- 1Guangzhou 11th People’s Hospital, Guangzhou Cadre and Talent Health Management Centre, Guangzhou, China
- 2State Key Laboratory of Dampness Syndrome of Chinese Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 3Guangdong Provincial Key Laboratory of Clinical Research on Traditional Chinese Medicine Syndrome, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
Purpose: To investigate the association between polymorphisms of the APOA5 rs2075291 and CIDEB rs2144492 loci and hypertriglyceridemia (HTG) in a population with Traditional Chinese Medicine (TCM) dampness syndrome.
Methods: A case-control study was conducted, enrolling 100 HTG patients and 100 age-matched controls with normal triglyceride levels from the physical examination cohort at Guangzhou 11th People’s Hospital (January–December 2023). Peripheral blood samples were collected to analyze APOA5 rs2075291 and CIDEB rs2144492 polymorphisms using PCR and sequencing. Lipid profiles were measured via an automated biochemical analyzer. Statistical analyses (chi-square tests, correlation analysis, and logistic regression) evaluated associations among gene polymorphisms, dampness syndrome, and HTG.
Results: The observation group showed significant differences in genotype frequencies of APOA5 rs2075291 (OR = 2.916, 95% CI:1.160–7.334, χ2p = 0.019) and CIDEB rs2144492 (OR = 1.688, 95% CI:0.886–3.141, χ2p = 0.042) versus the control group. Significant intergroup differences were also observed in allele frequencies of APOA5 rs2075291 (OR = 2.727, 95% CI:1.113–6.682, χ2p = 0.023) and CIDEB rs2144492 (OR = 1.837, 95% CI:1.040–3.244, χ2p = 0.034). Stratified by dampness syndrome status, in the dampness syndrome subgroup, the HTG group had a higher frequency of CIDEB rs2144492 TG/TT genotypes than controls, though the difference was not significant (OR = 2.065, 95% CI:0.816–5.226, χ2 p = 0.146). No significant difference in gene frequency was observed after FDR correction (p = 0.043, FDR threshold = 0.042). APOA5 rs2075291 showed no significant genotype/allele frequency differences (p > 0.05). In the non-dampness subgroup, FDR correction (p ≤ 0.033) revealed no significant differences in APOA5 rs2075291 genotype (OR = 4.083, 95% CI:0.977–17.063, χ2p = 0.041) or allele frequencies (p = 0.05), nor in CIDEB rs2144492 genotypes/allele frequencies (p > 0.05). Triglyceride levels did not differ significantly between dampness/non-dampness groups across genotypes (p > 0.05). Multivariate logistic regression identified male gender, higher BMI, dampness syndrome, and APOA5 rs2075291 genotype as independent risk factors for HTG (p < 0.05), while CIDEB rs2144492 trended toward significance (p = 0.05).
Conclusion: APOA5 rs2075291 and CIDEB rs2144492 polymorphisms are associated with hypertriglyceridemia. Dampness syndrome individuals with CIDEB rs2144492 variants may have increased HTG predisposition. Larger cohort studies are warranted to validate these findings and explore underlying mechanisms.
Introduction
Hypertriglyceridemia is a common lipid metabolism disorder and well-established risk factor for cardiovascular diseases (CVDs), including myocardial infarction and ischemic stroke (Xie, 2023; Jørgensen et al., 2013; Freiberg et al., 2008; Nordestgaard and Varbo, 2014). The etiology of HTG involves a complex interplay of genetic and environmental factors, with single nucleotide polymorphisms (SNPs) in genes such as APOA5 and CIDEB playing pivotal roles in triglyceride regulation (Xu Yn and Pan, 2022; Steinhagen-Thiessen et al., 2017; Kypreos and Zannis, 2006; Guardiola and Ribalta, 2017; Xu et al., 2012).
APOA5 encodes a 366-amino acid protein found in triglyceride-rich lipoproteins and high-density lipoprotein (HDL) particles (Zafar et al., 2019; Srivastava et al., 2015; Su et al., 2018). It is an effective regulator of plasma triglyceride (TG) and HDL cholesterol (HDL-C) levels (Ajjemami et al., 2015), and genetic variants in APOA5 are strong predictors of hypertriglyceridemia-related cardiovascular risk (Ding et al., 2012). Among APOA5 pathogenic mutations, the rs2075291 (Gly185Cys) variant is the most prevalent (Liu et al., 2024). A study (An et al., 2011) involving 406 Uyghur and 527 Han healthy physical examinees in Xinjiang, China, found that the distribution frequencies of the three genotypes of ApoA5 gene rs2075291 in the Uyghur group were 93.1% for the GG type, 6.7% for the GT type, and 0.25% for the TT type; those in the Han group were 90.7% for the GG type, 9.3% for the GT type, and no TT type. There was no statistically significant difference in the genotypic distribution between the two groups.
The CIDE family includes CIDEA, CIDEB and Fsp27 (CIDEC in humans) (Zhang et al., 2014), which were initially implicated in mammalian apoptosis (Park, 2015). However, subsequent research has revealed that CIDE proteins are critical regulators of multiple lipid metabolic pathways and lipid homeostasis (Lajnaf et al., 2023). CIDEB, an endoplasmic reticulum and lipid droplet-associated protein, located on human chromosome 14q11 (Ping et al., 2022), is involved in regulating lipid metabolism and related disorders (Xu et al., 2016). Recent studies have demonstrated that CIDEB promotes fatty acid synthesis, adipocyte formation, and hepatic triglyceride synthesis and storage (Li et al., 2010; Ng et al., 2021). A study (Liu and Zhan, 2016) on 528 Han Chinese individuals in Henan, China, found that the CIDEB rs2144492 locus is associated with TG. The CIDEB gene polymorphism and the ATCC haplotype of the CIDEB gene play a certain role in the risk of HTG.
In traditional Chinese medicine, dampness syndrome stems from impaired body fluid metabolism, presenting with symptoms like fatigue, abdominal distension, and a slippery tongue coating (Zhu Wf, 2011). Epidemiological evidence indicates that populations in humid regions (e.g., Lingnan) exhibit elevated triglyceride levels, which may be associated with dampness syndrome (Chen and Huang, 2022; Zhang Bc, 2020; Zhou et al., 2024). However, the link between TCM dampness syndrome and HTG-related genetic polymorphisms remains unclear. This study investigates APOA5 rs2075291 and CIDEB rs2144492 polymorphisms in HTG patients with dampness syndrome, exploring the combined impact of genetic and TCM-specific factors on lipid metabolism.
Participants and methods
Subjects
A total of 200 participants (100 HTG cases and 100 controls) were recruited from the physical examination cohort at Guangzhou 11th People’s Hospital between January and December 2023. To control for population stratification, participants were proportionally matched between the observation and control groups. Only Han Chinese individuals were included, excluding other ethnic groups to avoid genetic heterogeneity confounding the results. All participants completed the TCM Dampness Syndrome Assessment Scale. Ages ranged from 18 to 75 years (mean: 47.15 ± 11.93), with 134 males (67%; mean age 47.42 ± 10.63) and 66 females (33%; mean age 46.61 ± 14.29). This study was approved by the Ethics Committee of Guangzhou Cadre Health Management Centre(Ethics Number: JGZX-2023-06), and written informed consent was obtained from all participants.
Diagnostic criteria
1.HTG diagnosis (Wang ZW, 2024): Fasting TG ≥ 1.7 mmol/L(150 mg/dL). Controls: Total cholesterol (TC) < 5.2 mmol/L(200 mg/dL), low-density lipoprotein cholesterol (LDL-C) < 3.4 mmol/L (130 mg/dL), HDL-C≥ 1.0 mmol/L(40 mg/dL) and TG < 1.70 mmol/L(150 mg/dL). 2.Dampness syndrome diagnosis (Lu Ty and Cai, 2021): Evaluated using the TCM Dampness Syndrome Diagnostic and Evaluation Scale (National Key Laboratory of TCM Dampness Syndrome, Ministry-Province Co-Constructed). This 30-item self-assessment scale (total score 120 points) defines: No dampness syndrome 0–19 points; Dampness syndrome ≥20 points.
Inclusion and exclusion criteria
The inclusion criteria were as follows: ① completed the TCM Dampness Syndrome Evaluation Scale and obtained a score greater than or equal to 0; ② was able to provide written informed consent, cooperate with the completion of the questionnaire and provide a blood sample; ③ aged ≥20 years old and ≤75 years old. The exclusion criteria were as follows: ① Inability to cooperate with the study; ② History of mental disorders; ③ Pregnant or lactating women; ④ Patients with diabetes, hypothyroidism, nephrotic syndrome, liver/kidney diseases, heavy alcohol consumption, or those taking lipid-altering medications (statins/fibrates/omega-3 fatty acids, retinoids, steroids, beta-blockers, antiretrovirals).
Methods and data collection
Demographic, clinical, and biochemical data were retrieved from hospital records. Genotyping was performed via PCR and Sanger sequencing. All investigators specialized in TCM or integrated Chinese-Western medicine and were trained in the study’s standard operating procedures. Participants were randomly selected from outpatient attendees, with trained investigators assisting in questionnaire completion to ensure data integrity and reduce bias. Questionnaire components: ① General demographics (age, gender, etc.); ② Medical history; ③ TCM Dampness Syndrome Assessment Scale. Physical examinations: Height, weight, body mass index (BMI), blood pressure, waist circumference (WC), etc. Laboratory assessments: ① Biochemical markers: TC, TG, LDL-C, HDL-C, apolipoprotein AI, apolipoprotein B; ② Genetic polymorphisms: APOA5 rs2075291 and CIDEB rs2144492 loci. Genomic DNA extraction, PCR amplification, and sequencing were conducted by Guangzhou Aiji Biotechnology Co., Ltd.
Primer sequences: For ApoA5 rs2075291: Forward primer: 5′-CAGCAACTGAAGCCCTACACG-3′, Reverse primer: 5′-ATGCCGCTCACCAGCTCTCG-3′, Product length: 227 bp.
For CIDEB rs2144492: Forward primer: 5′-CTTATGGCTTCTCCAGTAGGT-3′, Reverse primer: 5′-GTATGTGTGTCTTTGGTGATGA-3′, Product length: 194 bp.
PCR reaction conditions: Initial denaturation at 94 C for 5 min; Denaturation at 94 C for 30 s; Annealing at 56 C for 30 s; Extension at 72 C for 30 s; 35 cycles in total; Final extension at 72 C for 5 min after the last cycle; Storage at 4 C.
The amplification products were analyzed by 1.5% agarose gel electrophoresis. The genotyping success rate and repeat concordance rate for APOA5 rs2075291 and CIDEB rs2144492 SNPs both reached 100%, satisfying quality control criteria.
Statistical analysis
Data were analyzed using SPSS 26.0. Measurement data were expressed as mean ± standard deviation. Subgroup comparisons were performed via t-tests or analysis of variance. Pearson correlation analysis was applied for normally distributed data, while Spearman correlation was used for non-normally distributed data. Allele and genotype frequencies (calculated via genotype counting) were compared using chi-square tests or Fisher’s exact test. The false discovery rate (FDR) was corrected via the Benjamini–Hochberg method, with corrected significant results reported. The Hardy-Weinberg equilibrium (HWE) was assessed for polymorphic locus genotype distributions. The additive model served as the primary model, with dominant/recessive models as secondary. Genotypes were coded as 0/1/2 under the additive model. Models reported the Area Under the Curve (AUC), Hosmer-Lemeshow test (HL) p-value, and maximum variance inflation factor (VIF), including APOA5×dampness and CIDEB × dampness interaction terms. Post-hoc power analysis was conducted using the expected minor allele frequency (MAF) and observed OR values. Binary logistic regression was used to identify factors associated with HTG, with p < 0.05 denoting statistical significance.
Results
Comparison of baseline characteristics between hypertriglyceridemia group and control group
As shown in Table 1, the HTG group had significantly higher waist circumference, BMI, TG, TC, LDL-C, apolipoprotein B, and dampness syndrome scores compared to controls (p < 0.05). Conversely, HDL-C and apolipoprotein AI were lower in the HTG group (p < 0.05). There was no significant age difference between groups (p = 0.152). Collinearity assessment for BMI and waist circumference showed a VIF of 1.
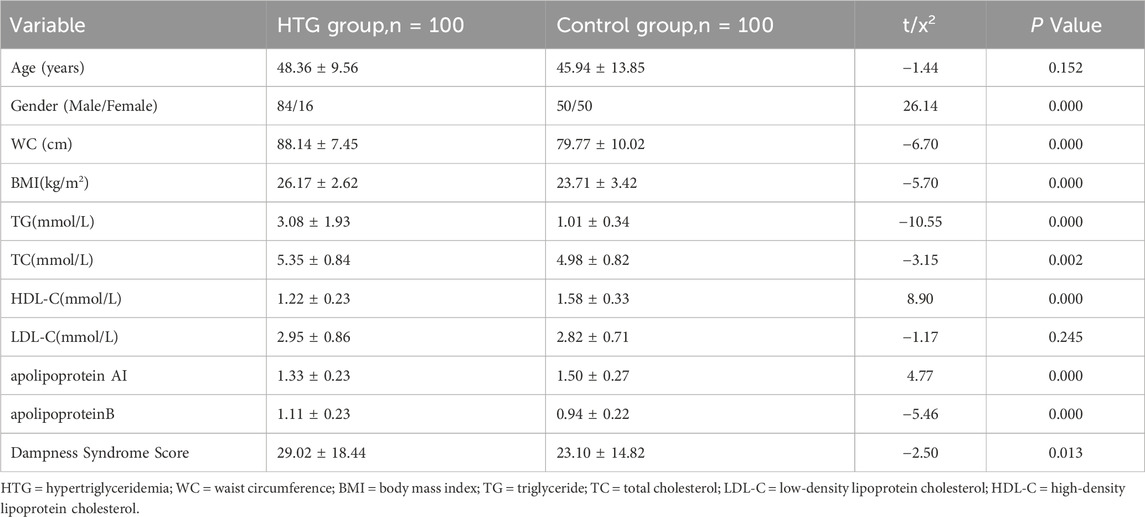
Table 1. Comparison of baseline characteristics between hypertriglyceridemia group and control group.
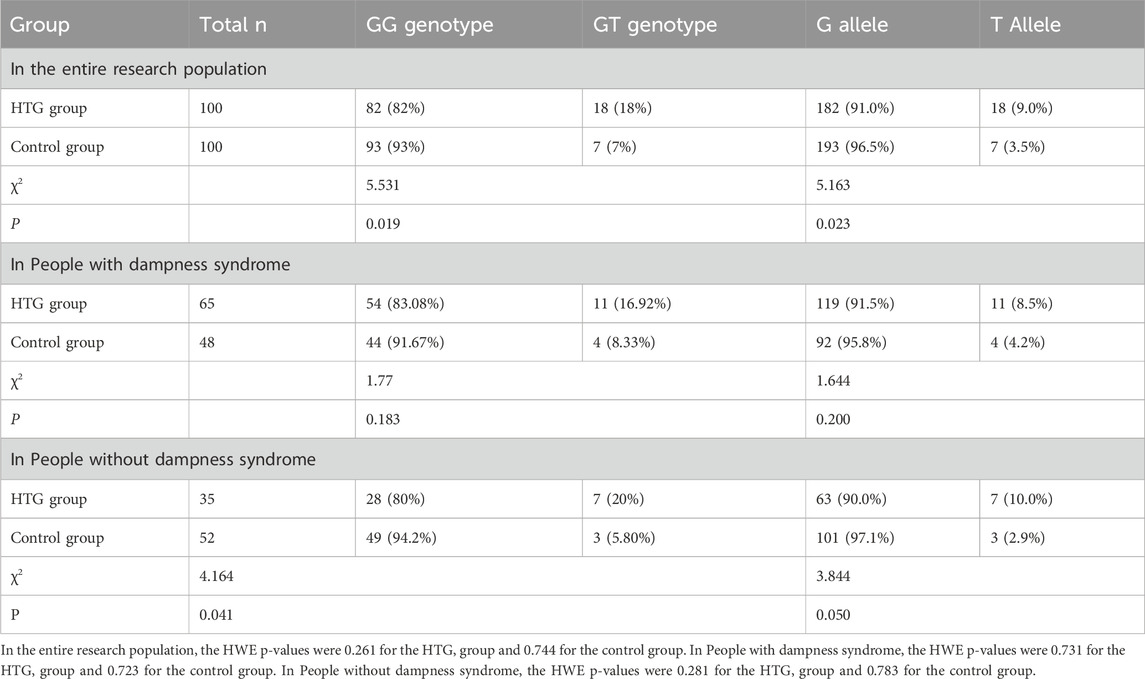
APOA5 rs2075291 genotype and allele frequency in different groups
The polymorphic genotypes of the APOA5 rs2075291 locus in both groups were in Hardy-Weinberg equilibrium (p > 0.05). An interaction was observed between APOA5 rs2075291 and dampness syndrome in the overall population (F = 5.796, p = 0.004). As presented in Table 2, after FDR correction (p ≤ 0.033), the genotype of APOA5 rs2075291 differed significantly between the HTG and control groups (OR = 2.916, 95% CI:1.160–7.334, χ2p = 0.019), with significant intergroup differences in allele frequencies (OR = 2.727, 95% CI:1.113–6.682, χ2p = 0.023). In the dampness syndrome subgroup, neither genotype (OR = 2.241, 95% CI:0.667–7.527, χ2p = 0.183) nor allele frequencies (p = 0.200) showed significant differences. In the non-dampness syndrome subgroup, the HTG group showed no significant difference from the control group in APOA5 rs2075291 genotype frequency (OR = 4.083, 95% CI:0.977–17.063, χ2 p = 0.041) or allele frequencies (p = 0.05).
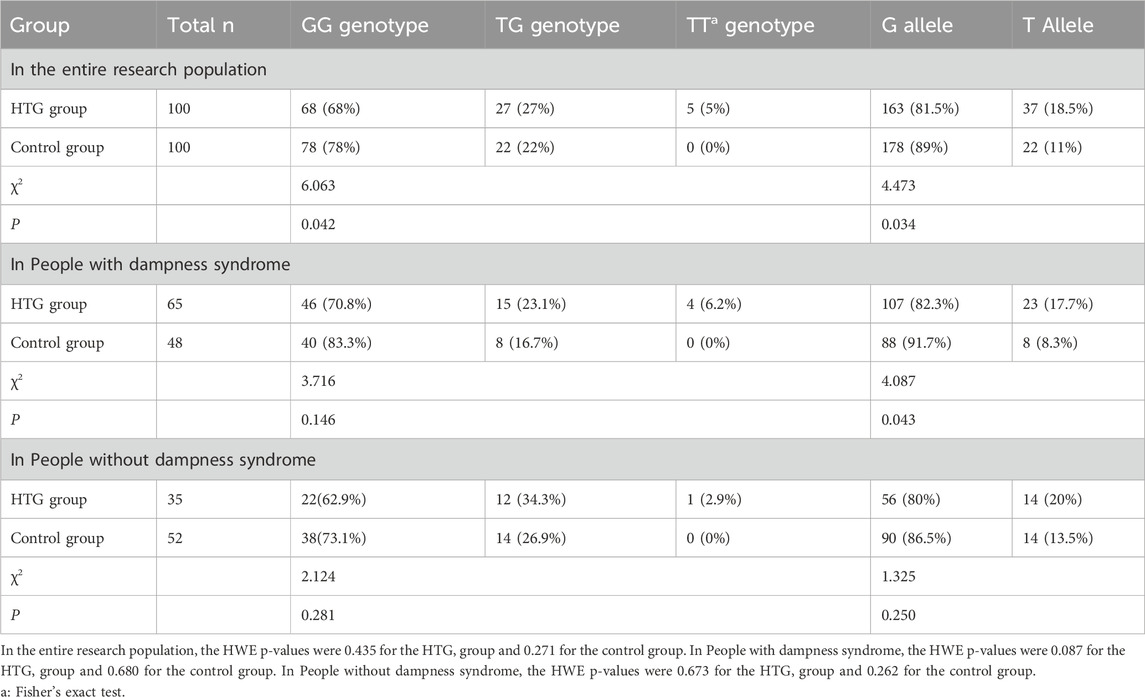
CIDEB rs2144492 genotype and allele frequency in different groups
The genotype distribution of the CIDEB rs2144492 locus polymorphism in both groups was in Hardy-Weinberg equilibrium (P > 0.05). An interaction between CIDEB rs2144492 and dampness syndrome was observed in the overall population (F = 5.796, p = 0.004). As shown in Table 3, after FDR correction (p ≤ 0.042), Fisher’s exact test showed significant differences in CIDE-B rs2144492 genotype (OR = 1.688, 95% CI:0.886–3.141, χ2p = 0.042) and allele frequency (OR = 1.837, 95% CI:1.040–3.244, χ2p = 0.034) between HTG and control groups. In the dampness syndrome subgroup, the HTG group exhibited a higher frequency of CIDEB rs2144492 TG/TT genotypes, though the difference did not reach statistical significance (OR = 2.065, 95% CI:0.816–5.226, χ2p = 0.146). Similarly, no significant difference was observed in allele frequencies between the two groups (p = 0.043). In the non-dampness subgroup, neither CIDEB rs2144492 genotype (OR = 1.604, 95% CI:0.639–4.023, χ2p = 0.281) nor allele frequency (p = 0.250) differed significantly between groups.
Comparison of mean triglyceride levels among different genotypes in dampness syndrome and non-dampness syndrome populations
Using log-transformed mean TG as the dependent variable, interactions of APOA5×dampness syndrome and CIDEB × dampness syndrome were tested. Interaction p-values were: APOA5×dampness syndrome (p = 0.322) and CIDEB × dampness syndrome (p = 0.966). As shown in Table 4, mean triglyceride levels did not differ significantly between dampness and non-dampness groups for APOA5 rs2075291 GG/GT genotypes or CIDEB rs2144492 GG/TG/TT genotypes (p > 0.05). An ANCOVA model for log-transformed TG was fitted, adjusting for age, gender, BMI, dampness syndrome, and genotype. Adjusted mean differences are reported in Table 4.
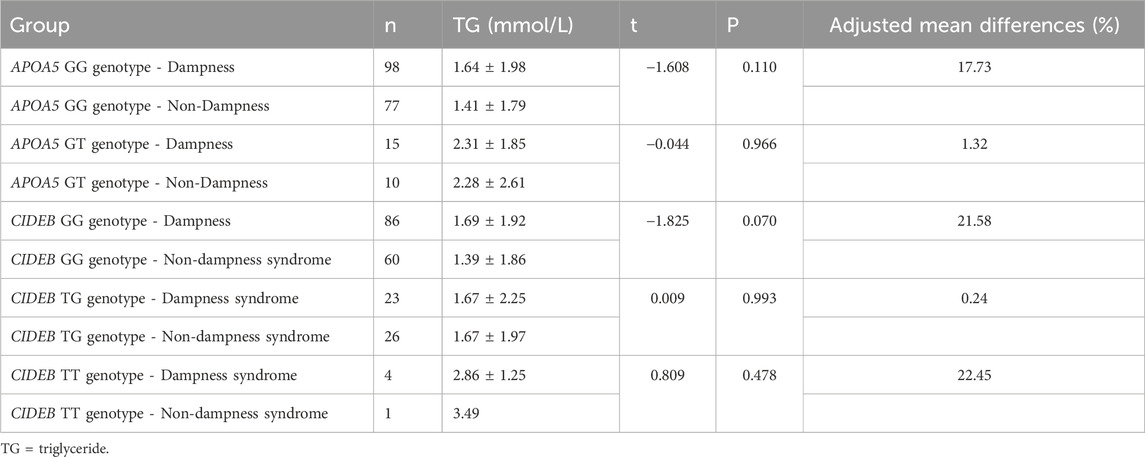
Table 4. Comparison of mean triglyceride levels among different genotypes in dampness syndrome and non-dampness syndrome populations.
Point-biserial correlation analysis of hypertriglyceridemia and related indicators in different groups
As shown in Table 5, in the overall population, HTG showed significant correlations with age, gender, body mass index, APOA5 rs2075291 genotype, and dampness syndrome (p < 0.05). In the dampness syndrome subgroup, HTG was significantly associated with gender and body mass index, whereas in the non-dampness syndrome subgroup, it was significantly correlated with gender, body mass index, and APOA5 rs2075291 genotype (p < 0.05 for all).
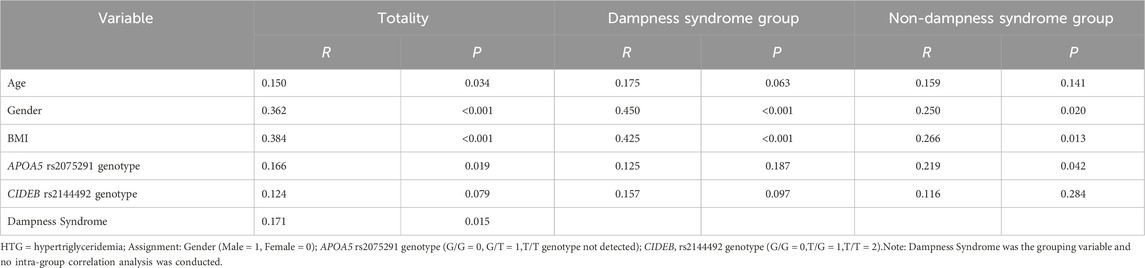
Table 5. Point-biserial Correlation Analysis of Hypertriglyceridemia and Related Indicators in different groups.
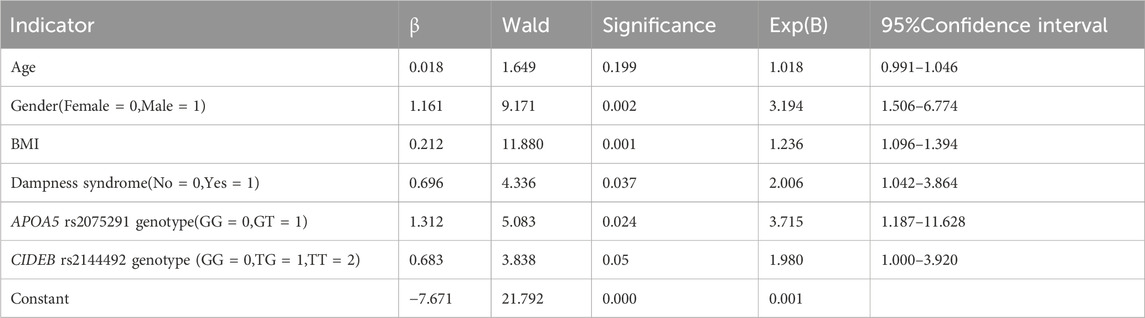
Binary logistic regression analysis of hypertriglyceridemia and related indicators
Variables were selected based on correlation analysis results and primary research objectives, with genotypes encoded additively. Binary multivariate logistic regression was performed, using HTG status (presence/absence) as the dependent variable and including dampness syndrome, APOA5 rs2075291 genotype (GG = 0, GT = 1), and CIDE-B rs2144492 genotype (GG = 0, TG = 1, TT = 2) as independent predictors. Collinearity assessment showed all tolerance values > 0.1 and max VIF = 1.310, indicating no multicollinearity. AUC values for variables (age = 0.586, gender = 0.670, APOA5 = 0.555, CIDEB = 0.556, BMI = 0.721, dampness syndrome = 0.585) all exceeded 0.5. The Hosmer-Lemeshow test (χ2 = 11.884, p = 0.156) indicated good model fit. Logistic regression identified male gender, higher BMI, dampness syndrome, and APOA5 rs2075291 genotype as independent HTG risk factors (p < 0.05), while CIDEB rs2144492 genotype trended toward significance (p = 0.05), as shown in Table 6.
Discussion
This study revealed that the genotype frequencies of APOA5 rs2075291 and CIDEB rs2144492 in the overall HTG group differed significantly from those in the control group. The pathogenesis of hypertriglyceridemia is influenced by both genetic and environmental factors. Single nucleotide polymorphism, the most prevalent form of genetic variation among individuals, refers to DNA sequence variations where a single nucleotide in a gene (or genome) differs among members of a biological species or within an individual’s paired chromosomes (Garelnabi et al., 2013). Previous studies have shown that secondary alleles of several common SNPs at the human ApoA5 gene locus are significantly associated with elevated plasma TG levels (Pennacchio et al., 2002; Kluger et al., 2008). Notably, the ApoA5 rs2075291 polymorphism has been found to be closely linked to TG levels in the Chinese population, but not in Caucasians (Kao et al., 2003; Hubácek et al., 2004). CIDEB influences gene expression across multiple metabolic pathways and signaling networks, including lipid droplet formation, adipogenesis, glycolysis, and gluconeogenesis (Xu et al., 2016; Gong et al., 2009; Chen et al., 2020). For instance, overexpression of CIDEB in goat mammary epithelial cells (GMECs) significantly upregulates genes involved in fatty acid synthesis, lipid droplet formation, and triacylglycerol (TAG) synthesis (He et al., 2024). Our findings confirm that polymorphisms at the APOA5 rs2075291 and CIDEB rs2144492 loci are associated with hypertriglyceridemia.
Previous studies have reported the impact of lipid regulators on SNPs. For example, interactions between dietary factors and SNPs within the ApoA1/ApoC3/ApoA4/ApoA5 gene cluster have been documented (Chen et al., 2009; Chien et al., 2009), suggesting that external factors may modulate the expression of gene polymorphisms. Dampness syndrome in traditional Chinese medicine represents a syndrome state developed in specific environmental contexts. According to TCM theory, this syndrome arises from both internal and external dampness pathogens. Earlier research (Zhang Bc, 2020) has shown that TG levels in patients with phlegm-dampness hyperlipidemia are significantly higher than those in other constitution groups. Our prior study (Zhou et al., 2024) further revealed a positive correlation between serum TG levels and the severity of dampness syndrome. Notably, the relationship between TCM dampness syndrome and gene polymorphisms and their combined effect on hypertriglyceridemia has not been previously reported.
This study investigates the association between APOA5 rs2075291 and CIDEB rs2144492 polymorphisms and HTG in a dampness syndrome population. Key findings include: in the dampness syndrome subgroup, the HTG group showed a higher count of CIDEB rs2144492 TG/TT genotypes than the control group, though the difference was not statistically significant (p = 0.146). Before FDR correction, the comparison of allele frequencies between the two groups showed a significant intergroup difference (p < 0.05), suggesting that CIDEB rs2144492 variants may enhance HTG susceptibility in individuals with dampness syndrome. This association was not observed in the non-dampness subgroup, implying a potential interaction between dampness syndrome and this gene locus in HTG pathogenesis. Correlation analysis revealed differential associations of HTG with APOA5 rs2075291 genotype between dampness and non-dampness groups. Multivariate logistic regression identified male gender, higher BMI, dampness syndrome, and APOA5 rs2075291 genotype as independent risk factors for HTG (p < 0.05), while CIDEB rs2144492 genotype trended toward significance (p = 0.05). Potential explanations for these findings include: 1. The associations of APOA5 rs2075291 or CIDEB rs2144492 with HTG may be modulated by TCM dampness syndrome; 2. The relatively small sample size may have limited the study’s statistical power to detect subtle associations; 3. HTG is a complex trait influenced by multiple genes (Steinhagen-Thiessen et al., 2017; Kypreos and Zannis, 2006; Guardiola and Ribalta, 2017; Xu et al., 2012) and environmental factors, contributing to heterogeneity in gene–disease associations.
This study reports for the first time the association between APOA5 rs2075291 and CIDEB rs2144492 polymorphisms and hypertriglyceridemia in a dampness syndrome population, offering critical insights for investigating triglyceride metabolism in TCM dampness syndrome cohorts. Baseline characteristic analysis showed significant group differences in gender distribution, BMI, and waist circumference between the observation and control groups. To minimize confounding by these baseline factors, gender and BMI were included as covariates in the multivariate logistic regression model. After covariate adjustment, the associations between APOA5 gene polymorphism, dampness syndrome, and HTG remained statistically significant.
Limitations
This study recruited participants from outpatient clinics, which may introduce selection bias. For example, outpatients differ from the general population in disease severity, treatment compliance, and help-seeking behaviors, potentially biasing the observed associations between APOA5/CIDEB polymorphisms and HTG. Additionally, the single-center outpatient sample limits external validity to populations with similar healthcare-seeking patterns and clinical profiles, hindering generalizability to non-visited or geographically distinct groups.
Post-hoc power analysis using expected MAF and observed ORs showed that with n = 200, the power was only ∼51% for APOA5 rs2075291 (MAF = 0.1, OR = 2.241) and ∼47.2% for CIDEB rs2144492 (MAF = 0.19, OR = 1.8)-both far below the 80% statistical benchmark. This indicates insufficient precision in effect estimation. For low-frequency variant association studies, small sample sizes may miss true effects or yield non-reproducible results due to random error. Study designs should pre-calculate sample size based on MAF and target OR to avoid unreliable conclusions. Future research could integrate community epidemiological surveys to comprehensively assess genotype-TCM dampness syndrome interactions in general populations.
Conclusion
This study confirms that APOA5 rs2075291 and CIDEB rs2144492 polymorphisms are associated with hypertriglyceridemia. Individuals with dampness syndrome carrying CIDEB rs2144492 variants may have an increased predisposition to HTG. These findings advance our understanding of the genetic underpinnings of HTG and may inform the development of personalized preventive strategies for at-risk populations.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://doi.org/10.17632/pcpg6pcs7f.1.
Ethics statement
The studies involving humans were approved by Ethics Committee of Guangzhou Cadre Health Management Centre. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
NL: Methodology, Writing – original draft. HZ: Resources, Visualization, Writing – review and editing. XaC: Investigation, Writing – review and editing, Data curation. SY: Data curation, Writing – review and editing, Investigation. XnC: Writing – review and editing, Resources, Visualization. GJ: Data curation, Investigation, Writing – review and editing. JY: Visualization, Resources, Writing – review and editing. JC: Formal Analysis, Validation, Writing – review and editing, Supervision. HZ: Supervision, Conceptualization, Writing – review and editing, Project administration, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was funded by the State Key Laboratory of Dampness Syndrome of Chinese Medicine Open Project, No. SZ2022KF19.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ajjemami, M., Ouatou, S., Charoute, H., Fakiri, M., Rhaissi, H., Benrahma, H., et al. (2015). Haplotype analysis of the apolipoprotein A5 gene in Moroccan patients with the metabolic syndrome. J. Diabetes Metab. Disord. 14, 29. doi:10.1186/s40200-015-0160-3
An, A. Y. S., Ma, Y. T., Xie, X., Yang, Y. n., Fu, Z. y., et al. (2011). Distributional characteristics of apolipoprotein A5 gene c.553G > T polymorphism and association with serum triglyceride in healthy Chinese Han and uighur people. Chin. Med. J. 91 (40), 2837–2840.
Chen, Y. J. G. B., and Huang, L. (2022). Three-year retrospective characteristic analysis of a 10,000-person natural person cohort in lingnan Region. J. Guangzhou Univ. Chin. Med. 39 (9), 1957–1963.
Chen, S. N., Cilingiroglu, M., Todd, J., Lombardi, R., Willerson, J. T., Gotto, A. M., et al. (2009). Candidate genetic analysis of plasma high-density lipoprotein-cholesterol and severity of coronary atherosclerosis. BMC Med. Genet. 10, 111. doi:10.1186/1471-2350-10-111
Chen, F. J., Yin, Y., and Chua, B. T. (2020). CIDE family proteins control lipid homeostasis and the development of metabolic diseases. Traffic 21 (1), 94–105. doi:10.1111/tra.12717
Chien, K. L., Hsu, H. C., Chen, Y. C., Su, T. C., Lee, Y. T., and Chen, M. F. (2009). Association between sequence variant of c.553 G > T in the apolipoprotein A5 gene and metabolic syndrome, insulin resistance, and carotid atherosclerosis. Transl. Res. 154 (3), 133–141. doi:10.1016/j.trsl.2009.06.005
Ding, Y., Zhu, M. A., Wang, Z. X., Zhu, J., Feng, J. B., and Li, D. S. (2012). Associations of polymorphisms in the apolipoprotein APOA1-C3-A5 gene cluster with acute coronary syndrome. J. Biomed. Biotechnol. 2012, 509420. doi:10.1155/2012/509420
Freiberg, J. J., Tybjaerg-Hansen, A., Jensen, J. S., and Nordestgaard, B. G. (2008). Nonfasting triglycerides and risk of ischemic stroke in the general population. Jama 300 (18), 2142–2152. doi:10.1001/jama.2008.621
Garelnabi, M., Lor, K., Jin, J., Chai, F., and Santanam, N. (2013). The paradox of ApoA5 modulation of triglycerides: evidence from clinical and basic research. Clin. Biochem. 46 (1-2), 12–19. doi:10.1016/j.clinbiochem.2012.09.007
Gong, J., Sun, Z., and Li, P. (2009). CIDE proteins and metabolic disorders. Curr. Opin. Lipidol. 20 (2), 121–126. doi:10.1097/MOL.0b013e328328d0bb
Guardiola, M., and Ribalta, J. (2017). Update on APOA5 genetics: toward a better understanding of its physiological impact. Curr. Atheroscler. Rep. 19 (7), 30. doi:10.1007/s11883-017-0665-y
He, Q., Yao, W., Wu, J., Xia, Y., Lei, Y., and Luo, J. (2024). Unveiling novel mechanism of CIDEB in Fatty acid synthesis through ChIP-Seq and functional analysis in dairy goat. Int. J. Mol. Sci. 25 (20), 11318. doi:10.3390/ijms252011318
Hubácek, J. A., Adámková, V., Ceska, R., Poledne, R., Horínek, A., and Vráblík, M. (2004). New variants in the apolipoprotein AV gene in individuals with extreme triglyceride levels. Physiol. Res. 53 (2), 225–228. doi:10.33549/physiolres.930546
Jørgensen, A. B., Frikke-Schmidt, R., West, A. S., Grande, P., Nordestgaard, B. G., and Tybjærg-Hansen, A. (2013). Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur. Heart J. 34 (24), 1826–1833. doi:10.1093/eurheartj/ehs431
Kao, J. T., Wen, H. C., Chien, K. L., Hsu, H. C., and Lin, S. W. (2003). A novel genetic variant in the apolipoprotein A5 gene is associated with hypertriglyceridemia. Hum. Mol. Genet. 12 (19), 2533–2539. doi:10.1093/hmg/ddg255
Kluger, M., Heeren, J., and Merkel, M. (2008). Apoprotein A-V: an important regulator of triglyceride metabolism. J. Inherit. Metab. Dis. 31 (2), 281–288. doi:10.1007/s10545-008-0863-4
Kypreos, K. E., and Zannis, V. I. (2006). LDL receptor deficiency or apoE mutations prevent remnant clearance and induce hypertriglyceridemia in mice. J. Lipid Res. 47 (3), 521–529. doi:10.1194/jlr.M500322-JLR200
Lajnaf, R., Feki, S., Ben Ameur, S., Attia, H., Kammoun, T., Ayadi, M. A., et al. (2023). Recent advances in selective allergies to Mammalian milk proteins not associated with Cow's milk proteins allergy. Food Chem. Toxicol. 178, 113929. doi:10.1016/j.fct.2023.113929
Li, J. Z., Lei, Y., Wang, Y., Zhang, Y., Ye, J., Xia, X., et al. (2010). Control of cholesterol biosynthesis, uptake and storage in hepatocytes by cideb. Biochim. Biophys. Acta 1801 (5), 577–586. doi:10.1016/j.bbalip.2010.01.012
Liu, L. P. Z. G., and Zhan, F. F. (2016). Study on the association between CIDEB/C gene polymorphism and hypertriglyceridemia. Chongqing Med. 45 (15), 2061–2064.
Liu, Y., Dai, S., Qin, S., Zhou, J., Wang, Z., and Yin, G. (2024). The pathogenic mutations of APOA5 in Chinese patients with hyperlipidemic acute pancreatitis. Lipids Health Dis. 23 (1), 44. doi:10.1186/s12944-024-02011-5
Lu Ty, X. Q., and Cai, J. X. (2021). Construction and preliminary optimization of the assessment scale for dampness syndrome in traditional Chinese medicine. J. Traditional Chin. Med. 62 (19), 1677–1683.
Ng, S. W. K., Rouhani, F. J., Brunner, S. F., Brzozowska, N., Aitken, S. J., Yang, M., et al. (2021). Convergent somatic mutations in metabolism genes in chronic liver disease. Nature 598 (7881), 473–478. doi:10.1038/s41586-021-03974-6
Nordestgaard, B. G., and Varbo, A. (2014). Triglycerides and cardiovascular disease. Lancet 384 (9943), 626–635. doi:10.1016/S0140-6736(14)61177-6
Park, H. H. (2015). Structural insight into CIDE domains: the janus face of CIDEs. Apoptosis 20 (2), 240–249. doi:10.1007/s10495-014-1067-z
Pennacchio, L. A., Olivier, M., Hubacek, J. A., Krauss, R. M., Rubin, E. M., and Cohen, J. C. (2002). Two independent apolipoprotein A5 haplotypes influence human plasma triglyceride levels. Hum. Mol. Genet. 11 (24), 3031–3038. doi:10.1093/hmg/11.24.3031
Ping, Z., Guo, Z., Lu, M., Chen, Y., and Liu, L. (2022). Association of CIDEB gene promoter methylation with overweight or obesity in adults. Aging (Albany NY) 14 (8), 3607–3616. doi:10.18632/aging.204032
Srivastava, R. K., Singh, P., Verma, P., Sethi, R., Verma, A., Ali, W., et al. (2015). Influence of APOA5 (rs662799 and rs3135506) gene polymorphism in acute myocardial infarction patients and its association with basic coronary artery disease risk factors. J. Appl. Pharm. Sci. 5 (6), 008–014. doi:10.7324/japs.2015.50602
Steinhagen-Thiessen, E., Stroes, E., Soran, H., Johnson, C., Moulin, P., Iotti, G., et al. (2017). The role of registries in rare genetic lipid disorders: review and introduction of the first global registry in lipoprotein lipase deficiency. Atherosclerosis 262, 146–153. doi:10.1016/j.atherosclerosis.2016.08.023
Su, X., Kong, Y., and Peng, D. Q. (2018). New insights into apolipoprotein A5 in controlling lipoprotein metabolism in obesity and the metabolic syndrome patients. Lipids Health Dis. 17 (1), 174. doi:10.1186/s12944-018-0833-2
Wang Zw, G. Y. (2024). Chinese lipid management guidelines primary edition. Chin. J. Circulation 39 (04), 313–321.
Xie, K. L. Y. (2023). Multidisciplinary expert consensus on clinical management of hypertriglyceridemia. Chin. J. Circulation 38 (06), 621–633.
Xu, L., Zhou, L., and Li, P. (2012). CIDE proteins and lipid metabolism. Arterioscler. Thromb. Vasc. Biol. 32 (5), 1094–1098. doi:10.1161/ATVBAHA.111.241489
Xu, W., Wu, L., Yu, M., Chen, F. J., Arshad, M., Xia, X., et al. (2016). Differential roles of cell death-inducing DNA fragmentation Factor-α-like effector (CIDE) proteins in promoting lipid droplet fusion and growth in subpopulations of hepatocytes. J. Biol. Chem. 291 (9), 4282–4293. doi:10.1074/jbc.M115.701094
Xu Yn, H. Y., and Pan, H. C. (2022). Research progress on genetic polymorphism of hypertriglyceridemia and its relationship with gut microbiota. J. Inn. Mong. Minzu Univ. Nat. Sci. Ed. 37 (05), 418–424.
Zafar, U., Khaliq, S., and Lone, K. P. (2019). Genetic association of apolipoprotein A5-1131T>C polymorphism with traits of metabolic syndrome. J. Coll. Physicians Surg. Pak 29 (7), 626–630. doi:10.29271/jcpsp.2019.07.626
Zhang Bc, L. Q. (2020). Research on the correlation between lipid levels and TCM constitution identification and classification in hyperlipidemia. Yunnan J. Traditional Chin. Med. Materia Medica 41 (6), 21–22.
Zhang, L. J., Wang, C., Yuan, Y., Wang, H., Wu, J., Liu, F., et al. (2014). Cideb facilitates the lipidation of chylomicrons in the small intestine. J. Lipid Res. 55 (7), 1279–1287. doi:10.1194/jlr.M046482
Zhou, H., Zhang, W., Cai, X., Yang, S., Liu, A., Zhou, X., et al. (2024). Unraveling the link between hypertriglyceridemia, dampness syndrome, and chronic diseases: a comprehensive observational study. Med. Baltim. 103 (33), e39207. doi:10.1097/MD.0000000000039207
Keywords: hypertriglyceridemia, dampness syndrome, APOA5 gene, CIDEB gene, singlenucleotide polymorphisms
Citation: Liu N, Zeng H, Cai X, Yang S, Chen X, Jiang G, Yuan J, Cai J and Zhou H (2025) Association of APOA5 rs2075291 and CIDEB rs2144492 polymorphisms with hypertriglyceridemia in individuals with traditional Chinese medicine dampness syndrome: a case-control study. Front. Genet. 16:1654501. doi: 10.3389/fgene.2025.1654501
Received: 01 August 2025; Accepted: 29 September 2025;
Published: 17 October 2025.
Edited by:
Nader Al-Dewik, Hamad Medical Corporation, QatarReviewed by:
M. Walid Qoronfleh, Q3 Research Institute, United StatesBalasubramani Gattu Linga, Department of Medicine, Saudi Arabia
Copyright © 2025 Liu, Zeng, Cai, Yang, Chen, Jiang, Yuan, Cai and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Zhou, emhvdWh1aV9qa3p4QDE2My5jb20=; Jianxiong Cai, bGFjdXM4MjZAZ3p1Y20uZWR1LmNu
 Na Liu1
Na Liu1 Hongli Zeng
Hongli Zeng Xiangsheng Cai
Xiangsheng Cai Hui Zhou
Hui Zhou