- Department of Orthopedics of the Second Hospital of Jilin University, Changchun, Jilin, China
Background: Osteonecrosis of the femoral head (ONFH) is a prevalent and challenging orthopedic condition that often leads to hip pain and dysfunction. Long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs) have emerged as potent regulators of gene expression that influence both transcriptional and post-transcriptional processes in ONFH pathogenesis. This study aimed to investigate the association between dysregulated lncRNAs and circRNAs and their functions in ONFH.
Methods: We performed a systematic literature review of PubMed, MEDLINE, and Web of Science for all publicly available data. We included papers published before 17 April 2024, to evaluate the regulatory role and differential expression of lncRNAs and circRNAs in ONFH.
Results: Forty-four eligible studies were retrieved from PubMed, MEDLINE, and Web of Science, including 19 expression profiling studies, 19 gene studies, and six therapeutic studies. A total of 37 circRNAs and 42 lncRNAs were identified using quantitative real-time PCR (qRT-PCR). Dynamic changes in lncRNA and circRNA expression are associated with the proliferation and apoptosis of bone marrow stem cells (BMSCs), bone marrow endothelial cells (BMECs), and necrotic bone tissues in ONFH. CircHIPK3 and circHGF act as miRNA sponges to disrupt the osteogenic-adipogenic equilibrium, whereas lncRNA SNHG1 and GAS5 directly suppress osteogenesis. Notably, HOX transcript antisense intergenic RNA (HOTAIR), LncAABR07053481, Miat, and LINC00473 play significant roles in ameliorating the abnormal differentiation of BMSCs and could be promising therapeutic targets for ONFH.
Conclusion: This systematic review discusses the current understanding of the involvement of lncRNAs and circRNAs in ONFH pathogenesis. Despite these promising findings, the limitations include heterogeneity in the study design and insufficient in vivo validation. This work consolidates ncRNA-mediated pathways in ONFH, offering novel targets for early diagnosis and RNA-based therapies, while advocating standardized multi-omics approaches in future research.
1 Introduction
ONFH results in substantial labor loss because of its high disability rate (Cui et al., 2016). Newly diagnosed cases of ONFH have remained relatively constant, with approximately 20,000 to 30,000 people affected each year in the United States, primarily among young adults aged between 20 and 40 years (Moya-Angeler et al., 2015; George and Lane, 2022). The condition typically progresses, leading to intense pain; ultimately, total hip arthroplasty becomes necessary for treating the advanced stages of ONFH (Wang et al., 2014a). ONFH is a complex pathological process, in which the interplay between various intrinsic and extrinsic factors leads to lesions in the intramedullary microvasculature. Following thrombosis, the femoral head becomes undernourished, resulting in osteoclast death. Recent findings have indicated that epigenetic regulatory mechanisms in MSCs significantly influence steroid-induced ONFH (SONFH) (Huang et al., 2023). However, the mechanisms underlying ONFH progression remain largely unclear, hindering progress in the diagnosis and therapeutic intervention of early-stage ONFH. Elucidating the underlying mechanisms represents a significant research focus that can inform strategies for the prevention, diagnosis, and treatment of ONFH.
Non-coding RNAs (ncRNAs) are vital regulatory factors in numerous biological processes such as cell death and disease pathogenesis (Liu et al., 2020). Studies have shown that ncRNAs, such as circular RNAs (circRNAs) and long non-coding RNAs (lncRNAs), are involved in the modulation of apoptosis, RNA pathways, and cell death regulation (Su et al., 2016). It is noteworthy that approximately 1.5% of the human genome is dedicated to protein-coding regions, with a vast expanse of the remaining sequences transcribed into non-coding RNAs that lack the capacity for protein coding (Lander, 2011). Although the contributions of ncRNAs to ONFH progression are increasingly being recognized owing to advances in high-throughput sequencing technologies and corresponding analytical methods (Wang A. et al., 2018; Xiang et al., 2020), a thorough evaluation of the relevant literature is yet to be conducted. Accordingly, we present an overview of the role of ncRNAs (especially circRNAs and lncRNAs) in the pathogenesis of non-traumatic ONFH.
2 Methods
2.1 Literature search protocol and search strategy
A systematic search of PubMed, MEDLINE, and Web of Science was performed for all publicly available data from the inception of the databases until 17 April 2024. The keywords and MeSH terms in our search strategy were the following keywords and combinations: “(lncRNA OR long non-coding RNA OR long ncRNA OR circular RNA OR circRNA OR ciRNA) AND (avascular necrosis OR aseptic necrosis OR osteonecrosis) AND (femoral head)”. We did not impose restrictions on the year of publication, publication status, or language, and we did not limit our inclusion to any specific study design; randomized and non-randomized clinical trials, cohort studies, and case-control studies were all considered. Additionally, we screened all articles referenced in the selected studies to identify the relevant literature. The study selection process was independently conducted by two authors (Wang and Lin), and any discrepancies were resolved through discussion.
2.2 Inclusion criteria and exclusion criteria
The inclusion criteria for this study were as follows: (a) investigations that obtained lncRNA and/or circRNA expression in patients with ONFH; (b) studies utilizing bone tissue, serum, plasma, or blood samples from individuals with ONFH; and (c) studies conducted on animals or ONFH cell lines. The exclusion criteria were as follows: (a) systematic reviews or meta-analyses, (b) studies without quantitative real-time PCR (qRT-PCR) to measure the expression of lncRNAs and/or circRNAs, and (c) correspondence to editors, technical notes, opinion-based studies, and single case reports. Abstracts from conferences that did not provide grouping information or sample sizes were excluded, as were those representing full-text articles already included in this study.
2.3 Data extraction and synthesis
Two authors (FFL and SCZ) independently evaluated the eligibility of the full-text articles and gathered essential information in a standardized format. lncRNA and circRNA data were obtained from the GEO database, microarray, and high-throughput sequencing (RNA-seq) datasets. The regulatory functions of lncRNAs and circRNAs were also considered. Other important information included the type of sample (bone tissue, serum, and plasma), the direction of lncRNA/circRNA expression (upregulated or downregulated), target miRNAs, and target mRNAs. Any inconsistencies in the data were addressed through discussions with a third review author (QYW).
3 Results
3.1 Summary of investigations into the dysregulated lncRNAs and circRNAs in ONFH
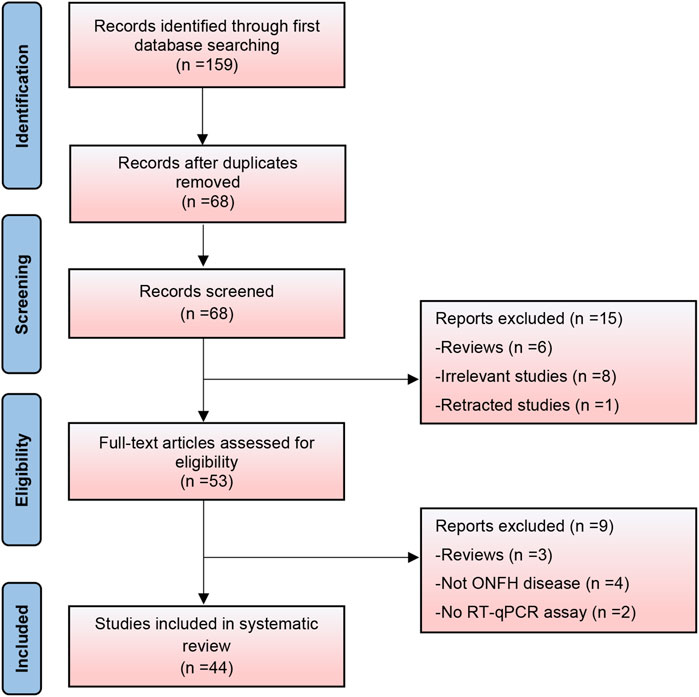
Based on the search strategy, 159 relevant studies were initially included. After removing duplicate records, 68 studies were analyzed. Following a thorough review of titles and abstracts, 15 studies were deemed ineligible and excluded. A comprehensive full-text review was conducted on the remaining 53 records, leading to the exclusion of an additional nine studies that failed to meet the predetermined inclusion criteria. Finally, 44 studies were included in this systematic review (Figure 1). It encompassed 19 expression profiling studies, 19 specific gene studies, and 6 therapeutic studies. Apart from two studies that reported the downregulation of lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) and one study that reported its upregulation, our systematic review identified 37 circular RNAs (26 upregulated and 11 downregulated) and 41 long non-coding RNAs (20 upregulated and 21 downregulated). Thirty-one studies were related to steroid-induced ONFH, two were associated with alcohol-induced ONFH, one with trauma-induced ONFH, four with non-traumatic ONFH (no details provided), and six did not specify the type of ONFH.
3.2 CircRNAs expression profiles in ONFH
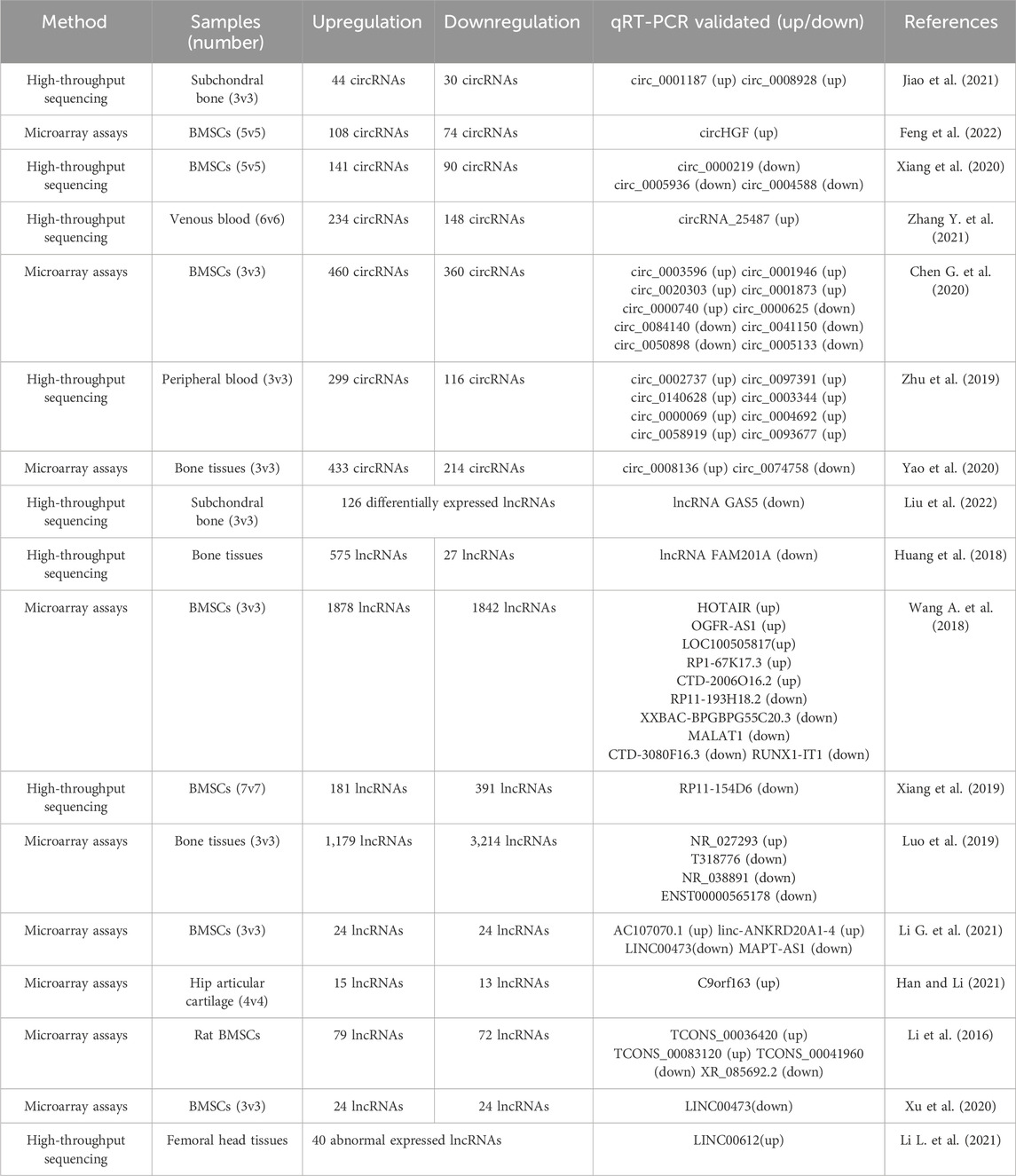
Seven studies, which included the validation of 27 circRNAs by qRT-PCR, revealed differentially expressed circRNAs in patients with ONFH. Of these, 18 circRNAs were upregulated and nine were downregulated (Table 1). These included four high-throughput sequencing studies and three microarray assays. Three studies focused on the differentially expressed circRNAs in bone marrow stem cells (BMSCs) of ONFH patients, two studies investigated the circRNA expression profile in the bone tissues of ONFH patients, and two studies focused on circRNA expression in peripheral blood.
Subchondral bone alterations have been reported to involve a cascade of gene expression changes, signaling pathway modifications, microarchitectural shifts, and histopathological variations that culminate in structural transformations across the entire joint (Zhang et al., 2012; Li et al., 2013; Chen et al., 2017). Regarding ONFH, although related research is limited, previous studies have characterized the degeneration of the hip articular cartilage associated with ONFH (Magnussen et al., 2005; Sonoda et al., 2017; Chen et al., 2019). Histological alterations in articular cartilage, including chondrocyte depletion, surface fibrillation, subchondral bone thickening, and elevated osteoclastic activity, can lead to differential gene expression within the articular cartilage (Im et al., 2000; Wang et al., 2014b; Liu et al., 2016). This suggests that these structural alterations are not merely physical deformities but also reflect underlying molecular shifts, indicating a more complex interplay between cartilage degradation and genetic regulation. Despite extensive research, the precise pathogenesis of ONFH in the subchondral bone remains elusive. Consequently, the subchondral bone is a significant area of study that provides an opportunity to directly reflect localized pathological changes associated with the disease.
Using next-generation sequencing, 74 differentially expressed circRNAs and 121 differentially expressed mRNAs were identified in the subchondral bone of patients with ONFH compared with the intertrochanteric region of patients with femur fractures (Jiao et al., 2021). The protein-protein interaction (PPI) network analysis revealed mRNAs with high connectivity degrees, including Collagen type I alpha 1 (COL1A1), Collagen type I alpha 2 (COL1A2), bone gamma carboxyglutamate protein (BGLAP), specificity protein-7(SP7), matrix metalloprotease 9 (MMP9), secreted protein acidic and rich in cysteine (SPARC) and insulin-like growth factor 1(IGF1). The interactions examined in the subchondral bone of ONFH patients play critical roles. circ_0000551 was proposed as a competing endogenous RNA (ceRNA) of hsa-miR-526b-5p and miR-6809-5p, which targets anoctamin-5 (ANO5). circ _0008928 and circ_0003915 have been proposed as ceRNAs of miR-150-5p, miR-500b-3p, and miR-619-5p, which target chromatin-modified protein 4C (CHMP4C). circ _0008928 acts as a sponge for miR-3605-5p by targeting the integrin-binding sialoprotein (IBSP) gene. However, these ceRNA mechanisms have not been empirically validated.
One study of the expression profiles in the osteonecrotic zone versus the normal zone of ONFH revealed that 647 circRNAs were differentially regulated, with 433 circRNAs highly expressed and 214 exhibiting low expression (Han et al., 2022). circ_0058122 expression was significantly elevated in dexamethasone (Dex)-treated human umbilical vein endothelial cells (HUVECs). Dex-induced apoptosis in HUVECs was reduced when circ_0058122 was silenced, whereas it increased in cells overexpressing hsa_circ_0058122. Furthermore, hsa_circ_0058122 functioned as a ceRNA for hsa-miR-7974, which targets and interacts with insulin-like growth factor binding protein 5 (IGFBP5).
3.3 Regulatory role of CircRNAs in BMSCs of ONFH
Steroid-induced endothelial dysfunction disrupts the blood supply to the femoral head, leading to the progressive upregulation of osteoclast-related proteins and localized bone tissue ischemia and necrosis (Maruyama et al., 2018; Wang Q. et al., 2018; Chen G. et al., 2020). The degradation of bone cells, coupled with an imbalance between osteogenic and osteoclastic activities, ultimately leads to deterioration and collapse of the bone structure (Piuzzi et al., 2019). Disruption of the BMSC differentiation equilibrium indicates a significant pathological alteration in this process (Powell et al., 2011; Chang et al., 2020). BMSCs with heightened adipogenic potential not only lose their regenerative capabilities but also result in the catastrophic accumulation of adipocytes and elevated internal bone pressure in the femoral head, thereby worsening the advancement of SONFH.
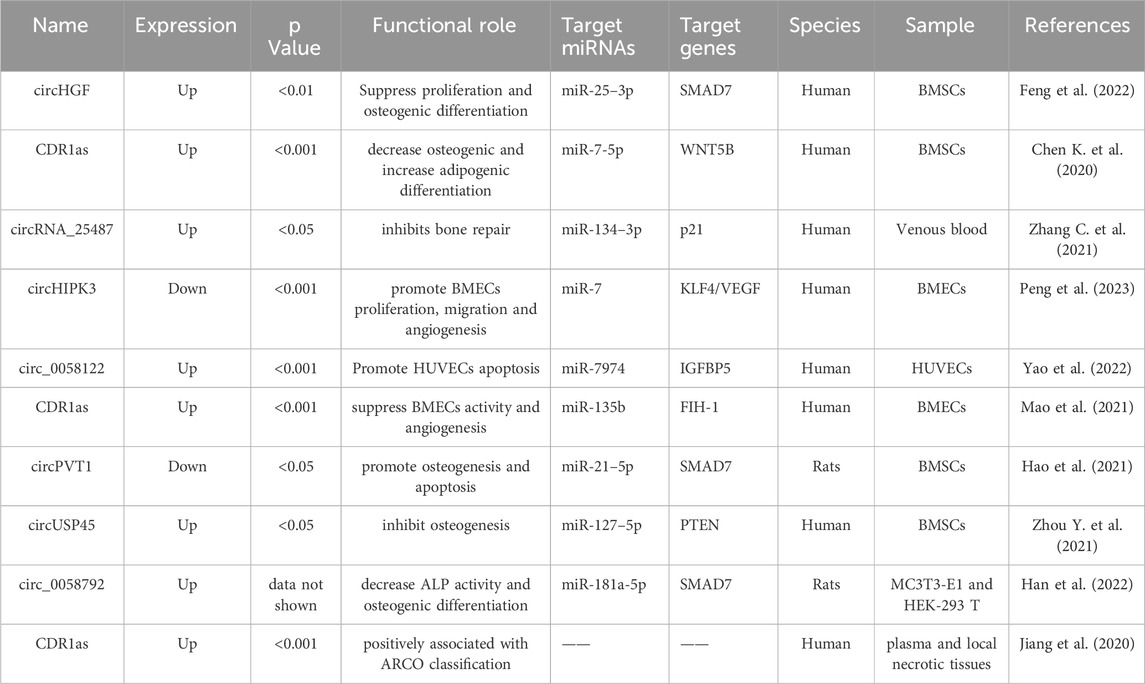
As shown in Table 2 and Figure 2, four circRNAs (circPVT1, circUSP45, circHGF, and CDR1as) regulated BMSCs. These circRNAs primarily affect cell proliferation and osteogenic differentiation. One study identified 182 differentially expressed circRNAs in ONFH-BMSCs, of which 108 were upregulated and 74 were downregulated (Feng et al., 2022). Additionally, among the upregulated circRNAs, circHGF was shown to suppress both the proliferation and osteogenic differentiation of BMSCs in ONFH by targeting the miR-25-3p/SMAD7 axis. Chen K. et al. (2020) screened circRNAs using microarray assays and identified 820 differentially expressed circRNAs in SONFH BMSCs. These included 460 upregulated and 360 downregulated circRNAs. Additionally, they found that the circRNA CDR1as was overexpressed in SONFH-BMSCs, which led to decreased osteogenic differentiation through the CDR1as-miR-7-5p-WNT5B axis.
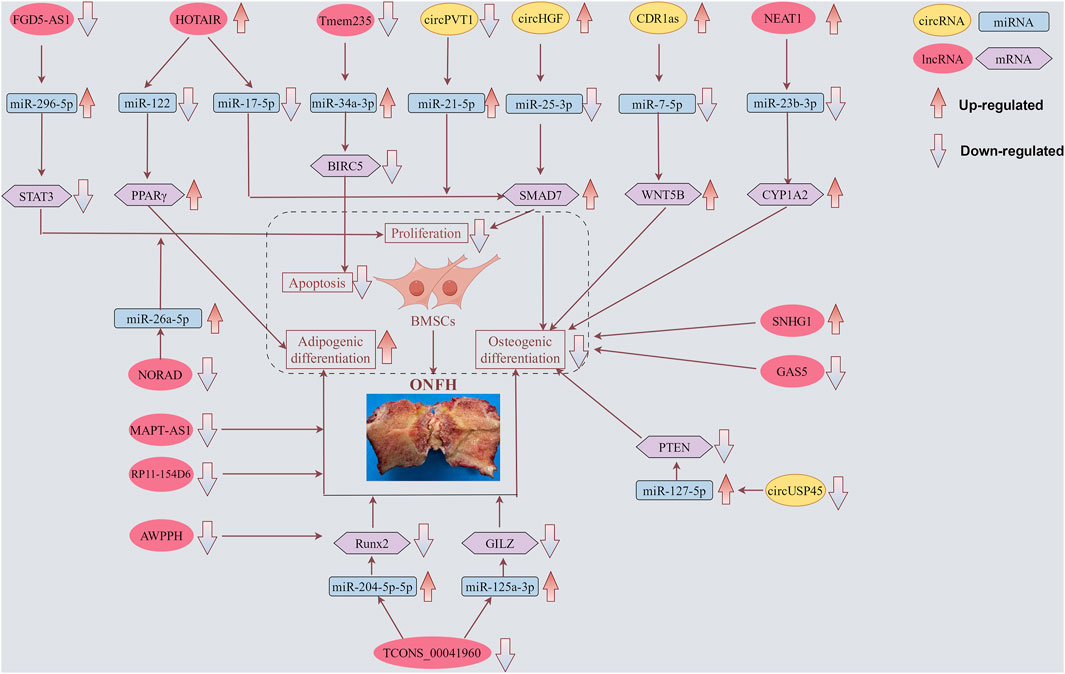
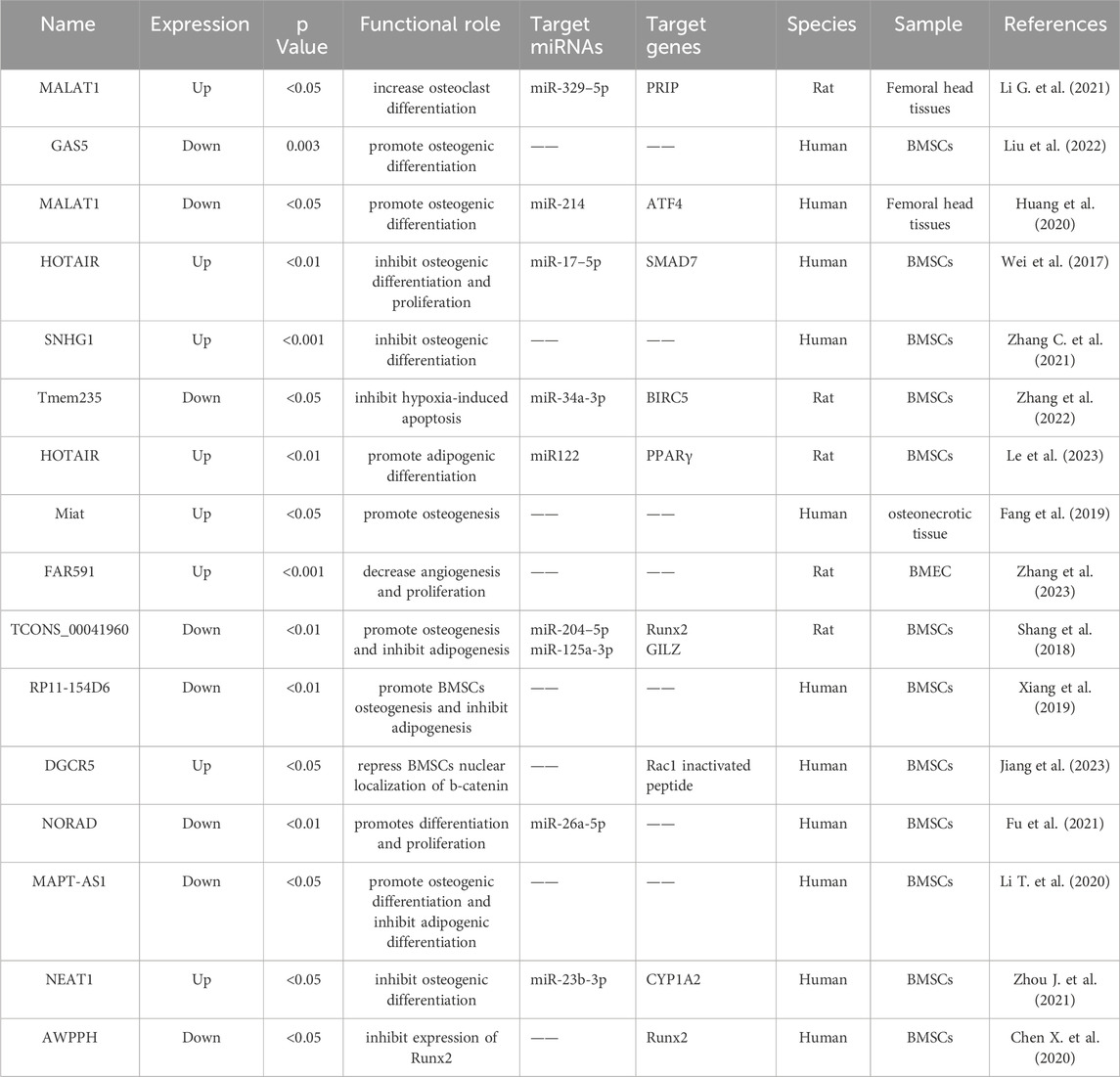
Figure 2. Functions of specific circRNAs and lncRNAs in proliferation, apoptosis, adipogenic and osteogenic differentiation of BMSCs. LncRNAs FGD5-AS1, HOTAIR, Tmem235, NEAT1, NORAD, and TCONS_00041960, as well as circRNAs CDR1as, circPVT1, circHGF, and circUSP45, regulate BMSCs through corresponding miRNAs, thereby contributing to the progression of ONFH. Meanwhile, lncRNAs MAPT-AS1 and RP11-154D6 directly promote adipogenic differentiation, whereas lncRNAs SNHG1 and GAS5 directly inhibit osteogenic differentiation. The yellow box symbolizes circRNA. The red box symbolizes lncRNA. The blue box symbolizes miRNA. The purple box symbolizes mRNA.
In one study, the circRNA expression profile of ONFH-BMSCs was determined using high-throughput sequencing. This analysis revealed 231 differentially expressed circRNAs, of which 141 were upregulated and 90 were downregulated. Of these, 215 circRNAs were derived from exonic regions, with only 16 originating from intronic or intergenic regions (Xiang et al., 2020). Intriguingly, the present study confirmed the time-dependent expression patterns of circ_0000219, circ_0004588, circ_0005936, miR-144-3p, and miR-1270 during osteogenic and adipogenic differentiation of BMSCs.
In addition to investigating the expression profiles of ONFH-BMSCs, studies have also been conducted on the regulatory roles and functions of specific circRNAs. The upregulation of circ_0066523 observed during the osteogenic induction of BMSCs indicated its role in promoting proliferation and differentiation by epigenetically silencing phosphatase and tensin homolog (PTEN), thereby activating the AKT pathway (Xin et al., 2021). This discovery paves the way for the identification of therapeutic targets relevant to osteoblast differentiation disorders such as ONFH.
3.4 Regulatory role of CircRNAs in BMECs of ONFH
Recent studies indicate that glucocorticoid-induced dysfunction of BMECs may result in changes in the microcirculation of the femoral head, potentially contributing significantly to the development of SONFH (Kerachian et al., 2009). BMECs modulate apoptosis during angiogenesis. An inverse relationship has been reported among angiogenic function, vascular integrity, and the extent of apoptosis. Prolonged exposure to glucocorticoids has been linked to the inhibition of angiogenesis, induction of cell apoptosis, and impairment of endothelial cell function (O'Connell and Genest Jr, 2001; Williams et al., 2006; Zhang et al., 2016). Previous research has indicated a decline in the angiogenic potential of BMECs coupled with an elevated propensity for apoptosis in patients with SONFH (Yu et al., 2020). Considering that ONFH involves compromised blood supply to the femoral head, circRNAs that govern angiogenesis could be critical. Altered expression of these circRNAs may impede the development of new blood vessels, subsequently affecting the delivery of essential nutrients and oxygen to the bone tissue.
Circular RNA homeodomain-interacting protein kinase 3 (circHIPK3) is a prevalent circular RNA that functions as a miRNA sponge, thereby modulating cellular processes, including angiogenesis, proliferation, migration, and apoptosis (Fu and Sun, 2021). CircHIPK3 is also involved in several pathophysiological processes, including fibrosis, tumorigenesis, and vascular endothelial damage (Zhou J. et al., 2021). In patients with ONFH, circHIPK3 expression was downregulated in necrotic tissue (M–W U test, U = 0, p < 0.001). Upregulation of circHIPK3 augmented the proliferative, migratory, and angiogenic capabilities of BMECs, while reducing their rate of apoptosis. However, these effects were reversed upon introduction of the miR-7 mimic (Peng et al., 2023).
In addition to its function in BMSCs, the influence of circCDR1as on angiogenesis in ONFH-associated BMECs has also been explored. The migration of BMECs was significantly enhanced in the circCDR1as silencing group compared to that in the negative control group. Furthermore, transfection with circCDR1as plasmids upregulated the protein expression of hypoxia inducible factor 1 (FIH-1) (p < 0.05), and conversely, decreased the expression of hypoxia inducible factor-1α (HIF-1α) and vascular endothelial growth factor (VEGF) compared to the NC group (p < 0.05) (Mao et al., 2021).
3.5 Regulatory role of plasma circRNAs in ONFH
The role of plasma circRNAs in ONFH is an emerging area of research that aims to elucidate the molecular mechanisms underlying this debilitating condition. In previous studies, significant alterations in specific mRNAs such as MMP9, Alpha-2-Macroglobulin, and CXC motif chemokine ligand 12 (CXCL12)/stromal cell-derived factor 1 (SDF-1) were detected in the serum of patients with ONFH (Ghale-Noie et al., 2018; Zheng et al., 2022; Li et al., 2023). These modifications in gene expression are instrumental in evaluating the severity of ONFH. However, studies focusing on circRNAs in the serum of ONFH patients are scarce.
Studies of plasma and local circRNA expression have garnered significant interest. This study enrolled ninety-nine patients diagnosed with nontraumatic ONFH and ninety-nine healthy individuals. Elevated expression of circCDR1as was observed in both the plasma and local necrotic tissues using RT-qPCR (Jiang et al., 2020). Additionally, this study suggests a positive correlation between the expression of both plasma and local circCDR1as and the Association Research Circulation Osseous (ARCO) classification system. Receiver operating characteristic (ROC) curve analysis implied that plasma levels of circCDR1as could serve as a potential biomarker for monitoring radiographic progression in patients with non-traumatic ONFH.
3.6 Research on lncRNA expression profiles in ONFH
Ten studies, which included the validation of 31 lncRNAs by qRT-PCR, revealed differentially expressed lncRNAs in patients with ONFH. Of these, 15 lncRNAs were upregulated, and 16 were downregulated (Table 1). These included four high-throughput sequencing studies and six microarray assays. Five studies focused on the differentially expressed lncRNAs in the BMSCs of patients with ONFH, and five studies investigated the lncRNA expression profile in the bone or cartilage tissues of patients with ONFH.
Using high-throughput RNA sequencing, Liu et al. (2022) detected the expression levels of lncRNAs and mRNAs in subchondral bone samples obtained from three patients diagnosed with ONFH and three with femoral neck fractures (FNF). A total of 126 and 959 differentially expressed lncRNAs and genes, respectively, were identified. More importantly, the expression of lncRNA GAS5 was strongly correlated with the osteogenic differentiation of BMSCs. This association was further substantiated by the notable downregulation of GAS5 in the subchondral trabecular bone tissue of patients diagnosed with ONFH and in ONFH rat models. The observed downregulation was statistically significant, as indicated by a log2FoldChange value of less than −1 and a p-value of 0.003. This study posits that lncRNA GAS5 could serve as an osteogenic biomarker for ONFH, offering a potential target for the early diagnosis and molecular therapy of ONFH. Huang et al. (2018) identified 575 upregulated and 27 downregulated lncRNAs in ONFH samples using RNA sequencing. The lncRNA FAM201A was significantly downregulated in ONFH samples compared with that in FNF samples, and the expression levels of FAM201A were found to be correlated with the progression of ONFH. Xiang et al. (Xiang et al., 2019) identified 181 upregulated and 391 downregulated lncRNAs in BMSCs derived from patients with steroid-induced ONFH using RNA sequencing analysis. Notably, the expression of lncRNA RP11-154D6 was significantly reduced in BMSCs from patients with ONFH. This reduction was associated with the promotion of osteogenic differentiation and inhibition of adipogenic differentiation in BMSCs.
In one of our previous studies utilizing microarray analysis (Wang A. et al., 2018), we reported that 1878 lncRNAs were significantly upregulated, whereas 1842 lncRNAs demonstrated statistically significant downregulation in BMSCs from patients with SONFH compared to the control group. The coding-non-coding co-expression (CNC) network and ceRNA network analyses indicated that lncRNA RP11-193H18.2, metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), and HOX transcript antisense intergenic RNA (HOTAIR) were associated with abnormal osteogenic and adipogenic differentiation of BMSCs in patients with steroid-induced SONFH. However, these regulatory relationships were not experimentally validated in this study. Additionally, using microarray analysis, Luo et al. (2019) revealed that there were 1,179 upregulated and 3,214 downregulated lncRNAs in the bone tissues of steroid-induced ONFH. The upregulated lncRNAs (NR_027293) and downregulated lncRNAs (T318776, NR_038891, and ENST00000565178) in the ONFH group were validated by RT-qPCR. Li T. et al. (2020) identified 24 downregulated and 24 upregulated lncRNAs in the BMSCs of patients with ONFH using microarray analysis. Among these dysregulated lncRNAs, the overexpression of MAPT antisense RNA 1 (MAPT-AS1) was observed to enhance osteogenesis while suppressing adipogenesis in BMSCs, as evidenced at both the cellular and mRNA levels.
3.7 Regulatory role of lncRNAs in BMSCs of ONFH
The role of lncRNAs in ONFH BMSCs is an intricate area of research that is yet to be fully understood. The significance of lncRNAs in the epigenetic regulation of BMSCs is becoming increasingly apparent as they potentially influence both osteogenic and adipogenic differentiation (Suh et al., 2005; Sheng et al., 2007; Xu et al., 2022). Dysregulation of these long non-coding transcripts may contribute to the development and progression of ONFH by altering normal cellular pathways that maintain bone health and remodeling. Further investigations into the specific mechanisms by which lncRNAs affect the differentiation of BMSCs may offer significant insights into the pathogenesis of ONFH. This may facilitate the development of innovative therapeutic strategies targeting these molecular pathways.
As shown in Table 3 and Figure 2, 11 lncRNAs (3 upregulated and 8 downregulated) have been reported to regulate BMSCs. These lncRNAs primarily affect cell proliferation, apoptosis, osteogenic differentiation, and lipogenic differentiation. HOTAIR, the first identified trans-acting lncRNA, has been observed to exhibit abnormally high expression levels in various tumor tissues and cell lines. These cancers include gastric cancer, breast cancer, hepatic carcinoma, ovarian cancer, and acute myeloid leukemia (Wu et al., 2014). Using microarray analysis, Xing et al. identified HOTAIR expression in osteoarthritic cartilage. Furthermore, its expression was significantly higher than that in normal cartilage samples (Xing et al., 2014). Wei et al. confirmed that HOTAIR suppressed miR-17-5p expression to regulate osteogenic differentiation and proliferation by interacting with miR-17-5p and SMAD7 (Wei et al., 2017). Le et al. (2023) demonstrated that the HOTAIR/miR-122/PPARγ signaling pathway mediated alcohol-induced ONFH in a rat model. Additionally, they found that sustained downregulation of miR-122 expression was responsible for the ongoing progression of alcohol-induced ONFH, even after cessation of alcohol intake.
In the context of ONFH, BMSCs are affected by various factors that can lead to reduced proliferation and increased apoptosis, contributing to the development and progression of this debilitating condition (Qi and Zeng, 2015; Wu F. et al., 2019). For instance, decreased proliferation of BMSCs can limit the ability of the bone to repair itself, leading to a weakened bone structure and potential collapse of the femoral head. Concurrently, an increase in BMSC apoptosis diminishes the pool of viable cells essential for bone regeneration. Dex suppresses the proliferation of human BMSCs and promotes apoptosis in a dose-dependent manner. Overexpression of FGD5-AS1 promoted cell proliferation and suppressed apoptosis in hBMSCs treated with Dex. Furthermore, FGD5-AS1 functions as a molecular sponge of miR-296-5p. Additionally, miR-296-5p directly targets the signal transducer and activator of transcription 3 (STAT3). Importantly, both miR-296-5p and STAT3 modulated the effects of lncRNA FGD5-AS1 on hBMSCs treated with Dex (Wu et al., 2022). These findings highlight the complex interplay between lncRNAs, miRNAs, and signaling pathways in regulating the response of hBMSCs to glucocorticoid treatment, which could have implications for therapeutic strategies targeting bone health and repair.
3.8 Regulatory role of lncRNAs in BMECs of ONFH
LncRNAs expressed in BMECs play a pivotal role in angiogenesis, which is crucial for maintaining the integrity of the femoral head. Angiogenesis, the process of forming new blood vessels from pre-existing ones, plays a crucial role in supplying essential nutrients and oxygen to the bone tissue (Li Z. et al., 2020). Dysregulated lncRNA expression in BMECs can impair angiogenesis, potentially leading to bone death, which is a defining characteristic of ONFH. Furthermore, glucocorticoids (GCs) have been found to induce apoptosis in BMECs and impede vascular regeneration (Zuo et al., 2020; Huang et al., 2021). Such occurrences precipitate dysfunction in bone microcirculation, thereby disrupting the synchrony between bone microcirculation and osteogenesis. This chain of events culminates in osteogenic damage and the onset of osteonecrosis.
Characterization of the expression profiles of lncRNAs in ONFH-BMECs is important. Zhang et al. (2023) conducted lncRNA/mRNA microarray and bioinformatics analyses using a model of GC-induced apoptosis in BMECs. A total of 105 lncRNAs exhibiting concentration-dependent expression changes owing to GC were identified, of which 46 were upregulated and 59 were downregulated. Among these, FAR591 was markedly upregulated during GC-induced BMEC apoptosis and femoral head necrosis (p < 0.001). Targeted deletion of FAR591 effectively abrogated GC-induced apoptosis of BMECs, thereby mitigating the deleterious effects of GCs on the femoral head microcirculation and inhibiting the pathogenesis and progression of steroid-induced ONFH.
3.9 Regulatory role of plasma lncRNAs in ONFH
Plasma lncRNAs have been identified as potential biomarkers for ONFH diagnosis and prognosis. The expression profiles of lncRNAs in the plasma of patients with ONFH differed significantly from those of healthy controls, suggesting their involvement in disease pathogenesis. Further research is necessary to elucidate the role of plasma lncRNAs in ONFH and their clinical utility as ONFH biomarkers.
Initially, MALAT1 was identified as a significant predictor of lung cancer (Ji et al., 2003). Previous studies have revealed that MALAT1 is significantly downregulated during compromised osteogenic differentiation of BMSCs in patients with steroid-induced ONFH. Furthermore, MALAT1 is capable of safeguarding human osteoblasts from dexamethasone-induced damage by engaging the Ppm1e-AMPK signaling pathway (Fan et al., 2018; Wang Q. et al., 2018). In SONFH, MALAT1 enhances osteogenic differentiation by modulating the activation of transcription factor 4 (ATF4) through the sequestration of miR-214 (Huang et al., 2020). Jin et al. (2021) further confirmed the significant downregulation of MALAT1 in both plasma and femoral head necroses. Notably, 104 patients with non-traumatic ONFH and 100 healthy controls were included in this study. The authors suggested that reduced levels of serum and local MALAT1 expression could potentially reflect disease severity in patients with nontraumatic ONFH.
AWPPH is a recently identified lncRNA that plays an oncogenic role in the development of hepatocellular and bladder cancers (Zhao et al., 2017; Zhu et al., 2018). Chen X. et al. (2020) were the first to report that AWPPH expression was significantly downregulated in patients with non-traumatic ONFH compared to healthy controls, as observed in both BMSCs and serum samples. Importantly, the authors proposed that AWPPH might contribute to ONFH development by enhancing the expression of Runx2.
3.10 Regulatory role of lncRNAs in ONFH therapy
In addition to the aforementioned investigations, six studies explored the treatment of ONFH by modulating lncRNAs. Two studies have reported that neohesperidin ameliorates ONFH by regulating the lncRNAs SNHG1 and HOTAIR (Yuan et al., 2020; Zhang C. et al., 2021). The other four studies investigated the regulation of lncRNAs (LncAABR07053481, Miat, and LINC00473) on abnormal differentiation of BMSC, which alleviates avascular necrosis (Fang et al., 2019; Xu et al., 2021; 2022; Wang et al., 2023). LINC00473 was found to be significantly downregulated in BMSCs and exhibited a protective role against dexamethasone-induced apoptosis by modulating the PEBP1/Akt/Bad/Bcl-2 signaling pathway (Xu et al., 2020; 2021). Transplantation of polylactic-co-glycolic acid (PLGA) hydrogels loaded with rat-derived BMSCs modified by LINC00473 significantly enhanced bone repair and regeneration in the necrotic region of the femoral head in a SONFH rat model (Xu et al., 2022). This discovery highlights the potential of LINC00473 as a molecular mediator with therapeutic relevance in mitigating glucocorticoid-induced osteonecrosis.
Huo Xue Tong Luo capsule (HXTL), a traditional Chinese medicinal formulation, has been found to significantly mitigate pain and halt the progression of necrosis, leading to short-to mid-term joint collapse. This effect results in a decreased rate of joint collapse, reduced need for total hip arthroplasty (THA), and fewer symptoms compared with the natural history of ONFH (Wei et al., 2019). Furthermore, a chromatin immunoprecipitation (ChIP) assay revealed that the HXTL capsule significantly enhanced the enrichment of H3K27me3 and diminished the presence of H3K4me3 in the promoter regions of lncRNA-miat (Fang et al., 2019). This indicates that the HXTL capsule suppressed lncRNA-miat expression through histone modification. Taken together, the HXTL capsule may foster osteogenesis to improve ONFH, at least in part, by suppressing the transcriptional expression of Miat.
4 Discussion
The refinement of microarray assays and high-throughput sequencing detection technologies, coupled with advanced data analysis techniques, ensures high readability of data (Shoemaker et al., 2001; Salzman et al., 2012; Zhao et al., 2020). This enables the swift retrieval of information from an extensive array of samples, facilitating their extensive use across diverse sectors, including genetic studies, pharmacodynamics, and disease diagnosis and treatment. In recent years, research involving high-throughput sequencing and gene chips pertinent to ONFH has increased, significantly propelling investigations into the etiology of the disease and unveiling the critical regulatory functions of lncRNAs. This systematic review elucidates the emerging roles of circRNAs and lncRNAs in ONFH, a significant orthopedic condition with a complex etiology. ONFH is characterized by bone cell death due to ischemia, which leads to structural failure and articular surface collapse if left untreated. Despite extensive research, the molecular mechanisms underlying ONFH remain unclear, hindering the development of targeted therapies.
Our findings highlight that circRNAs and lncRNAs are not merely transcriptional byproducts but also play critical regulatory roles in the cellular processes implicated in ONFH. CircRNAs arise from the splicing of one or two exons. This splicing event is characterized by the covalent bonding of the 3′and 5′ends, leading to the formation of a covalently closed, continuous loop. This unique structure sets circRNAs apart from other RNA species and contributes to their stability in cells (You et al., 2015; Chen et al., 2016; Greene et al., 2017). The functional roles of circRNAs are likely intertwined with their distinctive stabilities. Notably, previous studies have suggested that circRNAs can function as miRNA sponges, sequester miRNAs, or interact with functional proteins to regulate specific biological processes at either the transcriptional or post-transcriptional level (Hansen et al., 2013; Memczak et al., 2013; Müller and Appel, 2017). In this review, circPVT1, circUSP45, circHGF, and CDR1as were shown to regulate BMSC differentiation by acting as sponges.
LncRNAs can act as epigenetic regulators by binding to DNA or modifying histones, altering the chromatin structure, and affecting gene expression patterns (Xia et al., 2014; Shields et al., 2019). This may lead to the dysregulation of genes involved in bone metabolism and vascular health, contributing to bone tissue necrosis. HOTAIR, FGD5-AS1, Tmem235, NEAT1, NORAD, and TCONS_00041960 function as ceRNAs and influence the expression of genes related to osteogenesis and adipogenesis, key processes implicated in ONFH. LncRNAs can modulate signaling pathways that are essential for maintaining bone homeostasis, such as the Wnt/β-catenin, Hedgehog, and Notch pathways (Wu X. et al., 2019; Ma et al., 2021; Zhang X. et al., 2021; Zhao et al., 2023). The dysfunction of these pathways due to aberrant lncRNA activity can disrupt the equilibrium between bone formation and resorption, leading to the onset of ONFH.
The implications of these findings are twofold. First, they expand our understanding of ONFH beyond the traditional risk factors and provide a foundation for exploring novel therapeutic targets. Targeted interventions aimed at modulating specific circRNAs or lncRNAs could offer more precise and effective treatment options than the current empirical approaches. Second, given the unique expression patterns of these RNA species, they hold promise as diagnostic tools for the early detection and monitoring of ONFH, potentially enabling personalized medicine strategies. The dysregulated circRNAs and lncRNAs identified here hold promise as noninvasive biomarkers for early ONFH detection. Furthermore, RNA-targeted therapies such as antisense oligonucleotides or nanoparticle-delivered mimics can restore the balance of BMSC differentiation, offering alternatives to invasive surgical interventions.
5 Conclusion
Currently, advancements in the life sciences are deeply interconnected with the development of biodetection technologies. Over the past decade, extensive research has revealed significant roles of lncRNAs and circRNAs in numerous diseases. LncRNAs and circRNAs perform intricate regulatory functions in ONFH, suggesting their potential as novel diagnostic and therapeutic targets. Future research is needed to systematically identify these RNA molecules and their interaction networks, elucidate their specific mechanisms in the development of ONFH, and evaluate their effectiveness as biomarkers or therapeutic tools. However, it is also evident that there is a paucity of studies on the therapeutic effects of lncRNAs and circRNAs in ONFH, which should indeed constitute a significant focus for future research. Current studies are constrained by the small sample size, heterogeneity in ONFH subtypes, and the lack of in vivo validation of the proposed ceRNA networks. Future studies should prioritize the use of multicenter cohorts and functional assays. The conclusion now acknowledges translational barriers, such as RNA stability in clinical samples and off-target effects of RNA-based therapies. Further research is expected to provide more personalized and precise medical solutions for patients with ONFH.
Author contributions
FL: Conceptualization, Data curation, Investigation, Software, Writing – original draft. SZ: Data curation, Formal Analysis, Software, Writing – original draft. MY: Conceptualization, Data curation, Formal Analysis, Investigation, Writing – review and editing. QW: Conceptualization, Data curation, Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from Department of Science and Technology of Jilin Province (No. YDZJ202201ZYTS278, YDZJ202501ZYTS051) and scientific research project of Education Department of Jilin Province (No. JJKH20221070KJ).
Acknowledgments
The regulatory pattern diagrams (Figure 2) were crafted by Figdraw.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Chang, C., Greenspan, A., and Gershwin, M. E. (2020). The pathogenesis, diagnosis and clinical manifestations of steroid-induced osteonecrosis. J. Autoimmun. 110, 110102460. doi:10.1016/j.jaut.2020.102460
Chen, G., Wang, Q., Li, Z., Yang, Q., Liu, Y., Du, Z., et al. (2020). Circular RNA CDR1as promotes adipogenic and suppresses osteogenic differentiation of BMSCs in steroid-induced osteonecrosis of the femoral head. Bone 133, 133115258. doi:10.1016/j.bone.2020.115258
Chen, G., Zhong, L., Wang, Q., Li, Z., Shang, J., Yang, Q., et al. (2019). The expression of chondrogenesis-related and arthritis-related genes in human ONFH cartilage with different Ficat stages. PeerJ 7e6306 7, e6306. doi:10.7717/peerj.6306
Chen, K., Liu, Y., He, J., Pavlos, N., Wang, C., Kenny, J., et al. (2020). Steroid-induced osteonecrosis of the femoral head reveals enhanced reactive oxygen species and hyperactive osteoclasts. Int. J. Biol. Sci. 16 (11), 1888–1900. doi:10.7150/ijbs.40917
Chen, X., Han, P., Zhou, T., Guo, X., Song, X., and Li, Y. (2016). circRNADb: a comprehensive database for human circular RNAs with protein-coding annotations. Sci. Rep. 6, 634985. doi:10.1038/srep34985
Chen, X., Li, J., Liang, D., Zhang, L., and Wang, Q. (2020). LncRNA AWPPH participates in the development of non-traumatic osteonecrosis of femoral head by upregulating Runx2. Exp. Ther. Med. 19 (1), 153–159. doi:10.3892/etm.2019.8185
Chen, Y., Lin, S., Sun, Y., Guo, J., Lu, Y., Suen, C. W., et al. (2017). Attenuation of subchondral bone abnormal changes in osteoarthritis by inhibition of SDF-1 signaling. Osteoarthr. Cartil. 25 (6), 986–994. doi:10.1016/j.joca.2017.01.008
Cui, L., Zhuang, Q., Lin, J., Jin, J., Zhang, K., Cao, L., et al. (2016). Multicentric epidemiologic study on six thousand three hundred and ninety five cases of femoral head osteonecrosis in China. Int. Orthop. 40 (2), 267–276. doi:10.1007/s00264-015-3061-7
Fan, J. B., Zhang, Y., Liu, W., Zhu, X. H., Xu, D. W., Zhao, J. N., et al. (2018). Long non-coding RNA MALAT1 protects human osteoblasts from dexamethasone-induced injury via activation of PPM1E-AMPK signaling. Cell. Physiol. Biochem. 51 (1), 31–45. doi:10.1159/000495159
Fang, B., Li, Y., Chen, C., Wei, Q., Zheng, J., Liu, Y., et al. (2019). Huo Xue Tong Luo capsule ameliorates osteonecrosis of femoral head through inhibiting lncRNA-Miat. J. Ethnopharmacol. 238, 238111862. doi:10.1016/j.jep.2019.111862
Feng, X., Xiang, Q., Jia, J., Guo, T., Liao, Z., Yang, S., et al. (2022). CircHGF suppressed cell proliferation and osteogenic differentiation of BMSCs in ONFH via inhibiting miR-25-3p binding to SMAD7. Mol. Ther. Nucleic Acids 28, 2899–3113. doi:10.1016/j.omtn.2022.02.017
Fu, D., Yang, S., Lu, J., Lian, H., and Qin, K. (2021). LncRNA NORAD promotes bone marrow stem cell differentiation and proliferation by targeting miR-26a-5p in steroid-induced osteonecrosis of the femoral head. Stem Cell. Res. Ther. 12 (1), 18. doi:10.1186/s13287-020-02075-x
Fu, Y., and Sun, H. (2021). Biogenesis, cellular effects, and biomarker value of circHIPK3. Cancer Cell Int. 21 (1), 256. doi:10.1186/s12935-021-01956-2
George, G., and Lane, J. M. (2022). Osteonecrosis of the femoral head. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 6 (5), e21.00176. doi:10.5435/JAAOSGlobal-D-21-00176
Ghale-Noie, Z. N., Hassani, M., Kachooei, A. R., and Kerachian, M. A. (2018). High serum alpha-2-macroglobulin level in patients with osteonecrosis of the femoral head. Arch. Bone Jt. Surg. 6 (3), 219–224.
Greene, J., Baird, A. M., Brady, L., Lim, M., Gray, S. G., McDermott, R., et al. (2017). Circular RNAs: biogenesis, function and role in human diseases. Front. Mol. Biosci. 438, 38. doi:10.3389/fmolb.2017.00038
Han, N., and Li, Z. (2021). Non-coding RNA identification in osteonecrosis of the femoral head using competitive endogenous RNA network analysis. Orthop. Surg. 13 (3), 1067–1076. doi:10.1111/os.12834
Han, N., Qian, F., Niu, X., and Chen, G. (2022). Circ_0058792 regulates osteogenic differentiation through miR-181a-5p/Smad7 axis in steroid-induced osteonecrosis of the femoral head. Bioengineered 13 (5), 12807–12822. doi:10.1080/21655979.2022.2074617
Hansen, T. B., Jensen, T. I., Clausen, B. H., Bramsen, J. B., Finsen, B., Damgaard, C. K., et al. (2013). Natural RNA circles function as efficient microRNA sponges. Nature 495 (7441), 384–388. doi:10.1038/nature11993
Hao, Y., Lu, C., Zhang, B., Xu, Z., Guo, H., and Zhang, G. (2021). CircPVT1 up-regulation attenuates steroid-induced osteonecrosis of the femoral head through regulating miR-21-5p-mediated Smad7/TGFβ signalling pathway. J. Cell. Mol. Med. 25 (10), 4608–4622. doi:10.1111/jcmm.16294
Huang, C., Qing, L., Xiao, Y., Tang, J., and Wu, P. (2023). Insight into steroid-induced ONFH: the molecular mechanism and function of epigenetic modification in mesenchymal stem cells. Biomolecules 14 (1), 4. doi:10.3390/biom14010004
Huang, G., Zhao, G., Xia, J., Wei, Y., Chen, F., Chen, J., et al. (2018). FGF2 and FAM201A affect the development of osteonecrosis of the femoral head after femoral neck fracture. Gene 652, 65239–65247. doi:10.1016/j.gene.2018.01.090
Huang, X. Z., Huang, J., Li, W. Z., Wang, J. J., Song, D. Y., and Ni, J. D. (2020). LncRNA-MALAT1 promotes osteogenic differentiation through regulating ATF4 by sponging miR-214: implication of steroid-induced avascular necrosis of the femoral head. Steroids 154108533 154, 108533. doi:10.1016/j.steroids.2019.108533
Huang, Z., Wang, Q., Zhang, T., Fu, Y., and Wang, W. (2021). Hyper-activated platelet lysates prevent glucocorticoid-associated femoral head necrosis by regulating autophagy. Biomed. and Pharmacother. 139, 139111711. doi:10.1016/j.biopha.2021.111711
Im, G. I., Kim, D. Y., Shin, J. H., Cho, W. H., and Lee, C. J. (2000). Degeneration of the acetabular cartilage in osteonecrosis of the femoral head: histopathologic examination of 15 hips. Acta Orthop. Scand. 71 (1), 28–30. doi:10.1080/00016470052943847
Ji, P., Diederichs, S., Wang, W., Böing, S., Metzger, R., Schneider, P. M., et al. (2003). MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 22 (39), 8031–8041. doi:10.1038/sj.onc.1206928
Jiang, B., Zhu, S. H., Zeng, J. Y., and Mao, Z. (2020). Plasma and local expressions of CircRNA CDR1as are linked with disease severity in patients with non-traumatic osteonecrosis of femoral head. J. Orthop. Surg. Res. 15 (1), 592. doi:10.1186/s13018-020-02129-z
Jiang, W., Chen, Y., Sun, M., Huang, X., Zhang, H., Fu, Z., et al. (2023). LncRNA DGCR5-encoded polypeptide RIP aggravates SONFH by repressing nuclear localization of β-catenin in BMSCs. Cell Rep. 42 (8), 112969. doi:10.1016/j.celrep.2023.112969
Jiao, M., Tian, R., Liu, G., Liu, X., Wei, Q., Yan, J., et al. (2021). Circular RNA and messenger RNA expression profile and competing endogenous RNA network in subchondral bone in osteonecrosis of the femoral head. DNA Cell Biol. 40 (1), 61–69. doi:10.1089/dna.2020.5894
Jin, Y., Zhu, H. X., and Wei, B. F. (2021). Reduced serum and local LncRNA MALAT1 expressions are linked with disease severity in patients with non-traumatic osteonecrosis of the femoral head. Technol. Health Care 29 (3), 479–488. doi:10.3233/THC-202244
Kerachian, M. A., Séguin, C., and Harvey, E. J. (2009). Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action. J. Steroid Biochem. Mol. Biol. 114 (3-5), 121–128. doi:10.1016/j.jsbmb.2009.02.007
Lander, E. S. (2011). Initial impact of the sequencing of the human genome. Nature 470 (7333), 187–197. doi:10.1038/nature09792
Le, G., Lu, M., Li, L., and Luo, H. (2023). The Lnc-HOTAIR/miR122/PPARγ signaling mediated the occurrence and continuous development of alcohol-induced Osteonecrosis of the femoral head. Toxicol. Lett. 380, 38053–38061. doi:10.1016/j.toxlet.2023.04.002
Li, G., Ji, F., Guo, W., and Wei, B. (2023). Decreased serum MMP-9 levels in patients with nontraumatic osteonecrosis of the femoral head. BMC Musculoskelet. Disord. 24 (1), 240. doi:10.1186/s12891-023-06342-9
Li, G., Li, B., Li, B., Zhao, J., Wang, X., Luo, R., et al. (2021). The role of biomechanical forces and MALAT1/miR-329-5p/PRIP signalling on glucocorticoid-induced osteonecrosis of the femoral head. J. Cell. Mol. Med. 25 (11), 5164–5176. doi:10.1111/jcmm.16510
Li, G., Yin, J., Gao, J., Cheng, T. S., Pavlos, N. J., Zhang, C., et al. (2013). Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res. Ther. 15 (6), 223. doi:10.1186/ar4405
Li, L., Ding, Y., Liu, B., Wang, Z., Carlone, D. L., Yu, X., et al. (2021). Transcriptome landscape of the late-stage alcohol-induced osteonecrosis of the human femoral head. Bone 150116012 150, 116012. doi:10.1016/j.bone.2021.116012
Li, T., Xiao, K., Xu, Y., Ren, Y., Wang, Y., Zhang, H., et al. (2020). Identification of long non-coding RNAs expressed during the osteogenic differentiation of human bone marrow-derived mesenchymal stem cells obtained from patients with ONFH. Int. J. Mol. Med. 46 (5), 1721–1732. doi:10.3892/ijmm.2020.4717
Li, Z., Huang, C., Yang, B., Hu, W., Chan, M. T., and Wu, W. (2020). Emerging roles of long non-coding RNAs in osteonecrosis of the femoral head. Am. J. Transl. Res. 12 (9), 5984–5991.
Li, Z., Yu, X., and Shen, J. (2016). Long non-coding RNAs: emerging players in osteosarcoma. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 37 (3), 2811–2816. doi:10.1007/s13277-015-4749-4
Liu, G., Luo, S., Lei, Y., Jiao, M., Cao, R., Guan, H., et al. (2022). Osteogenesis-related long noncoding RNA GAS5 as a novel biomarker for osteonecrosis of femoral head. Front. Cell Dev. Biol. 10, 10857612. doi:10.3389/fcell.2022.857612
Liu, G. Z., Chen, C., Kong, N., Tian, R., Li, Y. Y., Li, Z., et al. (2020). Identification of potential miRNA biomarkers for traumatic osteonecrosis of femoral head. J. Cell Physiol. 235 (11), 8129–8140. doi:10.1002/jcp.29467
Liu, R., Liu, Q., Wang, K., Dang, X., and Zhang, F. (2016). Comparative analysis of gene expression profiles in normal hip human cartilage and cartilage from patients with necrosis of the femoral head. Arthritis Res. Ther. 18 (1), 98. doi:10.1186/s13075-016-0991-4
Luo, H., Lan, W., Li, Y., Lian, X., Zhang, N., Lin, X., et al. (2019). Microarray analysis of long-noncoding RNAs and mRNA expression profiles in human steroid-induced avascular necrosis of the femoral head. J. Cell Biochem. 120 (9), 15800–15813. doi:10.1002/jcb.28850
Ma, J., Ren, Y., Wang, B., Sun, W., Yue, D., and Wang, W. (2021). Progress of developmental mechanism of subtype H vessels in osteonecrosis of the femoral head. Zhongguo xiu fu chong jian wai ke za zhi 35 (11), 1486–1491. doi:10.7507/1002-1892.202103159
Magnussen, R. A., Guilak, F., and Vail, T. P. (2005). Cartilage degeneration in post-collapse cases of osteonecrosis of the human femoral head: altered mechanical properties in tension, compression, and shear. J. Orthop. Res. 23 (3), 576–583. doi:10.1016/j.orthres.2004.12.006
Mao, Z., Liu, G., Xiao, G. Y., Zhao, C., and Zou, Y. C. (2021). CircCDR1as suppresses bone microvascular endothelial cell activity and angiogenesis through targeting miR-135b/FIH-1 Axis. Orthop. Surg. 13 (2), 573–582. doi:10.1111/os.12883
Maruyama, M., Nabeshima, A., Pan, C. C., Behn, A. W., Thio, T., Lin, T., et al. (2018). The effects of a functionally-graded scaffold and bone marrow-derived mononuclear cells on steroid-induced femoral head osteonecrosis. Biomaterials 187, 18739–18746. doi:10.1016/j.biomaterials.2018.09.030
Memczak, S., Jens, M., Elefsinioti, A., Torti, F., Krueger, J., Rybak, A., et al. (2013). Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495 (7441), 333–338. doi:10.1038/nature11928
Moya-Angeler, J., Gianakos, A. L., Villa, J. C., Ni, A., and Lane, J. M. (2015). Current concepts on osteonecrosis of the femoral head. World J. Orthop. 6 (8), 590–601. doi:10.5312/wjo.v6.i8.590
Müller, S., and Appel, B. (2017). In vitro circularization of RNA. RNA Biol. 14 (8), 1018–1027. doi:10.1080/15476286.2016.1239009
O'Connell, B. J., and Genest, J. (2001). High-density lipoproteins and endothelial function. Circulation 104 (16), 1978–1983. doi:10.1161/hc3901.096667
Peng, P., He, W., Zhang, Y. X., Liu, X. H., Chen, Z. Q., and Mao, J. G. (2023). CircHIPK3 promotes bone microvascular endothelial cell proliferation, migration and angiogenesis by targeting miR-7 and KLF4/VEGF signaling in steroid-induced osteonecrosis of the femoral head. Adv. Clin. Exp. Med. 32 (1), 43–55. doi:10.17219/acem/153042
Piuzzi, N. S., Anis, H. K., and Muschler, G. F. (2019). Osteonecrosis of the femoral head with subchondral collapse. Cleve. Clin. J. Med. 86 (8), 511–512. doi:10.3949/ccjm.86a.19004
Powell, C., Chang, C., and Gershwin, M. E. (2011). Current concepts on the pathogenesis and natural history of steroid-induced osteonecrosis. Clin. Rev. Allergy Immunol. 41 (1), 102–113. doi:10.1007/s12016-010-8217-z
Qi, X., and Zeng, Y. (2015). Biomarkers and pharmaceutical strategies in steroid-induced osteonecrosis of the femoral head: a literature review. J. Int. Med. Res. 43 (1), 3–8. doi:10.1177/0300060514554724
Salzman, J., Gawad, C., Wang, P. L., Lacayo, N., and Brown, P. O. (2012). Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS. ONE 7 (2), e30733. doi:10.1371/journal.pone.0030733
Shang, G., Wang, Y., Xu, Y., Zhang, S., Sun, X., Guan, H., et al. (2018). Long non-coding RNA TCONS_00041960 enhances osteogenesis and inhibits adipogenesis of rat bone marrow mesenchymal stem cell by targeting miR-204-5p and miR-125a-3p. J. Cell Physiol. 233 (8), 6041–6051. doi:10.1002/jcp.26424
Sheng, H. H., Zhang, G. G., Cheung, W. H., Chan, C. W., Wang, Y. X., Lee, K. M., et al. (2007). Elevated adipogenesis of marrow mesenchymal stem cells during early steroid-associated osteonecrosis development. J. Orthop. Surg. Res. 215, 15. doi:10.1186/1749-799X-2-15
Shields, E. J., Petracovici, A. F., and Bonasio, R. (2019). lncRedibly versatile: biochemical and biological functions of long noncoding RNAs. Biochem. J. 476 (7), 1083–1104. doi:10.1042/BCJ20180440
Shoemaker, D. D., Schadt, E. E., Armour, C. D., He, Y. D., Garrett-Engele, P., McDonagh, P. D., et al. (2001). Experimental annotation of the human genome using microarray technology. Nature 409 (6822), 922–927. doi:10.1038/35057141
Sonoda, K., Motomura, G., Kawanami, S., Takayama, Y., Honda, H., Yamamoto, T., et al. (2017). Degeneration of articular cartilage in osteonecrosis of the femoral head begins at the necrotic region after collapse: a preliminary study using T1 rho MRI. Skelet. Radiol. 46 (4), 463–467. doi:10.1007/s00256-017-2567-z
Su, Y., Wu, H., Pavlosky, A., Zou, L. L., Deng, X., Zhang, Z. X., et al. (2016). Regulatory non-coding RNA: new instruments in the orchestration of cell death. Cell Death Dis. 7 (8), e2333. doi:10.1038/cddis.2016.210
Suh, K. T., Kim, S. W., Roh, H. L., Youn, M. S., and Jung, J. S. (2005). Decreased osteogenic differentiation of mesenchymal stem cells in alcohol-induced osteonecrosis. Clin. Orthop. Relat. Res. 431, 220–225. doi:10.1097/01.blo.0000150568.16133.3c
Wang, A., Ren, M., and Wang, J. (2018). The pathogenesis of steroid-induced osteonecrosis of the femoral head: a systematic review of the literature. Gene 671, 671103–671109. doi:10.1016/j.gene.2018.05.091
Wang, C., Peng, J., and Lu, S. (2014a). Summary of the various treatments for osteonecrosis of the femoral head by mechanism: a review. Exp. Ther. Med. 8 (3), 700–706. doi:10.3892/etm.2014.1811
Wang, C., Wang, X., Xu, X. L., Yuan, X. L., Gou, W. L., Wang, A. Y., et al. (2014b). Bone microstructure and regional distribution of osteoblast and osteoclast activity in the osteonecrotic femoral head. PLoS. ONE 9 (5), e96361. doi:10.1371/journal.pone.0096361
Wang, Q., Yang, Q., Chen, G., Du, Z., Ren, M., Wang, A., et al. (2018). LncRNA expression profiling of BMSCs in osteonecrosis of the femoral head associated with increased adipogenic and decreased osteogenic differentiation. Sci. Rep. 8 (1), 9127. doi:10.1038/s41598-018-27501-2
Wang, T., Xie, Z. H., Wang, L., Luo, H., Zhang, J., Dong, W. T., et al. (2023). LncAABR07053481 inhibits bone marrow mesenchymal stem cell apoptosis and promotes repair following steroid-induced avascular necrosis. Commun. Biol. 6 (1), 365. doi:10.1038/s42003-023-04661-0
Wei, B., Wei, W., Zhao, B., Guo, X., and Liu, S. (2017). Long non-coding RNA HOTAIR inhibits miR-17-5p to regulate osteogenic differentiation and proliferation in non-traumatic osteonecrosis of femoral head. PLoS. ONE 12 (2), e0169097. doi:10.1371/journal.pone.0169097
Wei, Q. S., Hong, G. J., Yuan, Y. J., Chen, Z. Q., Zhang, Q. W., and He, W. (2019). Huo Xue Tong Luo capsule, a vasoactive herbal formula prevents progression of asymptomatic osteonecrosis of femoral head: a prospective study. J. Orthop. Transl. 18, 1865–1873. doi:10.1016/j.jot.2018.11.002
Williams, T. A., Verhovez, A., Milan, A., Veglio, F., and Mulatero, P. (2006). Protective effect of spironolactone on endothelial cell apoptosis. Endocrinology 147 (5), 2496–2505. doi:10.1210/en.2005-1318
Wu, F., Jiao, J., Liu, F., Yang, Y., Zhang, S., Fang, Z., et al. (2019). Hypermethylation of Frizzled1 is associated with Wnt/β-catenin signaling inactivation in mesenchymal stem cells of patients with steroid-associated osteonecrosis. Exp. Mol. Med. 51 (2), 1–9. doi:10.1038/s12276-019-0220-8
Wu, X., Sun, W., and Tan, M. (2019). Noncoding RNAs in steroid-induced osteonecrosis of the femoral head. Biomed. Res. Int. 2019, 20198140595. doi:10.1155/2019/8140595
Wu, Y., Fang, L., Gao, Y., Zhao, Z., Zhou, L., and Zhang, G. (2022). lncRNA FGD5-AS1 regulates bone marrow stem cell proliferation and apoptosis by affecting miR-296-5p/STAT3 Axis in steroid-induced osteonecrosis of the femoral head. J. Healthc. Eng. 2022, 20229364467. doi:10.1155/2022/9364467
Wu, Y., Zhang, L., Wang, Y., Li, H., Ren, X., Wei, F., et al. (2014). Long noncoding RNA HOTAIR involvement in cancer. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 35 (10), 9531–9538. doi:10.1007/s13277-014-2523-7
Xia, T., Liao, Q., Jiang, X., Shao, Y., Xiao, B., Xi, Y., et al. (2014). Long noncoding RNA associated-competing endogenous RNAs in gastric cancer. Sci. Rep. 46088, 6088. doi:10.1038/srep06088
Xiang, S., Li, Z., and Weng, X. (2019). The role of lncRNA RP11-154D6 in steroid-induced osteonecrosis of the femoral head through BMSC regulation. J. Cell Biochem. 120 (10), 18435–18445. doi:10.1002/jcb.29161
Xiang, S., Li, Z., and Weng, X. (2020). Changed cellular functions and aberrantly expressed miRNAs and circRNAs in bone marrow stem cells in osteonecrosis of the femoral head. Int. J. Mol. Med. 45 (3), 805–815. doi:10.3892/ijmm.2020.4455
Xin, W., Yuan, S., Wang, B., Qian, Q., and Chen, Y. (2021). Hsa_circ_0066523 promotes the proliferation and osteogenic differentiation of bone mesenchymal stem cells by repressing PTEN. Bone Jt. Res. 10 (8), 526–535. doi:10.1302/2046-3758.108.BJR-2020-0127.R2
Xing, D., Liang, J. Q., Li, Y., Lu, J., Jia, H. B., Xu, L. Y., et al. (2014). Identification of long noncoding RNA associated with osteoarthritis in humans. Orthop. Surg. 6 (4), 288–293. doi:10.1111/os.12147
Xu, Y., Jiang, Y., Wang, Y., Jia, B., Gao, S., Yu, H., et al. (2022). LINC00473-modified bone marrow mesenchymal stem cells incorporated thermosensitive PLGA hydrogel transplantation for steroid-induced osteonecrosis of femoral head: a detailed mechanistic study and validity evaluation. Bioeng. Transl. Med. 7 (2), e10275. doi:10.1002/btm2.10275
Xu, Y., Jiang, Y., Wang, Y., Ren, Y., Zhao, Z., Wang, T., et al. (2020). LINC00473 regulated apoptosis, proliferation and migration but could not reverse cell cycle arrest of human bone marrow mesenchymal stem cells induced by a high-dosage of dexamethasone. Stem Cell Res. 48, 48101954. doi:10.1016/j.scr.2020.101954
Xu, Y., Jiang, Y., Wang, Y., Zhao, Z., and Li, T. (2021). LINC00473 rescues human bone marrow mesenchymal stem cells from apoptosis induced by dexamethasone through the PEBP1-mediated Akt/Bad/Bcl-2 signaling pathway. Int. J. Mol. Med. 47 (1), 171–182. doi:10.3892/ijmm.2020.4788
Yao, T., Wang, L., Ding, Z. F., and Yin, Z. S. (2022). hsa_circ_0058122 knockdown prevents steroid-induced osteonecrosis of the femoral head by inhibiting human umbilical vein endothelial cells apoptosis via the miR-7974/IGFBP5 axis. J. Clin. Lab. Anal. 36 (4), e24134. doi:10.1002/jcla.24134
Yao, T., Yin, Z. S., Huang, W., Ding, Z. F., and Cheng, C. (2020). Microarray profiling of circular RNAs in steroid-associated osteonecrosis of the femoral head: observational study. Medicine 99 (10), e19465. doi:10.1097/MD.0000000000019465
You, X., Vlatkovic, I., Babic, A., Will, T., Epstein, I., Tushev, G., et al. (2015). Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat. Neurosci. 18 (4), 603–610. doi:10.1038/nn.3975
Yu, H., Liu, P., Zuo, W., Sun, X., Liu, H., Lu, F., et al. (2020). Decreased angiogenic and increased apoptotic activities of bone microvascular endothelial cells in patients with glucocorticoid-induced osteonecrosis of the femoral head. BMC Musculoskelet. Disord. 21 (1), 277. doi:10.1186/s12891-020-03225-1
Yuan, S., Zhang, C., Zhu, Y., and Wang, B. (2020). Neohesperidin ameliorates steroid-induced osteonecrosis of the femoral head by inhibiting the histone modification of lncRNA HOTAIR. Drug Des. Dev. Ther. 14, 145419–145430. doi:10.2147/DDDT.S255276
Zhang, C., Yuan, S., Chen, Y., and Wang, B. (2021). Neohesperidin promotes the osteogenic differentiation of human bone marrow stromal cells by inhibiting the histone modifications of lncRNA SNHG1. Cell Cycle 20 (19), 1953–1966. doi:10.1080/15384101.2021.1969202
Zhang, F., Peng, W., Wang, T., Zhang, J., Dong, W., Wang, C., et al. (2022). Lnc Tmem235 promotes repair of early steroid-induced osteonecrosis of the femoral head by inhibiting hypoxia-induced apoptosis of BMSCs. Exp. Mol. Med. 54 (11), 1991–2006. doi:10.1038/s12276-022-00875-0
Zhang, F., Wei, L., Wang, L., Wang, T., Xie, Z., Luo, H., et al. (2023). FAR591 promotes the pathogenesis and progression of SONFH by regulating Fos expression to mediate the apoptosis of bone microvascular endothelial cells. Bone Res. 11 (1), 27. doi:10.1038/s41413-023-00259-8
Zhang, R., Fang, H., Chen, Y., Shen, J., Lu, H., Zeng, C., et al. (2012). Gene expression analyses of subchondral bone in early experimental osteoarthritis by microarray. PLoS. ONE 7 (2), e32356. doi:10.1371/journal.pone.0032356
Zhang, X. Y., Li, H. N., Chen, F., Chen, Y. P., Chai, Y., Liao, J. Z., et al. (2021). Icariin regulates miR-23a-3p-mediated osteogenic differentiation of BMSCs via BMP-2/Smad5/Runx2 and WNT/β-catenin pathways in osteonecrosis of the femoral head. Saudi Pharm. J. 29 (12), 1405–1415. doi:10.1016/j.jsps.2021.10.009
Zhang, Y., Jia, S., Wei, Q., Zhuang, Z., Li, J., Fan, Y., et al. (2021). CircRNA_25487 inhibits bone repair in trauma-induced osteonecrosis of femoral head by sponging miR-134-3p through p21. Regen. Ther. 16, 1623–1631. doi:10.1016/j.reth.2020.12.003
Zhang, Y., Yin, J., Ding, H., Zhang, C., and Gao, Y. S. (2016). Vitamin K2 ameliorates damage of blood vessels by glucocorticoid: a potential mechanism for its protective effects in glucocorticoid-induced osteonecrosis of the femoral head in a rat model. Int. J. Biol. Sci. 12 (7), 776–785. doi:10.7150/ijbs.15248
Zhao, J., Mu, L., Wang, Z., Fang, X., He, X., Zhang, X., et al. (2020). The potential roles of circular RNAs in osteonecrosis of the femoral head (Review). Mol. Med. Rep. 21 (2), 533–539. doi:10.3892/mmr.2019.10866
Zhao, X., Liu, Y., and Yu, S. (2017). Long noncoding RNA AWPPH promotes hepatocellular carcinoma progression through YBX1 and serves as a prognostic biomarker. Biochim. Biophys. Acta Mol. Basis Dis. 1863 (7), 1805–1816. doi:10.1016/j.bbadis.2017.04.014
Zhao, Y., Li, S., Feng, M., Zhang, M., Liu, Z., Yao, Y., et al. (2023). Effects of puerarin-loaded tetrahedral framework nucleic acids on osteonecrosis of the femoral head. Small 19 (41), e2302326. doi:10.1002/smll.202302326
Zheng, S. W., Sun, C. H., Wen, Z. J., Liu, W. L., Li, X., Chen, T. Y., et al. (2022). Decreased serum CXCL12/SDF-1 concentrations may reflect disease severity of non-traumatic osteonecrosis of femoral head. Clin. Chim. Acta 529, 52987–52995. doi:10.1016/j.cca.2022.02.009
Zhou, J., Wang, B., Bin, X., Xie, C., Li, B., Liu, O., et al. (2021). CircHIPK3: key player in pathophysiology and potential diagnostic and therapeutic tool. Front. Med. (Lausanne) 8, 8615417. doi:10.3389/fmed.2021.615417
Zhou, Y., Zhang, F., Xu, F., Wang, Q., Wu, J., Peng, W., et al. (2021). lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis. Open Life Sci. 16 (1), 969–980. doi:10.1515/biol-2021-0097
Zhu, F., Zhang, X., Yu, Q., Han, G., Diao, F., Wu, C., et al. (2018). LncRNA AWPPH inhibits SMAD4 via EZH2 to regulate bladder cancer progression. J. Cell Biochem. 119 (6), 4496–4505. doi:10.1002/jcb.26556
Zhu, Z., Du, W., Yu, H., Jin, H., and Tong, P. (2019). Expression profile analysis of differentially expressed circular RNAs in steroid-induced osteonecrosis of the femoral head. Dis. Markers 2019, 20198759642. doi:10.1155/2019/8759642
Keywords: lncRNA, circRNA, ONFH, BMSCs, BEMCs
Citation: Lin F, Zhou S, Yi M and Wang Q (2025) Emerging perspectives on osteonecrosis of the femoral head: the role of circular RNAs and long non-coding RNAs - a systematic review. Front. Genet. 16:1549684. doi: 10.3389/fgene.2025.1549684
Received: 21 December 2024; Accepted: 23 May 2025;
Published: 04 June 2025.
Edited by:
Michela Rossi, Bambino Gesù Children’s Hospital (IRCCS), ItalyReviewed by:
Bodhisattwa Banerjee, University of Vermont, United StatesAmy Brower, Creighton University, United States
Copyright © 2025 Lin, Zhou, Yi and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingyu Wang, d2FuZ3F5ZWR1QDE2My5jb20=
 Feifei Lin
Feifei Lin Qingyu Wang
Qingyu Wang