and mediators. Nontarget products identification using high-resolution mass spectrometry suggests that PFOA and PFOS degraded in ECOHR systems primarily through free-radical chain reactions. Reports on the use of laccase to reduce and manage the thatch layer on turf grass are also discussed in this review as another example of ECOHRs application. Laccase application at a rate of 2 U/cm2 once per month was found to be as effective as traditional thatch management methods, with the ECOHR effects leading to a reduction in the thatch thickness by 18%–22% in bermudagrass and 21%–30% in zoysiagrass. Overall, this review addresses the concept of ECOHRs, with the major enzymes and systems introduced, and highlights their possible environmental applications exemplified by PFAS remediation and thatch management.
1 Introduction
ECOHRs refer to a class of reactions critically involved in natural organic matter humification processes (Guggenberger, 2005). They are ubiquitous in soil systems and are usually catalyzed by extracellular enzymes produced by certain white/brown rot fungi. One natural function of these fungi is to degrade lignin, an integral part of the plant cell wall (Zavarzina et al., 2010). Lignin is the most persistent natural organic matter, comprising three-dimensional complex heteropolymer of aromatic alcohols. White/brown rot fungi are reported to be the most efficient lignin degraders in nature, with no other microorganisms being reported with a similar function, and thus play a unique role in plant organic matter turnover and carbon cycling (Atiwesh et al., 2022).
The special capability of white/brown rot fungi in degrading lignin is attributed to the extracellular enzymes that they produce, such as lignin peroxidases, manganese peroxidases, and laccases. These enzymes can effectively convert small-molecule chemicals containing phenolic or anilinic moieties into active intermediates, such as radicals or quinones. These intermediates are highly reactive, and undergo further reactions, including self-coupling and covalent bonding into natural organic matter. In the meantime, the active intermediates may also attack other inert chemicals, such as lignin, and thus cause their degradation and consequently be incorporated into the natural humification process (Abdel-Hamid et al., 2013). The small-molecule chemicals that serve as active intermediates during these enzymatic reactions are also referred to as mediators.
ECOHRs have been investigated for their potential applications, such as pulping, bleaching, dye decolorization, as well as remediation of water and soil contaminated with xenobiotics (Levin et al., 2019; Wei et al., 2021; Falade et al., 2018; Qayyum et al., 2009; Mohajershojaei et al., 2015; Mahmoodi et al., 2014; Mahmoodi and Saffar-Dastgerdi, 2020). It is regarded that the use of fungi or extracellular fungal enzymes in remediation has advantages due to their great resilience in environments of high pollutant concentrations, less stringent substrate specificity, and adaptability to extreme conditions (Chekroun et al., 2014; Aparici-Carratalá et al., 2023; Mehrotra et al., 2021).
This review introduces ECOHRs, including the major enzymes and catalytic mechanisms, and highlights their potential use as a novel strategy to address diverse environmental challenges, with PFAS remediation and turfgrass dethatching as examples. Where appropriate, knowledge gaps are identified with research needs discussed. This review is intended to facilitate new ideas in ECOHRs research and development of ECOHR-based strategies for pollutant remediation and ecosystem management.
2 ECOHRs
White/brown fungi belong to the second most abundant phylum, Basidiomycota (Harms et al., 2011). These fungi shift to secondary metabolism as a defense mechanism or under nutrient-deficient conditions and produce extracellular enzymes as well as small-molecule mediators, which, working together, can decompose complex compounds and are commonly involved in lignin degradation (Dashtban et al., 2009). These extracellular enzymes offer several advantages, including broad substrate specificity, facile harvesting and scalability (Schmidt-Dannert, 2016; Dashtban et al., 2009). Harvesting typically involves fungal fermentation in specific nutrient media that induce extracellular enzyme secretion into the culture broth, after which the broth is filtered and/or centrifuged to separate them from the mycelial biomass (Liu et al., 2016; Nüske et al., 2002). Moreover, purification of ECOHR enzymes is not required for remediation applications as seen in several studies, thereby enhancing cost-effectiveness and applicability (Ding et al., 2022; Luo et al., 2018b; Wen et al., 2010).
Laccase (EC 1.10.3.2) contains four copper atoms covalently bonded to the protein backbone through ten histidine residues and one cysteine residue, including one Type 1 (T1) copper, one Type 2 (T2) copper, and two Type 3 (T3) copper atoms that are coordinated by six histidine residues (Mot and Silaghi-Dumitrescu, 2012). The T2 and T3 coppers together form a trinuclear cluster (TNC), which is connected to the T1 site via a His-Cys-His triad (Quintanar et al., 2005). The binding pocket at the T1 site enables laccase to bind with a diverse range of substrates. A catalytic cycle with the laccase’s four coppers starting in the resting oxidized state is schematically shown in Figure 1 (Panel 3A). The substrate undergoes oxidation at the T1 site, with T1 copper reduced, and the electrons are shuttled until all copper ions reach the fully reduced state (Jones and Solomon, 2015). Subsequently, the trinuclear copper cluster (TNC) interacts with molecular oxygen (O2), initiating stepwise copper oxidation. T2 and one of the T3 copper ions are first oxidized, leading to the formation of a peroxide intermediate, followed by O–O bond cleavage via electron transfer from T1 and the oxidation of the second T3 copper, assisted by a proton from carboxylate residues near the TNC center, forming the native intermediate (Jones and Solomon, 2015). At this stage, if a substrate is present, it is oxidized by the native intermediate with electrons shuttled to all copper atoms to reach the fully reduced state again, releasing water as a byproduct, forming a catalytic cycle. In the absence of a substrate, the intermediate gradually decays to the resting oxidized form by releasing one water molecule, which may enter the catalytic cycle again when a substrate is present (Jones and Solomon, 2015).
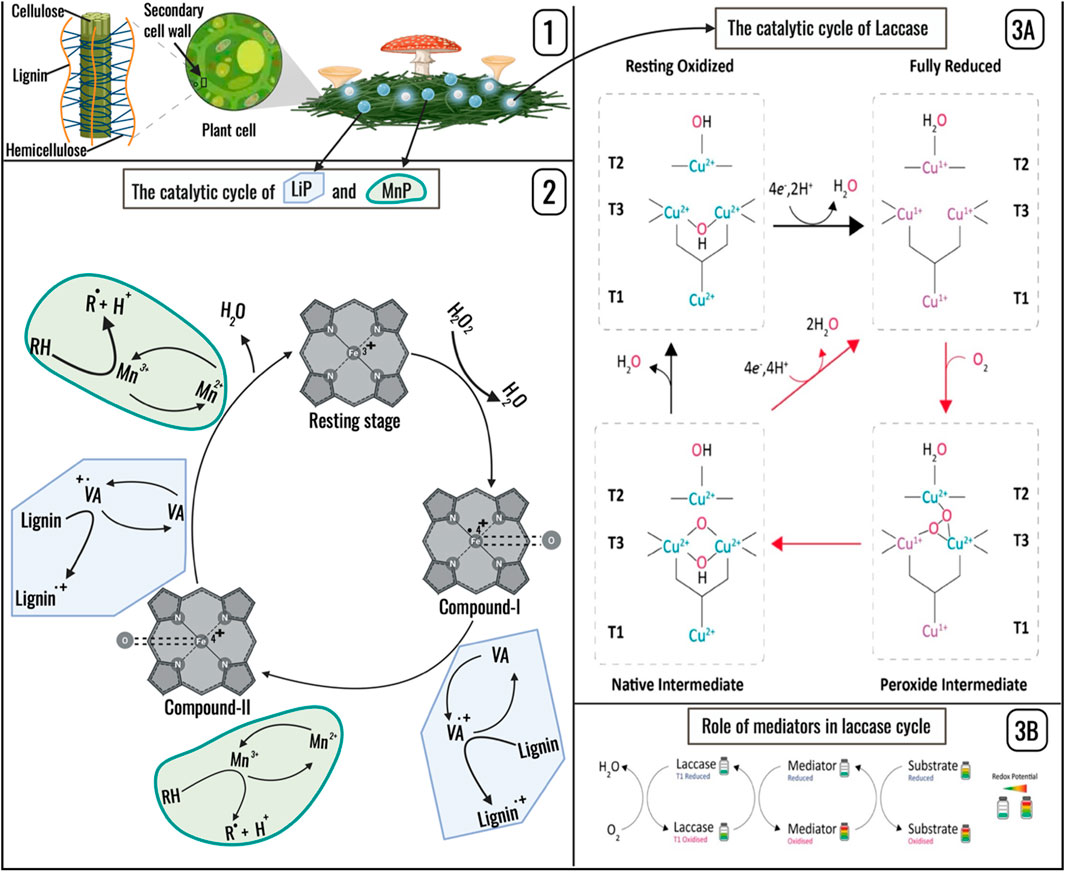
Figure 1. Schematic representation of the catalytic cycle of laccase, LiP and MnP; Panel 1 illustrates the position of lignin in a plant cell to highlight its protective role. Panel 2 depicts the catalytic cycle of LiP and MnP, with the grey drawings indicating the three peroxidase states common to LiP and MnP, while the two blobs in blue color showing the processes mediating LiP transformation and the two in green color showing the processes mediating MnP transformation: Both LiP and MnP are first oxidized to compound I by H2O2. Subsequently, LiP compound I converts to compound II by oxidizing veratryl alcohol to a radical cation, while MnP compound I converts to Compound II by oxidizing Mn+2. Another substrate molecule is oxidized, and the compound II return to the resting stage, completing the cycle. Panel 3A illustrates the catalytic cycle of laccase, transitioning from the resting state to the fully reduced state, passing through two intermediates: the peroxide intermediate and the native intermediate, and finally returning to the resting state. Panel 3B depicts a laccase-mediator system involving a cyclic interaction between a mediator compound and laccase, followed by its interaction with the substrate. This process enhances redox potential, enabling the transformation of contaminants with large molecular sizes and high redox potential (Martin, 2002; Abdel-Hamid et al., 2013; Aza and Camarero, 2023). (created with Biorender.com).
Laccase has a relatively low redox potential (0.4–0.8 V vs. normal hydrogen electrodes) but can interact with mediators to form reactive species that have higher redox potentials and thus enable reactions with a wider range of substrates (Lin et al., 2023). Mediators can be generated by lignin decomposition and are abundant in natural organic matter, such as syringaldehyde, coumaric acid, vanillin, and veratryl alcohol (Cañas and Camarero, 2010; Christopher et al., 2014). The mediators react with laccase to form highly reactive free radicals and/or quinones that can diffuse and oxidize substrates that are otherwise nonreactive to laccase. This process is cyclic, with the mediators continuously oxidized by laccase and the oxidized species reduced by the substrates (Figure 1, Panel 3B). The ideal mediators for laccase-mediator applications are non-toxic, highly effective, and responsive to the enzymatic reactions, to ensure continuous redox cycling. Recent studies have indicated that soybean meal (a soil amendment) contains phenolic compounds, such as vanillin, apocynin, and daidzein, thus providing an ideal, low-cost, natural source of laccase mediators for remediation purposes (Komorowicz et al., 2023; Sebastian et al., 2024; Chia et al., 2024; Liang et al., 2017).
Peroxidases (EC 1.11.1. X) are another class of enzymes able to mediate ECOHRs, for instance lignin peroxidase (LiP) and manganese peroxidase (MnP), which are involved in lignin degradation. Both LiP (EC 1.11.1.14) and MnP (EC 1.11.1.13) are heme-containing oxidoreductases in which Fe (III) is pentacoordinated with four heme tetrapyrrole nitrogens and one amino acid. They share a similar general catalytic mechanism; however, differ in substrate specificity and electron transfer route (Figure 1, Panel 2) (Rahi and Parmar, 2021). The catalytic cycle begins with the oxidation of the resting-state enzyme by H2O2, producing Compound I, an unstable two-electron oxidized intermediate characterized as an Fe (IV) = O porphyrin radical cation. This specie forms through electron transfer within the heme during peroxidase-mediated O–O bond cleavage, which also releases a water molecule. Subsequently, LiP and MnP follow one-electron oxidation, resulting in free radicals of their respective substrates, leading to the reduction of Compound I to Compound II (an Fe (IV) = O porphyrin complex). Finally, Compound II is further reduced back to the resting state upon oxidizing an additional substrate molecule to a free radical, accompanied by the release of another water molecule (Kumar and Chandra, 2020; Rahi and Parmar, 2021). A key difference between LiP and MnP lies in the substrate preference. LiP can directly oxidize aromatic substrates or utilize veratryl alcohol as a mediator to enhance oxidative efficiency (Kulikova et al., 2011), whereas MnP primarily oxidizes Mn2+ to Mn3+. Unlike LiP, MnP does not generate any metabolic mediator; instead, the Mn3+ product itself acts as a diffusible mediator after stabilization by organic acids such as oxalate. Another major distinction between LiP and MnP is the presence of a surface-exposed tryptophan residue (Trp171) in LiP, which enables the oxidation of distant aromatic substrates, a feature absent in MnP (Bernini et al., 2012; Kamitsuji et al., 2005). Versatile peroxidase (VP) exhibits qualities of both LiP and MnP which enable it to oxidize Mn2+ as well as low and high-molecular-weight substrates. The hybrid nature of VP is attributed to its unique structural feature with two distinct channels that carry out LiP and MnP functions, respectively (Ruiz-Duenas et al., 2009).
The ECOHR enzymes are pivotal in environmental catalysis; however, they also have some notable limitations. Laccase is restricted to low-redox-potential substrates, and requires mediators for non-phenolic oxidation, which can generate toxic byproducts (Gu et al., 2021), while this challenge can be addressed by using natural mediators, such as those from the soybean meal (Liang et al., 2017). LiP and MnP use hydrogen peroxide as the oxidant, which is itself hazardous, and MnP depends on organic acids to stabilize Mn3+, restricting the substrate range. Both LiP and MnP exhibit low natural production, limiting their large-scale applicability (Biko et al., 2020; Hofrichter, 2002). Laccase uses oxygen as the oxidant, and it can be produced by fermentation with high productivity, and thus offers great potential for various environmental applications. The environmental application of laccase-induced ECOHRs is discussed below with two examples.
3 ECOHRs application in PFAS remediation
PFAS are defined as fluorinated alkyl substances with at least one fully fluorinated methyl or methylene carbon atom (OECD, 2021), also known as “forever chemicals”. Perfluoroalkyl acids (PFAAs) are the most persistent subset of PFAS, with all alkyl carbons being fully fluorinated, having extreme thermal and chemical stability because of the strong carbon-fluorine (C-F) bonds (Lemal, 2004) and the protective helical conformation of the perfluorinated carbon chain (Battye et al., 2022; Patch et al., 2022). PFAS are extensively used in industrial and consumer applications, and their persistence has led to global environmental distribution (Wang et al., 2023; O'Hagan, 2008). Their bioaccumulative nature poses serious health risks, including increased incidences of kidney, prostate, and testicular cancers (Panieri et al., 2022; Zhang et al., 2010).
PFAS transformation under ECOHRs has been investigated in aqueous and soil matrices, primarily for PFOA and PFOS, the two most extensively detected PFAS in the environment (Table 1). PFOA, a representative PFAS, was investigated under ECOHRs induced by LMS (laccase-mediator system) (Table 1, Study 1) (Luo et al., 2015). After 157 days of treatment, PFOA exhibited an approximate 50% decrease in concentration, accompanied by 28.3% defluorination.
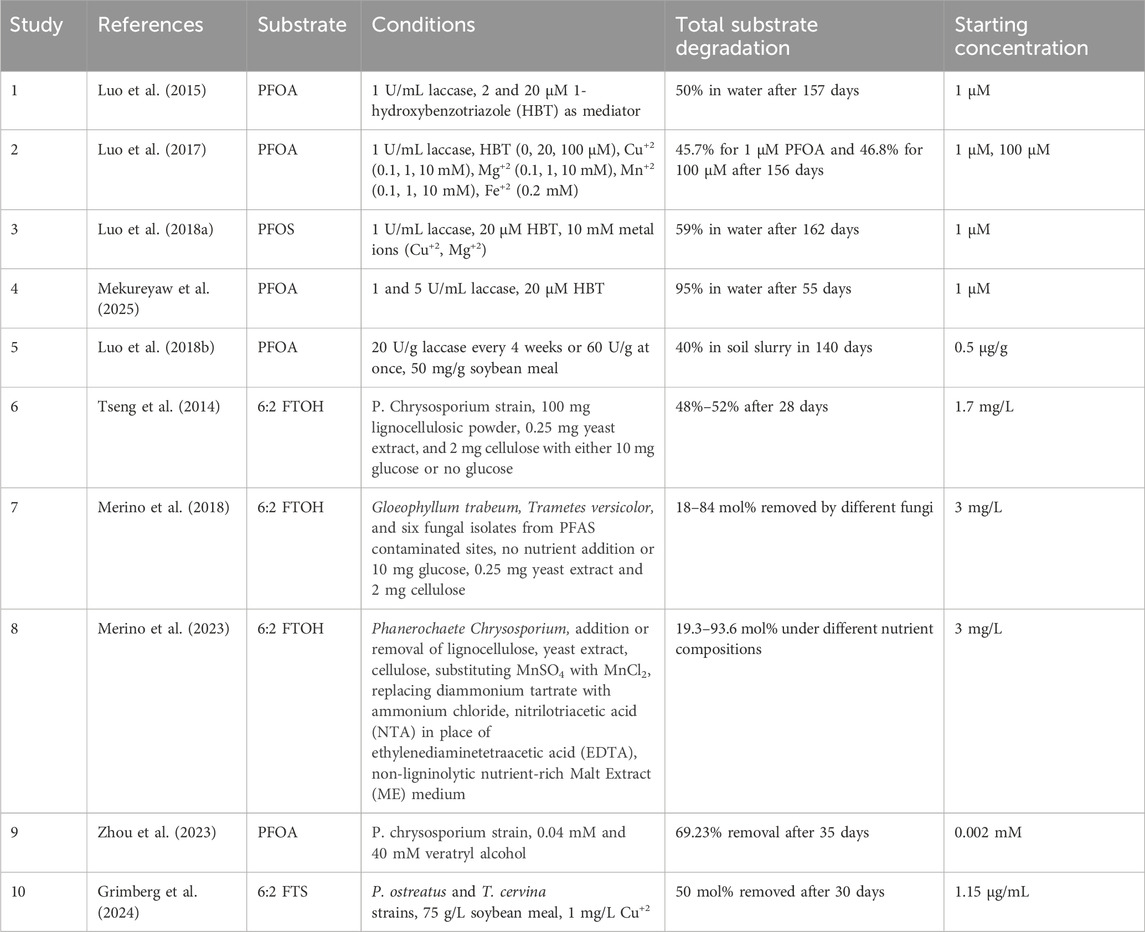
Table 1. Summary of studies examining PFAS degradation in ECOHRs with an overview of reported experimental conditions, PFAS initial concentrations, and degradation efficiencies.
High-resolution mass spectrometry (HRMS) identified PFOA transformation products, including alcohols, aldehydes, and aromatic compounds, which were either partially fluorinated or retained a perfluoroalkyl moiety. The reaction proceeds via electron transfer from the carboxyl headgroup to the HBT (1-hydroxybenzotriazole) radical, followed by Kolbe decarboxylation, hydrolysis, and elimination of fluorine to form one-carbon less perfluoroalkyl carboxylic acid (PFCA), and this CF2 unzipping process goes on until PFCA is mineralized. Products may also form by radical rearrangements, while cross-coupling with nonfluorinated organics generates partially fluorinated products (Luo et al., 2015). Recently, a concern has been raised that the observed PFAS concentration reduction in LMS may not be from their degradation but PFAS adsorption by the enzymes (Steffens et al., 2023). However, the experiments examining PFAS degradation in LMS were carried out with control treatments that had ruled out the contribution by PFAS adsorption (Luo et al., 2015; Luo et al., 2018b). PFOA was further investigated under variable treatment levels in LMS to assess ECOHR efficacy (Luo et al., 2017). Comparable degradation efficiency was observed even at a higher PFOA concentration (100 µM) under identical conditions (Table 1, Study 2). Additionally, increasing the laccase dosage (5 U/mL) (Table 1, Study 4) markedly improved PFOA degradation efficiency (95% in 55 days) (Mekureyaw et al., 2025). However, relatively high mediator concentration (100 µM) resulted in enzyme deactivation due to excess production of the HBT free radicals, which could induce radical quenching by self-coupling and thus reduce PFOA transformation (Table 1, Study 2). In addition, it was found that certain divalent cations, such as Cu2+ (10 mM), could enhance the degradation of PFOA by bridging laccase and PFOA, both of which are negatively charged (Luo et al., 2017). Another study (Table 1, Study 3) with PFOS as the model PFAS resulted in 59% degradation over 162 days with 47% defluorination and similar degradation products (Luo et al., 2018b). For the studies presented in Table 1 Study 1–5, it was proposed that the free radicals produced from the mediators by LMS initiated free-radical chain reactions leading to the observed products, involving C-C cleavage, decarboxylation, free radical rearrangements, and cross-coupling, evidenced by products identification with HRMS (Luo et al., 2017; Luo et al., 2015; Luo et al., 2018b). The complexation between multivalent cations and PFAA reduces the C-C bond energy and unravels the helical configuration, making PFAAs more susceptible to free-radical reactions during ECOHRs (Luo et al., 2018b). ECOHRs have also demonstrated effective transformation of PFOA in soil slurry via the amendment of laccase and soybean meal (Table 1, Study 5). Soybean meal contains high concentrations of small molecules that can serve as laccase mediators (Liang et al., 2017) and multivalent metal cations, both factors having been identified as essential to PFAA degradation by ECOHRs (Luo et al., 2018b). The experiment led to a 40% PFOA degradation via free-radical chain reactions similar to those by ECOHRs in the aqueous phase (Luo et al., 2018a).
PFAS transformation by ECOHRs has also been investigated with live fungal species, including wood rot fungi Trametopsis cervina, Pleurotus ostreatus, Phanerochaete chrysosporium, Trametes versicolor, and Gloeophyllum trabeum (Grimberg et al., 2024; Merino et al., 2018; Zhou et al., 2023) (Table 1, Study 6–10). The degradation mechanism involved fungal thallus adsorption followed by biotransformation mediated by extracellular ECOHR enzymes produced by the fungi.
PFAS initially (0–14 days) negatively affected the structure and activity of the enzymes, however in the next phase (14–35 days) fungal defense mechanism was activated to enhance cell survivability. Incubation time and growth medium composition have significant effects on enzyme activity levels and degradation pathways. The highest enzyme activities were observed at day 47 and 53 for Lip and MnP, respectively (Zhou et al., 2023). Limited supply of glucose produced sufficient metabolic energy and reducing power that led to greater production of 5:3 FTCA (fluorotelomer carboxylic acid) from 6:2 FTOH (fluorotelomer alcohol) degradation. Moreover, low glucose coupled with cellulose and yeast extract was able to divert the 6:2 FTOH degradation pathway from forming short PFAS products to 5:3 FTCA (Merino et al., 2023).
A life cycle assessment (LCA) of ECOHR-based remediation of PFAS or any other pollutants has not been reported and is needed in future research. LCA of some other enzymatic treatments has generally shown lower energy use, reduced ozone depletion, and fewer photochemical oxidants, underscoring their sustainability (Gabarrell et al., 2012); and switching electricity to steam for fungal medium sterilization could further improve energy use efficiency (Zhi Fu et al., 2005). ECOHR-based pollutant remediation is expected to benefit similarly as the other enzymatic treatment processes, and using crude enzyme for environmental applications eliminates the need for enzyme purification, further reducing costs (Nüske et al., 2002; Ding et al., 2022). However, a detailed techno-economic analysis is still needed in future research to elucidate capital and operational expenditures, and overall cost-effectiveness under real-world implementation scenarios.
4 ECOHRs applications in thatch management
Thatch forms as a thick organic matter layer between soil and turfgrass when the organic matter accumulation rate exceeds its degradation rate. It restricts hydraulic conductivity, oxygen diffusion, and reduces turfgrass quality. (McCarty et al., 2007; Sidhu et al., 2022; Braun et al., 2024). Lignin, a major structural component of the plant cell wall, forms a protective matrix embedding cellulose and hemicellulose (Figure 1, Panel 1) (Yadav and Chattopadhyay, 2023), thereby contributing substantially to thatch accumulation and limiting the effectiveness of conventional microbial management strategies. Its exceptional recalcitrance arises from a highly heterogeneous and cross-linked structure (Wong, 2009; Yadav and Chattopadhyay, 2023), hydrophobicity, and the abundance of phenolic hydroxyl groups (Zoghlami and Paës, 2019; Takeda et al., 2017). Functionally, lignin not only acts as a physical barrier but also inhibits cellulolytic enzyme activity, making it the principal rate-limiting factor in the microbial decomposition of organic matter (Brink et al., 2019).
Thatch is commonly managed through cultural practices like core aeration, sand topdressing, or supplementation of microbes and sugars to boost microbial activity. Core aeration and sand topdressing reduce the turf aesthetic and physical quality, leading to financial losses (Sidhu et al., 2022), while primary focus of microbial approach is cellulose and hemicellulose, which are structurally shielded by lignin. Conversely, ECOHRs induced by laccase application directly transform lignin; i.e., laccase catalyzes electron withdrawal from the phenolic hydroxyl group to form phenoxy radicals that lead to Cα-Cβ cleavage, alkyl-aryl cleavage, and Cα oxidation, thus disrupting the complex lignin structure and making labile carbohydrates accessible to microbial degraders (Wong, 2009; Zhao et al., 2025).
ECOHRs for thatch management have been tested in multiple studies at different scales by laccase application (Sidhu et al., 2022). It was found that ECOHRs induced significant dethatching effects, including decreased thatch thickness, reduced organic matter and monosaccharide content, and lower total lignin concentration. These effects led to improved hydraulic conductivity and thus facilitated sustainable, nondestructive thatch management (Sidhu et al., 2013b). Following successful greenhouse trials, a multi-year field study resulted in 18%–22% reduction in thatch layer thickness in bermudagrass and 21%–30% for zoysiagrass and identified the optimal treatment conditions (Sidhu et al., 2013a), demonstrating that a monthly application of 2 U cm-2 laccase for 6 months annually achieved dethatching efficiencies comparable to those obtained with conventional mechanical methods, thereby confirming the practical applicability of the approach under real-world conditions (Sidhu et al., 2014; Sidhu et al., 2019).
5 Conclusion
ECOHRs have been presented as a sustainable strategy with applications in dethatching and PFAS remediation. It transformed PFOA (40% in soil, ∼50% in water), PFOS (59% in water), and 6:2 FTOH (18–93.6 mol% under different conditions), as well as reduced the thatch layer in Bermuda and Zoysia grass by 18%–30%. Their potential for large-scale soil remediation and dethatching offers both environmental and economic benefits. However, field-scale validation of soil remediation with PFAS mixtures, co-contaminants, and variable concentrations remains a critical next step.
Author contributions
UM: Conceptualization, Data curation, Formal Analysis, Investigation, Visualization, Writing – original draft, Writing – review and editing. YW: Writing – review and editing. QH: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported in part by SERDP project ER23-3825 and HATCH funds.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Hamid, A. M., Solbiati, J. O., and Cann, I. K. (2013). Insights into lignin degradation and its potential industrial applicationsAdv. Appl. Microbiol., 82. 1–28. doi:10.1016/b978-0-12-407679-2.00001-6
Aparici-Carratalá, D., Esclapez, J., Bautista, V., Bonete, M.-J., and Camacho, M. (2023). Archaea: current and potential biotechnological applications. Res. Microbiol. 174, 104080. doi:10.1016/j.resmic.2023.104080
Atiwesh, G., Parrish, C. C., Banoub, J., and LE, T. A. T. (2022). Lignin degradation by microorganisms: a review. Biotechnol. Prog. 38, e3226. doi:10.1002/btpr.3226
Aza, P., and Camarero, S. (2023). Fungal laccases: fundamentals, engineering and classification update. Biomolecules 13, 1716. doi:10.3390/biom13121716
Battye, N. J., Patch, D. J., Roberts, D. M., O'Connor, N. M., Turner, L. P., Kueper, B. H., et al. (2022). Use of a horizontal ball mill to remediate per-and polyfluoroalkyl substances in soil. Sci. Total Environ. 835, 155506. doi:10.1016/j.scitotenv.2022.155506
Bernini, C., Pogni, R., Basosi, R., and Sinicropi, A. (2012). The nature of tryptophan radicals involved in the long-range electron transfer of lignin peroxidase and lignin peroxidase-like systems: insights from quantum mechanical/molecular mechanics simulations. Proteins Struct. Funct. Bioinforma. 80, 1476–1483. doi:10.1002/prot.24046
Biko, O. D., Viljoen-Bloom, M., and VAN Zyl, W. H. (2020). Microbial lignin peroxidases: applications, production challenges and future perspectives. Enzyme Microb. Technol. 141, 109669. doi:10.1016/j.enzmictec.2020.109669
Braun, R. C., Mandal, P., Nwachukwu, E., and Stanton, A. (2024). The role of turfgrasses in environmental protection and their benefits to humans: thirty years later. Crop Sci. 64, 2909–2944. doi:10.1002/csc2.21383
Brink, D. P., Ravi, K., Lidén, G., and Gorwa-Grauslund, M. F. (2019). Mapping the diversity of microbial lignin catabolism: experiences from the eLignin database. Appl. Microbiol. Biotechnol. 103, 3979–4002. doi:10.1007/s00253-019-09692-4
Cañas, A. I., and Camarero, S. (2010). Laccases and their natural mediators: biotechnological tools for sustainable eco-friendly processes. Biotechnol. Adv. 28, 694–705. doi:10.1016/j.biotechadv.2010.05.002
Chekroun, K. B., SáNCHEZ, E., Baghour, M., and Iss, J. (2014). The role of algae in bioremediation of organic pollutants, Nigera. 2360. ISSN.
Chelaliche, A. S., Benitez, S. F., Alvarenga, A. E., Zapata, P. D., and Fonseca, M. I. (2024). A comprehensive review on the application of mycoremediation in polychlorinated biphenyls treatment. Environ. Nanotechnol. Monit. and Manag. 22, 100974. doi:10.1016/j.enmm.2024.100974
Chia, X. K., Hadibarata, T., Kristanti, R. A., Jusoh, M. N. H., Tan, I. S., and Foo, H. C. Y. (2024). The function of microbial enzymes in breaking down soil contaminated with pesticides: a review. Bioprocess Biosyst. Eng. 47, 597–620. doi:10.1007/s00449-024-02978-6
Christopher, L. P., Yao, B., and Ji, Y. (2014). Lignin biodegradation with laccase-mediator systems. Front. Energy Res. 2, 12. doi:10.3389/fenrg.2014.00012
Dashtban, M., Schraft, H., and Qin, W. (2009). Fungal bioconversion of lignocellulosic residues; opportunities & perspectives. International Journal of Biological Sciences 5, 578.
Ding, Y., Cui, K., Liu, X., Xie, Q., Guo, Z., and Chen, Y. (2022). Lignin peroxidase-catalyzed direct oxidation of trace organic pollutants through a long-range electron transfer mechanism: using propranolol as an example. J. Hazard. Mater. 431, 128544. doi:10.1016/j.jhazmat.2022.128544
Falade, A. O., Mabinya, L. V., Okoh, A. I., and Nwodo, U. U. (2018). Ligninolytic enzymes: versatile biocatalysts for the elimination of endocrine-disrupting chemicals in wastewater. MicrobiologyOpen 7, e00722. doi:10.1002/mbo3.722
Gabarrell, X., Font, M., Vicent, T., Caminal, G., Sarrà, M., and Blánquez, P. (2012). A comparative life cycle assessment of two treatment technologies for the grey lanaset G textile dye: biodegradation by Trametes versicolor and granular activated carbon adsorption. Int. J. Life Cycle Assess. 17, 613–624. doi:10.1007/s11367-012-0385-z
Grimberg, F., Holsen, T. M., Fernando, S., and Wang, S. (2024). Biotransformation of 6: 2 fluorotelomer sulfonate (6: 2 FTS) in sulfur-rich media by Trametopsis cervina. Front. Environ. Sci. and Eng. 18, 107. doi:10.1007/s11783-024-1867-5
Gu, Y., Yuan, L., Jia, L., Xue, P., and Yao, H. (2021). Recent developments of a co-immobilized laccase–mediator system: a review. RSC Adv. 11, 29498–29506. doi:10.1039/d1ra05104k
Harms, H., Schlosser, D., and Wick, L. Y. (2011). Untapped potential: exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Microbiol. 9, 177–192. doi:10.1038/nrmicro2519
Hofrichter, M. (2002). Review: lignin conversion by manganese peroxidase (MnP). Enzyme Microb. Technol. 30, 454–466. doi:10.1016/s0141-0229(01)00528-2
Jones, S. M., and Solomon, E. I. (2015). Electron transfer and reaction mechanism of laccases. Cell. Mol. life Sci. 72, 869–883. doi:10.1007/s00018-014-1826-6
Kamitsuji, H., Watanabe, T., Honda, Y., and Kuwahara, M. (2005). Direct oxidation of polymeric substrates by multifunctional manganese peroxidase isoenzyme from Pleurotus ostreatus without redox mediators. Biochem. J. 386, 387–393. doi:10.1042/bj20040968
Komorowicz, M., Janiszewska-Latterini, D., Przybylska-Balcerek, A., and Stuper-Szablewska, K. (2023). Fungal biotransformation of hazardous organic compounds in wood waste. Molecules 28, 4823. doi:10.3390/molecules28124823
Kulikova, N., Klein, O., Stepanova, E., and Koroleva, O. (2011). Use of basidiomycetes in industrial waste processing and utilization technologies: fundamental and applied aspects (review). Appl. Biochem. Microbiol. 47, 565–579. doi:10.1134/s000368381106007x
Kumar, A., and Chandra, R. (2020). Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 6, e03170. doi:10.1016/j.heliyon.2020.e03170
Lemal, D. M. (2004). Perspective on fluorocarbon chemistry. ChemInform 35, chin.200417258–11. doi:10.1002/chin.200417258
Levin, L. N., Hernández-Luna, C. E., Niño-Medina, G., García-Rodríguez, J. P., López-Sadin, I., Méndez-Zamora, G., et al. (2019). Decolorization and detoxification of synthetic dyes by Mexican strains of trametes sp. Int. J. Environ. Res. Public Health 16, 4610. doi:10.3390/ijerph16234610
Liang, S., Luo, Q., and Huang, Q. (2017). Degradation of sulfadimethoxine catalyzed by laccase with soybean meal extract as natural mediator: mechanism and reaction pathway. Chemosphere 181, 320–327. doi:10.1016/j.chemosphere.2017.04.100
Lin, H., Yu, Z., Wang, Q., Liu, Y., Jiang, L., Xu, C., et al. (2023). Application of laccase catalysis in bond formation and breakage: a review. Catalysts 13, 750. doi:10.3390/catal13040750
Liu, J., Yu, Z., Liao, X., Liu, J., Mao, F., and Huang, Q. (2016). Scalable production, fast purification, and spray drying of native pycnoporus laccase and circular dichroism characterization. J. Clean. Prod. 127, 600–609. doi:10.1016/j.jclepro.2016.03.154
Luo, Q., Lu, J., Zhang, H., Wang, Z., Feng, M., Chiang, S.-Y. D., et al. (2015). Laccase-catalyzed degradation of perfluorooctanoic acid. Environ. Sci. and Technol. Lett. 2, 198–203. doi:10.1021/acs.estlett.5b00119
Luo, Q., Wang, Z., Feng, M., Chiang, D., Woodward, D., Liang, S., et al. (2017). Factors controlling the rate of perfluorooctanoic acid degradation in laccase-mediator systems: the impact of metal ions. Environ. Pollut. 224, 649–657. doi:10.1016/j.envpol.2017.02.050
Luo, Q., Liang, S., and Huang, Q. (2018a). Laccase induced degradation of perfluorooctanoic acid in a soil slurry. J. Hazard. Mater. 359, 241–247. doi:10.1016/j.jhazmat.2018.07.048
Luo, Q., Yan, X., Lu, J., and Huang, Q. (2018b). Perfluorooctanesulfonate degrades in a laccase-mediator system. Environ. Sci. and Technol. 52, 10617–10626. doi:10.1021/acs.est.8b00839
Mahmoodi, N. M., and Dastgerdi, H. (2020). Clean laccase immobilized nanobiocatalysts (graphene oxide-zeolite nanocomposites): from production to detailed biocatalytic degradation of organic pollutant. Appl. Catal. B Environ. Energy 268, 118443. doi:10.1016/j.apcatb.2019.118443
Mahmoodi, N. M., Arabloo, M., and Abdi, J. (2014). Laccase immobilized manganese ferrite nanoparticle: synthesis and LSSVM intelligent modeling of decolorization. Water Res. 67, 216–226. doi:10.1016/j.watres.2014.09.011
Martin, H. (2002). Review: lignin conversion by manganese peroxidase (MnP). Enz. Microb. Technol. 30, 454–466.
Mccarty, L. B., Gregg, M. F., and Toler, J. E. (2007). Thatch and mat management in an established creeping bentgrass golf green. Agron. J. 99, 1530–1537. doi:10.2134/agronj2006.0361
Mehrotra, T., Dev, S., Banerjee, A., Chatterjee, A., Singh, R., and Aggarwal, S. (2021). Use of immobilized bacteria for environmental bioremediation: a review. J. Environ. Chem. Eng. 9, 105920. doi:10.1016/j.jece.2021.105920
Mekureyaw, M. F., Junker, A. L., Bai, L., Petersen, A. E., Wei, Z., and Guo, Z. (2025). Biodegradation of perfluorooctanoic acid (PFOA) by laccase from Agaricus bisporus via oxidative decarboxylation. J. Hazard. Mater. Adv. 19, 100837. doi:10.1016/j.hazadv.2025.100837
Merino, N., Wang, M., Ambrocio, R., Mak, K., O'Connor, E., Gao, A., et al. (2018). Fungal biotransformation of 6: 2 fluorotelomer alcohol. Remediat. J. 28, 59–70. doi:10.1002/rem.21550
Merino, N., Wang, N., Gao, Y., Wang, M., and Mahendra, S. (2023). Roles of various enzymes in the biotransformation of 6: 2 fluorotelomer alcohol (6: 2 FTOH) by a white-rot fungus. J. Hazard. Mater. 450, 131007. doi:10.1016/j.jhazmat.2023.131007
Mohajershojaei, K., Mahmoodi, N. M., and Khosravi, A. (2015). Immobilization of laccase enzyme onto titania nanoparticle and decolorization of dyes from single and binary systems. Biotechnol. Bioprocess Eng. 20, 109–116. doi:10.1007/s12257-014-0196-0
Mot, A., and Silaghi-Dumitrescu, R. (2012). Laccases: complex architectures for one-electron oxidations. Biochem. Mosc. 77, 1395–1407. doi:10.1134/s0006297912120085
Nüske, J., Scheibner, K., Dornberger, U., Ullrich, R., and Hofrichter, M. (2002). Large scale production of manganese-peroxidase using agaric white-rot fungi. Enzyme Microb. Technol. 30, 556–561. doi:10.1016/s0141-0229(02)00013-3
O'Hagan, D. (2008). Understanding organofluorine chemistry. An introduction to the c–f bond. Chem. Soc. Rev. 37, 308–319. doi:10.1039/b711844a
Oecd, H. (2021). Reconciling terminology of the universe of per-and polyfluoroalkyl substances: recommendations and practical guidance. Paris, France. OECD Publishing Paris.
Panieri, E., Baralic, K., Djukic-Cosic, D., Buha Djordjevic, A., and Saso, L. (2022). PFAS molecules: a major concern for the human health and the environment. Toxics 10, 44. doi:10.3390/toxics10020044
Patch, D., O'Connor, N., Koch, I., Cresswell, T., Hughes, C., Davies, J. B., et al. (2022). Elucidating degradation mechanisms for a range of per-and polyfluoroalkyl substances (PFAS) via controlled irradiation studies. Sci. Total Environ. 832, 154941. doi:10.1016/j.scitotenv.2022.154941
Qayyum, H., Maroof, H., and Yasha, K. (2009). Remediation and treatment of organopollutants mediated by peroxidases: a review. Crit. Rev. Biotechnol. 29, 94–119. doi:10.1080/07388550802685306
Quintanar, L., Yoon, J., Aznar, C. P., Palmer, A. E., Andersson, K. K., Britt, R. D., et al. (2005). Spectroscopic and electronic structure studies of the trinuclear Cu cluster active site of the multicopper oxidase laccase: nature of its coordination unsaturation. J. Am. Chem. Soc. 127, 13832–13845. doi:10.1021/ja0421405
Rahi, D. K., and Parmar, A. S. (2021). Ligninolytic peroxidases: sources and applications. J. Adv. Sci. Res. 12, 69–80. doi:10.55218/jasr.s2202112310
Ruiz-Duenas, F. J., Morales, M., García, E., Miki, Y., Martínez, M. J, and Martínez, A. T. (2009). Substrate oxidation sites in versatile peroxidase and other basidiomycete peroxidases. Journal of Experimental Botany 60 (2), 441–452.
Schmidt-Dannert, C. (2016). Biocatalytic portfolio of Basidiomycota. Current Opinion in Chemical Biology 31, 40–49.
Sidhu, S. S., Huang, Q., Carrow, R. N., and Raymer, P. L. (2013a). Efficacy of fungal laccase to facilitate biodethatching in bermudagrass and zoysiagrass. Agron. J. 105, 1247–1252. doi:10.2134/agronj2012.0470
Sidhu, S. S., Huang, Q., Carrow, R. N., and Raymer, P. L. (2013b). Laccase mediated changes in physical and chemical composition properties of thatch layer in creeping bentgrass (Agrostis stolonifera L.). Soil Biol. Biochem. 64, 48–56. doi:10.1016/j.soilbio.2013.04.002
Sidhu, S. S., Huang, Q., Carrow, R. N., and Raymer, P. L. (2014). Optimizing laccase application on creeping bentgrass (Agrostis stolonifera L.) to facilitate biodethatching. Crop Sci. 54, 1804–1815. doi:10.2135/cropsci2013.09.0612
Sidhu, S. S., Huang, Q., Carrow, R. N., and Raymer, P. L. (2019). Short-term and residual effects of laccase application on creeping bentgrass thatch layer. Hortscience 54, 1610–1620. doi:10.21273/hortsci13970-19
Sidhu, S., Huang, Q. J., Carrow, R. N., Jesperson, D., Liu, J., and Raymer, P. L. (2022). A review of a novel enzyme system for the management of thatch and soil water repellency in turfgrass. Int. Turfgrass Soc. Res. J. 14, 450–461. doi:10.1002/its2.138
Steffens, S. D., Antell, E. H., Cook, E. K., Rao, G., Britt, R. D., Sedlak, D. L., et al. (2023). An artifact of perfluoroalkyl acid (PFAA) removal attributed to sorption processes in a laccase mediator system. Environ. Sci. and Technol. Lett. 10, 337–342. doi:10.1021/acs.estlett.3c00173
Takeda, Y., Koshiba, T., Tobimatsu, Y., Suzuki, S., Murakami, S., Yamamura, M., et al. (2017). Regulation of CONIFERALDEHYDE 5-HYDROXYLASE expression to modulate cell wall lignin structure in rice. Planta 246, 337–349. doi:10.1007/s00425-017-2692-x
Tseng, N., Wang, N., Szostek, B., and Mahendra, S. (2014). Biotransformation of 6: 2 fluorotelomer alcohol (6: 2 FTOH) by a wood-rotting fungus. Environ. Sci. and Technol. 48, 4012–4020. doi:10.1021/es4057483
Wang, Y., Munir, U., and Huang, Q. (2023). Occurrence of per-and polyfluoroalkyl substances (PFAS) in soil: sources, fate, and remediation. Soil and Environ. Health 1, 100004. doi:10.1016/j.seh.2023.100004
Wei, S., Liu, K., Ji, X., Wang, T., and Wang, R. (2021). Application of enzyme technology in biopulping and biobleaching. Cellulose 28, 10099–10116. doi:10.1007/s10570-021-04182-1
Wen, X., Jia, Y., and Li, J. (2010). Enzymatic degradation of tetracycline and oxytetracycline by crude manganese peroxidase prepared from phanerochaete chrysosporium. J. Hazard. Mater. 177, 924–928. doi:10.1016/j.jhazmat.2010.01.005
Wong, D. W. (2009). Structure and action mechanism of ligninolytic enzymes. Appl. Biochem. Biotechnol. 157, 174–209. doi:10.1007/s12010-008-8279-z
Yadav, S., and Chattopadhyay, D. (2023). Lignin: the building block of defense responses to stress in plants. J. Plant Growth Regul. 42, 6652–6666. doi:10.1007/s00344-023-10926-z
Zavarzina, A., Lisov, A., Zavarzin, A., and Leontievsky, A. (2010). Fungal oxidoreductases and humification in forest soils. Springer.
Zhang, T., Wu, Q., Sun, H. W., Zhang, X. Z., Yun, S. H., and Kannan, K. (2010). Perfluorinated compounds in whole blood samples from infants, children, and adults in China. Environ. Sci. and Technol. 44, 4341–4347. doi:10.1021/es1002132
Zhao, J., Zhu, M., Jin, W., Zhang, J., Fan, G., Feng, Y., et al. (2025). A comprehensive review of unlocking the potential of lignin-derived biomaterials: from lignin structure to biomedical application. J. Nanobiotechnology 23, 538. doi:10.1186/s12951-025-03604-7
Zhi Fu, G., Chan, A., and Minns, D. (2005). Preliminary assessment of the environmental benefits of enzyme bleaching for pulp and paper making (7 pp). Int. J. Life Cycle Assess. 10, 136–142. doi:10.1065/lca2004.06.162
Zhou, L., Li, W., Zhang, J., and Mao, H. (2023). Removal of perfluorooctanoic acid (PFOA) in the liquid culture of phanerochaete chrysosporium. Chemosphere 345, 140427. doi:10.1016/j.chemosphere.2023.140427
Keywords: enzyme catalyzed humification reactions (ECOHRs), PFAS, dethatching, remediation, laccase-mediator system, laccase, lignin peroxidase (LiP), manganese peroxidase (MnP)
Citation: Munir U, Wang Y and Huang Q (2025) Enzyme catalyzed oxidative humification reactions (ECOHRs): PFAS remediation and thatch management. Front. Environ. Eng. 4:1673461. doi: 10.3389/fenve.2025.1673461
Received: 25 July 2025; Accepted: 09 October 2025;
Published: 23 October 2025.
Edited by:
Sandhya Patidar, Heriot-Watt University, United KingdomReviewed by:
Andréa Miura Costa, Universidade Estadual de Santa Cruz (UESC), BrazilNiyaz Mohammad Mahmoodi, Institute for Color Science and Technology (ICST), Iran
Copyright © 2025 Munir, Wang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingguo Huang, cWh1YW5nQHVnYS5lZHU=
 Umar Munir
Umar Munir Yifei Wang
Yifei Wang Qingguo Huang
Qingguo Huang