- 1Department of Center for Reproductive Medicine, Tianjin Central Hospital of Gynaecology Obsterics, Tianjin, China
- 2Tianjin Institute of Gynaecology Obsteric, Tianjin Central Hospital of Gynaecology Obsterics, Tianjin, China
- 3Tianjin Key Laboratory of Human Development and Reproductive Regulation, Tianjin Central Hospital of Gynaecology Obsterics, Tianjin, China
Background:: Infertility is a multiplex disorder in the reproductive system. Unexplained infertility affects 2%-3% of reproductive-aged couples. Male factors contribute to about half of all infertility cases. About 15% of these cases are predicted to have a genetic etiology. With the wide application of whole exome sequencing (WES), more and more variations in male infertility have been identified.
Methods:: A patient diagnosed with asthenoteratozoospermia was involved in this study. WES was performed in the patient, and Sanger sequencing was used to confirm the variation. Mini-gene splicing assays were performed to validate the effect on the alternative splicing of the variation.
Results:: A novel heterozygous splice variant was identified in SYCP2 (c.2600+ 5G>C) in the patient ,which inherited from his phenotypically normal mother. SYCP2 encodes a protein critical for the synapsis of homologous chromosomes during meiosis I, and its disruption can impair spermatogenesis. Mini-gene splicing assays confirmed that this splicing variant impacted alternative splicing and that the stop codon appeared early, which was very likely to result in the loss of function of the protein and lead to the occurrence of male infertility.
Conclusion:: Our results suggested that the c.2600+5G>C variation in SYCP2 might be the genetic etiology for male infertility in this pedigree. This finding expanded the known genotype spectrum of male infertility and provided new etiological information for male infertility.
1 Introduction
Infertility is an important health problem with a multifactorial etiology that affects approximately 15% of couples who attempt pregnancy globally (Tamrakar and Bastakoti, 2019). In approximately 50% of these couples, a male factor plays an important role, which may exist either alone or in combination with female factors (Xie et al., 2018). Male infertility is a multifactorial pathological condition affecting approximately 7% of the male population (Li et al., 2024). The genetic factor of male infertility is highly complex, as testis and semen histological phenotypes are extremely heterogeneous, and at least 2000 genes are involved in spermatogenesis (Krausz and Riera-Escamilla, 2018). Understanding the genetic etiology of male infertility can provide genetic counselling and subsequent therapeutic interventions to patients, such as intracytoplasmic sperm injection (ICSI) and in vitro fertilization (IVF), as well as seeking donor sperm or adoption (Li et al., 2024). Therefore, identifying genetic variants associated with male infertility can provide patients with meaningful and actionable information.
The identification of novel candidate genes in infertile males has increased rapidly since the implementation of next-generation sequencing, including whole genome sequencing (WGS) and whole exome sequencing (WES). The molecular diagnostic project has become an important mean of clinical diagnosis. However, many genes have not yet accumulated sufficient evidence to be confidently implicated in male infertility.
Synaptonemal complex protein 2 (SYCP2) is a novel candidate gene associated with autosomal dominant male infertility and is located at 20q13.33 (Schilit et al., 2020). SYCP2 encodes synaptonemal complex protein 2, an axial element in the proteinaceous synaptonemal complex (SC) (Schalk et al., 1999). SC assembly contributes to the pairing and segregation of homologous chromosomes during meiosis (Page and Hawley, 2003). SYCP2 is important for spermatogenesis (Takemoto et al., 2020).
This study investigated the genetic cause of male infertility in a Chinese patient. WES and subsequent Sanger sequencing revealed a novel heterozygous variant in SYCP2 (c.2600 + 5G>C). This variation was inherited from the patient’s healthy mother. The variation was verified to impact the alternative splicing of SYCP2 and introduced an early stop codon that resulted in the prematuration and loss of function of SYCP2.
2 Materials and methods
2.1 Patients
A family with a proband diagnosed with male infertility was involved in this study. The family members who participated in this study were thoroughly informed about this study. This study was approved by the medical ethics committee of Tianjin Central Hospital of Gynecology Obstetrics (No. ZY2023001).
2.2 WES, variant interpretation, and sanger sequencing
Genomic DNA (gDNA) was isolated from peripheral blood via the DNeasy Blood and Tissue Kit (QIAGEN, Germany). WES was performed via the sequencing platform of the Beijing Genomics Institute (BGI). All steps were performed according to the manufacturer’s instructions. Exomes were hybridized and captured by the xGen Exome Research Panel of Integrated DNA Technologies (IDT, America) and sequenced on the MGI-2000. The average sequencing depth was 100x‒150x, and the data quality was Q20 ≥ 90% and Q30 ≥ 90%. The original data obtained by sequencing were filtered through fastp software (Chen et al., 2018) and then passed through BWA software (Houtgast et al., 2018). The sequenced reads were collected, filtered for quality, and aligned to the human reference genome (hg19/GRCh37). The sequenced variants were annotated via ANNOVAR software (Wang et al., 2010), and mutations were screened on the basis of patients’ clinical information, population databases, disease databases, and bioinformatic prediction tools. Candidate pathogenic variants were scored in accordance with the criteria set by the American College of Medical Genetics and Genomics (ACMG) (Richards et al., 2015) and confirmed by Sanger sequencing.
2.3 Mini-gene construction
To construct the mini gene, we amplified the SYCP2 fragment from the sectional intron26 (627 bp) to the sectional intron27 (84 bp) via nested polymerase chain reaction (PCR) with a primer pair and added the endonuclease recognition sequences of KpnI and XhoI to the front and end of the fragment, respectively. The detailed methods were presented in the supplementary materials. The PCR products were purified via alcohol and digested along with the vector pcMINI via the endonucleases KpnI and XhoI (New England Biolabs, America). The digested PCR products and vectors were purified via electrophoresis and ligated together with T4 ligase (New England Biolabs, America). Ligase products were transformed into DH5α competent cells, which were subsequently plated on LB plates coated with ampicillin. Single clones were then selected for proliferation and Sanger sequencing. The identified colonies were amplified, and plasmid DNA without endotoxin was extracted via the Rapid Plasmid Mini Kit (Simgen, China). The primers used in this study are listed in the Supplementary Material.
2.4 Cell transfection
Human embryonic kidney 293T (HEK293T) cells and HeLa cells were cultured in DMEM/high glucose (Gibco, America) supplemented with 10% FBS (Sigma, America) in a 37°C constant-temperature water bath incubator at 5% CO2. The cells were dissociated into single cells using trypsin-EDTA (Thermo Fisher, America) after they reached 80% confluence. The cells were counted, and 4 × 105 cells were seeded in 6-well plates 24 h before transfection. The cell medium was changed to Opti-MEM (Thermo Fisher, America) 2 h before transfection. Lipofectamine 2000 (Thermo Fisher, America) was incubated for 20 min at room temperature, the mixture was mixed thoroughly with 1 μg of plasmid, and then, the mixture was gently added to the cell medium. The cell medium was replaced with fresh culture medium supplemented with 10% FBS 12 h after transfection.
2.5 RT-PCR and sanger sequencing
Total RNA was extracted 48 h after transfection via TRIzol reagent (TaKaRa, China) following routine procedures. Reverse transcription‒polymerase chain reaction (RT-PCR) was performed via ABScript III RT Master Mix for qPCR with gDNA Remover (ABclonal, China) according to the manufacturer’s instructions. cDNA was used as a template for PCR with the primer pair F/R (Supplementary Material). The amplification products were subjected to electrophoresis on a 1% agarose gel and Sanger sequencing.
2.6 Bioinformatics analyses
The SYCP2 gene sequence was obtained from the NCBI Gene database (https://www.ncbi.nlm.nih.gov/gene/). The molecular structure of the cryo-EM structure of GATOR1 was viewed with Mol* View (Sehnal et al., 2021) and stored in the RCSB PDB (Berman et al., 2000).
3 Results
3.1 Clinical report for the patient
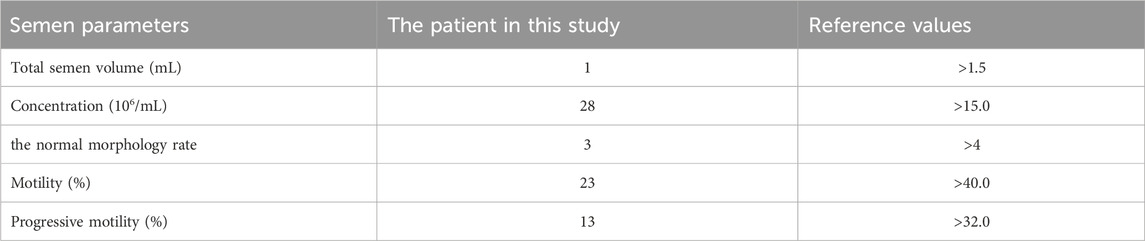
We sought to identify the genetic etiology of infertility for a male research participant who presented with a 2-year history of infertility at age 30. His assessment indicated asthenoteratozoospermia (AT) (the semen test results were shown in Table 1) in accordance with the WHO Laboratory Manual for the Examination and Handling of Human Semen, fifth edition. The patient displayed no dysmorphic features and had normal serum levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone. Y chromosome microdeletions were normal. The couple pursued ICSI as a treatment for male infertility. However, the couple did not become pregnant after ICSI treatment at the center. Despite multiple attempts at ICSI and despite successful fertilization, the couple was unable to obtain viable embryos. Eventually, the patient discontinued the treatment.
3.2 Genetic analysis identifies novel heterozygous SYCP2 variants in the patient
To explore the genetic factors contributing to the infertility of the patient, WES and subsequent validation through Sanger sequencing were performed. The patient was found to have a heterozygous variant in intron 27 of SYCP2, NM_014258.4:c.2600 + 5G>C (Figure 1A). Sanger sequencing via the primer SYCP2-F/R confirmed that this variant was inherited from the patient’s mother, who had a normal phenotype (Figure 1B).
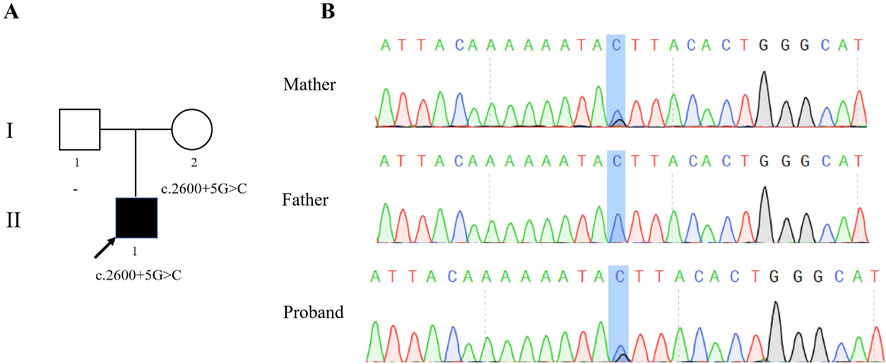
Figure 1. Identification of a heterozygous variation in SYCP2. (A) Pedigree of the family. The proband (II-1) was diagnosed with male infertility, as indicated by filled symbols with arrows in the pedigree. (B) Sanger sequencing of the proband and his parents revealed a c.2600 + 5G>C mutation in the SYCP2 gene, which was maternally inherited.
Notably, this variant was absent in several major population databases, including the 1KGP (1000 Genomes Project, Phase 3), ESP6500 (Genome Aggregation Database, V2), gnomAD (Genome Aggregation Database, r2.0.1) and ExAC (Exome Aggregation Consortium, r0.3.1) databases, highlighting its rarity within these populations. This is the first report of this phenomenon in this study. Bioinformatics analysis via “Ada” and “RF” scores predicted that this variant was likely to influence splicing (Supplementary Table S2). According to the ACMG guidelines, the novel variation c.2600 + 5G>C was designated as a variant of uncertain significance (VUS).
3.3 Functional splicing examination of the variant with mini-gene splicing assays
To validate the effect of the c.2600 + 5G>C variation on RNA alternative splicing, we conducted a mine gene splicing assay by constructing containing the wild-type (wt) and mutation-type (mut) target DNA fragments (Figure 2A). The vectors were confirmed by Sanger sequencing (Figure 2B). Plasmid DNA without endotoxin was transfected into HEK293T cells and HeLa cells. The total RNA of the transfected cells was extracted, and reverse transcription was performed to obtain cDNA. Agarose electrophoresis of the RT-PCR products revealed two distinct splicing patterns (Figures 2C,D). The full uncropped Gels image presented in Supplementary Figure S2. Sanger sequencing revealed that abnormal splicing occurred in cells transfected with the mutation plasmid and that the mutation C.2600 + 5G>C affected the normal splicing of gene mRNA (Figure 2E). The detection results of pcMINI and PCMINI-C were consistent, as shown inSupplementary Figure S1. There was one abnormal transcript after mutation: exon 27 skipping. The mutation caused exon 27 skipping, which was expressed in the cDNA as c.2530_2600del. Exon 27 skipping caused a subsequent frameshift and produced an early stop codon in exon 28, which might produce a truncated protein 844 aa in length. Thus, the variation was described as SYCP2:c.2600 + 5G>C (p.Lys845*).
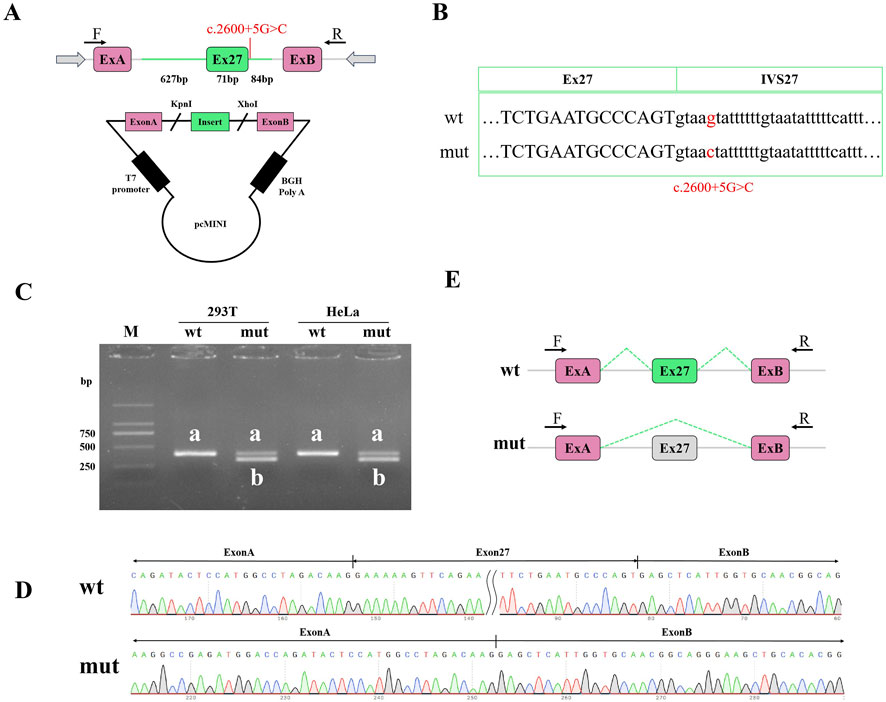
Figure 2. Examination of functional splicing of the variant with mini-gene splicing assays. (A) Schematic diagram of the constructed mini-gene. ExA and ExB are exonic sequences of the plasmid. (B) Sanger sequencing confirmed that the wild-type and mutant fragments were successfully introduced into the mini-gene construct. (C) RT-PCR was performed to verify alternative splicing in the wild-type and mutant groups. Abnormal splicing bands in the mutant groups were not detected in the HEK293T cells or the HeLa cells. Agarose gel electrophoresis revealed that the wt had only one band, and the labelled a and mut proteins produced two bands, labelled a and b. (D) The bands in a and b were identified via Sanger sequencing. Alternative splicing was affected by the c.2600 + 5G>C variation in SYCP2. PCR product sequencing revealed exon 27 skipping. (E) Alternative schematic diagram. wt, wild type; mut, mutant type; M, DL2000 DNA ladder.
3.4 Translation analysis and protein modelling
We further analysed the cDNA sequence of SYCP2 and found that the variation led to a frameshift and early appearance of the stop codon, which resulted in the prematuration of SYCP2 with 844 amino acid residues. Mutation of SYCP2 resulted in the loss of the whole coiled-coil (CC) domain (Figure 3). In rodents, SYCP2 directly interacts with SYCP1 and SYCP3 through its C-terminal domain and internal curly helix domain, respectively (Winkel et al., 2009). The deletion of the SYCP3-interacting domain of SYCP2 leads to severe defects in SC formation in mice, and males are sterile (Yang et al., 2006). The deletion of the CC domain meant that the protein was fundamentally changed and that its function was severely impaired.
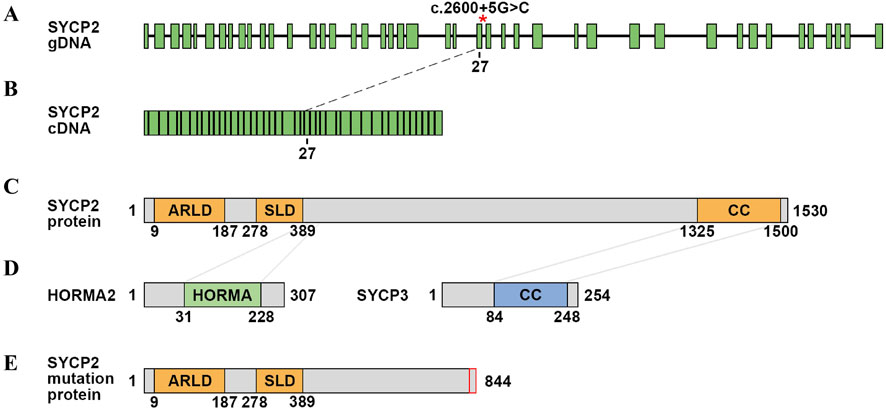
Figure 3. Schematic of the SYCP2 gene and protein domain. (A) gDNA structure of SYCP2. The red asterisk represents the variation site. (B) cDNA structure of SYCP2. (C) Schematic diagram of the SYCP2 domain. The SYCP2 protein contains the ARLD domain, SLD domain and CC domain. (D) Schematic diagram of the HORMA and SYCP3 structures. The connected parts interact with the SYCP2 domain. (E) Schematic diagram of the predicted protein structure of mutated SYCP2.
4 Discussion
Many Mendelian disorders are genetically heterogeneous, with a multitude of different disease genes in which a variety of disease-causing variants have been discovered (Zhao et al., 2015). With the development of next-generation sequencing (NGS), an increasing number of disease-causing genes have been discovered. Accurate molecular diagnosis can provide an important basis for genetic counselling, specific treatment and family planning, and prognosis management (Ellingford et al., 2015).
Male infertility is a common disorder among reproductive-aged couples. Understanding the precise causes may directly inform therapies for infertile couples (Schilit, 2019). In this study, we described a patient with a clinical presentation compatible with male infertility and discovered a novel germline splicing variant, c.2600 + 5G>C, in SYCP2. Our experimental results revealed that this mutation caused exon 27 skipping, leading to subsequent changes in the reading frame. A premature termination codon (PTC) was generated within exon 28, and a truncated protein with a length of 844 aa was likely to be generated, destroying the curly helix region, which could form a heterodimer with the SYCP3 protein and play an important role in the assembly of the synaptic complex and the chromosome coupling process (Takemoto et al., 2020). Mice lacking the coiled-coil domain of Sycp2 exhibit spermatocyte apoptosis and male-specific infertility (Yang et al., 2006). According to ACMG guidelines, the c.2600 + 5G>C variant was predicted to be likely pathogenic (LP), which was upgraded from VUS. The detection of the new mutation expanded the variation spectrum of SYCP2 and provided the basis for the subsequent treatment of this family. However, surprisingly, the concentration of the patient’s semen was normal. We suspected that meiotic errors such as aneuploidy or DNA fragmentation due to SYCP2 dysfunction could lead to failed fertilization, even if sperm were present in this patient.
The penetrance of the disorder may be unknown due to the ascertainment of affected cases (Li et al., 2024). Indeed, there was an azoospermic proband with a homozygous loss-of-function variant in SYCP2 whose father might have incomplete penetrance in the heterozygous state (Xu et al., 2023). Notably, in this study we found that female carrier was fertile, and maternally inherited SYCP2 variants had been seen in other reports (Li et al., 2024). In addition, disruption of Sycp2 gene has been shown to cause male infertility, but only female infertility in mouse model (Yang et al., 2006), suggesting that the relationship between SYCP2 and male infertility does not extend to female infertility. It was speculated that SYCP2 homologue SYCP2L may play an important role in female fertility (Li et al., 2024).
Mammalian RNA splicing is a delicate process whose precise coordination is not fully understood, but its regulation is critical for the proper expression of most genes and their isoforms (Zheng, 2004). Accurate pre-mRNA splicing is critical for proper protein translation and relies on the existence of consistent cis sequences that define exon‒intron boundaries and regulatory sequences recognized by splicing mechanisms (Wang et al., 2024). Variants that disrupt normal pre-mRNA splicing are increasingly recognized as major causes of monogenic disorders. Mutations in the canonical splice sequences usually lead to single-exon skipping, but the exact effect of specific splicing mutations on alternative pre-mRNA splicing needs further validation. Some nonclassical splicing events do not result in frameshifts, which might have a mild effect on protein function. In our study, the c.2600 + 5G>C variant in SYCP2 causes the skipping of exon 27, as expected, and results in a frameshift and early appearance of a stop codon, causing the SYCP2 protein to be prematurated and lose its normal function. The importance of understanding this process and being able to predict which variants alter splicing is therefore essential to understanding human disease.
SYCP2 (OMIM 604105) is located on chromosome 20q13.33 and spans 70 kb in length. The SYCP2 protein contains 1,530 amino acids. SYCP2 is a component of the synaptic complex and plays an important role in meiosis. At present, relatively few cases of SYCP2 gene mutations have been reported, with the majority being loss-of-function mutations. In a recent study, three frameshift variants in SYCP2 were identified in men with azoospermia, suggesting that heterozygous loss-of-function variants in SYCP2 might be responsible for the low sperm count and subsequent infertility (Schilit et al., 2020). Substantial experimental evidence supports the role of SYCP2 in male infertility, reinforcing the strong clinical validity of the classification of SYCP2 as a gene associated with autosomal dominant male infertility (Li et al., 2024).
Nevertheless, there were also several limitations to this study. The results of the mini-gene splicing assay were not further validated in patient samples and we did not confirm the presence of the truncated protein in the variant by western blot. Moreover, histological analysis of the patient’s testicles was not performed.
5 Conclusion
In conclusion, in this article, we describe a proband with male infertility harboring a novel splicing variation, c.2600 + 5G>C (p.Lys845*), in SYCP2 gene. The effect of this variation on alternative splicing and translation was confirmed by mini-gene splice assays. Our study provided a new source of evidence for the pathogenicity of splicing variation and expanded the phenotype and genotype spectrum of male infertility.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. The detailed original data is uploaded to figshare, DOI:10.6084/m9.figshare.28925081.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LC: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review and editing. ZnY: Conceptualization, Data curation, Methodology, Writing – review and editing. ZoY: Writing – review and editing. LH: Funding acquisition, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Jinmen medical staff of Tianjin.
Acknowledgments
The authors would like to express their sincere gratitude to the patients involved in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2025.1595720/full#supplementary-material
References
Berman, H. M., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T. N., Weissig, H., et al. (2000). The protein data bank. Nucleic Acids Res. 28 (1), 235–242. doi:10.1093/nar/28.1.235
Chen, S., Zhou, Y., Chen, Y., and Gu, J. (2018). fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34 (17), i884–i890. doi:10.1093/bioinformatics/bty560
Ellingford, J. M., Sergouniotis, P. I., Lennon, R., Bhaskar, S., Williams, S. G., Hillman, K. A., et al. (2015). Pinpointing clinical diagnosis through whole exome sequencing to direct patient care: a case of Senior-Loken syndrome. Lancet 385 (9980), 1916. doi:10.1016/S0140-6736(15)60496-2
Houtgast, E. J., Sima, V. M., Bertels, K., and Al-Ars, Z. (2018). Hardware acceleration of BWA-MEM genomic short read mapping for longer read lengths. Comput. Biol. Chem. 75, 54–64. doi:10.1016/j.compbiolchem.2018.03.024
Krausz, C., and Riera-Escamilla, A. (2018). Genetics of male infertility. Nat. Rev. Urol. 15 (6), 369–384. doi:10.1038/s41585-018-0003-3
Li, J., Schilit, S. L. P., Liang, S., Qin, N., Teng, X., and Zhang, J. (2024). Novel loss-of-function SYCP2 variants in infertile males upgrade the gene-disease clinical validity classification for SYCP2 and male infertility to strong. Genes (Basel) 15 (8), 1092. doi:10.3390/genes15081092
Page, S. L., and Hawley, R. S. (2003). Chromosome choreography: the meiotic ballet. Science 301 (5634), 785–789. doi:10.1126/science.1086605
Richards, S., Aziz, N., Bale, S., Bick, D., Das, S., Gastier-Foster, J., et al. (2015). Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of medical genetics and Genomics and the association for molecular pathology. Genet. Med. 17 (5), 405–424. doi:10.1038/gim.2015.30
Schalk, J. A., Offenberg, H. H., Peters, E., Groot, N. P., Hoovers, J. M., and Heyting, C. (1999). Isolation and characterization of the human SCP2 cDNA and chromosomal localization of the gene. Mamm. Genome 10 (6), 642–644. doi:10.1007/s003359901062
Schilit, S. L. P. (2019). Recent advances and future opportunities to diagnose male infertility. Curr. Sex. Health Rep. 11 (4), 331–341. doi:10.1007/s11930-019-00225-8
Schilit, S. L. P., Menon, S., Friedrich, C., Kammin, T., Wilch, E., Hanscom, C., et al. (2020). SYCP2 translocation-mediated dysregulation and frameshift variants cause human male infertility. Am. J. Hum. Genet. 106 (1), 41–57. doi:10.1016/j.ajhg.2019.11.013
Sehnal, D., Bittrich, S., Deshpande, M., Svobodová, R., Berka, K., Bazgier, V., et al. (2021). Mol* Viewer: modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 49 (W1), W431–W437. doi:10.1093/nar/gkab314
Takemoto, K., Imai, Y., Saito, K., Kawasaki, T., Carlton, P. M., Ishiguro, K. I., et al. (2020). Sycp2 is essential for synaptonemal complex assembly, early meiotic recombination and homologous pairing in zebrafish spermatocytes. PLoS Genet. 16 (2), e1008640. doi:10.1371/journal.pgen.1008640
Tamrakar, S. R., and Bastakoti, R. (2019). Determinants of infertility in couples. J. Nepal Health Res. Counc. 17 (1), 85–89. doi:10.33314/jnhrc.1827
Wang, K., Li, M., and Hakonarson, H. (2010). ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38 (16), e164. doi:10.1093/nar/gkq603
Wang, Y., Niu, W., Shi, H., Bao, X., Liu, Y., Lu, M., et al. (2024). A novel variation in DEPDC5 causing familial focal epilepsy with variable foci. Front. Genet. 15, 1414259. doi:10.3389/fgene.2024.1414259
Winkel, K., Alsheimer, M., Ollinger, R., and Benavente, R. (2009). Protein SYCP2 provides a link between transverse filaments and lateral elements of mammalian synaptonemal complexes. Chromosoma 118 (2), 259–267. doi:10.1007/s00412-008-0194-0
Xie, C., Chen, X., Liu, Y., Wu, Z., and Ping, P. (2018). Multicenter study of genetic abnormalities associated with severe oligospermia and non-obstructive azoospermia. J. Int. Med. Res. 46 (1), 107–114. doi:10.1177/0300060517718771
Xu, J., Sun, Y., Zhang, Y., Ou, N., Bai, H., Zhao, J., et al. (2023). A homozygous frameshift variant in SYCP2 caused meiotic arrest and non-obstructive azoospermia. Clin. Genet. 104 (5), 577–581. doi:10.1111/cge.14392
Yang, F., De La Fuente, R., Leu, N. A., Baumann, C., McLaughlin, K. J., and Wang, P. J. (2006). Mouse SYCP2 is required for synaptonemal complex assembly and chromosomal synapsis during male meiosis. J. Cell Biol. 173 (4), 497–507. doi:10.1083/jcb.200603063
Zhao, L., Wang, F., Wang, H., Li, Y., Alexander, S., Wang, K., et al. (2015). Next-generation sequencing-based molecular diagnosis of 82 retinitis pigmentosa probands from Northern Ireland. Hum. Genet. 134 (2), 217–230. doi:10.1007/s00439-014-1512-7
Keywords: male infertility, whole exome sequence, SYCP2, gene variation, mini-gene splicing assay
Citation: Liu C, Zhang Y, Zhao Y and Luo H (2025) A novel loss-of-function SYCP2 variant causes asthenoteratozoospermia in infertile males. Front. Genet. 16:1595720. doi: 10.3389/fgene.2025.1595720
Received: 18 March 2025; Accepted: 28 April 2025;
Published: 13 May 2025.
Edited by:
Musharraf Jelani, Islamia College Peshawar, PakistanCopyright © 2025 Liu, Zhang, Zhao and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haining Luo, MzAzMTcwMTJAbmFua2FpLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Cong Liu
Cong Liu Yinfeng Zhang1,2,3†
Yinfeng Zhang1,2,3† Haining Luo
Haining Luo