- 1State Key Laboratory of Integrative Sustainable Dryland Agriculture, Institute of Wheat Research, Shanxi Agricultural University, Linfen, China
- 2Crop Science, Institute of Crop Science and Resource Conservation (INRES), University of Bonn, Bonn, Germany
As a barrier for plants to contact with the outside world, epidermal wax plays an important role in resisting biotic and abiotic stresses. In this study, we analyzed the effect of wax content on leaf permeability by measuring the wax loss rate in the leaf. To further clarify the wax composition of the wheat epidermis and its molecular regulation mechanism, we applied untargeted lipidomic and transcriptome analysis on the leaf epidermis wax of Jimai 22 low-wax mutant (waxless) and multi-wax mutant (waxy). Our research showed that the mutant waxy has a slow loss rate, which can maintain higher leaf water content. 31 lipid subclasses and 1,367 lipid molecules were identified. By analyzing the wax differences of the two mutants, we found that the main lipid components of leaf epidermis wax in Jimai 22 were WE (C19-C50), DG (C27-C53), MG (C31-C35), and OAHFA (C31-C52). Carbon chain length analysis showed that, in wheat epidermis wax, WE was dominated by C44 molecules, DG was mainly concentrated in C47, C45, C37, and C31 molecules, C48 played a leading role in OAHFA, and C35 and C31 played a major role in MG. Among them, DG, MG, and OAHFA were detected in wheat leaf wax for the first time, and they were closely related to stress resistance. Compared with the waxy, 6,840 DEGs were detected in the mutant waxless, 3,181 DEGs were upregulated, and 3,659 DEGs were downregulated. The metabolic pattern of main waxy components in the wheat epidermis was constructed according to KEGG metabolic pathway and 46 related genes were screened, including KSC, TER, FAR, WSD1, CER1, MAH1, ALDH7A1, CYP704B1, ACOT1_2_4, CYP86, MGLL, GPAT, ALDH, DPP1, dgkA, plsC, and E2.3.1.158 related genes. The screened wax-related genes were confirmed to be highly reliable by qRT-PCR. In addition, we found TER gene TraesCS6B03G1132900LC in wheat mutant waxless leaves for the first time, which inhibited the synthesis of long-chain acyl-CoA (n+2) by downregulating its expression. These results provide valuable reference information for further study of wheat epidermis wax heredity and molecular regulation.
Introduction
As the first barrier for plants, epidermis wax plays an important role in resisting biotic and abiotic stresses (Aharoni et al., 2004; Franke et al., 2005; Bernard and Joubes, 2013). For example, it can limit the loss of non-stomatal water, improve the drought resistance of plants, help plants reduce mechanical damage, plant diseases, and insect pests, and protect plants from high temperature and strong ultraviolet radiation (Reina-Pinto and Yephremov, 2009; Yeats et al., 2012). Epidermal wax is a complex mixture of lipids composed of very-long-chain fatty acids (VLCFAs) and their derivatives (Zhang et al., 2005; Tafolla-Arellano et al., 2018). Existing studies have shown that the biosynthesis of epidermal wax begins with a waxy forerunner transformed by very-long-chain fatty acids C16 or C18 on the outer membrane of plastid epidermal cells. The carbon chains of C16 or C18 acyl-CoA and malonyl-CoA are lengthened by β-ketoacyl-CoA synthetase (KCS), β-ketoacyl-CoA reductase (KCR), β-hydroxyacyl-CoA dehydratase (HCD), and enoloyl-CoA reductase (ECR). Then, these very-long-chain fatty acids synthesize different waxy compounds by acyl reduction and decarbonylation (Samuels et al., 2008; Dong et al., 2019). Because of the complexity of waxy biosynthesis, the accurate determination of plant waxy components and content is helpful to infer the pathway of plant waxy biosynthesis accurately. However, most of the existing studies on epidermal wax components are based on gas chromatography-mass spectrometry (GC-MS), and some trace components cannot be detected, which leads to some limits in the study of the epidermal wax synthesis pathway.
Many genes involved in the wax synthesis and regulation have been found in Arabidopsis and rice (Racovita et al., 2016; Shaheenuzzamn et al., 2019). For instance, transcription factor WIN1/SHN1 in Arabidopsis upregulates the expression of epidermal wax synthesis genes CER1, CER2, CER4, KCS, CYP86A7, CYP86A4, GPAT4, LACS2, and HTH to induce epidermal wax accumulation (Kannangara et al., 2007). The overexpression of CER1 leads to the accumulation of alkanes (Bourdenx et al., 2011), and overexpression of CER4 (AtFAR3) induces the production of primary alcohols in C24:0-C30:0 (Rowland et al., 2006). Zhou et al. (2014) found that OsWR2 in rice, as a homologue of AtWIN1/AtSHN1TF, controls wax synthesis and accumulation by regulating the expression of very-long-chain fatty acid biosynthesis genes CER6/CUT1, FDH2, FAE, and LACS1 in the panicle. Due to the huge genomic information of wheat, there are few studies on the molecular regulation mechanism of wheat epidermal wax biosynthesis. It has been reported that W1-W5, Iw1, Iw2, and Iw3 are related to the wax synthesis of the wheat epidermis (Huang et al., 2017; Li et al., 2020). Li et al. (2021) studied the wax-deficient mutant w5 and found that the blockage of β-diketone biosynthesis inhibited waxy synthesis. Chai et al. (2018) cloned several TaFARs genes encoding fatty acyl-CoA reductase from wheat.
In view of the complexity of wheat epidermis wax composition and synthesis mechanism, the analysis of wheat wax deletion mutants is considered to be a tool to get many response genes. To study the regulation mechanism of wheat epidermis wax, low-wax mutants and multi-wax mutants obtained by ethyl methanesulfonate (EMS) mutagenesis of Jimai 22 were used as materials in this study. By means of mutual verification and joint analysis of untargeted liposome and transcriptome, the main components of wheat epidermis wax were identified, and new genes related to epidermal wax metabolism were excavated, such as TraesCS1D03G0373900, TraesCS1D03G0374000, TraesCS4B03G0019500, TraesCS7B03G1338900, TraesCS5B03G0557800, TraesCS5D03G0511400, and TraesCS7A03G0874000, which improved the possible molecular regulation mechanism of wheat epidermis wax synthesis. The purpose of this study is to provide valuable reference information for further study on the genetic and molecular mechanism of epidermis wax metabolism in wheat, which provides theoretical support for wheat breeding and genetic improvement.
Materials and Methods
Plant Materials
Jimai 22 is a high-yielding variety selected by the Crop Research Institute of Shandong Academy of Agricultural Sciences, containing wax in wild leaf epidermis. Combined with the experimental experience of our research group for many years, 0.6% (v/w) EMS was used to mutagenize Jimai 22 in this study, and then waxless and waxy were selected from the separated high-generation population. Compared with waxy, waxless showed less wax in the whole plant (Figures 1A,B). In October 2020, the mutants were planted in the Hancun Experimental Base of Wheat Research Institute of Shanxi Agricultural University (36°N, 111°E), with three replicates. The length was 2 m, the row spacing was 0.3 m, and 30 seeds per row were sown evenly. Wax content and transcriptome analysis were performed on flag leaves at the heading stage. Three biological repeats were performed for both waxless and waxy, and two samples with good repeatability were selected in transcriptome analysis.

FIGURE 1. Phenotype of low-wax mutant waxless and multi-wax mutant waxy. (A) The whole plant phenotype of waxless and waxy. (B) Leaf phenotype of waxless and waxy.
Determination of Water Loss Rate of Leaves
In order to analyze the effect of leaf epidermis wax on the water loss rate of wheat leaves, in the same period as transcriptome sequencing, the flag leaves at the heading stage of waxy and waxless mutants were fully soaked and dehydrated in the dark at room temperature for 10 h (stomata were completely closed). The leaf water loss rate was calculated by 0.001 mg analysis balance (AUW320, Japan) every hour. Each sample was repeated three times.
Untargeted Lipidomics Detection
Liposome was detected by liquid chromatogramphy-tandem mass spectrometry (LC-MS). The leaves were immersed in chloroform for 30 s to dissolve the epidermis wax and dried with nitrogen. Lipids were extracted according to the MTBE method (Pizarro et al., 2013). Briefly, samples were spiked with a suitable amount of internal lipid standards and then homogenized with 200 µl water and 240 µl methanol. After that, 800 µl of MTBE was added and the mixture was ultrasound-sonicated 20 min at 4°C followed by keeping still for 30 min at room temperature. The solution was centrifuged at 14,000 g for 15 min at 10°C and the upper organic solvent layer was obtained and dried under nitrogen. Reverse-phase chromatography was selected for LC separation using the CSH C18 column (1.7 µm, 2.1 × 100 mm, Waters). The lipid extracts were re-dissolved in 200 µl 90% isopropanol/acetonitrile, centrifuged at 14,000 g for 15 min; finally, 3 µl of the sample was injected. Solvent A was acetonitrile-water (6:4, v/v) with 0.1% formic acid and 0.1 Mm ammonium formate and solvent B was acetonitrile-isopropanol (1:9, v/v) with 0.1% formic acid and 0.1 Mm ammonium formate. The initial mobile phase was 30% solvent B at a flow rate of 300 μl/min. It was held for 2 min and then linearly increased to 100% solvent B in 23 min, followed by equilibrating at 5% solvent B for 10 min. Mass spectra were acquired by Q-Exactive Plus in positive and negative modes, respectively. ESI parameters were optimized and preset for all measurements as follows: source temperature was set at 300°C, capillary temp at 350°C, the ion spray voltage at 3000 V, S-Lens RF level at 50% and the scan range of the instruments at m/z 200–1800.
Extraction of RNA and Transcriptome Sequencing
The clean leaves of the two mutants were taken at the heading stage, wrapped in tin foil, frozen in liquid nitrogen quickly, and then frozen in the refrigerator at −80°C. The samples were sent to Beijing Baimaike Biotechnology Co., Ltd., for transcriptome sequencing. Total RNA was extracted using the Trizol method, and the concentration and purity of RNA were measured by NanoDrop 2000 (Thermo Fisher Science, Wilmington, DE). RNA integrity was evaluated using the RNA Nano 6000 analysis kit of Agilent Biological Analyzer 2,100 system (Agilent Technologies, CA, United States ). A total amount of 1 μg RNA per sample was used as input material for the RNA sample preparations. Sequencing libraries were generated using NEBNext Ultra TM RNA Library Prep Kit for Illumina (NEB, United States ) following the manufacturer’s recommendations and index codes were added to attribute sequences to each sample. In order to select cDNA fragments of preferentially 240 bp in length, the library fragments were purified with the AMPure XP system (Beckman Coulter, Beverly, United States ). Then, 3 μl USER Enzyme (NEB, United States ) was used with size-selected, adaptor-ligated cDNA at 37°C for 15 min followed by 5 min at 95°C before PCR. PCR was performed with Phusion High-Fidelity DNA polymerase, Universal PCR primers, and Index (X) Primer. At last, PCR products were purified (AMPure XP system) and library quality was assessed on the Agilent Bioanalyzer 2,100 system.
Real-Time Quantitative PCR Analysis
Primers were designed by Primer Premier Version 5.0 (Premier Biosoft International, Palo Alto, CA) and combined by Sangon (www.sangon.com) (Supplementary Table S1). LA-Taq enzyme from TaKaRa (www.takara.com.cn) was used for PCR amplification. PCR was performed in total volumes of 15 μl, including 3 pmol of each primer, 120 μM of each dNTP, 80 ng template DNA or cDNA, 0.75 unit La-Taq, and 7.5 μl of 2 × buffer (TaKaRa Biotechnology (Dalian) Co., Ltd., Product Code: DRR20AG). PCR was performed as follows: 95°C for 4 min, followed by 35 cycles of 95°C for 30 s, annealing (55–62°C) for 30 s, extension at 72°C (30 s–3 min), and 72°C for 30 s, with a final extension of 72°C for 10 min. Annealing temperatures and extension times depended upon individual primer sets and the length of expected PCR products.
Quantitative real-time PCR was performed using SYBR® Premix Ex Taq™ II (Takara) according to the manufacturer’s instructions on a 7,300 Real-time PCR System (Applied Biosystems), where the relative expression of each gene was calculated according to the 2−ΔΔCT method (Zheng et al., 2020). The glyceraldehyde-3-phosphate dehydrogenase gene was used as an endogenous reference for real-time PCR, and all analyses were performed with three technical and three biological replications.
Data Analysis
“Lipid Search” is a search engine for the identification of lipid species based on MS/MS math. Lipid Search contains more than 30 lipid classes and more than 1,500,000 fragment ions in the database. Both mass tolerances for precursor and fragment were set to 5 ppm. Under the positive and negative ion modes, the OPLS-DA model was constructed by SAMIC14.1 software, and the prediction rate Q2 of the model was obtained by 7-fold cross-validation. Q2 > 0.5 was taken as the reliable standard of the model; the multiple of variation analysis (Fold Change Analysis, FC) > 4 or < 0.25 and the variable importance for the projection (VIP) > 1, p value < 0.05, were used as the screening criteria to compare the overall differential expression multiples of lipid ions in leaves of waxless and waxy. Lipid differences were analyzed with SPSS19.0 and Microsoft Excel 2019, plotted with Origin 2018. The transcriptome data were further processed by the online platform of BMK Cloud (www.Biocloud.net). FPKM (Fragments Per Kilobase of exon model per Million mapped fragments) method was used to standardize the gene expression level. DESeq R package was used for differential analysis, and Fold Change ≥2 and FDR < 0.01 were used as screening criteria to determine the differentially expressed genes (DEGs) between waxless and waxy mutants.
Results and Analysis
Effect of Epidermis Wax on Water Loss Rate of Wheat Leaves
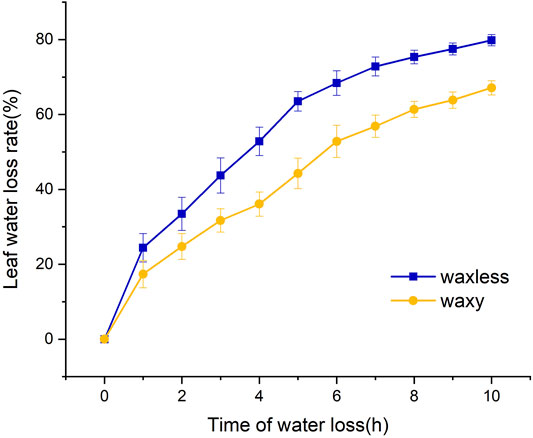
The leaf water loss rate of the two mutants was faster in the darkroom temperature environment. The wax content had a significant effect on the leaf dehydration rate. After natural dehydration for 1 h, the water loss rate of low-wax mutant leaves reached 24.4% and that of multi-wax mutant leaves reached 17.4%. The leaves of the two mutants were dehydrated rapidly within 1–5 h, and the water loss rate of waxless was significantly higher than that of waxy. After dehydration for 5 h, the water loss rate of waxless leaves reached 63.5%, while that of waxy was only 44.3%. There was a significant difference between the two mutants. Although the dehydration rate of the two mutants slowed down after 5 h of dehydration, the water loss rate of low-wax mutants was still significantly higher than that of wax-rich mutants at 10 h of dehydration (Figure 2). This shows that the mutant waxy has a slow dehydration rate and strong water retention capacity and can maintain higher leaf water content, indicating that leaf wax content plays an important role in maintaining leaf water content.
Identification and Analysis of Lipid Components in Leaf Epidermis
Positive and negative ion patterns of electrospray ionization (ESI) were used in this study. By UPLC analysis, 31 lipid subclasses and 1,367 lipid molecules were identified in the leaf epidermis of the two mutants (Figure 3A). The lipids with a high number of lipid species are triacylglycerol (TG), ceramide (Cer), diacylglycerol (DG), wax ester (WE), (O-acyl)-1-hydroxy fatty acid (OAHFA), monogalactosyl diacylglycerol (MGDG), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), cholesterol (ChE), cardiolipin (CL), phosphatidylinositol (PI), and phosphatidylserine (PS). Among them, TG, Cer, DG, WE, and OAHFA account for 83% of the total number of molecules (Figure 3B). Moreover, it was found that the main lipid components of wheat leaf epidermis were TG, Cer, DG, WE, and OAHFA.
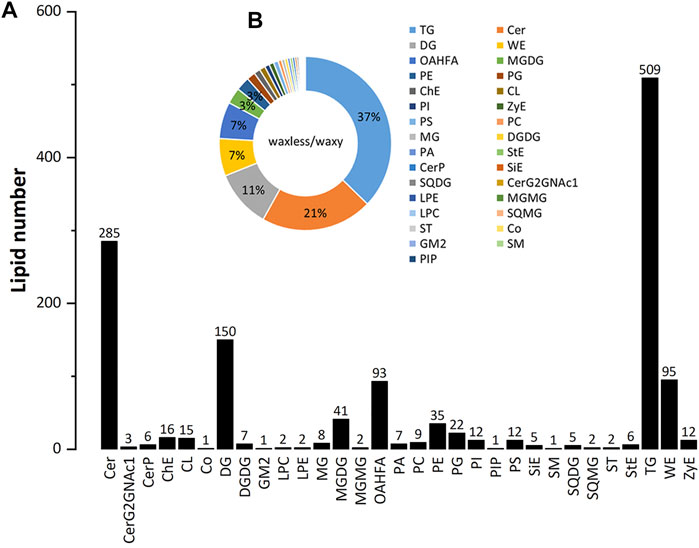
FIGURE 3. The statistical chart of numbers of lipid subclass in wheat leaves. (A) Abscissa is the detected lipid subclasses; ordinate is the number of lipids. (B) Different lipid subclasses are represented by different colors, and the proportion is expressed by the size of the color block area. For the corresponding relationship and proportion between each lipid subclass and color, see the legend on the right.
Analysis of Wax-Related Lipid Subclasses in Leaf Epidermis.
To test the repeatability of samples, we performed OPLS-DA analysis on all samples (Figure 4B). Each group of samples gathered closely and located in the middle of each group, indicating the liposome analysis showed high reproducibility. By comparing the contents of lipid subclasses between low-wax mutants and multi-wax mutants at the heading stage, significant differences in lipids were screened out. As shown in Figure 4A, 11 kinds of lipid substances with significant differences were found between the two mutants (VIP >1, p < 0.05). Among them, there are six lipid subclasses with high lipid content in waxless, namely, Cer, ZyE, PE, MGDG, CL, and digalactosyl diacylglycerol (DGDG). On the other hand, in waxy, five kinds of lipid classes were found to be DG, ChE, OAHFA, WE, and monoacylglycerol (MG) (VIP >1, p < 0.05). We infer that the more abundant and different lipids in the waxy are the main components of the wax in the epidermis of wheat compared with the waxless. That is the main lipid components of wheat leaf epidermis at the heading stage are DG, ChE, OAHFA, WE, and MG.
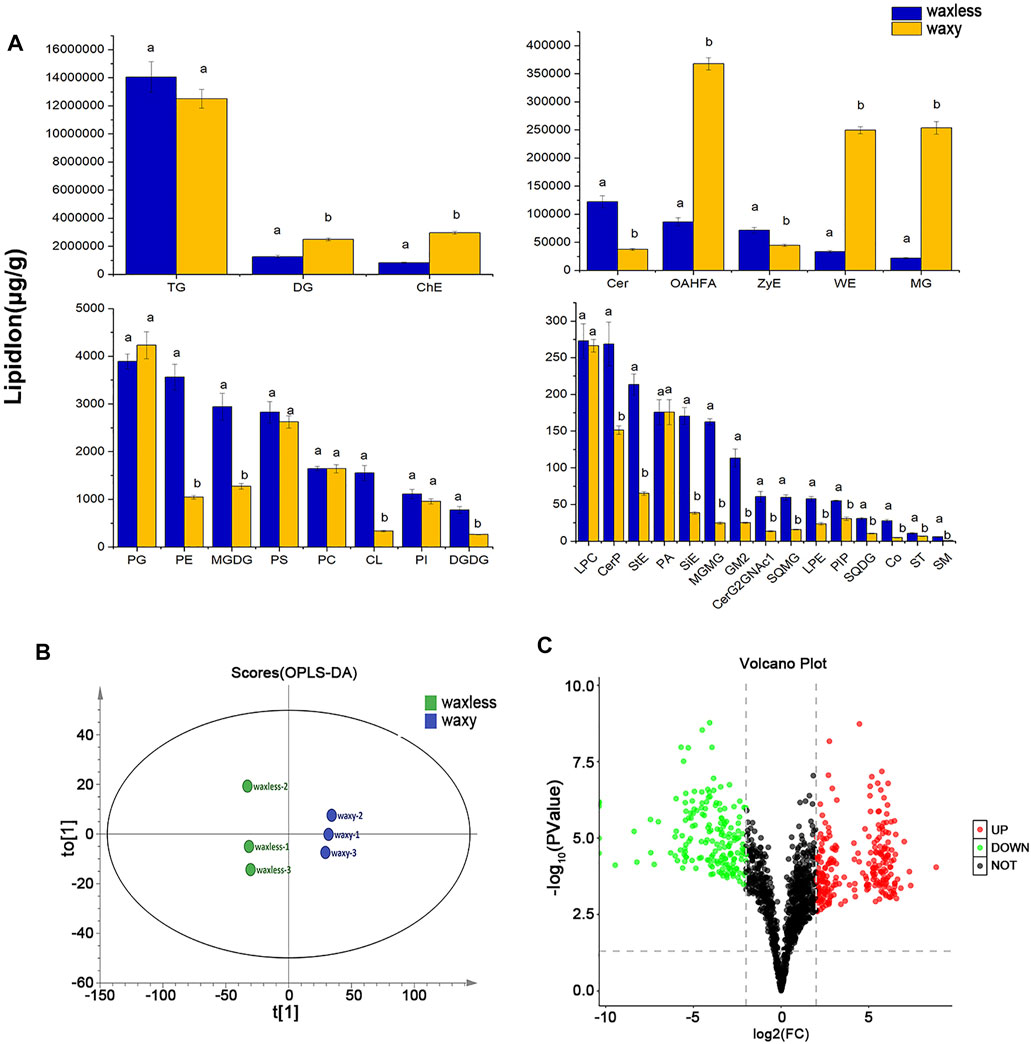
FIGURE 4. Differential expression of wax-related lipids between waxless and waxy. (A) Content of lipid subclasses in the leaf epidermis of two wheat mutants, abscissa is the lipid subclass, ordinate is the lipid subclass content, blue is the low-wax mutant waxless, yellow is the multi-wax mutant waxy, and the letters indicate significant differences (p < 0.05). (B) OPLS-DA score map, “t[1]” represents principal component 1, “to[1]” represents principal component 2, and the ellipse represents 95% confidence interval. Dots of the same color represent each biological weight in the group waxless and waxy. (C) Volcano plot, abscissa is log2FC (waxless/waxy) and ordinate is −log10 (p value). Each dot represents a lipid ion, red represents upregulated lipid ions, green represents downregulated lipid ions, and black indicates undifferentiated lipid ions (FC > 4 or < 0.25, VIP>1, p < 0.05).
Analysis of Lipid Ions Differences in Leaf Epidermis
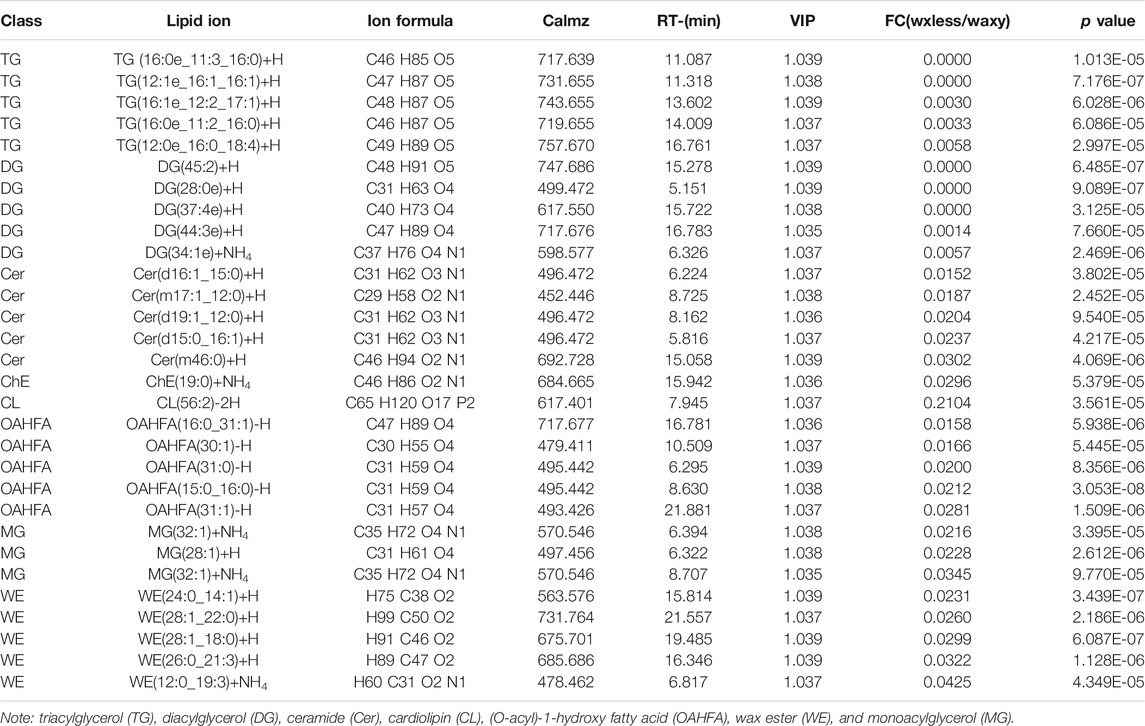
We analyzed the differences of 1,367 detected lipid ions (FC > 4 or <0.25, VIP >1, p < 0.05). Compared with the mutant waxy, the mutant waxless downregulated 177 lipid ions and upregulated 246 lipid ions. The results were shown in the form of a volcanic map (Figure 4C). 177 lipid ions related to wax metabolism in the epidermis of wheat leaves were screened (FC < 0.25, VIP >1, p < 0.05), belonging to eight lipid subclasses, including WE (C19-C50), DG (C27-C53), TG (C29-C73), MG (C31-C35), OAHFA (C31-C52), Cer (C29-C48), ChE (C46H86O2N1), and CL (C65H120O17P2) (Supplementary Table S2).
The main differential lipid ions in DG are DG(45:2)+H, DG(28:0e)+H and DG (37:4e)+H; ChE(19:0)+NH4 in ChE; OAHFA(16:0_31:1)-H, OAHFA(30:1)-H, and OAHFA(31:0)-H in OAHFA; WE(24:0_14:1)+H, WE(28:1_22:0)+H, WE(28:1_18:0)+H, WE(26:0_21:3)+H, and WE(12:0_19:3)+NH4 in WE; MG(32:1)+NH4, MG(28:1)+H, and MG(32:1)+NH4 in MG (Table 1). These may be the main lipid ions that affect the gloss phenotype of wheat leaf epidermis.
Analysis of Carbon Chain Length of Differential Lipids
In addition to the content of lipids, the carbon chain length of lipids is also an important factor that cannot be ignored. We added the lipid ions with the same carbon chain length and counted the lipid ions with diverse carbon chain lengths under different lipid subclasses to further analyze the main discrepant lipids in wheat epidermis wax (Figure 5). Compared with waxy mutants, the lipid ions significantly decreased in waxless mutants were WE (C29), WE (C31), WE (C44), WE (C46), DG (C47), DG (C45), DG (C43), DG (C37), DG (C36), DG (C31), OAHFA (C51), OAHFA (C50), OAHFA (C48), OAHFA (C31), MG (C35), MG (C31), and TG (C64).
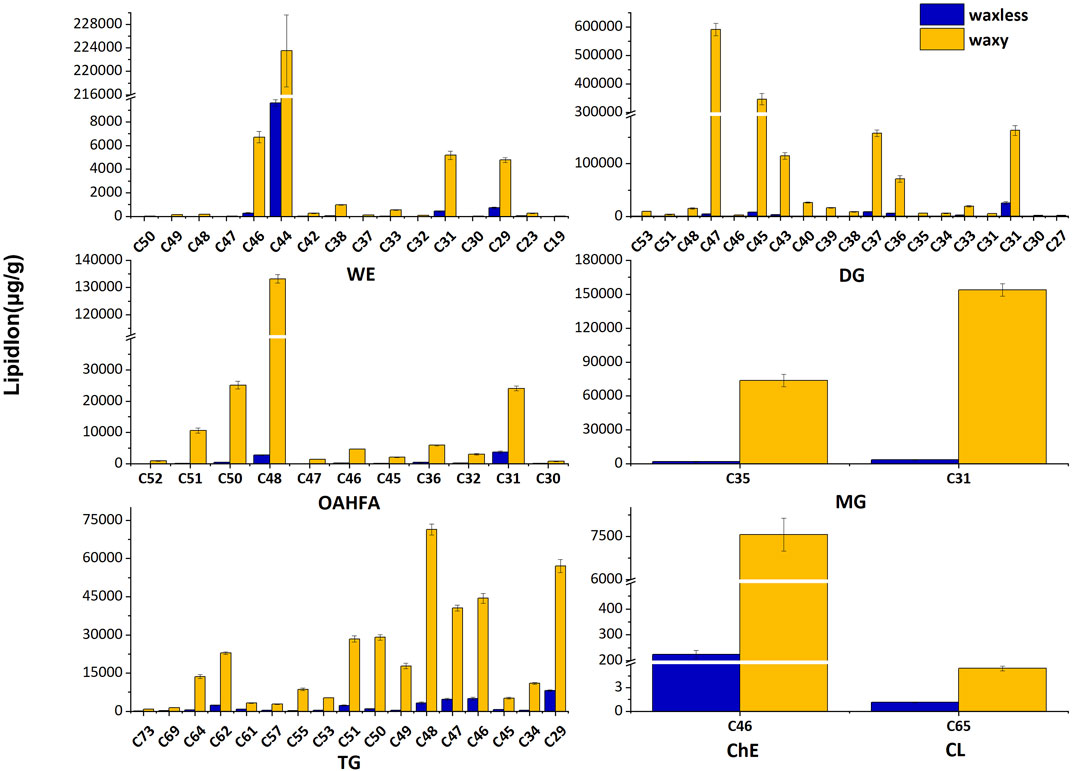
FIGURE 5. Carbon chain length distribution. Abscissa denotes the lipid molecules with different carbon chain lengths, ordinate indicates lipid ion content, blue is the low-wax mutant waxless, and yellow is the multi-wax mutant waxy.
These lipid ions affect the synthesis, transcription, and transport of wax through the change of carbon chain length, leading to the smooth green phenotype of wheat waxy deletion mutants. We found that WE in wheat leaf epidermis is mainly concentrated in C44-dominated wax ester molecules and also widely distributed in C46, C31, and C29 wax ester molecules but less in other chain lengths. DG in the wax mixture of the wheat epidermis is mainly concentrated in C47, C45, C37, and C31 molecules. In OAHFA, C48 plays a leading role, accompanied by a large number of C50-C51 and C31 molecules. C35 and C31 play significant roles in MG. On the other hand, in TG, the length of the carbon chain varies from C29 to C64, in which C48 plays a major role.
Gene Differential Expression Analysis
Through the analysis of leaf transcriptional groups of low-wax mutants and multi-wax mutants at the heading stage, correlation coefficients among different biological repetitive samples ranged from 0.890 to 0.926 (Figure 6A). Compared with the mutant waxy, 6,840 DEGs were detected in the mutant waxless, of which 3,181 genes were upregulated and 3,659 genes were downregulated (Fold Change ≥2, FDR <0.01) (Figure 6B). To understand the biological significance between low-wax mutant and multi-wax mutant DEGs, these genes were enriched by Gene Ontology (GO) analysis. We found that the biological processes were mainly enriched in the metabolic process (28.89%), cellular process (24.73%), single-organism process (14.96%), biological regulation (8.35%), and response to stimulus (6.46%). These genes are mainly distributed in the membrane, membrane part, cell, component cell part, and organelle. The molecular functions mainly include binding and catalytic activity (Supplementary Figure S1).
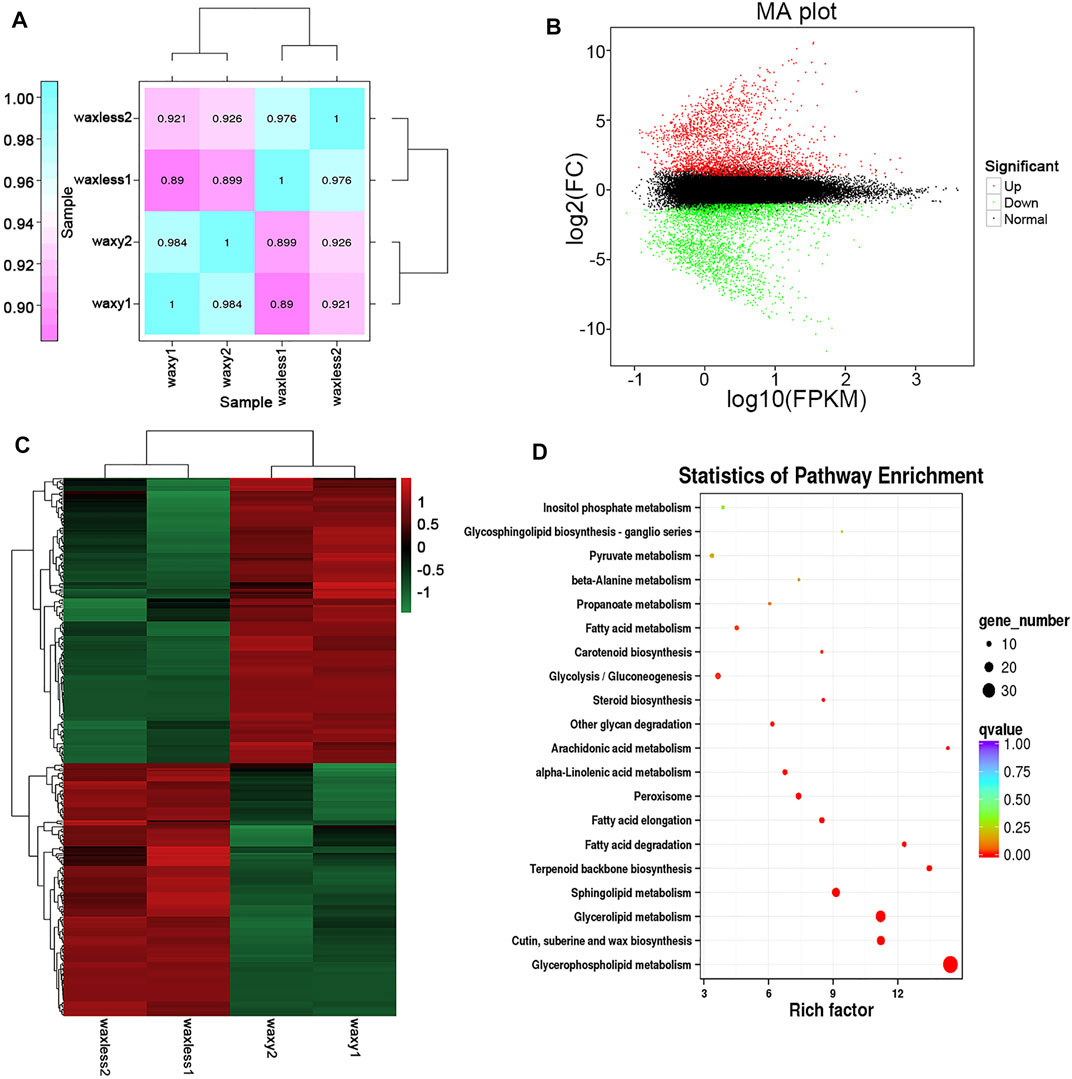
FIGURE 6. Transcriptome data analysis. (A) The expression quantity correlation heatmap of the sample. The numbers in each cell represent the correlation coefficients. (B) MA plot of DEGs, each dot in the MA map of differentially expressed genes represents a gene. Abscissa is value A: log10 (FPKM), ordinate is value M: log2FC(waxless/waxy), green dots represent the downregulated DEGs, red dots represent the upregulated DEGs, and black dots represent the non-differentially expressed genes. (C) The heatmap presentation of fold changes of 343 DEGs obtained from RNA-seq analysis. (D) Enrichment and scatter map of KEGG pathway of DEGs. Each circle in the figure represents a KEGG pathway, ordinate represents the name of the pathway, and abscissa is the enrichment factor. The greater the enrichment factor, the more significant the enrichment level of DEGs in this pathway. The color of the circle represents the qvalue. The size of the circle indicates the number of genes enriched in the pathway, and the larger the circle, the more the genes.
Furthermore, we annotated the lipid function of 3,659 DEGs, screened 343 DEGs, and enriched them using the Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis (Figures 6C,D). It was found that these genes are mainly concentrated in glycerophospholipid metabolism, cutin, suberine and wax biosynthesis, glycerolipid metabolism, sphingolipid metabolism, terpenoid backbone biosynthesis, fatty acid degradation, and fatty acid elongation pathway, which are consistent with the main lipid components that control the glossy phenotype of wheat leaves in this study.
Joint Analysis of Waxy and Differentially Expressed Genes
Combined with the determined lipid components, we marked the annotated genes in the corresponding pathway, constructed the metabolic model of the main lipid components in wheat epidermis according to the KEGG metabolic pathway (Figure 7), and screened 46 related genes (Table 2). Moreover, compared with the multi-wax mutant waxy, the genes annotated to the synthesis of wax ester (WE) in the low-wax mutant waxless were 3-ketoacyl-CoA synthase genes (KCS), very-long-chain enoyl-CoA reductase genes (TER), alcohol-forming fatty acyl-CoA reductase genes (FAR), wax-ester synthase genes (WSD1), aldehyde decarbonylase genes (CER1), and midchain alkane hydroxylase genes (MAH1). The downregulation of TER inhibits the conversion of long-chain 3-oxoacyl-CoA to long-chain acyl-CoA (n + 2), the downregulation of CER1 inhibits the transformation of upstream A long-chain aldehyde to A long-chain alkane, and the expression genes involved in KCS, FAR, WSD1, and MAH1 were both upregulated and downregulated.
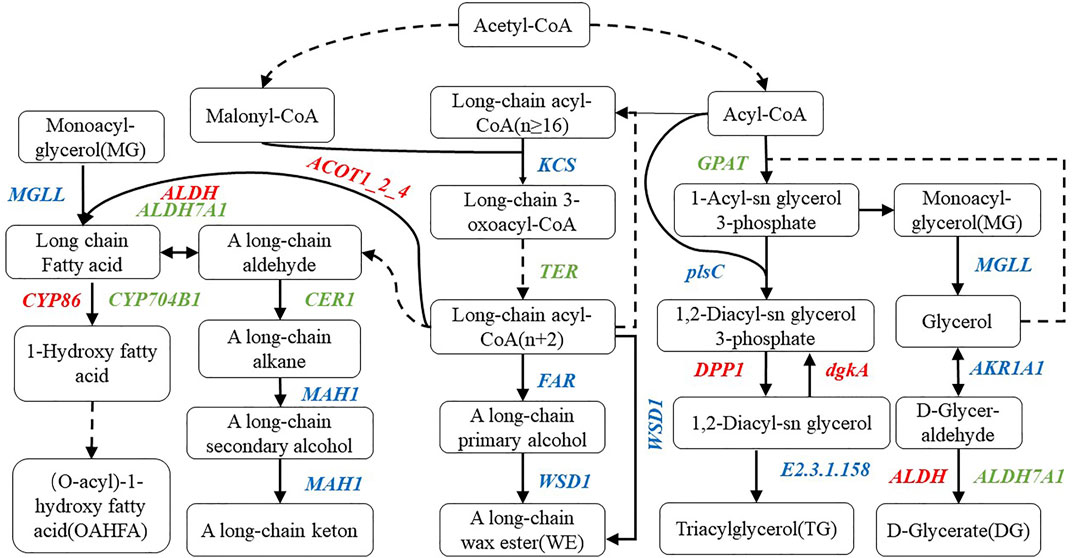
FIGURE 7. Metabolic pathway map of wax-related lipid synthesis in wheat epidermis. There are metabolic compounds in the box, red-marked enzymes are related to upregulated genes, green-marked enzymes are related to downregulated genes, and blue-marked enzymes are related to both upregulated and downregulated genes.
In the process of OAHFA synthesis, the upregulated expression of acyl-coenzyme A thioesterase 1/2/4 genes (ACOT1_2_4) promotes the synthesis of long-chain fatty acid, while the expression level of acylglycerol lipase genes (MGLL) was both upregulated and downregulated during the conversion of monoacyl-glycerol to long-chain fatty acid. Downregulation of aldehyde dehydrogenase family 7 member A1 genes (ALDH7A1) expression inhibited the transition between A long-chain aldehyde and long-chain fatty acid, while aldehyde dehydrogenase genes (ALDH) upregulated the transition between them. During the transformation of long-chain fatty acid into omega-hydroxy fatty acid, the expression of long-chain fatty acid omega-monooxygenase genes (CYP704B1) was downregulated, while that of fatty acid omega-hydroxylase genes (CYP86) was upregulated in the mutant waxless. Glycerol-3-phosphate acyltransferase genes (GPAT) and ALDH7A1 were downregulated in the process of glyceride metabolism of DG, MG, and TG. The downregulated expression of GPAT inhibits the synthesis of 1-acyl-sn glycerol 3-phosphate in waxless, which in turn reduces the synthesis of MG. The downregulated expression of ALDH7A1 in the low-wax mutant decreases the synthesis of DG. The upregulated genes are ALDH, diacylglycerol diphosphate phosphatase genes (DPP1), and diacylglycerol kinase genes (dgkA), while the expressions of 1-acyl-sn-glycerol-3-phosphate acyltransferase genes (plsC), phospholipid: diacylglycerol acyltransferase genes (E2.3.1.158), MGLL, and alcohol dehydrogenase genes (AKR1AI) were both upregulated and downregulated. These genes work together to reduce the content of TG in the low-wax mutation waxless. In order to verify the reliability of our data for screening wax-related genes, we randomly selected 13 differentially expressed genes for real-time quantitative PCR detection, and the results of qRT-PCR of 13 genes were consistent with those of transcriptome data (Supplementary Figure S2), indicating that the screened wax-related genes are highly reliable, which is helpful for further functional verification and cloning of wax-related genes.
Discussion
New Waxy Components of Wheat Epidermis Were Found by Untargeted Lipidomic Analysis
Epidermal wax is a complex lipid mixture composed of VLCFAs and their derivatives, which plays a special role in plant resistance to drought, diseases, and insect pests (Bernard and Joubes, 2013). At present, the GC-MS method is mainly used to determine the wax of epidermis (Rolim et al., 2015) and primary alcohols, secondary alcohols, aldehydes, alkanes, ketones, esters, triterpenes, sterols, and flavonoids (Zhang et al., 2005; Tafolla-Arellano et al., 2018). Based on the limitations of the determination method, there may still be many epidermal wax-related lipid components that have not been detected, which limits the study of the epidermal wax biosynthesis pathway. Lipidomics as a research model based on high-throughput analysis can systematically analyze the changes in lipid composition and expression in organisms. Perez-Navarro et al. (2019) used LC-MS technology to determine grape lipids and found new information on the composition of free fatty acids such as glycerol, glycerol phospholipids, and triterpenes in grape skins and seeds. Broughton et al. (2018) used electrospray ionization tandem mass spectrometry (ESI-MS/MS) to analyze the acyl parts of wax esters (WE) and sterol esters (SE) in common and mutant sunflower oils with different fatty acid profiles and discovered the methylsterol components in sunflower oil sterol esters (SE) for the first time.
Bianchi et al. (1980) studied the epidermal wax of common wheat in Chinese spring and found that its main components are n-alkanes, esters, aldehydes, free alcohols, β-diketones and hydroxy-β-diketones. Racovita et al. (2016) detected new waxy substances such as 2-alkyl alcohol, benzyl alcohol, phenylethyl alcohol, and hydroxyphenylethanol in wheat flag leaves and peduncles. Lavergne et al. (2018) analyzed the epidermis of wheat leaves and stems and found that the waxy components of the epidermis were alkanes (C20-C42), fatty acids (C7-C34), ketones (C9-C35), and primary alcohols (C22-C33). In this study, UHPLC-MS/MS analysis technique was used for the first time to detect the leaf epidermis wax of low-wax mutant waxless and multi-wax mutant waxy of Jimai 22. We found that the main lipid components of leaf epidermis wax of Jimai 22 were WE (C19-C50), DG (C27-C53), MG (C31-C35), OAHFA (C31-C52), TG (C29-C73), Cer (C29-C48), ChE (C46H86O2N1), and CL (C65H120O17P2). Among them, DG, MG and OAHFA were detected in wheat leaf wax for the first time, which improved the composition of wheat wax components. It is clear that the liposome analysis of wheat epidermis wax by LC-MS can more systematically study the changes and functions of related lipid subclasses and lipid molecules in the process of epidermal wax metabolism, which is helpful to improve the pathway and mechanism of epidermal wax synthesis.
New Components Such as Diacylglycerol, Monoacylglycerol, and (O-Acyl)-1-hydroxy Fatty Acid Are Closely Related to Stress Resistance
In this research, we found that the wax content was closely related to the water loss rate of leaves, which may be related to drought resistance (Zhang et al., 2013). Besides, DG, MG, and OAHFA were newly found in wheat epidermis wax. DG, as one of the main components of glycerol, plays a critical role in maintaining the stability of cell membrane (especially plasma membrane and chloroplast membrane) under high temperature and drought stress (Narayanan et al., 2016). It can maintain the fluidity of cell membrane by downregulating saturated DGs (4 and 10 double bonds) and u-regulating low unsaturated DGs (0–4 double bonds) (Navarro-Reig et al., 2019). MG, as a waxy substance, can form stable hydrated dispersions in water. Under salt stress, the ratio of MG unsaturated fatty acids to saturated fatty acids increased significantly in salt-tolerant varieties (Gogna et al., 2020). The MG in wheat epidermis wax found in this study is also mainly MG (32:1) + NH4 and MG (28:1) + H with low saturation. OAHFA acts as a surfactant in human tear film lipids and plays a key role in stabilizing tear film (Schuett and Millar, 2013; Marshall et al., 2016). Interestingly, we found OAHFA in wheat leaf epidermis wax for the first time and C48 molecules play a leading role. Its effects on wheat stress resistance and related mechanism need to be further studied.
In addition, as an important oil substance, WE plays a significant role in plant resistance to drought because of its hydrolytic resistance (Ivarson et al., 2017; Wang et al., 2021). The WE wax found by predecessors in sunflower is mainly concentrated in C32–C48 molecules (Broughton et al., 2018). In our study, we found that the WE wax in the epidermis of wheat leaves is mainly distributed in the molecular range of C29–C46 chain length, and the wax ester molecule dominated by C44 plays a role, which is similar to the results of previous studies.
Molecular Regulation Mechanism of Epidermal Wax Metabolism in Wheat
Previous studies have shown that the W1-W5 homologue of Arabidopsis CER protein in wheat increases the wax content of the epidermis by producing hydroxy-β-diketone and inducing the biosynthesis of β-diketone (Huang et al., 2017; Li et al., 2020). In this study, two CER1 genes, TraesCS1D03G0373900 and TraesCS1D03G0374000, were also detected in the low-wax mutants, which decreased the synthesis of A long-chain alkane by downregulating the expression of CER1. TaFAR1, TaFAR2, TaFAR3, TaFAR4, TaFAR5, and AtCER4 are homologous genes of CER4 in Arabidopsis. As a kind of alcohol-forming fatty acyl-CoA reductase, they induce the production of A long-chain primary alcohol and increase the wax content of wheat leaves (Wang W. et al., 2015; Wang et al., 2015b; Wang et al., 2015c; Wang et al., 2017). Besides, two FAR genes regulating primary alcohol synthesis, TraesCS4B03G0019500, and TraesCS7B03G1338900, were also found in the mutant waxless, which downregulated primary alcohol synthesis and promoted the smooth green appearance of the waxy wheat epidermis. In the research of low-wax mutants of wheat, Li et al. (2021) found that the expression levels of ACC1, LACS, KCS, and KCR were downregulated, which reduced the synthesis of VLCFAs. In our research, four KCS genes were also found to downregulate long-chain 3-oxoacyl-CoA synthesis in waxless. In addition, we found TER gene TraesCS6B03G1132900LC in wheat mutant waxless leaves for the first time, which inhibited the synthesis of long-chain acyl-CoA (n + 2) by downregulating its expression. This provides important reference information for enriching the molecular regulation mechanism of wheat epidermis wax synthesis.
Moreover, our study also found that the downregulation of two ALDH7A1 genes TraesCS5B03G0557800 and TraesCS5D03G0511400 inhibited the synthesis of long-chain fatty acid and DG, the downregulation of three CYP704B1 genes TraesCS3B03G0228000, TraesCS3B03G0938500, and TraesCS3D03G0167200 inhibited the synthesis of omega-hydroxy fatty acid, and the downregulation of GPAT genes TraesCS6A03G0083200 and TraesCS6B03G0120600 downregulated the expression of 1-acyl-sn glycerol 3-phosphate in DG, MG, and TG during glyceride metabolism, which inhibited the synthesis of 1-acyl-sn glycerol 3-phosphate in wax-free mutants. The downregulated expression of plsC gene TraesCS2B03G1160500 reduces the synthesis of 1,2-diacyl-sn glycerol 3-phosphate. Downregulation of phospholipid: diacylglycerol acyltransferase genes TraesCS7A03G0874000 expression inhibits the transformation from 1,2-diacyl-sn glycerol to TG. These genes are found for the first time to regulate waxy synthesis in the wheat epidermis. In-depth genomic comparison and functional identification of these genes are helpful in cloning wheat waxy functional genes and analyzing the regulatory mechanism of waxy metabolism. Our next research will focus on the functional verification of key genes to better explain the molecular regulation mechanism of wax in the leaf epidermis of wheat.
Conclusion
In this study, untargeted liposome detection and transcriptome analysis were carried out on the leaf epidermis wax of Jimai 22 low-wax mutant and multi-wax mutant. A total of 31 lipid subclasses and 1,367 lipid molecules were identified. The main lipid components of wheat leaf wax were identified as WE (C19-C50), DG (C27-C53), TG (C29-C73), MG (C31-C35), and OAHFA (C31-C52). DG, MG, and OAHFA were found in the epidermis wax of wheat leaf for the first time. Compared with the mutant waxy, a total of 6,840 DEGs were detected in the mutant waxless, of which 3,181 DEGs were upregulated and 3,659 DEGs were downregulated. According to KEGG metabolic pathway, the metabolic pattern of the main waxy components in the wheat epidermis was constructed and 46 related genes were screened, including KSC, TER, FAR, WSD1, CER1, MAH1, ALDH7A1, CYP704B1, ACOT1_2_4, ALDH, CYP86, MGLL, GPAT, DPP1, dgkA, plsC, and E2.3.1.158 related genes. This provides valuable reference information for further study of wheat epidermis wax inheritance and molecular regulation.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material. Mass spectrometry data has been uploaded to EBI Metabolights, accession number MTBLS3710. Sequencing data has been uploaded to NCBI SRA BioProject, accession number PRJNA777112.
Author Contributions
BY, LQ, and XM designed the study and wrote the article. HW and YF carried out the experiments and analyzed data. YW, BW, and YD carried out field experiments for screening material. All authors contributed to the article and approved the final article for publishing.
Funding
This study was supported by the State Key Laboratory of Integrative Sustainable Dryland Agriculture (in preparation), Shanxi Agricultural University (No. 202105D121008-2-1), and Research Program Sponsored by the Science Research of Shanxi Academy of Agricultural Sciences (Nos. YGJPY 2008, YCX2020YQ47, and 201801D221314).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Mingyi Zhang for his mutated materials (Institute of Wheat Research, Shanxi Agricultural University). We thank Applied Protein Technology Company (Shanghai) and Beijing Baimaike Company for their help and valuable advice on liposome detection and transcriptome sequencing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.757920/full#supplementary-material
References
Aharoni, A., Dixit, S., Jetter, R., Thoenes, E., van Arkel, G., and Pereira, A. (2004). The SHINE Clade of AP2 Domain Transcription Factors Activates Wax Biosynthesis, Alters Cuticle Properties, and Confers Drought Tolerance when Overexpressed in Arabidopsis[W]. Plant Cell 16, 2463–2480. doi:10.1105/tpc.104.022897
Bernard, A., and Joubès, J. (2013). Arabidopsis Cuticular Waxes: Advances in Synthesis, export and Regulation. Prog. Lipid Res. 52, 110–129. doi:10.1016/j.plipres.2012.10.002
Bianchi, G., Lupotto, E., Borghi, B., and Corbellini, M. (1980). Cuticular Wax of Wheat. Planta 148, 328–331. doi:10.1007/bf00388119
Bourdenx, B., Bernard, A., Domergue, F., Pascal, S., Léger, A., Roby, D., et al. (2011). Overexpression of Arabidopsis ECERIFERUM1 Promotes Wax Very-Long-Chain Alkane Biosynthesis and Influences Plant Response to Biotic and Abiotic Stresses. Plant Physiol. 156, 29–45. doi:10.1104/pp.111.172320
Broughton, R., Ruíz-Lopez, N., Hassall, K. L., Martínez-Force, E., Garcés, R., Salas, J. J., et al. (2018). New Insights in the Composition of Wax and Sterol Esters in Common and Mutant sunflower Oils Revealed by ESI-MS/MS. Food Chem. 269, 70–79. doi:10.1016/j.foodchem.2018.06.135
Chai, G., Li, C., Xu, F., Li, Y., Shi, X., Wang, Y., et al. (2018). Three Endoplasmic Reticulum-Associated Fatty Acyl-Coenzyme A Reductases Were Involved in the Production of Primary Alcohols in Hexaploid Wheat (Triticum aestivum L.). BMC Plant Biol. 18, 41. doi:10.1186/s12870-018-1256-y
Dong, X., Ji, J., Yang, L., Fang, Z., Zhuang, M., Zhang, Y., et al. (2019). Fine-mapping and Transcriptome Analysis of BoGL-3, a Wax-Less Gene in Cabbage (Brassica oleracea L. Var. Capitata). Mol. Genet. Genomics 294, 1231–1239. doi:10.1007/s00438-019-01577-5
Franke, R., Briesen, I., Wojciechowski, T., Faust, A., Yephremov, A., Nawrath, C., et al. (2005). Apoplastic Polyesters in Arabidopsis Surface Tissues - A Typical Suberin and a Particular Cutin. Phytochemistry 66, 2643–2658. doi:10.1016/j.phytochem.2005.09.027
Gogna, M., Choudhary, A., Mishra, G., Kapoor, R., and Bhatla, S. C. (2020). Changes in Lipid Composition in Response to Salt Stress and its Possible Interaction with Intracellular Na+-K+ Ratio in sunflower (Helianthus Annuus L.). Environ. Exp. Bot. 178, 104147. doi:10.1016/j.envexpbot.2020.104147
Huang, D., Feurtado, J. A., Smith, M. A., Flatman, L. K., Koh, C., and Cutler, A. J. (2017). Long Noncoding miRNA Gene Represses Wheat β-diketone Waxes. Proc. Natl. Acad. Sci. USA 114, E3149–E3158. doi:10.1073/pnas.1617483114
Ivarson, E., Iven, T., Sturtevant, D., Ahlman, A., Cai, Y., Chapman, K., et al. (2017). Production of Wax Esters in the Wild Oil Species Lepidium Campestre. Ind. Crops Prod. 108, 535–542. doi:10.1016/j.indcrop.2017.07.002
Kannangara, R., Branigan, C., Liu, Y., Penfield, T., Rao, V., Mouille, G., et al. (2007). The Transcription Factor WIN1/SHN1 Regulates Cutin Biosynthesis in Arabidopsis thaliana. Plant cell 19, 1278–1294. doi:10.1105/tpc.106.047076
Lavergne, F., Broeckling, C., Cockrell, D., Haley, S., Peairs, F., Jahn, C., et al. (2018). GC-MS Metabolomics to Evaluate the Composition of Plant Cuticular Waxes for Four Triticum aestivum Cultivars. Ijms 19 (2), 249. doi:10.3390/ijms19020249
Li, L., Qi, Z., Chai, L., Chen, Z., Wang, T., Zhang, M., et al. (2020). The Semidominant Mutation W5 Impairs Epicuticular Wax Deposition in Common Wheat (Triticum aestivum L.). Theor. Appl. Genet. 133, 1213–1225. doi:10.1007/s00122-020-03543-x
Li, L., Zhang, Z., Song, W., Su, Z., Zhang, Y., You, M., et al. (2021). The Essential Role of W5 in Wax Metabolism in Wheat (Triticum aestivum L.). J. Plant Biol. doi:10.1007/s12374-021-09325-2
Marshall, D. L., Saville, J. T., Maccarone, A. T., Ailuri, R., Kelso, M. J., Mitchell, T. W., et al. (2016). Determination of Ester Position in Isomeric (O -Acyl)-Hydroxy Fatty Acids by Ion Trap Mass Spectrometry. Rapid Commun. Mass. Spectrom. 30, 2351–2359. doi:10.1002/rcm.7715
Narayanan, S., Tamura, P. J., Roth, M. R., Prasad, P. V. V., and Welti, R. (2016). Wheat Leaf Lipids during Heat Stress: I. High Day and Night Temperatures Result in Major Lipid Alterations. Plant Cel Environ. 39, 787–803. doi:10.1111/pce.12649
Navarro-Reig, M., Tauler, R., Iriondo-Frias, G., and Jaumot, J. (2019). Untargeted Lipidomic Evaluation of Hydric and Heat Stresses on rice Growth. J. Chromatogr. B 1104, 148–156. doi:10.1016/j.jchromb.2018.11.018
Pérez-Navarro, J., Da Ros, A., Masuero, D., Izquierdo-Canas, P. M., Hermosín-Gutiérrez, I., Gomez-Alonso, S., et al. (2019). LC-MS/MS Analysis of Free Fatty Acid Composition and Other Lipids in Skins and Seeds of Vitis vinifera Grape Cultivars. Food Res. Int. 125, 108556. doi:10.1016/j.foodres.2019.108556
Pizarro, C., Arenzana-Rámila, I., Pérez-del-Notario, N., Pérez-Matute, P., and González-Sáiz, J.-M. (2013). Plasma Lipidomic Profiling Method Based on Ultrasound Extraction and Liquid Chromatography Mass Spectrometry. Anal. Chem. 85, 12085–12092. doi:10.1021/ac403181c
Racovita, R. C., Hen-Avivi, S., Fernandez-Moreno, J.-P., Granell, A., Aharoni, A., and Jetter, R. (2016). Composition of Cuticular Waxes Coating Flag Leaf Blades and Peduncles of Triticum aestivum Cv. Bethlehem. Phytochemistry 130, 182–192. doi:10.1016/j.phytochem.2016.05.003
Reina-Pinto, J. J., and Yephremov, A. (2009). Surface Lipids and Plant Defenses. Plant Physiol. Biochem. 47, 540–549. doi:10.1016/j.plaphy.2009.01.004
Rolim, A. E. H., Henrique-Araujo, R., Ferraz, E. G., de Araujo Alves Dultra, F. K., and Fernandez, L. G. (2015). Lipidomics in the Study of Lipid Metabolism: Current Perspectives in the Omic Sciences. Gene 554, 131–139. doi:10.1016/j.gene.2014.10.039
Rowland, O., Zheng, H., Hepworth, S. R., Lam, P., Jetter, R., and Kunst, L. (2006). CER4 Encodes an Alcohol-Forming Fatty Acyl-Coenzyme A Reductase Involved in Cuticular Wax Production in Arabidopsis. Plant Physiol. 142, 866–877. doi:10.1104/pp.106.086785
Samuels, L., Kunst, L., and Jetter, R. (2008). Sealing Plant Surfaces: Cuticular Wax Formation by Epidermal Cells. Annu. Rev. Plant Biol. 59, 683–707. doi:10.1146/annurev.arplant.59.103006.093219
Schuett, B. S., and Millar, T. J. (2013). An Investigation of the Likely Role of (O-Acyl) ω-hydroxy Fatty Acids in Meibomian Lipid Films Using (O-Oleyl) ω-hydroxy Palmitic Acid as a Model. Exp. Eye Res. 115, 57–64. doi:10.1016/j.exer.2013.06.016
Shaheenuzzamn, M., Liu, T. X., Shi, S. D., Wu, H. Q., and Wang, Z. H. (2019). Research Advances on Cuticular Waxes Biosynthesis in Crops: a Review. IJAB 21, 911–921. doi:10.17957/ijab/15.0975
Tafolla-Arellano, J. C., Baez-Sanudo, R., and Tiznado-Hernández, M. E. (2018). The Cuticle as a Key Factor in the Quality of Horticultural Crops. Scientia Horticulturae 232, 145–152. doi:10.1016/j.scienta.2018.01.005
Wang, M., Wu, H., Xu, J., Li, C., Wang, Y., and Wang, Z. (2017). Five Fatty Acyl-Coenzyme A Reductases Are Involved in the Biosynthesis of Primary Alcohols in Aegilops Tauschii Leaves. Front. Plant Sci. 8, 14. doi:10.3389/fpls.2017.01012
Wang, W., Zhang, Y., Xu, C., Ren, J., Liu, X., Black, K., et al. (2015a). Cucumber ECERIFERUM1 (CsCER1), Which Influences the Cuticle Properties and Drought Tolerance of Cucumber, Plays a Key Role in VLC Alkanes Biosynthesis. Plant Mol. Biol. 87, 219–233. doi:10.1007/s11103-014-0271-0
Wang, Y., Mao, H., Lv, Y., Chen, G., and Jiang, Y. (2021). Comparative Analysis of Total Wax Content, Chemical Composition and crystal Morphology of Cuticular Wax in Korla Pear under Different Relative Humidity of Storage. Food Chem. 339, 128097. doi:10.1016/j.foodchem.2020.128097
Wang, Y., Wang, J., Chai, G., Li, C., Hu, Y., Chen, X., et al. (2015b). Developmental Changes in Composition and Morphology of Cuticular Waxes on Leaves and Spikes of Glossy and Glaucous Wheat (Triticum aestivum L.). Plos One 10, e0141239. doi:10.1371/journal.pone.0141239
Wang, Y., Wang, M., Sun, Y., Wang, Y., Li, T., Chai, G., et al. (2015c). FAR5, a Fatty Acyl-Coenzyme A Reductase, Is Involved in Primary Alcohol Biosynthesis of the Leaf Blade Cuticular Wax in Wheat (Triticum aestivum L.). J. Exp. Bot. 66, 1165–1178. doi:10.1093/jxb/eru457
Yeats, T. H., Buda, G. J., Wang, Z., Chehanovsky, N., Moyle, L. C., Jetter, R., et al. (2012). The Fruit Cuticles of Wild Tomato Species Exhibit Architectural and Chemical Diversity, Providing a New Model for Studying the Evolution of Cuticle Function. Plant J. 69, 655–666. doi:10.1111/j.1365-313X.2011.04820.x
Zhang, J.-Y., Broeckling, C. D., Blancaflor, E. B., Sledge, M. K., Sumner, L. W., and Wang, Z.-Y. (2005). Overexpression of WXP1, a Putative Medicago Truncatula AP2 Domain-Containing Transcription Factor Gene, Increases Cuticular Wax Accumulation and Enhances Drought Tolerance in Transgenic Alfalfa (Medicago Sativa). Plant J. 42, 689–707. doi:10.1111/j.1365-313X.2005.02405.x
Zhang, Z., Wang, W., and Li, W. (2013). Genetic Interactions Underlying the Biosynthesis and Inhibition of β-Diketones in Wheat and Their Impact on Glaucousness and Cuticle Permeability. Plos One 8, e54129. doi:10.1371/journal.pone.0054129
Zheng, X., Liu, C., Qiao, L., Zhao, J., Han, R., Wang, X., et al. (2020). The MYB Transcription Factor TaPHR3-A1 Is Involved in Phosphate Signaling and Governs Yield-Related Traits in Bread Wheat. J. Exp. Bot. 71, 5808–5822. doi:10.1093/jxb/eraa355
Keywords: wheat, wax, liposome, transcriptome, molecular regulation
Citation: Wen H, Wang Y, Wu B, Feng Y, Dang Y, Yang B, Ma X and Qiao L (2021) Analysis of Wheat Wax Regulation Mechanism by Liposome and Transcriptome. Front. Genet. 12:757920. doi: 10.3389/fgene.2021.757920
Received: 13 August 2021; Accepted: 02 November 2021;
Published: 06 December 2021.
Edited by:
Jiang Libo, Beijing Forestry University, ChinaReviewed by:
Fei Tao, Northwest University, ChinaVandana Jaiswal, Institute of Himalayan Bioresource Technology (CSIR), India
Copyright © 2021 Wen, Wang, Wu, Feng, Dang, Yang, Ma and Qiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Yang, c3h4bXN5YjgzQDEyNi5jb20=; Xiaofei Ma, bm9uZ3h1ZTA2MTIzQDE2My5jb20=; Ling Qiao, cWlhb2xpbmdzbWlsZUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Hongwei Wen
Hongwei Wen Ying Wang1†
Ying Wang1† Yanru Feng
Yanru Feng Ling Qiao
Ling Qiao