- 1College of Arts and Sciences, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 2UNC Lineberger Comprehensive Cancer Center, School of Medicine, University of North Carolina, Chapel Hill, NC, United States
- 3Division of Pharmaceutical Outcomes and Policy, Eshelman School of Pharmacy, University of North Carolina, Chapel Hill, NC, United States
- 4Department of Public Health Science, College of Medicine, Medical University of South Carolina, Charleston, SC, United States
- 5Department of Behavioral, Social, and Health Education Science, Rollins School of Public Health, Emory University, Atlanta, GA, United States
- 6Department of Genetics, School of Medicine, University of North Carolina, Chapel Hill, NC, United States
- 7The Daffodil Centre, University of Sydney, A Joint Venture with Cancer Council NSW, Sydney, NSW, Australia
- 8Melanoma Institute Australia, University of Sydney, Sydney, NSW, Australia
- 9Health Sciences Library, University of North Carolina, Chapel Hill, NC, United States
Studies suggest that 1–3% of the general population in the United States unknowingly carry a genetic risk factor for a common hereditary disease. Population genetic screening is the process of offering otherwise healthy patients in the general population testing for genomic variants that predispose them to diseases that are clinically actionable, meaning that they can be prevented or mitigated if they are detected early. Population genetic screening may significantly reduce morbidity and mortality from these diseases by informing risk-specific prevention or treatment strategies and facilitating appropriate participation in early detection. To better understand current barriers, facilitators, perceptions, and outcomes related to the implementation of population genetic screening, we conducted a systematic review and searched PubMed, Embase, and Scopus for articles published from date of database inception to May 2020. We included articles that 1) detailed the perspectives of participants in population genetic screening programs and 2) described the barriers, facilitators, perceptions, and outcomes related to population genetic screening programs among patients, healthcare providers, and the public. We excluded articles that 1) focused on direct-to-consumer or risk-based genetic testing and 2) were published before January 2000. Thirty articles met these criteria. Barriers and facilitators to population genetic screening were organized by the Social Ecological Model and further categorized by themes. We found that research in population genetic screening has focused on stakeholder attitudes with all included studies designed to elucidate individuals’ perceptions. Additionally, inadequate knowledge and perceived limited clinical utility presented a barrier for healthcare provider uptake. There were very few studies that conducted long-term follow-up and evaluation of population genetic screening. Our findings suggest that these and other factors, such as prescreen counseling and education, may play a role in the adoption and implementation of population genetic screening. Future studies to investigate macro-level determinants, strategies to increase provider buy-in and knowledge, delivery models for prescreen counseling, and long-term outcomes of population genetic screening are needed for the effective design and implementation of such programs.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020198198
1 Introduction
Studies suggest that 1–3% of the general population in the United States carry a genetic risk factor for a common hereditary disease. Typically, genetic testing approaches for identifying these individuals are limited to testing those at high risk of hereditary disease (e.g., cascade testing for at-risk relatives of individuals with a diagnosis). Conversely, population genetic screening offers genetic testing (for common genomic variants) to otherwise healthy individuals to inform risk assessment, precision prevention and early detection of preventable, common diseases. A key example of population genetic screening is newborn screening, which is often celebrated as one of public health’s best accomplishments (Murray et al., 2018).
The Centers for Disease Control and Prevention Office of Genomics and Precision Health has prioritized population genetic screening for common disease conditions (Hereditary Breast and Ovarian Cancer, Lynch Syndrome, and familial hypercholesterolemia) as Tier 1 applications for genomics due to their “significant potential for positive impact on public health” (CDC, 2021). While clinical evidence is currently insufficient to recommend widespread screening in healthy populations (Hampel and de la Chapelle, 2011; Representatives of the Global Familial Hypercholesterolemia Community, 2020), clinical pilot programs are in place to understand cost-efficiency, implementation, and other health related outcomes of population genetic screening (Hay et al., 2021; Lacson et al., 2021; Smit et al., 2021). These pilot studies are on the rise and offer promising opportunities to build the necessary knowledge base for expanding population genetic screening.
Understanding the barriers, facilitators, perceptions, and outcomes to population genetic screening of healthy populations is critical for implementing screening programs in healthcare settings. Previous systematic reviews relating to population genetic screening focus on economic and informed choice evaluations (Rogowski, 2006; Ames et al., 2015). To address this need, we conducted a systematic review of current research literature to understand the barriers, facilitators, perceptions, and outcomes that will be vital for the successful translation of research to support population genetic screening (if found to be appropriate for scaling up).
2 Methods
2.1 Protocol and Registration
We adhered to the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) reporting guidelines (Moher et al., 2009) for this review (Supplementary Appendix SA). Details of the protocol for this systematic review were registered on PROSPERO and can be accessed at https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020198198 (Shen et al., 2022).
2.2 Search Strategy and Information Sources
We worked with a medical librarian (RC) to develop search strategies for the concept of population genetic screening in unknown- and average-risk populations in PubMed, Embase, and Scopus from date of database inception to 22 May 2020, when all searches were completed. Search filters were used to limit the results to original research articles written in English and to exclude preconception, prenatal, and carrier testing. The complete strategy for each of the searches can be found in Supplementary Appendix SB. We also manually examined the references of relevant literature reviews to identify additional studies that may have been missed by the database searches. All references were uploaded to Veritas Health Innovation Covidence systematic review software, 2021 (Veritas Health Innovation), a systematic review management system for study selection.
2.3 Eligibility Criteria
Conference abstracts, meeting reports, literature reviews, guidelines, and simulation modeling studies were excluded. Articles focusing on genetic literacy and research, hypothetical gene correlations, and those that lacked a methods section or relevant outcomes were also excluded. Finally, we excluded articles that focused on direct-to-consumer or high-risk genetic testing and articles that were published before 1 January 2000 to understand views of population genetic screening with the use of contemporary technology.
2.4 Study Selection
Each title and abstract were reviewed independently for eligibility by random sets of two reviewers (ES, SS, LP, CA, MD, KF, BH, LM, AS) and thematic issues were resolved by discussion. MR oversaw the process and formally resolved specific conflicts. Each full text was assessed independently by random sets of two reviewers (ES, SS, LP, CA, MD, BH, LM, AS) and thematic issues were resolved by discussion. KF oversaw this process and formally resolved specific conflicts. We included articles that detailed the perspectives of participants of population genetic screening programs and individuals asked about population genetic screening to capture all possible barriers, facilitators, perceptions, and outcomes from the position of patients, healthcare providers, and the public.
2.5 Data Items and Data Collection Process
Data extraction forms were developed in Covidence using the PICOS framework (Schardt et al., 2007) (see Supplementary Appendix SC) to collect information about each study’s population (patients, healthcare providers, and the public), intervention (disease area(s), whether population genetic screening was offered, and whether participants met with providers before or after screening), comparator group if applicable, outcomes (barriers, facilitators, perceptions, effectiveness measures), and setting (e.g., scale, country, type). We defined patients as healthy individuals with no known risk status who were seen in the healthcare system and the public as individuals who were selected from and represented the broader community. For studies that investigated more than three disease areas, we list their disease areas as “a variety of conditions” for simplicity. We note whether testing for monogenic or polygenic conditions were performed or proposed for consideration by the study. It can be noted that common genomic variants may vary from program to program.
We categorized effectiveness measures as Results (results of the actual screening), Follow-up, Change in Health Behavior, and Interpretation (ex: participants’ emotional responses, risk perception changes, etc.).
The extraction forms were developed based on a previous review (Srinivasan et al., 2020) and four sets of two reviewers independently piloted them on a subset of five articles to agree on a final version. ES, SS, and LP resolved disagreements in data extractions and discussed specific articles as needed. We separately examined articles that had implemented population genetic screening and those that had not implemented population genetic screening to account for contextual differences before analyzing these article types together. Barriers and facilitators were arranged according to the Social Ecological Model (Golden and Earp, 2012), which views health as being affected by interactions at the intrapersonal, interpersonal, and community levels. Perceptions were categorized into favorable, unfavorable, and in-between.
We initially aimed to understand barriers, facilitators, perceptions, and outcomes. It became apparent that barriers and facilitators were related to perceptions, and overall outcomes were quite diverse and hard to summarize across heterogeneous studies, therefore we focus our results on barriers and facilitators.
2.6 Risk of Bias in Individual Studies
Reviewers independently assessed the methodological quality of each study following the Mixed Method Appraisal Tool, version 2018 (Hong et al., 2018) for each study type (RCT, descriptive, observation, qualitative, or mixed methods). Meta-analysis was not conducted due to the high variation in study design, population, setting, and outcomes. Due to the small number of studies, we did not define a threshold with which to exclude “low quality” studies. To prevent highlighting any such studies, we ensured that our discussion points were present in multiple studies that mostly have an MMAT score of 3 or higher.
3 Results
3.1 Study Characteristics
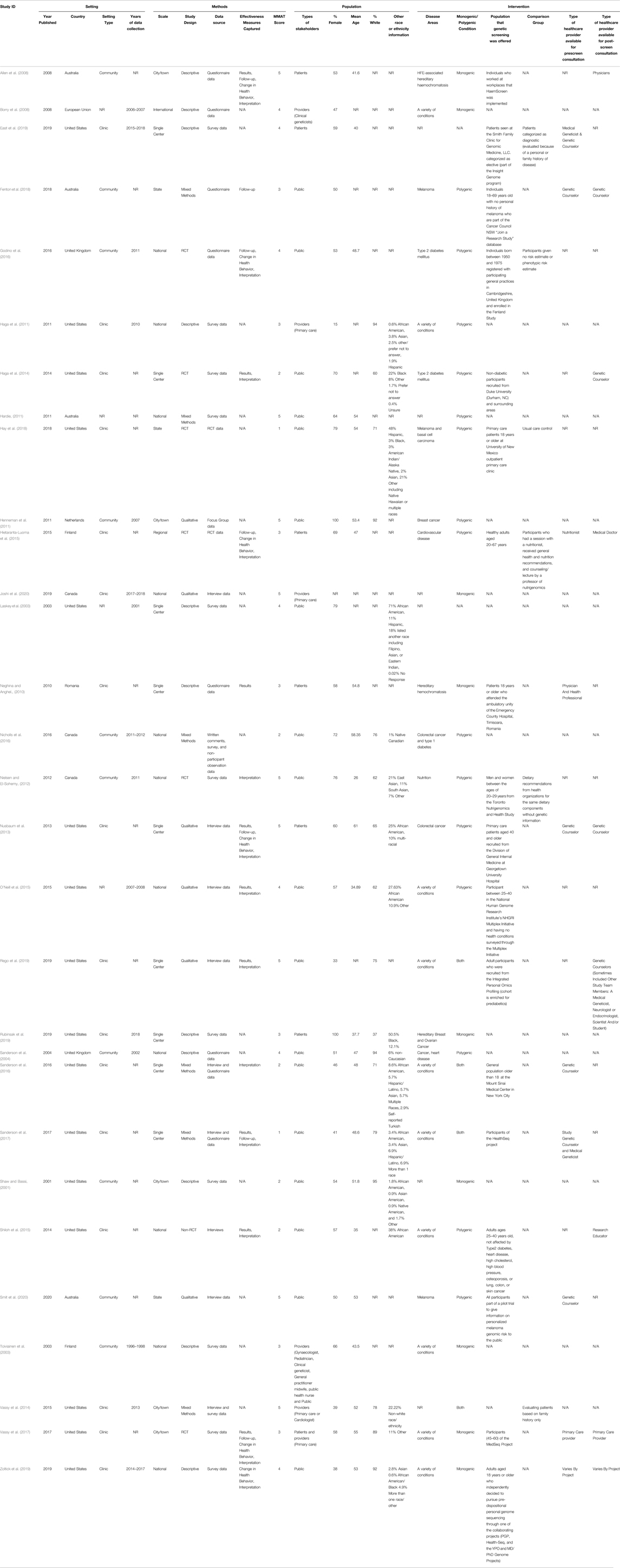
Characteristics of our included studies can be found in Table 1. Of the 4,821 unique studies that were identified through database searching, 323 articles were assessed for full-text eligibility (see Figure 1 for PRISMA diagram). Thirty articles were included. (Shaw and Bassi, 2001; Laskey et al., 2003; Toiviainen et al., 2003; Sanderson et al., 2004, 2017; Allen et al., 2008; Borry et al., 2008; Neghina and Anghel, 2010; Haga et al., 2011; Hardie, 2011; Henneman et al., 2011; Nielsen and El-Sohemy, 2012; Nusbaum et al., 2013; Haga et al., 2014; Vassy et al., 2014; Hietaranta-Luoma et al., 2015; O’Neill et al., 2015; Shiloh et al., 2015; Godino et al., 2016; Nicholls et al., 2016; Sanderson et al., 2016; Vassy et al., 2017; Fenton et al., 2018; Hay et al., 2018; East et al., 2019; Rego et al., 2019; Rubinsak et al., 2019; Zoltick et al., 2019; Joshi et al., 2020; Smit et al., 2020).
Most studies investigated the perspectives of the public (n = 18) (Shaw and Bassi, 2001; Laskey et al., 2003; Sanderson et al., 2004, 2017; Hardie, 2011; Henneman et al., 2011; Nielsen and El-Sohemy, 2012; Haga et al., 2014; O’Neill et al., 2015; Shiloh et al., 2015; Godino et al., 2016; Nicholls et al., 2016; Sanderson et al., 2016; Fenton et al., 2018; Hay et al., 2018; Rego et al., 2019; Zoltick et al., 2019; Smit et al., 2020), while six studies investigated the perspective of patients (Allen et al., 2008; Neghina and Anghel, 2010; Nusbaum et al., 2013; Hietaranta-Luoma et al., 2015; East et al., 2019; Rubinsak et al., 2019), only four investigated the perspective of providers (Borry et al., 2008; Haga et al., 2011; Vassy et al., 2014; Joshi et al., 2020), and two investigated multiple perspectives (Toiviainen et al., 2003; Vassy et al., 2017).
For the most part, studies reported key patient characteristics; however, eleven studies did not record race or ethnicity information (Toiviainen et al., 2003; Allen et al., 2008; Borry et al., 2008; Neghina and Anghel, 2010; Hardie, 2011; Hietaranta-Luoma et al., 2015; Godino et al., 2016; Fenton et al., 2018; East et al., 2019; Joshi et al., 2020; Smit et al., 2020) and one study did not record information about gender or sex (Joshi et al., 2020).
The included studies examined population genetic screening in the context of a variety of conditions, with the most common being melanoma (n = 2) (Fenton et al., 2018; Hay et al., 2018; Smit et al., 2020), Type 2 diabetes mellitus (n = 2) (Haga et al., 2014; Godino et al., 2016), hereditary haemochromatosis (n = 2) (Allen et al., 2008; Neghina and Anghel, 2010), and colorectal cancer (n = 2) (Nusbaum et al., 2013; Nicholls et al., 2016).
The majority (n = 18) implemented population genetic screening programs of some kind (Allen et al., 2008; Neghina and Anghel, 2010; Nielsen and El-Sohemy, 2012; Nusbaum et al., 2013; Haga et al., 2014; Hietaranta-Luoma et al., 2015; O’Neill et al., 2015; Shiloh et al., 2015; Godino et al., 2016; Sanderson et al., 2016; Sanderson et al., 2017; Vassy et al., 2017; Fenton et al., 2018; Hay et al., 2018; East et al., 2019; Rego et al., 2019; Zoltick et al., 2019; Smit et al., 2020), and the remaining 12 investigated individuals’ opinions on population genetic screening (Shaw and Bassi, 2001; Laskey et al., 2003; Toiviainen et al., 2003; Sanderson et al., 2004; Borry et al., 2008; Haga et al., 2011; Hardie, 2011; Henneman et al., 2011; Vassy et al., 2014; Nicholls et al., 2016; Rubinsak et al., 2019; Joshi et al., 2020).
Of those that implemented screening programs, many utilized genetic counseling either before screening (n = 5) (Neghina and Anghel, 2010; Sanderson et al., 2016; Sanderson et al., 2017; East et al., 2019; Smit et al., 2020), after screening (n = 4) (Allen et al., 2008; Haga et al., 2014; Shiloh et al., 2015; Rego et al., 2019), or both (n = 5) (Nusbaum et al., 2013; Hietaranta-Luoma et al., 2015; Vassy et al., 2017; Fenton et al., 2018; Zoltick et al., 2019). Four did not record counseling availability (Nielsen and El-Sohemy, 2012; O’Neill et al., 2015; Godino. et al., 2016; Hay et al., 2018).
The majority of studies (n = 16) were conducted in the US (Shaw and Bassi, 2001; Laskey et al., 2003; Haga et al., 2011; Nusbaum et al., 2013; Haga et al., 2014; Vassy et al., 2014; O’Neill et al., 2015; Shiloh et al., 2015; Sanderson et al., 2016; Sanderson et al., 2017; Vassy et al., 2017; Hay et al., 2018; East et al., 2019; Rego et al., 2019; Rubinsak et al., 2019; Zoltick et al., 2019) and were conducted in a clinical setting (n = 16) (Neghina and Anghel, 2010; Haga et al., 2011; Nusbaum et al., 2013; Haga et al., 2014; Vassy et al., 2014; Hietaranta-Luoma et al., 2015; Shiloh et al., 2015; Sanderson et al., 2016; Sanderson et al., 2017; Vassy et al., 2017; Hay et al., 2018; East et al., 2019; Rego et al., 2019; Rubinsak et al., 2019; Zoltick et al., 2019; Joshi et al., 2020) or the community setting (n = 10) (Shaw and Bassi, 2001; Toiviainen et al., 2003; Sanderson et al., 2004; Allen et al., 2008; Henneman et al., 2011; Nielsen and El-Sohemy, 2012; Godino et al., 2016; Nicholls et al., 2016; Fenton et al., 2018; Smit et al., 2020).
Included studies included a variety of study designs and received a range of MMAT scores. Of note, 23 studies received an MMAT score of 3 or greater (Laskey et al., 2003; Toiviainen et al., 2003; Sanderson et al., 2004; Allen et al., 2008; Borry et al., 2008; Neghina and Anghel, 2010; Hardie, 2011; Henneman et al., 2011; Nielsen and El-Sohemy, 2012; Nusbaum et al., 2013; Haga et al., 2014; Vassy et al., 2014; Hietaranta-Luoma et al., 2015; O’Neill et al., 2015; Godino et al., 2016; Vassy et al., 2017; Fenton et al., 2018; East et al., 2019; Rego et al., 2019; Rubinsak et al., 2019; Zoltick et al., 2019; Joshi et al., 2020; Smit et al., 2020), and only seven studies received an MMAT score below 3 (Shaw and Bassi, 2001; Haga et al., 2014; Shiloh et al., 2015; Nicholls et al., 2016; Sanderson et al., 2016; Sanderson et al., 2017; Hay et al., 2018).
3.2 Barriers
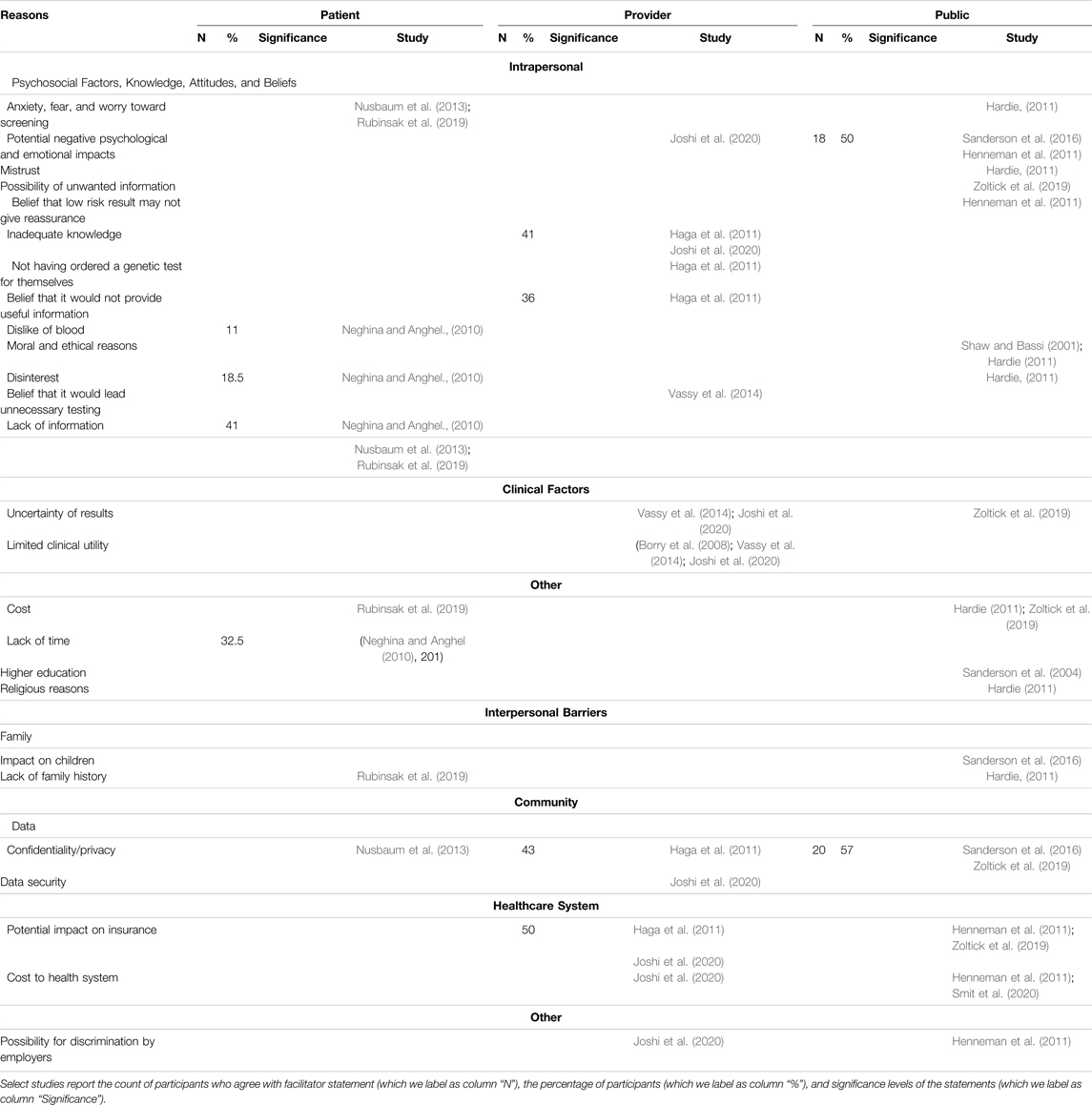
Intrapersonal, interpersonal, and community barriers are reported in Table 2 and below.
3.2.1 Intrapersonal Barriers
3.2.1.1 Psychosocial Factors, Knowledge, Attitudes, and Beliefs
Psychosocial factors such as anxiety, fear, and worry about screening (Hardie, 2011; Nusbaum et al., 2013; Rubinsak et al., 2019), dislike of blood (Neghina and Anghel, 2010), and potential negative psychological and emotional impacts (Henneman et al., 2011; Sanderson et al., 2016; Joshi et al., 2020) were reported as reasons to reject screening. Additional factors such as mistrust (Hardie, 2011), disinterest (Neghina and Anghel, 2010; Hardie, 2011), the possibility of receiving unwanted information (Zoltick et al., 2019), and the belief that a low-risk result may not give reassurance (Henneman et al., 2011) were reported barriers.
Two studies reported moral and ethical reasons, such as the fear of eugenics and a question of human mortality, as barriers (Shaw and Bassi, 2001; Hardie, 2011). Providers cited inadequate knowledge (Haga et al., 2011; Joshi et al., 2020), not having ordered a genetic test for themselves (Haga et al., 2011), their belief that it would not provide useful information (Haga et al., 2011), and their belief that it would lead to unnecessary future testing (Vassy et al., 2014) as barriers to participating in population genetic screening programs. Additionally, patients reported a lack of information about these programs (Neghina and Anghel, 2010; Nusbaum et al., 2013; Rubinsak et al., 2019).
3.2.1.2 Clinical Factors
Providers (Vassy et al., 2014; Joshi et al., 2020) and the public (Zoltick et al., 2019) cited the uncertainty of results as a barrier for interest and/or participation in screening programs with providers additionally reporting perceived limited clinical utility (Borry et al., 2008; Vassy et al., 2014; Joshi et al., 2020).
3.2.1.3 Other
Perceived cost of population genetic screening (Hardie, 2011; Rubinsak et al., 2019; Zoltick et al., 2019), religious reasons (Hardie, 2011), and higher education (Sanderson et al., 2004) among patients and the public were reported as other barriers for interest and/or participation as well as a lack of time (Neghina and Anghel, 2010).
3.2.2 Interpersonal Barriers
3.2.2.1 Family
A perceived potential for a negative impact on children (Sanderson et al., 2016) and a lack of family history (Hardie, 2011; Rubinsak et al., 2019) were negatively associated with interest and/or participation of population genetic screening among patients and the public.
3.2.3 Community Barriers
3.2.3.1 Data
Concerns related to confidentiality and privacy (Haga et al., 2011; Nusbaum et al., 2013; Sanderson et al., 2016; Zoltick et al., 2019) and data security (Joshi et al., 2020) were reported as barriers across stakeholders.
3.2.3.2 Healthcare System
Providers and the public reported that the potential impact of results on insurance (Haga et al., 2011; Henneman et al., 2011; Zoltick et al., 2019; Joshi et al., 2020) and the potential increased cost to the health system (Henneman et al., 2011; Joshi et al., 2020; Smit et al., 2020) would hinder their participation in population genetic screening.
3.2.3.3 Other
The possibility for discrimination by employers was reported by providers and the public (Henneman et al., 2011; Joshi et al., 2020).
3.3 Facilitators
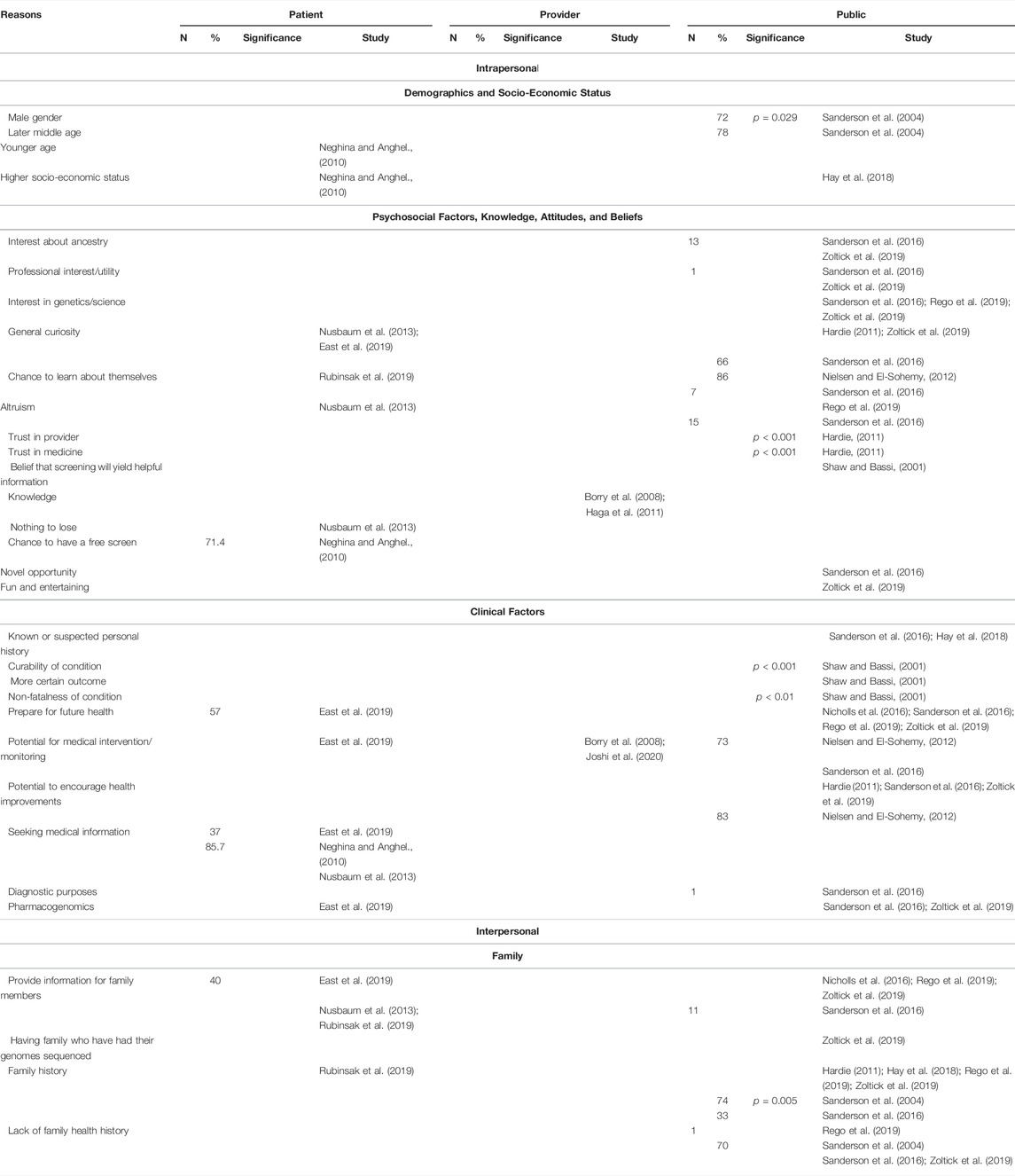
Intrapersonal, interpersonal, and community facilitators can be found in Table 3 and below.
3.3.1 Intrapersonal Facilitators
3.3.1.1 Demographics and Socio-Economic Status
One study (Sanderson et al., 2004) reported that male gender (p = 0.029) and later middle age were positively correlated with an interest in screening. On the other hand, another study (Neghina and Anghel, 2010) reported that younger age was a facilitator to uptake of screening. Higher socioeconomic status was additionally cited as a facilitator to participation (Neghina and Anghel, 2010; Hay et al., 2018).
3.3.1.2 Psychosocial Factors, Knowledge, Attitudes, and Beliefs
Attitudes related to having an interest about ancestry (Sanderson et al., 2016; Zoltick et al., 2019), professional interest (Sanderson et al., 2016; Zoltick et al., 2019), interest in genetics and/or science (Sanderson et al., 2016; Rego et al., 2019; Zoltick et al., 2019), and general curiosity (Hardie, 2011; Nusbaum et al., 2013; Sanderson et al., 2016; East et al., 2019; Zoltick et al., 2019) were reported facilitators for screening. Additional facilitators include altruism (Nusbaum et al., 2013; Sanderson et al., 2016; Rego et al., 2019) and the chance for participants to learn about themselves (Nielsen and El-Sohemy, 2012; Sanderson et al., 2016; Rubinsak et al., 2019).
Knowledge (Borry et al., 2008; Haga et al., 2011), the belief that screening will provide helpful information (Shaw and Bassi, 2001), trust in provider (Hardie, 2011) and trust in medicine (Hardie, 2011) were all associated with interest in population genetic screening, with the latter two being statistically significant.
Patients reported that the chance to have a free screen (Neghina and Anghel, 2010) and a “nothing to lose” attitude (Nusbaum et al., 2013) and the public reported that viewing population genetic screening as a novel opportunity (Sanderson et al., 2016) and a fun and entertaining activity (Zoltick et al., 2019) were facilitators for undergoing screening.
3.3.1.3 Clinical Factors
All stakeholders viewed the potential for medical intervention and/or monitoring (Borry et al., 2008; Nielsen and El-Sohemy, 2012; Sanderson et al., 2016; East et al., 2019; Joshi et al., 2020) as a facilitator to population genetic screening. The public reported that curability (p < 0.001) (Shaw and Bassi, 2001), non-fatalness of a condition (p < 0.01) (Shaw and Bassi, 2001), a more certain outcome (Shaw and Bassi, 2001), a known or suspected personal history (Sanderson et al., 2016; Hay et al., 2018), the potential to encourage health improvements through means such as behavioral changes (Hardie, 2011; Nielsen and El-Sohemy, 2012; Sanderson et al., 2016; Zoltick et al., 2019), and the use of results for future diagnostic purposes (Sanderson et al., 2016) were positively associated with interest and/or receipt of population genetic screening through a population-based context.
Additionally, patients reported their seeking medical information as a reason for receiving screening (Neghina and Anghel., 2010; Nusbaum et al., 2013; East et al., 2019). Patients and the public reported that the ability to prepare for future health (Nicholls et al., 2016; Sanderson et al., 2016; East et al., 2019; Rego et al., 2019; Zoltick et al., 2019) and the use of results for pharmacogenomics (Sanderson et al., 2016; East et al., 2019; Zoltick et al., 2019) were facilitators to population genetic screening.
3.3.2 Interpersonal Facilitators
3.3.2.1 Family
All interpersonal facilitators were related to participants’ family. Patients and the public reported that the ability to provide information to family members to them (Nusbaum et al., 2013; Nicholls et al., 2016; Sanderson et al., 2016; East et al., 2019; Rego et al., 2019; Rubinsak et al., 2019; Zoltick et al., 2019). Having family who have had their genomes sequenced facilitated participation as well (Zoltick et al., 2019).
Family history positively associated with both interest and/or participation in population genetic screening (Hardie, 2011; Sanderson et al., 2016; Hay et al., 2018; Rego et al., 2019; Rubinsak et al., 2019; Zoltick et al., 2019) and labeled as a statistically significant factor in one study (Sanderson et al., 2004). On the other hand, a lack of family health history was also reported as a facilitator for both interest and/or participation in four studies (Sanderson et al., 2004; Sanderson et al., 2016; Rego et al., 2019; Zoltick et al., 2019).
3.4 Perceptions
Perceptions are summarized in Supplementary Appendix SD.
3.5 Effectiveness Measures
Effectiveness measures are summarized in Supplementary Appendix SE.
4 Discussion
Overall, we identified multilevel barriers and facilitators for population genetic screening implementation. Psychosocial and attitudinal barriers, such as anxiety and worry toward screening and the possibility for negative psychological and emotional impacts, were the most reported individual-level barriers across stakeholders, even though studies to date have demonstrated limited impacts on psychological and emotional outcomes with any adverse responses dissipating over time (Hietaranta-Luoma et al., 2015; Hollands et al., 2016; Frieser et al., 2018; Smit et al., 2020).
Skeptical healthcare providers cited a perceived lack of clinical utility as a barrier, reporting that although they believe population genetic screening is valuable, they do not believe that it is ready for clinical use (Joshi et al., 2020). On the other hand, healthcare providers who supported population genetic screening reported the potential for results to inform medical intervention and/or monitoring as a reason for their support. Our findings are consistent with previous literature indicating that obtaining provider buy-in is needed for the implementation of large-scale screening (Peterson et al., 2016). Additionally, the current perception of clinical utility places value on genomic medicine in relation to informing treatment, and excludes other applications for screening such as risk prediction and prognosis (Joseph et al., 2016). The Association for Molecular Pathology (Joseph et al., 2016) recommends expanding the definition of clinical utility for molecular tools through approaches such as utilizing a modified ACCE model (CDC, 2019) and promoting patient-centered definitions of clinical utility. Our data suggests the need for interventions directed toward obtaining buy-in and expanding the definition of clinical utility to include the context of population genetic screening.
Studies also reported potential ethical issues, concerns relating to data management, and potential discrimination as barriers to interest in population genetic screening. These factors are especially important in the age of “big data” (Price and Cohen, 2019), and previous literature has called for the consideration of ethical questions in implementing population genetic screening (Murray et al., 2018). The BabySeq Project is assessing ethical, legal, and social implications (ELSI) relating to the ethical issues of result return (Friedman et al., 2017) and the medical, behavioral, and economic impacts (Holm et al., 2018) of newborn screening. These studies, along with essential ELSI questions raised by newborn screening (Goldenberg et al., 2019), may provide a potential framework that can be adapted for assessing ELSI considerations in evaluating general population genetic screening.
Many of our included studies investigated the general public’s perspective of population genetic screening. This presents an opportunity to focus on the roles of other stakeholders within the larger societal systems, such as healthcare providers and public health officials. Primary care providers, who will likely be the touchpoint for many interested in population genetic screening, reported inadequate knowledge as a barrier to ordering screening. In one study (Haga et al., 2011), roughly half of providers reported that they felt prepared to order population genetic screening. Previous literature has noted the limited evidence regarding the views and roles of healthcare providers in genomic medicine (Hann et al., 2017a; Hauser et al., 2018; Crellin et al., 2019), identified the importance of educational resources for provider preparedness to order and interpret results (Rohrer Vitek et al., 2017; Hauser et al., 2018; Smit et al., 2019), and described the integral role that public health officials will play in insuring proper implementation of population genetic screening (Molster et al., 2018). With few provider-based studies (most of which studied primary care providers) and no public health-based studies, we see a need for increased studies to investigate the viewpoints of these providers and develop the necessary educational interventions.
Furthermore, the current state of research in population genetic screening focuses on individuals, with most studies revealing barriers and facilitators to interest and/or participation in population genetic screening at an individual level. We identified few interpersonal facilitators and barriers and no community-level facilitators. All our included studies were designed to elucidate stakeholders’ views and attitudes. This leaves a large gap in the literature in understanding the complex interactions between communities, the healthcare system, and the public health system. The studies which revealed interpersonal and community factors conducted surveys or semi-structured interviews, suggesting a need for additional studies to explicitly investigate macro-level determinants for population genetic screening that are suited to quantitative methods.
Most (all but two) were conducted in racially/ethnically diverse countries (Australia, Canada, United States, and United Kingdom), however roughly one third did not include information on the race or ethnicity of individuals receiving population genetic screening. This is of particular importance as studies have found ethnic minorities to be generally more apprehensive toward genetic testing than white individuals (Hann et al., 2017b). Without data on race and ethnicity of study populations the generalizability of findings is unclear and we remain unable to monitor disparities in access to population genetic screening. This suggests a need for improved reporting of race/ethnicity in population genetic screening research and a need to focus on health equity.
In addition to this challenge, more general agreement on the terminology and reporting of race, ethnicity, and ancestry in genomic research with an eye toward reproducible, ethical, and equitable research is warranted (Flanagin et al., 2021). Though the National Human Genome Research Institute (NHGRI) boldly predicts that “research in human genomics will have moved beyond population descriptors based on historic social constructs such as race” by 2030 (Green et al., 2020), there are currently numerous challenges inherent in standardizing the use (or disuse) of race and ethnicity and other population descriptors in clinical genetics. Fortunately, the National Academies of Sciences, Engineering, and Medicine established a multi-disciplinary committee to examine the current use of population descriptors in genomics research and identify best practices for improving the use of the terminology in the future.
Many studies incorporated genetic counseling; however, they had varying forms of preintervention information content and delivery and only a few assessed the efficacy of different delivery methods. The best approach and timing for genetic counseling delivery has not yet been determined. To date, there is some evidence showing that different contexts will likely have different requirements (Evans and Manchanda, 2020). For example, while this review explicitly excluded reproductive genetic testing, population-wide screening will nonetheless have profound implications for individuals of reproductive age who would be at risk of passing a hereditary predisposition for a life-threatening condition to existing or future children. This provides an opportunity to implement studies specifically designed to investigate the best manner of prescreen education and counseling specific to the delivery context, such as health literacy levels, cultural considerations, reproductive age, and disease type.
Finally, out of the studies that implemented population genetic screening and collected post-intervention data, only one followed participants for more than 12 months (Allen et al., 2008). Without sufficient long-term data, it is difficult to assess the efficacy of the screening programs at the population level. There is a need for prospective cohort studies and randomized controlled trials to evaluate any long-term benefits, such as clinical and economic outcomes, to population-level genetic screening implementation (Murray et al., 2018, 2020). The BabySeq project provides a model for identifying these long-term outcomes (Holm et al., 2018), which may be adapted to the context of population genetic screening. Such studies will likely address our previous points of determining ELSI factors to population genetic screening and assessing the effects of prescreen education methods as well.
5 Limitations
There is a potential for bias as we reported missing items as “not reported” and did not contact authors for additional information. Articles varied as to which outcome was reported (barrier, facilitator, perception, and/or outcome), so some articles may be more represented than others. Our included studies did not assess effect sizes of barriers and facilitators on interest and/or uptake of population genetic screening, which prevented us from conducting a meta-analysis. Additionally, the heterogeneity in disease states and reported effectiveness measures prevented us from fully synthesizing the data. With all systematic reviews, there is the possibility that we missed relevant literature.
6 Conclusion
We found that 1) psychosocial, attitudinal, and belief-related factors present a barrier for stakeholders to participate in screening, 2) perceived limited clinical utility presents a barrier for provider uptake, 3) there is a need for additional studies investigating healthcare and public health provider roles and education, 4) research in population genetic screening has focused on stakeholder attitudes, and 5) there is a need for long-term follow-up studies and health equity-focused studies of population genetic screening. Future research should 1) evaluate the best manner for prescreen education and counseling for specific contexts, 2) examine provider buy-in and clinical utility expansion, 3) investigate the views of providers and develop educational resources, 4) investigate macro-level determinants of and address ELSI questions toward population genetic screening, and 5) assess the long-term outcomes of population genetic screening. Taken together this data can inform future interventions to improve the development and implementation of population genetic screening.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
ES, SS, and MR conceived of the study and designed the protocol. RC conducted database searches. ES, SS, LP, MD, KF, BH, and LM participated in the screening, full-text review, and data abstraction processes. AS and CA participated in the screening and full-text review. MR participated in the screening and data abstraction processes. ES synthesized the data and prepared the first draft of the manuscript. All authors read and approved the final manuscript.
Funding
CA was supported by KooCA253576 through the Medical University of South Carolina. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.865384/full#supplementary-material
References
Allen, K. J., Nisselle, A. E., Collins, V. R., Williamson, R., and Delatycki, M. B. (2008). Asymptomatic Individuals at Genetic Risk of Haemochromatosis Take Appropriate Steps to Prevent Disease Related to Iron Overload. Liver Int. 28, 363–369. doi:10.1111/j.1478-3231.2008.01661.x
Ames, A. G., Metcalfe, S. A., Archibald, A. D., Duncan, R. E., and Emery, J. (2015). Measuring Informed Choice in Population-Based Reproductive Genetic Screening: a Systematic Review. Eur. J. Hum. Genet. 23, 8–21. doi:10.1038/ejhg.2014.89
Borry, P., Goffin, T., Nys, H., and Dierickx, K. (2008). Attitudes Regarding Predictive Genetic Testing in Minors: a Survey of European Clinical Geneticists. Am. J. Med. Genet. 148c, 78–83. doi:10.1002/ajmg.c.30165
CDC (2019). ACCE Model List of 44 Targeted Questions. Available at: https://www.cdc.gov/genomics/gtesting/acce/acce_proj.htm (Accessed January 12, 2022).
CDC (2021). Tier 1 Genomics Applications and their Importance to Public Health. Available at: https://www.cdc.gov/genomics/implementation/toolkit/tier1.htm (Accessed December 28, 2021).
Crellin, E., McClaren, B., Nisselle, A., Best, S., Gaff, C., and Metcalfe, S. (2019). Preparing Medical Specialists to Practice Genomic Medicine: Education an Essential Part of a Broader Strategy. Front. Genet. 10, 789. doi:10.3389/fgene.2019.00789
East, K. M., Cochran, M., Kelley, W. V., Greve, V., Emmerson, K., and Raines, G. (2019). Understanding the Present and Preparing for the Future: Exploring the Needs of Diagnostic and Elective Genomic Medicine Patients. J. Genet. Couns. 28, 438–448. doi:10.1002/jgc4.1114
Evans, O., and Manchanda, R. (2020). Population-based Genetic Testing for Precision Prevention. Cancer Prev. Res. phila. Pa.) 13, 643. doi:10.1158/1940-6207.CAPR-20-0002
Fenton, G. L., Smit, A. K., Freeman, L., Badcock, C., Dunlop, K., Butow, P. N., et al. (2018). Development and Evaluation of a Telephone Communication Protocol for the Delivery of Personalized Melanoma Genomic Risk to the General Population. J. Genet. Couns. 27, 370–380. doi:10.1007/s10897-017-0183-7
Flanagin, A., Frey, T., and Christiansen, S. L. (2021). AMA Manual of Style Committee. Updated Guidance on the Reporting of Race and Ethnicity in Medical and Science Journals. JAMA 326 (7), 621–627. doi:10.1001/jama.2021.13304
Friedman, J. M., Cornel, M. C., Goldenberg, A. J., Lister, K. J., Sénécal, K., Vears, D. F., et al. (2017). Genomic Newborn Screening: Public Health Policy Considerations and Recommendations. BMC Med. Genomics 10, 9. doi:10.1186/s12920-017-0247-4
Frieser, M. J., Wilson, S., and Vrieze, S. (2018). Behavioral Impact of Return of Genetic Test Results for Complex Disease: Systematic Review and Meta-Analysis. Health Psychol. Off. J. Div. Health Psychol. Am. Psychol. Assoc. 37, 1134–1144. doi:10.1037/hea0000683
Godino, J. G., van Sluijs, E. M. F., Marteau, T. M., Sutton, S., Sharp, S. J., and Griffin, S. J. (2016). Lifestyle Advice Combined with Personalized Estimates of Genetic or Phenotypic Risk of Type 2 Diabetes, and Objectively Measured Physical Activity: A Randomized Controlled Trial. PLoS Med. 13. doi:10.1371/journal.pmed.1002185
Golden, S. D., and Earp, J. A. L. (2012). Social Ecological Approaches to Individuals and Their Contexts: Twenty Years of Health Education & Behavior Health Promotion Interventions. Health Educ. Behav. 39, 364–372. doi:10.1177/1090198111418634
Goldenberg, A. J., Lloyd-Puryear, M., Brosco, J. P., Therrell, B., Bush, L., Berry, S., et al. (2019). Including ELSI Research Questions in Newborn Screening Pilot Studies. Genet. Med. 21, 525–533. doi:10.1038/s41436-018-0101-x
Green, E. D., Gunter, C., Biesecker, L. G., Di Francesco, V., Easter, C. L., Feingold, E. A., et al. (2020). Strategic Vision for Improving Human Health at the Forefront of Genomics. Nature 586, 683–692. Epub 2020 Oct 28. PMID: 33116284; PMCID: PMC7869889. doi:10.1038/s41586-020-2817-4
Haga, S. B., Barry, W. T., Mills, R., Svetkey, L., Suchindran, S., Willard, H. F., et al. (2014). Impact of Delivery Models on Understanding Genomic Risk for Type 2 Diabetes. Public Health Genomics 17, 95–104. doi:10.1159/000358413
Haga, S. B., Carrig, M. M., O’Daniel, J. M., Orlando, L. A., Killeya-Jones, L. A., Ginsburg, G. S., et al. (2011). Genomic Risk Profiling: Attitudes and Use in Personal and Clinical Care of Primary Care Physicians Who Offer Risk Profiling. J. Gen. Intern Med. 26, 834–840. doi:10.1007/s11606-011-1651-7
Hampel, H., and de la Chapelle, A. (2011). The Search for Unaffected Individuals with Lynch Syndrome: Do the Ends Justify the Means? Cancer Prev. Res. phila. Pa 4, 1–5. doi:10.1158/1940-6207.CAPR-10-0345
Hann, K. E. J., Fraser, L., Side, L., Gessler, S., Waller, J., Sanderson, S. C., et al. (2017a). Health Care Professionals’ Attitudes towards Population-Based Genetic Testing and Risk-Stratification for Ovarian Cancer: a Cross-Sectional Survey. BMC Womens Health 17, 132. doi:10.1186/s12905-017-0488-6
Hann, K. E. J., Freeman, M., Fraser, L., Waller, J., Sanderson, S. C., Rahman, B., et al. (2017b). Awareness, Knowledge, Perceptions, and Attitudes towards Genetic Testing for Cancer Risk Among Ethnic Minority Groups: a Systematic Review. BMC Public Health 17, 503. doi:10.1186/s12889-017-4375-8
Hardie, E. A. (2011). Australian Community Responses to the Use of Genetic Testing for Personalised Health Promotion. Aust. J. Psychol. 63, 119–129. doi:10.1111/j.1742-9536.2011.00017.x
Hauser, D., Obeng, A. O., Fei, K., Ramos, M. A., and Horowitz, C. R. (2018). Views of Primary Care Providers on Testing Patients for Genetic Risks for Common Chronic Diseases. Health Aff. Proj. Hope 37, 793–800. doi:10.1377/hlthaff.2017.1548
Hay, J. L., Kaphingst, K. A., Buller, D., Schofield, E., Meyer White, K., Sussman, A., et al. (2021). Behavioral and Psychological Outcomes Associated with Skin Cancer Genetic Testing in Albuquerque Primary Care. Cancers 13, 4053. doi:10.3390/cancers13164053
Hay, J. L., Zielaskowski, K., Meyer White, K., Kaphingst, K., Robers, E., Guest, D., et al. (2018). Interest and Uptake of MC1R Testing for Melanoma Risk in a Diverse Primary Care Population a Randomized Clinical Trial. JAMA Dermatol 154, 684–693. doi:10.1001/jamadermatol.2018.0592
Henneman, L., Timmermans, D. R., Bouwman, C. M., Cornel, M. C., and Meijers-Heijboer, H. (2011). A Low Risk Is Still a Risk”: Exploring Women’s Attitudes towards Genetic Testing for Breast Cancer Susceptibility in Order to Target Disease Prevention. Public Health Genomics 14, 238–247. doi:10.1159/000276543
Hietaranta-Luoma, H.-L., Luomala, H. T., Puolijoki, H., and Hopia, A. (2015). Using ApoE Genotyping to Promote Healthy Lifestyles in Finland - Psychological Impacts: Randomized Controlled Trial. J. Genet. Couns. 24, 908–921. doi:10.1007/s10897-015-9826-8
Hollands, G. J., French, D. P., Griffin, S. J., Prevost, A. T., Sutton, S., King, S., et al. (2016). The Impact of Communicating Genetic Risks of Disease on Risk-Reducing Health Behaviour: Systematic Review with Meta-Analysis. BMJ 352, i1102. doi:10.1136/bmj.i1102
Holm, I. A., Agrawal, P. B., Ceyhan-Birsoy, O., Christensen, K. D., Fayer, S., Frankel, L. A., et al. (2018). The BabySeq Project: Implementing Genomic Sequencing in Newborns. BMC Pediatr. 18, 225. doi:10.1186/s12887-018-1200-1
Hong, Q., Pluye, P., Fabregues, S., Bartlett, G., Boardman, F., Cargo, M., et al. (2018). Mixed Methods Appraisal Tool (MMAT). version 2018. Montréal: Department of Family Medicine, McGill University.
Joseph, L., Cankovic, M., Caughron, S., Chandra, P., Emmadi, R., Hagenkord, J., et al. (2016). The Spectrum of Clinical Utilities in Molecular Pathology Testing Procedures for Inherited Conditions and Cancer: A Report of the Association for Molecular Pathology. J. Mol. Diagn. 18, 605–619. doi:10.1016/j.jmoldx.2016.05.007
Joshi, E., Mighton, C., Clausen, M., Casalino, S., Kim, T. H. M., Kowal, C., et al. (2020). Primary Care Provider Perspectives on Using Genomic Sequencing in the Care of Healthy Children. Eur. J. Hum. Genet. 28, 551–557. doi:10.1038/s41431-019-0547-6
Lacson, J. C. A., Doyle, S. H., Qian, L., Del Rio, J., Forgas, S. M., Valavanis, S., et al. (2021). A Randomized Trial of Precision Prevention Materials to Improve Primary and Secondary Melanoma Prevention Activities Among Individuals with Limited Melanoma Risk Phenotypes. Cancers 13, 3143. doi:10.3390/cancers13133143
Laskey, S. L., Williams, J., Pierre-Louis, J., O’Riordan, M., Matthews, A., and Robin, N. H. (2003). Attitudes of African American Premedical Students toward Genetic Testing and Screening. Genet. Med. 5, 49–54. doi:10.1097/00125817-200301000-00008
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. BMJ 339, b2535. doi:10.1136/bmj.b2535
Molster, C. M., Bowman, F. L., Bilkey, G. A., Cho, A. S., Burns, B. L., Nowak, K. J., et al. (2018). The Evolution of Public Health Genomics: Exploring its Past, Present, and Future. Front. Public Health 6. doi:10.3389/fpubh.2018.00247
Murray, M. F., Evans, J. P., Angrist, M., Chan, K., Uhlmann, W. R., Doyle, D. L., et al. (2018). A Proposed Approach for Implementing Genomics-Based Screening Programs for Healthy Adults. Washington, DC: NAM Perspectives. doi:10.31478/201812a
Murray, M. F., Evans, J. P., and Khoury, M. J. (2020). DNA-based Population Screening: Potential Suitability and Important Knowledge Gaps. JAMA 323, 307–308. doi:10.1001/jama.2019.18640
Neghina, A. M., and Anghel, A. (2010). Hereditary Hemochromatosis: Awareness and Genetic Testing Acceptability in Western Romania. Genet. Test. Mol. Biomark. 14, 847–850. doi:10.1089/gtmb.2010.0109
Nicholls, S. G., Etchegary, H., Carroll, J. C., Castle, D., Lemyre, L., Potter, B. K., et al. (2016). Attitudes to Incorporating Genomic Risk Assessments into Population Screening Programs: The Importance of Purpose, Context and Deliberation Donna Dickenson, Sandra Soo-Jin Lee, and Michael Morrison. BMC Med. Genomics 9. doi:10.1186/s12920-016-0186-5
Nielsen, D. E., and El-Sohemy, A. (2012). A Randomized Trial of Genetic Information for Personalized Nutrition. Genes Nutr. 7, 559–566. doi:10.1007/s12263-012-0290-x
Nusbaum, R., Leventhal, K.-G., Hooker, G. W., Peshkin, B. N., Butrick, M., Salehizadeh, Y., et al. (2013). Translational Genomic Research: Protocol Development and Initial Outcomes Following SNP Testing for Colon Cancer Risk. Transl. Behav. Med. 3, 17–29. doi:10.1007/s13142-012-0149-0
O’Neill, S. C., Tercyak, K. P., Baytop, C., Hensley Alford, S., and McBride, C. M. (2015). A New Approach to Assessing Affect and the Emotional Implications of Personal Genomic Testing for Common Disease Risk. Public Health Genomics 18, 104–112. doi:10.1159/000370101
Peterson, J. F., Field, J. R., Shi, Y., Schildcrout, J. S., Denny, J. C., McGregor, T. L., et al. (2016). Attitudes of Clinicians Following Large-Scale Pharmacogenomics Implementation. Pharmacogenomics J. 16, 393–398. doi:10.1038/tpj.2015.57
Price, W. N., and Cohen, I. G. (2019). Privacy in the Age of Medical Big Data. Nat. Med. 25, 37–43. doi:10.1038/s41591-018-0272-7
Rego, S., Dagan-Rosenfeld, O., Bivona, S. A., Snyder, M. P., and Ormond, K. E. (2019). Much Ado about Nothing: A Qualitative Study of the Experiences of an Average-Risk Population Receiving Results of Exome Sequencing. J. Genet. Couns. 28, 428–437. doi:10.1002/jgc4.1096
Representatives of the Global Familial Hypercholesterolemia Community (2020). Reducing the Clinical and Public Health Burden of Familial Hypercholesterolemia: A Global Call to Action. JAMA Cardiol. 5, 217–229. doi:10.1001/jamacardio.2019.5173
Rogowski, W. (2006). Genetic Screening by DNA Technology: a Systematic Review of Health Economic Evidence. Int. J. Technol. Assess. Health Care 22, 327–337. doi:10.1017/s0266462306051221
Rohrer Vitek, C. R., Abul-Husn, N. S., Connolly, J. J., Hartzler, A. L., Kitchner, T., Peterson, J. F., et al. (2017). Healthcare Provider Education to Support Integration of Pharmacogenomics in Practice: the eMERGE Network Experience. Pharmacogenomics 18, 1013–1025. doi:10.2217/pgs-2017-0038
Rubinsak, L. A., Kleinman, A., Quillin, J., Gordon, S. W., Sullivan, S. A., Sutton, A. L., et al. (2019). Awareness and Acceptability of Population-Based Screening for Pathogenic BRCA Variants: Do Race and Ethnicity Matter? Gynecol. Oncol. 154, 383–387. doi:10.1016/j.ygyno.2019.06.009
Sanderson, S. C., Linderman, M. D., Suckiel, S. A., Diaz, G. A., Zinberg, R. E., Ferryman, K., et al. (2016). Motivations, Concerns and Preferences of Personal Genome Sequencing Research Participants: Baseline Findings from the HealthSeq Project. Eur. J. Hum. Genet. 24, 14–20. doi:10.1038/ejhg.2015.118
Sanderson, S. C., Linderman, M. D., Suckiel, S. A., Zinberg, R., Wasserstein, M., Kasarskis, A., et al. (2017). Psychological and Behavioural Impact of Returning Personal Results from Whole-Genome Sequencing: the HealthSeq Project. Eur. J. Hum. Genet. 25, 280–292. doi:10.1038/ejhg.2016.178
Sanderson, S. C., Wardle, J., Jarvis, M. J., and Humphries, S. E. (2004). Public Interest in Genetic Testing for Susceptibility to Heart Disease and Cancer: a Population-Based Survey in the UK. Prev. Med. 39, 458–464. doi:10.1016/j.ypmed.2004.04.051
Schardt, C., Adams, M. B., Owens, T., Keitz, S., and Fontelo, P. (2007). Utilization of the PICO Framework to Improve Searching PubMed for Clinical Questions. BMC Med. Inf. Decis. Mak. 7, 16. doi:10.1186/1472-6947-7-16
Shaw, J. S., and Bassi, K. L. (2001). Lay Attitudes toward Genetic Testing for Susceptibility to Inherited Diseases. J. Health Psychol. 6, 405–423. doi:10.1177/135910530100600404
Shen, E. C., Swetha, S., Passero, L., Allen, C., Dixon, M., Foss, K., et al. (2022). Barriers and Facilitators for Universal Genetic Testing in Healthy Populations: A Systematic Review. Research Square.
Shiloh, S., deHeer, H. D., Peleg, S., Hensley Alford, S., Skapinsky, K., Roberts, J. S., et al. (2015). The Impact of Multiplex Genetic Testing on Disease Risk Perceptions. Clin. Genet. 87, 117–123. doi:10.1111/cge.12403
Smit, A. K., Allen, M., Beswick, B., Butow, P., Dawkins, H., Dobbinson, S. J., et al. (2021). Impact of Personal Genomic Risk Information on Melanoma Prevention Behaviors and Psychological Outcomes: a Randomized Controlled Trial. Genet. Med. Off. J. Am. Coll. Med. Genet. 23, 2394–2403. doi:10.1038/s41436-021-01292-w
Smit, A. K., Newson, A. J., Keogh, L., Best, M., Dunlop, K., Vuong, K., et al. (2019). GP Attitudes to and Expectations for Providing Personal Genomic Risk Information to the Public: a Qualitative Study. BJGP Open 3, bjgpopen18X101633. doi:10.3399/bjgpopen18X101633
Smit, A. K., Reyes-Marcelino, G., Keogh, L., Cust, A. E., and Newson, A. J. (2020). There Is a Lot of Good in Knowing, but There Is Also a Lot of Downs”: Public Views on Ethical Considerations in Population Genomic Screening. J. Med. Ethics medethics-2019, 105934. doi:10.1136/medethics-2019-105934
Srinivasan, S., Won, N. Y., Dotson, W. D., Wright, S. T., and Roberts, M. C. (2020). Barriers and Facilitators for Cascade Testing in Genetic Conditions: a Systematic Review. Eur. J. Hum. Genet. 28, 1631–1644. doi:10.1038/s41431-020-00725-5
Toiviainen, H., Jallinoja, P., Aro, A. R., and Hemminki, E. (2003). Medical and Lay Attitudes towards Genetic Screening and Testing in Finland. Eur. J. Hum. Genet. 11, 565–572. doi:10.1038/sj.ejhg.5201006
Vassy, J. L., Christensen, K. D., Schonman, E. F., Blout, C. L., Robinson, J. O., Krier, J. B., et al. (2017). The Impact of Whole-Genome Sequencing on the Primary Care and Outcomes of Healthy Adult Patients: A Pilot Randomized Trial. Ann. Intern Med. 167, 159–169. doi:10.7326/m17-0188
Vassy, J. L., Christensen, K. D., Slashinski, M. J., Lautenbach, D., Robinson, J. O., Blumenthal-Barby, J. A., et al. (2014). Someday it Will Be the Norm”: Physician Perceptions of the Clinical Utility of Whole Genome Sequencing. J. Gen. Intern. Med. 29, S4.
Veritas Health Innovation Covidence systematic review software (2021). Covidence. Available at: https://www.covidence.org/(Accessed December 28, 2021).
Keywords: population testing, universal genetic screening, healthy population screening, average risk, precision public health, perceptions, attitudes, outcomes
Citation: Shen EC, Srinivasan S, Passero LE, Allen CG, Dixon M, Foss K, Halliburton B, Milko LV, Smit AK, Carlson R and Roberts MC (2022) Barriers and Facilitators for Population Genetic Screening in Healthy Populations: A Systematic Review. Front. Genet. 13:865384. doi: 10.3389/fgene.2022.865384
Received: 29 January 2022; Accepted: 02 June 2022;
Published: 04 July 2022.
Edited by:
Yann Joly, McGill University, CanadaReviewed by:
Erica M. Bednar, University of Texas MD Anderson Cancer Center, United StatesBirgit Funke, Mount Sinai Genomics, Inc., United States
Copyright © 2022 Shen, Srinivasan, Passero, Allen, Dixon, Foss, Halliburton, Milko, Smit, Carlson and Roberts. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Megan C. Roberts, bWVnYW4ucm9iZXJ0c0B1bmMuZWR1
 Emily C. Shen
Emily C. Shen Swetha Srinivasan3
Swetha Srinivasan3 Lauren E. Passero
Lauren E. Passero Caitlin G. Allen
Caitlin G. Allen Brianna Halliburton
Brianna Halliburton Laura V. Milko
Laura V. Milko Megan C. Roberts
Megan C. Roberts