- Department of Biology and Laurier Institute for Water Science, Wilfrid Laurier University, Waterloo, ON, Canada
Native to the Atlantic Ocean, anadromous sea lamprey (Petromyzon marinus) likely invaded the Laurentian Great Lakes in the mid 1800's-early 1900's following construction of the Erie Canal. Initially restricted to Lake Ontario, and some smaller nearby lakes, they entered Lake Erie via the Welland Canal in the early 1900s. Sea lamprey quickly became established in Lake Erie (1921), from which they invaded the three upper Great Lakes. Along with overharvest, predation (parasitism) by blood-feeding sea lamprey devastated commercial, sport and Indigenous fisheries including lake trout (Salvelinus namaycush) and whitefish and ciscoes (Coregonus sp.) populations. To deal with the crisis, the Great Lakes Fishery Commission (GLFC) was founded in 1955 with a mandate to eradicate sea lamprey. Sea lamprey were not eradicated, but a comprehensive sea lamprey control (SLC) program brought populations under control using barriers (dams) and traps to prevent spawning by adult lampreys, and chemical control using lampricides that selectively targeted larval sea lamprey in nursery streams draining into the lakes. In this synthesis the sea lamprey invasion is explored through the lens of “invasion theory” to characterize the likely vectors that introduced sea lamprey into the Great Lakes ecosystem, and to establish what eco-physiological features of sea lamprey led to their establishment and spread. The weight of evidence suggests that pre-existing adaptations and a robust physiology facilitated the sea lamprey's invasion of the Great Lakes. Key features likely included: (i) facultative anadromy, which allowed them to complete their entire life cycle in fresh water, (ii) a generalist diet enabling them to feed on a wide variety of fishes, (iii) the high fecundity of females that expedited their spread, (iv) a resilient thermal physiology, and (v) the availability of similar, suitable spawning and nursery habitat to that found in their native ranges. Many of these features may make sea lamprey relatively resilient to climate change, with changes in water temperature, water quality and hydrology having both negative and positive effects on the distribution of invasive populations in the Great Lakes, and imperiled populations native to the Western and Eastern Atlantic Ocean, and the Mediterranean Sea.
Introduction
Sea lamprey (Petromyzon marinus) invaded the Laurentian Great Lakes of North America (hereafter Great Lakes) in the late 1800s and early 1900s, entering the basin via man-made canals (1–4). The combined effects of sea lamprey predation (parasitism) of fishes and overharvest devastated sport, commercial and Indigenous fisheries, causing serious ecological and socioeconomic harm (5, 6). In response to the crisis, the United States and Canada signed the Convention on Great Lakes Fisheries in 1954, leading to the creation of the Great Lakes Fishery Commission (GLFC) in 1955, which was given the mandate to co-manage fisheries in the Great Lakes and to eradicate sea lamprey (6). Sea lamprey were not eradicated, but sustained cooperative efforts coordinated by the GLFC between the two countries resulted in the implementation of a highly successful sea lamprey research and control program which resulted in the suppression of sea lamprey populations by more than 90 percent from peak levels in the 1950s, paving the way for the ongoing rehabilitation of the Great Lakes' ecosystem and its fisheries (6–9).
Sea lamprey control (SLC) measures that were implemented included an expansive network of barriers and traps to prevent sea lamprey from reaching spawning sites in the rivers and streams that drain into the Great Lakes (8, 10, 11). The identification of a chemical compound, 3-trifluoromethyl-4-nitrophenol (TFM), which was highly selective to sea lamprey was a game changer, and led to a chemical control program that used lampricides to target larval sea lamprey living burrowed in the soft sediments of stream and riverbeds (8, 12, 13). However, SLC could be potentially undermined by increases in temperature and adverse hydrological events arising from climate change (14).
Previous works, exploring the socioeconomic importance, biology, conservation and control of sea lamprey, have covered various aspects of lamprey physiology and ecology in the Great Lakes in recent years (8, 11, 12). However, less attention has been given to aspects of sea lamprey ecophysiology that allowed them to invade the Great Lakes in the first place (11). The objective of this synthesis is to examine the sea lamprey invasion through the lens of “invasion theory” to better understand what aspects of the sea lamprey's ecology and physiology may have allowed them to initially occupy and then become established in the Great Lakes. Throughout this synthesis, I propose that pre-existing physiological adaptations provided sea lamprey with the capacity to invade the temperate freshwaters of the Great Lakes. I also suggest that the sea lamprey's ecophysiology will make them resilient to climate change and the projected disturbances to air and water temperature, water quality and hydrology that are likely to occur in the coming decades. However, these future climate challenges could also exacerbate the challenges faced by imperiled native populations of sea lamprey in their more southerly distributions in the Western and Eastern Atlantic Ocean, and the Mediterranean Sea.
Lamprey phylogeny and life history
Lampreys (Order: Petromyzontiformes) and hagfishes (Order: Myxiniformes) are the only two extant groups of jawless fishes, known as agnathans (Infraphylum: Agnatha), and are commonly referred to as cyclostomes (round mouths) (15). The lampreys have an antitropical distribution, with populations in temperate waters of both the northern and southern hemisphere, but they are absent from tropical waters (16). There remain three extant families of lampreys, the Petromyzontidae, represented by approximately 35 species living in the Northern hemisphere (16), with the two remaining families, Geotriidae and Mordiciidae, represented by at least 5 species in the Southern hemisphere (17). The lampreys are thought to have diverged from the main vertebrate lineage approximately 450 million years ago, with the earliest fossil lamprey Priscomyzon riniensis dating back to the Devonian approximately 360 mya (18). Recent fossil evidence suggests that the predatory/parasitic lifestyle of lampreys may not have arisen until the mid-late Jurassic approximately 158–160 mya (19). All modern lamprey are thought to have descended from an anadromous predatory/parasitic ancestor, but the majority of modern lampreys are non-parasitic, and complete their entire lifecycle in freshwater, with 18 predatory/parasitic species including the sea lamprey (16).
The life cycle of modern lampreys includes a prolonged freshwater larval phase in which the larvae (often called ammocoetes) live burrowed in the soft, silty substrate of rivers and streams as suspension feeders, primarily ingesting detritus (>90 % of their diet), and lesser amounts of algae, diatoms and bacteria (20, 21). Water currents generated by a velar pump direct water through the oral hood of the larvae into the pharynx, which then exits via one of seven pairs of branchiopores (gill pores) on either side of the pharyngeal region (Figure 1A). Food particles are trapped by mucus secreted by an underlying endostyle, before being diverted to the esophagus (22, 23). This larval phase is thought to be a derived feature in modern lampreys, with recent fossil evidence suggesting that it did not arise until the late Triassic-early Jurassic approximately 200 mya (24). The multi-year larval phase is followed by a true metamorphosis into juvenile lamprey, often referred to as macrophthalmia, characterized by the formation of well-developed eyes, darkening and silvering of the body, and changes in the internal organs and metabolism, and the formation of a multi-toothed oral disc and dagger-like tongue (Figure 1B) which are used to attach to and feed on the blood of fishes and other vertebrates in predatory/parasitic species (25–27, 213).
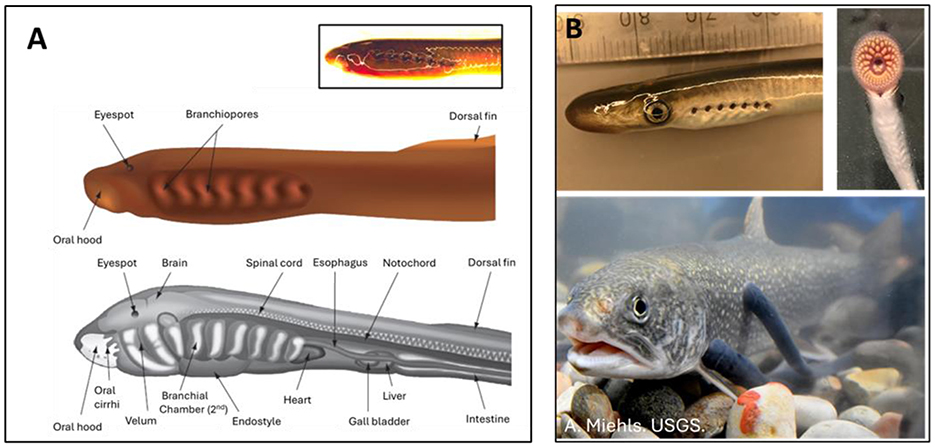
Figure 1. Anatomy and appearance of sea lamprey (Petromyzon marinus). (A) In suspension (filter) -feeding larval sea lamprey (ammocoetes) inward water currents are generated by contractions of a muscular velum (velar pump), which draws in water via the oral hood before it is directed to the pharyngeal chamber (not labeled) lying between seven pairs of branchiopores (gill pores) on either side of the anterior body. To promote gas exchange, water is expired across the gills in a unidirectional manner via pumping of the branchiopores. Food particles in the water are trapped by mucus secreted by the underlying endostyle and directed to the esophagus for digestion. Also note the presence of poorly developed eye spots and the light brown color of the ammocoete. (B) Following metamorphosis, the juvenile (macrophthalmia) phase is characterized by large well-developed eyes, and darker, silvery dorsolateral pigmentation. The feeding apparatus (B. Top right) comprises a suctorial oral disc containing numerous teeth used to latch onto fishes while feeding, and a rasping, dagger-like tongue used to gain access to the blood/tissue of their hosts. The gill are irrigated tidally by actively pumping water in and out of seven pairs of gill pouches, which is necessary when the juveniles are attached to their hosts, to rocky substrate, or when moving cobble when constructing redds (nests) for egg deposition and incubation. For further details, refer to the text or comprehensive reviews by Mallatt (22), Rovainen (23) and Renaud et al. (88). Attribution: (A) Modifed from Wilkie (114). (B) Lower panel. A. Miehls. US Geological Survey. Public domain.
In the anadromous sea lamprey, the larval phase last 3–7 years prior to metamorphosis, which lasts 3–4 months. Metamorphosis is followed by a 20–30 month parasitic juvenile phase in marine (sea water) environments, compared to 12–20 months in the landlocked, freshwater populations, before the maturing adults migrate up freshwater streams, spawn and then die (Figure 2) (28, 29). Unlike anadromous salmonids and other fishes, sea lamprey do not home to their natal streams, but instead identify suitable streams in which to spawn by searching for freshwater outflows and olfactory cues secreted by larvae or male sea lamprey, indicating that habitat is suitable for spawning (30–32, 68). Spawning takes place on redds constructed by the males using their oral discs, with females laying hundreds to thousands of eggs depending on the species (16, 28). In non-parasitic species of lamprey, feeding is by-passed following metamorphosis, with the mature adults remaining in fresh water, and then proceeding directly to spawning and death (15).
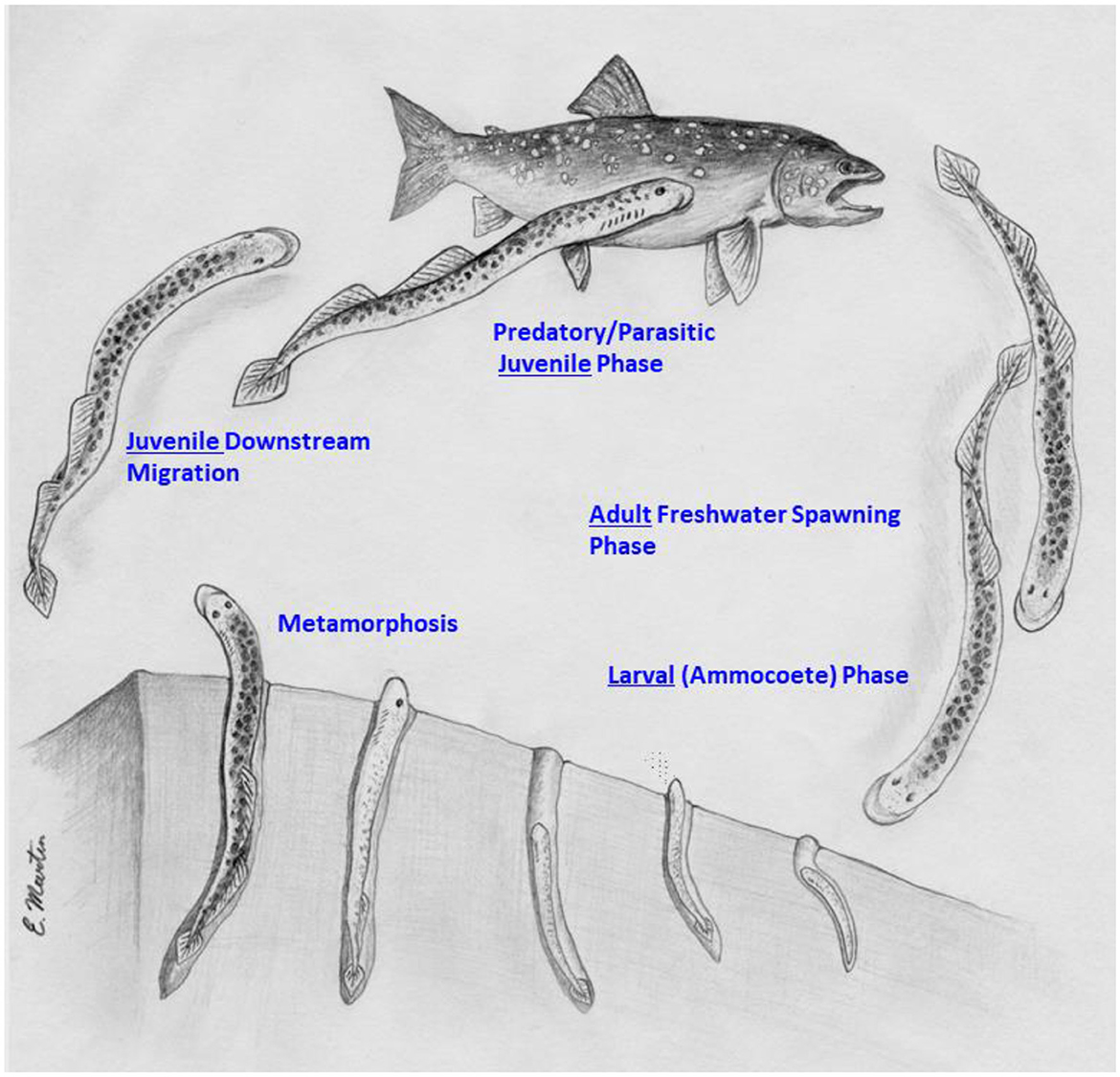
Figure 2. Life Cycle of the sea lamprey (Petromyzon marinus) in the Laurentian Great Lakes. As in all lampreys, the larval (ammocoete) phase of the sea lamprey life cycle typically lasts 3–7 years, followed by a 3–4 month period of metamorphosis. The “macrophthalmia” or juvenile phase takes place in the late fall or late winter-spring, after which the juvenile sea lamprey begin the parasitic, haematophagous (blood-feeding) phase of their life cycle, which typically last 12–18 months in the Great Lakes, and up to 2 years in marine environments, when the maturing adult sea lamprey stop feeding and begin their migration up freshwater streams before they spawn and die. Life stage categories follow the nomenclature proposed by Clemens (25). Attribution. Wilkie (114).
Invasion of the Great Lakes
Sea lamprey are native to the North Atlantic Ocean, with populations extending into the Baltic Sea and the Mediterranean Sea (see Hansen et al. (33) for review). Recent whole genome sequencing has uncovered three distinct populations of sea lamprey across the anadromous sea lamprey's native range: an East Atlantic/European population, a West Atlantic/North American population, and a Mediterranean population (211). The West Atlantic/North American population has a historical distribution ranging from northern Florida to Labrador, into the Gulf of St. Lawrence and upper St. Lawrence River, but populations in the most southern and northern ranges, and some extending further inland in the U.S., are now absent and possibly extinct (3, 33). Most likely, the sea lamprey that invaded the Great Lakes was from this population.
The invasion of novel habitat by an organism requires: (i) A vector by which the invader can reach the new territory, and it must have (ii) adequate propagule pressure, defined as the total number of animals and introductions taking place at an invasion site, and (iii) that the site has sufficient invasibility, making it susceptible to the establishment and spread of an introduced species (34–37). A conceptual framework incorporating these principles of invasion theory is provide in Figure 3.
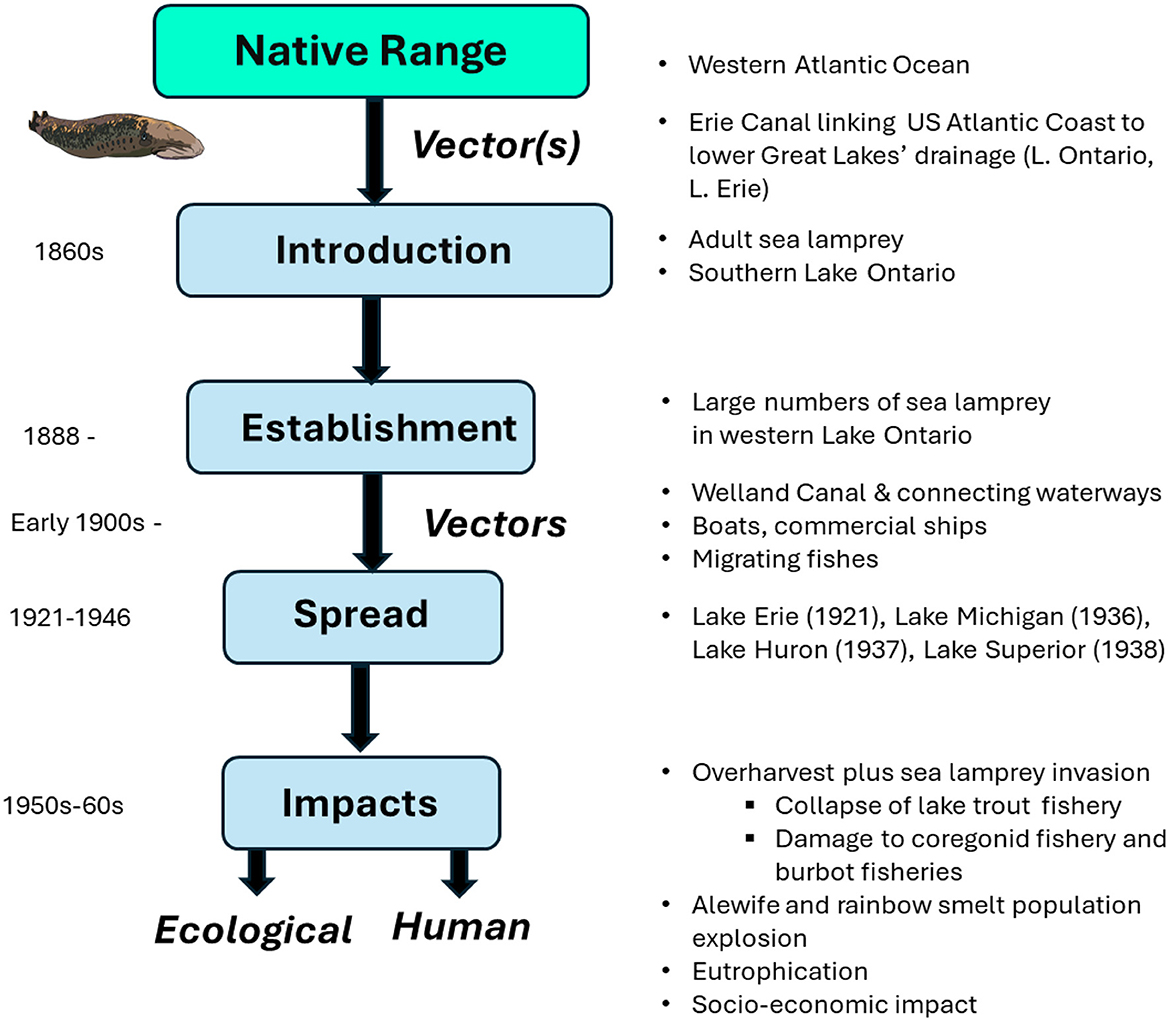
Figure 3. Conceptual framework of key events and timelines leading to the invasion of the Great Lakes by the sea lamprey (Petromyzon marinus). Sea lamprey are native to the western Atlantic Ocean. They may have been introduced into Lake Ontario via canals linking the US Atlantic Coast to the lower Great Lakes basin following the flooding of canals feeding water to the Erie Canal from rivers draining into the Atlantic Ocean. By the late 1890s, they had becoming established in Lake Ontario, and the spread via vectors including the Welland canal, allowing them to circumvent Niagara Falls and gain direct entry to Lake Erie, followed by the upper Great Lakes. The invasion had severe ecological and socio-economic impact. Combined with overharvest of commercial fishes, and the subsequent loss of top predators, populations of invasive alewife (Alosa pseudoharengus) and rainbow smelt (Osmerus mordax) exploded, leading to frequent mass die-offs, contributing to eutrophication of the lakes. Credit. - Adult sea lamprey drawing by M. Trzcinski, Wilfrid Laurier University.
Vector(s) of invasion
How sea lamprey got into Lake Ontario is still not completely resolved (2, 38–40). The first reliable reports of sea lamprey in Lake Ontario were in the 1880s, but Niagara Falls likely prevented them from entering Lake Erie via the Niagara River, the only natural waterway linking the two lakes (Figure 4A) (4, 41). Assuming the Lake Ontario populations of sea lamprey were due to an invasion event (but see below), the most likely vector(s) were the Erie Canal and the Susquehanna River (1, 2, 4, 39, 42). The original Erie Canal, completed in 1825, links Lake Erie to the Hudson River (Figure 4A), which was known to contain populations of anadromous sea lamprey in the 1800s (2). Although the Erie Canal lies south of and does not directly flow into Lake Ontario, its proximity in the lake's watershed likely led to the sea lamprey's eventual colonization (2). Eshenroder (2) proposed that sea lamprey may have been introduced into the Erie Canal via a diversion of the Hudson River, just west of the canal's starting point near Albany, New York (Figure 4A). From there, sea lamprey may have gained access to Lake Ontario via the Oswego River which emptied into the lake, via creeks connecting the river to the canal (Figure 4A). Tributaries of the Susquehanna River drainage, which flows into the Atlantic and were known to contain sea lamprey, may have also played a key role by introducing sea lamprey into the Lake Ontario drainage via feeder streams built in the early 1860s to supply water to the canal (2). This would have introduced sea lamprey into upstream tributaries of Oneida Lake, leading to its colonization, and subsequent migration of lamprey downstream into Lake Ontario via the Oswego River (Figure 4A). Downstream migrant juvenile sea lamprey may have also entered the Oswego via the Seneca River, which branches off the canal and drains into the river. The presence of sea lamprey in the more western reaches of the canal, due to the “breach” of the Susquehanna River, may have also attracted migratory adult sea lamprey from the Hudson River due to the secretion of migratory pheromones (discussed further below) by the earlier invaders (2). Within 2 decades of the “breach” of the Susquehanna system, sea lamprey were observed in Cayuga Lake (1875), Seneca Lake (1893), and Oneida Lake (1894), each of which eventually drain into Lake Ontario, along with high numbers of sea lamprey reported in the western end of that lake by 1888 (Figure 4A) (2, 43).
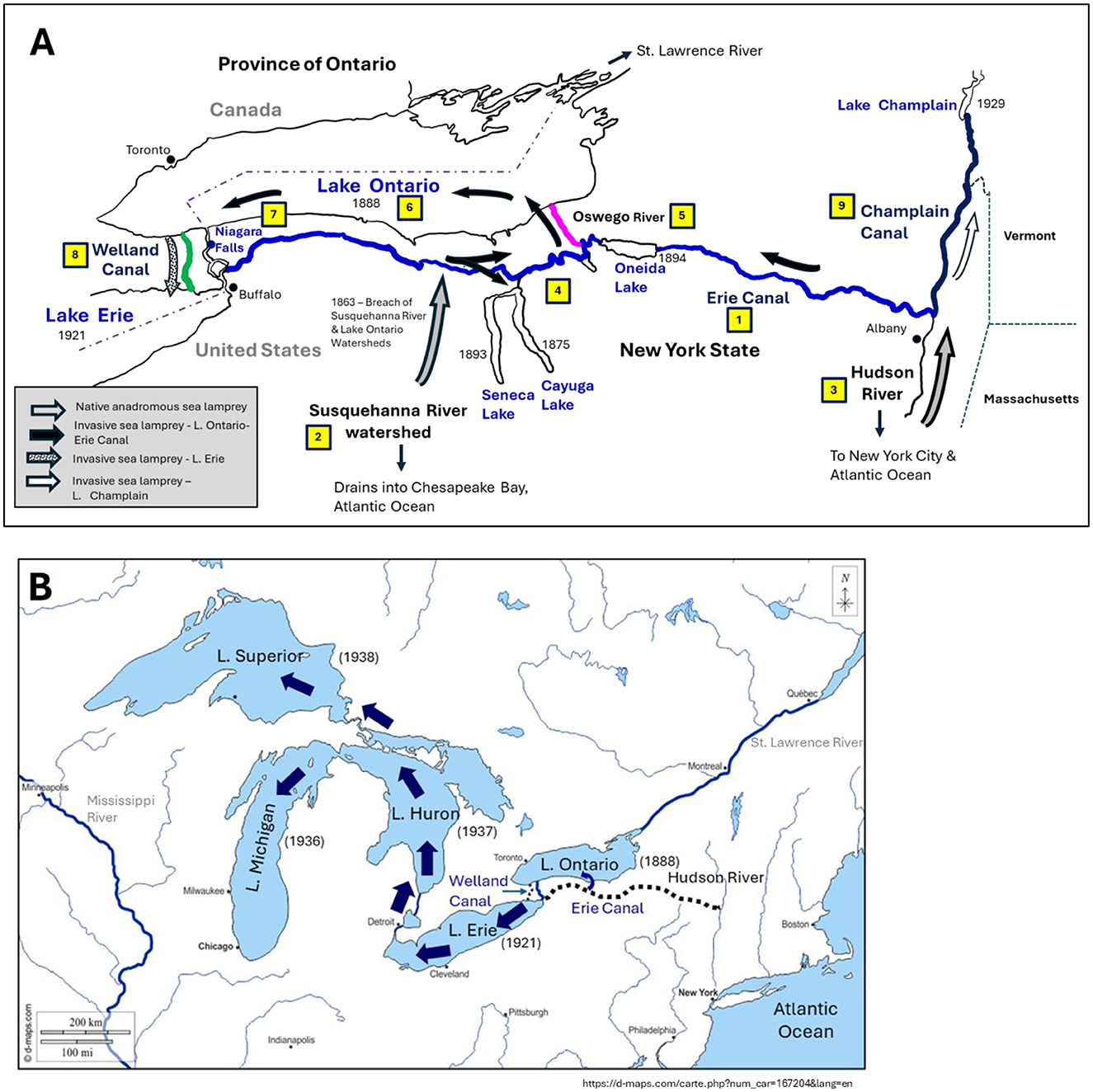
Figure 4. Proposed vectors and routes by which sea lamprey (Petromyzon marinus) invaded the Laurentian Great Lakes. (A) Anadromous sea lamprey are hypothesized to have accidentally entered the Lake Ontario watershed following the improvements to the Erie Canal (1; blue) in 1863, to which water was diverted from the Susquehanna River watershed (2), which drains into the Atlantic Ocean and was known to contain sea lamprey (2). At the same time, adult sea lamprey migrating up the Hudson River (3), could have been drawn into the Erie Canal by the improved flow in the canal, and perhaps by chemotaxes due to secretion of lamprey migratory pheromones upstream. Over the next two decades sea lamprey were observed in Seneca Lake, Cayuga Lake and Oneida Lake (4), which drain into Lake Ontario (6) via the Oswego River (5; magenta). Sea lamprey were observed in the western end of Lake Ontario by 1888, but Niagara Falls likely prevented them from entering Lake Erie via the Niagara River (7). Sea lamprey subsequently entered Lake Erie via the Welland canal (8; green), which by-passed Niagara Falls. Sea lamprey likely colonized Lake Champlain in New York-Vermont following construction of Champlain Canal (9; navy blue). (B) Sea lamprey were first reported in Lake Erie in 1921, followed by Lake's Huron (1937), Michigan (1936), and finally Lake Superior (1938). Numbers in (A) denote sequence of events. (A) Based on Eshendroder (2014). (B) Base map provided courtesy of d-maps.com. https://d-maps.com/carte.php?num_car=167218&lang=en.
Although this historical evidence suggests that sea lamprey was not present in Lake Ontario until the 1860s at the earliest, an alternative hypothesis is that sea lamprey are in fact native to its waters. Some of the argument is based on the unverified 1835 account of a sea lamprey in Duffin's Creek in what was then Upper Canada (now the province of Ontario) (44). Daniels (45) suggested that canal design, water flow and water quality was not conducive to sea lamprey migration via the Erie or other canal systems, instead arguing that sea lamprey were native to Lake Ontario, and were simply unnoticed for many years due to their small numbers in the lake. Subsequent genetic studies led to the hypothesis that sea lamprey may have been present in Lake Ontario since the end of the last glacial age (Wisconsian ~11,000 years ago), based on mitochondrial DNA and microsatellite markers which revealed significant differences in the allele and haplotype frequencies of Great Lake's sea lamprey compared to Atlantic coast populations, suggesting a long period of separation between the two populations (46, 47). However, if the initial invasion events involved relatively few individuals, the genomic variation between Great Lakes' and Atlantic' populations that were reported may have simply been a consequence of a genetic bottleneck due to low propagule pressure as suggested below (2, 48, 49). Given their high fecundity and voracious feeding habits, along with an abundance of large prey species including Atlantic salmon (Salmo salar) and lake trout (Salvelinus namaycush), it seems unlikely that the presence of sea lamprey in Lake Ontario, or the other smaller lakes, would have gone unnoticed as pointed out above. However, further population genetics studies using additional genetic markers and approaches are needed to provide more clarity on the sea lamprey's native vs. non-native status in Lake Ontario (38).
There is broad agreement that the dispersal of sea lamprey to Lake Erie was via the Welland Canal in the early twentieth century, which allowed sea lamprey to circumnavigate Niagara Falls (Figures 4A, B), probably while attached to the hulls of ships and/or onto migratory fishes while feeding (1, 2, 4, 38, 41). Sea lamprey were first reported in Lake Erie in 1921, from which they dispersed to the three upper Great Lakes. The first confirmed report of sea lamprey in Lake Michigan was in 1936, followed by Lake Huron in 1937, and finally Lake Superior in 1938 (Figure 4B) (4, 41). Today, sea lamprey occupy at least 500 streams draining into all 5 Great Lakes, plus populations in Lake Champlain, Lake Oneida and the Finger Lakes in upstate New York (Figure 4B) (50).
Propagule pressure
A route of introduction into Lake Ontario was clearly essential for sea lamprey in the incipient stages of the invasion. However, little is known about how sustained these colonization events were or how many animals were involved. One might reasonably surmise that propagule pressure would have had to be high and sustained over many years for sea lamprey to become established (34, 35, 49). Indeed, the long history of the Erie Canal and its proximity to the Lake Ontario drainage could have led to prolonged introductions of sea lamprey into Lake Ontario (1), but as noted above, this seems unlikely.
The sudden appearance of sea lamprey in Oneida Lake and two of the Finger Lakes (Seneca and Cayuga Lake), at around the same time as populations of sea lamprey were increasing in Lake Ontario in the 1880s, suggests that there was a sudden introduction of sea lamprey into these water bodies. As these lakes were well-studied and were commercially fished, it is unlikely that sea lamprey would not have been noticed beforehand (1, 2). This argues against any long-term, sustained propagule pressure. Similarly, the appearance of sea lamprey in nearby Lake Champlain did not likely occur until the early 1900s, following modifications to the Champlain Barge canal in 1916, which connected Lake Champlain to the Hudson River (Figure 4A) (2). Earlier accounts of sea lamprey in these waters could have been due to the misidentification of smaller silver lamprey (Ichthyomyzon unicuspis), which are also parasitic and native to these waters (2).
Docker and Potter (15) surmised that the odds of a few adult sea lamprey possessing a few alleles promoting freshwater survival, at low frequency, would have had little chance of establishment in a brief period of time (a few years or decades). This is true, but if anadromous juvenile sea lamprey already had the capacity to survive in fresh water, it could have facilitated the invasion even with low propagule pressure. Also, if we accept the “invasion by canal” scenario proposed by Eshenroder (1, 2), that the sea lamprey invasion of Lake Ontario was over a brief period and relatively recent, it seems unlikely that the propagule pressure would have been very high or sustained. However, the sea lamprey's high fecundity and ecophysiology may have pre-disposed them for survival in Lake Ontario and the Finger Lakes, making propagule pressure less important. However, high propagule pressure was likely key to the sea lamprey's rapid spread to Lake Erie and the upper Great Lakes.
Invasibility of the Great Lakes
Invasibility refers to the susceptibility of a habitat to the establishment and spread of a non-naive species (34, 35) and it is influenced by ecological filters, through which the introduced species must pass to become established in their new environment (51, 52, 71). Once the invading sea lamprey occupied Lake Ontario, their long-term persistence would have depended on their ability to navigate the ecological filters characteristic of the Great Lakes. Ecological filters can either be abiotic or biotic (51, 52). A key abiotic filter in the Great Lakes would have been the low ionic strength of the freshwater, to which juvenile sea lamprey would have had to tolerate as opposed to sea water, which would be their typical habitat (53, 54). Another would have been wide fluctuations in water temperature characteristic of Great Lakes' waters and their tributaries.
Suitable spawning habitat and nursery streams for larval sea lamprey would also have been essential, to provide food, ample oxygen, and protection from predators. Larval sea lamprey nursery habitat requires fine, silty (sandy) sediments to provide burrowing substrate, and organic detritus for food (“Type 1” substrate (20, 55)). They are typically found in moderate- to high-flow gradient streams, in pools and back-eddies with relatively slow flowing currents supplied with well-oxygenated water (55, 56). Older, larger larvae and metamorphosing animals tend to require coarser “type 2” substrate containing sand and some gravel (56, 57, 205). They tend not be found in hard-pack stream bottoms made of clay or bedrock [“Type 3” substrate (55)]. Upstream migrant adult lampreys, including sea lamprey, require rockier/gravel habitat in faster flowing and well-oxygenated water located upstream and immediately adjacent to larval habitat, with the cobble used for redd (nest) construction by males prior to spawning (58, 206).
Primary biotic filters would have been prey/host availability for juvenile sea lamprey, which was likely less varied in the Great Lakes than in marine habitats, where prey would not only have been more abundant but much larger (59). Major shifts in diet would not have been necessary for suspension feeding larval sea lamprey because detritus arising from allochthonous (land-derived) sources of decomposing plant and animal material would have been plentiful in the Great Lakes rivers and streams, as in Atlantic tributaries (20, 60). Predation by fishes on larval, juvenile, and adult lampreys, and competition with native lampreys for suitable habitat, could have also posed challenges, but burrows would have provided larval sea lamprey with the same degree of protection as in their native range. It is not coincidental that native lamprey populations are frequently found upstream of established larval sea lamprey populations (61).
To overcome Great Lakes' ecological filters, sea lamprey would have had to: (i) evolve in a similar environment to that of the invaded site, (ii) exhibit wide tolerance to environmental stressors, or (iii) have high genetic diversity and phenotypic plasticity (11). As described below, the primary factors behind its successful invasion of the Great Lakes may have been a combination of the sea lamprey's robust physiology and their life history, which would have precluded any need to undergo major genetic or phenotypic changes to thrive.
Life history
Many successful invasive species have short generation times, allowing them to adapt to new environments relatively quickly by natural selection (62, 63). However, the life history of sea lamprey typically spans 5–10 years, including the prolonged larval phase which typically lasts from 3 to 7 years depending upon a variety of abiotic and biotic variables including stream temperature, water quality, and productivity (64, 65, 212). However, the relatively slow growth of larval lampreys due to their low feeding rates and the poor nutritive value of their food (20, 21) is offset by the protection afforded by their burrowing lifestyle (66). The high fecundity of sea lamprey, which averages 70,000 eggs per female, compared to much smaller numbers in native American brook lamprey (Lethenteron appendix) and northern brook lamprey (Ichthyomyzon fossor), which average 19,000 and 1,200 eggs (67), respectively, would have likely offset the relatively low propagule pressure.
Another aspect of sea lamprey life history is that they do not home to natal streams, as do many other migratory fishes, most notably salmonids, and instead use a “most suitable” river strategy (32, 68). This non-philopatric nature of sea lamprey is likely related to their parasitic feeding habits in the juvenile stage in their native marine habitat whilst feeding or attached to large numerous species of migratory fishes such as salmonids, elasmobranchs, and even cetaceans (59, 69). Appropriate spawning habitat is located using a combination of behavioral and chemosensory methods providing the sea lamprey with coarse and fine-tuning methods to find the most suitable spawning habitat. As the sea lamprey mature they migrate toward the coastline, casting up and down in the water column and using a combination of barokinesis to sense changes in water pressure, and chemical odorants (“migratory pheromones”) to locate shorelines and suitable rivers for spawning (31, 70–72, 204). Meckley et al. (31), using radiotelemetry, determined that maturing adult sea lamprey used barokinesis to sense decreases in water pressure as water depth became shallower, allowing them to orient and migrate toward the shoreline. As they approach shorelines, they then rely on olfaction to sense migratory pheromones secreted by larval lampreys or by adult male sea lamprey to locate and select suitable rivers for spawning (30, 73, 207). Several olfactory compounds have been chemically characterized, with bile acids playing the primary role as migrating and mating pheromones, plus seminal secretions which also are important for female mate selection (30, 74, 75). In addition, chemical alarm cues including putrescine secreted by dead, dying or injured conspecifics and heterospecifc lampreys cause avoidance of affected streams (76). The olfactory system of sea lamprey and other lamprey species is highly developed, and capable of detecting certain bile salts in the picomolar (10−12 mol L−1) to femtomolar (10−15 mol L−1) range (77, 78).
Migrating adult sea lamprey are also sensitive to olfactory cues and migratory pheromones secreted by heterospecific lampreys (70, 79) including species native to the Great Lakes such as silver (Ichthyomyzon unicuspis), northern brook lamprey (Ichthyomyzon fossor) and American brook lamprey (Lethenteron appendix), which are widely distributed through the basin (80). This would have been critically important in the early stages of the invasion, as the secretion of migratory pheromones by native, heterospecific lampreys would have allowed early colonizing sea lamprey to identify prospective rivers and streams with suitable spawning and nursery habitat, in the absence of conspecific larvae (30). This conservation of a common suite of migratory pheromones likely reflects the common ancestry of modern lampreys, and a lack of selective pressure to develop more specific pheromones due the absence of any reproductive costs (70, 79, 81). Indeed, there are many examples of migratory parasitic lampreys and native, parasitic or non-parasitic lampreys co-existing in the same streams (61, 79). While the apparent lack of sea lamprey fidelity to migratory pheromones secreted by larvae lampreys may have contributed to their successful invasion of the Great Lakes, continued loss of larval habitat and the extirpation of native lamprey species elsewhere could potentially lead to a positive-feedback loop that undermines the ability of imperiled migratory lampreys to find suitable habitat for spawning (79).
Generalist diet and feeding
Generally considered vertebrate parasites or ectoparasites, juvenile sea lamprey might be more appropriately classified as predators, which is an “organism that consumes another organism”, and generally feeds on more than one organism whereas a true parasite typically spends its life cycle in a single host (82). Indeed, early laboratory studies suggested that a single, wild juvenile sea lamprey could kill up to 17 kg (~37 pounds) of fish during this life stage, underscoring its destructive effects in the Great Lakes (83).
In their native range in the Atlantic, juvenile sea lamprey have been reported to feed on numerous aquatic vertebrate species including teleost fishes and elasmobranchs (see reviews by Renaud and Cochran (59), Quintella et al. (69)). Indeed, sea lamprey have been reported to feed on numerous fine scaled and coarse scaled teleost fishes alike, and they can even penetrate the dermal denticle armor and feed on the blood of elasmobranchs (84–86). Several reports of sea lamprey feeding on cetaceans (59) are difficult to confirm, as they may be merely “hitching a ride”. Nevertheless, these examples highlight how the generalist diet of sea lampreys facilitated their invasion of the Great Lakes (11). In fresh water, parasitic juvenile sea lamprey have been reported to feed on at least 50 species of fishes, most notably top-predators found in the Great Lakes including lake trout, Atlantic salmon (S. salar), rainbow trout (Oncorhynchus mykiss), coho salmon (O. kisutch), chinook salmon (O. tshawytscha), various Coregonids including lake whitefish (Coregonus clupeaformis) and ciscoes (Coregonus sp.), northern pike (Esox lucius), muskellunge (E. masquinongy) and walleye (Sander vitreus), and fishes at lower trophic levels including white sucker (Catostomus commersonii), common carp (Cyprinus carpio), and lake sturgeon (Acipenser fulvescens) (59).
As sea lamprey populations exploded in the mid-twentieth century, particularly in the upper Great Lakes, the combination of sea lamprey predation and over-harvest exacerbated declining populations of top predatory fishes including the crash of lake trout populations in Lakes Superior and Michigan, and the extirpation of some species of ciscoes (4, 6, 8). However, the list of potential prey items is likely to expand, as we learn more about the diet of earlier stages of the juvenile phase, particularly just after metamorphosis (the macrophthalmia stage), when they are still very small (~5 g) and vulnerable to predation, and likely prefer small fishes in nearshore areas where the risk of predation is lower (33). The application of modern molecular methodologies such as DNA metabarcoding to examine the gut contents of juvenile lamprey in this elusive life stage could be particularly informative (87).
Being “haematophagous,” more than 98 % of the sea lamprey's diet is made up of blood (59). Adaptions for blood-feeding include its dagger like sub-lingual tongue and multi-toothed oral disc (Figure 1B), which equip sea lamprey with a versatile feeding apparatus to exploit a wide variety of large predatory fishes in the Great Lakes (88). Blood feeding is facilitated by buccal gland secretions, collectively referred to as lamphredin, which contains anti-coagulants and proteolytic enzymes, and also have anesthetic and immunosuppressive properties (89, 90). Recent studies on parasitized large male siscowet lake trout (S. namaycush siscowet; 1.9–4.1 kg) with penetrating wounds (termed Class A wounds) caused by sea lamprey feeding, revealed persistent changes in components of the clotting pathway that facilitated blood ingestion, including the inhibition of fibrin formation (91, 92).
Proteomic and metabolomic studies over the last 10–20 years have provided further insight into what bioactive components are present in lamphredin. These compounds include buccal gland secretory protein 1 (BGSP-1), and cathepsin-D, which inhibits the formation of the fibrin clot by promoting the breakdown of fibrinogen, its immediate precursor in the clotting cascade (93, 94). Lamphredin also contains SERPIN, which inhibits factor Xa in the coagulation cascade (90). Other components of lamphredin inhibit nociception by acting as anesthetics, cause immune system suppression, and various prostaglandins and kynurenines are hypothesized to cause smooth muscle relaxation to promote blood flow (93, 95).
Freshwater osmo- and ion-regulation by juvenile sea lamprey
Because juvenile sea lamprey are “hyper-adapted” to feed on the blood of fishes, it was a feature which allowed them to thrive following their invasion of the Great Lakes. However, in addition to nourishment and growth, feeding may have also played a critical role in facilitating osmoregulation in fresh water during the juvenile phase.
While sea lamprey in their native range spend the majority of their life in fresh water during their burrow-dwelling larval stages, metamorphosis, and then migrate up freshwater streams as adults to spawn, the parasitic juvenile phase is spent at sea where they remain for up to 2 years (33, 58). Hence, juvenile sea lamprey in the Great Lakes would have had to overcome the low ion content and osmolarity of freshwater not normally encountered during most of this life stage (96, 97). The possibility of sea lamprey rapidly evolving the ability to osmoregulate in FW seems remote given the likelihood of relatively low propagule pressure during the initial stages colonization of Lake Ontario in the 1860s [Eshenroder (2), Docker and Potter (15)—see above].
A more likely possibility was that the invasion of the Great Lakes was facilitated by juvenile sea lamprey that were pre-disposed to survive in freshwater (15, 53, 98, 99). In other words, sea lamprey might be considered “facultatively anadromous,” and capable of completing their entire life cycle in fresh water if necessary (54). Facultative anadromy has been reported in a number of teleost fishes including most notably brown trout (Salmo trutta), in which the decision to migrate to sea or remain in fresh water is based on genetic factors, physiological condition and factors such as food availability, predation risk, and environmental conditions (e.g., temperature, hydrological conditions) (100–103). Other anadromous fishes able to spend there entire life cycle in fresh water, include American shad (Alosa sapidissima), alewife (Alosa pseudoharengus), northern pike (Esox lucius), and salmonids such as freshwater dwelling sockeye salmon (Oncorhynchus nerka)—the kokanee, and Atlantic salmon (Salmo salar), not to mention numerous transplanted varieties of rainbow trout (O. mykiss), coho salmon (O. kisutch) and chinook salmon (O. tshawytscha) and other Pacific salmon found in the Great Lakes amongst other locations, but this is usually associated with lower sea water tolerance [e.g., (100, 104–106)]. An exhaustive literature review by Docker and Potter (15) revealed that there were no other instances of anadromous sea lamprey populations taking up permanent residence in fresh water, although there were instances of parasitic juvenile sea lamprey feeding in freshwater for several months. In the Great Lakes, however, migration back to sea water was not an option for introduced sea lamprey. In this context, the term facultative anadromy would apply from an eco-physiological perspective if it was a pre-existing phenotype.
Abundant food resources would have been important for juvenile sea lamprey to survive in fresh water, not only to fuel growth and development, but it would have helped to counter ion losses across the gill. Ingestion of blood from fishes would contribute to ionic and osmotic homeostasis, as both plasma osmolarity, and Na+ and Cl− concentrations would be comparable to concentrations found in juvenile sea lamprey (98, 107). Food ingestion has been shown to play an important, but often overlooked, contribution to ion homeostasis in fresh water fishes (208).
As juvenle sea lamprey fed, they would obviously become larger, resulting in a lower mass specific metabolic rate and lower O2 requirements (108). With lower respiratory demand, there would be less ventilatory water flow across the gill and less branchial blood perfusion, resulting in lower ion losses as the animals grew larger. The tight relationship between ion losses and metabolic rate, due to osmo-respiratory compromise (111) has been demonstrated in numerous teleost fishes (112), but has not yet been demonstrated in lampreys.
Gill mediated ion uptake and decreases in branchial permeability would have been critical for allowing sea lamprey to survive, indeed thrive, in the Great Lakes. A detailed description of gill-mediated ionoregulation and osmoregulation in lampreys is beyond the scope of this article, so I only focus on those processes that were likely critical for survival of juvenile sea lamprey in fresh water. Interested readers are referred to a recent and comprehensive review on this topic by Ferreira-Martins et al. (97). As hyper-osmoregulators in FW, the internal osmolarity and ion concentrations of sea lamprey body fluids are much higher than in their external environment, resulting in inwardly directed osmotic gradients favoring water uptake across the gills, and outwardly directed electrochemical gradients favoring the loss of Na+ and Cl− (96, 97). In fresh water, ion uptake at all stages of the lamprey life cycle is thought to be facilitated by the presence of intercalated ionocytes [also called mitochondria rich cells (MRC) or chloride cells], which are characterized by a complex tubular network arising from invagination of the basolateral membrane. These are similar to the FW ionocytes of teleosts, in which Na+ uptake is facilitated by Na+/K+-ATPase pumps (NKA pumps) located along on the basolateral membrane which maintain low intracellular Na+. This presumably facilitates Na+ uptake via an epithelial Na+ channel (ENaC), coupled to apically located H+-ATPase (aka. V-ATPase) which extrudes protons, thereby generating an inward electrochemical Na+ gradient across the apical membrane (96, 115, 116). A second type of freshwater ionocytes is found in larvae, ammocoete ionocytes (ammocoete MRC), which also express V-ATPases but lack the extensive tubular network and apical microvilli (96, 117, 209). The role of ammocoete ionocytes is not as clearly defined as intercalated cells, but recent evidence suggests that they might be involved in ion uptake and ammonia excretion (96). Of the two, the intercalated cells are probably most important for FW ionoregulation as they are retained so long as juvenile sea lamprey remain in FW but are lost following transfer to SW, before re-appearing during the adult FW migration stage (107, 115, 116).
As with anadromous teleosts, sea lamprey undergo “preparative adaptation” for sea water (54, 118) beginning around mid-metamorphosis (119). The process involves complete loss of ammocoete ionocytes, and lower numbers of intercalated cells, which are retained while juvenile lampreys remain in FW (119, 120). There is a massive increase in NKA mRNA expression, protein abundance and enzyme activity that begins during metamorphosis, peaking with SW exposure (119, 120). The subunit composition of the NKA, a pentameric protein, also changes with FW-SW transfer. In FW, subunits ATPA1 and ATP1B12 make up a FW NKA which is critical for Na+ absorption by intercalated cells, but in SW the ATP1B3 subunit predominates to facilitate Na+ excretion by the SW ionocytes (116, 121, 122). The SW ionocytes are arranged side-by-side in the interlamellar spaces of the gill, with an extensive network of basolateral infoldings and tubules, studded with numerous NKA pumps, resulting in intense fluorescence when examined using immunohistochemistry (96, 119, 120). Also located basolaterally are Na+/K+/2Cl− cotransporter proteins (NKCC1—aka. SLC12A2) by which the movement of Na+ into the ionocytes is accompanied by Cl−, which is thought to be excreted via a yet to be identified Cl− channel down its electrochemical (123).
Several studies have addressed SW tolerance in “landlocked” sea lamprey (53, 107, 124). Most recently, Norstog and McCormick (107) observed that the SW osmoregulatory capacity of landlocked juvenile sea lamprey was remarkably like that of anadromous populations (captured from Connecticut River). Unlike previous studies, landlocked juvenile sea lamprey from Lake Superior and Lake Champlain, as well anadromous populations, readily survived acute transfer to 30 ‰ salinity (~85% full strength SW), with the Lake Superior population also exhibiting 100 % survival in full strength SW (35‰). It was notable that mortalities were only experienced within the first 2 d of long-term (30 d) exposure, with the remainder of all populations tested surviving the full exposure period. In addition, the sea lamprey that had been held longest in captivity and had the highest mortality (40 %) were much smaller with significantly lower body masses (50 % of the Lake Superior lamprey) and lower Fulton condition factors than the other groups, suggesting that they may have lacked sufficient energy stores to cope with the additional demands of hyposmoregulation. This observation and previous observations (124) underscore the critical need (suggested above) for lampreys to feed following metamorphosis prior to entry into SW or for long-term survival in FW.
Juvenile sea lamprey also has the endocrine machinery in place needed for prolonged survival in freshwater. Gong et al. (122, 125) recently demonstrated that the hormonal control of FW acclimation in juvenile and adult sea lamprey involves a prolactin-like hormone (PRL-L), which is mainly produced in the pituitary gland, and triggers a switch in the subunit composition of the NKA from the SW subunit (ATP1B3) characteristic of SW ionocytes to the FW subunit type (ATP1A1; AP1B2). Prolactin plays a key role in FW osmoregulation in teleosts (126) which, in addition to regulating NKA expression, also plays a role in reducing gill ion and water permeability by changing tight junction composition and distribution (127). In juvenile sea lamprey, decreases in the mRNA expression of key claudins (cldn 10, cldn 14) are thought to increase gill ion permeability to facilitate Na+ excretion via paracellular pathways (128). However, the role of PRL-L in regulating branchial permeability via tight junctions remains to be determined. 11-deoxycortisol on the other hand, is upregulated upon exposure of freshwater acclimated sea lamprey to SW, demonstrating its dual role as mineralocorticoid and a glucocorticoid (129, 130).
The lack of (or subtle) differences detected by Norstog and McCormick (107) in SW osmoregulation amongst the Atlantic and landlocked populations of sea lamprey, their similar modes of ion regulation and the presence of SW ionocytes, and the high survival of some populations of sea lamprey (100 % Lake Superior) following FW-SW transfer lends support to the “facultative anadromy hypothesis” posed above, and by extension the “invasion by canal” hypothesis (2). Further support comes from observations of juvenile silver lamprey (Ichthyomyzon unicuspis), which are native to the Great Lakes, but do not express SW ionocytes (131). However, American brook lamprey, which are descendants of anadromous Arctic lamprey (Lethenteron camtschaticum) with a much shorter evolutionary history in FW than silver lamprey, have retained SW ionocytes, as demonstrated using transmission electron microscopy and immunohistochemistry (131, 132). Norstog and McCormick (107), have suggested that this retention of SW regulatory ability in landlocked sea lamprey could be due to “relaxed-selection” on traits required for saltwater survival. They suggested that such “relaxed selection” could be explained by the pleotropic effects of thyroid hormone and its many different physiological roles in sea lamprey, proposing that thyroid hormone could be acting as a brake on the loss of SW ionoregulatory capacity in Great Lakes' sea lamprey populations. Thyroid hormones plays a critical preparatory role for SW tolerance in salmonid fishes, and likely sea lamprey, and it is involved in triggering lamprey metamorphosis, along with regulating aspects of sea lamprey metabolism (26, 133).
The weight of evidence reviewed above suggests that juvenile sea lamprey had the gill machinery, and phenotypic flexibility to facilitate ion uptake and limit ion losses across the gill in FW. However, availability of suitable prey would have also been important to promote growth and supplement ion balance. These findings, along with their known capacity to feed on fishes in other FW environments for prolonged periods (15, 59), and that landlocked Great Lakes populations have equivalent capacity to tolerate SW (107), supports the hypothesis that “facultative anadromy” was key to their successful invasion of the Great Lakes.
Thermal physiology
The West Atlantic/North American population of sea lamprey (211) historically covers a wide range of thermal habitats from northern Florida to Newfoundland and Labrador (33). Seawater surface temperatures (SST) may range from just below 0 °C in the winter to the mid-teens in the summer in Atlantic Canada, whereas SST may range from 1 to 2 °C in the waters off the New England and Mid-Atlantic States up to the mid-twenties in the waters off northern Florida in summer (134, 135). As juvenile sea lamprey attach to large teleost fishes, elasmobranchs and marine mammals (59, 69), they may travel 100's or 1,000's of km in the Northwest Atlantic and be subjected to changes in temperature along latitudinal gradients, and depending on the habits of their prey, changes in water temperature with depth. Indeed, juvenile sea lamprey have been hauled from depths ranging from 100 m to more than 1 km, at which water temperatures ranged from −0.6 to ~4 °C (58). Thus, a robust thermal physiology for feeding juvenile sea lamprey, not to mention larval lamprey that occupy the rivers and streams draining into the Atlantic, would have been essential.
Larval sea lamprey occupy similar riverine ecological niches in the Great Lakes to those found in Atlantic Canada and the US Northeastern states, with similar thermal regimes in which water temperatures could be just above freezing in winter increasing to the mid- to high-teens and low twenties in summer (58, 80). Hence, temperature would have been an abiotic filter easily overcome in the Great Lakes' basin based on the sea lamprey's pre-existing thermal physiology. Indeed, spawning anadromous sea lamprey are known to migrate hundreds of kilometers inland via major Atlantic tributaries such as the St. Lawrence, Susquehanna, Potomac, Hudson, and Connecticut Rivers, with similar hydrological and thermal characteristic to the rivers draining into the Great Lakes (58).
Based on their preferred temperature, sea lamprey are considered “coldwater” guild fishes (preferred temperature < 19 °C) (136), but only a few studies have addressed sea lamprey thermal physiology. Laboratory thermal preference tests indicated that larval sea lamprey have a thermal niche falling between 17.8 and 21.8 °C (137), and many sea lamprey infested streams in the Great Lakes exceed 20 °C during the summer (64, 138, 139). Based on this, sea lamprey might be more appropriately classed as cold/cool water guild fishes (19–25 °C) (136). Nevertheless, thermal resilience would have been an important pre-existing physiological feature of larval sea lamprey given the spatial and temporal temperature variations that occur in rivers and streams in both Atlantic and Great Lakes' drainages. Indeed, annual variations in the surface temperature of the Great Lakes range from just above freezing in the spring, to just above 20 °C in Lake Superior and approximately 27 °C in Lake Ontario (140).
In general, ectotherms that are more physiologically resilient to changes in temperature tend to exhibit low thermal plasticity, with high thermal tolerance (141, 142). This appears to be true in sea lamprey based on the limited numbers of studies done. The upper incipient lethal temperature (UILT) of landlocked larval sea lamprey was determined to be 31.4 °C (Table 1), increasing very slightly, by 1.5 °C, between acclimation temperature of 5–25 °C, suggesting that sea lamprey exhibited low thermal plasticity (143). Thermal plasticity can be determined by calculating the acclimation response ratio (ARR), which reflects the change in thermal tolerance [e.g., UILT; critical thermal maxima (CTmax)] or performance (e.g., aerobic metabolic scope) per unit change in acclimation temperature (142, 144). An ARR of 1.0 would indicate an increase in thermal performance (or tolerance) of 1 °C for each 1 °C change in acclimation temperature, whereas an ARR of zero would indicate no effect of prior thermal acclimation and no thermal plasticity (Table 1). Based on their UILT's, the sea lamprey's very low ARR of 0.075, is consistent with very low thermal plasticity (143).
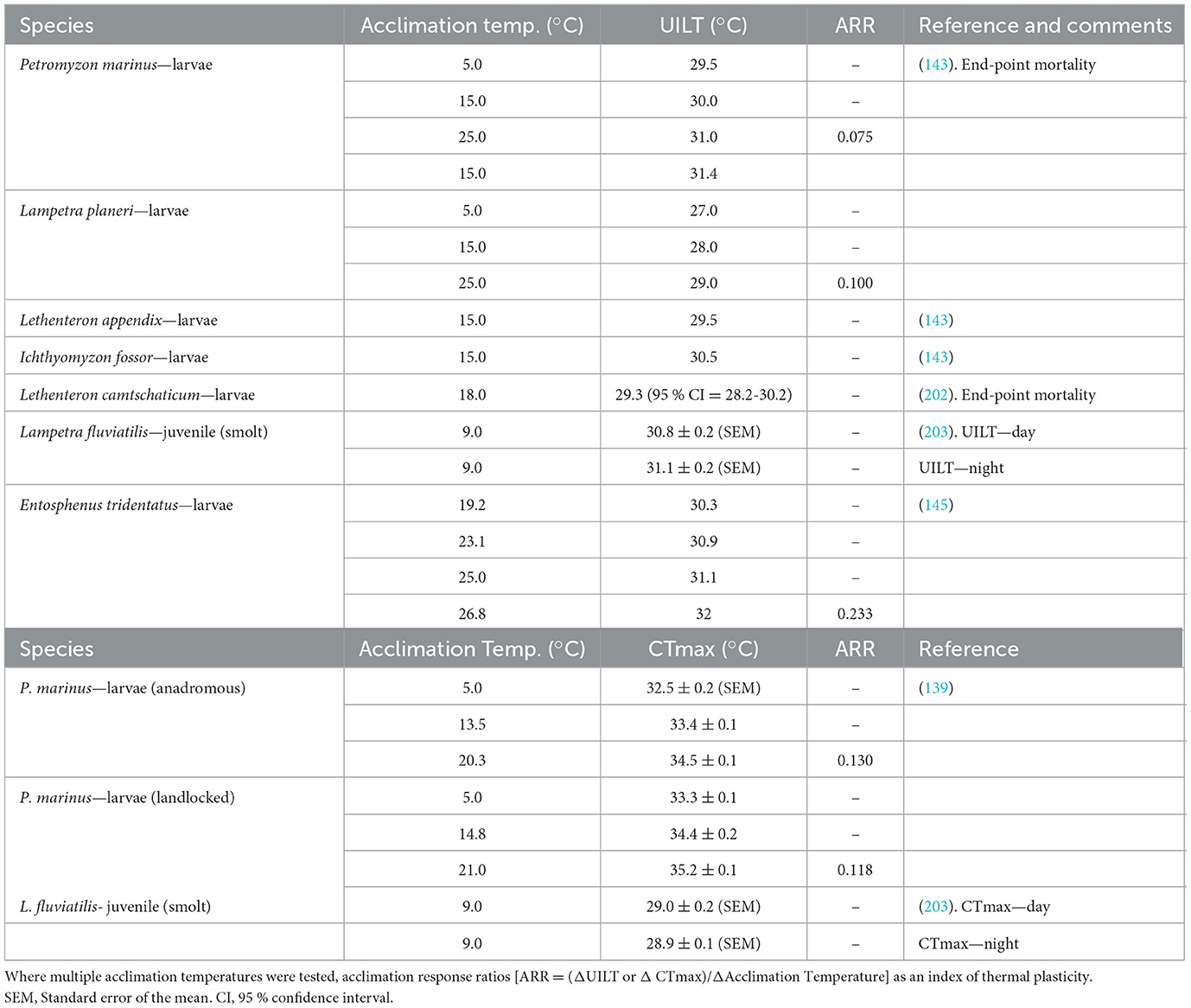
Table 1. Upper thermal tolerance limits of different species of lamprey expressed as either Upper Incipient Lethal Temperature (UILT) or Critical Thermal Maxima (CTmax).
These UILTs, calculated by Potter and Beamish (143), are relatively close to the sea lamprey CTmax, which is commonly used to calculate the upper thermal tolerance of ectotherms (142). Although there are few published CTmax values for lampreys, it is notable that the CTmax of anadromous populations of larval sea lamprey (collected from the Richibucto River, New Brunswick) are very similar to those landlocked populations from Great Lakes, with respective values of 33.4 and 34.4 °C at similar acclimation temperatures (13.5 and 15 °C, respectively) (139). This lack of variation in the CTmax also suggests a lack of divergence in the thermal tolerance of the two populations. Calculations of ARR yielded values of 0.12 for both the landlocked and anadromous populations, underscoring the lack of thermal plasticity in sea lamprey (139). The limited data acquired so far suggests that low thermal plasticity might be characteristic of many lamprey species, which all have upper thermal limits (CTmax or UILT) in the low thirty-degree Celsius range. Once exception appears to be the Pacific lamprey (Entosphenus tridentatus), which has an ARR falling near 0.23 (145) (Table 1).
This lack of thermal plasticity in larval sea lamprey is also reflected by the very high thermal thresholds needed to induce heat shock protein expression. Respective increases in water temperature of approximately 13–16 °C for sea lamprey, and 16–20 °C for American brook lamprey, were required to induce HSP70 and HSP90 expression in the gills (146), whereas 15–20 °C increases were needed to induce these HSPs in other tissues (liver, kidney, intestine) of sea lamprey (147). Given the very low metabolic rate of larval lamprey (110) and their limited rates of energy acquisition via feeding, one interpretation could be that such “emergency” measures (147) might be too costly to invoke on a regular basis in their thermally volatile habitats.
Less is known about the thermal physiology of juvenile and migrating adult sea lamprey, but further insight on their molecular, physiological and behavioral responses to changes in temperature could be very informative for predicting how climate change induced increases in water temperature affect sea lamprey population and distribution through the Great Lakes, and the risk of invasion into other water bodies in North America.
It remains an open question how similar the thermal physiology of these sea lamprey populations are to East Atlantic and Mediterranean populations of sea lamprey, where water temperature regimes are markedly different, particular in and around the Iberian Peninsula, where larval lamprey seldom experience temperatures less than 8 °C (33). Answering such questions could be critically important for the Mediterranean and Eastern Atlantic populations, which are threatened or endangered through much of the range (3, 148). Hence, better characterization of the thermal ecophysiology of imperiled sea lamprey populations will be essential for predicting how they will respond to climate change, habitat degradation, and further fragmentation of spawning migration routes and rearing habitat (33, 88, 145, 148, 149).
Sea lamprey control
Though the focus of this review is on the ecophysiology of the sea lamprey, the highly successful sea lamprey control program (SLCP) in the Laurentian Great Lakes and surrounding waters merits mention as one of the most successful invasive species control programs in the world (7, 8). The program integrates chemical control (pesticides) of larval sea lamprey populations using lampricides with barriers to block the upstream migration of adult, spawning lamprey, with various supplemental control methods including sterile male release to suppress egg fertilization, and more recently, chemical attractants and repellants to lure lamprey into traps. The SLCP has proven to be highly effective at suppressing Great Lake's sea lamprey population by more than 90 % from their peak levels in mid-twentieth century (Figure 5A) (8, 150, 151). The methods employed in the SLCP are based on a growing knowledge base of sea lamprey ecophysiology (8, 152). For instance, barriers not only deny adult sea lamprey access to spawning habitat, traps are also essential for assessment of Great Lakes' sea lamprey populations and where to direct SLCP efforts using lampricides (10, 57, 150). Chemical control using lampricides takes advantage of the relatively sessile larval stage of lamprey and knowledge of when they are most likely to undergo metamorphosis, thereby restricting treatments to times where the greatest numbers of sea lamprey in a stream are most likely to complete metamorphosis (151, 153). This approach maximizes treatment impact by killing multiple generations of larval sea lamprey, allowing lampricide treatments to take place over a typical cycle of every 3–4 years, optimizing human and financial resources (151, 153).
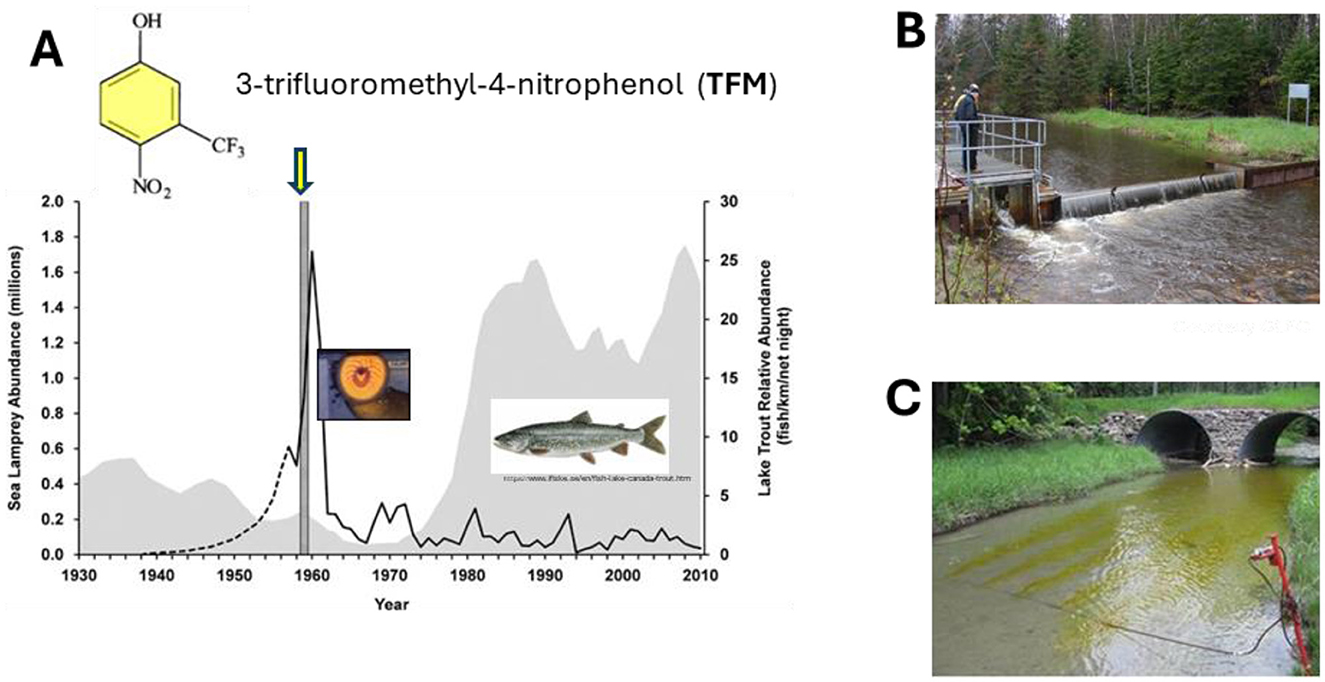
Figure 5. Sea lamprey control in the Great Lakes. (A) Changes in relative lake trout (Salvelinus namaycush) and sea lamprey (Petromyzon marinus) abundance from 1930 to 2010 in Lake Superior before and after population control measures were initiated using 3-trifluoromethyl-4-nitrophenol (TFM; yellow arrow) in 1959 (Modified from M.J. Siefkes. 2017. https://creativecommons.org/licenses/by/4.0/). (B) Low crest sea lamprey barrier (Photo by M. Moriarty, US Fish and Wildlife Service. Courtesy Great Lakes Fishery Commission). (C) Lampricide application to Duffin's Creek, Ontario (Photo by O. Birceanu. Wilfrid Laurier University. With permission).
The oral disc of juvenile and adult lampreys does not only serve as a means to attach to fishes during feeding, it is used by males to move stones when constructing redds for egg deposition and incubation following spawning, and for locomotion, including allowing them to maintain position when navigating in fast flowing waters and for climbing during migration (88, 154, 155). To prevent sea lamprey from crawling up and over barriers, many (~40 %) are equipped with overhanging lips (Figure 5B) (10, 156). Some barriers are also paired with traps, which not only block upstream migration but are used to quantify the relative number of spawning adults in the Great Lakes to estimate lake wide populations of sea lamprey, to estimate damage to fisheries, and where to target sea lamprey control efforts using chemical control methods (157).
Chemical control with lampricides is also based on prior assessment of larval sea lamprey populations and stage of development by biologists from the US Fish and Wildlife Service and Fisheries and Oceans Canada (4, 151, 157). The primary lampricide, 3-trifluoromethyl-4-nitrophenol (TFM) targets larval sea lamprey, which are more susceptible to the chemical than most non-target fishes due to their relatively low capacity to detoxify the compound (Figure 5C) (13). TFM is a phenolic compound which is detoxified in the liver using phase 2 biotransformation in which the compound is made more water soluble via the processes of glucuronidation and sulfation, making it less toxic and easier to excrete (158–161). Phase 1 processes involving cytochrome P450 enzymes may also play a minor role in TFM metabolism (158, 159, 162, 163). This lower capacity of larval lamprey to use glucuronidation is thought to be due to a lack of the enzyme UDP-glucuronosyltransferase (UGT), which is needed for glucuronidation of TFM (160).
Recent transcriptomics work using RNA sequencing suggests that sea lamprey only express genes of one UGT family, the UGT-2 family, which expresses four isoforms of the enzyme, whereas the highly TFM tolerant bluegill (Lepomis macrochirus) has genes coding for three families and seven different isoforms of UGTs, including the UGT-1 family which is thought to be involved in the detoxification of phenolic compounds such as TFM (162, 163). The UGTs are thought to have evolved to counter the phytochemical defenses of plants against herbivory (164–166). Given the hypercarnivorous diet of blood and tissue ingested by ancestral and modern parasitic lampreys, it may be that there was less selective pressure to evolve or retain these enzymes (152). Indeed, the abundance and diversity of UGTs is the lowest amongst hypercarnivorous mammals such as wild and domestic cats, hyenas and sea lions, compared to mesocarnivorous and omnivorous animals such as humans, some species of bears and canines, which have 9–12 functional isoforms of UGT-1 (165, 166).
Applications of TFM are often supplemented by the addition of niclosamide (0.5–2% of the TFM concentration), which interacts with TFM to increase its toxicity (151, 167). Co-application of TFM and niclosamide leads to greater accumulation of TFM in the liver of larval sea lamprey compared to TFM alone, suggesting that niclosamide impairs TFM detoxification (168).
Research is ongoing to develop alternate methods of sea lamprey control due to concerns about the possible evolution of lampricide resistance (62, 169) including the development of more environmentally friendly lampricides and genetic control methods to help counter this risk (113, 170, 171). However, lampricides will continue to be an important aspect of sea lamprey control for the foreseeable future until such technologies are developed, tested and validated. The need to continue using lampricides was recently demonstrated during the recent COVID-19 lockdowns which restricted travel and lampricide applications in 2020–21, resulting in an upwards surge in the Great Lakes' adult sea lamprey populations, particularly in Lakes Huron and Ontario in 2023 (172). With the resumption of lampricide treatments, sea lamprey populations have been supressed to near or below target levels, underscoring the critical importance of sea lamprey control for the ongoing protection of the Great Lakes' ecosystem (Figure 6) (9). However, an imminent, ongoing threat to sea lamprey control is climate change (14, 173).
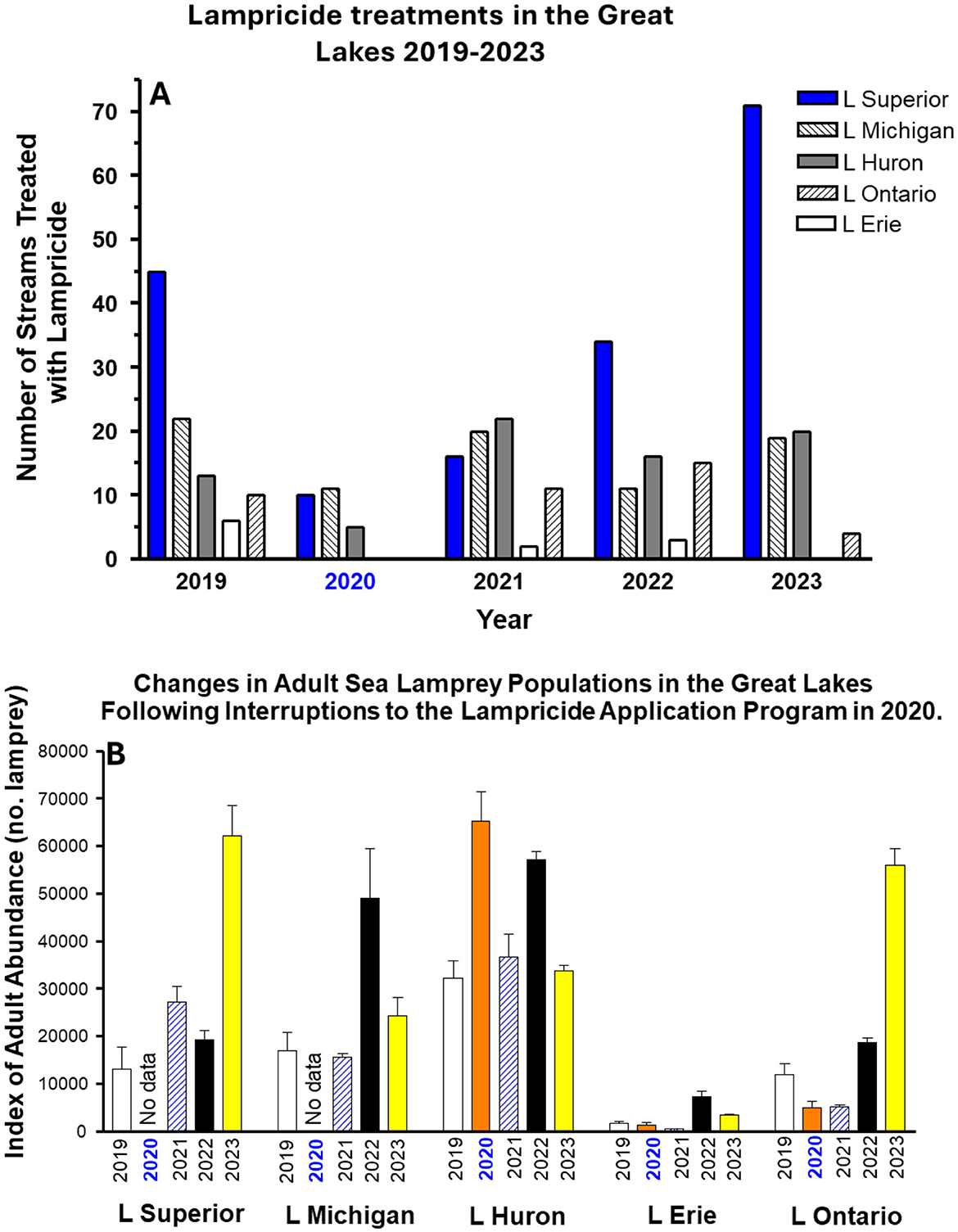
Figure 6. Effects of reduced lampricide application on adult sea lamprey populations in the Great Lakes 2019–2023. (A) Total lampricide (TFM, niclosamide) applications to each of the Great Lakes by year, between 2019 and 2023, and (B) corresponding estimated populations of adult sea lamprey in each of the lakes. Note the corresponding increases in adult abundance in 2022 and 2023 in Lakes Superior, Michigan, Erie and Ontario following reductions in TFM applications in 2020 and 2021. Data compiled from Sea Lamprey Control in the Great Lakes—Annual Report to the Great Lakes Fishery Commission (2019–2023), with permission. https://www.glfc.org/pubs/slcp/annual_reports/.
Impacts of climate change
The impacts of climate change on sea lamprey control efforts in the Great Lakes was comprehensively reviewed by Lennox et al. (14), Hume et al. (3), and Wang et al. (173). In the interest of brevity, I will only focus on how climate change could impact sea lamprey populations in the Great Lakes in the context of their ecophysiology.
Impacts of climate change on the Great Lakes Basin
Depending on the CO2 emissions scenario (high, moderate, low), average air temperatures in the Great Lakes basin are expected to rise by 1–3 °C by 2050 and 1.5–7 °C by 2100, with more severe elevations during heat waves (174, 175). With increasing air temperatures, lake, river and stream temperatures will also rise (176, 177), affecting fish and invertebrate populations and their distributions, with corresponding impacts on invasive species including sea lamprey (176, 178, 179).
With warmer temperatures there will be less ice-cover of the Great Lakes, with later ice formation in winter and earlier ice breakup in the spring, or near absence of ice cover (175, 178). This will lead to greater light penetration and decreased risk of hypoxia in winter, but increased risk of hypoxia due to more thermal stratification in summer, along with eutrophication (175, 180).
More frequent and intense storm events are also expected in the Great Lakes basin, with additional, more prolonged droughts in the summer, which could lead to large scale variation in discharge from rivers and streams (175, 181). Warmer temperatures are also conducive to greater eutrophication of surface waters including increased overall productivity of Great Lakes rivers and streams, in addition to more frequent algal blooms in lakes (175).
Another consequence of climate change could be freshwater acidification, as higher partial pressures of atmospheric CO2 equilibrate with surface waters, particularly in the lakes in which water pH is projected to drop by as much as 0.3–0.5 pH units by the end of the century (182). Rivers, streams, and small lakes are likely to be impacted less because their watershed to water surface area is greater than the much larger Great Lakes. As a result, smaller water bodies, rivers and streams would receive greater relative amounts of run-off and nutrient loading than large lakes and therefore changes in water chemistry arising from factors other than atmospheric CO2 (182). Lake Superior and its tributaries, which sit-atop the granite bedrock of Canadian Shield are more likely to be vulnerable to greater atmospheric CO2 due to its lower buffering capacity relative to the other Great Lakes, which are underlain by limestone comprised of CaCO3, providing an abundant source of and to buffer against CO2-induced decreases in water pH (182). Input of allochthonous-derived carbon will also be higher in rivers and streams, further buffering pH in these systems. It should also be kept in mind that temperature, pH, O2, and CO2 undergo marked temporal and spatial fluctuations in riverine systems due to photosynthesis by macrophytes and phytoplankton, changes in water flow and level, acidic precipitation, groundwater inputs, run-off and air temperature (183).
Larval sea lamprey and metamorphosis
Conventional wisdom and past observations suggest that warmer temperatures could result in faster growth of larval sea lamprey, leading to metamorphosis at earlier ages (3, 14). This would also depend on stream productivity, which would affect food availability, which varies widely amongst Great Lakes tributaries (65, 184, 185). Metamorphosis depends on the accumulation of sufficient lipid reserves to provide the larval sea lamprey with energy through the protracted (3–4 month) metamorphosis period, when the animals do not feed (186–188). In the Great Lakes, sea lamprey metamorphosis typically takes place when the larvae achieve a minimum length and mass of 120 mm and 3.0 g, respectively, with a corresponding Fulton's Condition Factor ≥1.5 (189, 212). Provided these conditions of body mass and lipid reserves are met, sea lamprey would be expected to enter metamorphosis at an earlier age as they grow faster as stream temperatures increase.
However, there would be an upper temperature limit to improved growth. Sutton and Bowen (20) reported that the feeding rate of larval sea lamprey peaked at 9–10 °C, with assimilation rates peaking between 16 and 17 °C. This may explain why larval sea lamprey growth performance was lower in larva reared at 22 °C compared to 15 °C in recent laboratory studies examining the effects of temperature on sea lamprey growth (190). In the latter study, larvae were fed comparable amounts of yeast, or yeast plus fish meal for 90 d, leading to greater lengths in the animals at 22 °C compared to the two lower temperatures, but there was no difference in weight gain between 15 °C and 22 °C, and CF was actually lower at 22 °C (190), suggesting that the accumulation of energy reserves (e.g., lipid and/or protein) was lower at the warmer temperature. Notably, 22 °C falls just above the thermal niche of larval sea lamprey (17.8–21.8 °C) (137).
The incidence and pace of metamorphosis could also be reduced if temperatures are greater than 25 °C, which is above the optimal temperature of 21 °C for metamorphosis to take place (191). This could lead to reduced rates of metamorphosis in the lower Great Lakes, where stream temperatures would be expected to be much warmer than those of the Upper Great Lakes. Although sea lamprey can withstand warmer temperatures, it seems doubtful that they would thrive at temperatures beyond their thermal niche (137). Dawson and Jones (64), comparing larval recruitment in two Lake Superior and two Lake Ontario streams, found that recruitment was lower in the one stream where water temperature fell above the known thermal niche of larval sea lamprey (137). It would be highly informative to expand on these studies to generate a deeper understanding of what the optimal temperature range is for lamprey metamorphosis to take place, which would better inform predictions of how they will respond to climate warming.
A lack of thermal plasticity (see above) could also undermine the viability of larval sea lamprey populations in the lower Great Lakes, which are expected to reach higher temperatures due to their lower latitude, volume and depth (177). Although their relatively high CTmax (32–34 °C) (139) and IULT (31.4 °C) (143) would allow larval sea lamprey to withstand occasional temperature pulses into the high 20s, it is doubtful if survival could be sustained for more than several days. It would also be costly, as standard metabolic rate would be expected to increase markedly at such temperatures, and the animals' thermal safety margin (TMS), the difference between their upper thermal tolerance (e.g., CTmax or IULT) minus environmental temperature, would be much lower as the climate warms. There is clearly a need for longer-term thermal performance studies that use relevant performance metrics such as metabolic rate, feeding and growth rates (192, 193) to better understand and predict how larval sea lamprey will respond to climate change-induced increases in water temperature.
The low thermal plasticity seen in larval lamprey based on their very low ARRs, not to mention the similar CTmax values of Atlantic vs. Great Lakes lamprey populations (139), suggests it is unlikely that there will be natural selection for higher thermal tolerance. Indeed, CTmax and IULTs are remarkably similar across many species of parasitic and non-parasitic species of lampreys (Table 1).
Parasitic juvenile and adult sea lamprey
Nearshore areas in all lakes are expected to be warmer in mid-summer (194). Warmer water temperatures in these areas could result in faster growth and increased feeding by juvenile sea lamprey the first few months following their downstream migration (89, 195). However, little is known about the thermal preferences of juvenile sea lamprey, or where they would initially forage, which is needed to better predict how they would respond to climate change in nearshore areas in the initial stages of this life stage. Decreased ice cover, including later freezing and earlier break up of lake ice, would also extend the effective growing season for juvenile sea lamprey (196). For instance, mean annual surface water temperatures in Lake Superior increased by 4–5 °C between 1960 and 2006, which was accompanied by a 25 % increase in the average body mass of adult sea lamprey (196). Similar warming trends in the other Great Lakes would therefore likely correspond to larger, more fecund adult females and greater impact on fisheries due to greater blood consumption over a longer period.
With a greater number of more intense storms in the basin due to climate change (175), particularly in the spring, there would also be greater risk of barrier and trap inundation (overflow), which would allow upstream migrant sea lamprey to circumvent barriers and potentially occupy new territory (3, 14). Hume et al. (3) also suggested that such high flow events could dilute sea lamprey chemical attractants, leading migrating sea lamprey to explore and potentially colonize new habitat. Together, with more favorable temperature conditions for larval growth in the upper Great Lakes compared to the lower lakes, sea lamprey populations could shift northward into the deeper, higher volume upper Great Lakes (3, 173). However, this would depend upon the presence of suitable larval rearing habitat, which are abundant in Lake Michigan and Lake Huron. The lower productivity of Lake Superior tributaries (65, 184) might constrain larval growth resulting in longer times to metamorphosis, but the total number of suitable streams for both larvae and spawning lamprey would likely offset any constraints on sea lamprey and population growth.
Impacts on sea lamprey control
Climate change could undermine efforts to keep sea lamprey populations at or below target levels in the Great Lakes by altering both their phenology and physiology (3, 14, 173). Higher growth rates at warmer temperatures leading to increased larval lamprey size could increase survival during lampricide applications due to the inverse relationship between TFM uptake and body mass (109). With higher growth rates, the yearly intervals over which sea lamprey metamorphosis occurs could be accelerated, requiring more frequent lampricide applications.
In recent years, evidence has accumulated that TFM sensitivity varies seasonally, with tolerance increasing through the summer as water temperatures increase (138, 197–199). This could potentially result in higher numbers of “residual” larvae that survive lampricide applications and then eventually metamorphose into juveniles and cause greater damage to fisheries. A need to use higher concentrations of TFM later in the summer compared to the spring could also result in greater risk of toxicity to non-target organisms. Although non-target fishes are generally much more tolerant to TFM, we know little of how their sensitivity to TFM will be affected at warmer temperatures. A need to increase lampricide consumption would also require greater financial and human resources. Shifting more treatments to the spring, when waters are cooler and sea lamprey more vulnerable to TFM, would be complicated by high water flows due to spring melt, but could conserve TFM in some instances depending on the hydrology of the stream. An increased frequency of hydrological disturbances due to storms or drought could also undermine treatment if water levels are too high or too low to apply lampricide effectively (210).
Lower pH due to increasing amounts of atmospheric dissolved CO2 in the water would likely have little direct impact on lampricide operations. Although lower pH makes TFM more bioavailable, by altering TFM's chemical speciation (13, 153), changes in dissolved CO2 in river and stream waters are likely to have less effect than allochthonous inputs of carbon and local geology on water pH, which would have greater effects on stream pH (182). Also, against the backdrop of regular daily and seasonal fluctuations in pH, CO2 and O2 due to biological processes [e.g., photosynthesis and respiration (183)] the effect of climate change-induced changes in water pH on lampricide effectivness would be minimal. It is also important to note that control agents continually monitor pH and adjust TFM application rates to match changes in water pH during lampricide treatments (151). Less is known about how climate change would affect niclosamide, which is often co-applied in lesser amounts (0.5–2.0 %) with TFM to increase its toxicity to sea lamprey but not non-target fishes (167).
The inundation of barriers and traps during extreme hydrological events is a clear and present danger in the Great Lakes and will be exacerbated with climate change. As noted, overflowing barriers could open-up novel habitat to upstream migrants, leading to spawning and subsequent colonization by larval sea lamprey. In response, additional resource would need to be invested to treat newly infested reaches of streams, which could potentially result in hundreds of additional kilometers requiring TFM applications, particularly in the upper Great Lakes.
The runs of spawning adult sea lamprey would likely take place earlier in the spring and be more prolonged with earlier ice out and warming (14). Adult sea lamprey typically congregate at the mouths of rivers and streams following ice-out and in response to migratory and sex pheromones. Their upstream migration begins when water temperatures reach 4–7 °C, with runs peaking as water temperatures approach 12 °C (200, 201). With climate change, river mouths are likely to reach these temperatures sooner, resulting in earlier spawning runs. This could potentially compromise SLC in two ways. First, it may be necessary to deploy temporary barriers or traps earlier in the year to prevent early maturing sea lamprey from migrating upstream to spawn. Second, if traps are not staffed and deployed before runs start, it could undermine adult collection and monitoring efforts, skewing adult index calculations of Great Lakes' sea lamprey populations, and to develop SLC treatment strategies (3, 14, 173).
Ultimately, research and investments in alternative methods of sea lamprey control will be necessary to mitigate the effects of climate change on sea lamprey control. The development of genetic control technologies such as daughterless technology or more environmentally friendly and sea lamprey specific lampricides will also be critically important in an era of increasing concern from the public and Indigenous communities about lampricides, and further restrictions on the use of pesticides in the environment (7, 97, 113, 170). Improved barrier design and construction, including more environmentally benign barriers that allow selective fish passage or more temporary barriers, that are more effective at blocking upstream migrant adult sea lamprey but with use restricted to the spring, will also be important (156).
Conclusions
The weight of evidence suggests that sea lamprey were introduced into the Great Lakes basin in the 1860s, but were not detected in Lake Ontario until the 1880s, likely due to low initial propagule pressure. The vector for the invasion was likely a breach of canals used to feed water into the Erie Canal, which connected Lake Erie to the Atlantic coast of the United States, followed by the Welland Canal which allowed sea lamprey to by-pass Niagara Falls and gain access to Lake Erie, and the upper Great Lakes. Sea lamprey were likely able to overcome abiotic and biotic filters posed by the lakes using pre-existing adaptations including facultative anadromy, which allowed them to spend the juvenile, predatory (parasitic) phase of their life cycle in fresh water; a generalist diet that allowed them to take advantage of the abundance of large salmonid and coregonid fishes in the Great Lakes; and a resilient thermal physiology. The robust thermal physiology of sea lamprey is likely to make them resilient to short-term elevations in water temperature, but questions remain about their long-term ability to survive in the watersheds of the Lower Great Lakes at higher temperatures, which could squeeze sea lamprey populations northward with climate change. Increased growth rates, body sizes and female fecundity due to warming waters in the upper Great Lakes may also result in greater damage to fisheries, without additional sea lamprey control measures. Continued research and development of alternate, innovative approaches for sea lamprey control will be needed to ensure that sea lamprey populations are kept under control in the face of climate change related elevations in water temperature, greater frequency and intensity of storms and hydrological disturbances, and changes in stream and lake productivity and water chemistry.
Author contributions
MW: Conceptualization, Investigation, Writing – original draft, Writing – review & editing.
Funding
The author declares that no financial support was received for the research and/or publication of this article.
Acknowledgments
The author wishes to acknowledge the support of the Great Lakes Fishery Commission for their financial assistance with the publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author declares that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Eshenroder RL. Comment: Mitochondrial DNA analysis indicates sea lampreys are indigenous to Lake Ontario. Transac Am Fisher Soc. (2009) 138:1178–89. doi: 10.1577/T08-035.1
2. Eshenroder RL. The role of the champlain canal and erie canal as putative corridors for colonization of Lake Champlain and Lake Ontario by Sea Lampreys. Transac Am Fisher Soc. (2014) 143:634–49. doi: 10.1080/00028487.2013.879818
3. Hume JB, Almeida PR, Buckley CM, Criger LA, Madenjian CP, Robinson KF, et al. Managing native and non-native sea lamprey (Petromyzon marinus) through anthropogenic change: a prospective assessment of key threats and uncertainties. J Great Lakes Res. (2021) 47:S704–22. doi: 10.1016/j.jglr.2020.08.015
4. Smith BR, Tibbles JJ. Sea lamprey (Petromyzon marinus) in Lakes Huron, Michigan and Superior—History of invasison and control-−1936–78. Can J Fisher Aquat Sci. (1980) 37:1780–801. doi: 10.1139/f80-222
5. Brant C. Great Lakes Sea Lamprey: The 70 Year War on a Biological Invader. Ann Arbor, MI: Univ Michigan Press (2019).
6. Gaden M, Brant OC, Lambe R. Why a Great Lakes Fishery Commission? The seven-decade pursuit of a Canada-U.S. fishery treaty. J Great Lakes Res. (2021) 47:S11–23. doi: 10.1016/j.jglr.2021.01.003
7. Gaden M, Brant C, Stedman RC, Cooke SJ, Young N, Lauber TB, et al. Shifting baselines and social license to operate: challenges in communicating sea lamprey control. J Great Lakes Res. (2021) 47:S800–8. doi: 10.1016/j.jglr.2021.01.016
8. Siefkes MJ. Use of physiological knowledge to control the invasive sea lamprey (Petromyzon marinus) in the Laurentian Great Lakes. Conserv Physiol. (2017) 5:cox031. doi: 10.1093/conphys/cox031
9. Cooke SJ, Baker CL, Hinderer JLM, Siefkes M, Barber JM, Steeves TB, et al. Ten lessons for controlling invasive species: wisdom from the long-standing sea lamprey control program on the Laurentian Great Lakes. Bioscience. (2025) 12. doi: 10.1093/biosci/biaf133
10. McLaughlin RL, Hallett A, Pratt TC, O'Connor LM, McDonald DG. Research to guide use of barriers, traps, and fishways to control sea lamprey. J Great Lakes Res. (2007) 33:7–19. doi: 10.3394/0380-1330(2007)33[7:RTGUOB]2.0.CO;2
11. Wilkie MP, Johnson NS, Docker MF. Invasive species control and management: the sea lamprey story. In:N. A. Fangue, S. J. Cooke, A. P. Farrell, C. J. Brauner, and E. J. Eliason, , editors. Conservation Physiology for the Anthropocene Part B: Issues and Applications, Vol. 39. (2022) (San Diego, CA: Elsevier Academic Press Inc). p. 489–579.
12. Birceanu O, Tessier LR, Huerta B, Li WM, McDonald A, Wilkie MP, et al. At the intersection between toxicology and physiology: what we have learned about sea lampreys and bony fish physiology from studying the mode of action of lampricides. J Great Lakes Res. (2021) 47:S673–89. doi: 10.1016/j.jglr.2021.07.007
13. Wilkie MP, Hubert TD, Boogaard MA, Birceanu O. Control of invasive sea lampreys using the piscicides TFM and niclosamide: toxicology, successes and future prospects. Aquat Toxicol. (2019) 211:235–52. doi: 10.1016/j.aquatox.2018.12.012
14. Lennox RJ, Bravener GA, Lin H-Y, Madenjian CP, Muir AM, Remucal CK, et al. Potential changes to the biology and challenges to the management of invasive sea lamprey Petromyzon marinusin the Laurentian Great Lakes due to climate change. Global Change Biol. (2020) 26:1118–37. doi: 10.1111/gcb.14957
15. Docker MF, Potter IC. Life history evolution in lampreys: alternative migratory and feeding types. In:M. F. Docker, , editor. Lampreys: Biology, Conservation and Control, Vol 2. Fish & Fisheries Series, Vol. 38 (2019) (Berlin: Springer). p. 287.
16. Renaud CB. Lampreys of the world. An annotated and illustrated catalogue of lamprey species known to date FAO Species Catalogue for Fishery Purposes No 5 (Rome:FAO). (2011). p. 109.
17. Miller AK, Baker C, Kitson JC, Yick JL, Manquel PEI, Alexander A, et al. The Southern Hemisphere lampreys (Geotriidae and Mordaciidae). Rev Fish Biol Fisher. (2021) 31:201–32. doi: 10.1007/s11160-021-09639-x
18. Gess R, Coates M, Rubidge B. A lamprey from the Devonian period of South Africa. Nature. (2006) 443:981–4. doi: 10.1038/nature05150
19. Wu FX, Janvier P, Zhang C. The rise of predation in Jurassic lampreys. Nat Commun. (2023) 14:6652. doi: 10.1038/s41467-023-42251-0
20. Sutton TM, Bowen SH. Significance of organic detritus in the diet of larval lamrpys in the Great Lakes basin. Can J Fisher Aquat Sci. (1994) 51:2380–7. doi: 10.1139/f94-239
21. Yap MR, Bowen SH. Feeding by northern brook lamprey (Ichthyomyzon fossor) on sestonic biofilm fragments: habitat selection results in ingestion of a higher quality diet. J Great Lakes Res. (2003) 29:15–25. doi: 10.1016/S0380-1330(03)70475-4
22. Mallatt J. Ventilation and the origin of jawed vertebrates: a new mouth. Zoo J Linnean Soc. (1996) 117:329–404. doi: 10.1111/j.1096-3642.1996.tb01658.x
23. Rovainen CM. Feeding and breathing in lampreys [Article; Proceedings Paper]. Brain Behav Evol. (1996) 48:297–305. doi: 10.1159/000113208
24. Miyashita T, Gess R, Tietjen K, Coates M. Non-ammocoete larvae of Palaeozoic stem lampreys. Nature. (2021) 591:408–12. doi: 10.1038/s41586-021-03305-9
25. Clemens B. A Call for standard terminology for lamprey life stages. Fisheries. (2019) 44:243–5. doi: 10.1002/fsh.10227
26. Manzon RG, Youson JH, Holmes JA. Lamprey metamorphosis. In:M. F. Docker, , editor. Lampreys: Biology, Conservation and Control, Vol. 37 (2015). p. 139–214.
27. Youson JH. Morphology and physiology of lamprey metamorphosis. Can J Fisher Aquat Sci. (1980) 37:1687–710. doi: 10.1139/f80-216
28. Beamish FWH, Potter IC. Biology of anadromous sea lamprey (Petromyzon marinus) in New Brunswick. J Zool. (1975) 177:57–72. doi: 10.1111/j.1469-7998.1975.tb05970.x
29. Bergstedt RA, Swink WD. Seasonal growth and duration of the parasitc life stage of the landlocked sea lamprey (Petromyzon marinus). Can J Fisher Aquat Sci. (1995) 52:1257–64. doi: 10.1139/f95-122
30. Buchinger TJ, Siefkes MJ, Zielinski BS, Brant CO, Li WM. Chemical cues and pheromones in the sea lamprey (Petromyzon marinus). Front Zool. (2015) 12:32. doi: 10.1186/s12983-015-0126-9
31. Meckley TD, Gurarie E, Miller JR, Wagner CM. How fishes find the shore: evidence for orientation to bathymetry from the non-homing sea lamprey. Can J Fisher Aquat Sci. (2017) 74:2045–58. doi: 10.1139/cjfas-2016-0412
32. Waldman J, Grunwald C, Wirgin I. Sea lamprey Petromyzon marinus: an exception to the rule of homing in anadromous fishes. Biol Lett. (2008) 4:659–62. doi: 10.1098/rsbl.2008.0341
33. Hansen MJ, Madenjian CP, Slade JW, Steeves TB, Almeida PR, Quintella BR, et al. Population ecology of the sea lamprey (Petromyzon marinus) as an invasive species in the Laurentian Great Lakes and an imperiled species in Europe. Rev Fish Biol Fisher. (2016) 26:509–35. doi: 10.1007/s11160-016-9440-3
34. Alpert TP, Bone E, Hozapfel C. Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspec Plant Ecol Evol Syst. (2000) 3:52–66. doi: 10.1078/1433-8319-00004
35. Leung B, Mandrak NE. The risk of establishment of aquatic invasive species: joining invasibility and propagule pressure. Proc Royal Soc B-Biol Sci. (2007) 274:2603–9. doi: 10.1098/rspb.2007.0841
36. Lockwood JL, Cassey P, Blackburn TM. The more you introduce the more you get: the role of colonization pressure and propagule pressure in invasion ecology. Divers Distrib. (2009) 15:904–10. doi: 10.1111/j.1472-4642.2009.00594.x
37. Williamson M, Fitter A. The varying success of invaders. Ecology. (1996) 77:1661–6. doi: 10.2307/2265769
38. Docker M, Bravener G, Garroway C, Hrodey P, Humed J, Johnson N, et al. A review of sea lamprey dispersal and population structure in the Great Lakes and the implications for control. J Great Lakes Res. (2021) 47:S549–69. doi: 10.1016/j.jglr.2021.09.015
39. Mandrak NE, Crossman EJ. Postglacial dispersal of freshwater fishes in Ontario. Can. J. Zoo. (1992) 70:2247–59. doi: 10.1139/z92-302
40. Waldman J, Daniels R, Hickerson M, Wirgin I. Mitochondrial DNA analysis indicates sea lampreys are indigenous to Lake Ontario: response to comment. Transac Am Fisher Soc. (2009) 138:1190–7. doi: 10.1577/T08-035R.1
41. Lawrie AH. Sea lamprey in Great Lakes. Transac Am Fisher Soc. (1970) 99:766–75. doi: 10.1577/1548-8659(1970)99<766:TSLITG>2.0.CO;2
42. Aron WI, Smith SH. Ship canals and aquatic ecosystems. Science. (1971) 174:13–20. doi: 10.1126/science.174.4004.13
43. Sturtevant RA, Mason DM, Rutherford ES, Elgin A, Lower E, Martinez F, et al. Recent history of nonindigenous species in the Laurentian Great Lakes; An update to Mills et al, 1993 (25 years later). J Great Lakes Res. (2019) 45:1011–35. doi: 10.1016/j.jglr.2019.09.010
44. Lark JGI. Early record of sea lamprey (Petromyzon marinus) from Lake Ontario. J Fisher Res Board Canada. (1973) 30:131–3. doi: 10.1139/f73-024
45. Daniels RA. Untested assumptions: The role of canals in the dispersal of sea lamprey, alewife, and other fishes in the eastern United States. Environ Biol Fishes. (2001) 60:309–29. doi: 10.1023/A:1011032907484
46. Waldman JR, Grunwald C, Roy NK, Wirgin II. Mitochondrial DNA analysis indicates sea lampreys are indigenous to Lake Ontario. Transac Am Fisher Soc. (2004) 133:950–60. doi: 10.1577/T03-104.1
47. Bryan MB, Zalinski D, Filcek KB, Libants S, Li W, Scribner KT, et al. Patterns of invasion and colonization of the sea lamprey (Petromyzon marinus) in North America as revealed by microsatellite genotypes. Mol Ecol. (2005) 14:3757–73. doi: 10.1111/j.1365-294X.2005.02716.x
48. Lockwood JL, Cassey P, Blackburn T. The role of propagule pressure in explaining species invasions. Trends Ecol Evol. (2005) 20:223–8. doi: 10.1016/j.tree.2005.02.004
49. Simberloff D. The role of propagule pressure in biological invasions. Ann Rev Ecol Evol Syst. (2009) 40:81–102. doi: 10.1146/annurev.ecolsys.110308.120304
50. Marsden JE, Siefkes MJ. Control of Invasive Sea Lamprey in the Great Lakes, Lake Champlain, and Finger Lakes of New York. Lampreys Biol Conserv Control. (2019) 38:411–79. doi: 10.1007/978-94-024-1684-8_5
51. Crowl TA, Crist TO, Parmenter RR, Belovsky G, Lugo AE. The spread of invasive species and infectious disease as drivers of ecosystem change. Front Ecol Environ. (2008) 6:238–46. doi: 10.1890/070151
52. Kelley AL. The role thermal physiology plays in species invasion. Conserv. Physiol. (2014) 2:cou045. doi: 10.1093/conphys/cou045
53. Beamish FWH, Strachan PD, Thomas E. Osmotic and ionic performance of anadromous sea lamprey, Petromyzon marinus. Compar Biochem Physiol A—Physiol. (1978) 60:435–43. doi: 10.1016/0300-9629(78)90013-0
54. Zydlewski J, Wilkie MP. Freshwater to seawater transitions in migratory fishes. In:S. D. McCormick, A. P. Farrell, C. J. Brauner, , editors. Euryhaline Fishes, Vol. 32. Academic Press (2013). p. 253–326. doi: 10.1016/b978-0-12-396951-4.00006-2
55. Sullivan WP, Lantry BF, Barber JM, Bishop DL, Bravener GA, Connerton MJ, et al. The path toward consistent achievement of sea lamprey abundance and lake trout marking targets in Lake Ontario, 2000-2019. J Great Lakes Res. (2021) 47:S523–48. doi: 10.1016/j.jglr.2021.06.002
56. Quintella BR, Andrade NO, Dias NM, Almeida PR. Laboratory assessment of sea lamprey larvae burrowing performance. Ecol Freshwater Fish. (2007) 16:177–82. doi: 10.1111/j.1600-0633.2006.00209.x
57. Dawson HA, Quintella BR, Almeida PR, Treble AJ, Jolley JC. The ecology of larval and metamorphosing lampreys. In:Lampreys: Biology, Conservation and Control, Vol 1, , ed. M F Docker. (2015) 75–137.
58. Beamish FWH. Biology of the North American anadromous sea lamprey, Petromyzon marinus. Can J Fisher Aquat Sci. (1980) 37:1924–43. doi: 10.1139/f80-233
59. Renaud CB, Cochran PA. Post-metamorphic feeding in lampreys. In:M. F. Docker, , editor. Lampreys: Biology, Conservation and Control, Vol. 38 (2019). p. 247–285.
60. Hayden B, Ferron M, Cunjak RA, Samways K. Fruit of the forest—larval sea lamprey Petromyzon marinusare fuelled by allochthonous resources. J Fish Biol. (2019) 95:781–92. doi: 10.1111/jfb.14064
61. Neave FB, Booth RMW, Philipps RR, Keffer DA, Bravener GA, Coombs N, et al. Changes in native lamprey populations in the Great Lakes since the onset of sea lamprey (Petromyzon marinus) control. J Great Lakes Res. (2021) 47:S378–87. doi: 10.1016/j.jglr.2020.10.005
62. Dunlop ES, McLaughlin R, Adams JV, Jones M, Birceanu O, Christie MR, et al. Rapid evolution meets invasive species control: the potential for pesticide resistance in sea lamprey. Can J Fisher Aquat Sci. (2018) 75:152–68. doi: 10.1139/cjfas-2017-0015
63. McMahon RF. Evolutionary and physiological adaptations of aquatic invasive animals: selection versus resistance. Can J Fisher Aquat Sci. (2002) 59:1235–44. doi: 10.1139/f02-105
64. Dawson H, Jones M. Factors affecting recruitment dynamics of Great Lakes sea lamprey (Petromyzon marinus) populations. J Great Lakes Res. (2009) 35:353–60. doi: 10.1016/j.jglr.2009.03.003
65. Young RJ, Kelso JRM, Weise JG. Occurrence, relative abundance, and size of landlocked sea lamprey (Petromyzon marinus) ammocoetes in relation to stream characteristics in the Great Lakes. Can J Fisher Aquat Sci. (1990) 47:1773–8. doi: 10.1139/f90-201
66. Griffith RW. Guppies, toadfish, lungfish, coelacanths and frogs—a scenario for the evolution of urea retention in fishes. Environ Biol Fishes. (1991) 32:199–218. doi: 10.1007/BF00007454
67. Docker MF. Lampreys: biology, conservation and control, volume 2. In:M. F. Docker, , editor. Fish and Fisheries Series, Vol. 38 (2019) (The Netherlands: Dordrecht and New York: Springer). p. 577.
68. Bergstedt RA, Seelye JG. Evidence for lack of homing by sea lampreys. Transac Am Fisher Soc. (1995) 124:235–9. doi: 10.1577/1548-8659(1995)124<0235:EFLOHB>2.3.CO;2
69. Quintella B, Clemens B, Sutton T, Lança M, Madenjian C, Happel A, et al. At-sea feeding ecology of parasitic lampreys. J Great Lakes Res. (2021) 47:S72–89. doi: 10.1016/j.jglr.2021.07.008
70. Fine JM, Vrieze LA, Sorensen PW. Evidence that petromyzontid lampreys employ a common migratory pheromone that is partially comprised of bile acids. J Chem Ecol. (2004) 30:2091–110. doi: 10.1023/B:JOEC.0000048776.16091.b1
71. Li WM, Sorensen PW, Gallaher DD. The oflactroy system of migratory adult sea lamprey (Petromyzon marinus) is specifically and acutely sensitive to unique bile-acids released by conspeceific larvae. J Gener Physiol. (1995) 105:569–87. doi: 10.1085/jgp.105.5.569
72. Sorensen PW, Hoye TR. A critical review of the discovery and application of a migratory pheromone inan invasive fish, the sea lamprey Petromyzon matinus L. J Fish Biol. (2007) 71:100–14. doi: 10.1111/j.1095-8649.2007.01681.x
73. Fissette SD, Buchinger TJ, Wagner CM, Johnson NS, Scott AM, Li W, et al. Progress towards integrating an understanding of chemical ecology into sea lamprey control. J Great Lakes Res. (2021) 47:S660–72. doi: 10.1016/j.jglr.2021.02.008
74. Fine JM, Sorensen PW. Isolation and biological activity of the multi-component sea lamprey migratory pheromone. J Chem Ecol. (2008) 34:1259–67. doi: 10.1007/s10886-008-9535-y
75. Sorensen PW, Vrieze LA, Fine JM. A multi-component migratory pheromone in the sea lamprey. Fish Physiol Biochem. (2003) 28:253–7. doi: 10.1023/B:FISH.0000030545.39156.2b
76. Mensch EL, Dissanayake AA, Nair MG, Wagner CM. The effect of putrescine on space use and activity in sea lamprey (Petromyzon marinus). Sci Rep. (2022) 12:17276. doi: 10.1038/s41598-022-22143-x
77. Johnson NS, Yun S-S, Thompson HT, Brant CO, Li W. A synthesized pheromone induces upstream movement in female sea lamprey and summons them into traps. Proc Natl Acad Sci U S A. (2009) 106:1021–6. doi: 10.1073/pnas.0808530106
78. Siefkes MJ, Winterstein SR, Li WM. Evidence that 3-keto petromyzonol sulphate specifically attracts ovulating female sea lamprey, Petromyzon marinus. Anim Behav. (2005) 70:1037–45. doi: 10.1016/j.anbehav.2005.01.024
79. Johnson NS, Buchinger TJ, Li WM. Reproductive ecology of lampreys. In:Lampreys: Biology, Conservation and Control, Vol 1, , ed. M F Docker. (2015) 265–303.
80. Scott WB, Crossman EJ. Freshwater Fishes of Canada. Ottawa, ON: Fisheries Research Board of Canada (1973).
81. Buchinger TJ, Li K, Huertas M, Baker CF, Jia L, Hayes MC, et al. Evidence for partial overlap of male olfactory cues in lampreys. J Exp Biol. (2017) 220:497–506. doi: 10.1242/jeb.149807
82. Raffel TR, Martin LB, Rohr JR. Parasites as predators: unifying natural enemy ecology. Trends Ecol Evol. (2008) 23:610–8. doi: 10.1016/j.tree.2008.06.015
83. Parker PS, Lennon RE. Biology of the sea lanprey in its parasitic phase. U S Fish and Wildlife Serv Res Rept. (1956) 44:1–32.
84. Gallant J, Harvey-Clark C, Myers RA, Stokesbury MJW. Sea lamprey attached to a Greenland shark in the St. Lawrence Estuary. Canada Northeast Nat. (2006) 13:35–8. doi: 10.1656/1092-6194(2006)13[35:SLATAG]2.0.CO;2
85. Jensen C, Schwartz FJ. Atlantic Ocean occurrence of the sea lamprey, Petromyzon marinus (Petromyzontiformes, Petromyzontidae). p. parastizatizing sandbar, Carcharhinus plumeus, and dusky, C. obscurus (Carcharhinusformes, Carharhinidae). p. sharks off North Carollina and South Carolina. Brimleyana. (1994) 21:69–72.
86. Wilkie MP, Turnbull S, Bird J, Wang YS, Claude JF, Youson JH, et al. Lamprey parasitism of sharks and teleosts: high capacity urea excretion in an extant vertebrate relic. Compar Biochem Physiol a-Mol Integr Physiol. (2004) 138:485–92. doi: 10.1016/j.cbpb.2004.06.001
87. Johnson N, Lewandoski S, Merkes C. Assessment of sea lamprey (Petromyzon marinus) diet using DNA metabarcoding of feces. Ecol Indicat. (2021) 125:107605. doi: 10.1016/j.ecolind.2021.107605
88. Renaud CB, Gill HS, Potter IC. Relationships between the diets and characteristics of the dentition, buccal glands and velar tentacles of the adults of the parasitic species of lamprey. J Zoo. (2009) 278:231–42. doi: 10.1111/j.1469-7998.2009.00571.x
89. Farmer GJ. Biology and physiology of feeding in adult lamprey. Can J Fisher Aquat Sci. (1980) 37:1751–61. doi: 10.1139/f80-220
90. Xiao R, Pang Y, Li QW. The buccal gland of Lampetra japonica is a source of diverse bioactive proteins. Biochimie. (2012) 94:1075–9. doi: 10.1016/j.biochi.2011.12.025
91. Bullingham OMN, Firkus TJ, Goetz FW, Murphy CA, Alderman SL. Lake charr (Salvelinus namaycush) clotting response may act as a plasma biomarker of sea lamprey (Petromyzon marinus) parasitism: implications for management and wound assessment. J Great Lakes Res. (2022) 48:207–18. doi: 10.1016/j.jglr.2021.11.005
92. Firkus TJ, Murphy CA, Adams JV, Treska TJ, Fischer G. Assessing the assumptions of classification agreement, accuracy, and predictable healing time of sea lamprey wounds on lake trout. J Great Lakes Res. (2021) 47:S368–77. doi: 10.1016/j.jglr.2020.07.016
93. Li BW, Gou M, Han JM, Yuan XF, Li YY, Li TS, et al. Proteomic analysis of buccal gland secretion from fasting and feeding lampreys (Lampetra morii). Proteome Sci. (2018) 16:9. doi: 10.1186/s12953-018-0137-5
94. Xiao R, Li QW, Perrett S, He RQ. Characterisation of the fibrinogenolytic properties of the buccal gland secretion from Lampetra japonica. Biochimie. (2007) 89:383–92. doi: 10.1016/j.biochi.2006.09.002
95. Gou M, Duan XY, Li J, Wang YC, Li QW, Pang Y, et al. Spatial metabolomics reveals the multifaceted nature of lamprey buccal gland and its diverse mechanisms for blood-feeding. Commun. Biol. (2023) 6:881. doi: 10.1038/s42003-023-05250-x
96. Bartels H, Potter IC. Cellular composition and ultrastructure of the gill epithelium of larval and adult lampreys—Implications for osmoregulation in fresh and seawater. J Exp Biol. (2004) 207:3447–62. doi: 10.1242/jeb.01157
97. Ferreira-Martins D, Wilson JM, Kelly SP, Kolosov D, McCormick SD. A review of osmoregulation in lamprey. J Great Lakes Res. (2021) 47:S59–71. doi: 10.1016/j.jglr.2021.05.003
98. Beamish FWH. Osmoregulation in juvenile and adult lampreys. Can J Fisher Aquat Sci. (1980) 37:1739–50. doi: 10.1139/f80-219
99. Hardisty MW. A comparison of gonadal development in ammocoetes of landlocked and anadromouos forms of sea lamprey Petromyzon marinus L. J Fish Biol. (1969) 1:153. doi: 10.1111/j.1095-8649.1969.tb03849.x
100. Diaz-Suarez A, Noreikiene K, Kisand V, Burimski O, Svirgsden R, Rohtla M, et al. Temporally stable small-scale genetic structure of Northern pike (Esox lucius) in the coastal Baltic Sea. Fisher Res. (2022) 254:106402. doi: 10.1016/j.fishres.2022.106402
101. Ferguson A, Reed TE, Cross TF, McGinnity P, Prodohl PA. Anadromy, potamodromy and residency in brown trout Salmo trutta: the role of genes and the environment. J Fish Biol. (2019) 95:692–718. doi: 10.1111/jfb.14005
102. Masson S, Lepais O, Manicki A, Prevost E, Chat J. Disentangling individual movement between populations from effective dispersal in the facultative anadromous Salmo trutta L. Ecol Freshwater Fish. (2018) 27:323–38. doi: 10.1111/eff.12349
103. Railsback SF, Harvey BC, White JL. Facultative anadromy in salmonids: linking habitat, individual life history decisions, and population-level consequences. Can J Fisher Aquat Sci. (2014) 71:1270–8. doi: 10.1139/cjfas-2014-0091
104. Holm E, Mandrak NE, Burridge ME. The ROM Gield Guide to Freshwater Fishes of Ontario. Toronto, On: Royal Ontario Museum. (2009).
105. Quinn TP, Wetzel LA, Hasselman DJ, Larsen K. Differences in life history patterns of American shad (Alosa sapi dissima) populations between ancestral, Atlantic coast, and non-native Pacific coast rivers of North America. Can J Fisher Aquat Sci. (2024) 81:862–78. doi: 10.1139/cjfas-2023-0286
106. Velotta JP, McCormick SD, O'Neill RJ, Schultz ET. Relaxed selection causes microevolution of seawater osmoregulation and gene expression in landlocked alewives. Oecologia. (2014) 175:1081–92. doi: 10.1007/s00442-014-2961-3
107. Norstog J, McCormick S. Landlocked populations have small but detectable differences in ionoregulatory physiology compared to anadromous sea lamprey, Petromyzon marinus. Can J Fisher Aquat Sci. (2023) 80:1579–94. doi: 10.1139/cjfas-2022-0242
108. Lewis SV. Respiration of lampreys. Can J Fisher Aquat Sci. (1980) 37:1711–22. doi: 10.1139/f80-217
109. Tessier LR, Long TAF, Wilkie MP. Influence of body size, metabolic rate and life history stage on the uptake and excretion of the lampricide 3-trifluoromethyl-4-nitrophenol (TFM) by invasive sea lampreys (Petromyzon marinus). Aquat Toxicol. (2018) 194:27–36. doi: 10.1016/j.aquatox.2017.10.020
110. Wilkie MP, Bradshaw PG, Joanis V, Claude JF, Swindell SL. Rapid metabolic recovery following vigorous exercise in burrow-dwelling larval sea lampreys (Petromyzon marinus). Physiol Biochem Zoo. (2001) 74:261–72. doi: 10.1086/319656
111. Gonzalez RJ, McDonald DG. The relationship between oxygen consumption and ion Ioss in a freshwater fish. J Exp Biol. (1992) 163:317–32. doi: 10.1242/jeb.163.1.317
112. Wood CM, Eom J. The osmorespiratory compromise in the fish gill. Compar Biochem Physiol a-Mol Integr Physiol. (2021) 254:110895. doi: 10.1016/j.cbpa.2021.110895
113. Ferreira-Martins D, Champer J, McCauley DW, Zhang Z, Docker MF. Genetic control of invasive sea lamprey in the Great Lakes. J Great Lakes Res. (2021) 47:S764–75. doi: 10.1016/j.jglr.2021.10.018
114. Wilkie MP. Lampreys: Energetics and development. In:AP Farrell, , editor. Encyclopedia of Fish Physiology: From genome to environment, Vol 1–3 (2011) (San Diego: Elsevier-Academic Press). p. 1779–87.
115. Ferreira-Martins D, Coimbra J, Antunes C, Wilson JM. Effects of salinity on upstream-migrating, spawning sea lamprey, Petromyzon marinus. Conserv Physiol (2016a) 4:cov064. doi: 10.1093/conphys/cov064
116. Ferreira-Martins D, McCormick SD, Campos A, Lopes-Marques M, Osorio H, Coimbra J, et al. A cytosolic carbonic anhydrase molecular switch occurs in the gills of metamorphic sea lamprey. Sci Rep. (2016) 6:33954. doi: 10.1038/srep33954
117. Youson JH, Freeman PA. Morphology of the gills of larval and parasitic adult sea lamprey, Petromyzon marinusL. J Morphol. (1976) 149:73–104. doi: 10.1002/jmor.1051490105
118. McCormick SD. Smolt physiology and endocrinology. Euryhaline Fishes. (2013) 32:199–251. doi: 10.1016/B978-0-12-396951-4.00005-0
119. Reis-Santos P, McCormick SD, Wilson JM. Ionoregulatory changes during metamorphosis and salinity exposure of juvenile sea lamprey (Petromyzon marinusL.). J Exp Biol. (2008) 211:978–88. doi: 10.1242/jeb.014423
120. Sunga J, Wilson JM, Wilkie MP. Functional re-organization of the gills of metamorphosing sea lamprey (Petromyzon marinus): preparation for a blood diet and the freshwater to seawater transition. J Compar Physiol B-Biochem Syst Environ Physiol. (2020) 190:701–15. doi: 10.1007/s00360-020-01305-1
121. Barany A, Shaughnessy CA, Fuentes J, Mancera JM, McCormick SD. Osmoregulatory role of the intestine in the sea lamprey (Petromyzon marinus). Am J Physiol Regulat Integr Comp Physiol. (2020) 318:R410–7. doi: 10.1152/ajpregu.00033.2019
122. Gong N, Ferreira-Martins D, Norstog J, McCormick S, Sheridan M. Discovery of prolactin-like in lamprey: role in osmoregulation and new insight into the evolution of the growth hormone/prolactin family. Proc Natl Acad Sci U S A. (2022) 119:e2212196119. doi: 10.1073/pnas.2212196119
123. Shaughnessy CA, McCormick SD. Functional characterization and osmoregulatory role of the Na+-K+-2Cl− cotransporter in the gill of sea lamprey (Petromyzon marinus). p. a basal vertebrate. Am J Physiol Regul Integr Compar Physiol. (2020) 318:R17–29. doi: 10.1152/ajpregu.00125.2019
124. Mathers JS, Beamish FWH. Changes in serum osmotic and ionic concentration in lanlocked Petromyzon marinus. Comp Biochem Physiol. (1974) 49:677–88. doi: 10.1016/0300-9629(74)90896-2
125. Gong NP, Ferreira-Martins D, McCormick SD, Sheridan MA. Divergent genes encoding the putative receptors for growth hormone and prolactin in sea lamprey display distinct patterns of expression. Sci Rep. (2020) 10:1674. doi: 10.1038/s41598-020-58344-5
126. Breves JP, McCormick SD, Karlstrom RO. Prolactin and teleost ionocytes: New insights into cellular and molecular targets of prolactin in vertebrate epithelia. Gener Compar Endocrinol. (2014) 203:21–8. doi: 10.1016/j.ygcen.2013.12.014
127. Kolosov D, Bui P, Donini A, Wilkie MP, Kelly SP. A role for tight junction-associated MARVEL proteins in larval sea lamprey (Petromyzon marinus) osmoregulation. J Exp Biol. (2017) 220:3657–70. doi: 10.1242/jeb.161562
128. Kolosov D, Bui P, Wilkie MP, Kelly SP. Claudins of sea lamprey (Petromyzon marinus)—organ-specific expression and transcriptional responses to water of varying ion content. J Fish Biol. (2020) 96:768–81. doi: 10.1111/jfb.14274
129. Barany A, Shaughnessy CA, McCormick SD. Corticosteroid control of Na+/K−-ATPase in the intestine of the sea lamprey (Petromyzon marinus). Gener Comp Endocrinol. (2021) 307:113756. doi: 10.1016/j.ygcen.2021.113756
130. Shaughnessy CA, Barany A, McCormick SD. 11-Deoxycortisol controls hydromineral balance in the most basal osmoregulating vertebrate, sea lamprey (Petromyzon marinus). Sci Rep. (2020) 10:12148. doi: 10.1038/s41598-020-69061-4
131. Bartels H, Docker MF, Fazekas U, Potter IC. Functional and evolutionary implications of the cellular composition of the gill epithelium of feeding adults of a freshwater parasitic species of lamprey, Ichthyomyzon unicuspis. Can J Zool. (2012) 90:1278–83. doi: 10.1139/z2012-089
132. Bartels H, Fazekas U, Youson JH, Potter IC. Changes in the cellular composition of the gill epithelium during the life cycle of a non-parasitic lamprey: functional and evolutionary implications. Can J Zool. (2011) 89:538–45. doi: 10.1139/z11-019
133. Björnsson BT, Stefansson SO, McCormick SD. Environmental endocrinology of salmon smoltification. Gener Compar Endocrinol. (2011) 170:290–8. doi: 10.1016/j.ygcen.2010.07.003
134. Greenan BJW, James TS, Loder JW, Pepin P, Azetsu-Scott K, Ianson D, et al. Changes in oceans surrounding Canada, Chapter 7. In:F. S. Annis and D. Lemmen, , editors. Canada's Changing Climate Report (2018).
135. Shearman RK Lentz SJ Long-term sea surface temperature variability along the US East Coast. J Phys Oceanogr. (2010) 40:1004–17. doi: 10.1175/2009JPO4300.1
136. Coker GA, Portt CB, Minns CK. Morphological and ecological characteristics of Canadian freshwater fishes. Can MS Rpt Fish Aquat Sci. (2001) 2554:iv+89p.
137. Holmes JA, Lin P. Thermal niche of larval sea lamprey, Petromyzon marinus. Can J Fisher Aquat Sci. (1994) 51:253–62. doi: 10.1139/f94-026
138. Hlina BL, Birceanu O, Robinson CS, Dhiyebi H, Wilkie MP. The relationship between thermal physiology and lampricide sensitivity in larval sea lamprey (Petromyzon marinus). J Great Lakes Res. (2021) 47:S272–84. doi: 10.1016/j.jglr.2021.10.002
139. Sutherby Sutherby J The effect of temperature on sea lamprey (Petromyzon marinus): ecological and cellular implications [Research University University of Manitoba]. Winnipeg, Manitoba, Canada. (2019).
140. Minor EC, Brinkley G. Alkalinity, pH, and pCO[[sb]]2[[/s]] in the Laurentian Great Lakes: an initial view of seasonal and inter-annual trends. J Great Lakes Res. (2022) 48:502–11. doi: 10.1016/j.jglr.2022.01.005
141. Somero GN. Comparative physiology: a crystal ball for predicting consequences of global change. Am J Physiol Regulat Integr Compar Physiol. (2011) 301:R1–R14. doi: 10.1152/ajpregu.00719.2010
142. Weaving H, Terblanche JS, Pottier P, English S. Meta-analysis reveals weak but pervasive plasticity in insect thermal limits. Nat Commun. (2022) 13:5292. doi: 10.1038/s41467-022-32953-2
143. Potter IC, Beamish FWH. Lethal temperatures in ammocoetes of 4 species of lampreys. Acta Zoo. (1975) 56:85–91. doi: 10.1111/j.1463-6395.1975.tb00084.x
144. Rohr JR, Civitello DJ, Cohen JM, Roznik EA, Sinervo B, Dell AI, et al. The complex drivers of thermal acclimation and breadth in ectotherms. Ecol Lett. (2018) 21:1425–39. doi: 10.1111/ele.13107
145. Whitesel TA, Sankovich PM. Climate projections and Pacific lamprey conservation: evidence that larvae in natural conditions may be resilient to climate warming. Biology. (2025) 14:74. doi: 10.3390/biology14010074
146. Wood LA, Brown IR, Youson JH. Characterization of the heat shock response in the gills of sea lampreys and a brook lamprey at different intervals of their life cycles. Compar Biochem Physiol a-Mol Integr Physiol. (1998) 120:509–18. doi: 10.1016/S1095-6433(98)10061-2
147. Wood LA, Brown IR, Youson JH. Tissue and developmental variations in the heat shock response of sea lampreys (Petromyzon marinus): effects of an increase in acclimation temperature. Compar Biochem Physiol a-Mol Integr Physiol. (1999) 123:35–42. doi: 10.1016/S1095-6433(99)00035-5
148. Lucas MC, Hume JB, Almeida PR, Aronsuu K, Habit E, Silva S, et al. Emerging conservation initiatives for lampreys: research challenges and opportunities. J Great Lakes Res. (2021) 47:S690–703. doi: 10.1016/j.jglr.2020.06.004
149. Mateus CS, Rodriguez-Munoz R, Quintella BR, Judite Alves M, Almeida PR. Lampreys of the Iberian Peninsula: distribution, population status and conservation. Endang Species Res. (2012) 16:183–98. doi: 10.3354/esr00405
150. Siefkes MJ, Johnson NS, Muir AM. A renewed philosophy about supplemental sea lamprey controls. J Great Lakes Res. (2021) 47:S742–52. doi: 10.1016/j.jglr.2021.03.013
151. Sullivan WP, Burkett DP, Boogaard MA, Criger LA, Freiburger CE, Hubert TD, et al. Advances in the use of lampricides to control sea lampreys in the Laurentian Great Lakes, 2000-2019. J Great Lakes Res. (2021) 47:S216–37. doi: 10.1016/j.jglr.2021.08.009
152. Borowiec BG, Docker MF, Johnson NS, Moser ML, Zielinski B, Wilkie MP, et al. Exploiting the physiology of lampreys to refine methods of control and conservation. J Great Lakes Res. (2021) 47:S723–41. doi: 10.1016/j.jglr.2021.10.015
153. McDonald DG, Kolar CS. Research to guide the use of lampricides for controlling sea lamprey. J Great Lakes Res. (2007) 33:20–34. doi: 10.3394/0380-1330(2007)33[20:RTGTUO]2.0.CO;2
154. Quintella BR, Pova I, Almeida PR. Swimming behaviour of upriver migrating sea lamprey assessed by electromyogram telemetry. J Appl Ichthyol. (2009) 25:46–54. doi: 10.1111/j.1439-0426.2008.01200.x
155. Reinhardt UG, Eidietis L, Friedl SE, Moser ML. Pacific lamprey climbing behavior. Can J Zoo. (2008) 86:1264–72. doi: 10.1139/Z08-112
156. Zielinski DP, Freiburger C. Advances in fish passage in the Great Lakes basin. J Great Lakes Res. (2021) 47:S439–47. doi: 10.1016/j.jglr.2020.03.008
157. Adams JV, Barber JM, Bravener GA, Lewandoski SA. Quantifying Great Lakes sea lamprey populations using an index of adults. J Great Lakes Res. (2021) 47:1103–12. doi: 10.1016/j.jglr.2021.04.009
158. Bussy U, Chung-Davidson Y-W, Buchinger T, Li K, Smith SA, Jones AD, et al. Metabolism of a sea lamprey pesticide by fish liver enzymes part A: identification and synthesis of TFM metabolites. Anal Bioanalyt Chem. (2018) 410:1749–61. doi: 10.1007/s00216-017-0830-8
159. Bussy U, Chung-Davidson Y-W, Buchinger T, Li K, Smith SA, Jones AD, et al. Metabolism of a sea lamprey pesticide by fish liver enzymes part B: method development and application in quantification of TFM metabolites formed in vivo. Anal. Bioanal Chem. (2018) 410:1763–74. doi: 10.1007/s00216-017-0831-7
160. Kane AS, Kahng MW, Reimschuessel R, Nhamburo PT, Lipsky MM. UDP-Glucuronosyltransferase kinetics for 3-trifluoromethyl-4-nitrophenol (TFM) in fish. Transac Am Fisher Soc. (1994) 123:217–22. doi: 10.1577/1548-8659(1994)123<0217:UGKFTN>2.3.CO;2
161. Lech JJ, Statham CN. Role of glucuronide formation in selective toxicity of 3-trifluoromethyl-4-nitrophenol (TFM) for sea lamprey—comparative aspects of TFM uptake and conjugation in sea lamprey and rainbow trout. Toxicol Appl Pharmacol. (1975) 31:150–8. doi: 10.1016/0041-008X(75)90063-0
162. Lawrence MJ, Grayson P, Jeffrey JD, Docker MF, Garroway CJ, Wilson JM, et al. Differences in the transcriptome response in the gills of sea lamprey acutely exposed to 3-trifluoromethyl-4-nitrophenol (TFM). p. niclosamide or a TFM: niclosamide mixture. Compar Biochem Physiol D-Genom Proteom. (2023) 48:101122. doi: 10.1016/j.cbd.2023.101122
163. Lawrence MJ, Grayson P, Jeffrey JD, Docker MF, Garroway CJ, Wilson JM, et al. Transcriptomic impacts and potential routes of detoxification in a lampricide-tolerant teleost exposed to TFM and niclosamide. Compar Biochem Physiol D-Genom Proteom. (2023) 46:101074. doi: 10.1016/j.cbd.2023.101074
164. Bock KW. Vertebrate UDP-glucuronosyltransferases: functional and evolutionary aspects. Biochem Pharmacol. (2003) 66:691–6. doi: 10.1016/S0006-2952(03)00296-X
165. Kondo M, Ikenaka Y, Nakayama SMM, Kawai YK, Ishizuka M. Duplication, loss, and evolutionary features of specific UDP-glucuronosyltransferase genes in carnivora (Mammalia, Laurasiatheria). Animals. (2022) 12:2954. doi: 10.3390/ani12212954
166. Shrestha B, Reed JM, Starks PT, Kaufman GE, Goldstone JV, Roelke ME, et al. Evolution of a major drug metabolizing enzyme defect in the domestic cat and other felidae: phylogenetic timing and the role of hypercarnivory. PLoS ONE. (2011) 6:e18046. doi: 10.1371/journal.pone.0018046
167. Dawson VK. Environmental fate and effects of the lampricide Bayluscide: a review. J Great Lakes Res. (2003) 29:475–92. doi: 10.1016/S0380-1330(03)70509-7
168. Lawrence MJ, Mitrovic D, Foubister D, Bragg LM, Sutherby J, Docker MF, et al. Contrasting physiological responses between invasive sea lamprey and non-target bluegill in response to acute lampricide exposure. Aquat Toxicol. (2021) 237:105848. doi: 10.1016/j.aquatox.2021.105848
169. Yin XS, Martinez AS, Perkins A, Sparks MM, Harder AM, Willoughby JR, et al. Incipient resistance to an effective pesticide results from genetic adaptation and the canalization of gene expression. Evol Appl. (2021) 14:847–59. doi: 10.1111/eva.13166
170. Lantz SR, Adair RA, Amberg JJ, Bergstedt R, Boogaard MA, Bussy U, et al. Next-generation lampricides: a three-stage process to develop improved control tools for invasive sea lamprey. Can J Fisher Aquat Sci. (2022) 79:692–702. doi: 10.1139/cjfas-2020-0316
171. Thresher RE, Jones M, Drake DAR. Evaluating active genetic options for the control of sea lamprey (Petromyzon marinus) in the Laurentian Great Lakes. Can J Fisher Aquat Sci. (2019) 76:1186–202. doi: 10.1139/cjfas-2018-0153
172. Marcy-Quay B, Lewandoski SA, Booth RMW, Connerton MJ, Jubar AK, Legard CD, et al. Sea lamprey control reduction during the COVID-19 pandemic corresponds to rapid increase in sea lamprey abundance. Fisheries. (2025) 50:355–65. doi: 10.1093/fshmag/vuaf020
173. Wang CJ, Hudson JM, Lassalle G, Whitesel TA. Impacts of a changing climate on native lamprey species: from physiology to ecosystem services. J Great Lakes Res. (2021) 47:S186–200. doi: 10.1016/j.jglr.2021.06.013
174. Angel JR, Kunkel KE. The response of Great Lakes water levels to future climate scenarios with an emphasis on Lake Michigan-Huron. J Great Lakes Res. (2010) 36:51–8. doi: 10.1016/j.jglr.2009.09.006
175. Bartolai AM, He L, Hurst AE, Mortsch L, Paehlke R, Scavia D, et al. Climate change as a driver of change in the Great Lakes St. Lawrence River Basin. J Great Lakes Res. (2015) 41:45–58. doi: 10.1016/j.jglr.2014.11.012
176. Hudson DT, Leach JA, Houle D. Thermal regimes of groundwater- and lake-fed headwater streams differ in their response to climate variability. Limnol Oceanogr Lett. (2023) 8:885–95. doi: 10.1002/lol2.10349
177. Shrestha RR, Pesklevits JC, Bonsal BR, Brannen R, Guo T, Hoffman S, et al. Rising summer river water temperature across Canada: spatial patterns and hydroclimatic controls. Environ Res Lett. (2024) 19:044058. doi: 10.1088/1748-9326/ad365f
178. Paukert C, Olden JD, Lynch AJ, Breshears DD, Chambers RC, Chu C, et al. Climate change effects on north american fish and fisheries to inform adaptation strategies. Fisheries. (2021) 46:449–64. doi: 10.1002/fsh.10668
179. Rahel FJ, Olden JD. Assessing the effects of climate change on aquatic invasive species. Conserv Biol. (2008) 22:521–33. doi: 10.1111/j.1523-1739.2008.00950.x
180. Assel RA. Classification of annual Great Lakes ice cycles: Winters of 1973-2002. J Clim. (2005) 18:4895–905. doi: 10.1175/JCLI3571.1
181. Rahimimovaghar M, Najafi MR, Shrestha RR, Liu YB. Successive warm-wet and warm-dry events in the Great Lakes Basin: future projections using CMIP6 models. Clim Dyn. (2025) 63:80. doi: 10.1007/s00382-024-07565-9
182. Phillips JC, McKinley GA, Bennington V, Bootsma HA, Pilcher DJ, Sterner RW, et al. The potential for CO2-induced acidification in freshwater: a Great Lakes case study. Oceanography. (2015) 28:136–45. doi: 10.5670/oceanog.2015.37
183. Hamid A, Bhat SU, Jehangir A. Local determinants influencing stream water quality. Appl Water Sci. (2019) 10:24. doi: 10.1007/s13201-019-1043-4
184. Dawson HA, Higgins-Weier CE, Steeves TB, Johnson NS. Estimating age and growth of invasive sea lamprey: a review of approaches and investigation of a new method. J Great Lakes Res. (2021) 47:S570–8. doi: 10.1016/j.jglr.2020.06.002
185. Johnson NS, Brenden TO, Swink WD, Lipps MA. Survival and metamorphosis of larval sea lamprey (Petromyzon marinus) residing in Lakes Michigan and Huron near river mouths. J Great Lakes Res. (2016) 42:1461–9. doi: 10.1016/j.jglr.2016.09.003
186. Holmes JA, Beamish FWH, Seelye JG, Sower SA, Youson JH. Long-term influence of water temperature, photoperiod, and food-deprivation on metamorphosis of sea lamprey Petromyzon marinus. Can J Fisher Aquat Sci. (1994) 51:2045–51. doi: 10.1139/f94-207
187. Kao YH, Youson JH, Holmes JA, Sheridan MA. Changes in lipolysis and lipogenesis in selected tissues of the landlocked lamprey, Petromyzon marinus, during metamorphosis. J Exp Zoo. (1997) 277:301–12. doi: 10.1002/(SICI)1097-010X(19970301)277:4<301::AID-JEZ4>3.0.CO;2-T
188. Lowe DR, Beamish FWH, Potter IC. Changes in proximate body composition of landlocked sea lamprey Petromyzon marinus (L) during larval life and metamorphois. J Fish Biol. (1973) 5:673–82. doi: 10.1111/j.1095-8649.1973.tb04503.x
189. Holmes JA, Youson JH. Fall condition factor and temperature influence the incidence of metamorphosis in sea lamrpeys, Petromyzon marinus. Can J Zool. (1994) 72:1134–40. doi: 10.1139/z94-151
190. Hume JB, Bennis S, Bruning T, Docker MF, Good S, Lampman R, et al. (2024). Evaluation of larval sea lamprey Petromyzon marinus growth in the laboratory: Influence of temperature and diet. Aquacult Res. (2024) 11:5547340. doi: 10.1155/2024/5547340
191. Holmes JA, Youson JH. Extreme and optimal temperatures for metamorphosis in sea lampreys. Transac Am Fisher Soc. (1998) 127:206–11. doi: 10.1577/1548-8659(1998)127
192. Lefevre S, Wang T, McKenzie DJ. The role of mechanistic physiology in investigating impacts of global warming on fishes. J Exp. Biol. (2021) 224:jeb238840. doi: 10.1242/jeb.238840
193. Schulte PM. The effects of temperature on aerobic metabolism: towards a mechanistic understanding of the responses of ectotherms to a changing environment. J Exp Biol. (2015) 218:1856–66. doi: 10.1242/jeb.118851
194. Trumpickas J, Shuter BJ, Minns CK, Cyr H. Characterizing patterns of nearshore water temperature variation in the North American Great Lakes and assessing sensitivities to climate change. J Great Lakes Res. (2015) 41:53–64. doi: 10.1016/j.jglr.2014.11.024
195. Farmer GJ, Beamish FWH, Robinson GA. Food consumption of adult landlocked sea lamprey, Petromyzon marinus L. Compar Biochem Physiol a—Physiol. (1975) 50:753–7. doi: 10.1016/0300-9629(75)90141-3
196. Cline TJ, Kitchell JF, Bennington V, McKinley GA, Moody EK, Weidel BC, et al. Climate impacts on landlocked sea lamprey: implications for host-parasite interactions and invasive species management. Ecosphere. (2014) 5:68. doi: 10.1890/ES14-00059.1
197. Muhametsafina A, Birceanu O, Hlina BL, Tessier LR, Wilkie MP. Warmer waters increase the larval sea lamprey's (Petromyzon marinus) tolerance to the lampricide 3-trifluoromethyl-4-nitrophenol (TFM). J Great Lakes Res. (2019) 45:921–33. doi: 10.1016/j.jglr.2019.07.011
198. Scholefield RJ, Slaght KS, Stephens BE. Seasonal variation in sensitivity of larval sea lampreys to the lampricide 3-Trifluoromethyl-4-Nitrophenol. North Am J Fisher Manage. (2008) 28:1609–17. doi: 10.1577/M06-178.1
199. Schueller J, Boogaard M, Kirkeeng C, Schloesser N, Wolfe S, Lettenberger A, et al. Seasonal differences in larval sea lamprey (Petromyzon marinus) sensitivity to the pesticide TFM. J Great Lakes Res. (2024) 50:102248. doi: 10.1016/j.jglr.2023.102248
200. Binder TR, McDonald DG. The role of temperature in controlling diel activity in upstream migrant sea lamprey (Petromyzon marinus). Can J Fisher Aquat Sci. (2008) 65:1113–21. doi: 10.1139/F08-070
201. McCann EL, Johnson NS, Hrodey PJ, Pangle KL. Characterization of sea lamprey stream entry using dual-frequency identification sonar. Transac Am Fisher Soc. (2018) 147:514–24. doi: 10.1002/tafs.10052
202. Arakawa H, Yanai S. Upper thermal tolerance of larval Arctic lamprey (Lethenteron camtschaticum). Ichthyol Res. (2021) 68:158–63. doi: 10.1007/s10228-020-00757-3
203. Golovanov VK, Nekrutov NS, Zvezdin AO, Smirnov AK, Tsimbalov IA. Thermoadaptation characteristics of European River Lamprey Lampetra fluviatilis smolts. J Ichthyol. (2019) 59:805–9. doi: 10.1134/S0032945219040052
204. Bjerselius R, Li WM, Teeter JH, Seelye JG, Johnsen PB, Maniak PJ, et al. (2000). Direct behavioral evidence that unique bile acids released by larval sea lamprey (Petromyzon marinus) function as a migratory pheromone. Can J Fisher Aquat Sci. 57, 557–569. doi: 10.1139/cjfas-57-3-557
205. Hansen MJ, Adams JV, Cuddy DW, Richards JM, Fodale MF, Larsons GL, et al. Optimizing larval assessment to support sea lamprey control in the Great Lakes. J Great Lakes Res. (2003) 29:766–82. doi: 10.1016/s0380-1330(03)70530-9
206. Moser ML, Almeida PR, King JJ, Pereira E. Passage and freshwater habitat requirements of anadromous lampreys: considerations for conservation and control. J Great Lakes Res. (2021) 47:S147–58. doi: 10.1016/j.jglr.2020.07.011
207. Buchinger TJ, Wang H, Li W, Johnson NS. Evidence for a receiver bias underlying female preference for a male mating pheromone in sea lamprey. Proc R Soc B-Biol Sci. (2013) 280:20131966. doi: 10.1098/rspb.2013.1966
208. Wood CM, Bucking C. The role of feeding in salt and water balance. In:M. Grosell, A. P. Farrell, C. J. Brauner, , editors. Multifunctional Gut of Fish, Vol. 30. (2011). p. 165–212. doi: 10.1016/s1546-5098(10)03005-0
209. Bartels H, Potter IC, Pirlich K, Mallatt J. Categorization of the mitochondria-rich cells in the gill epithelium of the freshwater phases in the life cycle of lampreys. Cell Tissue Res. (1998) 291:337–49. doi: 10.1007/s004410051003
210. Wilkie MP, Tessier LR, Boogaard M, O'Connor L, Birceanu O, Steeves TB, et al. Lampricide bioavailability and toxicity to invasive sea lamprey and non-target fishes: The importance of alkalinity, pH, and the gill microenvironment. J Great Lakes Res. (2021) 47:S407–20. doi: 10.1016/j.jglr.2021.09.005
211. Karachaliou E. Past & contemporary patterns of marine biodiversity: making inferences from anadromous sea lamprey population genomics & publicly archived genetic data (PhD thesis), University of Manitoba, Winnipeg, Manitoba, Canada (2025).
212. Youson JH, Holmes JA, Guchardi JA, Seelye JG, Beaver RE, Gersmehl JE, et al. Importance of condition factor and the influence of water temperature and s photoperiod on metamorphois of sea lamprey, Petromyzon marinus. Can J Fish Aquat Sci. (1993) 50:2448–56. doi: 10.1139/f93-269
Keywords: Petromyzon marinus, invasive species, facultative anadromy, thermal tolerance, lampricides, CTMax, fish gill, osmoregulation
Citation: Wilkie MP (2025) How the resilient ecophysiology of the sea lamprey allowed them to invade the Laurentian Great Lakes and could protect them from climate change. Front. Fish Sci. 3:1591515. doi: 10.3389/frish.2025.1591515
Received: 11 March 2025; Accepted: 29 August 2025;
Published: 27 October 2025.
Edited by:
Jane W. Behrens, Technical University of Denmark, DenmarkReviewed by:
Pedro Miguel Guerreiro, University of Algarve, PortugalMarius Dhamelincourt, INRAE Bretagne Normandie, France
Copyright © 2025 Wilkie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael P. Wilkie, bXdpbGtpZUB3bHUuY2E=
†ORCID: Michael P. Wilkie orcid.org/0000-0002-3844-8033
 Michael P. Wilkie
Michael P. Wilkie