- 1College of Computer Science and Technology, Jilin University, Changchun, China
- 2State Key Laboratory of Cancer Biology, National Clinical Research Center for Digestive Diseases, Xijing Hospital of Digestive Diseases, Fourth Military Medical University, Xi’an, China
- 3College of Life Sciences, Northwest University, Xi’an, China
- 4Department of Pediatric Oncology, The First Hospital of Jilin University, Changchun, China
Gastric cancer (GC) is a considerable global health burden. Accumulating evidence suggests that long non-coding RNAs (lncRNAs) are aberrantly expressed in many cancers and play important roles in GC. However, only a few lncRNAs have been functionally characterized. In this study, we identified that long intergenic non-protein coding RNA 941 (LINC00941) is a potential biomarker for diagnosis and prognosis from the cancer genome atlas (TCGA), and we found that the expression of LINC00941 is associated with tumor depth and distant metastasis in GC. Furthermore, functional enrichment analysis of LINC00941 co-expression network demonstrated that LINC00941 might be an essential regulator of tumor metastasis and cancer cell proliferation. To validate our findings, we utilized the loss-of-function analysis to reveal the biological function of LINC00941 in GC cells. Loss-of-function analysis revealed that silence of LINC00941 inhibits GC cells proliferation, migration, and invasion in vitro and modulates tumor growth in vivo. Our findings confirmed that LINC00941 plays an important oncogenic function in GC and may serve as a potential biomarker for diagnosis and prognosis of GC.
Introduction
Gastric cancer (GC) is one of the most frequently diagnosed cancer and the leading cause of cancer death in the world (Bray et al., 2018). In China, GC is a considerable health burden (Chen W. et al., 2018). The outlook for patients with metastatic GC is unfavorable, with median survival does usually not exceed 1 year (Van Cutsem et al., 2016). It is urgent to explore novel biomarkers and therapeutic targets for GC.
Long non-coding RNAs (lncRNAs) is an important subclass of non-coding RNAs (ncRNAs) and usually longer than 200 nucleotides with lack of protein-coding capability (Engreitz et al., 2016). Mounting evidence confirmed that lncRNAs are aberrantly expressed in many cancers and play key roles in promoting tumor initiation and progression (Lin and Yang, 2018). Several lncRNAs such as UCA1, MALAT1, HOXA11-AS, and ZEB1-AS1 have been proposed as individual diagnostic or prognostic biomarkers in GC (Sun et al., 2016; Li et al., 2017a,b; Wang et al., 2017). However, only a few lncRNAs have been functionally characterized and the mechanism by which lncRNAs regulate GC remains to be fully elucidated.
Long intergenic non-protein coding RNA 941 (LINC00941), also known as MSC-upregulated factor (lncRNA-MUF), is an lncRNA located in the 12p11.21 region of the human genome. In hepatocellular carcinoma, high expressed LINC00941 significantly promoted epithelial to mesenchymal transition (EMT) and malignant capacity (Yan et al., 2017). In lung adenocarcinoma, LINC00941 displayed prognostic values and regulated PI3K-AKT signaling pathway (Wang et al., 2018). In GC, LINC00941 is highly expressed in GC tissues and may participate in the process of GC (Luo et al., 2018). However, the precise biological function of LINC00941 in GC has not been characterized.
In this study, we confirmed that LINC00941 plays an important oncogenic function in GC by systematically integrating bioinformatics methods and in vitro/vivo studies. We identified that LINC00941 acts as a potential diagnostic and prognostic biomarker in GC, and the expression of LINC00941 is associated with tumor depth and distant metastasis based on the analysis of RNA-seq data from TCGA. In order to characterize the function of LINC00941 in GC, we applied weighted gene co-expression network analysis (WGCNA) (Langfelder and Horvath, 2008) to constructed LINC00941 co-expression network. Furthermore, through the functional enrichment analysis, we found that LINC00941 might be an essential regulator of tumor metastasis and cancer cell proliferation. To validate our findings, we utilized the loss-of-function analysis to reveal the biological function of LINC00941 in GC cells. Loss-of-function analysis confirmed that silence of LINC00941 inhibits GC cells proliferation, migration, and invasion in vitro and modulates tumor growth in vivo. Our results demonstrated that LINC00941 plays an important oncogenic function in GC and acts as a potential biomarker for diagnosis and prognosis of GC.
Materials and Methods
GC Data Collection and Processing
Gastric cancer data, including clinical information and gene expression data, were obtained from TCGA1 (Cancer Genome Atlas Research and Network, 2014). In this study, all samples with follow-up time exceeding 2000 days were excluded. GC data was processed as described in our previous work (Liu H. et al., 2018).
Identifying Cancer-Related, Metastasis-Related, and Survival-Related lncRNAs
In order to identify cancer-related lncRNAs, we utilized Mann–Whitney U-test to compare the expression values between cancer samples and normal samples. To identify metastasis-related lncRNAs, we used the same statistical method to compare the expression values between metastatic (M1) samples and non-metastatic (M0) samples. The cancer-related and metastasis-related lncRNAs with p-values < 0.05 and fold change ≥ 1 were considered significant. In order to identify survival-related lncRNAs, we divided samples into two groups (high and low) based on the median expression level of each lncRNA. We then utilized univariate Cox proportional-hazards regression model and log-rank test to identify survival-related lncRNAs. LncRNAs with p < 0.05 were considered survival related. Kaplan-Meier plot was used to represent the results.
LncRNA Co-expression Network Construction and Functional Enrichment Analysis
Long non-coding RNAs co-expression network construction was performed by combining lncRNAs with genes expression data using the R package WGCNA (Langfelder and Horvath, 2008). First, the expression matrix was constructed based on the Spearman’s rank correlation coefficient between all gene pairs. Then, the expression matrix was converted into an adjacency matrix (AM), and AM was further converted into a topological overlap matrix (TOM). Based on TOM, the average-linkage hierarchical clustering method was used to cluster the genes and dynamic tree cut algorithm was used to identify lncRNA co-expressed module that was set the minimum module size to 30. To determine the functions of lncRNA co-expressed module, the genes in the module were subjected to functional enrichment analysis by gene ontology (GO) (Antonazzo et al., 2017) and Kyoto Encyclopedia of Genes and Genomes (KEGG) (Kanehisa et al., 2017) analyses with DAVID 6.82 (Huang da et al., 2009). In this study, the result of functional enrichment analysis with FDR < 0.05 was considered statistically significant.
Cell Culture, RNA Extraction, and Real-Time PCR
The human GC cells (MKN45 and AGS) were purchased from American Type Culture Collection (ATCC). Cell culture, RNA extraction, and real-time PCR were performed as described in our previous work (Liu H. et al., 2018). Primer sets specific for LINC00941 were designed by RiboBio (RiboBio, China). The primer sequences of LINC00941 were as follows: LINC00941 forward, 5′- ACCACTACACTCAGCCAAATAC-3′, reverse, 5′ - GGCTATCAACTGTCTCCTTTAGAC-3′.
Cell Transfection
The short interfering RNA (siRNA) targeting LINC00941 were synthesized by RiboBio (RiboBio, China). The siRNA sequences for LINC00941 were as follows: LINC0 0941-si1: 5′- ACGCGTTGCATAACCTGA-3′, LINC00941-si2: 5′- GAGACAGTTGATAGCCAAA-3′. Oligonucleotide transfection was performed using Lipofectamine 2000 reagent (Invitrogen, United States). Short hairpin RNA (shRNA) directed against LINC00941 were synthesized by GeneChem (Shanghai, China) and were inserted into the vector. The sequence of the effective shRNA was as follows: sh-LINC00941: GAGACAGTTGATAGCCAAA. We utilized an empty vector as a negative control (VECTOR).
CCK-8, Colony Formation, Cell Migration, and Invasion Assays
CCK-8 and colony formation assays were performed as described in our previous work (Liu H. et al., 2018). Cell migration and invasion assays were measured using Transwell chamber (Corning, United States) with 8-μm pore membrane. Cells need to be starved for 12 h in serum-free medium before the experiment. For the migration assays, 5 × 105 cells were seeded into the top chamber of transwell. For the invasion assays, 1 × 105 cells were seeded top chamber of transwell with Matrigel (Corning, United States). After 24 h, non-invasive cells were removed, and that migrated or invaded cells were fixed and stained with 5% crystal violet (Beyotime Biotechnology, China). Cells were photographed and counted in five random fields at 100× magnification.
Protein Extraction and Western Blot Analysis
Cells were washed three times with PBS and collected in RIPA lysis buffer (Beyotime Biotechnology, China) supplemented with a protease inhibitor cocktail (Calbiochem, United States). Protein concentration was determined by staining with Coomassie Blue (Beyotime Biotechnology, China). After electrophoresis, the protein was transferred to a polyvinylidene fluoride membrane (Merck Millipore, Germany). After blocking with 0.1% Tween 20 (TBS-T) in Tris-buffered saline containing 5% skim milk for 1 h at room temperature, the primary monoclonal antibody was added to the membrane and incubated overnight at 4°C. The next day, the membrane was incubated with the corresponding secondary antibody for 1 h at room temperature and the signal was detected in a Bio-Rad ChemiDoc XRS imaging system. The ratio of the gray value of the target protein to the gray value of β-actin indicates the relative amount of protein. Primary antibodies were used as follows: anti-E-cadherin (1:1000; Cell Signaling Technology, United States), anti-Fibronectin (Cell Signaling Technology, United States) and anti-Snail1 (Cell Signaling Technology, United States), and anti-GAPDH (Santa Cruz Biotech, United States).
Tumorigenicity Assays in Nude Mice
Empty vector (negative control) and sh-LINC00941 stained cells SGC-7901 were injected subcutaneously into either side of the axillary region of male BALB/c nude mice (4–5 weeks old). Ten female BALB/c nude mice (5–6 weeks old) were randomly divided into two groups (sh-LINC00941 and VECTOR) and the mice were maintained under specific pathogen-free conditions. To establish the subcutaneous xenograft tumor model, 1 × 107 cells were injected into the right side of the back of nude mice. We measured and recorded nude mice body weight and tumor size weekly. After 4 weeks, all the nude mice were sacrificed under deep anesthesia. This study was carried out in strict accordance with the ethical standards of the Fourth Military Medical University. The tumor volumes were calculated using the following formula: tumor volume (mm3) = [length (mm) × width2 (mm) × π ]/6.
Results
LINC00941 Is a Potential Biomarker for Diagnosis and Prognosis of GC
We obtained 385 samples with gene expression profiles and clinical information from TCGA. By comparing 358 cancer samples with 27 normal samples, we identified 926 significantly cancer-related lncRNAs (fold change ≥ 1, p < 0.05, Mann-Whitney U-test, Supplementary Table S1). By comparing 24 metastatic (M1) samples with 316 non-metastatic (M0) samples, we identified 12 significantly metastasis-regulated lncRNAs (fold change = 1, p < 0.05, Mann-Whitney U-test, Supplementary Table S2). Then, we found 209 survival-related lncRNAs using univariate Cox proportional-hazards regression model (HR > 1, p < 0.05, log-rank test, Supplementary Table S3). As shown in Figure 1A, we identified that only LINC00941 is a survival-related (Figure 1B), cancer-related (Figure 1C), and metastasis-related lncRNA (Figure 1D). In addition, receiver operating characteristic (ROC) curve analysis was utilized to explore whether the expression level of LINC00941 could discriminate samples, and area under the ROC curve (AUC) was performed to calculate the diagnostic sensitivity and specificity. As shown in Figures 1E,F, the expression of LINC00941 discriminated cancer samples from normal samples (AUC = 0.7911, 95% CI: 0.7264–0.8559, p < 0.0001) and discriminated M1 samples from M0 samples (AUC = 0.6809, 95% CI: 0.5852–0.7766, p = 0.0031). Our findings indicated that LINC00941 is a potential biomarker for diagnosis and prognosis of GC.
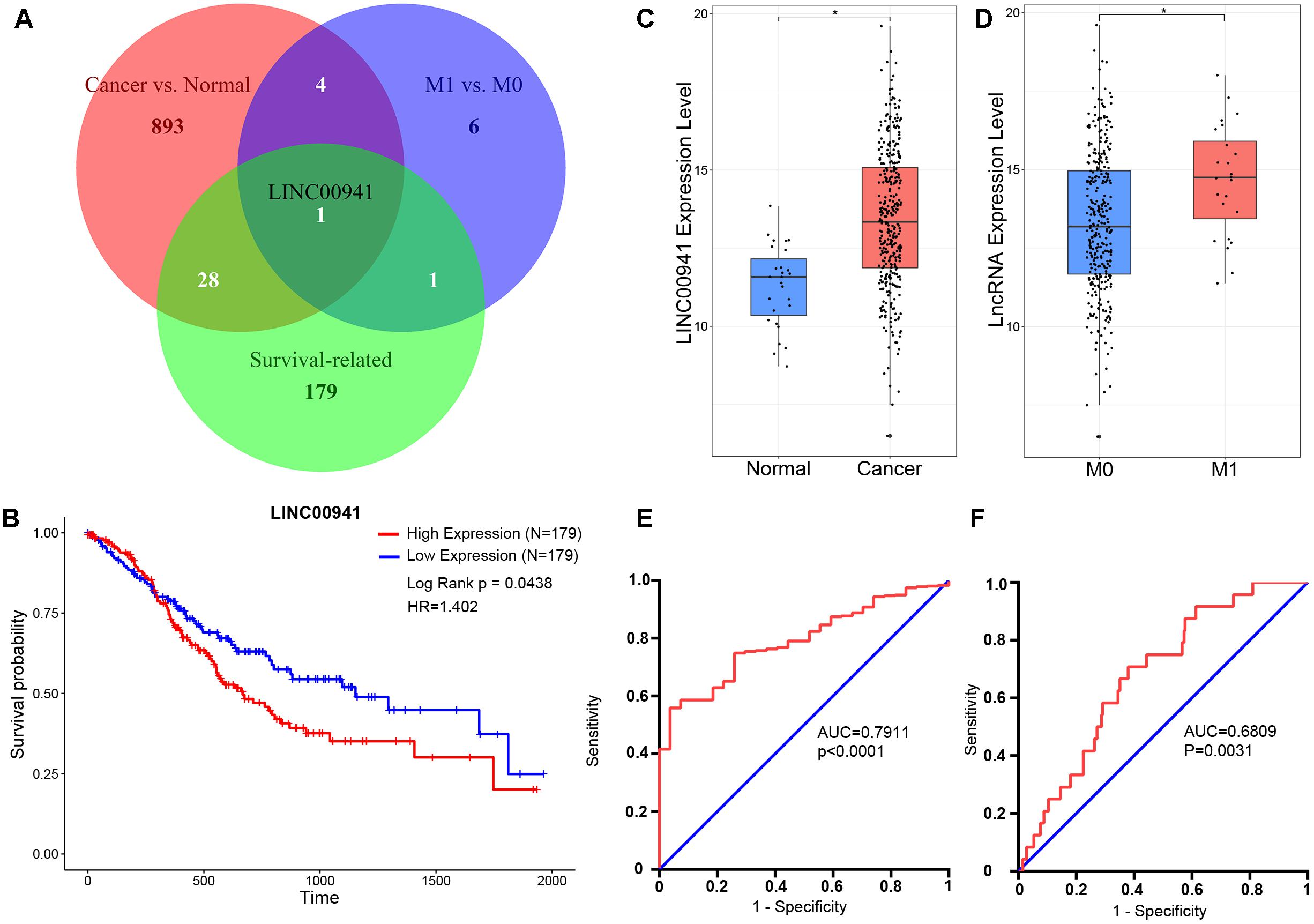
Figure 1. LINC00941 is potential diagnosis and prognosis biomarker for GC. (A) Venn diagram showed that only LINC00941 presented in the overlap of three data sets. (B) High expression of LINC00941 was associated with poor prognosis in GC. (C) LINC00941 were up-regulated in cancer samples, ∗p < 0.05. (D) LINC00941 was significantly up-regulated in M1 samples, ∗p < 0.05. (E) The ROC curve analysis for discriminative ability between cancer samples and normal samples. (F) The ROC curve analysis for discriminative ability between M1 samples and M0 samples.
The Expression of LINC00941 Is Associated With Tumor Depth and Distant Metastasis of Patients in GC
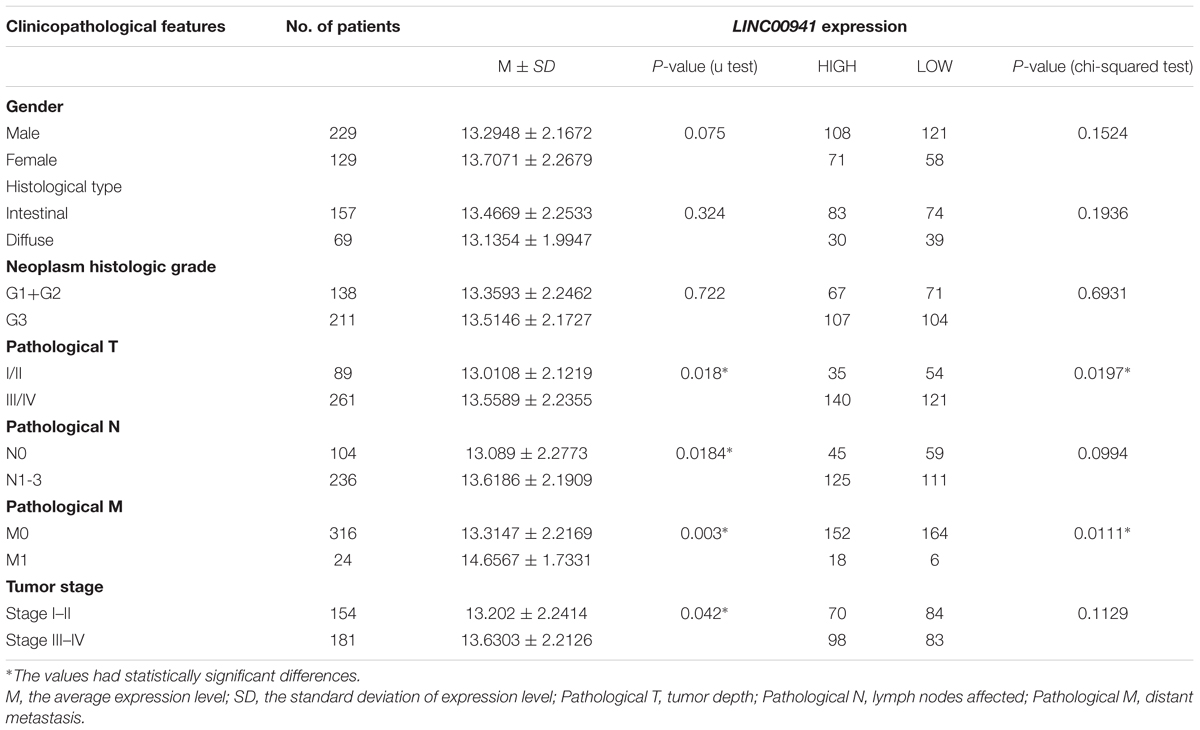
To explore the clinical value of LINC00941, Mann-Whitney U-test and Chi-square test were used to compare the different clinicopathological features in each group according to the expression of LINC00941 (Table 1 and Supplementary Table S4). The aberrantly expressed LINC00941 was observed in several clinicopathological features, such as tumor depth (p = 0.018, Mann-Whitney U-test), lymph node metastasis (p = 0.0184, Mann-Whitney U-test), distant metastasis (p = 0.003, Mann-Whitney U-test), and Tumor stage (p = 0.042, Mann-Whitney U-test). The results of chi-square test analysis demonstrated that the expression of LINC00941 had no significant correlation with clinicopathological features including gender, histological type, neoplasm histologic grade, lymph node metastasis, and tumor stage. However, the expression of LINC00941 expression was associated with tumor depth (p = 0.0197, chi-squared test) and distant metastasis (p = 0.0111, chi-squared test). By combining the results of two test methods, our results indicated that the expression of LINC00941 was associated with tumor depth and distant metastasis of patients in GC.
Construction and Functional Enrichment Analysis of LINC00941 Co-expression Network
In order to characterize the function of LINC00941 in GC, we applied WGCNA to constructe LINC00941 co-expression network by combining LINC00941 with dysregulated coding genes in GC samples (absolute fold change ≥ 1, p < 0.05, Mann-Whitney U-test, Supplementary Table S5). We selected the soft threshold power (β = 4) to ensure that the co-expression network was scale-free topology (Figure 2A). After hierarchical clustering and dynamic tree cutting, a total of 28 co-expression modules were identified (Figure 2B). We found that LINC00941 is clustered in “yellow” module, which contained 123 dysregulated coding genes (Supplementary Table S6). The genes in LINC00941 co-expression module was applied to explore the function of LINC00941 via the functional enrichment analysis. In this study, we identified 6 GO terms in BP and 4 KEGG pathways (Figures 2C,D and Supplementary Table S7). Some GO terms, such as extracellular matrix organization (GO: 0030198) and cell adhesion (GO: 0007155) are cell migration-related GO processes, which are associated with tumor metastasis. We detected that ECM-receptor interaction (KEGG: hsa04512) and Focal adhesion (KEGG: hsa04510) are metastasis-related pathways. PI3K-Akt signaling pathway (KEGG: hsa04151) is cell proliferation-related pathway. Our findings demonstrated that LINC00941 could be a potential regulator of tumor metastasis and cancer cell proliferation.
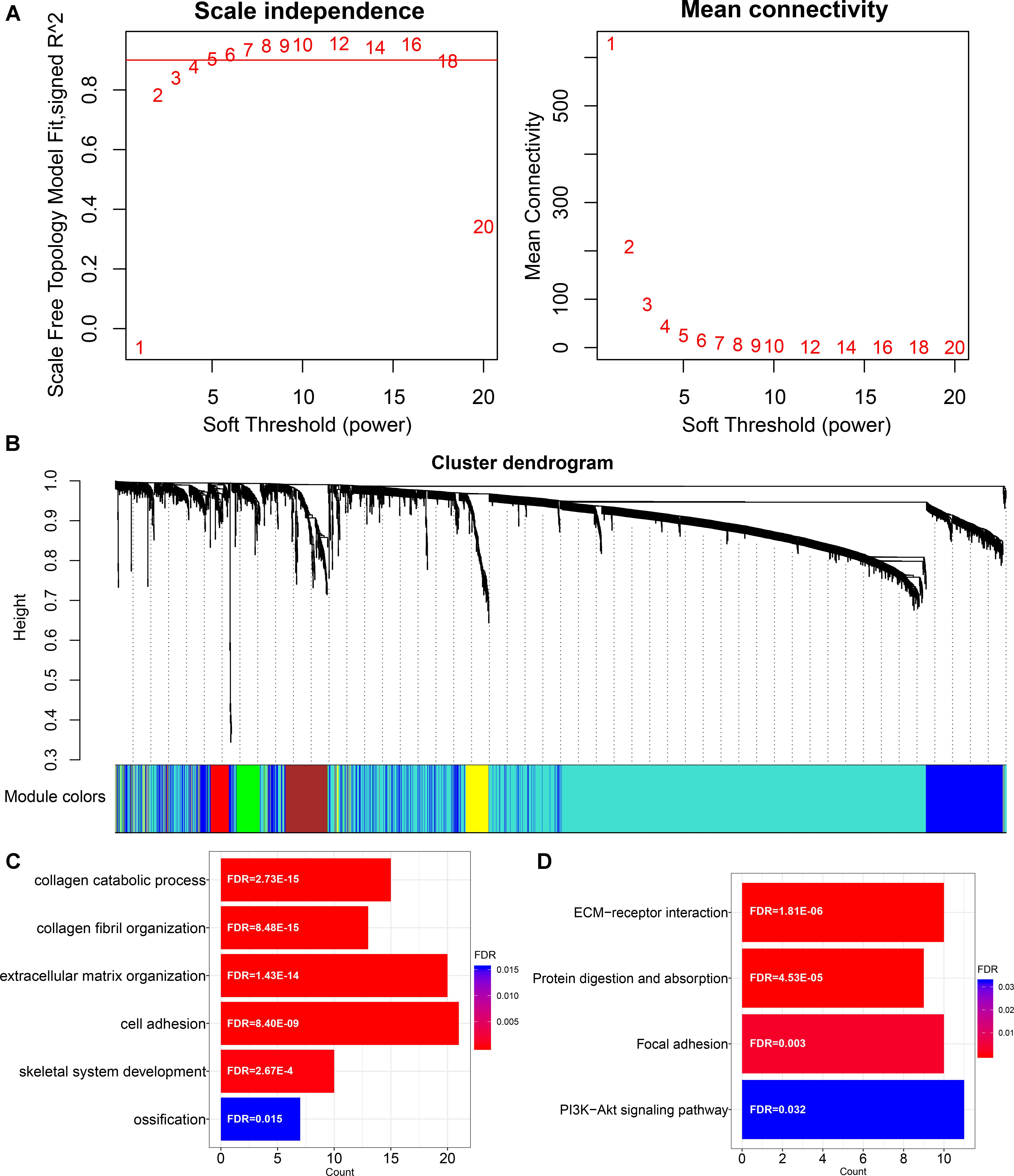
Figure 2. Construction and functional enrichment analysis of LINC00941 co-expression network. (A) Scale-free topology index and mean connectivity were used to determine the soft threshold. (B) Clustering dendrogram of LINC00941 co-expression network. The genes are assigned to different modules that are distinguished by different colors. (C) The GO terms in LINC00941 co-expression model in GC. (D) The KEGG pathways in LINC00941 co-expression model in GC.
Silence of LINC00941 Inhibits GC Cells Proliferation in vitro
To validate our findings, we designed two siRNAs, which can specifically target LINC00941. As shown in Figure 3A, LINC00941 was effectively knocked down by siRNAs in MKN45 and AGS cells. Then, we assessed whether silence of LINC00941 could affect the proliferation ability of MKN45 and AGS cells. CCK8 assay indicated that silence of LINC00941 significantly inhibited proliferation ability of MKN45 and AGS cells (Figure 3B). As shown in Figure 3C, colony formation ability of MKN45 and AGS cells was significantly decreased after silencing LINC00941. Our results confirmed that LINC00941 promotes GC cells proliferation in vitro.
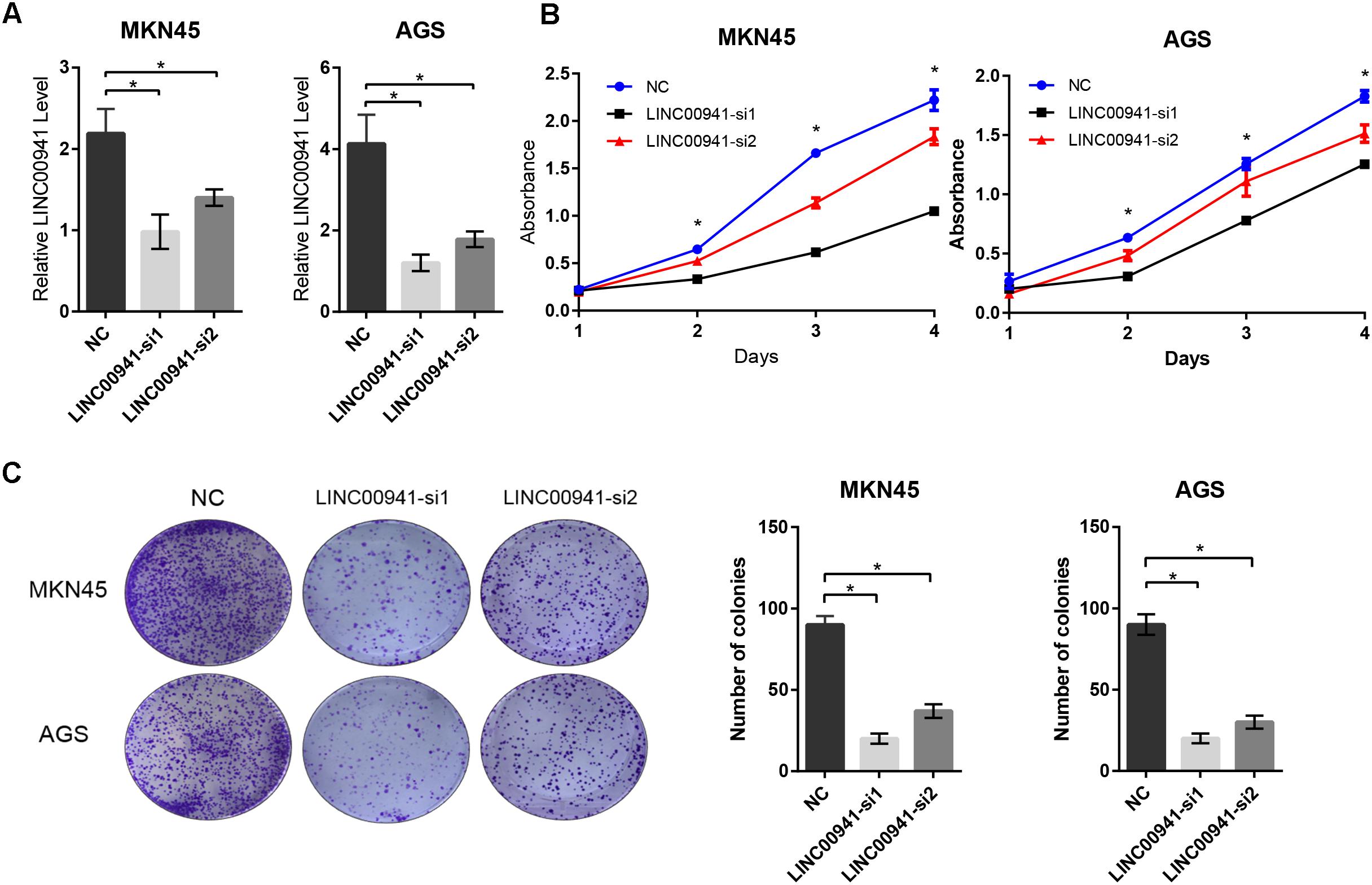
Figure 3. Silence of LINC00941 inhibits GC cells proliferation in vitro. (A) LINC00941 was knocked down by siRNAs in MKN45 and AGS cells. (B) Silence of LINC00941 significantly inhibited the proliferation ability of MKN45 and AGS cells. The effect on cell proliferation was assessed over 4 days using the CCK8. (C) Colony formation ability of MKN45 and AGS cells was significantly decreased after silencing LINC00941. Two-tailed Student’s t-test was used to analyze the significant differences, ∗p < 0.05.
Silence of LINC00941 Reduces GC Cells Migration and Invasion in vitro
To further analyze the effect of LINC00941 on GC metastasis, we utilized cell migration and invasion assays to explore the effect of silencing LINC00941 on metastasis ability of MKN45 and AGS cells. The results of transwell migration assay indicated that silence of LINC00941 inhibits the migration abilities of MKN45 and AGS cells (Figure 4A). The results of matrigel invasion assay indicated that silence of LINC00941 also inhibited the invasion abilities of GC cells (Figure 4B). Epithelial cell mesenchymal transition (EMT) is a process that is associated with tumor metastasis and lncRNAs could modulate cancer metastasis via affecting EMT (Xu et al., 2016; Grelet et al., 2017; Min et al., 2017). To explore the association between LINC00941 and EMT in GC metastasis, we measured the expression level of EMT markers, such as E-cadherin (CDH1), fibronectin (FN), and Snail1 (SNAI1), using western blot and qRT-PCR. The results confirmed that silence of LINC00941 decreased the protein and gene expression of CDH1, while increased the expression of FN and SNAI1 in MKN45 and AGS cells (Figure 4C). Therefore, our results demonstrated that LINC00941 might modulate GC cells metastatic properties via affecting EMT biomarker and could regulate GC cells migration and invasion in vitro.
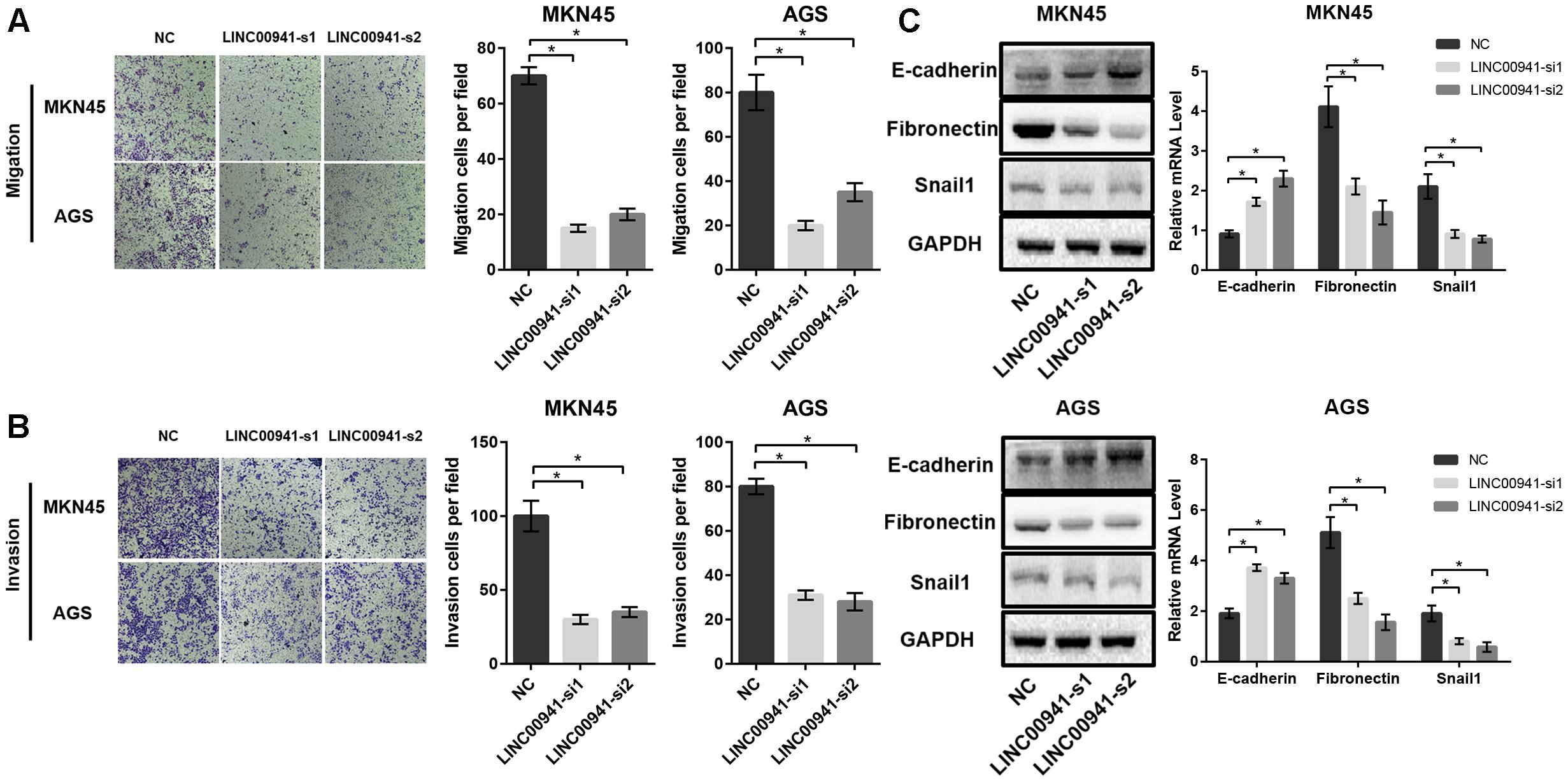
Figure 4. Silence of LINC00941 inhibits GC cells migration and invasion in vitro. (A) Silence of LINC00941 inhibits the migration abilities of MKN45 and AGS cells. (B) Silence of LINC00941 inhibits the invasive ability of MKN45 and AGS. (C) The expression of E-cadherin, Fibronectin, and Snail1 in MKN45 and AGS cells was assessed by western blot and qRT-PCR. GAPDH was used as a loading control. The expression was normalized to GAPDH. Two-tailed Student’s t-test was used to analyze the significant differences, ∗p < 0.05.
Silence of LINC00941 Suppresses Tumor Growth in vivo
To further validate the effect of LINC00941 on tumor growth, we used stably expressing MKN45 cells by infection with lentivirus expressing sh-LINC00941 and negative control. As shown in Figure 5A, LINC00941 was effectively knocked down by shRNA in MKN45 cells. We then evaluated the tumorigenic effects in BALB/c nude mice. The tumors in nude mice with sh-LINC00941-infected significantly smaller than the negative control (Figure 5B). We also measured the tumors weight and tumors volume of the two groups. We found that the tumor weight (Figure 5C) and the tumor volume (Figure 5D) of sh-LINC00941 were significantly smaller than the negative control. Thus, our findings confirmed that LINC00941 could modulate tumor growth in vivo.
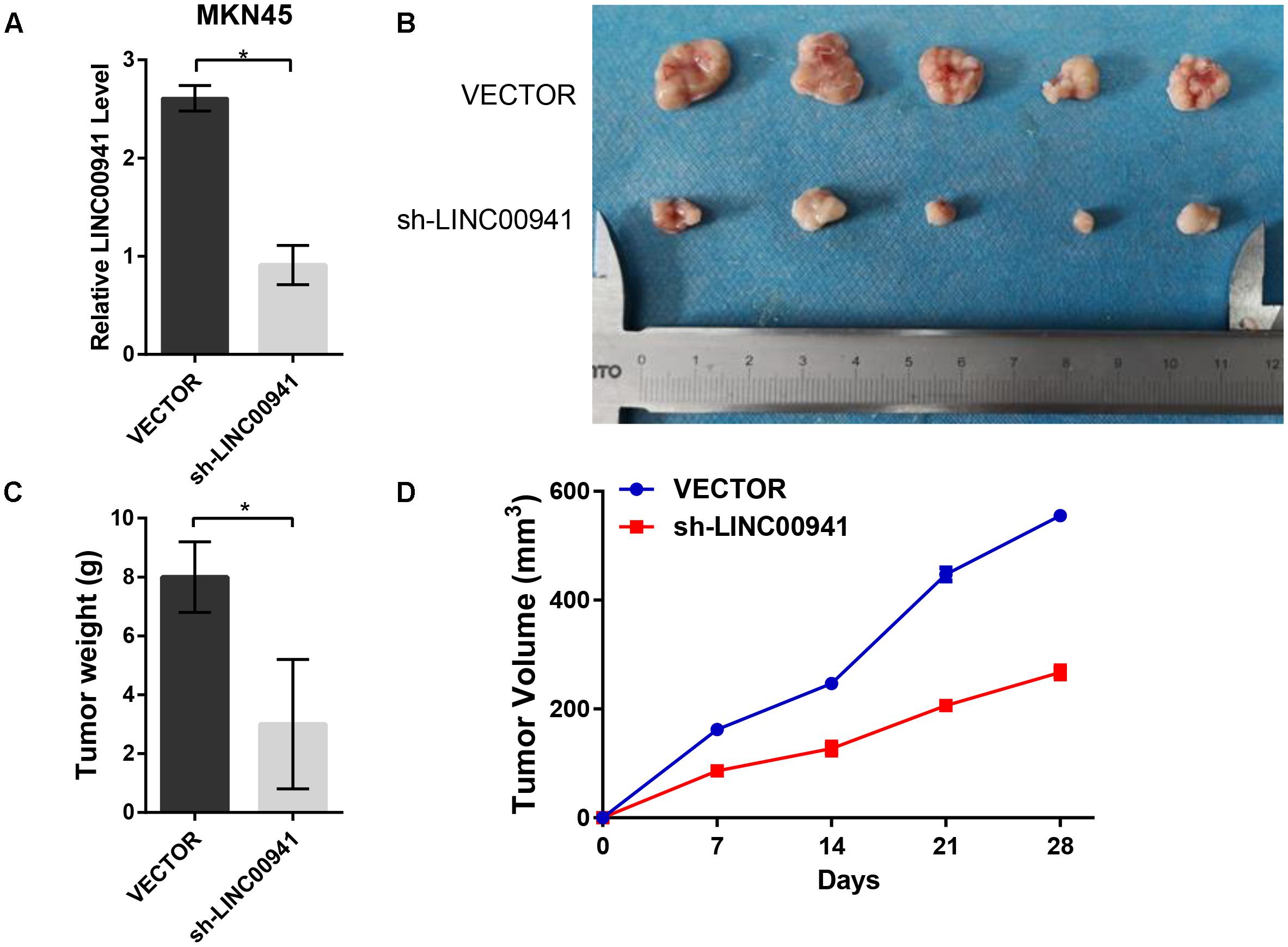
Figure 5. Silence of LINC00941 suppresses tumor growth in vivo. (A) LINC00941 was knocked down by shRNA in MKN45 cells. (B) The tumors in sh-LINC00941-infected nude mice significantly smaller than the negative control. (C) Tumor weights in nude mice with sh-LINC00941-infected were significantly lighter than the negative control. (D) Tumor volumes in nude mice with sh-LINC00941-infected significantly smaller than the negative control. Two-tailed Student’s t-test was used to analyze the significant differences, ∗p < 0.05.
Discussion
Non-coding RNAs represent more than 98% of the total human transcriptome (Yvonne et al., 2014) and the aberrantly expressed lncRNAs drive important cancer phenotypes (Schmitt and Chang, 2016). Few lncRNAs, such as UCA1, MALAT1, HOXA11-AS, and ZEB1-AS1 have been reported to play important roles in GC. However, only several lncRNAs have been functionally characterized in GC.
In this study, we systematically integrated GC clinical information and gene expression data from TCGA. By comparing cancer samples with normal samples, we identified 926 cancer-related lncRNAs, such as FEZF1-AS1 (Liu Y.W. et al., 2017), HOTAIR (Okugawa et al., 2014), HOXA11-AS (Sun et al., 2016), HOTTIP (Zhao et al., 2018), and LINC01234 (Chen X. et al., 2018) have been reported to play important roles in GC. By comparing metastatic samples with non-metastatic samples, we identified 12 metastasis-related lncRNAs, such as LINC00704 (Tracy et al., 2018), LINC00460 (Li et al., 2018), and LINC00520 (Henry et al., 2016) have been reported to associate with tumor metastasis. By using univariate Cox proportional-hazards regression model and ROC curve analysis, we identified that the LINC00941 may serve as a potential biomarker for diagnosis and prognosis of GC. Furthermore, we explored the associations between expression of LINC00941 and clinicopathological features, and we found that the expression of LINC00941 was associated with tumor depth and distant metastasis.
To further explore the function of LINC00941 in GC, LINC00941 co-expression network was constructed by combining LINC00941 with dysregulated coding genes in GC using R package WCGNA. After hierarchical clustering and dynamic tree cutting, LINC00941 co-expression module was identified. The dysregulated coding genes in the module was applied to explore the function of LINC00941 via the functional enrichment analysis. The results of GO terms and KEGG pathways were mainly enriched in cell proliferation, cell migration, and tumor metastasis. Our findings indicated that LINC00941 might play an important oncogenic function in GC. LINC00941 located in the 12p11.21 region of the human genome and also known as lncRNA-MUF. In lung adenocarcinoma, survival of patients was negatively associated with high expression of LINC00941 which modulates focal adhesion and PI3K-AKT signaling pathway (Wang et al., 2018). In hepatocellular carcinoma, high expression of LINC00941 significantly promoted EMT and malignant capacity tissues and correlated with poor prognosis (Yan et al., 2017). LINC00941 was differentially expressed in TGF-β1-activated A549 cells compared with those in normal controls (Liu H. et al., 2017) and was up-regulated upon treatment of colon cancer cells with chemotherapeutic drugs (Zinovieva et al., 2018). In GC, LINC00941 is up-regulated in tumor tissues (Luo et al., 2018). However, in GC, the precise biological function of LINC00941 have not been characterized.
To validate our findings, we utilized the loss-of-function analysis to reveal the biological function of LINC00941 in GC cells. CCK-8 and colony formation assays confirmed that LINC00941 could promote GC cells proliferation. Cell migration and invasion assays confirmed that LINC00941 could promote GC cells metastasis. Previous studies revealed that EMT is associated with tumor metastasis and lncRNAs could modulate cancer metastasis via affecting EMT (Xu et al., 2016; Grelet et al., 2017; Min et al., 2017). We further measured the expression level of EMT markers such as E-cadherin, Snail (Dong et al., 2012), and Fibronectin (Park and Schwarzbauer, 2014). The results demonstrated that LINC00941 could regulate GC cells metastasis via affecting EMT. Therefore, LINC00941 might modulate GC cells metastatic properties via affecting EMT biomarker. In this study, there are still some limitations and the specific biological mechanism by LINC00941 acts still needs to be investigated.
In summary, by systematically integrating bioinformatics and experimental methods, our findings firstly revealed that LINC00941 plays an important oncogenic function in GC. Our findings not only provide novel insights on the functional characterization of LINC00941 in GC, but can also provide a novel biomarker for diagnosis and prognosis of GC in future studies.
Ethics Statement
This study was carried out in accordance with the recommendations of the National Institutes of Health Laboratory Animal Care and Use Guidelines. The protocol was approved by the Xijing Hospital Institutional Review Board.
Author Contributions
YL and YN conceived of and directed the project. HL, NW, and ZZ designed and performed the experiments. HL and HG conducted the data analysis and interpreted the results. XZ and HZ revised the manuscript critically for important intellectual content. HL and NW wrote and edited the manuscript. All authors have reviewed the manuscript and approved it for publication.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 61471181, 81702966, and 81730016) and the Natural Science Foundation of Jilin Province (Grant Nos. 20140101194JC and 20150101056JC).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2019.00005/full#supplementary-material
Footnotes
References
Antonazzo, G., Attrill, H., Brown, N., Marygold, S. J., McQuilton, P., Ponting, L., et al. (2017). Expansion of the gene ontology knowledgebase and resources. Nucleic Acids Res. 45, D331–D338. doi: 10.1093/nar/gkw1108
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. doi: 10.3322/caac.21492
Cancer Genome Atlas Research and Network (2014). Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513, 202–209. doi: 10.1038/nature13480
Chen, W., Sun, K., Zheng, R., Zeng, H., Zhang, S., Xia, C., et al. (2018). Cancer incidence and mortality in China, 2014. Chin. J. Cancer Res. 30, 1–12. doi: 10.21147/j.issn.1000-9604.2018.01.01
Chen, X., Chen, Z., Yu, S., Nie, F., Yan, S., Ma, P., et al. (2018). Long noncoding RNA LINC01234 functions as a competing endogenous RNA to regulate CBFB expression by sponging miR-204-5p in gastric cancer. Clin. Cancer Res. 24, 2002–2014. doi: 10.1158/1078-0432.CCR-17-2376
Dong, C., Wu, Y., Yao, J., Wang, Y., Yu, Y., Rychahou, P. G., et al. (2012). G9a interacts with Snail and is critical for Snail-mediated E-cadherin repression in human breast cancer. J. Clin. Invest. 122, 1469–1486. doi: 10.1172/JCI57349
Engreitz, J. M., Ollikainen, N., and Guttman, M. (2016). Long non-coding RNAs: spatial amplifiers that control nuclear structure and gene expression. Nat. Rev. Mol. Cell Biol. 17, 756–770. doi: 10.1038/nrm.2016.126
Grelet, S., Link, L. A., Howley, B., Obellianne, C., Palanisamy, V., Gangaraju, V. K., et al. (2017). A regulated PNUTS mRNA to lncRNA splice switch mediates EMT and tumour progression. Nat. Cell Biol. 19, 1105–1115. doi: 10.1038/ncb3595
Henry, W. S., Hendrickson, D. G., Beca, F., Glass, B., Lindahl-Allen, M., He, L., et al. (2016). LINC00520 is induced by Src, STAT3, and PI3K and plays a functional role in breast cancer. Oncotarget 7, 81981–81994. doi: 10.18632/oncotarget.11962
Huang da, W., Sherman, B. T., and Lempicki, R. A. (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57. doi: 10.1038/nprot.2008.211
Kanehisa, M., Furumichi, M., Tanabe, M., Sato, Y., and Morishima, K. (2017). KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 45, D353–D361. doi: 10.1093/nar/gkw1092
Langfelder, P., and Horvath, S. (2008). WGCNA: an r package for weighted correlation network analysis. BMC Bioinformatics 9:559. doi: 10.1186/1471-2105-9-559
Li, K., Sun, D., Gou, Q., Ke, X., Gong, Y., Zuo, Y., et al. (2018). Long non-coding RNA linc00460 promotes epithelial-mesenchymal transition and cell migration in lung cancer cells. Cancer Lett. 420, 80–90. doi: 10.1016/j.canlet.2018.01.060
Li, Y., Wen, X., Wang, L., Sun, X., Ma, H., Fu, Z., et al. (2017a). LncRNA ZEB1-AS1 predicts unfavorable prognosis in gastric cancer. Surg. Oncol. 26, 527–534. doi: 10.1016/j.suronc.2017.09.008
Li, Y., Wu, Z., Yuan, J., Sun, L., Lin, L., Huang, N., et al. (2017b). Long non-coding RNA MALAT1 promotes gastric cancer tumorigenicity and metastasis by regulating vasculogenic mimicry and angiogenesis. Cancer Lett. 395, 31–44. doi: 10.1016/j.canlet.2017.02.035
Lin, C., and Yang, L. (2018). Long noncoding RNA in cancer: wiring signaling circuitry. Trends Cell Biol. 28, 287–301. doi: 10.1016/j.tcb.2017.11.008
Liu, H., Zhang, Z., Wu, N., Guo, H., Zhang, H., Fan, D., et al. (2018). Integrative analysis of dysregulated lncRNA-associated ceRNA network reveals functional lncRNAs in gastric cancer. Genes 9:303. doi: 10.3390/genes9060303
Liu, H., Zhao, X., Xiang, J., Zhang, J., Meng, C., Zhang, J., et al. (2017). Interaction network of coexpressed mRNA, miRNA, and lncRNA activated by TGF-β1 regulates EMT in human pulmonary epithelial cell. Mol. Med. Rep. 16, 8045–8054. doi: 10.3892/mmr.2017.7653
Liu, Y. W., Xia, R., Lu, K., Xie, M., Yang, F., Sun, M., et al. (2017). LincRNAFEZF1-AS1 represses p21 expression to promote gastric cancer proliferation through LSD1-Mediated H3K4me2 demethylation. Mol. Cancer 16:39. doi: 10.1186/s12943-017-0588-9
Luo, C., Tao, Y., Zhang, Y., Zhu, Y., Minyao, D. N., Haleem, M., et al. (2018). Regulatory network analysis of high expressed long non-coding RNA LINC00941 in gastric cancer. Gene 662, 103–109. doi: 10.1016/j.gene.2018.04.023
Min, F., Huang, Z., Zang, X., Lei, P., Wei, L., Chen, J., et al. (2017). Long noncoding RNA LINC00978 promotes cancer growth and acts as a diagnostic biomarker in gastric cancer. Cell Prolif. 51:e12425.
Okugawa, Y., Toiyama, Y., Hur, K., Toden, S., Saigusa, S., Tanaka, K., et al. (2014). Metastasis-associated long non-coding RNA drives gastric cancer development and promotes peritoneal metastasis. Carcinogenesis 35, 2731–2739. doi: 10.1093/carcin/bgu200
Park, J., and Schwarzbauer, J. E. (2014). Mammary epithelial cell interactions with fibronectin stimulate epithelial-mesenchymal transition. Oncogene 33, 1649–1657. doi: 10.1038/onc.2013.118
Schmitt, A. M., and Chang, H. Y. (2016). Long noncoding RNAs in cancer pathways. Cancer Cell 29, 452–463. doi: 10.1016/j.ccell.2016.03.010
Sun, M., Nie, F., Wang, Y., Zhang, Z., Hou, J., He, D., et al. (2016). LncRNA HOXA11-AS promotes proliferation and invasion of gastric cancer by scaffolding the chromatin modification factors PRC2, LSD1, and DNMT1. Cancer Res. 76, 6299–6310. doi: 10.1158/0008-5472.CAN-16-0356
Tracy, K. M., Tye, C. E., Ghule, P. N., Malaby, H. L. H., Stumpff, J., Stein, J. L., et al. (2018). Mitotically-associated lncRNA (MANCR) affects genomic stability and cell division in aggressive breast cancer. Mol. Cancer Res. 16, 587–598. doi: 10.1158/1541-7786.MCR-17-0548
Van Cutsem, E., Sagaert, X., Topal, B., Haustermans, K., and Prenen, H. (2016). Gastric cancer. Lancet 388, 2654–2664. doi: 10.1016/S0140-6736(16)30354-3
Wang, L., Zhao, H., Xu, Y., Li, J., Deng, C., Deng, Y., et al. (2018). Systematic identification of lincRNA-based prognostic biomarkers by integrating lincRNA expression and copy number variation in lung adenocarcinoma. Int. J. Cancer doi: 10.1002/ijc.31865 [Epub ahead of print].
Wang, Z. Q., He, C. Y., Hu, L., Shi, H. P., Li, J. F., Gu, Q. L., et al. (2017). Long noncoding RNA UCA1 promotes tumour metastasis by inducing GRK2 degradation in gastric cancer. Cancer Lett. 408, 10–21. doi: 10.1016/j.canlet.2017.08.013
Xu, Q., Deng, F., Qin, Y., Zhao, Z., Wu, Z., Xing, Z., et al. (2016). Long non-coding RNA regulation of epithelial-mesenchymal transition in cancer metastasis. Cell Death Dis. 7:e2254. doi: 10.1038/cddis.2016.149
Yan, X., Zhang, D., Wu, W., Wu, S., Qian, J., Hao, Y., et al. (2017). Mesenchymal stem cells promote hepatocarcinogenesis via lncRNA-MUF interaction with ANXA2 and miR-34a. Cancer Res. 77, 6704–6716. doi: 10.1158/0008-5472.CAN-17-1915
Yvonne, T., John, R., and Pier Paolo, P. (2014). The multilayered complexity of ceRNA crosstalk and competition. Nature 505, 344–352. doi: 10.1038/nature12986
Zhao, R., Zhang, Y., Zhang, X., Yang, Y., Zheng, X., Li, X., et al. (2018). Exosomal long noncoding RNA HOTTIP as potential novel diagnostic and prognostic biomarker test for gastric cancer. Mol. Cancer 17:68. doi: 10.1186/s12943-018-0817-x
Zinovieva, O. L., Grineva, E. N., Prokofjeva, M. M., Karpov, D. S., Zheltukhin, A. O., Krasnov, G. S., et al. (2018). Expression of long non-coding RNA LINC00973 is consistently increased upon treatment of colon cancer cells with different chemotherapeutic drugs. Biochimie 151, 67–72. doi: 10.1016/j.biochi.2018.05.021
Keywords: gastric cancer, non-coding RNA, LINC00941, biomarker, TCGA
Citation: Liu H, Wu N, Zhang Z, Zhong X, Zhang H, Guo H, Nie Y and Liu Y (2019) Long Non-coding RNA LINC00941 as a Potential Biomarker Promotes the Proliferation and Metastasis of Gastric Cancer. Front. Genet. 10:5. doi: 10.3389/fgene.2019.00005
Received: 02 November 2018; Accepted: 07 January 2019;
Published: 22 January 2019.
Edited by:
Quan Zou, University of Electronic Science and Technology of China, ChinaReviewed by:
Peng Zhang, University of Maryland, Baltimore, United StatesYing Wang, Xiamen University, China
Sushant Patil, The University of Chicago, United States
Copyright © 2019 Liu, Wu, Zhang, Zhong, Zhang, Guo, Nie and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongzhan Nie, eW9uZ3puaWVAZm1tdS5lZHUuY24= Yuanning Liu, bGl1eW5Aamx1LmVkdS5jbg==
†These authors have contributed equally to this work
 Haiming Liu
Haiming Liu Nan Wu2,3†
Nan Wu2,3† XiaoDan Zhong
XiaoDan Zhong Hao Zhang
Hao Zhang Yuanning Liu
Yuanning Liu