- 1The Key Laboratory of Environmental Pollution Monitoring and Disease Control, School of Public Health, Ministry of Education, Guizhou Medical University, Guiyang, China
- 2Xiangya School of Public Health, Central South University, Changsha, China
- 3School of Traditional Chinese Medicine Jilin Agricultural Science and Technology University, Changchun, China
- 4School of Life Sciences, Jilin University, Jilin, China
- 5Anorectal Center, The Affiliated Hospital to Changchun University of Chinese Medicine, Changchun, China
- 6School of Pharmacy, Changchun University of Traditional Chinese Medicine, Changchun, China
- 7Office of Infection Control, The Affiliated Hospital to Changchun University of Chinese Medicine, Changchun, China
- 8Proctology Department, Affiliated Hospital of Changchun University of Chinese Medicine, Changchun, China
- 9Research Center of Traditional Chinese Medicine, The Affiliated Hospital to Changchun University of Chinese Medicine, Changchun, China
Objective: Tuberculosis (TB) is the leading cause of mortality worldwide. Previous studies have reported that TB susceptibility can be caused by vitamin D deficiency, which is affected by polymorphisms in the vitamin D receptor (VDR) gene. However, these results have been inconsistent. Therefore, we performed a meta-analysis to investigate the association between VDR polymorphisms and TB susceptibility.
Methods: We systematically searched for relevant literature in PubMed, Embase, and Medline databases through December 31st, 2022. Inclusion and exclusion criteria were made to ensure that HIV-negative population is the targeted subjects. The pooled odds ratio (OR) and 95% confidence interval (CI) were then used to assess the strength of the association, and the quality of the included articles was evaluated using the Newcastle–Ottawa Scale. Potential sources of heterogeneity were evaluated based on subgroup and meta-regression analyses.
Results: In our meta-analysis, we found that the FokI polymorphism in the VDR gene was associated with increased TB susceptibility in the allele and recessive genotype models (OR f vs. F = 1.235, 95%CI: 1.035–1.475; OR ff vs. Ff + FF = 1.317, 95%CI: 1.005–1.727. Further subgroup analysis based on ethnicity demonstrated the association with the risk of TB in all genotype models of the FokI polymorphism for Han population. Meta-regression analysis also indicated that ethnicity could be a potential source of heterogeneity in the FokI and BsmI polymorphisms in the VDR gene. However, publication year was another source of heterogeneity for the TaqI polymorphism.
Conclusion: In summary, the FokI polymorphism in the VDR gene was found to increase the risk of TB in the HIV-negative population, both overall and in Asian populations. The findings presented in this paper could provide clues for preventing TB from the perspective of vitamin D supplementation, which is a controversial topic in the field of medicine and health.
Introduction
Tuberculosis (TB) is a communicable disease caused by the Mycobacterium tuberculosis complex (MTB). It is considered a major determinant of poor health and one of the leading causes of mortality, responsible for 1.6 million deaths worldwide in 2021 (Möller and Hoal, 2010). According to “Global Tuberculosis Report 2022” from the World Health Organization, approximately 10.6 million people worldwide were infected with TB in 2021, representing a 4.5% increase from the 10.1 million cases recorded in 2020. Similarly, the report cited an estimated 3.6% increase in TB incidence, to approximately 134 cases per 100,000 population, between 2021 and 2020 (WORLD HEALTH ORGANIZATION, 2022-10). These challenges highlight the serious issue associated with preventing and controlling TB epidemics.
The persisting association between MTB and its host implies that this pathogen has evolved extensive mechanisms to evade elimination by the immune system. Accordingly, it causes no substantial harm and is not transmitted until immune system responses decline due to co-infections or other factors. As a result, despite approximately one-quarter of the global population being infected with MTB, only 5%–10% of these individuals develop TB (Delgado et al., 2002; Dye et al., 1999). Moreover, the process of TB might also be associated with other factors, such as lifestyle, environment, and genetics (Hillerdal, 2000; Newport and Nejentsev, 2004; Nava-Aguilera et al., 2009). Among these, genetic factors of the host play a vital role in susceptibility or resistance to TB.
In recent decades, vitamin D has been shown to play an essential role in bone health (Ganmaa et al., 2020). Moreover, recent studies based on different populations have indicated that vitamin D deficiency increases the risk of developing TB. Vitamin D also plays a role in the biological modulation of the immune system in response to TB. Here, 1,25-dihydroxyvitamin D3 (the active form of vitamin D) is activated by 1
VDR is located on the long arm of chromosome 12q13 (Miyamoto et al., 1997). Polymorphisms of this gene are observed across various population groups, although the prevalence of specific VDR genotypes varies among populations. Several polymorphisms, including BsmI (rs1544410), ApaI (rs7975232), and TaqI (rs731236), at the 3′end of VDR with strong linkage disequilibrium have been examined. Despite having no impact on the structure of the expressed VDR protein, these three single nucleotide polymorphisms potentially have a role in regulating the expression of the VDR gene. Another gene polymorphism, FokI (rs2228570), is located in exon 2, at a translation initiation site, and is anticipated to alter the structure of the encoded protein (Figure 1) (Shaikh et al., 2016).

Figure 1. Genomic region and exon-intron structure of the vitamin D receptor (VDR) gene. (The VDR gene is placed on human chromosome 12q13.11, contains nine exons and encompasses various single nucleotide polymorphisms (SNPs) including FokI (F/f), BsmI (B/b), ApaI (A/a), TaqI (T/t). The presence of a T/C transition polymorphism (ATG to ACG) at the first of two potential translation initiation sites in exon II).
Multiple studies have investigated the potential effect of VDR gene polymorphisms on susceptibility to TB; however, the results of these studies have been inconsistent. This inconsistency could be due to various factors, such as small sample sizes, insufficient power to detect associations between VDR gene polymorphisms and susceptibility to TB, the study design, the ethnicity of the study population, and the genetic context. A meta-analysis, a statistical technique that combines multiple results from previous studies to increase the statistical power and improve the precision of the estimation of pooled data (Blettner et al., 1999), could thus be a good option for analyzing inconsistent results.
Several meta-analyses have been conducted to identify the potential association between VDR gene polymorphisms and TB susceptibility over the past decades; however, larger pooled datasets are required to improve the power of effect estimates. Furthermore, few studies have been performed to uncover the impact of VDR gene polymorphisms based on different ethnic backgrounds. Therefore, we performed a comprehensive meta-analysis to (1) systematically evaluate the relationship between VDR gene polymorphisms, including FokI, BsmI, ApaI, TaqI, and TB susceptibility, (2) explore the potential effect of VDR gene polymorphisms on TB susceptibility in various ethnic groups.
Materials and methods
Study selection
The PubMed, Embase, and MEDLINE databases were searched for studies to include in this meta-analysis. The keyword used were: “VDR”, “Vitamin D receptor”, “tuberculosis”, “gene”, and “polymorphism.” The reference lists of the review articles were also manually searched for additional pertinent publications. The article search was conducted for articles published until 30 December 2022.
Inclusion and exclusion criteria
The literature was included based on the following criteria: i) case-control studies assessing the association between VDR gene polymorphisms and TB risk; ii) all participants in the studies confirmed to be negative for human immunodeficiency virus (HIV-negative), which could be examined in accordance with a certain diagnostic criterion of laboratory or antibody tests; iii) sufficient data on alleles and genotypes for the case and control groups provided to calculate the odds ratios (ORs) and 95% confidence intervals (CIs). The exclusion criteria were as follows: i) studies of control groups with gene distributions that deviated from the Hardy–Weinberg equilibrium (HWE) (Mayo, 2008); ii) low-quality studies (i.e., Newcastle–Ottawa Scale (NOS) scores (Stang, 2010) below 6); iii) review articles, abstracts, animal experiments, letters, editorials, case reports, and non-English publications.
Data extraction and quality assessment
According to the predetermined data extraction sheet, the following data were extracted independently by two researchers (R.S. Tao and S.J. Xiao): first authors’ names, year of publication, country of origin, ethnicity, the total number of participants in the case and control groups, genotype and allele frequencies in the case and/or control groups, mean or range of age, genotyping method, and TB type. In case of discrepancies, a third reviewer (L.P. Wang) concluded on the extracted data. For quality assessments, the NOS was used. Studies were stratified into two categories, specifically low quality (scores 0–5) and high quality (scores ≥6).
Statistical analysis
A chi-square test was used to assess the deviation from the HWE in terms of allele and genotype frequencies in the control groups. The strength of the association between VDR polymorphisms and TB susceptibility was evaluated by calculating the pooled OR and its 95% CI. Data were extracted to build different comparison genotype models for the polymorphisms (i.e., FokI, BsmI, ApaI, TaqI) of the VDR gene, as follows: i) FokI, allele model (f vs. F), dominant model (ff + Ff vs. FF), recessive model (ff vs. Ff + FF), homozygote model (ff vs. FF); ii) BsmI, allele model (b vs. B), dominant model (bb + Bb vs. BB), recessive model (bb vs. Bb + BB), homozygote model (bb vs. BB); iii) ApaI, allele model (a vs. A), dominant model (aa + Aa vs. AA), recessive model (aa vs. Aa + AA), homozygote model (aa vs. AA); iv) TaqI, allele model (t vs. T), dominant model (tt + Tt vs. TT), recessive model (tt vs. Tt + TT), homozygote model (tt vs. TT). Heterogeneity among studies was measured based on the Q statistic (a p-value with a significance level of 0.05) and the I2 statistic, which was used to quantify the inconsistency between study results. Commonly, a fixed-effects model for the pooled OR is used for a Q statistic with p > 0.05 and I2 < 50%. Otherwise, a random-effects model is used to combine the data if p ≤ 0.05 and I2 ≥ 50%. Subgroup analysis was performed to evaluate the source of heterogeneity from the perspective of ethnicity, and meta-regression analysis was performed to explore the potential sources of heterogeneity based on the publication year and ethnicity. The stability of our results was assessed using a sensitivity analysis, and potential publication bias was evaluated using funnel plots and Egger’s test (Egger et al., 1997). The data for this study were analyzed using R language programming software (version 4.2.3).
Results
Characteristics of the eligible studies
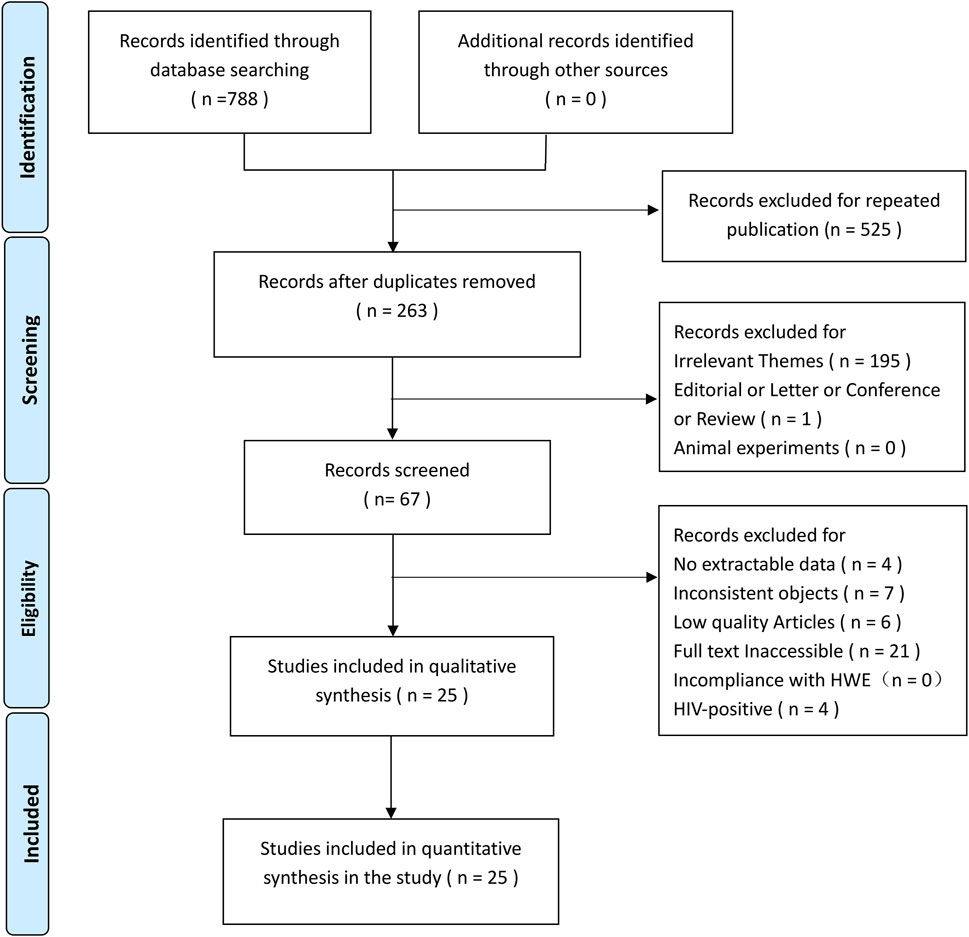
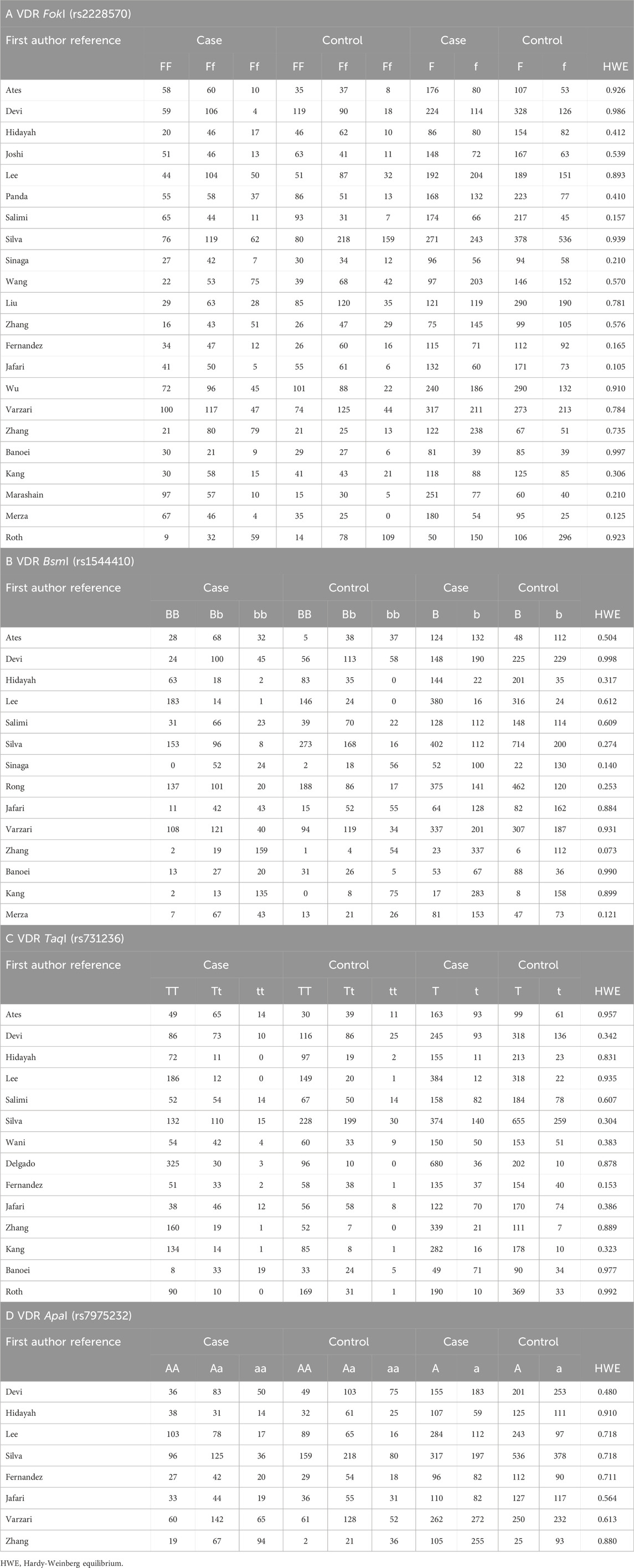
In total, 788 articles were identified via a systematic literature search of the PubMed, Embase, and MEDLINE databases. After screening using our inclusion and exclusion criteria, 25 eligible articles (Delgado et al., 2002; Liu et al., 2004; Roth et al., 2004; Merza et al., 2009; Banoei et al., 2010; Marashian et al., 2010; Zhang et al., 2010; Ates et al., 2011; Kang et al., 2011; Wu et al., 2013b; Joshi et al., 2014; Sinaga et al., 2014; Fernández-Mestre et al., 2015; Salimi et al., 2015; Jafari et al., 2016; Lee et al., 2016; Rong et al., 2017; Wang et al., 2017; Devi et al., 2018; Zhang et al., 2018; Panda et al., 2019; Silva-Ramírez et al., 2019; Hidayah et al., 2021; Varzari et al., 2021; Wani et al., 2021) were included (Figure 2: PRISMA flow diagram). Of these, 20 studies (Delgado et al., 2002; Liu et al., 2004; Merza et al., 2009; Banoei et al., 2010; Marashian et al., 2010; Zhang et al., 2010; Kang et al., 2011; Wu et al., 2013a; Joshi et al., 2014; Sinaga et al., 2014; Fernández-Mestre et al., 2015; Salimi et al., 2015; Lee et al., 2016; Rong et al., 2017; Wang et al., 2017; Devi et al., 2018; Zhang et al., 2018; Panda et al., 2019; Hidayah et al., 2021; Wani et al., 2021) were from Asia and the remaining five were from Europe (Varzari et al., 2021; Ates et al., 2011), North America (Silva-Ramírez et al., 2019), and South America (Jafari et al., 2016; Roth et al., 2004). The case and control groups consisted of 3,768 and 3,742 patients, respectively. Among these articles, 22 and eight studies provided information on the FokI and ApaI polymorphisms, respectively, and 15 studies provided information on the BsmI and TaqI polymorphisms. The NOS scores of the included studies ranged from 6 to 9. Tables 1, 2 summarize the basic characteristics of genotype and allele frequencies in the included studies, respectively.
Test of heterogeneity
No or low heterogeneity was detected for the ApaI polymorphism, which included allele (a vs. A, I2 = 23%, p = 0.25) homozygote (aa vs. AA, I2 = 13%, p = 0.33), recessive (aa vs. Aa + AA, I2 = 0%, p = 0.70), and dominant (aa + Aa vs. AA, I2 = 30%, p = 0.19) genotype models. Similarly, the TaqI polymorphism exhibited low heterogeneity in the recessive model (tt vs. Tt + TT, I2 = 33%, p = 0.12). Therefore, a fixed-effects model was applied to synthesize the OR for the ApaI polymorphism and a recessive model was used for the TaqI polymorphism. The remaining genotype models of VDR gene polymorphisms (i.e., FokI, BsmI, TaqI) were estimated to have substantial heterogeneity, indicating that a random-effects model could be applied to analyze the pooled OR.
Quantitative synthesis
Table 2 presents the results of the analysis. For the FokI VDR gene polymorphism, a significant association was observed with TB susceptibility in the allele model (f vs. F, OR = 1.235, 95%CI: 1.035–1.475, p = 0.019). A similar result was observed in the recessive model (ff vs. Ff + FF, OR = 1.317, 95%CI: 1.005–1.727, p = 0.046). However, no significant association with TB susceptibility was observed in the homozygote (ff vs. FF) and dominant (ff + Ff vs. FF) models. Regarding the other three VDR gene polymorphisms (BsmI, ApaI, TaqI), there was no evidence supporting a significant association between the four genotype models and TB susceptibility.
A subgroup analysis of ethnicity was performed according to the four genotype models of the VDR gene polymorphisms. In the Asian population, for the FokI polymorphism, high heterogeneity was observed in all genotype models. Therefore, to detect more specific source of the heterogeneity, we classified Asian ethnicity as Orang Indonesia, Indian, Han population, and Iranian according to the district of the study populations. There was low or no heterogeneity in all genotype models for Han population. As a result, a Fixed-effects model was suggested to pool ORs, which showed a significant association of the FokI polymorphism with TB susceptibility in the Han population. Similarly, there was evidence of a significant association with TB susceptibility in the dominant model (ff + Ff vs. FF) for Orang Indonesia (OR = 1.571, 95%CI = 1.002–2.462, Pheterogeneity = 0.25, I2 = 24%) and Indian (OR = 1.939, 95%CI = 1.488–2.528, Pheterogeneity = 0.35, I2 = 6%). However, there was no significant association of the homozygote model and recessive model with TB susceptibility for Iranian although low heterogeneity was observed. Furthermore, evidence of a significant association with TB susceptibility was found within three genotype models of the BsmI polymorphism in the Asian. One was a homozygote model (bb vs. BB) adopting a fixed-effects model to obtain a pooled OR = 1.751 (95%CI: 1.319–2.324) due to low heterogeneity (Pheterogeneity = 0.24, I2 = 21%). Another was the dominant model (bb + Bb vs. BB, Pheterogeneity < 0.01, I2 = 62%), in which it was a significant association with TB susceptibility in Indian (OR = 1.972, 95%CI = 1.351–2.878, generated from a fixed-effects model) with no heterogeneity (Pheterogeneity = 0.99, I2 = 0%). Besides, The result of the pooled ORs by a fixed-effects model displayed a significant association with TB in the allele model (b vs. B, OR = 1.265, 95%CI = 1.009–1.588, Pheterogeneity = 0.97, I2 = 0%) for Indian (Figures 3, 4). For the ApaI polymorphism, a significant association was found in the allele model (a vs. A, OR = 0.835, generated from a common-effects model, 95%CI: 0.712–0.979, Pheterogeneity < 0.32, I2 = 15%) and the homozygote model (aa vs. AA, OR = 0.702, generated from a common-effects model, 95%CI = 0.504–0.978, Pheterogeneity = 0.44, I2 = 0%) (Supplementary Figure S2).
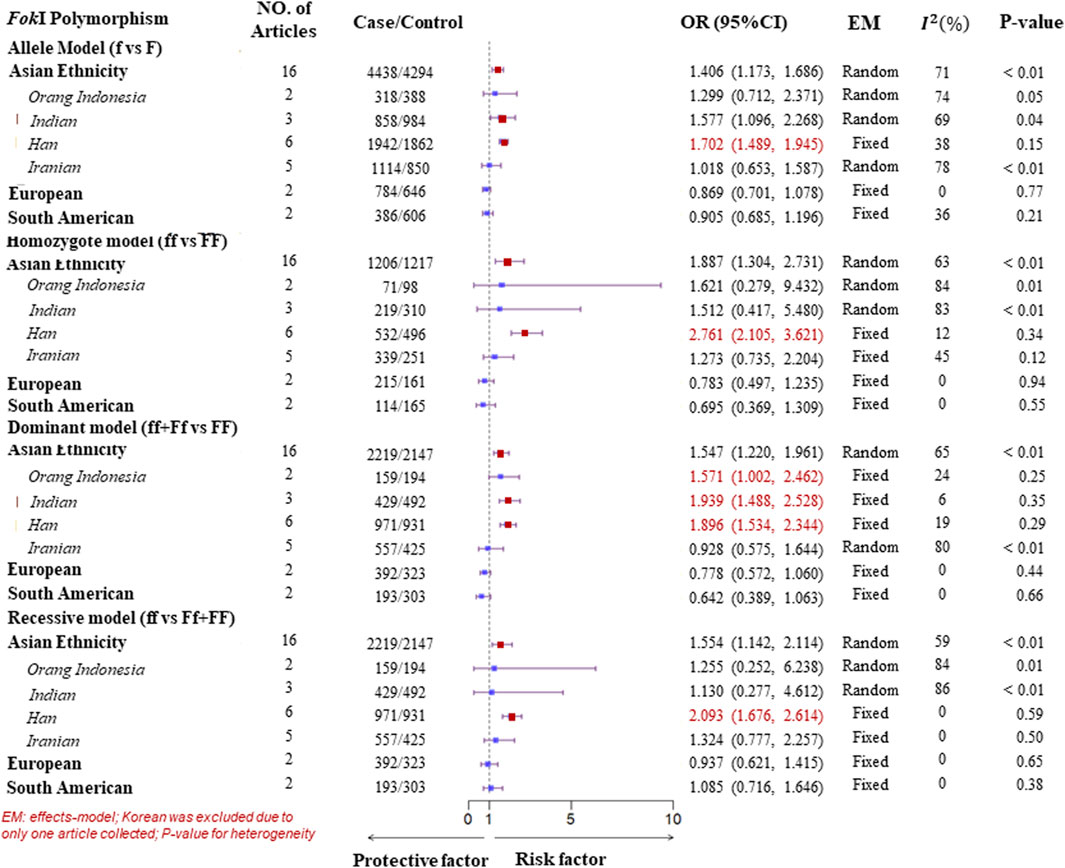
Figure 3. Subgroup analysis forest plot of four genotype models for the VDR gene FokI polymorphism on ethnicity.
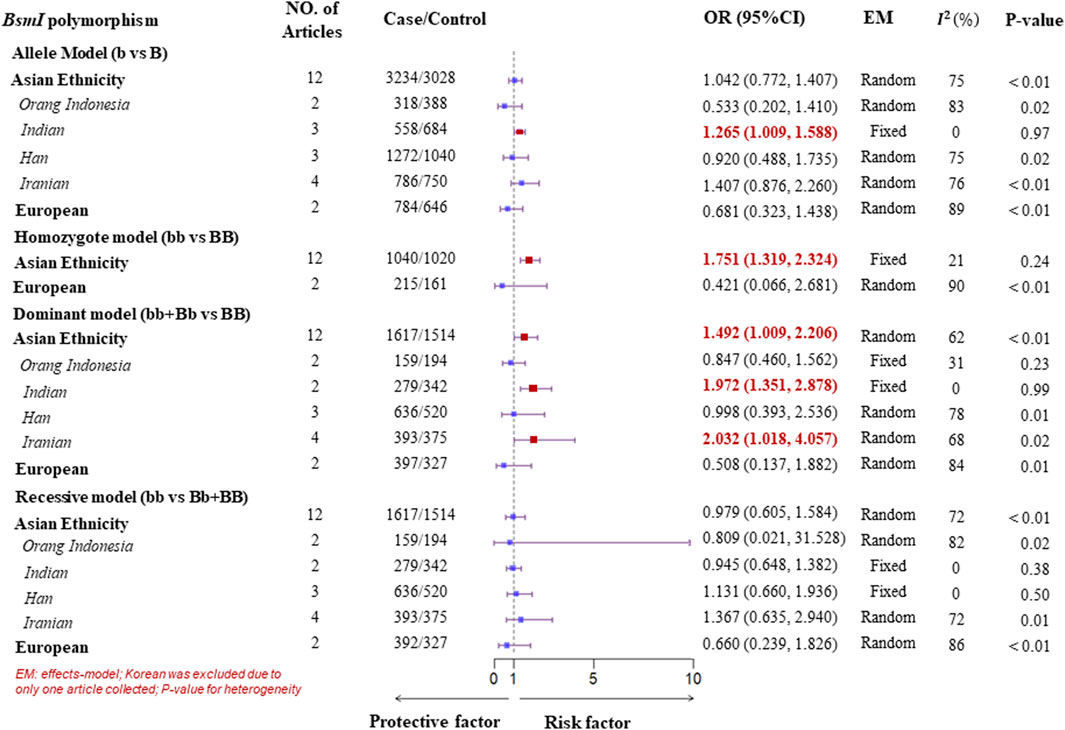
Figure 4. Subgroup analysis forest plot of four genotype models for the VDR gene BsmI polymorphism on ethnicity.
However, no significant association between VDR gene polymorphisms and TB susceptibility was found in the South American or European populations (Figures 3, 4; Supplementary Figures S1–4). The details of the pooled ORs, heterogeneity tests, and Egger’s test for publication bias are shown in Table 3.
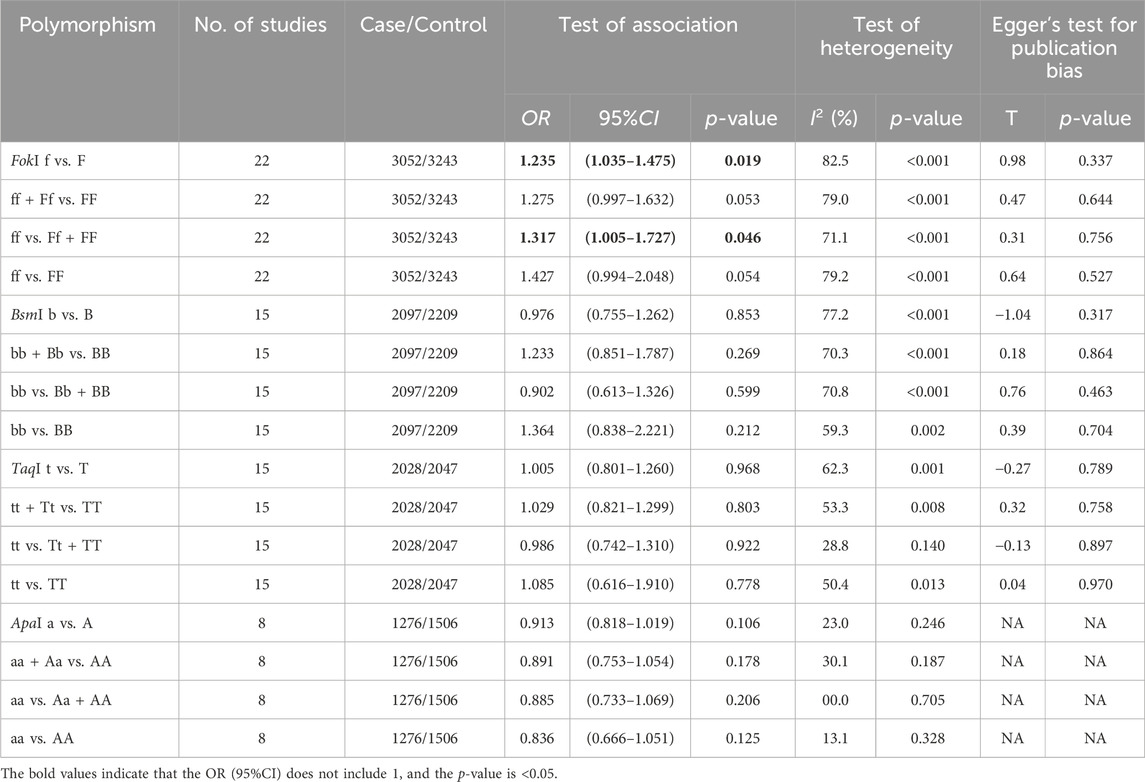
Table 3. The results of pooled ORs, test of heterogeneity and Egger’s test for publication bias in the four genotype models of VDR gene polymorphisms in the meta-analysis.
Sensitivity analysis
Sensitivity analysis was performed to evaluate the impact of an individual article on the pooled ORs using the leave-one-out method, which involves omitting a single article each time. The results indicated an obvious decrease in the heterogeneity and significance of the pooled OR within the homozygote model of the BsmI polymorphism after the deletion of one article (Jafari et al., 2016) (Supplementary Figure S5). Besides, no other individual article had a significant impact on the pooled OR or heterogeneity when omitted. This finding suggests that our results are relatively robust.
Publication bias
Publication bias was assessed using Egger’s test, which indicated no significant publication bias (p > 0.05) among in the included studies. Funnel plots were used to obtain the evidence of bias. No distinct asymmetry was found in the funnel plots, suggesting that there was no significant publication bias (Figure 5).
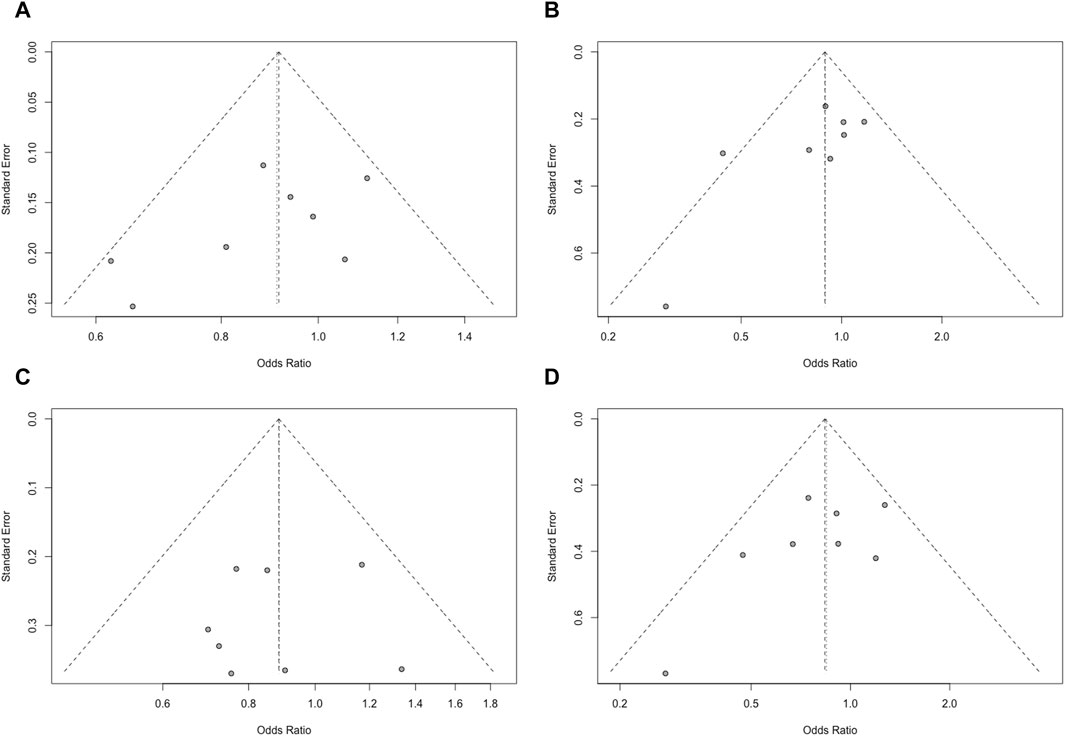
Figure 5. Funnel plot of the genotype models of the VDR gene ApaI polymorphism. [(A): allele (a vs A); (B): dominant (aa+Aa vs AA); (C): recessive (aa vs Aa+AA); (D): homozygote (aa vs AA)].
Meta-regression
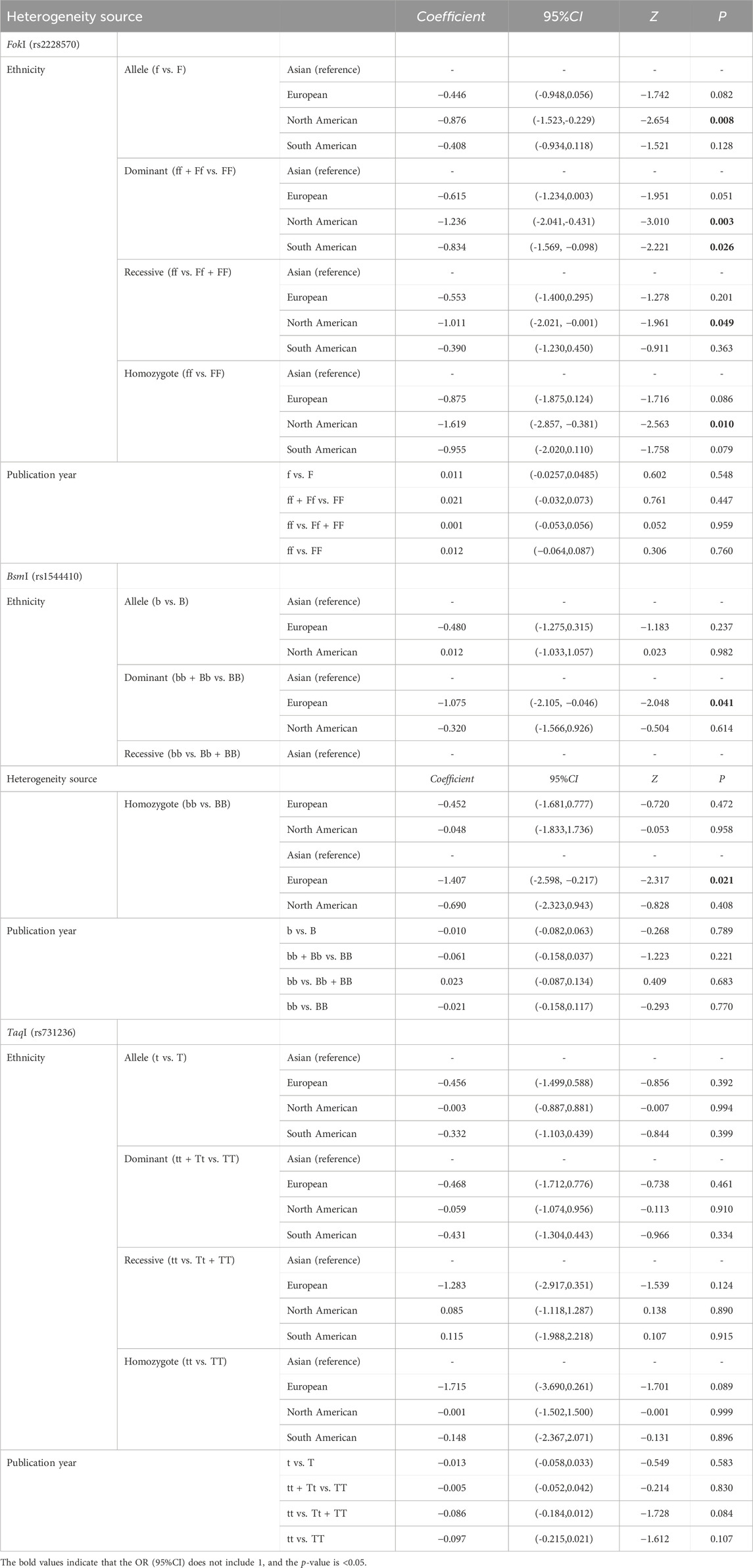
We finally performed a meta-regression analysis to explore the potential sources of heterogeneity among the VDR gene polymorphisms within the included articles. Our meta-regression analysis indicated that ethnicity could be a potential source of heterogeneity in the FokI and BsmI polymorphisms (i.e., within the homozygote and dominant models) of the VDR gene. However, the publication year was not the main source of heterogeneity. These details are presented in Table 4.
Discussion
The findings presented in this paper could provide clues for preventing TB from the perspective of vitamin D supplementation, which is a controversial topic in the field of medicine and health. In this meta-analysis, we pooled the results of 25 published articles to assess the association between various genotype models of VDR gene polymorphisms and TB susceptibility. We found that there was a significant association between an increased risk of developing TB and the allele (f vs. F) and recessive (ff vs. Ff + FF) models of the FokI polymorphism, whereas there was no evidence that the homozygote (ff vs. FF) and dominant (ff + Ff vs. FF) models were associated with TB risk. Further analysis based on Asian ethnicity revealed a significant association, in which all genotype models of the VDR FokI polymorphism contributed to the risk of developing TB in the Han population. It was observed there has likewise correlation for Orang Indonesia and Indian in the dominant model (ff + Ff vs. FF). However, a significant association between the ApaI polymorphism in VDR and a reduced risk of TB was found in the allele model (a vs. A) and the homozygote model (aa vs. AA). A possible reason for these inconsistent findings is that individuals are exposed to different environmental factors that could affect their genetic susceptibility to TB. However, further relevant studies are required to support this viewpoint.
Previous meta-analyses have evaluated the role of VDR gene polymorphisms in TB risk. Regarding the FokI polymorphism, some meta-analyses (Xu and Shen, 2019; Mohammadi et al., 2020) found no significant association between the FokI polymorphism and TB susceptibility. However, Cao et al. (2016) and Yadav et al. (2021) found evidence of an association in the homozygote (ff vs. Ff) and recessive (ff vs. Ff + FF) models. In addition, two meta-analyses (Chen et al., 2013; Huang et al., 2015) merely found that the f allele might contribute to the risk of TB in a recessive model (ff vs. Ff + FF), and our findings were consistent with this result. The FokI polymorphism, located in exon 2 at the translation initiation site of the VDR gene, produces two different receptor proteins. The F allele, linked to the expression of a shorter protein of 424 amino acids, displays higher transcriptional activity than another protein of 427 amino acids encoded by the f allele (Ruiz-Ballesteros et al., 2020). Therefore, the f allele of FokI could potentially decrease the activity of the VDR protein, thereby obstructing the interaction between active vitamin D and VDR, which might ultimately contribute to susceptibility to TB.
With respect to the BsmI polymorphism, no significant association was observed in this study. However, we found evidence to support an increased risk of TB in the homozygote model (bb vs. BB, OR = 1.751, 95%CI: 1.319–2.324) and the dominant model (bb + Bb vs. BB, OR = 1.492, 95%CI: 1.009–2.206) in Asian (Figure 4). More specific findings of Han population and Indian showed a significant association of the dominant model with the risk of TB. A similar meta-analysis performed by Wu et al. (2013a) demonstrated a significant association between the VDR gene BsmI polymorphism and a decreased TB risk within all four genotype models, and a similar association was found in Asians. One possible reason for the inconsistency in these findings is the lack of strict inclusion and exclusion criteria. For example, this previous study did not provide the criteria for excluding HIV-positive populations, as individuals with TB can be co-infected with this virus. Finally, the accuracy of a TB diagnosis is reduced in the HIV-positive population (Bell and Noursadeghi, 2018). Furthermore, a relevant assessment of the literature quality for case-control studies was not found in any previous study. Hence, low-quality articles could have generated biased results and might have further affected the pooled effects of the meta-analysis. Another reason could be the statistical power, which normally deviates with sample sizes; therefore, more relevant studies should be conducted in the future to examine our inconsistent results.
Regarding the association between the TaqI polymorphism in the VDR gene and the risk of TB, this association was not found within any of the four genotype models of the TaqI polymorphism Möller and Hoal, 2010. This is consistent with the results of a meta-analysis by Areeshi et al (Areeshi et al., 2017). A possible explanation for this is that the t allele of the VDR TaqI polymorphism is likely involved in the active disease process, whereas the variant does not act as a primary polymorphism with respect to TB infection. However, the TaqI polymorphism in the VDR gene was found to play a role in TB development in another meta-analysis performed by Xu and Shen (2019) in 2019; however, this meta-analysis did not exclude the HIV-positive population, which could have generated bias in terms of the pooled effect.
This meta-analysis had several strengths compared to previous studies. First, we performed the meta-analysis using relatively rigorous inclusion and exclusion criteria. Therefore, only high-quality articles including HIV-negative populations and those adhering to the HWE for gene distribution were eligible for the analysis. In other words, we avoided confounding factors that might have biased the pooled effect. Another strength is that we performed a meta-regression analysis of potential sources of heterogeneity among articles. Moreover, we anticipate performing more relevant analyses to explore other possible heterogeneity sources, such as the sample size or type of TB. However, our study has some limitations that should be acknowledged. First, despite the rigorous inclusion and exclusion criteria adhered to in this meta-analysis, our sample size was relatively small. Consequently, more studies with similar criteria are required to validate our pooled results. Second, in the present meta-analysis, we included articles published in English only, from three electronic databases (PubMed, Embase, and Medline). This could introduce a potential bias if studies in other languages or those indexed in other databases are missed. It should be noted that the impact of gene–environment interactions on the susceptibility to TB was also not considered in the present study. Furthermore, we anticipate performing Genome-wide association studies (GWAS) to identify a robust correlation between VDR gene polymorphisms and TB susceptibility in future research.
Conclusion
In summary, this meta-analysis adhered to strict inclusion and exclusion criteria to systematically evaluate the association between VDR gene polymorphisms and TB risk in the HIV-negative population. The FokI polymorphism was found to be associated with an increased risk of TB in the overall analysis. This indicates that the f allele could contribute more to TB risk than the F allele, particularly in Asians. However, the ApaI polymorphism was determined to play a protective role against TB. Further large-scale studies are required to classify the role of ethnicity and other potential factors in the relationship between VDR gene polymorphisms and TB susceptibility.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
RT: Conceptualization, Data curation, Formal Analysis, Investigation, Project administration, Writing–original draft, Writing–review and editing. SX: Conceptualization, Data curation, Formal Analysis, Investigation, Project administration, Writing–original draft, Writing–review and editing. LW: Conceptualization, Data curation, Formal Analysis, Investigation, Writing–original draft, Writing–review and editing. CH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–review and editing. HS: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–review and editing. RL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–review and editing. HL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–review and editing. WL: Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–review and editing, Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology. FH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–review and editing. JZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–review and editing. QL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by the Science and Technology Foundation of Guizhou Provincial Health Commission (grant number gzwkj2022-214); and the Education Science Planning Foundation of Guizhou Province (grant number 2022c025); and the “13th Five Year Plan” Science and Technology Project from the Education Department of Jilin Province (JJKH20200887KJ); and the Jilin Science and Technology Development Program Project: 20200404082YY; and the Guizhou Provincial Science and Technology Projects (Grant number ZK[2024]-134 general project); and the Science and Technology Fund Project of Guizhou Provincial Health Commission (Grant number gzwkj2025-435); and the Jilin Provincial Science and Technology Projects: 20220204072YY.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2024.1382957/full#supplementary-material
References
Areeshi, M. Y., Mandal, R. K., Wahid, M., Dar, S. A., Jawed, A., Lohani, M., et al. (2017). Vitamin D receptor ApaI (rs7975232) polymorphism confers decreased risk of pulmonary tuberculosis in overall and african population, but not in Asians: evidence from a meta-analysis. Ann. Clin. Lab. Sci. 47 (5), 628–637.
Ates, O., Dolek, B., Dalyan, L., Musellim, B., Ongen, G., and Topal-Sarikaya, A. (2011). The association between BsmI variant of vitamin D receptor gene and susceptibility to tuberculosis. Mol. Biol. Rep. 38 (4), 2633–2636. doi:10.1007/s11033-010-0404-8
Banoei, M. M., Mirsaeidi, M. S., Houshmand, M., Tabarsi, P., Ebrahimi, G., Zargari, L., et al. (2010). Vitamin D receptor homozygote mutant tt and bb are associated with susceptibility to pulmonary tuberculosis in the Iranian population. Int. J. Infect. Dis. 14 (1), e84–e85. doi:10.1016/j.ijid.2009.05.001
Bell, L. C. K., and Noursadeghi, M. (2018). Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat. Rev. Microbiol. 16 (2), 80–90. doi:10.1038/nrmicro.2017.128
Blettner, M., Sauerbrei, W., Schlehofer, B., Scheuchenpflug, T., and Friedenreich, C. (1999). Traditional reviews, meta-analyses and pooled analyses in epidemiology. Int. J. Epidemiol. 28 (1), 1–9. doi:10.1093/ije/28.1.1
Cao, Y., Wang, X., Cao, Z., and Cheng, X. (2016). Vitamin D receptor gene FokI polymorphisms and tuberculosis susceptibility: a meta-analysis. Arch. Med. Sci. 12 (5), 1118–1134. doi:10.5114/aoms.2016.60092
Chen, C., Liu, Q., Zhu, L., Yang, H., and Lu, W. (2013). Vitamin D receptor gene polymorphisms on the risk of tuberculosis, a meta-analysis of 29 case-control studies. PLoS One 8 (12), e83843. doi:10.1371/journal.pone.0083843
Delgado, J. C., Baena, A., Thim, S., and Goldfeld, A. E. (2002). Ethnic-specific genetic associations with pulmonary tuberculosis. J. Infect. Dis. 186 (10), 1463–1468. doi:10.1086/344891
Devi, K. R., Mukherjee, K., Chelleng, P. K., Kalita, S., Das, U., and Narain, K. (2018). Association of VDR gene polymorphisms and 22 bp deletions in the promoter region of TLR2Δ22 (-196-174) with increased risk of pulmonary tuberculosis: a case-control study in tea garden communities of Assam. J. Clin. Lab. Anal. 32 (7), e22562. doi:10.1002/jcla.22562
Dye, C., Scheele, S., Dolin, P., Pathania, V., and Raviglione, M. C. (1999). Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282 (7), 677–686. doi:10.1001/jama.282.7.677
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315 (7109), 629–634. doi:10.1136/bmj.315.7109.629
Fernández-Mestre, M., Villasmil, Á., Takiff, H., and Alcalá, Z. F. (2015). NRAMP1 and VDR gene polymorphisms in susceptibility to tuberculosis in Venezuelan population. Dis. Markers 2015, 860628. doi:10.1155/2015/860628
Ganmaa, D., Uyanga, B., Zhou, X., Gantsetseg, G., Delgerekh, B., Enkhmaa, D., et al. (2020). Vitamin D supplements for prevention of tuberculosis infection and disease. N. Engl. J. Med. 383 (4), 359–368. doi:10.1056/NEJMoa1915176
Hidayah, N., Djaharuddin, I., Ahmad, A., Natzir, R., Patellongi, I., Bukhari, A., et al. (2021). Association of vitamin D receptor polymorphism (rs2228570, rs1544410, rs7975232, and rs731236) and macrophage migration inhibitory factor-173 G/C (rs755622) with the susceptibility of active pulmonary tuberculosis in makassar, Indonesia. Open Access Macedonian J. Med. Sci. 9, 838–848. doi:10.3889/oamjms.2021.6859
Hillerdal, G. (2000). Environmental dangers: asbestos and tuberculosis. Respiration 67, (2), 134. doi:10.1159/000029498
Huang, L., Liu, C., Liao, G., Yang, X., Tang, X., and Chen, J. (2015). Vitamin D receptor gene FokI polymorphism contributes to increasing the risk of tuberculosis: an update meta-analysis. Med. Baltim. 94 (51), e2256. doi:10.1097/MD.0000000000002256
Jafari, M., Nasiri, M. R., Sanaei, R., Anoosheh, S., Farnia, P., Sepanjnia, A., et al. (2016). The NRAMP1, VDR, TNF-α, ICAM1, TLR2 and TLR4 gene polymorphisms in Iranian patients with pulmonary tuberculosis: a case-control study. Infect. Genet. Evol. 39, 92–98. doi:10.1016/j.meegid.2016.01.013
Joshi, L., Ponnana, M., Penmetsa, S. R., Nallari, P., Valluri, V., and Gaddam, S. (2014). Serum vitamin D levels and VDR polymorphisms (BsmI and FokI) in patients and their household contacts susceptible to tuberculosis. Scand. J. Immunol. 79 (2), 113–119. doi:10.1111/sji.12127
Kang, T. J., Jin, S. H., Yeum, C. E., Lee, S. B., Kim, C. H., Lee, S. H., et al. (2011). Vitamin D receptor gene TaqI, BsmI and FokI polymorphisms in Korean patients with tuberculosis. Immune Netw. 11 (5), 253–257. doi:10.4110/in.2011.11.5.253
L Bishop, E., Ismailova, A., Dimeloe, S., Hewison, M., and White, J. H. (2020). Vitamin D and immune regulation: antibacterial, antiviral, anti-inflammatory. JBMR Plus 5 (1), e10405. doi:10.1002/jbm4.10405
Lee, S. W., Chuang, T. Y., Huang, H. H., Liu, C. W., Kao, Y. H., and Wu, L. S. (2016). VDR and VDBP genes polymorphisms associated with susceptibility to tuberculosis in a Han Taiwanese population. J. Microbiol. Immunol. Infect. 49 (5), 783–787. doi:10.1016/j.jmii.2015.12.008
Liu, W., Cao, W. C., Zhang, C. Y., Tian, L., Wu, X. M., Habbema, J. D., et al. (2004). VDR and NRAMP1 gene polymorphisms in susceptibility to pulmonary tuberculosis among the Chinese Han population: a case-control study. Int. J. Tuberc. Lung Dis. 8 (4), 428–434.
Marashian, S. M., Farnia, P., Seyf, S., Anoosheh, S., and Velayati, A. A. (2010). Evaluating the role of vitamin D receptor polymorphisms on susceptibility to tuberculosis among iranian patients: a case-control study. Tuberk. Toraks 58 (2), 147–153.
Mayo, O. (2008). A century of Hardy-Weinberg equilibrium. Twin Res. Hum. Genet. 11 (3), 249–256. PMID: 18498203. doi:10.1375/twin.11.3.249
Merza, M., Farnia, P., Anoosheh, S., Varahram, M., Kazampour, M., Pajand, O., et al. (2009). The NRAMPI, VDR and TNF-alpha gene polymorphisms in Iranian tuberculosis patients: the study on host susceptibility. Braz J. Infect. Dis. 13 (4), 252–256. doi:10.1590/s1413-86702009000400002
Miyamoto, K., Kesterson, R. A., Yamamoto, H., Taketani, Y., Nishiwaki, E., Tatsumi, S., et al. (1997). Structural organization of the human vitamin D receptor chromosomal gene and its promoter. Mol. Endocrinol. 11 (8), 1165–1179. doi:10.1210/mend.11.8.9951
Mohammadi, A., Khanbabaei, H., Nasiri-Kalmarzi, R., Khademi, F., Jafari, M., and Tajik, N. (2020). Vitamin D receptor ApaI (rs7975232), BsmI (rs1544410), Fok1 (rs2228570), and TaqI (rs731236) gene polymorphisms and susceptibility to pulmonary tuberculosis in an Iranian population: a systematic review and meta-analysis. J. Microbiol. Immunol. Infect. 53 (6), 827–835. doi:10.1016/j.jmii.2019.08.011
Möller, M., and Hoal, E. G. (2010). Current findings, challenges and novel approaches in human genetic susceptibility to tuberculosis. Tuberc. (Edinb) 90 (2), 71–83. doi:10.1016/j.tube.2010.02.002
Nava-Aguilera, E., Andersson, N., Harris, E., Mitchell, S., Hamel, C., Shea, B., et al. (2009). Risk factors associated with recent transmission of tuberculosis: systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 13 (1), 17–26.
Newport, M. J., and Nejentsev, S. (2004). Genetics of susceptibility to tuberculosis in humans. Monaldi Arch. Chest Dis. 61 (2), 102–111. doi:10.4081/monaldi.2004.707
Panda, S., Tiwari, A., Luthra, K., Sharma, S. K., and Singh, A. (2019). Association of Fok1 VDR polymorphism with Vitamin D and its associated molecules in pulmonary tuberculosis patients and their household contacts. Sci. Rep. 9 (1), 15251. doi:10.1038/s41598-019-51803-8
Rong, H., Zhang, Q., and Zhang, Z. (2017). Host genetic effect on tuberculosis susceptibility in Chinese Uyghur. Front. Laboratory Med. 1 (1), 5–10. doi:10.1016/j.flm.2017.02.003
Roth, D. E., Soto, G., Arenas, F., Bautista, C. T., Ortiz, J., Rodriguez, R., et al. (2004). Association between vitamin D receptor gene polymorphisms and response to treatment of pulmonary tuberculosis. J. Infect. Dis. 190 (5), 920–927. doi:10.1086/423212
Ruiz-Ballesteros, A. I., Meza-Meza, M. R., Vizmanos-Lamotte, B., Parra-Rojas, I., and de la Cruz-Mosso, U. (2020). Association of vitamin D metabolism gene polymorphisms with autoimmunity: evidence in population genetic studies. Int. J. Mol. Sci. 21 (24), 9626. doi:10.3390/ijms21249626
Salimi, S., Farajian-Mashhadi, F., Alavi-Naini, R., Talebian, G., and Narooie-Nejad, M. (2015). Association between vitamin D receptor polymorphisms and haplotypes with pulmonary tuberculosis. Biomed. Rep. 3 (2), 189–194. doi:10.3892/br.2014.402
Shaikh, F., Baig, S., and Jamal, Q. (2016). Do VDR gene polymorphisms contribute to breast cancer? Asian Pac J. Cancer Prev. 17 (2), 479–483. doi:10.7314/apjcp.2016.17.2.479
Silva-Ramírez, B., Saenz-Saenz, C. A., Bracho-Vela, L. A., Peñuelas-Urquides, K., Mata-Tijerina, V., Escobedo-Guajardo, B. L., et al. (2019). Association between vitamin D receptor gene polymorphisms and pulmonary tuberculosis in a Mexican population. Indian J. Tuberc. 66 (1), 70–75. doi:10.1016/j.ijtb.2018.04.005
Sinaga, B. Y., Amin, M., Siregar, Y., and Sarumpaet, S. M. (2014). Correlation between Vitamin D receptor gene FOKI and BSMI polymorphisms and the susceptibility to pulmonary tuberculosis in an Indonesian Batak-ethnic population. Acta Med. Indones. 46 (4), 275–282.
Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25 (9), 603–605. doi:10.1007/s10654-010-9491-z
Varzari, A., Deyneko, I. V., Tudor, E., Grallert, H., and Illig, T. (2021). Synergistic effect of genetic polymorphisms in TLR6 and TLR10 genes on the risk of pulmonary tuberculosis in a Moldavian population. Innate Immun. 27 (5), 365–376. doi:10.1177/17534259211029996
Wang, G., Xie, L., Hu, J., Lu, H., Liu, X., Cao, Y., et al. (2017). Osteopontin, bone morphogenetic protein-4, and vitamin D receptor gene polymorphisms in the susceptibility and clinical severity of spinal tuberculosis. Cell Physiol. Biochem. 41 (5), 1881–1893. doi:10.1159/000471935
Wani, B. A., Shehjar, F., Shah, S., Koul, A., Yusuf, A., Farooq, M., et al. (2021). Role of genetic variants of Vitamin D receptor, Toll-like receptor 2 and Toll-like receptor 4 in extrapulmonary tuberculosis. Microb. Pathog. 156, 104911. doi:10.1016/j.micpath.2021.104911
WORLD HEALTH ORGANIZATION (2022). Global tuberculosis report2022[EB/OL]. Available at: https://www.who.int/publications/digital/global-tuberculosis-report-2021.
Wu, F., Zhang, W., Zhang, L., Wu, J., Li, C., Meng, X., et al. (2013a). NRAMP1, VDR, HLA-DRB1, and HLA-DQB1 gene polymorphisms in susceptibility to tuberculosis among the Chinese Kazakh population: a case-control study. Biomed. Res. Int. 2013, 484535. doi:10.1155/2013/484535
Wu, Y. J., Yang, X., Wang, X. X., Qiu, M. T., You, Y. Z., Zhang, Z. X., et al. (2013b). Association of vitamin D receptor BsmI gene polymorphism with risk of tuberculosis: a meta-analysis of 15 studies. PLoS One 8 (6), e66944. doi:10.1371/journal.pone.0066944
Xu, X., and Shen, M. (2019). Associations between vitamin D receptor genetic variants and tuberculosis: a meta-analysis. Innate Immun. 25 (5), 305–313. doi:10.1177/1753425919842643
Yadav, U., Kumar, P., and Rai, V. (2021). FokI polymorphism of the vitamin D receptor (VDR) gene and susceptibility to tuberculosis: evidence through a meta-analysis. Infect. Genet. Evol. 92, 104871. doi:10.1016/j.meegid.2021.104871
Zhang, H. Q., Deng, A., Guo, C. F., Wang, Y. X., Chen, L. Q., Wang, Y. F., et al. (2010). Association between FokI polymorphism in vitamin D receptor gene and susceptibility to spinal tuberculosis in Chinese Han population. Arch. Med. Res. 41 (1), 46–49. doi:10.1016/j.arcmed.2009.12.004
Keywords: tuberculosis, gene polymorphisms, VDR, vitamin D receptor, meta-analysis
Citation: Tao R, Xiao S, Wang L, Hu C, Suo H, Long R, Liu H, Luo W, Hong F, Zhao J and Li Q (2024) Association between vitamin D receptor gene polymorphisms and susceptibility to tuberculosis: a systematic review and meta-analysis. Front. Genet. 15:1382957. doi: 10.3389/fgene.2024.1382957
Received: 29 March 2024; Accepted: 29 July 2024;
Published: 20 August 2024.
Edited by:
John S. Spencer, Colorado State University, United StatesReviewed by:
Machao Li, National Institute for Communicable Disease Control and Prevention (China CDC), ChinaQing Li, Vanderbilt University Medical Center, United States
Copyright © 2024 Tao, Xiao, Wang, Hu, Suo, Long, Liu, Luo, Hong, Zhao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingjie Li, bHFqMTk4MTEwMDVAMTYzLmNvbQ==; Jingming Zhao, emptLTc4ODMzMTJAMTYzLmNvbQ==; Feng Hong, ZmhvbmdAZ21jLmVkdS5jbg==
†These authors have contributed equally to this work
 Rongshan Tao1†
Rongshan Tao1† Shujuan Xiao
Shujuan Xiao Feng Hong
Feng Hong Qingjie Li
Qingjie Li