- 1Department of Traditional Chinese Medicine, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children, Sichuan University, Chengdu, China
- 3Department of Gynaecology, Shanghai municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 4Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 5Department of Sports Medicine, Sichuan Orthopaedic Hospital, Chengdu, Sichuan, China
Background and Purpose: We aimed to explore the mechanisms and pathways of exercise-based interventions in the treatment of polycystic ovarian syndrome (PCOS).
Methods: In this literature review, studies related to exercise therapy for PCOS that were published in the past 20 years were searched, potentially effective active ingredients were screened, and gene prediction of active ingredients and diseases was conducted using the compound and GeneCards databases, respectively, to identify potential targets of exercised-related bioactive molecules in PCOS. Finally, hub genes and signaling pathways were predicted using bioinformatic methods.
Results: The review identified eight potential effective components were screened out, including irisin, 5α-reductase, kisspeptin, cocaine-and amphetamine-regulated transcript, nerve growth factor, nerve peptide Y, insulin-like growth factor-1, and interleukin-6. A total of 192 target genes for exercise-related components and PCOS were identified, including the hub genes TNF, IL6, IL1B, JUN, CCND1, and PSMA7.
Conclusion: The hub genes identified in this review indicate that exercise therapy in PCOS may affect the protease system, renin–angiotensin system, inflammatory signal transduction, neuroactive ligand–receptor interaction, and other pathways through the G protein-coupled receptor signaling pathway, neuropeptide signaling pathway, endocrine process, and other biological processes and regulate apoptosis, cell cycle, and intercellular communication.
1 Introduction
Polycystic ovarian syndrome (PCOS) is one of the most common reproductive disorders among women of childbearing age. PCOS is a systemic metabolic disease that is characterized by anovulation, infertility, obesity, and hirsutism, and is one of the primary causes of anovulatory infertility in adult women (Dapas and Dunaif, 2022). Exercise is the first-line treatment for PCOS, and meta-analyses have shown that exercise-based interventions can improve menstrual cycles and hirsutism as well as metabolic, body composition, and hormone-related indicators in patients with PCOS (Shan et al., 2021). Moreover, exercise can synergistically improve the efficacy of ovulation-promotion and assisted-reproduction treatments (Hakimi and Cameron, 2017). Furthermore, exercise can improve the body mass index, reproductive function, and hormonal status of patients with PCOS, with fewer adverse reactions and high patient acceptance (Chan et al., 2016). However, the specific mechanism through which exercise improves PCOS remains unclear. The application of bioinformatics is crucial for elucidating underlying mechanisms, as it allows for the systematic integration of fragmented information into a coherent molecular network.
This paper presents the results of a review of clinical and animal experimental studies on exercise therapy for PCOS that have been published over the past 20 years, summarizes the active compounds generated during those exercise interventions for PCOS, and, based on bioinformatics and network topology strategies, explores the main mechanism of action of exercise therapy for PCOS.
2 Data and methods
2.1 Data sources
Retrieval person and time: The first author conducted the literature search in September 2024.
Time limit for literature search: 2004-09-01 to 2024-09-01.
The PubMed, Embase, Cochrane Library, Web of Science database, CNKI, VIP database, Wanfang Database, and Chinese Biomedical Literature Database were searched.
Search terms: The English search terms included “Polycystic ovarian syndrome,” “Polycystic ovary,” “Stein*Leventhal Syndrome,” “Exercise,” “Training,” and “kinesitherapy.” The search was conducted by combining subject and entry words, and the references of the included articles were traced back to supplement the records that were obtained. The Chinese search terms included “polycystic ovary syndrome,” “polycystic ovary,” “polycystic ovary syndrome,” “exercise,” “training,” and “physical therapy.” Boolean logic words were used for the professional searches.
Manual search: Relevant magazines, conference papers, and materials were manually retrieved.
Search strategy: The PubMed search mode was undertaken as an example, as shown in Figure 1.
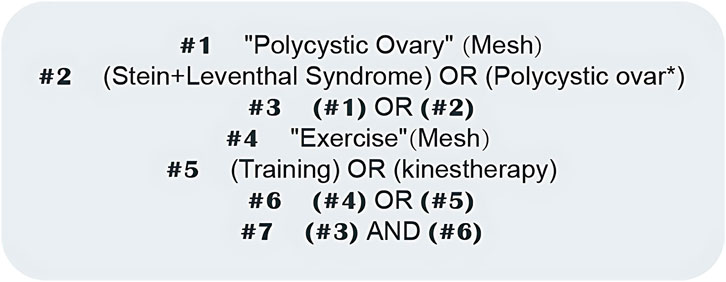
Figure 1. PubMed literature search strategy. Diagram showing the search terms and Boolean operators used for the systematic literature retrieval in PubMed.
2.2 Document inclusion criteria
All relevant studies, including clinical and animal experimental studies as well as observational and interventional research, that involved exercise intervention in PCOS were included without any restriction on the language.
2.3 Document exclusion criteria
The exclusion criteria included the following: (1) clinical studies that did not meet the 2003 Rotterdam Standard (Revised, 2003 consensus on diagnostic, 2004) or 2011 Chinese PCOS diagnostic criteria (Linlin and Zijiang, 2011) (e.g., only the ratio of anti-Muller hormone, menstrual cycle, luteinizing hormone (LH)/follicle-stimulating hormone (FSH) was used to diagnose PCOS); (2) animal experiments that did not include a description that confirmed the success of ovarian pathological section; (3) articles that pertained to complex interventions and reported inconclusive primary effects of exercise; (4) articles that did not include effective information on specific compounds/proteins at the mechanistic level; (5) articles that were literature review; (6) master and doctoral dissertations or conference papers; and (7) duplicate detection or duplicate publication.
2.4 Literature screening
Using the prespecified inclusion and exclusion criteria, two researchers independently searched the database and selected the appropriate literature; disagreements between researchers were resolved through negotiation, or if no consensus was reached, were resolved by a third researcher.
2.5 Data analysis
2.5.1 Screening of protein targets of active ingredients produced by the body after exercise
STITCH is an integrated database of small-molecule chemical interactions with proteins that comprises 390,000 chemicals and 3.6 million proteins from 1,133 organisms (Kuhn et al., 2014). Swiss Target Prediction was used to predict the most likely protein targets of small molecules with a high level of prediction performance (Daina et al., 2019). The SEA database integrates Compound Data Collection (ChEMBL), MDL Drug Data Report (MDDR), and other databases of compound and target information, and calculates compound similarity by using daylight molecular fingerprinting to cluster the targets of similar compounds (Keiser et al., 2007). Targets with a high affinity for the target compound were obtained from the abovementioned database. After combining the search results from the three databases, duplicate values were removed to identify the targets of the active compounds.
2.5.2 Screening of therapeutic targets for PCOS
GeneCards is a comprehensive database of human genes (Rebhan et al., 1997) that contains all annotated and predicted genetic information and integrates approximately 150 gene database resources, including genomic, transcriptomic, proteomic, genetic, clinical, and functional information. In this study, the GeneCards database was searched using the keyword “Polycystic Ovary Syndrome” and the related targets of PCOS were obtained.
2.5.3 Screening of targets related to exercise therapy for PCOS
We proceeded to develop two groups of targets using Venn tools (https://bioinfogp.cnb.csic.es/tools/venny/index.html), which showed the sports-related active ingredient targets with PCOS overlap between the target volume visualizations; the aspects in the overlap constitutes the core target of exercise therapy for PCOS. To clarify the relationship between the active ingredients produced by exercise and key targets, Cytoscape3.10.3 software was used to create a network diagram of the relationship between the active ingredients and targets (Shannon et al., 2003).
2.5.4 Construction of protein interaction network
Protein–protein networks (PPI) were constructed using STRING 11.5 (Szklarczyk et al., 2019). Possible targets of exercise therapy for PCOS were uploaded to the STRING tool to select humans as the species for retrieving the interacting genes/proteins. To clarify the relationship between active compounds and key targets, the search results were imported into Cytoscape3.10.3 software, and the CytoHubba plug-in was used for network topology attribute analysis; the results of multi-dimensions and algorithms were comprehensively compared to screen key genes (hub genes) (Chin et al., 2014).
2.5.5 Gene ontology and enrichment analysis of the kyoto genome encyclopedia
Gene ontology (GO) enrichment analysis (Thomas et al., 2022) and Kyoto Encyclopedia of Genes and Genomes (KEGG) (Kanehisa and Goto, 2000) were used, and the target effects were visualized using WebGestalt (https://www.webgestalt.org/) (Jm et al., 2024) and Hiplot tools (https://hiplot.com.cn/), which provided the basis for further functional research.
3 Results
3.1 Literature screening results
According to the search terms, 2,066 relevant studies were retrieved, from which irrelevant and duplicate studies were excluded; thus, 1,691 articles were screened by reading the literature. By applying the inclusion and exclusion criteria, 15 articles were selected, all of which were in English. All studies were animal experiments, 6 studies (Table 1) were found to have extractable compounds for subsequent analysis, from which 8 important active ingredients were selected, which were: irisin, 5α reductase, kisspeptin, amphetamine-regulated transcript, cocaine-and amphetamine-regulated transcript (CART), nerve growth factor (NGF), neuropeptide Y (NPY), insulin-like growth factor-1 (IGF-1), and interleukin-6 (IL-6) (Figure 2).
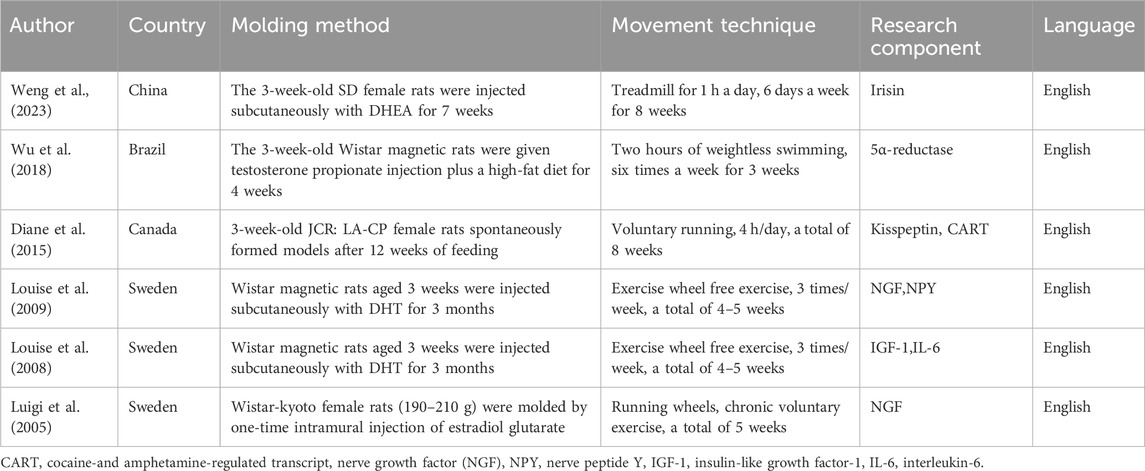
Table 1. Characteristics of included studies and extracted compounds. Summary of the six included animal studies, detailing the model establishment, exercise intervention, extracted active compounds, and other key characteristics.
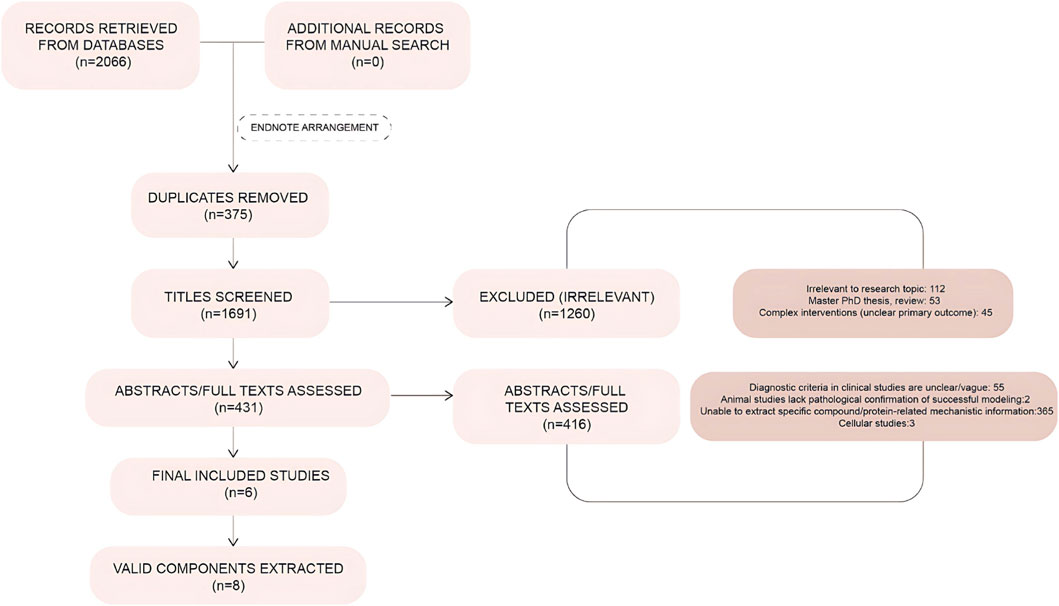
Figure 2. Literature screening flowchart. Flowchart illustrating the process of study identification, screening, and inclusion/exclusion based on predefined criteria.
3.2 Target screening of exercise therapy for PCOS
Using the STITCH, SEA, and Swiss Target Prediction databases, 328 genes that interacted with the active ingredients after acupuncture were obtained. 6684 PCOS-related genes were identified from the GeneCards database. After the human gene names in the two original documents were standardized and matched, 192 intersection targets with exercise-active ingredients and PCOS were mapped (Figure 3) to identify the potential therapeutic targets in PCOS. The “motion-active ingredient - target-disease” interaction network is shown in Figure 4, where the yellow triangular arrow represents exercise therapy, the red box represents PCOS, the orange hexagon represents the active ingredient, and the blue circle represents 192 potential therapeutic targets.
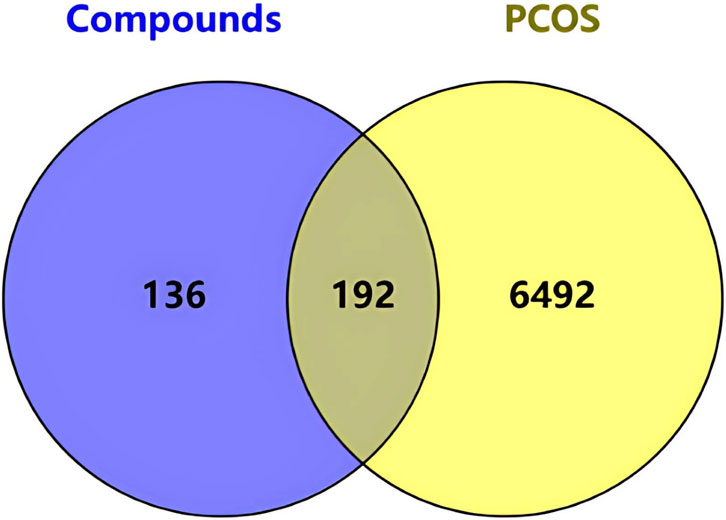
Figure 3. Venn diagram of overlapping targets. Venn diagram showing the intersection between exercise-related compound targets and PCOS-related genes, identifying 192 potential shared targets.
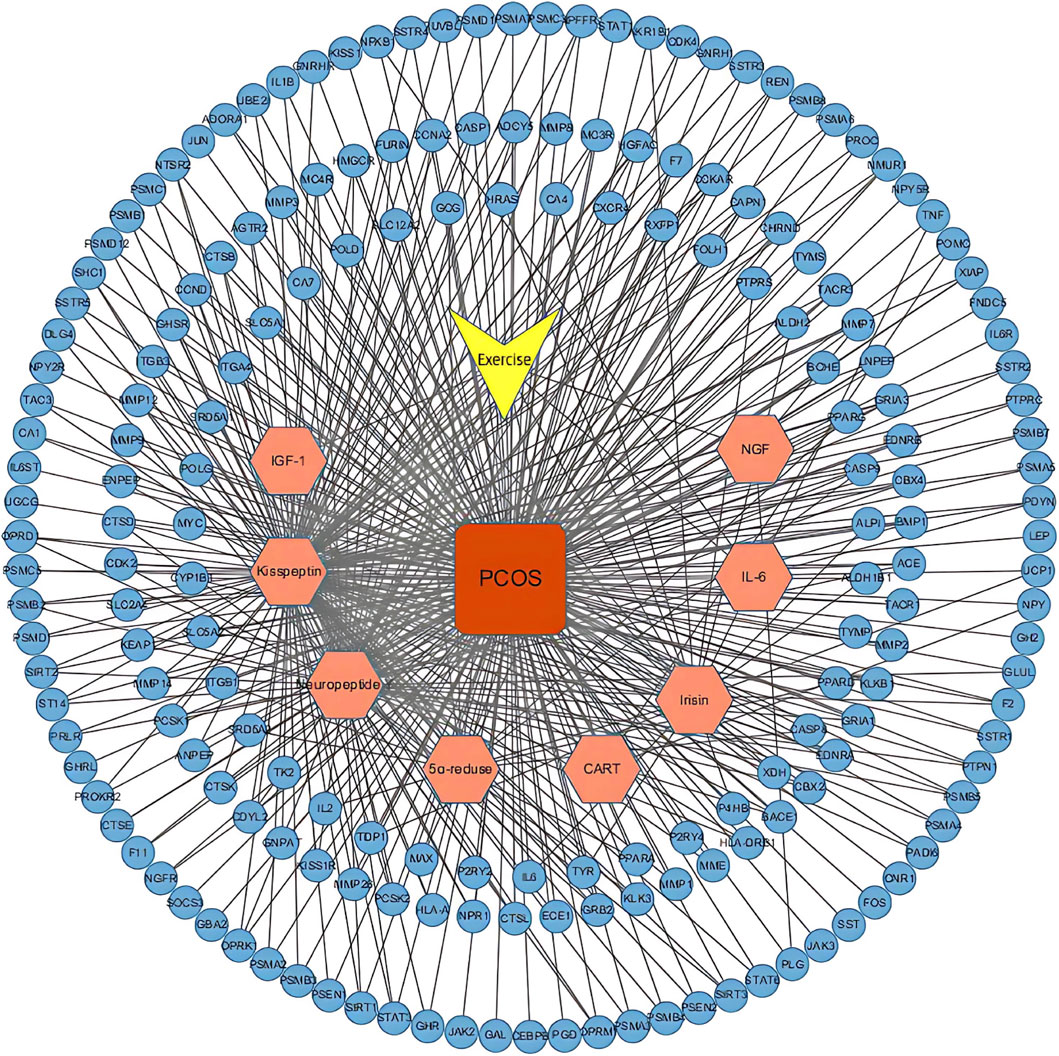
Figure 4. Active ingredient-potential target network. Network visualization depicting the interactions between exercise, the eight active ingredients (orange hexagons), PCOS (red square), and the 192 potential targets (blue circles).
3.3 Establishment and analysis of the PPI network
We constructed a PPI network based on the STRING database and visualized 187 nodes using Cytoscape. The Matthews correlation coefficient (MCC) method is an important iterative formula in the CytoHubba algorithm, providing a more accurate measure of node importance by iteratively calculating degree centrality while accounting for the node’s maximal clique. The topological network algorithm uses the CytoHubba plugin to assign values to each gene and to sort and screen hub genes and subnetworks. It was used to calculate the maximum clique centers of the nodes in the protein interaction network, as shown in Figure 5. TNF, CCND1 and PSMA7 showed a high correlation degree. In addition, we used various other algorithms to obtain hub genes (Table 2). Among different algorithms, TNF (appeared 7 times) ranked first, followed by IL6 (6 times), IL1B and JUN (all appeared 5 times).
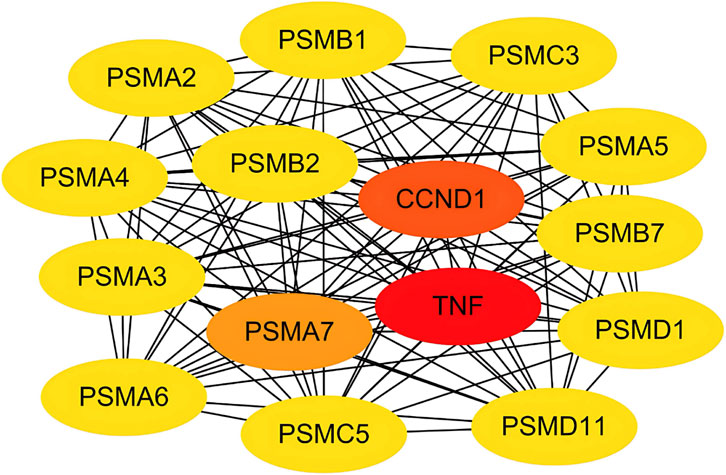
Figure 5. PPI network and hub genes. Protein-protein interaction network of the potential targets. Node color intensity corresponds to topological importance scores calculated by the MCC method, highlighting key hub genes like TNF and IL6.
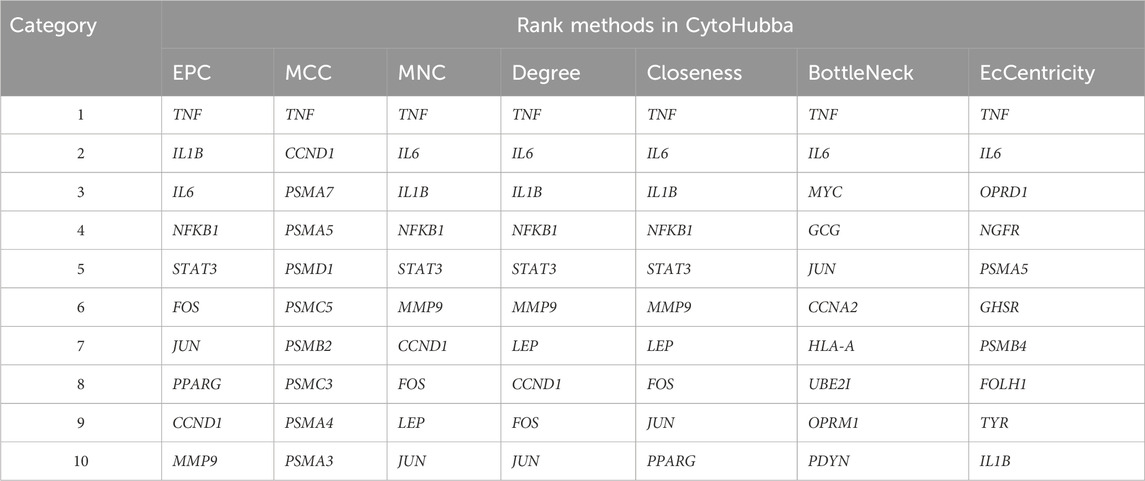
Table 2. Top 10 gene sets from different CytoHubba algorithms. Comparison of the highest-ranked genes identified by seven topological analysis algorithms in CytoHubba, demonstrating consistent hub genes like TNF and IL6 across methods.
3.4 GO and KEGG enrichment analysis
GO analysis can reflect the target function from three aspects: cellular components (CC), molecular functions (MF), and biological processes (BP). GO analysis identified 92 CC, 105 MF, and 177 BP. The first 20 CC, MF, BP, and KEGG lists were removed and visualized (Figure 6).
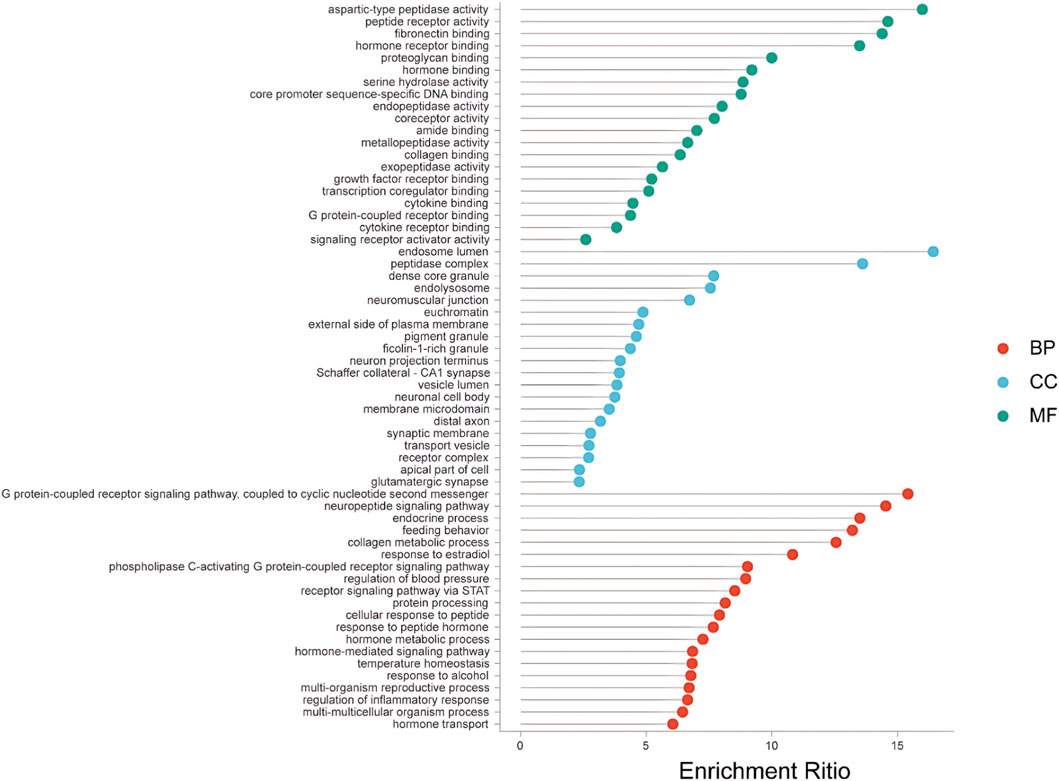
Figure 6. GO enrichment analysis. Bubble chart showing the significant enrichment terms in Biological Process (BP), Cellular Component (CC), and Molecular Function (MF) categories for the potential targets. Bubble size indicates the number of genes, and color indicates the enrichment significance.
At the BP level, the predicted target was the G protein-coupled receptor signaling pathway, which is mainly involved in the cyclic nucleotide second messenger. Coupled to cyclic nucleotide second messenger, neuropeptide signaling pathway, endocrine processes) and other biological processes have significant significance. At the CC level, the endosome lumen, peptidase complex, and dense core granules occupy a large proportion. The MF is closely associated with aspartic-type peptidase activity, peptide receptor activity, and fibronectin binding.
The potential KEGG enrichment results are shown in Figure 7. The KEGG signaling pathway involved the Proteasome, Renin-angiotensin system (RAS), prolactin signaling pathway, inflammatory bowel disease, growth hormone synthesis, secretion, and action (growth hormone synthesis, secretion and action, spinocerebellar ataxia, Th17 cell differentiation, neuroactive ligand-receptor interaction, and TNF signaling pathway.
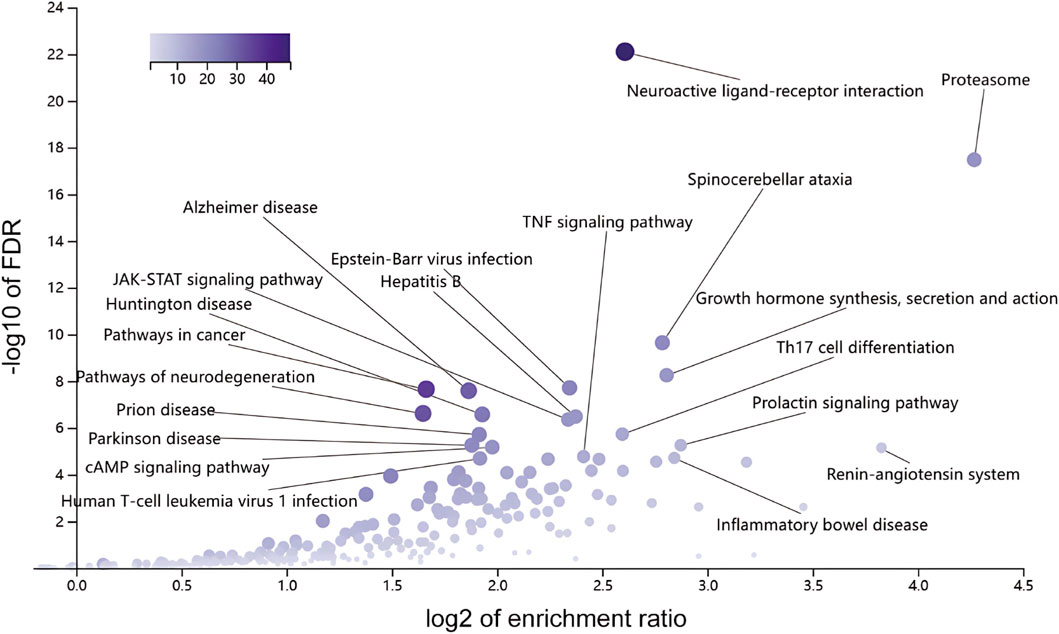
Figure 7. KEGG pathway enrichment analysis. Bar chart displaying the significantly enriched KEGG pathways for the potential targets, suggesting key mechanistic pathways like the Proteasome and Renin-angiotensin system.
Figure 8 lists the PPI interaction network shown on KEGG of the proteasome protease system, with 43 related proteins in total, among which PSMA4 and its family members may be the key proteins in this pathway, and the secreted protein IFNG is closely related to the protease system.
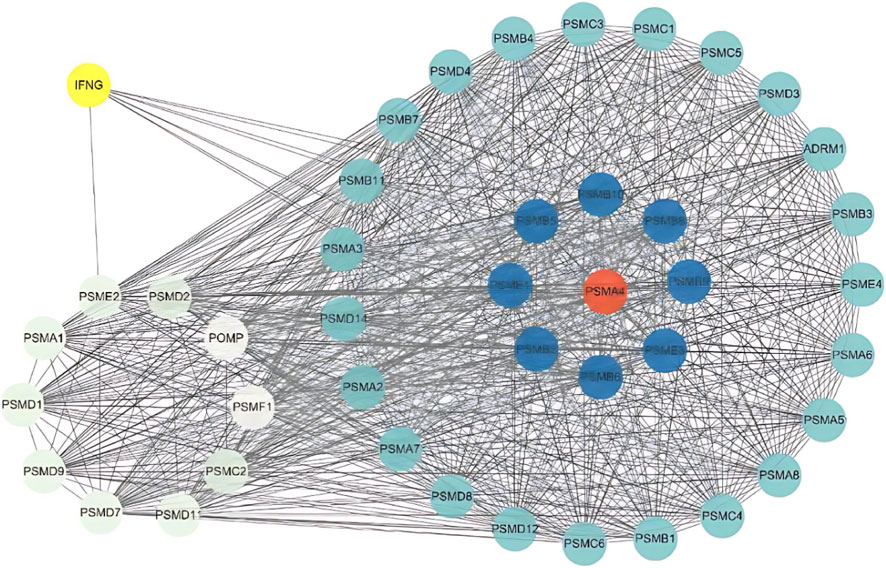
Figure 8. PPI subnetwork for the Proteasome pathway. Interaction subnetwork of proteins involved in the Proteasome pathway, highlighting key proteins like PSMA4 and IFNG.
4 Discussion
Numerous studies have shown that physical exercise has systemic effects, regulates allorgans, and promotes physical health, whereas inactivity can have serious negative effects on health throughout the life cycle (Harridge and Lazarus, 2017). In addition, exercise has potential mental health benefits (Venkatesh et al., 2020). This literature review identified eight potential active ingredients: irisin, 5α-reductase, kisspeptin, CART, NGF, IGF-1 and IL-6. Then, 192 related targets of exercise active ingredients and PCOS were obtained through screening of three compound databases and a comprehensive human gene database, and the key action targets and signaling pathways of exercise therapy for PCOS were predicted using bioinformatics. This study predicted that exercise might regulate the G protein-coupled receptor signaling pathway, neuropeptide signaling pathway, and endocrine processes through key targets such as TNF, IL6, IL1B, JUN, CCND1, PSMA7, etc., thereby affecting the protease, renin-angiotensin, inflammatory signaling, and neuroactive ligand-receptor interaction pathways.
The results of the “exercise-PCOS” co-target PPI network showed that exercise interfered with PCOS and 192 targets, among which TNF, IL-6, IL1B and JUN proteins had relatively strong interactions. TNF, IL-6, and IL1B are associated with various inflammatory disease states and are involved in the regulation of various biological processes, including cell proliferation, differentiation, apoptosis, and lipid metabolism (M Ller and Villiger, 2006). It is well known that chronic inflammation is a typical feature of PCOS, and studies have found that PCOS patients (whether obese or not) have significantly higher levels of circulating inflammatory factors in their blood than healthy women (Nehir et al., 2016), treatment with etanercept, a TNF-α inhibitor, can significantly inhibit androgen increase and follicular dysplasia in letrozole-induced PCOS rats (Lang et al., 2019). Furthermore, moderate exercise has anti-inflammatory effects in various diseases, and meta-analyses have shown that regular exercise can reduce levels of inflammation-related markers in autoimmune diseases, such as C-reactive protein, TNF-α, and IL-6 (Pedersen and Saltin, 2015). Therefore, exercise therapy for PCOS may regulate inflammation and immune status. JUN is the protein-coding gene of c-Jun, and c-Jun is the most widely studied protein in the activator protein-1 complex, which is involved in a variety of cellular activities such as proliferation, apoptosis, survival, tumorigenesis, and tissue morphogenesis. c-Jun has always been at the center of molecular networks, with mysterious functional properties that regulate multiple levels of the body in a complex manner. It can crosstalk, amplify, and integrate different signals for tissue development and disease (Qinghang and Xia, 2011). c-Jun N-terminal kinase (JNK) is involved in the regulation of oxidative stress-induced apoptosis in mouse follicular granuloid cells (Qianna et al., 2016). In addition, many studies have shown that under oxidative stress, phosphorylation of JNK in mammalian ovarian granulosa cells increases and the JNK/FoxO1 signaling pathway is activated, leading to apoptosis of ovarian granulosa cells (Xuan et al., 2023) (Kong et al., 2022). These results indicate that phosphorylation of JNK and activation of the JNK/FoxO1 signaling pathway in mammalian ovarian granulosa cells under oxidative stress led to the apoptosis of ovarian granulosa cells. Recent studies have shown that exercise can regulate the mitochondrial unfolded protein response through the JNK signaling pathway to ensure mitochondrial protein stability and function (Gaspar et al., 2023) and that exercise enhances the expression of c-Fos and c-Jun in the hypothalamus, mid-dorsum, and hippocampus (Hong et al., 2014), suggesting that the effect of exercise on JNK may be systemic. Studies have shown that among the various topological analysis methods of the CytoHubba plug-in, the MCC method has better performance in predicting the accuracy of essential proteins and can provide a more comprehensive and accurate evaluation of node centrality (Chicco et al., 2021). Therefore, we also pay special attention to the other two Hub genes predicted by MCC method. PSMA7 is a protein-coding gene that encodes the alpha subunit of the 20S proteasome core complex, which is involved in protein degradation through the ubiquitin-proteasome pathway and plays an important role in cell proliferation, cell cycle control, transcriptional regulation, immune and stress responses, cell differentiation, and apoptosis. PSMA7 can play a role in cellular stress by regulating hypoxia-inducing factor-1 α (Du et al., 2009). No studies have found that this gene is related to motility and PCOS; however, a Japanese study suggested that PSMA7 might be involved in the regulation of germ cell survival during spermatogenesis (Shimizu et al., 2014). CCND1 encodes a highly conserved family of cyclins whose members are characterized by significant periodicity in protein abundance throughout the cell cycle and whose activity is required for cell cycle G1/S transition. CCND is misregulated in many cancer types, and the carcinogenic properties of cyclins and cyclin-dependent kinases they activate are well established (Qie and Diehl, 2016). Previous studies have shown that the expression of CCND1 and CCND3 is downregulated in PCOS mouse models (Tong et al., 2024), and may be an important cause of follicular dysplasia in patients with PCOS. At the same time, exercise was found to rejuvenate dormant skeletal muscle stem cells in aged mice by restoring CCND1 activity and inhibiting TNF-β signaling (Brett et al., 2020). In summary, the abovementioned genes are closely related and affect each other in the process of exercise therapy for PCOS, and are concentrated in the four major phenotypes of inflammation, apoptosis, oxidative stress, and the cell cycle, which are worthy of further exploration.
The results of GO analysis showed that the mechanism of action of exercise therapy for PCOS is a multi-target, multi-layer approach and involves multiple biological processes. First, BP levels affect the G protein-coupled receptor and neuropeptide signaling pathways. NPY is a neurotransmitter widely distributed in the central nervous system and plays an important role in the regulation of animal physiology and behavior. They constitute the largest and most diverse neuronal messengers that mediate neuroendocrine signaling and extrasynaptic communication in the nervous system. The secreted neuropeptides mainly bind to G protein-coupled receptors on neighboring neurons or distant target cells. To regulate various physiological processes and brain functions, such as eating, sleep, arousal, reproduction, and learning (Jiang et al., 2024). Studies on C. elegans have found that hermaphrodite-specific neurons release neuropeptides that cause them to dramatically increase their movement before laying eggs, affecting some reproductive behaviors in response to environmental influences (Hardaker et al., 2001). Hypothalamic neurons expressing NPY and agoutine chromoprotein-related peptides (NPY/AgRP neurons) are closely associated with fertility regulation. Selective activation of NPY/AgRP neurons significantly improves the frequency of hypothalamic gonadotropin-releasing hormone (GnRH)/LH pulses in patients (Coutinho et al., 2019). Kisspeptin is a neuropeptide produced by the KISS1 gene. Altered kisspeptin signal transduction can lead to abnormal GnRH pulse secretion that induces an elevated LH/FSH ratio, which leads to PCOS progression. Studies have shown that both swimming (Arisha and Moustafa, 2019) and running (Kacar et al., 2023) can inhibit kisspeptin secretion, and thereby inhibit GnRH secretion to improve reproductive function. In addition, BP suggested that exercise intervention in PCOS is related to the improvement of the biological process of endocrine. PCOS, in most patients, is complicated by insulin resistance and related hyperinsulinemia, and this phenomenon is prevalent even in non-obese patients with PCOS. Hyperandrogenism and insulin resistance have a bidirectional relationship, which forms a vicious cycle in the endocrine status of patients with PCOS (Moghetti and Tosi, 2021). Exercise training is considered an effective stimulus for improving insulin resistance (Sun and Ding, 2020). In insulin resistance, the adaptive response to exercise training induces an improvement in glucose tolerance and enhanced sensitivity of skeletal muscle insulin to glucose transport. Although the specific mechanism has not been fully elucidated, recent studies have suggested that it may be closely related to the upregulation of specific components of the glucose transport system in muscles and the alleviation of mitochondrial dysfunction (Mthembu et al., 2022; Siemers et al., 2023).
The results of the KEGG analysis showed that exercise interfered with PCOS mainly in the protease system. The proteasome has a wide range of functions that can cleavecell proteins into peptides and play a regulatory role in various biological processes. Previous studies showed that exercise improves proteasome function and promotes synaptic plasticity in mice with brain injury, and this mechanism may be related to improved oxidative stress levels (Szabo et al., 2010). Recent studies have suggested that exercise can enhance hippocampal proteasome activity, and thereby contribute to neurogenesis. This benefit is reversed after the use of the proteasome inhibitor MG132, which suggests that exercise can significantly enhance the activity of the proteasome system in the central nervous system (Niu et al., 2020). Currently, there is a lack of direct evidence regarding the relationship between the protease system and PCOS. However, PCOS is associated with insulin resistance and obesity. In obese mouse models, immunoglobulin superfamilies containing leucine-rich repeats (Islr) control ubiquitin-independent proteasome degradation of INα by interacting with Pmsa4, a key gene in the protease system, and regulating insulin sensitivity (Zhang et al., 2023). In addition, although no direct evidence has been found in human studies, the effects of exercise and physical exercise on the RAS of the second enrichment pathway have been confirmed in various animal models of diseases (Gomes-Santos et al., 2014; Ren et al., 2016; Liu et al., 2022; Chang et al., 2022). Physical exercise can benefit from the regulation of the ACE-AngII-AT1 axis of the RAS (Nunes-Silva et al., 2017). In addition to genes related to proliferation, some genes enriched in the prolactin signaling pathway, such as JAK2, NFKB1, PRLR, SHC1, SOCS3, STAT1, and STAT3, are related to cell–cell interaction communication, and this suggests that exercise may affect reproductive system function through the exercise-nervous system via cell–cell communication via neurohumoral signaling.
5 Limitation
Only eight representative active substances produced after exercise were included in this study, and this research was limited by the current research level, which is characterized by a lack of human data research. The selection of model animals in the included literature as well as the exercise mode and duration were not completely uniform, which may have caused certain interference in the results of the analysis.
Nevertheless, this review employed bioinformatics and network topology analyses to explore the biological mechanisms of exercise therapy for PCOS, and the insights from this research provide a basis for future experimental research.
Author contributions
XY: Writing – original draft. HY: Writing – original draft. DS: Writing – original draft. QZ: Writing – original draft, Data curation. CZ: Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This work was supported by the Science and Technology Plan Project of Sichuan Province (Key Research and Development Project, No. 2024YFFK0300).
Acknowledgments
AcknowledgementsWe thank Bullet Edits Limited for linguistic editing and proofreading of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2025.1634588/full#supplementary-material
References
Arisha, A. H., and Moustafa, A. (2019). Potential inhibitory effect of swimming exercise on the Kisspeptin-GnRH signaling pathway in male rats. Theriogenology 133, 87–96. doi:10.1016/j.theriogenology.2019.04.035
Brett, J. A.-O., Arjona, M. A.-O., Ikeda, M. A.-O., Quarta, M., de Morrée, A., Egner, I. M., et al. (2020). Exercise rejuvenates quiescent skeletal muscle stem cells in old mice through restoration of Cyclin D1. Nat. Metab. 2 (4), 307–317. doi:10.1038/s42255-020-0190-0
Chan, Z., Lina, S., and Cong, Q. (2016). Clinical observation of Chinese medicine combined with exercise therapy in the treatment of polycystic ovary syndrome. Chin. J. Sports Med. 35 (04), 359–361. (in Chinese).
Chang, Y. S., Lin, C. L., Lee, C. W., Lin, H. C., Wu, Y. T., and Shih, Y. H. (2022). Exercise normalized the hippocampal renin-angiotensin system and restored spatial memory function, neurogenesis, and blood-brain barrier permeability in the 2K1C-Hypertensive mouse. Int. J. Mol. Sci. 23 (10), 5531. doi:10.3390/ijms23105531
Chicco, D., T, T. N., and Jurman, G. (2021). The matthews correlation coefficient (MCC) is more reliable than balanced accuracy, bookmaker informedness, and markedness in two-class confusion matrix evaluation. BioData Min. 14 (1), 13. doi:10.1186/s13040-021-00244-z
Chin, C. H., Chen, S. H., Wu, H. H., Ho, C. W., Ko, M. T., and Lin, C. Y. (2014). cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 8 (Suppl. 4), S11. doi:10.1186/1752-0509-8-S4-S11
Coutinho, E. A., Prescott, M., Hessler, S., Marshall, C. J., Herbison, A. E., and Campbell, R. E. (2019). Activation of a classic hunger circuit slows luteinizing hormone pulsatility. Neuroendocrinology 110 (7-8), 671–687. doi:10.1159/000504225
Daina, A., Michielin, O., and Zoete, V. (2019). SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic acids Res. 47 (W1), W357–w64. doi:10.1093/nar/gkz382
Dapas, M., and Dunaif, A. (2022). Deconstructing a syndrome: genomic insights into PCOS causal mechanisms and classification. Endocr. Rev. 43 (6), 927–965. doi:10.1210/endrev/bnac001
Diane, A., Kupreeva, M., Borthwick, F., Proctor, S. D., Pierce, W. D., and Vine, D. F. (2015). Cardiometabolic and reproductive benefits of early dietary energy restriction and voluntary exercise in an obese PCOS-prone rodent model. J. Endocrinol. 226 (3), 193–206. doi:10.1530/JOE-14-0711
Du, H., Huang, X., Wang, S., Wu, Y., Xu, W., and Li, M. (2009). PSMA7, a potential biomarker of diseases. Protein peptide Lett. 16 (5), 486–489. doi:10.2174/092986609788167824
Gaspar, R. S., Katashima, C. K., Crisol, B. M., Carneiro, F. S., Sampaio, I., Silveira, L. D. R., et al. (2023). Physical exercise elicits UPR(mt) in the skeletal muscle: the role of c-Jun N-terminal kinase. Mol. Metab. 78, 101816. doi:10.1016/j.molmet.2023.101816
Gomes-Santos, I. L., Fernandes, T., Couto, G. K., Ferreira-Filho, J. C. A., Salemi, V. M. C., Fernandes, F. B., et al. (2014). Effects of exercise training on circulating and skeletal muscle renin-angiotensin system in chronic heart failure rats. PloS one 9 (5), e98012. doi:10.1371/journal.pone.0098012
Hakimi, O., and Cameron, L. C. (2017). Effect of exercise on ovulation: a systematic review. Sports Med. Auckl. NZ 47 (8), 1555–1567. doi:10.1007/s40279-016-0669-8
Hardaker, L. A., Singer, E., Kerr, R., Zhou, G., Schafer, W. R., et al. (2001). Serotonin modulates locomotory behavior and coordinates egg-laying and movement in Caenorhabditis elegans. J. Neurobiol. 49 (4), 303–313. doi:10.1002/neu.10014
Harridge, S. D., and Lazarus, N. R. (2017). Physical activity, aging, and physiological function. Physiol. (Bethesda, Md) 32 (2), 152–161. doi:10.1152/physiol.00029.2016
Hong, H., Kim, C. J., Kim, J. D., and Seo, J. H. (2014). β-glucan reduces exercise-induced stress through downregulation of c-Fos and c-Jun expression in the brains of exhausted rats. Mol. Med. Rep. 9 (5), 1660–1666. doi:10.3892/mmr.2014.2005
Jiang, T., Zheng, T., Li, R., Sun, J., Luan, X., and Wang, M. (2024). The role of NPY signaling pathway in diagnosis, prognosis and treatment of stroke. Neuropeptides 104, 102412. doi:10.1016/j.npep.2024.102412
Jm, E., Y, L., Id, O., Zhu, Q., Pico, A. R., and Zhang, B. (2024). WebGestalt 2024: faster gene set analysis and new support for metabolomics and multi-omics. Nucleic acids Res. 52 (W1), W415–W421. doi:10.1093/nar/gkae456
Kacar, E., Bulmus, O., Ercan, Z., Buran Kavuran, I., Zorlu, G., Tan, F., et al. (2023). Treadmill exercise has healing effects on obesity-induced sexual behavior disorder through kisspeptin and kiss1R expression in male rats. Cell Mol. Biol. (Noisy-le-grand) 69 (3), 8–12. doi:10.14715/cmb/2023.69.3.2
Kanehisa, M., and Goto, S. (2000). KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28 (1), 27–30. doi:10.1093/nar/28.1.27
Keiser, M. J., Roth, B. L., Armbruster, B. N., Ernsberger, P., Irwin, J. J., and Shoichet, B. K. (2007). Relating protein pharmacology by ligand chemistry. Nat. Biotechnol. 25 (2), 197–206. doi:10.1038/nbt1284
Kong, C., Su, J., Wang, Q., Liu, K., Fu, R., and Sui, S. (2022). Signaling pathways of Periplaneta americana peptide resist H(2)O(2)-induced apoptosis in pig-ovary granulosa cells through FoxO1. theriogenology 183, 108–119. doi:10.1016/j.theriogenology.2022.02.004
Kuhn, M., Szklarczyk, D., Fau - Pletscher-Frankild, S., Pletscher-Frankild, S., Fau - Blicher, T. H., Jensen, L. J., et al. (2014). STITCH 4: integration of protein-chemical interactions with user data. Nucleic Acids Res. 42 (Database issue), D401–D407. doi:10.1093/nar/gkt1207
Lang, Q., Yidong, X., Xueguang, Z., Sixian, W., Wenming, X., and Tao, Z. (2019). ETA-mediated anti-TNF-α therapy ameliorates the phenotype of PCOS model induced by letrozole. PloS one 14 (6), e0217495. doi:10.1371/journal.pone.0217495
Linlin, C., and Zijiang, C. (2011). Introduction of diagnostic criteria and diagnostic guidelines for polycystic ovary syndrome (in Chinese). Int. J. Reproductive Health/Family Plan. 30 (5), 405–408.
Liu, Q., Chen, L., Liang, X., Cao, Y., Zhu, X., Wang, S., et al. (2022). Exercise attenuates angiotensinⅡ-induced muscle atrophy by targeting PPARγ/miR-29b. J. sport health Sci. 11 (6), 696–707. doi:10.1016/j.jshs.2021.06.002
Louise, M., Iingibjörg, J., Agneta, H., Lönn, M., and Stener-Victorin, E. (2008). Low-frequency electro-acupuncture and physical exercise improve metabolic disturbances and modulate gene expression in adipose tissue in rats with dihydrotestosterone-induced polycystic ovary syndrome. Endocrinology 149 (7), 3559–3568. doi:10.1210/en.2008-0053
Louise, M., Stefan, C., Malin, L., and Stener-Victorin, E. (2009). Acupuncture and exercise restore adipose tissue expression of sympathetic markers and improve ovarian morphology in rats with dihydrotestosterone-induced PCOS. 296 (4), 1124–1131. doi:10.1152/ajpregu.90947.2008
Luigi, M., Cajander, S., Thomas, L., Naylor, A. S., Aloe, L., Holmäng, A., et al. (2005). Effect of exercise on ovarian morphology and expression of nerve growth factor and alpha(1)- and beta(2)-adrenergic receptors in rats with steroid-induced polycystic ovaries. J. Neuroendocrinol. 17 (12), 846–858. doi:10.1111/j.1365-2826.2005.01378.x
M Ller, B., and Villiger, P. M. (2006). Inhibition of IL-1, IL-6, and TNF-alpha in immune-mediated inflammatory diseases. Springer seminars Immunopathol. 27 (4), 391–408. doi:10.1007/s00281-006-0012-9
Moghetti, P., and Tosi, F. (2021). Insulin resistance and PCOS: chicken or egg? J. Endocrinol. investigation 44 (2), 233–244. doi:10.1007/s40618-020-01351-0
Mthembu, S. X. H., Mazibuko-Mbeje, S. E., Ziqubu, K., Nyawo, T. A., Obonye, N., Nyambuya, T. M., et al. (2022). Impact of physical exercise and caloric restriction in patients with type 2 diabetes: skeletal muscle insulin resistance and mitochondrial dysfunction as ideal therapeutic targets. Life Sci. 15 (297), 120467. doi:10.1016/j.lfs.2022.120467
Nehir, A. A., Bastu, E., Demiral, I., Bulut, H., Dogan, M., and Buyru, F. (2016). Relationship between hyperandrogenism, obesity, inflammation and polycystic ovary syndrome. Gynecol. Endocrinol. official J. Int. Soc. Gynecol. Endocrinol. 32 (9), 709–713. doi:10.3109/09513590.2016.1155208
Niu, X., Zhao, Y., Yang, N., Zhao, X., Zhang, W., Bai, X., et al. (2020). Proteasome activation by insulin-like growth factor-1/nuclear factor erythroid 2-related factor 2 signaling promotes exercise-induced neurogenesis. Stem cells Dayt. Ohio 38 (2), 246–260. doi:10.1002/stem.3102
Nunes-Silva, A., Rocha, G. C., Magalhaes, D. M., Vaz, L. N., Salviano de Faria, M. H., and Simoes E Silva, A. C. (2017). Physical exercise and ACE2-Angiotensin-(1-7)-Mas receptor axis of the renin angiotensin System. Protein peptide Lett. 24 (9), 809–816. doi:10.2174/0929866524666170728151401
Pedersen, B. K., and Saltin, B. (2015). Exercise as medicine - evidence for prescribing exercise as therapy in 26. Scand. J. Med. Sci. Sports Suppl 3, 1–72. doi:10.1111/sms.12581
Qianna, W., Zequn, L., Bojiang, Li, Liu, K., Wu, W., and Liu, H. (2016). Oxidative stress induces mouse follicular granulosa cells apoptosis via JNK/FoxO1 pathway. - PLoS One 11 (12), e0167869. doi:10.1371/journal.pone.0167869
Qie, S., and Diehl, J. A. (2016). Cyclin D1, cancer progression, and opportunities in cancer treatment. J. Mol. Med. Berlin, Ger. 94 (12), 1313–1326. doi:10.1007/s00109-016-1475-3
Qinghang, M., and Xia, Y. (2011). c-Jun, at the crossroad of the signaling network. Protein Cell 2 (11), 889–898. doi:10.1007/s13238-011-1113-3
Rebhan, M., Chalifa-Caspi, V., Prilusky, J., and Lancet, D. (1997). GeneCards: integrating information about genes, proteins and diseases. Trends Genet. TIG 13 (4), 163. doi:10.1016/s0168-9525(97)01103-7
Ren, C. Z., Yang, Y. H., Sun, J. C., Wu, Z. t., Zhang, R. w., Shen, D., et al. (2016). Exercise training improves the altered renin-angiotensin system in the rostral ventrolateral medulla of hypertensive rats. Oxidative Med. Cell. Longev. 2016, 7413963. doi:10.1155/2016/7413963
Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (2004). Fertil. Steril. 81 (1), 19–25. doi:10.1016/j.fertnstert.2003.10.004
Shan, L., Xiaoling, S., Shizheng, D., Haixiang, S., Jing, W., Ning, W., et al. (2021). Summary of the best evidence for lifestyle management in patients with polycystic ovary syndrome (in Chinese). J. reproductive Med. 30 (9), 1180–1188.
Shannon, P., Markiel, A., Ozier, O., Baliga, N. S., Wang, J. T., Ramage, D., et al. (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13 (11), 2498–2504. doi:10.1101/gr.1239303
Shimizu, N., Ueno, K., Fau - Kurita, E., Kurita, E., Fau - Shin, S.-W., Amano, T., et al. (2014). Possible role of ZPAC, zygote-specific proteasome assembly chaperone, during spermatogenesis in the mouse. J. Reprod. Dev. 60 (3), 179–186. doi:10.1262/jrd.2014-003
Siemers, K. A.-O., Klein, A. K., and Baack, M. A.-O. X. (2023). Mitochondrial dysfunction in PCOS: insights into reproductive organ pathophysiology. Int. J. Mol. Sci. 24 (17), 13123. doi:10.3390/ijms241713123
Sun, Y., and Ding, S. (2020). ER-Mitochondria contacts and insulin resistance modulation through exercise intervention. Int. J. Mol. Sci. 21 (24), 9587. doi:10.3390/ijms21249587
Szabo, Z., Ying, Z., Radak, Z., and Gomez-Pinilla, F. (2010). Voluntary exercise May engage proteasome function to benefit the brain after trauma. Brain Res. 1341, 25–31. doi:10.1016/j.brainres.2009.01.035
Szklarczyk, D., Gable, A. L., Lyon, D., Junge, A., Wyder, S., Huerta-Cepas, J., et al. (2019). STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic acids Res. 47 (D1), D607-D613–d13. doi:10.1093/nar/gky1131
Thomas, P. D., Ebert, D., Muruganujan, A., Mushayahama, T., Albou, L. P., and Mi, H. (2022). PANTHER: making genome-scale phylogenetics accessible to all. Protein Sci. a Publ. Protein Soc. 31 (1), 8–22. doi:10.1002/pro.4218
Tong, X., Hu, Z., Zhou, H., Zhang, Y., Zhang, Y. L., Zhang, S., et al. (2024). Testosterone-induced H3K27 deacetylation participates in granulosa cell proliferation suppression and pathogenesis of polycystic ovary syndrome. Am. J. Pathol. 194 (12), 2326–2340. doi:10.1016/j.ajpath.2024.08.012
Venkatesh, A., Edirappuli, S. D., Zaman, H. P., and Zaman, R. (2020). The effect of exercise on mental health: a focus on inflammatory mechanisms. Psychiatr. Danub. 32 (Suppl. 1), 105–113.
Weng, Y., Zhang, Y., Wang, D., Wang, R., Xiang, Z., Shen, S., et al. (2023). Exercise-induced irisin improves follicular dysfunction by inhibiting IRE1α-TXNIP/ROS-NLRP3 pathway in PCOS. J. ovarian Res. 16 (1), 151. doi:10.1186/s13048-023-01242-x
Wu, C., Jiang, F., Wei, K., and Jiang, Z. (2018). Exercise activates the PI3K-AKT signal pathway by decreasing the expression of 5α-reductase type 1 in PCOS rats. Sci. Rep. 8 (1), 7982. doi:10.1038/s41598-018-26210-0
Xuan, Z., Dingding, Z., Hongmin, Li, Liu, Z., Yang, Y., Li, J., et al. (2023). Melatonin-mediated suppression of mtROS-JNK-FOXO1 pathway alleviates hypoxia-induced apoptosis in Porcine granulosa cells. Antioxidants (Basel) 12 (10), 1881. doi:10.3390/antiox12101881
Keywords: polycystic ovary syndrome, exercise, bioinformatics, mechanism, target
Citation: Yang X, Yang H, Shen D, Zhang Q and Zhu C (2025) Bioinformatics identification of key genes and therapeutic targets for exercise intervention in polycystic ovary syndrome. Front. Genet. 16:1634588. doi: 10.3389/fgene.2025.1634588
Received: 24 May 2025; Accepted: 03 November 2025;
Published: 20 November 2025.
Edited by:
Nan Sheng, Jilin University, ChinaReviewed by:
Vennila Suriyagandhi, Bharathidasan University, IndiaNasri Nasri, Universitas Hasanuddin, Indonesia
Copyright © 2025 Yang, Yang, Shen, Zhang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chan Zhu, emMzNDUzNEBzY3UuZWR1LmNu
†These authors have contributed equally to this work
 Xiaodie Yang
Xiaodie Yang Hong Yang
Hong Yang Dongyi Shen
Dongyi Shen Qiang Zhang5
Qiang Zhang5