- 1Department of Gynecological Oncology, Tianjin Central Hospital of Gynecology and Obstetrics, Tianjin, China
- 2Clinical School of Obstetrics and Gynecology Center, Tianjin Medical University, Tianjin, China
Primary primitive neuroectodermal tumor (PNET) in the female tract is rare. Recently, a case of cervical PNET was diagnosed in our hospital. A 29-year-old pregnant woman presented with a cystic-solid cervical mass at the 7th week of gestation. The mass grew rapidly during follow-up and ruptured at the 22nd week. A biopsy was performed on the mass. Pathological examination revealed a malignant neoplasm composed of small cells which exhibited positive immunohistochemical (IHC) staining for CD99, SYN, and FLI1. Fluorescence in situ hybridization (FISH) displayed the presence of EWS-FLI1 fusion gene resulting from the chromosomal translocation t (11;22, q24;q12), which confirmed the diagnosis of cervical PNET. The reverse transcription-polymerase chain reaction (RT-PCR) results showed type 2 EWS-FLI1 fusion occurred in this tumor, suggesting a poor prognosis. The patient underwent surgical resection and was given adjuvant chemotherapy followed by pelvic radiotherapy. PNET arising from the genital tract, especially in the uterine cervix, is very rare and presents a diagnostic challenge. FISH and RT-PCR analysis are helpful for the diagnosis of such a tumor at an unusual site, as in the present case.
Introduction
Primary primitive neuroectodermal tumor (PNET) is a highly malignant tumor characterized by neuroectodermal and neural crest cells’ origin, most of which arise from the central nervous system, soft tissues, and bones (Rajwanshi et al., 2009). In more than 90% of PNET cases, chromosomal translocations result in the fusion between the EWS gene (also known as EWSR1, Ewing sarcoma breakpoint region 1) and a member of the ETS family of transcription factors, such as FLI1 and ERG (Song et al., 2012).
In this article, we presented a case of cervical PNET diagnosed during pregnancy whose tumor grew very rapidly. The patient terminated her pregnancy, and was treated with surgical resection, adjuvant chemotherapy, followed by pelvic radiotherapy. To gain a deeper understanding of this rare disease, we reviewed PNET-related literature and found 26 cases of primary cervical PNET, including 5 cases occurring during pregnancy.
Case report
A 29-year-old female, gravida 1 para 0, was referred to her obstetrician at the 7th week of gestation. An ultrasonography examination revealed a cystic-solid mass measuring 5 cm × 4 cm in the cervix. Her medical and family history were not significant. No additional examinations or treatments were performed because of her pregnancy. During follow-up, the ultrasonography examination demonstrated that the mass grew rapidly, and at the 20th week, the patient was admitted to our department because she began to complain of low back pain. An MRI was performed and it was found that the mass grew to 11 cm × 10 cm × 10 cm in just 2 months. The levels of CA-125, CEA, SCC, and NSE were normal. Considering the pregnancy, the patient refused biopsy and chose to continue observation.
However, at the 22nd week of gestation, the patient was referred to the emergency department because of vaginal watery discharge. Gynecological examination showed that there was a rupture on the surface of the tumor and fluid was flowing out of the rupture, which was thicker than amniotic fluid. The result of amniotic fluid crystallization was negative. In addition, MRI showed the cystic-solid mass was smaller (10 cm × 8 cm × 7 cm) than before. Bimanual pelvic examination revealed the tumor was in the cervix, without invading the vagina, or adjacent organs (Figure 1). The patient underwent a biopsy of the cervical mass, and it was found that the tumor consisted of small round cells and had extensive necrosis. IHC showed that tumor cells expressed CD99, synaptophysin (SYN), friend leukemia integration 1 (FLI1), and P16. However, no expression of cytokeratin (CAM5.2), P53, chromogranin A (CgA), CD56, or neuron specific enolase (NSE) was detected. Ki-67 proliferation index was about 80% (Figure 2). A pathological diagnosis of cervical PNET was suspected. Next, we performed FISH to detect the presence of the chromosomal translocation t (11;22, q24;q12). A section of 4 μm was cut from paraffin-embedded biopsy blocks and stained with hematoxylin-eosin in order to confirm the presence of tumor cells and to choose the appropriate area for the hybridization procedures. A specific translocation separation probe kit (Vysis, United States ) was used, which consisted of two probes; one directly labeled with red fluorescence and bound to 3′ end of the FLI1 (11q24) gene, and the second directly labeled with green fluorescence and bound to 5′ end of the EWSR1 (22q12) gene. According to the manufacturer’s instructions, 200 nuclei with fluorescence signal were randomly selected to observe the number of nuclei with hybridization signal by using a Zeiss Axioplan fluorescence microscope (Zeiss, Germany). Nuclei with the EWS-FLI1 fusion gene showed yellow signals, while nuclei without gene translocation showed separate red and green signal. The criterion for a positive result of the EWS-FLI1 fusion gene was that more than 10% of the nuclei showed a fusion signal. FISH demonstrated that more than 35% of the cells were positive, indicating the presence of the EWS-FLI1 fusion gene (Figure 3A). In order to clarify the type of fusion, we performed RT-PCR test. Total RNA was isolated using TRIzol (Gibico, United States ) and the concentrations were measured by NanoDrop-2000c (Thermo Fisher Scientific, United States ). Transcriptor high fidelity cDNA synthesis kit (Takara, Japan) was used for reverse transcription of 1 μg of RNA from the sample. RT-PCR was carried out using SYBR Green Master Mix (Roche, Switzerland) in a Quantstudio3 real-time thermal cycler (Thermo Fisher Scientific, United States ). Expression data were normalized to GAPDH. The primer sequences used for RT-PCR analysis of EWS-FLI1 were as follows; 5′- CCAAGTCAATATAGCCAACAG-3′ and 5′-GGCCAGAATTCATGTTATTGC-3’. RT-PCR products were analyzed on a 2% agarose gel electrophoresis. The results showed the RT-PCR product of the tumor was 166bp, which indicated that the 7th exon of EWS was fused to the 5th exon of FLI1 (Figures 3B,C). These results confirmed the diagnosis of cervical PNET.
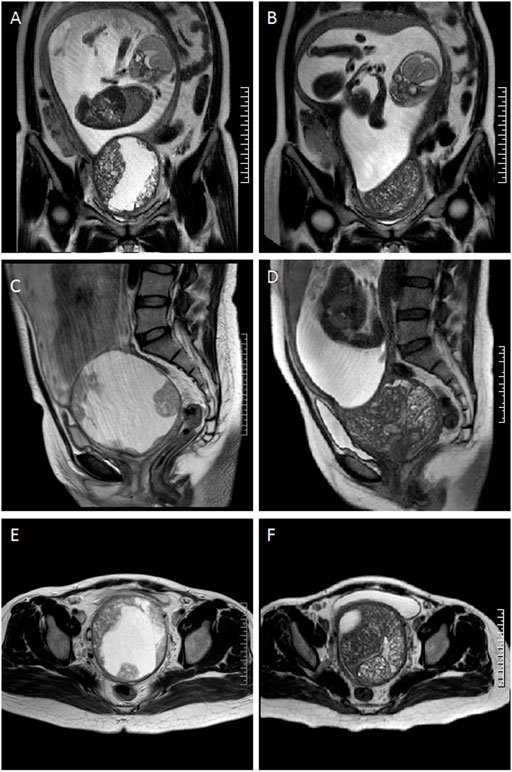
FIGURE 1. Comparison of the magnetic resonance images of the tumor at the coronal, sagittal, and axial plane before and after rupture. (A), (C) and (E) tumor at 20 weeks (before rupture) was shown. (B), (D) and (F) tumor at 22 weeks (after rupture) was shown.

FIGURE 2. (A,B) Microscopic view of the tumor of the cervix with H&E staining, revealing that tumors were composed of a monotonous population of small round cells. IHC staining was positive for (C) CD99, (D) FLI-1, (E) Ki-67, and (F) p16. Magnification details: (A) ×100; (B)200×; (C) 200×; (D) 400×; (E) 200×; and (F) 200×.
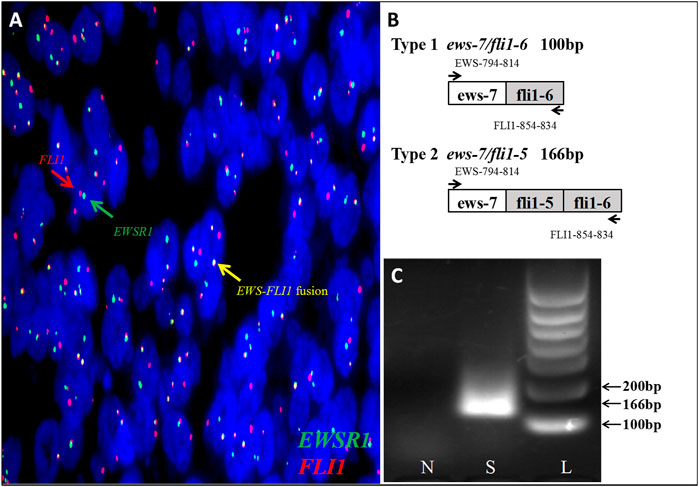
FIGURE 3. (A) FISH testing demonstrated that more than 35% of cells were positive indicating EWS-FLI1 gene rearrangement (FLI1 gene, red; EWSR1 gene, green; and EWS-FLI1 fusion gene, yellow). (B)Fusion of EWS -exon 7 to FLI1-exon 6 (ews-7/fli1-6) creates a PCR product of 100 bp, while fusion of EWS -exon 7 to FLI1-exon 5 (ews-7/fli1-5) creates a PCR product of 166 bp. (C)Investigation of the EWS-FLI1 fusion transcripts in this patient. The primers (EWS- and FLI1-) were used in subsequent PCR reactions of RT-PCR assays performed on RNA isolated from paraffin-embedded tumor tissue to search for fusion transcripts with different exon combinations (EWS-exon 7 fused to either FLI1-exon 5 or -exon 6). The patient’s sample was found positive for a EWS-FLI1 product at the 166bp. S: sample; N: normal cervix tissue; L: 100bp DNA ladder.
Chest computed tomography, abdominal and pelvic MRI did not reveal lymph nodes or distant metastases. The patient and her family were informed of the highly invasive nature and poor prognosis of the disease, and they decided to terminate the pregnancy and receive surgical treatment. Subsequently, the patient underwent a type C1 radical hysterectomy, bilateral salpingo-oophorectomy, and pelvic lymphadenectomy. There was a complete fetus in the uterine cavity, and the cervical tumor was 9 cm × 8 cm × 8 cm in stereoscopic size, invading more than two-thirds of the cervical interstitium and extending to the parametrium. No lymph node metastasis was found. However, there was lymphovascular invasion. Histological and IHC staining supported the previous diagnosis of PNET, and according to the International Federation of Gynecology and Obstetrics, the clinical stage was determined asⅡB. After surgery, the patient received adjuvant chemotherapy with pirarubicin, ifosfamide, and cisplatin for six cycles, followed by pelvic radiotherapy of 50 Gy. She remained free of disease for thirty-one months after the diagnosis.
Discussion
PNET belongs to a group of small round cell tumors that are most commonly found in the central nervous system. In the female reproductive tracts, the ovaries are the most common sites (De Nola et al., 2018). To date, only 26 cases of cervical PNET have been reported in the English literature (Table 1). The age of reported patients ranged from 19 to 59 years old, with a median age of 34. The main symptoms of cervical PNET include irregular vaginal bleeding, lower abdominal pain, uterine enlargement, and increasing mass size. Cervical PNET in pregnant women is even rare, with only five cases reported. Tsao et al. is credited with the first description of cervical PNET in a pregnant woman (Tsao et al., 2001). The authors described a 24-year-old woman in her 8th week of pregnancy presented with a cervical mass about 8 cm × 7 cm and underwent tissue biopsy with further diagnosis of PNET. A radical hysterectomy with bilateral ovarian transposition and periaortic lymphadenectomy was performed, followed by chemotherapy and radiotherapy. The patient remained disease free for 24 months after the treatment. Two cases were described by Kyriazoglou et al. and Khosla et al. (Khosla et al., 2014; Kyriazoglou et al., 2019). In both instances, the patients (9 weeks of pregnancy and 10 weeks of pregnancy, respectively) presented with a cervical mass of about 8 cm × 7.6 cm and measuring 5 cm in the greatest dimension, respectively. Both patients underwent radical hysterectomy and received adjuvant chemotherapy and radiotherapy. A follow-up of 42 and 33 months, respectively, showed both patients to be alive after initial diagnosis. In addition to the reports of cervical PNET in pregnancy, Feng et al. and Al-Nueimy et al. also described one case each, found at 14 weeks of pregnancy and during cesarean section at term, respectively (Al-Nueimy, 2014; Feng et al., 2021). Both patients presented with cervical masses of 3 cm × 3 cm and 6 cm × 4.5 cm, respectively. A radical hysterectomy with bilateral adnexectomy was performed, without lymphadenectomy. Follow-up information obtained only in the Feng et al. report, showed the patient to be alive 36 months after her diagnosis. Our case was the largest PNET ever reported, and we observed that the tumor grew rapidly during pregnancy and even spontaneous rupture occurred. Some reports show that PNET enlargement is more obvious during pregnancy (Torga et al., 2015; Miao et al., 2018). However, the association between the change of hormones during pregnancy and tumor growth needs further confirmation.
The diagnosis of PNET mainly depends on the histopathological morphology, IHC examination, and molecular genetic analysis. In hematoxylin-eosin staining, the tumor is composed of small round cells of uniform size with indistinct cell boundaries and little cytoplasm. Homer-Wright rosettes, a characteristic manifestation of PNET, are seen in approximately 30–70% of cases (Tsao et al., 2001). The IHC markers currently used in the diagnosis of PNET include CD99 (also designated as MIC2 or HBA-71), neurofilament proteins, NSE, vimentin, FLI-1, and SYN (Patel et al., 2020). CD99 represents the most specific marker for the diagnosis of PNET, and in our case, it was intensely and diffusely expressed on tumor cell membranes. Occasionally, the expressions of neuroendocrine markers such as NSE, CgA, SYN, CD56, and S-100 are different, indicating neuroectoderm differentiation. The epithelial marker desmin might be focal positive. A characteristic chromosomal translocation t (11;22) (q24;q12), resulting in the EWS-FLI1 fusion, was found in more than 90% cases, and other fusion genes include ERG, ETV1, E1AF, and FEV (Dedeurwaerdere et al., 2002; Teicher et al., 2011). Different combinations of chromosome breakpoints lead to strong heterogeneity at the molecular level. In general, breakpoints in EWS occur between introns 7–9, while breakpoints in FLI1 occur between introns 3–9. Frequently, the 7th exon of EWS is fused to the 6th exon (60%) or to the 5th exon (20%) of FLI1, creating type 1 and type 2 EWS-FLI1 fusions, respectively. Other variations of the EWS-FLI1 fusion transcript have a lower incidence. Two independent retrospective studies showed the types of EWS-FLI1 fusion associated with different clinical outcomes. Patients without metastatic disease usually had type 1 EWS-FLI1 fusion, and higher event-free and overall survival rates than patients with type 2 fusion (De Alava et al., 2000; van Doorninck et al., 2010). These studies suggest that the EWS-FLI1 fusion type can be used to predict outcome at the time of diagnosis. The diagnostic criteria proposed by Schmidt et al. is the combination of tumor histomorphological characteristics, plus the positive immunohistochemical marker CD99 and the positive of two or more different neural markers (Schmidt et al., 1991). Boldorini et al. considered that FISH and RT-PCR detection of fusion genes are the gold standards, especially for the detection of PNET in rare sites (Boldorini et al., 2010). In our case, the IHC marker CD99 was diffusely positive, SYN and FLI-1 were partially expressed, and we confirmed the presence of the EWS-FLI1 fusion gene by FISH. And RT-PCR results indicated that the patient displayed type 2 EWS-FLI1 fusion, indicating a dismal prognosis and poor clinical outcome. Currently, positron emission tomography/computed tomography (PET/CT) after injection of Gadolinium-68-labeled somatostatin receptor binding Dota-octreotide (Dotanoc) is the preferred technique for functional imaging of neuroendocrine tumors, because the better histologically differentiated forms show an overexpression of somatostatin receptors at their cell surfaces. The specificity and sensitivity were 100 and 96%, respectively (Ambrosini et al., 2011). Gadolinium-68-Dotanoc PET/CT showed great advantages in the diagnosis and staging of neuroendocrine tumors. However, this method has not been widely carried out in China. In addition, PET/CT is radioactive, which limits its use in pregnant women. We performed chest computed tomography, abdominal and pelvic MRI instead to assess the staging of this patient. The results showed no lymph nodes or distant metastases.
Primary PNET of the female reproductive system is relatively rare in clinical cases, and is a highly aggressive malignant tumor with high mortality and short-term recurrence and metastasis. Because cervical PNET belongs to the family of Ewing’s sarcoma, the current treatment strategy is surgery combined with adjuvant chemotherapy. The role of radiation is unclear. More than 80% of patients who only underwent surgery would develop tumor relapse. The overall survival was significantly improved by combining surgery with chemotherapy (Snijders-Keilholz et al., 2005). Common chemotherapy drugs include cyclophosphamide, ifosfamide, vincristine, actinomycin-D, and doxorubicin. There is no standard chemotherapy regimen for PNET, and some recent case reports have shown longer disease-free intervals after treatment with platinum and etoposide (Thorn et al., 2016). Radiotherapy is usually used for patients with inoperable tumors and/or positive surgical margins, as well as for those with poor histological results (Wang et al., 2017). In the future, specific antibodies, such as antibodies of IGF-1, and cellular immunotherapy may be applied to inhibit PNET growth (Toretsky et al., 2001). In our case, after total hysterectomy and bilateral salpingo-oophorectomy and pelvic lymphadenectomy, we applied pirarubicin, ifosfamide, and cisplatinum chemotherapy every 3 weeks for six cycles and regional radiotherapy.
The overall prognosis of PNET is very poor, with a 5-year overall survival rate of less than 30%. Late stage, insufficient surgical resection and adverse effects of chemotherapy appear to be associated with unfavorable prognosis (Odunsi et al., 2004). At the time of writing, the patient had completed the treatment and experienced significant remission. Previously, two patients suffering from cervical PNET were treated with the same chemotherapy regiment in our department, and they remained free of disease for sixty and seventy-five months, respectively.
Conclusion
PNET in the female reproductive tract is an extremely rare disease. In this article, we reported a case of cervical PNET complicated by pregnancy. The diagnosis of PNET depends on pathology, immunohistochemistry, and genetic analysis. Molecular analysis may significantly contribute to the final diagnosis of PNET occurring in this unusual location. In addition, the type of EWS-FLI1 fusion may be related to clinical outcomes. At present, the effective treatment for PNET is surgery combined with chemotherapy and radiotherapy. The rapid growth of PNET during pregnancy may be related to hormonal changes and further research is needed.
Data availability statement
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Tianjin Central Hospital of Gynecology Obstetrics. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
QP and DW designed and organized the study. ZJ was responsible for the genetic counseling of the patient. LX collected and prepared the archived PNET tissue. DW wrote the first draft of the manuscript. QP supervised the study. All authors contributed to the manuscript and approved the submitted version.
Funding
This study was financially supported by the Tianjin Health Research Project (no. KJ20098) and Tianjin Municipal Science and Technology Commission Grant (no. 21JCYBJC00310).
Acknowledgments
The authors thank the patient’s family for providing background information and allowing them to publish this grand round. The authors report no conflicts of interest in this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad, I., Chufal, K. S., Bhargava, A., and Bashir, I. (2017). Primitive neuroectodermal tumour of the cervix: a rare diagnosis. BMJ Case Rep. 2017, bcr2016217461. doi:10.1136/bcr-2016-217461
Al-Nueimy, W. A. M. S. (2014). Primitive neuroectodermal tumor of the cervix uteri – a case report. Jordan Med. J. 48 (4), 285–291.
Ambrosini, V., Campana, D., Tomassetti, P., Grassetto, G., Rubello, D., Fanti, S., et al. (2011). PET/CT with 68Gallium-DOTA-peptides in NET: an overview. Eur. J. Radiol. 80 (2), e116–9. doi:10.1016/j.ejrad.2010.07.022
Arora, N., Kalra, A., Kausar, H., Ghosh, T. K., and Majumdar, A. (2012). Primitive neuroectodermal tumour of uterine cervix - a diagnostic and therapeutic dilemma. J. Obstet. Gynaecol. 32 (7), 711–713. doi:10.3109/01443615.2012.689028
Benbrahim, Z., Arifi, S., Daoudi, K., Serraj, M., Amara, B., Benjelloun, M. C., et al. (2013). Askin's tumor: a case report and literature review. World J. Surg. Oncol. 1110, 10. doi:10.1186/1477-7819-11-10
Boldorini, R., Riboni, F., Cristina, S., Allegrini, S., Valentini, S., Muscara, M., et al. (2010). Primary vulvar ewing's sarcoma/primitive neuroectodermal tumor in a post-menopausal woman: a case report. Pathol. Res. Pract. 206 (7), 476–479. doi:10.1016/j.prp.2009.07.006
Cenacchi, G., Pasquinelli, G., Montanaro, L., Cerasoli, S., Vici, M., Bisceglia, M., et al. (1998). Primary endocervical extraosseous Ewing's sarcoma/PNET. Int. J. Gynecol. Pathol. 17 (1), 83–88. doi:10.1097/00004347-199801000-00015
De Alava, E., Panizo, A., Antonescu, C. R., Huvos, A. G., Pardo-Mindan, F. J., Barr, F. G., et al. (2000). Association of EWS-FLI1 type 1 fusion with lower proliferative rate in Ewing's sarcoma. Am. J. Pathol. 156 (3), 849–855. doi:10.1016/S0002-9440(10)64953-X
De Nola, R., Di Naro, E., Schonauer, L. M., Lucarelli, G., Battaglia, M., Fiore, M. G., et al. (2018). Clinical management of a unique case of PNET of the uterus during pregnancy, and review of the literature. Med. Baltim. 97 (2), e9505. doi:10.1097/MD.0000000000009505
Dedeurwaerdere, F., Giannini, C., Sciot, R., Rubin, B. P., Perilongo, G., Borghi, L., et al. (2002). Primary peripheral PNET/ewing's sarcoma of the dura: a clinicopathologic entity distinct from central PNET. Mod. Pathol. 15 (6), 673–678. doi:10.1038/modpathol.3880585
Farzaneh, F., Rezvani, H., Boroujeni, P. T., and Rahimi, F. (2011). Primitive neuroectodermal tumor of the cervix: a case report. J. Med. Case Rep. 5489, 489. doi:10.1186/1752-1947-5-489
Feng, X., Zhang, L., Tan, Y., Feng, A., Luo, F., Xu, M., et al. (2021). Primitive neuroectodermal tumor of the cervix diagnosed during pregnancy: a rare case report with discussion. BMC Pregnancy Childbirth 21 (1), 382. doi:10.1186/s12884-021-03859-6
Goda J, M. B. P. P. (2006). Primitive neuroectodermal tumour of the cervix: A rare entity. Internet J. Radiology 6 (1), 1–5.
Gupta, V., Raju, K., Sridhar, D., Ahmed, S. M., and Fonseca, D. (2020). Primary ewing's sarcoma/primitive neuroectodermal tumour of the cervix: a rare tumour. Indian J. Surg. Oncol. 11 (1), 162–165. doi:10.1007/s13193-019-00965-y
Horn, L. C., Fischer, U., and Bilek, K. (1997). Primitive neuroectodermal tumor of the cervix uteri. A case report. Gen. Diagn. Pathol. 142 (3-4), 227–230.
Khosla, D., Rai, B., Patel, F. D., Sreedharanunni, S., Dey, P., Sharma, S. C., et al. (2014). Primitive neuroectodermal tumor of the uterine cervix diagnosed during pregnancy: a rare case with review of literature. J. Obstet. Gynaecol. Res. 40 (3), 878–882. doi:10.1111/jog.12238
Kyriazoglou, A., Tsironis, G., Liontos, M., Papakosta, A., Mahaira, L., Thomakos, N., et al. (2019). Ewing's sarcoma of the cervix: A case report of an unusual diagnosis in pregnancy treated with surgery, adjuvant vide and radiotherapy. Oncol. Lett. 17 (6), 5529–5535. doi:10.3892/ol.2019.10267
Li, B., Ouyang, L., Han, X., Zhou, Y., Tong, X., Zhang, S., et al. (2013). Primary primitive neuroectodermal tumor of the cervix. Onco Targets Ther. 6, 707–711. doi:10.2147/OTT.S45889
Malpica, A., and Moran, C. A. (2002). Primitive neuroectodermal tumor of the cervix: a clinicopathologic and immunohistochemical study of two cases. Ann. Diagn. Pathol. 6 (5), 281–287. doi:10.1053/adpa.2002.35739
Mashriqi, N., Gujjarlapudi, J. K., Sidhu, J., Zur, M., and Yalamanchili, M. (2015). Ewing's sarcoma of the cervix, a diagnostic dilemma: a case report and review of the literature. J. Med. Case Rep. 9255, 255. doi:10.1186/s13256-015-0733-2
Masoura, S., Kourtis, A., Kalogiannidis, I., Kotoula, V., Anagnostou, E., Angelidou, S., et al. (2012). Primary primitive neuroectodermal tumor of the cervix confirmed with molecular analysis in a 23-year-old woman: A case report. Pathol. Res. Pract. 208 (4), 245–249. doi:10.1016/j.prp.2012.01.004
Miao, C., Yang, J., Xue, J., Zhu, J., Chen, W., Qin, Y., et al. (2018), "Renal ewing sarcoma/primitive neuroectodermal tumor in a pregnant woman who underwent robot-assisted laparoscopic nephrectomy: a case report and literature review", Onco. Targets. Ther., 11. 16839-16843. doi:10.2147/OTT.S155523
Odunsi, K., Olatinwo, M., Collins, Y., Withiam-Leitch, M., Lele, S., Spiegel, G. W., et al. (2004). Primary primitive neuroectodermal tumor of the uterus: a report of two cases and review of the literature. Gynecol. Oncol. 92 (2), 689–696. doi:10.1016/j.ygyno.2003.09.029
Patel, D., Nandu, N. S., and Reddy, A. (2020). Extraosseus ewing's sarcoma in pancreas: A review. Cureus 12 (4), e7505. doi:10.7759/cureus.7505
Pauwels, P., Ambros, P., Hattinger, C., Lammens, M., Dal Cin, P., Ribot, J., et al. (2000). Peripheral primitive neuroectodermal tumour of the cervix. Virchows Arch. 436 (1), 68–73. doi:10.1007/pl00008201
Rajendra, K. T., Saxena, M., Goyal, H., Siddiqui, S., Kumar Singh, R., Singh Gothwal, R., et al. (2020). Primitive neuroectodermal tumor of cervix: Report of a rare case and review of literature. Cancer Res. J. (N. Y. N. Y). 8 (4), 62. doi:10.11648/j.crj.20200804.12
Rajwanshi, A., Srinivas, R., and Upasana, G. (2009). Malignant small round cell tumors. J. Cytol. 26 (1), 1–10. doi:10.4103/0970-9371.54861
Sato, S., Yajima, A., Kimura, N., Namiki, T., Furuhashi, N., and Sakuma, H. (1996). Peripheral neuroepithelioma (peripheral primitive neuroectodermal tumor) of the uterine cervix. Tohoku J. Exp. Med. 180 (2), 187–195. doi:10.1620/tjem.180.187
Schmidt, D., Herrmann, C., Jurgens, H., and Harms, D. (1991). Malignant peripheral neuroectodermal tumor and its necessary distinction from Ewing's sarcoma. A report from the Kiel Pediatric Tumor Registry. Cancer 68 (10), 2251–2259. doi:10.1002/1097-0142(19911115)68:10<2251::aid-cncr2820681025>3.0.co;2-x
Snijders-Keilholz, A., Ewing, P., Seynaeve, C., and Burger, C. W. (2005). Primitive neuroectodermal tumor of the cervix uteri: a case report -- changing concepts in therapy. Gynecol. Oncol. 98 (3), 516–519. doi:10.1016/j.ygyno.2005.05.020
Song, H. C., Sun, N., Zhang, W. P., and Huang, C. R. (2012). Primary Ewing's sarcoma/primitive neuroectodermal tumor of the urogenital tract in children. Chin. Med. J. 125 (5), 932–936.
Teicher, B. A., Bagley, R. G., Rouleau, C., Kruger, A., Ren, Y., Kurtzberg, L., et al. (2011). Characteristics of human Ewing/PNET sarcoma models. Ann. Saudi Med. 31 (2), 174–182. doi:10.4103/0256-4947.78206
Thorn, D., Mamot, C., Krasniqi, F., Metternich, F., and Prestin, S. (2016). Multimodality treatment in ewing's sarcoma family tumors of the maxilla and maxillary sinus: Review of the literature. Sarcoma 2016, 3872768. doi:10.1155/2016/3872768
Toretsky, J. A., Steinberg, S. M., Thakar, M., Counts, D., Pironis, B., Parente, C., et al. (2001). Insulin-like growth factor type 1 (IGF-1) and IGF binding protein-3 in patients with Ewing sarcoma family of tumors. Cancer 92 (11), 2941–2947. doi:10.1002/1097-0142(20011201)92:11<2941::aid-cncr10072>3.0.co;2-c
Torga, G., Barrow, W., and Han, M. (2015). Rare renal incidentaloma in pregnancy: An unusual primitive neuroectodermal tumor presentation. Urol. Case Rep. 3 (2), 12–14. doi:10.1016/j.eucr.2014.12.004
Tsao, A. S., Roth, L. M., Sandler, A., and Hurteau, J. A. (2001). Cervical primitive neuroectodermal tumor. Gynecol. Oncol. 83 (1), 138–142. doi:10.1006/gyno.2001.6339
van Doorninck, J. A., Ji, L., Schaub, B., Shimada, H., Wing, M. R., Krailo, M. D., et al. (2010). Current treatment protocols have eliminated the prognostic advantage of type 1 fusions in ewing sarcoma: a report from the children's oncology group. J. Clin. Oncol. 28 (12), 1989–1994. doi:10.1200/JCO.2009.24.5845
Wang, X., Gao, Y., Xu, Y., Liu, Y., and Qu, P. (2017). Primary primitive neuroectodermal tumor of the cervix: A report of two cases and review of the literature. Mol. Clin. Oncol. 6 (5), 697–700. doi:10.3892/mco.2017.1193
Keywords: primary primitive neuroectodermal tumor, cervix, pregnant woman, molecular analysis, literature review
Citation: Wei D, Jianguo Z, Xiao L and Pengpeng Q (2022) Primary primitive neuroectodermal tumor of the cervix confirmed with molecular analysis in a pregnant woman: A case report and literature review. Front. Genet. 13:871531. doi: 10.3389/fgene.2022.871531
Received: 08 February 2022; Accepted: 27 June 2022;
Published: 10 August 2022.
Edited by:
Bruna De Felice, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Lucia Taja-Chayeb, National Institute of Cancerology (INCAN), MexicoThekke Rajeev, K S Hegde Medical Academy, India
Copyright © 2022 Wei, Jianguo, Xiao and Pengpeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qu Pengpeng, cXUucGVuZ3BlbmdAaG90bWFpbC5jb20=
†These authors have contributed equally to this work
 Ding Wei
Ding Wei Zhao Jianguo1†
Zhao Jianguo1† Qu Pengpeng
Qu Pengpeng