- 1Institute for Advanced Study, Chengdu University, Chengdu, Sichuan, China
- 2Key Laboratory of Coarse Cereal Processing, Ministry of Agriculture and Rural Affairs, School of Food and Biological Engineering, Chengdu University, Chengdu, Sichuan, China
- 3School of Modern Agriculture, Meishan Vocational and Technical College, Meishan, Sichuan, China
- 4Office of Academic Affairs, Chengdu University, Chengdu, Sichuan, China
Drought stress poses a significant threat to maize productivity, highlighting the need to elucidate molecular mechanisms underlying drought tolerance. Previous studies identified ZmLBD33 as a regulator of drought stress responses that interacts with the cell wall-loosening gene ZmEXPB7. To elucidate the function of ZmEXPB7 in drought tolerance, we conducted heterologous expression studies in Arabidopsis. The results demonstrated that ZmEXPB7 expression was rapidly induced under PEG6000-simulated stress, reaching peak levels within 1 h. Overexpression of ZmEXPB7 in Arabidopsis significantly enhanced drought tolerance, improved root growth, and increased survival rates under osmotic and soil drought conditions. Transgenic plants exhibited reduced water loss, decreased stomatal density, and enhanced stomatal closure. DAB and NBT staining demonstrated that the ROS accumulated in ZmEXPB7-overexpressed Arabidopsis. A physiological index assay also revealed that SOD and POD activities in ZmEXPB7-overexpressed Arabidopsis were lower than those in wild-type Arabidopsis. These findings indicate that ZmEXPB7 positively regulates drought tolerance by modulating stomatal aperture and H2O2 signaling. This study highlights the crucial role of expansin genes in stress adaptation and positions ZmEXPB7 as a potential target for engineering drought-resilient crops.
1 Introduction
Global maize-growing regions are facing increasing threats from drought stress, which has severely impacted maize yields. Abnormal droughts have caused significant yield reductions in America, particularly in areas with light-textured soils (Lobell et al., 2013). In Thailand, climate change has led to prolonged droughts, significantly reducing the suitability of the region for maize cultivation (Amnuaylojaroen et al., 2021). Drought impacts in China’s major maize-producing areas exhibit distinct regional and temporal characteristics. In Northeast China, the limited precipitation during the growing season, with a pronounced concentration in the latter phenological stages, has significantly impaired maize growth and development (Wan et al., 2022). In the Huang-Huai-Hai region, drought conditions have worsened, with precipitation from May 2024 onward being 70% below the annual average. In core production areas such as central Hebei, northern Henan, and southwestern Shandong, approximately 63% of farmland had relative soil water content below 55% in the 0–40 cm soil layer (classified as moderate to severe drought), severely impeding sowing and seedling emergence (Yanping, 2025). The FAO notes that the frequency of such drought events has increased by 34% over the past decade, directly threatening national food security (FAO, 2023).
Drought stress significantly and multifacetedly affects maize growth, development, and yield. First, drought stress directly inhibits maize morphogenesis. Drought leads to reduced plant height, decreased leaf area, and diminished dry matter accumulation. It also delays the development of tassels and ears, prolongs the anthesis-silking interval (ASI), impairs pollination and fertilization, reduces floret differentiation, and increases kernel abortion, ultimately lowering kernel number per ear (Yang et al., 2018; Guo et al., 2021; Wang et al., 2019). Second, drought disrupts maize water balance and photosynthesis, triggering stomatal closure to reduce transpiration while simultaneously lowering photosynthetic efficiency (Tuzet et al., 2010; Mura and Vangimalla, 2020). The accumulation of reactive oxygen species (ROS) exacerbates membrane lipid peroxidation, increases malondialdehyde (MDA) content, and causes abnormal activity of antioxidant enzymes such as superoxide dismutase (SOD) and peroxidase (POD), further damaging cell structures (Faize et al., 2011). During drought stress responses, abscisic acid (ABA) and hydrogen peroxide (H2O2) serve as critical signaling molecules, with their levels regulated by transcription factors (Zhu, 2016; Kubila et al., 2017; Miller et al., 2010). Drought-induced changes in ABA activate receptors on guard cell membranes, catalyzing H2O2 production via nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which in turn activates calcium channels and triggers protein kinase cascades, further regulating anion channels to induce cell water loss and stomatal closure (Kwak et al., 2003; Pei et al., 2000; Zhang et al., 2001).
Expansin protein family represents a crucial group of cell wall-modifying agents that facilitate acid growth through targeted cleavage of hydrogen bonds linking matrix polysaccharides. These proteins are widely present in plants and feature highly conserved domains, including an N-terminal signal peptide, a catalytic domain (DPBB_1, Domain A), and a C-terminal pollen allergen domain (Domain B), with Domain B potentially involved in substrate recognition (Cosgrove, 2000a; Yennawar et al., 2006). Based on sequence homology and phylogenetic relationships, the expansin family is divided into four subfamilies: EXPA (α-expansin), EXPB (β-expansin), EXLA, and EXLB, with EXPA and EXPB being widely present and functionally well-characterized in plants (Kende et al., 2004). Genomic studies reveal significant interspecies variation in the number of Expansin genes: 93 Expansin genes have been identified in maize, including 40 EXPA, 47 EXPB, and 6 EXLA genes, with no EXLB genes detected. These genes are predominantly clustered on chromosomes, with their expansion primarily driven by segmental and tandem duplication events (Jiang et al., 2018). The promoters of Expansin genes are enriched with hormone-responsive elements (e.g., ABA-responsive ABRE and auxin-responsive TGA-element) and stress-responsive elements (e.g., MYB-binding sites), suggesting their involvement in transcriptional regulatory networks governing development and stress responses (Sampedro and Cosgrove, 2005).
Expansin genes exhibit diverse functions in plants, primarily participating in vegetative organs development, pollen tube growth, seed germination, and fruit development, while also playing important roles in responses to biotic and abiotic stresses. Expansin genes promote cell elongation and division by facilitating cell wall loosening: AtEXPA1 influences asymmetric division of pericycle cells and radial expansion of lateral root primordia, thereby affecting lateral root development in Arabidopsis (Ramakrishna et al., 2019). In Arabidopsis, AtLBD18/AtASL20 directly binds to the EXPANSINA14 (AtEXPA14) promoter to activate its expression and promote lateral root growth (Lee and Kim, 2013). In rice, OsEXPA10 expression in root tips is essential for root cell elongation, and its absence impedes root growth (Che et al., 2016). Heterologous expression of ClEXPA1 and ClEXPA2 in tobacco increases pith parenchyma cell size and stem thickness, indirectly affecting cellulose metabolism (Wang et al., 2011). OsEXPA7 influences yield and quality by participating in the jasmonic acid metabolic pathway (Zhang et al., 2024). TaEXPA6 affects grain weight and increases yield (Vicentin et al., 2023). SlEXP1 synergizes with endoglucanase SlCEL2 to promote fruit softening by enhancing cell wall disassembly (Su et al., 2024). In maize, ZmEXPB15 regulates kernel size and weight by coordinating nucellus degradation and early endosperm development, with its expression controlled by transcription factors ZmNAC11 and ZmNAC29 (Sun et al., 2022).
Additionally, Expansin plays significant role in biotic and abiotic stress responses. AtEXPA3, AtEXPA6, AtEXPA8, AtEXPA10, and AtEXPA16 participate in nematode-induced syncytium formation in roots (Wieczorek et al., 2006). OsEXPA10 has dual functions: it regulates growth while also participating in biotic stress responses. Overexpression of OsEXPA10 promotes rice growth but increases susceptibility to brown planthopper infestation and rice blast, whereas knockdown reduces plant height and grain size while enhancing resistance to pests (Tan et al., 2018). In soybean, GmEXPA11, which is transcriptionally regulated by GmPTF1, interacts with GmNOD20 to synergistically promote nodule enlargement and enhance nitrogen fixation efficiency (Xing et al., 2025). GmEXPB2 increases root cortex cell proliferation and expansion, stimulates root hair formation and nodule development, and optimizes root system architecture, thereby enhancing phosphorus acquisition efficiency in soybean (Yang et al., 2021). TaEXPB23 enhances salt tolerance by improving water retention and lowering osmotic potential in tobacco (Han et al., 2012). TaEXPA2 interacts with TaMPS to stimulate lateral root formation, increase cellular water retention, and mitigate oxidative stress by reducing ROS levels, ultimately conferring drought tolerance in wheat (Yang et al., 2020). In maize, overexpression of ZmEXPA4 and ZmEXPA5 alleviates drought-induced increases in the anthesis-silking interval and improves yield (Liu et al., 2021; Tao et al., 2023).
Our previous studies identified ZmLBD33 as a regulator of drought stress responses that interacts with the cell wall-loosening protein ZmEXPB7. To elucidate the function of ZmEXPB7 in drought tolerance, we conducted heterologous expression studies in Arabidopsis. Our results demonstrated that ZmEXPB7 expression was rapidly induced under PEG6000 stress, reaching peak levels within 1 h. Overexpressing ZmEXPB7 seedlings displayed significantly drought tolerance in Arabidopsis, which correlated with enhanced root system architecture - including increased root length, surface area, and tip number - facilitating more efficient soil water exploration. The transgenic seedlings also exhibited reduced stomatal density and aperture, resulting in decreased water loss rates. These findings position ZmEXPB7 as a dual-function regulator that modulates both root morphology and stomatal behavior to enhance drought tolerance. Our study provides mechanistic insights into expansin-mediated stress adaptation and identifies ZmEXPB7 as a promising target for developing drought-resistant maize cultivars.
2 Materials and methods
2.1 Expression levels of ZmEXP7 under drought stress
To investigate the role of ZmEXP7 under drought stress, maize seedlings at the one-leaf stage were transplanted into Hoagland nutrient solution and cultured until the three-leaf stage. The seedlings were then subjected to drought stress by treating with 20% (w/v) PEG6000 dissolved directly in the culture solution (Li et al., 2017; Ma et al., 2018). Roots were collected at 0 h, 1 h, 3 h, 6 h, 12 h, 24 h, and 48 h after PEG6000 treatment, immediately frozen in liquid nitrogen, and stored at −80 °C. All samples were collected at the terminal time point. Uniformly grown, healthy maize seedlings were selected for sampling. Each experiment included at least three biological replicates.
2.2 RNA extraction and quantitative real-time PCR
Total RNA was extracted following the instructions of the Plant Total RNA Isolation Kit (FOREGENE, RE-05014). Specific primers for ZmEXPB7 were designed to span intronic regions, based on the B73 reference genome. Quantitative real-time PCR (RT-PCR) was performed using Zme1F1α and Zm18S as internal reference genes. The reaction system was prepared according to the SYBR Green Fast qPCR Mix kit (ABclonal, RM21203) manual, and RT-PCR amplification was conducted on a Bio-Rad CFX96 PCR instrument following the protocol. The experimental results were analyzed using the 2−ΔΔCT method. All experiments included three biological replicates.
2.3 Drought experiments with ZmEXPB7-Overexpressing Arabidopsis
The ZmEXPB7 coding sequence was cloned into the pBI121-mcherry expression vector to generate the ZmEXPB7-mcherry vector. The ZmEXPB7 gene was overexpressed in wild-type Arabidopsis using the floral dip method. After obtaining homozygous seeds, seedlings with high expression levels were selected for phenotypic characterization experiments.
Sterilized seeds of the overexpressing Arabidopsis homozygous lines and wild-type were sown on 0, 200, 250, 300 mM mannitol medium. After vernalization at 4 °C for 72 h, seeds were transferred to short-day conditions for growth.
2.3.1 Germination rate
Radicle emergence was recorded as germination, and the number of germinated seeds for each genotype was counted until no further changes were observed per 12 h under each stress condition.
2.3.2 Survival rate
After germination 5 days, seedlings were scored for greening under different stress treatments. Albino seedlings and ungerminated seeds were classified as dead, while green seedlings were counted as survival.
2.3.3 Mannitol stress assay
After 5 days of growth on normal medium, well-grown and uniform lines were transplanted to freshly prepared 0, 200 mM, 250 mM, 300 mM mannitol medium for vertical culture. After 7 days, differences between genotypes were observed, photographed, and root images were acquired using an EPSON 11000XL root scanner. Root traits were analyzed using WinRhizo Pro2013 software.
2.3.4 Drought stress assay
Seven-day-old seedlings grown on normal medium were transplanted into soil. After 1 month of growth in soil, the growth of different genotypes under normal conditions was photographed and recorded. Seedlings from different genotypes were subjected to a 7-day water-withholding period, after which clear wilting symptoms were recorded. Survival rates were quantified after 3 days of rewatering.
2.4 Water loss rate measurement in Arabidopsis
The fresh weight of the aerial parts was measured using one-month-old Arabidopsis seedlings. The seedlings were then placed on a laboratory bench, and their weights were recorded at predetermined time points (0.5 h, 1 h, 2 h, 3 h). The rate of water loss was determined at designated time intervals using the following equation: water loss rate (%) = [(W0 - Wt)/W0] × 100.
2.5 Stomatal aperture measurement in Arabidopsis
The middle portion of the fourth leaf from one-month-old Arabidopsis plants was placed on a laboratory bench and dehydrated for 1 h. Both normal and dehydrated Arabidopsis leaves were fixed in Carnoy’s fixative (absolute ethanol: glacial acetic acid = 3:1) for 24 h. The leaves were then dehydrated sequentially in 30%, 50%, 70%, 80%, 85%, 90%, 95%, and 100% ethanol for 30 min each. The dehydrated leaves were placed in clearing solution (chloral hydrate: water: glycerol = 8:3:1) until transparent. Stomata were observed under a light microscope, and their length and width were measured using ImageJ. Stomatal aperture was expressed as the ratio of stomatal width to length.
2.6 NBT and DAB staining
Arabidopsis leaves were immersed in NBT staining solution (0.01 g NBT powder dissolved in 10 mL of 50 mM phosphate buffer, pH 7.8) or DAB staining solution (0.1% DAB in HCl solution, pH 3.8, protected from light). After vacuum infiltration for 30 min, the leaves were kept in the dark at 22 °C for 10 h. The leaves were then decolorized in decolorizing solution (acetic acid: glycerol: ethanol = 1:1:3) by boiling for 5 min and stored in 95% ethanol for slide preparation and observation.
2.7 H2O2 content measurement
Fresh tissue (0.1 g) was flash-frozen in liquid nitrogen and ground to a powder. After adding 1 mL of 0.1% TCA and mixing thoroughly, the sample was centrifuged at 4 °C and 12,000 r/min for 15 min. Then, 500 µL of the supernatant was mixed with an equal volume of PBS buffer and 1 mL of 1 M KI solution. The mixture was incubated at 30 °C in the dark with shaking (150 r/min) for 1 h, and the absorbance was measured at 390 nm. A standard curve was prepared using 300 μmol/L H2O2 as the stock solution. H2O2 content (µmol/g FW) was calculated using: C × Vt/(FW × V1). Measurements were performed with three biological replicates, each containing three technical replicates.
2.8 Antioxidant enzyme activity assay
2.8.1 Enzyme extraction
Fresh leaf samples (0.1 g) were homogenized in liquid nitrogen and extracted with 1 mL of ice-cold 50 mM phosphate buffer (pH 7.8) containing 1% PVP, 2 mM DTT, and 0.1 mM EDTA. After incubation on ice for 10 min, the homogenate was centrifuged at 12,000 × g for 15 min at 4 °C, and the supernatant was used for enzyme assays.
2.8.2 Superoxide dismutase (SOD) activity
The reaction mixture (3 mL) contained 50 mM phosphate buffer (pH 7.8), 130 mM methionine, 750 μM NBT, 100 μM EDTA-Na2, 20 μM riboflavin, and 50 μL enzyme extract. One control tube was wrapped in aluminum foil and kept in the dark throughout the experiment, while another and the sample tubes were illuminated at an intensity of 4,000 lx for 20 min using fluorescent lamps. Absorbance was measured at 560 nm. One unit (U) of SOD activity was defined as the amount of enzyme required to inhibit 50% of the photochemical reduction of NBT.
2.8.3 Peroxidase (POD) activity
The reaction mixture (3 mL) contained 0.3% (v/v) H2O2, 0.2% (v/v) guaiacol, 50 mM phosphate-buffered saline (PBS, pH 7.0), and 50 μL of enzyme extract. The reaction was initiated by adding the enzyme extract, and the increase in absorbance at 470 nm was recorded every 30 s for 5 min at 25 °C. One unit (U) of POD activity was defined as an increase of 0.01 in absorbance per minute under the assay conditions.
2.8.4 Catalase (CAT) activity
The reaction mixture (3 mL) contained 0.3% (v/v) H2O2 in 50 mM phosphate buffer (pH 7.0) and 50 μL of enzyme extract. The decrease in absorbance at 240 nm was monitored at 25 °C for 3 min, with readings taken at 30-s intervals. One unit (U) of CAT activity was defined as a decrease of 0.1 in absorbance per minute.
Enzyme activities were expressed as U/(g min) (POD, CAT) or U/g (SOD) based on fresh weight. All assays were performed with three biological and technical replicates.
2.9 Yeast two-hybrid assay
The ZmEXPB7 coding sequence was cloned into the pGBKT7 vector (EXPB7-BD) and the full-length fragment of ZmLBD33 was cloned into the pGADT7 vector (LBD33-AD). Yeast cells harboring the ZmEXPB7-BD and ZmLBD33-AD, or ZmEXPB7-BD and pGADT7, or ZmLBD33-AD and pGBKT7 were respectively diluted to three concentrations and grown on nonselective (SD/-Trp/-Leu) or selective (SD/-Trp/-Leu/-His/-Ade) medium. A pGBKT7-53 and pGADT7-T combination was used as a positive control. A pGBKT7-Lam and pGADT7-T combination was used as a negative control.
2.10 Bimolecular fluorescence complementation (BiFC) assay
The ZmEXPB7 coding sequence was cloned into the pXYc104 vector (yielding ZmEXPB7-104, containing the C-terminal fragment of the fluorescent protein), while the ZmLBD33 gene was inserted into the pXYn106 vector (producing ZmLBD33-106, harboring the N-terminal fragment). For transient co-expression, both constructs were introduced into Agrobacterium tumefaciens strain GV3101 and co-infiltrated into Nicotiana benthamiana leaves. Following 36–48 h of incubation under controlled conditions, reconstituted fluorescence was visualized using confocal laser scanning microscopy.
2.11 Co-immunoprecipitation (Co-IP)
Agrobacterium tumefaciens strains harboring either ZmEXPB7-mCherry or ZmLBD33-eGFP/pCAMBIA2300 (control) plasmids were mixed (1:1) and co-infiltrated into Nicotiana benthamiana leaves. After 36 h, infiltrated leaf tissues were flash-frozen, homogenized in ice-cold extraction buffer (50 mM Tris-HCl pH 7.9, 120 mM NaCl, 5 mM EDTA, 1 mM PMSF, 10 mM DTT, 0.1% NP-40, protease inhibitors), and centrifuged (12,000 × g, 15 min, 4 °C). The supernatant was incubated with anti-GFP magnetic beads (Abclonal, AE079) for 2 h at 4 °C. Beads were washed, resuspended in 2× SDS loading buffer, and boiled (95 °C, 15 min). Input and immunoprecipitated samples were analyzed by immunoblotting using anti-GFP (Abcam, ab290) and anti-mCherry (ImmunoWay, YM3212) antibodies.
2.12 Western blot analysis
Proteins were separated by SDS-PAGE using a 10% separating gel, followed by electrophoretic transfer onto a methanol-activated PVDF membrane using a semi-dry transfer system. The membrane was blocked with 5% skim milk for 2 h at room temperature, then incubated overnight at 4 °C with primary antibody (diluted as recommended). After TBST washes, HRP-conjugated secondary antibody (1:10,000) was applied for 2 h at room temperature. Protein bands were visualized using ECL substrate and chemiluminescence detection after rigorous TBST washing steps.
3 Results
3.1 Interaction between ZmLBD33 and ZmEXPB7 proteins
Yeast library screening revealed that ZmEXPB7 is an interacting protein of ZmLBD33. To validate whether ZmEXPB7 indeed interacts with ZmLBD33, ZmEXPB7-BD was co-transformed with ZmLBD33-AD or the pGADT7 empty vector into yeast Y2HGold strain. On double dropout (-Trp/-Leu) medium, the growth status of the co-transformants was similar to that of the positive and negative controls. On quadruple dropout (-Trp/-Leu/-His/-Ade) medium, yeast strains co-transformed with ZmEXPB7-BD and ZmLBD33-AD activated the expression of the downstream reporter genes His and Ade, growing normally like the positive control. This confirmed the interaction between ZmEXPB7 and ZmLBD33 (Figure 1A).
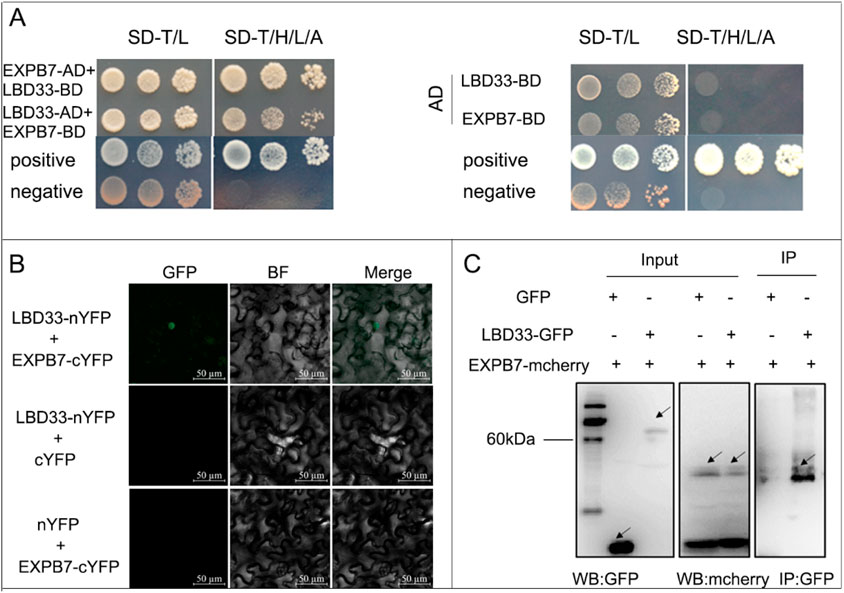
Figure 1. Interaction between the ZmLBD33 and ZmEXPB7. (A) ZmLBD33 and ZmEXPB7 interactions in the yeast two-hybrid assay. Yeast cells co-transformed with ZmLBD33-AD and ZmEXPB7-BD were grown on SD/–Trp/–His/–Leu/Ade medium for 5 days at 30 °C. (B) Bimolecular fluorescence complementation (BiFC) assay confirming the interaction between ZmEXPB33 and ZmEXPB7 in Nicotiana benthamiana leaves. YFP fluorescence was observed 48 h after agroinfiltration. Scale bar = 50 µm. (C) Co-immunoprecipitation (Co-IP) assay detecting the interaction between ZmLBD33 and ZmEXPB7 in vivo. Proteins were extracted from leaves expressing ZmLBD33-GFP and ZmEXPB7-mcherry and immunoprecipitated with anti-GFP beads, followed by immunoblotting using anti-mcherry antibody.
To validate the interaction between ZmLBD33 and ZmEXPB7 in plant, the ZmEXPB7-cYFP construct was co-infiltrated with Agrobacterium carrying ZmLBD33-nYFP into tobacco leaves at a 1:1 ratio. After 36 h, green fluorescence signals were observed under a microscope. Fluorescence signals were detected in the nuclei of tobacco leaf cells co-expressing ZmLBD33-nYFP and ZmEXPB7-cYFP (Figure 1B). In contrast, no fluorescence was observed when ZmLBD33-nYFP was co-infiltrated with cYFP or nYFP was co-infiltrated with ZmEXPB7-cYFP. The BiFC assay demonstrated that ZmLBD33 interacts with ZmEXPB7 proteins in planta, and the interaction occurs in the nucleus.
To further confirm the interaction between ZmLBD33 and ZmEXPB7 in living plant cells, the ZmEXPB7-mcherry construct was transformed into Agrobacterium and co-infiltrated with ZmLBD33-eGFP Agrobacterium at a 1:1 ratio. A negative control was prepared by co-infiltrating pCAMBIA2300-eGFP with ZmEXPB7-mcherry. After 36 h of infiltration, fluorescence was observed, and samples were collected for protein extraction under non-denaturing conditions. Co-immunoprecipitation was performed using GFP antibody, followed by Western blot detection with mcherry and GFP antibodies. As shown in the figure (Figure 1C), mcherry antibody detected bands corresponding to ZmEXPB7-mcherry, and GFP antibody detected bands corresponding to GFP and ZmLBD33-GFP, confirming successful expression of both proteins. In the samples immunoprecipitated with GFP antibody-conjugated beads, mcherry antibody detected ZmEXPB7-mcherry only in the ZmLBD33-GFP sample, with no bands detected in the pCAMBIA2300-GFP negative control, ruling out nonspecific binding between ZmEXPB7-mcherry and GFP. These results further demonstrate the interaction between ZmLBD33 and ZmEXPB7 in planta.
3.2 Expression pattern analysis of ZmEXPB7
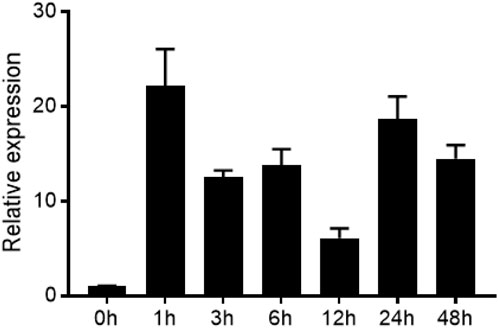
Given the established protein-protein interaction between ZmEXPB7 and ZmLBD33, we sought to determine whether these proteins exhibit functional conservation in their biological roles. Using qRT-PCR with Zme1F1α and Zm18S as the internal reference, we analyzed the expression pattern of ZmEXPB7 under PEG6000-simulated drought stress at different time points. ZmEXPB7 expression was significantly induced under PEG6000 stress, peaking at 1 h post-treatment (Figure 2), indicating its responsiveness to osmotic stress.
Expression levels of RNA transcripts were quantified under PEG6000 treatment using quantitative real-time PCR, with Zme1F1α and Zm18S as internal reference genes. Data were analyzed via the 2−ΔΔCT method and are presented as means ± SD (n = 3).
3.3 Drought resistance of ZmEXPB7-Overexpressing Arabidopsis
The ZmEXPB7-mcherry expression vector was transformed into wild-type Arabidopsis via floral dip method. Kanamycin screening identified 12 positive transformants. RNA was extracted to screen for high-expression lines, and three lines with high expression levels (OE2, OE4, OE8) were chosen for further studies (Figure 3A).
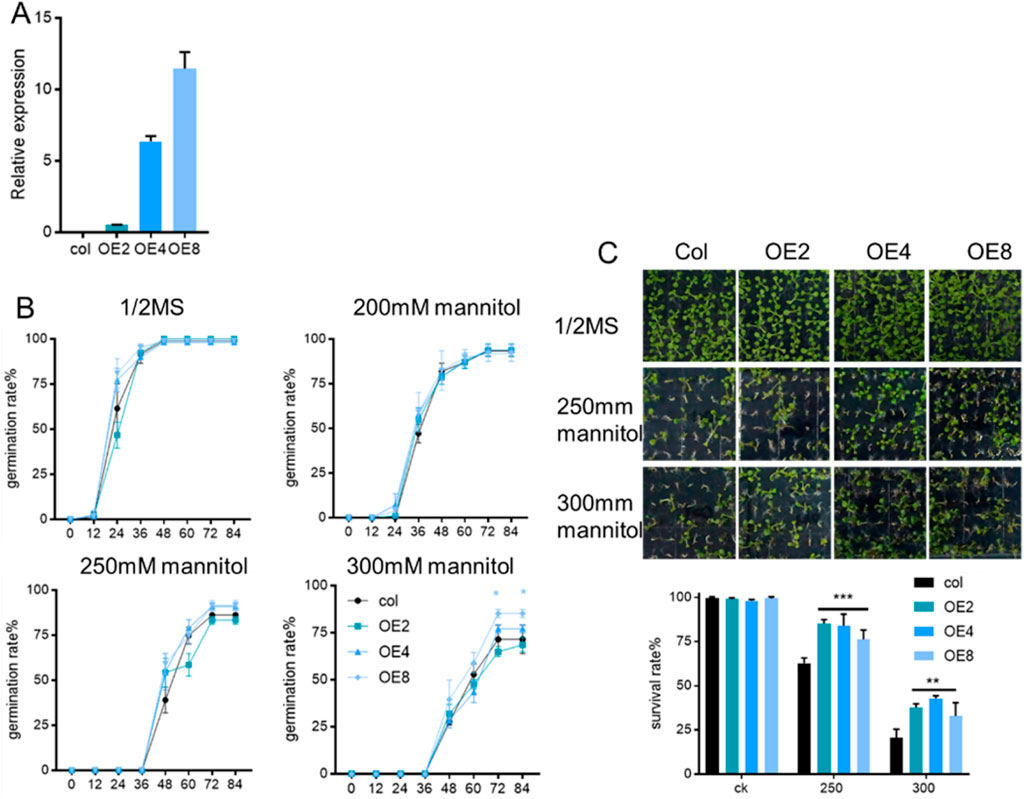
Figure 3. Germination and survival rate of ZmEXPB7 overexpressing Arabidopsis under different concentration mannitol. (A) Expression level of ZmEXPB7 overexpressed Arabidopsis lines; (B) Germination rate of ZmEXPB7 overexpression Arabidopsis under different concentration mannitol; (C) Survival rate of ZmEXPB7 overexpression Arabidopsis under different concentration mannitol. Each experiment had three repeats, each repeat used 70 seeds. All data represent means ± SD. Asterisks represented the significance compare to wild type by using one-way ANOVA, *, p < 0.05, **, p < 0.01, ***, p < 0.001.
We investigated the resistance of ZmEXPB7-overexpressing and wild-type Arabidopsis to mannitol-induced osmotic stress during seed germination and seedling stages. Elevated mannitol concentrations induced a progressive inhibition of Arabidopsis thaliana seed germination, resulting in a statistically significant delay in germination (Figure 3B). The germination rates of ZmEXPB7-overexpressing and wild-type Arabidopsis showed no significant differences on normal 1/2 MS medium or 1/2MS + 200 mM mannitol medium. However, on 1/2MS + 250 mM and 300 mM mannitol media, ZmEXPB7-overexpressing Arabidopsis exhibited higher germination rates than wild-type seedlings, although only OE8 reached statistical significance (Figure 3B).
Five days after germination, the number of normally developed seedlings and albino seedlings was counted on mannitol stress media, with albino seedlings and ungerminated seeds recorded as dead. Under normal conditions, no significant difference in survival rates was observed between ZmEXPB7-overexpressing Arabidopsis thaliana and wild-type seedlings. However, in half MS supplemented with 250 mM and 300 mM mannitol, the survival rate of ZmEXPB7-overexpressing lines showed a statistically significant increase compared to wild-type (Figure 3C). Notably, under 300 mM mannitol stress, the survival count of ZmEXPB7-overexpressing seedlings was approximately two-fold higher than that of wild-type seedlings.
To further observe the phenotypic changes of ZmEXPB7-overexpressing Arabidopsis under mannitol stress, 5-day-old seedlings grown on normal medium were transferred to mannitol-containing media for 7 days. Analysis of total root length, root surface area, and root tip number revealed that ZmEXPB7-overexpressing Arabidopsis exhibited longer roots than wild-type under both normal and mannitol stress conditions. No significant differences in root surface area or tip number were observed on normal or 200 mM mannitol. However, under 250 mM and 300 mM mannitol stress, ZmEXPB7-overexpressing Arabidopsis showed significantly higher root surface area and tip numbers than wild-type seedlings, indicating enhanced resistance to high osmotic stress (Figure 4).
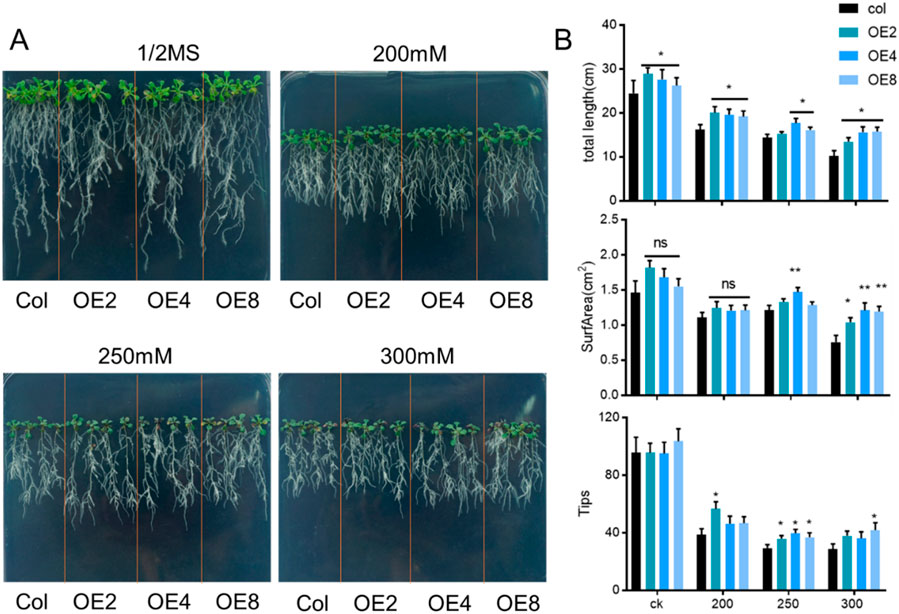
Figure 4. Phenotype of ZmEXPB7 overexpressed Arabidopsis under different mannitol treatment. (A) Phenotype of ZmEXPB7 overexpressed Arabidopsis under normal condition, 200 mM, 250 mM, 300 mM mannitol concentration; (B) Data analysis of total root length, root surf area, root tips under normal condition, 200 mM, 250 mM, 300 mM mannitol in ZmEXPB7 overexpressed Arabidopsis. Each experiment had three repeats, each time had at least 12 seedlings. All data represent means ± SD. Asterisks represented the significance compare to wild type by using one-way ANOVA, *, p < 0.05, **, p < 0.01, ***, p < 0.001.
To further confirmed the drought resistance of ZmEXPB7-overexpressing Arabidopsis, overexpressing and wild-type seedlings were grown in soil for 4 weeks before water was withheld. Phenotypes were observed until wilting occurred, and survival rates were recorded 3 days after rehydration. After 4 weeks of growth, ZmEXPB7-overexpressing Arabidopsis exhibited larger rosettes and more leaves than wild-type, with significantly higher aerial fresh weight (Figures 5A,B). At 7 days of drought stress, wild-type leaves showed wilting earlier than ZmEXPB7-overexpressing Arabidopsis. After 3 days of rehydration, wild-type survival was only 20%, while ZmEXPB7-overexpressing lines (except OE2) showed significantly higher survival rates (Figures 5C,D). Together, these results demonstrate that ZmEXPB7 overexpression enhances drought tolerance under both osmotic and soil drought stress.
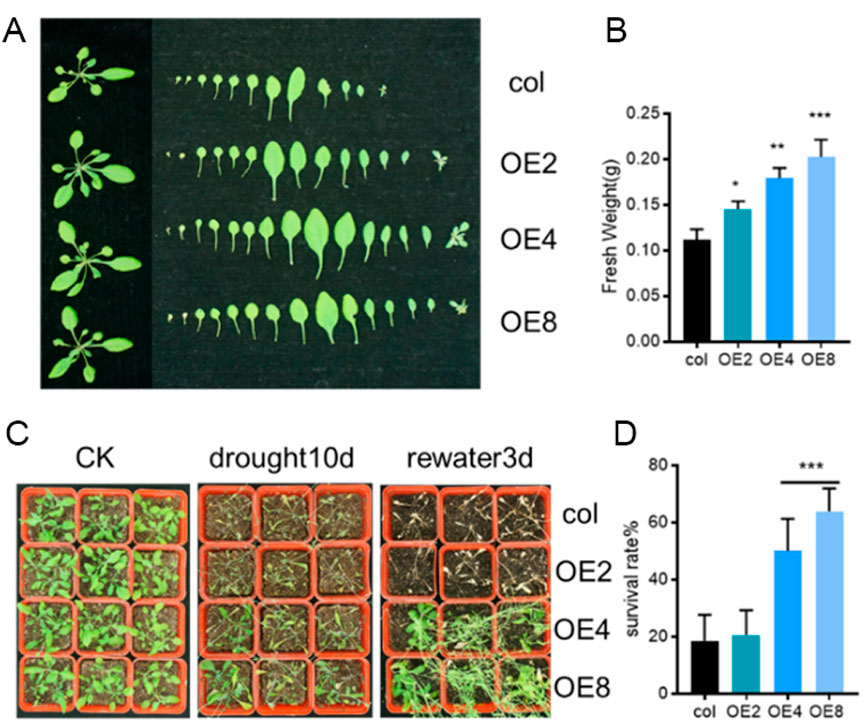
Figure 5. Decrease of stomatal number and aperture contributed to the tolerant of ZmEXPB7 overexpressed Arabidopsis. (A,B) Phenotype and fresh weight of wild-type and ZmEXPB7-overexpressing Arabidopsis lines under normal growth conditions. Data represent mean ± SE (n = 12). (C,D) Phenotype and survival rate of wild-type and ZmEXPB7-overexpressing lines after drought stress treatment. Drought stress was applied by withholding water for 7 days followed by rewatering for 3 days. Survival rates were calculated as the percentage of seedlings recovering after rewatering. Data represent mean ± SE (n = 3). Statistical significance was determined by one-way ANOVA; asterisks indicate significant differences compared with WT (*, p < 0.05, **, p < 0.01, ***, p < 0.001).
3.4 ZmEXPB7 decreased the water loss rate by reducing the stomatal density and aperture
To elucidate the relationship between drought resistance and water loss in ZmEXPB7-overexpressing Arabidopsis, detached leaves from 4-week-old seedlings were used for water loss rate measurement. Wild-type leaves showed higher water loss rates at all time points than ZmEXPB7-overexpressing leaves (Figure 6A). Analysis of stomatal density and aperture in the middle of the fourth leaf revealed that ZmEXPB7-overexpressing Arabidopsis had lower stomatal density and significantly smaller stomatal aperture after 1 h of detachment than wild-type (Figures 6B–D), suggesting that reduced stomatal density and aperture slowed water loss, contributing to drought tolerance.
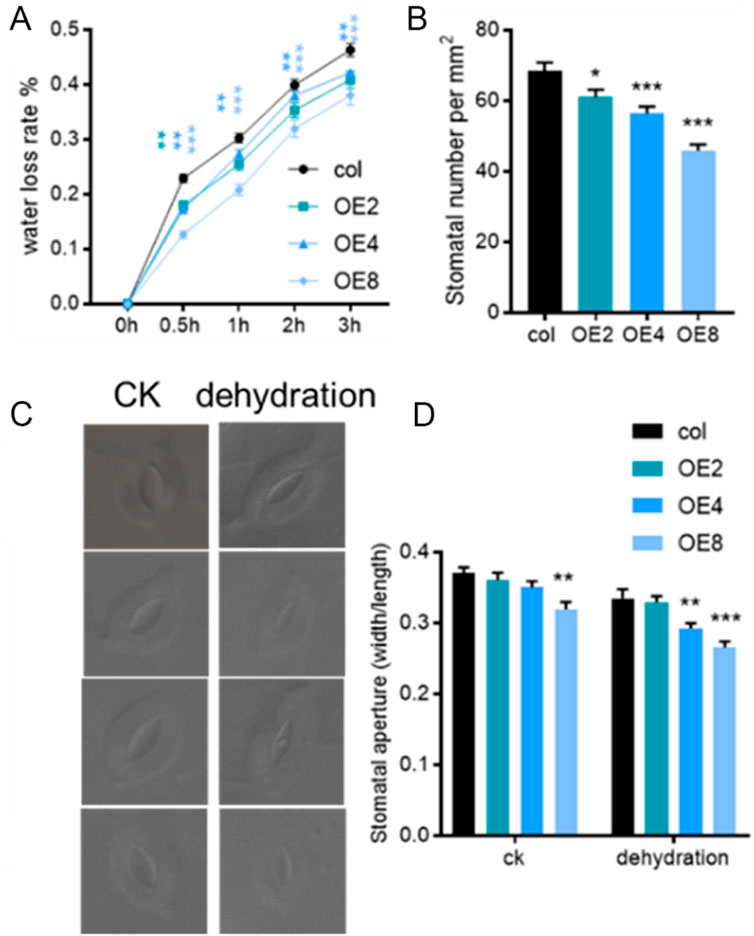
Figure 6. Water loss rate, stomatal number and aperture of leaves in ZmEXPB7 transgenic Arabidopsis. (A) Water loss rate at different time points. (B) Stomatal number of fourth leaf medium. (C,D) Stomatal aperture of leaves after detaching 1 h. Asterisks represented the significance compare to wild type by using one-way ANOVA, *, p < 0.05, **, p < 0.01, ***, p < 0.001.
3.5 Overexpression of ZmEXPB7 improved ROS accumulation in Arabidopsis
Drought stress often accompanies reactive oxygen species (ROS) production. NBT and DAB staining were used to detect H2O2 levels in ZmEXPB7-overexpressing and wild-type leaves, with darker staining indicating higher H2O2 accumulation. NBT and DAB staining was darker in ZmEXPB7-overexpressing than wild-type leaves (Figures 7A,B), with more pronounced differences under drought stress. KI-based H2O2 quantification confirmed that ZmEXPB7-overexpressing Arabidopsis accumulated more H2O2 than wild-type under both normal and drought conditions (Figure 7C). Concurrently, antioxidant enzyme (SOD, POD, CAT) activities were lower in ZmEXPB7-overexpressing lines, potentially explaining their higher H2O2 levels (Figures 7D–F). H2O2 may act as a signaling molecule to induce stomatal closure.
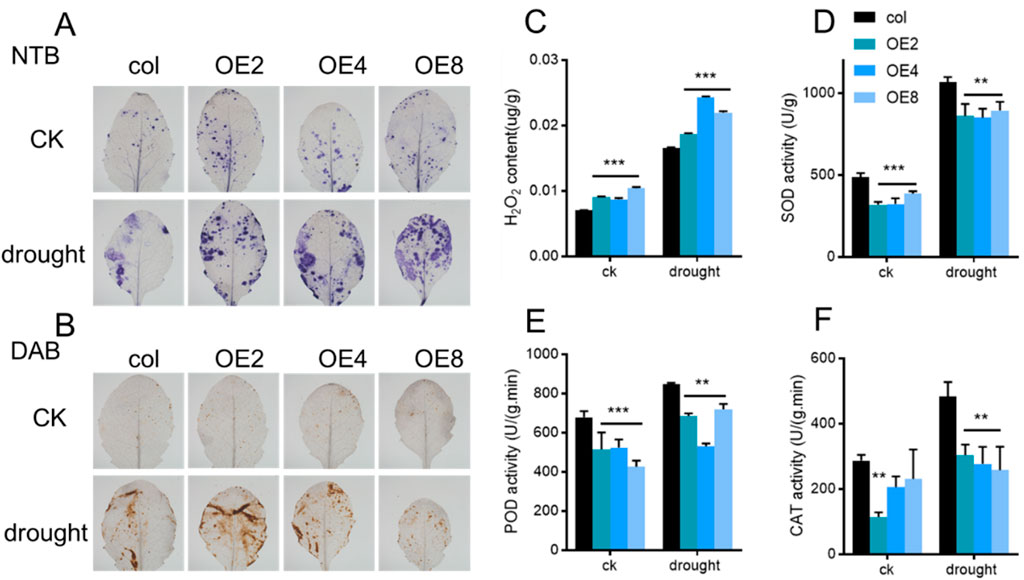
Figure 7. Staining ROS and determination antioxidant enzyme of leaf in ZmEXPB7 overexpressed Arabidopsis. (A,B) NBT and DAB staining of fourth leaf in ZmEXPB7 overexpressed Arabidopsis; (C) H2O2 content measurement in ZmEXPB7 overexpressed Arabidopsis leaves. (D–F) Enzyme activity of SOD, POD and CAT. Asterisks represented the significance compare to wild type by using one-way ANOVA, *, p < 0.05, **, p < 0.01, ***, p < 0.001.
4 Disccusion
Expansin is cell wall-loosening proteins widely involved in cell wall modification and play critical roles in plant growth and development, including vegetative growth (Wang et al., 2011), root elongation (Guo et al., 2011; Lin et al., 2011),flower development (Cosgrove, 2000b),fruit ripening and softening (Shi et al., 2021), and seed yield enhancement (Bae et al., 2014). Heterologous expression of Cunninghamia lanceolata ClEXPA1 and ClEXPA2 in tobacco increases plant height, stem thickness, leaf number, carpel thickness, pith parenchyma cell size, and xylem cell wall thickness, directly or indirectly affecting cellulose metabolism (Wang et al., 2011). OsEXPA10 is essential for root tip elongation (Che et al., 2016),while soybean GmEXPB2 increases the size and number of cortex cells in root meristem and elongation zones, enhances root hair density, modifies root architecture, and promotes nodule growth and development (Li et al., 2015). In this study, heterologous expression of ZmEXPB7 in Arabidopsis significantly increased total root length (Figure 4), suggesting that ZmEXPB7 plays a critical role in promoting root development. The altered root architecture may enhance drought tolerance through two potential mechanisms: (1) well-developed roots can penetrate deeper soil layers, thereby improving water uptake efficiency; and (2) an expanded root surface area facilitates more efficient water exchange at the root-soil interface under drought conditions. These morphological adaptations collectively contribute to enhanced drought tolerance from a root system perspective.
Beyond their established roles in modulating plant growth and development, expansin proteins are increasingly recognized as key players in abiotic stress responses. Previous studies have demonstrated that certain expansins, such as TaEXPB23 and TaEXPA2, contribute to drought tolerance primarily through water retention capacity (Han et al., 2012; Yang et al., 2020). Similarly, overexpression of ZmEXPA4 and ZmEXPA5 in maize has been shown to mitigate drought-induced yield loss by shortening the anthesis-silking interval (Liu et al., 2021; Tao et al., 2023). In contrast, our study provides compelling evidence that ZmEXPB7 confers drought resistance via a novel dual mechanism encompassing both root remodeling and direct stomatal regulation. ZmEXPB7 expression was rapidly induced by PEG-simulated drought stress, peaking within 1 h of treatment (Figure 2), indicating its involvement in early drought signaling. Transgenic maize overexpressing ZmEXPB7 exhibited significantly enhanced survival under both osmotic and drought conditions (Figures 3C, 4, 5C), consistent with the general protective role of expansins. However, we further uncovered a previously unreported function of ZmEXPB7 in stomatal regulation. Overexpression lines showed reduced stomatal density and aperture (Figure 6), resulting in decreased water loss from detached leaves. While previously reported drought-tolerant expansins primarily confer stress resistance through a single mechanism centered on root system modulation, our study uniquely demonstrates that ZmEXPB7 exerts its drought-tolerance function via a dual regulatory pathway involving both root architecture improvement and stomatal regulation. This distinction marks a key innovation compared to existing research on expansin-mediated drought responses.
We propose that ZmEXPB7 may influence stomatal formation and kinetics by modulating the mechanical properties of guard cell walls, potentially through interactions with key transcriptional regulators of stomatal development, such as SPCH (SPEECHLESS) and MUTE (Xiang et al., 2021). These genes are central to the initiation and progression of stomatal lineage cells, and their expression or activity may be indirectly affected by expansin-mediated cell wall modifications. This hypothesized link between expansin action and stomatal developmental programs represents a significant conceptual advance, distinguishing ZmEXPB7 from other drought-responsive expansins that function primarily through root-based mechanisms. Thus, our work not only identifies ZmEXPB7 as a critical regulator in maize drought response but also provides new insights into the multifaceted mechanisms by which expansins coordinate stress resilience at both the root and leaf levels. Notably, under drought stress, ZmEXPB7-overexpressing plants exhibited unique redox characteristics: elevated H2O2 levels but reduced SOD, POD, and CAT antioxidant enzyme activities (Figure 7). This seemingly paradoxical phenomenon may reflect the dual role of ROS signaling, where H2O2 in this context may act more as a secondary messenger to activate stress response pathways, while the ZmEXPB7-ZmLBD33 module may finely regulate ROS homeostasis to balance stress responses and growth inhibition.
The LBD gene family is a plant-specific group of transcription factors involved in development and abiotic stress responses. AtLBD18 plays a crucial role as a transcriptional activator in promoting lateral root formation in Arabidopsis by directly regulating EXPANSIN genes. Studies demonstrate that AtLBD18 binds to the EXPANSIN14 (AtEXP14) promoter, activating its expression to facilitate lateral root emergence (Kim and Lee, 2013). Additionally, AtLBD18, along with its homolog AtASL20, upregulates EXPANSINA17 (AtEXP17) during auxin signaling, further enhancing lateral root development (Lee and Kim, 2013). These findings highlight LBD18’s central role in the gene regulatory network of lateral root formation, where it directly modulates EXPANSIN family members, including AtEXP14 and AtEXP17, to coordinate cell wall loosening and root primordia emergence (Lee et al., 2013). This study confirmed the interaction between ZmLBD33 and ZmEXPB7 through yeast two-hybrid and bimolecular fluorescence complementation assays (Figure 1), indicating their collaborative involvement in maize drought stress responses. However, the mechanistic details of their joint action in drought responses require further investigation. Moreover, ZmEXPB7 overexpression produced multiple beneficial phenotypes: promoting root development, optimizing stomatal behavior, and enhancing cell wall mechanical strength. The synergistic effects of these traits ultimately improved overall drought tolerance, demonstrating ZmEXPB7’s potential as a preferred target for drought-resistant maize breeding and providing important theoretical foundations for crop drought resistance improvement.
5 Conclusion
In conclusion, our study reveals that ZmEXPB7 enhances drought tolerance in Arabidopsis through dual mechanisms: improving root architecture for efficient water uptake and reducing stomatal density to minimize water loss. The interaction between ZmEXPB7 and ZmLBD33, along with elevated H2O2 levels and altered antioxidant enzyme activities, suggests a role for ROS signaling in stress adaptation. These findings highlight ZmEXPB7 as a key regulator of drought responses and a promising target for breeding drought-resistant maize varieties. Subsequent efforts are focused on validating these findings in maize through CRISPR-Cas9 gene editing and overexpression studies, which will further elucidate its agronomic potential.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
JX: Investigation, Methodology, Validation, Writing – original draft. XW: Investigation, Writing – original draft. XY: Methodology, Writing – original draft. CL: Validation, Writing – original draft. JH: Conceptualization, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The Natural Science Foundation of Sichuan Province, grant number 2023NSFSC1172; Chengdu University Research Startup Fund 2081925044.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2025.1688658/full#supplementary-material
References
Amnuaylojaroen, T., Chanvichit, P., Janta, R., and Surapipith, V. (2021). Projection of rice and maize productions in northern Thailand under climate change scenario RCP8.5. Agriculture 11 (1), 23. doi:10.3390/agriculture11010023
Bae, J. M., Kwak, M. S., Noh, S. A., Oh, M. J., Kim, Y. S., and Shin, J. S. (2014). Overexpression of sweetpotato expansin cDNA (IbEXP1) increases seed yield in Arabidopsis. Transgenic Res. 23 (4), 657–667. doi:10.1007/s11248-014-9804-1
Che, J., Yamaji, N., Shen, R. F., and Ma, J. F. (2016). An Al-inducible expansin gene, OsEXPA10 is involved in root cell elongation of rice. Plant J. 88 (1), 132–142. doi:10.1111/tpj.13237
Cosgrove, D. J. (2000a). Loosening of plant cell walls by expansins. Nature 407 (6802), 321–326. doi:10.1038/35030000
Cosgrove, D. J. (2000b). New genes and new biological roles for expansins. Curr. Opin. Plant Biol. 3 (1), 73–78. doi:10.1016/s1369-5266(99)00039-4
Faize, M., Burgos, L., Faize, L., Piqueras, A., Nicolas, E., Barba-Espin, G., et al. (2011). Involvement of cytosolic ascorbate peroxidase and Cu/Zn-superoxide dismutase for improved tolerance against drought stress. J. Exp. Bot. 62 (8), 2599–2613. doi:10.1093/jxb/erq432
FAO (2023). The state of food security and nutrition in the world 2023. Food Agric. Organ. U. N. Available online at: https://www.fao.org/statistics/zh/.
Guo, W., Zhao, J., Li, X., Qin, L., Yan, X., and Liao, H. (2011). A soybean β-expansin gene GmEXPB2 intrinsically involved in root system architecture responses to abiotic stresses. Plant J. 66 (3), 541–552. doi:10.1111/j.1365-313X.2011.04511.x
Guo, J., Qu, L., Hu, Y., Lu, W., and Lu, D. (2021). Proteomics reveals the effects of drought stress on the kernel development and starch formation of waxy maize. BMC Plant Biol. 21 (1), 434. doi:10.1186/s12870-021-03214-z
Han, Y. y., Li, A., Li, F., Zhao, M., and Wang, W. (2012). Characterization of a wheat (Triticum aestivum L.) expansin gene, TaEXPB23, involved in the abiotic stress response and phytohormone regulation. Plant Physiology Biochem. 54, 49–58. doi:10.1016/j.plaphy.2012.02.007
Jiang, Z., Li, F., and Zhao, M. (2018). Analysis of location and expression pattern of maize expansin gene family. Curr. Biotechnol. 8 (1), 8. doi:10.19586/j.2095-2341.2017.0030
Kende, H., Bradford, K., Brummell, D., Cho, H. T., Cosgrove, D., Fleming, A., et al. (2004). Nomenclature for members of the expansin superfamily of genes and proteins. Plant Mol. Biol. 55 (3), 311–314. doi:10.1007/s11103-004-0158-6
Kim, J., and Lee, H. W. (2013). Direct activation of EXPANSIN14 by LBD18 in the gene regulatory network of lateral root formation in Arabidopsis. Plant Signal Behav. 8 (2), e22979. doi:10.4161/psb.22979
Kubilay, Y., and Zeki, K. (2017). Gene regulation network behind drought escape, avoidance and tolerance strategies in black poplar (Populus nigra L.). Plant Physiology and Biochem. Paris 115, 183–199. doi:10.1016/j.plaphy.2017.03.020
Kwak, J. M., Mori, I. C., Pei, Z. M., Leonhardt, N., Torres, M. A., Dangl, J. L., et al. (2003). NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. Embo J. 22 (11), 2623–2633. doi:10.1093/emboj/cdg277
Lee, H. W., and Kim, J. (2013). EXPANSINA17 up-regulated by LBD18/ASL20 promotes lateral root formation during the auxin response. Plant and Cell Physiology 54 (10), 1600–1611. doi:10.1093/pcp/pct105
Lee, H. W., Kim, M. J., Kim, N. Y., Lee, S. H., and Kim, J. (2013). LBD18 acts as a transcriptional activator that directly binds to the EXPANSIN14 promoter in promoting lateral root emergence of Arabidopsis. Plant J. 73 (2), 212–224. doi:10.1111/tpj.12013
Li, X., Zhao, J., Tan, Z., Zeng, R., and Liao, H. (2015). GmEXPB2, a cell wall β-expansin, affects soybean nodulation through modifying root architecture and promoting nodule formation and development. Plant Physiol. 169 (4), 2640–2653. doi:10.1104/pp.15.01029
Li, J., Li, Y., Yin, Z., Jiang, J., Zhang, M., Guo, X., et al. (2017). OsASR5 enhances drought tolerance through a stomatal closure pathway associated with ABA and H2 O2 signalling in rice. Plant Biotechnol. J. 15 (2), 183–196. doi:10.1111/pbi.12601
Lin, C., Choi, H.-S., and Cho, H.-T. (2011). Root hair-specific EXPANSIN A7 is required for root hair elongation in Arabidopsis. Mol. Cells 31 (4), 393–397. doi:10.1007/s10059-011-0046-2
Liu, B., Zhang, B., Yang, Z., Liu, Y., Yang, S., Shi, Y., et al. (2021). Manipulating ZmEXPA4 expression ameliorates the drought-induced prolonged anthesis and silking interval in maize. Plant Cell 33 (6), 2058–2071. doi:10.1093/plcell/koab083
Lobell, D. B., Hammer, G. L., McLean, G., Messina, C., Roberts, M. J., and Schlenker, W. (2013). The critical role of extreme heat for maize production in the United States. Nat. Clim. Change 3 (5), 497–501. doi:10.1038/nclimate1832
Ma, H., Liu, C., Li, Z., Ran, Q., Xie, G., Wang, B., et al. (2018). ZmbZIP4 contributes to stress resistance in maize by regulating ABA synthesis and root development. Plant Physiol. 178 (2), 753–770. doi:10.1104/pp.18.00436
Miller, G., Suzuki, N., Ciftci-Yilmaz, S., and Mittler, R. (2010). Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, cell and Environ. 33 (4), 453–467. doi:10.1111/j.1365-3040.2009.02041.x
Mura, J. D., and Vangimalla, R. R. (2020). Stomatal closure response to soil drying at different vapor pressure deficit conditions in maize. Plant Physiology Biochem. 154, 714–722. doi:10.1016/j.plaphy.2020.07.023
Pei, Z.-M., Murata, Y., Benning, G., Thomine, S., Klüsener, B., Allen, G. J., et al. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406 (6797), 731–734. doi:10.1038/35021067
Ramakrishna, P., Ruiz Duarte, P., Rance, G. A., Schubert, M., Vordermaier, V., Vu, L. D., et al. (2019). EXPANSIN A1-mediated radial swelling of pericycle cells positions anticlinal cell divisions during lateral root initiation. Proc. Natl. Acad. Sci. 116 (17), 8597–8602. doi:10.1073/pnas.1820882116
Sampedro, J., and Cosgrove, D. J. (2005). The expansin superfamily. Genome Biol. 6 (12), 242. doi:10.1186/gb-2005-6-12-242
Shi, Y., Vrebalov, J., Zheng, H., Xu, Y., Yin, X., Liu, W., et al. (2021). A tomato LATERAL ORGAN BOUNDARIES transcription factor, SlLOB1, predominantly regulates cell wall and softening components of ripening. Proc. Natl. Acad. Sci. U. S. A. 118 (33), e2102486118. doi:10.1073/pnas.2102486118
Su, G., Lin, Y., Wang, C., Lu, J., Liu, Z., He, Z., et al. (2024). Expansin SlExp1 and endoglucanase SlCel2 synergistically promote fruit softening and cell wall disassembly in tomato. Plant Cell 36 (3), 709–726. doi:10.1093/plcell/koad291
Sun, Q., Li, Y., Gong, D., Hu, A., Zhong, W., Zhao, H., et al. (2022). A NAC-EXPANSIN module enhances maize kernel size by controlling nucellus elimination. Nat. Commun. 13 (1), 5708. doi:10.1038/s41467-022-33513-4
Tan, J., Wang, M., Shi, Z., and Miao, X. (2018). OsEXPA10 mediates the balance between growth and resistance to biotic stress in rice. Plant Cell Rep. 37 (7), 993–1002. doi:10.1007/s00299-018-2284-7
Tao, K., Li, Y., Hu, Y., Li, Y., Zhang, D., Li, C., et al. (2023). Overexpression of ZmEXPA5 reduces anthesis-silking interval and increases grain yield under drought and well-watered conditions in maize. Mol. Breed. 43 (12), 84. doi:10.1007/s11032-023-01432-x
Tuzet, A., Perrier, A., and Leuning, R. (2010). A coupled model of stomatal conductance, photosynthesis and transpiration. Plant, Cell and Environ. 26 (7), 1097–1116. doi:10.1046/j.1365-3040.2003.01035.x
Vicentin, L., Canales, J., and Calderini, D. (2023). The effect of TaExpA6 and TaGW2 gene expression on grain weight and the trade-off with grain number in wheat. Plant Biology Europe 2023. Marseille, France. doi:10.13140/RG.2.2.18440.43525
Wan, W., Liu, Z., Li, J., Xu, J., Wu, H., and Xu, Z. (2022). Spatiotemporal patterns of maize drought stress and their effects on biomass in the Northeast and North China Plain from 2000 to 2019. Agric. For. Meteorology 315, 108821. doi:10.1016/j.agrformet.2022.108821
Wang, G., Gao, Y., Wang, J., Yang, L., Song, R., Li, X., et al. (2011). Overexpression of two cambium-abundant Chinese fir (Cunninghamia lanceolata) alpha-expansin genes ClEXPA1 and ClEXPA2 affect growth and development in transgenic tobacco and increase the amount of cellulose in stem cell walls. Plant Biotechnol. J. 9 (4), 486–502. doi:10.1111/j.1467-7652.2010.00569.x
Wang, B., Liu, C., Zhang, D., He, C., Zhang, J., and Li, Z. (2019). Effects of maize organ-specific drought stress response on yields from transcriptome analysis. BMC Plant Biol. 19 (1), 335. doi:10.1186/s12870-019-1941-5
Wieczorek, K., Golecki, B., Gerdes, L., Heinen, P., Szakasits, D., Durachko, D. M., et al. (2006). Expansins are involved in the formation of nematode-induced syncytia in roots of Arabidopsis thaliana. Plant J. 48 (1), 98–112. doi:10.1111/j.1365-313X.2006.02856.x
Xiang, Y., Sun, X., Bian, X., Wei, T., Han, T., Yan, J., et al. (2021). The transcription factor ZmNAC49 reduces stomatal density and improves drought tolerance in maize. J. Exp. Bot. 72 (4), 1399–1410. doi:10.1093/jxb/eraa507
Xing, X., Du, H., Yang, Z., Zhang, H., Shao, Z., Li, N., et al. (2025). GmEXPA11 facilitates nodule enlargement and nitrogen fixation via interaction with GmNOD20 under regulation of GmPTF1 in soybean. Plant Sci. 355, 112469. doi:10.1016/j.plantsci.2025.112469
Yang, L., Fountain, J. C., Ji, P., Ni, X., Chen, S., Lee, R. D., et al. (2018). Deciphering drought-induced metabolic responses and regulation in developing maize kernels. Plant Biotechnol. J. 16, 1616–1628. doi:10.1111/pbi.12899
Yang, J., Zhang, G., An, J., Li, Q., Chen, Y., Zhao, X., et al. (2020). Expansin gene TaEXPA2 positively regulates drought tolerance in transgenic wheat (Triticum aestivum L.). Plant Sci. 298, 110596. doi:10.1016/j.plantsci.2020.110596
Yang, Z., Gao, Z., Zhou, H., He, Y., Liu, Y., Lai, Y., et al. (2021). GmPTF1 modifies root architecture responses to phosphate starvation primarily through regulating GmEXPB2 expression in soybean. Plant J. 107 (2), 525–543. doi:10.1111/tpj.15307
Yanping, S. Z. (2025). Analysis and suggestions for defense countermeasures of summer drought in North China, Northwest China, Huang-Huai Region and other northern regions in 2024. China Flood and Drought Manag. 35 (4), 21–27. doi:10.16867/j.issn.1673-9264.2024426
Yennawar, N. H., Li, L. C., Dudzinski, D. M., Tabuchi, A., and Cosgrove, D. J. (2006). Crystal structure and activities of EXPB1 (Zea m 1), a beta-expansin and group-1 pollen allergen from maize. Proc. Natl. Acad. Sci. U. S. A. 103 (40), 14664–14671. doi:10.1073/pnas.0605979103
Zhang, X., Zhang, L., Dong, F., Gao, J., Galbraith, D. W., and Song, C. P. (2001). Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 126 (4), 1438–1448. doi:10.1104/pp.126.4.1438
Zhang, X., Wang, Y., Liu, M., Yan, P., Niu, F., Ma, F., et al. (2024). OsEXPA7 encoding an expansin affects grain size and quality traits in rice (oryza sativa L.). Rice (N Y) 17 (1), 36. doi:10.1186/s12284-024-00715-x
Keywords: ZmEXPB7, drought tolerance, stomatal regulation, reactive oxygen species (ROS), maize
Citation: Xiong J, Wang X, Ye X, Liu C and Han J (2025) ZmEXPB7, a β-expansin gene, contributes to drought tolerance in Arabidopsis. Front. Genet. 16:1688658. doi: 10.3389/fgene.2025.1688658
Received: 19 August 2025; Accepted: 11 September 2025;
Published: 26 September 2025.
Edited by:
Zhiqiang Wang, Chengdu agriculture college, ChinaReviewed by:
Zhengqiao Liao, Mianyang Normal University, ChinaYufei Wang, Shanghai Jiao Tong University, China
Copyright © 2025 Xiong, Wang, Ye, Liu and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Xiong, eGlvbmdqaW5nQGNkdS5lZHUuY24=; Jialiang Han, aGFuamlhbGlhbmdAY2R1LmVkdS5jbg==
 Jing Xiong
Jing Xiong Xianqiu Wang3
Xianqiu Wang3 Xueling Ye
Xueling Ye Changying Liu
Changying Liu