- Department of Agriculture and Natural Resources, Delaware State University, Dover, DE, United States
Vegetatively propagated polyploid crops such as potato, strawberry, sugarcane, and banana play a crucial role in global agriculture by meeting essential nutritional and food demands. The quality of the economically important traits in these crops is significantly affected by global climate change. However, their complex genomes and clonal propagation nature pose significant challenges for traditional breeding to improve quality and climate-resilient traits. Transgenics and genome editing offer promising solutions in crop improvement to enhance yield, quality, and biotic and abiotic stress tolerance. Despite these advancements, several challenges persist, such as a lack of genotype-independent transformation protocols, random transgene integration, unintended mutations, and somaclonal variation. The complexity of polyploid genomes also necessitates optimizing editing tools to improve precision and efficiency. Regulatory hurdles and public acceptance further influence the commercial success of genetically engineered crops. Employing efficient transgene-free genome-editing platforms can help to overcome the regulatory hurdles and accelerate breeding even in heterozygous backgrounds. This review reports the recent progress, obstacles, and prospects of transgenics and genome editing in vegetatively propagated crops, namely, potato, strawberry, banana, and sugarcane, focusing on quality and climate-resilient traits and methods to address technical challenges and navigate regulatory hurdles. The reported advancements in genetic engineering approaches for addressing challenges in improving the vegetatively propagated polyploid crops have tremendous potential in ensuring food security and agricultural sustainability in the face of climate change.
1 Introduction
Polyploidy is one of the most important forces of evolution and speciation in plants (Heslop-Harrison et al., 2023). Polyploids have more than two sets of chromosomes in their genome, and based on their origin, polyploids are divided into two distinct categories: autopolyploid and allopolyploid. In general, autopolyploids, such as potato (Solanum tuberosum L.) and bananas (Musa spp.), contain multiple sets of the same chromosomes originating from the same species as a result of intraspecific genome duplication (Sattler et al., 2016; Heslop-Harrison et al., 2023). In contrast, allopolyploids have multiple sets of the same chromosomes derived from different species of the same genus through hybridization and subsequent genome duplication (Sattler et al., 2016; Heslop-Harrison et al., 2023). Among cultivated polyploids, many of them are allopolyploids, including wheat (Triticum aestivum), sugarcane (Saccharum spp), strawberry (Fragaria × ananassa), soybean (Glycine max), grape (Vitis vinifera), apple (Malus domestica), and many others.
Polyploids exhibit complex genetic architecture and inheritance compared to diploids. They are characterized by high gene copy numbers, heterozygosity, genome size, gene redundancy, homoeologous recombination, repetitive sequences, complex meiotic behaviors, dosage effects, and altered gene expression patterns (Birchler et al., 2005; Comai, 2005; Osabe et al., 2012; Te Beest et al., 2012; Meirmans et al., 2018; Soares et al., 2021; Healey et al., 2024). The complex genetics of polyploids pose challenges in the varietal development process for breeding new cultivars, and common problems include infertility, hybrid sterility, and inbreeding depression (Levin, 2002; Comai, 2005; Paterson, 2005; Udall and Wendel, 2006; Sattler et al., 2016; Bradshaw, 2024).
Despite breeding difficulties, polyploids have numerous advantages in adaptation and biomass production compared to diploids (Udall and Wendel, 2006; Osabe et al., 2012; Sattler et al., 2016). High heterozygosity and genetic variability make polyploids more vigorous and improve their buffering capacity in response to various biotic and abiotic stresses compared to their diploid counterparts (Adams and Wendel, 2005; Van de Peer et al., 2009). Most polyploids have both asexual and sexual modes of reproduction, which enable the indefinite multiplication of transgressive segregants with desired characteristics obtained through hybridization between different parental lines (Chahal and Gosal, 2002; Comai, 2005; Osabe et al., 2012; Sattler et al., 2016; Schiessl et al., 2019). Vegetative propagation is the primary mode of reproduction widely used to cultivate polyploid crops such as potato, sugarcane, strawberry, banana, and apple. This allows these crops to maintain favorable heterozygosity and pass on hybrid superiority for many generations, making it easier to maintain true-to-type plants (Comai, 2005; Udall and Wendel, 2006). It is interesting to note that most vegetatively propagated polyploids show high levels of outcrossing and exhibit a perennial nature (McKey et al., 2010).
Classical breeding and marker-assisted breeding have played significant roles in improving yield, quality, and stress resistance in vegetatively propagated polyploid crop plants (Collard and Mackill, 2008; Bharadwaj, 2015; Sattler et al., 2016; Wolter et al., 2019). Although classical breeding is highly appealing, it is time-consuming and resource-intensive (Acquaah, 2015; Wolter et al., 2019). Additionally, factors such as self-incompatibility, hybrid sterility, infertility, and limited availability of variation and novel alleles for traits of interest in the natural gene pool severely constrain clonal crop improvement through traditional and marker-assisted breeding (Acquaah, 2015; Bharadwaj, 2015). Transgenic-based breeding and genome editing speed up crop improvement by introducing novel genes from different organisms and creating new alleles for existing traits, respectively, which are highly challenging to achieve using conventional techniques (Kamthan et al., 2016; Gao, 2021; Marone et al., 2023).
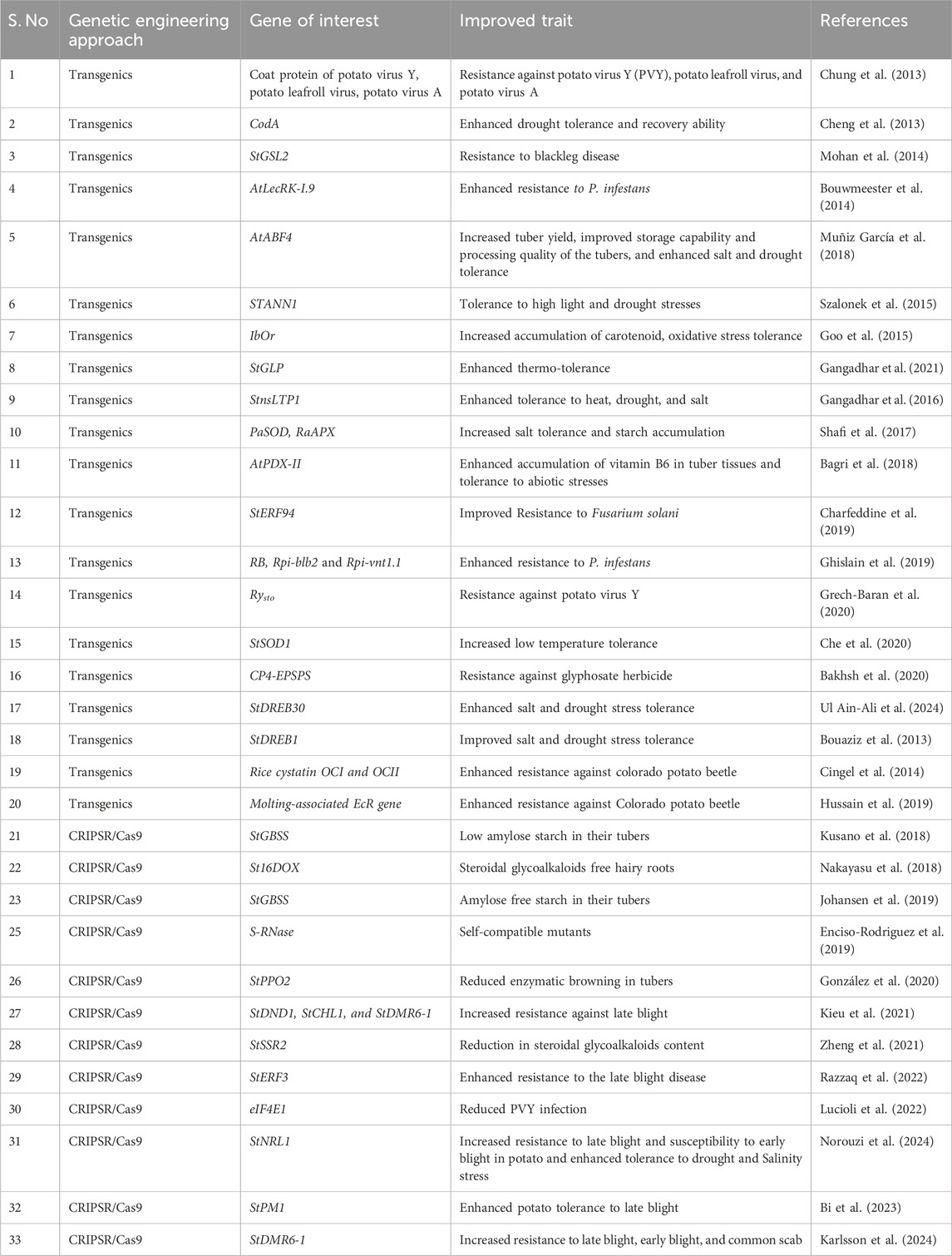
Climate change profoundly impacts crop production worldwide, with developing countries particularly vulnerable to its adverse effects (Kogo et al., 2021; Malhi et al., 2021; Yuan et al., 2024). The shifting precipitation patterns, rising temperatures, and increased frequency of extreme weather events such as unprecedented rainfall and severe droughts are significantly affecting crop production and threatening global food security (Kogo et al., 2021; Malhi et al., 2021; Yuan et al., 2024). These changes also facilitate the emergence and spread of new virulent strains of plant pathogens and insect pests, further exacerbating the situation (Malhi et al., 2021; Skendžić et al., 2021; Singh et al., 2023). Whereas several research and review articles have individually described transgenic and gene editing events in the context of climate resilience and quality related traits in vegetatively propagated crops (Tripathi L. et al., 2019; Schaart et al., 2021; Tiwari et al., 2022; Lakhani et al., 2023; Surya Krishna et al., 2023a; Vondracek et al., 2024). However, there is a lack of comprehensive review addressing both approaches collectively. The current review aims to bridge this existing gap by providing an integrated overview of both transgenic and gene editing studies relevant to these crops. The current review cited the literature from 2002 to 2025 based on articles, book chapters, and websites. The information was sourced from Google Scholar, PubMed, Web of Science, and other databases. The listed transgenic events and gene-edited lines provided based on literature between 2011 and 2025. This review highlights the importance of transgenics and genome editing approaches, mainly CRISPR/Cas (Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated proteins), in developing climate-resilient and quality-enhanced cultivars in the era of climate change, focusing on four major clonally propagated polyploid crops: potato, banana, strawberry, and sugarcane (Figure 1).
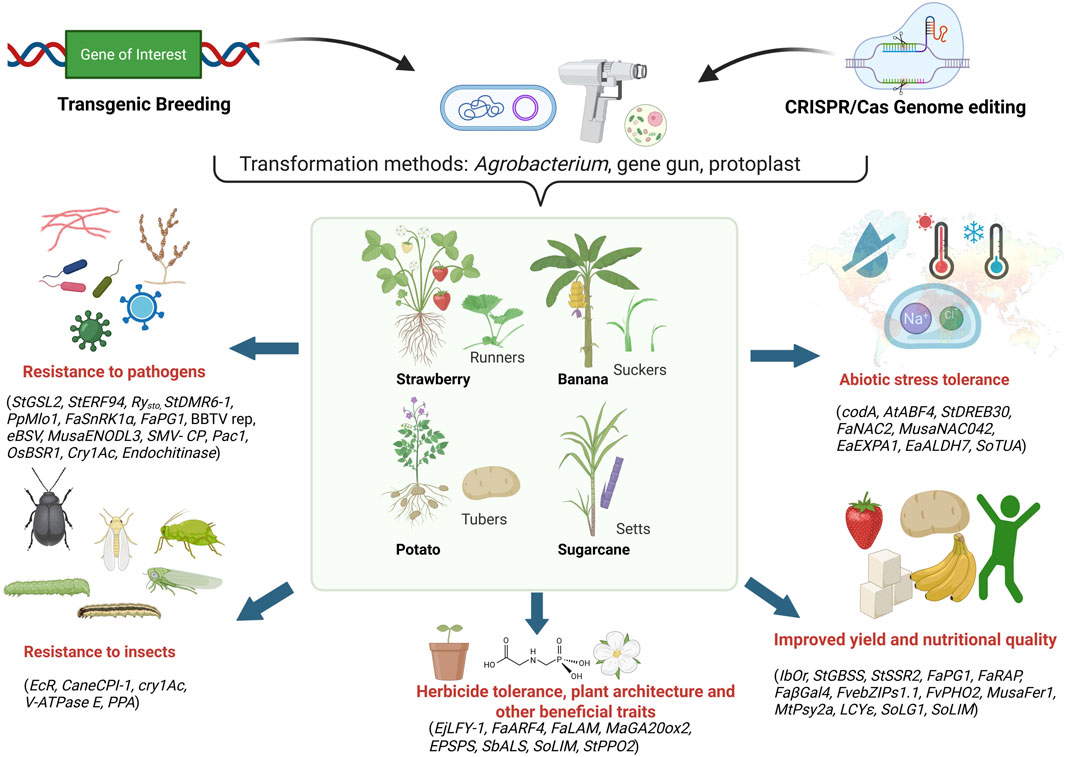
Figure 1. Application of transgenics and genome editing for improving abiotic stress tolerance, biotic stress resistance, yield, quality and other beneficial traits in vegetatively propagated polyploids by targeting genes of interest. Runners, suckers, tubers, and setts represent the commercial propagation materials of strawberry, banana, potato, and sugarcane, respectively Image created using Biorender (https://BioRender.com).
2 Potato
Potato (Solanum tuberosum L.) is the third most important food crop in the world, following wheat and rice, and is consumed widely across the globe (Halterman et al., 2016; del Mar Martínez-Prada et al., 2021). Significant progress has been made in developing transgenic and genome-edited potatoes with improved yield, pest and disease resistance, and enhanced quality (Nahirñak et al., 2022). In potato, the transformation efficiency was reported from 3% to 60%, which is highly dependent on type of genotype, explant and transformation method (Craze et al., 2018; Nahirñak et al., 2022). Agrobacterium-mediated transformation is the most commonly used method in potato genetic engineering (Craze et al., 2018; Nahirñak et al., 2022). Since 1995, various transgenic potato events have been developed for commercial purposes. First, Monsanto introduced the transgenic potato, NewLeaf, which conferred resistance to the Colorado potato beetle through the introduction of the cry3A gene in the U.S. and Canada (Halterman et al., 2016; del Mar Martínez-Prada et al., 2021). By 1998, enhanced versions of NewLeaf were created by stacking additional transgenes, such as PLRV replicase, Helicase, and PVY coat protein, providing resistance to potato leafroll virus and potato virus Y, alongside the cry3A gene (Halterman et al., 2016; del Mar Martínez-Prada et al., 2021).
Amylopectin from potatoes has various applications in bioplastic, textile, and ethanol industries; separating it from the amylose adds more processing cost, and chemical treatments lead to environmental pollution. Techniques such as RNA interference (RNAi) and CRISPR/Cas9 have successfully silenced or knocked out the granule-bound starch synthase (GBSS) genes responsible for amylose production, enabling the development of amylose-free potatoes for industrial applications (Brummell et al., 2015; Andersson et al., 2017; 2018; Kusano et al., 2018). Acrylamide, a carcinogenic compound formed from a reaction between natural sugars and the amino acid asparagine during high-temperature cooking (e.g., frying, roasting, or baking), poses health risks (Bethke and Bussan, 2013). Using RNAi, Zhu et al. (2014) silenced the vacuolar invertase (VInv) gene to develop low-acrylamide potatoes by lowering the reducing sugar formation in the tubers and making potatoes more suitable for French fries. In 2015, the U.S. approved the commercialization of J.R. Simplot’s Innate® 1.0 potatoes, which exhibit reduced acrylamide formation and black spot bruising through the downregulation of Asn1 and Ppo2 genes (del Mar Martínez-Prada et al., 2021; ISAAA, 2025). By 2017, Innate® 2.0 potatoes further incorporated traits such as resistance to late blight pathogens, reduced bruising and asparagine content, and enhanced cold storage ability (del Mar Martínez-Prada et al., 2021; ISAAA, 2025).
CRISPR/Cas-mediated genome editing has also significantly advanced potato improvement. For instance, the knockout of the St16DOX gene eliminates toxic steroidal glycoalkaloids (Nakayasu et al., 2018). CRISPR/Cas9-mediated knockout of an S gene, StPM1 gene in potato enhanced the resistance to Phytophthora infection without affecting growth and development (Bi et al., 2023). StPM1 negatively regulates plant immunity against invading pathogens by aiding vacuolar-mediated degradation of StRbohC, an NADPH oxidase that is involved in oxidative burst under infection conditions (Bi et al., 2023). Furthermore, the knockout of StNPR3 gene showed resistance to potato zebra chip disease through salicylic acid (SA) mediated defense and jasmonic acid (JA) catabolism (Ramasamy et al., 2024). StNPR3-edited lines showed higher expression of marker genes for plant defense such as NPR1, WRKY6, PR1 and PR3 and exhibited enhanced SA accumulation under both uninfected and infected conditions (Ramasamy et al., 2024). Veillet et al. (2019) employed cytidine base editors to modify ALS genes, further demonstrating the efficacy of base editing in potatoes. A recent review by Kumari et al. (2024), specifically focused on genome editing in potato, provides detailed information on the current status and applications of genome editing in this crop. We provided an overall list of events related to quality and stress tolerance in Table 1 which highlights the application of transgenics and genome editing techniques for potato crop improvement.
3 Strawberry
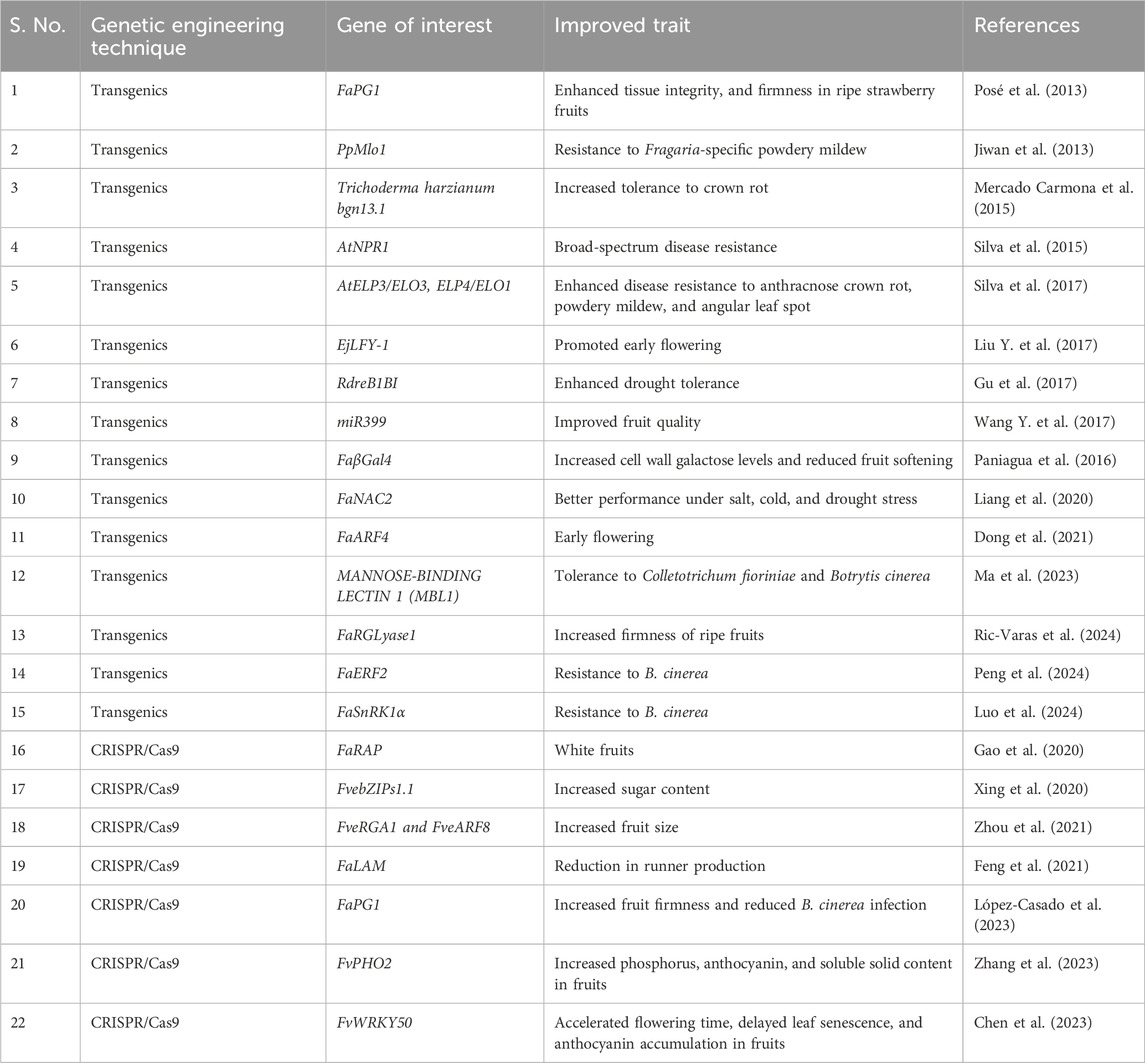
Strawberry is one of the economically important fruit crops, widely grown and consumed in different parts of the world for its organoleptic properties and nutritional value (Biswas et al., 2019). Cultivated strawberry (Fragaria × ananassa) is an allo-octoploid (2n = 8x = 56) originated from the interspecific hybridization between diploid progenitors: Fragaria vesca and Fragaria iinumae (Jin et al., 2023; Song et al., 2024). Due to the high ploidy level and heterozygous nature, breeding strawberries through classical methods for targeted trait improvement is a daunting task. Overexpressing or functional downregulation of a gene of interest for a particular trait improvement has been more promising compared to classical breeding methods, and the desired transgenic events or mutants can be multiplied using either runners or through tissue culture (López-Casado et al., 2023; Zhang et al., 2023; Luo et al., 2024). In addition to crop improvement, genetic engineering approaches also serve as an efficient tool for validating the QTLs or genes identified through genomics and transcriptomics.
Transgenics have been successfully employed for quality improvement and disease resistance in strawberry (Wang Y. et al., 2017; Li et al., 2021; Zhang et al., 2021; Luo et al., 2024). Agrobacterium-mediated transformation is commonly used for both the development of transgenic events and genome-edited (GE) lines, with an efficiency ranging between 2.9%–100% depending on the genotype and explants used for genetic transformation (Schaart, 2014; Vondracek et al., 2024). Transgenic plants overexpressing miR399a showed increased phosphorus uptake and improved total sugar, soluble solid, and vitamin C contents (Wang Y. et al., 2017). The fungal diseases, gray mold caused by Botrytis cinerea and anthracnose by Colletotrichum species, are the devastating fruit rot diseases in strawberry that severely affect fruit yield and quality (Petrasch et al., 2019; Ji et al., 2022). Zhang et al. (2021) reported that overexpression of FaMAPK5 and FaMAPK10 genes showed increased resistance to gray mold fungus and enhanced production of antioxidants. FaSnRK1α gene overexpression in strawberry plants induced the expression of SA biosynthetic genes, FaPAL1 and FaPAL2, which helped to obtain the elevated levels of SA and thus led to the enhanced resistance to B. cinerea (Luo et al., 2024). Further, Under B. cinerea infection, FaSnRK1α-OE lines had increased concentrations of SA allowing FaSnRK1α to interact with TGA and WRKY33.2 transcription factors to induce the expression of pathogen-related genes, namely, PR1.1, FaPR1.2, FaPR1.5, FaPR4.2, FaPR4.3, and FaPR5, which ultimately improved fruit resistance to B. cinerea (Luo et al., 2024). In addition to biotic stresses, strawberry production is significantly affected by abiotic stresses including, extreme temperatures, drought, and salinity, that affect growth, physiology, fruit yield, and quality (Ghaderi et al., 2018; Menzel, 2021; Ullah et al., 2024; Rodríguez-Aguirre et al., 2025). Heterologous expression of At-rty improved drought stress tolerance in strawberry due to accumulation of indolylacetic acid (IAA) and abscisic acid (ABA) (Li et al., 2021). The transgenic lines showed improved water use efficiency and reduced water loss along with the enhanced activity of antioxidants under drought conditions (Li et al., 2021).
Despite the octoploid nature of the cultivated strawberry, genome editing has also shown some significant success in enhancing disease resistance and altering the fruit properties. For example, CRISPR/Cas9 induced PG1 mutants in octoploid strawberry have shown less B. cinerea infection, increased fruit firmness, and altered fruit shape (López-Casado et al., 2023). Martín-Pizarro et al. (2019) edited the FaTM6 genes using CRISPR/Cas9 GE to study the role of TM6 MADS-box gene in the octoploid strawberry. The mutants showed abnormal flower morphology, decreased pollen production, and receptacle growth. CRISPR/Cas9 GE has been effectively used to manipulate the coloration of strawberry fruits. The RAP gene plays a crucial role in anthocyanin formation in strawberries, which gives the characteristic red color (Luo et al., 2018; Gao et al., 2020). It encodes a glutathione S-transferase (GST) protein that facilitates the transport of anthocyanins from the cytosol to the vacuole, where they accumulate to give the fruit its red color (Luo et al., 2018; Gao et al., 2020). The knockout of multiple copies of FaRAP gene has produced white colored strawberry fruits (Gao et al., 2020). The application of transgenics and genome editing techniques for strawberry crop improvement is given in Table 2. The recent reviews focused on strawberry biotechnology programs, genomic resources, transgenics, and genome editing have been listed and discussed, along with the prerequisites for crop improvement (Mukherjee and Gantait, 2024; Vondracek et al., 2024). Genetic engineering and genome editing have huge potential in improving strawberry crop production by enhancing fruit quality, producing novel fruit types, and developing stress-resilient strawberry cultivars.
4 Banana
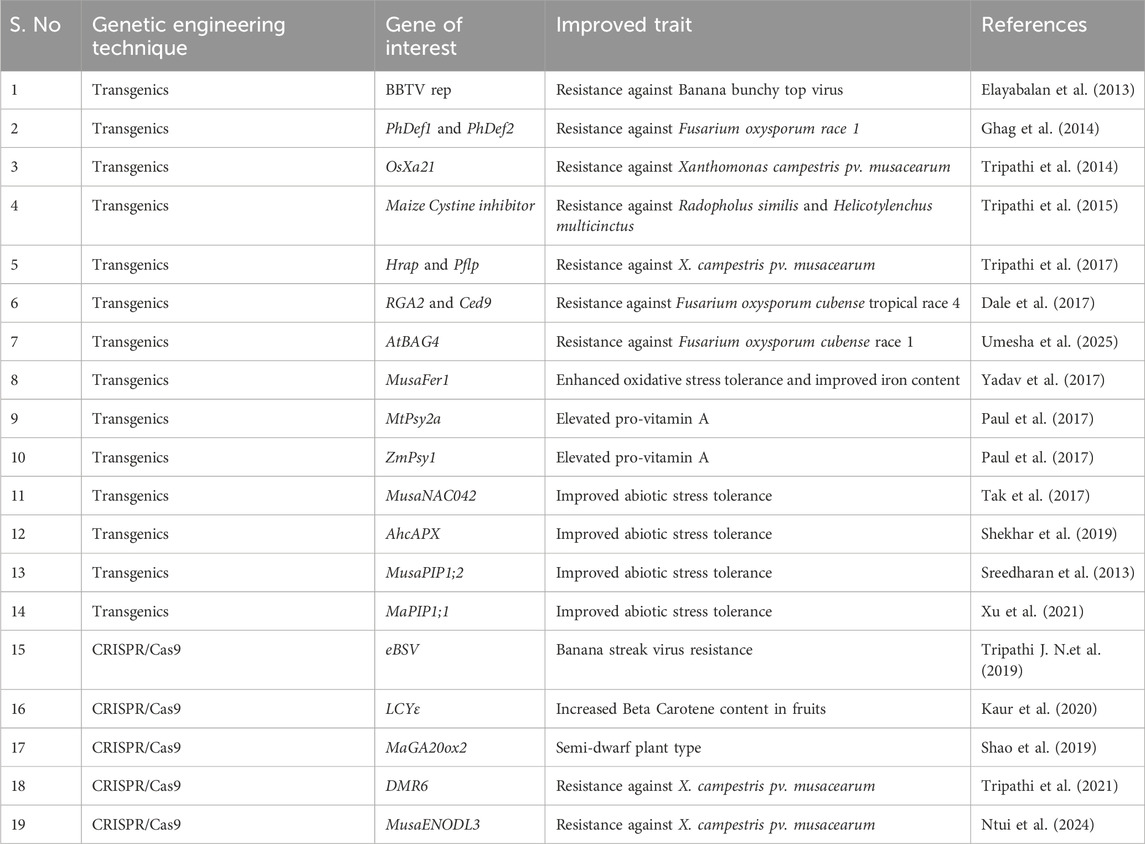
Banana (Musa spp.) is one of the important fruit crops consumed globally from the tropics to temperate regions and plays a significant role in satisfying the nutrient needs of people (Tripathi et al., 2024b). Most edible are diploid or triploid hybrids from Musa acuminata (A-genome) alone or from hybridization with Musa balbisiana (B-genome) (Perrier et al., 2011). Most commercial cultivars are seedless triploids, such as the commercially important Cavendish dessert banana (AAA) and the staple cooking African plantains (AAB) (Sardos et al., 2016). The gametic sterility, coupled with selection for edible pulp enhancement, led to parthenocarpic fruits during the domestication process (Perrier et al., 2011; Sardos et al., 2016). Bananas are propagated primarily through suckers and the meristem tip culture. Meristem tip culture produces clean seed material without any disease infection (Saraswathi et al., 2024).
Despite their major role in food security, bananas are one of the least genetically improved crops through breeding, due to their parthenocarpic nature, sterility, heterozygosity, and polyploid nature (Nansamba et al., 2020; Ganapathi et al., 2021; Tripathi et al., 2024b). In this scenario, genetic engineering offers endless opportunities for their crop improvement (Ganapathi et al., 2021; Tripathi et al., 2024b). Transgenic bananas have been effective against various biotic and abiotic stresses. Agrobacterium-mediated transformation is a majorly reported method for banana genetic modification due to its advantages of stable integration and lower copy number insertions (Cheng et al., 2024). Transformation efficiencies was ranging from as low as 2% to as high as 100%, depending on the explant, transformation method, and genotype (Liu J. et al., 2017; Dong et al., 2020). RNAi transgenic bananas expressing acetylcholinesterase genes from the aphid Pentalonia nigronervosa, showed reduced banana aphid infestation (Jekayinoluwa et al., 2021). Banana aphids are the vectors of banana bunchy top virus (BBTV), the causal agent of banana bunchy top disease. Besides BBTV, one of the devastating diseases of banana is fusarium wilt caused by the fungus, Fusarium oxysporum f. sp. Cubense. Transgenic lines overexpressing Ced9 anti-apoptosis gene derived from the nematode Caenorhabditis elegans (Paul et al., 2011) and RGA2 (Dale et al., 2017), a putative nucleotide-binding and leucine-rich repeat (NB-LRR)-type resistance (R) gene, from a seedling of Musa acuminata ssp. malaccensis showed resistance to fusarium wilt disease. On the other hand, banana transgenic lines have been very successful in combating abiotic stresses. Overexpression of aquaporin genes such as MusaPIP1;2 and MaPIP2-7 exhibited an improved stress tolerance to various abiotic stresses such as drought, cold, and salinity (Xu et al., 2020; 2021). The transgenic lines achieved enhanced stress tolerance and exhibited elevated proline, soluble sugar, chlorophyll, K+/Na+ ratio, and ABA content with lower ion leakage and malondialdehyde (MDA) content under stress conditions (Xu et al., 2020; 2021). Genetic engineering has not only proven to be effective against biotic and abiotic stresses but also improved nutritional content. For example, golden bananas developed through the expression of a Fe’i banana-derived phytoene synthase 2a (MtPsy2a) gene and maize phytoene synthase 1 (ZmPsy1) gene showed elevated pro-vitamin A in the fruits under field conditions (Paul et al., 2017).
Genome editing has been applied for creating stress-tolerant mutants and functional genomics studies in banana crop improvement programs. Mutating downy mildew resistance 6 (DMR6), a susceptibility gene encoding 2-oxoglutarate Fe(II)-dependent oxygenase (2OGO), induced the resistance to Banana Xanthomonas Wilt (BXW), bacterial disease, under field conditions without showing any off-target effects (Tripathi et al., 2021). DMR6 acts as a suppressor of plant immunity and is upregulated under pathogen infection (Tripathi et al., 2021). CRISPR/Cas9-based genome-editing technology was applied to inactivate endogenous banana streak virus (eBSV) in the B genome of plantain (AAB), and these GE lines showed 75% less viral symptoms under water stress conditions in comparison to the control plants, indicating the inactivation of eBSV in GE lines (Tripathi J. N. et al., 2019). CRISPR/Cas9 GE was used to develop the β-carotene-enriched banana cultivar by mutating the fifth exon of the lycopene epsilon-cyclase (LCYε) gene (Kaur et al., 2020). Metabolic profiling of the GE fruits showed enhanced accumulation of β-carotene content up to 6-fold, suggesting the potential of CRISPR technology in improving the nutritional quality of the bananas without the aid of transgenes (Kaur et al., 2020). Some of the recent reviews primarily focused on banana improvement through biotechnological applications, providing an elaborate discussion on banana genetic resources and genetic engineering (Cheng et al., 2024; Tripathi et al., 2024b). Table 3 highlights the application of transgenics and genome editing techniques for banana crop improvement.
5 Sugarcane
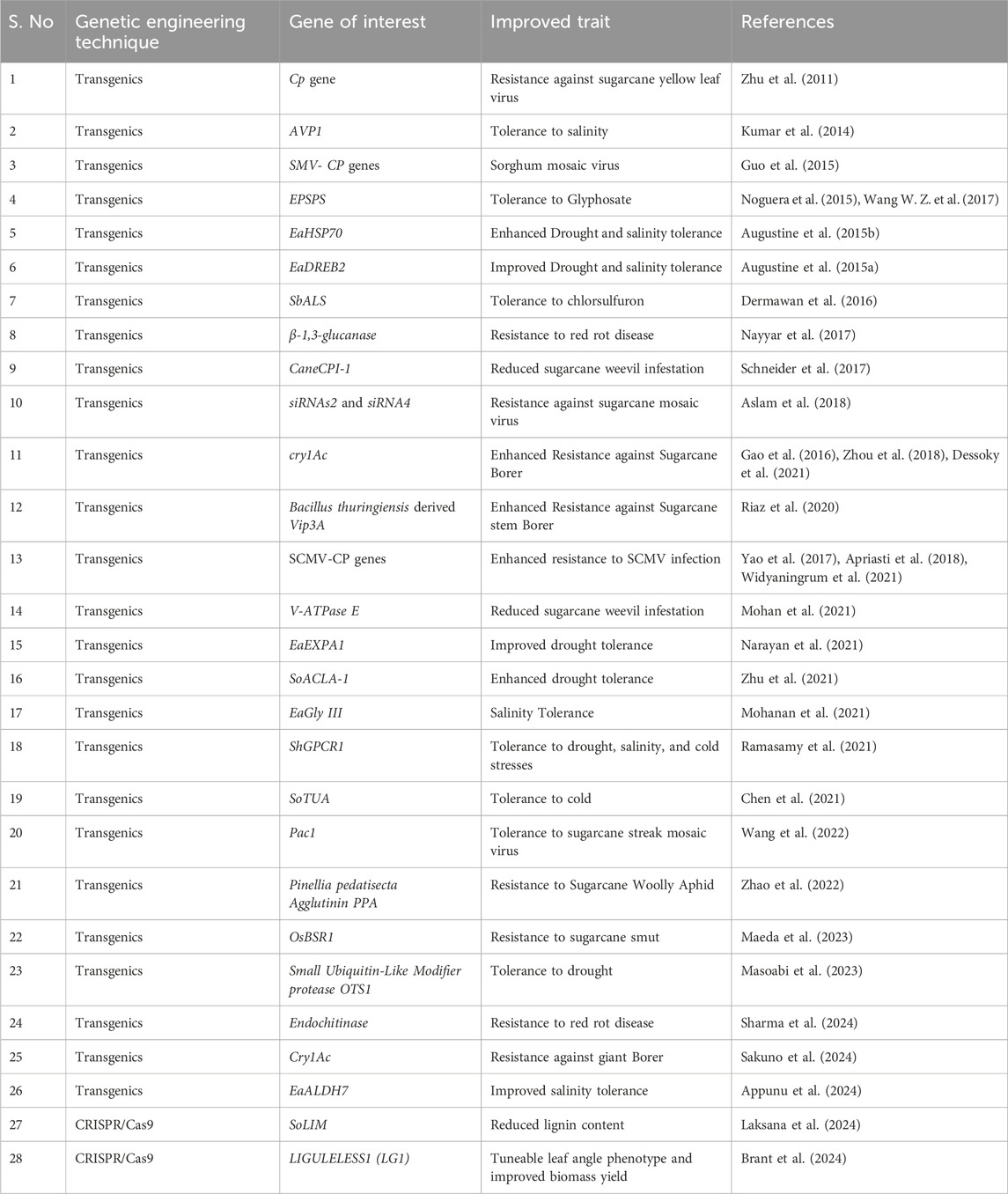
Sugarcane is an important crop primarily cultivated in the tropical and subtropical regions of the world (Dinesh Babu et al., 2022; Surya Krishna et al., 2023a). It plays a crucial role in satisfying global sugar demands, accounting for 80% of global sugar production (Dinesh Babu et al., 2022). Beyond sugar production, sugarcane is the major raw material for bioethanol production, a cleaner alternative to fossil fuels, and byproducts such as molasses and bagasse, which are used in various industries ranging from alcohol production to electricity generation (Surya Krishna et al., 2023a). Sugarcane possesses a highly complicated genetic architecture that makes crop improvement and genetic studies challenging. Unlike other cultivated polyploids, sugarcane is an aneuploid with an interspecific origin, with chromosomes ranging from 80 to 130 (Vieira et al., 2018; Piperidis and D’Hont, 2020; Healey et al., 2024). Modern cultivars (Saccharum spp.) are interspecific hybrids with tolerance to the aneuploid constitution, producing offspring with unique chromosome combinations when propagated through seeds (Vieira et al., 2018; Healey et al., 2024). In this context, genetic engineering techniques offer a unique advantage by enabling the introduction or modification of genes for specific traits in superior clones without altering other traits, a process that is highly challenging to achieve through conventional or marker-assisted breeding (Verma et al., 2022; Surya Krishna et al., 2023a).
Despite the crop’s large genome size and high ploidy level, transgenics have successfully improved biotic and abiotic stress tolerance (Nayyar et al., 2017; Ramasamy et al., 2021; Appunu et al., 2024; Chinnaswamy et al., 2024; Sharma et al., 2024). Particle bombardment and Agrobacterium-mediated transformation have been widely used for sugarcane genetic transformation (Surya Krishna et al., 2023a). Transformation efficiency of sugarcane is lower than most other crops and is affected by various factors such as explants, genotypes, transformation methods, and others (Verma et al., 2022). Red rot and viral diseases pose significant threats to sugarcane production and affect juice quality (Surya Krishna et al., 2023a; 2023b). Transgenic events overexpressing the β-1,3-glucanase gene and endochitinase gene from Trichoderma spp. have shown resistance against the red rot pathogen infection (Nayyar et al., 2017; Sharma et al., 2024). Virus resistant events have been created through the coat protein genes of the sugarcane yellow leaf virus and sugarcane mosaic virus (Budeguer et al., 2021; Surya Krishna et al., 2023b). Additionally, Bt technology has been successfully employed in sugarcane to develop transgenic events resistant to sugarcane borers (Gao et al., 2016; Wang W. Z. et al., 2017; Cristofoletti et al., 2018; Dessoky et al., 2021). Many abiotic stress tolerance events have been developed, and the overexpression of transcription factors such as TERF1 and EaNF-YB2 showed improved drought stress tolerance (Rahman et al., 2021; Chinnaswamy et al., 2024). The improved proline and antioxidant activity achieved the improved stress tolerance in these transgenic lines and effective management of hydrogen peroxide and MDA under water deficit conditions (Rahman et al., 2021; Chinnaswamy et al., 2024). Next to drought, salinity stress is a significant abiotic stressor that affects sugarcane production. Overexpression of genes such as EaGly III (Mohanan et al., 2021) and EaALDH7 (Appunu et al., 2024) have been proven to enhance sugarcane resilience to salinity stress. Furthermore, transgenic events overexpressing the sugarcane G-protein-coupled receptor (ShGPCR1) exhibited tolerance to multiple abiotic stresses, such as drought, salinity, and cold (Ramasamy et al., 2021). GPCRs play a crucial role in plant stress tolerance by acting as molecular switches that transmit signals upon exposure to drought, salinity, and temperature extremes, to the cellular machinery (Ramasamy et al., 2021; Majumdar et al., 2023). Upon activation, G-proteins interact with various effectors and secondary messengers, regulating the expression of stress-responsive genes, including those coding for antioxidative enzymes and osmoprotectants, thus leading to acquired stress tolerance (Ramasamy et al., 2021; Majumdar et al., 2023).
Despite the complex genetic nature of sugarcane, genome editing has been successful in inducing targeted mutations in multiple alleles of marker genes such as magnesium chelatase subunit I (MgCh) (Eid et al., 2021) and acetolactate synthase (ALS) genes (Oz et al., 2021). Laksana et al. (2024) successfully produced genome-edited lines with low lignin content, making them suitable for second-generation bioethanol production by editing the SoLIM transcription factor. CRISPR/Cas9 genome editing of multiple copies of the LIGULELESS1 (LG1) gene resulted in tunable leaf angle phenotypes, and the mutants showed a high biomass yield and tillers under field conditions (Brant et al., 2024). Despite the complexity of sugarcane’s genome, genetic engineering and gene editing have tremendous potential to accelerate the breeding process, as recent reports demonstrate its success in improving various traits (Surya Krishna et al., 2023a; Kumar et al., 2024; Brant et al., 2025). The application of transgenics and genome editing techniques for sugarcane crop improvement is given in Table 4.
6 Current challenges and future directions
Crop improvement through inter and intraspecific crosses and genome-assisted breeding has been promising in crop plants (Ahmar et al., 2020; Gaikwad et al., 2020). Despite their huge success in diploids and seed-propagated crops, it has been highly challenging to obtain the same success in polyploids due to the complex genetic architecture. Polyploids have multiple homologous or homeologous copies of each chromosome, resulting in multiple alleles at each genetic locus, making it difficult to track the introgressed genes or to precisely recover the genetic background of the recipient parent during backcrossing (Jiang et al., 2000; Heslop-Harrison et al., 2023). The segregation of traits in polyploids does not follow simple mendelian ratios due to polysomic inheritance (in autopolyploids) or disomic inheritance with sub-genome interactions (in allopolyploids) (Feldman and Levy, 2009; Parisod et al., 2010; Leal-Bertioli et al., 2018). This makes it notably more challenging to recover the target trait or maintain heterozygosity for the polygenic traits. During introgression, repeated backcrossing may lead to loss of desirable heterozygosity, negatively affecting agronomic performance and adaptability. On the other hand, cross-compatibility barriers, such as low fertility in hybrids, hybrid sterility, incomplete pairing of homologous chromosomes, or failure of chromosome doubling, genomic instability, and epigenetic modifications, make the situation even more complicated (Birchler et al., 2005; Comai, 2005; Osabe et al., 2012; Te Beest et al., 2012; Soares et al., 2021; Healey et al., 2024). Considering the complexities associated with utilizing traditional breeding methods for polyploid crop improvement, genetic engineering serves as a viable alternative for the targeted improvement of vegetatively propagated polyploids for various agronomic traits, including yield, quality, and stress resistance (Kamthan et al., 2016; Gao, 2021; Marone et al., 2023). The vegetative propagation allows for clonal replication of desirable GE mutants and transgenic events, preserving traits without the segregation and variation associated with sexual reproduction (Schaart et al., 2021). This unique advantage makes genetic engineering techniques more promising for polyploid breeding. However, it also has some drawbacks, such as the need for field gene banks for germplasm maintenance, pathogen accumulation in plant material, and the increased susceptibility of monocultures to pest or disease epidemics (Chahal and Gosal, 2002; Acquaah, 2015).
Although the clonal propagation allows the fixation of desirable alleles in a single generation, when it comes to removing the Cas9 and other transgenes from the desirable GE mutants leaving the mutation unaffected is an arduous task. Transformation techniques leading to genetically modified plants reduce public acceptance of targeted mutagenized (edited) plants (Nguyen et al., 2023). However, the issue of stable transgene integration can be addressed by transient transformations with ribonucleoprotein complexes (RNPs) that degrade rapidly once they enter the cell and possess less off-target effects (Gu et al., 2021; He et al., 2022; Surya Krishna et al., 2023a). Scoring GE mutants is challenging in polyploid genomes as multiple mutation types can occur within a single gene, as each gene copy may harbor different mutations in the targeted region. In addition, the presence of somaclonal variation in the GE mutants could make the evaluation of genome-editing lines challenging (Fossi et al., 2019; Li et al., 2019; Graham et al., 2020). The somaclonal variation is more prevalent in the protoplast-generated plants (Fossi et al., 2019; Li et al., 2019). The other significant challenges are lower transformation efficiency, genotype dependency, lower editing efficiency, low in vitro regeneration ability, and more off-target effects, underscoring the need for genotype-independent, multiplex, and DNA-free delivery systems (May et al., 2023). Some of the important factors that significantly impact GE efficiency are guide RNA selection, promoters for guide RNA and Cas expression and crop specific codon optimization of the Cas gene. The most commonly used promoter to drive Cas9 expression in potato genome editing is the CaMV35S (Kusano et al., 2018; Nakayasu et al., 2018; Enciso-Rodriguez et al., 2019; Kieu et al., 2021; Bi et al., 2023; Karlsson et al., 2024; Norouzi et al., 2024). Similarly, CaMV35 is also used in strawberry (Gao et al., 2020; Feng et al., 2021), banana (Kaur et al., 2020; Tripathi et al., 2021; Ntui et al., 2024) and sugarcane (Brant et al., 2024). However, other promoters such as pUbi (Shao et al., 2019; Xing et al., 2020; Chen et al., 2023), PcUbi (Tripathi J. N. et al., 2019; López-Casado et al., 2023), AtUBQ (Zhou et al., 2021), and PPDK (Johansen et al., 2019; Lucioli et al., 2022) have also been used for Cas9 expression. For guide RNA expression in these crops, endogenous U6 promoters or U6 promoters from rice and Arabidopsis are commonly used.
Several techniques based on PCR and sequencing are available to detect mutations in the GE lines. PCR based approaches such as PCR-RFLP, T7E1, heteroduplex mobility assays (HMA), Competition-based PCR, high-resolution melting analysis, and digital droplet PCR enable the rapid preliminary screening of GE mutants in an inexpensive and rapid way (Jung and Altpeter, 2016; Lomov et al., 2019; Camerlengo et al., 2020). However, these techniques only provide information about the presence or absence of a mutation but fail to indicate the type of mutation. The sequencing of the targeted loci in GE mutants provides detailed information about the types of mutations, namely, base substitutions or Insertions and Deletions (INDELs) and the allelic nature of the genes targeted. Generally, the sanger sequencing of amplicons of the targeted regions has been carried out to identify the nature and frequency of the induced mutation (Grohmann et al., 2019; Yun et al., 2022). Despite the advantages of sanger sequencing, multiple alleles of the polyploids complicate the interpretation of sequencing results, as it leads to overlapping peaks in the electropherogram, making it difficult to read the sequence accurately (Zischewski et al., 2017; Cardi et al., 2023). Polyploids often have heterozygous mutations spread across different allelic copies, which makes it difficult to resolve heterozygous loci using sanger sequencing (Griffin et al., 2011). In polyploids, one or two mutant alleles may be overshadowed by multiple copies of the wild-type alleles. This lowers the mutant allele frequency in the sequencing signal, making it hard to detect point mutations. However, next-generation sequencing technologies address the limitations associated with sanger sequencing by offering better resolution and coverage, enabling them to detect mutations in multiple alleles as well as off-target mutations (Griffin et al., 2011; Zischewski et al., 2017; Motazedi et al., 2018; Li et al., 2019; Cardi et al., 2023). For instance, Brant et al. (2024) used CRISPR Amplicon Next-Generation Sequencing to identify the mutation in the multiple alleles of LIGULELESS1 gene is sugarcane. Similarly, Martín-Pizarro et al. (2019) sequenced the GE mutants of strawberry for TM6 MADS-box gene using high-throughput paired-end amplicon sequencing. In the case of transgenics, transgene integration is random in the genome. PCR-based screening followed by southern blot hybridization enables the identification of transgenic events with stable transgene integration (Liu Y. et al., 2017; Widyaningrum et al., 2021; Vennapusa et al., 2022; Jiang et al., 2023; Umesha et al., 2025). However, the next-generation sequencing of transgenic events allows us to find out the exact location of the transgene integration as well as the gene copy numbers (Park et al., 2017; Xu et al., 2024). It also enables us to study the potential off-target effects based on the site of the transgene integration, whether in the gene-rich or non-genic regions of the genome. Despite the progress made in the crops discussed in this review, genome editing remains under-deployed in other vegetatively propagated polyploids such as sweet potato, yam, and taro, which are important food crops in some tropical countries (Divya et al., 2024; Tripathi et al., 2024a). Transgenics have been successful in improving potato, banana, strawberry, and sugarcane for biotic and abiotic stress tolerance, nutritional content, and quality. In contrast, genome editing has shown notable progress in improving these crops for disease resistance. However, genome editing has shown few improvements in insect resistance, abiotic stress tolerance, and post-harvest applications. The complex regulatory networks involved in stress response and the highly complex nature of these traits hinder the improvement in abiotic stress tolerance ability by targeted editing of one or a few genes in these crops (Zhang et al., 2022; Kumar et al., 2023).
7 Genetically engineered crops and regulations
Transgenic events and site-directed nuclease (SDN) events are the two outcomes of genetic engineering techniques that enable the targeted crop improvement for a particular trait. Transgenic events involve the integration of foreign DNA into a plant genome to express novel traits that are not present in the gene pool of a crop species. Classic examples of commercialized transgenic crops are potatoes, sugarcane, cotton, corn, canola and soybean, with the first two (potato and sugarcane) being vegetatively propagated polyploids (Castagnola and Jurat-Fuentes, 2012; Koul et al., 2024; ISAAA, 2025). While transgenic technologies have successfully introduced significant improvements in crop traits, the foreign DNA from different organisms has led to public skepticism and stringent regulatory oversight, particularly regarding environmental and food safety concerns in many parts of the world (Lucht, 2015; Smyth, 2017; Koul et al., 2024).
On the other hand, genome-editing tools such as CRISPR/Cas9, TALENs (Transcription Activator-Like Effector Nucleases), and ZFNs (Zinc Finger Nucleases) make targeted modifications at specific genomic loci, offering higher accuracy and reduced off-target effects. SDN products are classified into three main categories SDN 1, SDN 2, and SDN 3 based on the nature of the genomic modifications (Figure 2) (Sprink et al., 2016; Zannoni, 2019). SDN-1 involves small base deletions insertions or substitutions at the target site by harnessing the power of non-homologous end joining (NHEJ) without the use of foreign DNA template (Sprink et al., 2016; Zannoni, 2019). SDN-2 introduces precise nucleotide substitutions or modifications using short donor DNA templates (Sprink et al., 2016; Zannoni, 2019). The products of SDN-1 and SDN-2 are transgene-free and indistinguishable from natural mutations. SDN-3 enables the site-specific integration of a long stretch of DNA sequences, producing plants similar to transgenics or cisgenics (Sprink et al., 2016; Zannoni, 2019). Genome-edited crops such as high oleic acid soybean, less bitter mustard greens, and high GABA tomato are on the market, offering consumers products with enhanced qualities achieved through precise genetic modifications (Koul et al., 2024; Genetic Literacy Project, 2025). As a result, SDN1-based crops are subject to less stringent regulations in many jurisdictions, leading to wider acceptance among the public and policymakers.
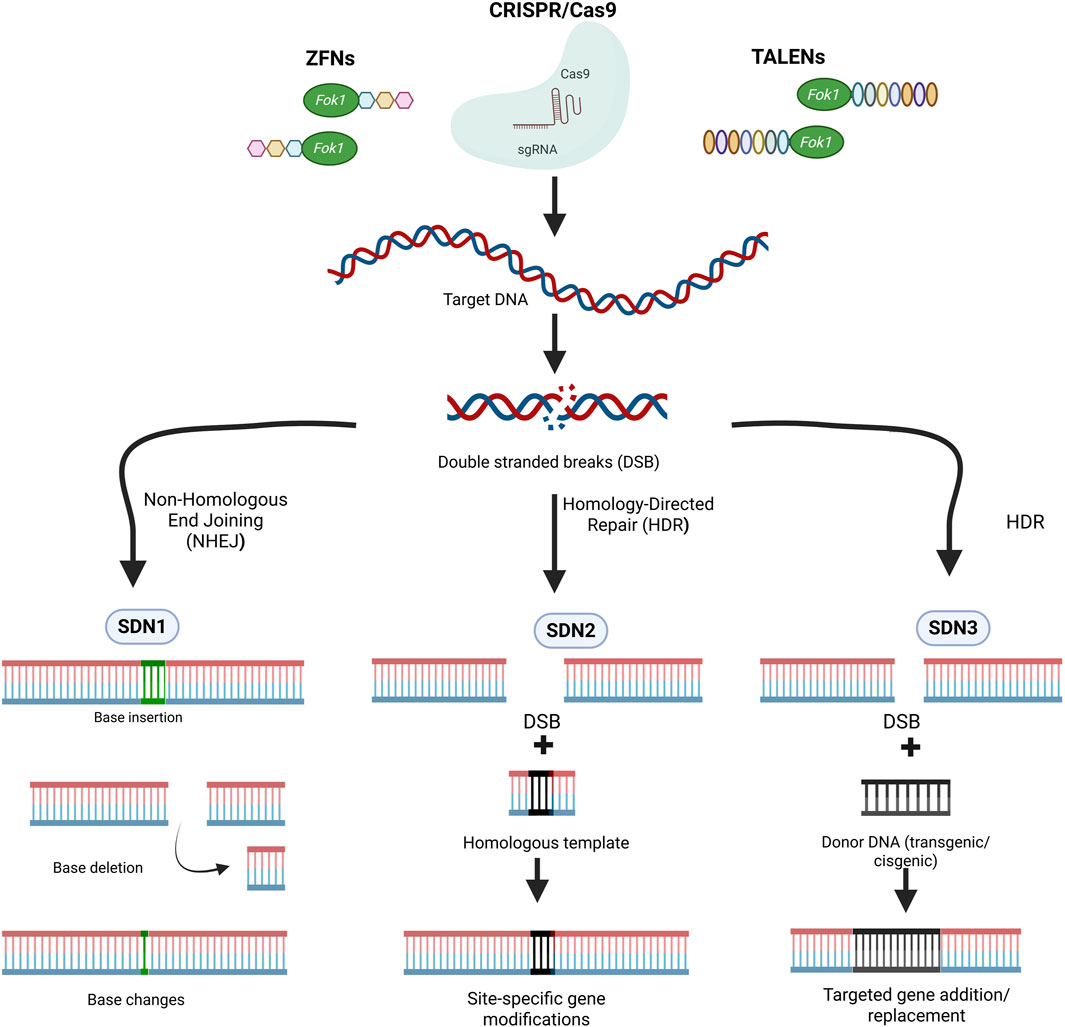
Figure 2. Classification of SDN (Site-Directed Nucleases) products for genome editing based on the nature of genomic modifications. (ZFN, Zinc Finger Nuclease; TALENs, Transcription Activator-Like Effector Nucleases; CRISPR/Cas9, Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated protein 9). Image created using Biorender (https://BioRender.com).
Regulations for new breeding techniques (NBTs) are rapidly evolving worldwide. GMO’s are primarily regulated in two ways as process-based regulation and product-based regulation (Sprink et al., 2016). Product-based regulations are followed by countries such as the United States, Canada, Japan, and a few others, where the guidelines and rules focus on the safety, efficacy, and impact of the final GMO products rather than the process used to create them (Sprink and Wilhelm, 2024; Genetic Literacy Project, 2025). Process-based regulation is followed by countries such as India, Australia, Brazil, and most others where the guidelines and rules focus on the techniques and methods used to create the GMOs rather than solely on the characteristics of the final product (Sprink and Wilhelm, 2024; Genetic Literacy Project, 2025). The United States, Canada, China, Japan, Brazil, and the Philippines have approved the SDN-1 GE events for commercial cultivation, and some other countries are under field trials (Koul et al., 2024; Sprink and Wilhelm, 2024; Genetic Literacy Project, 2025). Despite the commercial success of genome-edited seed crops like tomato, soybean, and mustard, the market success of vegetatively propagated GE mutants like potatoes, sugarcane, and bananas is yet to be seen (Koul et al., 2024; Genetic Literacy Project, 2025). The legal environment surrounding genome-edited plants presents a multifaceted challenge, with diverse national approaches impacting the pace and direction of research and innovation (Metje-Sprink et al., 2020; Koul et al., 2024; Sprink and Wilhelm, 2024).
8 Public perception of genetically engineered crops
Consumer attitudes towards these technologies exhibit significant variability due to limited public understanding, a lack of trust in information sources, and ethical and societal concerns (Wunderlich and Gatto, 2015; Cui and Shoemaker, 2018; Woźniak-Gientka et al., 2022; Medani et al., 2024). A primary concern is the potential for unintended health consequences, such as allergies, toxicity, or long-term health effects (Cui and Shoemaker, 2018; Woźniak-Gientka et al., 2022; Catherine et al., 2024). While extensive research has generally shown genetically modified (GM) foods to be safe, these concerns persist (Atherton, 2002; Hallman et al., 2013). Concerns include the potential for horizontal gene flow to wild plants, the impact on biodiversity, and the development of herbicide-resistant superweeds and pesticide residues in the plant products (Bawa and Anilakumar, 2013; Vennapusa et al., 2018; Gbashi et al., 2021; Medani et al., 2024). Some individuals have ethical concerns about interfering god’s creation by manipulating the genetic makeup of organisms and dependence on industries for seeds (Hossain and Onyango, 2004; Frewer et al., 2013; Scott et al., 2018; Medani et al., 2024). Many consumers lack trust in regulatory agencies, the food industry, and even scientists regarding GMOs (Ghasemi et al., 2013; Turker et al., 2013; Akbari et al., 2019). This lack of trust stems from past controversies, conflicting information, and perceived industry bias (Dean and Shepherd, 2007; Sorgo et al., 2012; Scott et al., 2016; Cui and Shoemaker, 2018; Robayo-Avendaño et al., 2018).
Public adoption of GM crops discussed in this review varied notably based on the socio-economic status, health benefits, environmental attributes, and awareness of GM technology (Kikulwe et al., 2011; Muringai et al., 2020; Vermeulen et al., 2020). A survey among Canadian consumers regarding the acceptance of gene-edited versus genetically modified potatoes showed that consumers preferred gene-edited products over GM products (Muringai et al., 2020). Some consumers are even interested in paying more for health benefits such as low acrylamide content when fried and for positive environmental attributes (Muringai et al., 2020). A study on South African consumers’ perception and acceptance of sugar made from genetically modified sugarcane showed that the acceptance reduced with socio-economic status and age (Vermeulen et al., 2020). Those who support GM sugar were willing to pay a higher premium amount, especially when environmental benefits were emphasized (Vermeulen et al., 2020). A consumer survey on the attitudes and perceptions of GM bananas in Uganda showed that over 90% of respondents agreed they would purchase GM bananas if they offered better taste or nutrition and were priced the same as conventional bananas (Kikulwe et al., 2011). However, acceptance dropped to 39% if GM bananas were priced higher than non-GMOs (Kikulwe et al., 2011). A study on consumer preference towards specialty labelled products among Utah consumers revealed that non-GMO labeling of strawberry provides tangible market advantages through consumer willingness to pay premiums, even if modest (Curtis et al., 2024). The consumer preference pattern suggests that non-GMO strawberries will likely maintain competitive advantages over GM alternatives, particularly when combined with other valued attributes like organic certification or local production (Curtis et al., 2024). The willingness to pay premiums for non-GMO labeling suggests underlying consumer concerns about genetic modification.
Bridging the gap between scientific consensus and public perception of GM technologies requires clear communication, transparency, and inclusive engagement (Gbashi et al., 2021; Woźniak-Gientka et al., 2022; Catherine et al., 2024). Simplifying concepts through multimedia tools and integrating biotechnology into education can foster understanding and trust (Gbashi et al., 2021; Catherine et al., 2024; Medani et al., 2024). Highlighting GM success stories, such as improved yield and nutritional qualities and reduced pesticide use, provides concrete proof of their benefits. Community outreach, open dialogues on ethics and safety, and leveraging trusted voices like scientists and farmers can address misconceptions and build confidence (Gbashi et al., 2021; Woźniak-Gientka et al., 2022; Catherine et al., 2024; Medani et al., 2024). These strategies can collectively enhance public trust and facilitate the responsible adoption of GM technologies. Furthermore, the global regulatory policy environment for GE crops (non-transgenics) is still emerging. This evolving landscape can shape innovation in crop improvement and influence its overall socioeconomic benefits to society (Schaart et al., 2021; Kalaitzandonakes et al., 2023; Lakhani et al., 2023).
9 Conclusion
While transgenics remain valuable for introducing novel genes and complex traits, genome editing has emerged as an efficient and precise platform for genome modifications, often with fewer regulatory hurdles. Both technologies enable targeted modifications in complex genomes, effectively overcoming traditional breeding challenges associated with ploidy levels and asexual reproduction in vegetatively propagated crops. By focusing on key traits such as biotic and abiotic stress resistance, yield improvement, and quality enhancement, genetic engineering and genome editing can significantly accelerate the development of superior cultivars in vegetatively propagated polyploid crops. As evidenced by past achievements, these approaches can be used to breed crops with desirable traits in minimal time compared to conventional breeding methods. Overall, transgenic and genome editing technologies play complementary roles in breeding clonally propagated polyploids, offering promising solutions to meet future food security challenges under changing climate scenarios.
Author contributions
SS: Writing – original draft, Conceptualization. AV: Writing – review and editing, Conceptualization. KM: Conceptualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors thank the United States Department of Agriculture (USDA-NIFA) for funding support through the award number 2020-38821-31083 and McIntire-Stennis award DELXMS2020-2025 to Delaware State University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acquaah, G. (2015). “Conventional plant breeding principles and techniques,” in Advances in plant breeding strategies: breeding, biotechnology and molecular tools. Editors J. M. Al-Khayri, S. M. Jain, and D. V. Johnson (Cham: Springer International Publishing), 115–158. doi:10.1007/978-3-319-22521-0_5
Adams, K. L., and Wendel, J. F. (2005). Polyploidy and genome evolution in plants. Curr. Opin. Plant Biol. 8, 135–141. doi:10.1016/j.pbi.2005.01.001
Ahmar, S., Gill, R. A., Jung, K.-H., Faheem, A., Qasim, M. U., Mubeen, M., et al. (2020). Conventional and molecular techniques from simple breeding to speed breeding in crop plants: recent advances and future outlook. Int. J. Mol. Sci. 21, 2590. doi:10.3390/ijms21072590
Akbari, M., Ardekani, Z. F., Pino, G., and Maleksaeidi, H. (2019). An extended model of Theory of Planned Behavior to investigate highly-educated Iranian consumers’ intentions towards consuming genetically modified foods. J. Clean. Prod. 227, 784–793. doi:10.1016/j.jclepro.2019.04.246
Andersson, M., Turesson, H., Nicolia, A., Fält, A.-S., Samuelsson, M., and Hofvander, P. (2017). Efficient targeted multiallelic mutagenesis in tetraploid potato (Solanum tuberosum) by transient CRISPR-Cas9 expression in protoplasts. Plant Cell Rep. 36, 117–128. doi:10.1007/s00299-016-2062-3
Andersson, M., Turesson, H., Olsson, N., Fält, A., Ohlsson, P., Gonzalez, M. N., et al. (2018). Genome editing in potato via CRISPR-Cas9 ribonucleoprotein delivery. Physiol. Plant. 164, 378–384. doi:10.1111/ppl.12731
Appunu, C., Krishna, S. S., Chandar, S. H., Valarmathi, R., Suresha, G. S., Sreenivasa, V., et al. (2024). Overexpression of EaALDH7, an aldehyde dehydrogenase gene from Erianthus arundinaceus enhances salinity tolerance in transgenic sugarcane (Saccharum spp. Hybrid). Plant Sci. 348, 112206. doi:10.1016/j.plantsci.2024.112206
Apriasti, R., Widyaningrum, S., Hidayati, W. N., Sawitri, W. D., Darsono, N., Hase, T., et al. (2018). Full sequence of the coat protein gene is required for the induction of pathogen-derived resistance against sugarcane mosaic virus in transgenic sugarcane. Mol. Biol. Rep. 45, 2749–2758. doi:10.1007/s11033-018-4326-1
Aslam, U., Tabassum, B., Nasir, I. A., Khan, A., and Husnain, T. (2018). A virus-derived short hairpin RNA confers resistance against sugarcane mosaic virus in transgenic sugarcane. Transgenic Res. 27, 203–210. doi:10.1007/s11248-018-0066-1
Atherton, K. T. (2002). Safety assessment of genetically modified crops. Toxicology 181, 421–426. doi:10.1016/S0300-483X(02)00485-7
Augustine, S. M., Ashwin Narayan, J., Syamaladevi, D. P., Appunu, C., Chakravarthi, M., Ravichandran, V., et al. (2015a). Overexpression of EaDREB2 and pyramiding of EaDREB2 with the pea DNA helicase gene (PDH45) enhance drought and salinity tolerance in sugarcane (Saccharum spp. hybrid). Plant Cell Rep. 34, 247–263. doi:10.1007/s00299-014-1704-6
Augustine, S. M., Narayan, J. A., Syamaladevi, D. P., Appunu, C., Chakravarthi, M., Ravichandran, V., et al. (2015b). Erianthus arundinaceus HSP70 (EaHSP70) overexpression increases drought and salinity tolerance in sugarcane (Saccharum spp. hybrid). Plant Sci. 232, 23–34. doi:10.1016/j.plantsci.2014.12.012
Bagri, D. S., Upadhyaya, D. C., Kumar, A., and Upadhyaya, C. P. (2018). Overexpression of PDX-II gene in potato (Solanum tuberosum L.) leads to the enhanced accumulation of vitamin B6 in tuber tissues and tolerance to abiotic stresses. Plant Sci. 272, 267–275. doi:10.1016/j.plantsci.2018.04.024
Bakhsh, A., Hussain, T., Rahamkulov, I., Demirel, U., and Çalışkan, M. E. (2020). Transgenic potato lines expressing CP4-EPSP synthase exhibit resistance against glyphosate. PCTOC 140, 23–34. doi:10.1007/s11240-019-01708-1
Bawa, A., and Anilakumar, K. (2013). Genetically modified foods: safety, risks and public concerns—a review. J. Food Sci. Technol. 50, 1035–1046. doi:10.1007/s13197-012-0899-1
Bethke, P. C., and Bussan, A. J. (2013). Acrylamide in processed potato products. Am. J. Potato Res. 90, 403–424. doi:10.1007/s12230-013-9321-4
Bharadwaj, D. N. (2015). Polyploidy in crop improvement and evolution. Plant Biol. Biotechnol. Vol. Plant divers. Organ. Funct. improv., 619–638. doi:10.1007/978-81-322-2286-6_24
Bi, W., Liu, J., Li, Y., He, Z., Chen, Y., Zhao, T., et al. (2023). CRISPR/Cas9-guided editing of a novel susceptibility gene in potato improves Phytophthora resistance without growth penalty. Plant Biotechnol. J. 22, 4–6. doi:10.1111/pbi.14175
Birchler, J. A., Riddle, N. C., Auger, D. L., and Veitia, R. A. (2005). Dosage balance in gene regulation: biological implications. Trends Genet. 21, 219–226. doi:10.1016/j.tig.2005.02.010
Biswas, A., Melmaiee, K., Elavarthi, S., Jones, J., and Reddy, U. (2019). Characterization of strawberry (Fragaria spp.) accessions by genotyping with SSR markers and phenotyping by leaf antioxidant and trichome analysis. Sci. Hortic. 256, 108561. doi:10.1016/j.scienta.2019.108561
Bouaziz, D., Pirrello, J., Charfeddine, M., Hammami, A., Jbir, R., Dhieb, A., et al. (2013). Overexpression of StDREB1 transcription factor increases tolerance to salt in transgenic potato plants. Mol. Biotechnol. 54, 803–817. doi:10.1007/s12033-012-9628-2
Bouwmeester, K., Han, M., Blanco-Portales, R., Song, W., Weide, R., Guo, L., et al. (2014). The Arabidopsis lectin receptor kinase Lec RK-I. 9 enhances resistance to Phytophthora infestans in Solanaceous plants. Plant Biotechnol. J. 12, 10–16. doi:10.1111/pbi.12111
Bradshaw, J. E. (2024). “Potato genetics for crop improvement,” in Approaches for potato crop improvement and stress management (Springer), 1–27. doi:10.1007/978-981-97-1223-6_1
Brant, E. J., Eid, A., Kannan, B., Baloglu, M. C., and Altpeter, F. (2024). The extent of multiallelic, co-editing of LIGULELESS1 in highly polyploid sugarcane tunes leaf inclination angle and enables selection of the ideotype for biomass yield. Plant Biotechnol. J. 22, 2660–2671. doi:10.1111/pbi.14380
Brant, E., Zuniga-Soto, E., and Altpeter, F. (2025). RNAi and genome editing of sugarcane: progress and prospects. Plant J. 121, e70048. doi:10.1111/tpj.70048
Brummell, D. A., Watson, L. M., Zhou, J., McKenzie, M. J., Hallett, I. C., Simmons, L., et al. (2015). Overexpression of starch branching enzyme II increases short-chain branching of amylopectin and alters the physicochemical properties of starch from potato tuber. BMC Biotechnol. 15, 28–14. doi:10.1186/s12896-015-0143-y
Budeguer, F., Enrique, R., Perera, M. F., Racedo, J., Castagnaro, A. P., Noguera, A. S., et al. (2021). Genetic transformation of sugarcane, current status and future prospects. Front. Plant Sci. 12, 768609. doi:10.3389/fpls.2021.768609
Camerlengo, F., Frittelli, A., Sparks, C., Doherty, A., Martignago, D., Larré, C., et al. (2020). CRISPR-Cas9 multiplex editing of the α-amylase/trypsin inhibitor genes to reduce allergen proteins in durum wheat. Front. Sustain. Food Syst. 4, 104. doi:10.3389/fsufs.2020.00104
Cardi, T., Murovec, J., Bakhsh, A., Boniecka, J., Bruegmann, T., Bull, S. E., et al. (2023). CRISPR/Cas-mediated plant genome editing: outstanding challenges a decade after implementation. Trends Plant Sci. 28, 1144–1165. doi:10.1016/j.tplants.2023.05.012
Castagnola, A. S., and Jurat-Fuentes, J. L. (2012). Bt crops: past and future. 283–304. doi:10.1007/978-94-007-3021-2_15
Catherine, K. N., Mugiira, B. R., and Muchiri, N. J. (2024). Public perception of genetically modified organisms and the implementation of biosafety measures in Kenya. Adv. Agric. 2024, 5544617. doi:10.1155/2024/5544617
Chahal, G., and Gosal, S. (2002). Principles and procedures of plant breeding: biotechnological and conventional approaches. Alpha Science Int’l Ltd. Boca Raton, FL: CRC Press.
Charfeddine, M., Samet, M., Charfeddine, S., Bouaziz, D., and Gargouri Bouzid, R. (2019). Ectopic expression of StERF94 transcription factor in potato plants improved resistance to Fusarium solani infection. Plant Mol. Biol. Rep. 37, 450–463. doi:10.1007/s11105-019-01171-4
Che, Y., Zhang, N., Zhu, X., Li, S., Wang, S., and Si, H. (2020). Enhanced tolerance of the transgenic potato plants overexpressing Cu/Zn superoxide dismutase to low temperature. Sci. Hortic. 261, 108949. doi:10.1016/j.scienta.2019.108949
Chen, J., Khan, Q., Sun, B., Tang, L., Yang, L., Zhang, B., et al. (2021). Overexpression of sugarcane SoTUA gene enhances cold tolerance in transgenic sugarcane. Agron. J. 113, 4993–5005. doi:10.1002/agj2.20618
Chen, Y., Liu, L., Feng, Q., Liu, C., Bao, Y., Zhang, N., et al. (2023). FvWRKY50 is an important gene that regulates both vegetative growth and reproductive growth in strawberry. Hortic. Res. 10, uhad115. doi:10.1093/hr/uhad115
Cheng, Y.-J., Deng, X.-P., Kwak, S.-S., Chen, W., and Eneji, A. E. (2013). Enhanced tolerance of transgenic potato plants expressing choline oxidase in chloroplasts against water stress. Bot. Stud. 54, 30–39. doi:10.1186/1999-3110-54-30
Cheng, C., Wu, S., Deng, G., Sheng, O., Yi, G., and Yang, Q. (2024). Recent advances and future directions in banana molecular biology and breeding. Mol. Hortic. 4, 42. doi:10.1186/s43897-024-00122-2
Chinnaswamy, A., Sakthivel, S. K., Channappa, M., Ramanathan, V., Shivalingamurthy, S. G., Peter, S. C., et al. (2024). Overexpression of an NF-YB gene family member, EaNF-YB2, enhances drought tolerance in sugarcane (Saccharum Spp. Hybrid). BMC Plant Biol. 24, 1246. doi:10.1186/s12870-024-05932-6
Chung, B. N., Yoon, J.-Y., and Palukaitis, P. (2013). Engineered resistance in potato against potato leafroll virus, potato virus A and potato virus Y. Virus Genes 47, 86–92. doi:10.1007/s11262-013-0904-4
Cingel, A., Savić, J., Ćosić, T., Zdravković-Korać, S., Momčilović, I., Smigocki, A., et al. (2014). Pyramiding rice cystatin OCI and OCII genes in transgenic potato (Solanum tuberosum L.) for resistance to Colorado potato beetle (Leptinotarsa decemlineata Say). Euphytica 198, 425–438. doi:10.1007/s10681-014-1119-z
Collard, B. C., and Mackill, D. J. (2008). Marker-assisted selection: an approach for precision plant breeding in the twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 363, 557–572. doi:10.1098/rstb.2007.2170
Comai, L. (2005). The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 6, 836–846. doi:10.1038/nrg1711
Craze, M., Bates, R., Bowden, S., and Wallington, E. J. (2018). Highly efficient agrobacterium-mediated transformation of potato (Solanum tuberosum) and production of transgenic microtubers. Curr. Protoc. Plant Biol. 3, 33–41. doi:10.1002/cppb.20065
Cristofoletti, P. T., Kemper, E. L., Capella, A. N., Carmago, S. R., Cazoto, J. L., Ferrari, F., et al. (2018). Development of transgenic sugarcane resistant to sugarcane borer. Trop. Plant Biol. 11, 17–30. doi:10.1007/s12042-018-9198-y
Cui, K., and Shoemaker, S. P. (2018). Public perception of genetically-modified (GM) food: a nationwide Chinese consumer study. Npj Sci. Food 2, 10. doi:10.1038/s41538-018-0018-4
Curtis, K., Langford, M., and Pignatari, M. (2024). Utah consumer preferences and willingness to pay for specialty labeled fruit products [Fact sheet]. Available online at: https://digitalcommons.usu.edu/extension_curall/2430/.
Dale, J., James, A., Paul, J.-Y., Khanna, H., Smith, M., Peraza-Echeverria, S., et al. (2017). Transgenic Cavendish bananas with resistance to Fusarium wilt tropical race 4. Nat. Commun. 8, 1496. doi:10.1038/s41467-017-01670-6
Dean, M., and Shepherd, R. (2007). Effects of information from sources in conflict and in consensus on perceptions of genetically modified food. Food Qual. prefer. 18, 460–469. doi:10.1016/j.foodqual.2006.05.004
del Mar Martínez-Prada, M., Curtin, S. J., and Gutiérrez-González, J. J. (2021). Potato improvement through genetic engineering. Gm. Crops Food 12, 479–496. doi:10.1080/21645698.2021.1993688
Dermawan, H., Karan, R., Jung, J., Zhao, Y., Parajuli, S., Sanahuja, G., et al. (2016). Development of an intragenic gene transfer and selection protocol for sugarcane resulting in resistance to acetolactate synthase-inhibiting herbicide. PCTOC 126, 459–468. doi:10.1007/s11240-016-1014-5
Dessoky, E. S., Ismail, R. M., Elarabi, N. I., Abdelhadi, A. A., and Abdallah, N. A. (2021). Improvement of sugarcane for borer resistance using Agrobacterium mediated transformation of cry1Ac gene. Gm. Crops Food 12, 47–56. doi:10.1080/21645698.2020.1809318
Dinesh Babu, K. S., Janakiraman, V., Palaniswamy, H., Kasirajan, L., Gomathi, R., and Ramkumar, T. R. (2022). A short review on sugarcane: its domestication, molecular manipulations and future perspectives. Genet. Resour. Crop Evol. 69, 2623–2643. doi:10.1007/s10722-022-01430-6
Divya, K., Thangaraj, M., and Krishna Radhika, N. (2024). CRISPR/Cas9: an advanced platform for root and tuber crops improvement. Front. Genome 5, 1242510. doi:10.3389/fgeed.2023.1242510
Dong, T., Bi, F., Huang, Y., He, W., Deng, G., Gao, H., et al. (2020). Highly efficient biolistic transformation of embryogenic cell suspensions of banana via a liquid medium selection system. HortScience 55, 703–708. doi:10.21273/HORTSCI14884-20
Dong, X., Li, Y., Guan, Y., Wang, S., Luo, H., Li, X., et al. (2021). Auxin-induced auxin response factor4 activates APETALA1 and FRUITFULL to promote flowering in woodland strawberry. Hortic. Res. 8, 115. doi:10.1038/s41438-021-00550-x
Eid, A., Mohan, C., Sanchez, S., Wang, D., and Altpeter, F. (2021). Multiallelic, targeted mutagenesis of magnesium chelatase with CRISPR/Cas9 provides a rapidly scorable phenotype in highly polyploid sugarcane. Front. Genome Ed. 3, 654996. doi:10.3389/fgeed.2021.654996
Elayabalan, S., Kalaiponmani, K., Subramaniam, S., Selvarajan, R., Panchanathan, R., Muthuvelayoutham, R., et al. (2013). Development of Agrobacterium-mediated transformation of highly valued hill banana cultivar Virupakshi (AAB) for resistance to BBTV disease. World J. Microbiol. Biotechnol. 29, 589–596. doi:10.1007/s11274-012-1214-z
Enciso-Rodriguez, F., Manrique-Carpintero, N. C., Nadakuduti, S. S., Buell, C. R., Zarka, D., and Douches, D. (2019). Overcoming self-incompatibility in diploid potato using CRISPR-Cas9. Front. Plant Sci. 10, 376. doi:10.3389/fpls.2019.00376
Feldman, M., and Levy, A. A. (2009). Genome evolution in allopolyploid wheat—a revolutionary reprogramming followed by gradual changes. J. Genet. Genomics 36, 511–518. doi:10.1016/S1673-8527(08)60142-3
Feng, J., Cheng, L., Zhu, Z., Yu, F., Dai, C., Liu, Z., et al. (2021). GRAS transcription factor loss of axillary meristems is essential for stamen and runner formation in wild strawberry. Plant Physiol. 186, 1970–1984. doi:10.1093/plphys/kiab184
Fossi, M., Amundson, K., Kuppu, S., Britt, A., and Comai, L. (2019). Regeneration of Solanum tuberosum plants from protoplasts induces widespread genome instability. Plant Physiol. 180, 78–86. doi:10.1104/pp.18.00906
Frewer, L. J., van der Lans, I. A., Fischer, A. R., Reinders, M. J., Menozzi, D., Zhang, X., et al. (2013). Public perceptions of agri-food applications of genetic modification–a systematic review and meta-analysis. Trends Food Sci. Technol. 30, 142–152. doi:10.1016/j.tifs.2013.01.003
Gaikwad, K. B., Rani, S., Kumar, M., Gupta, V., Babu, P. H., Bainsla, N. K., et al. (2020). Enhancing the nutritional quality of major food crops through conventional and genomics-assisted breeding. Front. Nutr. 7, 533453. doi:10.3389/fnut.2020.533453
Ganapathi, T., Negi, S., Tak, H., and Bapat, V. (2021). Transgenic banana: current status, opportunities and challenges. Genet. Modif. Crops Curr. Status Prospects Chall. 2, 111–128. doi:10.1007/978-981-15-5932-7_5
Gangadhar, B. H., Sajeesh, K., Venkatesh, J., Baskar, V., Abhinandan, K., Yu, J. W., et al. (2016). Enhanced tolerance of transgenic potato plants over-expressing non-specific lipid transfer protein-1 (StnsLTP1) against multiple abiotic stresses. Front. Plant Sci. 7, 1228. doi:10.3389/fpls.2016.01228
Gangadhar, B. H., Mishra, R. K., Kappachery, S., Baskar, V., Venkatesh, J., Nookaraju, A., et al. (2021). Enhanced thermo-tolerance in transgenic potato (Solanum tuberosum L.) overexpressing hydrogen peroxide-producing germin-like protein (GLP). Genomics 113, 3224–3234. doi:10.1016/j.ygeno.2021.07.013
Gao, C. (2021). Genome engineering for crop improvement and future agriculture. Cell 184, 1621–1635. doi:10.1016/j.cell.2021.01.005
Gao, S., Yang, Y., Wang, C., Guo, J., Zhou, D., Wu, Q., et al. (2016). Transgenic sugarcane with a cry1Ac gene exhibited better phenotypic traits and enhanced resistance against sugarcane borer. PLoS One 11, e0153929. doi:10.1371/journal.pone.0153929
Gao, Q., Luo, H., Li, Y., Liu, Z., and Kang, C. (2020). Genetic modulation of RAP alters fruit coloration in both wild and cultivated strawberry. Plant Biotechnol. J. 18, 1550–1561. doi:10.1111/pbi.13317
Gbashi, S., Adebo, O., Adebiyi, J. A., Targuma, S., Tebele, S., Areo, O. M., et al. (2021). Food safety, food security and genetically modified organisms in Africa: a current perspective. Biotechnol. Genet. Eng. Rev. 37, 30–63. doi:10.1080/02648725.2021.1940735
Genetic Literacy Project (2025). Glob. Gene ed. Regul. Tracker. Available online at: https://crispr-gene-editing-regs-tracker.geneticliteracyproject.org/#jet-tabs-control-1401 (Accessed February 15, 2025).
Ghaderi, N., Hatami, M. R., Mozafari, A., and Siosehmardeh, A. (2018). Change in antioxidant enzymes activity and some morpho-physiological characteristics of strawberry under long-term salt stress. Physiol. Mol. Biol. Plants 24, 833–843. doi:10.1007/s12298-018-0535-2
Ghag, S. B., Shekhawat, U. K., and Ganapathi, T. R. (2014). Host-induced post-transcriptional hairpin RNA-mediated gene silencing of vital fungal genes confers efficient resistance against F usarium wilt in banana. Plant Biotechnol. J. 12, 541–553. doi:10.1111/pbi.12158
Ghasemi, S., Karami, E., and Azadi, H. (2013). Knowledge, attitudes and behavioral intentions of agricultural professionals toward genetically modified (GM) foods: a case study in Southwest Iran. Sci. Eng. Ethics 19, 1201–1227. doi:10.1007/s11948-012-9383-6
Ghislain, M., Byarugaba, A. A., Magembe, E., Njoroge, A., Rivera, C., Román, M. L., et al. (2019). Stacking three late blight resistance genes from wild species directly into African highland potato varieties confers complete field resistance to local blight races. Plant Biotechnol. J. 17, 1119–1129. doi:10.1111/pbi.13042
González, M. N., Massa, G. A., Andersson, M., Turesson, H., Olsson, N., Fält, A.-S., et al. (2020). Reduced enzymatic browning in potato tubers by specific editing of a polyphenol oxidase gene via ribonucleoprotein complexes delivery of the CRISPR/Cas9 system. Front. Plant Sci. 10, 1649. doi:10.3389/fpls.2019.01649
Goo, Y.-M., Han, E.-H., Jeong, J. C., Kwak, S.-S., Yu, J., Kim, Y.-H., et al. (2015). Overexpression of the sweet potato IbOr gene results in the increased accumulation of carotenoid and confers tolerance to environmental stresses in transgenic potato. C. R. Biol. 338, 12–20. doi:10.1016/j.crvi.2014.10.006
Graham, N., Patil, G. B., Bubeck, D. M., Dobert, R. C., Glenn, K. C., Gutsche, A. T., et al. (2020). Plant genome editing and the relevance of off-target changes. Plant Physiol. 183, 1453–1471. doi:10.1104/pp.19.01194
Grech-Baran, M., Witek, K., Szajko, K., Witek, A. I., Morgiewicz, K., Wasilewicz-Flis, I., et al. (2020). Extreme resistance to Potato virus Y in potato carrying the Rysto gene is mediated by a TIR-NLR immune receptor. Plant Biotechnol. J. 18, 655–667. doi:10.1111/pbi.13230
Griffin, P. C., Robin, C., and Hoffmann, A. A. (2011). A next-generation sequencing method for overcoming the multiple gene copy problem in polyploid phylogenetics, applied to Poa grasses. BMC Biol. 9, 19–18. doi:10.1186/1741-7007-9-19
Grohmann, L., Keilwagen, J., Duensing, N., Dagand, E., Hartung, F., Wilhelm, R., et al. (2019). Detection and identification of genome editing in plants: challenges and opportunities. Front. Plant Sci. 10, 236. doi:10.3389/fpls.2019.00236
Gu, X., Gao, Z., Yan, Y., Wang, X., Qiao, Y., and Chen, Y. (2017). RdreB1BI enhances drought tolerance by activating AQP-related genes in transgenic strawberry. Plant Physiol. biochem. 119, 33–42. doi:10.1016/j.plaphy.2017.08.013
Gu, X., Liu, L., and Zhang, H. (2021). Transgene-free genome editing in plants. Front. Genome 3, 805317. doi:10.3389/fgeed.2021.805317
Guo, J., Gao, S., Lin, Q., Wang, H., Que, Y., and Xu, L. (2015). Transgenic sugarcane resistant to Sorghum mosaic virus based on coat protein gene silencing by RNA interference. Biomed. Res. Int. 2015, 861907. doi:10.1155/2015/861907
Hallman, W. K., Cuite, C. L., and Morin, X. (2013). Public perceptions of labeling genetically modified foods. Rutgers University. Available online at:. doi:10.7282/T33N255N
Halterman, D., Guenthner, J., Collinge, S., Butler, N., and Douches, D. (2016). Biotech potatoes in the 21st century: 20 years since the first biotech potato. Am. J. Potato Res. 93, 1–20. doi:10.1007/s12230-015-9485-1
He, Y., Mudgett, M., and Zhao, Y. (2022). Advances in gene editing without residual transgenes in plants. Plant Physiol. 188, 1757–1768. doi:10.1093/plphys/kiab574
Healey, A., Garsmeur, O., Lovell, J., Shengquiang, S., Sreedasyam, A., Jenkins, J., et al. (2024). The complex polyploid genome architecture of sugarcane. Nature 628, 804–810. doi:10.1038/s41586-024-07231-4
Heslop-Harrison, J., Schwarzacher, T., and Liu, Q. (2023). Polyploidy: its consequences and enabling role in plant diversification and evolution. Ann. Bot. 131, 1–10. doi:10.1093/aob/mcac132
Hossain, F., and Onyango, B. (2004). Product attributes and consumer acceptance of nutritionally enhanced genetically modified foods. Int. J. Consum. Stud. 28, 255–267. doi:10.1111/j.1470-6431.2004.00352.x
Hussain, T., Aksoy, E., Çalışkan, M. E., and Bakhsh, A. (2019). Transgenic potato lines expressing hairpin RNAi construct of molting-associated EcR gene exhibit enhanced resistance against Colorado potato beetle (Leptinotarsa decemlineata, Say). Transgenic Res. 28, 151–164. doi:10.1007/s11248-018-0109-7
ISAAA (2025). GM approv. Database. Available online at: https://www.isaaa.org/gmapprovaldatabase/default.asp (Accessed February 14, 2025).
Jekayinoluwa, T., Tripathi, J. N., Dugdale, B., Obiero, G., Muge, E., Dale, J., et al. (2021). Transgenic expression of dsRNA targeting the Pentalonia nigronervosa acetylcholinesterase gene in banana and plantain reduces aphid populations. Plants 10, 613. doi:10.3390/plants10040613
Ji, Y., Li, X., Gao, Q.-H., Geng, C., and Duan, K. (2022). Colletotrichum species pathogenic to strawberry: discovery history, global diversity, prevalence in China, and the host range of top two species. Phytopathol. Res. 4, 42. doi:10.1186/s42483-022-00147-9
Jiang, C., Chee, P. W., Draye, X., Morrell, P. L., Smith, C. W., and Paterson, A. H. (2000). Multilocus interactions restrict gene introgression in interspecific populations of polyploid Gossypium (cotton). Evolution 54, 798–814. doi:10.1111/j.0014-3820.2000.tb00081.x
Jiang, L., Mu, R., Wang, Z., Liu, S., and Lu, D. (2023). Silencing P25, HC-Pro and Brp1 of potato virus (viroid) using artificial microRNA confers resistance to PVX, PVY and PSTVd in transgenic potato. Potato Res. 66, 231–244. doi:10.1007/s11540-022-09580-x
Jin, X., Du, H., Zhu, C., Wan, H., Liu, F., Ruan, J., et al. (2023). Haplotype-resolved genomes of wild octoploid progenitors illuminate genomic diversifications from wild relatives to cultivated strawberry. Nat. Plants 9, 1252–1266. doi:10.1038/s41477-023-01473-2
Jiwan, D., Roalson, E. H., Main, D., and Dhingra, A. (2013). Antisense expression of peach mildew resistance locus O (PpMlo1) gene confers cross-species resistance to powdery mildew in Fragaria x ananassa. Transgenic Res. 22, 1119–1131. doi:10.1007/s11248-013-9715-6
Johansen, I. E., Liu, Y., Jørgensen, B., Bennett, E. P., Andreasson, E., Nielsen, K. L., et al. (2019). High efficacy full allelic CRISPR/Cas9 gene editing in tetraploid potato. Sci. Rep. 9, 17715. doi:10.1038/s41598-019-54126-w
Jung, J. H., and Altpeter, F. (2016). TALEN mediated targeted mutagenesis of the caffeic acid O-methyltransferase in highly polyploid sugarcane improves cell wall composition for production of bioethanol. Plant Mol. Biol. 92, 131–142. doi:10.1007/s11103-016-0499-y
Kalaitzandonakes, N., Willig, C., and Zahringer, K. (2023). The economics and policy of genome editing in crop improvement. Plant Genome 16, e20248. doi:10.1002/tpg2.20248
Kamthan, A., Chaudhuri, A., Kamthan, M., and Datta, A. (2016). Genetically modified (GM) crops: milestones and new advances in crop improvement. Theor. Appl. Genet. 129, 1639–1655. doi:10.1007/s00122-016-2747-6
Karlsson, M., Kieu, N. P., Lenman, M., Marttila, S., Resjö, S., Zahid, M. A., et al. (2024). CRISPR/Cas9 genome editing of potato StDMR6-1 results in plants less affected by different stress conditions. Hortic. Res. 11, uhae130. doi:10.1093/hr/uhae130
Kaur, N., Alok, A., Kumar, P., Kaur, N., Awasthi, P., Chaturvedi, S., et al. (2020). CRISPR/Cas9 directed editing of lycopene epsilon-cyclase modulates metabolic flux for β-carotene biosynthesis in banana fruit. Metab. Eng. 59, 76–86. doi:10.1016/j.ymben.2020.01.008
Kieu, N. P., Lenman, M., Wang, E. S., Petersen, B. L., and Andreasson, E. (2021). Mutations introduced in susceptibility genes through CRISPR/Cas9 genome editing confer increased late blight resistance in potatoes. Sci. Rep. 11, 4487. doi:10.1038/s41598-021-83972-w
Kikulwe, E. M., Wesseler, J., and Falck-Zepeda, J. (2011). Attitudes, perceptions, and trust. Insights from a consumer survey regarding genetically modified banana in Uganda. Appetite 57, 401–413. doi:10.1016/j.appet.2011.06.001
Kogo, B. K., Kumar, L., and Koech, R. (2021). Climate change and variability in Kenya: a review of impacts on agriculture and food security. Environ. Dev. Sustain. 23, 23–43. doi:10.1007/s10668-020-00589-1
Koul, B., Pudhuvai, B., Bhanot, M., and Tiwari, S. (2024). “Updates on global status of transgenic and genome-edited crops,” in Genetic engineering of crop plants for food and health security. Editors S. Tiwari, and B. Koul (Singapore: Springer Nature Singapore), 2, 469–510. doi:10.1007/978-981-97-3119-0_19
Kumar, T., Khan, M. R., Abbas, Z., and Ali, G. M. (2014). Genetic improvement of sugarcane for drought and salinity stress tolerance using Arabidopsis vacuolar pyrophosphatase (AVP1) gene. Mol. Biotechnol. 56, 199–209. doi:10.1007/s12033-013-9695-z
Kumar, M., Prusty, M. R., Pandey, M. K., Singh, P. K., Bohra, A., Guo, B., et al. (2023). Application of CRISPR/Cas9-mediated gene editing for abiotic stress management in crop plants. Front. Plant Sci. 14, 1157678. doi:10.3389/fpls.2023.1157678
Kumar, T., Wang, J.-G., Xu, C.-H., Lu, X., Mao, J., Lin, X.-Q., et al. (2024). Genetic engineering for enhancing sugarcane tolerance to biotic and abiotic stresses. Plants 13, 1739. doi:10.3390/plants13131739
Kumari, N., Kumar, A., Sharma, S., Thakur, P., Chadha, S., and Dhiman, A. (2024). CRISPR/Cas system for the traits enhancement in potato (Solanum tuberosum L.): present status and future prospectives. J. Plant Biochem. Biotechnol. 33, 108–128. doi:10.1007/s13562-024-00878-0
Kusano, H., Ohnuma, M., Mutsuro-Aoki, H., Asahi, T., Ichinosawa, D., Onodera, H., et al. (2018). Establishment of a modified CRISPR/Cas9 system with increased mutagenesis frequency using the translational enhancer dMac3 and multiple guide RNAs in potato. Sci. Rep. 8, 13753. doi:10.1038/s41598-018-32049-2
Lakhani, H., Thakur, N., and Tiwari, S. (2023). Genome editing for vegetatively propagated crops improvement: a new horizon of possibilities. J. Plant Biochem. Biotechnol. 32, 718–729. doi:10.1007/s13562-022-00819-9
Laksana, C., Sophiphun, O., and Chanprame, S. (2024). Lignin reduction in sugarcane by performing CRISPR/Cas9 site-direct mutation of SoLIM transcription factor. Plant Sci. 340, 111987. doi:10.1016/j.plantsci.2024.111987
Leal-Bertioli, S. C., Godoy, I. J., Santos, J. F., Doyle, J. J., Guimarães, P. M., Abernathy, B. L., et al. (2018). Segmental allopolyploidy in action: increasing diversity through polyploid hybridization and homoeologous recombination. Am. J. Bot. 105, 1053–1066. doi:10.1002/ajb2.1112
Levin, D. A. (2002). The role of chromosomal change in plant evolution. USA: Oxford University Press. doi:10.1093/oso/9780195138597.001.0001
Li, J., Manghwar, H., Sun, L., Wang, P., Wang, G., Sheng, H., et al. (2019). Whole genome sequencing reveals rare off-target mutations and considerable inherent genetic or/and somaclonal variations in CRISPR/Cas9-edited cotton plants. Plant Biotechnol. J. 17, 858–868. doi:10.1111/pbi.13020
Li, M., Yang, Y., Raza, A., Yin, S., Wang, H., Zhang, Y., et al. (2021). Heterologous expression of Arabidopsis thaliana rty gene in strawberry (Fragaria× ananassa Duch.) improves drought tolerance. BMC Plant Biol. 21, 57–20. doi:10.1186/s12870-021-02839-4
Liang, J., Zheng, J., Wu, Z., and Wang, H. (2020). Strawberry FaNAC2 enhances tolerance to abiotic stress by regulating proline metabolism. Plants 9, 1417. doi:10.3390/plants9111417
Liu, J., Gao, P., Sun, X., Zhang, J., Sun, P., Wang, J., et al. (2017). Efficient regeneration and genetic transformation platform applicable to five Musa varieties. Electron. J. Biotechnol. 25, 33–38. doi:10.1016/j.ejbt.2016.11.002
Liu, Y., Zhao, Q., Meng, N., Song, H., Li, C., Hu, G., et al. (2017). Over-expression of EjLFY-1 leads to an early flowering habit in strawberry (Fragaria× ananassa) and its asexual progeny. Front. Plant Sci. 8, 496. doi:10.3389/fpls.2017.00496
Lomov, N., Viushkov, V., Petrenko, A., Syrkina, M., and Rubtsov, M. (2019). Methods of evaluating the efficiency of CRISPR/Cas genome editing. Mol. Biol. 53, 862–875. doi:10.1134/S0026893319060116
López-Casado, G., Sánchez-Raya, C., Ric-Varas, P. D., Paniagua, C., Blanco-Portales, R., Muñoz-Blanco, J., et al. (2023). CRISPR/Cas9 editing of the polygalacturonase FaPG1 gene improves strawberry fruit firmness. Hortic. Res. 10, uhad011. doi:10.1093/hr/uhad011
Lucht, J. M. (2015). Public acceptance of plant biotechnology and GM crops. Viruses 7, 4254–4281. doi:10.3390/v7082819
Lucioli, A., Tavazza, R., Baima, S., Fatyol, K., Burgyan, J., and Tavazza, M. (2022). CRISPR-Cas9 targeting of the eIF4E1 gene extends the potato virus y resistance spectrum of the Solanum tuberosum l. cv. desirée. Front. Microbiol. 13, 873930. doi:10.3389/fmicb.2022.873930
Luo, H., Dai, C., Li, Y., Feng, J., Liu, Z., and Kang, C. (2018). Reduced Anthocyanins in Petioles codes for a GST anthocyanin transporter that is essential for the foliage and fruit coloration in strawberry. J. Exp. Bot. 69, 2595–2608. doi:10.1093/jxb/ery096
Luo, J., Yu, W., Xiao, Y., Zhang, Y., and Peng, F. (2024). FaSnRK1α mediates salicylic acid pathways to enhance strawberry resistance to Botrytis cinerea. Hortic. Plant J. 10, 131–144. doi:10.1016/j.hpj.2023.05.006
Ma, L., Haile, Z. M., Sabbadini, S., Mezzetti, B., Negrini, F., and Baraldi, E. (2023). Functional characterization of MANNOSE-BINDING LECTIN 1, a G-type lectin gene family member, in response to fungal pathogens of strawberry. J. Exp. Bot. 74, 149–161. doi:10.1093/jxb/erac396
Maeda, S., Ackley, W., Yokotani, N., Sasaki, K., Ohtsubo, N., Oda, K., et al. (2023). Enhanced resistance to fungal and bacterial diseases due to overexpression of BSR1, a rice RLCK, in sugarcane, tomato, and torenia. Int. J. Mol. Sci. 24, 3644. doi:10.3390/ijms24043644
Majumdar, P., Torres Rodríguez, M. D., and Pandey, S. (2023). Role of heterotrimeric G-proteins in improving abiotic stress tolerance of crop plants. J. Plant Growth Regul. 42, 6681–6698. doi:10.1007/s00344-023-10965-6
Malhi, G. S., Kaur, M., and Kaushik, P. (2021). Impact of climate change on agriculture and its mitigation strategies: a review. Sustainability 13, 1318. doi:10.3390/su13031318
Marone, D., Mastrangelo, A. M., and Borrelli, G. M. (2023). From transgenesis to genome editing in crop improvement: applications, marketing, and legal issues. Int. J. Mol. Sci. 24, 7122. doi:10.3390/ijms24087122
Martín-Pizarro, C., Triviño, J. C., and Posé, D. (2019). Functional analysis of the TM6 MADS-box gene in the octoploid strawberry by CRISPR/Cas9-directed mutagenesis. J. Exp. Bot. 70, 885–895. doi:10.1093/jxb/ery400
Masoabi, M., Burger, N. F. V., Botha, A.-M., Le Roux, M. L., Vlok, M., Snyman, S., et al. (2023). Overexpression of the Small Ubiquitin-Like Modifier protease OTS1 gene enhances drought tolerance in sugarcane (Saccharum spp. hybrid). Plant Biol. 25, 1121–1141. doi:10.1111/plb.13585
May, D., Paldi, K., and Altpeter, F. (2023). Targeted mutagenesis with sequence-specific nucleases for accelerated improvement of polyploid crops: progress, challenges, and prospects. Plant Genome 16, e20298. doi:10.1002/tpg2.20298
McKey, D., Elias, M., Pujol, B., and Duputié, A. (2010). The evolutionary ecology of clonally propagated domesticated plants. New Phytol. 186, 318–332. doi:10.1111/j.1469-8137.2010.03210.x
Medani, K. R., Neill, A., Garrod, G., Ojo, M., and Hubbard, C. (2024). Societal perceptions and attitudes towards genetically modified (GM) crops, feed, and food products in the Middle East, North Africa, and Turkey (MENAT) region: a systematic literature review. Food Qual. prefer. 117, 105148. doi:10.1016/j.foodqual.2024.105148
Meirmans, P. G., Liu, S., and van Tienderen, P. H. (2018). The analysis of polyploid genetic data. J. Hered. 109, 283–296. doi:10.1093/jhered/esy006
Menzel, C. (2021). Higher temperatures decrease fruit size in strawberry growing in the subtropics. Horticulturae 7, 34. doi:10.3390/horticulturae7020034
Mercado Carmona, J., Barceló Muñoz, M., Pliego, C., Rey, M., Caballero Repullo, J., Muñoz-Blanco, J., et al. (2015). Expression of the β-1, 3-glucanase gene bgn13. 1 from Trichoderma harzianum in strawberry increases tolerance to crown rot diseases but interferes with plant growth. Transgenic Res. 24, 979–989. doi:10.1007/s11248-015-9895-3
Metje-Sprink, J., Sprink, T., and Hartung, F. (2020). Genome-edited plants in the field. Curr. Opin. Biotechnol. 61, 1–6. doi:10.1016/j.copbio.2019.08.007
Mohan, S., Meiyalaghan, S., Latimer, J. M., Gatehouse, M. L., Monaghan, K. S., Vanga, B. R., et al. (2014). GSL2 over-expression confers resistance to Pectobacterium atrosepticum in potato. Theor. Appl. Genet. 127, 677–689. doi:10.1007/s00122-013-2250-2
Mohan, C., Shibao, P. Y. T., de Paula, F. F. P., Toyama, D., Vieira, M. A. S., Figueira, A., et al. (2021). hRNAi-mediated knock-down of Sphenophorus levis V-ATPase E in transgenic sugarcane (Saccharum spp interspecific hybrid) affects the insect growth and survival. Plant Cell Rep. 40, 507–516. doi:10.1007/s00299-020-02646-5
Mohanan, M. V., Pushpanathan, A., Padmanabhan, S., Sasikumar, T., Jayanarayanan, A. N., Selvarajan, D., et al. (2021). Overexpression of Glyoxalase III gene in transgenic sugarcane confers enhanced performance under salinity stress. J. Plant Res. 134, 1083–1094. doi:10.1007/s10265-021-01300-9
Motazedi, E., Finkers, R., Maliepaard, C., and de Ridder, D. (2018). Exploiting next-generation sequencing to solve the haplotyping puzzle in polyploids: a simulation study. Brief. Bioinform. 19, 387–403. doi:10.1093/bib/bbw126
Mukherjee, E., and Gantait, S. (2024). Strawberry biotechnology: a review on progress over past 10 years. Sci. Hortic. 338, 113618. doi:10.1016/j.scienta.2024.113618
Muñiz García, M. N., Cortelezzi, J. I., Fumagalli, M., and Capiati, D. A. (2018). Expression of the Arabidopsis ABF4 gene in potato increases tuber yield, improves tuber quality and enhances salt and drought tolerance. Plant Mol. Biol. 98, 137–152. doi:10.1007/s11103-018-0769-y
Muringai, V., Fan, X., and Goddard, E. (2020). Canadian consumer acceptance of gene-edited versus genetically modified potatoes: a choice experiment approach. Can. J. Agric. Econ. Can. Agroeconomie 68, 47–63. doi:10.1111/cjag.12221
Nahirñak, V., Almasia, N. I., González, M. N., Massa, G. A., Décima Oneto, C. A., Feingold, S. E., et al. (2022). State of the art of genetic engineering in potato: from the first report to its future potential. Front. Plant Sci. 12, 768233. doi:10.3389/fpls.2021.768233
Nakayasu, M., Akiyama, R., Lee, H. J., Osakabe, K., Osakabe, Y., Watanabe, B., et al. (2018). Generation of α-solanine-free hairy roots of potato by CRISPR/Cas9 mediated genome editing of the St16DOX gene. Plant Physiol. biochem. 131, 70–77. doi:10.1016/j.plaphy.2018.04.026
Nansamba, M., Sibiya, J., Tumuhimbise, R., Karamura, D., Kubiriba, J., and Karamura, E. (2020). Breeding banana (Musa spp.) for drought tolerance: a review. Plant Breed. 139, 685–696. doi:10.1111/pbr.12812
Narayan, J. A., Chakravarthi, M., Nerkar, G., Manoj, V., Dharshini, S., Subramonian, N., et al. (2021). Overexpression of expansin EaEXPA1, a cell wall loosening protein enhances drought tolerance in sugarcane. Ind. Crops Prod. 159, 113035. doi:10.1016/j.indcrop.2020.113035
Nayyar, S., Sharma, B. K., Kaur, A., Kalia, A., Sanghera, G. S., Thind, K. S., et al. (2017). Red rot resistant transgenic sugarcane developed through expression of β-1, 3-glucanase gene. PLoS One 12, e0179723. doi:10.1371/journal.pone.0179723
Nguyen, T. H., Ben Taieb, S., Moritaka, M., Ran, L., and Fukuda, S. (2023). Public Acceptance of foods derived from genome editing technology: a review of the technical, social and regulatory aspects. J. Int. Food Agribus. Mark. 35, 397–427. doi:10.1080/08974438.2021.2011526
Noguera, A., Enrique, R., Perera, M. F., Ostengo, S., Racedo, J., Costilla, D., et al. (2015). Genetic characterization and field evaluation to recover parental phenotype in transgenic sugarcane: a step toward commercial release. Mol. Breed. 35, 115–15. doi:10.1007/s11032-015-0300-y
Norouzi, M., Nazarain-Firouzabadi, F., Ismaili, A., Ahmadvand, R., and Poormazaheri, H. (2024). CRISPR/Cas StNRL1 gene knockout increases resistance to late blight and susceptibility to early blight in potato. Front. Plant Sci. 14, 1278127. doi:10.3389/fpls.2023.1278127
Ntui, V. O., Tripathi, J. N., Shah, T., and Tripathi, L. (2024). Targeted knockout of early nodulin-like 3 (MusaENODL3) gene in banana reveals its function in resistance to Xanthomonas wilt disease. Plant Biotechnol. J. 22, 1101–1112. doi:10.1111/pbi.14248
Osabe, K., Kawanabe, T., Sasaki, T., Ishikawa, R., Okazaki, K., Dennis, E. S., et al. (2012). Multiple mechanisms and challenges for the application of allopolyploidy in plants. Int. J. Mol. Sci. 13, 8696–8721. doi:10.3390/ijms13078696
Oz, M. T., Altpeter, A., Karan, R., Merotto, A., and Altpeter, F. (2021). CRISPR/Cas9-mediated multi-allelic gene targeting in sugarcane confers herbicide tolerance. Front. Genome Ed. 3, 673566. doi:10.3389/fgeed.2021.673566
Paniagua, C., Blanco-Portales, R., Barceló-Muñoz, M., García-Gago, J. A., Waldron, K. W., Quesada, M. A., et al. (2016). Antisense down-regulation of the strawberry β-galactosidase gene FaβGal4 increases cell wall galactose levels and reduces fruit softening. J. Exp. Bot. 67, 619–631. doi:10.1093/jxb/erv462
Parisod, C., Holderegger, R., and Brochmann, C. (2010). Evolutionary consequences of autopolyploidy. New Phytol. 186, 5–17. doi:10.1111/j.1469-8137.2009.03142.x
Park, D., Park, S.-H., Ban, Y. W., Kim, Y. S., Park, K.-C., Kim, N.-S., et al. (2017). A bioinformatics approach for identifying transgene insertion sites using whole genome sequencing data. BMC Biotechnol. 17, 67. doi:10.1186/s12896-017-0386-x
Paterson, A. H. (2005). Polyploidy, evolutionary opportunity, and crop adaptation. Genet. Adapt. 123, 191–196. doi:10.1007/s10709-003-2742-0
Paul, J., Becker, D. K., Dickman, M. B., Harding, R. M., Khanna, H. K., and Dale, J. L. (2011). Apoptosis-related genes confer resistance to Fusarium wilt in transgenic ‘Lady Finger’bananas. Plant Biotechnol. J. 9, 1141–1148. doi:10.1111/j.1467-7652.2011.00639.x
Paul, J., Khanna, H., Kleidon, J., Hoang, P., Geijskes, J., Daniells, J., et al. (2017). Golden bananas in the field: elevated fruit pro-vitamin A from the expression of a single banana transgene. Plant Biotechnol. J. 15, 520–532. doi:10.1111/pbi.12650
Peng, Y., Liang, M., Zhang, X., Yu, M., Liu, H., Cheng, Z., et al. (2024). FaERF2 activates two β-1, 3-glucanase genes to enhance strawberry resistance to Botrytis Cinerea. Plant Sci. 347, 112179. doi:10.1016/j.plantsci.2024.112179
Perrier, X., De Langhe, E., Donohue, M., Lentfer, C., Vrydaghs, L., Bakry, F., et al. (2011). Multidisciplinary perspectives on banana (Musa spp.) domestication. Proc. Natl. Acad. Sci. 108, 11311–11318. doi:10.1073/pnas.1102001108
Petrasch, S., Knapp, S. J., Van Kan, J. A., and Blanco-Ulate, B. (2019). Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol. 20, 877–892. doi:10.1111/mpp.12794
Piperidis, N., and D’Hont, A. (2020). Sugarcane genome architecture decrypted with chromosome-specific oligo probes. Plant J. 103, 2039–2051. doi:10.1111/tpj.14881
Posé, S., Paniagua, C., Cifuentes, M., Blanco-Portales, R., Quesada, M. A., and Mercado, J. A. (2013). Insights into the effects of polygalacturonase FaPG1 gene silencing on pectin matrix disassembly, enhanced tissue integrity, and firmness in ripe strawberry fruits. J. Exp. Bot. 64, 3803–3815. doi:10.1093/jxb/ert210
Rahman, M. A., Wu, W., Yan, Y., and Bhuiyan, S. A. (2021). Overexpression of. Crop Pasture Sci. 72, 268–279. doi:10.1071/CP20161
Ramasamy, M., Damaj, M. B., Vargas-Bautista, C., Mora, V., Liu, J., Padilla, C. S., et al. (2021). A sugarcane G-protein-coupled receptor, ShGPCR1, confers tolerance to multiple abiotic stresses. Front. Plant Sci. 12, 745891. doi:10.3389/fpls.2021.745891
Ramasamy, M., Rajkumar, M. S., Bedre, R., Irigoyen, S., Berg-Falloure, K., Kolomiets, M. V., et al. (2024). Genome editing of NPR3 confers potato resistance to Candidatus Liberibacter spp. Plant Biotechnol. J. 22, 2635–2637. doi:10.1111/pbi.14378
Razzaq, H. A., Ijaz, S., Haq, I. U., and Khan, I. A. (2022). Functional inhibition of the StERF3 gene by dual targeting through CRISPR/Cas9 enhances resistance to the late blight disease in Solanum tuberosum L. Mol. Biol. Rep. 49, 11675–11684. doi:10.1007/s11033-022-07958-1
Riaz, S., Nasir, I. A., Bhatti, M. U., Adeyinka, O. S., Toufiq, N., Yousaf, I., et al. (2020). Resistance to Chilo infuscatellus (Lepidoptera: pyraloidea) in transgenic lines of sugarcane expressing Bacillus thuringiensis derived Vip3A protein. Mol. Biol. Rep. 47, 2649–2658. doi:10.1007/s11033-020-05355-0
Ric-Varas, P., Paniagua, C., López-Casado, G., Molina-Hidalgo, F. J., Schückel, J., Knox, J. P., et al. (2024). Suppressing the rhamnogalacturonan lyase gene FaRGLyase1 preserves RGI pectin degradation and enhances strawberry fruit firmness. Plant Physiol. biochem. 206, 108294. doi:10.1016/j.plaphy.2023.108294
Robayo-Avendaño, A., Galindo-Mendoza, M. G., Yáñez-Estrada, L., and Aldama-Aguilera, C. (2018). Measurement of public perception of GMOs with a likert-type scale. Agrociencia 52, 767–781.
Rodríguez-Aguirre, E., Badillo-Márquez, A. E., Aguilar-Lasserre, A. A., and Flores-Asis, R. (2025). An agent-based model to evaluate agricultural vulnerability and risk facing climate change in strawberry production. Crop Sci. 65, e21100. doi:10.1002/csc2.21100
Sakuno, C. I. R., Francischini, F. J. B., Komada, K. M. A., Basso, M., and Huang, F. (2024). Transgenic sugarcane expressing Bacillus thuringiensis Cry1Ac protein offers new possibilities for controlling the giant borer, Telchin licus (Drury), a pest difficult to control. Crop Prot. 175, 106469. doi:10.1016/j.cropro.2023.106469
Saraswathi, M., Bathrinath, M., Kannan, G., Karthi, C., Mahendran, J., Sankar, C., et al. (2024). Production of quality planting material in commercial banana cvs. Rasthali (Silk, AAB) and Neypoovan (Neypoovan, AB) through farmer-friendly macropropagation technique and their field evaluation. South Afr. J. Bot. 167, 410–418. doi:10.1016/j.sajb.2024.02.047
Sardos, J., Perrier, X., Doležel, J., Hřibová, E., Christelová, P., Van Den Houwe, I., et al. (2016). DArT whole genome profiling provides insights on the evolution and taxonomy of edible Banana (Musa spp.). Ann. Bot. 118, 1269–1278. doi:10.1093/aob/mcw170
Sattler, M. C., Carvalho, C. R., and Clarindo, W. R. (2016). The polyploidy and its key role in plant breeding. Planta 243, 281–296. doi:10.1007/s00425-015-2450-x
Schaart, J. G. (2014). Agrobacterium-mediated transformation of strawberry. Bio-Protoc. 4, e1022. doi:10.21769/BioProtoc.1022
Schaart, J. G., van de Wiel, C. C., and Smulders, M. J. (2021). Genome editing of polyploid crops: prospects, achievements and bottlenecks. Transgenic Res. 30, 337–351. doi:10.1007/s11248-021-00251-0
Schiessl, S.-V., Katche, E., Ihien, E., Chawla, H. S., and Mason, A. S. (2019). The role of genomic structural variation in the genetic improvement of polyploid crops. Crop J. 7, 127–140. doi:10.1016/j.cj.2018.07.006
Schneider, V. K., Soares-Costa, A., Chakravarthi, M., Ribeiro, C., Chabregas, S. M., Falco, M. C., et al. (2017). Transgenic sugarcane overexpressing CaneCPI-1 negatively affects the growth and development of the sugarcane weevil Sphenophorus levis. Plant Cell Rep. 36, 193–201. doi:10.1007/s00299-016-2071-2
Scott, S. E., Inbar, Y., and Rozin, P. (2016). Evidence for absolute moral opposition to genetically modified food in the United States. Perspect. Psychol. Sci. 11, 315–324. doi:10.1177/1745691615621275
Scott, S. E., Inbar, Y., Wirz, C. D., Brossard, D., and Rozin, P. (2018). An overview of attitudes toward genetically engineered food. Annu. Rev. Nutr. 38, 459–479. doi:10.1146/annurev-nutr-071715-051223
Shafi, A., Pal, A. K., Sharma, V., Kalia, S., Kumar, S., Ahuja, P. S., et al. (2017). Transgenic potato plants overexpressing SOD and APX exhibit enhanced lignification and starch biosynthesis with improved salt stress tolerance. Plant Mol. Biol. Rep. 35, 504–518. doi:10.1007/s11105-017-1041-3
Shao, X., Wu, S., Dou, T., Zhu, H., Hu, C., Huo, H., et al. (2019). Using CRISPR/Cas9 genome editing system to create MaGA20ox2 gene-modified semi-dwarf banana. Plant Biotechnol. J. 18, 17–19. doi:10.1111/pbi.13216
Sharma, A. S., Kalia, A., Sharma, A., Sidhu, M. S., Sanghera, G. S., Chhabra, G., et al. (2024). Expression of Trichoderma spp. endochitinase gene improves red rot disease resistance in transgenic sugarcane. Plos One 19, e0310306. doi:10.1371/journal.pone.0310306
Shekhar, S., Rustagi, A., Kumar, D., Yusuf, M. A., Sarin, N. B., and Lawrence, K. (2019). Groundnut AhcAPX conferred abiotic stress tolerance in transgenic banana through modulation of the ascorbate–glutathione pathway. Physiol. Mol. Biol. Plants 25, 1349–1366. doi:10.1007/s12298-019-00704-1
Silva, K. J. P., Brunings, A., Peres, N. A., Mou, Z., and Folta, K. M. (2015). The Arabidopsis NPR1 gene confers broad-spectrum disease resistance in strawberry. Transgenic Res. 24, 693–704. doi:10.1007/s11248-015-9869-5
Silva, K. J. P., Brunings, A. M., Pereira, J. A., Peres, N. A., Folta, K. M., and Mou, Z. (2017). The Arabidopsis ELP3/ELO3 and ELP4/ELO1 genes enhance disease resistance in Fragaria vesca L. BMC Plant Biol. 17, 230. doi:10.1186/s12870-017-1173-5
Singh, B. K., Delgado-Baquerizo, M., Egidi, E., Guirado, E., Leach, J. E., Liu, H., et al. (2023). Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 21, 640–656. doi:10.1038/s41579-023-00900-7
Skendžić, S., Zovko, M., Živković, I. P., Lešić, V., and Lemić, D. (2021). The impact of climate change on agricultural insect pests. Insects 12, 440. doi:10.3390/insects12050440
Smyth, S. J. (2017). Genetically modified crops, regulatory delays, and international trade. Food Energy Secur 6, 78–86. doi:10.1002/fes3.100
Soares, N. R., Mollinari, M., Oliveira, G. K., Pereira, G. S., and Vieira, M. L. C. (2021). Meiosis in polyploids and implications for genetic mapping: a review. Genes 12, 1517. doi:10.3390/genes12101517
Song, Y., Peng, Y., Liu, L., Li, G., Zhao, X., Wang, X., et al. (2024). Phased gap-free genome assembly of octoploid cultivated strawberry illustrates the genetic and epigenetic divergence among subgenomes. Hortic. Res. 11, uhad252. doi:10.1093/hr/uhad252
Sorgo, A., Jausovec, N., Jausovec, K., and Puhek, M. (2012). The influence of intelligence and emotions on the acceptability of genetically modified organisms. Electron. J. Biotechnol. 15, 1. doi:10.2225/vol15-issue1-fulltext-1
Sprink, T., and Wilhelm, R. (2024). “Genome editing in biotech regulations worldwide,” in A roadmap for plant genome editing. Editors A. Ricroch, D. Eriksson, D. Miladinović, J. Sweet, K. Van Laere, and E. Woźniak-Gientka (Cham: Springer), 425–435. doi:10.1007/978-3-031-46150-7_25
Sprink, T., Eriksson, D., Schiemann, J., and Hartung, F. (2016). Regulatory hurdles for genome editing: process-vs. product-based approaches in different regulatory contexts. Plant Cell Rep. 35, 1493–1506. doi:10.1007/s00299-016-1990-2
Sreedharan, S., Shekhawat, U. K., and Ganapathi, T. R. (2013). Transgenic banana plants overexpressing a native plasma membrane aquaporin Musa PIP 1; 2 display high tolerance levels to different abiotic stresses. Plant Biotechnol. J. 11, 942–952. doi:10.1111/pbi.12086
Surya Krishna, S., Harish Chandar, S. R., Ravi, M., Valarmathi, R., Lakshmi, K., Prathima, P. T., et al. (2023a). Transgene-free genome editing for biotic and abiotic stress resistance in sugarcane: prospects and challenges. Agronomy 13, 1000. doi:10.3390/agronomy13041000
Surya Krishna, S., Viswanathan, R., Valarmathi, R., Lakshmi, K., and Appunu, C. (2023b). CRISPR/Cas-Mediated genome editing approach for improving virus resistance in sugarcane. Sugar Tech. 25, 735–750. doi:10.1007/s12355-023-01252-5
Szalonek, M., Sierpien, B., Rymaszewski, W., Gieczewska, K., Garstka, M., Lichocka, M., et al. (2015). Potato annexin STANN1 promotes drought tolerance and mitigates light stress in transgenic Solanum tuberosum L. plants. PloS One 10, e0132683. doi:10.1371/journal.pone.0132683
Tak, H., Negi, S., and Ganapathi, T. (2017). Banana NAC transcription factor MusaNAC042 is positively associated with drought and salinity tolerance. Protoplasma 254, 803–816. doi:10.1007/s00709-016-0991-x
Te Beest, M., Le Roux, J. J., Richardson, D. M., Brysting, A. K., Suda, J., Kubešová, M., et al. (2012). The more the better? The role of polyploidy in facilitating plant invasions. Ann. Bot. 109, 19–45. doi:10.1093/aob/mcr277
Tiwari, J. K., Buckseth, T., Challam, C., Zinta, R., Bhatia, N., Dalamu, D., et al. (2022). CRISPR/Cas genome editing in potato: current status and future perspectives. Front. Genet. 13, 827808. doi:10.3389/fgene.2022.827808
Tripathi, J. N., Lorenzen, J., Bahar, O., Ronald, P., and Tripathi, L. (2014). Transgenic expression of the rice Xa21 pattern-recognition receptor in banana (Musa sp.) confers resistance to Xanthomonas campestris pv. musacearum. Musacearum. Plant Biotechnol. J. 12, 663–673. doi:10.1111/pbi.12170
Tripathi, L., Babirye, A., Roderick, H., Tripathi, J. N., Changa, C., Urwin, P. E., et al. (2015). Field resistance of transgenic plantain to nematodes has potential for future African food security. Sci. Rep. 5, 8127. doi:10.1038/srep08127
Tripathi, L., Atkinson, H., Roderick, H., Kubiriba, J., and Tripathi, J. N. (2017). Genetically engineered bananas resistant to Xanthomonas wilt disease and nematodes. Food Energy Secur 6, 37–47. doi:10.1002/fes3.101
Tripathi, J. N., Ntui, V. O., Ron, M., Muiruri, S. K., Britt, A., and Tripathi, L. (2019). CRISPR/Cas9 editing of endogenous banana streak virus in the B genome of Musa spp. overcomes a major challenge in banana breeding. Commun. Biol. 2, 46. doi:10.1038/s42003-019-0288-7
Tripathi, L., Ntui, V. O., and Tripathi, J. N. (2019). Application of genetic modification and genome editing for developing climate-smart banana. Food Energy Secur 8, e00168. doi:10.1002/fes3.168
Tripathi, J. N., Ntui, V. O., Shah, T., and Tripathi, L. (2021). CRISPR/Cas9-mediated editing of DMR6 orthologue in banana (Musa spp.) confers enhanced resistance to bacterial disease. Plant Biotechnol. J. 19, 1291–1293. doi:10.1111/pbi.13614
Tripathi, J. N., Muiruri, S., and Tripathi, L. (2024a). Advancements and challenges in gene editing for improvement of vegetatively propagated crops. Curr. Opin. Plant Biol. 82, 102653. doi:10.1016/j.pbi.2024.102653
Tripathi, J. N., Ntui, V. O., and Tripathi, L. (2024b). Precision genetics tools for genetic improvement of banana. Plant Genome 17, e20416. doi:10.1002/tpg2.20416
Turker, T., Kocak, N., Aydin, I., İstanbulluoğlu, H., Yildiran, N., Turk, Y. Z., et al. (2013). Determination of knowledge, attitude, behavior about genetically modified organisms in nursing school students. Gülhane Tip. Derg. 55, 297. doi:10.5455/gulhane.33326
Udall, J. A., and Wendel, J. F. (2006). Polyploidy and crop improvement. Crop Sci. 46. doi:10.2135/cropsci2006.07.0489tpg
Ul Ain-Ali, Q., Munir, F., Tahir, M., Amir, R., and Gul, A. (2024). Cloning and overexpression of the DREB30 gene enhances drought and osmotic stress tolerance in transgenic potato. J. Plant Interact. 19, 2364656. doi:10.1080/17429145.2024.2364656
Ullah, I., Toor, M. D., Yerlikaya, B. A., Mohamed, H. I., Yerlikaya, S., Basit, A., et al. (2024). High-temperature stress in strawberry: understanding physiological, biochemical and molecular responses. Planta 260, 118. doi:10.1007/s00425-024-04544-6
Umesha, M., Sunisha, C., Chandrashekar, N., Usharani, T., Sowmya, H., and Sriram, S. (2025). Root-specific expression of anti-apoptotic gene AtBAG4 for engineering Fusarium wilt resistance in banana cv. Rasthali. Rasthali. Trop. Plant Biol. 18, 14. doi:10.1007/s12042-024-09383-z
Van de Peer, Y., Maere, S., and Meyer, A. (2009). The evolutionary significance of ancient genome duplications. Nat. Rev. Genet. 10, 725–732. doi:10.1038/nrg2600
Veillet, F., Perrot, L., Chauvin, L., Kermarrec, M.-P., Guyon-Debast, A., Chauvin, J.-E., et al. (2019). Transgene-free genome editing in tomato and potato plants using agrobacterium-mediated delivery of a CRISPR/Cas9 cytidine base editor. Int. J. Mol. Sci. 20, 402. doi:10.3390/ijms20020402
Vennapusa, A. R., Faleco, F., Vieira, B., Samuelson, S., Kruger, G. R., Werle, R., et al. (2018). Prevalence and mechanism of atrazine resistance in waterhemp (Amaranthus tuberculatus) from Nebraska. Weed Sci. 66, 595–602. doi:10.1017/wsc.2018.38
Vennapusa, A. R., Agarwal, S., Rao Hm, H., Aarthy, T., Babitha, K. C., Thulasiram, H. V., et al. (2022). Stacking herbicide detoxification and resistant genes improves glyphosate tolerance and reduces phytotoxicity in tobacco (Nicotiana tabacum L.) and rice (Oryza sativa L.). Plant Physiol. biochem. 189, 126–138. doi:10.1016/j.plaphy.2022.08.025
Verma, K. K., Song, X.-P., Budeguer, F., Nikpay, A., Enrique, R., Singh, M., et al. (2022). Genetic engineering: an efficient approach to mitigating biotic and abiotic stresses in sugarcane cultivation. Plant Signal. Behav. 17, 2108253. doi:10.1080/15592324.2022.2108253
Vermeulen, H., Delport, M., Louw, M., Gouse, M., and Miller, T. (2020). Consumer acceptance of sugar derived from genetically modified sugarcane in South Africa. AgBioForum 22. Available online at: https://agbioforum.org/consumer-acceptance-of-sugar-derived-from-genetically-modified-sugarcane-in-south-africa-2/.
Vieira, M. L. C., Almeida, C. B., Oliveira, C. A., Tacuatiá, L. O., Munhoz, C. F., Cauz-Santos, L. A., et al. (2018). Revisiting meiosis in sugarcane: chromosomal irregularities and the prevalence of bivalent configurations. Front. Genet. 9, 213. doi:10.3389/fgene.2018.00213
Vondracek, K., Altpeter, F., Liu, T., and Lee, S. (2024). Advances in genomics and genome editing for improving strawberry (Fragaria× ananassa). Front. Genet. 15, 1382445. doi:10.3389/fgene.2024.1382445
Wang, W. Z., Yang, B. P., Feng, X. Y., Cao, Z. Y., Feng, C. L., Wang, J. G., et al. (2017). Development and characterization of transgenic sugarcane with insect resistance and herbicide tolerance. Front. Plant Sci. 8, 1535. doi:10.3389/fpls.2017.01535
Wang, Y., Zhang, J., Cui, W., Guan, C., Mao, W., and Zhang, Z. (2017). Improvement in fruit quality by overexpressing miR399a in woodland strawberry. J. Agric. Food Chem. 65, 7361–7370. doi:10.1021/acs.jafc.7b01687
Wang, W., Wang, J., Feng, X., Shen, L., Feng, C., Zhao, T., et al. (2022). Breeding of virus-resistant transgenic sugarcane by the integration of the Pac1 gene. Front. Sustain. Food Syst. 6, 925839. doi:10.3389/fsufs.2022.925839
Widyaningrum, S., Pujiasih, D. R., Sholeha, W., Harmoko, R., and Sugiharto, B. (2021). Induction of resistance to sugarcane mosaic virus by RNA interference targeting coat protein gene silencing in transgenic sugarcane. Mol. Biol. Rep. 48, 3047–3054. doi:10.1007/s11033-021-06325-w
Wolter, F., Schindele, P., and Puchta, H. (2019). Plant breeding at the speed of light: the power of CRISPR/Cas to generate directed genetic diversity at multiple sites. BMC Plant Biol. 19, 176. doi:10.1186/s12870-019-1775-1
Woźniak-Gientka, E., Agata, T., Milica, P., Anna, B., Dennis, E., Nick, V., et al. (2022). Public perception of plant gene technologies worldwide in the light of food security. Gm. Crops Food 13, 218–241. doi:10.1080/21645698.2022.2111946
Wunderlich, S., and Gatto, K. A. (2015). Consumer perception of genetically modified organisms and sources of information. Adv. Nutr. 6, 842–851. doi:10.3945/an.115.008870
Xing, S., Chen, K., Zhu, H., Zhang, R., Zhang, H., Li, B., et al. (2020). Fine-tuning sugar content in strawberry. Genome Biol. 21, 230–14. doi:10.1186/s13059-020-02146-5
Xu, Y., Hu, W., Liu, J., Song, S., Hou, X., Jia, C., et al. (2020). An aquaporin gene MaPIP2-7 is involved in tolerance to drought, cold and salt stresses in transgenic banana (Musa acuminata L.). Plant Physiol. biochem. 147, 66–76. doi:10.1016/j.plaphy.2019.12.011
Xu, Y., Liu, J., Jia, C., Hu, W., Song, S., Xu, B., et al. (2021). Overexpression of a banana aquaporin gene MaPIP1; 1 enhances tolerance to multiple abiotic stresses in transgenic banana and analysis of its interacting transcription factors. Front. Plant Sci. 12, 699230. doi:10.3389/fpls.2021.699230
Xu, W., Liang, J., Wang, F., and Yang, L. (2024). Comparative evaluation of gene copy number estimation techniques in genetically modified crops: insights from Southern blotting, qPCR, dPCR and NGS. Plant Biotechnol. J. 22, 3456–3458. doi:10.1111/pbi.14466
Yadav, K., Patel, P., Srivastava, A. K., and Ganapathi, T. (2017). Overexpression of native ferritin gene MusaFer1 enhances iron content and oxidative stress tolerance in transgenic banana plants. PLoS One 12, e0188933. doi:10.1371/journal.pone.0188933
Yao, W., Ruan, M., Qin, L., Yang, C., Chen, R., Chen, B., et al. (2017). Field performance of transgenic sugarcane lines resistant to sugarcane mosaic virus. Front. Plant Sci. 8, 104. doi:10.3389/fpls.2017.00104
Yuan, X., Li, S., Chen, J., Yu, H., Yang, T., Wang, C., et al. (2024). Impacts of global climate change on agricultural production: a comprehensive review. Agronomy 14, 1360. doi:10.3390/agronomy14071360
Yun, J.-Y., Yu, S., Bang, S., Kim, J.-Y., Lee, S. H., and Lee, B. (2022). Identification of CRISPR-induced mutations in plants: with a focus on the next-generation sequencing assay. J. Plant Biol. 65, 435–443. doi:10.1007/s12374-022-09368-z
Zannoni, L. (2019). Evolving regulatory landscape for genome-edited plants. CRISPR J. 2, 3–8. doi:10.1089/crispr.2018.0016
Zhang, Y., Long, Y., Liu, Y., Yang, M., Wang, L., Liu, X., et al. (2021). MAPK5 and MAPK10 overexpression influences strawberry fruit ripening, antioxidant capacity and resistance to Botrytis cinerea. Planta 255, 19. doi:10.1007/s00425-021-03804-z
Zhang, H., Zhu, J., Gong, Z., and Zhu, J.-K. (2022). Abiotic stress responses in plants. Nat. Rev. Genet. 23, 104–119. doi:10.1038/s41576-021-00413-0
Zhang, C., Liu, Y., Wang, B., Li, H., Zhang, J., Ma, Y., et al. (2023). CRISPR/Cas9 targeted knockout FvPHO2 can increase phosphorus content and improve fruit quality of woodland strawberry. Sci. Hortic. 317, 112078. doi:10.1016/j.scienta.2023.112078
Zhao, M., Zhou, Y., Su, L., Li, G., Huang, Z., Huang, D., et al. (2022). Expression of Pinellia pedatisecta agglutinin PPA gene in transgenic sugarcane led to stomata patterning change and resistance to sugarcane woolly aphid, Ceratovacuna lanigera Zehntner. Int. J. Mol. Sci. 23, 7195. doi:10.3390/ijms23137195
Zheng, Z., Ye, G., Zhou, Y., Pu, X., Su, W., and Wang, J. (2021). Editing sterol side chain reductase 2 gene (StSSR2) via CRISPR/Cas9 reduces the total steroidal glycoalkaloids in potato. Life 14, 401–413. doi:10.1080/26895293.2021.1925358
Zhou, D., Liu, X., Gao, S., Guo, J., Su, Y., Ling, H., et al. (2018). Foreign cry1Ac gene integration and endogenous borer stress-related genes synergistically improve insect resistance in sugarcane. BMC Plant Biol. 18, 342–14. doi:10.1186/s12870-018-1536-6
Zhou, J., Sittmann, J., Guo, L., Xiao, Y., Huang, X., Pulapaka, A., et al. (2021). Gibberellin and auxin signaling genes RGA1 and ARF8 repress accessory fruit initiation in diploid strawberry. Plant Physiol. 185, 1059–1075. doi:10.1093/plphys/kiaa087
Zhu, Y. J., McCafferty, H., Osterman, G., Lim, S., Agbayani, R., Lehrer, A., et al. (2011). Genetic transformation with untranslatable coat protein gene of sugarcane yellow leaf virus reduces virus titers in sugarcane. Transgenic Res. 20, 503–512. doi:10.1007/s11248-010-9432-3
Zhu, X., Richael, C., Chamberlain, P., Busse, J. S., Bussan, A. J., Jiang, J., et al. (2014). Vacuolar invertase gene silencing in potato (Solanum tuberosum L.) improves processing quality by decreasing the frequency of sugar-end defects. PloS One 9, e93381. doi:10.1371/journal.pone.0093381
Zhu, K., Huang, C., Phan, T.-T., Yang, L.-T., Zhang, B.-Q., Xing, Y.-X., et al. (2021). Overexpression of SoACLA-1 gene confers drought tolerance improvement in sugarcane. Plant Mol. Biol. Rep., 1–12. doi:10.1007/s11105-020-01263-6
Keywords: polyploids, vegetative propagation, genome editing, transgenics, climate resilience, quality, food security
Citation: Sakthivel SK, Vennapusa AR and Melmaiee K (2025) Enhancing quality and climate resilient traits in vegetatively propagated polyploids: transgenic and genome editing advancements, challenges and future directions. Front. Genet. 16:1599242. doi: 10.3389/fgene.2025.1599242
Received: 24 March 2025; Accepted: 28 July 2025;
Published: 11 August 2025.
Edited by:
Songwen Zhang, University of Washington, United StatesReviewed by:
Ranjeet Ranjan Kumar, Indian Agricultural Research Institute (ICAR), IndiaSudhakar Reddy Palakolanu, International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), India
Copyright © 2025 Sakthivel, Vennapusa and Melmaiee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kalpalatha Melmaiee, a21lbG1haWVlQGRlc3UuZWR1
 Surya Krishna Sakthivel
Surya Krishna Sakthivel Amaranatha Reddy Vennapusa
Amaranatha Reddy Vennapusa Kalpalatha Melmaiee
Kalpalatha Melmaiee