- 1Jiangxi Province Key Laboratory of Oil Crops Genetic Improvement, Institute of Crops, Jiangxi Academy of Agricultural Sciences, Nanchang, China
- 2Institute of Food Crops, Hubei Academy of Agricultural Sciences, Wuhan, China
- 3College of Agriculture, Yangtze University, Jingzhou, China
GATAs, a type of zinc finger protein transcription factors, can bind to DNA regulatory regions to control the expression of target genes, thereby affecting plant growth and development under normal conditions or environmental stress. However, the GATA gene family has not been identified in sweet potato. In this study, a total of 35, 33, 34, 39, 63, and 56 GATA genes were identified in sweet potato, Ipomoea aquatica, Ipomoea cairica, Ipomoea nil, Ipomoea triloba, and Ipomoea trifida, respectively. Phylogenetic analysis categorized the GATA genes into six groups according to their distinct features, and this classification was validated by the structural characteristics of exons/introns and conserved motif analysis. The cis-acting elements located in the promoter regions were also found to be enriched with biotic and abiotic responsive elements, which may play a pivotal role in plant stress adaptation. Then the gene duplication events and synteny between the genome of sweet potato and those of Ipomoea aquatica, Ipomoea cairica, Ipomoea nil, Ipomoea triloba, and Ipomoea trifida were analyzed, which provided insights into evolutionary mechanisms. Moreover, expression pattern analysis was performed on IbGATA genes, many of which were significantly induced by multiple types of abiotic stress, which may render these genes candidates for molecular breeding strategies in sweet potato. Overall, this experiment conducted a systematic exploration of GATA genes by investigating their evolutionary relationships, structural characteristics, functional properties, and expression patterns, thereby establishing a theoretical foundation for further in-depth research on the features of the GATA gene family.
1 Introduction
Transcription factors (TFs) are a class of protein factors that regulate gene expression by binding to specific cis-acting regulatory elements in the promoter regions of downstream target genes, which play important roles in plant development and stress response (Strader et al., 2022). There are abundant and diverse TFs distributed in plants. The study of TFs is important for understanding the genetic regulation of gene expression in multiple metabolic pathways in plants. A growing family of TFs have been identified in plants, such as MYB (myeloblastosis) (Dubos et al., 2010; Chen et al., 2019; Millard et al., 2019; Zhang et al., 2023), bZIP (basic leucine zipper) (Liu et al., 2023), AP2/ERF (APETALA2/ethylene responsive factor) (Feng et al., 2020; Ma et al., 2024), bHLH (basic helix–loop–helix) (Gao and Dubos, 2024; Lei et al., 2024), NAC (NAM, ATAF1/2, CUC) (Yan et al., 2021; Yan et al., 2023), WRKY (Chen et al., 2018; Mahiwal et al., 2024), and GATA (Du et al., 2022).
GATA TFs are transcription regulatory factors found in animals, plants, and fungi that recognize the DNA sequence W-G-A-T-A-R through a single type IV zinc finger and regulate the transcriptional levels of the target genes (Patient and McGhee, 2002). GATA TFs were first discovered and reported to bind globin gene promoters in chickens and participate in the hematopoietic process. Subsequent studies have shown that GATA TFs in animals contain two C-X2-C-X17-20-C-X2-C zinc finger domains, in which only the C-terminal zinc finger structure binds to DNA, whereas the N-terminal zinc finger structure regulates the specific binding of the C-terminal zinc finger to DNA and participates in the process of development, differentiation, and cell proliferation. Most of the GATA TFs in fungi contain only one zinc finger domain, divided into two classes, namely C-X2-C-X17-C-X2-C or C-X2-C-X18-C-X2-C domains, which play a key role in a variety of biological processes, such as light induction, circadian rhythm, siderophore biosynthesis, main-type switching, and nitrogen cycling. In plants, the first known GATA TF was identified in tobacco (Nicotiana tabacum) and named NTL1 because it is a homolog of the NIT2 protein found in Neurospora crassa (Daniel-Vedele and Caboche, 1993). GATA TFs have since been studied in numerous plants, such as rice (Oryza sativa) (Reyes et al., 2004), tomato (Lycopersicon esculentum) (Zhao et al., 2021), soybean (Glycine max) (Zhang et al., 2020), and potato (Solanum tuberosum) (Aksoy et al., 2024).
Most plant GATA proteins have a single C-X2-C-X18-C-X2-C zinc finger structural domain, whereas only a few GATA proteins have C-X2-C-X20-C-X2-C or two zinc finger structural domains. It has been shown that GATA TFs play important roles in regulating plant growth and development and nitrogen metabolism, as well as in mediating responses to biotic and abiotic stresses. For instance, the Arabidopsis GATA TF BME3 mediates the developmental processes of seeds from dormancy to germination and positively regulates seed germination (Liu et al., 2005). In Arabidopsis thaliana, GATA TF ZIM regulates hypocotyl and petiole elongation, whereas overexpression of GATA TF TaZIM-A1 in Triticum aestivum leads to delayed flowering and decreased thousand-grain weight (Shikata et al., 2004; Liu et al., 2019). GATA TFs have been found to play important roles in plant photomorphogenesis, of which AtGATA2 is an important positive regulator of photomorphogenesis, which can directly bind to the promoter of photoresponsive genes and brassinosteroid (BR) genes to regulate their expression (Luo et al., 2010). In Arabidopsis thaliana, GATA TFs GNC (GATA, nitrate-inducible, carbon metabolism-involved) and GNL (GNC-like) regulate chlorophyll synthesis, flowering time, and cold resistance (Richter et al., 2013). GNC and GNL help balance the phototropic and gravitropic growth responses in Arabidopsis thaliana (Sala et al., 2023). In rice, overexpression of OsGATA6 resulted in delayed heading, increased grain number, and decreased grain size, which potentially increases rice yield (Zhang et al., 2022). OsGATA8 increases seed size and stress resistance in both Arabidopsis and rice by regulating the expression of critical genes involved in stress tolerance, scavenging of reactive oxygen species, and chlorophyll biosynthesis (Nutan et al., 2020). Moreover OsGATA8 has also been found to be a key coordinator of uptake and tiller formation in rice. OsGATA8 negatively regulates nitrogen uptake by repressing the transcription of the ammonium transport gene OsAMT3.2. At the same time, it promotes the formation of tillers by inhibiting the transcription of OsTCP19 (Wu et al., 2024). The OsGATA16 positive regulator controls chlorophyll biosynthesis and chloroplast development by directly binding to the promoter regions of OsHEMA, OsCHLH, OsPORA, OsPORB, and OsFtsZ and upregulates their expression. Meanwhile, it improves cold tolerance at the seedling stage in rice by binding to the promoter region of OsWRKY45-1 and repressing its expression (Lim et al., 2024; Zhang et al., 2021). The functions of GATA TFs have also been discovered and identified in other plants. In potato, StGATA2 enhances the ability of potato to resist heat damage (Zhu et al., 2023). In tomato, SlGATA17 promotes drought tolerance of transgenic tomato by enhancing the activity of the phenylpropanoid biosynthesis pathway (Zhao et al., 2021). PdGNC and PdGATA19 regulate photosynthesis, growth, and drought resistance in poplars (Shen et al., 2021; An et al., 2020).
Sweet potato (Ipomoea batatas L.), the seventh most valuable crop in the world, is a fundamental source of calories, protein, vitamins, and minerals for humans (Yang et al., 2017). Sweet potato is widely cultivated in various countries and regions around the world and plays a vital role in food security, hunger eradication, nutrition provision, and poverty reduction in poverty-stricken areas for its adaptability and resilience to different planting environments and soil conditions (Wu et al., 2018). Sweet potato is usually cultivated in marginal areas such as desert margins, coastal mudflats, and hilly area, and drought and salt stress are limiting factors inhibiting its growth and yield. The sweet potato stress tolerance-related TFs IbMYB308 (Wang et al., 2022), IbC3H18 (Zhang et al., 2019), IbBBX24 (Zhang et al., 2022), and IbNAC3 (Meng et al., 2023) have been reported successively. With the rapid development of sequencing technology, more and more families of TFs have been identified in plants. Currently, GATA gene families have been identified in many plants, including Arabidopsis thaliana (Kim et al., 2021), Triticum aestivum (Feng et al., 2022), Capsicum annuum (Yu et al., 2021), Solanum tuberosum (Zhang et al., 2024), and Setaria italica (Lai et al., 2022). Based on the whole-genome-wide analyses, 33, 64, and 96 GATA family genes were identified in Sorghum bicolor, Glycine max, and Brassica napus, respectively (Yao et al., 2023; Zhang et al., 2015; Zhu et al., 2020). However, the identification, classification, evolution, and function of the GATA gene family remain unclear in sweet potato.
In this study, the GATA gene family in the whole genome of sweet potato was identified using bioinformatic methods. Then the physicochemical properties, chromosomal distributions, gene structure, conserved motifs, duplication events, phylogenetic relationships, and expression profiles of GATA genes in different tissues and multiple adversity stresses were analyzed. It provides a theoretical basis for further studying the functions of GATA gene family members and provides references for molecular breeding of sweet potato.
2 Materials and methods
2.1 Identification of GATA genes in Ipomoea species
The whole-genome sequence and annotation files of Ipomoea batatas, Ipomoea trifida, and Ipomoea triloba were downloaded from the Ipomoea Genome Hub (https://sweetpotato.com/) and Sweetpotato Genomics Resource (http://sweetpotato.uga.edu/), respectively. The whole genome information of Ipomoea cairica, Ipomoea aquatica, and Ipomoea nil was downloaded from Plant GARDEN (https://plantgarden.jp/). The genome annotations of Arabidopsis thaliana were downloaded from TAIR (https://www.arabidopsis.org/). To identify the sweet potato GATA genes, the Arabidopsis GATA gene family was obtained from PlantTFDB 5.0 (https://planttfdb.gao-lab.org/). The IbGATA proteins were identified using two screening methods. First, based on the amino acid sequence of 30 AtGATA members in Arabidopsis, BLAST (E-value ≤ 1e-5) searches were performed in sweet potato protein sequences to identify candidate IbGATA proteins. Then the hidden Markov model file of the GATA protein domain (PF00320) was downloaded from the Pfam dataset (http://pfam.xfam.org/), and the whole sweet-potato protein sequence was retrieved using HMMER 3.3.2 software, and the screening threshold was set at an E-value ≤ 1e-5. Finally, duplicate redundant sequences were eliminated by combining the search results of both methods. To ensure the reliability of the candidate sequences, the integrity of their conserved domains was verified using SMART (http://smart.embl-heidelberg.de/) and the NCBI-CDD search program (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). Proteins that were absent in the GATA structural domain were manually eliminated to obtain the final IbGATA proteins. The important physicochemical properties of the identified proteins, such as protein sequence length, molecular weight (MW), and theoretical isoelectric point (pI), were analyzed using the online ExPASy program (https://www.expasy.org/). The subcellular localization predictions of IbGATA proteins were predicted on the online website Cell-PLoc 2.0 (http://www.csbio.sjtu.edu.cn/bioinf/Cell-PLoc-2/).
2.2 Gene structure, protein motifs, and conserved domain analysis of GATA genes
Gene structure information of IbGATAs was extracted from sweet potato gff3 files, and a visual map of the gene structure was mapped using TBtools software (Chen et al., 2023). Conserved motifs in IbGATA proteins were discovered using the online tool MEME (https://meme-suite.org/meme/tools/meme); the maximum number of motifs was set to 10, and the remaining parameters were set to default values. The conserved domain of IbGATA proteins was verified using the NCBI-CDD database (https://www.ncbi.nlm.nih.gov/cdd/).
2.3 Cis-acting elements in the promoter region of IbGATA genes
The promoter sequences 2,000 bp upstream of the start codon of IbGATA genes were extracted from sweet potato genome data using TBtools software (Chen et al., 2023). Then cis-acting elements on the promoter sequences were predicted and screened using the online website PlantCARE (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/) and visualized on TBtools software (Chen et al., 2023).
2.4 Phylogenetic analysis of GATA genes
Multiple sequence alignment of GATA proteins from Arabidopsis thaliana, Oryza sativa, Ipomoea cairica, Ipomoea aquatica, Ipomoea trifida, Ipomoea triloba, and Ipomoea nil with identified GATA proteins from sweet potato was performed using ClustalW in MEGA X (Kumar et al., 2018). The obtained aligned sequences were submitted to MEGA X software (Kumar et al., 2018) for phylogenetic analysis, and the phylogenetic tree self-expansion value was set to 1,000, with the rest set to default. Afterward, the obtained phylogenetic tree was embellished and modified in Evolview (Subramanian et al., 2019).
2.5 Chromosomal localization and collinearity analysis of GATA genes
The information about GATA gene positions in chromosomes was obtained from gff3 files of Ipomoea species and then mapped on the chromosomes using TBtools software (Chen et al., 2023). The syntenic relationship of orthologous GATA genes between Ipomoea batatas and other Ipomoea species, Ipomoea aquatica, Ipomoea cairica, Ipomoea nil, Ipomoea triloba, and Ipomoea trifida, was analyzed using MCScanX software (Chen et al., 2023).
2.6 Ka/Ks analysis of duplicate and synonymous GATA genes
The non-synonymous substitution rate (Ka), synonymous substitution rate (Ks), and the ratio (Ka/Ks) of duplicate and homologous GATA gene pairs of different Ipomoea species were calculated using TBtools software (Chen et al., 2023).
2.7 RNA extraction and qRT-PCR of GATA genes in sweet potato
Sweet potato cultivar Ganshu 8 was used as the experimental material. The 6-week-old potato seedlings, approximately 25 cm long, were cut from the field and cultured in 1/2 Hoagland solution for 7 days to keep them alive. The root, stem, leaf, and petiole of Ganshu 8 were measured to analyze the expression specificity of IbGATA genes in different tissues. One-week-old seedlings in 1/2 Hoagland solution were treated with 200 mM NaCl and 20% PEG-6000 to evaluate the response of IbGATA genes to abiotic stress. The leaves were collected at 0, 6, 12, and 24 h posttreatment, and the untreated plants were used as controls (Zhang et al., 2019). Total RNA from sweet potato leaves was extracted using the FastPure® Plant Total RNA Isolation Kit (Wuhan, China) according to the manufacturer’s instructions. The first-strand cDNA was synthesized using EasyScript® All-in-One First-Strand cDNA Synthesis SuperMix for qPCR (one-step gDNA removal) (Wuhan, China). Each 20 μL contained 4 μL 5× EasyScript® Uni All-in-One SuperMix for qPCR, 1 μL gDNA remover, 1 μg total RNA, and variable RNase-free water. The cycling conditions for PCR were as follows: 42°C for 15 min and then 80°C for 5 s. qRT-PCR was performed using TransStart® Tip Green qPCR SuperMix (Wuhan, China), and each 20 μL mixture contained 10 μL TransStart® Tip Green qPCR SuperMix, 0.8 μL each specific primer, 7.4 μL nuclease-free water, and 1 μL cDNA. The qRT-PCR program comprised preheating at 94°C for 2 min, followed by 45 cycles of denaturation at 94°C for 5 s and annealing at 58°C for 30 s. The expression levels of IbGATA genes were detected using qRT-PCR analysis conducted on the LightCycler® 96 system (Roche, United States). Each experiment had three biological replicates and three technical replicates, and the relative expression levels of GATA genes were calculated using the 2−ΔΔCt method (Zhang et al., 2019). The sweet potato β-actin gene was used as an internal reference gene. The primers used for qRT-PCR in this study are listed in Supplementary Table S1.
2.8 Statistical analysis
Data analysis in this study was performed using Microsoft Excel 2019 and SPSS 26 software. Significance of differences between treatments was determined using one-way ANOVA. An LSD test was used to calculate p-values, and p < 0.01 indicates significant differences.
3 Results
3.1 Identification of GATAs in Ipomoea species
A total of 35 IbGATA genes were identified in the whole genome of sweet potato, and 33, 34, 39, 63, and 56 GATA genes were identified from Ipomoea aquatica, Ipomoea cairica, Ipomoea nil, Ipomoea triloba, and Ipomoea trifida, respectively (Supplementary Table S2). The amino acid length, MW, theoretical pI, instability index, aliphatic index, and grand average of hydropathicity (GRAVY) of Ipomoea species are shown in Supplementary Table S1. These GATA genes were named IbGATA1 to IbGATA35, IaGATA1 to IaGATA33, IcGATA1 to IcGATA34, InGATA1 to InGATA39, ItbGATA1 to IbGATA63, and ItfGATA1 to ItfGATA56. In the GATA protein of Ipomoea batatas, the length of protein sequences and MW ranged from 142 to 546 aa and 16212.38 to 191512.3 Da, respectively, and the average length and MW were 304 aa and 38161.27 Da, respectively. GRAVY ranged from −1.080 to −0.339, and pI ranged from 5.60 to 10.33. Subcellular localization prediction results showed that all IbGATAs may have nuclear localization signals.
The average lengths of proteins in I. aquatica, I. cairica, I. nil, I. triloba, and I. trifida were 328, 351, 291, 304, and 308 aa, respectively. Subcellular localization predictions showed that GATA proteins may have nuclear localization signals, except IcGATA22.
3.2 Phylogenetic analysis of GATA genes
To explore the phylogenetic relationship of the GATA genes in Ipomoea species, a phylogenetic tree was constructed using 316 GATA amino acid sequences from Arabidopsis thaliana, Oryza sativa, and Ipomoea species (Figure 1). It shows that among the different species, the evolutionary tree was clustered into three distinct groups, namely Ⅰ–Ⅲ, with group Ⅲ containing four subclasses: Ⅲ-Ⅰ, Ⅲ-Ⅱ, Ⅲ-Ⅲ, and Ⅲ-Ⅳ. Except for group Ⅱ, all other groups contained I. batatas, I. aquatica, I. cairica, I. nil, I. triloba, I. trifida, Arabidopsis thaliana, and Oryza sativa GATA proteins, suggesting that the characteristics of the GATA gene family emerged prior to the divergence of these species. Among these six groups, groups Ⅲ–Ⅳ exhibited the largest number of GATA proteins, reaching 91, followed by group Ⅲ-Ⅱ (72 GATA proteins), Ⅲ-Ⅰ (65 GATA proteins), Ⅲ-Ⅲ (53 GATA proteins), Ⅰ (33 GATA proteins), and Ⅱ (2 GATA proteins).
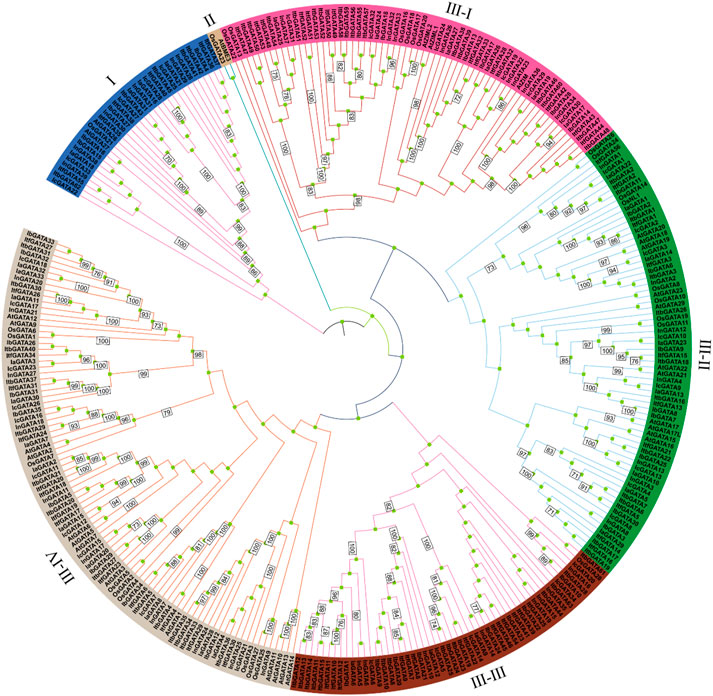
Figure 1. Phylogenetic tree of GATA genes in I. batatas, I. aquatica, I. cairica, I. nil, I. triloba, I. trifida, Arabidopsis thaliana, and Oryza sativa.
3.3 Conserved motif and gene structure analysis of GATA proteins
The conserved motifs and gene structure of the GATA family in Ipomoea species were analyzed using the MEME online tool to investigate their functional evolution (Figure 2). A total of eight motifs (motif 1 to motif 8) were identified in the six Ipomoea species GATA proteins. In Ipomoea batatas, motif 1 is the most prevalent. Analysis of the motif distribution in each protein revealed that with the exception of IbGATA15 (containing only one motif), all other IbGATA proteins possess two or more motifs. Furthermore, motif 1 exists in all IbGATAs, suggesting that motif 1 constitutes an evolutionarily critical domain in the IbGATA genes. In the exon/intron structure, it was found that with the exception of IbGATA10, IbGATA23, and IbGATA24, which contain only one exon, all other genes possess two or more exons and introns. This study further analyzed the distribution of motifs and the structural characterization of GATA genes in I. aquatica, I. cairica, I. nil, I. triloba, and I. trifida (Figures 2B–F). This study aims to establish a foundational framework for elucidating the structural characteristics of GATA genes in sweet potato and other Ipomoea species.
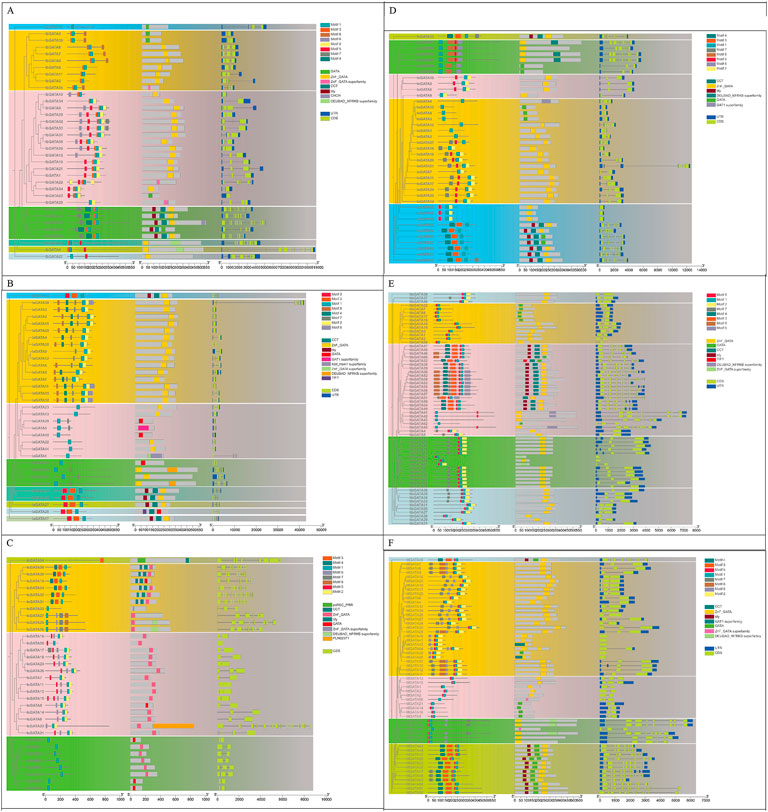
Figure 2. Evolutionary relationship, conserved motifs, protein conserved domains, and gene structure of GATA proteins in Ipomoea species. (A–F) I. batatas, I. aquatica, I. cairica, I. nil, I. triloba, and I. trifida.
3.4 Chromosomal location and duplication analysis of GATA genes
The information about the chromosomal locations of the GATA genes was extracted from the Ipomoea species genome annotation file, and a chromosomal distribution map of GATA genes was generated. A total of 35, 29, 34, 39, 63, and 56 GATA genes were mapped throughout the chromosomes of I. batatas, I. aquatica, I. cairica, I. nil, I. triloba, and I. trifida (Figure 3). There were four GATA genes that were mapped to unassembled scaffolds in I. cairica (Figure 3C). The distribution of GATA genes across the chromosomes in I. batatas is uneven; there were 1, 5, 1, 2, 4, 3, 2, 2, 1, 2, 2, 4, 3, and 3 GATA genes mapped on chromosomes 1, 2, 3, 4, 5, 7, 8, 9, 10, 11, 12, 13, 14, and 15 of I. batatas, respectively, whereas no GATA genes were mapped on chromosome 5 (Figure 3A). The phenomenon of unbalanced chromosomal distribution of GATA genes has also been observed in I. aquatica, I. cairica, I. nil, I. triloba, and I. trifida (Figures 3B–F).
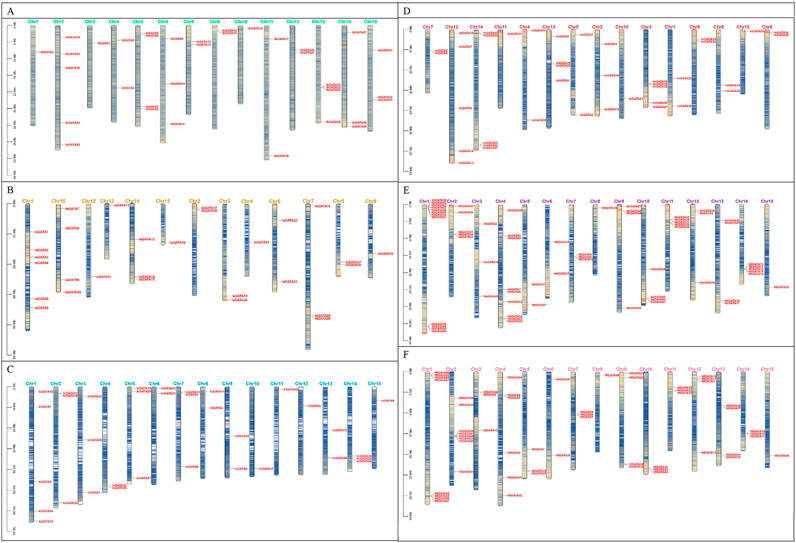
Figure 3. Chromosome localization of GATA genes in Ipomoea species. (A–F) Ipomoea batatas, I. aquatica, I. cairica, I. nil, I. triloba, and I. trifida.
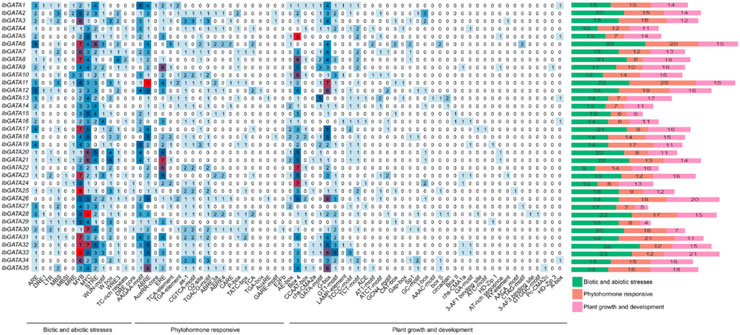
3.5 Cis-acting elements in promoter regions of IbGATA genes
To further elucidate the biological functions of the GATA gene family in I. batatas, the promoter sequences of the IbGATA genes were analyzed. Various cis-acting elements existed in the promoter region of the IbGATA genes, such as ABRE, MYB, Box 4, G box, and other elements (Figure 4). The cis-acting elements were divided into three types: abiotic and biotic stresses, phytohormone responsive, and plant growth and development. Among the all cis-acting elements, MYB has the highest distribution in 35 IbGATA genes, which was 153 in total, followed by MYC and Box 4, with 129 and 105, respectively. On the whole, the numbers of biotic and abiotic stresses were significantly greater than those of phytohormone responsive and plant growth and development elements. The results demonstrate that the sweet potato GATA gene family may be more sensitive to stress response.
3.6 Syntenic analysis of GATA genes in Ipomoea species
To further systematically elucidate the evolutionary mechanisms of the IbGATA family, the collinearity of IbGATA gene pairs among the genomes of I. aquatica, I. cairica, I. nil, I. triloba, and I. trifida was compared (Figure 5). The results showed that IbGATA formed 63, 64, 57, 67, and 67 collinearity gene pairs with IaGATA, IcGATA, InGATA, ItbGATA, and ItfGATA, respectively.
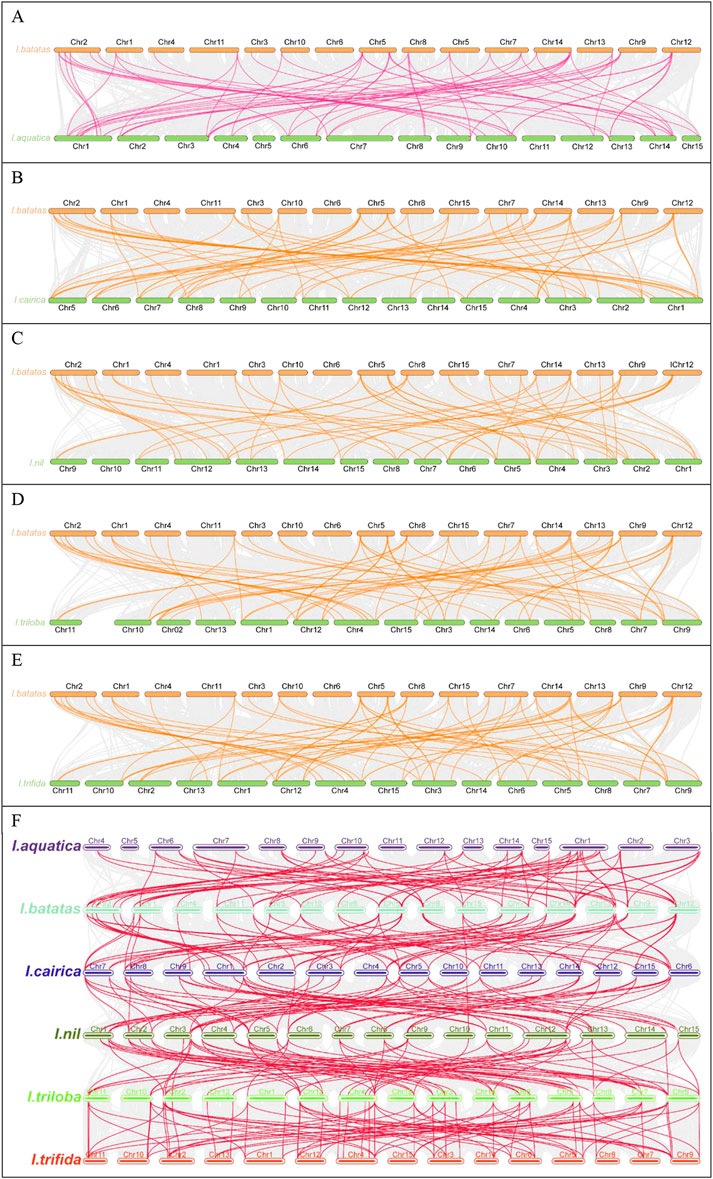
Figure 5. Collinearity analysis of the GATA genes between Ipomoea species. (A) Ipomoea batatas and I. aquatica; (B) Ipomoea batatas and I. cairica; (C) Ipomoea batatas and I. nil; (D) Ipomoea batatas and I. triloba; (E) Ipomoea batatas and I. trifida. (F) Schematic representation of syntenic genes among I. batatas, I. aquatica, I. cairica, I. nil, I. triloba, and I. trifida.
Multiple IbGATA genes have been identified as homologous genes to single IaGATA, IcGATA, InGATA, ItbGAA, and ItfGATA genes. In addition, there are multiple IaGATA, IcGATA, InGATA, ItbGAA, and ItfGATA genes that are homogeneous to a single IbGATA gene. These results indicate that the GATA gene families of sweet potato and I. aquatica, I. cairica, I. nil, I. triloba, and I. trifida share a close evolutionary relationship, and these genes may have similar functions.
The segmental duplication events of IbGATA genes were identified using MCScanX and BLASTp searches (Figure 6). It was found that there were 18 pairs of IbGATA genes in the sweet potato chromosome, of which 17 pairs of genes were segmental duplication events in IbGATA genes and IbGATA22/IbGATA23 was a tandem duplication event. The segmental duplication events of GATA genes in other Ipomoea species were identified, and the results were similar to that of IbGATA genes (Figure 6).
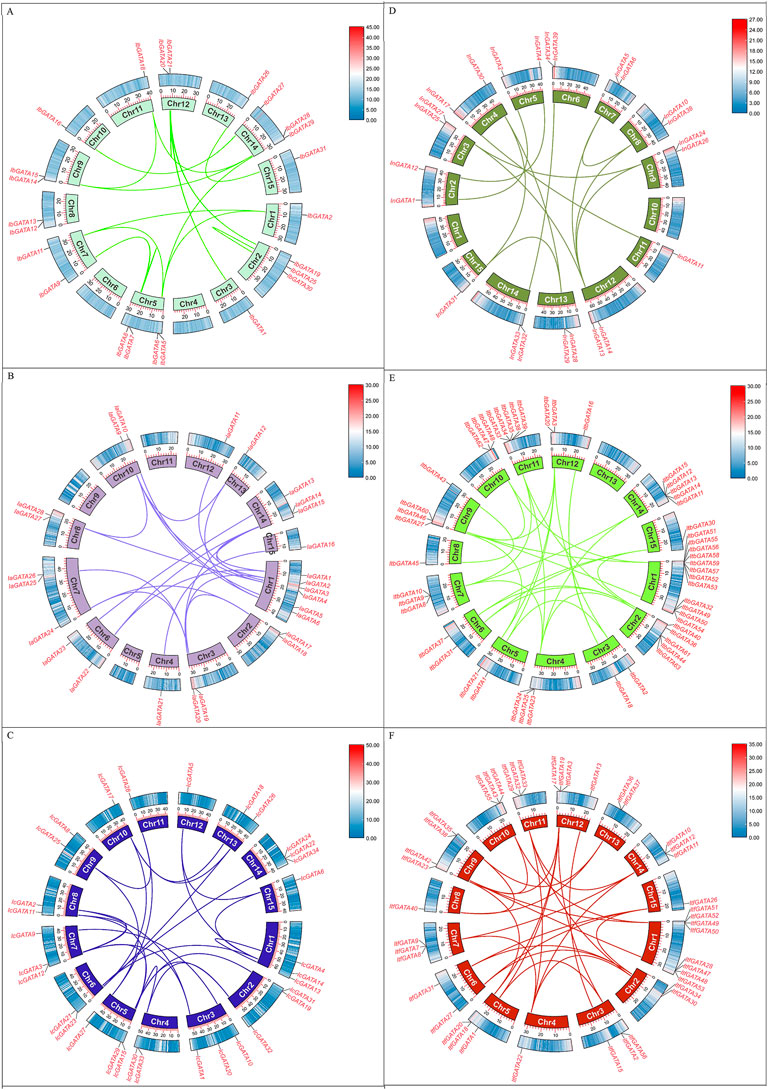
Figure 6. Schematic diagram of GATA gene collinearity analysis in Ipomoea species. (A–F) I. batatas, I. aquatica, I. cairica, I. nil, I. triloba, and I. trifida.
3.7 Ka/Ks analysis of duplicated and syntenic GATA genes
To determine whether the GATA genes are under positive selection, the Ka/Ks analysis of syntenic GATA genes within six Ipomoea species was conducted. In the six Ipomoea species, all of the syntenic GATA genes possessed a Ka/Ks ratio <1 (Supplementary Table S3). These results suggest that syntenic GATA genes were subject to purifying selection in the genome during speciation.
3.8 Expression analysis of IbGATA genes through qRT-PCR
To further investigate the expression characteristics of GATA genes, 17 IbGATA genes were chosen in order to study their dynamic expression patterns in different tissues and in response to drought and salt stress (Supplementary Table S2). The expression of IbGATA genes in the root, stem, leaf, and petiole was analyzed through qRT-PCR (Figure 7A). The results showed that IbGATA expression levels vary across different tissues, and IbGATA1, IbGATA3, IbGATA9, IbGATA10, IbGATA13, IbGATA18, IbGATA20, IbGATA21, IbGATA23, and IbGATA32 were highly expressed in the leaf of sweet potato. IbGATA7 showed strong upregulated expression in the root. IbGATA12 was highly expressed in the petioles. Many IbGATA genes, such as IbGATA13 and IbGATA21, had similar expression.
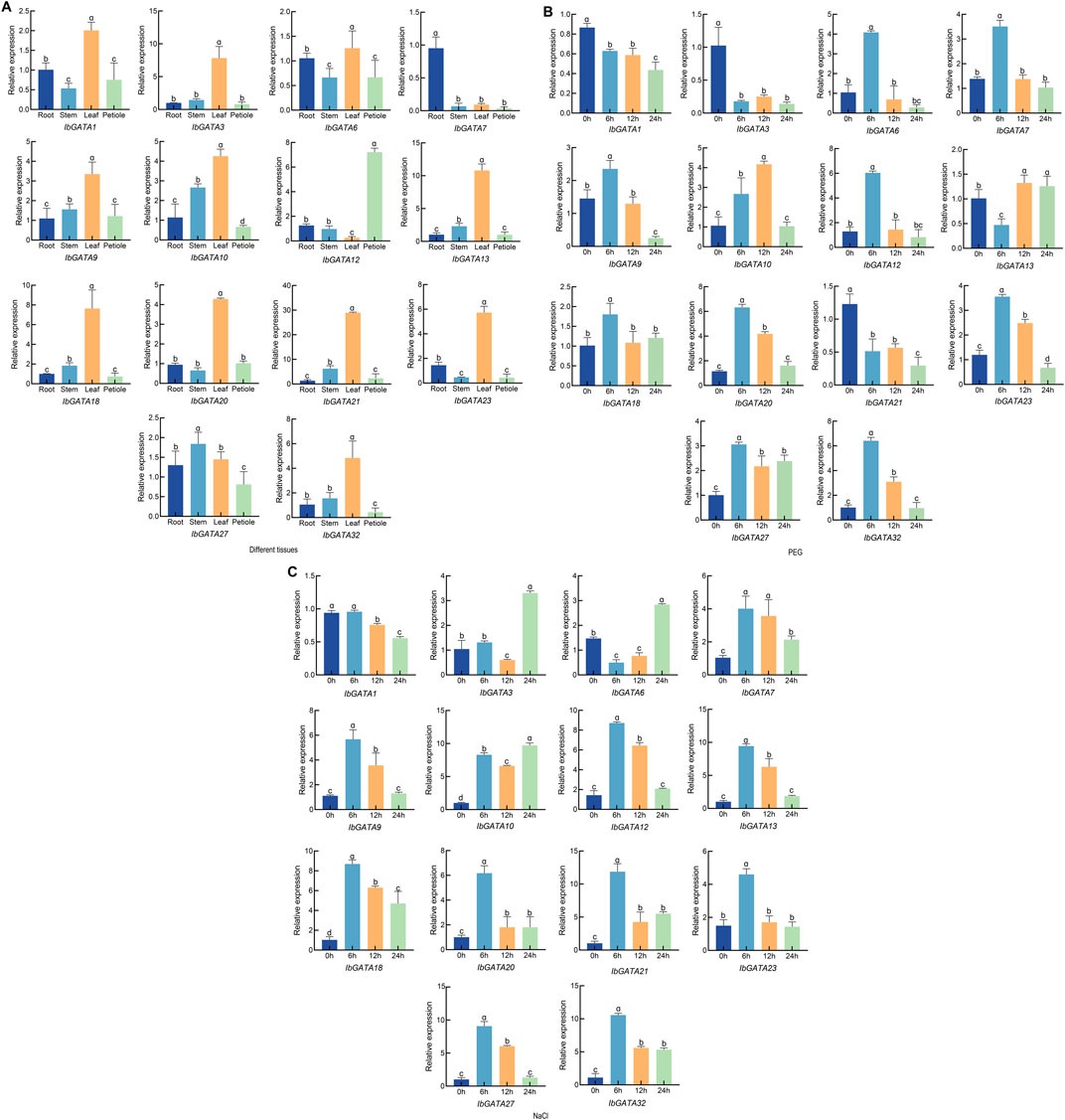
Figure 7. The expression profile of the IbGATA gene in sweet potato was detected through qRT-PCR. (A) Relative gene expression levels in different tissues: root, stem, leaf, and petiole. (B) Relative gene expression levels under drought (20% PEG-6000) treatment over the same time periods (0, 6, 12, and 24 h). The control group was treated with distilled water. (C) Relative gene expression levels under salt (200 mM NaCl) treatment over the same time periods (0, 6, 12, and 24 h). Data represent the mean of three biological replicates ±SD (n = 3). Error lines indicate standard deviations. Different lowercase letters (a, b, c, and d) on the bars indicate significant differences at p < 0.01.
The expression levels of IbGATA genes in drought and salt stress were analyzed through qRT-PCR (Figures 7B,C). The expression of IbGATA6, IbGATA7, IbGATA9, IbGATA10, IbGATA12, IbGATA18, IbGATA20, IbGATA23, IbGATA27, and IbGATA32 was upregulated under drought stress. The expression of IbGATA6, IbGATA7, IbGATA9, IbGATA12, IbGATA18, IbGATA20, IbGATA23, IbGATA27, and IbGATA32 was highest at 6 h. In addition, IbGATA6, IbGATA7, IbGATA9, IbGATA12, IbGATA20, and IbGATA32 exhibited similar expression patterns under drought conditions. The expression levels of IbGATA1, IbGATA3, and IbGATA21 exhibited a downward trend under drought stress (Figure 7B). Under salt stress, the expression of IbGATA1 was downregulated. By contrast, for example, IbGATA7 and IbGATA18 exhibited upregulated expression patterns under salt stress. The expression of IbGATA3 and IbGATA6 reached the highest value at 24 h (Figure 7C). These findings indicate that IbGATA genes play a significant role in drought and salt stress responses.
4 Discussion
GATA TFs have been demonstrated to play important roles in different plant biological processes such as seedling development, signal transduction, nitrogen and carbon metabolism, light regulation, and abiotic stresses (drought, cold, and salinity) (Schwechheimer et al., 2022). The GATA gene family has been identified and studied in a variety of plants, including rice (Oryza sativa) (Reyes et al., 2004), tomato (Lycopersicon esculentum) (Zhao et al., 2021), soybean (Glycine max) (Zhang et al., 2020), and potato (Solanum tuberosum) (Aksoy et al., 2024). However, primarily due to the fact that widely cultivated sweet potato varieties are highly heterozygous autopolyploid hexaploids with complex genetic analysis challenges and relatively scarce genomic databases, a genome-wide study of the GATA gene family has not yet been conducted in sweet potato and other Ipomoea species. With the completion of genome sequencing for sweet potato and an increasing number of Ipomoea species, these data provide valuable resources for the identification of gene families and genome-wide bioinformatic analyses in sweet potato and other Ipomoea species.
In this study, 260 GATA genes were identified from sweet potato and other Ipomoea species using bioinformatic technology. The number of GATA genes were 35, 33, 34, 39, 63, and 56 in sweet potato, Ipomoea aquatica, Ipomoea cairica, Ipomoea nil, Ipomoea triloba, and Ipomoea trifida, respectively. The expression of these GATA genes was similar to that in rice and Arabidopsis. The GATA gene counts in sweet potato diverges from those of other species, exemplified by Triticum aestivum (79) (Zheng et al., 2024), Dimocarpus longan Lour (24) (Zheng et al., 2024), and Setaria italica (28) (Lai et al., 2022), demonstrating lineage-specific expansion patterns of GATA gene families among plant taxa. In addition, the GATA genes can be divided into six groups, among which group Ⅲ-Ⅳ has the most members, whereas group Ⅱ has the fewest GATAs. The current study provides valuable insights for the future functional characterization of GATA genes and contributes to increased adaptive capacity in plants.
In plants, exon/intron structures of GATA genes showed a low concentration. In sweet potato, exon numbers in GATA genes range from 1 to 8 and exhibit lineage-specific divergence compared to those in I. aquatica, I. cairica, I. nil, I. triloba, and I. trifida. The exon number in Ipomoea is very similar to that of wheat (Feng et al., 2022). The conserved motif analysis revealed that all 35 IbGATA family members contain motif 1, indicating that this motif is crucial for the function of IbGATA proteins. Additionally, different subfamilies contain distinct types of conserved motifs, leading to functional diversification during evolution. In contrast, the conserved motifs of GATA TFs within the same subfamily are generally identical, indicating that these GATA proteins are likely to have similar functions. In brief, IbGATA proteins within the same subfamily share similar conserved motifs, gene structures, and phylogenetic relationships, which enhances the reliability of the subfamily classification of IbGATA genes in this study.
Cis-acting elements are specific binding sites for TFs, regulating the precise initiation sites and efficiency of gene transcription (Moriwaki et al., 2022). Previous studies have shown that GATA TFs can regulate light signal transduction by binding to elements related to plant growth and development, thereby modulating the light responsiveness within GATA promoter sequences (Luo et al., 2010). It has been found that CrGATA1 could activate the promoters of light-responsive vindoline pathway genes, and the expression of CrGATA1 and vindoline pathway genes was greatly induced in Catharanthus roseus under light conditions (Liu et al., 2019). In this research, plant growth and development elements, such as light-responsive elements, were widely distributed in IbGATA genes, suggesting that IbGATA genes could regulate light-response processes in sweet potato. Additionally, the majority of IbGATA gene promoters contain hormone-responsive elements, as well as low-temperature and drought stress-responsive elements. The previous studies have found that overexpression of BdGATA13 in transgenic Arabidopsis enhanced drought tolerance compared to the wild type, and BdGATA13 also promoted primary root development under gibberellins (GAs) treatment (Guo et al., 2021). All 35 IbGATA genes contain many cis-acting elements related to adverse stress and hormone regulation, which suggests that IbGATA genes not only play a vital role in regulating plant growth and development but may also be involved in abiotic stress and hormone regulation.
Gene duplication events are crucial for the expansion and functional diversification of gene families during the evolutionary process (Qiao et al., 2019). In this research, gene duplication events have occurred in GATA genes of sweet potato and other Ipomoea species during evolution. These findings suggest that segmental duplication events likely represent the predominant mechanism underlying the expansion of the GATA gene family during evolution. The collinearity analysis showed that the genomes of sweet potato, Ipomoea aquatica, Ipomoea cairica, Ipomoea nil, Ipomoea triloba, and Ipomoea trifida have many homologous gene pairs in the GATA gene family. The results indicate a closer phylogenetic relationship between the GATA gene families of sweet potato and other Ipomoea species.
The previous study indicates gene expression patterns can, to some extent, reveal gene function. It has been found that the DlGATA genes were strongly upregulated in roots and stems (Zheng et al., 2024). The expression of TaGATA genes varies in different tissues of wheat (Feng et al., 2022). In this study, the expression patterns of IbGATA genes in different tissues exhibit differential expression. This study revealed a significant variation in the expression levels of IbGATA genes across different tissues. For example, IbGATA1 and IbGATA9 exhibited markedly higher expression in leaves, IbGATA7 showed elevated expression in roots, and IbGATA12 displayed the highest expression in petioles (Figure 7A), suggesting that distinct IbGATA genes may function in tissue-specific contexts.
Drought and salt stress are abiotic stress factors that limit the normal growth and development of crops, posing serious threats to land productivity and biomass yield. In tomato, overexpression of SlGATA17 increases drought tolerance in transgenic plants (Zhao et al., 2021). Overexpression of TaGATA62 and TaGATA73 genes significantly enhanced the drought and salt tolerance of yeast and Arabidopsis (Du et al., 2022). In this study, IbGATA7, IbGATA9, and IbGATA21 were upregulated under drought and salt stress, suggesting that these genes may function in drought and salt stress signaling pathways contributing to plant drought and salinity tolerance.
5 Conclusion
This study systematically analyzed the GATA gene family in Ipomoea species, including gene structure, predicted physical and chemical properties, conserved domains, collinearity, and evolutionary tree. A phylogenetic tree was constructed using GATA sequences from sweet potato, Ipomoea aquatica, Ipomoea cairica, Ipomoea nil, Ipomoea triloba, Ipomoea trifida, rice, and Arabidopsis, and the sweet potato GATA genes were divided into six groups. In most subfamilies, the exon/intron architecture and motif configurations demonstrated evolutionary conservation. These GATA genes were unevenly distributed on 15 chromosomes, and the segmental duplication events were analyzed. The expression characteristics of GATA gene family members in various tissues of sweet potato and their stress-responsive expression patterns have been systematically validated through qRT-PCR analysis. This study revealed that GATA TFs play pivotal roles in regulating plant growth and development and mediating stress adaptation mechanisms. In summary, this study systematically deciphered the expression patterns and functional characteristics of the GATA gene family in sweet potato and other Ipomoea species, offering critical data support for an in-depth understanding of the biological functions of this TF family.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
CW: Writing – original draft, Data curation, Writing – review and editing. ML: Data curation, Conceptualization, Writing – review and editing, Methodology, Investigation. MX: Conceptualization, Writing – review and editing, Data curation, Software. YP: Formal Analysis, Data curation, Methodology, Writing – review and editing. HP: Writing – review and editing. JD: Conceptualization, Data curation, Software, Investigation, Writing – review and editing. WW: Writing – review and editing, Visualization, Resources, Funding acquisition, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the Basic Research and Talent Training Program of Jiangxi Academy of Agricultural Sciences (JXSNKYJCRC202422), China Agriculture Research System of MOF and MARA (CARS-10-SYZ06), and Seed Industry High-Quality Development of Hubei Province (HBZY2023B002 and HBZY2023B002-4).
Acknowledgments
The authors thank the reviewers for their comments on the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2025.1635749/full#supplementary-material
References
Aksoy, E., Yavuz, C., Yagiz, A. K., Unel, N. M., and Baloglu, M. C. (2024). Genome-wide characterization and expression analysis of GATA transcription factors under combination of light wavelengths and drought stress in potato. Plant Direct 8 (4), e569. doi:10.1002/pld3.569
An, Y., Zhou, Y. Y., Han, X., Shen, C., Wang, S., Liu, C., et al. (2020). The GATA transcription factor GNC plays an important role in photosynthesis and growth in poplar. J. Exp. Bot. 71 (6), 1969–1984. doi:10.1093/jxb/erz564
Chen, C., Zhang, K. X., Khurshid, M., Li, J. B., He, M., Georgiev, M. I., et al. (2019). MYB transcription repressors regulate plant secondary metabolism. Crit. Rev. Plant Sci. 38 (3), 159–170. doi:10.1080/07352689.2019.1632542
Chen, C. J., Wu, Y., Li, J. W., Wang, X., Zeng, Z. H., Xu, J., et al. (2023). TBtools-II: a “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 16 (11), 1733–1742. doi:10.1016/j.molp.2023.09.010
Chen, F., Hu, Y., Vannozzi, A., Wu, K. C., Cai, H. Y., Qin, Y., et al. (2018). The WRKY transcription factor family in model plants and crops. Crit. Rev. Plant Sci. 36 (5-6), 311–335. doi:10.1080/07352689.2018.1441103
Daniel-Vedele, F., and Caboche, M. (1993). A tobacco cDNA clone encoding a GATA-1 zinc finger protein homologous to regulators of nitrogen metabolism in fungi. Mol. Gen. Genet. 240 (3), 365–373. doi:10.1007/bf00280388
Du, X., Lu, Y. X., Sun, H. C., Duan, W. J., Hu, Y. K., and Yan, Y. M. (2022). Genome-wide analysis of wheat GATA transcription factor genes reveals their molecular evolutionary characteristics and involvement in salt and drought tolerance. Int. J. Mol. S. C. 24 (1), 27. doi:10.3390/ijms24010027
Dubos, C., Stracke, R., Grotewold, E., Weisshaar, B., Martin, C., and Lepiniec, L. (2010). MYB transcription factors in arabidopsis. Trends Plant Sci. 15 (10), 573–581. doi:10.1016/j.tplants.2010.06.005
Feng, K., Hou, X. L., Xing, G. M., Liu, J. X., Duan, A. Q., Xu, Z. S., et al. (2020). Advances in AP2/ERF super-family transcription factors in plant. Crit. Rev. Biotechnol. 40 (6), 750–776. doi:10.1080/07388551.2020.1768509
Feng, X., Yu, Q., Zeng, J. B., He, X. Y., and Liu, W. X. (2022). Genome-wide identification and characterization of GATA family genes in wheat. BMC Plant Biol. 22 (1), 372. doi:10.1186/s12870-022-03733-3
Gao, F., and Dubos, C. (2024). The arabidopsis bHLH transcription factor family. Trends Plant Sci. 29 (6), 668–680. doi:10.1016/j.tplants.2023.11.022
Guo, J., Bai, X. H., Dai, K. L., Yuan, X. Y., Guo, P. Y., Zhou, M. X., et al. (2021). Identification of GATA transcription factors in Brachypodium distachyon and functional characterization of BdGATA13 in drought tolerance and response to gibberellins. Front. Plant Sci. 12, 763665. doi:10.3389/fpls.2021.763665
Kim, M., Xi, H., and Park, J. (2021). Genome-wide comparative analyses of GATA transcription factors among 19 Arabidopsis ecotype genomes: intraspecific characteristics of GATA transcription factors. PLoS One 16 (5), e0252181. doi:10.1371/journal.pone.0252181
Kumar, S., Stecher, G., Li, M., Knyaz, C., and Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35 (6), 1547–1549. doi:10.1093/molbev/msy096
Lai, D. L., Yao, X., Yan, J., Gao, A. J., Yang, H., Xiang, D. B., et al. (2022). Genome-wide identification, phylogenetic and expression pattern analysis of GATA family genes in foxtail millet (Setaria italica). BMC Genomics 23 (1), 549. doi:10.1186/s12864-022-08786-0
Lei, P., Jiang, Y. X., Zhao, Y., Jiang, M. Q., Ji, X. M., Ma, L., et al. (2024). Functions of basic helix-loop-helix (bHLH) proteins in the regulation of plant responses to cold, drought, salt, and iron deficiency: a comprehensive review. J. Agric. Food Chem. 72 (19), 10692–10709. doi:10.1021/acs.jafc.3c09665
Lim, C., Kim, Y., Shim, Y., Cho, S. H., Yang, T. J., Song, Y. H., et al. (2024). Rice OsGATA16 is a positive regulator for chlorophyll biosynthesis and chloroplast development. Plant J. 117 (2), 599–615. doi:10.1111/tpj.16517
Liu, H., Li, T., Wang, Y. M., Zheng, J., Li, H. F., Hao, C. Y., et al. (2019). TaZIM-A1 negatively regulates flowering time in common wheat (Triticum aestivum L.). J. Integr. Plant Biol. 61 (3), 359–376. doi:10.1111/jipb.12720
Liu, H. T., Tang, X., Zhang, N., Li, S. G., and Si, H. J. (2023). Role of bZIP transcription factors in plant salt stress. Int. J. Mol. Sci. 24 (9), 7893. doi:10.3390/ijms24097893
Liu, P. P., Koizuka, N., Martin, R. C., and Nonogaki, H. (2005). The BME3 (blue micropylar end 3) GATA zinc finger transcription factor is a positive regulator of Arabidopsis seed germination. Plant J. 44 (6), 960–971. doi:10.1111/j.1365-313X.2005.02588.x
Liu, Y., Patra, B., Pattanaik, S., Wang, Y., and Yuan, L. (2019). GATA and phytochrome interacting factor transcription factors regulate light-induced vindoline biosynthesis in Catharanthus roseus. Plant Physiol. 180 (3), 1336–1350. doi:10.1104/pp.19.00489
Luo, X. M., Lin, W. H., Zhu, S. W., Zhu, J. Y., Sun, Y., Fan, X. Y., et al. (2010). Integration of light- and brassinosteroid-signaling pathways by a GATA transcription factor in Arabidopsis. Dev. Cell 19 (6), 872–883. doi:10.1016/j.devcel.2010.10.023
Ma, Z. M., Hu, L. J., and Jiang, W. Z. (2024). Understanding AP2/ERF transcription factor responses and tolerance to various abiotic stresses in plants: a comprehensive review. Int. J. Mol. Sci. 25 (2), 893. doi:10.3390/ijms25020893
Mahiwal, S., Pahuja, S., and Pandey, G. K. (2024). Review: structural-Functional relationship of WRKY transcription factors: unfolding the role of WRKY in plants. Int. J. Biol. Macromol. 257 (Pt 2), 128769. doi:10.1016/j.ijbiomac.2023.128769
Meng, X. Q., Liu, S. Y., Zhang, C. B., He, J. N., Ma, D. F., Wang, X., et al. (2023). The unique sweet potato NAC transcription factor IbNAC3 modulates combined salt and drought stresses. Plant Physiol. 191 (1), 747–771. doi:10.1093/plphys/kiac508
Millard, P. S., Kragelund, B. B., and Burow, M. (2019). R2R3 MYB transcription factors - functions outside the DNA-Binding domain. Trends Plant Sci 24 (10), 934–946. doi:10.1016/j.tplants.2019.07.003
Moriwaki, K., Yanagisawa, S., Iba, K., and Negi, J. (2022). Two independent cis-acting elements are required for the guard cell-specific expression of SCAP1, which is essential for late stomatal development. Plant J. 110 (2), 440–451. doi:10.1111/tpj.15679
Nutan, K. K., Singla-Pareek, S. L., and Pareek, A. (2020). The saltol QTL-Localized transcription factor OsGATA8 plays an important role in stress tolerance and seed development in arabidopsis and rice. J. Exp. Bot. 71 (2), 684–698. doi:10.1093/jxb/erz368
Patient, R. K., and McGhee, J. D. (2002). The GATA family (vertebrates and invertebrates). Curr. Opin. Genet. Dev. 12 (4), 416–422. doi:10.1016/s0959-437x(02)00319-2
Qiao, X., Li, Q. H., Yin, H., Qi, K. J., Li, L., Wang, R. Z., et al. (2019). Gene duplication and evolution in recurring polyploidization-diploidization cycles in plants. Genome Biol. 20 (1), 38. doi:10.1186/s13059-019-1650-2
Reyes, J. C., Muro-Pastor, M. I., and Florencio, F. J. (2004). The GATA family of transcription factors in arabidopsis and rice. Plant Physiol. 134 (4), 1718–1732. doi:10.1104/pp.103.037788
Richter, R., Behringer, C., Zourelidou, M., and Schwechheimer, C. (2013). Convergence of auxin and gibberellin signaling on the regulation of the GATA transcription factors GNC and GNL in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U. S. A. 110 (32), 13192–13197. doi:10.1073/pnas.1304250110
Sala, J., Mosesso, N., Isono, E., and Schwechheimer, C. (2023). Arabidopsis thaliana B-GATA factors repress starch synthesis and gravitropic growth responses. New Phytol. 239 (3), 979–991. doi:10.1111/nph.18992
Schwechheimer, C., Schroder, P. M., and Blaby-Haas, C. E. (2022). Plant GATA factors: their biology, phylogeny, and phylogenomics. Annul. Rev. Plant Biol. 73, 123–148. doi:10.1146/annurev-arplant-072221-092913
Shen, C., Zhang, Y., Li, Q., Liu, S. J., He, F., An, Y., et al. (2021). PdGNC confers drought tolerance by mediating stomatal closure resulting from NO and H2O2 production via the direct regulation of PdHXK1 expression in Populus. New Phytol. 230 (5), 1868–1882. doi:10.1111/nph.17301
Shikata, M., Matsuda, Y., Ando, K., Nishii, A., Takemura, M., Yokota, A., et al. (2004). Characterization of arabidopsis ZIM, a member of a novel plant-specific GATA factor gene family. J. Exp. Bot. 55 (397), 631–639. doi:10.1093/jxb/erh078
Strader, L., Weijers, D., and Wagner, D. (2022). Plant transcription factors - being in the right place with the right company. Curr. Opin. Plant Biol. 65, 102136. doi:10.1016/j.pbi.2021.102136
Subramanian, B., Gao, S. H., Lercher, M. J., Hu, S. N., and Chen, W. H. (2019). Evolview v3: a webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 47 (W1), W270–W275. doi:10.1093/nar/gkz357
Wang, C., Wang, L. J., Lei, J., Chai, S. S., Jin, X. J., Zou, Y. Y., et al. (2022). IbMYB308, a sweet potato R2R3-MYB gene, improves salt stress tolerance in transgenic tobacco. Genes (Basel) 13 (8), 1476. doi:10.3390/genes13081476
Wu, S., Lau, K. H., Cao, Q. H., Hamilton, J. P., Sun, H., Zhou, C., et al. (2018). Genome sequences of two diploid wild relatives of cultivated sweet potato reveal targets for genetic improvement. Nat. Commun. 9 (1), 4580. doi:10.1038/s41467-018-06983-8
Wu, W., Dong, X. O., Chen, G. M., Lin, Z. X., Chi, W. H., Tang, W. J., et al. (2024). The elite haplotype OsGATA8-H coordinates nitrogen uptake and productive tiller formation in rice. Nat. Genet. 56 (7), 1516–1526. doi:10.1038/s41588-024-01795-7
Yan, H. F., Ma, G. H., Teixeira da Silva, J. A., Qiu, L. H., Xu, J., Zhou, H. W., et al. (2021). Genome-wide identification and analysis of NAC transcription factor family in two diploid wild relatives of cultivated sweet potato uncovers potential NAC genes related to drought tolerance. Front. Genet. 12, 744220. doi:10.3389/fgene.021.744220
Yan, P. S., Du, Q. G., Chen, H., Guo, Z. F., Wang, Z. H., Tang, J. H., et al. (2023). Biofortification of iron content by regulating a NAC transcription factor in maize. Science 382 (6675), 1159–1165. doi:10.1126/science.adf3256
Yang, J., Moeinzadeh, M. H., Kuhl, H., Helmuth, J., Xiao, P., Haas, S., et al. (2017). Haplotype-resolved sweet potato genome traces back its hexaploidization history. Nat. Plants 3 (9), 696–703. doi:10.1038/s41477-017-0002-z
Yao, X., Lai, D. L., Zhou, M. L., Ruan, J. J., Ma, C., Wu, W. J., et al. (2023). Genome-wide identification, evolution and expression pattern analysis of the GATA gene family in Sorghum bicolor. Front. Plant Sci. 14, 1163357. doi:10.3389/fpls.2023.1163357
Yu, C. Y., Li, N., Yin, Y. X., Wang, F., Gao, S. H., Jiao, C. H., et al. (2021). Genome-wide identification and function characterization of GATA transcription factors during development and in response to abiotic stresses and hormone treatments in pepper. J. App Genet. 62 (2), 265–280. doi:10.1007/s13353-021-00618-3
Zhang, C. F., Jiao, C., Sun, X. P., and Li, X. L. (2023). A MYB transcription factor atlas provides insights into the evolution of environmental adaptations in plants. Int. J. Mol. Sci. 24 (3), 2566. doi:10.3390/ijms24032566
Zhang, C. J., Hou, Y. Q., Hao, Q. N., Chen, H. F., Chen, L. M., Yuan, S. L., et al. (2015). Genome-wide survey of the soybean GATA transcription factor gene family and expression analysis under low nitrogen stress. PLoS One 10 (4), e0125174. doi:10.1371/journal.pone.0125174
Zhang, C. J., Huang, Y. Q., Xiao, Z. Y., Yang, H. L., Hao, Q. N., Yuan, S. L., et al. (2020). A GATA transcription factor from soybean (glycine Max) regulates chlorophyll biosynthesis and suppresses growth in the transgenic Arabidopsis thaliana. Plants (Basel). 9 (8), 1036. doi:10.3390/plants9081036
Zhang, H., Gao, X. R., Zhi, Y. H., Li, X., Zhang, Q., Niu, J. B., et al. (2019). A non-tandem CCCH-type zinc-finger protein, IbC3H18, functions as a nuclear transcriptional activator and enhances abiotic stress tolerance in sweet potato. New Phytol. 223 (4), 1918–1936. doi:10.1111/nph.15925
Zhang, H., Wang, Z., Li, X., Gao, X. R., Dai, Z. R., Cui, Y. F., et al. (2022). The IbBBX24-IbTOE3-IbPRX17 module enhances abiotic stress tolerance by scavenging reactive oxygen species in sweet potato. New Phytol. 233 (3), 1133–1152. doi:10.1111/nph.17860
Zhang, H. J., Wu, T., Li, Z., Huang, K., Kim, N. E., Ma, Z. M., et al. (2021). OsGATA16, a GATA transcription factor, confers cold tolerance by repressing OsWRKY45-1 at the seedling stage in rice. Rice (N Y) 14 (1), 42. doi:10.1186/s12284-021-00485-w
Zhang, X., Fan, R., Yu, Z., Du, X. Y., Yang, X. Y., Wang, H. T., et al. (2024). Genome-wide identification of GATA transcription factors in tetraploid potato and expression analysis in differently colored potato flesh. Front. Plant Sci. 15, 1330559. doi:10.3389/fpls.2024.1330559
Zhang, Y. J., Zhang, Y., Zhang, L. L., He, J. X., Xue, H. W., Wang, J. W., et al. (2022). The transcription factor OsGATA6 regulates rice heading date and grain number per panicle. J. Exp. Bot. 73 (18), 6133–6149. doi:10.1093/jxb/erac247
Zhao, T. T., Wu, T. R., Pei, T., Wang, Z. Y., Yang, H. H., Jiang, J. B., et al. (2021). Overexpression of SlGATA17 promotes drought tolerance in transgenic tomato plants by enhancing activation of the phenylpropanoid biosynthetic pathway. Front. Plant S. C. 12, 634888. doi:10.3389/fpls.2021.634888
Zheng, K. H., Lu, J. Y., He, X. Y., Lan, S. X., Zhai, T. K., Cao, S. J., et al. (2024). Genome-wide identification and expression analysis of GATA family genes in Dimocarpus longan Lour. Int. J. Mol. Sci. 25 (2), 731. doi:10.3390/ijms25020731
Zhu, W. H., Guo, Y. Y., Chen, Y. K., Wu, D. Z., and Jiang, L. X. (2020). Genome-wide identification, phylogenetic and expression pattern analysis of GATA family genes in Brassica napus. BMC Plant Biol. 20 (1), 543. doi:10.1186/s12870-020-02752-2
Keywords: sweet potato (Ipomoea batatas L.), GATA transcription factor, genome-wide, abiotic stress, drought and salt stress
Citation: Wang C, Lan M, Xiao M, Peng Y, Pan H, Deng J and Wu W (2025) Genome-wide identification of GATA family genes in sweet potato (Ipomoea batatas L.) and their expression patterns under abiotic stress. Front. Genet. 16:1635749. doi: 10.3389/fgene.2025.1635749
Received: 27 May 2025; Accepted: 17 June 2025;
Published: 09 July 2025; Corrected: 23 July 2025.
Edited by:
Meng Kou, Xuzhou Institute of Agricultural Sciences in Jiangsu Xuhuai District, ChinaReviewed by:
Lei Zhang, Jiangsu Normal University, ChinaChang Ye, Chinese Academy of Agricultural Sciences, China
Hongpan Wang, Chinese Academy of Sciences (CAS), China
Copyright © 2025 Wang, Lan, Xiao, Peng, Pan, Deng and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wensheng Wu, MTM3NTU2MTM1MjRAMTYzLmNvbQ==
 Chong Wang
Chong Wang Mengjiao Lan1
Mengjiao Lan1