- Affiliated Foshan Maternity and Child Healthcare Hospital, Guangdong Medical University, Foshan, Guangdong, China
Background: PATL2 deficiency is a significant cause of female infertility. Although multiple PATL2 missense variants have been reported in prior studies, a number of these variants remain classified as variants of uncertain significance (VUS).
Methods: We present a patient of primary infertility characterized by oocyte maturation disorders and fertilization failure. Comprehensive genetic analysis was conducted through whole-exome sequencing (WES) to identify pathogenic variants, followed by Sanger sequencing for familial co-segregation analysis. Reverse transcription (RT-PCR), cDNA sequencing and quantitative RT-PCR were performed to validate the effect of the variant on pre-mRNA splicing.
Results: We identified compound heterozygous variants in the PATL2 gene by WES: a pathogenic splice-site splicing variant (c.223-14_223-2del) and a missense variant (c.877G>T) initially classified as a VUS. Sanger sequencing confirmed that the proband carried biallelic variants, whereas her sisters with either wild-type genotypes or a single heterozygous variant exhibited normal fertility, supporting the co-segregation of the identified variants. Critically, RNA assays demonstrated that the missense variant c.877G>T disrupts canonical splicing of PATL2, resulting in exon 12 skipping.
Conclusion: This study provides the first experimental evidence that a PATL2 missense variant (c.877G>T) can exert its pathogenicity through aberrant splicing, supporting its pathogenic reclassification and elucidating a genotype-phenotype correlation for PATL2 missense variants through functional assays.
1 Introduction
Infertility is an important issue in human reproductive health. According to a newly published report by WHO, approximately 17.5% of the adult population experiences infertility. With the widespread application of assisted reproductive technology, including in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI), most infertile couples are able to have their own babies. However, recurrent IVF/ICSI failure still persists in some patients. Such failures can be result from problems related to oocyte production which can be caused by various factors, including defects in gonadal differentiation and ovary development, oocyte maturation and gamete recognition, or early embryonic development (Solovova and Chernykh, 2022). Oocyte maturation involves the stages from germinal vesicle (GV) to metaphase I (MI) and ultimately to metaphase II (MII) oocytes, is a fundamental prerequisite for reproduction (Pei et al., 2023). Oocyte maturation disorder is a rare syndrome characterized by the repeated production of a majority of immature oocytes, causing primary infertility, repetitive production of immature oocytes, inability of in vitro maturation (IVM) to stimulate maturation, and recurrent fertilization failure after IVF/ICSI (Beall et al., 2010). Oocyte maturation is a well-organized complex process. In recent years, emerging evidence highlights the pivotal role of genetic factors in oocyte maturation disorder, fertilization failure, early embryonic development arrest, revealing multiple disease-causing genes (Fei and Zhou, 2022).
The PATL2 gene encodes Protein PAT1 homolog 2, an RNA-binding protein, is more highly and specifically expressed in human germinal vesicle, metaphase I, and polar body I oocytes than in various somatic tissues. PATL2 is highly expressed before germinal vesicle breakdown (GVBD). As the oocyte matures, PATL2 is gradually degraded in the cytoplasm of oocytes (Chen et al., 2017). PATL2 acts as a translational repressor during oocyte maturation (Nakamura et al., 2010; Christou-Kent et al., 2018), is regulated maternal mRNA expression in immature oocytes. Variants in human PATL2 gene lead to oocyte/zygote/embryo maturation arrest-4 (OZEMA4, OMIM: 617743). These findings indicate that temporal control of PATL2 expression levels is critical for the normal maturation of oocytes.
In the present study, we identified compound heterozygous variants of PATL2 in a patient with primary infertility: a pathogenic splice-site variant (c.223-14_223-2del; p.Arg75Valfs*21) and a missense variant (c.877G>T; p.D293Y) initially classified as a VUS. We first demonstrated that the missense variant c.877G>T disrupted canonical splicing, inducing exon 12 skipping in the mRNA transcript and producing an in-frame 18-amino acid deletion (p.293_310del) in the N-terminal PAT1 domain. Given the critical role of the PAT1 domain in RNA binding (Nakamura et al., 2010), this deletion might potentially impair the protein’s function. Our findings offered evidence for re-evaluating the pathogenicity of the c.877G>T variant, and provided new insight into the pathogenic mechanism of missense variants in the PATL2 gene.
2 Methods
2.1 Evaluation of oocyte and embryo phenotypes
Oocytes were obtained from the proband and a normal individual undergoing in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI). Morphological assessments of oocyte maturation, fertilization, and embryonic development were performed using time lapse imaging. The study was approved by the Medical Ethics Committee of Foshan Maternity and Child Healthcare Hospital, Guangdong Medical University (No. FSFY-MEC-2025-028). The participants provided their written informed consent to participate in this study.
2.2 Whole-exome sequencing and pathogenicity analysis
Genomic DNA (gDNA) was extracted with QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany) from peripheral blood of proband and other family members using standard methods. Candidate variants were identified by whole-exome sequencing (WES). Briefly, DNA was fragmented and processed using the NadPrep DNA Universal Library Preparation Kit (Nanodigmbio, Nanjing, China) for whole-genome and exome library construction. Whole-exome enrichment was performed with the Exome Plus Panel v2.0 (Nanodigmbio, Nanjing, China) targeting exon and known pathogenic regions. Libraries were sequenced on the DNBSEQ-T7 platform (MGI Tech, Shenzhen, China). The sequencing data were converted to FASTQ format. Clean reads were aligned to the UCSC hg19 reference genome using BWA software, with duplicate reads removed via Picard. Variants, including SNVs and InDels, were identified using GATK HaplotypeCaller. Variants were annotated with ANNOVAR. The online software SpliceAI, dbscSNV, MaxEntScan, SPiCE, CADD splice were used to predict the influence on the splicing. Pathogenicity analysis of variants was performed according to the ACMG/AMP practice guidelines.
2.3 Variant validation and co-segregation analysis
Sanger sequencing was used to verify the candidate variants in the proband and perform segregation analysis in the other family members. The following Sanger sequencing primer pairs were used: PATL2-c223-F/R and PATL2-c877-F/R.
2.4 RT-PCR, cDNA sequencing and quantitative RT-PCR
Total RNA was isolated from the peripheral blood leukocytes of the proband and control individuals using TRIzol reagent (Invitrogen, MA, United States). RNA was reverse-transcribed using a TaKaRa PrimeScript reagent kit (TaKaRa, Dalian, China) according to the manufacturer’s protocol. To investigate the aberrant splicing of the variant of PATL2, we amplified PATL2 cDNA spanning exons 10 to 14 in the proband and a normal control individual (NC) using primers PATL2-E10-F and PATL2-E14-R. Then, the obtained PCR products were analyzed by gel electrophoresis on a 1% agarose gel. Individual bands were excised from the gel, eluted using the Gel Extraction Purification Kit (QIAGEN, Hilden, Germany) and the further Sanger sequencing was performed on the cDNA purified product. Quantitative RT-PCR (qPCR) was conducted using SYBR Premix Ex Taq (Takara, Dalian, China) according to the manufacturer’s protocol. GAPDH served as an endogenous control. The 2−ΔΔCt method was used to analyze the relative gene expression. All sequencing and qPCR primers used in this study are listed in Supplementary Table S1.
3 Clinical description and results
3.1 Clinical characterization
The proband, a 30-year-old female, and her 28-year-old husband had been diagnosed with primary infertility for 1 year. Both had normal karyotypes. Following two unsuccessful intrauterine insemination (IUI) cycles, the couple underwent two consecutive IVF/ICSI cycles, neither of which yielded viable embryos. In these two cycles, we used a time-lapse embryo incubator for cultivation and imaging, with images captured every 15 min for 7 consecutive days of continuous observation. In the first cycle, 16 oocytes were retrieved. Then IVF fertilization was conducted, but total fertilization failure occurred. After 16-18 h in vitro, 1 germinal vesicle (GV) stage oocyte, 2 metaphase I (MI) stage oocytes, and 13 metaphase II (MII) oocytes were observed. Strikingly, all MII oocytes displayed morphological abnormalities, notably enlarged or double polar bodies (Figure 1A). Subsequently, Late rescue ICSI was conducted on 8 MII oocytes, but no pronuclei formation was observed, 1 oocyte showed abnormal cleavage. Eventually, no embryo was obtained, and the cycle was canceled. In the second cycle, 18 oocytes were retrieved, 8 at the GV stage, 4 at the MI stage, and 6 at the MII stage. Similar to the first cycle, all MII oocytes again exhibited polar body defects (Figure 1A). ICSI was performed on 6 MII oocytes, but no pronuclei formation was observed on D1. 4 oocytes showed abnormal cleavage on D2 and D3 (Figure 1C). Eventually, no embryos were obtained, and the cycle was canceled. Notably, 6 oocytes were arrested at the GV stage, 4 oocytes were arrested at the MI stage after 65 h in vitro (Figure 1B). The proband has five sisters and two brothers range from 20 to 40 years old, all of whom exhibit normal fertility without reproductive complications.
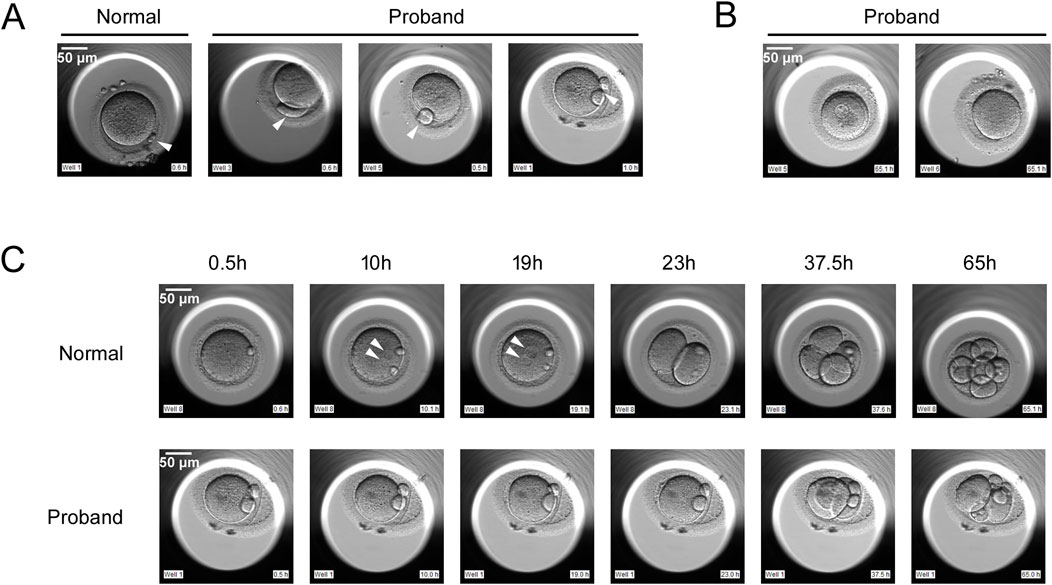
Figure 1. Phenotypes of oocytes from normal individual and the proband with PATL2 variants. (A) The morphology of metaphase II (MII) oocytes from a normal individual and the proband. The proband had MII oocytes with abnormal polar body, as indicated by the arrows. (B) Oocytes arrested at metaphase I (MI) stage and germinal vesicle (GV) stage after 65 h in vitro. (C) Development of fertilized eggs from a normal individual and the proband. In the proband’s MII oocytes subjected to ICSI, no pronucleus formation was observed, and abnormal cleavage was present. The arrows indicate the pronucleus. Scale bar, 50 μm.
3.2 Identification of variants in PATL2 gene
WES was performed to elucidate the genetic basis of infertility in the proband (individual II-4). Bioinformatic analysis identified two heterozygous variants in the PATL2 gene (NM_001387263.1): a splicing variant c.223-14_223-2del (p. R75Vfs*21) and a missense variant c.877G>T (p.D293Y). These variants may be responsible for the phenotypes of the proband. No pathogenic variants were detected in other known female infertility or oocyte development-associated genes. The c.223-14_223-2del variant was classified as pathogenic based on prior literature and database annotations (Chen et al., 2017; Huang et al., 2018). While the c.877G>T variant was initially classified as a VUS according to ACMG/AMP guidelines (PM2_Supporting, PM3_Supporting, PP3), the supporting evidence is as follows: 1. The c.877G>T variant has an allele frequency of 0% in population databases and has been undetected in all major control cohorts (e.g., gnomAD, ExAC, 1,000 Genomes, NHLBI ESP) (PM2_Supporting); 2. In a female infertility patient, it cannot be determined whether this variant and another variant c.223-14_223-2del (a known pathogenic variant) are in cis or trans configuration (PM3_Supporting) (Wu et al., 2019); 3. Multiple in silico prediction tools (e.g., SpliceAI, dbscSNV, MaxEntScan, SPiCE, CADD splice) suggest that this variant may disrupt mRNA splicing (PP3).
Then, Sanger sequencing was used to verify the candidate variants in the proband and to perform segregation analysis among other family members (Figure 2A). The Sanger sequencing results indicated that the proband’s two sisters (II-3, II-6) exhibited wild-type genotypes (WT/WT) at both loci, two other sisters (II-1, II-5) were heterozygous carriers of the c.223-14_223-2del variant, the youngest sister (II-7) was heterozygous carriers of the c.877G>T variant (Figure 2B). All the sisters of proband retained fertility, consistent with an autosomal recessive inheritance pattern and supporting co-segregation of the compound heterozygous variants with the infertility phenotype. Following validation, the variant c.877G>T lies in trans to the pathogenic variant c.223-14_223-2del, meeting PM3 criteria for pathogenic evidence. Combining segregation data from affected and unaffected individuals yielded a LOD score of 0.62, meeting PP1 criteria for pathogenic evidence (Oza et al., 2018).
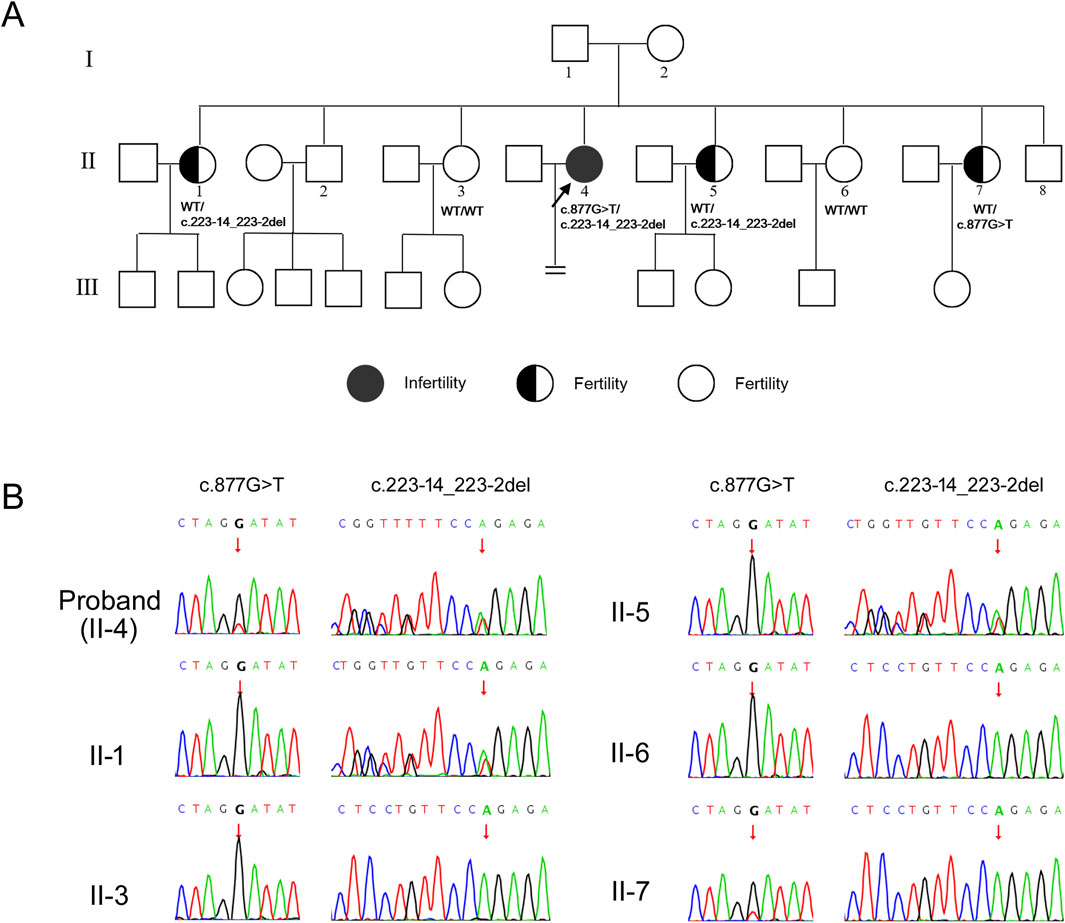
Figure 2. Identification of variants in PATL2 gene. (A) Pedigrees of the affected family. Black circle represents affected individuals. Half black circles represent carriers. Clear circles represent unaffected individuals. The black arrow indicates the proband. The “ = ” sign indicates infertility. Corresponding Sanger sequencing results are presented below the family members. (B) Sequencing chromatograms of family members. The figure illustrates carrying status of the variant c.877G>T and c.223-14_223-2del among the family members. The arrows in the chromatograms indicate the positions of the variants.
3.3 Confirmation of the aberrant splicing
The missense variant c.877G>T was located on the first base of exon 12 in the PATL2 gene (NM_001387263.1), adjacent to the splicing acceptor site of intron 11-a region critical for splicing fidelity (Figure 3A). Multiple in silico prediction tools (e.g., SpliceAI, dbscSNV, MaxEntScan, SPiCE, CADD splice) suggest that this variant may disrupt mRNA splicing, SpliceAI suggested the score of acceptor loss was 0.43 (threshold ≥ 0.2), indicated that the variant (c.877G>T) was likely to change the acceptor spot for mRNA splicing. To assess the impact of the variant on PATL2 transcript processing, total RNA was isolated from the proband and normal control individual. The RT-PCR amplification of PATL2 transcripts spanning exons 10-14 revealed two distinct bands in the lane of proband (bands A and B), contrasting with a single product (band A) observed in the lane of control sample (Figure 3B). Sanger sequencing of gel-purified products demonstrated that band A corresponded to the canonical splicing pattern (exon11-exon12-exon13), but band B was an aberrant splicing band which skipped complete exon12 (exon11-exon13) (Figure 3C). Exon12 contain 54 base pair. So, this splicing defect led to 18-amino acid in-frame deletion (p.293_310del) in N-terminus of PAT1 domain of PATL2 (Figure 3A). We subsequently used qPCR to investigate the abundance of aberrant splicing variants at the mRNA level. Primers targeting exon7-exon9 (shared by both alleles) were used to evaluate the total expression of PATL2, while mutant-specific primers (forward: upstream of the deletion region [exon 9]; reverse: within the deletion region [exon12]) specifically amplified the other allele. The results showed that the total PATL2 mRNA level in the proband was slightly increased compared with normal controls, but the difference was not statistically significant. The mRNA level of the exon 9-exon12 region in the proband was approximately half that of normal control (Figure 3D).These findings indicated that aberrant splicing may not result in degradation of PATL2 mRNA. Collectively, RNA assays demonstrated that the c.877G>T variant results in truncation of <10% of the full-length PATL2 protein, meeting the criteria for PVS1_Moderate according to the ACMG/AMP guidelines.
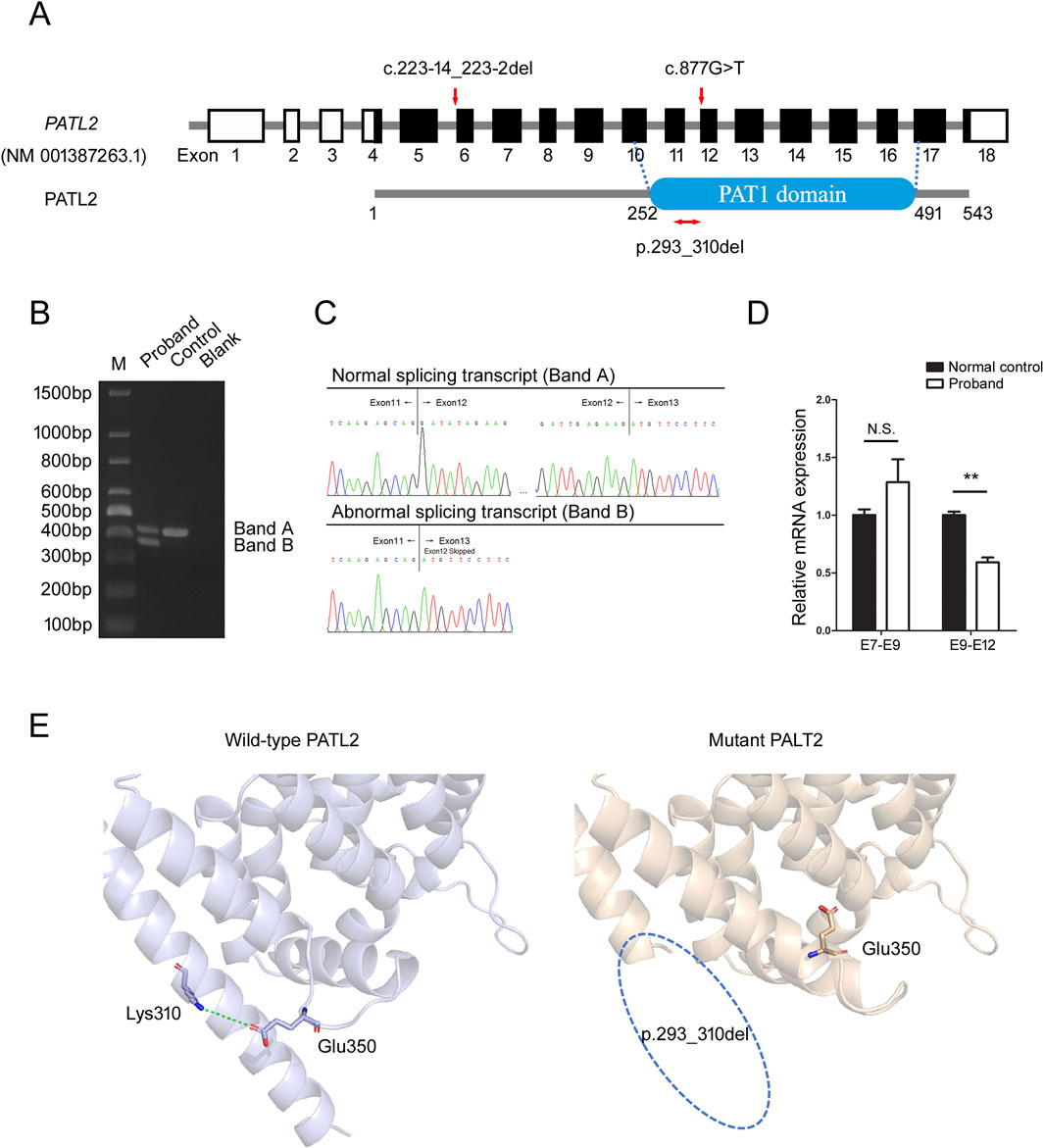
Figure 3. Identification of aberrant splicing in PATL2. (A) The positions of variants and functional domains are indicated in the gene structures. The missense variant c.877G>T leads to in-frame deletion (p.293_310del) in PATL2 is marked in red. (B) Agarose gel electrophoresis of RT-PCR products reveals two distinct amplification products: Band a corresponds to the wild-type transcript (normal splicing), while Band b represents the aberrant splicing isoform. M means the DNA marker. (C) Chromatograms demonstrate the precise splicing patterns corresponding to the RT-PCR products shown in (B), confirming the existence of both normal and aberrant transcripts. (D) Quantitative RT-PCR analysis of relative PATL2 mRNA expression levels in the proband and normal female controls (NC). E7-E9 represents amplification of the exon7-exon9 region; E9-E12 represents amplification of the exon9-exon12 region. N.S.: Not Significant (P > 0.05), **P < 0.01 (t-test), Error bars represent mean ± SEM (n = 3). (E) Structural modeling of wild-type and mutant PATL2 proteins using AlphaFold3. The wild-type PATL2 is presented in gray, while the mutant PALT2 is presented in wheat color. Blue dotted circle: a segment of the α-helix within the PAT1 domain of the mutant PALT2 is absent.
To illustrate the structural location of the deleted amino acids within the protein, we employed AlphaFold3 (Abramson et al., 2024) for structural modeling of wild-type and mutant PATL2 proteins, which revealed that this variant caused a deletion of an alpha-helix in the PAT1 domain. Further analysis demonstrated that a critical salt bridge interaction between Lys310 and Glu350 in the wild-type protein was disrupted by the Asp293-Lys310 deletion (Figure 3E). While these protein structure predictions have not been experimentally validated and serve only as supporting evidence, they can provide valuable insights for subsequent research.
4 Discussion
The PATL2 gene encodes an RNA-binding protein known as Protein PAT1 homolog 2 that regulates maternal mRNA homeostasis. P100 in Xenopus oocytes is the orthologue of human PATL2, which was regarded as a mRNA-binding protein (mRNP) associated with other mRNPs, such as Xp54, xRAP55, and CPEB. plays a role in regulating the translation of specific maternal mRNAs required for the progression of Xenopus oocyte maturation (Nakamura et al., 2010). Consistent with this role, Patl2 knockout mice exhibit oocyte and zygote morphological and developmental defects. Alongside significant downregulation of gene expression associated with oocyte maturation, including CDC25a and SohIh2 in PATL2-absence mouse oocytes (Christou-Kent et al., 2018). In humans, biallelic PATL2 variants, including homozygous or compound heterozygous variants lead to infertility due to oocyte germinal vesicle (GV) arrest or MI arrest, fertilization failure, and early embryo developmental arrest (Huo et al., 2022; Ye et al., 2024). All of these researches indicated that the PATL2 play a pivotal role in oocyte maturation.
In this study, we identified compound heterozygous PATL2 variants in a patient with primary infertility due to oocyte maturation disorders and fertilization failure: a pathogenic splice-site variant (c.223-14_223-2del, p.R75Vfs*21) and a missense variant (c.877G>T, p.D293Y). The latter was only reported in one patient, and the patient carried heterozygous variants c.877G>T and c.223-14_223-2del with an unknown inheritance pattern (Wu et al., 2019). Thus, the pathogenicity of the variant c.877G>T was not well defined. To understand the molecular pathogenesis of the missense variant c.877G>T, we performed RNA assay and confirmed that the c.877G>T variant caused aberrant splicing of the PATL2 transcript characterized by exon 12 skipping (Δ54 bp), resulting in an in-frame 18-amino acid deletion (p.293_310del) in the N-terminus of PAT1 domain. The PAT1 domain is a critical functional domain responsible for RNA binding. Another variation that is different at the DNA level but leads to the same protein-level deletion has also been reported. The canonical splice-site variant PATL2: c.877-1G>A was previously reported as pathogenic in patients with primary infertility due to oocyte maturation arrest (OMA). In two unrelated OMA patients, this variant was experimentally proven to cause exon 12 skipping, resulting in an in-frame 18-amino acid deletion (p.293_310del) within the N-terminal PAT1 domain (Sun et al., 2022; Zhu et al., 2022; Zhou et al., 2024). These results also demonstrate that the p.293_310del impairs the function of PATL2, supporting the pathogenicity of the c.877G>T variant. Several PATL2 missense variants reported in prior studies were documented to either reduce their expression levels or enhance degradation (Liu et al., 2020; Cao et al., 2021; Yu et al., 2024). In this study, we first verified that the missense variant c.877G>T in PALT2 disrupts canonical splicing, leading to oocyte maturation disorders and fertilization failure.
Owing to the clinical and genetic variability associated with oocyte maturation disorders, WES is considered the primary genetic detection method. WES enables the efficient identification of genetic variants within genes. However, many variants, especially missense variants detected by WES, remain poorly understood and are often classified as “variants of uncertain significance (VUS)”. The mechanisms by which missense variants lead to abnormal protein functions are diverse, making it challenging to definitively assess their pathogenicity. Therefore, experimental validation of the functional impacts of these variants is essential to clarify their clinical relevance. In this study, we provided additional evidence for pathogenicity assessment of the PATL2 missense variant c.877G>T through experimental validation and familial co-segregation analysis, supporting our proposal to reclassify this variant as “likely pathogenic” according to the ACMG/AMP guidelines (PM2_Supporting, PM3, PVS1_Moderate (RNA), and PP1). These findings offer more information to support genetic counseling and personalized treatment for the proband. Currently, there is no established therapeutic regimen for this disorder, and oocyte donation may serve as a potential fertility option. Collectively, our results underscore the importance of integrating functional assays with genetic analyses to elucidate the pathogenicity of genetic variants and the mechanisms responsible for clinical phenotypes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of Foshan Maternity and Child Healthcare Hospital (No. FSFY-MEC-2025-028). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HL: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Writing – original draft. YL: Investigation, Methodology, Writing – original draft. WM: Data curation, Formal Analysis, Methodology, Writing – original draft. TY: Investigation, Methodology, Writing – original draft. LD: Methodology, Visualization, Writing – original draft. YC: Data curation, Visualization, Writing – original draft. SF: Resources, Software, Writing – original draft. GL: Resources, Validation, Writing – review and editing. JZ: Validation, Writing – review and editing. GS: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the Medical Research Project of Foshan Health Bureau (grant number 20250057); National Health Commission Key Laboratory of Chronobiology open project (grant number NHCC-2024-03) and Foshan Science and Technology Bureau self-funded medical science and technology research projects (grant number 2420001003739).
Acknowledgments
We are grateful to the patient’s family.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2025.1611138/full#supplementary-material
References
Abramson, J., Adler, J., Dunger, J., Evans, R., Green, T., Pritzel, A., et al. (2024). Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 630 (8016), 493–500. doi:10.1038/s41586-024-07487-w
Beall, S., Brenner, C., and Segars, J. (2010). Oocyte maturation failure: a syndrome of bad eggs. Fertil. Steril. 94 (7), 2507–2513. doi:10.1016/j.fertnstert.2010.02.037
Cao, Q., Zhao, C., Wang, C., Cai, L., Xia, M., Zhang, X., et al. (2021). The recurrent mutation in PATL2 inhibits its degradation thus causing female infertility characterized by oocyte maturation defect through regulation of the Mos-MAPK pathway. Front. Cell Dev. Biol. 9, 628649. doi:10.3389/fcell.2021.628649
Chen, B., Zhang, Z., Sun, X., Kuang, Y., Mao, X., Wang, X., et al. (2017). Biallelic mutations in PATL2 cause female infertility characterized by oocyte maturation arrest. Am. J. Hum. Genet. 101 (4), 609–615. doi:10.1016/j.ajhg.2017.08.018
Christou-Kent, M., Kherraf, Z. E., Amiri-Yekta, A., Le Blévec, E., Karaouzène, T., Conne, B., et al. (2018). PATL2 is a key actor of oocyte maturation whose invalidation causes infertility in women and mice. EMBO Mol. Med. 10 (5), e8515. doi:10.15252/emmm.201708515
Fei, C. F., and Zhou, L. Q. (2022). Gene mutations impede oocyte maturation, fertilization, and early embryonic development. Bioessays 44 (10), e2200007. doi:10.1002/bies.202200007
Huang, L., Tong, X., Wang, F., Luo, L., Jin, R., Fu, Y., et al. (2018). Novel mutations in PATL2 cause female infertility with oocyte germinal vesicle arrest. Hum. Reprod. 33 (6), 1183–1190. doi:10.1093/humrep/dey100
Huo, M., Zhang, Y., Shi, S., Shi, H., Liu, Y., Zhang, L., et al. (2022). Gene spectrum and clinical traits of nine patients with oocyte maturation arrest. Front. Genet. 13, 772143. doi:10.3389/fgene.2022.772143
Liu, Z., Zhu, L., Wang, J., Luo, G., Xi, Q., Zhou, X., et al. (2020). Novel homozygous mutations in PATL2 lead to female infertility with oocyte maturation arrest. J. Assist. Reprod. Genet. 37 (4), 841–847. doi:10.1007/s10815-020-01698-6
Nakamura, Y., Tanaka, K. J., Miyauchi, M., Huang, L., Tsujimoto, M., and Matsumoto, K. (2010). Translational repression by the oocyte-specific protein P100 in xenopus. Dev. Biol. 344 (1), 272–283. doi:10.1016/j.ydbio.2010.05.006
Oza, A. M., DiStefano, M. T., Hemphill, S. E., Cushman, B. J., Grant, A. R., Siegert, R. K., et al. (2018). Expert specification of the ACMG/AMP variant interpretation guidelines for genetic hearing loss. Hum. Mutat. 39 (11), 1593–1613. doi:10.1002/humu.23630
Pei, Z., Deng, K., Xu, C., and Zhang, S. (2023). The molecular regulatory mechanisms of meiotic arrest and resumption in oocyte development and maturation. Reprod. Biol. Endocrinol. 21 (1), 90. doi:10.1186/s12958-023-01143-0
Solovova, O. A., and Chernykh, V. B. (2022). Genetics of oocyte maturation defects and early embryo development arrest. Genes 13 (11), 1920. doi:10.3390/genes13111920
Sun, L., Tong, K., Liu, W., Tian, Y., Yang, S., Zhou, D., et al. (2022). Identification and characterization of a novel homozygous splice site variant of PATL2 causing female infertility due to oocyte germinal vesicle arrest. Front. Genet. 13, 967288. doi:10.3389/fgene.2022.967288
Wu, L., Chen, H., Li, D., Song, D., Chen, B., Yan, Z., et al. (2019). Novel mutations in PATL2: expanding the mutational spectrum and corresponding phenotypic variability associated with female infertility. J. Hum. Genet. 64 (5), 379–385. doi:10.1038/s10038-019-0568-6
Ye, Z., Li, D., Niu, X., Yang, A., Pan, Z., Yu, R., et al. (2024). Identification novel mutations and phenotypic spectrum expanding in PATL2 in infertile women with IVF/ICSI failure. J. Assist. Reprod. Genet. 41 (5), 1233–1243. doi:10.1007/s10815-024-03071-3
Yu, A., Huang, Z., Shi, H., Lin, Y., Cai, X., Ke, Z., et al. (2024). Identification of a novel mutation in PATL2 gene associated with the germinal vesicle arrest of oocytes. Biochem. Biophys. Rep. 40, 101886. doi:10.1016/j.bbrep.2024.101886
Zhou, L., Yang, M., Mei, M., Mai, Z., Li, X., Deng, K., et al. (2024). Exploring the role of non-canonical splice site variants in aberrant splicing associated with reproductive genetic disorders. Clin. Genet. 106 (6), 750–756. doi:10.1111/cge.14604
Keywords: infertility, oocyte maturation disorders, PATL2, missense variant, aberrant splicing
Citation: Li H, Lin Y, Ma W, Yu T, Dong L, Chen Y, Fan S, Luo G, Zhang J and Song G (2025) Identification of a PATL2 missense variant (c.877G>T) disrupting canonical splicing and contributing to female infertility. Front. Genet. 16:1611138. doi: 10.3389/fgene.2025.1611138
Received: 13 April 2025; Accepted: 01 July 2025;
Published: 09 July 2025.
Edited by:
Yiping Shen, Harvard Medical School, United StatesCopyright © 2025 Li, Lin, Ma, Yu, Dong, Chen, Fan, Luo, Zhang and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ge Song, c29uZ2dlcHBAMTI2LmNvbQ==
 Hongyan Li
Hongyan Li Yue Lin
Yue Lin Weixu Ma
Weixu Ma Ting Yu
Ting Yu Lingfeng Dong
Lingfeng Dong Yankun Chen
Yankun Chen Shuming Fan
Shuming Fan Guoqun Luo
Guoqun Luo Jingwen Zhang
Jingwen Zhang Ge Song
Ge Song