- 1Institute of Marine Research, Animal Welfare Science Group, Matredal, Norway
- 2Department of Biology, Norwegian University of Science and Technology, Trondheim, Norway
This study investigated the contribution of catecholamines to stress regulation in Atlantic salmon, with the goal of clarifying inconsistencies between the classical model of cortisol control in teleosts and recent observations that challenge it. According to the traditional theory, cortisol secretion is driven primarily by adrenocorticotropic hormone (ACTH) through activation of the hypothalamic–pituitary–interrenal (HPI) axis. However, several studies in salmonids have reported that elevations in cortisol can occur in the absence of, or prior to, measurable increases in ACTH. To examine whether catecholamines influence cortisol production we performed ex vivo incubations of head kidney tissue either with ACTH (10−6 M, 10−8 M, and 10−10 M), or catecholamine (adrenaline and noradrenaline, 10−6 M, 10−8 M for both) and monitored cortisol production up to 60 min post-incubation. The results confirmed that ACTH elicited a cortisol response, but not catecholamines. However, when head kidneys were incubated with combinations of ACTH (10−6 M) and catecholamines (adrenaline or noradrenaline, 10−7 M each) there was a massive increase in cortisol (by ~2.4-fold) production far exceeding that of ACTH alone. These findings suggest that catecholamines are unlikely to function as independent stimulators of cortisol synthesis but will enhance the responsiveness or sensitivity of interrenal cells to ACTH. Such a synergistic interaction could represent an adaptive mechanism enabling rapid cortisol elevation during acute stress, thereby helping to reconcile discrepancies between ACTH and cortisol profiles reported in vivo. Overall, this work provides new insight into the interplay between sympathetic activation and endocrine regulation in teleost fish.
Highlights
• Combined exposure to ACTH and catecholamines enhances both the magnitude and speed of cortisol release in head kidney cells compared with that of ACTH alone.
• ACTH is confirmed as the primary driver of cortisol secretion in Atlantic salmon head kidney tissue.
• Catecholamines alone exert minimal effects, with only adrenaline inducing a modest cortisol increase.
Introduction
The term stress is defined as the behavioral and physiological response to a perceived threat (1). The response can be triggered by a variety of factors, including changes in water quality (2, 3), handling, predation threats, and exposure to pollutants or pathogens (4, 5). The traditional view is that the stress response activates two distinct pathways: the brain–sympathetic–chromaffin (BSC) axis and the hypothalamic–pituitary–interrenal (HPI) axis (5, 6). Activation of the BSC axis begins in the preoptic area of the brain and proceeds via the sympathetic nervous system, which sends neuroendocrine signals through cholinergic fibers. These fibers innervate organs such as the heart, muscles, and gastrointestinal tract, inducing immediate physiological responses. A long sympathetic nerve fiber also innervates the head kidney in teleost fish, where it stimulates chromaffin cells to release catecholamines (here referred to as adrenaline and noradrenaline, also known as epinephrine and norepinephrine) into the circulation (7–9). In the second pathway, the HPI axis, the hypothalamic cells of the nucleus preopticus stimulate the synthesis and release of adrenocorticotropic hormone (ACTH) from the pituitary. ACTH is then transported via the bloodstream to the head kidney, where it stimulates interrenal cells to synthesize and secrete glucocorticoids—primarily cortisol—into circulation. Because cortisol synthesis requires ACTH stimulation and de novo synthesis, its secretion is considerably slower than that of catecholamines. However, once elevated, cortisol concentrations can remain high for several hours, depending on the nature and duration of the stressor (6, 10–12).
There are several lines of evidence suggesting that this classical model is widely conserved across animal species. In mammals such as rats, ACTH levels rise rapidly after exposure to stress, followed by an increase in corticosterone (the rodent analog of cortisol) (13), with both peaking around 30 min after the onset of stress and then returning to baseline by 120 min (14, 15). This supports a direct cause–effect mechanism between ACTH release and cortisol synthesis. Likewise, in teleosts such as rainbow trout (Oncorhynchus mykiss) and coho salmon (Oncorhynchus kisutch), ACTH rises rapidly and before the cortisol response, further supporting the classical model (16, 17). However, in other teleosts, these links are not so clear. In tilapia (Oreochromis niloticus), the increase in cortisol following stress is not preceded or accompanied by an elevation in ACTH (18). This could suggest that factors other than, or in addition to, ACTH may be linked to the cortisol response. In Atlantic salmon parr, Madaro et al. (11) found no significant increase in ACTH until 1–4 h following stress and considerably later than the cortisol production, which increased as early as 10 min after exposure to the stressor. Similarly, in a study comparing triploid and diploid salmon, ACTH peaked between 15 and 30 min post-stress and considerably later than the increase in cortisol (12). These findings, in line with observations in tilapia, raise the question of whether factors other than, or in addition to, ACTH may be involved in the initial triggering of the cortisol production in Atlantic salmon. One possible hypothesis is that catecholamines, which appear in circulation immediately following stress, may be involved through paracrine signaling.
In teleost fish, interrenal and chromaffin cells are closely associated anatomically along the walls of the posterior cardinal vein in the head kidney (19–21). This anatomical proximity could suggest paracrine communication during the early stages of the stress response (22, 23). In support of this, results from sea bass (Dicentrarchus labrax) have shown that catecholamines can stimulate cortisol release from the head kidney tissue in vitro (24). However, results differ between studies, and there appear to be clear species differences in the response of the head kidney to catecholamines. For instance, in carp (Cyprinus carpio L.), catecholamines have been reported to inhibit cortisol release from interrenal cells (25), while catecholamines had no effect on cortisol release in sea bream (Sparus auratus) (24).
Considering this, the present study aimed to address discrepancies between classical theories and previous observations in Atlantic salmon regarding the regulation of cortisol release following stress. A central focus was the potential paracrine interaction between chromaffin and interrenal cells within the head kidney. To this end, ex vivo experiments were conducted to test whether catecholamines, either alone or in combination with ACTH, can stimulate cortisol production in head kidney tissue.
Materials and methods
Ex vivo head kidney incubations
Animals
Two ex vivo experiments were performed using Atlantic salmon farmed at the Institute of Marine Research in Matre (Masfjorden, Norway). For the first incubation trial, establishing a dose–response curve to cortisol production following exposure to ACTH or catecholamines, five fish housed in a circular tank of 3 m diameter were used, with a mean weight of 1,334.05 ± 191.75 g and a mean fork length of 49.45 ± 2.44 cm. For the second incubation trial, the combined effects of ACTH and catecholamines, six fish housed in a sea cage (Smørdalen sea cage research facility, 61°N of the Institute of Marine Research) were used, with a mean weight of 1,860 ± 413.7 g and a mean fork length of 52.58 ± 3.9 cm.
Induction trial preparation
Fish and tissue samples were processed in the same manner for the two incubation trials. Specifically, fish were euthanized by immersion in an anesthetic bath containing an overdose of anesthetic (1 g/L; MS-222 Tricaine Methanesulfonate, FINQUEL VET., MSD Animal Health, Norway), followed by percussion to the head. Each fish was then measured for length and weight. The head kidney was quickly dissected and placed in 50 ml Falcon tubes containing Hepes–Ringer solution (15 mM Hepes) kept on ice (0–4 °C) and prepared according to Rotllant et al. (24), consisting of NaCl (171 mM), KCl (2 mM), CaCl2 (2 mM), 0.25% (w/v) glucose, and 0.03% (w/v) bovine serum albumin. The pH of the medium was measured using an InoLab 7110 pH meter (WTW, Weilheim, Germany) and adjusted with 2 M NaOH as needed (final pH 7.38). Medium osmolality was then measured with a Fiske® 210 Micro-Sample Osmometer (Advanced Instruments, Norwood, MA, USA) to ensure a final value of approximately 350 mOsm/kg. Head kidney tissues were collected and kept on ice until the next dissection step, which involved removing the surrounding membranes, rinsing away blood, and finely slicing the tissue in a Petri dish containing fresh Hepes–Ringer solution. All procedures were performed under aseptic conditions using sterile instruments and solutions, and tissues were maintained at 0–4 °C to minimize enzymatic degradation and preserve viability during preparation and pre-incubation. Fragments then were then transferred to cell culture flasks (CELLSTAR®, Greiner Bio-One GmbH, Germany) or wells (Thermo Fisher Scientific, Roskilde, Denmark) for further processing and left to rest for 150 min in order to reach stabilized basal levels of cortisol (24, 26, 27). During this incubation the tissue was incubated in a Hepes–Ringer (1:10 tissue: medium) at 16 °C in a temperature-controlled incubator equipped with a Rotamax 120 shaker (Heidolph Scientific, Germany) to ensure gentle movement. The media pH was measured using universal indicator paper (pH 0–14). Media samples were collected every 50 min for cortisol quantification, and then the entire media was replaced with fresh solution. In the final 50-min phase (100 min after incubation began), tissue pools were carefully divided in small fragments which were weighed and placed into 24-well plates for the first incubation trial or into 6-well plates for the second incubation trial, as detailed in the following sections. The level of residual cortisol release measured before induction is displayed in Supplementary Figure S1. For both experiments, a stock solution of ACTH (Apollo Scientific, Stockport, UK) was prepared by dissolving ACTH in Hepes–Ringer solution to a concentration of 10−4 M. Stock solutions of adrenaline and noradrenaline (ThermoFisher GmbH, Kandel, Germany) were prepared at an initial concentration of 10−2 M. Approximately 2 ml of 1 M hydrochloric acid (HCl) was added during preparation to aid solubilization (final pH of the stock solutions ~3.5).
First incubation trial
Head kidney fragments (0.15–0.27 g) were transferred into 60 individual wells across three plates, one plate for each neurohormone, with samples arranged in groups of six replicates. In the ACTH plate, 24 wells were assigned to four treatment groups: ACTH at 10−6 M, 10−8 M, and 10−10 M, plus a control group in which the ACTH medium was replaced with Ringer's solution. In the other two plates—one for adrenaline and one for noradrenaline–18 samples per plate were assigned to three treatment groups: 10−6 M, 10−8 M, and a control group. Each well initially contained 1 ml of fresh Hepes–Ringer solution, with additional medium added according to tissue weight to maintain a consistent 1:10 tissue-to-medium ratio. Samples were incubated for an additional 50 min, completing the 150-min stabilization period. At the start of the induction, the medium was replaced with fresh medium containing the specified concentrations, and incubation continued for 60 min. During this period, 200 μl of medium was sampled every 20 min (20, 40, and 60 min) and replaced with fresh Hepes–Ringer solution. Collected samples were transferred to pre-labeled 0.6 ml tubes and stored at −80 °C until cortisol analysis.
Second incubation trial
In the second trial a larger amount of tissue was employed in larger wells. The head kidney pool was initially divided into two 6-well plates, with approximately 1 g of tissue per well in 10 ml of Hepes–Ringer solution. After 150 min of the stabilization period, the head kidney pool was then divided into four 6-well plates, one per treatment group, totaling six replicates of each treatment. Each well contained approximately 0.5 g of tissue in 5 ml of Hepes–Ringer solution (1:10 ratio). At the start of the induction, the medium was replaced with fresh medium solution containing (i) ACTH (10−6 M) alone; (ii) ACTH + adrenaline (5 × 10−7 M); (iii) ACTH + noradrenaline (5 × 10−7 M); (iv) adrenaline + noradrenaline (5 × 10−7 M); and (v) control group (Hepes–Ringer only). The incubation lasted for 120 min, during which media samples were collected at 10, 20, 40, 60, 90, and 120 min. At the 10-min timepoint, 100 μl of media was collected from each well; for all subsequent timepoints, 50 μl samples were collected. The samples were transferred to pre-labeled 0.6 ml tubes and stored at −80 °C until cortisol analysis. Unlike the first induction trial, no additional media was added during the treatment phase.
Plasma analyses
Prior to analysis, samples were thawed on ice and thoroughly mixed using a vortex mixer to ensure uniformity. Cortisol concentrations were measured using an enzyme-linked immunosorbent assay (ELISA; DEH3388, Demeditec Diagnostics GmbH, Kiel, Germany) with a standard detection range of 10–800 ng/ml, the sample size was 10 μl per well. Immunodetection quantification was performed using a Sunrise microplate reader (Tecan, Austria) at 450 nm.
Statistical analysis
Comparison between different concentrations in the first incubation trial for each timepoint were corrected by Tukey's multiple comparisons test. The cortisol concentration comparisons of each time point with the first measurement are corrected by Dunnett's multiple comparisons test. For all tests, p < 0.05 was considered significant.
The data on the second incubation trial were tested by a linear mixed-effects model fitted to examine the effects of treatment, timepoint, and their interaction on cortisol release. The model included sample ID as a random effect to account for repeated measures and used an AR(1) correlation structure to model temporal autocorrelation across timepoints within each subject. Statistical analyses were performed with RStudio (R version 4.5.1) and using the nlme (version 3.1-168), MuMIn (version 1.48.11), and emmeans (version 1.11.2) packages. All graphs were produced using GraphPad Prism (GraphPad Software, version 10.4.2, San Diego, USA). All data are presented as mean ± SEM; otherwise.
Results
Ex vivo: first incubations with single hormones
Effect of ACTH on cortisol production
Incubating head kidney tissue with increasing amounts of ACTH led to general dose-dependent cortisol production throughout the 60 min of incubation (Figure 1a). The lowest increase was found with ACTH 10−10 M, and significant increase in cortisol was found only after 40 min, 35.92 ± 5.180 nanograms per gram (ng/g; p = 0.045), and peaking at 53.88 ± 7.66 ng/g after 60 min. The media with the highest concentration of ACTH (10−6 M) induced a significantly increased cortisol production after 20 min (56.85 ± 13.36 ng/g compared to the T = 0 level of 23.91 ± 7.16 ng/g; p = 0.04) peaking at 216.9 ± 47.64 ng/g after 60 min (p = 0.019 compared to 0 min). The 10−8 M group cortisol release after 60 min was intermediate between the two groups, peaking at 155.6 ± 63.10 ng/g after 60 min (p = 0.166 compared to 0 min).
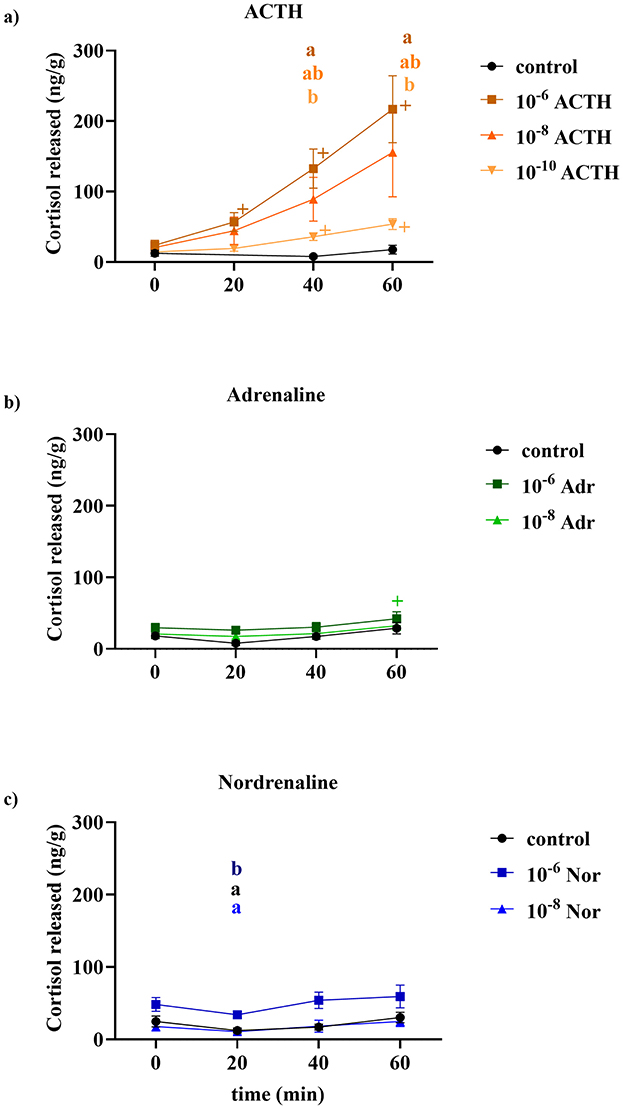
Figure 1. Cortisol release (ng/g tissue) over time (min) in response to different concentrations of (a) ACTH, (b) adrenaline (Adr), and (c) noradrenaline (Nor). ACTH was tested at 10−6, 10−8, and 10−10 M, while adrenaline and noradrenaline were tested at 10−6 and 10−8 M. Each treatment was compared to a control group. In the ACTH group the control was exclude for statistical analyses due to the 20 min time point being missing. Data are presented as mean ± SEM (n = 6 per group per time point). The “+” indicates a significant difference between values at each time point compared with their value at 10 min. Different letters at a given time point indicate significant differences between treatment groups (p < 0.05).
Effect of catecholamines on cortisol production
When head kidney tissue samples were added with media containing only adrenaline 10−6 or 10−8 M (Figure 1b), there was little or no change in cortisol release compared to the control group. At 60 min there was a small but still significant increase in cortisol concentration (69.97 ± 47.64 ng/g compared to the 4.5 ± 7.16 ng/g at 0 min; p = 0.0260). Similarly, noradrenaline alone had no effect on cortisol release from the head kidney tissue (Figure 1c). At 20 min, the medium collected from tissue exposed to 10−6 M noradrenaline had significantly higher cortisol concentration than the other two groups (p = 0.03 when compared with control and p = 0.01 if compared with 10−8 M). However, this difference is more likely attributable to residual cortisol present in the tissue rather than to an induction effect by noradrenaline, as cortisol levels in the 10−6 M noradrenaline group at 20 min (34.16 ± 4.29 ng/g) did not differ significantly from baseline levels at the start of induction (48.31 ± 9.498 ng/g; p = 0.16). Moreover, the cortisol levels measured at 60 min post-induction (59.49 ± 15.75 ng/g) did not differ significantly from baseline value (p = 0.76).
Ex vivo: second incubations, single and combined hormones
Combined effect of ACTH and catecholamines on cortisol production
Figure 2 shows the effect of catecholamines only, ACTH only, and the combination of ACTH and catecholamines on inducing cortisol release by head kidney tissue. For each set of incubations, the increase was relatively linear over time. The combined effect of adrenaline and noradrenaline on stimulating a cortisol release was very low and not statistically relevant (p = 0.57), and cortisol levels rose from 48.9 ± 22.59 ng/g at 10 min to 185 ± 63.38 ng/g after 120 min. The effect of ACTH alone was more pronounced, peaking at 574.5 ± 137.48 ng/g after 120 min. Combining either catecholamine with ACTH resulted in an approximately 2.4-fold increase in cortisol release, peaking at 1,357.07 ± 262.09 ng/g and 1,160.38 ± 262.09 ng/g after 120 min for the combinations of ACTH with adrenaline and ACTH with noradrenalin, respectively. At 20 min after incubation started, only the combinations of ACTH with adrenaline or noradrenaline showed a significant increase in cortisol levels (231.6 ± 5.94 ng/g and 347 ± 74.45 ng/g, respectively) compared to 10 min after induction (89.6 ± 19.66 ng/g and 118.45 ± 25.20 ng/g, respectively). In the ACTH group, cortisol levels began to rise at 40 min (217.64 ± 47.36 ng/g; p = 0.079) but only in a significant manner 60 min post-induction (284.8 ± 68.98 ng/g; p = 0.019). Cortisol continued to increase similarly in the groups where ACTH was combined with either adrenaline or noradrenaline throughout the trial.
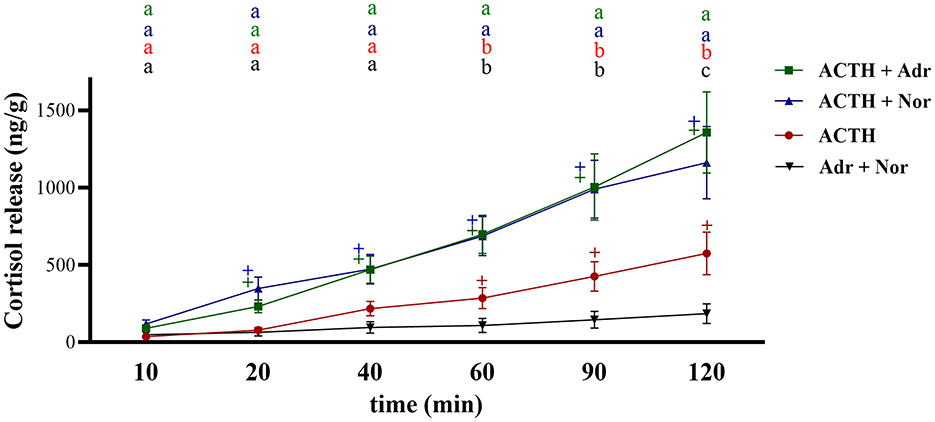
Figure 2. Cortisol release (ng/g tissue) over time (min) from head kidney fragment in response to the induction of medium containing ACTH alone, ACTH in combination with adrenaline (ACTH + Adr), ACTH in combination with noradrenaline (ACTH+Nor), or adrenaline and noradrenaline (Adr+Nor). ACTH concentration in the media was 10−6 M, while it was 10−7 for adrenaline and noradrenaline. The “+” indicates a significant difference between ACTH (red), ACTH+Adr (green), ACTH+Nor (blue), and Adr+Nor (black) values at each time point compared with their values at 10 min. Letters indicate significant differences between the treatments at each time point. Values are considered different when p < 0.05.
Discussion
This study aimed to elucidate the role of catecholamines in regulating the stress response in Atlantic salmon and to address discrepancies between the traditional model of stress regulation in fish and emerging evidence challenging it. In the present paper, we examined whether catecholamines, either alone or in combination with ACTH, could directly stimulate cortisol production ex vivo.
The results clearly confirm the established role of ACTH stimulating cortisol release in salmon in a dose-dependent manner. This mechanism in fish (6, 10) is similar to that of other vertebrates, including mammals (28–30), in that the stress-related HPI axis is a principal regulator of cortisol production. This regulation occurs through a cascade in which the hypothalamus secretes corticotropin-releasing factor, which stimulates pituitary ACTH release, ultimately driving cortisol synthesis and secretion by interrenal cells (6). Sumpter et al. (16), showed that handling and confinement first induced a steady increase in plasma ACTH levels, followed by a delayed cortisol release in both coho salmon (Oncorhynchus kisutch) and rainbow trout (Oncorhynchus mykiss). In addition, ACTH can also stimulate cortisol production in other tissues. For example, Samaras and Pavlidis (27) showed that in vitro ACTH administration increased cortisol release in isolated scales of sea bass. This effect was reversed when the scales were incubated with metyrapone, an inhibitor of cortisol synthesis. However, recent studies in Atlantic salmon have shown that increases in ACTH do not always clearly precede cortisol release following stress (11, 12), challenging the classical view that ACTH alone triggers cortisol production through the HPI axis. Similarly, in tilapia, the increase in cortisol following stress was not preceded or accompanied by an elevation in plasma ACTH (18). One possible explanation for this could be a paracrine stimulation of cortisol production by catecholamines as observed by Rotllant et al. (24).
In the current trial, catecholamines alone showed limited capacity to stimulate cortisol secretion. Only adrenaline produced a very small increase at 60 min post-induction at the highest concentration tested, whereas noradrenaline had no effect. These results suggest possible species-specific differences in paracrine regulation, as the pattern observed in Atlantic salmon differed from that reported by Rotllant et al. (24) in sea bass and sea bream. In sea bream, neither adrenaline nor noradrenaline affected cortisol release, while in sea bass, both catecholamines stimulated cortisol secretion. Because the hormone concentrations applied here were comparable to those used by Rotllant et al. (24), it remains possible that higher doses might elicit a stronger stimulatory response.
The most compelling finding of the present study was the pronounced increase in cortisol production when head kidneys were co-incubated with both ACTH and catecholamines. While ACTH alone elicited a moderate cortisol response, the presence of catecholamines enhanced both the magnitude and speed of cortisol release. This suggests that catecholamines may increase the sensitivity or responsiveness of interrenal cells to ACTH, rather than acting as independent stimulants. This mechanism appears biologically plausible, as cortisol in teleost fish, unlike in mammals, often serves dual roles as both a glucocorticoid and a mineralocorticoid due to the absence of aldosterone (6, 31, 32). Consequently, cortisol is involved not only in stress responses but also in lower concentrations in osmoregulation, ion balance, metabolic processes, and energy balance (31, 33–35). An experiment by Gesto et al. (36) on rainbow trout suggested a possible paracrine role of catecholamines in the head kidney, contributing to enhanced cortisol production. Interestingly, the authors reported that intraperitoneal injection of propranolol increased plasma catecholamine secretion and cortisol levels in stressed fish compared to stressed-only fish or controls. Rotllant et al. (24), in their study showed that the stimulatory effect of catecholamines on cortisol production occurs through β-adrenoceptor activation of the adenylate cyclase–protein kinase A signaling pathway, ultimately enhancing cortisol synthesis. In Gesto et al.'s (36) study, however, propranolol treatment—expected to block β-adrenoceptor-mediated effects—did not prevent cortisol production. This suggests the involvement of alternative pathways beyond β-adrenoceptor signaling.
The combined action of ACTH and catecholamines may therefore represent an important adaptive mechanism to rapidly adjust cortisol levels in response to stress. These findings suggest that sympathetic activation via the catecholamines—adrenaline and noradrenaline—play a key role in rapidly stimulating cortisol release during an acute stress episode, even when circulating ACTH levels are relatively low. By contrast, ACTH alone triggered a lower cortisol response, implying that it may be more important for maintaining basal cortisol levels and supporting stress-dependent physiological regulation when sympathetic activation is minimal. Although much of this proposed mechanism remains speculative, this potential synergistic interaction could help explain the temporal mismatch reported by Balm et al. (18) and Madaro et al. (11, 12), in which cortisol levels rise rapidly and remain elevated after acute stress, even though ACTH peaks at a later stage. In the current trial we tested only a single concentration of catecholamines in combination with ACTH. It would be interesting to investigate whether varying catecholamine concentrations can differentially modulate the magnitude of cortisol release. Such experiments would provide valuable insight into the capacity of the sympathetic system to directly fine-tune the stress response in Atlantic salmon and to assess whether similar mechanisms operate in other species.
Conclusion
This study provides new insights into the role of catecholamines in regulating the stress response of Atlantic salmon. The ex vivo experiments showed that catecholamines alone, at the concentrations tested, had negligible to minor effects on cortisol release, suggesting that, at least in Atlantic salmon, their immediate direct paracrine action on interrenal cells is limited. However, when combined with ACTH, both adrenaline and noradrenaline significantly increased cortisol secretion far beyond that induced by ACTH alone. This synergistic effect, reported here for the first time in Atlantic salmon, indicates that catecholamines may act as modulators of interrenal sensitivity to ACTH rather than as independent stimulants. Such an interaction emphasizes the importance of sympathetic activation in shaping the early cortisol response. As a preliminary ex vivo study, these findings warrant in vivo validation under controlled acute stress scenarios. Further studies should also investigate whether the current observations in Atlantic salmon are consistent across other fish species.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
AM: Formal analysis, Methodology, Supervision, Data curation, Software, Conceptualization, Writing – review & editing, Visualization, Writing – original draft, Investigation. VB: Formal analysis, Data curation, Conceptualization, Methodology, Writing – review & editing, Writing – original draft, Investigation, Software, Visualization. RO: Conceptualization, Visualization, Methodology, Writing – original draft, Supervision, Writing – review & editing, Investigation, Software, Data curation, Formal analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding for this work was provided Institute of Marine Research Project 14930 (Fish Welfare in aquaculture) and NTNU.
Acknowledgments
The authors wish to extend their gratitude to Karen Anita Kvestad for her invaluable and meticulous assistance in the laboratory, and to Liv Søfteland for sharing her input and suggestions on the tissue culture procedures. We also deeply appreciate the support provided by Christine Sørfonn, Thea Svendsen, and Ivar Helge Matre at the Matre Research Facility for their assistance with fish rearing and sampling.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frish.2025.1708976/full#supplementary-material
Supplementary Figure 1 | Stabilization of cortisol release (ng/g tissue) during the pre-treatment phase. Data represent mean ± SEM from n = 21 samples at 50 and 100 min, and n = 72 samples at 150 min. Cortisol levels decreased steadily over time, indicating a baseline by 150 min.
References
1. Schreck CB, Tort L. 1 - The concept of stress in fish. In: Schreck CB, Tort L, Farrell AP, Brauner CJ, , editors. Fish Physiology. London, UK: Academic Press (2016). pp. 1–34. doi: 10.1016/B978-0-12-802728-8.00001-1
2. Canosa LF, Bertucci JI. The effect of environmental stressors on growth in fish and its endocrine control. Front Endocrinol. (2023) 14:1109461. doi: 10.3389/fendo.2023.1109461
3. Zhang K, Ye Z, Qi M, Cai W, Saraiva JL, Wen Y, et al. Water quality impact on fish behavior: a review from an aquaculture perspective. Rev Aquac. (2025) 17:e12985. doi: 10.1111/raq.12985
4. Tort L. Stress and immune modulation in fish. Spec. Issue Teleost Fish Immunol. (2011) 35:1366–75. doi: 10.1016/j.dci.2011.07.002
5. Yuan M, Fang Q, Lu W, Wang X, Hao T, Chong C.-M, et al. Stress in fish: neuroendocrine and neurotransmitter responses. Fishes. (2025) 10:307. doi: 10.3390/fishes10070307
6. Wendelaar Bonga SE. The stress response in fish. Physiol Rev. (1997) 77:591–625. doi: 10.1152/physrev.1997.77.3.591
7. Fabbri E, Moon TW. Adrenergic signaling in teleost fish liver, a challenging path. Comp Biochem Physiol B Biochem Mol Biol. (2016) 199:74–86. doi: 10.1016/j.cbpb.2015.10.002
8. Reid SG. Control of Catecholamine Storage and Release in Teleost Fish. Ottawa, ON: University of Ottawa (Canada) (1995).
9. Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. (2009) 10:397–409 doi: 10.1038/nrn2647
10. Gorissen M, Flik G. The endocrinology of the stress response in fish: an adaptation-physiological view. In:Schreck CB, Tort L, Farrell AP, Brauner CJ, , editors. Fish Physiology, Vol. 35. London: Academic Press (2016). p 75-111. doi: 10.1016/B978-0-12-802728-8.00003-5
11. Madaro A, Nilsson J, Whatmore P, Roh H, Grove S, Stien LH, et al. Acute stress response on Atlantic salmon: a time-course study of the effects on plasma metabolites, mucus cortisol levels, and head kidney transcriptome profile. Fish Physiol Biochem. (2023) 49:97–116. doi: 10.1007/s10695-022-01163-4
12. Madaro A, Lai F, Fjelldal PG, Hansen T, Gelebart V, Muren B, et al. Comparing physiological responses of acute and chronically stressed diploid and triploid Atlantic salmon (Salmo salar). Aquac Rep. (2024) 36:102041. doi: 10.1016/j.aqrep.2024.102041
13. Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. (2016) 6:603–21. doi: 10.1002/j.2040-4603.2016.tb00694.x
14. Lovelock DF, Deak T. Acute stress imposed during adolescence has minimal effects on hypothalamic-pituitary-adrenal (HPA) axis sensitivity in adulthood in female Sprague Dawley rats. Physiol Behav. (2020) 213:112707. doi: 10.1016/j.physbeh.2019.112707
15. Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, et al. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab. (2005) 289:E823–8. doi: 10.1152/ajpendo.00122.2005
16. Sumpter JP, Dye HM, Benfey TJ. The effects of stress on plasma ACTH, αMSH, and cortisol levels in salmonid fishes. Gen Comp Endocrinol. (1986) 62:377–85. doi: 10.1016/0016-6480(86)90047-X
17. Pickering AD, Pottinger TG, Sumpter JP, Carragher JF, Le Bail PY. Effects of acute and chronic stress on the levels of circulating growth hormone in the rainbow trout, Oncorhynchus mykiss. Gen Comp Endocrinol. (1991) 83:86–93. doi: 10.1016/0016-6480(91)90108-I
18. Balm PHM, Pepels PPLM, Helfrich S, Hovens MLM, Bonga SW. Adrenocorticotropic hormone in relation to interrenal function during stress in tilapia (Oreochromis mossambicus). Gen Comp Endocrinol. (1994) 96:347–60. doi: 10.1006/gcen.1994.1190
19. Gaber W, Abdel-Maksoud FM. Interrenal tissue, chromaffin cells and corpuscles of Stannius of Nile tilapia (Oreochromis niloticus). Microscopy. (2019) 68:195–206. doi: 10.1093/jmicro/dfy146
20. Grassi Milano E, Basari F, Chimenti C. Adrenocortical and adrenomedullary homologs in eight species of adult and developing teleosts: morphology, histology, and immunohistochemistry. Gen Comp Endocrinol. (1997) 108:483–96. doi: 10.1006/gcen.1997.7005
21. Nandi J. New Arrangement of interrenal and chromaffin tissues of teleost fishes. Science. (1961) 134:389–90. doi: 10.1126/science.134.3476.389
22. Reid SG, Vijayan MM, Perry SF. Modulation of catecholamine storage and release by the pituitary-interrenal axis in the rainbow trout, Oncorhynchus mykiss. J Comp Physiol B. (1996) 165:665–76. doi: 10.1007/BF00301135
23. Martorell-Ribera J, Koczan D, Tindara Venuto M, Viergutz T, Brunner RM, Goldammer T, et al. Experimental handling challenges result in minor changes in the phagocytic capacity and transcriptome of head-kidney cells of the salmonid fish coregonus maraena. Front Vet Sci. (2022) 9:889635. doi: 10.3389/fvets.2022.889635
24. Rotllant J, Ruane NM, Dinis MT, Canario AVM, Power DM. Intra-adrenal interactions in fish: catecholamine stimulated cortisol release in sea bass (Dicentrarchus labrax L.). Comp Biochem Physiol A Mol Integr Physiol. (2006) 143:375–81. doi: 10.1016/j.cbpa.2005.12.027
25. Gfell B, Kloas W, Hanke W. Neuroendocrine effects on adrenal hormone secretion in carp (Cyprinus carpio). Gen Comp Endocrinol. (1997) 106:310–9. doi: 10.1006/gcen.1996.6870
26. Conde-Sieira M, Alvarez R, López-Patiño MA, Míguez JM, Flik G, Soengas JL. ACTH-stimulated cortisol release from head kidney of rainbow trout is modulated by glucose concentration. J Exp Biol. (2013) 216:554–67. doi: 10.1242/jeb.076505
27. Samaras A, Pavlidis M. Fish scales produce cortisol upon stimulation with ACTH. Animals. (2022) 12:3510. doi: 10.3390/ani12243510
28. Birnie MT, Conway-Campbell BL. Dynamics of ACTH and cortisol secretion and implications for disease. Endocr Rev. (2020) 41:bnaa002. doi: 10.1210/endrev/bnaa002
29. Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. (2005) 67:259–84. doi: 10.1146/annurev.physiol.67.040403.120816
30. Mbiydzenyuy NE. and Qulu LA. Stress, hypothalamic-pituitary-adrenal axis, hypothalamic-pituitary-gonadal axis, and aggression. Metab Brain Dis. (2024) 39:1613–36. doi: 10.1007/s11011-024-01393-w
31. Mommsen TP, Vijayan MM, Moon TW. Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fish. (1999) 9:211–68. doi: 10.1023/A:1008924418720
32. Wu CY, Lee TH, Tseng DY. Mineralocorticoid receptor mediates cortisol regulation of ionocyte development in tilapia (Oreochromis mossambicus). Fishes. (2023) 8:283. doi: 10.3390/fishes8060283
33. Cowan M, Azpeleta C, López-Olmeda JF. Rhythms in the endocrine system of fish: a review. J Comp Physiol B. (2017) 187:1057–89. doi: 10.1007/s00360-017-1094-5
34. McCormick SD. The hormonal control of osmoregulation in teleost fish. Life Sci. (2011) 1:1466–73. doi: 10.1016/B978-0-12-374553-8.00212-4
35. McCormick SD, Taylor ML, Regish AM. Cortisol is an osmoregulatory and glucose-regulating hormone in Atlantic sturgeon, a basal ray-finned fish. J Exp Biol. (2020) 223:jeb220251. doi: 10.1242/jeb.220251
Keywords: adrenaline, noradrenaline, cortisol, ex vivo study, head kidney
Citation: Madaro A, Basso VW and Olsen RE (2025) Catecholamines as central modulators of the stress response. A preliminary study in Atlantic salmon (Salmo salar) head kidney cells. Front. Fish Sci. 3:1708976. doi: 10.3389/frish.2025.1708976
Received: 19 September 2025; Revised: 20 October 2025;
Accepted: 04 November 2025; Published: 26 November 2025.
Edited by:
D. K. Meena, Central Inland Fisheries Research Institute (ICAR), IndiaReviewed by:
Michael James Murray, Monterey Bay Aquarium, United StatesPande Gde Sasmita Julyantoro, Udayana University, Indonesia
Nitesh Kumar Yadav, Maharana Pratap University of Agriculture and Technology, India
Copyright © 2025 Madaro, Basso and Olsen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angelico Madaro, YW5nZWxpY28ubWFkYXJvQGhpLm5v
 Angelico Madaro
Angelico Madaro Victoria Warth Basso
Victoria Warth Basso Rolf Erik Olsen
Rolf Erik Olsen