- 1Henan Luoyang Orthopedic Hospital (Henan Provincial Orthopedic Hospital), Zhengzhou, Henan, China
- 2Luoyang Postgraduate Training Department, Henan University of Chinese Medicine, Zhengzhou, Henan, China
- 3Department of Spinal Surgery, The First Affiliated Hospital of Hainan Medical University, Haikou, Hainan, China
- 4School of Clinical Medicine, Guilin Medical University, Guilin, Guangxi, China
- 5The First College for Clinical Medicine, Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China
Osteosarcoma (OS) is a primary solid malignant tumor that occurs most frequently in the metaphysis of long bones. More likely to happen to children and adolescents. OS has high mortality and disability rate. However, the etiology and pathogenesis of OS have not been fully understood till now. Due to the lack of effective biomarkers, OS cannot be precisely detected in the early stage. With the application of next-generation and high-throughput sequencing, more and more abnormally expressed non-coding RNAs(ncRNAs) have been identified in OS. Growing evidences have suggested the ncRNAs, such as microRNAs (miRNAs), long non-coding RNAs (lncRNAs), circular RNAs (circRNAs), have played an important role in the tumorigenesis and progression of OS. Thus, they can be served as novel biomarkers for diagnosis, treatment and prognosis. This review summarized the application of ncRNA as biomarkers in OS in detail, and discussed the limitation and future improvement of the potential biomarkers.
Introduction
Osteosarcoma (OS), as a common kind of primary malignant tumor of bone, has a high mortality (Cersosimo et al., 2020), accounting for about 2.4% of children with malignant tumors and 20% of all primary bone cancers. Depending on statistics, there are about 800 new cases in the United States each year, among which about 50% are children and adolescents (Ward et al., 2014). Studies have shown that the incidence of OS in men is higher than that in women, which is 1.27 times that of women. (Lee J. A. et al., 2021). OS occurs in the epiphysis of long bones more frequently, such as the proximal tibia, distal femur, proximal humerus, and other parts of the fastest growing bone (Zhao et al., 2018). OS has a high disability and mortality, and is prone to metastasis (Zheng et al., 2020). The prognosis of patients with early metastasis are very poor, the 5-year survival rate of patients with metastasis is lower than 20% (Thanindratarn et al., 2019). However, the early symptoms of OS are not typical, it was usually in the late stage when found even with metastasis, resulting in the high mortality rate. Early diagnosis and treatment are very important for the prognosis of OS. With the continuous improvement of the diagnosis and treatment methods, a variety of therapies have emerged, including targeted therapy and immunotherapy, which have effectively reduced the overall mortality of the patients. Nevertheless, the prognosis is still far from ideal. Therefore, it is urgent to carry out in-depth research on the related molecular mechanisms of the occurrence and development. By looking for new and effective biomarkers for detection, diagnosis, therapy and prognosis, the disability rate and mortality rate can be reduced in some extent, so as to prolong the survival time of the patients.
There are a wide kinds of tumor markers. At present, the discovered species include nucleic acid, protein, glucose, small molecular metabolites, cytokines, circulating tumor cells (CTCs), and so on, which play an important role in the diagnosis, treatment and prognosis of tumors. In the past few decades, bioinformatics has provided new ideas for the functions of early diagnosis and treatment with its advantages of simplicity, non-invasive and high efficiency, further play an important role in early diagnosis and prognosis evaluation. Compared with protein biomarkers, ncRNAs tumor markers have the advantage of replicability, stable expression, less affected by in vivo and in vitro stimulation. In clinic, the commonly used ncRNAs tumor markers of OS include microRNA (miRNA), long non-coding RNA (lncRNA), and circular RNA (circRNA) (Inamoto et al., 2018; Yang et al., 2021). Biomarkers have become a new potential due to their advantages of easy sample acquisition, less trauma to the human body, higher sensitivity and specificity. These biomarkers have played significant roles in non-invasive diagnosis and detection during the occurrence, development and prognosis of tumor. Non-coding RNA (ncRNAs), because of its own advantages, has become a major participant in tumor markers, such as in hepatocellular carcinoma, lung cancer, breast cancer, et al. (Wong et al., 2018; Iqbal et al., 2019; Shen et al., 2020).
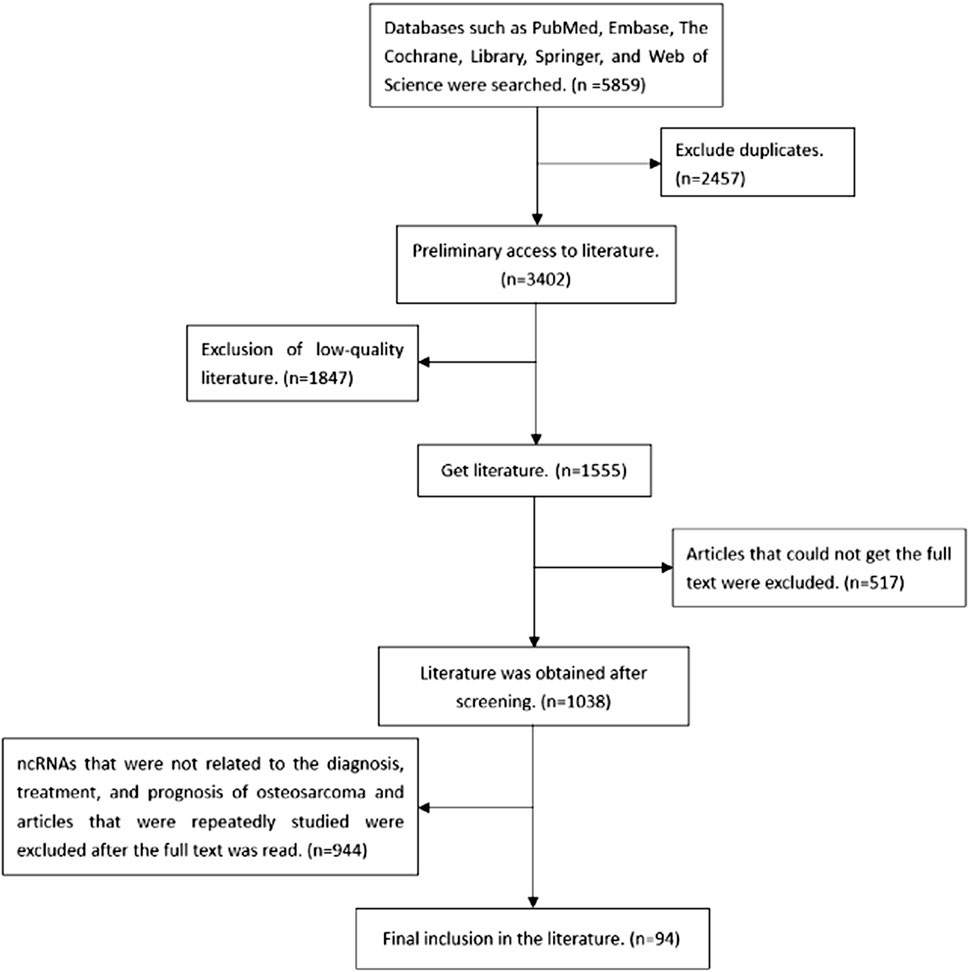
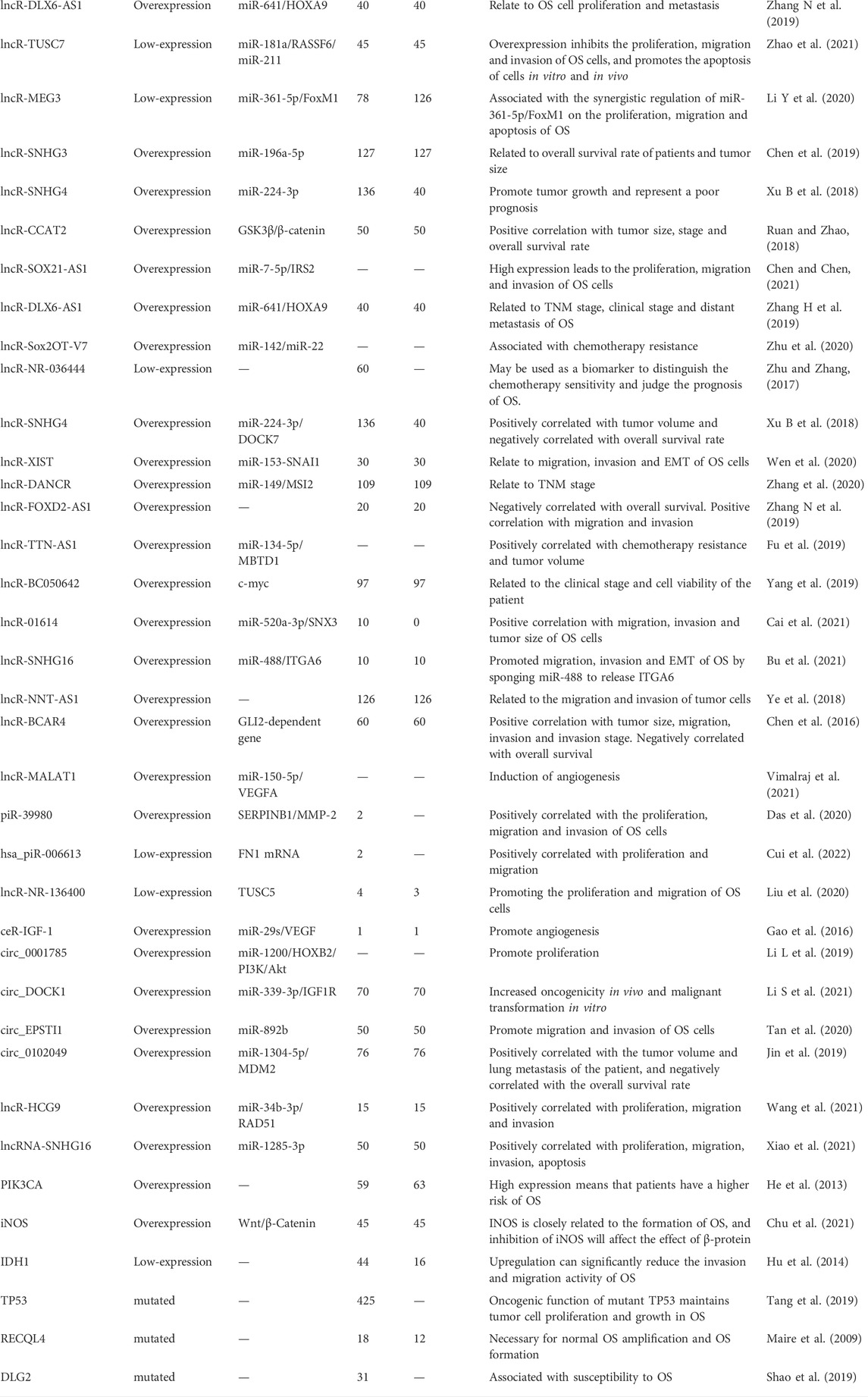
At present, the researches focused on ncRNAs tumor markers and relevant action pathways have revealed the important roles in the occurrence, metastasis and prognosis. These biomarkers can help to determine the accuracy of the diagnosis, decide on the appropriate treatment time, make personalized treatment plans, and explore new therapeutic targets. Many ncRNAs biomarkers and corresponding protein products have been reported to be overexpressed or under-expressed in OS, such as miR-223 (Xu et al., 2013), COP9 signalosome subunit 3 (COPS3) (Zhang et al., 2018), lncRNA small nucleolar RNA host gene 4 (lncRNA SNHG4) (Xu R et al., 2018), miR-142 (Shabani et al., 2019), etc. Although the reports on types and functions of ncRNAs in OS are still limited, they might express some diagnostic, stage, treatment and prognosis targets. ncRNAs such as miRNAs, lncRNAs, circRNAs studied before were summarized in this review. miRNA is a small ncRNA molecule, which mainly regulates gene expression by degrading mRNA or inhibiting the translation process after transcription. The discovery of lncRNA mainly comes from microarray technology, the second generation high-throughput transcriptome sequencing technology, single cell sequencing technology, etc., and plays an important role in maintaining the activities of living cells. circRNA is a wide range of ncRNA that is stable in nature, highly conservative and specific. In this paper, by searching the relevant databases, we searched the ncRNA related to the occurrence and development of OS, and expounded its role in the occurrence and development of OS. The specific literature screening process is shown in Figure 1. The specific roles of some of the relevant ncRNAs are presented in Table 1.
In this review, the roles of ncRNAs who had the potential as biomarkers in the diagnosis, stage, treatment, and prognosis of OS were discussed in depth, which has great potential to provide reference for relevant studies.
Diagnostic biomarkers
Diagnosing OS at the early stage can cure diseases faster and earlier, significantly improve the prognosis of patients. Abnormal expression of ncRNA biomarkers can be used as potential biomarkers for diagnosis of OS. For miRNAs, there were significant differences in serum and plasma levels of miR-21 between patients with OS and healthy people, which found can be used as biomarkers of OS. Studies have shown that miR-21 was over-expressed in OS tissues and cell lines, and it had great potential as a biomarker for OS diagnosis. Cong et al. (2018) found that the serum miR-124 level in patients with OS was significantly lower than that in the periostitis group and the healthy control group. The level of miR-124 normalized after tumor resection, and the area under the area under curve (AUC) for serum miR-124 was 0.846, with sensitivity of 79.8% and specificity of 86.0%, which proved that miR-124 had the potential to be the biomarker for diagnosis of OS. Wang et al. (2014) found that compared with the corresponding para-carcinoma tissue, the miR-143 level was significantly decreased in OS, which proved that miR-143 can be used as diagnostic biomarker of OS.
Fraxetin (FXT), had been reported to be associated with the development of various tumors (Yao et al., 2022). Through examining the expression of FTX in 25 OS and the adjacent tissues, Huang et al. (2020) found that FTX were significantly upregulated in OS tissues, and knocking out FTX gene could inhibit the survival rate, invasion, and migration force of OS cells, as well as promote the apoptosis of OS cells. LncRNA can not only directly regulate OS cells, but also affect the growth, proliferation, apoptosis and invasion of OS cells through the regulation of mRNA or miRNA, playing a key regulatory role in the occurrence and development of OS. The research studied by Li et al. (2017) found that lncRNA can be regulated in the occurrence and development of OS by competing endogenous RNAs (ceRNA), Wnt/β-Catenin and other pathways. In short, lncRNA can be served as biomarkers for early screening of OS, but their specificity and sensitivity need to be further verified clinically.
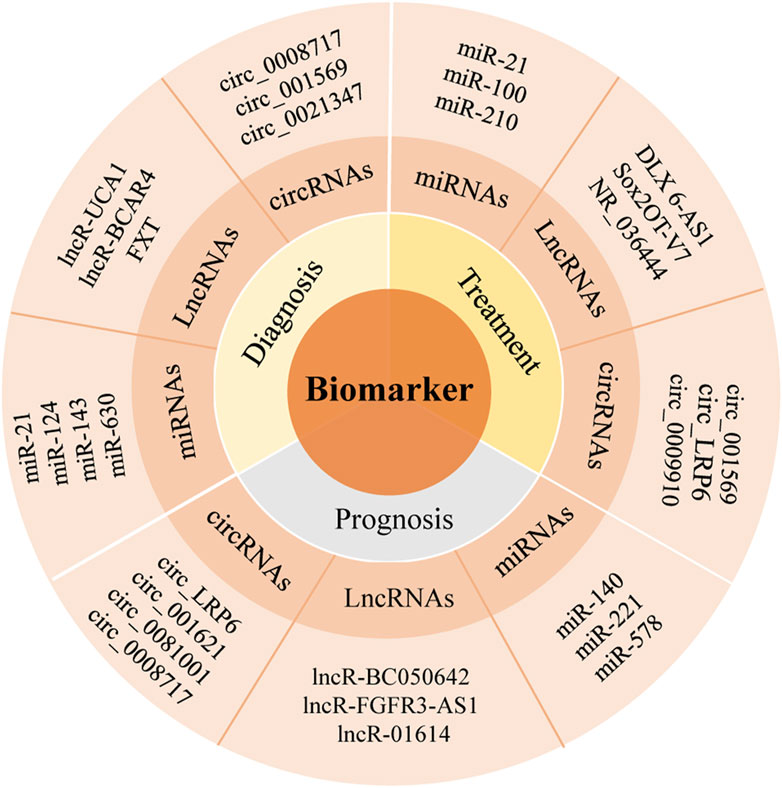
When it comes to circRNAs, recently more and more studies have shown that circRNAs can be used as a kind of biomarkers in the early diagnosis of OS, with high accuracy and specificity. Zhou et al. (2018) reported that circ_0008717 owned high expression in OS tissues, compared with the corresponding paracancerous tissues (Figure 2). Molecular sponge could bind to miR-203 to exert its carcinogenic effect. Its AUC was 0.782 (95% CI: 0.682–0.862), sensitivity was 0.80, and specificity was 0.73, which could be used as a potential biomarker for the diagnosis of OS. The commonly used biomarkers for clinical OS diagnosis are serum alkaline phosphatase (AKP) and lactate dehydrogenase (LDH), but the comparison of diagnostic efficacy between these biomarkers and OS biomarkers has not been reported. Lei and Xiang (2020) found that hsa_circ_0003074 was highly expressed in plasma of OS patients and had a high efficacy in distinguishing OS patients from healthy volunteers, with the AUC of 0.93, higher than LDH (AUC = 0.83) and alkaline phosphatase (ALP) (AUC = 0.88). Thus, circRNAs also had the potential to be a diagnostic biomarker, which need further studies.
It can be observed that many articles have proved that ncRNAs also has the potential as a diagnostic marker. In addition to the function in diagnosis, the levels of OS ncRNAs biomarkers also play an important role in determining the disease stage of OS at diagnosis. Abnormal expression of OS related genes and biomarkers can induce the activity, proliferation and differentiation of osteoblasts. The abnormal degree can directly reflect the pathological staging of patients and affect the prognosis. Enneking staging has become an important basis of OS surgical staging which can better remind the prognosis, and help to select an appropriate treatment. However, research on the relationship between ncRNAs biomarkers and tumor staging is still very lacking. A variety of ncRNAs biomarkers are closely related to the clinical stage of Enneking and have been widely used to determine the clinical stage of OS. The more malignant the lesion is, the higher Enneking stage and higher possibility of distant metastasis the tumor have.
It was found that the expression of lncRNA GNAS antisense (lncRNA GNAS-AS1) could significantly increase in OS cells and tissues, which were positively correlated with Enneking stage and distant metastasis. Its high expression often predicted the short overall survival time (Mi et al., 2021). Through regulating miR-490-3p, lncRNA GNAS-AS1 could play a major role in cell proliferation, migration and invasion, which could be an independent prognostic predictor of OS, providing a new therapeutic strategy for OS (Mi et al., 2021). The expression of circRNA-mitochondrial tRNA translation optimization 1 (circ_MTO1) in OS is low, its expression level is significantly correlated with Enneking staging, and its high expression in the tumor meant that the Enneking staging was lower in OS patients who had better neoadjuvant chemotherapy response and longer disease-free survival (DFS) (Shi et al., 2021). Except for the above, the increased expression of circ_001569 in OS was positively related to tumor size, Enneking stage or Tumor Node Metastasis (TNM) stage and lung metastasis (Xiao et al., 2020). The results reported by Wang et al. (2019) showed that circ_0021347 was significantly downregulated in OS tissues and cell lines, negatively correlated with TNM staging and positively correlated with patient survival. circ_0021347 can target B7 Homolog3 (B7-H3), which showed a strong negative correlation with the expression of B7-H3 in OS and exerted anti-cancer effect by negatively regulating the expression of B7-H3.
In a word, the emergence of ncRNAs biomarkers in patients provides a reliable non-invasive method for the diagnosis and clinical staging of OS. However, when exploring new serum biomarkers in the future, the sensitivity and specificity of existing biomarkers should not be ignored, so as to provide accurate guidance information for clinical practice.
Therapeutic biomarkers
Neoadjuvant chemotherapy is the standard treatment at present, and its survival rate has been greatly improved. However, the survival rate of patients with lung metastasis and chemotherapy resistance is still very low. Although the introduction of neoadjuvant chemotherapy has greatly improved the 5-year survival rate of OS, a large number of patients still have poor response to chemotherapy. Even after surgical resection and chemotherapy, there still exist a high risk of local recurrence or distant metastasis, leading to poor prognosis. It is necessary to develop new therapeutic methods for OS clinically, which require us to clarify the molecular mechanisms of the pathogenesis and development of OS. The screening of new molecular markers is important for the prognosis and treatment of OS.
The ncRNAs have good stability, which can be used as potential cancer biomarkers and treatment targets (Rong et al., 2017). Previous studies have explained the mechanism of OS from multiple drug resistance-related genes, miRNAs, circRNAs and other aspects. Significant changes of miRNAs expression profile in drug-resistant OS cells indicate that miRNAs can participate in the development of drug resistance by regulating various targets and signaling pathways. The treatment based on miRNAs mainly included blocking the expression of oncogenic miRNAs and restoring the expression of tumor-inhibiting miRNAs genes. For instance, miR-21, which was highly expressed in many cancer types, had higher expression level in the sera of OS than that in the healthy, which has been used clinically as a biomarker of chemotherapy sensitivity and prognosis (Yuan et al., 2012; Hua et al., 2017; Zhao H et al., 2019). Related studies have proved that transforming growth factor-β1 (TGF-β1) inhibitor treatment reduced the inhibitory effects of miR-21 knockdown on OS cell proliferation. miR-21 inhibition may inhibit OS cell proliferation by targeting PTEN and regulating the TGF-β1 signaling pathway (Hu et al., 2018). In addition, the miRNAs profile is associated with tumor response which can be a preferred tool for predicting tumor susceptibility to treatment.
With the deepening understanding of genetic biomarkers, the therapeutic effect of lncRNAs has become a hot research topic in recent years, which is superior to known protein encoded gene biomarkers in predicting drug responses. At present, the abnormal expression of lncRNAs has been found in many patients. These abnormal expressions can affect the processes of drug outflow, apoptosis, DNA repair, cell cycle, proliferation, autophagy, etc. At the same time, these abnormal expressions participate in the chemotherapy resistance of OS by regulating the expression of different target genes and related signaling pathways. Distal-less homeobox 6 antisense 1 (DLX 6-AS1), which was highly expressed in OS patients, exerted its capability of inhibiting the proliferation, invasion and metastasis of OS cells by targeting the miR-641/homeobox protein Hox-A9 (HOXA9) signaling pathway (Zhang H et al., 2019). Upregulation of SOX2 overlapping transcript lncRNA transcript variant 7 (Sox2OT-V7) in OS can directly target miR-142/miR-22 to inhibit its expression, especially in OS tissues and cell lines that were resistant to chemotherapy. When knocking out Sox2OT-V7 in OS cells, the drug-resistant U2OS/Dox cells can be re-sensitive to chemotherapy drugs (Zhu et al., 2020). Small nucleolar RNA host genes 4 (SNHG 4) can play a role in the occurrence and development of OS through the miR-224-3p/DOCK7 pathway, which provide a new method in the treatment of OS (Xu B et al., 2018). Lee A. M. et al. (2021) identified a positive correlation between the expression of lncRNA and anti-sense non-coding RNA in the INK4 locus (ANRIL) and the resistance of two therapeutic drugs for OS, cisplatin and doxorubicin. It was found that the drug resistance of MG-63/DXR cells transfected with lncRNA NR_036444 decreased significantly, the proportion of cells in the G (1) phase increased. The proportion of cells in the later stage of apoptosis also increased, which might play an important role in regulating the drug resistance to doxorubicin, and might become a useful biomarker to evaluate chemotherapy sensitivity and predict prognosis of OS in the future (Zhu and Zhang, 2017).
CircRNAs can play positive or negative regulatory roles in the occurrence and development of OS. In the fact, circRNAs can further participate in the regulation of chemotherapy resistance and metastasis of OS through “sponging” miRNAs as a tumor activating or inhibiting factor. The treatment based on miRNAs mainly included blocking the expression of oncogenic miRNAs and restoring the expression of tumor-inhibiting miRNAs genes. The carcinogenic effects of circ_0009910 were partially dependent on the JAK2/STAT3 pathway, which was involved in the apoptosis and proliferation of cells, thus affecting the progression of OS (Richardson et al., 2010). And circ_001569 may promote resistance through the Wnt/β-catenin pathway (Wu et al., 2014).
Drug resistance is the main limiting factor for the effectiveness of cancer treatment. Some drugs can quickly relieve the tumor, but on the one hand, they can also produce resistance, thus increasing the difficulty of treatment (Vasan et al., 2019). At present, more and more ncRNAs have been found to be associated with drug resistance of OS (Raei et al., 2021). The reversal of targeted ncRNAs is likely to be a potential method for reversing drug resistance of OS, which requires us to consider how to select the key ncRNAs from a large number of candidates ncRNAs. In the future, we should actively carry out clinical trials or transformation research based on targeted ncRNAs therapy, clarify the detailed mechanism of ncRNAs in OS drug resistance, and further apply them to clinical treatment of OS.
Prognostic biomarkers
OS has a poor prognosis due to chemo-resistance and/or metastases. ncRNAs biomarkers of OS can affect the prognosis in many ways. Clarifying the relationship between ncRNAs biomarkers and the prognosis of OS, can help to optimize the current treatment plan and choose the right time of surgery and chemotherapy.
A variety of miRNAs also have great significance for the prognosis and progression of OS. The expression of miR-140 was related to the chemotherapy sensitivity of OS xenografts, which was involved in a wide range of chemotherapy resistance mechanisms by inhibiting HDAC4-mediated decrease in cell proliferation in G (1) and G (2) stages and participating in chemotherapy resistance. miR-140 could inhibit the proliferation of U2OS cells, but its inhibitory effect on MG-63 cell was weak (Song et al., 2009). miR-223 has proved to be a potential prognostic marker for a variety of cancers in the past. Recently, some scholars have observed its function in OS. They found that miR-223 may be related to OS metastasis, and it has great potential as a potential biomarker for diagnosis and prognosis of OS (AUC:0.926) (Dong et al., 2016).
LncRNAs can play important roles in drug sensitivity and cancer metastasis. lncR-BC050642 was significantly increased in OS tissues and cell lines, playing an important role in promoting the proliferation of OS cells, who can also be an independent biomarker of OS prognosis (Yang et al., 2019). In OS tissues, the significantly upregulated lncR-01614 indicated a worse prognosis for patients. After knocking out lncR-01614, the proliferation, invasion and metastasis of OS cells could be inhibited. This effect was achieved through the miR-520a-3p/SNX3 regulatory axis, which had the potential to be a new clinical prognostic marker for OS (Cai et al., 2021). Titin-antisense RNA1 (TTN-AS1) could act as a carcinogen, playing a role in cancer through the miR-134-5p/MBTD1. Its high expression was also significantly related to the poor prognosis, and it had a potential to be a biomarker (Fu et al., 2019).
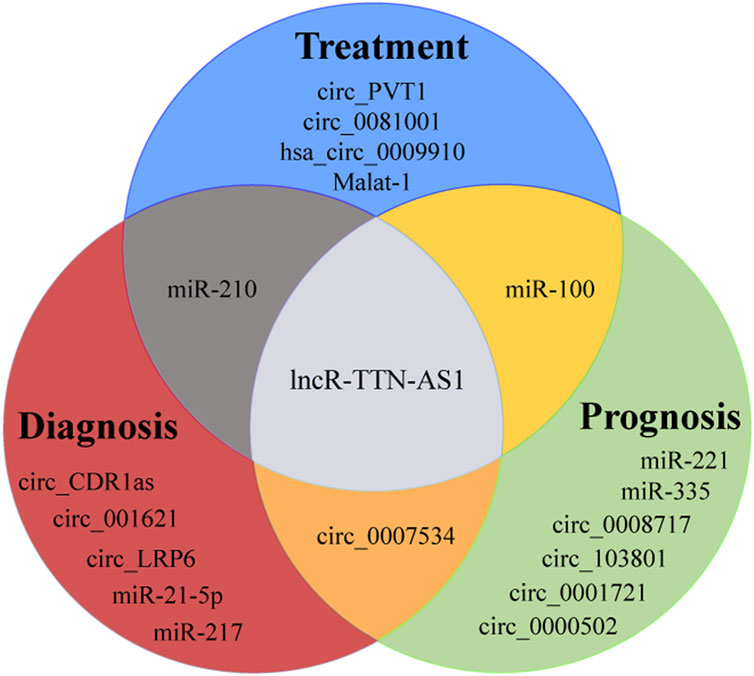
CircRNA LRP6 (circ_LRP6) was highly expressed in OS, which can promote the progress of OS by inhibiting the expression of APC and KLF2. Its high expression usually indicated the short-term disease-free survival time and overall survival time (Zheng et al., 2019) (Figure 2). Studies have established an experimental system to study the in vivo and in vitro effects of the interaction between circ_001621/miR-578/VEGF, and the results show that circ_001621 played an important role in OS, and can increase the malignant degree, directly inhibit the expression of miR-578, significantly upregulate the expressions of VEGF, cyclin dependent kinase 4 (CDK4) and matrix metalloprotein 9 (MMP-9) in MG-63 and U2OS cells. Further it can promote the proliferation and migration of OS cells and provide a new therapeutic target for advanced OS (Ji et al., 2020) (Figure 3). It was found that circ_0008717 and lung metastasis are independent prognostic factors through Cox multivariate regression analysis. After knocking down the expression of circ_0008717, the proliferation, migration and invasion of tumor cells can be reduced, and apoptosis can be promoted (Shen et al., 2022). circ_0081001 was another potential biomarker of OS prognosis, which was selected from chemotherapy and chemotherapy-sensitive OS cell lines, showing high levels in advanced OS, chemotherapy-resistant and lung metastasis (Wei et al., 2021) (Figures 2, 3).
Although some ncRNAs as prognostic biomarkers have been identified in OS, the outcome was not fully validated. As there have been some studies on the prognostic markers of OS, there is no practical application. The function of biomarkers for the diagnosis and prognosis of OS, especially patients with metastasis, may be an urgently needed tool for early diagnosis and identifying potential therapeutic targets.
Perspectives and future opportunities
The early symptoms of OS are not typical. It is easy to metastasize, and the treatment often lagging behind with poor effect. Although the diagnosis and treatment of OS have made continuous progress in recent years, the 10-year survival rate of patients with metastatic OS is still less than 20% (Yang et al., 2020). As a result, new early diagnosis and treatment methods of OS are urgently needed. Biomarkers refer to biochemical indicators that can mark changes or possible changes in the structure or function of systems, organs, tissues, cells, and subcellular. The development of biomarkers provides new insights for the early diagnosis and treatment of diseases, which can assist clinicians in initial diagnosis especially for patients with metastatic OS, guide the treatment method, judge disease stages, evaluate the safety and effectiveness of new drugs or therapies in target population, providing a series of key genes and approaches for elucidating the molecular mechanism of OS. Therefore, it is urgent to search for useful biomarkers and therapeutic targets for clinical application. ncRNAs can be produced in the early stage of disease, which is an important part of epigenetics research. It can be used as a key regulatory factor to regulate the expression of related genes and participate in the processes of cell development, differentiation, proliferation, transcription, post-transcriptional modification, apoptosis, and cell metabolism (de la Fuente et al., 2012). Moreover, it can naturally connect related genetic networks to affect various basic protein effect factors that drive specific cellular biological responses and determine cell fate (Wei et al., 2017). As a carcinogen or anti-cancer factor, it plays an important role in the occurrence and development of a variety of cancers, with a certain stability and richness. ncRNAs can enter the circulatory system, which is expected to become a potential biomarker of OS (Slack and Chinnaiyan, 2019).
The application of these ncRNAs in the research and development of anti-OS drugs, related therapeutic targets and biomarkers in the field of OS was discussed in detail in this paper. At present, the newly discovered tumor biomarkers are one of the hot spots in oncology research. The research on ncRNAs-encoded peptides or proteins has opened up a new research field for the diagnosis and treatment of tumors. As for OS, ncRNAs can be produced in the early stage of the disease, with certain stability and richness. When them entered the circulatory system, they can play an important role in cell function and affect its clinical manifestations to a certain extent. It is expected to become an effective diagnosis and treatment method. However, current research on ncRNAs also has the following problems. Firstly, the related research on ncRNAs and OS is limited and not in-depth. The current research mainly focuses on basic in vitro experiments. Further animal experiments and clinical trials are needed to confirm it in the future. Secondly, the targeted anticancer drugs of ncRNAs can weaken or even eliminate drug resistance of the patients, and further enhance the therapeutic effect. However, these drugs still have the disadvantages of insufficient specificity and utilization rate. The absorption and biodistribution of some drugs still need further solution. There is still a long way to go before the approval and commercialization of ncRNAs-targeted anticancer drugs. Thirdly, ncRNAs have many targets and complex regulatory networks. At present, most research on ncRNAs are independent and scattered. The lack of specificity in the diagnosis and treatment of OS targeting ncRNAs still needs to be resolved. Finally, there still exist problems such as small sample size in the current research on the relationship between ncRNAs and OS, and lacking of epidemiological evaluation and functional exploration of candidate biomarkers. Only a few ncRNAs have been fully studied, and the possibility of its clinical application is still uncertain. In addition, most ncRNAs cannot be detected by standard methods such as quantitative PCR. It is an urgent to find new detection optimization methods and advanced laboratory platform conditions.
In summary, the expression of the biomarker is significantly related to the tumor size, distant metastasis, TNM stage, and Enneking surgical stage of the patients, which has the ability to distinguish OS from the healthy. It is important to look for a reliable clinical biomarker in the field of the early diagnosis and treatment of OS. These biomarkers are helpful to clarify the molecular mechanisms related to OS, and may become new targets for OS diagnosis and treatment. The higher the level of biological evolution, the higher the proportion of ncRNAs in the genome. Constituting almost 60% of the transcriptional output in human cells, ncRNAs have been shown to regulate cellular processes and pathways in developmental and pathological contexts (Anastasiadou et al., 2018). Therefore, searching for more types of ncRNAs and its more extensive and diverse biological functions and action mechanisms are our current main tasks. The role of these ncRNAs in the proliferation, occurrence and development of OS cells should be deeply studied, so as to better predict and diagnose OS, provide meaningful theoretical guidance for individualized treatment and drug research. In the future, ncRNAs may continue to better help researchers and clinicians find molecular features, help in differential diagnosis, personalized treatment and determine prognosis et al. Although the applications of ncRNAs are still at an early stage, it deserves expectation can be put into clinical practice especially those candidates that can be readily detected. In the follow-up study, we would further verify the efficacy of diagnosis, treatment and prognosis of OS in large samples, and combine multiple ncRNAs to build a model to assist the diagnosis of clinical OS. Benign biomarkers should affect biological function of the disease, and the impact of promising ncRNA on the function of OS will be further explored. In addition, the biomarkers found in urine or blood by non-invasive procedures is ideal better than collecting tissues. Furthermore, there are great challenges associated with RNA-based therapeutics. On the one hand, RNA drugs can easily approach the targets based on nucleotide hybridization. On the other hand, the delivery method is a challenging process. As a promising new biomarker, ncRNA research is undergoing a rapid change and entering a new area of development. To keep up the pace, better techniques for the detect of new useful ncRNAs need to be implemented, and the evaluation of those potential biomarker should be validated in a larger sample size. In the near future, our team will still be committed to the above studies of OS. Discovering new targets by high throughput sequencing methods, and follow-up studies on pathways are our research goals which can promise the revolution of OS. It is believed that with the further popularization and deepening of research, there will be new thoughts on the early prevention, accurate diagnosis and safer and more effective treatment of OS, and further application of ncRNAs to the diagnosis and treatment of other types of cancer.
Author contributions
JL conceived the study. LF and ZZ conducted the study and drafted the application sections, they contributed equally to this work. YL contributed to the writing and review of the manuscript. All authors read, revised and approved the final manuscript.
Funding
This work was supported by the Innovation Fund of National Clinical Research Center for Orthopedics, Sports Medicine, and Rehabilitation (2021-NCRC-CXJJ-PY-13), Young Elite Scientists Sponsorship Program by CAST (2021-QNRC2-A06), the Major Project of TCM research in Henan Province (2019ZY2156), the Major Project of Science and Technology in Henan Province (222102310428), and the project of health guiding plan in Luoyang (2101043A).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anastasiadou, E., Jacob, L. S., and Slack, F. J. (2018). Non-coding RNA networks in cancer. Nat. Rev. Cancer 18, 5–18. doi:10.1038/nrc.2017.99
Bu, J., Guo, R., Xu, X. Z., Luo, Y., and Liu, J. F. (2021). LncRNA SNHG16 promotes epithelial-mesenchymal transition by upregulating ITGA6 through miR-488 inhibition in osteosarcoma. J. Bone Oncol. 27, 100348. doi:10.1016/j.jbo.2021.100348
Cai, Q., Zhao, X., Wang, Y., Li, S., Wang, J., Xin, Z., et al. (2021). LINC01614 promotes osteosarcoma progression via miR-520a-3p/SNX3 axis. Cell. Signal. 83, 109985. doi:10.1016/j.cellsig.2021.109985
Cao, C., and Shu, X. (2021). Suppression of circ_0008932 inhibits tumor growth and metastasis in osteosarcoma by targeting miR-145-5p. Exp. Ther. Med. 22, 1106. doi:10.3892/etm.2021.10540
Cersosimo, F., Lonardi, S., Bernardini, G., Telfer, B., Mandelli, G. E., Santucci, A., et al. (2020). Tumor-associated macrophages in osteosarcoma: From mechanisms to therapy. Int. J. Mol. Sci. 21, E5207. doi:10.3390/ijms21155207
Chen, B., Huang, Z., Zhang, Y., Chen, Y., and Li, Z. (2015). MicroRNA-145 suppresses osteosarcoma metastasis via targeting MMP16. Cell. Physiol. biochem. 37, 2183–2193. doi:10.1159/000438575
Chen, F., Mo, J., and Zhang, L. (2016). Long noncoding RNA BCAR4 promotes osteosarcoma progression through activating GLI2-dependent gene transcription. Tumour Biol. 37, 13403–13412. doi:10.1007/s13277-016-5256-y
Chen, H., and Chen, J. (2021). LncRNA SOX21-AS1 promotes the growth and invasiveness of osteosarcoma cells through miR-7-5p/IRS2 regulatory network. Arch. Med. Res. 52, 294–303. doi:10.1016/j.arcmed.2020.11.007
Chen, J., and Chen, Z. (2020). Downregulation of miR-19a inhibits the proliferation and promotes the apoptosis of osteosarcoma cells by regulating the JAK2/STAT3 pathway. Oncol. Lett. 20, 173. doi:10.3892/ol.2020.12033
Chen, J., Wu, Z., and Zhang, Y. (2019). LncRNA SNHG3 promotes cell growth by sponging miR-196a-5p and indicates the poor survival in osteosarcoma. Int. J. Immunopathol. Pharmacol. 33, 2058738418820743. doi:10.1177/2058738418820743
Chen, R., Li, X., He, B., and Hu, W. (2017). MicroRNA-410 regulates autophagy-related gene ATG16L1 expression and enhances chemosensitivity via autophagy inhibition in osteosarcoma. Mol. Med. Rep. 15, 1326–1334. doi:10.3892/mmr.2017.6149
Chen, Y., Huang, T., Yang, X., Liu, C., Li, P., Wang, Z., et al. (2018). MicroRNA106a regulates the proliferation and invasion of human osteosarcoma cells by targeting VNN2. Oncol. Rep. 40, 2251–2259. doi:10.3892/or.2018.6601
Cheng, S., Zheng, J., Liu, X., Shi, J., Gong, F., Zhang, X., et al. (2021). Knockdown of 91 H suppresses the tumorigenesis of osteosarcoma via inducing methylation of CDK4 promoter. Technol. Cancer Res. Treat. 20, 1533033821990006. doi:10.1177/1533033821990006
Chu, W., Cao, L., Daokun, G., and Zhao, J. (2021). iNOS promotes the development of osteosarcoma via wnt/β-catenin pathway. J. Immunol. Res. 2021, 4549221. doi:10.1155/2021/4549221
Cong, C., Wang, W., Tian, J., Gao, T., Zheng, W., and Zhou, C. (2018). Identification of serum miR-124 as a biomarker for diagnosis and prognosis in osteosarcoma. Cancer Biomark. 21, 449–454. doi:10.3233/CBM-170672
Cui, P., Xin, D., Li, F., Deng, L., and Gao, Y. (2022). Butorphanol suppresses the proliferation and migration of osteosarcoma by promoting the expression of piRNA hsa_piR_006613. Front. Oncol. 12, 775132. doi:10.3389/fonc.2022.775132
Das, B., Jain, N., and Mallick, B. (2020). piR-39980 promotes cell proliferation, migration and invasion, and inhibits apoptosis via repression of SERPINB1 in human osteosarcoma. Biol. Cell. 112, 73–91. doi:10.1111/boc.201900063
de la Fuente, M., Valera, S., and Martinez-Guitarte, J. L. (2012). ncRNAs and thermoregulation: a view in prokaryotes and eukaryotes. FEBS Lett. 586, 4061–4069. doi:10.1016/j.febslet.2012.10.018
Deng, N., Li, L., Gao, J., Zhou, J., Wang, Y., Wang, C., et al. (2018). Hsa_circ_0009910 promotes carcinogenesis by promoting the expression of miR-449a target IL6R in osteosarcoma. Biochem. Biophys. Res. Commun. 495, 189–196. doi:10.1016/j.bbrc.2017.11.028
Dong, J., Liu, Y., Liao, W., Liu, R., Shi, P., and Wang, L. (2016). miRNA-223 is a potential diagnostic and prognostic marker for osteosarcoma. J. Bone Oncol. 5, 74–79. doi:10.1016/j.jbo.2016.05.001
Fu, D., Lu, C., Qu, X., Li, P., Chen, K., Shan, L., et al. (2019). LncRNA TTN-AS1 regulates osteosarcoma cell apoptosis and drug resistance via the miR-134-5p/MBTD1 axis. Aging (Albany NY) 11, 8374–8385. doi:10.18632/aging.102325
Gao, S., Cheng, C., Chen, H., Li, M., Liu, K., and Wang, G. (2016). IGF1 3'UTR functions as a ceRNA in promoting angiogenesis by sponging miR-29 family in osteosarcoma. J. Mol. Histol. 47, 135–143. doi:10.1007/s10735-016-9659-2
Guan, H., Xu, H., Chen, J., Wu, W., Chen, D., Chen, Y., et al. (2021). Circ_0001721 enhances doxorubicin resistance and promotes tumorigenesis in osteosarcoma through miR-758/TCF4 axis. Cancer Cell. Int. 21, 336. doi:10.1186/s12935-021-02016-5
He, M. L., Wu, Y., Zhao, J. M., Wang, Z., and Chen, Y. B. (2013). PIK3CA and AKT gene polymorphisms in susceptibility to osteosarcoma in a Chinese population. Asian pac. J. Cancer Prev. 14, 5117–5122. doi:10.7314/apjcp.2013.14.9.5117
He, S., Wang, Z., Tang, H., Dong, J., Qu, Y., and Lv, J. (2019). MiR-217 inhibits proliferation, migration, and invasion by targeting SIRT1 in osteosarcoma. Cancer biother. Radiopharm. 34, 264–270. doi:10.1089/cbr.2017.2394
Hu, X., Li, L., Lu, Y., Yu, X., Chen, H., Yin, Q., et al. (2018). miRNA-21 inhibition inhibits osteosarcoma cell proliferation by targeting PTEN and regulating the TGF-β1 signaling pathway. Oncol. Lett. 16, 4337–4342. doi:10.3892/ol.2018.9177
Hu, X., Liu, Y., Qin, C., Pan, Z., Luo, J., Yu, A., et al. (2014). Up-regulated isocitrate dehydrogenase 1 suppresses proliferation, migration and invasion in osteosarcoma: In vitro and in vivo. Cancer Lett. 346, 114–121. doi:10.1016/j.canlet.2013.12.020
Hua, Y., Jin, Z., Zhou, F., Zhang, Y. Q., and Zhuang, Y. (2017). The expression significance of serum MiR-21 in patients with osteosarcoma and its relationship with chemosensitivity. Eur. Rev. Med. Pharmacol. Sci. 21, 2989–2994.
Huang, S., Zhu, X., Ke, Y., Xiao, D., Liang, C., Chen, J., et al. (2020). LncRNA FTX inhibition restrains osteosarcoma proliferation and migration via modulating miR-320a/TXNRD1. Cancer Biol. Ther. 21, 379–387. doi:10.1080/15384047.2019.1702405
Huang, Y. Z., Zhang, J., Shao, H. Y., Chen, J. P., and Zhao, H. Y. (2015). MicroRNA-191 promotes osteosarcoma cells proliferation by targeting checkpoint kinase 2. Tumour Biol. 36, 6095–6101. doi:10.1007/s13277-015-3290-9
Inamoto, T., Uehara, H., Akao, Y., Ibuki, N., Komura, K., Takahara, K., et al. (2018). A panel of MicroRNA signature as a tool for predicting survival of patients with urothelial carcinoma of the bladder. Dis. Markers 2018, 5468672. doi:10.1155/2018/5468672
Iqbal, M. A., Arora, S., Prakasam, G., Calin, G. A., and Syed, M. A. (2019). MicroRNA in lung cancer: Role, mechanisms, pathways and therapeutic relevance. Mol. Asp. Med. 70, 3–20. doi:10.1016/j.mam.2018.07.003
Ji, X., Shan, L., Shen, P., and He, M. (2020). Circular RNA circ_001621 promotes osteosarcoma cells proliferation and migration by sponging miR-578 and regulating VEGF expression. Cell. Death Dis. 11, 18. doi:10.1038/s41419-019-2204-y
Jiang, X., and Chen, D. (2021). Circular RNA hsa_circ_0000658 inhibits osteosarcoma cell proliferation and migration via the miR-1227/IRF2 axis. J. Cell. Mol. Med. 25, 510–520. doi:10.1111/jcmm.16105
Jin, Y., Li, L., Zhu, T., and Liu, G. (2019). Circular RNA circ_0102049 promotes cell progression as ceRNA to target MDM2 via sponging miR-1304-5p in osteosarcoma. Pathol. Res. Pract. 215, 152688. doi:10.1016/j.prp.2019.152688
Ju, L., Zhou, Y. M., and Yang, G. S. (2016). Up-regulation of long non-coding RNA BCAR4 predicts a poor prognosis in patients with osteosarcoma, and promotes cell invasion and metastasis. Eur. Rev. Med. Pharmacol. Sci. 20, 4445
Kun-Peng, Z., Xiao-Long, M., and Chun-Lin, Z. (2018). Overexpressed circPVT1, a potential new circular RNA biomarker, contributes to doxorubicin and cisplatin resistance of osteosarcoma cells by regulating ABCB1. Int. J. Biol. Sci. 14, 321–330. doi:10.7150/ijbs.24360
Lee, J. A, J. A., Lim, J., Jin, H. Y., Park, M., Park, H. J., Park, J. W., et al. (2021). Osteosarcoma in adolescents and Young adults. Cells 10. 2684. doi:10.3390/cells10102684
Lee. A. M, A. M., Ferdjallah, A., Moore, E., Kim, D. C., Nath, A., Greengard, E., et al. (2021). Long non-coding RNA ANRIL as a potential biomarker of chemosensitivity and clinical outcomes in osteosarcoma. Int. J. Mol. Sci. 22, 11168. doi:10.3390/ijms222011168
Lei, S., and Xiang, L. (2020). Up-regulation of circRNA hsa_circ_0003074 expression is a reliable diagnostic and prognostic biomarker in patients with osteosarcoma. Cancer Manag. Res. 12, 9315–9325. doi:10.2147/CMAR.S262093
Li, B., and Li, X. (2018). Overexpression of hsa_circ_0007534 predicts unfavorable prognosis for osteosarcoma and regulates cell growth and apoptosis by affecting AKT/GSK-3β signaling pathway. Biomed. Pharmacother. 107, 860–866. doi:10.1016/j.biopha.2018.08.086
Li, H., Pan, R., Lu, Q., Ren, C., Sun, J., Wu, H., et al. (2020). MicroRNA1455p inhibits osteosarcoma cell proliferation by targeting E2F transcription factor 3. Int. J. Mol. Med. 45, 1317. doi:10.3892/ijmm.2020.4504
Li, Z., Dou, P., Liu, T., and He, S. (2017). Application of long noncoding RNAs in osteosarcoma: Biomarkers and therapeutic targets. Cell. Physiol. biochem. 42, 1407–1419. doi:10.1159/000479205
Li, L, L., Guo, L., Yin, G., Yu, G., Zhao, Y., and Pan, Y. (2019). Upregulation of circular RNA circ_0001721 predicts unfavorable prognosis in osteosarcoma and facilitates cell progression via sponging miR-569 and miR-599. Biomed. Pharmacother. 109, 226–232. doi:10.1016/j.biopha.2018.10.072
Li, L, L., Pei, S., and Sun, N. (2020). MEG3 targets miR-184 and Wnt/β-catenin and modulates properties of osteosarcoma. Front. Biosci. 25, 1901–1912. doi:10.2741/4884
Li, S, S., Liu, F., Zheng, K., Wang, W., Qiu, E., Pei, Y., et al. (2021). CircDOCK1 promotes the tumorigenesis and cisplatin resistance of osteogenic sarcoma via the miR-339-3p/IGF1R axis. Mol. Cancer 20, 161. doi:10.1186/s12943-021-01453-0
Li, S, S., Pei, Y., Wang, W., Liu, F., Zheng, K., and Zhang, X. (2019). Circular RNA 0001785 regulates the pathogenesis of osteosarcoma as a ceRNA by sponging miR-1200 to upregulate HOXB2. Cell. Cycle 18, 1281–1291. doi:10.1080/15384101.2019.1618127
Li, Y, Y., Liu, J. J., Zhou, J. H., Chen, R., and Cen, C. Q. (2020). LncRNA HULC induces the progression of osteosarcoma by regulating the miR-372-3p/HMGB1 signalling axis. Mol. Med. 26, 26. doi:10.1186/s10020-020-00155-5
Li, Z. Q, Z. Q., Wang, Z., Zhang, Y., Lu, C., Ding, Q. L., Ren, R., et al. (2021). CircRNA_103801 accelerates proliferation of osteosarcoma cells by sponging miR-338-3p and regulating HIF-1/Rap1/PI3K-Akt pathway. J. Biol. Regul. Homeost. Agents 35, 1021–1028. doi:10.23812/20-725-A
Liu, C., Han, X., Li, B., Huang, S., Zhou, Z., Wang, Z., et al. (2021). MALAT-1 is associated with the doxorubicin resistance in U-2OS osteosarcoma cells. Cancer Manag. Res. 13, 6879–6889. doi:10.2147/CMAR.S304922
Liu, L., Zheng, M., Wang, X., Gao, Y., and Gu, Q. (2020). LncRNA NR_136400 suppresses cell proliferation and invasion by acting as a ceRNA of TUSC5 that is modulated by miR-8081 in osteosarcoma. Front. Pharmacol. 11, 641. doi:10.3389/fphar.2020.00641
Liu, X., Zhang, C., Wang, C., Sun, J., Wang, D., Zhao, Y., et al. (2018). miR-210 promotes human osteosarcoma cell migration and invasion by targeting FGFRL1. Oncol. Lett. 16, 2229–2236. doi:10.3892/ol.2018.8939
Liu, Y., Zhu, S. T., Wang, X., Deng, J., Li, W. H., Zhang, P., et al. (2016). MiR-100 inhibits osteosarcoma cell proliferation, migration, and invasion and enhances chemosensitivity by targeting IGFIR. Technol. Cancer Res. Treat. 15, NP40–48. doi:10.1177/1533034615601281
Ma, W., Xue, N., Zhang, J., Wang, D., Yao, X., Lin, L., et al. (2021). circUBAP2 regulates osteosarcoma progression via the miR2043p/HMGA2 axis. Int. J. Oncol. 58, 298–311. doi:10.3892/ijo.2021.5178
Maire, G., Yoshimoto, M., Chilton-MacNeill, S., Thorner, P. S., Zielenska, M., and Squire, J. A. (2009). Recurrent RECQL4 imbalance and increased gene expression levels are associated with structural chromosomal instability in sporadic osteosarcoma. Neoplasia 11, 260–268. 263p following 268. doi:10.1593/neo.81384
Mi, Z., Dong, Y., Wang, Z., and Ye, P. (2021). Biomarker potential of lncRNA GNAS-AS1 in osteosarcoma prognosis and effect on cellular function. J. Orthop. Surg. Res. 16, 470. doi:10.1186/s13018-021-02611-2
Qi, H., Sun, Y., Jiang, Y., and Li, X. (2018). Upregulation of circular RNA circ_0000502 predicts unfavorable prognosis in osteosarcoma and facilitates cell progression via sponging miR-1238. J. Cell. Biochem. 120, 8475–8482. doi:10.1002/jcb.28134
Raei, N., Safaralizadeh, R., Hesseinpourfeizi, M., Yazdanbod, A., Pourfarzi, F., and Latifi-Navid, S. (2021). Crosstalk between lncRNAs and miRNAs in gastrointestinal cancer drug resistance. Life Sci. 284, 119933. doi:10.1016/j.lfs.2021.119933
Richardson, P., Mitsiades, C., Laubach, J., Schlossman, R., Ghobrial, I., Hideshima, T., et al. (2010). Lenalidomide in multiple myeloma: An evidence-based review of its role in therapy. Core Evid. 4, 215–245. doi:10.2147/ce.s6002
Rong, D., Sun, H., Li, Z., Liu, S., Dong, C., Fu, K., et al. (2017). An emerging function of circRNA-miRNAs-mRNA axis in human diseases. Oncotarget 8, 73271–73281. doi:10.18632/oncotarget.19154
Ruan, R., and Zhao, X. L. (2018). LncRNA CCAT2 enhances cell proliferation via GSK3β/β-catenin signaling pathway in human osteosarcoma. Eur. Rev. Med. Pharmacol. Sci. 22, 2978–2984. doi:10.26355/eurrev_201805_15053
Shabani, P., Izadpanah, S., Aghebati-Maleki, A., Baghbani, E., Baghbanzadeh, A., Fotouhi, A., et al. (2019). Role of miR-142 in the pathogenesis of osteosarcoma and its potential as therapeutic approach. J. Cell. Biochem. 120, 4783–4793. doi:10.1002/jcb.27857
Shao, Y. W., Wood, G. A., Lu, J., Tang, Q. L., Liu, J., Molyneux, S., et al. (2019). Cross-species genomics identifies DLG2 as a tumor suppressor in osteosarcoma. Oncogene 38, 291–298. doi:10.1038/s41388-018-0444-4
Shen, S., Jiang, M., Deng, W., Liu, X., Xiong, J., Zeng, Z., et al. (2022). Circ_0008717 promotes renal cell carcinoma progression by upregulating FBXO17 via targeting miR-217. J. Gene Med., e3418. doi:10.1002/jgm.3418
Shen, Y., Peng, X., and Shen, C. (2020). Identification and validation of immune-related lncRNA prognostic signature for breast cancer. Genomics 112, 2640–2646. doi:10.1016/j.ygeno.2020.02.015
Shi, Z., Wen, Y., Zhang, S., and Cheng, X. (2021). Circular RNA MTO1 intercorrelates with microRNA-630, both associate with Enneking stage and/or pathological fracture as well as prognosis in osteosarcoma patients. J. Clin. Lab. Anal. 35, e23987. doi:10.1002/jcla.23987
Slack, F. J., and Chinnaiyan, A. M. (2019). The role of non-coding RNAs in oncology. Cell. 179, 1033–1055. doi:10.1016/j.cell.2019.10.017
Song, B., Wang, Y., Xi, Y., Kudo, K., Bruheim, S., Botchkina, G. I., et al. (2009). Mechanism of chemoresistance mediated by miR-140 in human osteosarcoma and colon cancer cells. Oncogene 28, 4065–4074. doi:10.1038/onc.2009.274
Sun, J., Wang, X., Fu, C., Wang, X., Zou, J., Hua, H., et al. (2016). Long noncoding RNA FGFR3-AS1 promotes osteosarcoma growth through regulating its natural antisense transcript FGFR3. Mol. Biol. Rep. 43, 427–436. doi:10.1007/s11033-016-3975-1
Sun, X., Dai, G., Yu, L., Hu, Q., Chen, J., and Guo, W. (2018). miR-143-3p inhibits the proliferation, migration and invasion in osteosarcoma by targeting FOSL2. Sci. Rep. 8, 606. doi:10.1038/s41598-017-18739-3
Sun, X. H., Geng, X. L., Zhang, J., and Zhang, C. (2015). miRNA-646 suppresses osteosarcoma cell metastasis by downregulating fibroblast growth factor 2 (FGF2). Tumour Biol. 36, 2127–2134. doi:10.1007/s13277-014-2822-z
Tan, X., Tan, D., Li, H., Lin, Y., Wen, Z., and Zeng, C. (2020). circEPSTI1 acts as a ceRNA to regulate the progression of osteosarcoma. Curr. Cancer Drug Targets 20, 288–294. doi:10.2174/1568009619666191107140948
Tang, F., Min, L., Seebacher, N. A., Li, X., Zhou, Y., Hornicek, F. J., et al. (2019). Targeting mutant TP53 as a potential therapeutic strategy for the treatment of osteosarcoma. J. Orthop. Res. 37, 789–798. doi:10.1002/jor.24227
Tang, J., Zhao, H., Cai, H., and Wu, H. (2015). Diagnostic and prognostic potentials of microRNA-27a in osteosarcoma. Biomed. Pharmacother. 71, 222–226. doi:10.1016/j.biopha.2015.01.025
Thanindratarn, P., Dean, D. C., Nelson, S. D., Hornicek, F. J., and Duan, Z. (2019). Advances in immune checkpoint inhibitors for bone sarcoma therapy. J. Bone Oncol. 15, 100221. doi:10.1016/j.jbo.2019.100221
Vasan, N., Baselga, J., and Hyman, D. M. (2019). A view on drug resistance in cancer. Nature 575, 299–309. doi:10.1038/s41586-019-1730-1
Vimalraj, S., Subramanian, R., and Dhanasekaran, A. (2021). LncRNA MALAT1 promotes tumor angiogenesis by regulating MicroRNA-150-5p/VEGFA signaling in osteosarcoma: In-vitro and in-vivo analyses. Front. Oncol. 11, 742789. doi:10.3389/fonc.2021.742789
Wang, L., Li, S., Qi, L., and Ling, L. (2021). Long noncoding RNA HCG9 promotes osteosarcoma progression through RAD51 by acting as a ceRNA of miR-34b-3p. Mediat. Inflamm. 2021, 9978882. doi:10.1155/2021/9978882
Wang, L., Zhang, G. C., Kang, F. B., Zhang, L., and Zhang, Y. Z. (2019). hsa_circ0021347 as a potential target regulated by B7-H3 in modulating the malignant characteristics of osteosarcoma. Biomed. Res. Int. 2019, 9301989. doi:10.1155/2019/9301989
Wang, Q., Cai, J., Wang, J., Xiong, C., and Zhao, J. (2014). MiR-143 inhibits EGFR-signaling-dependent osteosarcoma invasion. Tumour Biol. 35, 12743–12748. doi:10.1007/s13277-014-2600-y
Ward, E., DeSantis, C., Robbins, A., Kohler, B., and Jemal, A. (2014). Childhood and adolescent cancer statistics. Ca. Cancer J. Clin. 64, 83–103. doi:10.3322/caac.21219
Wei, J. W., Huang, K., Yang, C., and Kang, C. S. (2017). Non-coding RNAs as regulators in epigenetics (Review). Oncol. Rep. 37, 3–9. doi:10.3892/or.2016.5236
Wei, W., Ji, L., Duan, W., and Zhu, J. (2021). Circular RNA circ_0081001 knockdown enhances methotrexate sensitivity in osteosarcoma cells by regulating miR-494-3p/TGM2 axis. J. Orthop. Surg. Res. 16, 50. doi:10.1186/s13018-020-02169-5
Wen, J. F., Jiang, Y. Q., Li, C., Dai, X. K., Wu, T., and Yin, W. Z. (2020). LncRNA-XIST promotes the oxidative stress-induced migration, invasion, and epithelial-to-mesenchymal transition of osteosarcoma cancer cells through miR-153-SNAI1 axis. Cell. Biol. Int. 44, 1991–2001. doi:10.1002/cbin.11405
Wen, Y., Li, B., He, M., Teng, S., Sun, Y., and Wang, G. (2021). circHIPK3 promotes proliferation and migration and invasion via regulation of miR637/HDAC4 signaling in osteosarcoma cells. Oncol. Rep. 45, 169–179. doi:10.3892/or.2020.7833
Wong, C. M., Tsang, F. H., and Ng, I. O. (2018). Non-coding RNAs in hepatocellular carcinoma: Molecular functions and pathological implications. Nat. Rev. Gastroenterol. Hepatol. 15, 137–151. doi:10.1038/nrgastro.2017.169
Wu, J., Liao, Q., He, H., Zhong, D., and Yin, K. (2014). TWIST interacts with beta-catenin signaling on osteosarcoma cell survival against cisplatin. Mol. Carcinog. 53, 440–446. doi:10.1002/mc.21991
Wu, Y., Xie, Z., Chen, J., Chen, J., Ni, W., Ma, Y., et al. (2019). Circular RNA circTADA2A promotes osteosarcoma progression and metastasis by sponging miR-203a-3p and regulating CREB3 expression. Mol. Cancer 18, 73. doi:10.1186/s12943-019-1007-1
Wu, H, H., Luo, Y. X., Hu, W., Zhao, M. L., Bie, J., Yang, M., et al. (2021). MicroRNA-382-5p inhibits osteosarcoma development and progression by negatively regulating VEZF1 expression. Oncol. Lett. 22, 752. doi:10.3892/ol.2021.13013
Wu, Z, Z., Zhou, Z., Zhang, W., and Yu, Y. (2021). MiR-21-5p inhibition attenuates Warburg effect and stemness maintenance in osteosarcoma cells via inactivation of Wnt/β-catenin signaling. Acta Biochim. Pol. 68, 725–732. doi:10.18388/abp.2020_5631
Xiao, B., Zhang, X., Li, X., and Zhao, Z. (2020). Circ_001569 regulates FLOT2 expression to promote the proliferation, migration, invasion and EMT of osteosarcoma cells through sponging miR-185-5p. Open Life Sci. 15, 476–487. doi:10.1515/biol-2020-0050
Xiao, X., Jiang, G., Zhang, S., Hu, S., Fan, Y., Li, G., et al. (2021). LncRNA SNHG16 contributes to osteosarcoma progression by acting as a ceRNA of miR-1285-3p. BMC Cancer 21, 355. doi:10.1186/s12885-021-07933-2
Xie, Y., Deng, H., Wei, R., Sun, W., Qi, Y., Yao, S., et al. (2019). Overexpression of miR-335 inhibits the migration and invasion of osteosarcoma by targeting SNIP1. Int. J. Biol. Macromol. 133, 137–147. doi:10.1016/j.ijbiomac.2019.04.016
Xu, J., Yao, Q., Hou, Y., Xu, M., Liu, S., Yang, L., et al. (2013). MiR-223/Ect2/p21 signaling regulates osteosarcoma cell cycle progression and proliferation. Biomed. Pharmacother. 67, 381–386. doi:10.1016/j.biopha.2013.03.013
Xu, B, B., Yang, T., Wang, Z., Zhang, Y., Liu, S., and Shen, M. (2018). CircRNA CDR1as/miR-7 signals promote tumor growth of osteosarcoma with a potential therapeutic and diagnostic value. Cancer Manag. Res. 10, 4871–4880. doi:10.2147/CMAR.S178213
Xu, R, R., Feng, F., Yu, X., Liu, Z., and Lao, L. (2018). LncRNA SNHG4 promotes tumour growth by sponging miR-224-3p and predicts poor survival and recurrence in human osteosarcoma. Cell. Prolif. 51, e12515. doi:10.1111/cpr.12515
Yang, C., Tian, Y., Zhao, F., Chen, Z., Su, P., Li, Y., et al. (2020). Bone microenvironment and osteosarcoma metastasis. Int. J. Mol. Sci. 21, E6985. doi:10.3390/ijms21196985
Yang, T., Guo, J. P., Li, F., Xiu, C., Wang, H., and Duan, X. L. (2021). Long noncoding RNADUXAP8 regulates TOP2A in the growth and metastasis of osteosarcoma via microRNA635. Mol. Med. Rep. 24, 511. doi:10.3892/mmr.2021.12150
Yang, Y., Fei, M., Zhou, X., Li, Y., and Jin, D. (2019). The potential value of lncRNA-BC050642 in osteosarcoma origination and outcomes. Artif. Cells Nanomed. Biotechnol. 47, 1859–1866. doi:10.1080/21691401.2019.1611593
Yang, Z., Zhang, Y., Zhang, X., Zhang, M., Liu, H., Zhang, S., et al. (2015). Serum microRNA-221 functions as a potential diagnostic and prognostic marker for patients with osteosarcoma. Biomed. Pharmacother. 75, 153–158. doi:10.1016/j.biopha.2015.07.018
Yao, H., Li, X., Pan, X., Xu, J., Zhao, S., Su, Z., et al. (2022). Fraxetin exerts anticancer effect in glioma by suppressing MiR-21-3p. Drug Dev. Res. 83, 501–511. doi:10.1002/ddr.21881
Yao, X. Y., Liu, J. F., Luo, Y., Xu, X. Z., and Bu, J. (2021). LncRNA HOTTIP facilitates cell proliferation, invasion, and migration in osteosarcoma by interaction with PTBP1 to promote KHSRP level. Cell. Cycle 20, 283–297. doi:10.1080/15384101.2020.1870820
Ye, H., Lin, J., Yao, X., Li, Y., Lin, X., and Lu, H. (2018). Overexpression of long non-coding RNA NNT-AS1 correlates with tumor progression and poor prognosis in osteosarcoma Cell. Physiol. biochem. 45, 1904–1914. doi:10.1159/000487966
Yuan, J., Chen, L., Chen, X., Sun, W., and Zhou, X. (2012). Identification of serum microRNA-21 as a biomarker for chemosensitivity and prognosis in human osteosarcoma. J. Int. Med. Res. 40, 2090–2097. doi:10.1177/030006051204000606
Zhang, F., Yan, T., Guo, W., Sun, K., Wang, S., Bao, X., et al. (2018). Novel oncogene COPS3 interacts with Beclin1 and Raf-1 to regulate metastasis of osteosarcoma through autophagy. J. Exp. Clin. Cancer Res. 37, 135. doi:10.1186/s13046-018-0791-6
Zhang, W., Li, J. Z., Tai, Q. Y., Tang, J. J., Huang, Y. H., and Gao, S. B. (2020). LncRNA DANCR regulates osteosarcoma migration and invasion by targeting miR-149/MSI2 axis. Eur. Rev. Med. Pharmacol. Sci. 24, 6551–6560. doi:10.26355/eurrev_202006_21639
Zhang, Z., Wu, X., Han, Q., and Huang, Z. (2021). Downregulation of long non-coding RNA UCA1 represses tumorigenesis and metastasis of osteosarcoma via miR-513b-5p/E2F5 axis. Anticancer. Drugs 32, 602–613. doi:10.1097/CAD.0000000000001034
Zhang, H, H., Lu, Y., Wang, J., Zhang, T., Dong, C., Li, X., et al. (2019). Downregulation of the long noncoding RNA FOXD2AS1 inhibits cell proliferation, migration and invasion in osteosarcoma. Mol. Med. Rep. 20, 292–302. doi:10.3892/mmr.2019.10254
Zhang, N, N., Meng, X., Mei, L., Zhao, C., and Chen, W. (2019). LncRNA DLX6-AS1 promotes tumor proliferation and metastasis in osteosarcoma through modulating miR-641/HOXA9 signaling pathway. J. Cell. Biochem. 120, 11478–11489. doi:10.1002/jcb.28426
Zhao, A., Liu, W., Cui, X., Wang, N., Wang, Y., Sun, L., et al. (2021). lncRNA TUSC7 inhibits osteosarcoma progression through the miR181a/RASSF6 axis. Int. J. Mol. Med. 47, 583–594. doi:10.3892/ijmm.2020.4825
Zhao, J., Zhang, C., Gao, Z., Wu, H., Gu, R., and Jiang, R. (2018). Long non-coding RNA ASBEL promotes osteosarcoma cell proliferation, migration, and invasion by regulating microRNA-21. J. Cell. Biochem. 119, 6461–6469. doi:10.1002/jcb.26671
Zhao, D, D., Wang, S., Chu, X., and Han, D. (2019). LncRNA HIF2PUT inhibited osteosarcoma stem cells proliferation, migration and invasion by regulating HIF2 expression. Artif. Cells Nanomed. Biotechnol. 47, 1342–1348. doi:10.1080/21691401.2019.1596934
Zhao, H, H., Yan, P., Wang, J., Zhang, Y., Zhang, M., Wang, Z., et al. (2019). Clinical significance of tumor miR-21, miR-221, miR-143, and miR-106a as biomarkers in patients with osteosarcoma. Int. J. Biol. Markers 34, 184–193. doi:10.1177/1724600819843537
Zheng, C., Tang, F., Min, L., Hornicek, F., Duan, Z., and Tu, C. (2020). PTEN in osteosarcoma: Recent advances and the therapeutic potential. Biochim. Biophys. Acta. Rev. Cancer 1874, 188405. doi:10.1016/j.bbcan.2020.188405
Zheng, S., Qian, Z., Jiang, F., Ge, D., Tang, J., Chen, H., et al. (2019). CircRNA LRP6 promotes the development of osteosarcoma via negatively regulating KLF2 and APC levels. Am. J. Transl. Res. 11, 4126
Zhou, X., Natino, D., Qin, Z., Wang, D., Tian, Z., Cai, X., et al. (2018). Identification and functional characterization of circRNA-0008717 as an oncogene in osteosarcoma through sponging miR-203. Oncotarget 9, 22288–22300. doi:10.18632/oncotarget.23466
Zhu, K. P., and Zhang, C. L. (2017). Sensitivity of doxorubicin-resistant osteosarcoma cells to doxorubicin regulated by long non-coding RNA NR_036444. Zhonghua Zhong Liu Za Zhi 39, 250–255. doi:10.3760/cma.j.issn.0253-3766.2017.04.003
Keywords: osteosarcoma, non-coding RNAs, biomarker, diagnosis, treatment, prognosis
Citation: Fan L, Zhong Z, Lin Y and Li J (2022) Non-coding RNAs as potential biomarkers in osteosarcoma. Front. Genet. 13:1028477. doi: 10.3389/fgene.2022.1028477
Received: 26 August 2022; Accepted: 07 October 2022;
Published: 19 October 2022.
Edited by:
Jared C. Roach, Institute for Systems Biology (ISB), United StatesReviewed by:
Wangyang Zheng, Third Affiliated Hospital of Harbin Medical University, ChinaMenggang Zhang, First Affiliated Hospital of Zhengzhou University, China
Copyright © 2022 Fan, Zhong, Lin and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jitian Li, aml0aWFubGVlQGhvdG1haWwuY29t
†These authors have contributed equally to this work
 Lijuan Fan
Lijuan Fan Zhenhao Zhong
Zhenhao Zhong Yubo Lin
Yubo Lin Jitian Li
Jitian Li